Spectral Separation of Multifluorescence Labels with the LSM 510 META
|
|
|
- Naomi Curtis
- 6 years ago
- Views:
Transcription
1 Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their multifarious environment. For conventional microscopical detection methods it can be hard even to separate the signals emitted by green and yellow fluorochromes without overlaps. Emission Fingerprinting with the LSM 510 META is a completely new technique for the detection, spectral separation and visualization of multiple fluorescent labels. The technique precisely separates widely overlapping emission spectra and reliably eliminates broad-band autofluorescences. The potential of the LSM 510 META is demonstrated by application of Emission Fingerprinting to a sample tagged with four fluorescent proteins (Cyan FP, Cyan/Green FP, Green FP and Yellow FP). The tropical rain forest displays many hues of green. -coded projections of spectral image stacks of CFP-, CGFP-, GFP- and YFP-labeled cells. Quadruple-labeled cell; wavelength-coded projection of the spectral image stack. Pseudocolor-coded, spectrally unmixed quadruple labeling. Everything simply green? Not at all!
2 The Problem Multiple fluorescence labeling is an established method for the simultaneous visualization of different structural or functional features in biomedical samples. The investigation of complex structures and physiological processes requires many different sample parameters to be detected. Therefore there is a great demand for microscopical systems that can simultaneously visualize as many different fluorescent dyes as possible. Inevitably, this means that one has to accept increasingly broader overlaps between emission spectra having typical widths between 50 and 100 nm. Even if a spectral band width of 350 to 700 nm is available for detection, crosstalk between the emission signals will occur as soon as more than three dyes are to be visualized at a time. In conventional detection systems, a spectral band is selected that detects the strongest possible signal of the desired dye and the weakest possible signal of the spectrally adjacent dyes. It is obvious that this compromise will fail under the requirements outlined above. The problem aggravates if the samples are to be labeled with fluorescent proteins (XFPs). This labeling method has the advantage that live cells or even entire organisms can express XFPs bound to proteins. Since fluorescent proteins are non-toxic, they allow physiological processes to be visualized in live cells directly (Miteli & Spector, 1997). On the other hand, the spectral variation of the fluorescent proteins available at present is narrower than that of classical fluorochromes. Because of the widely overlapping emission spectra of CFP, GFP and YFP, triple labelings with these XFPs cannot be visualized separately by conventional methods (Fig. 1). 100 [%] [nm] Fig. 1: Overlapping emission spectra of various fluorescent proteins (Dark blue: BFP, Light blue: CFP, Green: GFP, Yellow: YFP, Red: DS Red). Source: 2
3 The Solution The LSM 510 META solves the problem by an entirely new approach. It uses a method termed Emission Fingerprinting, by which spectrally separated images are obtained in three steps: 1. Acquisition Lambda-Stacks 2. Definition of reference spectra 3. Spectral separation of the raw data This article will describe in detail how the LSM 510 META precisely separates and visualizes the emission signals of dyes with widely or almost completely overlapping emission spectra. Fig. 2: LSM 510 META in combination with the Axiovert 200 M (side- and baseport configuration), Axioplan 2 Imaging MOT and Axioskop FS (with Non Descanned Detection; NDD) microscopes. Samples and Imaging Double labeling with GFP and FITC: Fixed NIH 3T3 cell culture with cover slip. Cell nuclei labeled with GFP-histone B2, actin filaments labeled with FITCphalloidine. Sample provided by courtesy of Mary Dickinson of the Caltech Institute, Pasadena, USA (see page 8). Quadruple labeling with CFP, CGFP, GFP and YFP: Fixed HE 393 cell culture with cover slip. The cells were transfected with four different fluorescent proteins labeling different cellular structures (CFP cytoplasm, CGFP nuclei, GFP cell membrane, YFP mitochondria). In addition to the quadruple-labeled samples, four single-labeled ones were available for acquiring the reference spectra. Sample provided by courtesy of A. Miyawaki of the Riken Institute, Wako, Japan (see page 10). Imaging: LSM 510 META UV/VIS confocal laser scanning microscope with spectral detector, release 3.0. Objective lens: 63x/1.4 Oil Plan-Apochromat. Excitation laser lines: 458 nm (70%) and 488 nm (4%). Main dichroic beamsplitter: NT 80/20. Thickness of optical section: 0.8 µm at 543 nm. 3
4 How Does Emission Fingerprinting Work? The Principle 1. Acquisition of Lambda-Stacks Emission Fingerprinting is a completely novel approach to analyzing multiple fluorescences. Rather than attempting to separate individual spectral bands, this technique detects and spectrally resolves the total fluorescent light emitted by the sample. For the subsequent spectral separation, the technique uses the profile of the spectrum rather than the intensity of a spectral band. This results in pseudocolor-coded multichannel fluorescence images, each of which contains the emission signal of a single channel, i.e. of a single dye. This also applies to fluorochromes having almost completely overlapping emission spectra. The entire process of Emission Fingerprinting consists of three steps: Lambda Stacks are the raw data of Emission Fingerprinting. As a rule, these are three-dimensional image stacks having the coordinates X, Y and lambda (λ). Each individual image of the stack represents a spectrally defined band of the emission signal, i.e. the information sensed by one element of the META detector (Fig. 3). The spectral bandwidth of a channel is 10.7 nm at minimum. For the investigation of three-dimensional objects or dynamic processes, the acquisition of Lambda Stacks can be combined with that of Z stacks and/or time series. A B λ X Y Fig. 3: Acquisition of a Lambda Stack; three-dimensional image stack with the dimensions X, Y and lambda. (A) Sample. (B) Lambda Stack. 4
5 2. Definition of Reference Spectra Reference spectra are the emission spectra of individual fluorochromes under sample conditions. These spectra are required for the spectral separation of multiple fluorescence labels. Reference spectra can be acquired by either of two methods, depending on the nature of the sample: If, in a Lambda Stack, regions can be defined which are labeled by a single dye only, the reference spectra of these dyes can be established directly from the Lambda Stack of the sample. This method is applicable especially if the sample has morphologically distinct structural elements with different tags (Fig. 4). A D C B Fig. 4: Acquisition of reference spectra from a sample containing spatially non-overlapping tags. (A) Sample. (B) Lambda Stack. (C) Lambda-coded projection with regions of interest (ROIs). (D) Spectral signatures of the dyes. 5
6 In samples not containing regions with single tags, reference spectra have to be obtained in another way. This is the case where the labeled structures are either very small or overlap. Also, various tags may be localized in the same subcellular compartment (e.g., proteins that contain a target sequence for the nucleus, marked with different fluorochromes). Under such conditions, reference spectra can be defined by the acquisition of Lambda Stacks of several samples, each of which has been labeled with a single dye. Each sample thus provides a single reference spectrum; all of them are filed in a spectral database (Fig. 5). Fig. 5: Acquisition of reference spectra from a sample containing spatially overlapping tags. (A) Sample and three reference samples. (B) Lambda Stacks. (C) Lambda-coded projections with regions of interest (ROIs). (D) Spectral signatures of the dyes. A B C D 6
7 S(λ) sum = Intensity S(λ) + Intensity S(λ) + dye A dye A dye B dye B Intensity dye C S(λ) dye C 3. Spectral Separation of the Raw Data For spectral separation, Lambda Stacks of the sample and the reference spectra of the dyes to be expected in the sample are required. The Linear Unmixing software function provided for spectral separation activates a linear algorithm (Landsfort et al., 2001), which computes, for each pixel, the intensities of the emission signal of the dyes used. Consider a pixel representing a sample point at which the three dyes A, B and C with spectra S(λ) dye A, B and C overlap, the cumulative spectrum measured there [S(λ) sum ] can be expressed as shown in the equation above. With the known reference spectra [S(λ) dye A, B and C ], the equation can be solved to find the intensities A, B and C. The result is presented by means of a pseudocolorcoded multichannel image, with each channel representing exactly one dye. The examples on the following pages show that the method is also applicable to dyes whose spectra overlap almost completely. Fig. 6 provides an overview of the Emission Fingerprinting procedure. Sample Acquisition of a Lambda-Stack Can ROIs with single dye labeling be defined in the Lambda Stack projection? Yes Extraction of reference spectra Lambda-Stack No Extraction of the reference spectrum Reference sample Acquisition of a Lambda-Stack Lambda-Stack Spectral database Linear Unmixing Reference sample Acquisition of a Lambda-Stack Extraction of the reference spectrum Lambda-Stack Multichannel image Reference sample Acquisition of a Lambda-Stack Lambda-Stack Extraction of the reference spectrum Fig. 6: Emission Fingerprinting procedure (schematic). 7
8 A Emission Fingerprinting in Practice Two examples illustrate how widely overlapping A comparison between the two channels demon- emission signals can be separated by means of the strates the precision of the method. Although the Linear Unmixing function. spectra of the two labels overlap almost completely, no crosstalk between the channels is observed. The SYTOX Green channel only shows nuclei, whereas GFP and FITC double labeling the FITC channel only shows actin filaments. Fig. 7 Nuclei of the cells were stained with SYTOX Green, D shows the enlarged superimposition image of the whereas the actin filaments were stained with FITC- SYTOX Green and FITC channels. coupled phalloidine (specimen: M. Dickinson, Biological Imaging Center, Caltech, Pasadena, USA). As B both tags mark clearly separate, morphologically distinct structures, it was possible to extract the spectra required for separation directly from the Lambda Stack of the double-labeled sample. In the first step, an eight-channel Lambda Stack was obtained ( nm, spectral bandwidth per channel 10.7 nm) (Fig. 7 A). In the lambda-coded projection (Fig. 7 B), the individual images of the Lambda Stack are color-coded according to their respective wave- C lengths, and projected in superimposed fashion. This presentation illustrates the problem of spectrally overlapping emission signals. With lambda coding alone, no statement is possible as to which dye is present in which part of the sample. Two regions of interest were selected, with only the cell nucleus selected in one (SYTOX Green labeling, ROI 1, red), and only the actin filaments in the other (FITC D labeling, ROI 2, green). The left part of the window shows the mean spectral distribution of the intensities of these ROIs. Either of the two spectra represents the fingerprint of a fluorescent label under sample conditions (the spectral location of the two maxima differs by only 7 nm!). The spectra thus obtained are then subjected to spectral separation. With the Linear Unmixing function selected, the software computes a two-channel image (Fig. 7 C), in which either channel contains only the pixels corresponding to the spectral fingerprint of ROI 1 or ROI 2, respectively. The colors used for the ROI frames and spectral curves have been used for coding the respective channels. 8 Fig. 7: Emission Fingerprinting of a sample labeled with SYTOX Green and FITC. (A) Lambda Stack. (B) Lambda-coded projection with ROIs and emission spectra. (C) Two-channel image resulting from Linear Unmixing. Below: Superimposition of the two channels. (D) Enlarged superimposition of the two channels. 9
9 CFP, CGFP, GFP and YFP quadruple labeling Whereas in the previous example reference spectra were obtained directly from the Lambda Stack, here is an example of the use of a spectral reference database for spectral separation. The sample is a HeLa cell culture in which four different cellular targets were labeled with the fluorescent proteins CFP (cytoplasm), CGFP (nucleus), GFP (cytoplasmic membrane), and YFP (mitochondria) (specimen: A. Miyawaki, Riken, Japan). Given the high degree of spatial overlap between the tags, the Linear Unmixing procedure required the acquisition of reference spectra from samples labeled with a single dye each. A Lambda Stack was acquired from each sample (spectral range nm, 16 channels of 10.7 nm bandwidth each) (Fig. 8 A). The lambdacoded projections of these stacks (Fig. 8 B) are suitable for defining ROIs from which the reference spectra can be collected (Fig. 8 B,C). With the Save to dye database dialog, the spectra can now be filed in a spectral reference database (Fig. 8 D) where they are available for Linear Unmixing later. In the next step, a Lambda Stack of the quadruplelabeled sample is acquired (Fig. 8 E,F). Irrespective of the presentation mode selected - Gallery (Fig. 8 E) or Lambda coded (Fig. 8 F) - no information about which cellular structures are labeled with which dyes can be extracted here. Even with maximum flexibility A B Fig. 8: Emission Fingerprinting of a sample labeled with CFP, CGFP, GFP and YFP. (A) Lambda Stacks of the single-labeled reference samples. (B) Lambda-coded projections with ROIs and emission spectra. (C) Enlarged presentation of the GFP emission spectrum from (B) with Save to dye database function for storing the spectrum in the reference database. C 10
10 in the definition of bandpass filters, the widely overlapping emissions would not allow any gain in information. To optimize the subsequent Linear Unmixing, the spectral distribution of the background signals was also determined and saved it to the database as an additional reference spectrum. The Lambda Stack of the quadruple-labeled sample is spectrally separated by means of the Linear Unmixing dialog in the Process menu. Selection of the Lambda Stack to be separated (Fig. 8 D, small window at top left) is followed by loading the previously established reference spectra and the background spectrum from the database. The presentation of the overlapping spectra again illustrates the impossibility of separating the emission E signals with bandpass filters. The Linear Unmixing algorithm is started with Apply (Fig. 8 D). The result of Linear Unmixing is a multichannel image in which each channel exactly represents one tag (Fig. 8 G). The first four channels show the emission signals of CFP, CGFP, GFP and YFP (channel arrangement: top row left to right, then bottom row). Channel 5 is the background signal. Channel 6 (shown enlarged in Fig. 8 H) is a superimposition of channels 1 through 5. The various channels are distinguished by freely selected pseudocolors. Only in this way is it possible to distinguish between the differently labeled structures; cytoplasm, nuclei, membrane structures and mitochondria can now be identified. In the channel superimposition, additional background correction was performed by the deactivation of channel 5. F (D) Spectral reference database with the selected spectra of CFP, CGFP, GFP, YFP and background (black). (E) Lambda Stack of the quadruple-labeled sample. (F) Lambda-coded projection of the quadruple-labeled sample. (G) 5-channel image after spectral separation (Linear Unmixing), inclusive of background channel. (H) Pseudocolored superimposition of the various channels: Cytoplasm blue, nuclei green, cell membrane yellow, mitochondria red, deactivated background black. D G H 11
11 Outlook The LSM 510 META opens up a new dimension in confocal fluorescence microscopy. Experimental designs such as double labeling with Sytox Green and GFP, whose signal peaks could not be detected separately so far, will become routine. As the example has shown, real quadruple-fluorescence-labeled samples can be unmixed without any problems. The LSM 510 META can unmix the spectral signatures of up to eight fluorochromes. Looking ahead, we can already envisage samples that will exhaust the capabilities of the system and thus stimulate the development of yet another generation of microscopes. REFERENCES Miteli, T., Spector, D.L. (1997) Applications of green fluorescent protein in cell biology and biotechnology. Nature Biotechnol. 15, 961. Sawano, A., Miyawaki A. (2000) Directed evolution of green fluorescent protein by a new versatile PCR strategy for site directed and semi-random mutagenesis. Nucleic Acids Res Aug 15, 28 (16), E 78. Landsford, R., Bearman, G. and Fraser, S.E. (2001) Resolution of multiple green fluorescent protein color variants and dyes using two photon microscopy. Journal of Biomedical Optics 6, For further details, please contact: Dickinson, M.E., Bearman, G., Tille, S., Landsford and Fraser, S.E. (2001) Multi-spectral imaging and Linear Unmixing add a whole new dimension to Laser scanning fluorescence microscopy Bio Techniques 31/6, Carl Zeiss Advanced Imaging Microscopy Jena GERMANY Phone: Telefax: micro@zeiss.de Subject to change. Printed on environment-friendly paper, bleached without the use of chlorine e/10.02
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Microscopy from Carl Zeiss
 Microscopy from Carl Zeiss LSM 710 In Tune with Your Application Enjoy new freedom in selecting fluorescent dyes with In Tune, the new laser system for the LSM 710. Whatever the wavelength, you can match
Microscopy from Carl Zeiss LSM 710 In Tune with Your Application Enjoy new freedom in selecting fluorescent dyes with In Tune, the new laser system for the LSM 710. Whatever the wavelength, you can match
Widefield Microscopy Bleed-Through
 In widefield microscopy the excitation wavelengths which illuminate the sample, and the emission wavelengths which reach the CCD camera are selected throughout a filter cube. A filter cube consists of
In widefield microscopy the excitation wavelengths which illuminate the sample, and the emission wavelengths which reach the CCD camera are selected throughout a filter cube. A filter cube consists of
The new LSM 700 from Carl Zeiss
 The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Multiphoton excitation spectra in biological samples
 Journal of Biomedical Optics 8(3), 329 338 (July 2003) Multiphoton excitation spectra in biological samples Mary E. Dickinson California Institute of Technology Beckman Institute Biological Imaging Center
Journal of Biomedical Optics 8(3), 329 338 (July 2003) Multiphoton excitation spectra in biological samples Mary E. Dickinson California Institute of Technology Beckman Institute Biological Imaging Center
Microscopy from Carl Zeiss. DirectFRAP. News from the Cell. The New Class of Laser Manipulation for the Analysis of Cell Dynamics
 Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
The LSM 5 Family Laser Scanning Microscopes Localizing, Imaging and Analyzing Biomolecules
 Microscopy from Carl Zeiss The LSM 5 Family Laser Scanning Microscopes Localizing, Imaging and Analyzing Biomolecules The LSM 5 Family The fifth generation of Carl Zeiss Laser Scanning Microscopes offer
Microscopy from Carl Zeiss The LSM 5 Family Laser Scanning Microscopes Localizing, Imaging and Analyzing Biomolecules The LSM 5 Family The fifth generation of Carl Zeiss Laser Scanning Microscopes offer
Nodes of regulation in cellular systems
 Nodes of regulation in cellular systems cell membrane signal transduction ligands receptors oligomerization transport signal transduction modified protein Golgi transcription factor transport ER transport
Nodes of regulation in cellular systems cell membrane signal transduction ligands receptors oligomerization transport signal transduction modified protein Golgi transcription factor transport ER transport
Special Techniques 1. Mark Scott FILM Facility
 Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Spectral imaging fluorescence microscopy
 Oxford, GTC Genes 1356-9597 September 79 Blackwell to UK Cells Science, 2002 Ltd Ltd COMMENTARY Spectral imaging fluorescence microscopy T Haraguchi imaging et al. fluorescence microscopy Tokuko Haraguchi
Oxford, GTC Genes 1356-9597 September 79 Blackwell to UK Cells Science, 2002 Ltd Ltd COMMENTARY Spectral imaging fluorescence microscopy T Haraguchi imaging et al. fluorescence microscopy Tokuko Haraguchi
SUPPLEMENTARY FIGURES
 SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING. Marc Verhaegen
 HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
Contents. SCHOOL of FLUORESCENCE. For more information, go to lifetechnologies.com/imagingbasics
 MPSF educator packet This packet contains illustrations and figures from the Molecular Probes School of Fluorescence website. They illustrate concepts from the basic physical properties that underlie fluorescence
MPSF educator packet This packet contains illustrations and figures from the Molecular Probes School of Fluorescence website. They illustrate concepts from the basic physical properties that underlie fluorescence
MICROSCOPY. "micro" (small) "scopeo" (to watch)
 MICROSCOPY "micro" (small) "scopeo" (to watch) THE RELATIVE SIZES OF MOLECULES, CELLS AND ORGANISMS THE RELATIVE SIZES OF MOLECULES, CELLS AND ORGANISMS MICROSCOPY 1590 2012 MICROSCOPY THE LIGHT Light:
MICROSCOPY "micro" (small) "scopeo" (to watch) THE RELATIVE SIZES OF MOLECULES, CELLS AND ORGANISMS THE RELATIVE SIZES OF MOLECULES, CELLS AND ORGANISMS MICROSCOPY 1590 2012 MICROSCOPY THE LIGHT Light:
Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers
 Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Confocal Microscopy & Imaging Technology. Yan Wu
 Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
How to use the SP5 confocal microscope
 How to use the SP5 confocal microscope Mailfert Sébastien (mailfert@ciml.univ-mrs.fr) Tel : 9126 Imaging Immunity (ImagImm) photonic microscopy facility Centre d Immunologie de Marseille-Luminy 2016 /
How to use the SP5 confocal microscope Mailfert Sébastien (mailfert@ciml.univ-mrs.fr) Tel : 9126 Imaging Immunity (ImagImm) photonic microscopy facility Centre d Immunologie de Marseille-Luminy 2016 /
Confocal Microscopy of Electronic Devices. James Saczuk. Consumer Optical Electronics EE594 02/22/2000
 Confocal Microscopy of Electronic Devices James Saczuk Consumer Optical Electronics EE594 02/22/2000 Introduction! Review of confocal principles! Why is CM used to examine electronics?! Several methods
Confocal Microscopy of Electronic Devices James Saczuk Consumer Optical Electronics EE594 02/22/2000 Introduction! Review of confocal principles! Why is CM used to examine electronics?! Several methods
EDUCATION EXPERIMENT. Fluorescence of an Unknown Fluorophore. Introduction.
 EDUCATION EXPERIMENT Fluorescence of an Unknown Fluorophore Introduction www.oceanoptics.com info@oceanoptics.com Fluorescent tags have become a powerful tool in research and diagnostics, capable of revealing
EDUCATION EXPERIMENT Fluorescence of an Unknown Fluorophore Introduction www.oceanoptics.com info@oceanoptics.com Fluorescent tags have become a powerful tool in research and diagnostics, capable of revealing
Innovations To Meet Your Needs
 Innovations To Meet Your Needs Cooled CCD Camera 1340 x 1037 pixel resolution for greatest image quality 12-bit precision provides 3 orders of linear dynamic range Windows and Power Macintosh Software
Innovations To Meet Your Needs Cooled CCD Camera 1340 x 1037 pixel resolution for greatest image quality 12-bit precision provides 3 orders of linear dynamic range Windows and Power Macintosh Software
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
The analysis of fluorescence microscopy images for FRET detection
 The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
D e c N o. 2 8
 D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng
 Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Spectral imaging and its use in the measurement of Förster resonance energy transfer in living cells
 Laboratory Techniques in Biochemistry and Molecular Biology, Volume 33 FRET and FLIM Techniques T. W. J. Gadella (Editor) CHAPTER 8 Spectral imaging and its use in the measurement of Förster resonance
Laboratory Techniques in Biochemistry and Molecular Biology, Volume 33 FRET and FLIM Techniques T. W. J. Gadella (Editor) CHAPTER 8 Spectral imaging and its use in the measurement of Förster resonance
Chapter 4. the biological community to assay for protein-protein interactions. FRET describes the
 31 Chapter 4 Determination of nachr stoichiometry using Normalized Försters Resonance Energy Transfer (NFRET) Försters resonance energy transfer (FRET) has become a technique widely used in the biological
31 Chapter 4 Determination of nachr stoichiometry using Normalized Försters Resonance Energy Transfer (NFRET) Försters resonance energy transfer (FRET) has become a technique widely used in the biological
Partha Roy
 Fluorescence microscopy http://micro.magnet.fsu.edu/primer/index.html Partha Roy 1 Lecture Outline Definition of fluorescence Common fluorescent reagents Construction ti of a fluorescence microscope Optical
Fluorescence microscopy http://micro.magnet.fsu.edu/primer/index.html Partha Roy 1 Lecture Outline Definition of fluorescence Common fluorescent reagents Construction ti of a fluorescence microscope Optical
Supporting Information for. Electrical control of Förster energy transfer.
 1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
Compensation: Fundamental Principles
 Flow Cytometry Seminar Series 2017 : Fundamental Principles Spillover correction in multicolor flow cytometry 28.02.2017 http://www.cytometry.uzh.ch Contents Fluorescence and its detection Absorption and
Flow Cytometry Seminar Series 2017 : Fundamental Principles Spillover correction in multicolor flow cytometry 28.02.2017 http://www.cytometry.uzh.ch Contents Fluorescence and its detection Absorption and
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Imaging facilities at WUR
 Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
11/19/2013. Janine Zankl FACS Core Facility 13. November Cellular Parameters. Cellular Parameters. Monocytes. Granulocytes.
 DEPARTEMENT BIOZENTRUM Janine Zankl FACS Core Facility 13. November 2013 Cellular Parameters Granulocytes Monocytes Basophils Neutrophils Lymphocytes Eosinophils Cellular Parameters 1 What Is Flow Cytometry?
DEPARTEMENT BIOZENTRUM Janine Zankl FACS Core Facility 13. November 2013 Cellular Parameters Granulocytes Monocytes Basophils Neutrophils Lymphocytes Eosinophils Cellular Parameters 1 What Is Flow Cytometry?
Super Resolution Imaging Solution Provider. Imaging Future
 Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
BASICS OF FLOW CYTOMETRY
 BASICS OF FLOW CYTOMETRY AUTHOR: Ana Isabel Vieira APPROVAL: Henrique Veiga Fernandes Ana Sílvia Gonçalves SOP.UCF.002 03-09-2015 Pag. 1/9 Overview Flow: Fluid Cyto: Cell Metry: Measurement Flow cytometry
BASICS OF FLOW CYTOMETRY AUTHOR: Ana Isabel Vieira APPROVAL: Henrique Veiga Fernandes Ana Sílvia Gonçalves SOP.UCF.002 03-09-2015 Pag. 1/9 Overview Flow: Fluid Cyto: Cell Metry: Measurement Flow cytometry
Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone
 Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone Sven Terclavers LSM780 System overview The Scan Module - Core of the LSM 780 1 V/tunable PTC laser ports (405/440, cw/ps;
Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone Sven Terclavers LSM780 System overview The Scan Module - Core of the LSM 780 1 V/tunable PTC laser ports (405/440, cw/ps;
Sapphire. Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING
 Sapphire Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING Breakthrough image capture and analysis The Sapphire Biomolecular Imager is a next generation laser scanning system that provides
Sapphire Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING Breakthrough image capture and analysis The Sapphire Biomolecular Imager is a next generation laser scanning system that provides
More on fluorescence
 More on fluorescence Last class Fluorescence Absorption emission Jablonski diagrams This class More on fluorescence Common fluorophores Jablonski diagrams to spectra Properties of fluorophores Excitation
More on fluorescence Last class Fluorescence Absorption emission Jablonski diagrams This class More on fluorescence Common fluorophores Jablonski diagrams to spectra Properties of fluorophores Excitation
Lambda Square Mapping and FLIM
 Lambda Square Mapping and FLIM Explore Photonic Landscapes with the Leica TCS SP5 X A 620 B C Excitation/nm 470 485 Emission/nm 695 Full spectral analysis of images and lifetime measurements User guidance
Lambda Square Mapping and FLIM Explore Photonic Landscapes with the Leica TCS SP5 X A 620 B C Excitation/nm 470 485 Emission/nm 695 Full spectral analysis of images and lifetime measurements User guidance
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
3D Transfection System
 3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
Fluorescence Microscopy: A Biological Perspective
 Fluorescence Microscopy: A Biological Perspective From nanometre to metre: the scale of life Instrumentation and accessible scale limits the questions that can be addressed in biology Why are there limits?
Fluorescence Microscopy: A Biological Perspective From nanometre to metre: the scale of life Instrumentation and accessible scale limits the questions that can be addressed in biology Why are there limits?
Fluorescence Microscopy
 Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Localization Microscopy
 Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Hong et al.,
 Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
F* techniques: FRAP, FLIP, FRET, FLIM,
 F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
Confocal Microscopes. Evolution of Imaging
 Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
The most extensively used technique for tissue analysis is light microscopy.
 Fluorescence Theory Quantum yield Wavelength shift Ligand interactions Membrane interactions Using quenchning effects Fluorescence in-vivo Localization Distance measurements FRET The most extensively used
Fluorescence Theory Quantum yield Wavelength shift Ligand interactions Membrane interactions Using quenchning effects Fluorescence in-vivo Localization Distance measurements FRET The most extensively used
Real-Time Multiplex Kinase Phosphorylation Sensors in Living Cells
 Real-Time Multiplex Kinase Phosphorylation Sensors in Living Cells Nur P. Damayanti 1,2,5, Kevin Buno 3, Yi Cui 1,2,6, Sherry L. Voytik-Harbin 2-4, Roberto Pili 5, Jennifer Freeman 7 Joseph M. K. Irudayaraj
Real-Time Multiplex Kinase Phosphorylation Sensors in Living Cells Nur P. Damayanti 1,2,5, Kevin Buno 3, Yi Cui 1,2,6, Sherry L. Voytik-Harbin 2-4, Roberto Pili 5, Jennifer Freeman 7 Joseph M. K. Irudayaraj
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Two-Photon Microscopy for Deep Tissue Imaging of Living Specimens
 for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
INTRODUCTION TO FLOW CYTOMETRY
 DEPARTEMENT BIOZENTRUM INTRODUCTION TO FLOW CYTOMETRY F ACS C ore F acility Janine Zankl FACS Core Facility 3. Dezember 2015, 4pm Cellular Parameters Granulocytes Monocytes Basophils Lymphocytes Neutrophils
DEPARTEMENT BIOZENTRUM INTRODUCTION TO FLOW CYTOMETRY F ACS C ore F acility Janine Zankl FACS Core Facility 3. Dezember 2015, 4pm Cellular Parameters Granulocytes Monocytes Basophils Lymphocytes Neutrophils
Single cell molecular profiling using Quantum Dots. Technical Journal Club Rahel Gerosa
 Single cell molecular profiling using Quantum Dots Technical Journal Club 01.10.2013 Rahel Gerosa Molecular Profiling Powerful technique to study complex molecular networks underlying physiological and
Single cell molecular profiling using Quantum Dots Technical Journal Club 01.10.2013 Rahel Gerosa Molecular Profiling Powerful technique to study complex molecular networks underlying physiological and
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Dino-Lite knowledge & education. Fluorescence Microscopes
 Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
[A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal:
![[A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal: [A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal:](/thumbs/93/114465749.jpg) [A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal: Broadcast An entire city should be of a sufficiently
[A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal: Broadcast An entire city should be of a sufficiently
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
Selecting Reagents for Multicolor Flow Cytometry
 HotLines Platinum Edition f a l l 0 0 6 Selecting Reagents for Multicolor Flow Cytometry By Holden Maecker and Joe Trotter The availability of flow cytometers capable of detecting 6, 8, and more colors
HotLines Platinum Edition f a l l 0 0 6 Selecting Reagents for Multicolor Flow Cytometry By Holden Maecker and Joe Trotter The availability of flow cytometers capable of detecting 6, 8, and more colors
ab CytoPainter ER Staining Kit Red Fluorescence
 ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
Each question may have MULTIPLE correct answers. Select all that are correct.
 Knowledge Assessment Flow Cytometry Workshop, Part 1 April 20, 2015 Each question may have MULTIPLE correct answers. Select all that are correct. 1. Tandem dyes are a. highly stable fluorophores after
Knowledge Assessment Flow Cytometry Workshop, Part 1 April 20, 2015 Each question may have MULTIPLE correct answers. Select all that are correct. 1. Tandem dyes are a. highly stable fluorophores after
Fluorescence Microscopy
 Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Sep No.20 CONFOCAL APPLICATION LETTER. resolution. Emission. Wavelength LAS AF APPLICATION WIZARD FRET SENSITIZED EMISSION
 CONFOCAL APPLICATION LETTER Sep. 2006 No.20 resolution Green Channel Red Channel Emission Wavelength LAS AF APPLICATION WIZARD FRET SENSITIZED EMISSION FRET with Leica TCS SP5 (LAS AF) S1 Donor S1 Acceptor
CONFOCAL APPLICATION LETTER Sep. 2006 No.20 resolution Green Channel Red Channel Emission Wavelength LAS AF APPLICATION WIZARD FRET SENSITIZED EMISSION FRET with Leica TCS SP5 (LAS AF) S1 Donor S1 Acceptor
Design for Manufacturability (DFM) in the Life Sciences
 T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
204 Part 3.3 SUMMARY INTRODUCTION
 204 Part 3.3 Chapter # METHODOLOGY FOR BUILDING OF COMPLEX WORKFLOWS WITH PROSTAK PACKAGE AND ISIMBIOS Matveeva A. *, Kozlov K., Samsonova M. Department of Computational Biology, Center for Advanced Studies,
204 Part 3.3 Chapter # METHODOLOGY FOR BUILDING OF COMPLEX WORKFLOWS WITH PROSTAK PACKAGE AND ISIMBIOS Matveeva A. *, Kozlov K., Samsonova M. Department of Computational Biology, Center for Advanced Studies,
Challenges to measuring intracellular Ca 2+ Calmodulin: nature s Ca 2+ sensor
 Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
CytoPainter Golgi Staining Kit Green Fluorescence
 ab139483 CytoPainter Golgi Staining Kit Green Fluorescence Instructions for Use Designed for the detection of Golgi bodies by microscopy This product is for research use only and is not intended for diagnostic
ab139483 CytoPainter Golgi Staining Kit Green Fluorescence Instructions for Use Designed for the detection of Golgi bodies by microscopy This product is for research use only and is not intended for diagnostic
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility
 Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Live cell microscopy
 Live cell microscopy 1. Why do live cell microscopy? 2. Maintaining living cells on a microscope stage. 3. Considerations for imaging living cells. 4. Fluorescence labeling of living cells. 5. Imaging
Live cell microscopy 1. Why do live cell microscopy? 2. Maintaining living cells on a microscope stage. 3. Considerations for imaging living cells. 4. Fluorescence labeling of living cells. 5. Imaging
Experts in Femtosecond Laser Technology. DermaInspect. Non-invasive multiphoton tomography of human skin
 Experts in Femtosecond Laser Technology DermaInspect Non-invasive multiphoton tomography of human skin In vivo optical biopsies with subcellular spatial resolution based on near infrared femtosecond laser
Experts in Femtosecond Laser Technology DermaInspect Non-invasive multiphoton tomography of human skin In vivo optical biopsies with subcellular spatial resolution based on near infrared femtosecond laser
In situ semi-quantitative assessment of single cell viability by resonance
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) In situ semi-quantitative assessment
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) In situ semi-quantitative assessment
2004 Debye Lecture 4 C. B. Murray. Quantum Dot Applications: Sun Screen. Solar Cells. Bio-tagging. Solid State Lighting?
 2004 Debye Lecture 4 C. B. Murray Quantum Dot Applications: Sun Screen Solar Cells Bio-tagging Solid State Lighting? Quantum Dot Solar cells Nanocrystal Solar Cells Double-labeling of mitochondria
2004 Debye Lecture 4 C. B. Murray Quantum Dot Applications: Sun Screen Solar Cells Bio-tagging Solid State Lighting? Quantum Dot Solar cells Nanocrystal Solar Cells Double-labeling of mitochondria
Workflow Spheroids: 3D tissue model in cancer research
 Workflow Spheroids: 3D tissue model in cancer research Irmtraud Steinmetz Updated by: Marco Meijering Application Support Specialist EMEA 2D cell culture and animal models for Cancer research 2D- cell
Workflow Spheroids: 3D tissue model in cancer research Irmtraud Steinmetz Updated by: Marco Meijering Application Support Specialist EMEA 2D cell culture and animal models for Cancer research 2D- cell
Con-focal and Multi-photon Microscope Experiment Fundamental. Qian Hu, Lab of Laser Scanning Confocal & Two-Photon Microscopy, ION, CAS
 Con-focal and Multi-photon Microscope Experiment Fundamental Qian Hu, Lab of Laser Scanning Confocal & Two-Photon Microscopy, ION, CAS 1. Light is Electromagnetic Wave ν = c / λ 2. Image of a Point Source
Con-focal and Multi-photon Microscope Experiment Fundamental Qian Hu, Lab of Laser Scanning Confocal & Two-Photon Microscopy, ION, CAS 1. Light is Electromagnetic Wave ν = c / λ 2. Image of a Point Source
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Spectral Imaging and Linear Unmixing in Light Microscopy
 Adv Biochem Engin/Biotechnol (2005) 95: 245 265 DOI 10.1007/b102216 Springer-Verlag Berlin Heidelberg 2005 Spectral Imaging and Linear Unmixing in Light Microscopy Timo Zimmermann ( ) Advanced Light Microscopy
Adv Biochem Engin/Biotechnol (2005) 95: 245 265 DOI 10.1007/b102216 Springer-Verlag Berlin Heidelberg 2005 Spectral Imaging and Linear Unmixing in Light Microscopy Timo Zimmermann ( ) Advanced Light Microscopy
Cell analysis and bioimaging technology illustrated
 Cell analysis and bioimaging technology illustrated The Cell Analysis Center Scientific Bulletin Part 1 Sysmex has been studying and exploring principles of automated haematology analysers, making full
Cell analysis and bioimaging technology illustrated The Cell Analysis Center Scientific Bulletin Part 1 Sysmex has been studying and exploring principles of automated haematology analysers, making full
Supplementary Figure 1. Design of linker truncation library between Nluc and either Venus or mneongreen
 1 2 3 Supplementary Figure 1. Design of linker truncation library between Nluc and either Venus or mneongreen 4 5 6 7 8 9 10 The cdna of C-terminally deleted FPs (mneongreen or Venus) mutants and N-terminally
1 2 3 Supplementary Figure 1. Design of linker truncation library between Nluc and either Venus or mneongreen 4 5 6 7 8 9 10 The cdna of C-terminally deleted FPs (mneongreen or Venus) mutants and N-terminally
Illumatool ΤΜ Tunable Light System: A Non-Destructive Light Source For Molecular And Cellular Biology Applications. John Fox, Lightools Research.
 Illumatool ΤΜ Tunable Light System: A Non-Destructive Light Source For Molecular And Cellular Biology Applications. John Fox, Lightools Research. Fluorescent dyes and proteins are basic analytical tools
Illumatool ΤΜ Tunable Light System: A Non-Destructive Light Source For Molecular And Cellular Biology Applications. John Fox, Lightools Research. Fluorescent dyes and proteins are basic analytical tools
Theremino DNA Meter. Fluorometer for DNA Measurements. Ana Rodriguez (MUSE) - Lodovico Lappetito (Theremino)
 Theremino DNA Meter Fluorometer for DNA Measurements Ana Rodriguez (MUSE) - Lodovico Lappetito (Theremino) Theremino_DNAMeter_Hardware_ENG - 11/01/2016 Pag. 1 Table of Contents Principle of Operation...
Theremino DNA Meter Fluorometer for DNA Measurements Ana Rodriguez (MUSE) - Lodovico Lappetito (Theremino) Theremino_DNAMeter_Hardware_ENG - 11/01/2016 Pag. 1 Table of Contents Principle of Operation...
RaftNote. Imaging and Sorting Living Cells Stained with Vital Dyes on Cell Microsystems CytoSort Array and Automated CellRaft AIR System
 RaftNote Applications and protocols for use with CellRaft Technology Imaging and Sorting Living Cells Stained with Vital Dyes on Cell Microsystems CytoSort Array and Automated CellRaft AIR System Jacquelyn
RaftNote Applications and protocols for use with CellRaft Technology Imaging and Sorting Living Cells Stained with Vital Dyes on Cell Microsystems CytoSort Array and Automated CellRaft AIR System Jacquelyn
Imaging of Cells using fluorescents dyes. By: Josué A. Benjamín Rivera September 27, 2018
 Imaging of Cells using fluorescents dyes By: Josué A. Benjamín Rivera September 27, 2018 1 History Sir William Henry Perkin BRITISH CHEMIST In 1856, at the age of 18, William Henry Perkin set out with
Imaging of Cells using fluorescents dyes By: Josué A. Benjamín Rivera September 27, 2018 1 History Sir William Henry Perkin BRITISH CHEMIST In 1856, at the age of 18, William Henry Perkin set out with
Sapphire. Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING
 Sapphire Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING Breakthrough image capture and analysis The Sapphire Biomolecular Imager is a next generation laser scanning system that provides
Sapphire Biomolecular Imager THE NEXT GENERATION OF LASER-BASED IMAGING Breakthrough image capture and analysis The Sapphire Biomolecular Imager is a next generation laser scanning system that provides
ab CytoPainter ER Staining Kit Red Fluorescence
 ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
ab139482 CytoPainter ER Staining Kit Red Fluorescence Instructions for Use Designed to detect Human endoplasmic reticulum by microscopy. This product is for research use only and is not intended for diagnostic
Fluorescent protein. Origin of fluorescent proteins. Structural and biophysical analysis of FPs. Application in molecular biology and biotechnology
 Origin of fluorescent proteins types of FPs mechanism of fluorescence Fluorescent protein Structural and biophysical analysis of FPs Application in molecular biology and biotechnology Variety of organisms
Origin of fluorescent proteins types of FPs mechanism of fluorescence Fluorescent protein Structural and biophysical analysis of FPs Application in molecular biology and biotechnology Variety of organisms
A quantitative protocol for intensity-based live cell FRET imaging.
 A quantitative protocol for intensity-based live cell FRET imaging. Kaminski CF, Rees EJ, Schierle GS. Methods Mol Biol. 2014; 1076:445-454. Department of Chemical Engineering and Biotechnology, Pembroke
A quantitative protocol for intensity-based live cell FRET imaging. Kaminski CF, Rees EJ, Schierle GS. Methods Mol Biol. 2014; 1076:445-454. Department of Chemical Engineering and Biotechnology, Pembroke
Intercalation-based single-molecule fluorescence assay to study DNA supercoil
 Intercalation-based single-molecule fluorescence assay to study DNA supercoil dynamics Mahipal Ganji, Sung Hyun Kim, Jaco van der Torre, Elio Abbondanzieri*, Cees Dekker* Supplementary information Supplementary
Intercalation-based single-molecule fluorescence assay to study DNA supercoil dynamics Mahipal Ganji, Sung Hyun Kim, Jaco van der Torre, Elio Abbondanzieri*, Cees Dekker* Supplementary information Supplementary
CyFlow Space Your flexible flow cytometer
 CyFlow Space Your flexible flow cytometer www.sysmex-partec.com CyFlow Space its flexibility gives you the space you need for your work Analysing cells and particles, be it from blood, plasma, tissue,
CyFlow Space Your flexible flow cytometer www.sysmex-partec.com CyFlow Space its flexibility gives you the space you need for your work Analysing cells and particles, be it from blood, plasma, tissue,
A Brief History of Light Microscopy And How It Transformed Biomedical Research
 A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION
 APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis
 Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis Bilge Karaçalı, PhD Department of Electrical and Electronics Engineering Izmir Institute of Technology Outline Experimental design
Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis Bilge Karaçalı, PhD Department of Electrical and Electronics Engineering Izmir Institute of Technology Outline Experimental design
cell and tissue imaging by fluorescence microscopy
 cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
FLUORESCENCE. Matyas Molnar and Dirk Pacholsky
 FLUORESCENCE Matyas Molnar and Dirk Pacholsky 1 Information This lecture contains images and information from the following internet homepages http://micro.magnet.fsu.edu/primer/index.html http://www.microscopyu.com/
FLUORESCENCE Matyas Molnar and Dirk Pacholsky 1 Information This lecture contains images and information from the following internet homepages http://micro.magnet.fsu.edu/primer/index.html http://www.microscopyu.com/
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
The CQ1 Confocal Quantitative Image Cytometer and its Application to Biological Measurement
 The CQ1 Confocal Quantitative Image Cytometer and its Application to Biological Measurement Hirofumi Sakashita *1 Koji Ohashi *1 Kazuo Ozawa *2 ohei Tsubouchi *1 The CQ1 confocal quantitative image cytometer,
The CQ1 Confocal Quantitative Image Cytometer and its Application to Biological Measurement Hirofumi Sakashita *1 Koji Ohashi *1 Kazuo Ozawa *2 ohei Tsubouchi *1 The CQ1 confocal quantitative image cytometer,
PALM/STORM, BALM, STED
 PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
Fast, three-dimensional super-resolution imaging of live cells
 Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
T H E J O U R N A L O F C E L L B I O L O G Y
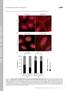 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
Supplementary Material
 Supplementary Material Supplementary Method Sample preparation and image data acquisition Osteoclast - bone data Femoral bones were excised, fixed with 4%(v/v) paraformaldehyde buffered with 0.1 M phosphate
Supplementary Material Supplementary Method Sample preparation and image data acquisition Osteoclast - bone data Femoral bones were excised, fixed with 4%(v/v) paraformaldehyde buffered with 0.1 M phosphate
