Accredited Laboratory
|
|
|
- Jade Burke
- 6 years ago
- Views:
Transcription
1 Accredited Laboratory A2LA has accredited St. Paul, MN for technical competence in the field of Biological Testing This laboratory is accredited in accordance with the recognized International Standard ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories. This accreditation demonstrates technical competence for a defined scope and the operation of a laboratory quality management system (refer to joint ISO-ILAC-IAF Communiqué dated 8 January 2009). Presented this 6 th day of December President and CEO For the Accreditation Council Certificate Number Valid to January 31, 2019 For the tests to which this accreditation applies, please refer to the laboratory s Biological Scope of Accreditation.
2 SCOPE OF ACCREDITATION TO ISO/IEC 17025: Executive Drive St. Paul, MN Deborah Erickson Phone: BIOLOGICAL Valid To: January 31, 2019 Certificate Number: In recognition of the successful completion of the A2LA evaluation process, accreditation is granted to this laboratory to perform the following biological tests: In-Vitro Tests Bacterial Mutagenicity Test (Ames Assay) In Vitro Mouse Lymphoma Assay The In Vivo Mouse Micronucleus Assay In Vitro Chromosome Aberration Analysis Using Chinese Hamster Ovary (CHO) Cells Complement Activation SC5b-9 (TCC) Assay Complement Activation C3a Assay Platelet and Leukocyte Count Assay In Vitro Hemocompatability Assay Partial Thromboplastin Time (PTT) Assay ASTM Hemolysis Assay Prothrombin Time Assay NIH Hemolysis Testing: Extract Method and Direct Contact Methods Agarose Overlay Cytotoxicity Test Extract Colony Assay Cytotoxicity Test Direct Cell Contact Cytotoxicity Test Minimum Essential Medium (MEM) Elution Assay MTT Assay Neutral Red Uptake Assay ISO : Current Edition, Biological evaluation of medical devices - Part 3: Tests for genotoxicity carcinogenicity and reproductive toxicity ISO : Current Edition, Biological evaluation of medical devices - Part 4: Selection of test for interactions with blood ISO : Current Edition, Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity (A2LA Cert. No ) 12/06/2016 Page 1 of 3
3 In-Life Studies Subcutaneous Implant Test Intramuscular Implant 28 Day Osteoinduction Assay in Mice or Rats Japanese Primary Skin Irritation Japanese Intracutaneous Reactivity Test USP Intracutaneous Injection Test ISO Intracutaneous Reactivity Test ISO Guinea Pig Maximization Sensitization Test Buehler Sensitization Test Primary Skin Irritation Guinea Pig Maximization Sensitization Test - Method for Japanese Ministry of Health, Labor and Welfare Vaginal Mucosal Irritation Study Acute Systemic Toxicity Test Subacute/Subchronic Toxicity Test Rabbit Pyrogen Test In Vivo Assay for Viral Contaminants Sample Preparation Procedures Japanese Extraction Procedures and Alternate Preparation Methods Preparation of Biomaterials for Extraction Preparation of Biomaterials for Implant Tests Preparation of Biomaterials for Agarose Overlay, Primary Skin Irritation, and Repeated Patch Dermal Sensitization Preparation of Biomaterials for Hemocompatability Tests Sample Preparation for USP Rabbit Pyrogen Test and the Material Mediated Rabbit Pyrogen Test ISO : Current Edition, Biological Intramuscular evaluation of medical devices - Part 6: Tests For local effects after implantation ISO : Current Edition, Biological evaluation of medical devices - Part 10: Tests for irritation and delayed-type hypersensitivity ISO : Current Edition, Biological evaluation of medical devices - Part 11: Tests for systemic toxicity Guidance for Industry Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications (FDA, 2010) Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals (1993) FDA European Pharmacopeia Current Edition; Tests for extraneous agents in viral vaccines ISO : Current Edition, Biological Evaluation of medical devices - Part 12: Sample preparation and reference materials (A2LA Cert. No ) 12/06/2016 Page 2 of 3
4 Mycoplasma with Mycoplasmastasis with Mycoplasmastasis including Spiroplasma melliferum including M. putrefaciens and DNA Fluorochrome Staining with Mycoplasmastasis including S. melliferum including M. putrefaciens Research Products Test for the Presence of Mycoplasma with Mycoplasmastasis with Mycoplasmastasis including S. melliferum European Pharmacopeia Current Edition; Mycoplasma Testing (EP) 21 CFR Subpart D; Mycoplasma Testing U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research (CBER) Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals United States Pharmacopeia Current Edition: USP <63> (A2LA Cert. No ) 12/06/2016 Page 3 of 3
5 Accredited Laboratory A2LA has accredited St. Paul, MN for technical competence in the field of Chemical Testing This laboratory is accredited in accordance with the recognized International Standard ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories. This accreditation demonstrates technical competence for a defined scope and the operation of a laboratory quality management system (refer to joint ISO-ILAC-IAF Communiqué dated 8 January 2009). Presented this 6 th day of December President and CEO For the Accreditation Council Certificate Number Valid to January 31, 2019 For the tests to which this accreditation applies, please refer to the laboratory s Chemical Scope of Accreditation.
6 SCOPE OF ACCREDITATION TO ISO/IEC 17025: Executive Drive St. Paul, MN Deborah Erickson Phone: CHEMICAL Valid To: January 31, 2019 Certificate Number: In recognition of the successful completion of the A2LA evaluation process, accreditation is granted to this laboratory to perform the following tests on medical devices including, but not limited to: polymers, metals & alloys, ceramics, drug compounds, and natural macromolecules: Test Technology Inductively Coupled Plasma / Mass Spectrometry (ICP/MS) Inductively Coupled Plasma / Optical Emission Spectrometry (ICP/OES) Ultra Performance Liquid Chromatography / Mass Spectrometry (UPLC/MS) Gas Chromatography / Mass Spectrometry (GC/MS) Residual Solvent Headspace Gas Chromatography / Mass Spectrometry (HSGC/MS) Test Method Extraction (A2LA Cert. No ) 12/06/2016 Page 1 of 1
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that American Preclinical Services 8945 Evergreen
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that American Preclinical Services 8945 Evergreen
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Toxikon Corporation 15-25 Wiggins Avenue
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Toxikon Corporation 15-25 Wiggins Avenue
BIOCOMPATIBILITY IN THIS SECTION EXPERT SOLUTIONS FOR PRODUCT DEVELOPMENT. Cytotoxicity Testing
 EXPERT SOLUTIONS FOR PRODUCT DEVELOPMENT BIOCOMPATIBILITY Medical devices and their component materials may leach compounds or have surface characteristics that may produce undesirable effects when used
EXPERT SOLUTIONS FOR PRODUCT DEVELOPMENT BIOCOMPATIBILITY Medical devices and their component materials may leach compounds or have surface characteristics that may produce undesirable effects when used
BIOCOMPATIBILITY IN THIS SECTION. Cytotoxicity Testing
 BIOCOMPATIBILITY Medical devices and their component materials may leach compounds or have surface characteristics that may produce undesirable effects when used clinically. The selection and evaluation
BIOCOMPATIBILITY Medical devices and their component materials may leach compounds or have surface characteristics that may produce undesirable effects when used clinically. The selection and evaluation
Medical Device Testing. Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark
 Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
P103b Annex: Policy on Estimating Measurement Uncertainty for Life Sciences Testing Labs
 Page 1 of 10 A2LA has compiled information for classifying some common types of test methods to meet the A2LA Policy on Measurement Uncertainty for Testing Laboratories. The A2LA Policy is intended to
Page 1 of 10 A2LA has compiled information for classifying some common types of test methods to meet the A2LA Policy on Measurement Uncertainty for Testing Laboratories. The A2LA Policy is intended to
Public Interest Incorporated Foundation BioSafety Research Center
 Public Interest Incorporated Foundation BioSafety Research Center http://www.anpyo.or.jp Ver. 201801 Building #1(Test and research annex) Building #2 (Administrat ion office) Building #3 (Toxicity test
Public Interest Incorporated Foundation BioSafety Research Center http://www.anpyo.or.jp Ver. 201801 Building #1(Test and research annex) Building #2 (Administrat ion office) Building #3 (Toxicity test
Testing and Evaluation Strategies for the Biological Evaluation of Medical Devices submitted for CE Mark and FDA Approval
 Testing and Evaluation Strategies for the Biological Evaluation of Medical Devices submitted for CE Mark and FDA Approval Today, millions of medical devices are used worldwide to treat or support patients.
Testing and Evaluation Strategies for the Biological Evaluation of Medical Devices submitted for CE Mark and FDA Approval Today, millions of medical devices are used worldwide to treat or support patients.
Our Business. in vitro / in vivo. in vitro / in vivo ADME. in vitro / in vivo. Toxicology. Cell Battery V79. Analytical Services. Service.
 GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
FDA/ISO MHLW-Japan USP Microbiology. 15 Wiggins Avenue, Bedford, MA
 Medical Device Testing Guide A resource for sample submissions, test descriptions, sample requirements, and turnaround times for biocompatibility testing according to: FDA/ISO 10993 MHLW-Japan USP Microbiology
Medical Device Testing Guide A resource for sample submissions, test descriptions, sample requirements, and turnaround times for biocompatibility testing according to: FDA/ISO 10993 MHLW-Japan USP Microbiology
4 Principles for the valuation of the biological evaluation of materials and medical devices
 1 Purpose This checklist supplements the process description device certification and regulates the details and approach for the evaluation of the biological evaluation of medical devices in the context
1 Purpose This checklist supplements the process description device certification and regulates the details and approach for the evaluation of the biological evaluation of medical devices in the context
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EXOVA PHARMA US LLC. 9240 Santa Fe Springs Rd. Santa Fe Springs, CA 90670 Eric Lindsay Phone: (562) 948-2225 x 70300 CHEMICAL Valid To: March 31, 2020 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EXOVA PHARMA US LLC. 9240 Santa Fe Springs Rd. Santa Fe Springs, CA 90670 Eric Lindsay Phone: (562) 948-2225 x 70300 CHEMICAL Valid To: March 31, 2020 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NATIONAL INSTITUTE FOR AVIATION RESEARCH (NIAR) at WICHITA STATE UNIVERSITY National Institute for Aviation Research Building 1845 Fairmount
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NATIONAL INSTITUTE FOR AVIATION RESEARCH (NIAR) at WICHITA STATE UNIVERSITY National Institute for Aviation Research Building 1845 Fairmount
Biological evaluation of medical devices --
 Translated English of Chinese Standard: GB/T16886.1-2011 Translated by: www.chinesestandard.net Wayne Zheng et al. Email: Sales@ChineseStandard.net ICS 11.040.01 C 30 NATIONAL STANDARD OF THE PEOPLE S
Translated English of Chinese Standard: GB/T16886.1-2011 Translated by: www.chinesestandard.net Wayne Zheng et al. Email: Sales@ChineseStandard.net ICS 11.040.01 C 30 NATIONAL STANDARD OF THE PEOPLE S
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NIAR (NATIONAL INSTITUTE FOR AVIATION RESEARCH) National Institute for Aviation Research Building 1845 Fairmount Street Wichita, KS
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NIAR (NATIONAL INSTITUTE FOR AVIATION RESEARCH) National Institute for Aviation Research Building 1845 Fairmount Street Wichita, KS
Accredited Laboratory A2LA has accredited WUXI APPTEC, INC. Marietta, GA for technical competence in the field of
 Accredited Laboratory A2LA has accredited Marietta, GA for technical competence in the field of Biological Testing This laboratory is accredited in accordance with the recognized International Standard
Accredited Laboratory A2LA has accredited Marietta, GA for technical competence in the field of Biological Testing This laboratory is accredited in accordance with the recognized International Standard
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EXOVA INC., DBA ELEMENT MATERIALS TECHNOLOGY Products Warren Laboratory 1920 Concept Dr. Warren, MI 48091-1385 N Jeri Laird Phone: 248 458 5900 x 73258 Fax:
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EXOVA INC., DBA ELEMENT MATERIALS TECHNOLOGY Products Warren Laboratory 1920 Concept Dr. Warren, MI 48091-1385 N Jeri Laird Phone: 248 458 5900 x 73258 Fax:
ANALYTICAL CHEMISTRY TESTING: Outsourced: Upcoming Projects: Names /Contracts for this area CDA / MSA Audited:
 To better understand your outsourcing needs, please complete the following: COMPANY NAME: Current Customer ( ) DIVISION: Prospect ( ) YOUR NAME: TITLE: ADDRESS: ANALYTICAL CHEMISTRY TESTING: Outsourced:
To better understand your outsourcing needs, please complete the following: COMPANY NAME: Current Customer ( ) DIVISION: Prospect ( ) YOUR NAME: TITLE: ADDRESS: ANALYTICAL CHEMISTRY TESTING: Outsourced:
Biocompatibility, FDA and ISO 10993
 Introduction to BioMEMS & Medical Microdevices Biocompatibility, FDA and ISO 10993 Companion lecture to the textbook: Fundamentals of BioMEMS and Medical Microdevices, by, http://saliterman.umn.edu/ ISO
Introduction to BioMEMS & Medical Microdevices Biocompatibility, FDA and ISO 10993 Companion lecture to the textbook: Fundamentals of BioMEMS and Medical Microdevices, by, http://saliterman.umn.edu/ ISO
SAFETY RESEARCH INSTITUTE FOR CHEMICAL COMPOUNDS CO., LTD.
 SAFETY RESEARCH INSTITUTE FOR CHEMICAL COMPOUNDS CO., LTD. HISTORICAL BACKGROUND I. PREDECESSOR CONCERN (SAFETY STUDY ASSOCIATION) The origin of our company is a group of researchers, Safety Study Association,
SAFETY RESEARCH INSTITUTE FOR CHEMICAL COMPOUNDS CO., LTD. HISTORICAL BACKGROUND I. PREDECESSOR CONCERN (SAFETY STUDY ASSOCIATION) The origin of our company is a group of researchers, Safety Study Association,
ISO Definition of a Medical Device
 Introduction to BioMEMS & Medical Microdevices Biocompatibility, FDA and ISO 10993 Prof., http://saliterman.umn.edu/ ISO Definition of a Medical Device Any instrument, apparatus, appliance, material or
Introduction to BioMEMS & Medical Microdevices Biocompatibility, FDA and ISO 10993 Prof., http://saliterman.umn.edu/ ISO Definition of a Medical Device Any instrument, apparatus, appliance, material or
ATI FLAT ROLLED PRODUCTS Brackenridge, PA for technical competence in the field of
 A2LA has accredited Brackenridge, PA for technical competence in the field of Mechanical Testing This laboratory is accredited in accordance with the recognized International Standard ISO/IEC 17025:2005
A2LA has accredited Brackenridge, PA for technical competence in the field of Mechanical Testing This laboratory is accredited in accordance with the recognized International Standard ISO/IEC 17025:2005
ISO Definition of a Medical Device
 Introductory Medical Device Prototyping Biocompatibility, http://saliterman.umn.edu/ Department of Biomedical Engineering, University of Minnesota ISO Definition of a Medical Device Any instrument, apparatus,
Introductory Medical Device Prototyping Biocompatibility, http://saliterman.umn.edu/ Department of Biomedical Engineering, University of Minnesota ISO Definition of a Medical Device Any instrument, apparatus,
Medical Devices INNOVATIVE SOLUTION FOR THE HEALTHCARE
 Medical Devices INNOVATIVE SOLUTION FOR THE HEALTHCARE Medical Devices The new Regulation 745/2017 defined as Medical Device any instrument, apparatus, appliance, software, implant, reagent, material or
Medical Devices INNOVATIVE SOLUTION FOR THE HEALTHCARE Medical Devices The new Regulation 745/2017 defined as Medical Device any instrument, apparatus, appliance, software, implant, reagent, material or
Deborah Rice, CMAR. BAXTER HEALTHCARE, Round Lake, IL
 Deborah Rice, CMAR 11234 Wildridge St. Westchester, IL 60154 Phone (708) 269-8890 e-mail ricedeborah7@aol.com SUMMARY Certified Manager of Animal Resources, and a registered technologist with extensive
Deborah Rice, CMAR 11234 Wildridge St. Westchester, IL 60154 Phone (708) 269-8890 e-mail ricedeborah7@aol.com SUMMARY Certified Manager of Animal Resources, and a registered technologist with extensive
SERVA DNA Stain Clear G Cat. No
 1 SERVA DNA Stain Clear G Cat. No. 39804 1. Introduction Ethidium bromide (EtBr) is most commonly used nucleic acid stain in molecular biology laboratories. It has been proved to be strong carcinogen and
1 SERVA DNA Stain Clear G Cat. No. 39804 1. Introduction Ethidium bromide (EtBr) is most commonly used nucleic acid stain in molecular biology laboratories. It has been proved to be strong carcinogen and
510(k) Summary. January 10, 2014
 510(k) Premnarket Notification: Nerve Cull' JAN 1 0 2014 510(k) Summary January 10, 2014 Cook Biotech Incorporated Nerve Cuff Manufacturer Name: Official Contact: Cook Biotech Incorporated 1425 Innovation
510(k) Premnarket Notification: Nerve Cull' JAN 1 0 2014 510(k) Summary January 10, 2014 Cook Biotech Incorporated Nerve Cuff Manufacturer Name: Official Contact: Cook Biotech Incorporated 1425 Innovation
Colorants in Medical Devices:
 Colorants in Medical Devices: The Spectrum of Current Regulatory Expectations John Iannone Program Manager/ Technical Specialist Overview» Company Introduction» Why use Colorants in Devices?» Regulatory
Colorants in Medical Devices: The Spectrum of Current Regulatory Expectations John Iannone Program Manager/ Technical Specialist Overview» Company Introduction» Why use Colorants in Devices?» Regulatory
GB/T / ISO :2007
 Translated English of Chinese Standard: GB/T16886.6-2015 www.chinesestandard.net Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE'S REPUBLIC OF CHINA ICS 11.040.01 C 30 GB/T 16886.6-2015 /
Translated English of Chinese Standard: GB/T16886.6-2015 www.chinesestandard.net Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE'S REPUBLIC OF CHINA ICS 11.040.01 C 30 GB/T 16886.6-2015 /
ISO INTERNATIONAL STANDARD. Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity
 INTERNATIONAL STANDARD ISO 10993-5 Third edition 2009-06-01 Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity Évaluation biologique des dispositifs médicaux Partie 5: Essais
INTERNATIONAL STANDARD ISO 10993-5 Third edition 2009-06-01 Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity Évaluation biologique des dispositifs médicaux Partie 5: Essais
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. DNV GL PVEL, LLC Fifth Street Berkeley, CA Mr. John Watts Phone: ELECTRICAL
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DNV GL PVEL, LLC 1 1360 Fifth Street Berkeley, CA 94710 Mr. John Watts Phone: 585 690 3621 ELECTRICAL Valid To: January 31, 2020 Certificate Number: 3252.02
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DNV GL PVEL, LLC 1 1360 Fifth Street Berkeley, CA 94710 Mr. John Watts Phone: 585 690 3621 ELECTRICAL Valid To: January 31, 2020 Certificate Number: 3252.02
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 4155 Lisa Drive Tipp City, OH 45371 Craig Riviello Phone: 937 669 4500 MECHANICAL Valid To: December 31, 2017 Certificate Number: 2633.01 In recognition of
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 4155 Lisa Drive Tipp City, OH 45371 Craig Riviello Phone: 937 669 4500 MECHANICAL Valid To: December 31, 2017 Certificate Number: 2633.01 In recognition of
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 4155 Lisa Drive Tipp City, OH 45371 Craig Riviello Phone: 937 669 4500 CHEMICAL Valid To: November 30, 2017 Certificate Number: 2633.02 In recognition of the
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 4155 Lisa Drive Tipp City, OH 45371 Craig Riviello Phone: 937 669 4500 CHEMICAL Valid To: November 30, 2017 Certificate Number: 2633.02 In recognition of the
S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use
 S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use Peter Kasper BfArM and S2(R1)EWP International Conference on Harmonisation of
S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use Peter Kasper BfArM and S2(R1)EWP International Conference on Harmonisation of
ISO EP, USP ISO EP, USP ISO EP, USP ISO EP, USP ISO MSDA ISO MSDA
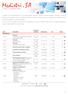 Laboratory Price List 2018 In addition to the tests described in the list below, Medistri SA offers complete validations (examples: verification of cleaning processes with markers, search for residues
Laboratory Price List 2018 In addition to the tests described in the list below, Medistri SA offers complete validations (examples: verification of cleaning processes with markers, search for residues
Archived Content. This content was archived on February 4, 2017.
 This content was archived on February 4, 2017. Archived Content Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after
This content was archived on February 4, 2017. Archived Content Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after
United States Pharmacopeia: Revision Process for USP Biocompatibility General Chapters <87>, <88> and <1031>
 United States Pharmacopeia: Revision Process for USP Biocompatibility General Chapters , and Daniel L. Norwood, M.S.P.H, Ph.D. USP Packaging and Distribution Expert Committee Executive
United States Pharmacopeia: Revision Process for USP Biocompatibility General Chapters , and Daniel L. Norwood, M.S.P.H, Ph.D. USP Packaging and Distribution Expert Committee Executive
Legacy Evaluations. Ensuring your commercialized products meet the current regulations
 Legacy Evaluations Ensuring your commercialized products meet the current regulations legacy evaluations: the process Gap Evaluation Testing Risk ssessment Scalable Programs Proven Performance TRINING
Legacy Evaluations Ensuring your commercialized products meet the current regulations legacy evaluations: the process Gap Evaluation Testing Risk ssessment Scalable Programs Proven Performance TRINING
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. TSP / USB LABORATORY South Main Street Houston, TX Lester Burgess Phone:
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 TSP / USB LABORATORY 1 12895 South Main Street Houston, TX 77035 Lester Burgess Phone: 713 726 1000 MECHANICAL Valid To: June 30, 2018 Certificate Number: 0929.01
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 TSP / USB LABORATORY 1 12895 South Main Street Houston, TX 77035 Lester Burgess Phone: 713 726 1000 MECHANICAL Valid To: June 30, 2018 Certificate Number: 0929.01
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. Q LABORATORIES, INC Harrison Avenue Cincinnati, OH David Goins Phone:
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 1400 Harrison Avenue Cincinnati, OH 45214 David Goins Phone: 513-471-1300 BIOLOGICAL Valid To: July 31, 2018 Certificate Number: 3026.02 In recognition of the
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 1400 Harrison Avenue Cincinnati, OH 45214 David Goins Phone: 513-471-1300 BIOLOGICAL Valid To: July 31, 2018 Certificate Number: 3026.02 In recognition of the
Risk Management of Biological Evaluation What s the Future for ISO 10993?
 Risk Management of Biological Evaluation What s the Future for ISO 10993? ISO 10993 High level guidance on how to conduct a biological evaluation Detailed test methods for investigation of different aspects
Risk Management of Biological Evaluation What s the Future for ISO 10993? ISO 10993 High level guidance on how to conduct a biological evaluation Detailed test methods for investigation of different aspects
BIOCOMPATIBILITY TESTING AT PACIFIC BIOLABS
 BIOCOMPATIBILITY TESTING AT PACIFIC BIOLABS For over 25 years, Pacific BioLabs has conducted biocompatibility testing for the medical device and pharmaceutical industries. Our staff toxicologists have
BIOCOMPATIBILITY TESTING AT PACIFIC BIOLABS For over 25 years, Pacific BioLabs has conducted biocompatibility testing for the medical device and pharmaceutical industries. Our staff toxicologists have
Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system.
 Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system. Application for seeking approval of the Central Government for conduct of clinical trial in
Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system. Application for seeking approval of the Central Government for conduct of clinical trial in
Midori Green Advance DNA Stain Safety Report
 Publishing date: 30.09.2011 Midori Green Advance DNA Stain Safety Report IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name Catalog number Supplier Information in case of emergency Midori Green
Publishing date: 30.09.2011 Midori Green Advance DNA Stain Safety Report IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name Catalog number Supplier Information in case of emergency Midori Green
Cooperation in the field of toxicology, analytics, R&D and production
 Cooperation in the field of toxicology, analytics, R&D and production Where you can find us 2 2 VUOS PARTNER FOR COOPERATION History 3 3 VUOS PARTNER FOR COOPERATION Organizational structure 1. R&D and
Cooperation in the field of toxicology, analytics, R&D and production Where you can find us 2 2 VUOS PARTNER FOR COOPERATION History 3 3 VUOS PARTNER FOR COOPERATION Organizational structure 1. R&D and
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 MONTOI, S.A. DE C.V. Libramiento Noreste Km.27 Escobedo, CP 66052 Nuevo Leon, Mexico Rodolfo Caballero Phone: 52-818154030 Email: Rodolfo.Caballero@Mattel.com
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 MONTOI, S.A. DE C.V. Libramiento Noreste Km.27 Escobedo, CP 66052 Nuevo Leon, Mexico Rodolfo Caballero Phone: 52-818154030 Email: Rodolfo.Caballero@Mattel.com
Experimental techniques: The Skin; Biocompatibility. Dr. Gábor Erős
 Experimental techniques: The Skin; Biocompatibility Dr. Gábor Erős Structure of the skin Possibilities - Human trials - Animal experiments - In vitro examinations Cosmetic products and their ingredients
Experimental techniques: The Skin; Biocompatibility Dr. Gábor Erős Structure of the skin Possibilities - Human trials - Animal experiments - In vitro examinations Cosmetic products and their ingredients
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 JSC RECEIVING INSPECTION AND TEST FACILITY Building 15, M/S NT315 Houston, TX 77058 Deborah Applegate Phone: 281 483 0288 ELECTRICAL Valid Until: December 31,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 JSC RECEIVING INSPECTION AND TEST FACILITY Building 15, M/S NT315 Houston, TX 77058 Deborah Applegate Phone: 281 483 0288 ELECTRICAL Valid Until: December 31,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. WN GLOBAL LABORATORY South Main Street Houston, TX Lester Burgess Phone:
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 WN GLOBAL LABORATORY 1 12895 South Main Street Houston, TX 77035 Lester Burgess Phone: 713 726 1000 MECHANICAL Valid To: June 30, 2020 Certificate Number: 0929.01
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 WN GLOBAL LABORATORY 1 12895 South Main Street Houston, TX 77035 Lester Burgess Phone: 713 726 1000 MECHANICAL Valid To: June 30, 2020 Certificate Number: 0929.01
YY/T Translated English of Chinese Standard: YY/T
 Translated English of Chinese Standard: YY/T0606.9-2007 www.chinesestandard.net Sales@ChineseStandard.net YY PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.40 C 45 YY/T 0606.9-2007
Translated English of Chinese Standard: YY/T0606.9-2007 www.chinesestandard.net Sales@ChineseStandard.net YY PHARMACEUTICAL INDUSTRY STANDARD OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.40 C 45 YY/T 0606.9-2007
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 SOUTHERN CALIBRATION & SERVICE, INC. 590 W. Crossville Road, Suite 102 Roswell, GA 30075 Charles Dilcher Phone: 800 241 4622 CALIBRATION
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 SOUTHERN CALIBRATION & SERVICE, INC. 590 W. Crossville Road, Suite 102 Roswell, GA 30075 Charles Dilcher Phone: 800 241 4622 CALIBRATION
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. Q LABORATORIES, INC Harrison Avenue Cincinnati, OH David Goins Phone: CHEMICAL
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 1400 Harrison Avenue Cincinnati, OH 45214 David Goins Phone: 513-471-1300 CHEMICAL Valid To: October 31, 2018 Certificate Number: 3026.01 In recognition of
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 1400 Harrison Avenue Cincinnati, OH 45214 David Goins Phone: 513-471-1300 CHEMICAL Valid To: October 31, 2018 Certificate Number: 3026.01 In recognition of
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. Q LABORATORIES, INC Harrison Avenue Cincinnati, OH David Goins Phone: CHEMICAL
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 1400 Harrison Avenue Cincinnati, OH 45214 David Goins Phone: 513-471-1300 CHEMICAL Valid To: September 30, 2018 Certificate Number: 3026.01 In recognition of
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 1400 Harrison Avenue Cincinnati, OH 45214 David Goins Phone: 513-471-1300 CHEMICAL Valid To: September 30, 2018 Certificate Number: 3026.01 In recognition of
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. WN GLOBAL LABORATORY South Main Street Houston, TX Lester Burgess Phone:
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 WN GLOBAL LABORATORY 1 12895 South Main Street Houston, TX 77035 Lester Burgess Phone: 713 726 1000 MECHANICAL Valid To: June 30, 2020 Certificate Number: 0929.01
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 WN GLOBAL LABORATORY 1 12895 South Main Street Houston, TX 77035 Lester Burgess Phone: 713 726 1000 MECHANICAL Valid To: June 30, 2020 Certificate Number: 0929.01
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 R. J. REYNOLDS TOBACCO COMPANY 1 Product Services Analytical Testing Laboratories 950 Reynolds Blvd. Winston Salem, NC 27105 Jannell Rowe Phone: 336 741 4121
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 R. J. REYNOLDS TOBACCO COMPANY 1 Product Services Analytical Testing Laboratories 950 Reynolds Blvd. Winston Salem, NC 27105 Jannell Rowe Phone: 336 741 4121
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 10993-14 First edition 2001-11-15 Biological evaluation of medical devices Part 14: Identification and quantification of degradation products from ceramics Évaluation biologique
INTERNATIONAL STANDARD ISO 10993-14 First edition 2001-11-15 Biological evaluation of medical devices Part 14: Identification and quantification of degradation products from ceramics Évaluation biologique
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EUROFINS TESTING TECHNOLOGY (SHENZHEN) CO., LTD. 4/F, Building #3 Runheng Electronic Park Luxian Road 2 nd Road, Xin an Street, Bao an District Shenzhen City,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EUROFINS TESTING TECHNOLOGY (SHENZHEN) CO., LTD. 4/F, Building #3 Runheng Electronic Park Luxian Road 2 nd Road, Xin an Street, Bao an District Shenzhen City,
ISO INTERNATIONAL STANDARD. Biological evaluation of medical devices Part 2: Animal welfare requirements
 INTERNATIONAL STANDARD ISO 10993-2 Second edition 2006-07-15 Biological evaluation of medical devices Part 2: Animal welfare requirements Évaluation biologique des dispositifs médicaux Partie 2: Exigences
INTERNATIONAL STANDARD ISO 10993-2 Second edition 2006-07-15 Biological evaluation of medical devices Part 2: Animal welfare requirements Évaluation biologique des dispositifs médicaux Partie 2: Exigences
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL TECHNICAL SYSTEMS Massachusetts Division 1146 Massachusetts Avenue Boxborough, MA 01719 David Potpinka Phone: 732 936 0800 MECHANICAL Valid to: September
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL TECHNICAL SYSTEMS Massachusetts Division 1146 Massachusetts Avenue Boxborough, MA 01719 David Potpinka Phone: 732 936 0800 MECHANICAL Valid to: September
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CHUN YU WORKS & CO., LTD. QUALITY ASSURANCE DEPARTMENT TESTING LABORATORY 100, Ta-Pao St. Kangshan, Kaohsiung, 82063 Taiwan, R.O.C. Su Mien Chang Phone: +886
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CHUN YU WORKS & CO., LTD. QUALITY ASSURANCE DEPARTMENT TESTING LABORATORY 100, Ta-Pao St. Kangshan, Kaohsiung, 82063 Taiwan, R.O.C. Su Mien Chang Phone: +886
Safety Testing of Chinese Hamster Ovary (CHO) Cell Banks
 Safety Testing of Chinese Hamster Ovary (CHO) Cell Banks Biosafety testing within the manufacturing process should be established in the early stages of drug development. We offer biosafety testing services
Safety Testing of Chinese Hamster Ovary (CHO) Cell Banks Biosafety testing within the manufacturing process should be established in the early stages of drug development. We offer biosafety testing services
Biological evaluation of medical devices. Part 6: Tests for local effects after implantation
 INTERNATIONAL STANDARD ISO 10993-6 Third edition 2016-12-01 Biological evaluation of medical devices Part 6: Tests for local effects after implantation Évaluation biologique des dispositifs médicaux Partie
INTERNATIONAL STANDARD ISO 10993-6 Third edition 2016-12-01 Biological evaluation of medical devices Part 6: Tests for local effects after implantation Évaluation biologique des dispositifs médicaux Partie
Biocompatibility: a risk based approach
 Biocompatibility: a risk based approach Legal framework The regulatory requirements include: Demonstration of safety Demonstration of efficacy Positive balance of risk and benefit The regulatory requirements
Biocompatibility: a risk based approach Legal framework The regulatory requirements include: Demonstration of safety Demonstration of efficacy Positive balance of risk and benefit The regulatory requirements
Inspections, Compliance, Enforcement, and Criminal Investigations
 Seite 1 von 6 Inspections, Compliance, Enforcement, and Criminal Investigations Sitek Research Laboratories 5/21/09 Department of Health and Human Services Public Health Service Food and Drug Administration
Seite 1 von 6 Inspections, Compliance, Enforcement, and Criminal Investigations Sitek Research Laboratories 5/21/09 Department of Health and Human Services Public Health Service Food and Drug Administration
A Life Cycle Approach to Raw Material Qualification for Cell and Gene Therapy Products
 A Life Cycle Approach to Raw Material Qualification for Cell and Gene Therapy Products Angela Whatley, Ph.D. Office of Tissues and Advanced Therapies CBER/FDA CMC Strategy Forum on Cell & Gene Therapies
A Life Cycle Approach to Raw Material Qualification for Cell and Gene Therapy Products Angela Whatley, Ph.D. Office of Tissues and Advanced Therapies CBER/FDA CMC Strategy Forum on Cell & Gene Therapies
Safety Testing of Chinese Hamster Ovary (CHO) Cell Banks
 Safety Testing of Chinese Hamster Ovary (CHO) Cell Banks About Sartorius Stedim BioOutsource Sartorius Stedim BioOutsource Ltd. is a leading provider of contract testing services to the global biopharmaceutical
Safety Testing of Chinese Hamster Ovary (CHO) Cell Banks About Sartorius Stedim BioOutsource Sartorius Stedim BioOutsource Ltd. is a leading provider of contract testing services to the global biopharmaceutical
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DAYTON T. BROWN, INC. 1175 Church Street Bohemia, NY 11716-5031 Mary Alice Der Aris Phone: 631 244 6315 / 1 800 TEST456 Fax: 631 589 4046 Email: test@dtbtest.com
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DAYTON T. BROWN, INC. 1175 Church Street Bohemia, NY 11716-5031 Mary Alice Der Aris Phone: 631 244 6315 / 1 800 TEST456 Fax: 631 589 4046 Email: test@dtbtest.com
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NATIONAL CALIBRATION INC. 2380 Wyecroft Rd. Unit 11 Oakville, ON L6L 6W1 CANADA Douglas Fox Phone: 905 330-5419 CALIBRATION Valid To:
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NATIONAL CALIBRATION INC. 2380 Wyecroft Rd. Unit 11 Oakville, ON L6L 6W1 CANADA Douglas Fox Phone: 905 330-5419 CALIBRATION Valid To:
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NEWAGE TESTING INSTRUMENTS, INC. 8600 Somerset Drive Largo, FL 33773 Michael Guglicelli Phone: 727 538 6015 CALIBRATION Valid To: July
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 NEWAGE TESTING INSTRUMENTS, INC. 8600 Somerset Drive Largo, FL 33773 Michael Guglicelli Phone: 727 538 6015 CALIBRATION Valid To: July
ISO INTERNATIONAL STANDARD. Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity
 INTERNATIONAL STANDARD ISO 10993-5 Third edition 2009-06-01 Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity Évaluation biologique des dispositifs médicaux Partie 5: Essais
INTERNATIONAL STANDARD ISO 10993-5 Third edition 2009-06-01 Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity Évaluation biologique des dispositifs médicaux Partie 5: Essais
An NGO Perspective on Regulatory Acceptance of Non-animal Data and Related Issues. Martin Stephens, Ph.D. The Humane Society of the United States
 An NGO Perspective on Regulatory Acceptance of Non-animal Data and Related Issues Martin Stephens, Ph.D. The Humane Society of the United States Animal Use Numbers Rabbits 222,167 Guinea pigs 203,098 Hamsters
An NGO Perspective on Regulatory Acceptance of Non-animal Data and Related Issues Martin Stephens, Ph.D. The Humane Society of the United States Animal Use Numbers Rabbits 222,167 Guinea pigs 203,098 Hamsters
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 10993-13 Second edition 2010-06-15 Biological evaluation of medical devices Part 13: Identification and quantification of degradation products from polymeric medical devices
INTERNATIONAL STANDARD ISO 10993-13 Second edition 2010-06-15 Biological evaluation of medical devices Part 13: Identification and quantification of degradation products from polymeric medical devices
Test Report. The Report of Acute Oral Toxicity Test
 Test Report Sample No.: 2011KF0435 Test Item: Safe Nucleic Acid Stain Sponsor: Yekta Tajhiz Azma The Report of Acute Oral Toxicity Test Test item: Safe Nucleic Acid Stain Sample No.: 2011KF0435 Subject
Test Report Sample No.: 2011KF0435 Test Item: Safe Nucleic Acid Stain Sponsor: Yekta Tajhiz Azma The Report of Acute Oral Toxicity Test Test item: Safe Nucleic Acid Stain Sample No.: 2011KF0435 Subject
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 VALE CANADA LTD. COPPER CLIFF ANALYTICAL SERVICES 18 Rink Street Copper Cliff, Ontario P0M 1N0 Canada Mr. Claude Serre Phone: 705 682 7501 CHEMICAL Valid To:
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 VALE CANADA LTD. COPPER CLIFF ANALYTICAL SERVICES 18 Rink Street Copper Cliff, Ontario P0M 1N0 Canada Mr. Claude Serre Phone: 705 682 7501 CHEMICAL Valid To:
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 ELEMENT MATERIALS TECHNOLOGY DENVER 1530 Vista View Drive Longmont, CO 80504 Sandra Frank Phone 561 529 1488 MECHANICAL Valid To: July 31, 2018 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 ELEMENT MATERIALS TECHNOLOGY DENVER 1530 Vista View Drive Longmont, CO 80504 Sandra Frank Phone 561 529 1488 MECHANICAL Valid To: July 31, 2018 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005. SEMBLEX CORPORATION 388 Carol Lane Campus Elmhurst, IL Ray Lafferty Phone: MECHANICAL
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 SEMBLEX CORPORATION 388 Carol Lane Campus Elmhurst, IL 60126 Ray Lafferty Phone: 630 617 5159 MECHANICAL Valid To: April 30, 2019 Certificate Number: 0794.01
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 SEMBLEX CORPORATION 388 Carol Lane Campus Elmhurst, IL 60126 Ray Lafferty Phone: 630 617 5159 MECHANICAL Valid To: April 30, 2019 Certificate Number: 0794.01
Standard Guide for Determining the Impact of Extractables from Non-Metallic Materials on the Safety of Biotechnology Products 1
 Designation: 00 Standard Guide for Determining the Impact of Extractables from Non-Metallic Materials on the Safety of Biotechnology Products 1 This standard is issued under the fixed designation ; the
Designation: 00 Standard Guide for Determining the Impact of Extractables from Non-Metallic Materials on the Safety of Biotechnology Products 1 This standard is issued under the fixed designation ; the
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CHEMITOX, INC. YAMANASHI TESTING CENTER 18349 Egusa, Sutama-cho Hokuto-shi, Yamanashi-Ken, Japan Ms. Kuniko Ito (Authorized Representative) Phone: 81 551 42
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CHEMITOX, INC. YAMANASHI TESTING CENTER 18349 Egusa, Sutama-cho Hokuto-shi, Yamanashi-Ken, Japan Ms. Kuniko Ito (Authorized Representative) Phone: 81 551 42
Use of International Standard ISO , "Biological Evaluation of Medical Devices Part 1: Evaluation and Testing"
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Use of International Standard ISO- 10993, "Biological Evaluation of Medical Devices Part 1: Evaluation and Testing" Draft Guidance
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Use of International Standard ISO- 10993, "Biological Evaluation of Medical Devices Part 1: Evaluation and Testing" Draft Guidance
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
ISO/TS TECHNICAL SPECIFICATION
 TECHNICAL SPECIFICATION ISO/TS 10993-20 First edition 2006-08-01 Biological evaluation of medical devices Part 20: Principles and methods for immunotoxicology testing of medical devices Évaluation biologique
TECHNICAL SPECIFICATION ISO/TS 10993-20 First edition 2006-08-01 Biological evaluation of medical devices Part 20: Principles and methods for immunotoxicology testing of medical devices Évaluation biologique
ONAMER M. PRESERVATIVE and ANTIMICROBIAL ONAMER
 ONAMER M PRESERVATIVE and ANTIMICROBIAL ONAMER M Stepan Lipid Nutrition is a division of Stepan Company which manufactures lipid and polymer based ingredients. HO OH SUMMARY Our quaternary ammonium polymer
ONAMER M PRESERVATIVE and ANTIMICROBIAL ONAMER M Stepan Lipid Nutrition is a division of Stepan Company which manufactures lipid and polymer based ingredients. HO OH SUMMARY Our quaternary ammonium polymer
JUL Sponsor/Applicant Name and Address Penumbra, Inc Harbor Bay Parkway Alameda, CA 94502, USA
 JUL 0 3 2014-510(k) Summary ILH 134f (as required by 21 CER 807.92) Pursuant to Section 12, Part (a)(i)(3a) of the Safe Medical Devices Act of 1990, Penumbra Inc. is providing the summary of Substantial
JUL 0 3 2014-510(k) Summary ILH 134f (as required by 21 CER 807.92) Pursuant to Section 12, Part (a)(i)(3a) of the Safe Medical Devices Act of 1990, Penumbra Inc. is providing the summary of Substantial
Satisfying ISO and US FDA Biocompatibility requirements for Breathing Gas Pathways in Healthcare Application
 Satisfying ISO 18562 and US FDA Biocompatibility requirements for Breathing Gas Pathways in Healthcare Application Audrey Turley, B.S., RM (NRCM), CBA Biocompatibility Expert 801-290-7907 aturley@nelsonlabs.com
Satisfying ISO 18562 and US FDA Biocompatibility requirements for Breathing Gas Pathways in Healthcare Application Audrey Turley, B.S., RM (NRCM), CBA Biocompatibility Expert 801-290-7907 aturley@nelsonlabs.com
ISO INTERNATIONAL STANDARD. Biological evaluation of medical devices Part 17: Establishment of allowable limits for leachable substances
 INTERNATIONAL STANDARD ISO 10993-17 First edition 2002-12-01 Biological evaluation of medical devices Part 17: Establishment of allowable limits for leachable substances Évaluation biologique des dispositifs
INTERNATIONAL STANDARD ISO 10993-17 First edition 2002-12-01 Biological evaluation of medical devices Part 17: Establishment of allowable limits for leachable substances Évaluation biologique des dispositifs
Services for Medical Devices. Expert solutions for product development from concept to commercialization
 Services for Medical Devices Expert solutions for product development from concept to commercialization Services for Medical Devices WuXi AppTec s comprehensive testing programs and expert guidance help
Services for Medical Devices Expert solutions for product development from concept to commercialization Services for Medical Devices WuXi AppTec s comprehensive testing programs and expert guidance help
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL CERTIFIED TESTING LABORATORIES, INC. (NCTL-YORK) 5 Leigh Drive York, PA 17406 Amy Becker Phone: 717 846 1200 abecker@nctlinc.com MECHANICAL Valid To:
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL CERTIFIED TESTING LABORATORIES, INC. (NCTL-YORK) 5 Leigh Drive York, PA 17406 Amy Becker Phone: 717 846 1200 abecker@nctlinc.com MECHANICAL Valid To:
Leachable and Extractable Testing
 Leachable and Extractable Testing A Primer on Regulations and Methods As presented to By: Anthony Grilli, MS General Manager SGS LSS NJ Laboratory 973 244 2435 Anthony.Grilli@SGS.com Summary Why perform
Leachable and Extractable Testing A Primer on Regulations and Methods As presented to By: Anthony Grilli, MS General Manager SGS LSS NJ Laboratory 973 244 2435 Anthony.Grilli@SGS.com Summary Why perform
SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006. : Fazor. Version 2.0 Revision Date Print Date
 1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Trade name : 1.2 Relevant identified uses of the substance or mixture and uses advised against Use of the
1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Trade name : 1.2 Relevant identified uses of the substance or mixture and uses advised against Use of the
SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006 : FAZOR 60 SG. Version 2.0 Revision Date Print Date
 1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Trade name : 1.2 Relevant identified uses of the substance or mixture and uses advised against Use of the
1. Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Trade name : 1.2 Relevant identified uses of the substance or mixture and uses advised against Use of the
SAFETY DATA SHEET <#####> RABON 50% WETTABLE POWDER INSECTICIDE Version 1.1 Revision Date 02/20/2013
 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING Product information Product Name: MSDS Number: 122000008516 Company BAYER HEALTHCARE LLC Animal Health Division 12707 Shawnee Mission
1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING Product information Product Name: MSDS Number: 122000008516 Company BAYER HEALTHCARE LLC Animal Health Division 12707 Shawnee Mission
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 10993-9 Second edition 2009-12-15 Biological evaluation of medical devices Part 9: Framework for identification and quantification of potential degradation products Évaluation
INTERNATIONAL STANDARD ISO 10993-9 Second edition 2009-12-15 Biological evaluation of medical devices Part 9: Framework for identification and quantification of potential degradation products Évaluation
NAMSA is a Medical Research Organization (MRO), accelerating product development
 NAMSA is a Medical Research Organization (MRO), accelerating product development through integrated laboratory, clinical and consulting services. Driven by our regulatory expertise, NAMSA's MRO Approach
NAMSA is a Medical Research Organization (MRO), accelerating product development through integrated laboratory, clinical and consulting services. Driven by our regulatory expertise, NAMSA's MRO Approach
IMPLANT LINE PRODUCT GUIDE
 IMPLANT LINE GUIDE LONG TERM COMMITMENT LIQUID SILICONE RUBBERS @ MED-4810 11 700 (4.8) 45 (0.3) @ 200 1,075 65 (11.5) 5 m / 150 MED-4820 20 910 (6.3) 65 (0.5) @ 200 875 125 (22.0) 5 m / 150 Our signature
IMPLANT LINE GUIDE LONG TERM COMMITMENT LIQUID SILICONE RUBBERS @ MED-4810 11 700 (4.8) 45 (0.3) @ 200 1,075 65 (11.5) 5 m / 150 MED-4820 20 910 (6.3) 65 (0.5) @ 200 875 125 (22.0) 5 m / 150 Our signature
Supplies and Reagents
 Supplies and Reagents PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 4 th /5 th, 2006 NHLBI-sponsored PACT Group Guidance for Industry INDs Approaches to Complying with CGMP During
Supplies and Reagents PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 4 th /5 th, 2006 NHLBI-sponsored PACT Group Guidance for Industry INDs Approaches to Complying with CGMP During
SCOPE OF ACCREDITATION TO ISO/IEC
 SCOPE OF ACCREDITATION TO ISO/IEC 17025-2005 RADIOMETRICS MIDWEST CORPORATION 12 East Devonwood Avenue Romeoville, IL 60446 Joseph Strzelecki Phone: 815 293 0772 ELECTRICAL (EMC) Valid To: February 29,
SCOPE OF ACCREDITATION TO ISO/IEC 17025-2005 RADIOMETRICS MIDWEST CORPORATION 12 East Devonwood Avenue Romeoville, IL 60446 Joseph Strzelecki Phone: 815 293 0772 ELECTRICAL (EMC) Valid To: February 29,
PHARMACEUTICAL TESTING
 WHITEHOUSE, NJ PHARMACEUTICAL TESTING Pharmaceutical Expertise for GMP & CMC Testing Our Pharmaceutical Expertise With more than 20 years of experience in a variety of industries, our Whitehouse, New Jersey
WHITEHOUSE, NJ PHARMACEUTICAL TESTING Pharmaceutical Expertise for GMP & CMC Testing Our Pharmaceutical Expertise With more than 20 years of experience in a variety of industries, our Whitehouse, New Jersey
HDGreen Safe DNA Dye Safety Test Reports
 HDGreen Safe DNA Dye Safety Test Reports IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name HDGreen Safe DNA Dye Catalog number ISII-HDGreen Supplier Information in case of emergency Intas Science
HDGreen Safe DNA Dye Safety Test Reports IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name HDGreen Safe DNA Dye Catalog number ISII-HDGreen Supplier Information in case of emergency Intas Science
