Intermediate Filaments in Motion: Observations of Intermediate Filaments in Cells Using Green Fluorescent Protein-Vimentin V
|
|
|
- Bartholomew Booth
- 6 years ago
- Views:
Transcription
1 Molecular Biology of the Cell Vol. 10, , May 1999 Intermediate Filaments in Motion: Observations of Intermediate Filaments in Cells Using Green Fluorescent Protein-Vimentin V Jayme L. Martys, Chung-Liang Ho,* Ronald K. H. Liem, and Gregg G. Gundersen Video Essay Departments of Anatomy and Cell Biology and Pathology, Columbia University College of Physicians and Surgeons, New York, NY Submitted October 15, 1998; Accepted February 18, 1999 Monitoring Editor: W. James Nelson INTRODUCTION Intermediate filaments (IFs) are the least understood of the three major cytoskeletal elements. One of the difficulties of studying the functions of IFs in vitro is that the IF proteins are, unlike microtubules (MTs) and microfilaments (MFs), quite insoluble in nondenaturing buffers and the assembly and disassembly of IFs can therefore not be studied under physiological conditions. Despite this limitation, in vitro studies have revealed important aspects of IF assembly (see Herrmann and Aebi, 1998), but they have not been able to address specific questions about IF dynamics in vivo. IFs are known to undergo dynamic changes in distribution and organization during cell growth, polarization, and differentiation. These changes in distribution occur without complete disassembly of IFs into individual subunits. Thus, unlike MTs or MFs, whose dynamics are driven by the concentration of the soluble subunits, IFs are dynamic despite the fact that there is very little IF protein found in the soluble fraction. By injection of rhodamine-labeled vimentin or expression of tagged vimentin, it has been shown that IF turnover occurs along the length of the filaments, unlike the turnover of MTs or MFs, which only add or subtract subunits from the filament ends. One question that arises from these results is whether the turnover of the IF array is the result of an insoluble intermediate and whether one can visualize these intermediates in vivo. MTs and perhaps other cellular structures are required to form the extended array of IFs in cells. Drugs that induce breakdown of MTs cause collapse of IFs to the perinuclear region (Goldman, 1971; Hynes and Destree, 1978; Wang and Choppin, 1981; Masurovsky V Online version of this essay contains video material for Figures 1 7. Online version available at * Present address: Department of Pathology, National Cheng- Kung University, Tainan, Taiwan, Republic of China. et al., 1982; Gurland and Gundersen, 1995). Two separate studies have demonstrated that microinjection of tubulin antibodies promotes collapse of IFs to the perinuclear region (Blose and Feramisco 1984; Gurland and Gundersen, 1995). This apparent association of IFs with MTs has also been shown to require kinesin as a linker molecule, because microinjection of kinesin antibodies (Gyoeva and Gelfand, 1991) or tubulin fragments that interfere with kinesin binding to MTs (Kreitzer et al., 1999) promote collapse of the IF array. Furthermore, a kinesin protein has been shown to bind directly to vimentin filaments in vitro (Liao and Gundersen, 1988). These results suggest that IFs may be extended in cells by MT-based motor proteins. To study IF assembly and dynamics in vivo, we have used stable cell lines expressing green fluorescent protein (GFP)-vimentin (Ho et al., 1998). We were particularly interested in understanding how IFs are extended in cells and whether intermediates in the turnover and assembly of IFs can be detected. In this assay, we summarize some of our previously published results and include examples that support the idea that IFs move along MTs and that small fragments of IFs may be intermediates in the turnover of the IF array. Since our initial report (Ho et al., 1998), similar findings have been reported from the Goldman laboratory (Prahlad et al. 1998; Yoon et al., 1998). VIDEO MOVIES Movie 1: The IF Array Is Dynamic To analyze the behavior of vimentin IFs in living cells, a chimeric protein was constructed. The enhanced form of GFP was fused to the amino-terminal end of vimentin and expressed in NIH3T3 cells. GFP-vimentin constituted 20% of the cellular vimentin in the stably transfected NIH3T3 cells (for more detail, see Ho et al., 1998). Recordings of 4 h were possible with little photobleaching of GFP fluorescence, making 1999 by The American Society for Cell Biology 1289
2 J.L. Martys et al. Figure 1. Movie 1: The IF array is dynamic. Shown is the behavior of the IF array in an NIH3T3 cell expressing GFP-vimentin. The movements of the IF array in this movie are typical of other arrays we have recorded. Bar, 15 m. Figure 2. Movie 2: IFs in a mitotic cell form cage-like structures around dividing nuclei. The organization of GFP-vimentin in a dividing NIH3T3 cell is shown. The IF array forms cage-like structures around the daughter nuclei, and it is connected by a bridge. GFP-vimentin a good reporter for observing IF behavior in living cells. For experiments presented in this essay, we studied cells at the edge of a wounded monolayer, because these cells become highly polarized when they migrate into the wound, and we suspect that IFs undergo corresponding rearrangements (Gurland and Gundersen, 1995). Specific conditions have been described in detail by Ho et al. (1998). Initial low-magnification recordings of GFP-vimentin expressing cells revealed the dynamic behavior of the IF array. IFs were observed to change curvature and orientation over the course of several minutes. In longer recordings it was possible to visualize a wavelike motion for IFs. Presented in Movie 1 (Ho et al., 1998) is a recording where many of the IFs were seen to shift and exhibit this wavy motion. This recording was 90 min in length (frame interval, 2.5 min). The still figure presented (Figure 1) is the first image from Movie 1, and the arrow indicates an IF that bends in a wave-like motion. It is important to note that larger bundles of IFs did not appear to bend as much as the smaller IF bundles. In general, individual IFs were observed to be extending toward the edge of the cell, while concurrently the entire array appeared to be pulled inward toward the nucleus. The movement toward the nucleus is similar to what has been described as centripetal transport of other cytoskeletal elements (Theriot and Mitchison, 1992; Mikhailov and Gundersen, 1995). The nucleus in this cell moved dramatically at the beginning of the movie and distorted the IFs. Some IFs also appeared to move with the nucleus, suggesting that they may be tethered to the nuclear envelope. This movie illustrates the usefulness of using GFP-vimentin, because these types of movements would not have been observed using other methods. Movie 2: IFs in a Mitotic Cell Form Cage-like Structures around Dividing Nuclei The ability to detect IFs in living cells with GFP fusion proteins offered us the opportunity to examine the IF network of mitotic cells in three dimensions using confocal microscopy. To obtain optimal three-dimensional resolution, optical sections of 0.5 m were scanned eight times. Additionally, to avoid flattening, the cells were imaged in coverslip dishes in which a hole had been punched in the bottom and a coverslip had been placed on the bottom of the dish. Confocal microscopy was performed with a Zeiss LSM 410 scanning laser confocal attachment mounted on a Zeiss Axiovert 100 TV inverted fluorescence microscope (Carl Zeiss, Jena, Germany). The stack of images for all the scanned z-planes was processed using Zeiss LSM software to produce the final three-dimensional image. The fixed cell shown in Movie 2 was in telophase (Figure 2); from Ho et al., 1998, cover photo). Through the 360 rotation, we clearly see cables of filaments that appear to run through the midbody connecting the two daughter cells. These data are compatible with the idea that the vimentin network does not completely disassemble during mitosis but rather remains in cage-like structures around the dividing nuclear material. The GFP-vimentin stable cell lines should be useful to explore further the dynamics and rearrangements of IFs during mitosis. Movie 3: Individual Vimentin IFs Extend, Retract, and Translocate The dynamic behavior of the IF array must be dependent on movements of individual IF bundles. To ana Molecular Biology of the Cell
3 Intermediate Filament Dynamics Figure 3. Movie 3: Vimentin IFs extend, retract, and translocate. IFs at the edge of the cell undergo continuous changes in position. The filaments extend, retract, and shift. Shown is the first frame of the movie, which demonstrates the movements of IFs at the edge of the cell. Bar, 10 m. lyze movements of individual filament bundles, we focused on the leading edge of migrating cells (Movie 3, from Ho et al., 1998; Figure 3). During the recording process, the focus was maintained on the edge of the cell, sometimes at the expense of focus on the entire array. The intensity of the image had to be scaled so that the filaments (or bundles of filaments) at the edge of the cell were detectable. Images were processed every 2.5 min over the course of 2.5 h. Several types of movements were observed for individual filament bundles in the cells we observed. Extension and retraction of several IFs at the edge of the cell were detected. The average rate of filament extension measured for vimentin IFs was 0.61 m/min. Translocation, defined as a lateral shift in a filament without obvious change in length, could also be observed for many filaments at the edges of the cells. In addition to the movements of intact IFs, we observed the presence of vimentin fragments at the edge of the cell. Further analysis of vimentin fragments revealed that they also exhibit dynamic behavior. These movements are better described in Movies 4 and 6. Movie 4: Fountains the Movements of IF Fragments In some cells, we observed an abundance of fragments in the cell periphery, particularly at the leading edge of the cell (Movie 4). The movie presented is from the region enclosed in the box in Figure 4D. Movie 4 is almost 50 min, with images acquired every 2 min. This movie shows fragments that moved in a linear manner toward the edge of the cell and then turned and moved back toward the cell body. In this movie, individual fragments were continuously generated during the entire sequence. Sequentially formed fragments followed the same general paths on their way out toward the leading edge of the cell. The overall path followed by these fragments resembled a fountain, and so we have dubbed them vimentin fountains. The fragments within these fountains moved at an average rate of 15 m/min. This is 25 times faster than that seen for extension of individual IF bundles. We have seen numerous vimentin fragments in 50% of the cells at the wound edge, suggesting that this is a common feature of IF dynamics in these cells. We do not know whether fragments only form at the edge of the cell or, rather, can only be detected there. On occasion we have observed them along the lateral edges of the cell. One concern with the fragments is that they are artifacts produced either by the GFP-vimentin chimera or by photo damage during fluorescent imaging. To address this question, we examined parental NIH3T3 cells that were fixed in 20 C methanol and immunofluorescently stained for endogenous vimentin. The parental cell line exhibited immunofluorescently detectable fragments near the leading edge (Figure 4C) that were similar in number and size to those detected in the GFP-vimentin cell line by either direct GFP fluorescence (Figure 4A) or immunofluorescence (Figure 4B). This demonstrates that GFP fragments were not a product of GFP-vimentin expression or of the imaging conditions. Movie 5: GFP-Vimentin Fragments Intermediates of IF Turnover One possible explanation for the presence of the GFPvimentin fragments is that they are an intermediate in the turnover of vimentin filaments. In agreement with this possibility, we observed the apparent joining of two fragments onto the end of a single filament bundle (Movie 5A). Figure 5A shows selected frames from a recording in which the fragments have been outlined. Over the course of the 13-min movie, the two fragments were seen to align with a longer IF that appeared to come from the IF array. These aligned fragments then extended together toward the edge of the cell. Although we cannot be certain that the fragments actually annealed into a single IF bundle, the unified extension of these coaligned fragments is consistent with this possibility. It is interesting to speculate that IFs may increase in length through en bloc incorporation of fragments at the ends of filaments and that the fragments we have observed are an intermediate in IF formation. We have also detected the formation of two smaller fragments from a larger IF segment (Movie 5B). At this time we are not able to rule out the possibility that the Vol. 10, May
4 J.L. Martys et al. Figure 4. Movie 4: Fountains the movements of IF fragments. IF fragments are present in GFP-vimentin expressing cells and in the parental NIH3T3 cells. (A and B) GFP-vimentin expressing NIH3T3 cells. (A) GFP fluorescence. (B) Indirect immunofluorescent pattern of the same cells fixed and stained as described by Gurland and Gundersen (1995) using a polyclonal antibody (1274) (Wang et al., 1984). Arrows point to two of the many fragments present. (C) NIH3T3 cells fixed and stained as in B. Arrows point to IF fragments present in the parental cells. (D) First frame of a movie in which the IF fragments display dynamic movements. The movie is from the region enclosed in the box and is played four times. There is a pause only after the first time through the movie. Bars: (A, B, and D) 10 mm; (C) 5 mm. apparent formation of fragments in this recording may be explained by an initial coalignment of two separate fragments that subsequently separate from each because of movements at different speeds. Yet, it is reasonable to suggest that vimentin fragment intermediates may form through this mechanism. We speculate that if one end of the fragment is immobilized and kinesin pulls on the other, or if there is an uneven distribution of kinesin, small fragments of IFs may be formed. The observations presented in these two movies, although not conclusive, suggest that IF fragments are an important component of IF dynamics, possibly an intermediate, and may be directly involved in IF turnover and rearrangement. Prahlad et al. (1998) ob1292 served similar IF fragments, termed squiggles, before the formation of a filamentous IF array in spreading cells. Movie 6: Movement of GFP-Vimentin Fragments Depends on Intact MTs There is considerable evidence that MTs play an important role in the distribution of IFs. We wanted to know whether MTs played a direct role in the observed movements of GFP-vimentin filaments or GFPvimentin fragments that we observed in our recordings. To address this question, we recorded the movements of IFs and fragments before and after Molecular Biology of the Cell
5 Intermediate Filament Dynamics Figure 5. Movie 5: GFP-vimentin fragments possible intermediates in IF turnover. (A) The coalignment of two GFP-vimentin fragments with an IF is observed. Every other frame from the first 10 min of the movie is shown, with the IFs of interest outlined. In the first frame the fragments of interest are outlined as they are in the movie. Bar, 5 m. (B) We observed the formation of two fragments from a longer fragment. The six frames of the movie are shown. As in A, the fragment(s) of interest are outlined. Bar, 5 m. Vol. 10, May
6 J.L. Martys et al. perfusion of 20 m nocodazole. In Movie 6, the effect of nocodazole on both filament extension and fragment movement is presented (Figure 6). The movements of IFs were recorded for 20 min during perfusion with nocodazole. Before nocodazole addition, the filaments can be seen extending and moving as in the other movies presented above. Additionally, both outward and inward movements of vimentin fragments were observed. During nocodazole perfusion (4 ml at 1 ml/min), imaging was difficult, and there is a break of 4 min between the last frame before perfusion and the first frame after perfusion. After perfusion the recording was continued for another 35 min with images acquired every minute. Almost immediately after perfusion, the dynamic behavior of both the IF bundles and fragments decreased. The filaments no longer extended, and the fragments stopped moving forward toward the edge of the cell. Movement in the direction of the nucleus continued as the IF array began to collapse toward the perinuclear region. This experiment and an earlier one reported by Ho et al. (1998) show that both the slower-moving intact IFs and the faster-moving IF fragments depend on MTs for their outward movements. Movie 7: Collapse of the IF Array Occurs through Redistribution of Intact Filaments There are a number of cellular treatments (microinjection of antibodies to IFs, tubulin, or kinesin or treatment with MT antagonists) that promote collapse of the IF array to a perinuclear location (Goldman, 1971; Hynes and Destree, 1978; Wang and Choppin, 1981; Klymkowsky, 1982; Masurovsky et al., 1982; Blose and Feramisco, 1984; Gyoeva and Gelfand, 1991; Gurland and Gundersen, 1995). From these earlier studies, it was unclear whether the rearrangement represented a redistribution of intact filaments or some dynamic disassembly reassembly process as has been observed for MTs and MFs. In Movie 7, we examined the collapse of the IF array, in real time, by microinjecting anti-ifa antibody, which had previously been shown to collapse IFs (Klymkowsky, 1982) (Figure 7). Cells at a wound edge were injected with anti-ifa and imaged as previously described (see Ho et al., 1998). The arrow in the still image presented as the start of the movie points to an IF that is observed to move in bulk to an area on the other side of the nucleus. The GFP-vimentin IF array did not disassemble but instead appeared to be moved back toward the center of the cell. Control experiments (see Ho et al., 1998) demonstrated that the cell remained spread and extended and that the MT array was not disrupted. Although anti-ifa can induce disassembly of IFs in vitro, the use of time-lapse recording in this experiment demonstrates that the collapse of IFs to a perinuclear area in vivo involves Figure 6. Movie 6: Movement of GFP-vimentin fragments depends on intact MTs. Vimentin fragments stop moving in the presence of nocodazole. Shown are a number of fragments at the edge of the cell. The fragments move during the first part of the recording (before perfusion), as shown by the change in fragment location from 0 to 21 min. After perfusion of 20 M nocodazole (NOC, bottom right), the fragments stop moving toward the edge of the cell and are only seen moving away from the edge, as is shown by the different fragment distributions at 25 and 55 min. Bar, 10 m Molecular Biology of the Cell
7 Intermediate Filament Dynamics Figure 7. Movie 7: Collapse of the IF array occurs through redistribution of intact filaments. Anti-IFA antibody was microinjected into cells expressing GFP-vimentin, and the resultant redistribution of the IF array was recorded. Bar, 10 m. bulk movement of the IFs rather than a disassembly reassembly process. SUMMARY In the movies presented in this essay we have demonstrated that IFs are dynamic in nature. Movements of IFs include both extension and retraction and are dependent on the presence of intact MTs. Furthermore, we have characterized a possible intermediate of IF turnover, IF fragments. These fragments are also dynamic and can be visualized moving at the leading edge of cells migrating into a wound edge. Interestingly, the movement of these fragments is also dependent on intact MTs, suggesting that MTs may play a role in turnover of IFs. ACKNOWLEDGMENTS The confocal images were taken by Teresa Swayne. Her efforts are greatly appreciated. This work was supported by National Institutes of Health grants NS (to R.K.H.L.) and GM (to G.G.G.). J.L.M. is a postdoctoral trainee supported by National Research Service Award training grant GM REFERENCES Blose, S.H., Meltzer, D.I., and Feramisco, J.R. (1984). 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J. Cell Biol. 98, Goldman, R.D. (1971). The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J. Cell Biol. 51, Gurland, G., and Gundersen, G.G. (1995). Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J. Cell Biol. 131, Gyoeva, F.K., and Gelfand, V.I. (1991). Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature 353, Herrmann, H., and Aebi, U. (1998). Intermediate filament assembly: fibrillogenesis is driven by decisive dimer-dimer interactions. Curr. Opin. Struct. Biol. 8, Ho, C.-L., Martys, J.L., Mikhailov, A., Gundersen, G.G., and Liem, R.K.H. (1998). Novel features of intermediate filament dynamics revealed by green fluorescent protein chimeras. J. Cell Sci. 111, Hynes, R.O., and Destree, A.T. (1978). 10 nm filaments in normal and transformed cells. Cell 13, Klymkowsky, M.W. (1982). Vimentin and keratin intermediate filament systems in cultured PtK2 epithelial cells are interrelated. EMBO J. 1, Kreitzer, G., Liao, G., and Gundersen, G.G. (1999). Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo through a kinesin-dependent mechanism. Mol. Biol. Cell 10, Lamb, N., Fernandes, A., Feramisco, J.R., and Welch, W.J. (1989). Modulation of vimentin containing intermediate filament distribution and phosphorylation in living fibroblasts by the camp-dependent protein kinase. J. Cell Biol. 108, Liao, G., and Gundersen, G.G. (1988). Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. J. Biol. Chem. 273, Masurovsky, E.B., Peterson, E.R., Crain, S.M., and Horowitz, S.B. (1982). Taxol-induced microtubule formations in fibroblasts of fetal mouse dorsal root ganglion-spinal cord cultures. Biol. Cell 46, Mikhailov, A.L., and Gundersen, G.G. (1995). The centripetal transport of microtubules in motile cells. Cell Motil. Cytoskeleton 32, Prahlad, V., Yoon, M., Moir, R.D., Vale, R.D., and Goldman, R.D. (1998). Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143, Theriot, J.A., and Mitchison, T.S. (1992). Comparison of actin and cell surface dynamics in motile fibroblasts. J. Cell Biol. 119, Wang, E., Cairncross, J.G., and Liem, R.K. (1984). Identification of glial filament protein and vimentin in the same intermediate filament system in human glioma cells. Proc. Natl. Acad. Sci. USA 81, Wang, E., and Choppin, P.W. (1981). Effect of vanadate on intracellular distribution and function of 10-nm filaments. Proc. Natl. Acad. Sci. USA 78, Yoon, M., Moir, R.D., Prahlad, V., and Goldman, R.D. (1998). Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, Vol. 10, May
Chapter 15. Cytoskeletal Systems. Lectures by Kathleen Fitzpatrick Simon Fraser University Pearson Education, Inc.
 Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
Chapter 15 Cytoskeletal Systems Lectures by Kathleen Fitzpatrick Simon Fraser University Table 15-1 - Microtubules Table 15-1 - Microfilaments Table 15-1 Intermediate Filaments Table 15-3 Microtubules
CHAPTER 16 THE CYTOSKELETON
 CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
Three major types of cytoskeleton
 The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
T H E J O U R N A L O F C E L L B I O L O G Y
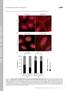 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Feng et al., http://www.jcb.org/cgi/content/full/jcb.201408079/dc1 Figure S1. A modest elevation of disulfide-bonded K14 in primary mouse
Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules. Chapter 17. Microtubules
 Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Chapter 15. Mechanism of Vesicle Formation A reminder: two things I said that we should to keep an eye on for each of the components of the cytoskeleton: The role of polymerization and depolymerization
Microtubules. Forms a sheet of protofilaments that folds to a tube Both tubulins bind GTP
 Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Microtubules Polymerize from a-tubulin/ß-tubulin dimers Hollow tube of 13 protofilaments In vitro- filament number varies In vivo- always 13 protofilaments Forms a sheet of protofilaments that folds to
Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc.
 15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
15/05/2012 Super-resolution imaging: early days w/ Video-enhanced DIC, TIRF, PALM, STORM, etc. Prof. Dr. Rainer Duden duden@bio.uni-luebeck.de 1 Using conventional light microscopy resolution is limited
The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit
 Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Cell Reports, Volume 5 Supplemental Information The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina During Interphase and Mitotic Exit Andrey Poleshko, Katelyn M. Mansfield, Caroline
Intermediate filaments (IF)1 are major cytoskeletal
 Motile Properties of Vimentin Intermediate Filament Networks in Living Cells Miri Yoon, Robert D. Moir, Veena Prahlad, and Robert D. Goldman Northwestern University Medical School, Department of Cell and
Motile Properties of Vimentin Intermediate Filament Networks in Living Cells Miri Yoon, Robert D. Moir, Veena Prahlad, and Robert D. Goldman Northwestern University Medical School, Department of Cell and
October 24, BIOS 95: Cell Division and Chromosome Dynamics
 October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 1 SUPPLEMENTARY INFORMATION 2 WWW.NATURE.COM/NATURECELLBIOLOGY SUPPLEMENTARY INFORMATION WWW.NATURE.COM/NATURECELLBIOLOGY 3 SUPPLEMENTARY INFORMATION
Figure legends for supplement
 Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Figure legends for supplement Supplemental Figure 1 Characterization of purified and recombinant proteins Relevant fractions related the final stage of the purification protocol(bingham et al., 1998; Toba
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
Cytoskeleton. B. Balen
 Cytoskeleton B. Balen structural backbone of the cell supports the fragile plasma membrane provides mechanical linkages that let the cell bear stresses without being ripped apart Importance and function
Cytoskeleton B. Balen structural backbone of the cell supports the fragile plasma membrane provides mechanical linkages that let the cell bear stresses without being ripped apart Importance and function
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Supplemental material Thompson et al., http://www.jcb.org/cgi/content/full/jcb.200909067/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Modification-specific antibodies do not detect unmodified
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Biophysics of Molecules
 Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
Biophysics of Molecules Cytoskeletal filaments, Actin polymerization and Actin Treadmilling Part 2 (26.11.2012) Dr. Carsten Grashoff MPI of Biochemistry E-mail: cgrasho@biochem.mpg.de Lecture Outline The
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
(phosphatase tensin) domain is shown in dark gray, the FH1 domain in black, and the
 Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
Supplemental Figure 1. Predicted Domain Organization of the AFH14 Protein. (A) Schematic representation of the predicted domain organization of AFH14. The PTEN (phosphatase tensin) domain is shown in dark
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Hong et al.,
 Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Supplemental material JCB Hong et al., http://www.jcb.org/cgi/content/full/jcb.201412127/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Analysis of purified proteins by SDS-PAGE and pull-down assays. (A) Coomassie-stained
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
SUPPLEMENTARY INFORMATION
 DOI: 1.18/ncb46 a Damage Fracture s s 7 5 16 1 9 26 5 µm 5 µm b Position of photo-damage site in cells 15 Counts 1 5-14 -12-1 -8-6 -4-2 Distance from damage site to tip (µm) Supplementary Figure 1 Effect
DOI: 1.18/ncb46 a Damage Fracture s s 7 5 16 1 9 26 5 µm 5 µm b Position of photo-damage site in cells 15 Counts 1 5-14 -12-1 -8-6 -4-2 Distance from damage site to tip (µm) Supplementary Figure 1 Effect
Rapid Movements of Vimentin on Microtubule Tracks: Kinesin-dependent Assembly of Intermediate Filament Networks
 Rapid Movements of Vimentin on Microtubule Tracks: Kinesin-dependent Assembly of Intermediate Filament Networks Veena Prahlad,* Miri Yoon,* Robert D. Moir,* Ronald D. Vale, and Robert D. Goldman* *Department
Rapid Movements of Vimentin on Microtubule Tracks: Kinesin-dependent Assembly of Intermediate Filament Networks Veena Prahlad,* Miri Yoon,* Robert D. Moir,* Ronald D. Vale, and Robert D. Goldman* *Department
MCBII. Points this page
 1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
1. What makes intermediate filaments (IFs) an inefficient track for motor proteins (4 pts)? A. The outer surface of IFs is hydrophobic. B. IFs are nonpolar structures. C. IFs contain coiled coil domains.
In vitro EGFP-CALI comprehensive analysis. Liang CAI. David Humphrey. Jessica Wilson. Ken Jacobson. Dept. of Cell and Developmental Biology
 In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
5 HARNESSING THE PURSE STRING FOR
 107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced
 Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
Biophysical Journal, Volume 113 Supplemental Information Acetylated Microtubules Are Preferentially Bundled Leading to Enhanced Kinesin-1 Motility Linda Balabanian, Christopher L. Berger, and Adam G. Hendricks
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental Material
 Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
Supplemental Material 1 Figure S1. Phylogenetic analysis of Cep72 and Lrrc36, comparative localization of Cep72 and Lrrc36 and Cep72 antibody characterization (A) Phylogenetic alignment of Cep72 and Lrrc36
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
CELL BIOLOGY - CLUTCH CH CYTOSKELETON AND CELL MOVEMENT.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
!! www.clutchprep.com CONCEPT: OVERVIEW OF THE CYTOSKELETON The cytoskeleton is an intricate of protein filaments that extend throughout the cytoplasm It is a highly organized and dynamic structure (constantly
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
3D Transfection System
 3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
Resolution of Microscopes Visible light is nm Dry lens(0.5na), green(530nm light)=0.65µm=650nm for oil lens (1.4NA) UV light (300nm) = 0.13µm f
 Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
CYTOSKELETON, MOTORPROTEINS.
 CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
CYTOSKELETON, MOTORPROTEINS. SCIENCE PHOTO LIBRARY Tamás Huber 15. 10. 2012. CYTOSKELETON: Dynamic scaffold in eukaryotic cells Three main filament systems: A. Intermediate filaments B. Microtubules C.
Biosensors. DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses)
 Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
Biosensors DNA Microarrays (for chemical analysis) Protein Sensors (for identifying viruses) DNA Microarrays 40 000 detectors in parallel, each detecting a specific DNA sequence. Combinatorial Chemistry
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin
 Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin (WGA-Alexa555) was injected into the extracellular perivitteline
Fig. S1. Endocytosis of extracellular cargo does not depend on dia. (A) To visualize endocytosis, fluorescently labelled wheat germ agglutinin (WGA-Alexa555) was injected into the extracellular perivitteline
SUPPLEMENTARY INFORMATION
 Clustering of a kinesin-14 motor enables processive retrograde microtubule-based transport in plants NATURE PLANTS www.nature.com/natureplants 1 2 NATURE PLANTS www.nature.com/natureplants NATURE PLANTS
Clustering of a kinesin-14 motor enables processive retrograde microtubule-based transport in plants NATURE PLANTS www.nature.com/natureplants 1 2 NATURE PLANTS www.nature.com/natureplants NATURE PLANTS
Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends.
 SUPPLEMENTAL DATA Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends. Supplemental Experimental Procedures Dominant negative nesprin KASH (DN KASH) plasmid construction-
SUPPLEMENTAL DATA Supplemental Data includes Supplemental Experimental Procedures, 4 Figures and 5 Movie Legends. Supplemental Experimental Procedures Dominant negative nesprin KASH (DN KASH) plasmid construction-
Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true?
 Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true? A. COPII and COPI vesicles are transported with dynein. B. COPII and COPI vesicles
Golgi are located near the MTOC while the ER is spread throughout the cytoplasm, so which of the following is probably true? A. COPII and COPI vesicles are transported with dynein. B. COPII and COPI vesicles
- ex. polarized epithelial cells: certain organelles are arranged in defined order from apical to basal end of cell
 THE CYTOSKELETON - vertebrates: skeleton ; eukaryotic cells: cytoskeleton (analogous) - consists of hardened elements that support soft tissues of body - mediates bodily movements - 3 elements of cytoskeleton
THE CYTOSKELETON - vertebrates: skeleton ; eukaryotic cells: cytoskeleton (analogous) - consists of hardened elements that support soft tissues of body - mediates bodily movements - 3 elements of cytoskeleton
me239 mechanics of the cell homework biopolymers - motivation 3.1 biopolymers - motivation 3. biopolymers biopolymers biopolymers
 3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
Supporting Online Material Material and Methods
 Supporting Online Material Material and Methods Live-cell DIC recordings PtK 1 cells (ATCC, Manassas, VA) were filmed on a Nikon (Nikon Instruments, Melville, NY) inverted microscope equipped with 60x
Supporting Online Material Material and Methods Live-cell DIC recordings PtK 1 cells (ATCC, Manassas, VA) were filmed on a Nikon (Nikon Instruments, Melville, NY) inverted microscope equipped with 60x
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
T H E J O U R N A L O F C E L L B I O L O G Y
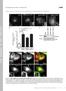 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Allison et al., http://www.jcb.org/cgi/content/full/jcb.201211045/dc1 Figure S1. Spastin depletion causes increased endosomal tubulation.
Biophysics of contractile ring assembly
 Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
Supplemental material JCB. Pitaval et al., Cell shape and ciliogenesis Pitaval et al.
 Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called.
 Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Name: Quiz name: Quiz 7 ate: 1. The microtubule wall is composed of globular proteins arranged in longitudinal rows called. microfilaments protofilaments prototubules microtubular subunits 2. Which of
Phalloidin-induced actin polymerization in the cytoplasm of
 Proc. Nati. Acad. Sci. USA Vol. 74, No. 12, pp. 5613-5617, December 1977 Cell Biology Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth
Proc. Nati. Acad. Sci. USA Vol. 74, No. 12, pp. 5613-5617, December 1977 Cell Biology Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth
Molecular Cell Biology
 Molecular Cell Biology Biophysics of Helical Polymers Intermediate Filaments Michael Greenberg Department of Biochemistry and Molecular Biophysics greenberg@biochem.wustl.edu Intermediate Filaments Diversity
Molecular Cell Biology Biophysics of Helical Polymers Intermediate Filaments Michael Greenberg Department of Biochemistry and Molecular Biophysics greenberg@biochem.wustl.edu Intermediate Filaments Diversity
Supplementary Figures 1-6
 1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
1 Supplementary Figures 1-6 2 3 4 5 6 7 Supplementary Fig. 1: GFP-KlpA forms a homodimer. a, Hydrodynamic analysis of the purified full-length GFP-KlpA protein. Fractions from size exclusion chromatography
System of protein filaments in the cytoplasm of. eukaryotic cell that gives the cell shape and capacity for
 Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
SUPPLEMENTARY INFORMATION Dynamic Phosphorylation of HP1 Regulates Mitotic Progression in Human Cells Supplementary Figures Supplementary Figure 1. NDR1 interacts with HP1. (a) Immunoprecipitation using
BME Engineering Molecular Cell Biology. The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament
 BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
SONOMA STATE UNIVERSITY DEPARTMENT OF BIOLOGY BIOLOGY 344: CELL BIOLOGY Fall 2013
 SONOMA STATE UNIVERSITY DEPARTMENT OF BIOLOGY BIOLOGY 344: CELL BIOLOGY Fall 2013 Instructor Murali C. Pillai, PhD Office 214 Darwin Hall Telephone (707) 664-2981 E-mail pillai@sonoma.edu Website www.sonoma.edu/users/p/pillai
SONOMA STATE UNIVERSITY DEPARTMENT OF BIOLOGY BIOLOGY 344: CELL BIOLOGY Fall 2013 Instructor Murali C. Pillai, PhD Office 214 Darwin Hall Telephone (707) 664-2981 E-mail pillai@sonoma.edu Website www.sonoma.edu/users/p/pillai
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Charras et al.,
 JCB: SUPPLEMENTAL MATERIAL Charras et al., http://www.jcb.org/cgi/content/full/jcb.200602085/dc1 Supplemental information Constitutive expression of ezrin mutants modulates the proportion of blebbing cells
JCB: SUPPLEMENTAL MATERIAL Charras et al., http://www.jcb.org/cgi/content/full/jcb.200602085/dc1 Supplemental information Constitutive expression of ezrin mutants modulates the proportion of blebbing cells
Transcription factor dynamics in the nucleus
 Transcription factor dynamics in the nucleus March 21, 2018 Luca Giorgetti txnlecture2018@fmi.ch Transcription factors bind to enhancer and promoter regions Adapted from Streubel & Bracken EMBO J 2015
Transcription factor dynamics in the nucleus March 21, 2018 Luca Giorgetti txnlecture2018@fmi.ch Transcription factors bind to enhancer and promoter regions Adapted from Streubel & Bracken EMBO J 2015
Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb
 Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
Fig. S1 Sequence of C-terminal tail regions of myosin heavy chains in class XI of Nicotiana benthamiana (Nb myosin XI-2, -F and K) and BY-2 cell (Nt 170-kD myosin and Nt 175-kD myosin). Amino acids identical
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Ono et al., http://www.jcb.org/cgi/content/full/jcb.201208008/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Chromatin-binding properties of MCM2 and condensin II during
Supplemental material Ono et al., http://www.jcb.org/cgi/content/full/jcb.201208008/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Chromatin-binding properties of MCM2 and condensin II during
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
The microtubule-associated tau protein has intrinsic acetyltransferase activity. Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and
 SUPPLEMENTARY INFORMATION: The microtubule-associated tau protein has intrinsic acetyltransferase activity Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and Virginia M.Y. Lee Cohen
SUPPLEMENTARY INFORMATION: The microtubule-associated tau protein has intrinsic acetyltransferase activity Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and Virginia M.Y. Lee Cohen
Chapter One. Construction of a Fluorescent α5 Subunit. Elucidation of the unique contribution of the α5 subunit is complicated by several factors
 4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 CELL BIOLOGY BIO-2B06 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 CELL BIOLOGY BIO-2B06 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
Modeling cytoskeleton self-assembly
 Modeling cytoskeleton self-assembly Dimitrios Vavylonis Department of Physics, Lehigh University BIOS 10 Lehigh University Oct 30, 2009 Cell organization How does the cell achieve internal organization?
Modeling cytoskeleton self-assembly Dimitrios Vavylonis Department of Physics, Lehigh University BIOS 10 Lehigh University Oct 30, 2009 Cell organization How does the cell achieve internal organization?
The cytoskeletal system
 The cytoskeletal system Types, polymerization, characteristics and mechanical properties of cytoskeletal filaments. Associated protein. 24.09.2013. Cytoskeleton The cellular "scaffold" or "skeleton" within
The cytoskeletal system Types, polymerization, characteristics and mechanical properties of cytoskeletal filaments. Associated protein. 24.09.2013. Cytoskeleton The cellular "scaffold" or "skeleton" within
cell and tissue imaging by fluorescence microscopy
 cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
Live Analysis of precisely timed drug injections in the Drosophila embryo.
 Barbara Fasulo and William Sullivan Live Analysis of precisely timed drug injections in the Drosophila embryo. In: Confocal Microscopy Methods and Protocols. Series: Methods in Molecular Biology Pub Date:
Barbara Fasulo and William Sullivan Live Analysis of precisely timed drug injections in the Drosophila embryo. In: Confocal Microscopy Methods and Protocols. Series: Methods in Molecular Biology Pub Date:
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Supplemental material Prashar et al., http://www.jcb.org/cgi/content/full/jcb.201304095/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. FBT phagocytosis in cells expressing PM-GFP. (A) T-PC
Microtubule Cytoskeleton and Cell Patterning
 Microtubule Cytoskeleton and Cell Patterning 10:00 Anna Akhmanova Microtubule Dynamics 11:00 Lukas Kapitein Motors and Transport 12:00 12:30 Microscopy Facility Tour 13:00-14:30 Lunch and Self-study Jacobson
Microtubule Cytoskeleton and Cell Patterning 10:00 Anna Akhmanova Microtubule Dynamics 11:00 Lukas Kapitein Motors and Transport 12:00 12:30 Microscopy Facility Tour 13:00-14:30 Lunch and Self-study Jacobson
me239 mechanics of the cell me239 mechanics of the cell 3.1 biopolymers - motivation 3.2 biopolymers - polymerization
 4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
Methanol fixation allows better visualization of Kal7. To compare methods for
 Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
Supplementary Data Methanol fixation allows better visualization of Kal7. To compare methods for visualizing Kal7 in dendrites, mature cultures of dissociated hippocampal neurons (DIV21) were fixed with
THE JOURNAL OF CELL BIOLOGY
 Supplemental Material THE JOURNAL OF CELL BIOLOGY Toso et al., http://www.jcb.org/cgi/content/full/jcb.200809055/dc1 Figure S1. Control experiments for sirna depletions used in Figs. 1 3. The phenotype
Supplemental Material THE JOURNAL OF CELL BIOLOGY Toso et al., http://www.jcb.org/cgi/content/full/jcb.200809055/dc1 Figure S1. Control experiments for sirna depletions used in Figs. 1 3. The phenotype
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Ab-DeliverIN TM - Antibody Delivery Reagent Results
 Ab-DeliverIN TM - Antibody Delivery Reagent Results OZ Biosciences is delighted to announce the launching of the innovative Ab-DeliverIN TM - antibody delivery reagent. Ab-DeliverIN TM is a lipid based
Ab-DeliverIN TM - Antibody Delivery Reagent Results OZ Biosciences is delighted to announce the launching of the innovative Ab-DeliverIN TM - antibody delivery reagent. Ab-DeliverIN TM is a lipid based
Description: Nuclear morphology and dynamics in nontargeting sirna transfected cells. HeLa Kyoto
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Tables Title of file for HTML: Supplementary Movie 1 Description: Nuclear morphology and dynamics
Journal of Cell Science Supplementary Material
 Figure S4 A His-(RBCPS)T15 His-(_BCPS) T15 His-PXN His-PXN+T15 His-PXN+ (RB_PS)T15 Myc-Ub IP:Ni-NTA 6M GuHCL WB: anti-myc WB: anti-his (Ub)n His-PXN His-(RBCPS)T15 His-(_BCPS)T15 + + + + + + + + + -100
Figure S4 A His-(RBCPS)T15 His-(_BCPS) T15 His-PXN His-PXN+T15 His-PXN+ (RB_PS)T15 Myc-Ub IP:Ni-NTA 6M GuHCL WB: anti-myc WB: anti-his (Ub)n His-PXN His-(RBCPS)T15 His-(_BCPS)T15 + + + + + + + + + -100
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
NIH Public Access Author Manuscript Proc IEEE Int Symp Biomed Imaging. Author manuscript; available in PMC 2011 July 29.
 NIH Public Access Author Manuscript Published in final edited form as: Proc IEEE Int Symp Biomed Imaging. 2011 June 9; 2011(March 30 2011-April 2 2011): 1330 1333. doi: 10.1109/ISBI.2011.5872646. AUTOMATED
NIH Public Access Author Manuscript Published in final edited form as: Proc IEEE Int Symp Biomed Imaging. 2011 June 9; 2011(March 30 2011-April 2 2011): 1330 1333. doi: 10.1109/ISBI.2011.5872646. AUTOMATED
T H E J O U R N A L O F C E L L B I O L O G Y
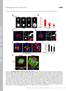 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
Figure S1 is related to Figure 1B, showing more details of outer segment of
 Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
Supplemental Information Supplementary Figure legends and Figures Figure S1. Electron microscopic images in Sema4A +/+ and Sema4A / retinas Figure S1 is related to Figure 1B, showing more details of outer
TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS. Kathryn P. Trogden
 TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS Kathryn P. Trogden A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill
TOG PROTEINS ARE SPATIALLY REGULATED BY RAC-GSK3β TO CONTROL INTERPHASE MICROTUBULE DYNAMICS Kathryn P. Trogden A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Colchicine. Colchicine. a b c d e
 α1-tub Colchicine Laulimalide RB3 β1-tub Vinblastine α2-tub Taxol Colchicine β2-tub Maytansine a b c d e Supplementary Figure 1 Structures of microtubule and tubulin (a)the cartoon of head to tail arrangement
α1-tub Colchicine Laulimalide RB3 β1-tub Vinblastine α2-tub Taxol Colchicine β2-tub Maytansine a b c d e Supplementary Figure 1 Structures of microtubule and tubulin (a)the cartoon of head to tail arrangement
Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only)
 Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only) I. Protein Synthesis: creation of new proteins a. Much of the cellular machinery is devoted to synthesizing
Human Anatomy & Physiology I Dr. Sullivan Unit IV Cellular Function Chapter 4, Chapter 27 (meiosis only) I. Protein Synthesis: creation of new proteins a. Much of the cellular machinery is devoted to synthesizing
Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information
 Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information 1. Supplementary Figure S1-S10: Pages 2-11 2. Supplementary References:
Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate toward tumor blood vessels Supplementary Information 1. Supplementary Figure S1-S10: Pages 2-11 2. Supplementary References:
Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology. Chad Zimprich January 2015
 Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology Chad Zimprich January 2015 Presentation verview HaloTag Fusion Technology Design Functionality
Tracking Cellular Protein Localization and Movement in Cells with a Flexible Fluorescent Labeling Technology Chad Zimprich January 2015 Presentation verview HaloTag Fusion Technology Design Functionality
Practice Exam 3 MCBII
 1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
1. Which is not a feature of lamin intermediate filaments (IFs)? (4 pts) A. They are protein polymers. B. They are used by motor proteins to traffic cargo. C. They contain coiled coil domains. D. They
Force Measurement Software Module
 Microscopy from Carl Zeiss Force Measurement Software Module Optical tweezers are used to manipulate microscopic particles such as cells or sub-cellular structures. The Force Measurement (FM) module expands
Microscopy from Carl Zeiss Force Measurement Software Module Optical tweezers are used to manipulate microscopic particles such as cells or sub-cellular structures. The Force Measurement (FM) module expands
