US FDA: CMC Issues for INDs
|
|
|
- Derrick Lee
- 6 years ago
- Views:
Transcription
1 ISBTC Global Regulatory Summit October 29, 2008 US FDA: CMC Issues for INDs Keith Wonnacott, Ph.D. US Food and Drug Administration Center for Biologics Evaluation and Research Office of Cellular, Tissue, and Gene Therapies
2 Basis for regulation of INDs Statutes: THE LAW --- passed by Congress and signed by the President 42 USC 262 (United States Code) Regulations: details of the law --- written by the Agency and approved by the Executive Branch 21 CFR 312 (Code of Federal Regulations) Guidance: the Agency s interpretation of the Regulations --- written and approved within the Agency 68 FR (Federal Register)
3
4 Drug and Biologics Law 1902 Biologics Control Act authorizes regulations to ensure purity and safety of serums, vaccines, and similar products 1938 Food, Drug, and Cosmetic Act required new drugs to be shown safe before marketing. It also authorized factory inspections and other provisions PHS Act incorporates provisions for biologics regulation. Outlines licensing requirements that are independent from pre-marketing requirements for drugs FD&C Act Amendments required drug manufacturers to prove the effectiveness of their products before marketing them and allowed FDA to regulate investigational studies.
5 Center for Veterinary Medicine Center for Food Safety and Applied Nutrition Center for Drug Evaluation and Research FDA National Center for Toxicological Research Center for Devices and Radiological Health Center for Biologics Evaluation and Research
6 Premarket approval pathways Biologics BLA Biologics License Application Drugs NDA New Drug Application ANDA Abbreviated New Drug Application Devices PMA Premarketing Application HDE Humanitarian Device Exemption Combination products
7 Drug and Biologics Marketing Regulations 21 CFR parts 210, 211, 225, & 226 Good manufacturing practices for drugs and biologics 21 CFR parts 600, 601, & 610 Biological products regulations 21 CFR parts 201, 202, 203, 314 Prescription drug regulations 21 CFR part 25 Environmental impact considerations
8 Investigational studies Biologics IND Investigational New Drug application Drugs IND Investigational New Drug application Devices IDE Investigational Device Exemption Combination products
9 Regulations For INDs 21 CFR 312 Investigational New Drug Application
10 What are the phases of investigation? Preclinical Phase 1 Phase 2 Phase 3 Marketing Phase 4 Phase I: Designed to evaluate safety and side effects Phase II: Designed to evaluate safety and explore efficacy and dose ranging Phase III: Expanded study designed to obtain efficacy and safety data for approval Phase IV: Post marketing commitments to monitor safety and efficacy
11 What are the elements of an IND application? Form FDA CFR (a)(1) Table of Contents 21 CFR (a)(2) Introductory statement and general investigational plan 21 CFR (a)(3) Investigator s brochure 21 CFR (a)(5) Protocols 21 CFR (a)(6) Chemistry, manufacturing, and control data 21 CFR (a)(7) Pharmacology and toxicology data 21 CFR (a)(8) Previous human experience 21 CFR (a)(9) Additional information 21 CFR (a)(10)
12 Initial IND Review Process Sponsor FDA Receipt Review Decision Letter Sponsor Prepare and Submit IND Project Manager Clinical Manufacturing Preclinical Supervisory Concurrence Proceed With Trial or Address Deficiencies Other (as needed) 30 days 30 days
13 IND Review criteria Clinical Hold Criteria (21 CFR ) Risks are unreasonable and significant Investigators not qualified Investigator brochure false or misleading Insufficient information to assess risk Gender exclusion for a condition that occurs in both men and women
14 CMC Information for INDs: Starting material Choose an appropriate starting material Appropriate screening and testing of donors See DE rule and guidance Perform all required testing on your cell bank Sterility, mycoplasma, viral testing (in vivo, in vitro, specific viruses) Identity testing (species origin, markers, activity, purity, etc) Stability (maintain desired activity upon thaw and passage) Propagation, containers, labeling, storage Ensure that your starting material is consistent Make sure your cell line is stable Account for and determine how to control individual patient variability
15 CMC Information for INDs: Manufacturing Choose and qualify your reagents Ensure that reagents will perform as desired in the manufacturing process Ensure clinical quality reagents (safety, purity, potency/activity) Document reagent quality (in-house testing or COA) Establish adequate facility and equipment performance standards and monitoring plans
16 CMC Information for INDs: Manufacturing process Choose a robust manufacturing process Establish standard operating procedures (SOPs) Establish batch records Qualify your facilities Do performance runs to ensure process consistency Establish procedures to prevent: Product mix-ups Product cross-contamination Ensure process safety Qualify procedure for killing of tumorigenic cells Validate viral clearance methods
17 CMC Information for INDs: Product quality testing In-process testing Design to assess process and product quality Final product testing Perform on the final product, not intermediate Establish proper specifications Ensure the safety and consistency of product lots Base acceptance criteria on experience Determine the criteria for acceptable product Should agree with current FDA standards
18 CMC Information for INDs: Other Include a plan for post infusion sterility failure: Notification of physician and patient. Identification and sensitivity testing of the microbial contaminant. Description of additional patient monitoring that will be conducted as a result of the event. Investigation and potential corrective action Reporting of the incident to the IRB and FDA Have a quality assurance program Provide stability data to justify storage and holding times Make sure cross-references are accurate and relevant
19 Common Cell and Gene Therapy IND Deficiencies Analysis of FDA comments in ~100 Clinical Hold Letters issued Cytotherapy. 2008;10(3):312-6 INDs Submitted To FDA That Are Placed On Clinical Hold: Experience of the Office of Cellular, Tissue, and Gene Therapies. Keith Wonnacott, Deborah Lavoie, Robert Fiorentino, Maritza McIntyre, Ying Huang, Steven Hirschfeld.
20 Summary FDA regulates both investigational and marketing applications Information about the process in US is contained in regulations and guidance documents Detailed manufacturing information is needed during product development Communicate with the FDA throughout your product development
21 Biologic Products Standards U.S. Food and Drug Administration GMPs for Drugs and Biologics Drug GMPs Cell and Tissue GMPs 21 CFR CFR CFR CFR 610 Device GMPs
22 Manufacturing Expectations During Product Development IND Submitted BLA Submitted Pre-IND IND BLA Development Preclinical Phase I Phase II Phase III Marketing Safety Quality Principles of GMP apply throughout clinical manufacturing Regulatory requirements depend on the stage of development
23 FINAL RULE: GMP and Investigational New Drugs ( Exempts most Phase 1 studies from compliance with regulations in 21 CFR Part 211 EXCEPT when drug: (1) has already been made available for use in Phase 2 or 3 study, or (2) has been lawfully marketed GMP will still be required; appropriate to early IND Development of well-defined written procedures Adequate control of equipment/manufacturing environment Accurate/consistent data recording from manufacturing (including testing) Guidance published
24 Ensuring GMP Compliance CMC Review CGMP Inspection Source Material Components Manufacturing Process Process Controls (Safety-related) Analytical Methods/ Specifications Stability Companion Personnel Quality Control Facilities Equipment Laboratory Control Component Control Production Control Distribution Records Labeling
25 Contact Information Cellular product manufacturing questions Keith Wonnacott, Ph.D General CBER Issues Office of Communication, Training & Manufacturers Assistance Manufacturers Assistance and Technical Training Branch Telephone: or Internet:
Structure and Mandate of FDA
 Structure and Mandate of FDA Leonard Sacks, M.D. Office of Medical Policy Center for Drug Evaluation and Research FDA FDA Clinical Investigator Training Course November 13, 2018 Mission of regulatory agencies
Structure and Mandate of FDA Leonard Sacks, M.D. Office of Medical Policy Center for Drug Evaluation and Research FDA FDA Clinical Investigator Training Course November 13, 2018 Mission of regulatory agencies
Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization.
 Philadelphia, 2013 Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization. Brian Barnes, Ph.D. VP, Clinical and Regulatory Affairs
Philadelphia, 2013 Point-of-Care Cell Processing Devices A Manufacturer s Perspective Clinical trials, regulatory compliance, and commercialization. Brian Barnes, Ph.D. VP, Clinical and Regulatory Affairs
Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited.
 Statutory Framework for Biologics Drugs Investigational Use Application IND Pre-Market Approval Applications 505(b)(1) NDA 505(b)(2) NDA 505(j) ANDA Over-the-Counter (OTC) Non- Rx Drugs Monograph Biologics
Statutory Framework for Biologics Drugs Investigational Use Application IND Pre-Market Approval Applications 505(b)(1) NDA 505(b)(2) NDA 505(j) ANDA Over-the-Counter (OTC) Non- Rx Drugs Monograph Biologics
Regulatory Issues in Human Subjects Research
 Regulatory Issues in Human Subjects Research Ian McNiece, PhD University of Miami Human Subjects Research Require IRB approval Studies of new drugs or applications of drugs require an FDA approved IND
Regulatory Issues in Human Subjects Research Ian McNiece, PhD University of Miami Human Subjects Research Require IRB approval Studies of new drugs or applications of drugs require an FDA approved IND
A Life Cycle Approach to Raw Material Qualification for Cell and Gene Therapy Products
 A Life Cycle Approach to Raw Material Qualification for Cell and Gene Therapy Products Angela Whatley, Ph.D. Office of Tissues and Advanced Therapies CBER/FDA CMC Strategy Forum on Cell & Gene Therapies
A Life Cycle Approach to Raw Material Qualification for Cell and Gene Therapy Products Angela Whatley, Ph.D. Office of Tissues and Advanced Therapies CBER/FDA CMC Strategy Forum on Cell & Gene Therapies
Development of Regenerative Medicine Products: FDA Perspectives
 資料 3-3 Development of Regenerative Medicine Products: FDA Perspectives Steven R. Bauer, Ph.D. Chief, Cellular and Tissue Therapies Branch Office of Cellular, Tissue and Gene Therapies Center for Biologics
資料 3-3 Development of Regenerative Medicine Products: FDA Perspectives Steven R. Bauer, Ph.D. Chief, Cellular and Tissue Therapies Branch Office of Cellular, Tissue and Gene Therapies Center for Biologics
Regulatory Approval of Modern Gene-Based Cancer Immunotherapies CAR T Cells A product perspective
 Regulatory Approval of Modern Gene-Based Cancer Immunotherapies CAR T Cells A product perspective ASQ509 Biomed/Biotech SIG 2/1/18 Xiaobin Victor Lu Division of Cellular and Gene Therapies Office of Tissues
Regulatory Approval of Modern Gene-Based Cancer Immunotherapies CAR T Cells A product perspective ASQ509 Biomed/Biotech SIG 2/1/18 Xiaobin Victor Lu Division of Cellular and Gene Therapies Office of Tissues
CGMP Requirements for Investigational Products
 PREP #6 CGMP Requirements for Investigational Products Ji-Eun Kim, RPh, PhD Research Pharmacist Regulatory Affairs Office of Research Compliance December 6, 2016 1 CME Disclosure Statement Northwell Health
PREP #6 CGMP Requirements for Investigational Products Ji-Eun Kim, RPh, PhD Research Pharmacist Regulatory Affairs Office of Research Compliance December 6, 2016 1 CME Disclosure Statement Northwell Health
Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited.
 Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
Expanded Access and the Individual Patient IND
 Expanded Access and the Individual Patient IND Research Wednesdays April 26, 2017 Erika Segear Johnson, PhD, RAC Associate Director of Regulatory Affairs Office of Regulatory Affairs and Quality Office
Expanded Access and the Individual Patient IND Research Wednesdays April 26, 2017 Erika Segear Johnson, PhD, RAC Associate Director of Regulatory Affairs Office of Regulatory Affairs and Quality Office
Agency Information Collection Activities; Submission for Office of Management and Budget
 This document is scheduled to be published in the Federal Register on 02/12/2019 and available online at https://federalregister.gov/d/2019-01962, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 02/12/2019 and available online at https://federalregister.gov/d/2019-01962, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Office for Human Subject Protection. University of Rochester
 POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
POLICY 1. Purpose Outline the responsibilities and regulatory requirements when conducting human subject research that involves the use of drugs, agents, biological products, or nutritional products (e.g.,
Guidance for Industry
 Guidance for Industry Cooperative Manufacturing Arrangements for Licensed Biologics DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Guidance for Industry Cooperative Manufacturing Arrangements for Licensed Biologics DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Cell Therapy Product Manufacturing Considerations. July 17, 2017 CMC Strategy Forum Mo Heidaran, Ph.D.
 Cell Therapy Product Manufacturing Considerations July 17, 2017 CMC Strategy Forum Mo Heidaran, Ph.D. Office of Tissues and Advanced Therapies FDA/CBER Overview Establishing Manufacturing Control Applying
Cell Therapy Product Manufacturing Considerations July 17, 2017 CMC Strategy Forum Mo Heidaran, Ph.D. Office of Tissues and Advanced Therapies FDA/CBER Overview Establishing Manufacturing Control Applying
Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development
 Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development No. 1 of 10 1. regulates all the drugs products manufactured and sold in the USA. (A) EMEA (B) IND (C) FDA (D) NDA (E) OSHA
Toxicology - Problem Drill 24: Toxicology Studies in Pharmaceutical Development No. 1 of 10 1. regulates all the drugs products manufactured and sold in the USA. (A) EMEA (B) IND (C) FDA (D) NDA (E) OSHA
Inspections, Compliance, Enforcement, and Criminal Investigations
 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Celltex Therapeutics Corporation
Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Celltex Therapeutics Corporation
How to put together an IND application
 How to put together an IND application Judit Milstein, Chief, Project Management Staff Judit.milstein@fda.hhs.gov Eithu Lwin, Regulatory Health Project Manager Eithu.Lwin@fda.hhs.gov Division of Transplant
How to put together an IND application Judit Milstein, Chief, Project Management Staff Judit.milstein@fda.hhs.gov Eithu Lwin, Regulatory Health Project Manager Eithu.Lwin@fda.hhs.gov Division of Transplant
REGULATORY ISSUES: HOW TO APPLY FOR AN IND. Penny Jester and Maaike Everts
 REGULATORY ISSUES: HOW TO APPLY FOR AN IND Penny Jester and Maaike Everts Outline 2 What is the purpose of an IND? What types of INDs are there? When do you need one? How do you apply for one? How do you
REGULATORY ISSUES: HOW TO APPLY FOR AN IND Penny Jester and Maaike Everts Outline 2 What is the purpose of an IND? What types of INDs are there? When do you need one? How do you apply for one? How do you
GUIDANCE FOR SPONSORS, INDUSTRY, RESEARCHERS, INVESTIGATORS, AND FOOD AND DRUG ADMINISTRATION STAFF
 GUIDANCE FOR SPONSORS, INDUSTRY, RESEARCHERS, INVESTIGATORS, AND FOOD AND DRUG ADMINISTRATION STAFF Certifications To Accompany Drug, Biological Product, and Device Applications/Submissions:Compliance
GUIDANCE FOR SPONSORS, INDUSTRY, RESEARCHERS, INVESTIGATORS, AND FOOD AND DRUG ADMINISTRATION STAFF Certifications To Accompany Drug, Biological Product, and Device Applications/Submissions:Compliance
Guidelines: c. Providing the investigational drug for the requested use will not interfere with the initiation, conduct, or completion of clinical
 Guidelines for the Submission of an Expanded Access IND to Permit Diagnosis, Monitoring or Treatment of Intermediate-size Patient Populations with an Investigational Drug or a REMS-restricted, Approved
Guidelines for the Submission of an Expanded Access IND to Permit Diagnosis, Monitoring or Treatment of Intermediate-size Patient Populations with an Investigational Drug or a REMS-restricted, Approved
Question Yes No. 1. Is the study interventional (a clinical trial)? Study Type data element is Interventional
 Checklist for Evaluating Whether a Clinical Trial or Study is an Applicable Clinical Trial (ACT) Under 42 CFR 11.22(b) for Clinical Trials Initiated on or After January 18, 2017 1 (NOT FOR SUBMISSION 2
Checklist for Evaluating Whether a Clinical Trial or Study is an Applicable Clinical Trial (ACT) Under 42 CFR 11.22(b) for Clinical Trials Initiated on or After January 18, 2017 1 (NOT FOR SUBMISSION 2
To document the review procedures for a submission regarding compassionate/treatment use of investigational drugs, biologics and devices.
 UNIVERSITY OF TENNESSEE GRADUATE SCHOOL OF MEDICINE INSTITUTIONAL REVIEW BOARD COMPASSIONATE/TREATMENT USE OF MEDICAL DRUGS, BIOLOGICS AND DEVICES I. PURPOSE To document the review procedures for a submission
UNIVERSITY OF TENNESSEE GRADUATE SCHOOL OF MEDICINE INSTITUTIONAL REVIEW BOARD COMPASSIONATE/TREATMENT USE OF MEDICAL DRUGS, BIOLOGICS AND DEVICES I. PURPOSE To document the review procedures for a submission
CBER Regulation of Devices for Cell Therapy
 CBER Regulation of Devices for Cell Therapy Richard D. McFarland, Ph.D., M.D. Associate Director for Policy Office of Cellular, Tissue and Gene Therapies Center for Biologics Evaluation and Research Food
CBER Regulation of Devices for Cell Therapy Richard D. McFarland, Ph.D., M.D. Associate Director for Policy Office of Cellular, Tissue and Gene Therapies Center for Biologics Evaluation and Research Food
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Thomas E. Young,
Page 1 of 5 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Thomas E. Young,
CMC Considerations for Stem Cell-based. Donald W. Fink, Jr., Ph.D.
 Food and Drug Administration Center for Biologics Evaluation and Research CMC Considerations for Stem Cell-based Products Donald W. Fink, Jr., Ph.D. Phone: (301) 827-5153 E-Mail: donald.fink@fda.hhs.gov
Food and Drug Administration Center for Biologics Evaluation and Research CMC Considerations for Stem Cell-based Products Donald W. Fink, Jr., Ph.D. Phone: (301) 827-5153 E-Mail: donald.fink@fda.hhs.gov
FDA: The New Animal Drug Approval Process and Cell Based Products
 FDA: The New Animal Drug Approval Process and Cell Based Products Lynne Boxer, DVM March 29, 2017 Division of Therapeutic Drugs for Non Food Animals Office of New Animal Drug Evaluation Center for Veterinary
FDA: The New Animal Drug Approval Process and Cell Based Products Lynne Boxer, DVM March 29, 2017 Division of Therapeutic Drugs for Non Food Animals Office of New Animal Drug Evaluation Center for Veterinary
3/17/2017. Scope. Guidance 218: Cell Based Products for Animal Use. FDA: The New Animal Drug Approval Process and Cell Based Products
 FDA: The New Animal Drug Approval Process and Cell Based Products Lynne Boxer, DVM March 29, 2017 Division of Therapeutic Drugs for Non Food Animals Office of New Animal Drug Evaluation Center for Veterinary
FDA: The New Animal Drug Approval Process and Cell Based Products Lynne Boxer, DVM March 29, 2017 Division of Therapeutic Drugs for Non Food Animals Office of New Animal Drug Evaluation Center for Veterinary
Regulatory considerations for manufacturing and testing of investigational chimeric antigen receptor (CAR) T-cell products
 Regulatory considerations for manufacturing and testing of investigational chimeric antigen receptor (CAR) T-cell products Xiaobin Victor Lu Product Reviewer Gene Therapies Branch DCGT/OCTGT/CBER/FDA MEASUREMENT
Regulatory considerations for manufacturing and testing of investigational chimeric antigen receptor (CAR) T-cell products Xiaobin Victor Lu Product Reviewer Gene Therapies Branch DCGT/OCTGT/CBER/FDA MEASUREMENT
Presentation Outline
 IND Application Process: For The New Clinical Investigator John M. Centanni Department of Medicine University of Wisconsin Presentation Outline Journey from basic to clinical research Introduction to FDA
IND Application Process: For The New Clinical Investigator John M. Centanni Department of Medicine University of Wisconsin Presentation Outline Journey from basic to clinical research Introduction to FDA
Amarex Clinical Research Washington DC metro area A Product Development Services Company. From Lab to Market Approval
 Amarex Clinical Research Washington DC metro area A Product Development Services Company Regulatory Strategy From Lab to Market Approval Strategy Implementation Pre-Clinical Assays Global Clinical Trials
Amarex Clinical Research Washington DC metro area A Product Development Services Company Regulatory Strategy From Lab to Market Approval Strategy Implementation Pre-Clinical Assays Global Clinical Trials
Supplies and Reagents
 Supplies and Reagents PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 4 th /5 th, 2006 NHLBI-sponsored PACT Group Guidance for Industry INDs Approaches to Complying with CGMP During
Supplies and Reagents PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 4 th /5 th, 2006 NHLBI-sponsored PACT Group Guidance for Industry INDs Approaches to Complying with CGMP During
Drug Development - Points to Consider
 Drug Development - Points to Consider The Regulator View ד"ר עפרה אקסלרוד מנהלת המכון לביקורת ותקנים של חומרי רפואה והאכיפה אוקטובר 2018 וס. מנהל מערך הרקוחות Keep in mind Know your product Know the regulatory
Drug Development - Points to Consider The Regulator View ד"ר עפרה אקסלרוד מנהלת המכון לביקורת ותקנים של חומרי רפואה והאכיפה אוקטובר 2018 וס. מנהל מערך הרקוחות Keep in mind Know your product Know the regulatory
Guidance for Industry
 Guidance for Industry Cooperative Manufacturing Arrangements for Licensed Biologics Additional copies of this guidance are available from the Office of Communication, Training and Manufacturers Assistance
Guidance for Industry Cooperative Manufacturing Arrangements for Licensed Biologics Additional copies of this guidance are available from the Office of Communication, Training and Manufacturers Assistance
Phase Appropriate GMPs for IMPs. Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017
 Phase Appropriate GMPs for IMPs Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017 1 Lets start with References https://mhrainspectorate.blog.gov.uk/2016/0 5/20/manufacture-of-investigationalmedicinal-products-frequently-askedquestions/
Phase Appropriate GMPs for IMPs Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017 1 Lets start with References https://mhrainspectorate.blog.gov.uk/2016/0 5/20/manufacture-of-investigationalmedicinal-products-frequently-askedquestions/
11.0 FDA-Regulated Research Research Involving Investigational Drugs and Biologics
 11.0 FDA-Regulated Research The IRB evaluates the safety or efficacy of all drugs and devices used in research. Studies involving unapproved or investigational drugs or devices will be reviewed to ensure
11.0 FDA-Regulated Research The IRB evaluates the safety or efficacy of all drugs and devices used in research. Studies involving unapproved or investigational drugs or devices will be reviewed to ensure
IDENTIFYING & MANAGING GCP COMPLIANCE RISKS FOR THE PHARMACEUTICAL, BIOTECH & DEVICE INDUSTRIES
 IDENTIFYING & MANAGING GCP COMPLIANCE RISKS FOR THE PHARMACEUTICAL, BIOTECH & DEVICE INDUSTRIES Karen Weaver Epstein, Becker & Green Health Care & Life Sciences Practice APPLICABLE REGULATIONS 21 CFR 312
IDENTIFYING & MANAGING GCP COMPLIANCE RISKS FOR THE PHARMACEUTICAL, BIOTECH & DEVICE INDUSTRIES Karen Weaver Epstein, Becker & Green Health Care & Life Sciences Practice APPLICABLE REGULATIONS 21 CFR 312
Comparative Study of Regulatory Requirements for Biologics Filing in United States and European Union
 Comparative Study of Regulatory Requirements for Biologics Filing in United States and European Union Mr. Shashi Kumar Yadav Assistant Professor Sri Indu Institute of Pharmacy Hyderabad Outline Introduction
Comparative Study of Regulatory Requirements for Biologics Filing in United States and European Union Mr. Shashi Kumar Yadav Assistant Professor Sri Indu Institute of Pharmacy Hyderabad Outline Introduction
Regulation of Microbiota- Based Products
 Regulation of Microbiota- Based Products LCDR Matthew Steele, PhD Team Leader, Regulatory Review Branch 1 Division of Vaccines and Related Products Applications CBER/OVRR My presentation is an informal
Regulation of Microbiota- Based Products LCDR Matthew Steele, PhD Team Leader, Regulatory Review Branch 1 Division of Vaccines and Related Products Applications CBER/OVRR My presentation is an informal
MCW Office of Research Standard Operating Procedure
 MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DEVICES Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW Faculty and Staff involved
MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DEVICES Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW Faculty and Staff involved
Beth Hutchins, PhD PhRMA ICH Gene Therapy Discussion Group
 ICH Considerations on General Principles to Address the Risk of Inadvertent Germline Integration of Gene Therapy Vectors and Current Topics on Gene Therapy in USA Beth Hutchins, PhD PhRMA ICH Gene Therapy
ICH Considerations on General Principles to Address the Risk of Inadvertent Germline Integration of Gene Therapy Vectors and Current Topics on Gene Therapy in USA Beth Hutchins, PhD PhRMA ICH Gene Therapy
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only. Document issued on: July 14, 2011
 Draft Guidance for Industry and Food and Drug Administration Staff In Vitro Companion Diagnostic Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document issued
Draft Guidance for Industry and Food and Drug Administration Staff In Vitro Companion Diagnostic Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document issued
Supplies and Reagents
 Supplies and Reagents PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 10 th -11 th, 2007 NHLBI-sponsored PACT Group Guidance for Industry INDs Approaches to Complying with CGMP During
Supplies and Reagents PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 10 th -11 th, 2007 NHLBI-sponsored PACT Group Guidance for Industry INDs Approaches to Complying with CGMP During
FDA Regulation of Companion Diagnostics
 FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
11.0 FDA-Regulated Research Research Involving Investigational Drugs and Biologics
 11.0 FDA-Regulated Research The IRB evaluates the safety or efficacy of all drugs and devices used in research. Studies involving unapproved or investigational drugs or devices will be reviewed to ensure
11.0 FDA-Regulated Research The IRB evaluates the safety or efficacy of all drugs and devices used in research. Studies involving unapproved or investigational drugs or devices will be reviewed to ensure
Paths to Market & FDA Product Lifecycle Regulation. Patricia Kaeding Design of Medical Devices University of Minnesota April 14, 2005
 Paths to Market & FDA Product Lifecycle Regulation Patricia Kaeding Design of Medical Devices University of Minnesota April 14, 2005 FDA Organization U.S. Food & Drug Administration Headed by a Commissioner
Paths to Market & FDA Product Lifecycle Regulation Patricia Kaeding Design of Medical Devices University of Minnesota April 14, 2005 FDA Organization U.S. Food & Drug Administration Headed by a Commissioner
CGMP for Phase 1 INDs
 CGMP for Phase 1 INDs Laurie P. Norwood Deputy Director Division of Manufacturing and Product Quality Office of Compliance and Biologics Quality Center for Biologics Evaluation and Research 1 Overview
CGMP for Phase 1 INDs Laurie P. Norwood Deputy Director Division of Manufacturing and Product Quality Office of Compliance and Biologics Quality Center for Biologics Evaluation and Research 1 Overview
11.0 FDA-Regulated Research
 11.0 FDA-Regulated Research The HSC evaluates the safety or efficacy of all drugs and devices used in research. Studies involving unapproved or investigational drugs or devices will be reviewed to ensure
11.0 FDA-Regulated Research The HSC evaluates the safety or efficacy of all drugs and devices used in research. Studies involving unapproved or investigational drugs or devices will be reviewed to ensure
FDA Perspective on the Preclinical Evaluation of Biological Therapies for Cancer
 FDA Perspective on the Preclinical Evaluation of Biological Therapies for Cancer Yongjie Zhou, M.D., Ph.D. FDA/CBER/OCTGT/DCEPT Yongjie.zhou@fda.hhs.gov isbtc Global Regulatory Summit October 29, 2008
FDA Perspective on the Preclinical Evaluation of Biological Therapies for Cancer Yongjie Zhou, M.D., Ph.D. FDA/CBER/OCTGT/DCEPT Yongjie.zhou@fda.hhs.gov isbtc Global Regulatory Summit October 29, 2008
FDA approval of emergency expanded access use may be requested by telephone, facsimile, or other means of electronic communications.
 Guidelines for the Submission of an Expanded Access IND to Permit Diagnosis, Monitoring or Treatment of an Individual Patient with an Investigational Drug or a REMS-restricted, Approved Drug Guidelines:
Guidelines for the Submission of an Expanded Access IND to Permit Diagnosis, Monitoring or Treatment of an Individual Patient with an Investigational Drug or a REMS-restricted, Approved Drug Guidelines:
Draft Guidance for Industry, Clinical Laboratories, and FDA Staff. In Vitro Diagnostic Multivariate Index Assays
 Draft Guidance for Industry, Clinical Laboratories, and FDA Staff In Vitro Diagnostic Multivariate Index Assays DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
Draft Guidance for Industry, Clinical Laboratories, and FDA Staff In Vitro Diagnostic Multivariate Index Assays DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
COVERAGE ELIGIBILITY OF SERVICES ASSOCIATED WITH A CLINICAL TRIAL
 Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
1 Purpose. 2 Procedure. Title: FDA-Regulated Research. SOP Number: 1301 Effective Date: June 2, Previous Version Dates:
 Previous Version Dates: Title: FDA-Regulated Research SOP Number: 1301 Effective Date: June 2, 2017 1 Purpose FDA regulations apply to research that involves a FDA-regulated test article in a clinical
Previous Version Dates: Title: FDA-Regulated Research SOP Number: 1301 Effective Date: June 2, 2017 1 Purpose FDA regulations apply to research that involves a FDA-regulated test article in a clinical
CANADA (HEALTH CANADA)
 LEADERS 1 THE GMP GAZETTE TM February 2015 HPFBI CANADA (HEALTH CANADA) Blood Establishment Registration Application: Form and Instructions - (FRM-0353) Who s affected? Anyone who plans to perform the
LEADERS 1 THE GMP GAZETTE TM February 2015 HPFBI CANADA (HEALTH CANADA) Blood Establishment Registration Application: Form and Instructions - (FRM-0353) Who s affected? Anyone who plans to perform the
Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited.
 Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited. Special Review Designations & Approval Pathways Special Designations
Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited. Special Review Designations & Approval Pathways Special Designations
Early Development Best Practices for Stability- Regulatory Perspective
 Early Development Best Practices for Stability- Regulatory Perspective IQ Workshop, Feb. 4-5, 2014, Washington, D.C. Ramesh Sood, Ph.D. Division Director (Acting) Office of New Drug Quality Assessment
Early Development Best Practices for Stability- Regulatory Perspective IQ Workshop, Feb. 4-5, 2014, Washington, D.C. Ramesh Sood, Ph.D. Division Director (Acting) Office of New Drug Quality Assessment
IND Development Process Published on ResearchGo UCLA (
 IND Development Process IND Development Overview The Pre-IND Process The IND Study Protocol Preparing the Initial IND Submission Filing the IND Maintaining the IND IND Templates, Education and Useful Links
IND Development Process IND Development Overview The Pre-IND Process The IND Study Protocol Preparing the Initial IND Submission Filing the IND Maintaining the IND IND Templates, Education and Useful Links
Quality Issues for Clinical Trial Materials: The Chemistry, Manufacturing and Controls (CMC) Review
 Quality Issues for Clinical Trial Materials: The Chemistry, Manufacturing and Controls (CMC) Review Presented by Erika E. Englund, Ph.D. Slides courtesy of Dorota Matecka, Ph.D. Office of Pharmaceutical
Quality Issues for Clinical Trial Materials: The Chemistry, Manufacturing and Controls (CMC) Review Presented by Erika E. Englund, Ph.D. Slides courtesy of Dorota Matecka, Ph.D. Office of Pharmaceutical
"From Bedside to Bench and Back: regulatory requirements for collaborations between pharma industry and academia
 "From Bedside to Bench and Back: regulatory requirements for collaborations between pharma industry and academia Damir Hamamdžić D.V.M., Ph.D. Office of Research Regulatory Affairs Rutgers University RWJMS
"From Bedside to Bench and Back: regulatory requirements for collaborations between pharma industry and academia Damir Hamamdžić D.V.M., Ph.D. Office of Research Regulatory Affairs Rutgers University RWJMS
13 FDA-Regulated Research
 13 FDA-Regulated Research FDA regulations apply to research that involves a FDA-regulated test article in a clinical investigation involving human subjects as defined by the FDA regulations. For FDA-regulated
13 FDA-Regulated Research FDA regulations apply to research that involves a FDA-regulated test article in a clinical investigation involving human subjects as defined by the FDA regulations. For FDA-regulated
Investigator-Initiated INDs
 Investigator-Initiated INDs Marjorie Small, RN, CCRC Office of Clinical Research 23 May 2011 PPHS/IRB Research Grand Rounds Outline of Presentation I. What is an IND? II. Code of Federal Regulations III.
Investigator-Initiated INDs Marjorie Small, RN, CCRC Office of Clinical Research 23 May 2011 PPHS/IRB Research Grand Rounds Outline of Presentation I. What is an IND? II. Code of Federal Regulations III.
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations. Audrey Chang, PhD, Senior Director Development Services
 Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
The Children s Hospital of Philadelphia Committees for the Protection of Human Subjects Policies and Procedures Determination of IND/IDE Requirement
 Page: 1 of 8 I. PURPOSE II. III. IV. The purpose of this Standard Operating Procedure is to delineate when an investigator must obtain an Investigational New Drug (IND) or Investigational Device Exemption
Page: 1 of 8 I. PURPOSE II. III. IV. The purpose of this Standard Operating Procedure is to delineate when an investigator must obtain an Investigational New Drug (IND) or Investigational Device Exemption
Guidance for Industry
 Guidance for Industry Comparability Protocols - Protein Drug Products and Biological Products - Chemistry, Manufacturing, and Controls Information DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry Comparability Protocols - Protein Drug Products and Biological Products - Chemistry, Manufacturing, and Controls Information DRAFT GUIDANCE This guidance document is being distributed
How to Avoid Common Deficiencies in INDs and NDAs. Ramesh Raghavachari, Ph.D. Branch Chief, Branch IX ONDQA/OPS/CDER
 How to Avoid Common Deficiencies in INDs and NDAs Ramesh Raghavachari, Ph.D. Branch Chief, Branch IX ONDQA/OPS/CDER 1 Structure of FDA Office of Commissioner Chief Scientist FOODS Medical Products & Tobacco
How to Avoid Common Deficiencies in INDs and NDAs Ramesh Raghavachari, Ph.D. Branch Chief, Branch IX ONDQA/OPS/CDER 1 Structure of FDA Office of Commissioner Chief Scientist FOODS Medical Products & Tobacco
KFDA Regulatory Framework on Biopharmaceuticals - Focus on Biosimilar
 KFDA Regulatory Framework on Biopharmaceuticals - Focus on Biosimilar Kyung-Min Baek, Ph.D. Recombinant Protein Products Division Korea Food and Drug Administration(KFDA) Biopharmaceuticals A biopharmaceutical
KFDA Regulatory Framework on Biopharmaceuticals - Focus on Biosimilar Kyung-Min Baek, Ph.D. Recombinant Protein Products Division Korea Food and Drug Administration(KFDA) Biopharmaceuticals A biopharmaceutical
Justification of Specifications (JOS): What Product and Process Development was all About. Christopher A Bravery
 Justification of Specifications (JOS): What Product and Process Development was all About Christopher A Bravery cbravery@advbiols.com 1 The Importance of Characterisation 2 http://www.advbiols.com/documents/importance
Justification of Specifications (JOS): What Product and Process Development was all About Christopher A Bravery cbravery@advbiols.com 1 The Importance of Characterisation 2 http://www.advbiols.com/documents/importance
Title: Review of Medical Devices Page: 1 of 5 Written by:
 THE NORTH SHORE MEDICAL CENTER Institutional Review Board (IRB) POLICIES AND PROCEDURES IRB Policy Number: 031.1 Title: Review of Medical Devices Page: 1 of 5 Written by: Approved by: Laura W. Knight,
THE NORTH SHORE MEDICAL CENTER Institutional Review Board (IRB) POLICIES AND PROCEDURES IRB Policy Number: 031.1 Title: Review of Medical Devices Page: 1 of 5 Written by: Approved by: Laura W. Knight,
Medical Device Labeling HealthPack 2004 Program
 Medical Device Labeling HealthPack 2004 Program Elizabeth Kempen Overview Regulatory Agencies and Pathways Labeling Regulations General Medical Device Labeling Requirements Electronic Labeling FDA s Current
Medical Device Labeling HealthPack 2004 Program Elizabeth Kempen Overview Regulatory Agencies and Pathways Labeling Regulations General Medical Device Labeling Requirements Electronic Labeling FDA s Current
Clinical trial applications in the EU and US
 Clinical trial applications in the EU and US Alain Patat, M.D. Translational Development Wyeth Research Paris, France AGAH-Club Phase 1 1 st Symposium Strasbourg 17-18 March 2005 Overview of the presentation
Clinical trial applications in the EU and US Alain Patat, M.D. Translational Development Wyeth Research Paris, France AGAH-Club Phase 1 1 st Symposium Strasbourg 17-18 March 2005 Overview of the presentation
PHARM 532 Regulation of Pharmaceuticals II
 FDA s Regulatory Structuret PHARM 532 Regulation of Pharmaceuticals II Spring 2009 Center(s) for Biologics Evaluation & Research (CBER) 1 Drug Evaluation & Research (CDER) 2 Device & Radiological Health
FDA s Regulatory Structuret PHARM 532 Regulation of Pharmaceuticals II Spring 2009 Center(s) for Biologics Evaluation & Research (CBER) 1 Drug Evaluation & Research (CDER) 2 Device & Radiological Health
FDA s Role in Vaccine Supply
 IOM Committee on Review of Priorities of the National Vaccine Plan Stakeholder Workshop #1 24 July 2008 FDA s Role in Vaccine Supply Norman W. Baylor, Ph.D., Director Office of Vaccines Research and Review
IOM Committee on Review of Priorities of the National Vaccine Plan Stakeholder Workshop #1 24 July 2008 FDA s Role in Vaccine Supply Norman W. Baylor, Ph.D., Director Office of Vaccines Research and Review
Food and Drug Administration (FDA) 101
 Food and Drug Administration (FDA) 101 What is the Food and Drug Administration (FDA)? The FDA is an agency within the U.S. Department of Health and Human Services that is responsible for protecting the
Food and Drug Administration (FDA) 101 What is the Food and Drug Administration (FDA)? The FDA is an agency within the U.S. Department of Health and Human Services that is responsible for protecting the
Topics Covered. FDA s Role in Expediting the Development of Novel Medical Products. How a Regulatory Agency Comes into Existence 3/5/2018
 FDA s Role in Expediting the Development of Novel Medical Products Peter Marks, M.D., Ph.D. Director Center for Biologics Evaluation and Research Topics Covered Brief history of FDA Expediting product
FDA s Role in Expediting the Development of Novel Medical Products Peter Marks, M.D., Ph.D. Director Center for Biologics Evaluation and Research Topics Covered Brief history of FDA Expediting product
Investigational New Drug Development Steps for CRCs
 Investigational New Drug Development Steps for CRCs Natalie Nardone, PhD Program Manager, Department of Medicine CRC Instructor, Office of Clinical Research Contact: natalie.nardone@ucsf.edu Learning Objectives
Investigational New Drug Development Steps for CRCs Natalie Nardone, PhD Program Manager, Department of Medicine CRC Instructor, Office of Clinical Research Contact: natalie.nardone@ucsf.edu Learning Objectives
Established Conditions: Reportable CMC Changes for Approved Drug and Biologic Products Guidance for Industry
 Established Conditions: Reportable CMC Changes for Approved Drug and Biologic Products Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Established Conditions: Reportable CMC Changes for Approved Drug and Biologic Products Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Expanded Access (including Emergency Use & Humanitarian Use Devices)
 Expanded Access (including Emergency Use & Humanitarian Use Devices) Bertha delanda, CIP IRB Training Specialist Research Compliance Office March 2012 What Is Expanded Access? Permits use of an investigational
Expanded Access (including Emergency Use & Humanitarian Use Devices) Bertha delanda, CIP IRB Training Specialist Research Compliance Office March 2012 What Is Expanded Access? Permits use of an investigational
Product Testing & Release. PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 4 th /5 th, 2006
 Product Testing & Release PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 4 th /5 th, 2006 Product Testing Used to determine Safety, Purity, Identity, Potency, etc. Suitability of
Product Testing & Release PACT Workshop: Design & Operation of GMP Cell Therapy Facilities April 4 th /5 th, 2006 Product Testing Used to determine Safety, Purity, Identity, Potency, etc. Suitability of
UT SOUTHWESTERN MEDICAL CENTER AT DALLAS INSTITUTIONAL REVIEW BOARD. Emergency Use of an Investigational Drug, Biologic or Device
 Emergency Use of an Investigational Drug, Biologic or Device Introduction The emergency use provision in the FDA regulations (21 CFR 56.102d) is an exemption from prior review and approval by the IRB for
Emergency Use of an Investigational Drug, Biologic or Device Introduction The emergency use provision in the FDA regulations (21 CFR 56.102d) is an exemption from prior review and approval by the IRB for
Bioresearch Monitoring Inspections in Vitro Diagnostics Devices
 Seite 1 von 7 U.S. Food and Drug Administration Protecting and Promoting Your Health Bioresearch Monitoring Inspections in Vitro Diagnostics Devices TABLE OF CONTENTS Introduction Nature, Scope, & Purpose
Seite 1 von 7 U.S. Food and Drug Administration Protecting and Promoting Your Health Bioresearch Monitoring Inspections in Vitro Diagnostics Devices TABLE OF CONTENTS Introduction Nature, Scope, & Purpose
The Right Prescription for Working with Investigational Drug Service at BMC
 The Right Prescription for Working with Investigational Drug Service at BMC Andrew Schoch Pharmacy Intern Northeastern University Class of 2010 Hyeseon Hong, Pharm.D. IDS Pharmacy Manager What is Investigational
The Right Prescription for Working with Investigational Drug Service at BMC Andrew Schoch Pharmacy Intern Northeastern University Class of 2010 Hyeseon Hong, Pharm.D. IDS Pharmacy Manager What is Investigational
A Life-cycle Approach to Dose Finding Studies
 A Life-cycle Approach to Dose Finding Studies Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA This presentation reflects the views of the author
A Life-cycle Approach to Dose Finding Studies Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA This presentation reflects the views of the author
Guidance. Investigational New Drug Applications for Positron Emission Tomography (PET) Drugs GUIDANCE
 Guidance Investigational New Drug Applications for Positron Emission Tomography (PET) Drugs GUIDANCE U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance Investigational New Drug Applications for Positron Emission Tomography (PET) Drugs GUIDANCE U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Medidée Services SA. Nano-Tera.ch. 05 February 2015 part 8. PMA, 510k, IDE. Pierre-Alain Sommer
 Nano-Tera.ch 05 February 2015 part 8 PMA, 510k, IDE Pierre-Alain Sommer Pierre-alain.sommer@medidee.com www.medidee.com Nano-Tera 2015 05.02.2015 USA/FDA Pre Market Approval System - PMA, Pre Market Notifcation
Nano-Tera.ch 05 February 2015 part 8 PMA, 510k, IDE Pierre-Alain Sommer Pierre-alain.sommer@medidee.com www.medidee.com Nano-Tera 2015 05.02.2015 USA/FDA Pre Market Approval System - PMA, Pre Market Notifcation
Introduction to the FDA. Historical Context. Changes over Time FDA - BE-200 9/23/2004. Copyright C. S. Tritt, Ph.D. 1
 Introduction to the FDA C. S. Tritt, Ph.D. October 21, 2003 Historical Context In the early 1900 s people were being ripped off, made sick and even killed by bad food, patent medicines and cosmetics with
Introduction to the FDA C. S. Tritt, Ph.D. October 21, 2003 Historical Context In the early 1900 s people were being ripped off, made sick and even killed by bad food, patent medicines and cosmetics with
March 4, Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
HCT/P Regulation vs 361 Products
 HCT/P Regulation - 351 vs 361 Products Presented by: Paul Gadiock February 15, 2017 Arent Fox LLP Washington, DC New York, NY Los Angeles, CA San Francisco, CA 1 Presentation Overview Introduction Public
HCT/P Regulation - 351 vs 361 Products Presented by: Paul Gadiock February 15, 2017 Arent Fox LLP Washington, DC New York, NY Los Angeles, CA San Francisco, CA 1 Presentation Overview Introduction Public
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective
 Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
Global Clinical Trials in Korea
 Global Clinical Trials in Korea In-Sook Park Department of Drug Evaluation Korea Food & Drug Administration Contents Regulatory changes relevant to Clinical Trials in Korea Current Status of Clinical Trials
Global Clinical Trials in Korea In-Sook Park Department of Drug Evaluation Korea Food & Drug Administration Contents Regulatory changes relevant to Clinical Trials in Korea Current Status of Clinical Trials
Investigational New Devices
 Page 1 of 14 Investigational New Devices I. Purpose This document describes the policies and procedures for conducting studies involving investigational new devices at Stanford Hospital & Clinics (SHC)
Page 1 of 14 Investigational New Devices I. Purpose This document describes the policies and procedures for conducting studies involving investigational new devices at Stanford Hospital & Clinics (SHC)
ORC Sponsor-Investigator IDE Checklist
 A sponsor-investigator assumes BOTH investigator and sponsor responsibilities as outlined in the FDA Code of Federal Regulations 21CFR812. This means that such investigators have additional responsibilities.
A sponsor-investigator assumes BOTH investigator and sponsor responsibilities as outlined in the FDA Code of Federal Regulations 21CFR812. This means that such investigators have additional responsibilities.
Chapter 2 The Regulatory Environment
 Chapter 2 The Regulatory Environment The relevance and importance of the regulatory environment cannot be overemphasized. 2.1 Introduction This chapter introduces the regulatory environment in which new
Chapter 2 The Regulatory Environment The relevance and importance of the regulatory environment cannot be overemphasized. 2.1 Introduction This chapter introduces the regulatory environment in which new
MCW Office of Research Standard Operating Procedure
 MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DRUGS AND BIOLOGICS Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW/FH Faculty
MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DRUGS AND BIOLOGICS Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW/FH Faculty
Role of Academic Investigators in Drug Development
 DTRCS Regulatory Education Seminar, June 12, 2007 Role of Academic Investigators in Drug Development Howard Lee, MD, PhD Associate Adjunct Professor Director, Center for Drug Terminology Sponsor Investigator
DTRCS Regulatory Education Seminar, June 12, 2007 Role of Academic Investigators in Drug Development Howard Lee, MD, PhD Associate Adjunct Professor Director, Center for Drug Terminology Sponsor Investigator
Subpart H [Reserved] Subpart I Expanded Access to Investigational Drugs for Treatment. 21 CFR Ch. I ( Edition)
![Subpart H [Reserved] Subpart I Expanded Access to Investigational Drugs for Treatment. 21 CFR Ch. I ( Edition) Subpart H [Reserved] Subpart I Expanded Access to Investigational Drugs for Treatment. 21 CFR Ch. I ( Edition)](/thumbs/89/100764462.jpg) 312.300 (c) Disposition of unused drug. The person who ships the drug under paragraph (a) of this section shall assure the return of all unused supplies of the drug from individual investigators whenever
312.300 (c) Disposition of unused drug. The person who ships the drug under paragraph (a) of this section shall assure the return of all unused supplies of the drug from individual investigators whenever
The Application of Pharmaceutical cgmp to Live Bacterial Products. James Harris Business Development Manager
 The Application of Pharmaceutical cgmp to Live Bacterial Products James Harris Business Development Manager The Objective CMC expectations of a cgmp live bacterial biopharmaceutical project Overview Define
The Application of Pharmaceutical cgmp to Live Bacterial Products James Harris Business Development Manager The Objective CMC expectations of a cgmp live bacterial biopharmaceutical project Overview Define
Outcomes in Mesenchymal Stem Cell Manufacturing. Athena Russell, MT(AAB) Human Cellular Therapy Laboratory Mayo Clinic Jacksonville, FL
 Outcomes in Mesenchymal Stem Cell Manufacturing Athena Russell, MT(AAB) Human Cellular Therapy Laboratory Mayo Clinic Jacksonville, FL Background HCTL established in 1992 to support BMT programs of Mayo
Outcomes in Mesenchymal Stem Cell Manufacturing Athena Russell, MT(AAB) Human Cellular Therapy Laboratory Mayo Clinic Jacksonville, FL Background HCTL established in 1992 to support BMT programs of Mayo
Drug Development & the FDA Pharmacy 309 November 2005
 Goals & Objectives Drug Development & the FDA Pharmacy 309 November 2005 Tom Hazlet, Pharm.D., Dr.P.H. H375S 206.616.2732 thazlet@u... Be able to describe major regulatory events in the drug & biologic,
Goals & Objectives Drug Development & the FDA Pharmacy 309 November 2005 Tom Hazlet, Pharm.D., Dr.P.H. H375S 206.616.2732 thazlet@u... Be able to describe major regulatory events in the drug & biologic,
Ancillary Materials for Cell & Tissue Therapies Definitions, US Regulatory Approach, and USP s Risk-Tiered Approach
 USP/ISCT Workshop 2012 Seattle, WA, USA Ancillary Materials for Cell & Tissue Therapies Definitions, US Regulatory Approach, and USP s Risk-Tiered Approach Elizabeth Read, MD Head of Product Development
USP/ISCT Workshop 2012 Seattle, WA, USA Ancillary Materials for Cell & Tissue Therapies Definitions, US Regulatory Approach, and USP s Risk-Tiered Approach Elizabeth Read, MD Head of Product Development
DMTC Technology Readiness Levels Guideline
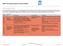 Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
The FDA/CDRH Perspective in the Regulation of Surgical Mesh
 The FDA/CDRH Perspective in the Regulation of Surgical Mesh David Krause, Ph.D., Branch Chief Plastic & Reconstructive Surgery Branch Division of Surgical, Orthopedic & Restorative Devices Office of Device
The FDA/CDRH Perspective in the Regulation of Surgical Mesh David Krause, Ph.D., Branch Chief Plastic & Reconstructive Surgery Branch Division of Surgical, Orthopedic & Restorative Devices Office of Device
