Current Trends in Sterile Manufacturing. by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS)
|
|
|
- Sibyl Watson
- 6 years ago
- Views:
Transcription
1 Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS)
2 Executive Summary Drug developers go to great lengths to assure that their products are safe as well as efficacious. For injectable drugs, in particular, a sterile manufacturing and filling environment is necessary to minimize the risk of product contamination. Historically, manufacturers have used clean rooms to achieve this. For decades, the clean room approach was sufficient, and the risk/reward ratio did not justify change. In recent years, however, the emergence of expensive biologics and a changing regulatory environment have made other options, including sterile isolators, increasingly viable. As today s manufacturers look at new products and aging facilities, they have an opportunity to re-evaluate their manufacturing processes, their risks and their options. In selecting a solution, the manufacturer must weigh a number of factors. This paper addresses the evolution of sterile manufacturing, looks in detail at currently available options and considerations, and provides a glimpse at how sterile manufacturing might continue to progress. History Hippocrates said, First, do no harm. In their role as health care providers, drug developers go to great lengths to assure that their products are as safe as possible as well as efficacious. For injectable drugs, in particular, a sterile manufacturing and filling environment is a necessary part of providing a safe product to the patient. With injectable drugs, product contamination is of utmost concern, because the product is introduced systemically. Potential contaminants must be controlled, not only during product manufacturing, but also during the process of filling the vials, ampules or syringes. In the 1970s, pharmaceutical manufacturers began to use clean rooms to control contamination. Clean room technology uses filtered, high-pressure air to push particles down to the floor in order to keep them from contaminating the vial. Theoretically, recirculating the filtered air eventually should remove all particles; however, the presence of operators means potential for particle generation. By keeping the operator outside the sterile field, isolation chambers, or isolators, significantly reduce the potential for contamination. Introduced in the 1990s, the technology provides a barrier with a Class 100 or better space inside a sealed isolator, which can be located in a Class 10,000 room. Although isolators were rather quickly embraced in Europe, it is only recently garnering real attention in the United States for several reasons. First, the capital cost is high compared to a clean room. Second, one company had patented Vapor Hydrogen Peroxide (VHP) cleaning, but the decontamination cycle initially was 18 hours. That was not an issue for vaccine manufacturers running long production cycles, but it represented an unacceptable level of downtime for many others. A handful of companies, most notably the LUMS Group (Lilly, Upjohn and Merck) deployed it in the 90s with limited success, and others were reluctant to follow. Today, the business drivers are different. Decades-old clean rooms are aging; wall systems built with inferior materials are breaking down and beginning to particulate. In addition, the emergence of high-value APIs has upped the ante in sterile manufacturing, and few, if any, facilities are available to manufacture them. The cost of these products has reduced manufacturers risk tolerance. Generic products produced by chemistry cost a few dollars per vial. Today s biologics can cost $100, $1,000 or more. Investing the capital in a new facility is a less difficult decision than it was a decade or more ago. What s more, the VHP cycle has been cut to three hours, and the U.S. Food and Drug Administration (FDA) is recognizing the isolator technology process as validatible and reproducible. Clean rooms Traditional clean room technology uses laminar flow motion high-velocity air forced down vertically to drive particulate past the filling process line, ideally to the floor. By doing this, it is possible to achieve Grade A or Class 100 space that is, 100 particles per 1 cubic foot of air. In semiconductor manufacturing, the air is forced down through perforations in the floor and then recirculated. In pharmaceutical manufacturing, however, the perforations in the floor provide a potential breeding ground for bacteria, particularly if fluids become trapped beneath the floor. Instead, return registers around the walls are
3 used to recirculate the air. This approach makes it difficult to obtain unidirectional laminar flow motion, as the air tends to seek corners instead of flowing straight down. Over the years, the technology has been refined. Making rooms narrower with no more than four inside corners makes it easier to optimize airflow. Air is pushed through HEPA filters at higher velocities, exceeding 90 feet per minute, to drive the particles downward. Horizontal surface that could collect dust can be minimized. For example, fire extinguisher boxes and electrical outlets should be flush with the wall to eliminate any ledges. Operators remain the weak link in the clean room system. Effective training on gowning, degowning and other procedures can help reduce the risk that an operator will introduce contamination, but some risk always remains. Conversely, a clean room does not provide a barrier to protect the operators from exposure to potent products, which could contaminate a gown and expose the operator during de-gowning. Clean rooms are also expensive to operate. Energy costs to operate an air system at 90 fpm are significant. Additionally, the time operators must spend gowning and de-gowning is not productive. The economics vary by project; however, several confidential client studies have shown an approximately six-month payback when comparing capital expenditures to operational savings. Isolation technology Isolation technology has matured to the point where it can be reliably deployed. One factor that makes isolation more attractive than it was in the 90s is a shorter decontamination cycle. Steris s patent on VHP expired, leaving the door open for innovations from other vendors. Both SKAN and Bosch put engineers to work on the challenge. As a result of their changes to isolator designs and air handling systems, VHP cycles are as short as three to four hours. What s more, the process can be validated and reproduced reliably, and as a result, the FDA is increasingly more comfortable with the technology. Advantages: Parenteral manufacturing center A recently expanded parenteral manufacturing center demonstrates many of the advantages of today s isolation technology. The 20-year-old facility required additional capacity for both liquid and lyophilized products. The goal was to have four independent product cells, each with capabilities for formulation, primary packaging and ancillary support services, such as autoclaves, parts washers, etc. When evaluating technologies for the new aseptic facilities, a multidisciplinary team considered industry benchmarking, quality and regulatory expectations. It made facility visits and assessed technologies. Based on its findings, the team decided to pursue isolation technology rather than convention clean rooms. Next, the team looked at isolator technology versus restricted access barrier (RABS) technology, a relatively new approach that places an aseptic filler in a Class 10,000 room. RABS are appealing due to lower capital costs, but the technology has several problems. RABS are not pressurized the way isolators are; when the door is opened, it creates an opportunity for contamination. RABS do not use VHP decontamination, but must be opened up and cleaned manually, thereby increasing the environmental monitoring burden. The problems might be addressed by using laminar flow with Grade A air over the door, but in order for this approach to be effective, the space must be small, the returns must be close to the HEPA filter, and the HEPA filters must be larger than four feet. Eventually, the space becomes a Grade A room which defeats the purpose. With a true isolator, it is possible to leverage Grade C support spaces. A study conducted by Johnson & Johnson found that lifecycle costs for RABS are higher than for clean rooms or for isolators. Ultimately, the team recommended isolator technology. It offers an improved compliance profile and protected both the product and personnel. It eliminates the need for Class A support space and gowning rooms, and reduced expected operating costs. Finally, its validated VHP cycle provides more effective and reproducible decontamination than manual sanitization. The process called for a continuous line for liquid and lyophilized products and fixed, automatic loading into four lyophilizers. A number of different configurations were possible; computer modeling was used to optimize the line configuration select the number of cappers. Based on the operating rules, production scheduling,
4 equipment and facility design, and quality assurance concerns, the model suggested that a T configuration would offer a 50-percent increase in capacity and justify the added cost of a third capper. Vendors were selected early in the design phase. Bosch manufactured the filling line; SKAN manufactured the isolators, and BOC Edwards manufactured the freeze driers and loading system. The addition was adapted to the equipment; a three-story design allows use of a two-story lyophilizer and improves maintenance access. The production cells are Grade C throughout for efficient personnel and material flows and to reduce the need for airlocks and gowning rooms. Like the addition, rooms were designed around optimized manufacturing process flows, rather than the opposite. Corridors to the cells are Pharmaceutical Unclassified, with controlled access. Advantages: Clinical supplies operation (CSO) An isolator also was the technology of choice for a recently completed CSO expansion facility for both oral solid dosages and parenteral liquid fill batches. The goal was to create a flexible facility with the ability to perform multi-product clinical scale manufacturing that handles solvent-based and potent compound operations formulation and finishing while adhering to current good manufacturing practice (cgmp) and European guidelines. The project represents the first FDA-approved clinical sterile continuous process isolation facility in the United States. It utilizes state-of-the-art isolation/containment technologies, including closed system processing equipment, contained material transfer systems and isolated equipment and operations. Use of isolation technology made it possible to create a multipurpose sterile line to manufacture chemical, biologic, flammable and explosive potent compounds up to Band 5 while protecting personnel from exposure and product from contamination. In addition, cutting-edge wireless Process and Analytical Technology (PAT) also helped minimize human interface by reducing the frequency of entry and exit from the process rooms. Single-pot processors further reduced contamination risk. Finally, IPS developed a formal integrated program and practices for conducting a risk-based assessment approach to commissioning/qualification. This innovative approach helped minimize the project schedule and eliminate delays when evaluating the impact of system conditions or components on product quality. The approach also resulted in a Facility of the Year award from ISPE, INTERPHEX and Pharmaceutical Processing magazine. The CSO/DPTC (Drug Product Technology Center) expansion project was selected from a group of nineteen nominees from six countries as 2008 Category Winner in Equipment Innovation award. Options and considerations Today, manufacturers can select equipment from a number of vendors, as listed below: Isolators - SKAN - Bosch - Steris Filling equipment - Bosch - Groninger - Inova Lyophilizers and loading devices - BOC Edwards Stopper processing systems - Steritect - Gettinge Beta bags - La Calhene - Central Research Isolator facilities can be laid out in a number of ways, depending on process requirements and other considerations. The isolator can be located in a traditional suite, or it can be located in an isolator cell with a Grade C corridor, as it was for the CSO facility. It can be located in a process cell ballroom, as was done for the parenteral manufacturing site, or it can be added to an existing traditional suite. The choice of layout is based on consideration of several variables, including the number of products, the number of cells and the overall building layout. Another advantage of isolator technology is that it makes it possible to segregate functions and locate all aseptic processing in a single zone. For example, all process mechanical equipment
5 can be located on the first floor, product manufacturing on the second and HVAC on the third. Each zone has its own distinct set of procedures. Color-coding the zones can help facilitate compliance. From the outset, the company s operating philosophy will drive the decision-making process. To minimize risk, the optimal approach is to select the best equipment available for the task and to have it sized, and then design the space around the equipment. This approach saves time. It is not necessary to specify every detail of the equipment, as long as the specifications include any necessary options and indicate that the equipment meet all applicable regulations. In today s pharmaceutical manufacturing environment, isolators clearly offer an advantage. The primary obstacle to widespread acceptance is the relative lack of experience with the technology. This lack of confidence is a barrier, but it is not insurmountable, as long as the organization is open to developing and training on new SOPs that ensure a well-defined and defendable flow of material. Looking ahead With the growing importance of biologics in the pharmaceutical industry, and the continued aging of the existing manufacturing infrastructure, the need for new sterile manufacturing capacity can only increase. Isolator technology is already widely accepted in Europe and is beginning to make inroads in the United States. As the base of experience builds, so will confidence in the technology on the part of manufacturers and regulators alike, and isolators will become an increasingly clear choice for sterile manufacturing Sterling G. Kline, R.A., has more than 35 years of experience in strategic planning, master planning, programming, design, construction management and facilities management for the health care, pharmaceutical and biotech industries. He has held senior management positions at engineering and construction service firms and has also served as director of engineering for several major pharmaceutical manufacturers. Mr. Kline has completed pharmaceutical projects ranging from major R&D facilities including discovery, development and kilo labs; clinical manufacturing pilot plants for sterile and oral solid dosage, including potent compounds; to manufacturing facilities for sterile products using vials, syringes and devices incorporating both traditional and isolation technology, and oral solid dosage products utilizing traditional and isolation technology. He has also completed pharmaceutical support facilities including office, warehouse, quality lab, utility, parking, and hazardous material holding buildings. A member of ISPE, he holds a degree in Civil Engineering from Northeastern University.
Why change is inevitable in aseptic manufacturing?
 Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite
 APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
Overview of Inspection Issues with Legacy Products. Barbara Breithaupt Consumer Safety Officer
 Overview of Inspection Issues with Legacy Products Barbara Breithaupt Consumer Safety Officer 1 Overview of the presentation The role of the Quality Unit will be discussed in the context of the inspection
Overview of Inspection Issues with Legacy Products Barbara Breithaupt Consumer Safety Officer 1 Overview of the presentation The role of the Quality Unit will be discussed in the context of the inspection
INSPECTOR S OBSERVATIONS AND WARNING LETTER
 Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
MONZA ST5 Project. Alberto Penati Patheon Monza EU Engineering Director. The world leader in serving science
 MONZA ST5 Project Alberto Penati Patheon Monza EU Engineering Director The world leader in serving science SUMMARY 1. Monza Site Overview 2. Design Data 3. Philosophy and approach on installation 4. Challenges
MONZA ST5 Project Alberto Penati Patheon Monza EU Engineering Director The world leader in serving science SUMMARY 1. Monza Site Overview 2. Design Data 3. Philosophy and approach on installation 4. Challenges
GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR
 GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR 2 GETINGE ISOFLEX THE ANSWER TO YOUR DAILY NEEDS FOR STERILE HANDLING In any sterile operation, maintaining the sterility of goods -- whether you have purchased them
GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR 2 GETINGE ISOFLEX THE ANSWER TO YOUR DAILY NEEDS FOR STERILE HANDLING In any sterile operation, maintaining the sterility of goods -- whether you have purchased them
Environmental Monitoring of Aseptic Processing Areas - 1
 Environmental Monitoring of Aseptic Processing Areas - 1 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Training Course Agenda Overview of Environmental Monitoring
Environmental Monitoring of Aseptic Processing Areas - 1 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Training Course Agenda Overview of Environmental Monitoring
Media Fill A Process Simution. Presented By Shikha Chauhan
 Media Fill A Process Simution Presented By Shikha Chauhan 21 CFR 211.113 Validation of Aseptic Processing and Sterilization Process Simulation / Media Fill Filtration Efficacy Sterilization of Equipment,
Media Fill A Process Simution Presented By Shikha Chauhan 21 CFR 211.113 Validation of Aseptic Processing and Sterilization Process Simulation / Media Fill Filtration Efficacy Sterilization of Equipment,
Contract Manufacturing Services for Pre-filled Syringes
 Contract Manufacturing Services for Pre-filled Syringes From Standard Solutions to Complex Products Stefan Czvitkovich, PhD Director Product Partnering Sterile Pharmaceuticals Central Europe CPhI woldwide
Contract Manufacturing Services for Pre-filled Syringes From Standard Solutions to Complex Products Stefan Czvitkovich, PhD Director Product Partnering Sterile Pharmaceuticals Central Europe CPhI woldwide
FLEXIBLE CONTAINMENT
 FLEXIBLE CONTAINMENT Collaboration Partners Bectochem produces a comprehensive range of pharmaceutical process equipment & turnkey solids dosage and liquid manufacturing systems. Introducing barrier isolator
FLEXIBLE CONTAINMENT Collaboration Partners Bectochem produces a comprehensive range of pharmaceutical process equipment & turnkey solids dosage and liquid manufacturing systems. Introducing barrier isolator
USP Chapter 823 USP 32 (old) vs. USP 35 (new)
 USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
Gloveless Robotic Technologies in Aseptic. Personalized Cytotoxic. Association. Drugs. Sergio Mauri FEDEGARI GROUP
 Gloveless Robotic Technologies in Aseptic PDA: Manufacturing A Global for Personalized Cytotoxic Association Sergio Mauri FEDEGARI GROUP Drugs Presentation outline Pharma outlook trends and the aseptic
Gloveless Robotic Technologies in Aseptic PDA: Manufacturing A Global for Personalized Cytotoxic Association Sergio Mauri FEDEGARI GROUP Drugs Presentation outline Pharma outlook trends and the aseptic
Trends in Sterile Manufacturing Technologies
 Trends in Sterile Manufacturing Technologies ISPE Thailand Annual Meeting Charlotte Enghave Fruergaard 2013.07.18 Where we come from 1930s Danish Novo and Nordisk Gentofte (later Novo Nordisk) employed
Trends in Sterile Manufacturing Technologies ISPE Thailand Annual Meeting Charlotte Enghave Fruergaard 2013.07.18 Where we come from 1930s Danish Novo and Nordisk Gentofte (later Novo Nordisk) employed
FDA s Guidance for Industry
 Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Webinar. The New EU-GMP Annex 1 draft Dupont. GOP-Innovations your Partner for Practical Training and e-learning. 8 June 2018
 Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
HVAC and Risk Management RACI Meeting 7 th December Agenda. Design. Project Management. Context. Qualification. Construction
 HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids
 Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
Table of Contents. Welcome to Dishman. Our Mission. Our Focus: Client Satisfaction
 Welcome to Dishman Our Mission Dishman Group continually invests in the global pharmaceutical industry, ensuring Dishman s business can provide pharmaceutical customers with high-value, high-quality products
Welcome to Dishman Our Mission Dishman Group continually invests in the global pharmaceutical industry, ensuring Dishman s business can provide pharmaceutical customers with high-value, high-quality products
September 2, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 September 2, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Draft Guidance: Current Good Manufacturing Practice Interim
September 2, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Draft Guidance: Current Good Manufacturing Practice Interim
Baxa Corporation. Technical Paper. Environmental Controls for Sterile Compounding
 Baxa Corporation Environmental Controls for Sterile Compounding Technical Paper A guidance document on developing sterile preparation areas to meet the USP requirements for low and medium risk compounded
Baxa Corporation Environmental Controls for Sterile Compounding Technical Paper A guidance document on developing sterile preparation areas to meet the USP requirements for low and medium risk compounded
While the global pharmaceutical industry tends to be conservative
 A new line at Handai Biken exemplifies the creative use of isolator technology found throughout Japan s pharmaceutical industry. By Jim Akers, Akers Kennedy and Associates, Kazuhito Tanimoto, Shibuya Kogyo
A new line at Handai Biken exemplifies the creative use of isolator technology found throughout Japan s pharmaceutical industry. By Jim Akers, Akers Kennedy and Associates, Kazuhito Tanimoto, Shibuya Kogyo
Allergy Laboratories, Inc. 10/4/13
 Allergy Laboratories, Inc. 10/4/13 SEP 04, 2013 Department of Health and Human Services Public Health Service Food and Drug Administration Office of Regulatory Affairs 12420 Parklawn Drive ELEM-2152 Rockville,
Allergy Laboratories, Inc. 10/4/13 SEP 04, 2013 Department of Health and Human Services Public Health Service Food and Drug Administration Office of Regulatory Affairs 12420 Parklawn Drive ELEM-2152 Rockville,
PDA: A Global. Association. Contamination Control: Particles, Bio-contamination, Aseptic manufacturing. PDA Parenteral 2014 Munich Conference
 PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
LAMINAR FLOW BENCH GUIDELINES
 LAMINAR FLOW BENCH GUIDELINES 4.2.6 Personnel should not be a source of contamination. 4.2.7 Directional airflow within production or packing areas should assist in preventing contamination. Airflows should
LAMINAR FLOW BENCH GUIDELINES 4.2.6 Personnel should not be a source of contamination. 4.2.7 Directional airflow within production or packing areas should assist in preventing contamination. Airflows should
LOADING SYSTEMS LOADING AND UNLOADING SYSTEMS FOR FREEZE DRYERS
 LOADING SYSTEMS LOADING AND UNLOADING SYSTEMS FOR FREEZE DRYERS LOADING SYSTEMS Automatic loading and unloading systems minimize the risk of contamination through human intervention in the loading and
LOADING SYSTEMS LOADING AND UNLOADING SYSTEMS FOR FREEZE DRYERS LOADING SYSTEMS Automatic loading and unloading systems minimize the risk of contamination through human intervention in the loading and
PDA: A Global. Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG. Maturing Aseptic Technologies
 Maturing Aseptic Technologies PDA: A Global create more flexible Facilities Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG Content Changing Environment for Parenteral Manufacturing
Maturing Aseptic Technologies PDA: A Global create more flexible Facilities Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG Content Changing Environment for Parenteral Manufacturing
arrier Isolation Technology
 B PharmaED s Register by November 15 th and receive a $300 Discount! arrier Isolation Technology New Technologies, Case Studies and Current Applications in the Usage of Isolator Systems JANUARY 12-13,
B PharmaED s Register by November 15 th and receive a $300 Discount! arrier Isolation Technology New Technologies, Case Studies and Current Applications in the Usage of Isolator Systems JANUARY 12-13,
Advancements on implementation of single use technology in vaccine manufacturing
 Advancements on implementation of single use technology in vaccine manufacturing October 25 th, 2013 DCVMN Meeting Rio de Janeiro, Brazil Outline Overview of single use products Applications of single
Advancements on implementation of single use technology in vaccine manufacturing October 25 th, 2013 DCVMN Meeting Rio de Janeiro, Brazil Outline Overview of single use products Applications of single
HISTORY AND MILESTONES
 HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
COMPANY PROFILE. Dishman offers innovation, security of supply and value for money. Dishman Group. Research EARLY STAGE API. Preclinical.
 COMPANY PROFILE Dishman is a global outsourcing partner for the pharmaceutical industry offering a portfolio of development, scale-up and services. Dishman improves its customers businesses by providing
COMPANY PROFILE Dishman is a global outsourcing partner for the pharmaceutical industry offering a portfolio of development, scale-up and services. Dishman improves its customers businesses by providing
Creating a Centralized Compounding Pharmacy within a Health System
 SALAS BLANCAS Amy Benner, PharmD Director of Central and Shared Services Scripps Health. Robert Miller, MS Quality Manager Scripps Health Ensuring the production of accurate and safe sterile compounded
SALAS BLANCAS Amy Benner, PharmD Director of Central and Shared Services Scripps Health. Robert Miller, MS Quality Manager Scripps Health Ensuring the production of accurate and safe sterile compounded
Guidance for Industry Process Validation: General Principles and Practices; A Contract Manufacturing Organization's Approach
 May -3, 202 Javits Center New York, NY Guidance for Industry Process Validation: General Principles and Practices; A Contract Manufacturing Organization's Approach Sandra Lueken Director of Quality Baxter
May -3, 202 Javits Center New York, NY Guidance for Industry Process Validation: General Principles and Practices; A Contract Manufacturing Organization's Approach Sandra Lueken Director of Quality Baxter
ISPE Aseptic Conference February 2014 Washington, D.C.
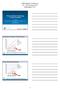 Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
The importance of fill & finish in the commercialization of your cell & gene therapy
 OVERCOMING DOWNSTREAM BIOPROCESSING BOTTLENECKS INNOVATOR INSIGHT The importance of fill & finish in the commercialization of your cell & gene therapy Jean-Sébastien is Head of Sales at Aseptic Technologies,
OVERCOMING DOWNSTREAM BIOPROCESSING BOTTLENECKS INNOVATOR INSIGHT The importance of fill & finish in the commercialization of your cell & gene therapy Jean-Sébastien is Head of Sales at Aseptic Technologies,
Where Quality Meets Flexibility
 Where Quality Meets Flexibility is an industry leading 503B Outsourcing Facility providing sterile and non-sterile compounding services to hospitals, surgery centers, clinics, researchers & patients nationwide.
Where Quality Meets Flexibility is an industry leading 503B Outsourcing Facility providing sterile and non-sterile compounding services to hospitals, surgery centers, clinics, researchers & patients nationwide.
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE. Fundamentals of Sterile Compounding (8 lessons/8 hours CE)
 Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE CriticalPoint s Sterile Compounding elearning curriculum is written by industry experts and covers both Chapter and
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE CriticalPoint s Sterile Compounding elearning curriculum is written by industry experts and covers both Chapter and
INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY
 INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY John Klostermyer, Ph.D. ISPE Product Show Track 3 Session 3 September 26, 2018 AGENDA 1. BACKGROUND INFORMATION 2. BENEFITS 3. PROCESS 4. BEST
INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY John Klostermyer, Ph.D. ISPE Product Show Track 3 Session 3 September 26, 2018 AGENDA 1. BACKGROUND INFORMATION 2. BENEFITS 3. PROCESS 4. BEST
Curriculum Vitae. SENIOR VICE PRESIDENT JAMES P. KULLA Mobile Phone:
 Curriculum Vitae SENIOR VICE PRESIDENT JAMES P. KULLA Mobile Phone: 240.413.3350 Summary of Qualifications Jim has been active in the medical products industry since 1967. Jim founded a company in 1980
Curriculum Vitae SENIOR VICE PRESIDENT JAMES P. KULLA Mobile Phone: 240.413.3350 Summary of Qualifications Jim has been active in the medical products industry since 1967. Jim founded a company in 1980
Quality is Our Promise.
 Quality is Our Promise. Our goal at KRS Global Biotechnology is to provide the highest quality pharmaceutical preparations. We accomplish this with an unrivaled quality assurance and quality control program
Quality is Our Promise. Our goal at KRS Global Biotechnology is to provide the highest quality pharmaceutical preparations. We accomplish this with an unrivaled quality assurance and quality control program
Annex A2. Guidance on Process Validation Scheme for Aseptically Processed Products
 Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D.
 Basic GMP Requirement PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D. Topic Process validation What and Why? Principle of process validation Manufacturing process validation Aseptic process validation
Basic GMP Requirement PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D. Topic Process validation What and Why? Principle of process validation Manufacturing process validation Aseptic process validation
Design Considerations for TGA Licensed Pharmacies. Presented by Ashley Isbel 11 th August 2015
 Design Considerations for TGA Licensed Pharmacies Presented by Ashley Isbel 11 th August 2015 Focus of the session Primarily of relevance to sterile compounding facilities, particularly those producing
Design Considerations for TGA Licensed Pharmacies Presented by Ashley Isbel 11 th August 2015 Focus of the session Primarily of relevance to sterile compounding facilities, particularly those producing
STANDARDS FOR COMPOUNDING PHARMACIES
 STANDARDS FOR COMPOUNDING PHARMACIES APPLICATION NOTE LC-139 (US) Introduction This publication provides excerpts from some of the many guidelines and standards that pertain to compounding pharmacies.
STANDARDS FOR COMPOUNDING PHARMACIES APPLICATION NOTE LC-139 (US) Introduction This publication provides excerpts from some of the many guidelines and standards that pertain to compounding pharmacies.
Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM
 Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM DCVMN Workshop, Hyderabad, 4-8 April 2016 G. Somasundaram Associate Director - Technology Management Overview Key Regulatory
Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM DCVMN Workshop, Hyderabad, 4-8 April 2016 G. Somasundaram Associate Director - Technology Management Overview Key Regulatory
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE. Fundamentals of Sterile Compounding (8 lessons/8 hours CE)
 Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE Fundamentals of Sterile Compounding (8 lessons/8 hours CE) The History of Compounding and USP Sterile Compounding Chapters
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE Fundamentals of Sterile Compounding (8 lessons/8 hours CE) The History of Compounding and USP Sterile Compounding Chapters
Prequalification Team WHO PUBLIC INSPECTION REPORT Vaccine Manufacturer
 Part 1: General information Name of Manufacturer Production Block Physical address Contact address Prequalification Team WHO PUBLIC INSPECTION REPORT Vaccine Manufacturer. Clean Utilities in the Basement.
Part 1: General information Name of Manufacturer Production Block Physical address Contact address Prequalification Team WHO PUBLIC INSPECTION REPORT Vaccine Manufacturer. Clean Utilities in the Basement.
Partner with the Global Leader in Drug Delivery Systems
 3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems Northridge, CA, USA Manufacturing Facility Experts at Commercializing Innovation 3M: Transforming New Ideas into Thousands
3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems Northridge, CA, USA Manufacturing Facility Experts at Commercializing Innovation 3M: Transforming New Ideas into Thousands
Custom processing services
 Custom processing services Cleaning excellence for your critical environment The only thing in your container is what you add Save wasted resources dedicate your time to the manufacturing process, not
Custom processing services Cleaning excellence for your critical environment The only thing in your container is what you add Save wasted resources dedicate your time to the manufacturing process, not
Aseptic Fill / Finish Facility Design for Compliance
 Aseptic Fill / Finish Facility Design for Compliance Herman F. Bozenhardt Vantage Consulting Group October 2014 @ Copyright by Bozenhardt Consulting Services LLC 2014, Agenda Review Compliance Drivers
Aseptic Fill / Finish Facility Design for Compliance Herman F. Bozenhardt Vantage Consulting Group October 2014 @ Copyright by Bozenhardt Consulting Services LLC 2014, Agenda Review Compliance Drivers
FDA's Perspectives on Cross- Contamination in CMO Facilities, Considering High Risk Products
 FDA's Perspectives on Cross- Contamination in CMO Facilities, Considering High Risk Products Bo Chi, Ph.D. Biotech Manufacturing Assessment Branch DGMPA/OMPQ/OC/CDER 1 Outline Regulatory framework Regulatory
FDA's Perspectives on Cross- Contamination in CMO Facilities, Considering High Risk Products Bo Chi, Ph.D. Biotech Manufacturing Assessment Branch DGMPA/OMPQ/OC/CDER 1 Outline Regulatory framework Regulatory
White Paper. Risk Management in Environmental Monitoring: Should you be spotting it sooner?
 White Paper Risk Management in Environmental Monitoring: Should you be spotting it sooner? Executive Overview Quality Risk Management is a comparatively new approach in the pharmaceutical manufacturing
White Paper Risk Management in Environmental Monitoring: Should you be spotting it sooner? Executive Overview Quality Risk Management is a comparatively new approach in the pharmaceutical manufacturing
USP CHAPTER <797> INTRODUCTION RISK LEVELS
 USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
Compounding Aseptic Isolators (CAI) James T Wagner
 Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
Presentation Title Author Name
 Presentation Title Author Name 1 An Introduction to West Pharmaceutical Services: West Pharmaceutical Services, Inc. is a leading manufacturer of packaging components and delivery systems for injectable
Presentation Title Author Name 1 An Introduction to West Pharmaceutical Services: West Pharmaceutical Services, Inc. is a leading manufacturer of packaging components and delivery systems for injectable
US Regulatory Requirements: Clinical & Clinical Research Production, SPECT & PET Radiopharmaceuticals (RPs)
 US Regulatory Requirements: Clinical & Clinical Research Production, SPECT & PET Radiopharmaceuticals (RPs) 20 th International Symposium Radiopharmaceutical Sciences Jeju, Korea Pre-Symposium Workshop
US Regulatory Requirements: Clinical & Clinical Research Production, SPECT & PET Radiopharmaceuticals (RPs) 20 th International Symposium Radiopharmaceutical Sciences Jeju, Korea Pre-Symposium Workshop
Single-Use Final Fill: Benefits and Considerations
 Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
Guidance for Industry
 Guidance for Industry for the Submission Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry for the Submission Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products Center for Drug Evaluation and Research (CDER) Center
Containment Booths. FOR MORE INFORMATION ON CONTAINMENT BOOTHS CALL +44 (0) OR
 www.extract-technology.com ENGINEERED CONTAINMENT SOLUTIONS Containment Booths AT Extract Technology, we provide complete containment solutions based around an innovative range of Downflow Containment
www.extract-technology.com ENGINEERED CONTAINMENT SOLUTIONS Containment Booths AT Extract Technology, we provide complete containment solutions based around an innovative range of Downflow Containment
Modular Turnkey Concept for Pharmaceutical Sterile Formulation Facilities
 Presented at the World Batch Forum European Conference Mechelen, Belgium 11-13 October 2004 900 Fox Valley Drive, Suite 204 Longwood, FL 32779-2552 +1.407.774.5764 Fax: +1.407.774.6751 E-mail: info@wbf.org
Presented at the World Batch Forum European Conference Mechelen, Belgium 11-13 October 2004 900 Fox Valley Drive, Suite 204 Longwood, FL 32779-2552 +1.407.774.5764 Fax: +1.407.774.6751 E-mail: info@wbf.org
Introduction and Background
 Introduction and Background Validation of Biotech Facilities Pacific Biotech Alliance Robert J. Mackey Pacific Biotech Alliance is an established consulting firm in the Pacific Northwest that specializes
Introduction and Background Validation of Biotech Facilities Pacific Biotech Alliance Robert J. Mackey Pacific Biotech Alliance is an established consulting firm in the Pacific Northwest that specializes
An Introduction to Double Dragon Consulting
 An Introduction to Double Dragon Consulting www.doubledragonconsulting.com 1 June 2012 About DDC Our Experience: All dosage forms, aseptic processes, OTC Remediation of 483 observations, warning letters,
An Introduction to Double Dragon Consulting www.doubledragonconsulting.com 1 June 2012 About DDC Our Experience: All dosage forms, aseptic processes, OTC Remediation of 483 observations, warning letters,
PRAXIS. A publication by Bioengineering AG
 PRAXIS A publication by Bioengineering AG Portrait of Rentschler Biotechnologie GmbH, a globally active service company that supports its clients in the development, production, and registration of biopharmaceuticals.
PRAXIS A publication by Bioengineering AG Portrait of Rentschler Biotechnologie GmbH, a globally active service company that supports its clients in the development, production, and registration of biopharmaceuticals.
GUIDE TO INSPECTIONS OF MICROBIOLOGICAL PHARMACEUTICAL QUALITY CONTROL LABORATORIES
 GUIDE TO INSPECTIONS OF MICROBIOLOGICAL PHARMACEUTICAL QUALITY CONTROL LABORATORIES Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA,
GUIDE TO INSPECTIONS OF MICROBIOLOGICAL PHARMACEUTICAL QUALITY CONTROL LABORATORIES Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA,
Ampio Pharmaceuticals Production and Office Facility Englewood, Colorado
 Related Experience Ampio Pharmaceuticals Production and Office Facility Englewood, Colorado About the Project Design and construction of a 19,000-SF pharmaceutical production facility, which included cleanrooms,
Related Experience Ampio Pharmaceuticals Production and Office Facility Englewood, Colorado About the Project Design and construction of a 19,000-SF pharmaceutical production facility, which included cleanrooms,
Facility and Operational Issues for Cord Blood Banks: FDA Draft Guidance
 Facility and Operational Issues for Cord Blood Banks: FDA Draft Guidance Mary Malarkey, Director Office of Compliance and Biologics Quality ISCT 7 th Annual Somatic Cell Therapy Symposium, September 26-28,
Facility and Operational Issues for Cord Blood Banks: FDA Draft Guidance Mary Malarkey, Director Office of Compliance and Biologics Quality ISCT 7 th Annual Somatic Cell Therapy Symposium, September 26-28,
GMP considerations when designing an API plant. Presented by Cameron Roberts, Senior Engineer 4 July, 2016
 GMP considerations when designing an API plant Presented by Cameron Roberts, Senior Engineer 4 July, 2016 Introduction API manufacturing is too broad a topic to cover all scenarios so I will draw from
GMP considerations when designing an API plant Presented by Cameron Roberts, Senior Engineer 4 July, 2016 Introduction API manufacturing is too broad a topic to cover all scenarios so I will draw from
Good Manufacturing Practices Purpose and Principles of GMP. Tony Gould
 Good Manufacturing Practices Purpose and Principles of GMP Tony Gould Why GMP? Provides a high level assurance that medicines are manufactured in a way that ensures their safety, efficacy and quality Medicines
Good Manufacturing Practices Purpose and Principles of GMP Tony Gould Why GMP? Provides a high level assurance that medicines are manufactured in a way that ensures their safety, efficacy and quality Medicines
ANNEX 1: COMMENT SUBMISSION DOCUMENT
 Submission Comments on: GMP Annex 1: Proposal for amendments to the environmental classification table for particles and associated text, amendment to section 42 concerning acceptance criteria for media
Submission Comments on: GMP Annex 1: Proposal for amendments to the environmental classification table for particles and associated text, amendment to section 42 concerning acceptance criteria for media
Pharmaceutical Compounding Sterile and Nonsterile Preparations. Jeanne Sun, PharmD
 Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
Vetter Development Service
 A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE
 XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
Vetter Commercial Manufacturing
 A unique blend of capabilities for aseptic fill and finish Vetter Commercial Manufacturing Delivering results across the production chain Answers that work 1 Manufacturing quality is just the beginning.
A unique blend of capabilities for aseptic fill and finish Vetter Commercial Manufacturing Delivering results across the production chain Answers that work 1 Manufacturing quality is just the beginning.
For sterile products manufacturing, Lyoseal is an innovative technology. presented by Mr. Tony Bouzerar from BioCorp offers us detailed
 For sterile products manufacturing, Lyoseal is an innovative technology to improve quality problems with final drug product due to crimping issues and inadequate stopper seating. It eliminates aluminum
For sterile products manufacturing, Lyoseal is an innovative technology to improve quality problems with final drug product due to crimping issues and inadequate stopper seating. It eliminates aluminum
Supplementary Training Modules on Good Manufacturing Practice. Validation. WHO Technical Report Series, No. 937, Annex 4.
 Supplementary Training Modules on Good Manufacturing Practice Validation WHO Technical Report Series, No. 937, 2006. Annex 4. Validation Slide 1 of 48 August 2006 Validation! Part 1. General overview on
Supplementary Training Modules on Good Manufacturing Practice Validation WHO Technical Report Series, No. 937, 2006. Annex 4. Validation Slide 1 of 48 August 2006 Validation! Part 1. General overview on
GETINGE ISOCYT FREJA THE SAFEST WAY TO PROTECT BOTH PATIENT AND PHARMACIST. Always with you
 GETINGE ISOCYT FREJA THE SAFEST WAY TO PROTECT BOTH PATIENT AND PHARMACIST Always with you PROTECT THE PATIENT. PROTECT THE PHARMACIST. PROTECT THE PLANET. Cytotoxic drugs are an occupational health hazard.
GETINGE ISOCYT FREJA THE SAFEST WAY TO PROTECT BOTH PATIENT AND PHARMACIST Always with you PROTECT THE PATIENT. PROTECT THE PHARMACIST. PROTECT THE PLANET. Cytotoxic drugs are an occupational health hazard.
Overview of Inspection Issues with Legacy Products
 Overview of Inspection Issues with Legacy Products Presented by Simone E. Pitts Consumer Safety Officer, USFDA Team Biologics simone.pitts@fda.hhs.gov Objective To provide some examples of recent inspectional
Overview of Inspection Issues with Legacy Products Presented by Simone E. Pitts Consumer Safety Officer, USFDA Team Biologics simone.pitts@fda.hhs.gov Objective To provide some examples of recent inspectional
Animal cell and tissue culture. Lab 1
 Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
Competence in Lyophilization Systems
 Competence in Lyophilization Systems 2 COMPETENCE IN LYOPHILIZATION SYSTEMS COMPETENCE IN LYOPHILIZATION SYSTEMS 3 Competence in Lyophilization Systems GEA is one of the world s market leaders in Freeze
Competence in Lyophilization Systems 2 COMPETENCE IN LYOPHILIZATION SYSTEMS COMPETENCE IN LYOPHILIZATION SYSTEMS 3 Competence in Lyophilization Systems GEA is one of the world s market leaders in Freeze
Overview of Regulatory Requirements for API and Formulations
 Overview of Regulatory Requirements for API and Formulations Sangeeta Sardesai 4-Dec-2010 Definition - Regulatory Requirement The restrictions, licenses, and laws applicable to a product or business, imposed
Overview of Regulatory Requirements for API and Formulations Sangeeta Sardesai 4-Dec-2010 Definition - Regulatory Requirement The restrictions, licenses, and laws applicable to a product or business, imposed
WHAT IS A CLEAN ROOM?
 WHAT IS A CLEAN ROOM? A clean room is an extremely sophisticated work environment, designed and built with the express purpose of providing a high level of cleanliness for a production or research facility.
WHAT IS A CLEAN ROOM? A clean room is an extremely sophisticated work environment, designed and built with the express purpose of providing a high level of cleanliness for a production or research facility.
On behalf of the PHSS Pharmaceutical and Healthcare Sciences Society (UK).
 31 st March 2015 Submission of comments on ' Concept paper on the revision of annex 1 of the guidelines on good manufacturing practice manufacture of sterile medicinal products EMA/INS/GMP/735037/2014
31 st March 2015 Submission of comments on ' Concept paper on the revision of annex 1 of the guidelines on good manufacturing practice manufacture of sterile medicinal products EMA/INS/GMP/735037/2014
EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT
 1 EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT UNDERSTAND THE PROGRESSION OF REGULATORY THINKING AND HOW TO SUCCESSFULLY IMPLEMENT THE PROPOSED CHANGES BALTIMORE, MD MAY 6TH AND 7TH, 2019 UNIVERSITY
1 EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT UNDERSTAND THE PROGRESSION OF REGULATORY THINKING AND HOW TO SUCCESSFULLY IMPLEMENT THE PROPOSED CHANGES BALTIMORE, MD MAY 6TH AND 7TH, 2019 UNIVERSITY
Formulation Development & CTM Manufacturing Services
 Formulation Development & CTM Manufacturing Services VxP Pharma Purdue Research Park 5225 Exploration Drive Indianapolis, IN 46241 Tel: 317.759.2299 Fax: 317.713.2950 VxP Pharmaprovides an extensive range
Formulation Development & CTM Manufacturing Services VxP Pharma Purdue Research Park 5225 Exploration Drive Indianapolis, IN 46241 Tel: 317.759.2299 Fax: 317.713.2950 VxP Pharmaprovides an extensive range
MINIMUM REQUIREMENTS FOR A VENDOR
 MINIMUM REQUIREMENTS FOR A VENDOR When outsourcing the production of sterile products the first step in vendor evaluation is to see if they meet the minimum requirements. We ve developed a group of questions
MINIMUM REQUIREMENTS FOR A VENDOR When outsourcing the production of sterile products the first step in vendor evaluation is to see if they meet the minimum requirements. We ve developed a group of questions
Microbiological Consideration for Non-Sterile Pharmaceutical
 May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
As advanced therapies such as oncolytic viruses and gene
 Designing and Operating Flexible, High-Containment Vaccine Manufacturing Thomas Page A new facility type integrates next-generation mobile cleanroom systems. Thomas Page, PhD is vice-president, Engineering
Designing and Operating Flexible, High-Containment Vaccine Manufacturing Thomas Page A new facility type integrates next-generation mobile cleanroom systems. Thomas Page, PhD is vice-president, Engineering
SynCo Bio Partners B.V. & Wacker Biotech GmbH: THE MICROBIAL CMO
 CREATING TOMORROW`S SOLUTIONS SynCo Bio Partners B.V. & Wacker Biotech GmbH: THE MICROBIAL CMO Human Microbiota: Proof of Concept to Production Işil Tüzün Weber Process Engineer Technical Operations Title
CREATING TOMORROW`S SOLUTIONS SynCo Bio Partners B.V. & Wacker Biotech GmbH: THE MICROBIAL CMO Human Microbiota: Proof of Concept to Production Işil Tüzün Weber Process Engineer Technical Operations Title
tempris sensor Tempris a powerful PAT Tool Pave your way to Pharma 4.0 with
 tempris sensor technology Pave your way to Pharma 4.0 with Tempris a powerful PAT Tool process control with wireless, battery-free real-time temperature measurement WHY USE TEMPRIS as a PAT Tool for product
tempris sensor technology Pave your way to Pharma 4.0 with Tempris a powerful PAT Tool process control with wireless, battery-free real-time temperature measurement WHY USE TEMPRIS as a PAT Tool for product
Microbial contamination is a
 B I O P R O C E S S TECHNICAL Barrier Vial Technology A Global Approach to the Aseptic Filling Process Diego López-Alvarez, Sergi Roura, and J.A. Garcia Microbial contamination is a concern and a constant
B I O P R O C E S S TECHNICAL Barrier Vial Technology A Global Approach to the Aseptic Filling Process Diego López-Alvarez, Sergi Roura, and J.A. Garcia Microbial contamination is a concern and a constant
GOOD MANUFACTURING PRACTICE
 GOOD MANUFACTURING PRACTICE Introduction The first WHO draft text on good manufacturing practices (GMP) was prepared in 1967 by a group of consultants at the request of the Twentieth World Health Assembly
GOOD MANUFACTURING PRACTICE Introduction The first WHO draft text on good manufacturing practices (GMP) was prepared in 1967 by a group of consultants at the request of the Twentieth World Health Assembly
ISPE Baseline Pharmaceutical Engineering Guide for New and Renovated Facilities Volume 2: Oral Solid Dosage Forms (Revision) Executive Summary
 Reprinted from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE September/October 2008, Vol. 28 No. 5 This executive summary provides an overview of the second edition of the ISPE Baseline Guide:
Reprinted from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE September/October 2008, Vol. 28 No. 5 This executive summary provides an overview of the second edition of the ISPE Baseline Guide:
Sam Halaby OSD Subject Matter Expert
 OSD Subject Matter Expert Education B.S., Chemical Engineering - Clarkson University B.S., Interdisciplinary Engineering and Management - Clarkson University Professional Associations Parenteral Drug Association
OSD Subject Matter Expert Education B.S., Chemical Engineering - Clarkson University B.S., Interdisciplinary Engineering and Management - Clarkson University Professional Associations Parenteral Drug Association
PHCT 401 Aseptic Processes & Techniques
 PHCT 401 Aseptic Processes & Techniques Principles of Aseptic Techniques Aseptic Processing Sterility Testing Laminar flow air cleaning Quality Control Tests Personnel Principles of Aseptic Techniques
PHCT 401 Aseptic Processes & Techniques Principles of Aseptic Techniques Aseptic Processing Sterility Testing Laminar flow air cleaning Quality Control Tests Personnel Principles of Aseptic Techniques
Alkermes Contract Pharma Services
 Alkermes Contract Pharma Services Highly Potent, Poorly Soluble Product Manufacturing Contract, with 100% OTIF #501 April 2013 Fidelma Callanan Senior Director, Marketing and Commercial Development Contents
Alkermes Contract Pharma Services Highly Potent, Poorly Soluble Product Manufacturing Contract, with 100% OTIF #501 April 2013 Fidelma Callanan Senior Director, Marketing and Commercial Development Contents
Brief Introduction of Aburaihan Company Organization Policy Organization Chart Qualified Human Resource Production Department Engineering Department
 1 Contents Brief Introduction of Aburaihan Company Organization Policy Organization Chart Qualified Human Resource Production Department Engineering Department Sanitation and Environment Protection Documentation
1 Contents Brief Introduction of Aburaihan Company Organization Policy Organization Chart Qualified Human Resource Production Department Engineering Department Sanitation and Environment Protection Documentation
Review Validation of aseptic processes for pharmaceuticals
 OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
Actavis Italy. Nerviano Plant
 Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
Microbiology Testing: USP requirements for Sterile and Nonsterile Preparations Webinar Q&A
 USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
Conducting Supplier Audits: Ensure Validation Compliance
 Conducting Supplier Audits: Ensure Validation Compliance Presented by: Gamal Amer, Ph. D. Principal Premier Compliance Services, Inc. All rights reserved. Do not copy without permission. 1 Outline Why
Conducting Supplier Audits: Ensure Validation Compliance Presented by: Gamal Amer, Ph. D. Principal Premier Compliance Services, Inc. All rights reserved. Do not copy without permission. 1 Outline Why
Environmental Control Requirements. Gordon Farquharson July 2017
 Environmental Control Requirements Gordon Farquharson July 2017 Learning Objectives Learn how contamination control standards and guidance sets requirements for Critical Parameters ISO-14644-1 Classification
Environmental Control Requirements Gordon Farquharson July 2017 Learning Objectives Learn how contamination control standards and guidance sets requirements for Critical Parameters ISO-14644-1 Classification
