Cancer Vanguard. Biosimilars Trust Policy Template
|
|
|
- Gabriel Malone
- 6 years ago
- Views:
Transcription
1 Cancer Vanguard Biosimilars Trust Policy Template Aim of this document: The document provides generic guidance and outline for the development of local trust policies in relation to the adoption of biosimilars in trusts. All trusts work in slightly different ways and have different processes with the goal of achieving similar outcomes. The document aims to highlight key points in the use and adoption of biosimilars, which can then be developed and adapted to individual trust needs and processes as appropriate. The focus of the template is on haematological and solid tumour biosimilars, however it can be adapted to biosimilars used in all therapy areas. The policy is seen as an overarching policy which will link into specific SOPs for individual biosimilar medicines. Summary: What are biologics? Medicines that are made or derived from a biological source and as such are complex with inherent variability in there structure. As biological medicines are derived from living cells or organisms there is always a small degree of variability in the manufacturing process, thus biologics may show a degree of variation from batch to batch of the product. This is also the case for biosimilars. What are biosimilars? Biosimilars are highly similar to the biological originator medicine (already licensed), shown by non-clinical studies (in vivo and in vitro analysis) and clinical studies to show no clinically meaningful differences from the originator biological medicine in relation to quality, safety and efficacy. To note: Biosimilar medicines are not considered as generic to the originator biological medicines the two are similar and not identical. However in relation to licensing they have met stringent regulatory requirements based on a comprehensive scientific comparability exercise such that they do not have any clinically meaningful differences from the reference medicines in terms of quality, safety and efficacy. Page 1 of 14
2 Table of contents Section Page number 1. Background & Scope 4 2. Definitions 5 3. Duties & Responsibilities 5 4. Introduction of a new biosimilar Considerations to be taken prior to adoption of biosimilar 6 & Internal governance requirements Commissioner Position Informing and involving patients in introduction 7 & Prescribing requirements IT readiness Patient Registration and Consent Pharmacovigilance and monitoring Clinical outcomes monitoring Monitoring patient satisfaction Pharmacy Purchasing requirements Tracking of savings 9 & Evaluation of Service impact of biosimilar introduction Bibliography Linked documents 11 Appendix 1. Adoption time lines chart 12 Appendix 2. Biosimilars uptake tracker 13 Page 3 of 14
3 1. Background & scope The policy has been developed in line with recommendations [insert detail here, this may include different organisation e.g. Cancer Vanguard Guidance, BOPA position statement on biosimilars, NICE & others]. The use of biosimilars in cancer is set to increase exponentially in the next few years as patents of originator biologics expire. The adoption of biosimilars will help provide much needed savings to the NHS, which may be utilised to further benefit patient care (however introduction should not be driven purely by financial considerations). The purpose of the policy is to aid this early adoption process in order that the benefits can be realised early. The use of biosimilars will not alter the care provided to patients, with the patient seeing no change in the treatment experience. Detailed guidance will be provided on the following topics: Adoption process for biosimilars including: o Considerations prior to adoption o Approach to homecare if required o Existing versus New Patients o Governance requirements and local approval o Informing and involving patients in introduction o Prescribing requirements o IT readiness o Pharmacovigilance and monitoring o Clinical outcomes monitoring o Tracking of any savings The policy is overarching and should be used in conjunction with individual SOPs developed for the introduction and use of specific biosimilars at the trust. Page 4 of 14
4 2. Definitions: Biological medicine medicine derived from living cells or organisms, consisting of large highly complex molecular entities which may be difficult to characterise. Biosimilar medicine a biological product that is highly similar but not identical, to the licensed originator biological medicine and shows no clinically meaningful difference in terms of quality safety and efficacy. Generic medicine - is identical or bioequivalent to a brand name drug in dosage form, safety, strength, route of administration, quality, performance characteristics and intended use. Extrapolation the decision by the Regulator whether to extend the efficacy and safety data from an indication for which a biosimilar has been clinically tested to other conditions for which the reference product is approved. Interchangeability the medical practice of changing one medicine for another that is expected to achieve the same clinical effect in a given clinical setting and in any patient on the initiative or with the agreement of the prescriber. 3. Duties & Responsibilities This policy applies to medical, nursing, pharmacy staff and other key staff involved in any aspects of providing biosimilar medicines to patients. Lead Consultant (and clinical team) Support the proposed biosimilar introduction, in the agreed patient groups and endorse the DTC submission on behalf of the clinical unit Carry out initial patient consultation with patients in lead up to biosimilar medicine adoption (see also specialist nurses and pharmacists) Pharmacy Department Coordinate and manage an effective implementation programme Specialist and unit pharmacists to be able to provide information on biosimilars to HCPs and patients Provide required detail for management of trust prescribing systems and aseptics unit work sheets (if required) Reporting on uptake of the biosimilar medicine following any biosimilar introduction and reporting financial savings realised from adoption Procurement of the selected biosimilar Page 5 of 14
5 If the biosimilar is to be delivered via a Homecare delivery service, coordination and requirements of a biosimilar introduction will need to be considered Specialist nurses and specialist pharmacists (who have direct involvement with relevant patients) Carry out initial consultation with patients in lead up to biosimilar medicine adoption Be available to answer patient questions and provide information regarding biosimilar medicines to patients and other HCPs should it be required 4. Core elements: Introduction of a new biosimilar This section provides potential requirements to be taken into consideration when reviewing initial adoption of a biosimilar. 4.1 Considerations to be taken prior to adoption General: Points to be included in policy should include: Does the biosimilar have the required licensed indications? Anticipated launch date and supply chain details Patient groups to be included: Adult and paediatric setting? Will the biosimilar be intended for all indications or only specific indications? Process to be adopted: o to be introduced by the Trust for existing patients o to be introduced by the Trust for new patients o both of the above Are the biosimilar presentations i.e. strengths, concentration & preparation the same as for the originator? Are the biosimilar stability once prepared and storage conditions the same as the originator? Are the biosimilar administration requirements the same as for the originator i.e. route and duration of administration? Are the required clinical outcomes data available prior to review by the trusts DTC? Are a number of biosimilars medicines for the same originator biological medicine anticipated to be launched around the same time by different manufacturers? If so a decision will need to be made on which will be adopted, and when, with an aim to avoid Page 6 of 14
6 further changes in the short-term which may introduce risk and damage patient confidence (see also section 4.10 pharmacy purchasing). Possible resource implications of the adoption process. These may include: o o o o o patient counselling requirements MDT education and training requirements possible administration route change e.g. SC to IV possible administration duration change is the biological medicine given in the Homecare setting and will this have to be reviewed (e.g. for initial dosing or patient self-administration training) 4.2 Internal governance requirements DTC submission as per local requirements. The submission should include points highlighted in Commissioner position Prior to undertaking the change from originator biological medicine to a new biosimilar medicine, the position of the commissioner e.g. NHSE should be sought, with adoption meeting the requirements of any NHSE initiative such as CQUINs within the required timeline. 4.4 Informing and involving patients in introduction Local decision on requirement to inform new patients once the biosimilar has been approved and adopted at the trust. For new patients this is not a change but a recommended treatment by a clinician. Required at point of initial adoption to inform and educate currently-treated patients. How this is carried out will be dependent on the biological medicine in question e.g. how often it is prescribed, in what setting it is given (IP, OP, or Homecare), and how the clinics are set up. Possible methods for informing and involving patients may include: o focus groups prior to adoption o one to one patient consultation by trained clinician, nurse or pharmacist in lead up to the adoption (feasibility will be dependent on how the clinic is coordinated at the trust) o the utilisation of a patient information leaflet with Q&A section and contact details of relevant HCP if patients wish to discuss further o patient letter to be sent out to patients explaining: 1. the planned change Page 7 of 14
7 2. how the decision has been undertaken 3. that clinical efficacy and safety have not been affected 4. that significant financial benefits will be achieved for the NHS and/or the Trust. 4.5 Prescribing requirements & interchangeability It is recommended that biosimilar medicines be prescribed by brand name for example, International Non-proprietary Name (INN) (Brand name ) i.e. Filgrastim (Zarzio ) (see section 4.6. for electronic prescribing systems). Prescribing by brand reduces the risk of one biosimilar brand being substituted for another without a review and due consideration by the prescribing clinician/team. This does not mean that a biosimilar medicine cannot be changed from one brand to another, however this needs to be done as part of a clinically led management process. Biosimilars are interchangeable. Interchangeability is the practice of changing one medicine for another that is expected to achieve the same clinical effect. The decision to interchange is one that again requires review and due consideration by the prescribing clinician/team and approval via the local DTC. Batch number must also be recorded as with all biologic medicines in case of requirement to report an ADR (a local process dependent on prescribing systems should be adopted to ensure this). 4.6 IT readiness Points to be included in policy should include: If the originator biological medicine and biosimilar are both to continue to be used at the trust (e.g. in change over period or for different indications) the pharmacy systems clearly need to differentiate between the two (i.e. is brand name in the profile name). Systems will include dispensing and in some cases aseptic unit systems. 4.7 Patient Registration and consultation/ shared decision making Following the adoption of a biosimilar at the trust it will be a local decision on how patients need to be consulted if a biosimilar change is to take place mid treatment. All new patients will follow the standard consent process as with the reference originator medicine. Page 8 of 14
8 4.8 Pharmacovigilance and monitoring All biological medicines require additional monitoring for safety and any suspected adverse drug should be reported using the MHRA yellow card scheme, with the provision of the brand and the batch number. 4.9 Clinical outcomes monitoring As with all biologic medicines collection of clinical outcomes should take place, and after an agreed time period assessed to ensure quality of outcomes Monitoring patient satisfaction. A patient experience survey in the form of a short questionnaire may be carried out pre and post implementation of biosimilar to ensure that the patient experience has not been negatively impacted following the introduction of the biosimilar medicine. The finding may also assist in supporting future biosimilar adoptions if shared with patients and MDTs Pharmacy Purchasing requirements Close liaison with regional procurement leads should take place, in order to keep up to date with new biosimilar medicines: - anticipated launch dates - planned tenders and timelines - product specifications - pricing information 4.12 Tracking of savings and biosimilar adoption rate Following implementation of a biosimilar medicine tracking of: the drug acquisition cost savings should be monitored and recorded on a monthly basis to calculate savings achieved from the change (see appendix 3. for example). breakdown of: o number of new patients on the biosimilar o number of patients changed to the biosimilar medicine part-way through current treatment, for the approved indication o reasons identified for those patients that have not been changed o metrics and indicators in line with any NHSE requirements e.g. Medicines Optimisation and CQUINs Page 9 of 14
9 4.13 Evaluation of Service impact on the Trust of adopting a biosimilar Data should be collected through-out the change process in order to ascertain the resource impact of adopting the biosimilar in both new and mid-treatment change patients (refer to Service impact tool on Biosimilar adoption process time line for further information). Page 10 of 14
10 5. Bibliography (papers utilised to complete this guidance & may further assist in development of local guidance): 1. A clinicians guide to biosimilars in oncology. Hope S et al. Cancer treatment reviews 46. (2016) pp Available form < 2. Basingstoke, Southampton and Winchester District Prescribing Committee (2016). Biosimilar medicines position statement and guidance. Available from < biosimilar-medicines-position-statement-and-guidance-june-2016/file> 3. BOPA. Draft Position Statement on Implementation of Biosimilar Monoclonal Antibodies (2017). Available from <awaited> 4. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology derived proteins as active substance: non-clinical and clinical. December Available from < C pdf> 5. NHSE (2015). What is a biosimilar? Available from < Page 11 of 14
11 6. Linked documents This will be different for individual trust and may include: Standard Operating Procedures for individual biosimilars Medicines Management Policy Page 12 of 14
12 Appendix 1. Biosimilar Adoption Process Timeline Page 13 of 14
13 Appendix 2. Biosimilars uptake tracker requirements (to note NHSE may provide a tracker in relation to any CQUIN requirements). The tracker will be used to provide details of biosimilar uptake and any associated savings made. Data from the tracker can be used to assist in reporting that CQUIN requirements on a local or national level have been met. It should initially be completed on a monthly basis. Once the adoption process has stabilised following initial uptake this may go to a quarterly review, although this should be agreed locally. From initial adoption the tracker can be used to gauge success of the adoption programme, and predict when uptake by all patients anticipated to receive the biosimilar will be achieved. Detail of reasons why patients may not be receiving the biosimilar can be also be ascertained. Information that should be tracked includes: Indication for which biosimilar has been approved for use* (if more than one possible indication for use) Number of vials of biosimilar used per month OR Number of mgs (or other mass unit) used per month (if manufactured by a 3 rd party provider) Number of patients treated using biosimilar Number of vials of originator biologic used per month (for same indication*) OR Number of mgs (or other mass unit) used per month (if manufactured by a 3 rd party provider) Number of patients treated using originator biologic Page 14 of 14
Regional Medicines Optimisation Committee Briefing Best Value Biologicals: Adalimumab Update 2
 Regional Medicines Optimisation Committee Briefing Best Value Biologicals: Adalimumab Update 2 Adalimumab RMOC Update 1: https://www.sps.nhs.uk/articles/rmoc-briefing-paper-on-adalimumab/ The purpose of
Regional Medicines Optimisation Committee Briefing Best Value Biologicals: Adalimumab Update 2 Adalimumab RMOC Update 1: https://www.sps.nhs.uk/articles/rmoc-briefing-paper-on-adalimumab/ The purpose of
NCCP Guidance on the use of Biosimilar Medicines in Cancer Treatment
 NCCP Guidance on the use of Biosimilar Medicines in Cancer Treatment Version Date published Amendment Approved By 1 August 2017 Working Group 2 September 2017 Inclusion of link to HPRA information for
NCCP Guidance on the use of Biosimilar Medicines in Cancer Treatment Version Date published Amendment Approved By 1 August 2017 Working Group 2 September 2017 Inclusion of link to HPRA information for
The introduction of biosimilars into clinical practice
 Commissioning Chemotherapy Services The introduction of biosimilars into clinical practice Jatinder Harchowal Chief Pharmacist / Clinical Director Royal Marsden NHS Foundation Trust Medicines lead for
Commissioning Chemotherapy Services The introduction of biosimilars into clinical practice Jatinder Harchowal Chief Pharmacist / Clinical Director Royal Marsden NHS Foundation Trust Medicines lead for
NHS England, National QIPP Delivery Programme, Medicines Management & Optimisation
 NHS England, National QIPP Delivery Programme, Medicines Management & Optimisation Biosimilars National Surveys; CCGs and NHS Trusts Summary Report - April 2018 1.0 Background This report summarises the
NHS England, National QIPP Delivery Programme, Medicines Management & Optimisation Biosimilars National Surveys; CCGs and NHS Trusts Summary Report - April 2018 1.0 Background This report summarises the
FIP STATEMENT OF POLICY Pharmacist's authority in pharmaceutical product selection: therapeutic interchange and substitution
 Pharmacist's authority in pharmaceutical product selection: therapeutic interchange and substitution Purpose: The purpose of this document is to provide a set of recommendations on therapeutic interchange
Pharmacist's authority in pharmaceutical product selection: therapeutic interchange and substitution Purpose: The purpose of this document is to provide a set of recommendations on therapeutic interchange
Purchasing for Safety Policy for Pharmaceuticals January Page 1 of 8 (Purchasing for Safety Policy for Pharmaceuticals)
 Purchasing for Safety Policy for s January 2010 Page 1 of 8 (Purchasing for Safety Policy for s) Policy Title Purchasing for Safety Policy for s Policy Reference Number Acute10/006 Implementation Date
Purchasing for Safety Policy for s January 2010 Page 1 of 8 (Purchasing for Safety Policy for s) Policy Title Purchasing for Safety Policy for s Policy Reference Number Acute10/006 Implementation Date
Policy Position. Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs)
 Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs) Geneva, April 2016 Appropriate use of biotherapeutics including SBPs - SBPs, also known as biosimilars, are developed to
Pharmacy-mediated interchangeability for Similar Biotherapeutic Products (SBPs) Geneva, April 2016 Appropriate use of biotherapeutics including SBPs - SBPs, also known as biosimilars, are developed to
Roche Position 1 on Similar Biotherapeutic Products Biosimilars
 Roche Position 1 on Similar Biotherapeutic Products Biosimilars Similar Biotherapeutic Products Biosimilars Innovative biotherapeutic products (e.g.monoclonal antibodies) are losing market exclusivity,
Roche Position 1 on Similar Biotherapeutic Products Biosimilars Similar Biotherapeutic Products Biosimilars Innovative biotherapeutic products (e.g.monoclonal antibodies) are losing market exclusivity,
Tendering for Medicines and Services with CMU. Kevan Wind, Medicines Procurement Specialist Pharmacist, London & East of England
 Tendering for Medicines and Services with CMU Kevan Wind, Medicines Procurement Specialist Pharmacist, London & East of England What we will cover 1. Pricing and Procurement of Medicines in the NHS. 2.
Tendering for Medicines and Services with CMU Kevan Wind, Medicines Procurement Specialist Pharmacist, London & East of England What we will cover 1. Pricing and Procurement of Medicines in the NHS. 2.
What next? Manufacture the biosimilar product
 What next? Manufacture the biosimilar product Design manufacturing process to match QTPP Full quality dossier required. Use state of the art technologies In accordance with relevant ICH and CHMP guidelines
What next? Manufacture the biosimilar product Design manufacturing process to match QTPP Full quality dossier required. Use state of the art technologies In accordance with relevant ICH and CHMP guidelines
How to Deliver NHS Hospital Medicines Optimisation CQUIN & Implement Biosimilar Monoclonal Antibodies. Guide to GE3: Hospital Medicines Optimisation
 Guide to GE3: Hospital Medicines Optimisation Biosimilars Monoclonal Antibodies Implementation Resource Pack The development of this guide has been funded by an unconditional educational grant from Napp
Guide to GE3: Hospital Medicines Optimisation Biosimilars Monoclonal Antibodies Implementation Resource Pack The development of this guide has been funded by an unconditional educational grant from Napp
Medicines optimisation & the model hospital
 Medicines optimisation & the model hospital - Securing value from medicines Andrew Davies, Professional Lead for Hospital Pharmacy, NHS Improvement Andrew.davies@nhs.net @HospChiefPharm Commissioning Chemotherapy
Medicines optimisation & the model hospital - Securing value from medicines Andrew Davies, Professional Lead for Hospital Pharmacy, NHS Improvement Andrew.davies@nhs.net @HospChiefPharm Commissioning Chemotherapy
Biosimilars contracting in the NHS. Maggie Dolan Regional Pharmacy Procurement Specialist South East Coast
 Biosimilars contracting in the NHS Maggie Dolan Regional Pharmacy Procurement Specialist South East Coast Aim Introduction Managing the Medicine Budget in the NHS The Biosimilar Medicine Challenges Questions
Biosimilars contracting in the NHS Maggie Dolan Regional Pharmacy Procurement Specialist South East Coast Aim Introduction Managing the Medicine Budget in the NHS The Biosimilar Medicine Challenges Questions
5 key characteristics
 Biosimilars 5 key characteristics Biologics are medicinal products derived from living organisms. 1 Their use has impacted the treatment of a variety of diseases. 1 Patents for many biologics will soon
Biosimilars 5 key characteristics Biologics are medicinal products derived from living organisms. 1 Their use has impacted the treatment of a variety of diseases. 1 Patents for many biologics will soon
HARROGATE & RURAL DISTRICT AREA PRESCRIBING COMMITTEE (HaRD APC) Application for a new product to be added to the formulary
 Guidance on completing the form Your submission should be comprehensive and indicate which, if any, information has been supplied by a pharmaceutical company. The application must be completed with the
Guidance on completing the form Your submission should be comprehensive and indicate which, if any, information has been supplied by a pharmaceutical company. The application must be completed with the
BRITISH GENERIC MANUFACTURERS ASSOCIATION
 BRITISH GENERIC MANUFACTURERS ASSOCIATION Response by the British Generic Manufacturers Association (BGMA) to the Department of Health Consultation on Amendments to the Statutory Scheme to Control the
BRITISH GENERIC MANUFACTURERS ASSOCIATION Response by the British Generic Manufacturers Association (BGMA) to the Department of Health Consultation on Amendments to the Statutory Scheme to Control the
CURRENT POLICY ON ACCESS TO MEDICINES AND HEALTH IN EUROPE. The role of Biosimilar Medicines
 CURRENT POLICY ON ACCESS TO MEDICINES AND HEALTH IN EUROPE The role of Biosimilar Medicines Marc A. Mahl, MD MBA(INSEAD) President, Medicines for Europe Baltimore, 05 th September 2018 patients quality
CURRENT POLICY ON ACCESS TO MEDICINES AND HEALTH IN EUROPE The role of Biosimilar Medicines Marc A. Mahl, MD MBA(INSEAD) President, Medicines for Europe Baltimore, 05 th September 2018 patients quality
ACG Public Forum. Join ACG, the FDA, and EMA for a discussion on biosimilars and IBD. Monday, 12:45 pm 2:15 pm
 ACG Public Forum Join ACG, the FDA, and EMA for a discussion on biosimilars and IBD Monday, 12:45 pm 2:15 pm ACG 2017: FDA-EMA workshop on biosimilars Joachim Musaus EMA Product Lead Gastroenterology Human
ACG Public Forum Join ACG, the FDA, and EMA for a discussion on biosimilars and IBD Monday, 12:45 pm 2:15 pm ACG 2017: FDA-EMA workshop on biosimilars Joachim Musaus EMA Product Lead Gastroenterology Human
ASBM Biosimilars. Canada Prescribers and Biosimilars October, Kevin Olson, CEO Industry Standard Research
 ASBM Biosimilars Canada Prescribers and Biosimilars October, 2017 Kevin Olson, CEO Industry Standard Research KevinO@ISRreports.com Table of contents Page 3 Methodology 5 Sample Characteristics 6 Executive
ASBM Biosimilars Canada Prescribers and Biosimilars October, 2017 Kevin Olson, CEO Industry Standard Research KevinO@ISRreports.com Table of contents Page 3 Methodology 5 Sample Characteristics 6 Executive
New Model for Immunoglobulin Assessment Panels Implementation Pack
 Improving Value in Specialised Services New Model for Immunoglobulin Assessment Panels Implementation Pack Insert Author Name: Leena Sevak & Rob Coster Version: 0.1 10 th October 2017 This pack provides
Improving Value in Specialised Services New Model for Immunoglobulin Assessment Panels Implementation Pack Insert Author Name: Leena Sevak & Rob Coster Version: 0.1 10 th October 2017 This pack provides
Chemically synthesized proteins referencing biological medicinal products
 Chemically synthesized proteins referencing biological medicinal products A EuropaBio white paper Calling for: - Equal assessment transparency - Equal measures for traceability and adverse event reporting
Chemically synthesized proteins referencing biological medicinal products A EuropaBio white paper Calling for: - Equal assessment transparency - Equal measures for traceability and adverse event reporting
Regulation of Biosimilars in Canada
 Regulation of Biosimilars in Canada Session 2: Global Regulatory Trends of Biosimilars GBC 2018 June 28, 2018 Stephanie Hardy Office of Policy and International Collaboration Biologics and Genetic Therapies
Regulation of Biosimilars in Canada Session 2: Global Regulatory Trends of Biosimilars GBC 2018 June 28, 2018 Stephanie Hardy Office of Policy and International Collaboration Biologics and Genetic Therapies
Biosimilar Medicines: A National Prescribing Framework. May 2015
 Biosimilar Medicines: A National Prescribing Framework May 2015 Healthcare Improvement Scotland 2015 First published May 2015 Healthcare Improvement Scotland is committed to equality and diversity. We
Biosimilar Medicines: A National Prescribing Framework May 2015 Healthcare Improvement Scotland 2015 First published May 2015 Healthcare Improvement Scotland is committed to equality and diversity. We
fact sheet 3 Introduction to Biosimilars & Regulatory Requirements
 3 fact sheet 3 Introduction to Biosimilars & Regulatory Requirements International Alliance of Patients Organizations CAN Mezzanine 49-51 East Road London N1 6AH United Kingdom International Federation
3 fact sheet 3 Introduction to Biosimilars & Regulatory Requirements International Alliance of Patients Organizations CAN Mezzanine 49-51 East Road London N1 6AH United Kingdom International Federation
The EU Market Environment for Biosimilar Medicines
 1 The EU Market Environment for Biosimilar Medicines 12 th Annual IGPA Conference Sep 30-Oct 2, 2009 Montreal Suzette Kox Senior Director Scientific Affairs European Generic medicines Association 2 Outlines
1 The EU Market Environment for Biosimilar Medicines 12 th Annual IGPA Conference Sep 30-Oct 2, 2009 Montreal Suzette Kox Senior Director Scientific Affairs European Generic medicines Association 2 Outlines
Workshop on Access to and Uptake of Biosimilar Medicinal Products
 EUROPEAN COMMISSION Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs Consumer, Environmental and Health Technologies Biotechnology and Food Supply Chain Workshop on Access to
EUROPEAN COMMISSION Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs Consumer, Environmental and Health Technologies Biotechnology and Food Supply Chain Workshop on Access to
Biosimilars an update
 Biosimilars an update Darren Roberts Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists Clinical pharmacology and toxicology, St Vincent s Hospital (Sydney) Nephrology,
Biosimilars an update Darren Roberts Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists Clinical pharmacology and toxicology, St Vincent s Hospital (Sydney) Nephrology,
POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS. October 31, 2006
 POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS October 31, 2006 POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS 1 This is a joint position statement of
POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS October 31, 2006 POLICY POSITION ON NAMING OF BIOTECHNOLOGY-DERIVED THERAPEUTIC PROTEINS 1 This is a joint position statement of
Re: Therapeutic Goods Administration, Consultation: Nomenclature of Biological Medicines
 September 7, 2017 Biological Science Section Therapeutic Goods Administration (TGA) PO Box 100 Woden ACT 2606 Re: Therapeutic Goods Administration, Consultation: Nomenclature of Biological Medicines Dear
September 7, 2017 Biological Science Section Therapeutic Goods Administration (TGA) PO Box 100 Woden ACT 2606 Re: Therapeutic Goods Administration, Consultation: Nomenclature of Biological Medicines Dear
Identification and traceability of biological products. Dr Peter De Veene EU QPPV, Roche On behalf of EBE
 Identification and traceability of biological products Dr Peter De Veene EU QPPV, Roche On behalf of EBE Outline Biopharmaceuticals & Pharmacovigilance Traceability & Pharmacovigilance Legal Requirement
Identification and traceability of biological products Dr Peter De Veene EU QPPV, Roche On behalf of EBE Outline Biopharmaceuticals & Pharmacovigilance Traceability & Pharmacovigilance Legal Requirement
BIOSIMILARS OF PROTEIN BASED DRUGS: CURRENT STATUS AND FUTURE DIRECTIONS
 BIOSIMILARS OF PROTEIN BASED DRUGS: CURRENT STATUS AND FUTURE DIRECTIONS George Dranitsaris B.Pharm. PhD Consultant in Health Economics and Biostatistics What is a Biosimilar? Protein based drugs are made
BIOSIMILARS OF PROTEIN BASED DRUGS: CURRENT STATUS AND FUTURE DIRECTIONS George Dranitsaris B.Pharm. PhD Consultant in Health Economics and Biostatistics What is a Biosimilar? Protein based drugs are made
NCCP GUIDANCE DOCUMENT DOSE BANDING FOR SYSTEMIC ANTICANCER THERAPY (SACT)
 NCCP GUIDANCE DOCUMENT DOSE BANDING FOR SYSTEMIC ANTICANCER THERAPY (SACT) This is a draft document subject to agreement Version Date Amendment Approved By 1 05/04/16 Initial Draft 1 Table of Contents
NCCP GUIDANCE DOCUMENT DOSE BANDING FOR SYSTEMIC ANTICANCER THERAPY (SACT) This is a draft document subject to agreement Version Date Amendment Approved By 1 05/04/16 Initial Draft 1 Table of Contents
Submission of comments on Guideline on Similar Biological Medicinal Products (CHMP/437/04 Rev1)
 October 31, 2013 Submission of comments on Guideline on Similar Biological Medicinal Products (CHMP/437/04 Rev1) Comments from: Name of organisation or individual Biotechnology Industry Organization (BIO)
October 31, 2013 Submission of comments on Guideline on Similar Biological Medicinal Products (CHMP/437/04 Rev1) Comments from: Name of organisation or individual Biotechnology Industry Organization (BIO)
The importance of interchangeability in the procurement of medications: Biosimilar case
 The importance of interchangeability in the procurement of medications: Biosimilar case ALIMS Congres, Kragujevac, November 6, 2015 Prof. Borut Štrukelj, PhD Brief CV Current position: professor, Pharmaceutical
The importance of interchangeability in the procurement of medications: Biosimilar case ALIMS Congres, Kragujevac, November 6, 2015 Prof. Borut Štrukelj, PhD Brief CV Current position: professor, Pharmaceutical
ICON MEDICINE FORMULARY PROCESS
 1 ICON MEDICINE FORMULARY PROCESS DISCLAIMER The ICON Formulary process is an ethical and scientific cost sensitive exercise performed as described in this document. ICON or Isimo do not have any influence
1 ICON MEDICINE FORMULARY PROCESS DISCLAIMER The ICON Formulary process is an ethical and scientific cost sensitive exercise performed as described in this document. ICON or Isimo do not have any influence
Medicines Agency EMA & Biosimilar update: Trends from marketing authorisation applications, scientific advice procedures and policies
 EMA & Biosimilar update: Trends from marketing authorisation applications, scientific advice procedures and policies Presented by: Peter Richardson Head of Quality Office Specialised Scientific Disciplines
EMA & Biosimilar update: Trends from marketing authorisation applications, scientific advice procedures and policies Presented by: Peter Richardson Head of Quality Office Specialised Scientific Disciplines
BOARD PAPER - NHS ENGLAND. Purpose of Paper: To inform the Board of the development of an NHS England Personalised Medicine Strategy.
 Paper: PB.24.09.15/05 Title: Personalised Medicine Strategy. From: Sir Bruce Keogh, National Medical Director. BOARD PAPER - NHS ENGLAND Purpose of Paper: To inform the Board of the development of an NHS
Paper: PB.24.09.15/05 Title: Personalised Medicine Strategy. From: Sir Bruce Keogh, National Medical Director. BOARD PAPER - NHS ENGLAND Purpose of Paper: To inform the Board of the development of an NHS
NEW YORK STATE BAR ASSOCIATION FOOD, DRUG AND COSMETIC LAW SECTION AND HEALTH LAW SECTION COMMITTEE ON MEDICAL RESEARCH AND BIOTECHNOLOGY
 NEW YORK STATE BAR ASSOCIATION FOOD, DRUG AND COSMETIC LAW SECTION AND HEALTH LAW SECTION COMMITTEE ON MEDICAL RESEARCH AND BIOTECHNOLOGY MEMORANDUM IN SUPPORT OF AN AMENDMENT TO NEW YORK S PHARMACY LAW
NEW YORK STATE BAR ASSOCIATION FOOD, DRUG AND COSMETIC LAW SECTION AND HEALTH LAW SECTION COMMITTEE ON MEDICAL RESEARCH AND BIOTECHNOLOGY MEMORANDUM IN SUPPORT OF AN AMENDMENT TO NEW YORK S PHARMACY LAW
BGMA Associate Membership
 BGMA Associate Membership Making medicines affordable Promoting Innovation November 2011 GENERICS -THE FACTS Generic medicines meet the same standards of quality, safety and efficacy as originator brands.
BGMA Associate Membership Making medicines affordable Promoting Innovation November 2011 GENERICS -THE FACTS Generic medicines meet the same standards of quality, safety and efficacy as originator brands.
FDA Public Hearing: Approval Pathway for Biosimilar. Products. November 2-3, 2010
 FDA Public Hearing: Approval Pathway for Biosimilar and Interchangeable Biological Products November 2-3, 2010 1 The Biotechnology Industry Organization Over 1,100 members, including biotechnology companies,
FDA Public Hearing: Approval Pathway for Biosimilar and Interchangeable Biological Products November 2-3, 2010 1 The Biotechnology Industry Organization Over 1,100 members, including biotechnology companies,
Professor Ahmed H Al-jedai, PharmD, MBA, BCPS, FCCP, FAST, Saudi Arabia
 GaBI Scientific Meetings First GCC Stakeholder Meeting on Approval Process, Interchangeability/Substitution and Safety of Biosimilars 20 November 2017, Holiday Inn Izdihar Riyadh, Saudi Arabia Professor
GaBI Scientific Meetings First GCC Stakeholder Meeting on Approval Process, Interchangeability/Substitution and Safety of Biosimilars 20 November 2017, Holiday Inn Izdihar Riyadh, Saudi Arabia Professor
Guideline on the Regulation of Therapeutic Products in New Zealand
 Guideline on the Regulation of Therapeutic Products in New Zealand Part 10: Requirements for information for prescribers and consumers Edition 7.0 March 2017 Section 1: Legislation Section summary This
Guideline on the Regulation of Therapeutic Products in New Zealand Part 10: Requirements for information for prescribers and consumers Edition 7.0 March 2017 Section 1: Legislation Section summary This
Trial oversight SOP for HEY-sponsored CTIMPs
 R&D Department Trial oversight SOP for HEY-sponsored CTIMPs Hull And East Yorkshire Hospitals NHS Trust 2010 All Rights Reserved No part of this document may be reproduced, stored in a retrieval system
R&D Department Trial oversight SOP for HEY-sponsored CTIMPs Hull And East Yorkshire Hospitals NHS Trust 2010 All Rights Reserved No part of this document may be reproduced, stored in a retrieval system
THE BIOSIMILARS LANDSCAPE: STRATEGIES FOR CLINICAL AND COMMERCIAL SUCCESS
 THE BIOSIMILARS LANDSCAPE: STRATEGIES FOR CLINICAL AND COMMERCIAL SUCCESS What all developers need to know Alicia Baker Director, Global Regulatory Affairs Strategy, Covance John Carlsen, MHA Vice President,
THE BIOSIMILARS LANDSCAPE: STRATEGIES FOR CLINICAL AND COMMERCIAL SUCCESS What all developers need to know Alicia Baker Director, Global Regulatory Affairs Strategy, Covance John Carlsen, MHA Vice President,
Biosimilar Adoption Process Timeline
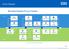 Biosimilar Adoption Process Timeline Pharmacy-Led Horizon Scanning Evaluate Service Impact Complete Preparation Calculate Actual Budget Impact Quantify Opportunity Agree Adoption Process Optimise Reference
Biosimilar Adoption Process Timeline Pharmacy-Led Horizon Scanning Evaluate Service Impact Complete Preparation Calculate Actual Budget Impact Quantify Opportunity Agree Adoption Process Optimise Reference
Effective Prescribing Programme - optimising the safe and effective use of biological medicines Case studies
 Effective Prescribing Programme - optimising the safe and effective use of biological medicines Case studies Introduction These case studies outline the experiences, challenges and lessons learned to date
Effective Prescribing Programme - optimising the safe and effective use of biological medicines Case studies Introduction These case studies outline the experiences, challenges and lessons learned to date
Managing the introduction of new generic medicines: Guidance for Chief Pharmacists and Trust medicines procurement leads.
 Introduction Managing the introduction of new generic medicines: Guidance for Chief Pharmacists and Trust medicines procurement leads. The loss of exclusivity and launches of new generic medicines allows
Introduction Managing the introduction of new generic medicines: Guidance for Chief Pharmacists and Trust medicines procurement leads. The loss of exclusivity and launches of new generic medicines allows
IMP Management and Accountability
 This is a controlled document. The master document is posted on the JRCO website and any print-off of this document will be classed as uncontrolled. Researchers and their teams may print off this document
This is a controlled document. The master document is posted on the JRCO website and any print-off of this document will be classed as uncontrolled. Researchers and their teams may print off this document
CMC Strategy Forum Japan November Piyanan Boonprasirt Bureau of Drug Control Thailand Food and Drug Administration
 CMC Strategy Forum Japan 2015 9-10 November 2015 Piyanan Boonprasirt Bureau of Drug Control Thailand Food and Drug Administration 1 1. Background 2. Organization Chart 3. Biological Products 4. Regulation
CMC Strategy Forum Japan 2015 9-10 November 2015 Piyanan Boonprasirt Bureau of Drug Control Thailand Food and Drug Administration 1 1. Background 2. Organization Chart 3. Biological Products 4. Regulation
Managing the introduction of new generic medicines: Guidance for Chief Pharmacists and Trust medicines procurement leads.
 Introduction Managing the introduction of new generic medicines: Guidance for Chief Pharmacists and Trust medicines procurement leads. The loss of exclusivity and launches of new generic medicines allows
Introduction Managing the introduction of new generic medicines: Guidance for Chief Pharmacists and Trust medicines procurement leads. The loss of exclusivity and launches of new generic medicines allows
Advance Topics in Pharmacoepidemiology. Risk Management. Conflict of Interest Declaration. Benefit Harm Profile?
 Advance Topics in Pharmacoepidemiology Risk Management 2012 Mid-Year ISPE Meeting Miami, April 21-23, 2012 Ariel E., Arias MD, PhD - Fac. Pharmacy; Université de Montréal - Biologics & Genetic Therapies
Advance Topics in Pharmacoepidemiology Risk Management 2012 Mid-Year ISPE Meeting Miami, April 21-23, 2012 Ariel E., Arias MD, PhD - Fac. Pharmacy; Université de Montréal - Biologics & Genetic Therapies
Nomenclature for Biological and Biotechnological Substances, including Biosimilars/Generic Biopharmaceuticals
 Nomenclature for Biological and Biotechnological Substances, including Biosimilars/Generic Biopharmaceuticals Gordon Johnston, RPh., M.S. VP Regulatory Affairs Generic Pharmaceutical Association WHO Headquarters
Nomenclature for Biological and Biotechnological Substances, including Biosimilars/Generic Biopharmaceuticals Gordon Johnston, RPh., M.S. VP Regulatory Affairs Generic Pharmaceutical Association WHO Headquarters
Role of HIT Vendors in Promoting Safe Use of Biosimilars
 Role of HIT Vendors in Promoting Safe Use of Biosimilars Session #312, February 22, 2017 Stella Stergiopoulos, MS, MPH; Senior Project Manager, Tufts CSDD Thomas Felix, MD, Medical Director, R&D Policy;
Role of HIT Vendors in Promoting Safe Use of Biosimilars Session #312, February 22, 2017 Stella Stergiopoulos, MS, MPH; Senior Project Manager, Tufts CSDD Thomas Felix, MD, Medical Director, R&D Policy;
MAKING OUTCOME-BASED PAYMENTS A REALITY IN THE NHS RESEARCH STUDY: PHASE INTRODUCTION. Research Brief
 MAKING OUTCOME-BASED PAYMENTS A REALITY IN THE NHS RESEARCH STUDY: PHASE 1 1.0 INTRODUCTION Cancer Research UK (CRUK) believes cancer patients should have access to the best, evidence-based innovative
MAKING OUTCOME-BASED PAYMENTS A REALITY IN THE NHS RESEARCH STUDY: PHASE 1 1.0 INTRODUCTION Cancer Research UK (CRUK) believes cancer patients should have access to the best, evidence-based innovative
Supply of aseptically - prepared doses of IMPs across legal boundaries. Edition 1. December 2017
 Supply of aseptically - prepared doses of IMPs across legal boundaries Edition 1 December 2017 Endorsed and supported by: NHS Pharmaceutical Quality Assurance Committee 2017 with National Pharmacy Clinical
Supply of aseptically - prepared doses of IMPs across legal boundaries Edition 1 December 2017 Endorsed and supported by: NHS Pharmaceutical Quality Assurance Committee 2017 with National Pharmacy Clinical
Demand Management Plan for Immunoglobulin Use
 Demand Management Plan for Immunoglobulin Use BACKGROUND Availability of immunoglobulin to the NHS Therapeutic immunoglobulin, a blood product, is used effectively in the treatment of a wide range of diseases.
Demand Management Plan for Immunoglobulin Use BACKGROUND Availability of immunoglobulin to the NHS Therapeutic immunoglobulin, a blood product, is used effectively in the treatment of a wide range of diseases.
EUROPEAN GENERIC MEDICINES ASSOCIATION
 biosimilars biosimilars contents Introduction... 6 What this Handbook Covers Whom this Handbook is for Executive Summary... 8 The Importance of Biosimilar Medicines... 11 For Patients For Clinicians For
biosimilars biosimilars contents Introduction... 6 What this Handbook Covers Whom this Handbook is for Executive Summary... 8 The Importance of Biosimilar Medicines... 11 For Patients For Clinicians For
Internal Business Case Template & Guidance
 Internal Business Case Template & Guidance Creation of Chief Pharmacist Information Officer role at UHBristol 1. INTRODUCTION AND SUMMARY This document defines the need, strategic context and preferred
Internal Business Case Template & Guidance Creation of Chief Pharmacist Information Officer role at UHBristol 1. INTRODUCTION AND SUMMARY This document defines the need, strategic context and preferred
What do physicians expect from a new drug?
 What do physicians expect from a new drug? New drug Novel activity Efficacy Safety Affordable cost Approval of new biologic drugs Is consistently increasing New Biotech Drug and Vaccine Approvals/ New
What do physicians expect from a new drug? New drug Novel activity Efficacy Safety Affordable cost Approval of new biologic drugs Is consistently increasing New Biotech Drug and Vaccine Approvals/ New
NCCP GUIDANCE DOCUMENT DOSE BANDING FOR SYSTEMIC ANTICANCER THERAPY (SACT) Version Date Amendment Approved By 1 03/06/16 Version 1 Working Group
 NCCP GUIDANCE DOCUMENT DOSE BANDING FOR SYSTEMIC ANTICANCER THERAPY (SACT) Version Date Amendment Approved By 1 03/06/16 Version 1 Working Group 1 Table of Contents Acknowledgements... 3 1 Background...
NCCP GUIDANCE DOCUMENT DOSE BANDING FOR SYSTEMIC ANTICANCER THERAPY (SACT) Version Date Amendment Approved By 1 03/06/16 Version 1 Working Group 1 Table of Contents Acknowledgements... 3 1 Background...
Re: Docket Number FDA-2017-D-0154 Considerations in Demonstrating Interchangeability With a Reference Product; Guidance for Industry, Draft Guidance
 PO Box 3691 Arlington, VA 22203 (703) 971-1700 May 22, 2017 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville,
PO Box 3691 Arlington, VA 22203 (703) 971-1700 May 22, 2017 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville,
Goldman Sachs Key Debates In Biosimilars Conference
 Goldman Sachs Key Debates In Biosimilars Conference Diem Nguyen Regional President North America, Global Established Pharmaceuticals April 2, 2015 1 2 Forward Looking Statements Our discussions during
Goldman Sachs Key Debates In Biosimilars Conference Diem Nguyen Regional President North America, Global Established Pharmaceuticals April 2, 2015 1 2 Forward Looking Statements Our discussions during
Guide for Marketing Authorisation Holders on Direct Healthcare Professional Communications
 Guide for Marketing Authorisation Holders on Direct Healthcare Professional Communications 1 INTRODUCTION A Direct Healthcare Professional Communication (DHPC) aims to promote safe and effective use of
Guide for Marketing Authorisation Holders on Direct Healthcare Professional Communications 1 INTRODUCTION A Direct Healthcare Professional Communication (DHPC) aims to promote safe and effective use of
Pharmacovigilance for biotherapeutics: Partnering for patient safety
 International Federation of Pharmaceutical Manufacturers & Associations Pharmacovigilance for biotherapeutics: Partnering for patient safety Fermin Ruiz de Erenchun IFPMA Biotherapeutics Group Chair (F.
International Federation of Pharmaceutical Manufacturers & Associations Pharmacovigilance for biotherapeutics: Partnering for patient safety Fermin Ruiz de Erenchun IFPMA Biotherapeutics Group Chair (F.
Pharmacist Rana Musa Al-ali (Malkawi) MSc (Pharmaceutical Quality Assurance) Registration Department/JFDA
 Pharmacist Rana Musa Al-ali (Malkawi) MSc (Pharmaceutical Quality Assurance) Registration Department/JFDA 1 2 ND MENA Regulatory Conference On Bioequivalence, Biowaivers, Bioanalysis, Dissolution & Biosimilars
Pharmacist Rana Musa Al-ali (Malkawi) MSc (Pharmaceutical Quality Assurance) Registration Department/JFDA 1 2 ND MENA Regulatory Conference On Bioequivalence, Biowaivers, Bioanalysis, Dissolution & Biosimilars
Biosimilars in the EU
 Biosimilars in the EU Information guide for healthcare professionals Prepared jointly by the European Medicines Agency and the European Commission Table of contents Foreword 2 Summary 3 Biological medicines:
Biosimilars in the EU Information guide for healthcare professionals Prepared jointly by the European Medicines Agency and the European Commission Table of contents Foreword 2 Summary 3 Biological medicines:
Optimising medicines use, value and funding
 Optimising medicines use, value and funding Dr Keith Ridge, Chief Pharmaceutical Officer December 2017 Medicines Achieving are the an NHS important Five Year part of NHS care and Forward help many View
Optimising medicines use, value and funding Dr Keith Ridge, Chief Pharmaceutical Officer December 2017 Medicines Achieving are the an NHS important Five Year part of NHS care and Forward help many View
GUIDELINES FOR THE DOSE BANDING OF CANCER CHEMOTHERAPY
 GUIDELINES FOR THE DOSE BANDING OF CANCER CHEMOTHERAPY Quality and safety for every patient every time Document Control Prepared By Issue Date Approved By Review Date Version Contributors Comments/ Amendment
GUIDELINES FOR THE DOSE BANDING OF CANCER CHEMOTHERAPY Quality and safety for every patient every time Document Control Prepared By Issue Date Approved By Review Date Version Contributors Comments/ Amendment
BIOSIMILAR AWARENESS INITIATIVE PROJECT MANAGEMENT PLAN
 BIOSIMILAR AWARENESS INITIATIVE PROJECT MANAGEMENT PLAN Date: June 2016 This document and its attachments are UNCLASSIFIED PRINCE2 Project Initiation Documentation TABLE OF CONTENTS 1 PROJECT DEFINITION...
BIOSIMILAR AWARENESS INITIATIVE PROJECT MANAGEMENT PLAN Date: June 2016 This document and its attachments are UNCLASSIFIED PRINCE2 Project Initiation Documentation TABLE OF CONTENTS 1 PROJECT DEFINITION...
Electronic Prescribing and Medicines Administration Project Overview
 Electronic Prescribing and Medicines Administration Project Overview 1. Project Background The purpose of the project is to implement an Electronic Prescribing and Medicines Administration (epma) system
Electronic Prescribing and Medicines Administration Project Overview 1. Project Background The purpose of the project is to implement an Electronic Prescribing and Medicines Administration (epma) system
A Physician s consideration towards Biosimilars. João Eurico Fonseca
 A Physician s consideration towards Biosimilars João Eurico Fonseca Disclosure I received unrestricted research grants or acted as a speaker for Abbvie, Amgen, BMS, Celtrion, Celgene, Janssen, MSD, Novartis,
A Physician s consideration towards Biosimilars João Eurico Fonseca Disclosure I received unrestricted research grants or acted as a speaker for Abbvie, Amgen, BMS, Celtrion, Celgene, Janssen, MSD, Novartis,
GVP Module X - additional monitoring of medicines
 GVP Module X - additional monitoring of medicines 6 th Stakeholders forum on the implementation of the new pharmacovigilance legislation Mick Foy, Group Manager - MHRA An agency of the European Union Contents
GVP Module X - additional monitoring of medicines 6 th Stakeholders forum on the implementation of the new pharmacovigilance legislation Mick Foy, Group Manager - MHRA An agency of the European Union Contents
Rasha Sayed Salama, MD, PhD, UAE
 GaBI Scientific Meetings 10 October 2018, Le Meridien Dubai, United Arab Emirates 2nd MENA Stakeholder Meeting on Regulatory Approval, Clinical Settings, Interchangeability and Pharmacovigilance of Biosimilars
GaBI Scientific Meetings 10 October 2018, Le Meridien Dubai, United Arab Emirates 2nd MENA Stakeholder Meeting on Regulatory Approval, Clinical Settings, Interchangeability and Pharmacovigilance of Biosimilars
Use of immunotherapy for cancer treatment
 Use of immunotherapy for cancer treatment Pre- and post-licensing considerations Professor Angela Thomas, Pediatric Hematologist Vice Chair Commission on Human Medicines Chair Clinical Trials, Biologicals
Use of immunotherapy for cancer treatment Pre- and post-licensing considerations Professor Angela Thomas, Pediatric Hematologist Vice Chair Commission on Human Medicines Chair Clinical Trials, Biologicals
Chin Koerner Executive Director US Regulatory and Development Policy
 Chin Koerner Executive Director US Regulatory and Development Policy Novartis Pharmaceuticals Corporation 1700 Rockville Pike Suite 510 Rockville, MD 20852 Tel 301.468.5607 Fax 301.468.5614 Email: Chin.Koerner@novartis.com
Chin Koerner Executive Director US Regulatory and Development Policy Novartis Pharmaceuticals Corporation 1700 Rockville Pike Suite 510 Rockville, MD 20852 Tel 301.468.5607 Fax 301.468.5614 Email: Chin.Koerner@novartis.com
Thijs J Giezen, PharmD, MSc, PhD The Netherlands
 Thijs J Giezen, PharmD, MSc, PhD The Netherlands Hospital Pharmacist, Foundation Pharmacy for Hospitals in Haarlem, The Netherlands Member of the Biosimilar Medicinal Product Working Party of European
Thijs J Giezen, PharmD, MSc, PhD The Netherlands Hospital Pharmacist, Foundation Pharmacy for Hospitals in Haarlem, The Netherlands Member of the Biosimilar Medicinal Product Working Party of European
Application form UNIVERSITY OF NOTTINGHAM MEDICAL SCHOOL ETHICS COMMITTEE
 Application form UNIVERSITY OF NOTTINGHAM MEDICAL SCHOOL ETHICS COMMITTEE In completing this form please refer to the attached Notes of Guidance Application for approval of all studies involving Healthy
Application form UNIVERSITY OF NOTTINGHAM MEDICAL SCHOOL ETHICS COMMITTEE In completing this form please refer to the attached Notes of Guidance Application for approval of all studies involving Healthy
Implications for Preclinical and Clinical Programs. Novartis Pharmaceuticals Oncology Business Unit June 2, 2011
 EU Biosimilarityi il it Guidance Implications for Preclinical and Clinical Programs Shefali Kakar Novartis Pharmaceuticals Oncology Business Unit June 2, 2011 Biologics are more complex than small molecules
EU Biosimilarityi il it Guidance Implications for Preclinical and Clinical Programs Shefali Kakar Novartis Pharmaceuticals Oncology Business Unit June 2, 2011 Biologics are more complex than small molecules
Published 07 February 2011 Page January 2011
 filgrastim 12 million units (120microgram) / 0.2mL, 30 million units (300microgram) / 0.5mL, 48 million units (480microgram) / 0.5mL solution for injection/infusion in pre-filled syringe (Nivestim) SMC
filgrastim 12 million units (120microgram) / 0.2mL, 30 million units (300microgram) / 0.5mL, 48 million units (480microgram) / 0.5mL solution for injection/infusion in pre-filled syringe (Nivestim) SMC
Management and Accountability of Investigational Medicinal Products in the King s Clinical Research Facility
 Management and Accountability of Investigational Medicinal Products in the King s Clinical Research Facility Document Detail Document type Standard Operating Procedure CRF-STU-SOP-1: Management and Accountability
Management and Accountability of Investigational Medicinal Products in the King s Clinical Research Facility Document Detail Document type Standard Operating Procedure CRF-STU-SOP-1: Management and Accountability
Biosimilar. Medicinal Products. What you Need to Know about
 What you Need to Know about Biosimilar Medicinal Products Process on Corporate Responsibility in the Field of Pharmaceuticals Access to Medicines in Europe A Consensus Information Document Enterprise and
What you Need to Know about Biosimilar Medicinal Products Process on Corporate Responsibility in the Field of Pharmaceuticals Access to Medicines in Europe A Consensus Information Document Enterprise and
Early Access to Medicines Scheme (EAMS)
 Early Access to Medicines Scheme (EAMS) Safe and Timely Access to Medicines for Patients (STAMP) Dr Daniel O Connor May 2015 Expert Medical Assessor MHRA Early Access to Medicines A proposal for an Early
Early Access to Medicines Scheme (EAMS) Safe and Timely Access to Medicines for Patients (STAMP) Dr Daniel O Connor May 2015 Expert Medical Assessor MHRA Early Access to Medicines A proposal for an Early
FDA s Implementation of the Legal and Regulatory Framework for Biosimilars
 FDA s Implementation of the Legal and Regulatory Framework for Biosimilars Sally Howard Deputy Commissioner for Policy, Planning, and Legislation 1 What are therapeutic biologics? Many biologics treat
FDA s Implementation of the Legal and Regulatory Framework for Biosimilars Sally Howard Deputy Commissioner for Policy, Planning, and Legislation 1 What are therapeutic biologics? Many biologics treat
Biosimilars 2016 Balancing Fast Uptake with Industry Sustainability. Dan Ionescu Head of Pricing and Market Access Sandoz Biopharmaceuticals
 Biosimilars 2016 Balancing Fast Uptake with Industry Sustainability Dan Ionescu Head of Pricing and Market Access Sandoz Biopharmaceuticals 10 years on, biosimilars have had a strong impact on global healthcare
Biosimilars 2016 Balancing Fast Uptake with Industry Sustainability Dan Ionescu Head of Pricing and Market Access Sandoz Biopharmaceuticals 10 years on, biosimilars have had a strong impact on global healthcare
Version Number: 004. On: June 2018 Review Date: June 2021 Distribution: Essential Reading for: Information for: Page 1 of 10
 Introduction of Novel Therapeutic Interventions Policy CONTROLLED DOCUMENT CATEGORY: CLASSIFICATION: PURPOSE Controlled Number: Document Policy Governance To set out the agreed policy for the submission
Introduction of Novel Therapeutic Interventions Policy CONTROLLED DOCUMENT CATEGORY: CLASSIFICATION: PURPOSE Controlled Number: Document Policy Governance To set out the agreed policy for the submission
KFDA Regulatory Framework on Biopharmaceuticals - Focus on Biosimilar
 KFDA Regulatory Framework on Biopharmaceuticals - Focus on Biosimilar Kyung-Min Baek, Ph.D. Recombinant Protein Products Division Korea Food and Drug Administration(KFDA) Biopharmaceuticals A biopharmaceutical
KFDA Regulatory Framework on Biopharmaceuticals - Focus on Biosimilar Kyung-Min Baek, Ph.D. Recombinant Protein Products Division Korea Food and Drug Administration(KFDA) Biopharmaceuticals A biopharmaceutical
9. EEA Qualified Pharmacy Technician Assessment Framework
 9. EEA Qualified Pharmacy Technician Assessment Framework Subject Description 1 Chemistry to include: The structure and classification of inorganic chemicals, nuclear and electronic structure of atoms,
9. EEA Qualified Pharmacy Technician Assessment Framework Subject Description 1 Chemistry to include: The structure and classification of inorganic chemicals, nuclear and electronic structure of atoms,
OREGON HEALTH AUTHORITY DRUG USE REVIEW/PHARMACY AND THERAPEUTICS COMMITTEE. OPERATING PROCEDURES Updated: March 2018
 OREGON HEALTH AUTHORITY DRUG USE REVIEW/PHARMACY AND THERAPEUTICS COMMITTEE OPERATING PROCEDURES Updated: March 2018 MISSION: To encourage safe, effective, and innovative drug policies that promote high
OREGON HEALTH AUTHORITY DRUG USE REVIEW/PHARMACY AND THERAPEUTICS COMMITTEE OPERATING PROCEDURES Updated: March 2018 MISSION: To encourage safe, effective, and innovative drug policies that promote high
INTRODUCTION TO PILOT PROJECT FOR WHO PREQUALIFICATION (PQ) OF BIOSIMILARS FOR CANCER TREATMENT. Guido Pantè, PhD
 INTRODUCTION TO PILOT PROJECT FOR WHO PREQUALIFICATION (PQ) OF BIOSIMILARS FOR CANCER TREATMENT Guido Pantè, PhD 1 The Proposal To initiate a pilot WHO prequalification process for similar biotherapeutic
INTRODUCTION TO PILOT PROJECT FOR WHO PREQUALIFICATION (PQ) OF BIOSIMILARS FOR CANCER TREATMENT Guido Pantè, PhD 1 The Proposal To initiate a pilot WHO prequalification process for similar biotherapeutic
Re: Comments on January 2017 Draft Guidance: Considerations in Demonstrating Interchangeability with a Reference Product (Docket No.
 800 17th Street, NW Suite 1100, Washington, DC 20006 May 1, 2017 Dr. Stephen Ostroff Acting Commissioner Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Re: Comments on
800 17th Street, NW Suite 1100, Washington, DC 20006 May 1, 2017 Dr. Stephen Ostroff Acting Commissioner Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Re: Comments on
NUH PHARMACY CLINICAL TRIALS. Sheila Hodgson, Lead Pharmacist, Clinical Trials June 30th 2014
 NUH PHARMACY CLINICAL TRIALS Sheila Hodgson, Lead Pharmacist, Clinical Trials June 30th 2014 Dedicated pharmacy trials staff Lead Pharmacist, Service Co-ordinator Pharmacists: Band 7 and 8a Technicians:
NUH PHARMACY CLINICAL TRIALS Sheila Hodgson, Lead Pharmacist, Clinical Trials June 30th 2014 Dedicated pharmacy trials staff Lead Pharmacist, Service Co-ordinator Pharmacists: Band 7 and 8a Technicians:
Factors Supporting a Sustainable European Biosimilar Medicines Market PROJECT SUMMARY FOR EXTERNAL COMMUNICATION
 Market Access Factors Supporting a Sustainable European Biosimilar Medicines Market PROJECT SUMMARY FOR EXTERNAL COMMUNICATION A study undertaken by GfK Market Access on behalf of the European Biosimilars
Market Access Factors Supporting a Sustainable European Biosimilar Medicines Market PROJECT SUMMARY FOR EXTERNAL COMMUNICATION A study undertaken by GfK Market Access on behalf of the European Biosimilars
MCW Office of Research Standard Operating Procedure
 MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DRUGS AND BIOLOGICS Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW/FH Faculty
MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DRUGS AND BIOLOGICS Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW/FH Faculty
Standard Operating Procedure for Development, Implementation, Management and
 Standard Operating Procedure for Development, Implementation, Management and Review of Documents produced by NSAG (NOSCAN Systemic Anti-Cancer Therapy Advisory Group) Lead Author/Co-ordinator: Mark Parsons,
Standard Operating Procedure for Development, Implementation, Management and Review of Documents produced by NSAG (NOSCAN Systemic Anti-Cancer Therapy Advisory Group) Lead Author/Co-ordinator: Mark Parsons,
Louise Brook Clinical Trials Quality Monitor. Date
 Details: Author: Louise Brook Clinical Trials Quality Monitor SOP Pages: 12 Version No. of replaced SOP: Effective date of replaced SOP: NA NA Approval: Version No: of the SOP being approved. Name of person
Details: Author: Louise Brook Clinical Trials Quality Monitor SOP Pages: 12 Version No. of replaced SOP: Effective date of replaced SOP: NA NA Approval: Version No: of the SOP being approved. Name of person
Submission to the Department of Health (Fed) regarding. Biosimilar Uptake Drivers Implementation. This joint submission is made by:
 Submission to the Department of Health (Fed) regarding Biosimilar Uptake Drivers Implementation This joint submission is made by: The Society of Hospital Pharmacists of Australia (SHPA) SHPA is the national
Submission to the Department of Health (Fed) regarding Biosimilar Uptake Drivers Implementation This joint submission is made by: The Society of Hospital Pharmacists of Australia (SHPA) SHPA is the national
Guideline on Similar Biological Medicinal Products
 1 2 3 22 May 2013 CHMP/437/04 Rev 1 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Draft 7 Draft agreed by Biosimilar Medicinal Products Working Party and Biologics Working Party March 2013
1 2 3 22 May 2013 CHMP/437/04 Rev 1 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Draft 7 Draft agreed by Biosimilar Medicinal Products Working Party and Biologics Working Party March 2013
Interchangeability: What is Next? Analysis of the concept & of the FDA Draft Guidance
 Interchangeability: What is Next? Analysis of the concept & of the FDA Draft Guidance Hillel Cohen, Executive Director of Scientific Affairs Leading on Biosimilars: The 2017 AAM Biosimilars Council Conference
Interchangeability: What is Next? Analysis of the concept & of the FDA Draft Guidance Hillel Cohen, Executive Director of Scientific Affairs Leading on Biosimilars: The 2017 AAM Biosimilars Council Conference
RD SOP32 Gaining MHRA Approval
 RD SOP32 Gaining MHRA Approval Version Number: 1.0 Name of originator/author: Dr Lloyd Gregory Name of responsible committee: R&I Committee Name of executive lead: Medical Director & Director of Quality
RD SOP32 Gaining MHRA Approval Version Number: 1.0 Name of originator/author: Dr Lloyd Gregory Name of responsible committee: R&I Committee Name of executive lead: Medical Director & Director of Quality
The role of the Hospital Pharmacist: Availability of medicines above price-only procurement
 The role of the Hospital Pharmacist: Availability of medicines above price-only procurement Thijs J. Giezen, PhD, PharmD, MSc Hospital Pharmacist, Foundation Pharmacy for Hospitals in Haarlem, Haarlem,
The role of the Hospital Pharmacist: Availability of medicines above price-only procurement Thijs J. Giezen, PhD, PharmD, MSc Hospital Pharmacist, Foundation Pharmacy for Hospitals in Haarlem, Haarlem,
