Gene therapy in rare anemias
|
|
|
- Milton McKinney
- 6 years ago
- Views:
Transcription
1 Gene therapy in rare anemias Jose Carlos Segovia Differentiation and Cytometry Unit. Hematopoietic Innovative Therapies Division CIEMAT-CIBER / IIS-FJD Madrid - Spain 6 th EUROPEAN SYMPOSIUM ON RARE ANAEMIAS 1 st Dutch-Belgian meeting for patients and health professionals 21 st - 22 nd November 2015 Amsterdam - The Netherlands
2 Disclosures Company name Research support Employee Consultant Stockholder Speakers bureau Advisory board Other
3 This talk is applicable for: Thalassemia s Sickle cell disease Membrane disorders (e.g. sferocytosis) Enzym defects (e.g. PKD, G6PD) PNH Other forms of hemolytic disease Definite X X X Probable X X
4 What is the aim of a Gene Therapy? To introduce in the cell nucleic acids with the correct information that will allow, transiently or permanently, the expression of the protein of interest The wild type version of the defective protein that produces de erythroid disease
5 How does ex vivo gene therapy work? 1. Cells are extracted from the patient 2. A virus is modified to avoid its reproduction maintaining its ability to enter the cell (vector) Gene 3. The gene of interest (healthy PKLR) is inserted in the vector 6. Modified cells are injected in the patient and start to produce the healthy protein Modified cell 4. Patient cells and modified viruses are put in contact 5. Gene modification (correction) occurs in the cells
6 Which are candidate diseases for gene therapy? Monogenic diseases: Mutations in one known gene generate the disease Recessive inheritance: Both copies of the gene are mutated Allogeneic bone marrow transplantation cures the disease
7 Which cells should be modified? Erythrocytes / Red Blood Cells Short half-life: 120 days Need to be replenish Hematopoietic Stem Cell (HSC) - Lives all life long - Generates new HSC - Its job is to produce all blood cells - They are in the Bone Marrow but can be moved to the blood
8 How does it work? Viral vector carrying the healthy RPK gene Defective erythrocytes Healthy erythrocytes
9 The gene therapy drug AUTOLOGOUS HEMATOPOIETIC STEM CELLS CARRYING THE CORRECTED GENE Healthy RPK gene How can it be tested?
10 Mouse model
11 Mouse model for Pyruvate Kinase Deficiency Erythrocyte parameters Healthy mouse Mouse strain PKD mouse RBC 10,5 x /L 6,39 x /L HGB 13,8 g/dl 9,7 g/dl HCT 46,2% 38,9% Healthy PKD Min-Oo et al. Nature Genetics (2003) Healthy PKD
12 The treatment Chemo- and/or Radio-therapy Are they corrected? Autologous transplantation
13 The results: No anemia 14 Erythrocytes (1x10 12 /L) Mouse: WT B19 B19 B19 Vector: EGFP RPK 30 dpt 60 dpt 90 dpt Meza N et al, Molecular Therapy (2009)
14 % of Reticulocytes The results: No reticulocytosis dpt 60 dpt 90 dpt Mouse: WT B19 B19 B19 Vector: EGFP RPK Meza N et al, Molecular Therapy (2009)
15 The results: Late progenitors balance correction Progenitores eritroides PROERYTHROBLASTS BASOPHILIC ERYTHROBLASTS POLYCHROMATOPHILIC ERYTHROBLASTS ORTHOCHROMATIC ERYTHROBLASTS RETYCULOCYTES AND ERYTHROCYTES Meza N et al, Molecular Therapy (2009)
16 Erythropoietin levels estabilization E r ythroid values of control and transplanted RPK deficient mice Animals Hg level (g/dl) Hct MCV (fl) Plasma Fe level ( m g/dl) Plasma erytrthropoietin level (pg/dl) WT B EGFP RPK Meza N et al, Molecular Therapy (2009)
17 The results: No splenomegaly Transplanted with corrected cells Healthy PKD Spleen morphometry Transplanted with corrected cells B19 Healthy Meza N et al, Molecular Therapy (2009) Spleen weight (mg)
18 Reduction of iron deposits Meza N et al, Molecular Therapy (2009)
19 Has gene therapy been applied in humans?
20 Gene therapy clinical trials in hematopoiesis Kaufman et al, EMBO Mol Med (2014)
21 β-thalassaemia ex vivo gene therapy Transfusion-independent 12 months after gene therapy Cavazzana-Calvo et al, Nature (2010)
22 β-thalassaemia ex vivo gene therapy Cavazzana-Calvo et al, Nature (2010)
23 β-thalassaemia ex vivo gene therapy Hematopoiesis turned to be oligoclonal Cavazzana-Calvo et al, Nature (2010)
24 New β-globin gene therapy trials Subject Number Age of consent Characteristics of subjects with β-thalassemia major prbc CD34+VCN in Transfusion Cell Sex Genotype drug Requirement source (ml/kg/year) a substance CD34+ Cell Dose (x10 6 /kg) Follow up Female β 0 /β F 139 Apheresis M Male β 0 /β F 188 Apheresis M Not yet Male β 0 /β Apheresis 0.8 treated Indication for transplant Age of Consent Characteristics of subject with severe sickle cell disease (1204) prbc CD34+VCN in Transfusion Cell Sex Genotype drug Requirement source substance (ml/kg/year) Multiple VOCs 13 Male β 0 /β ACS Silent cerebral infarct X CD34+ Cell Dose (x10 6 /kg) Follow up Bone Marrow 1.2/ M a Mean prbc requirement per year, over the past 2 years prior to consent b VCN = number of vector copies per diploid genome c ACS = acute chest syndrome Cavazzana-Calvo et al, EHA Meeting (2015)
25 Is it safe?
26 Gene therapy clinical trials in hematopoiesis Target cell/injection Disease Transgene Vector HSC SCID-X1 IL2Rγc Gammaretroviral WAS WASP Gammaretroviral Modified from Kaufman et al, EMBO Mol Med (2014)
27 What about PKD?
28 Orphan Drug Designation in August, 2014 EU/3/14/1130 Lentiviral vector carrying the healthy RPK gene Healthy RPK gene 1 Design and development of the THERAPEUTIC VIRUS 4 ORPHAN DRUG designation Registered drug 6 Gene therapy development 2 In vitro validation 3 Preclinical studies in mouse models (EFFICACY and SAFETY) 5 CLINICAL TRIALS
29 ENERCA The consortium PATIENTS SIDE DRUG SIDE P2: R van Wijk UMCU Utrecht- The Netherlands P4: JL Vives HClinB Barcelona - Spain P6: S Pissard INSERM Paris - France P3: P Bianchi IRCCS Milan - Italy P5: L Ribeiro CHUC Coimbra - Portugal P7: I Badell HPS Barcelona - Spain P1: JC Segovia CIBER Madrid - Spain P8: J Sevilla SERMAS Madrid - Spain P9: GMP viral production facility P11: J Mountford Univ Glasgow Glasgow UK P10: Farzin Farzaneh KCL London UK VIRAL VECTOR PLATFORM EU PKD Patient s groups / Spanish Association for Rare Diseases
30 Who could be enrolled in the Clinical Trial? French study (preliminary results) No data 12 % (7) Never transfused 30 %(18) Continuous Transfusion 23 %(14) No Surgery 36 % (5) No Surgery 48 %(10) Surgery Spleen surgery 64 % (9) Transfusion ended 35 %(21) Surgery 52 % (11) Courtesy of S. Pissard, Paris France Medium value of post surgery increase in Hb : 2 g/dl
31 Patient s support
32 Patient s support
33 Patient s support 832 answers UK; 35 Venezuela; 1 USA; 135 Australia; 44 Belgium; 38 Canada; 27 Cyprus; 1 Denmark; 81 Ecuador; 1 The Netherlands; 93 France; 125 Sweden; 1 Spain; 164 Saudi Arabia; 1 Rumania; 1 Portugal; 4 Germany; 1 Greece; 7 Italy; Mexico; 62 1 Ireland; 2 Norway; 1 Latvia; 1 Pakistan; 1 Poland; 1 New Zealand; 3
34 Patient s support I'm a patient with PKD 4% (37) Link to PKD Other 23% (195) I'm a family member of someone with PKD 16% (131) I'm a parent of a child/adult with PKD 6% (48) I'm a friend of someone with PKD 51% (421)
35 Susana Navarro M. García-Gómez Raquel Chinchón Sergio López Differentiation and Cytometry Unit María G. Bravo Israel Orman José Bustos Oscar Quintana Sara Fañanás Rebeca Sanchez Omaira Alberquilla
36 Acknowledgements Juan A. Bueren Hematopoietic Innovative Therapies King s College London (London) M. Antoniou Agios Pharmaceuticals (Boston) Sh. Jin C. Kung P. Kosinski HSR-TIGET (Milan) E. Montini A. Calabria F. Benedicenti Hannover University A. Schambach H. Clinic (Barcelona) J.L. Vives-Corrons M. Mañu PKreset Ca Granda OMP (Milan) P. Bianchi A. Zanella H.U. Utrecht (Utrecht) R v. Wijk E.J. v. Beers H.I.U. Niño Jesús (Madrid) J. Sevilla H. Sant Pau (Barcelona) I. Badell INSERM (Paris) S. Pissard Hospitals L. Riveiro (Portugal) MedCare CIEMAT Laure Gelsin Patients and Healthy donors EpiSevi 2.0
37
38 Gene therapy clinical trials in hematopoiesis Target cell/injection Disease Transgene Vector HSC SCID-X1 IL2Rγc Gammaretroviral WAS WASP Gammaretroviral Modified from Kaufman et al, EMBO Mol Med (2014)
39 Target patients (PKD Natural History Study) 6/75 EHA Learning Center. Grace R. Jun 12, 2015;
Pros and Cons of Gene Therapy in ß-Thalassemia
 Pros and Cons of Gene Therapy in ß-Thalassemia ß-Thalassemia: established and potential new therapeutic approaches Many ß-thalassemic patients are treated with blood transfusion and iron chelation. However,
Pros and Cons of Gene Therapy in ß-Thalassemia ß-Thalassemia: established and potential new therapeutic approaches Many ß-thalassemic patients are treated with blood transfusion and iron chelation. However,
Phase I/II Gene therapy trial of Fanconi anemia patients with a new Orphan Drug consisting of a lentiviral vector carrying the FANCA
 Phase I/II Gene therapy trial of Fanconi anemia patients with a new Orphan Drug consisting of a lentiviral vector carrying the FANCA gene: A Coordinated International Action J. Bueren Hematopoietic Innovative
Phase I/II Gene therapy trial of Fanconi anemia patients with a new Orphan Drug consisting of a lentiviral vector carrying the FANCA gene: A Coordinated International Action J. Bueren Hematopoietic Innovative
Stem Cells Regenerative Medicine Congress September 15, 2014
 Stem Cells Regenerative Medicine Congress 2014 September 15, 2014 Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use
Stem Cells Regenerative Medicine Congress 2014 September 15, 2014 Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use
Orchard Therapeutics. Overcoming the complex challenges associated with ex vivo gene therapies. Adrien Lemoine VP Business Development & Operations
 Orchard Therapeutics Overcoming the complex challenges associated with ex vivo gene therapies Adrien Lemoine VP Business Development & Operations Orchard at a glance Who we are Our mission Team Academic
Orchard Therapeutics Overcoming the complex challenges associated with ex vivo gene therapies Adrien Lemoine VP Business Development & Operations Orchard at a glance Who we are Our mission Team Academic
From regulation to reality challenges in translation of gene therapy and cell-based medicinal products
 From regulation to reality challenges in translation of gene therapy and cell-based medicinal products Gene therapy case study: ADA-SCID Alessandro Aiuti Jonathan Appleby HSR-TIGET is focused on the implementation
From regulation to reality challenges in translation of gene therapy and cell-based medicinal products Gene therapy case study: ADA-SCID Alessandro Aiuti Jonathan Appleby HSR-TIGET is focused on the implementation
Gene therapy. Findings by Alert
 Gene therapy Published Mar 21, 2000 Version 1 Findings by Alert Research in gene therapy has increased dramatically during the past 15 years, particularly in the United States. The research has encompassed
Gene therapy Published Mar 21, 2000 Version 1 Findings by Alert Research in gene therapy has increased dramatically during the past 15 years, particularly in the United States. The research has encompassed
Making Hope A Reality bluebird style. November, 2017 Nasdaq : BLUE
 Making Hope A Reality bluebird style November, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information. The
Making Hope A Reality bluebird style November, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information. The
Preclinical pharmacokinetics and pharmacodynamics of AG-519, an allosteric pyruvate kinase activator
 S830 Preclinical pharmacokinetics and pharmacodynamics of AG-519, an allosteric pyruvate kinase activator Yue Chen, Raj Nagaraja, Kha Le, Penelope A Kosinski, Gavin Histen, Charles Kung, Hyeryun Kim, Chandra
S830 Preclinical pharmacokinetics and pharmacodynamics of AG-519, an allosteric pyruvate kinase activator Yue Chen, Raj Nagaraja, Kha Le, Penelope A Kosinski, Gavin Histen, Charles Kung, Hyeryun Kim, Chandra
Q4 and Full Year 2017 Conference Call. February 22, 2018
 Q4 and Full Year 2017 Conference Call February 22, 2018 Agenda Welcome McDavid Stilwell VP, Corporate Communications and Investor Relations Q4 2017 and Full Year Review and Recent Highlights Dr. Sandy
Q4 and Full Year 2017 Conference Call February 22, 2018 Agenda Welcome McDavid Stilwell VP, Corporate Communications and Investor Relations Q4 2017 and Full Year Review and Recent Highlights Dr. Sandy
Gene Therapy for Fanconi Anaemia Hope or hype? Dr Phil Ancliff Great Ormond Street Hospital / UCL Institute of Child Health Twycross Zoo October 2017
 Gene Therapy for Fanconi Anaemia Hope or hype? Dr Phil Ancliff Great Ormond Street Hospital / UCL Institute of Child Health Twycross Zoo October 2017 Gene therapy in Seattle Grace Miranda Bailey had the
Gene Therapy for Fanconi Anaemia Hope or hype? Dr Phil Ancliff Great Ormond Street Hospital / UCL Institute of Child Health Twycross Zoo October 2017 Gene therapy in Seattle Grace Miranda Bailey had the
Gene Therapy of FA: Premises and Promises
 Gene Therapy of FA: Premises and Promises Jakub Tolar tolar003@umn.edu Treat marrow failure Prevent leukemia Reduce endocrine problems Lower risk of head/neck cancer Improve quality of life A unified trial
Gene Therapy of FA: Premises and Promises Jakub Tolar tolar003@umn.edu Treat marrow failure Prevent leukemia Reduce endocrine problems Lower risk of head/neck cancer Improve quality of life A unified trial
Corporate Presentation. March 2018
 Corporate Presentation March 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
Corporate Presentation March 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
BioCentury Future Leaders Conference. March 20, 2015
 BioCentury Future Leaders Conference March 20, 2015 Forward Looking Statement This presentation includes certain estimates and other forward-looking statements within the meaning of Section 21E of the
BioCentury Future Leaders Conference March 20, 2015 Forward Looking Statement This presentation includes certain estimates and other forward-looking statements within the meaning of Section 21E of the
Sangamo Therapeutics Announces Presentations at 2017 Annual meeting of the American Society of Gene & Cell Therapy
 April 24, 2017 Sangamo Therapeutics Announces Presentations at 2017 Annual meeting of the American Society of Gene & Cell Therapy RICHMOND, Calif., April 24, 2017 /PRNewswire/ -- Sangamo Therapeutics,
April 24, 2017 Sangamo Therapeutics Announces Presentations at 2017 Annual meeting of the American Society of Gene & Cell Therapy RICHMOND, Calif., April 24, 2017 /PRNewswire/ -- Sangamo Therapeutics,
3Rs in use of laboratory animals for testing and developing regenerative medicine. Frank Staal
 3Rs in use of laboratory animals for testing and developing regenerative medicine Frank Staal Stem Cell Biology, Dept. Immunohematology November 2016 Replacement in regenerative medicine 3 Rs = Replacement,
3Rs in use of laboratory animals for testing and developing regenerative medicine Frank Staal Stem Cell Biology, Dept. Immunohematology November 2016 Replacement in regenerative medicine 3 Rs = Replacement,
Peripheral Blood Stem Cell Collections in the Age of Gene Therapy
 Peripheral Blood Stem Cell Collections in the Age of Gene Therapy John P Manis MD Boston Children s Hospital Brigham and Women s Hospital Dana Farber Cancer Institute Boston MA No Disclosures Conflict
Peripheral Blood Stem Cell Collections in the Age of Gene Therapy John P Manis MD Boston Children s Hospital Brigham and Women s Hospital Dana Farber Cancer Institute Boston MA No Disclosures Conflict
CQAs for C> Products to Enable Comparability Assessment. Ben Thompson Snr Director, Biopharmaceutical CMC RA GlaxoSmithKline
 CQAs for C> Products to Enable Comparability Assessment Ben Thompson Snr Director, Biopharmaceutical CMC RA GlaxoSmithKline Overview Demonstrate the value of defining CQAs early in product development
CQAs for C> Products to Enable Comparability Assessment Ben Thompson Snr Director, Biopharmaceutical CMC RA GlaxoSmithKline Overview Demonstrate the value of defining CQAs early in product development
Cellular Therapy Products & NDC vs. ISBT128 Coding/Labeling
 Cellular Therapy Products & NDC vs. ISBT128 Coding/Labeling William E. Janssen, Ph.D. Director, Cell Therapies Facility Moffitt Cancer Center Tampa, Florida What is ISBT 128? Information standard for blood,
Cellular Therapy Products & NDC vs. ISBT128 Coding/Labeling William E. Janssen, Ph.D. Director, Cell Therapies Facility Moffitt Cancer Center Tampa, Florida What is ISBT 128? Information standard for blood,
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform
 This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements
Bringing curative gene therapies to patients with rare, undertreated diseases
 Bringing curative gene therapies to patients with rare, undertreated diseases January 2018 Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements in this presentation
Bringing curative gene therapies to patients with rare, undertreated diseases January 2018 Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements in this presentation
Cell and Gene Therapy Catapult clinical trials database
 Cell and Gene Therapy Catapult clinical trials database Executive summary The number of cell and gene therapy clinical trials in the UK continues to increase with 85 ongoing trials at present, representing
Cell and Gene Therapy Catapult clinical trials database Executive summary The number of cell and gene therapy clinical trials in the UK continues to increase with 85 ongoing trials at present, representing
This study is currently recruiting participants. Verified July 2012 by Memorial Sloan-Kettering Cancer Center
 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies Treatment of ß-Thalassemia Major With Autologous CD34+ Hematopoietic Progenitor Cells Transduced
Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies Treatment of ß-Thalassemia Major With Autologous CD34+ Hematopoietic Progenitor Cells Transduced
Current SCID Gene Transfer Experience
 Current Perspectives on Gene Transfer for X-SCID Recombinant DNA Advisory Committee Safety Symposium March 15, 2005 Discussion Questions Current SCID Gene Transfer Experience 1. What data is available
Current Perspectives on Gene Transfer for X-SCID Recombinant DNA Advisory Committee Safety Symposium March 15, 2005 Discussion Questions Current SCID Gene Transfer Experience 1. What data is available
Medical Topics: Gene Therapy. E. Anne Jackson, FSA MAAA July 30, 2018
 Medical Topics: Gene Therapy E. Anne Jackson, FSA MAAA July 30, 2018 Agenda Terminology Crash course in the science Existing FDA-approved gene therapies Underwriting implications 2 Terms related to high
Medical Topics: Gene Therapy E. Anne Jackson, FSA MAAA July 30, 2018 Agenda Terminology Crash course in the science Existing FDA-approved gene therapies Underwriting implications 2 Terms related to high
Dr. Gaurav Shah Chief Executive Officer & President
 Bloomberg Biotech Innovations Conference April 9 th,2018 Dr. Gaurav Shah Chief Executive Officer & President Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements
Bloomberg Biotech Innovations Conference April 9 th,2018 Dr. Gaurav Shah Chief Executive Officer & President Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements
PART 1 (COUNCIL DECISION 2002/813/EC)
 PART 1 (COUNCIL DECISION 2002/813/EC) SUMMARY NOTIFICATION INFORMATION FORMAT FOR THE RELEASE OF GENETICALLY MODIFIED ORGANISMS OTHER THAN HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 11 OF DIRECTIVE 2001/18/EC
PART 1 (COUNCIL DECISION 2002/813/EC) SUMMARY NOTIFICATION INFORMATION FORMAT FOR THE RELEASE OF GENETICALLY MODIFIED ORGANISMS OTHER THAN HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 11 OF DIRECTIVE 2001/18/EC
ICH Considerations Oncolytic Viruses ONCOLYTIC VIRUSES (EMEA/CHMP/ICH/607698/2008) TRANSMISSION TO CHMP November 2008
 European Medicines Agency October 2009 EMEA/CHMP/ICH/607698/2008 ICH Considerations Oncolytic Viruses ONCOLYTIC VIRUSES (EMEA/CHMP/ICH/607698/2008) TRANSMISSION TO CHMP November 2008 TRANSMISSION TO INTERESTED
European Medicines Agency October 2009 EMEA/CHMP/ICH/607698/2008 ICH Considerations Oncolytic Viruses ONCOLYTIC VIRUSES (EMEA/CHMP/ICH/607698/2008) TRANSMISSION TO CHMP November 2008 TRANSMISSION TO INTERESTED
The Regulatory Environment for Therapeutic Vaccines. Thomas Hinz Head, Section Therapeutic Vaccines Paul Ehrlich Institute, Germany
 The Regulatory Environment for Therapeutic Vaccines Thomas Hinz Head, Section Therapeutic Vaccines Paul Ehrlich Institute, Germany hinth@pei.de 1 Topics Addressed Regulatory environment - EU - Germany
The Regulatory Environment for Therapeutic Vaccines Thomas Hinz Head, Section Therapeutic Vaccines Paul Ehrlich Institute, Germany hinth@pei.de 1 Topics Addressed Regulatory environment - EU - Germany
Important Information
 October 218 Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements in this release concerning Rocket s future expectations, plans and prospects, including without
October 218 Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements in this release concerning Rocket s future expectations, plans and prospects, including without
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
The Cell and Gene Therapy Catapult UK clinical trials database
 The Cell and Gene Therapy Catapult UK clinical trials database The UK clinical trials database covers cell and gene therapy clinical trial activities that the Cell and Gene Therapy Catapult (CGT Catapult)
The Cell and Gene Therapy Catapult UK clinical trials database The UK clinical trials database covers cell and gene therapy clinical trial activities that the Cell and Gene Therapy Catapult (CGT Catapult)
Corporate Presentation. January 7, 2019
 Corporate Presentation January 7, 2019 1 Acceleron Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS ABOUT THE COMPANY S STRATEGY, FUTURE PLANS and prospects, including statements
Corporate Presentation January 7, 2019 1 Acceleron Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS ABOUT THE COMPANY S STRATEGY, FUTURE PLANS and prospects, including statements
Gene Therapy for Lung Diseases. Deborah Gill Gene Medicine Research Group Oxford University
 Gene Therapy for Lung Diseases Deborah Gill Gene Medicine Research Group Oxford University Who am I? What do I do? - crash course in genetics - cystic fibrosis lung disease - gene therapy - anything else?
Gene Therapy for Lung Diseases Deborah Gill Gene Medicine Research Group Oxford University Who am I? What do I do? - crash course in genetics - cystic fibrosis lung disease - gene therapy - anything else?
Immuno-Oncology Program
 Immuno-Oncology Program 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform
Immuno-Oncology Program 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform
ABOUT GLYCOSTEM. Company Overview
 ABOUT GLYCOSTEM The company is a clinical stage biotech company established in the Netherlands in 2007. The company s headquarters and new state-of-the-art lab and production facilities are based at Pivot
ABOUT GLYCOSTEM The company is a clinical stage biotech company established in the Netherlands in 2007. The company s headquarters and new state-of-the-art lab and production facilities are based at Pivot
Regulatory Challenges and Threats in Orphan Drug Development Are Ultra Orphans Easy?
 Regulatory Challenges and Threats in Orphan Drug Development Are Ultra Orphans Easy? EPLS September 24, 2014 Ulrich Granzer Granzer Regulatory Consulting & Services The Challenge: What needs to be done
Regulatory Challenges and Threats in Orphan Drug Development Are Ultra Orphans Easy? EPLS September 24, 2014 Ulrich Granzer Granzer Regulatory Consulting & Services The Challenge: What needs to be done
ICH CONSIDERATIONS Oncolytic Viruses
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
Genetics and Genomics in Medicine Chapter 9 Questions
 Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
For more information about how to cite these materials visit
 Author(s): David Ginsburg, M.D., 2012 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): David Ginsburg, M.D., 2012 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Jefferies 2018 Healthcare Conference June 7, Gaurav Shah, M.D. Chief Executive Officer and President
 Jefferies 2018 Healthcare Conference June 7, 2018 Gaurav Shah, M.D. Chief Executive Officer and President Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements
Jefferies 2018 Healthcare Conference June 7, 2018 Gaurav Shah, M.D. Chief Executive Officer and President Important Information Cautionary Statement Regarding Forward-Looking Statements Various statements
10/07/2018. Liver directed gene therapy. - An overview. DNA/RNA therapies for Wilson s disease. The liver, organ of choice. Highly vascularised
 DNA/RNA therapies for Wilson s disease Julien Baruteau - Great Ormond Street Institute of Child Health, University College London, UK Metabolic Medicine, Great Ormond Street Hospital, London, UK PLAN Landscape
DNA/RNA therapies for Wilson s disease Julien Baruteau - Great Ormond Street Institute of Child Health, University College London, UK Metabolic Medicine, Great Ormond Street Hospital, London, UK PLAN Landscape
Milano, September 7 th 2016
 Milano, September 7 th 2016 Dear IRDiRC Consortium Assembly, I would be delighted to share with the IRDIRC Therapeutic Scientific Committee my knowledge and expertise in both fields of regulatory affairs
Milano, September 7 th 2016 Dear IRDiRC Consortium Assembly, I would be delighted to share with the IRDIRC Therapeutic Scientific Committee my knowledge and expertise in both fields of regulatory affairs
SANGAMO THERAPEUTICS, INC. (Exact name of registrant as specified in its charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
Solutions to Problem Set 6
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Name: Question 1 Solutions to 7.012 Problem Set 6 The
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Name: Question 1 Solutions to 7.012 Problem Set 6 The
Principles and perspectives of gene therapy
 Principles and perspectives of gene therapy Prof. Józef Dulak, PhD, DSc Department of Medical Biotechnology Faculty of Biochemistry, Biophysics and Biotechnology Room 3.025/3.07 Phone 664-63-75 Email:
Principles and perspectives of gene therapy Prof. Józef Dulak, PhD, DSc Department of Medical Biotechnology Faculty of Biochemistry, Biophysics and Biotechnology Room 3.025/3.07 Phone 664-63-75 Email:
Basic Concepts and History of Genetic Engineering. Mitesh Shrestha
 Basic Concepts and History of Genetic Engineering Mitesh Shrestha Genetic Engineering AKA gene manipulation, gene cloning, recombinant DNA technology, genetic modification, and the new genetics. A technique
Basic Concepts and History of Genetic Engineering Mitesh Shrestha Genetic Engineering AKA gene manipulation, gene cloning, recombinant DNA technology, genetic modification, and the new genetics. A technique
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Oncology for Scientists Christopher Choi, PhD Director, TCPF Assistant Professor of Oncology
 Oncology for Scientists Christopher Choi, PhD Director, TCPF Assistant Professor of Oncology Immunological Battle Ground Tumor immunity Tumor growth The tumor environment is an immunological battle ground:
Oncology for Scientists Christopher Choi, PhD Director, TCPF Assistant Professor of Oncology Immunological Battle Ground Tumor immunity Tumor growth The tumor environment is an immunological battle ground:
Mark Rothera President & Chief Executive Officer. January 9, 2019
 Mark Rothera President & Chief Executive Officer January 9, 2019 Forward Looking Statements Certain information set forth in this presentation and in statements made orally during this presentation contains
Mark Rothera President & Chief Executive Officer January 9, 2019 Forward Looking Statements Certain information set forth in this presentation and in statements made orally during this presentation contains
Biosafety Committees and Biological Materials Oversight: Past, Present and Future for Clinical Research
 Biosafety Committees and Biological Materials Oversight: Past, Present and Future for Clinical Research HealthCare Compliance Association / 06-03-2014 Chris Jenkins, PhD, MPH, RBP, CHMM Overview Biosafety
Biosafety Committees and Biological Materials Oversight: Past, Present and Future for Clinical Research HealthCare Compliance Association / 06-03-2014 Chris Jenkins, PhD, MPH, RBP, CHMM Overview Biosafety
Germline Gene Therapy
 Germline Gene Therapy US275 Scientific Ethics John R. Hoffman Arcadia University 1 Germline gene therapy is the placement of new DNA into a fertilized egg. http://genmed.yolasite.com/basics-of-gene-therapy.php
Germline Gene Therapy US275 Scientific Ethics John R. Hoffman Arcadia University 1 Germline gene therapy is the placement of new DNA into a fertilized egg. http://genmed.yolasite.com/basics-of-gene-therapy.php
The prospects of gene editing for EB
 The prospects of gene editing for EB Marcel F. Jonkman m.f.jonkman@umcg.nl DEBRA International Congress 2016, Zagreb, 23 September 2016 Disclosure Monitor for Roche Shares in Philae Pharmaceuticals DEBRA
The prospects of gene editing for EB Marcel F. Jonkman m.f.jonkman@umcg.nl DEBRA International Congress 2016, Zagreb, 23 September 2016 Disclosure Monitor for Roche Shares in Philae Pharmaceuticals DEBRA
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform
 This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements
Meet the New CEO of the Dutch Biotech Making a Cure for Blood Cancer
 Meet the New CEO of the Dutch Biotech Making a Cure for Blood Cancer Written by Clara Rodríguez Fernández Arthur Lahr, CEO of Kiadis Pharma, explains the promising cell therapy the company is developing
Meet the New CEO of the Dutch Biotech Making a Cure for Blood Cancer Written by Clara Rodríguez Fernández Arthur Lahr, CEO of Kiadis Pharma, explains the promising cell therapy the company is developing
Mid-term review. Recitation5 03/31/2014. Stem Cell Biology and Function W4193 1
 Mid-term review Recitation5 03/31/2014 Stem Cell Biology and Function W4193 1 What to expect in the midterm2 It will be all so far but mainly whatever was NOT covered by the previous mid-term- not a huge
Mid-term review Recitation5 03/31/2014 Stem Cell Biology and Function W4193 1 What to expect in the midterm2 It will be all so far but mainly whatever was NOT covered by the previous mid-term- not a huge
CHANGES TO A GENE THERAPY MEDICINAL PRODUCT: REGULATORY VIEWS
 CHANGES TO A GENE THERAPY MEDICINAL PRODUCT: REGULATORY VIEWS Maria Cristina Galli, Ph.D. Istituto Superiore di Sanità Roma, Italy EATRIS ATMP platform chair ISCT 2016 Global Regulatory Perspective Workshop
CHANGES TO A GENE THERAPY MEDICINAL PRODUCT: REGULATORY VIEWS Maria Cristina Galli, Ph.D. Istituto Superiore di Sanità Roma, Italy EATRIS ATMP platform chair ISCT 2016 Global Regulatory Perspective Workshop
ROLE OF OECD AND THE TEST GUIDELINES PROGRAMME IN THE REGULATORY ACCEPTANCE OF ALTERNATIVE METHODS
 ROLE OF OECD AND THE TEST GUIDELINES PROGRAMME IN THE REGULATORY ACCEPTANCE OF ALTERNATIVE METHODS Anne Gourmelon Principal Administrator OECD Test Guidelines Programme Environmental, Health and Safety
ROLE OF OECD AND THE TEST GUIDELINES PROGRAMME IN THE REGULATORY ACCEPTANCE OF ALTERNATIVE METHODS Anne Gourmelon Principal Administrator OECD Test Guidelines Programme Environmental, Health and Safety
Press Release. Interim Data Summary
 Print Page Close Window Press Release bluebird bio Reports Interim Clinical Data from Starbeam Study of Lenti-D at AAN 2016 Annual Meeting First clinical data to be presented from Phase 2/3 Starbeam Study;
Print Page Close Window Press Release bluebird bio Reports Interim Clinical Data from Starbeam Study of Lenti-D at AAN 2016 Annual Meeting First clinical data to be presented from Phase 2/3 Starbeam Study;
Second Quarter 2016 Financial Results. August 4, 2016
 Second Quarter 2016 Financial Results August 4, 2016 Cautionary Note Regarding Forward-Looking Statements This presentation and various remarks we make during this presentation contain forward-looking
Second Quarter 2016 Financial Results August 4, 2016 Cautionary Note Regarding Forward-Looking Statements This presentation and various remarks we make during this presentation contain forward-looking
The Cell and Gene Therapy Catapult UK clinical trials database
 The Cell and Gene Therapy Catapult UK clinical trials database The UK clinical trials database covers cell and gene therapy clinical trial activities that the Cell and Gene Therapy Catapult (CGT Catapult)
The Cell and Gene Therapy Catapult UK clinical trials database The UK clinical trials database covers cell and gene therapy clinical trial activities that the Cell and Gene Therapy Catapult (CGT Catapult)
Homologous chromosomes fail to separate. Meiosis I: Nondisjunction
 Chromosomal Mutations of Number Nondisjunction ( not coming apart ) is when chromosomes do not separate properly during meiosis 1 or 2. The result of nondisjunction during Meiosis 1 is that 2 gametes will
Chromosomal Mutations of Number Nondisjunction ( not coming apart ) is when chromosomes do not separate properly during meiosis 1 or 2. The result of nondisjunction during Meiosis 1 is that 2 gametes will
Regulation of advanced blood cell therapies
 Regulation of advanced blood cell therapies www.pei.de Clinical trials using cell-based products Substantially manipulated cells and cells for non-homologous use Quality, safety and non-clinical aspects
Regulation of advanced blood cell therapies www.pei.de Clinical trials using cell-based products Substantially manipulated cells and cells for non-homologous use Quality, safety and non-clinical aspects
FINAL PROGRAM. September 13 to September 17, 2007 Thessaloniki, Halkidiki, Greece
 FINAL PROGRAM IV th Conference on Stem Cell Gene Therapy September 13 to September 17, 2007 Thessaloniki, Halkidiki, Greece THURSDAY, SEPTEMBER 13, 2007 6:00 pm NO HOST COCKTAILS 7:00 pm WELCOME RECEPTION
FINAL PROGRAM IV th Conference on Stem Cell Gene Therapy September 13 to September 17, 2007 Thessaloniki, Halkidiki, Greece THURSDAY, SEPTEMBER 13, 2007 6:00 pm NO HOST COCKTAILS 7:00 pm WELCOME RECEPTION
European Regenerative Medicine Firms & Their Strategic Approaches. Michael Morrison University of York
 European Regenerative Medicine Firms & Their Strategic Approaches Michael Morrison University of York OVERVIEW Creating the European RM Universe of firms Characterizing the European RM Universe Strategic
European Regenerative Medicine Firms & Their Strategic Approaches Michael Morrison University of York OVERVIEW Creating the European RM Universe of firms Characterizing the European RM Universe Strategic
Regulatory Pathways for Rare Diseases
 Regulatory Pathways for Rare Diseases Celia M. Witten, Ph.D., M.D. Deputy Director, FDA Center for Biologics Evaluation and Research Emerging Technologies for Rare Diseases: Clinical and Regulatory Case
Regulatory Pathways for Rare Diseases Celia M. Witten, Ph.D., M.D. Deputy Director, FDA Center for Biologics Evaluation and Research Emerging Technologies for Rare Diseases: Clinical and Regulatory Case
19F MRI cellular tracer preserves the differentiation potential of hematopoietic stem cells. Brooke Helfer, PhD Celsense, Inc Pittsburgh PA
 19F MRI cellular tracer preserves the differentiation potential of hematopoietic stem cells Brooke Helfer, PhD Celsense, Inc Pittsburgh PA Financial disclosure I have the following relationships to disclose:
19F MRI cellular tracer preserves the differentiation potential of hematopoietic stem cells Brooke Helfer, PhD Celsense, Inc Pittsburgh PA Financial disclosure I have the following relationships to disclose:
Hemophilia and Gene Therapy
 Hemophilia and Gene Therapy Jackie Chu June 4, 2008 Overview Hemophilia, the disease Gene therapy Hemophilia as a target for gene therapy Gene delivery systems Clinical trials New methods Future of gene
Hemophilia and Gene Therapy Jackie Chu June 4, 2008 Overview Hemophilia, the disease Gene therapy Hemophilia as a target for gene therapy Gene delivery systems Clinical trials New methods Future of gene
Iron metabolism. And. Anemia in chronic kidney disease
 Iron metabolism And Anemia in chronic kidney disease Chittima Sirijerachai Iron distribution Intracellular iron Intracellular ferrous iron Heme iron compound : Hb, myoglobin Iron containing enzyme Intracellular
Iron metabolism And Anemia in chronic kidney disease Chittima Sirijerachai Iron distribution Intracellular iron Intracellular ferrous iron Heme iron compound : Hb, myoglobin Iron containing enzyme Intracellular
Target Selection in the area of Gene Therapy Harald Petry, PhD
 Target Selection in the area of Gene Therapy Harald Petry, PhD Chief Scientific Officer Forward-looking statements This presentation contains forward-looking statements that involve substantial risks and
Target Selection in the area of Gene Therapy Harald Petry, PhD Chief Scientific Officer Forward-looking statements This presentation contains forward-looking statements that involve substantial risks and
Jefferies 2016 Healthcare Conference Engineering Genetic Cures
 Jefferies 2016 Healthcare Conference Engineering Genetic Cures Edward Lanphier Founder Sangamo BioSciences, Inc. June 8, 2016 Forward Looking Statements This presentation contains forward-looking statements
Jefferies 2016 Healthcare Conference Engineering Genetic Cures Edward Lanphier Founder Sangamo BioSciences, Inc. June 8, 2016 Forward Looking Statements This presentation contains forward-looking statements
Where are we with gene therapy?
 Where are we with gene therapy? Session 9: Gene Based Therapies Professor Alan Boyd PFPM 7 th February 2018 Faculty of Pharmaceutical Medicine, Royal Colleges of Physicians, UK Topics to be covered Gene
Where are we with gene therapy? Session 9: Gene Based Therapies Professor Alan Boyd PFPM 7 th February 2018 Faculty of Pharmaceutical Medicine, Royal Colleges of Physicians, UK Topics to be covered Gene
Update from the Center for Biologics Evaluation and Research (CBER) Peter Marks, M.D., Ph.D. GMP By The Sea 2017
 Update from the Center for Biologics Evaluation and Research (CBER) Peter Marks, M.D., Ph.D. GMP By The Sea 2017 Outline Products regulated Significance of complex biologics Product and process Cutting
Update from the Center for Biologics Evaluation and Research (CBER) Peter Marks, M.D., Ph.D. GMP By The Sea 2017 Outline Products regulated Significance of complex biologics Product and process Cutting
Making cancer a chronic disease. Troels Jordansen
 Making cancer a chronic disease Troels Jordansen Glycostem corporate summary Clinical stage company developing allogeneic cellular products for cancer immunotherapy using NK-cells Founded in 2007 Until
Making cancer a chronic disease Troels Jordansen Glycostem corporate summary Clinical stage company developing allogeneic cellular products for cancer immunotherapy using NK-cells Founded in 2007 Until
Cell Processing at CAGT. Overview. Adrian P. Gee Center for Cell & Gene Therapy. CAGT Introduction
 Cell Processing at CAGT Adrian P. Center for Cell & Gene Therapy CAGT Introduction Overview Facility Description Products prepared Flow cytometry Quality Control Quality Assurance 1 Center for Cell & Gene
Cell Processing at CAGT Adrian P. Center for Cell & Gene Therapy CAGT Introduction Overview Facility Description Products prepared Flow cytometry Quality Control Quality Assurance 1 Center for Cell & Gene
SANGAMO THERAPEUTICS, INC. (Exact name of registrant as specified in its charter)
 . UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Form 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December
. UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Form 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December
European Haemophilia Safety Surveillance System (EUHASS) Mike Makris Sheffield, UK
 European Haemophilia Safety Surveillance System (EUHASS) Mike Makris Sheffield, UK Pharmacovigilance Voluntary by health professionals and patients Often not used Too busy, not aware of the scheme Believe
European Haemophilia Safety Surveillance System (EUHASS) Mike Makris Sheffield, UK Pharmacovigilance Voluntary by health professionals and patients Often not used Too busy, not aware of the scheme Believe
Biology BIOL5 Unit 5 Control in cells and in organisms Friday 25 June pm to 3.45 pm For this paper you must have: Time allowed
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2010 Question 1 2 Mark Biology
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2010 Question 1 2 Mark Biology
Chapter 5 Learning Objectives
 Schedule and Announcements Go over Exam 1 Look at Elodea (plant cells) Start Chapter 5 Quiz Thursday over lab material Science Café 2 Friday Don t forget- research plan for project is due Friday September
Schedule and Announcements Go over Exam 1 Look at Elodea (plant cells) Start Chapter 5 Quiz Thursday over lab material Science Café 2 Friday Don t forget- research plan for project is due Friday September
Results to be Presented at LDN WORLD Symposium in February Initiation of Repeat-Dose Pompe Study Anticipated in 3Q13
 Amicus Therapeutics Announces Positive Results from All Four Cohorts in Phase 2 Chaperone-Enzyme Replacement Therapy (ERT) Co-Administration Study for Pompe Disease Strong Proof-of-Concept Data for Chaperone
Amicus Therapeutics Announces Positive Results from All Four Cohorts in Phase 2 Chaperone-Enzyme Replacement Therapy (ERT) Co-Administration Study for Pompe Disease Strong Proof-of-Concept Data for Chaperone
CAP CONTEXT INDICATORS
 CAP CONTEXT INDICATORS 2014-2020 24. AGRICULTURAL TRAINING OF FARM MANAGERS 2017 update CONTEXT INDICATOR 24: AGRICULTURAL TRAINING OF FARM MANAGERS Learning by doing is still the main form of for the
CAP CONTEXT INDICATORS 2014-2020 24. AGRICULTURAL TRAINING OF FARM MANAGERS 2017 update CONTEXT INDICATOR 24: AGRICULTURAL TRAINING OF FARM MANAGERS Learning by doing is still the main form of for the
Agios Pharmaceuticals, Inc.
 Agios Pharmaceuticals, Inc. The people pictured here are some of the many friends and family of Agios employees affected by cancer. All of us at Agios are passionate about transforming patients lives.
Agios Pharmaceuticals, Inc. The people pictured here are some of the many friends and family of Agios employees affected by cancer. All of us at Agios are passionate about transforming patients lives.
Mutagenesis and Expression of Mammalian Clotting Factor IX. Mark McCleland The Children s Hospital of Philadelphia and Lycoming College
 Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Overview of the clinical protocol
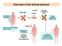 Overview of the clinical protocol CD34+ cells Vector + Cytokines Maximize % Transduced HSCs Bone Marrow or PB Harvest Testing and Release While Frozen (2x10 8 unsorted BM cells/kg kept for rescue) Bone
Overview of the clinical protocol CD34+ cells Vector + Cytokines Maximize % Transduced HSCs Bone Marrow or PB Harvest Testing and Release While Frozen (2x10 8 unsorted BM cells/kg kept for rescue) Bone
Advanced-therapy medicinal products: new competencies in hospital pharmacy Seminar PH4. Relevant Financial Relationships - None
 EAHP March 2016 Advanced-therapy medicinal products: new competencies in hospital pharmacy Seminar PH4 Dr. Lenka Taylor Pharmacy University Hospital Heidelberg Disclosure Relevant Financial Relationships
EAHP March 2016 Advanced-therapy medicinal products: new competencies in hospital pharmacy Seminar PH4 Dr. Lenka Taylor Pharmacy University Hospital Heidelberg Disclosure Relevant Financial Relationships
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL. PHARMACEUTICAL COMMITTEE 22 October 2012
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, EMA PHARM 608 PHARMACEUTICAL COMMITTEE 22 October 2012 Subject: Hospital exemption
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, EMA PHARM 608 PHARMACEUTICAL COMMITTEE 22 October 2012 Subject: Hospital exemption
What are the origins of medical practice? Humans have been involved with medical biotechnology
 Name: Score: / Quiz 8 on Medical Biotechnology Part 1 What are the origins of medical practice? Humans have been involved with medical biotechnology A. since the cloning of the insulin gene in the 1980s
Name: Score: / Quiz 8 on Medical Biotechnology Part 1 What are the origins of medical practice? Humans have been involved with medical biotechnology A. since the cloning of the insulin gene in the 1980s
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) <DRAFT>
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 17 November 2005 Doc. Ref. EMEA/273974/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 17 November 2005 Doc. Ref. EMEA/273974/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE
INO Therapeutics is now
 INO Therapeutics is now The materials contained herein are a description of our business. They do not purport to be all inclusive or contain all information that you desire. This document includes or may
INO Therapeutics is now The materials contained herein are a description of our business. They do not purport to be all inclusive or contain all information that you desire. This document includes or may
Gene Therapy: The Basics. Mark A. Kay MD PhD Dennis Farrey Family Professor Stanford University
 Gene Therapy: The Basics Mark A. Kay MD PhD Dennis Farrey Family Professor Stanford University Definition of gene therapy Gene therapy is the introduction of nucleic acids (e.g. DNA/genes) into somatic
Gene Therapy: The Basics Mark A. Kay MD PhD Dennis Farrey Family Professor Stanford University Definition of gene therapy Gene therapy is the introduction of nucleic acids (e.g. DNA/genes) into somatic
Jefferies 2017 Healthcare Conference
 Jefferies 2017 Healthcare Conference June 9, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information. The
Jefferies 2017 Healthcare Conference June 9, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information. The
Epoetin alfa. Epogen, Procrit (epoetin alfa), Retacrit (epoetin alfa epbx) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.06 Subject: Epoetin alfa Page: 1 of 8 Last Review Date: September 20, 2018 Epoetin alfa Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.85.06 Subject: Epoetin alfa Page: 1 of 8 Last Review Date: September 20, 2018 Epoetin alfa Description
An Overview of Chimeric Antigen Receptor T-cells: CAR-T-ing Away Cancer
 An Overview of Chimeric Antigen Receptor T-cells: CAR-T-ing Away Cancer Maxwell Brown, PharmD Clinical Pharmacy Manager, Hematopoietic Stem Cell Transplantation NewYork-Presbyterian/Weill Cornell Medical
An Overview of Chimeric Antigen Receptor T-cells: CAR-T-ing Away Cancer Maxwell Brown, PharmD Clinical Pharmacy Manager, Hematopoietic Stem Cell Transplantation NewYork-Presbyterian/Weill Cornell Medical
Iron Deficiency Anemia
 Diagnosis of Iron Deficiency Anemia Using the Abaxis VetScan HM2 Hematology System Contributing Authors: Mary Anna Thrall, BA, DVM, MS, DACVP; Barton Gillespie, BS, DVM Ross University School of Veterinary
Diagnosis of Iron Deficiency Anemia Using the Abaxis VetScan HM2 Hematology System Contributing Authors: Mary Anna Thrall, BA, DVM, MS, DACVP; Barton Gillespie, BS, DVM Ross University School of Veterinary
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website or on the website www.clinicalstudyresults.org hosted by the Pharmaceutical Research and
Daily Agenda. Make Checklist: Think Time Replication, Transcription, and Translation Quiz Mutation Notes Download Gene Screen for ipad
 Daily Agenda Make Checklist: Think Time Replication, Transcription, and Translation Quiz Mutation Notes Download Gene Screen for ipad Genetic Engineering Students will be able to exemplify ways that introduce
Daily Agenda Make Checklist: Think Time Replication, Transcription, and Translation Quiz Mutation Notes Download Gene Screen for ipad Genetic Engineering Students will be able to exemplify ways that introduce
Apheresis M. Flores, BSN, RN, HP (ASCP)
 Apheresis M. Flores, BSN, RN, HP (ASCP) Objectives: 1. Discuss Apheresis and key concepts 2. Discuss : Cell Collection Extracorporeal Photopheresis (ECP) 1 What is Apheresis? Apheresis: Greek word meaning
Apheresis M. Flores, BSN, RN, HP (ASCP) Objectives: 1. Discuss Apheresis and key concepts 2. Discuss : Cell Collection Extracorporeal Photopheresis (ECP) 1 What is Apheresis? Apheresis: Greek word meaning
HUMAN GENOME EDITING FAQs
 HUMAN GENOME EDITING FAQs DEFINITIONS } What is a genome? } What is genome editing and how does it work? } What is the difference between somatic cell and germline genome editing? } What are the genome
HUMAN GENOME EDITING FAQs DEFINITIONS } What is a genome? } What is genome editing and how does it work? } What is the difference between somatic cell and germline genome editing? } What are the genome
Information Day Leeds, UK 27 October 2015 Treating and managing disease
 The societal challenge 'Health, demographic change and well-being' Work programme 2016-2017 Information Day Leeds, UK 27 October 2015 Treating and managing disease Image credit: Adam Fagen Rallying for
The societal challenge 'Health, demographic change and well-being' Work programme 2016-2017 Information Day Leeds, UK 27 October 2015 Treating and managing disease Image credit: Adam Fagen Rallying for
2111: ALL Post-HCT. Add/ Remove/ Modify. Manual Section. Date. Description. Comprehensive Disease- Specific Manuals
 2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
DARATUMUMAB, A CD38 MONOCLONAL ANTIBODY IN PATIENTS WITH MULTIPLE MYELOMA Data from the dose-escalation part of the FIH study Abstract #8512
 DARATUMUMAB, A CD38 MONOCLONAL ANTIBODY IN PATIENTS WITH MULTIPLE MYELOMA Data from the dose-escalation part of the FIH study Abstract #8512 Henk Lokhorst, Torben Plesner, Peter Gimsing, Hareth Nahi, Monique
DARATUMUMAB, A CD38 MONOCLONAL ANTIBODY IN PATIENTS WITH MULTIPLE MYELOMA Data from the dose-escalation part of the FIH study Abstract #8512 Henk Lokhorst, Torben Plesner, Peter Gimsing, Hareth Nahi, Monique
