MEDPOR. Orbital Floor
|
|
|
- Norma Griffith
- 6 years ago
- Views:
Transcription
1 MEDPOR Orbital Floor
2 Orbital Fracture Repair Available in a variety of shapes, sizes, and configurations, MEDPOR and MEDPOR TITAN Implants provide surgeons with an expanding range of options for reconstruction and augmentation of orbital fractures. MEDPOR Biomaterial has been used in over 400,000 surgical procedures and referenced in over 350 published articles since The interconnecting, omni-directional pore structure of the biocompatible porous material may allow for fibrovascular in-growth and integration of the patient s tissue. 1. MEDPOR BARRIER Implants are designed to reduce tissue attachment to the non-porous BARRIER surface of the implants. MEDPOR TITAN was the first craniofacial implant to combine high-density polyethylene sheets and titanium mesh in a single implant for increased flexibility, shape retention, radiographic visualization and strength. 1. MEDPOR TITAN FAN Available in two configurations, with or without a BARRIER MTM Titanium mesh embedded within porous, high-density polyethylene MTB Titanium mesh embedded within a porous polyethylene matrix with a solid, barrier surface on one side, potentially allowing for fibrovascular ingrowth only on the porous side of the implant Combines high-density polyethylene sheets and titanium mesh in a single implant for increased flexibility, shape retention, radiographic visualization and strength MTM 40mm 62mm 0.85mm MTB 40mm 62mm 1.0mm 2
3 Micro Thin Sheets CAT# A B THICKNESS mm 50mm 0.25mm mm 50mm 0.35mm mm 50mm 0.40mm mm 50mm 0.45mm mm 50mm 0.45mm Ultra Thin Sheets CAT# A B THICKNESS mm 50mm 0.85mm mm 76mm 0.85mm mm 127mm 0.85mm mm 178mm 0.85mm Sheets CAT# A B THICKNESS mm 50mm 1.5mm mm 76mm 1.5mm mm 127mm 1.5mm mm 50mm 3.0mm 3
4 BARRIER Single Channel Sheets 9527 BARRIER Microplate 38mm 50mm 0.85mm Single Channel Sheet BARRIER Channel Sheets 9531 BARRIER Miniplate 40mm 52mm 2.3mm Channel Sheet 9532 BARRIER Microplate 40mm 52mm 2.3mm Channel Sheet BARRIER Sheets 8305 Orbital Floor Implant 38mm 50mm 1.0mm 9305 Orbital Floor Implant 38mm 50mm 1.6mm 8312 Rectangle 50mm 76mm 1.0mm 9312 Rectangle 50mm 76mm 1.6mm Enophthalmos Wedge 9541 Regular Left 22mm 31mm 7.0mm 9542 Regular Right 22mm 31mm 7.0mm 9543 Large Left 28mm 40mm 7.5mm 9544 Large Right 28mm 40mm 7.5mm 4
5 MEDPOR TITAN MAX Orbital Floor and Wall (OFW) CAT# DESCRIPTION A B C THICKNESS MTM 41mm 42mm 1.0mm 0.85mm MTB - Left 41mm 42mm 1.0mm 1.0mm MTB - Right 41mm 42mm 1.0mm 1.0mm US Patent 7,655,047 MEDPOR TITAN Orbital Floor and Wall (OFW) CAT# DESCRIPTION A B C THICKNESS MTM 41mm 42mm 0.5mm 0.85mm MTB Left 41mm 42mm 0.5mm 1.0mm MTB Right 41mm 42mm 0.5mm 1.0mm BTB 41mm 42mm 0.5mm 0.6mm US Patent 7,655,047 MEDPOR TITAN Implants MTM MTB BTB - Titanium mesh embedded within porous, high-density polyethylene. - Titanium mesh embedded within a porous polyethylene matrix with a solid, barrier surface on one side, potentially allowing for fibrovascular ingrowth only on the porous side of the implant. - Titanium mesh embedded within solid, non-porous high-density polyethylene MTM 76mm 50mm 0.85mm MTM 38mm 50mm 0.85mm MTM 38mm 50mm 1.5mm MTM 76mm 50mm 1.5mm BTB 38mm 50mm 0.6mm BTB 76mm 50mm 0.6mm MTB 38mm 50mm 1.0mm MTB 76mm 50mm 1.0mm MTB 38mm 50mm 1.6mm MTB 76mm 50mm 1.6mm 5
6 Stryker Australia 8 Herbert Street St Leonards NSW 2065 Australia Phone: +61 (2) Fax: +61 (2) Stryker New Zealand 515 Mt. Wellington Highway Auckland 1060 New Zealand Phone: +64 (9) Fax: +64 (9) This document is intended solely for the use of healthcare professionals. A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery. The information presented in this brochure is intended to demonstrate a Stryker product. Always refer to the package insert, product label and/or user instructions including the instructions for cleaning and sterilization (if applicable) before using any Stryker products. Products may not be available in all markets. Product availability is subject to the regulatory or medical practices that govern individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area. This document is not for the distribution in the USA. Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Strykyer, HydroSet. All other trademarks are trademarks of their respective owners or holders. The products listed above are CE marked Literature Number SSP LOT C0214 Copyright 2014 Stryker Printed in Australia
More than surface deep
 Tritanium PL Posterior Lumbar Cage More than surface deep Featuring Tritanium In-Growth Technology: 1 TM Built to fuse Tritanium In-Growth Technology1 Stryker s proprietary Tritanium In-Growth Technology,
Tritanium PL Posterior Lumbar Cage More than surface deep Featuring Tritanium In-Growth Technology: 1 TM Built to fuse Tritanium In-Growth Technology1 Stryker s proprietary Tritanium In-Growth Technology,
EasyClip Xpress Sterile staple solution. Operative technique
 EasyClip Xpress Sterile staple solution Operative technique Sterile staple solution Table of contents Indications 3 Operative technique 4 This publication sets forth detailed recommended procedures for
EasyClip Xpress Sterile staple solution Operative technique Sterile staple solution Table of contents Indications 3 Operative technique 4 This publication sets forth detailed recommended procedures for
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength
 The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
SCIENCE. VALUE. INNOVATION.
 SCIENCE. VALUE. INNOVATION. OUR STORY TELA Bio, a surgical reconstruction company, was created out of the desire to do things differently. Combining the drive and innovation of a start-up with a seasoned
SCIENCE. VALUE. INNOVATION. OUR STORY TELA Bio, a surgical reconstruction company, was created out of the desire to do things differently. Combining the drive and innovation of a start-up with a seasoned
LITe LIF Less invasive posterior lumbar interbody fusion procedure
 LITe LIF Less invasive posterior lumbar interbody fusion procedure LITe LIF Access LITe Midline Retractor Fixation Xia CT Interbody Tritanium PL Cage Instruments LITe Decompression 2.0 Power CD3 and RemB
LITe LIF Less invasive posterior lumbar interbody fusion procedure LITe LIF Access LITe Midline Retractor Fixation Xia CT Interbody Tritanium PL Cage Instruments LITe Decompression 2.0 Power CD3 and RemB
RegenKit THT. Autologous Platelet Rich Plasma (A-PRP) Reference Guide. Biologics. Concentrated on Healing
 RegenKit THT Autologous Platelet Rich Plasma (A-PRP) Reference Guide Biologics Concentrated on Healing Step I: Collect Whole Blood RegenKit THT Simple 4-Step Process e single use RegenKit THT is designed
RegenKit THT Autologous Platelet Rich Plasma (A-PRP) Reference Guide Biologics Concentrated on Healing Step I: Collect Whole Blood RegenKit THT Simple 4-Step Process e single use RegenKit THT is designed
HydroSet. Injectable HA Bone Substitute
 HydroSet Injectable HA Bone Substitute The Latest Advancement in Bone Substitute Technology Fast Setting Injectable or Manual Implantation Osteoconductive Excellent Wet-field Characteristics Isothermic
HydroSet Injectable HA Bone Substitute The Latest Advancement in Bone Substitute Technology Fast Setting Injectable or Manual Implantation Osteoconductive Excellent Wet-field Characteristics Isothermic
ENT navigation system. Product catalog
 ENT navigation system Product catalog Your superior experience starts with our people TGS Target Guided Surgery The next step in image guided surgery Scopis TGS is a next-generation solution for navigated
ENT navigation system Product catalog Your superior experience starts with our people TGS Target Guided Surgery The next step in image guided surgery Scopis TGS is a next-generation solution for navigated
A Legacy of Serving the Surgical Community
 Precisely. A Legacy of Serving the Surgical Community For over half a century, Stryker has been developing products based on the expressed needs of leading practitioners. You will often find a Stryker
Precisely. A Legacy of Serving the Surgical Community For over half a century, Stryker has been developing products based on the expressed needs of leading practitioners. You will often find a Stryker
Let s Talk. Science... The Science of: > > > Motion13. Wear1. Triathlon. Knee System
 Let s Talk Science... The Science of: > Motion13 > > Fit6 Wear1 Triathlon Knee System The Science of Increased Motion High flexion knee system designed for mobility with stability through 150 degrees of
Let s Talk Science... The Science of: > Motion13 > > Fit6 Wear1 Triathlon Knee System The Science of Increased Motion High flexion knee system designed for mobility with stability through 150 degrees of
The information contained in this document is intended for healthcare professionals only.
 The information contained in this document is intended for healthcare professionals only. Orthopaedics Modular Taper Junction and Neck Strength in Total Hip Arthroplasty Re s tora ti on Modular Revi s
The information contained in this document is intended for healthcare professionals only. Orthopaedics Modular Taper Junction and Neck Strength in Total Hip Arthroplasty Re s tora ti on Modular Revi s
CRANIOS REINFORCED BONE VOID FILLER
 CRANIOS REINFORCED BONE VOID FILLER The tougher bone cement TECHNICAL MONOGRAPH INTRODUCTION CRANIOS REINFORCED Bone Void Filler contains reinforcing fibers that impart an increase in toughness* to reduce
CRANIOS REINFORCED BONE VOID FILLER The tougher bone cement TECHNICAL MONOGRAPH INTRODUCTION CRANIOS REINFORCED Bone Void Filler contains reinforcing fibers that impart an increase in toughness* to reduce
The Power in You. TM
 RegenKit THT Autologous Platelet Rich Plasma (A-PRP) The Power in You. TM Biologics Concentrated on Healing RegenKit THT Autologous Platelet Rich Plasma (A-PRP) The RegenKit THT is designed to be used
RegenKit THT Autologous Platelet Rich Plasma (A-PRP) The Power in You. TM Biologics Concentrated on Healing RegenKit THT Autologous Platelet Rich Plasma (A-PRP) The RegenKit THT is designed to be used
Advancing the Open Ventral Hernia Repair Experience
 Advancing the Open Ventral Hernia Repair Experience SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Absorbable Fixation System Optimized Design for Open Ventral
Advancing the Open Ventral Hernia Repair Experience SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Absorbable Fixation System Optimized Design for Open Ventral
BIOFIBER BIOFIBER CM AMBIENT TEMPERATUR. Absorbable Scaffold. Collagen Coated. Absorbable Scaffold
 BIOFIBER Absorbable Scaffold BIOFIBER CM Collagen Coated Absorbable Scaffold A B S O R B A B L E AMBIENT TEMPERATUR B I O L O G I C S C AAND F F OCRYOPRESERVE L D I N G S OSTORAGE L U T I O N S OPTION
BIOFIBER Absorbable Scaffold BIOFIBER CM Collagen Coated Absorbable Scaffold A B S O R B A B L E AMBIENT TEMPERATUR B I O L O G I C S C AAND F F OCRYOPRESERVE L D I N G S OSTORAGE L U T I O N S OPTION
Specifically designed for abdominal wall repair STRUCTURAL
 Specifically designed for abdominal wall repair STRUCTURAL The Better Approach To a Better Allograft Better Standards: Board of Directors comprised primarily of surgeons 3-years run by surgeons, for surgeons
Specifically designed for abdominal wall repair STRUCTURAL The Better Approach To a Better Allograft Better Standards: Board of Directors comprised primarily of surgeons 3-years run by surgeons, for surgeons
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: B I O M A T E R I A L en English INSTRUCTIONS FOR USE FOR GORE SYNECOR INTRAPERITONEAL BIOMATERIAL INDICATIONS The GORE SYNECOR Intraperitoneal Biomaterial device is intended
INSTRUCTIONS FOR USE FOR: B I O M A T E R I A L en English INSTRUCTIONS FOR USE FOR GORE SYNECOR INTRAPERITONEAL BIOMATERIAL INDICATIONS The GORE SYNECOR Intraperitoneal Biomaterial device is intended
For personal use only
 Quarterly Cash Flow Report June 2016 Perth, Australia; 29 April 2016: Orthocell Limited s Quarterly Cash Flow Report for the quarter ended 30 June 2016 is attached. Orthocell is a commercial-stage, regenerative
Quarterly Cash Flow Report June 2016 Perth, Australia; 29 April 2016: Orthocell Limited s Quarterly Cash Flow Report for the quarter ended 30 June 2016 is attached. Orthocell is a commercial-stage, regenerative
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM INTRAPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM INTRAPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted
Sterile Titanium Implants for Craniofacial Osteosynthesis
 Sterile Titanium Implants for Craniofacial Osteosynthesis Sterile Titanium Implants for Craniofacial Osteosynthesis Daily routine in the hospital s OR means meeting challenges continuously. Especially
Sterile Titanium Implants for Craniofacial Osteosynthesis Sterile Titanium Implants for Craniofacial Osteosynthesis Daily routine in the hospital s OR means meeting challenges continuously. Especially
ISO INTERNATIONAL STANDARD. Implants for surgery Metallic materials Classification of microstructures for alpha+beta titanium alloy bars
 INTERNATIONAL STANDARD ISO 20160 First edition 2006-05-01 Corrected version 2006-09-15 Implants for surgery Metallic materials Classification of microstructures for alpha+beta titanium alloy bars Implants
INTERNATIONAL STANDARD ISO 20160 First edition 2006-05-01 Corrected version 2006-09-15 Implants for surgery Metallic materials Classification of microstructures for alpha+beta titanium alloy bars Implants
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 7198 Second edition 2016-08-01 Cardiovascular implants and extracorporeal systems Vascular prostheses Tubular vascular grafts and vascular patches Implants cardiovasculaires
INTERNATIONAL STANDARD ISO 7198 Second edition 2016-08-01 Cardiovascular implants and extracorporeal systems Vascular prostheses Tubular vascular grafts and vascular patches Implants cardiovasculaires
Disclosures. New therapies for spinal fusions. Cells, Molecules and Surfaces" Hyun Bae, MD
 Hyun Bae, MD Professor of Surgery Department of Surgery Director of Education Cedars Spine Center Medical Director Disclosures Depuy- Consultant Stryker- Consultant Nuvasive- Consultant Prosidyan- SAB
Hyun Bae, MD Professor of Surgery Department of Surgery Director of Education Cedars Spine Center Medical Director Disclosures Depuy- Consultant Stryker- Consultant Nuvasive- Consultant Prosidyan- SAB
ISO INTERNATIONAL STANDARD. Implants for surgery Metallic materials Part 14: Wrought titanium 15-molybdenum 5-zirconium 3-aluminium alloy
 INTERNATIONAL STANDARD ISO 5832-14 First edition 2007-10-15 Implants for surgery Metallic materials Part 14: Wrought titanium 15-molybdenum 5-zirconium 3-aluminium alloy Implants chirurgicaux Matériaux
INTERNATIONAL STANDARD ISO 5832-14 First edition 2007-10-15 Implants for surgery Metallic materials Part 14: Wrought titanium 15-molybdenum 5-zirconium 3-aluminium alloy Implants chirurgicaux Matériaux
One Surgeon. One Patient. FLH /11
 FLH 219 03/11 This publication has been issued by: European Central Marketing Waterton Industrial Estate Bridgend South Wales CF31 3XA +44 [0] 1656 655221 +44 [0] 1656 645454 Responsible Manufacturer:
FLH 219 03/11 This publication has been issued by: European Central Marketing Waterton Industrial Estate Bridgend South Wales CF31 3XA +44 [0] 1656 655221 +44 [0] 1656 645454 Responsible Manufacturer:
INDUSTRY OVERVIEW SOURCE OF INFORMATION
 The information presented in this section, including certain facts, statistics and data, is derived from various official government publications and other publications and from the market research report
The information presented in this section, including certain facts, statistics and data, is derived from various official government publications and other publications and from the market research report
ALLODERM SELECT ALLODERM SELECT RESTORE
 ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
NORIAN DRILLABLE TECHNICAL MONOGRAPH. Fiber reinforced calcium phosphate phate bone void filler
 NORIAN DRILLABLE Fiber reinforced calcium phosphate phate bone void filler TECHNICAL MONOGRAPH INDICATIONS & CONTRAINDICATIONS Drillable Drillable is intended for bony voids or defects of the extremities
NORIAN DRILLABLE Fiber reinforced calcium phosphate phate bone void filler TECHNICAL MONOGRAPH INDICATIONS & CONTRAINDICATIONS Drillable Drillable is intended for bony voids or defects of the extremities
strength of science Now with sizes up to 25 cm x 45 cm XCM BIOLOGIC Tissue Matrix
 strength of science XCM BIOLOGIC Tissue Matrix Now with sizes up to 25 cm x 45 cm Your trusted partner providing a complete portfolio of biologic meshes. DePuy Synthes Companies of Johnson & Johnson offer
strength of science XCM BIOLOGIC Tissue Matrix Now with sizes up to 25 cm x 45 cm Your trusted partner providing a complete portfolio of biologic meshes. DePuy Synthes Companies of Johnson & Johnson offer
ISO INTERNATIONAL STANDARD. Implants for surgery Metallic materials Classification of microstructures for alpha+beta titanium alloy bars
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 20160 First edition 2006-05-01 Implants for surgery Metallic materials Classification of microstructures for alpha+beta titanium alloy bars Implants
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 20160 First edition 2006-05-01 Implants for surgery Metallic materials Classification of microstructures for alpha+beta titanium alloy bars Implants
Trabecular Metal Technology
 Trabecular Metal Technology Alexandre Nehme Departement of Orthopedic Surgery. Saint Georges University Hospital. Beirut. The cellular structure of Tantallum (Trabecular Metal) resembles bone and approximates
Trabecular Metal Technology Alexandre Nehme Departement of Orthopedic Surgery. Saint Georges University Hospital. Beirut. The cellular structure of Tantallum (Trabecular Metal) resembles bone and approximates
For personal use only
 Quarterly Cash Flow Report March 31 2015 Perth, Australia; 30 April 2015: Commercial stage regenerative medicine company Orthocell Limited s Quarterly Cash Flow Report for the quarter ended March 31 2015
Quarterly Cash Flow Report March 31 2015 Perth, Australia; 30 April 2015: Commercial stage regenerative medicine company Orthocell Limited s Quarterly Cash Flow Report for the quarter ended March 31 2015
Ask your surgeon for more information about Natrelle and visit GummyImplants.com
 The #1 surgeon-preferred gummy implant collection in the US* Two-stage breast reconstruction starts with Natrelle 133 Series Tissue Expanders Natrelle 133 Series Tissue Expanders are made to match Natrelle
The #1 surgeon-preferred gummy implant collection in the US* Two-stage breast reconstruction starts with Natrelle 133 Series Tissue Expanders Natrelle 133 Series Tissue Expanders are made to match Natrelle
FORWARD-LOOKING STATEMENTS
 FORWARD-LOOKING STATEMENTS The statements made by Kensey Nash and its representatives in this presentation will include certain forward-looking statements, including financial forecasts, that are based
FORWARD-LOOKING STATEMENTS The statements made by Kensey Nash and its representatives in this presentation will include certain forward-looking statements, including financial forecasts, that are based
Power Digital Imaging
 OfficePACS Power Digital Imaging Power Unleashed Organize According to Workflow PowerGroup allows physicians to organize studies by key fields and customize the PatientFinder to the way they work. Find
OfficePACS Power Digital Imaging Power Unleashed Organize According to Workflow PowerGroup allows physicians to organize studies by key fields and customize the PatientFinder to the way they work. Find
Specialist capability for all generation systems
 Generation We have a solid track record in construction and maintenance of all types of generation facilities including coal, gas, geothermal, hydro and wind energy. We have experience in the latest technology
Generation We have a solid track record in construction and maintenance of all types of generation facilities including coal, gas, geothermal, hydro and wind energy. We have experience in the latest technology
Medical Device Design & Manufacturing
 Medical Device Design & Manufacturing Comprehensive product development and additive manufacturing solutions to support implant and instrument performance Precision Healthcare Solutions Empowering the
Medical Device Design & Manufacturing Comprehensive product development and additive manufacturing solutions to support implant and instrument performance Precision Healthcare Solutions Empowering the
Affinity. A Paradigm Shift in Skeletal Reconstruction
 Affinity A Paradigm Shift in Skeletal Reconstruction INTRODUCING TRS AFFINITY SKELETAL RECONSTRUCTION BREAKTHROUGH Tissue Regeneration Systems (TRS ) is a start-up medical device company commercializing
Affinity A Paradigm Shift in Skeletal Reconstruction INTRODUCING TRS AFFINITY SKELETAL RECONSTRUCTION BREAKTHROUGH Tissue Regeneration Systems (TRS ) is a start-up medical device company commercializing
LAP-BAND System Access Port II Kit
 LAP-BAND System Access Port II Kit DIRECTIONS FOR USE (DFU) Access Port Needle Access Port Priming Needle Access Port II End Plug Band Priming Needle Stainless Steel Connector Tubing LAP-BAND System Access
LAP-BAND System Access Port II Kit DIRECTIONS FOR USE (DFU) Access Port Needle Access Port Priming Needle Access Port II End Plug Band Priming Needle Stainless Steel Connector Tubing LAP-BAND System Access
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 14630 Fourth edition 2012-12-01 Non-active surgical implants General requirements Implants chirurgicaux non actifs Exigences générales Reference number ISO 14630:2012(E) ISO
INTERNATIONAL STANDARD ISO 14630 Fourth edition 2012-12-01 Non-active surgical implants General requirements Implants chirurgicaux non actifs Exigences générales Reference number ISO 14630:2012(E) ISO
Pre-Congress Bone-tec 2013 Clinical Translational Workshop
 Pre-Congress Bone-tec 2013 Clinical Translational Workshop Sat, Dec 14, 830-1600, Tan Tock Seng Hospital, Main Building, Level 3, Seminar Rm 1 & 2, 11 Jalan Tan Tock Seng, Singapore 308433 Clinical translational
Pre-Congress Bone-tec 2013 Clinical Translational Workshop Sat, Dec 14, 830-1600, Tan Tock Seng Hospital, Main Building, Level 3, Seminar Rm 1 & 2, 11 Jalan Tan Tock Seng, Singapore 308433 Clinical translational
TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing. Technical Q&A
 TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing Technical Q&A TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing
TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing Technical Q&A TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing
ISO INTERNATIONAL STANDARD. Implants for surgery Ceramic materials Part 1: Ceramic materials based on high purity alumina
 INTERNATIONAL STANDARD ISO 6474-1 First edition 2010-02-15 Implants for surgery Ceramic materials Part 1: Ceramic materials based on high purity alumina Implants chirurgicaux Produits céramiques Partie
INTERNATIONAL STANDARD ISO 6474-1 First edition 2010-02-15 Implants for surgery Ceramic materials Part 1: Ceramic materials based on high purity alumina Implants chirurgicaux Produits céramiques Partie
ISO 5817 INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 5817 Second edition 2003-10-01 Welding Fusion-welded joints in steel, nickel, titanium and their alloys (beam welding excluded) Quality levels for imperfections Soudage Assemblages
INTERNATIONAL STANDARD ISO 5817 Second edition 2003-10-01 Welding Fusion-welded joints in steel, nickel, titanium and their alloys (beam welding excluded) Quality levels for imperfections Soudage Assemblages
OSCILLATING SAW BLADES PAT. PENDING SPEZIAL TOOTHING
 MEDICAL ADVANCED TECHNOLOGY OSCILLATING SAW BLADES PAT. PENDING SPEZIAL TOOTHING GUARANTEES THE PERFECT CUT BY LOW PRESSURE ON THE BONE RISK OF THERMAL NECROSIS IS HIGHLY REDUCED introducing ourselve Manufacturer
MEDICAL ADVANCED TECHNOLOGY OSCILLATING SAW BLADES PAT. PENDING SPEZIAL TOOTHING GUARANTEES THE PERFECT CUT BY LOW PRESSURE ON THE BONE RISK OF THERMAL NECROSIS IS HIGHLY REDUCED introducing ourselve Manufacturer
ISO INTERNATIONAL STANDARD. Implants for surgery Metallic materials Part 1: Wrought stainless steel
 INTERNATIONAL STANDARD ISO 5832-1 Fourth edition 2007-06-15 Implants for surgery Metallic materials Part 1: Wrought stainless steel Implants chirurgicaux Matériaux métalliques Partie 1: Acier inoxydable
INTERNATIONAL STANDARD ISO 5832-1 Fourth edition 2007-06-15 Implants for surgery Metallic materials Part 1: Wrought stainless steel Implants chirurgicaux Matériaux métalliques Partie 1: Acier inoxydable
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 13175-3 First edition 2012-10-01 Implants for surgery Calcium phosphates Part 3: Hydroxyapatite and beta-tricalcium phosphate bone substitutes Implants chirurgicaux Phosphates
INTERNATIONAL STANDARD ISO 13175-3 First edition 2012-10-01 Implants for surgery Calcium phosphates Part 3: Hydroxyapatite and beta-tricalcium phosphate bone substitutes Implants chirurgicaux Phosphates
POLYSITE Low Profile Hybrid Ports. Comfort your patient deserves. Strength you demand.
 POLYSITE Low Profile Hybrid Ports Comfort your patient deserves. Strength you demand. The comfort your patient deserves. POLYSITE Hybrid Ports Port combines lightweight plastic body for comfort and a strong
POLYSITE Low Profile Hybrid Ports Comfort your patient deserves. Strength you demand. The comfort your patient deserves. POLYSITE Hybrid Ports Port combines lightweight plastic body for comfort and a strong
TECHNICAL BULLETIN TB161
 DATE; FRIDAY, 13 JULY 2018 TECHNICAL BULLETIN TB161 ISSUES WITH RESIN BACKED TILES TB161.004 ARDEX Australia 2010-2018 INTRODUCTION & SCOPE We are all familiar with natural stone tiles and the great variety
DATE; FRIDAY, 13 JULY 2018 TECHNICAL BULLETIN TB161 ISSUES WITH RESIN BACKED TILES TB161.004 ARDEX Australia 2010-2018 INTRODUCTION & SCOPE We are all familiar with natural stone tiles and the great variety
Histoacryl A revolution in mesh fixation
 Histoacryl A revolution in mesh fixation Closure Technologies Histoacryl A revolution in mesh fixation A new indication for a classic product Histoacryl has been used for more than 40 years in operating
Histoacryl A revolution in mesh fixation Closure Technologies Histoacryl A revolution in mesh fixation A new indication for a classic product Histoacryl has been used for more than 40 years in operating
Date July 16, 2015 Court Intellectual Property High Court Case number 2015 (Gyo-Ke) 10002
 Date July 16, 2015 Court Intellectual Property High Court Case number 2015 (Gyo-Ke) 10002 Fourth Division A case in which, with respect to a trademark consisting of a combination of a figure and a color
Date July 16, 2015 Court Intellectual Property High Court Case number 2015 (Gyo-Ke) 10002 Fourth Division A case in which, with respect to a trademark consisting of a combination of a figure and a color
The Orthopaedic Research Laboratory (ORL) performs research in the field of orthopaedics.
 The Orthopaedic Research Laboratory (ORL) performs research in the field of orthopaedics. The biomechanical section focusses on bone, soft tissues and on pre-clinical testing of implants. We are specialized
The Orthopaedic Research Laboratory (ORL) performs research in the field of orthopaedics. The biomechanical section focusses on bone, soft tissues and on pre-clinical testing of implants. We are specialized
Ultraporous Acetabular Surfaces are Needed PRO
 Ultraporous Acetabular Surfaces are Needed PRO Wayne G. Paprosky, MD, FACS Medical Director, Cadence Health Joint Replacement Institute Central DuPage Hospital Professor RUSH University Medical Center
Ultraporous Acetabular Surfaces are Needed PRO Wayne G. Paprosky, MD, FACS Medical Director, Cadence Health Joint Replacement Institute Central DuPage Hospital Professor RUSH University Medical Center
Instructions for Use READY TO USE
 READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
YOUR PARTNER. In The Global Life Sciences Market. Value Plastics Fluid Management Components Biomaterial Delivery Devices Avalon Catheter Solutions
 YOUR PARTNER In The Global Life Sciences Market Value Plastics Fluid Management Components Biomaterial Delivery Devices Avalon Catheter Solutions COMMITMENT To Quality and Consistency With a solid reputation
YOUR PARTNER In The Global Life Sciences Market Value Plastics Fluid Management Components Biomaterial Delivery Devices Avalon Catheter Solutions COMMITMENT To Quality and Consistency With a solid reputation
Comfort your patient deserves. Strength you demand.
 POLYSITE LOW PROFILE HYBRID PORTS Comfort your patient deserves. Strength you demand. We never settle because you never settle. Throughout our 35-year history, clinicians and patients have benefited from
POLYSITE LOW PROFILE HYBRID PORTS Comfort your patient deserves. Strength you demand. We never settle because you never settle. Throughout our 35-year history, clinicians and patients have benefited from
Micro-Electrode Arrays (MEA), Micro-Wire Arrays (MWA)
 MicroProbes for Life Science Micro-Electrode Arrays (MEA), Micro-Wire Arrays (MWA) User Instructions The distance between contacts sites could be between 0.1-1.0 mm 2 mm (recommended) Different lengths
MicroProbes for Life Science Micro-Electrode Arrays (MEA), Micro-Wire Arrays (MWA) User Instructions The distance between contacts sites could be between 0.1-1.0 mm 2 mm (recommended) Different lengths
Forefoot. Foot & Ankle. Foot & Ankle. Midfoot. Bone Graft Substitutes Soft Tissue Repair. Rearfoot. Ankle
 Forefoot Foot & Ankle Midfoot Rearfoot Foot & Ankle Bone Graft Substitutes Soft Tissue Repair Ankle Vitoss Vitoss is the #1 selling Synthetic Bone Graft for one good reason... IT WORKS. 1 Human clinical
Forefoot Foot & Ankle Midfoot Rearfoot Foot & Ankle Bone Graft Substitutes Soft Tissue Repair Ankle Vitoss Vitoss is the #1 selling Synthetic Bone Graft for one good reason... IT WORKS. 1 Human clinical
New creative textile construction using innovative braiding technology and materials
 White Paper New creative textile construction using innovative braiding technology and materials DSM and Meister Provide Foundation for Medical Device Design Philippe Gédet, Engineer, Meister & Cie AG
White Paper New creative textile construction using innovative braiding technology and materials DSM and Meister Provide Foundation for Medical Device Design Philippe Gédet, Engineer, Meister & Cie AG
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET Product Name: Polierpaste Cleaning Paste Manufacturer Australian Supplier: New Zealand Supplier: Name: Henke-Sass, Wolf Gmbh Stryker Australia Stryker New Zealand Address: Keltenstraße
MATERIAL SAFETY DATA SHEET Product Name: Polierpaste Cleaning Paste Manufacturer Australian Supplier: New Zealand Supplier: Name: Henke-Sass, Wolf Gmbh Stryker Australia Stryker New Zealand Address: Keltenstraße
ISO INTERNATIONAL STANDARD. Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy
 INTERNATIONAL STANDARD ISO 5832-12 Second edition 2007-05-01 Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy Implants chirurgicaux Matériaux métalliques Partie
INTERNATIONAL STANDARD ISO 5832-12 Second edition 2007-05-01 Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy Implants chirurgicaux Matériaux métalliques Partie
ISO INTERNATIONAL STANDARD. Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy
 INTERNATIONAL STANDARD ISO 5832-12 Second edition 2007-05-01 Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy Implants chirurgicaux Matériaux métalliques Partie
INTERNATIONAL STANDARD ISO 5832-12 Second edition 2007-05-01 Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy Implants chirurgicaux Matériaux métalliques Partie
CapSure Permanent Fixation System
 CapSure Permanent Fixation System Permanent Fixation Redefined Advancing the Fixation Experience Recipient of 2015 SLS Innovations of the Year recognition. SOFT TISSUE REPAIR Right Procedure. Right Product.
CapSure Permanent Fixation System Permanent Fixation Redefined Advancing the Fixation Experience Recipient of 2015 SLS Innovations of the Year recognition. SOFT TISSUE REPAIR Right Procedure. Right Product.
YY Translated English of Chinese Standard: YY NATIONAL STANDARD OF THE
 Translated English of Chinese Standard: YY0847-2011 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF THE YY PEOPLE S REPUBLIC OF CHINA ICS 11.040.99 C 40 YY 0847-2011 Medical endoscopes
Translated English of Chinese Standard: YY0847-2011 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF THE YY PEOPLE S REPUBLIC OF CHINA ICS 11.040.99 C 40 YY 0847-2011 Medical endoscopes
BROCHURE. Explore the CuattroDR Digital Radiography Portfolio
 BROCHURE Explore the CuattroDR Digital Radiography Portfolio Watson Health IBM Corporation 2017 Introduction Updating your radiography equipment doesn t have to mean a full system replacement. With CuattroDR,
BROCHURE Explore the CuattroDR Digital Radiography Portfolio Watson Health IBM Corporation 2017 Introduction Updating your radiography equipment doesn t have to mean a full system replacement. With CuattroDR,
Reinforce with Confidence. PERFORMANCE through innovation
 Reinforce with Confidence PERFORMANCE through innovation Reduces Leaks. Most clinical data literature supporting leak reduction 1 GORE SEAMGUARD Bioabsorbable Staple Line Reinforcement has the most published
Reinforce with Confidence PERFORMANCE through innovation Reduces Leaks. Most clinical data literature supporting leak reduction 1 GORE SEAMGUARD Bioabsorbable Staple Line Reinforcement has the most published
Biological Prostheses
 SAPIENZA UNIVERSITA DI ROMA EHS Dipartimento di Chirurgia Generale Paride Stefanini U.O.C. di Chirurgia Generale D e Day Surgery (Direttore: Prof. P. Negro) PRG M Chirurgia della Parete Addominale (Prof.
SAPIENZA UNIVERSITA DI ROMA EHS Dipartimento di Chirurgia Generale Paride Stefanini U.O.C. di Chirurgia Generale D e Day Surgery (Direttore: Prof. P. Negro) PRG M Chirurgia della Parete Addominale (Prof.
ISO INTERNATIONAL STANDARD. Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy
 INTERNATIONAL STANDARD ISO 5832-12 Second edition 2007-05-01 Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy Implants chirurgicaux Matériaux métalliques Partie
INTERNATIONAL STANDARD ISO 5832-12 Second edition 2007-05-01 Implants for surgery Metallic materials Part 12: Wrought cobalt-chromium-molybdenum alloy Implants chirurgicaux Matériaux métalliques Partie
3034 Owen Drive Antioch, TN symmetrysurgical.com 2018 Symmetry Surgical, Inc.
 Who We Are Symmetry Surgical develops and delivers high-quality surgical instrumentation to healthcare providers around the world. Our portfolio of more than 20,000 instruments includes proven, trusted
Who We Are Symmetry Surgical develops and delivers high-quality surgical instrumentation to healthcare providers around the world. Our portfolio of more than 20,000 instruments includes proven, trusted
Proven reliability. P e r f o r m a n c e t h r o u g h e x p e r i e n c e
 Proven reliability P e r f o r m a n c e t h r o u g h e x p e r i e n c e Proven visceral protection Versatile material easily trimmable to fit defect CORDUROY surface for tissue ingrowth Available with
Proven reliability P e r f o r m a n c e t h r o u g h e x p e r i e n c e Proven visceral protection Versatile material easily trimmable to fit defect CORDUROY surface for tissue ingrowth Available with
RESEARCH REPOSITORY.
 RESEARCH REPOSITORY This is the author s final version of the work, as accepted for publication following peer review but without the publisher s layout or pagination. The definitive version is available
RESEARCH REPOSITORY This is the author s final version of the work, as accepted for publication following peer review but without the publisher s layout or pagination. The definitive version is available
Ventrio ST Hernia Patch featuring Sepra Technology
 Ventrio ST Hernia Patch featuring Sepra Technology Sepra Technology An extensively studied barrier with more than 10 publications and used clinically since 2007. Unique hydrogel barrier swells to minimize
Ventrio ST Hernia Patch featuring Sepra Technology Sepra Technology An extensively studied barrier with more than 10 publications and used clinically since 2007. Unique hydrogel barrier swells to minimize
February 22, Dear Dr. Cox:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Integra LifeSciences Corp.
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Integra LifeSciences Corp.
AS/NZS :2014. Guide to the protection of structural steel against atmospheric corrosion by the use of protective coatings AS/NZS 2312.
 Australian/New Zealand Standard AS/NZS 2312.1:2014 Guide to the protection of structural steel against atmospheric corrosion by the use of protective coatings Part 1: Paint coatings Superseding AS/NZS
Australian/New Zealand Standard AS/NZS 2312.1:2014 Guide to the protection of structural steel against atmospheric corrosion by the use of protective coatings Part 1: Paint coatings Superseding AS/NZS
White Paper: Designing Functional Fabrics for Today s Heart Valves and Beyond
 White Paper: Designing Functional Fabrics for Today s Heart Valves and Beyond Ryan Heniford Business Development Director Peter Gabriele Director, Emerging Technology How fabrics can be used in heart valves,
White Paper: Designing Functional Fabrics for Today s Heart Valves and Beyond Ryan Heniford Business Development Director Peter Gabriele Director, Emerging Technology How fabrics can be used in heart valves,
2018 PHYSICIAN CODING GUIDE ENB PROCEDURE
 2018 PHYSICIAN CODING GUIDE ENB PROCEDURE The following coding scenarios are intended for illustrative purposes only and do not reflect every ENB coding scenario available, therefore, reimbursement will
2018 PHYSICIAN CODING GUIDE ENB PROCEDURE The following coding scenarios are intended for illustrative purposes only and do not reflect every ENB coding scenario available, therefore, reimbursement will
HANDLING OF WRIGHT DISPOSABLE PROPHECY ANKLE INSTRUMENTS
 HANDLING OF WRIGHT DISPOSABLE PROPHECY ANKLE INSTRUMENTS 150870-0 English (en) The following languages are included in this packet: For additional information and translations please contact the manufacturer
HANDLING OF WRIGHT DISPOSABLE PROPHECY ANKLE INSTRUMENTS 150870-0 English (en) The following languages are included in this packet: For additional information and translations please contact the manufacturer
MOBILE OPERATING TABLE BETACLASSIC
 MOBILE OPERATING TABLE BETACLASSIC This document is intended to provide information to an international audience outside of the US. The Gold Standard Surgical Workplaces BETACLASSIC 3 BETACLASSIC ECONOMIC
MOBILE OPERATING TABLE BETACLASSIC This document is intended to provide information to an international audience outside of the US. The Gold Standard Surgical Workplaces BETACLASSIC 3 BETACLASSIC ECONOMIC
Precision in fixation
 For more information, visit Medartis at www.medartis.com or contact us directly: Medartis AG Hochbergerstrasse 60E CH-4057 Basel P +41 61 633 34 34 F +41 61 633 34 00 www.medartis.com Precision in fixation
For more information, visit Medartis at www.medartis.com or contact us directly: Medartis AG Hochbergerstrasse 60E CH-4057 Basel P +41 61 633 34 34 F +41 61 633 34 00 www.medartis.com Precision in fixation
EXTREMITY C-ARM IMAGING. Fluoroscan InSight The Original High Definition Extremity C-arm
 EXTREMITY C-ARM IMAGING Fluoroscan InSight The Original High Definition Extremity C-arm Versatility to Perform Your Extremity Procedures. The new Fluoroscan InSight with IQ Enhanced software delivers superb
EXTREMITY C-ARM IMAGING Fluoroscan InSight The Original High Definition Extremity C-arm Versatility to Perform Your Extremity Procedures. The new Fluoroscan InSight with IQ Enhanced software delivers superb
Biologic Prosthetics & Beyond
 & Beyond & Beyond: Overview Introduction Prosthetics Overview UCSF Postgraduate Course in General Surgery San Francisco, CA May 18, 2013 Review of Clinical Data Summary Recommendations Hobart W. Harris,
& Beyond & Beyond: Overview Introduction Prosthetics Overview UCSF Postgraduate Course in General Surgery San Francisco, CA May 18, 2013 Review of Clinical Data Summary Recommendations Hobart W. Harris,
The technologies described in this document are in development and are not available for clinical use
 R...utilizes a model of direct collaboration with surgeons and interventionalists to develop the next generation of synthetic cardiovascular devices. From expandable vascular grafts to synthetic transcatheter
R...utilizes a model of direct collaboration with surgeons and interventionalists to develop the next generation of synthetic cardiovascular devices. From expandable vascular grafts to synthetic transcatheter
Meso BioMatrix Acellular Peritoneum Matrix RECOMMENDED TECHNIQUE GUIDE. Recreating harmonious bodies for a new vision of rebirth.
 Meso BioMatrix Acellular Peritoneum Matrix RECOMMENDED TECHNIQUE GUIDE Recreating harmonious bodies for a new vision of rebirth. 1 DISCOVER SEBBIN Sebbin is a 30 years-established company with a peerless
Meso BioMatrix Acellular Peritoneum Matrix RECOMMENDED TECHNIQUE GUIDE Recreating harmonious bodies for a new vision of rebirth. 1 DISCOVER SEBBIN Sebbin is a 30 years-established company with a peerless
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 10675-1 First edition 2008-03-01 Non-destructive testing of welds Acceptance levels for radiographic testing Part 1: Steel, nickel, titanium and their alloys Essais non destructifs
INTERNATIONAL STANDARD ISO 10675-1 First edition 2008-03-01 Non-destructive testing of welds Acceptance levels for radiographic testing Part 1: Steel, nickel, titanium and their alloys Essais non destructifs
Orthopaedic Implant Micromotion Sensing Using An Eddy Current Sensor
 Orthopaedic Implant Micromotion Sensing Using An Eddy Current Sensor RAJAS KHOKLE, KARU ESSELLE, MICHAEL HEIMLICH, DESMOND BOKOR DEPARTMENT OF ENGINEERING Faculty of Science and Engineering Motivation
Orthopaedic Implant Micromotion Sensing Using An Eddy Current Sensor RAJAS KHOKLE, KARU ESSELLE, MICHAEL HEIMLICH, DESMOND BOKOR DEPARTMENT OF ENGINEERING Faculty of Science and Engineering Motivation
The Ventrio ST Hernia Patch Featuring Sepra Technology. Efficient:
 Ventrio ST Hernia Patch featuring SEPRA Technology NEW Sepra Technology Based on the technology used in Seprafilm with more than 13 years of proven clinical success. Unique hydrogel barrier swells to minimise
Ventrio ST Hernia Patch featuring SEPRA Technology NEW Sepra Technology Based on the technology used in Seprafilm with more than 13 years of proven clinical success. Unique hydrogel barrier swells to minimise
DermaMatrix Acellular Dermis. Comparative testing.
 DermaMatrix Acellular Dermis. Comparative testing. Natural, like native tissue Minimally processed Strong and flexible DermaMatrix Acellular Dermis. Human dermal collagen matrix. Unique process DermaMatrix
DermaMatrix Acellular Dermis. Comparative testing. Natural, like native tissue Minimally processed Strong and flexible DermaMatrix Acellular Dermis. Human dermal collagen matrix. Unique process DermaMatrix
Neovasc Tissue Products. July 2011
 Neovasc Tissue Products July 2011 Neovasc Inc. Neovasc Inc. is a medical device company focused on products used to treat heart disease and related conditions Founded: 2000 Location: Employees: Facility:
Neovasc Tissue Products July 2011 Neovasc Inc. Neovasc Inc. is a medical device company focused on products used to treat heart disease and related conditions Founded: 2000 Location: Employees: Facility:
Scheduled maintenance and rectification works. Systems integration and comprehensive reporting
 Distribution We have significant experience in asset management, maintenance and construction for overhead and underground electricity distribution networks. This enables us to consistently add-value,
Distribution We have significant experience in asset management, maintenance and construction for overhead and underground electricity distribution networks. This enables us to consistently add-value,
Trabecular Metal Revision Shell. Surgical Technique IMAGE TO COME. The Best Thing Next to Bone
 Trabecular Metal Revision Shell Surgical Technique IMAGE TO COME The Best Thing Next to Bone Trabecular Metal Revision Shell Surgical Technique Initial and Long-Term Stability Fully-interconnected trabecular
Trabecular Metal Revision Shell Surgical Technique IMAGE TO COME The Best Thing Next to Bone Trabecular Metal Revision Shell Surgical Technique Initial and Long-Term Stability Fully-interconnected trabecular
ADVANCING THE ART OF SINGLE-STAGE REPAIR
 ADVANCING THE ART OF SINGLE-STAGE REPAIR PREPERITONEAL PLACEMENT GORE BIO-A WEB PTFE Knit GORE BIO-A WEB OVERVIEW GORE SYNECOR Preperitoneal Biomaterial is designed specifically for preperitoneal, retromuscular
ADVANCING THE ART OF SINGLE-STAGE REPAIR PREPERITONEAL PLACEMENT GORE BIO-A WEB PTFE Knit GORE BIO-A WEB OVERVIEW GORE SYNECOR Preperitoneal Biomaterial is designed specifically for preperitoneal, retromuscular
Proven 1-4, Safe Performance. Product overview
 Proven 1-4, Safe Performance Product overview DePuy Polyethylene: proven 1-4, safe performance Polyethylene has been used as an orthopaedic bearing material for more than 4 years. Over this period of time
Proven 1-4, Safe Performance Product overview DePuy Polyethylene: proven 1-4, safe performance Polyethylene has been used as an orthopaedic bearing material for more than 4 years. Over this period of time
ECHA DECLARATION OF INTEREST
 ECHA DECLARATION OF INTEREST There is a conflict of interest where the impartiality and objectivity of a decision, opinion or recommendation of the Agency and/or its bodies, is or might in the public perception
ECHA DECLARATION OF INTEREST There is a conflict of interest where the impartiality and objectivity of a decision, opinion or recommendation of the Agency and/or its bodies, is or might in the public perception
Computer-Aided Surgical Navigation Coding Guide Neurosurgery. May 1, 2009
 Computer-Aided Surgical Navigation Coding Guide Neurosurgery May 1, 2009 Please direct any questions to: Kim Brew Manager, Reimbursement and Therapy Access Medtronic Surgical Technologies (904) 279-7569
Computer-Aided Surgical Navigation Coding Guide Neurosurgery May 1, 2009 Please direct any questions to: Kim Brew Manager, Reimbursement and Therapy Access Medtronic Surgical Technologies (904) 279-7569
ISO INTERNATIONAL STANDARD. Cardiovascular implants Endovascular devices Part 3: Vena cava filters
 INTERNATIONAL STANDARD ISO 25539-3 First edition 2011-12-01 Cardiovascular implants Endovascular devices Part 3: Vena cava filters Implants cardiovasculaires Dispositifs endovasculaires Partie 3: Filtres
INTERNATIONAL STANDARD ISO 25539-3 First edition 2011-12-01 Cardiovascular implants Endovascular devices Part 3: Vena cava filters Implants cardiovasculaires Dispositifs endovasculaires Partie 3: Filtres
2 stryker 2010 annual review. Dear Shareholders,
 2 stryker 2010 annual review Dear Shareholders, You can measure a company s performance in countless ways. You can slice financial data into earnings, margins and cash flow, or you can track dividends
2 stryker 2010 annual review Dear Shareholders, You can measure a company s performance in countless ways. You can slice financial data into earnings, margins and cash flow, or you can track dividends
EXTREMITY C-ARM IMAGING. Fluoroscan InSight The Original High Definition Extremity C-arm
 EXTREMITY C-ARM IMAGING Fluoroscan InSight The Original High Definition Extremity C-arm Versatility to Perform Your Extremity Procedures. The new Fluoroscan InSight with IQ Enhanced software delivers superb
EXTREMITY C-ARM IMAGING Fluoroscan InSight The Original High Definition Extremity C-arm Versatility to Perform Your Extremity Procedures. The new Fluoroscan InSight with IQ Enhanced software delivers superb
SurgiMend. Product Summary
 SurgiMend Collagen Matrix for Soft Tissue Reconstruction Product Summary Because we are committed to limiting uncertainty, Integra offers SurgiMend in 1, 2, 3 or 4 mm thicknesses and in over sixty configurations,
SurgiMend Collagen Matrix for Soft Tissue Reconstruction Product Summary Because we are committed to limiting uncertainty, Integra offers SurgiMend in 1, 2, 3 or 4 mm thicknesses and in over sixty configurations,
Medi-Therm Precise. Easy. Versatile.
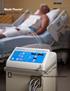 Medi-Therm Precise. Easy. Versatile. Medi-Therm Hyper/Hypothermia System Patient warming and cooling are often critical factors in achieving positive outcomes. The Stryker Medi-Therm system can be used
Medi-Therm Precise. Easy. Versatile. Medi-Therm Hyper/Hypothermia System Patient warming and cooling are often critical factors in achieving positive outcomes. The Stryker Medi-Therm system can be used
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 14607 Third edition 2018-04 Corrected version 2018-08 Non-active surgical implants Mammary implants Particular requirements Implants chirurgicaux non actifs Implants mammaires
INTERNATIONAL STANDARD ISO 14607 Third edition 2018-04 Corrected version 2018-08 Non-active surgical implants Mammary implants Particular requirements Implants chirurgicaux non actifs Implants mammaires
