REGISTRATION DOCUMENT FOR RECOMBINANT DNA RESEARCH
|
|
|
- Grace Perkins
- 6 years ago
- Views:
Transcription
1 EHRS Date Received: Reg. Doc. No.: REGISTRATION DOCUMENT FOR RECOMBINANT DNA RESEARCH Principal Investigator: Penn ID#: Position Title: School: Department: Mailing Address: Mail Code: Telephone: FAX: Date of Request: Location of lab(s): PROJECT INFORMATION A. Project Title: B. Names of individuals participating in this project: Name Penn ID C. Provide a brief description of proposed research: D. Attach copy of (grant) abstract. E. DUAL USE RESEARCH Check any categories below that apply to your project: Renders a useful vaccine ineffective Adds antibiotic resistance affecting response to a clinically useful drug Enhances pathogen virulence Increases pathogen transmissibility Widens a pathogen s host range Lets a pathogen evade diagnostic or detection modalities Weaponization (e.g., environmental stabilization of pathogens) Check here if none of the above apply TRAINING A. Have you read the most current NIH guidelines for research involving rdna? No Yes B. Have the PI and ALL personnel participating in this research completed Penn s Online Recombinant DNA Training? No Yes C. Are you knowledgeable about the appropriate Biosafety Level for this project: No Yes
2 NIH GUIDELINES SECTION III This section describes experiments covered by the NIH Guidelines. Check the appropriate registration category(s) for your experiment: (Note: No research may be initiated for categories A through D below until ALL required approvals are received.) III-A. Experiments that Require Institutional Biosafety Committee Approval, RAC Review, and NIH Director Approval Before Initiation. 1. Major Actions (see Section IV-C-1-b-(1) of the NIH guidelines). 1a. Deliberate transfer of drug resistance trait to microorganisms that are unknown to acquire the trait naturally, if such acquisition could compromise use of the drug to control disease agents in humans, veterinary medicine or agriculture. III-B. Experiments that Require NIH/OBA and Institutional Biosafety Committee Approval Before Initiation. 1. Experiments Involving the Cloning of Toxin Molecules with LD50 of Less than 100 Nanograms Per Kilogram Body Weight. III-C. Experiments that Require Institutional Biosafety Committee and Institutional Review Board Approvals and NIH/OBA Registration Before Initiation 1. Experiments Involving the Deliberate Transfer of Recombinant DNA or DNA or RNA Derived from Recombinant DNA into One or More Human Subjects (human gene transfer). III-D. Experiments that Require Institutional Biosafety Committee Approval Before Initiation 1. Experiments Using Risk Group 2, Risk Group 3, Risk Group 4 or Restricted Agents as Host-Vector Systems. 2. Experiments in which DNA from Risk Group 2, Risk Group 3, Risk Group 4, or Restricted Agents is Cloned into Nonpathogenic Prokaryotic or Lower Eukaryotic Host-Vector Systems. 3. Experiments Involving the Use of Infectious DNA or RNA Viruses or Defective DNA or RNA Viruses in the Presence of Helper Virus in Tissue Culture Systems. 4. Experiments Involving Whole Animals. (Do NOT check if ONLY generating or crossing transgenic rodents [III-E-3].) 5. Experiments Involving Whole Plants. 6. Experiments Involving More than 10 Liters of Culture. 7. Experiments Involving Influenza Viruses. (Consult with EHRS for guidance. BSL-3 containment may apply.) III-E. Experiments that Require Institutional Biosafety Committee Notice Simultaneous with Initiation. 1. Experiments Involving the Formation of Recombinant DNA Molecules Containing No More than Two-Thirds of the Genome of any Eukaryotic Virus. 2. Experiments Involving Whole Plants 3. Experiments Involving Transgenic Rodents This registration is for (check the one section that applies): CROSSING two different transgenic rodent lines to produce a new transgenic strain Fill out Section 1, ONLY CREATING transgenic rodents Fill out Section 2, ONLY GENERATION of rdna Fill out Section 3, ONLY USE of rdna (including rdna received from Vector Core, gifted, etc.) Fill out Section 4, ONLY Both GENERATION and USE of rdna Fill out Section 5, ONLY
3 SIGNATURE PAGE Your signature below indicates that you acknowledge all requirements and restrictions of the most current NIH guidelines for the Biosafety Level you have indicated above, unless modified by the IBC; that you accept responsibility for the safe conduct of the experiments conducted at this Biosafety Level; and that you have informed all associated personnel of the conditions required for this work. Signature of Principal Investigator: Date: Sponsorship (*Required only if investigator is not a member of the Standing or Associated Faculty) Faculty Sponsor* (PRINT): Faculty Sponsor* (SIGNATURE): Date: --DO NOT WRITE BELOW THIS LINE-- Comments: IBC ACTION Acceptance Exemption Rejection Date: Signature of IBC Representative: Print Name:
4 SECTION 1. CROSSING TRANSGENIC RODENTS Complete this section if you are breeding two different transgenic rodent strains to generate a new transgenic strain. Example: Breeding of knockouts from two different transgenic strains. Existing Transgenic Line A Existing Transgenic Line B Newly Bred Line C Genotype of New Transgenic BIOSAFETY CONTAINMENT LEVEL A. This project will be conducted at Biosafety Level: B. This project will be conducted at Animal Biosafety Level: N/A 1 2 3
5 SECTION 2. CREATING TRANSGENIC RODENTS Complete this section if you are using rdna ONLY to create transgenic rodents. It is not necessary to fill out any of the other sections (DO NOT fill out any generation or use sections). Example: Creating any transgenic rodent. A. Genus, species, of parent strain: B. Transgenic strain identification: TRANSGENE A. Specify the nature of the gene sequence inserted into the recombinant vector: Promoter Gene Name Source of gene (genus, species) Biological Activity of Sequence B. If any of the above genes are from a viral source, is it more than 2/3 of the viral genome? No Yes, specify: C. Will a deliberate attempt be made to obtain expression of the foreign gene encode in the recombinant DNA or RNA? No Yes D. Describe the method of gene transfer: BIOSAFETY CONTAINMENT LEVEL C. This project will be conducted at Biosafety Level: D. This project will be conducted at Animal Biosafety Level: N/A 1 2 3
6 SECTION 3. GENERATION OF rdna Complete this section if you are generating rdna materials in your laboratory, but are NOT using them. Example: You generate an rdna vector for a collaborating researcher. TRANSGENE A. Specify the nature of the gene sequence inserted into the recombinant vector: Promoter Gene Name Source of gene (genus, species) Biological Activity of Sequence B. If any of the above genes are from a viral source, is it more than 2/3 of the viral genome? No Yes, specify: HOST-VECTOR SYSTEM A. Identify name of vector: B. Identify vector system: Naked DNA or RNA Bacterial Plasmid...PLEASE ATTACH MAP(S) OF PLASMID. Viral Vector...PLEASE ATTACH MAP(S) OF EXPRESSION CASSETTE. Adeno-associated virus (AAV) Adenovirus Lentivirus Identify generation of vector system: Retrovirus Other Describe: C. List host cell line or packaging cells for recombinant vector propagation: D. If this is a viral vector system: 1. What % of the viral genome remains: 2. Is this vector replication competent? No Yes 3. Is a helper virus required for replication? No Yes, specify: BIOSAFETY CONTAINMENT LEVEL A. This project will be conducted at Biosafety Level: B. This project will be conducted at Animal Biosafety Level: N/A 1 2 3
7 SECTION 4. USE OF rdna Complete this section if you are using rdna materials in your laboratory. This includes all rdna constructs that you have received from another source. Example: The Vector Core or collaborator from another institution makes an rdna construct for your lab and you will be using it in tissue culture, animals, etc. TARGET RECIPIENT Indicate the recipient(s) of the rdna (check all that apply). Animal only (specify species and if mouse, strain): Tissue Culture only (specify cell line name and source): Modified tissue culture cell lines into animals Specify cell line name and source: Specify animal species/mouse strain: Plant cells: Plants: Gene therapy, specify target host (s): Human Animal species/mouse strain: DNA vaccine, specify target recipients (s): Human Animal species/mouse strain: RECOMBINANT MATERIAL A. Identify name of vector: HIV based lentiviral vector B. Type of vector: Naked DNA or RNA Bacterial Plasmid...PLEASE ATTACH MAP(S) OF PLASMID. Viral Vector...PLEASE ATTACH MAP(S) OF EXPRESSION CASSETTE. Adeno-associated virus (AAV) Adenovirus Lentivirus Identify generation of vector system: second Retrovirus Other Describe: C. List host cell line or packaging cells for recombinant vector propagation:hek293t D. If this is a viral vector: 1) What % of the viral genome remains: 28% 2) Is this vector replication competent? No Yes
8 TRANSGENE A. Specify the nature of the gene sequence inserted into the recombinant vector: Gene Name Source of gene (genus, species) Biological Activity of Sequence egfp Jellyfish. Aequorea victoria enhanced green fluorescent protein cdna; used as a reporter gene; protein exhibits bright green fluorescence when exposed to blue light CRE Bacteriophage P1 cre recombinase cdna; encodes a type I topoisomerase from P1 bacteriophage that catalyzes site-specific recombination of DNA between loxp sites CFTR human cystic fibrosis transmembrane regulator cdna; encodes a ABC transporter-class ion channel that transports chloride and thiocyanate ions across epithelial cell membranes. Mutations of the CFTR gene affect functioning of the chloride ion channels in these cell membranes, leading to cystic fibrosis and congenital absence of the vas deferens B. If any of the above genes are from a viral source, is it more than 2/3 of the viral genome? No Yes, specify: C. Will a deliberate attempt be made to obtain expression of the foreign gene encoded in the recombinant DNA or RNA? No Yes BIOSAFETY CONTAINMENT LEVEL A. This project will be conducted at Biosafety Level: B. This project will be conducted at Animal Biosafety Level: N/A 1 2 3
9 SECTION 5. Both GENERATION and USE OF rdna Complete this section if you are both generating and using rdna in your laboratory. Example: You generate an rdna construct and use it in tissue culture, animals, etc. TRANSGENE A. Specify the nature of the gene sequence inserted into the recombinant vector: Promoter Gene Name Source of gene (genus, species) Biological Activity of Sequence B. If any of the above genes are from a viral source, is it more than 2/3 of the viral genome? No Yes, specify: C. Will a deliberate attempt be made to obtain expression of the foreign gene encoded in the recombinant DNA or RNA? No Yes HOST-VECTOR SYSTEM A. Identify name of vector: B. Identify vector system: Naked DNA or RNA Bacterial Plasmid Viral Vector Adeno-associated virus (AAV) Adenovirus Lentivirus Identify generation of vector system: Retrovirus Other Describe: C. List host cell line or packaging cells for recombinant vector propagation: D. If this is a viral vector system: 1. What % of the viral genome remains: 2. Is this vector replication competent? No Yes E. Is a helper virus required for replication? No Yes, specify:
10 TARGET RECIPIENT Indicate the recipient(s) of the rdna (check all that apply). Animal only (specify species and if mouse, strain): Tissue Culture only (specify cell line name and source): Tissue culture cell lines into animals Specify cell line name and source: Specify animal species/mouse strain: Plant cells: Plants: Gene therapy, specify target host (s): Human Animal species/mouse strain: DNA vaccine, specify target recipients (s): Human Animal species/mouse strain: BIOSAFETY CONTAINMENT LEVEL A. This project will be conducted at Biosafety Level: B. This project will be conducted at Animal Biosafety Level: N/A 1 2 3
REGISTRATION DOCUMENT FOR RECOMBINANT & SYNTHETIC DNA RESEARCH
 IBC Date Received: Reg. Doc. No.: REGISTRATION DOCUMENT FOR RECOMBINANT & SYNTHETIC DNA RESEARCH Principal Investigator: --------------------------------------- Position Title: -------------- Department:
IBC Date Received: Reg. Doc. No.: REGISTRATION DOCUMENT FOR RECOMBINANT & SYNTHETIC DNA RESEARCH Principal Investigator: --------------------------------------- Position Title: -------------- Department:
University of Delaware Department of Environmental Health & Safety Recombinant DNA Registration
 Registration #: University of Delaware Department of Environmental Health & Safety Recombinant DNA Registration Directions: Please complete this form to register recombinant DNA research with the University
Registration #: University of Delaware Department of Environmental Health & Safety Recombinant DNA Registration Directions: Please complete this form to register recombinant DNA research with the University
NIH Guidelines for rdna Research: Tutorial for Completing Penn s rdna Registration Document
 NIH Guidelines for rdna Research: Tutorial for Completing Penn s rdna Registration Document Revision March 3, 2011 Penn s rdna Registration Document Dual Use Research Definition: Research that yields information
NIH Guidelines for rdna Research: Tutorial for Completing Penn s rdna Registration Document Revision March 3, 2011 Penn s rdna Registration Document Dual Use Research Definition: Research that yields information
Registration Document For Biohazards
 Protocol #: Registration Document For Biohazards All applicants are required to complete the following sections: Principal Investigator Information Location of Study Section A: General Administrative Information
Protocol #: Registration Document For Biohazards All applicants are required to complete the following sections: Principal Investigator Information Location of Study Section A: General Administrative Information
Tutorial for Completing Penn s r s DNA Registration Document
 NIH GUIDELINES FOR RESEARCH INVOLVING RECOMBINANT OR SYNTHETIC NUCLEIC ACID MOLECULES NIH GUIDELINES Tutorial for Completing Penn s r s DNA Registration Document Revision March 7, 2013 Penn s r s DNA Registration
NIH GUIDELINES FOR RESEARCH INVOLVING RECOMBINANT OR SYNTHETIC NUCLEIC ACID MOLECULES NIH GUIDELINES Tutorial for Completing Penn s r s DNA Registration Document Revision March 7, 2013 Penn s r s DNA Registration
Instructions and Certifications (Failure to follow may result in a delay in processing) ORC Use Only. Date Received: IBC Determination:
 ORC Use Only Date Received: IBC Determination: Exempt Experiment Determination for Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines); Registration for Experiments that Require Institutional
ORC Use Only Date Received: IBC Determination: Exempt Experiment Determination for Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines); Registration for Experiments that Require Institutional
Biosafety and the NIH Guidelines
 Biosafety and the NIH Guidelines This section will explore: Why the NIH Guidelines are important The definition of recombinant or synthetic nucleic acid research Content of the NIH Guidelines Section III
Biosafety and the NIH Guidelines This section will explore: Why the NIH Guidelines are important The definition of recombinant or synthetic nucleic acid research Content of the NIH Guidelines Section III
Biological Research Registration Form
 Biological Research Registration Form The University of Oregon requires Institutional Biosafety Committee review and approval of research involving recombinant or synthetic nucleic acids (rsna), organisms
Biological Research Registration Form The University of Oregon requires Institutional Biosafety Committee review and approval of research involving recombinant or synthetic nucleic acids (rsna), organisms
Safe Operating Procedure
 Safe Operating Procedure RECOMBINANT OR SYNTHETIC NUCLEIC ACIDS IBC AND OTHER REVIEW REQUIREMENTS (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) (Revised
Safe Operating Procedure RECOMBINANT OR SYNTHETIC NUCLEIC ACIDS IBC AND OTHER REVIEW REQUIREMENTS (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) (Revised
BIOSAFETY REGISTRATION FORM
 BRF 10/2015 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax: (215) 707-9100, Email: ibc@temple.edu research.temple.edu/research-compliance
BRF 10/2015 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax: (215) 707-9100, Email: ibc@temple.edu research.temple.edu/research-compliance
SECTION I CITIZENSHIP: CITIZENSHIP: SECTION II
 APPLICATION FOR INSTITUTIONAL BIOSAFETY COMMITTEE REVIEW AND APPROVAL South Dakota School of Mines and Technology (Protocol Submission Form) This form must be submitted for ALL research or teaching activities
APPLICATION FOR INSTITUTIONAL BIOSAFETY COMMITTEE REVIEW AND APPROVAL South Dakota School of Mines and Technology (Protocol Submission Form) This form must be submitted for ALL research or teaching activities
BIOSAFETY REGISTRATION FORM
 BRF 5/2015 Page 1 of 5 COMMITTEE USE ONLY 1. PERSONNEL TEMPLE UNIVERSITY Office of the Vice Provost for Research Division of Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax:
BRF 5/2015 Page 1 of 5 COMMITTEE USE ONLY 1. PERSONNEL TEMPLE UNIVERSITY Office of the Vice Provost for Research Division of Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax:
Please refer to The University of Chicago Biosafety Manual for supplementary information about the concepts presented in this training module.
 1 Please refer to The University of Chicago Biosafety Manual for supplementary information about the concepts presented in this training module. http://biologicalsafety.uchicago.edu/page/university-chicago-biosafety-manual
1 Please refer to The University of Chicago Biosafety Manual for supplementary information about the concepts presented in this training module. http://biologicalsafety.uchicago.edu/page/university-chicago-biosafety-manual
National Institutes of Health (NIH)
 Recombinant DNA (rdna) is regulated by the National Institutes of Health (NIH). LCSC, along with all other institutions which use rdna in research, is required to provide training to the researchers who
Recombinant DNA (rdna) is regulated by the National Institutes of Health (NIH). LCSC, along with all other institutions which use rdna in research, is required to provide training to the researchers who
COMMITTEE USE ONLY IBC REGISTRATION
 BRF September 2016 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # ASSOCIATED ACUP/IRB# Research Integrity and Compliance Institutional Biosafety Committee APPROVAL BIOSAFETY REGISTRATION FORM Tel: (215)
BRF September 2016 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # ASSOCIATED ACUP/IRB# Research Integrity and Compliance Institutional Biosafety Committee APPROVAL BIOSAFETY REGISTRATION FORM Tel: (215)
The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines)
 The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) Northern Arizona University Office of Regulatory Compliance Shelley Jones, Director of Biological
The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) Northern Arizona University Office of Regulatory Compliance Shelley Jones, Director of Biological
Application for Research Involving Biological Materials and Recombinant DNA
 BATES COLLEGE Institutional Biosafety Committee Application for Research Involving Biological Materials and Recombinant DNA INSTRUCTIONS: All submissions must be typed. E-mail completed applications to
BATES COLLEGE Institutional Biosafety Committee Application for Research Involving Biological Materials and Recombinant DNA INSTRUCTIONS: All submissions must be typed. E-mail completed applications to
Calvin College Biosafety Application
 SECTION 1: GENERAL INFORMATION Calvin College Biosafety Application Applicant Name: Campus Address: Email Address: Campus Phone #: Project Title: APPLICATION TYPE: Research Teaching Course #(s) PROTOCOL
SECTION 1: GENERAL INFORMATION Calvin College Biosafety Application Applicant Name: Campus Address: Email Address: Campus Phone #: Project Title: APPLICATION TYPE: Research Teaching Course #(s) PROTOCOL
Registration for the Use of Biological Materials
 Form 01: Please return to: Registration for the Use of Biological Materials Environmental Health and Safety (EHS) 135 College St., Suite 100 Tel. 737-2121 Fax 785-7588 OEHS Use Only BSL: BBP State Select
Form 01: Please return to: Registration for the Use of Biological Materials Environmental Health and Safety (EHS) 135 College St., Suite 100 Tel. 737-2121 Fax 785-7588 OEHS Use Only BSL: BBP State Select
Recombinant or Synthetic Nucleic Acid Molecules
 Overview of the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules A scientificallyresponsive
Overview of the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules A scientificallyresponsive
Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Study Update / Change Request
 Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Study Update / Change Request 1 Submit the renewal electronically via email attachment to: IBCOFFICE@swmail.sw.org 2 Fax a Signed copy
Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Study Update / Change Request 1 Submit the renewal electronically via email attachment to: IBCOFFICE@swmail.sw.org 2 Fax a Signed copy
GVSU BIOSAFETY APPLICATION
 SECTION 1: GENERAL INFORMATION GVSU BIOSAFETY APPLICATION Applicant Name: Campus Address: Email Address: Campus Phone #: Project Title: APPLICATION TYPE: Research Teaching Course #( s) PROTOCOL TYPE: New
SECTION 1: GENERAL INFORMATION GVSU BIOSAFETY APPLICATION Applicant Name: Campus Address: Email Address: Campus Phone #: Project Title: APPLICATION TYPE: Research Teaching Course #( s) PROTOCOL TYPE: New
HOW CAN WE FACILITATE UNDERSTANDING OF THE r-dna GUIDELINES TO THE SCIENTIFIC COMMUNITY? Esmeralda Meyer Kalpana Rengarajan Patty Olinger
 HOW CAN WE FACILITATE UNDERSTANDING OF THE r-dna GUIDELINES TO THE SCIENTIFIC COMMUNITY? Esmeralda Meyer Kalpana Rengarajan Patty Olinger October 13, 2015 Topics 1. Objective of the NIH Guidelines for
HOW CAN WE FACILITATE UNDERSTANDING OF THE r-dna GUIDELINES TO THE SCIENTIFIC COMMUNITY? Esmeralda Meyer Kalpana Rengarajan Patty Olinger October 13, 2015 Topics 1. Objective of the NIH Guidelines for
Yale University Biological Safety Committee
 EHS Use Only Protocol#: Yale University Biological Safety Committee Send original to: Yale Environmental Health and Safety Occupational Health and Safety Section 135 College Street, Suite 100 New Haven,
EHS Use Only Protocol#: Yale University Biological Safety Committee Send original to: Yale Environmental Health and Safety Occupational Health and Safety Section 135 College Street, Suite 100 New Haven,
Skidmore College Institutional Biosafety Committee (IBC) Protocol Registration Form
 Skidmore College Institutional Biosafety Committee (IBC) Protocol Registration Form Please return completed form to Loretta Greenholtz, 424 Palamountain Hall. IBC Reg. No: Risk Group: General Instructions:
Skidmore College Institutional Biosafety Committee (IBC) Protocol Registration Form Please return completed form to Loretta Greenholtz, 424 Palamountain Hall. IBC Reg. No: Risk Group: General Instructions:
Appendix 4 (3208) USUHS Institutional Biosafety Committee (IBC) Project Registration
 Appendix 4 (3208) USUHS Institutional Biosafety Committee (IBC) Project Registration Please use the attached application for requesting a review and approval of activities involving recombinant DNA (rdna)
Appendix 4 (3208) USUHS Institutional Biosafety Committee (IBC) Project Registration Please use the attached application for requesting a review and approval of activities involving recombinant DNA (rdna)
IBC protocol Risk Assessment and Determination of NIH Guidelines
 IBC protocol Risk Assessment and Determination of NIH Guidelines The following are points to consider when reviewing all protocols for risk, recommended containment conditions, and determine applicable
IBC protocol Risk Assessment and Determination of NIH Guidelines The following are points to consider when reviewing all protocols for risk, recommended containment conditions, and determine applicable
Syracuse University Institutional Biosafety Committee Protocol Application Form
 Syracuse University Institutional Biosafety Committee Protocol Application Form The Syracuse University Institutional Biosafety Committee (IBC) has been established to protect the health of University
Syracuse University Institutional Biosafety Committee Protocol Application Form The Syracuse University Institutional Biosafety Committee (IBC) has been established to protect the health of University
Instructions for Biosafety Application Research and Sponsored Programs 201J University Hall Wright State University Dayton, OH (937)
 Instructions for Biosafety Application Research and Sponsored Programs 201J University Hall Wright State University Dayton, OH 45435 (937) 775-2425 Please use the attached application for requesting a
Instructions for Biosafety Application Research and Sponsored Programs 201J University Hall Wright State University Dayton, OH 45435 (937) 775-2425 Please use the attached application for requesting a
Recombinant DNA and Research with Animals
 Recombinant DNA and Research with Animals Recombinant DNA and Research with Animals Jan V. Parker-Thornburg, Ph.D. Director, Transgenic Animal Core Facility MD Anderson Cancer Center Stephen H. Hughes,
Recombinant DNA and Research with Animals Recombinant DNA and Research with Animals Jan V. Parker-Thornburg, Ph.D. Director, Transgenic Animal Core Facility MD Anderson Cancer Center Stephen H. Hughes,
Biosafety Training NIH Guidelines for Research Involving Recombinant DNA. About the Course. Who Should Complete this Course?
 Biosafety Training NIH Guidelines for Research Involving Recombinant DNA The University of Iowa Date(s) Revised: 01/2012 NIH GUIDELINES FOR RESEARCH INVOLVING RECOMBINANT DNA MOLECULES (NIH GUIDELINES)
Biosafety Training NIH Guidelines for Research Involving Recombinant DNA The University of Iowa Date(s) Revised: 01/2012 NIH GUIDELINES FOR RESEARCH INVOLVING RECOMBINANT DNA MOLECULES (NIH GUIDELINES)
Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Initial Application
 Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Initial Application 1 Submit the renewal electronically via email attachment to: IBCOFFICE@swmail.sw.org 2 Submit the Investigator s
Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Initial Application 1 Submit the renewal electronically via email attachment to: IBCOFFICE@swmail.sw.org 2 Submit the Investigator s
NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules
 NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules Department of Environment, Health and Safety 10/2017 This is the print version of the Recombinant or Synthetic Nucleic
NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules Department of Environment, Health and Safety 10/2017 This is the print version of the Recombinant or Synthetic Nucleic
What goes into my Biological Inventory?
 What goes into my Biological Inventory? What Information is Required for an Effective Risk Assessment? According to the Laboratory Biosafety Guidelines (2004), the risk group of an organism is determined
What goes into my Biological Inventory? What Information is Required for an Effective Risk Assessment? According to the Laboratory Biosafety Guidelines (2004), the risk group of an organism is determined
VECTOR SAFETY INFORMATION. AAV Vectors: Material Information
 VECTOR SAFETY INFORMATION AAV Vectors: Material Information AAV vectors contain recombinant transgene sequences (e.g. encoding reporter or therapeutic genes) flanked by the AAV inverted terminal repeats
VECTOR SAFETY INFORMATION AAV Vectors: Material Information AAV vectors contain recombinant transgene sequences (e.g. encoding reporter or therapeutic genes) flanked by the AAV inverted terminal repeats
Overview of the NIH Guidelines for Research Involving Recombinant DNA Molecules
 Overview of the NIH Guidelines for Research Involving Recombinant DNA Molecules A scientifically responsive document that will continue to evolve Has undergone multiple revisions since 1976 Latest version
Overview of the NIH Guidelines for Research Involving Recombinant DNA Molecules A scientifically responsive document that will continue to evolve Has undergone multiple revisions since 1976 Latest version
INSTITUTIONAL BIOSAFETY COMMITTEE
 IBC Proposal No.: IBC- Recombinant DNA: Infectious agents: Toxins or Exempt- Containment Level tumorigenic material: Covered by Section III.C Section III.D. Section III.E. INSTITUTIONAL BIOSAFETY COMMITTEE
IBC Proposal No.: IBC- Recombinant DNA: Infectious agents: Toxins or Exempt- Containment Level tumorigenic material: Covered by Section III.C Section III.D. Section III.E. INSTITUTIONAL BIOSAFETY COMMITTEE
University of Lethbridge GUIDELINES FOR USE OF ADENO-ASSOCIATED VIRAL VECTORS (AAV VECTORS)
 GUIDELINES FOR USE OF ADENO-ASSOCIATED VIRAL VECTORS (AAV VECTORS) Abstract All laboratories using AAV vectors must adhere to this code of practice December 2013 Purpose: To provide guidelines for safely
GUIDELINES FOR USE OF ADENO-ASSOCIATED VIRAL VECTORS (AAV VECTORS) Abstract All laboratories using AAV vectors must adhere to this code of practice December 2013 Purpose: To provide guidelines for safely
Touro University of California Mare Island, Vallejo
 Touro University of California Mare Island, Vallejo Institutional Biosafety Committee INITIAL REVIEW FORM Date Received: For IBC use only: Exempt Approved Approved with contingency(ies) Revisions required
Touro University of California Mare Island, Vallejo Institutional Biosafety Committee INITIAL REVIEW FORM Date Received: For IBC use only: Exempt Approved Approved with contingency(ies) Revisions required
18.0 INSTITUTIONAL BIOSAFETY COMMITTEE
 18.0 INSTITUTIONAL BIOSAFETY COMMITTEE 18.1 INTRODUCTION... 1 18.2 BIOLOGICAL SAFETY COMMITTEE SCOPE AND MISSION STATEMENT... 1 18.3 DEFINITION OF A BIOLOGICAL AGENT... 2 18.4 REVIEW CLASSIFICATION...
18.0 INSTITUTIONAL BIOSAFETY COMMITTEE 18.1 INTRODUCTION... 1 18.2 BIOLOGICAL SAFETY COMMITTEE SCOPE AND MISSION STATEMENT... 1 18.3 DEFINITION OF A BIOLOGICAL AGENT... 2 18.4 REVIEW CLASSIFICATION...
Institutional Biosafety Committee. Policies and Procedures
 Institutional Biosafety Committee Policies and Procedures September 2011 CONTENTS 1. INTRODUCTION 1.1 UNLV Institutional Biosafety Committee Roles and Responsibilities 1.2 The Foundation for IBC Review:
Institutional Biosafety Committee Policies and Procedures September 2011 CONTENTS 1. INTRODUCTION 1.1 UNLV Institutional Biosafety Committee Roles and Responsibilities 1.2 The Foundation for IBC Review:
WHO SHOULD FILL THE FORM This page need not be submitted only for reading
 Project Categories WHO SHOULD FILL THE FORM This page need not be submitted only for reading 1) Category I: Routine rdna projects that do not need elaboration: Experiments involving non-pathogenic and
Project Categories WHO SHOULD FILL THE FORM This page need not be submitted only for reading 1) Category I: Routine rdna projects that do not need elaboration: Experiments involving non-pathogenic and
Biosafety Level Host Range Propagation Comments
 Guidelines BSL for Commonly used Viral Vectors Version 1.0 Office of Animal Care and Institutional Biosafety (OACIB) 1737 West Polk Street (MC 672) 206 Administrative Office Building Chicago, IL 60612
Guidelines BSL for Commonly used Viral Vectors Version 1.0 Office of Animal Care and Institutional Biosafety (OACIB) 1737 West Polk Street (MC 672) 206 Administrative Office Building Chicago, IL 60612
Cornell University. Charge to the Institutional Biosafety Committee
 Cornell University Charge to the Institutional Biosafety Committee AUTHORIZATION Cornell University shall have an Institutional Biological Safety Committee established under the authority of The Office
Cornell University Charge to the Institutional Biosafety Committee AUTHORIZATION Cornell University shall have an Institutional Biological Safety Committee established under the authority of The Office
3. At least one individual with expertise in animal containment practices.
 Title: Approval Date: May 15, 2015 Effective Date: May 15, 2015 A. Purpose The (IBC) reviews recombinant or synthetic nucleic acid molecules research (r/s DNA) conducted at or sponsored by the University
Title: Approval Date: May 15, 2015 Effective Date: May 15, 2015 A. Purpose The (IBC) reviews recombinant or synthetic nucleic acid molecules research (r/s DNA) conducted at or sponsored by the University
Biosafety Committees and Biological Materials Oversight: Past, Present and Future for Clinical Research
 Biosafety Committees and Biological Materials Oversight: Past, Present and Future for Clinical Research HealthCare Compliance Association / 06-03-2014 Chris Jenkins, PhD, MPH, RBP, CHMM Overview Biosafety
Biosafety Committees and Biological Materials Oversight: Past, Present and Future for Clinical Research HealthCare Compliance Association / 06-03-2014 Chris Jenkins, PhD, MPH, RBP, CHMM Overview Biosafety
Bacteria Reproduce Asexually via BINARY FISSION
 An Introduction to Microbial Genetics Today: Intro to Microbial Genetics Lunch pglo! Bacteria Reproduce Asexually via BINARY FISSION But, Bacteria still undergo GENETIC RECOMBINATION (combining DNA from
An Introduction to Microbial Genetics Today: Intro to Microbial Genetics Lunch pglo! Bacteria Reproduce Asexually via BINARY FISSION But, Bacteria still undergo GENETIC RECOMBINATION (combining DNA from
Microbial Biotechnology agustin krisna wardani
 Microbial Biotechnology agustin krisna wardani 1. The Structure of Microbes Microbes (microorganisms) are tiny organisms that are too small to be seen individually by the naked eye and must be viewed with
Microbial Biotechnology agustin krisna wardani 1. The Structure of Microbes Microbes (microorganisms) are tiny organisms that are too small to be seen individually by the naked eye and must be viewed with
REQUEST TO USE INFECTIOUS AGENTS Yale Biological Safety Committee
 EHS Protocol #: Send original to: Yale Environmental Health and Safety 135 College Street, Suite 100 New Haven, CT 06510 Phone Fax: 203-785-7588 REQUEST TO USE INFECTIOUS AGENTS Yale Biological Safety
EHS Protocol #: Send original to: Yale Environmental Health and Safety 135 College Street, Suite 100 New Haven, CT 06510 Phone Fax: 203-785-7588 REQUEST TO USE INFECTIOUS AGENTS Yale Biological Safety
Southeast Missouri State University Biosafety Form
 Southeast Missouri State University Biosafety Form Shaded Areas for EHSC Use Only Form Reviewed by: Date: Form Routed to: Date: 1. Protocol Title: 2. Principal Investigator E-mail Address Department Title
Southeast Missouri State University Biosafety Form Shaded Areas for EHSC Use Only Form Reviewed by: Date: Form Routed to: Date: 1. Protocol Title: 2. Principal Investigator E-mail Address Department Title
The Molecular Virology Support Core: Adenoviral Vectors and Beyond
 The : Adenoviral Vectors and Beyond Christoph A. Kahl, Ph.D. Viral Vector Workshop, May 5th, 2011 Outline 1) Overview of the MVSC 2) Adenoviral Vectors 3) Expertise and Services Overview What does the
The : Adenoviral Vectors and Beyond Christoph A. Kahl, Ph.D. Viral Vector Workshop, May 5th, 2011 Outline 1) Overview of the MVSC 2) Adenoviral Vectors 3) Expertise and Services Overview What does the
APPENDIX D - BIOLOGICAL ASSESSMENT QUESTIONNAIRE & BIOPROCESS SAFETY CHECKLIST
 Guidelines for Process Safety in Bioprocess Manufacturing Facilities by Center for Chemical Process Safety Copyright 2011 American Institute of Chemical Engineers, Inc. APPENDIX D - BIOLOGICAL ASSESSMENT
Guidelines for Process Safety in Bioprocess Manufacturing Facilities by Center for Chemical Process Safety Copyright 2011 American Institute of Chemical Engineers, Inc. APPENDIX D - BIOLOGICAL ASSESSMENT
NOTES - CH 15 (and 14.3): DNA Technology ( Biotech )
 NOTES - CH 15 (and 14.3): DNA Technology ( Biotech ) Vocabulary Genetic Engineering Gene Recombinant DNA Transgenic Restriction Enzymes Vectors Plasmids Cloning Key Concepts What is genetic engineering?
NOTES - CH 15 (and 14.3): DNA Technology ( Biotech ) Vocabulary Genetic Engineering Gene Recombinant DNA Transgenic Restriction Enzymes Vectors Plasmids Cloning Key Concepts What is genetic engineering?
Downstate Biotechnology Incubator Application
 Downstate Biotechnology Incubator Application Business Name: SECTION I: CONTACT INFORMATION Contact Person First Name Last Name Phone #: Mobile Phone #: Email: SECTION II: BUSINESS INFORMATION Current
Downstate Biotechnology Incubator Application Business Name: SECTION I: CONTACT INFORMATION Contact Person First Name Last Name Phone #: Mobile Phone #: Email: SECTION II: BUSINESS INFORMATION Current
RISK ASSESSMENT OF GENETICALLY MODIFIED MICRO-ORAGNISMS: A FORMAT THAT OFFERS ONE POSSIBLE WAY OF ACHIEVING GOOD PRACTICE
 RISK ASSESSMENT OF GENETICALLY MODIFIED MICRO-ORAGNISMS: A FORMAT THAT OFFERS ONE POSSIBLE WAY OF ACHIEVING GOOD PRACTICE 1 Regulation 6 of the Genetically Modified Organisms (Contained Use) Regulations
RISK ASSESSMENT OF GENETICALLY MODIFIED MICRO-ORAGNISMS: A FORMAT THAT OFFERS ONE POSSIBLE WAY OF ACHIEVING GOOD PRACTICE 1 Regulation 6 of the Genetically Modified Organisms (Contained Use) Regulations
Chapter 9. Biotechnology and DNA Technology
 Chapter 9 Biotechnology and DNA Technology SLOs Compare and contrast biotechnology, recombinant DNA technology, and genetic engineering. Identify the roles of a clone and a vector in making recombined
Chapter 9 Biotechnology and DNA Technology SLOs Compare and contrast biotechnology, recombinant DNA technology, and genetic engineering. Identify the roles of a clone and a vector in making recombined
Environmental Health & Safety Policy Manual. Laboratory Inspection Program
 Environmental Health & Safety Policy Manual Date: 6/30/2011 Revised: 1/17/2013 Policy # EHS 400.13 Laboratory Inspection Program 1.0 PURPOSE This policy establishes guidelines for performing safety inspections
Environmental Health & Safety Policy Manual Date: 6/30/2011 Revised: 1/17/2013 Policy # EHS 400.13 Laboratory Inspection Program 1.0 PURPOSE This policy establishes guidelines for performing safety inspections
Research Laboratory Information Principal Investigator: Project Title: Department: Office Phone: Laboratory Safety Representative:
 Research Laboratory Information Principal Investigator: Project Title: Department: Office Phone: Email: Laboratory Safety Representative: Laboratory Safety Representative Phone: Laboratory Safety Representative
Research Laboratory Information Principal Investigator: Project Title: Department: Office Phone: Email: Laboratory Safety Representative: Laboratory Safety Representative Phone: Laboratory Safety Representative
The NIH rdna Guidelines Explained
 Clemson University IBC Page 1 of 8 The NIH rdna Guidelines Explained The next few pages were written to extract the essence of the NIH guideline requirements and put them into readable form. Since many
Clemson University IBC Page 1 of 8 The NIH rdna Guidelines Explained The next few pages were written to extract the essence of the NIH guideline requirements and put them into readable form. Since many
Genome Engineering. Brian Petuch
 Genome Engineering Brian Petuch Guiding Principles An understanding of the details of gene engineering is essential for understanding protocols when making recommendations on: Risk assessment Containment
Genome Engineering Brian Petuch Guiding Principles An understanding of the details of gene engineering is essential for understanding protocols when making recommendations on: Risk assessment Containment
Name Biol Group Number. ALE 11. The Genetics of Viruses, Control of Gene Expression, and Recombinant DNA Technology
 Name Biol 211 - Group Number ALE 11. The Genetics of Viruses, Control of Gene Expression, and Recombinant DNA Technology Chapter 19: The Genetics of Viruses (pp. 381-395, Biology by Campbell/Reece, 8 th
Name Biol 211 - Group Number ALE 11. The Genetics of Viruses, Control of Gene Expression, and Recombinant DNA Technology Chapter 19: The Genetics of Viruses (pp. 381-395, Biology by Campbell/Reece, 8 th
Biotechnology and DNA Technology
 11/27/2017 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College CHAPTER 9 Biotechnology and DNA Technology Introduction to Biotechnology Learning Objectives Compare
11/27/2017 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College CHAPTER 9 Biotechnology and DNA Technology Introduction to Biotechnology Learning Objectives Compare
Tues 1/21. Today: Virus movie clip, ek paragraph for ch 20. Next class: collect Ch. 20 Guided Reading
 Tues 1/21 Today: Virus movie clip, ek paragraph for ch 20. Next class: collect Ch. 20 Guided Reading Pg. 104 Ch. 20 Guided Reading Pg. 105 EK Paragraph 3C3 Wed. 1/22 Collect-Ch 20 Guided Reading Today:
Tues 1/21 Today: Virus movie clip, ek paragraph for ch 20. Next class: collect Ch. 20 Guided Reading Pg. 104 Ch. 20 Guided Reading Pg. 105 EK Paragraph 3C3 Wed. 1/22 Collect-Ch 20 Guided Reading Today:
Risk Assessment: Microorganisms and Materials Containing Recombinant DNA
 Assessment: Microorganisms and Materials Containing Recombinant DNA Srisin Khusmith Professor, Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University Committee, Technical
Assessment: Microorganisms and Materials Containing Recombinant DNA Srisin Khusmith Professor, Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University Committee, Technical
POLICY SUMMARY FORM. Policy Name: Recombinant DNA and/or Infectious Biohazards in Teaching and Research
 POLICY SUMMARY FORM Policy Name: Recombinant DNA and/or Infectious Biohazards in Teaching and Research Policy Number: 8.9 Is this policy new, being reviewed/revised, or deleted? Review/Revise Date of last
POLICY SUMMARY FORM Policy Name: Recombinant DNA and/or Infectious Biohazards in Teaching and Research Policy Number: 8.9 Is this policy new, being reviewed/revised, or deleted? Review/Revise Date of last
Unit 2: Metabolism and Survival Sub-Topic (2.7) Genetic Control of Metabolism (2.8) Ethical considerations in the use of microorganisms
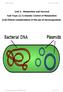 Unit 2: Metabolism and Survival Sub-Topic (2.7) Genetic Control of Metabolism (2.8) Ethical considerations in the use of microorganisms Duncanrig Secondary JHM&MHC 2015 Page 1 of 18 On completion of this
Unit 2: Metabolism and Survival Sub-Topic (2.7) Genetic Control of Metabolism (2.8) Ethical considerations in the use of microorganisms Duncanrig Secondary JHM&MHC 2015 Page 1 of 18 On completion of this
Biosc10 schedule reminders
 Biosc10 schedule reminders Review of molecular biology basics DNA Is each person s DNA the same, or unique? What does DNA look like? What are the three parts of each DNA nucleotide Which DNA bases pair,
Biosc10 schedule reminders Review of molecular biology basics DNA Is each person s DNA the same, or unique? What does DNA look like? What are the three parts of each DNA nucleotide Which DNA bases pair,
Baculovirus Adenovirus Adeno-associated virus Lentivirus/Retrovirus Many more: Vaccinia virus, Semliki Forest virus
 Recombinant Viruses Viruses produced by recombinant DNA technology Naturally occuring recombinant virus (= when more than one virus strain infects the same cell and genetic information is exchanged) Common
Recombinant Viruses Viruses produced by recombinant DNA technology Naturally occuring recombinant virus (= when more than one virus strain infects the same cell and genetic information is exchanged) Common
Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines)
 Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines) Jacqueline Corrigan-Curay, J.D. M.D. Acting Director Office of Biotechnology Activities National Institute
Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines) Jacqueline Corrigan-Curay, J.D. M.D. Acting Director Office of Biotechnology Activities National Institute
2054, Chap. 14, page 1
 2054, Chap. 14, page 1 I. Recombinant DNA technology (Chapter 14) A. recombinant DNA technology = collection of methods used to perform genetic engineering 1. genetic engineering = deliberate modification
2054, Chap. 14, page 1 I. Recombinant DNA technology (Chapter 14) A. recombinant DNA technology = collection of methods used to perform genetic engineering 1. genetic engineering = deliberate modification
Reporting An Untoward Event
 Reporting An Untoward Event Robert J. Hashimoto, CBSP, RBP, SM(NRCM) University of California-Berkeley American Biological Safety Association Anaheim, California November 2, 2011 Introduction The National
Reporting An Untoward Event Robert J. Hashimoto, CBSP, RBP, SM(NRCM) University of California-Berkeley American Biological Safety Association Anaheim, California November 2, 2011 Introduction The National
BCH 462 Competent Cells Formation and Transformation of Competent Cells with plasmid DNA.
 Lab#2 BCH 462 Competent Cells Formation and Transformation of Competent Cells with plasmid DNA. Outlines: 1-Insertion of foreign gene to the plasmid. 2-Competent cell. 3-Transformation of bacterial cell.
Lab#2 BCH 462 Competent Cells Formation and Transformation of Competent Cells with plasmid DNA. Outlines: 1-Insertion of foreign gene to the plasmid. 2-Competent cell. 3-Transformation of bacterial cell.
Molecular Cloning. Restriction Enzymes and Ligases
 Tools in Genetic engineering The science of using living systems to benefit humankind is called biotechnology. Technically speaking, the domestication of plants and animals through farming and breeding
Tools in Genetic engineering The science of using living systems to benefit humankind is called biotechnology. Technically speaking, the domestication of plants and animals through farming and breeding
Biology Test Review Microorganisms
 Name: Period: Biology Test Review Microorganisms Use your booklet, notes, & quizzes to complete this review. 1. Define the following terms using a few key words: a. Host cell - victim of the virus b. Retrovirus
Name: Period: Biology Test Review Microorganisms Use your booklet, notes, & quizzes to complete this review. 1. Define the following terms using a few key words: a. Host cell - victim of the virus b. Retrovirus
IBC Approval at TAMU What? Why? How?
 IBC Approval at TAMU What? Why? How? Biosafety Program Presented by Christine T. McFarland, Ph.D. Director, Biosafety, BSO, RO Why is IBC approval necessary? System Regulation 15.99.06: Use of Biohazards
IBC Approval at TAMU What? Why? How? Biosafety Program Presented by Christine T. McFarland, Ph.D. Director, Biosafety, BSO, RO Why is IBC approval necessary? System Regulation 15.99.06: Use of Biohazards
UNIVERSITY OF EXETER
 UNIVERSITY OF EXETER Risk Assessment Of Genetically Modified Micro-organisms Where The Primary Risk Posed Is To Human Health Rather Than To The Environment This risk assessment form is for use in cases
UNIVERSITY OF EXETER Risk Assessment Of Genetically Modified Micro-organisms Where The Primary Risk Posed Is To Human Health Rather Than To The Environment This risk assessment form is for use in cases
Chapter 9 Genetic Engineering
 Chapter 9 Genetic Engineering Biotechnology: use of microbes to make a protein product Recombinant DNA Technology: Insertion or modification of genes to produce desired proteins Genetic engineering: manipulation
Chapter 9 Genetic Engineering Biotechnology: use of microbes to make a protein product Recombinant DNA Technology: Insertion or modification of genes to produce desired proteins Genetic engineering: manipulation
Basic Concepts and History of Genetic Engineering. Mitesh Shrestha
 Basic Concepts and History of Genetic Engineering Mitesh Shrestha Genetic Engineering AKA gene manipulation, gene cloning, recombinant DNA technology, genetic modification, and the new genetics. A technique
Basic Concepts and History of Genetic Engineering Mitesh Shrestha Genetic Engineering AKA gene manipulation, gene cloning, recombinant DNA technology, genetic modification, and the new genetics. A technique
Introducing new DNA into the genome requires cloning the donor sequence, delivery of the cloned DNA into the cell, and integration into the genome.
 Key Terms Chapter 32: Genetic Engineering Cloning describes propagation of a DNA sequence by incorporating it into a hybrid construct that can be replicated in a host cell. A cloning vector is a plasmid
Key Terms Chapter 32: Genetic Engineering Cloning describes propagation of a DNA sequence by incorporating it into a hybrid construct that can be replicated in a host cell. A cloning vector is a plasmid
Laboratory Risk Assessment Form. PART A: Research/Investigation. Principal Investigator: Department: Location of Research: Funding agency: Agent Used:
 Laboratory Risk Assessment Form PART A: Research/Investigation Principal Investigator: Department: Location of Research: Funding agency: Agent Used: Material Safety Data Sheet (MSDS) available? Risk Group
Laboratory Risk Assessment Form PART A: Research/Investigation Principal Investigator: Department: Location of Research: Funding agency: Agent Used: Material Safety Data Sheet (MSDS) available? Risk Group
Frequently Asked Questions (FAQs) of Interest to IBCs
 National Institutes of Health Office of Biotechnology Activities Frequently Asked Questions (FAQs) of Interest to IBCs 1. NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules
National Institutes of Health Office of Biotechnology Activities Frequently Asked Questions (FAQs) of Interest to IBCs 1. NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules
Lecture 25 (11/15/17)
 Lecture 25 (11/15/17) Reading: Ch9; 328-332 Ch25; 990-995, 1005-1012 Problems: Ch9 (study-guide: applying); 1,2 Ch9 (study-guide: facts); 7,8 Ch25 (text); 1-3,5-7,9,10,13-15 Ch25 (study-guide: applying);
Lecture 25 (11/15/17) Reading: Ch9; 328-332 Ch25; 990-995, 1005-1012 Problems: Ch9 (study-guide: applying); 1,2 Ch9 (study-guide: facts); 7,8 Ch25 (text); 1-3,5-7,9,10,13-15 Ch25 (study-guide: applying);
Legal arguments to keep plants. such as cisgenesis outside the GMO regulation
 Legal arguments to keep plants from novel breeding techniques such as cisgenesis outside the GMO regulation dr Henk J Schouten Wageningen University and Research Centre & Inova Fruit bv Content Definition
Legal arguments to keep plants from novel breeding techniques such as cisgenesis outside the GMO regulation dr Henk J Schouten Wageningen University and Research Centre & Inova Fruit bv Content Definition
To UPCI Lentiviral Facility USERS
 IBC Application Form University of Pittsburgh - Institutional Biosafety Committee Use this form as of 14 February 2011 for: New research proposals involving the use of recombinant materials Modifications
IBC Application Form University of Pittsburgh - Institutional Biosafety Committee Use this form as of 14 February 2011 for: New research proposals involving the use of recombinant materials Modifications
TYPES OF DEALINGS WITH GMOS CLASSIFIED AS NOTIFIABLE LOW RISK DEALINGS (NLRDS)
 September 2011 TYPES OF DEALINGS WITH GMOS CLASSIFIED AS NOTIFIABLE LOW RISK DEALINGS (NLRDS) Excerpt from the Gene Technology Regulations 2001 (Statuty Rules 2001 No. 106 as amended) (the Regulations),
September 2011 TYPES OF DEALINGS WITH GMOS CLASSIFIED AS NOTIFIABLE LOW RISK DEALINGS (NLRDS) Excerpt from the Gene Technology Regulations 2001 (Statuty Rules 2001 No. 106 as amended) (the Regulations),
Chapter 13: Biotechnology
 Chapter Review 1. Explain why the brewing of beer is considered to be biotechnology. The United Nations defines biotechnology as any technological application that uses biological system, living organism,
Chapter Review 1. Explain why the brewing of beer is considered to be biotechnology. The United Nations defines biotechnology as any technological application that uses biological system, living organism,
WARM UP. 1. Take out your laptop and Chapter 12 Notes 2. Log in to Google Classroom 3. Wait for me to post the quick quiz!
 WARM UP 1. Take out your laptop and Chapter 12 Notes 2. Log in to Google Classroom 3. Wait for me to post the quick quiz! AGENDA Warm up- Quick Quiz Chapter 13 Notes: Genetic Technology Genetic Engineering
WARM UP 1. Take out your laptop and Chapter 12 Notes 2. Log in to Google Classroom 3. Wait for me to post the quick quiz! AGENDA Warm up- Quick Quiz Chapter 13 Notes: Genetic Technology Genetic Engineering
IBC Instructional Registration Document Rev. 10/2016
 FOR OFFICE USE ONLY: IBC PROTOCOL # STATUS: ANIMAL WORK: CONTAINMENT BSL: RECOMBINANT DNA: TRAINING COMPLETE: University of North Dakota Institutional Biosafety Committee (IBC) Instructional Registration
FOR OFFICE USE ONLY: IBC PROTOCOL # STATUS: ANIMAL WORK: CONTAINMENT BSL: RECOMBINANT DNA: TRAINING COMPLETE: University of North Dakota Institutional Biosafety Committee (IBC) Instructional Registration
Researchers use genetic engineering to manipulate DNA.
 Section 2: Researchers use genetic engineering to manipulate DNA. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the different tools and processes used in genetic
Section 2: Researchers use genetic engineering to manipulate DNA. K What I Know W What I Want to Find Out L What I Learned Essential Questions What are the different tools and processes used in genetic
KANSAS STATE UNIVERSITY INSTITUTIONAL BIOSAFETY COMMITTEE STANDARD OPERATING PROCEDURES
 KANSAS STATE UNIVERSITY INSTITUTIONAL BIOSAFETY COMMITTEE STANDARD OPERATING PROCEDURES I. INTRODUCTION The Kansas State University (K State) Institutional Biosafety Committee, hereafter referred to as
KANSAS STATE UNIVERSITY INSTITUTIONAL BIOSAFETY COMMITTEE STANDARD OPERATING PROCEDURES I. INTRODUCTION The Kansas State University (K State) Institutional Biosafety Committee, hereafter referred to as
Lecture Series 10 The Genetics of Viruses and Prokaryotes
 Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Chapter 8: Recombinant DNA. Ways this technology touches us. Overview. Genetic Engineering
 Chapter 8 Recombinant DNA and Genetic Engineering Genetic manipulation Ways this technology touches us Criminal justice The Justice Project, started by law students to advocate for DNA testing of Death
Chapter 8 Recombinant DNA and Genetic Engineering Genetic manipulation Ways this technology touches us Criminal justice The Justice Project, started by law students to advocate for DNA testing of Death
Recombinant DNA Technology in Today s Medicine
 Recombinant DNA Technology in Today s Medicine Shelley M. Martineau Jessica A. Matthews Catherine C. Miller Carol D. Riley Institute of TIP Productions, Inc. Part A Overview rdna Objectives Steps in rdna
Recombinant DNA Technology in Today s Medicine Shelley M. Martineau Jessica A. Matthews Catherine C. Miller Carol D. Riley Institute of TIP Productions, Inc. Part A Overview rdna Objectives Steps in rdna
Overview of the Current NIH Guidelines for Research Involving Recombinant DNA Molecules. Kathryn Harris
 Overview of the Current NIH Guidelines for Research Involving Recombinant DNA Molecules Kathryn Harris NIH Guidelines for Research Involving Recombinant DNA Molecules A scientificallyresponsive document
Overview of the Current NIH Guidelines for Research Involving Recombinant DNA Molecules Kathryn Harris NIH Guidelines for Research Involving Recombinant DNA Molecules A scientificallyresponsive document
VECTOR SYSTEMS. Vector Systems
 VECTOR SYSTEMS Vector Systems The vector systems currently available through Penn Vector Core are those derived from adenoassociated virus (AAV), adenovirus and lentivirus. The Core offers AAV-based vectors
VECTOR SYSTEMS Vector Systems The vector systems currently available through Penn Vector Core are those derived from adenoassociated virus (AAV), adenovirus and lentivirus. The Core offers AAV-based vectors
Chapter 5. Microbial Biotechnology. PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.
 PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 5 Microbial Biotechnology Lectures by Lara Dowland Chapter Contents 5.1 The Structure
PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 5 Microbial Biotechnology Lectures by Lara Dowland Chapter Contents 5.1 The Structure
number Done by Corrected by Doctor Hamed Al Zoubi
 number 3 Done by Neda a Baniata Corrected by Waseem Abu Obeida Doctor Hamed Al Zoubi Note: it is important to refer to slides. Bacterial genetics *The main concepts we will talk about in this lecture:
number 3 Done by Neda a Baniata Corrected by Waseem Abu Obeida Doctor Hamed Al Zoubi Note: it is important to refer to slides. Bacterial genetics *The main concepts we will talk about in this lecture:
CHAPTER 2A HOW DO YOU BEGIN TO CLONE A GENE? CHAPTER 2A STUDENT GUIDE 2013 Amgen Foundation. All rights reserved.
 CHAPTER 2A HOW DO YOU BEGIN TO CLONE A GENE? 35 INTRODUCTION In the Program Introduction, you learned that the increase in diabetes in the United States has resulted in a great demand for its treatment,
CHAPTER 2A HOW DO YOU BEGIN TO CLONE A GENE? 35 INTRODUCTION In the Program Introduction, you learned that the increase in diabetes in the United States has resulted in a great demand for its treatment,
Rochester Institute of Technology Institutional Biosafety Committee (IBC) Guidelines for Committee Authority, Structure and Responsibilities
 1. Purpose The purpose of this document is to provide details of the responsibilities, scope of authority and structure of the. 2. Mission The mission of the Institutional Biosafety Committee is to: 2.1.
1. Purpose The purpose of this document is to provide details of the responsibilities, scope of authority and structure of the. 2. Mission The mission of the Institutional Biosafety Committee is to: 2.1.
Regulation of metabolic pathways
 Regulation of metabolic pathways Bacterial control of gene expression Operon: cluster of related genes with on/off switch Three Parts: 1. Promoter where RNA polymerase attaches 2. Operator on/off, controls
Regulation of metabolic pathways Bacterial control of gene expression Operon: cluster of related genes with on/off switch Three Parts: 1. Promoter where RNA polymerase attaches 2. Operator on/off, controls
