ABSENCE OF THE Lyt-2-,L3T4 + LINEAGE OF T CELLS IN MICE TREATED NEONATALLY WITH ANTI-I-A CORRELATES WITH ABSENCE OF INTRATHYMIC
|
|
|
- Lenard Baldwin
- 6 years ago
- Views:
Transcription
1 ABSENCE OF THE Lyt-2-,L3T4 + LINEAGE OF T CELLS IN MICE TREATED NEONATALLY WITH ANTI-I-A CORRELATES WITH ABSENCE OF INTRATHYMIC I-A-BEARING ANTIGEN-PRESENTING CELL FUNCTION BY ADAM. KRUISBEEK,* JAMES J. MOND,* B. J. FOWLKES, JULIE A. CARMEN,* SANDRA BRIDGES, *m AND DAN L. LONGO* From the *Medicine Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20205; the $Department of Medicine, Univormed Services University of the Health Sciences; the Laborato~'y of Microbial Immunity, National Institute of AUergy and Infectious Diseases, National Institutes of Health; and the ~Centro di Endocrinologia ed Oncologia Sperimentale del C. N. R, Naples, Italy Experiments with radiation-induced bone marrow chimeras and thymus-reconstituted nude mice have shown that the repertoire of T cells is influenced greatly by the major histocompatibility complex (MHC) 1 genes expressed in their developmental milieu. For class I MHC-restricted T cells, there appear to be both thymic and extrathymic influences on the repertoire (1-8), while for class II MHC-restricted T cells, the I region gene phenotype of the thymus appears to determine entirely the self-restriction specificity (9-13). The precise mechanism by which the thymus shapes the T cell receptor repertoire is unclear, but it appears that bone marrow-derived, medullary, Ia-bearing, thymic, antigenpresenting ceils (APC) play a critical role in determining class II MHC-restriction specificity (14-16). 2 In these experiments, the process that generates the T cell repertoire is not readily accessible to experimental manipulation. The recent development of a model system in which mice are treated from birth with anti-i-a has allowed us to look at the process of T cell development more closely. In addition to its effects on B cell development (17), chronic anti-i-a treatment early in life has This work was supported by U. S. Public Health Service research grant R 08347, National Science Foundation grant PCM , and the Office of Naval Research grant G28353, N RR33041, and in part by the Italian National Research Council (CNR) Special Project "Oncology." Address correspondence to A. Kruisbeek; Medicine Branch, National Cancer Institute, National Institutes of Health, Building 10, Room 12N226, Bethesda, MD, Abbreviations used in this paper: APC, antigen-presenting cell; BSA, bovine serum albumin; CTL, cytotoxic T lymphocyte; CTLL, IL-2-dependent line of cytotoxic T lymphocyte; E/R, Eagle's/RPMI special medium; FACS, fluorescence-activated cell sorter; FCS, fetal calf serum; FITC, fluorescein isothiocyanate; IL-2, interleukin 2; KLH, keyhole limpet hemocyanin; mab, monoclonal antibody; MHC, major histocompatibility complex; MLC, mixed lymphocyte culture; NMS, normal mouse serum; PI, propidium iodide; TR, Texas Red. Bone marrow-derived thymic antigen presenting cells determine self-recognition of Ia-restricted T lymphocytes. D. L. Longo, A. M. Kruisbeek, M. L. Davis, and L. A. Matis. Manuscript submitted for publication. Journal of Experimental Medicine Volume 161, May
2 1030 ABSENCE OF Lyt-2-,L3T4 + CELLS IN ANTI-I-A-TREATED MICE profound effects on T cell development (18), 3 despite the fact that murine T cells do not express I-A. Thymic and splenic Ia-antigen expression is strongly reduced in mice treated neonatally with anti-i-a (17, 18). In vitro cytotoxic T lymphocyte (CTL) responses to alloantigens and trinitrophenyl-modified-self antigens by spleen cells from such mice could only be generated in the presence of an exogenous source of interleukin 2 (IL-2) (18), implying that T cells capable of generating helper factors in vitro are defective in anti-i-a-treated mice. Finally, recent studies 3 demonstrated abrogation of allo-class II-specific and self-class II-restricted T cell proliferative responses in both thymus and spleen of anti-i-a-treated mice. These data (18) 3 suggested that class II MHC-restricted T cells were either missing or functionally defective in mice treated from birth with anti-i-a. In an effort to distinguish whether class II-restricted T cells were absent or defective in this model, we examined the effects of neonatal anti-i-a treatment on the L3T4 and Lyt-2 surface phenotype of thymic and splenic T cells, and on their class II-specific T cell functions. In addition, we tested the hypothesis that the T cell defects reported here and previously (18) ~ were somehow related to the reduced thymic Ia antigen expression (18) by assessing the relationship of the class II-specific T cell defects to the function of thymic APC. Identifying the L3T4 and Lyt-2 phenotype of T cells should be a reliable measure for presence or absence of a particular T cell subset because of the striking correlation between cell surface phenotype and MHC-subclass specificity. The expression of Lyt-2,3 molecules on mature mouse T cells usually correlates with class I MHC specificity (19-23), while expression of the L3T4 antigen correlates with class II MHC specificity (24-29). How this impressively strong association between T cell surface marker expression and MHC-subclass specificity is acquired in the developing T cell repertoire is unknown. In the thymus, all precursors of alloreactive IL-2-producing T cells are contained within the subset of phenotypically "mature" Lyt-2-,L3T4 + T cells (30), while all alloreactive CTL precursors are recovered in the Lyt-2 + subset of "mature" T cells (31). We report here that the functional defects in class II-restricted T cells in anti- I-A-treated mice are correlated with an absence of the Lyt-2-,L3T4 + T cell subset, and with absence of functional thymic APC. The Lyt-2+,L3T4 - class I- restricted T cell subset develops normally in these mice. When anti-i-a treatment is stopped, thymic and splenic APC function return to normal, and the Lyt- 2-,L3T4 +, class II-restricted T cell subset reappears, first in the thymus, with return of these cells to the periphery lagging behind. Collectively, these findings suggest that development of Lyt-2-,L3T4 +, class II-restricted T cells is dependent on I-A-bearing thymic APC, and that the Lyt-2+,L3T4 -, class I-specific T cell subset develops by a distinct mechanism, unaffected by anti-i-a. Materials and Methods Mice and Anti-I-A Treatment. Neonatal C57BL/6 (H-2 b) mice were treated within 24 h of birth with protein A-purified anti-i-a b (hybridoma Y-3P [32], a kind gift from Dr. 3 In vivo treatment of neonatal mice with anti-i-a antibodies interferes with the development of the class I-, class II- and M I s-reactive proliferating T cell subset, A. M. Kruisbeek, S. Bridges, J. Carmen, D. L. Longo, and J. J. Mond. J. lmmunol. In press.
3 KRUISBEEK ET AL Charles A. Janeway, Yale University, New Haven, CT) and treated every 48 h thereafter. Intraperitoneal injections of 600, 800, and 1,000 #g of antibody were given during the first, second, and third week of life, respectively. Mouse strains to be used as donors for stimulator cells were DBA/2, B6.C.H-2 b~"l, D 1.LP, B10.D2 (The Jackson Laboratory, Bar Harbor, ME) and B6.C.H-2 bm~2 (bred in our facilities from breeding pairs provided by Drs. Richard Hodes, NIH, and Ted Hansen, Washington University, St. Louis, MO). H- 2 and Mls phenotypes of these strains are: DBA/2:H-2 d, Mlsa; B10.D2:H-2 d, Mlsb; B6.C.H-2bml: H-2 b (K bm~ mutant), Mlsb; DI.LP: H-2 b, Mlsa; B6.C. H-2bm12:H-2 b (I b"~2 mutant), Mls b. Mice treated with anti-i-a were killed at 3 wk of age, either after continuous antibody treatment, or after 1 wk of treatment and 2 wk with no treatment. Generation of T Cell Proliferative Responses in Mixed Lymphocyte Culture (MLC). Responder thymocytes were cultured at cells/well, and spleen cells at 4 l0 b cells/well, with varying concentrations (2, 4, and 8 105) of 2,000 rad irradiated ailogeneic stimulator spleen cells in a final volume of 0.2 ml of complete tissue culture medium consisting of half RPM I 1640, half Eagle's Hanks' amino acids medium (33) supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, UT), 2 mm glutamine, penicillin (100 U/ml), streptomycin (100 mg/ml), 2-mercaptoethanol (5 10 -~ M), and sodium pyruvate (0.11 mg/ml). This medium is referred to as E/R. Triplicate cultures were set up in round-bottom (thymocytes) or flat-bottom (spleen cells) 96-well microtiter plates. On day 4 for thymocytes or day 3 for spleen cells, 1 t~ci of [SH]thymidine (sp act 6.7 Ci/mmol; New England Nuclear, Boston, MA) was added per well, and cultures were harvested h later on an automated sample harvester. ['H]Thymidine incorporation was measured in a liquid scintillation counter, and data are expressed as the arithmetic mean cpm. SEM were <20%. Fluorescence Staining and Two-color Fluorescence-activated Cell Sorter (FACS) Analysis. Spleen cell and thymocyte suspensions from normal and anti-i-a-treated mice were prepared in Hank's balanced salt solution (without phenol red) containing 0.1% bovine serum albumin (BSA) and 0.1% sodium azide (FACS buffer). Cells (106]30 #i buffer) were incubated on ice for 30 min with 10 #l of the appropriate reagent, and washed twice after each incubation. For two-color FACS analysis of L3T4 and Lyt-2, the following sequence was used for thymocytes: (a) anti-lyt-2.2 culture supernatants (mouse IgG2a, clone 19/178 [34]); (b) Texas Red (TR)-Iabeled, affinity-purified goat anti-mouse Ig (crossreactive with rat Ig) (35); (c) normal mouse serum (NMS); (d) anti-l3t4 culture supernatant (rat IgG2b, clone GK-1.5 [24, 27]); (e) fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG (not crossreactive with mouse IgG) (Cappel Laboratories, Cochranville, PA). The sequence used for spleen cells was (a) anti-l3t4 culture supernatant; (b) FITCconjugated mouse anti-rat g (mouse IgG2a, clone Marl8.5 [36]); (c) rat lg fraction (purified from rat serum); (d) biotin-labeled anti-lyt-2 (rat IgG2a, clone [37] purified by ion-exchange and gel filtration); or (e) TR-avidin. NMS or rat Ig, which had the capacity to react with both primary antibodies, was used to block unoccupied binding sites on bound second antibody. Control cells, either unstained or stained with second antibody alone, were used to obtain background fluorescence levels. In later experiments, not shown here, we used, with equal success, the following sequence for both thymocytes and spleen cells: (a) biotin-labeled anti-l3t4; (b) TR-avidin; (c) FITC-conjugated anti- Lyt-2. Flow microfluorometry analysis was performed on a FACS II (Becton-Dickinson and Co., Mountain View, CA), and analyzed by a PDP 11/34 computer (Digital Equipment, Marlboro, MA), using programs previously described (38). All data were collected using log amplifiers and displayed on a log~0 scale of increasing green and red fluorescence intensity, without defining units. Subpopulations in stained samples that did not differ from the unstained control were scored as negative. Dead cells were rejected from analysis by forward light scatter (39) and propidium iodide staining (PI) by electronic gating. 10 #1 of PI at 50 mg/ml in 0.1% sodium citrate (40) were added to the cell sample just prior to FACS-analysis. Generation of IL-2 in Limiting-dilution Microculture. A modification of the method described by Miller and Stutman (41) was used. Responder C57BL/6 spleen cells or
4 1032 ABSENCE OF Lyt-2-,L3T4 + CELLS IN ANTI-I-A-TREATED MICE thymocytes were, in some experiments, depleted of Lyt-2-bearing cells by anti-lyt-2.2 monoclonal antibodies (mab) and complement treatment to eliminate cells that may use IL-2 (42). Stimulator cells were nonirradiated resident peritoneal cells from C57BL/10 X B10.D2 F~ nude mice or C57BL/6 X bml2 F~ mice treated wtih anti-thy-l.2 mab plus complement to eliminate T cells; these stimulator cells were shown to be devoid of T cells by their inability to generate a concanavalin A-induced proliferative response (data not shown). Varying numbers of untreated or Lyt-2-depleted responder cells were cultured in round-bottom wells of 96-well microtiter plates with Fl stimulator cells for 5 d. After centrifugation of the plates at 500 g, 100 #i of supernatant was removed from each well, and tested for its IL-2 activity by measuring its ability to support growth of CTLL cells (an IL-2-dependent CTL line). The supernatants to be tested were cultured with CTLL cells in fiat-bottom wells of 96-well microtiter plates. After 24 h, cultures were labeled with 1/~Ci of [SH]thymidine for 4-6 h, and harvested and processed as "described above for MLC cultures. Supernatants of microcultures were defined as positive when the IL-2 activity exceeded by >3 SD the mean [3H]thymidine incorporation of CTLL cells grown in control supernatants (i.e., obtained from 5-d cultures of stimulator cells only). Minimal estimates of the precursor frequencies were calculated by plotting the percent negative cultures against cell dose, according to the Poisson model, and by using the minimum X 2 method of estimating the frequency, as described by Taswell (43). Measurement of APC Function for l-region-spec~c T Cells with Antigen-reactive T Cell Clones. Antigen-reactive T cell clones were derived from draining lymph nodes of mice immunized with keyhole limpet hemocyanin (KLH) (lot , Calbiochem-Behring Corp., San Diego, CA) 8-10 d earlier (50 #g KLH per hind foot pad in an emulsion with complete Freund's adjuvant) as previously described (44). The cells were cloned by limiting dilution 2 d after the third stimulation with antigen and syngeneic 3,300 rad irradiated spleen cells. Alloreactive T cell clones were derived from secondary MLC; both antigenspecific and alloreactive T cell clones were maintained by alternating 4-d periods of stimulation and 7-d periods of rest with syngeneic irradiated spleen cells. I-region specificity was determined by mapping using recombinant mouse strains, and by inhibition of proliferation with mab of the appropriate specificity. All clones were independent of exogenous IL-2 for their proliferation. T cell clones recovered at the end of the rest period were used to assay APC function of thymocytes or spleen cells from control or anti-i-a-treated mice. 10 ~ cloned T cells were cocultured with various doses of 2,000 rad irradiated APC and, when appropriate, KLH, in a final volume of 0.2 ml of E/R in flat-bottom, 96-well microtiter plates. [3H] Thymidine incorporation was assessed on day 2 or 3 as described above for MLC. APC were light-density cells collected from a discontinuous BSA gradient consisting of a 35% and an 11% layer, spun at 25,000 g. Cells from the interface between the layers were washed twice in E/R without FCS. This enrichment for light-density cells is specifically necessary to measure thymic APC function (14). Results Lyt-2-,L3T4 + T Cells Are Not Present in Spleen or Thymus from Anti-I-A-treated Mice. The key question to be addressed regarding the defects in anti-i-atreated mice is whether the absence of T cell functions generally associated with class li recognition (18) 3 is due to the absence of a subpopulation of T cells, or to functional defects in the subpopulation of T cells responsible for class II recognition. This question may be answered by analyzing expression of the cell surface markers associated with T cells' specificity for the class I and class II MHC subregions. Expression of the L3T4 marker correlates with specificity for class II-MHC gene products (23-27). Lyt-2 expression, on the other hand, usually correlates with T cell specificity for class I-MHC gene products (21-23). We studied expression of the L3T4 and the Lyt-2 antigens on spleen cells and
5 KRUISBEEK ET AL thymocytes from 3-wk-old anti-i-a-treated mice, using two-color flow cytometric analysis. The results obtained with spleen cells using GK-1.5 (recognizing LBT4) and anti-lyt-2 (Fig. l) show, in both adult and 3-wk-oid normal spleen cells (Fig. 1, top and bottom), distinct populations of Lyt-2-,L3T4 + cells, and Lyt-2+,L3T4 - cells. In contrast, the subset of Lyt-2-,L3T4 + cells is entirely absent in spleen cells from 3-wk-old anti-i-a-treated mice (Fig. 1, middle). In a series of five experiments, 6.2 _+ 0.05% (mean +SE) of normal spleen cells expressed the Lyt- 2-,LBT4 + phenotype, while in spleen cells from anti-i-a-treated mice, only 0.9 ~ ~ ~ "-t NORMAL SPLEEN (3 weeks om) 1~000 C3 O loo ANTI-I-A SPLEEN (3 weeks old) I I I I 1~000 ~'%-"(~ L I....J NORMAL ADULT SPLEEN k~ q~ L~-~---- J I ~00 Ioglo GREEN FL (L3T4) FIGURE 1. Two-color FAGS analysis of Lyt-2 (red fluorescence, ordinate) and L3T4 (green fluorescence, abscissa) on spleen cells from control (top) and anti-i-ab-treated (middle) C57BL/6 mice. Spleen cells from 2-3 control or anti-i-a-treated mice were pooled and stained, first with FITC-anti-L3T4, then washed and stained with biotin-labeled anti-lyt-2, followed by Texas Red-avidin. For comparison, a similar analysis of adult (10-wk-old) spleen cells is given (bottom). IL-2 production by spleen cells from these same mice is shown in Fig. 3.
6 1034 ABSENCE OF Lyt-2-,L3T4 + CELLS IN ANTI-l-A-TREATED MICE + 0.2% Lyt-2-,L3T4 + cells were found. This suggests that the absence of alloclass II-specific and self-class II-restricted proliferative responses in spleen cells from anti-i-a-treated mice ~ is due to absence of the Lyt-2-,L3T4 + T cell subset responsible for these functions, rather than functional defects in such cells. Since thymocytes from anti-i-a treated mice also exhibit inability to generate allo-class II-specific and self-class II-restricted proliferative responses, 3 it is likely that anti-i-a affects intrathymic T cell development as well. Two-color flow cytometry analysis of thymocytes supports tbis notion. In both adult (Fig. 2, bottom) and 3-wk-old normal thymocytes (Fig. 2, top), four subpopulations are detected after staining for L3T4 and Lyt-2:Lyt-2+,L3T4 + (representing the cortical type, immature T cells [30, 31 ]), Lyt-2+,L3T4 - (representing functional CTL precursors [31 ]); Lyt-2-,L3T4 + (representing functional class II-restricted 1.ooo NORMAL THYMUS (3 weeks old) 0o o3 : 9 10 ANTI-I-A THYMUS (3 weeks oldj I L......,-i-J 1,ooo 100 NORMAL ADULT THYMUS,o I L...,.f_,J IOglO GREEN FL (L3T4) FIGURE 2. Two-color FACS analysis of Lyt-2 (red fluorescence, ordinate) and L3T4 (green fluorescence, abscissa) on thymocytes from control (top) and anti-l-altreated (middle) C57BL/6 mice. Legend as in Fig. 1.1L-2 production by thymocytes from these same mice is shown in Fig. 4. 1J~0
7 KRUISBEEK ET AL IL-2-producing cells [30, 31]); and Lyt-2-,L3T4-, a small subpopulation including the dull Lyt-1 +,Lyt-2- (dly 1) precursor thymocyte (45). A striking difference emerged when thymocytes from 3-wk-old anti-i-a-treated mice were tested: the subpopulation of Lyt-2-, L3T4 + T cells is completely absent (Fig. 2, middle), with no apparent change in the other subpopu ations. In a series of five experiments, % Lyt-2-,L3T4 + cells are detected in normal thymus, while in anti-i-a-treated mice, only % of these cells was present. Thus, anti-i- A treatment selectively affected development of the Lyt-2-,L3T4 + T cell subset. Previous publications (23-27, 30) ascribed both antigen plus class II MHCrestricted or class II-alioantigen-specific proliferation and IL-2 production to Lyt-2-,L3T4 + cells. We therefore wished to determine whether the absence of the Lyt-2-,L3T4 + T cell subset in anti-i-a-treated mice was accompanied by lack of IL-2 production. Alternatively, anti-i-a treatment could have led to loss of the L3T4 marker, without loss of functions associated with this subset. Using a microculture system developed by others (41), IL-2 production in individual microcu]tures of normal and anti-i-a-treated spleen cells in response to stimulation by semiallogeneic stimulator cells was determined. Under such circumstances, most of the IL-2 produced is released by Lyt-2- cells, which respond preferentially to class II differences (30, 41, 42). Responder cells were pretreated with anti-lyt-2 plus complement to remove cells that do not produce, but may use IL-2 (42). Recovery of spleen cells after such treatment was 70-80% from both normal and anti-i-a-treated spleen cells. As shown in Fig. 3, when the supernatants from individual microcultures were tested for IL-2, spleen cells from anti-i-a-treated mice exhibited an obvious decrease in the number of positive cultures. The frequency of IL-2-producing cells under these conditions was 1/189 for normal spleen cells, compared with 1/5,810 for spleen cells from anti-i-a-treated mice (mean of 10 separate experiments; see also Table I). The normal spleen cell values are within the range of those previously reported (4 I, 42, 46) for adult spleen cells. Similar results, i.e., a strong decrease in the frequency of IL-2-producing precursors in anti-i-a-treated mice, were observed when responder cells were not depleted of Lyt-2 cells (data not shown). We choose to present data with Lyt-2- responder cells only, because under such circumstances more accurate frequency determinations are obtained (42), presumably because Lyt-2 + cells may use IL-2. A similar analysis with thymocytes was found to be technically difficult: after treatment of normal thymocytes with anti-lyt-2 plus complement, 6-10% of the cells could be recovered, while, with tbymocytes from anti-i-a-treated mice, often only very few cells survived the treatment. Nonetheless, data representative of four interpretable experiments with Lyt-2- thymocytes are presented. A limiting-dilution analysis of the Lyt-2- thymocyte population of normal and anti- I-A-treated mice indicated (Fig. 4) that virtually no microcultures in the anti-i- A-treated group released detectable levels of IL-2; in three out of four experiments, a frequency of<l/104 was found (see also Table I). In contrast, even low numbers of normal thymocytes are, as reported earlier (30, 31), able to give rise to easily measurable levels of IL-2: for four experiments, an average frequency of 1/286 (see also Table I) of IL-2-producing precursors was observed. Comparable results were obtained with untreated responder thymocytes (data not
8 1036 ABSENCE OF Lyt-2-,L3T4 + CELLS IN ANTI-I-A-TREATED MICE Anti-I-A Spleen Control Spleen _* ;,: o. o: 10,0(]0 -. ::.. Z _o :.:. :!"! rf- O a. n- O _z :~00-1,000 - " ~. ii: : :. :: :.. ",:.!il:!i ~i... ::! :.. :il ::. :::"..! * :.'o..:,.=: t,. :: -..: :, : "-k!: :. ::. ".", 300 I , ' ' 80O 1,600 ' 3200 ' NUMBER OF RESPONDER CELLS PER WELL FIGURE 3. Limiting-dilution analysis of IL-2 production in the spleen of control and anti-i- Ab-treated C57BL/6 mice. Groups of 36 replicate microcultures were established of varying numbers of Lyt-2- responder spleen cells, and Y cell-depleted (B10 x B10.D2) nude peritoneal stimulator cells. On day 5, 100 #1 of supernatants was harvested from each culture, and tested for ability to support growth of CTLL cells. Each dot represents IL-2 activity of an individual microwell. Positive wells were defined as those in which the activity exceeded the mean of control wells receiving no responder cells (solid line) by more than three SD of the mean (broken line). For frequency values, see Table I. shown). Taken together, these data demonstrate that in vivo anti-i-a treatment results in a T cell function defect strictly confined to the Lyt-2-,L3T4 + subset. Such cells are markedly decreased in either thymus or spleen from anti-i-atreated mice, and functions associated with this subset, i.e., class II-MHCrestricted T cell proliferation 3 and IL-2 production are strongly diminished, or absent. Correlation of Defective Proliferative T Cell Response with Defective Thymic APC Function in Anti-I-A-treated Mice. It was previously observed (18) that Ia antigen expression in thymuses from anti-i-a-treated mice was strongly diminished. It has also been shown (14, 15) 2 that one pathway of acquisition of T cell I-region recognition is through interaction in the thymus with a bone marrow-derived cell that functions as an APC. In view of the absence of class II-restricted T cell responses 3 and lack of the Lyt-2-,L3T4 + subset in anti-i-a-treated mice, it was of interest to analyze their thymic APC function. Splenic APC function was probed as well, because splenic Ia antigen expression is completely absent in
9 KRUISBEEK ET AL TABLE I Frequency of ll-2-producing Cells among Lyt-2- Thymocytes and Spleen Cells from Control and Anti-I-A-treated Mice Exp. Lyt-2- responder cells T cell-depleted lator cells stimu- Frequency of IL-2-producing cells* 1' Control spleen B10 B10.D2 F1 1/400 Anti-I-A spleen 1/2,808 Control thymus 1/356 Anti-I-A thymus 1/9,535 2 Control spleen 1/89 Anti-I-A spleen 1/3,081 Control thymus 1/280 Anti-I-A thymus < 1 / Control spleen 1 / 112 Anti-I-A spleen 1/8,486 Control thymus 1/153 Anti-I-A thymus < 1 / Control spleen B6 bml2 F~ 1/110 Anti-I-A spleen 1/4,100 Control thymus 1/349 Anti-I-A thymus <1/104 * In all experiments a linear semilogarithmic relationship between the proportion of negative cultures and the dose of responder cells was observed. * Experiment also shown in Figs. 3 and 4. An experiment with an only allo-i-region difference was included to illustrate that most IL-2 production under the circumstances used is a reflection of I- region recognition. these mice (17). The results in Fig. 5 show that spleen cells and thymic ceils from anti-i-ab-treated B6 (H-2 b) mice were defective in presenting KLH to an I-A b plus KLH-specific T cell clone. Similar results were obtained with two other I- A b plus KLH-specific T cell clones, and with two ailoantigen-specific T cell clones that recognize I-A b antigens (data not shown). This strongly argues that anti-i-a-treated mice lack functional Ia-bearing APC in both the thymus and the spleen. The failure of cells from anti-i-a-treated mice to induce proliferation in several different clones suggest that the APC lack I-A, rather than expressing altered I-A molecules. Mixing experiments, using APC from both normal and anti-i-a-treated mice, excluded induction of suppressor cells as the cause for the abrogation of APC function in anti-i-a-treated mice (data not shown). 4 The possible contribution of defective thymic and/or splenic APC function to the observed defect in class II-restricted T cell proliferative responses was also studied. B6 mice were treated for 1 wk with anti-la b, then left untreated for 2 wk, and subsequently tested for their ability to generate thymic and splenic proliferative responses in MLC. Fig. 6 shows a typical pattern of the effect of this treatment schedule. While splenic responses are still considerably suppressed in such mice, and, in fact, indistinguishable from mice treated continuously for 4 In vivo treatment of adult mice with anti-i-a antibodies: Disappearance of splenic antigen presenting cell function concommitant with modulation of splenic cell surface I-A and I-E antigens A. M. Kruisbeek, J. A. Titus, D. A. Stephany, B. L. Gause, and D. L. Longo. J. Iramunol. In press.
10 1038 ABSENCE OF Lyt-2-,L3T4 CELLS IN ANTI-I-A-TREATED MICE Anti-I-A Thymus Control Thymus 30,000 - v z o 10,000 - ~- 3~100-2 e~ O z oo.:: ~ [,~Y..~: :.~'....:.!;~i ::i" ". :... :. ::.; o.,!~.. =.:.. o. *'oo ".=: i".. : ::, :,, ~.= ::- : o,. ;.fi" 5: " 300 NUMBER OF RESPONDER CELLS PER CULTURE FIGURE 4. Limiting-dilution analysis of IL-2 production by thymocytes from control and anti-i-ab-treated C57BL/6 mice, Conditions as described in legend to Fig. 3, except that thymocytes instead of spleen cells were used as responder cells. 3 wk with anti-i-a, 3 after 2 wk of recovery, thymocyte proliferative responses had returned to almost normal values. Note that 1 wk of treatment is sufficient to suppress both thymocyte and splenic MLC responses (data not shown). In addition, the number of Lyt-2-,L3T4 + T cells in the thymus returned to normal values 2 wk after anti-i-a treatment was terminated (data not shown). Thymic and splenic APC function were also tested 2 wk after termination of anti-i-a treatment. As shown in Fig. 7, thymic and splenic APC function from these mice is indistinguishable from that of untreated mice, even at low APC numbers. The mice used for the experiment in Fig. 7 are the same as those used for Fig. 6. Thus, an interesting picture emerges: when thymic APC function is reduced (Fig. 5), i.e., after 3 wk of continuous anti-ia treatment, splenic and thymic class II-specific MLC responses, 3 as well as IL-2 production in response to an allogeneic stimulus (Figs. 3 and 4) are reduced, and Lyt-2-,L3T4 + cells are absent (Figs. 1 and 2.) However, when thymic APC function has become normal (i.e., 2 wk after treatment has been stopped), thymic MLC responses (Fig. 6) and Lyt- 2-,L3T4 + cell numbers are returning to normal values, while splenic MLC responses are still diminished (Fig. 6). Return of splenic APC function is not correlated with return of splenic T cell responses. We interpret these findings as indicating that the generation of the T cell subset responsible for class II
11 KRUISBEEK ET AL x 10 s APC 5 x 104 APC z O_ 4O ~ Sp een 20 / Spleen O Thymus 10 ~Sp~n Thymus -r Spleen ~o ~._..~.~ I nymus ~ Thymus 0 2, KLH in ~gtrnt ContToI-B6 thymus/spleen o Anti-I-A-B6 thymus/spleen (2 weeks of treatrne~'lt) FIGURE 5. Splenic and thymic APC from B6 mice treated from birth with anti-i-a b cannot present antigen (KLH) to KLH plus I-Ab-specific T cell clones. 104 cloned T cells from a KLH plus l-ab-specific clone were cultured with the indicated doses of KLH, and either 2 x l0 s or 5 x 104 2,000 rad irradiated APC, collected from the interphase of a BSA gradient consisting of a 35% and an 11% BSA layer. Cultures were labeled with 1 /~Ci [SH]thymidine on day 2, and harvested on day 3. [SH]Thymidine incorporation is expressed as the mean of triplicate cultures; standard deviation values were <14%. Control cpm values were 536 _+ 67 for T cells alone, and ranged from 560 to 869 cpm for the various types and numbers of APC. recognition depends on thymic, rather than splenic APC function, and that after anti-ia treatment, new T cells capable of Ia recognition must be generated in the thymus before such cells can be detected in the periphery. Discussion Although the MHC phenotype of the nonlymphoid cells in the thymus has been implicated in the process that selects the MHC restriction specificities of T cells during development (1-15), the precise intrathymic events determining acquisition of MHC restriction specificities remain unresolved. Some of the most basic questions regarding the development of class I- and class II-specific T cells are: With which MHC-bearing nonlymphoid cells do developing T cells interact? Do cells destined to become class II-specific T cells interact with intrathymic elements different than those required for development of precursors of the class I-specific repertoire? Do intrathymic events determine the strong association between expression of L3T4 or Lyt-2 and, respectively, MHC subclass specificity for class II or class I? In the experiments reported here, an attempt is made to better understand development of MHC restriction specificities through interference with intrathymic expression of MHC antigens, specifically I-A antigens. This paper also describes the first experiments to have examined whether development of Lyt-2-,L3T4 + T cells can be separated from development of the Lyt-2+,L3T4 - T cell subset. We present evidence that, in mice treated from birth with anti-i-a, the thymus and spleen do not contain Lyt-2-,L3T4 + T cells, as defned by dual-parameter FACS analysis. We also investigated whether absence of Lyt2-,L3T4 T cells was accompanied by decreased IL-2 production, and found that anti-i-a-treated mice develop only very low levels of IL-2-producing precursors. Therefore, absence of the Lyt-2-,L3T4 subset of T cells was defined both by marker
12 1040 ABSENCE OF Lyt-2-,L3T4 CELLS IN ANTI-I-A-TREATED MICE _ # _~ 10 K+I {BI0.GD) / - I(bm12} Reaponder Cells: Spleen 81 I I i i i 8 z I.-,- 10 J Reeponder Cells: Thymus NUMBER OF STIMULATOR SPLEEN CELLS (x 10 "5) 4 x 10 s Contrc4 B6-responder cetls o 4 x 10 s Anti-I-A b B6-responder cells (1 week treatment, 2 weeks no treatment) z 4 x 10 5 Anti-I-A b B6-responder cells (3 weeks treatment) FIGURE 6. Thymocyte proliferative responses to allogeneic stimulator cells recover before spleen cell responses in B6 mice treated neonatally with anti-l-a b, after the treatment is stopped spleen cells or 6 x 105 thymocytes from control and anti-i-a-treated C57BL/6 mice were cultured with the indicated numbers of 2,000 tad irradiated allogeneic spleen cells, in a volume of 0.2 ml. Mice were either treated for 1 wk with anti-i-a b, then left untreated for 2 wk before being tested (O), or treated continuously for 3 wk (Z~). 1 wk after treatment was terminated, thymocyte and spleen cell responses of mice from the same litter were still completely suppressed (data not shown). Spleen cell cultures were harvested on day 4; thymocyte cultures were harvested on day 5, each after a h labeling period in the presence of 1 #Ci of [SH]thymidine. Control values in cultures containing stimulator cells only hml varied from 700 to 1,400 cpm. Responses to a class I alloantigen difference (H-2 stimulator cells) or to Mls-disparate stimulator cells (D1.LP) behaved similarly, spleen cells being still almost entirely suppressed, and thymocytes exhibiting considerable recovery (data not shown). analysis and by function. The presence of the lineage of Lyt-2+,L3T4 - cells was similarly defined both functionally (i.e., normal CTL precursors were present in these mice) (18), and through marker analysis: the dual-parameter FACS patterns demonstrate that the number of Lyt-2+,L3T4 - T cells in thymus and spleen is not affected by neonatal anti-i-a treatment. Several points can be made regarding these findings. First, completion of the generation of the two major compartments of the T cell repertoire is clearly not a single event: development of the Lyt-2-,L3T4 + lineage can be selectively abrogated by a manipulation (i.e., neonatal anti-i-a treatment) that leaves the Lyt-2+,L3T4 - lineage unaffected. Our experiments do not distinguish whether the Lyt-2-,L3T4 + subset does not develop at all, or does develop, initially, but fails to expand to detectable levels. Perhaps it needs interaction with intrathymic I-A-bearing cells (see below) or factors produced by such cells as a signal to expand.
13 KRUISBEEK ET AL Z o_ <~ s0 2 x 10SAPC --.f~,'o%ep*ee n 5 x 104APC Zo Thymus 10 ~Spleen Thymus >- 20 -,r I..I I I O Control 136 thymus/spleen KLH (in/kg/ml) o Anti-l-A b B6 thymus/spleen (1 week treatment, 2 weeks no treatment) FIGURE 7. In B6 mice treated for 1 wk after birth with anti-i-a b, APC function in spleen and thymus has returned to normal values 2 wk after the treatment is terminated. Conditions were as described in legend to Fig. 5. Thymus and spleen cell suspensions were from the same mice whose T cell proliferative responses are shown in Fig. 6. Control cpm values were 468 _+ 71 for cloned T cells alone, and varied from 289 to 761 for the various types and numbers of APC. Second, expression of the L3T4 + marker is, in and of itself, not a feature dependent on intrathymic I-A-bearing cells, because normal levels (~80%) of cells staining positively for both L3T4 and Lyt-2 are found in the thymus of anti- I-A-treated mice, in accord with previous reports on the size of this "immature," cortical thymocyte population (30) in the normal thymus. There is currently no consensus on the relationship between the immature Lyt-2+,L3T4 + subset and the lineages of Lyt-2+,L3T4 - and Lyt-2-,L3T4 + cells (45, 47-49); it is not clear whether precursors for either one or both of these lineages are contained within the Lyt-2+,L3T4 + subset. Several studies (45, 49, 50) identified a dull Lyt-1 +, Lyt-2-,L3T4- precursor cell of the Lyt-2+,L3T4 + subset, while recent 5 in vivo transfer studies demonstrated that this Lyt-1 + dull precursor cell can give rise to all three other thymocyte subsets: Lyt-2+,L3T4+; Lyt-2-,L3T4+; and Lyt- 2+,L3T4 -. Our findings suggest that generation of the subsets of Lyt-2+,L3T4 + and Lyt-2+,L3T4 - cells is not dependent on I-A-bearing elements affected by anti-i-a treatment, while generation of the Lyt-2-,L3T4 + subset is. Models such as this one may aid in further dissecting the intrathymic differentiation pathways leading to the various T cell subsets. Third, the question arises whether, in fact, all IL-2-producing T cells, regardless of their MHC subregion specificity, are affected by anti-i-a treatment. In our experiments, IL-2 production was induced by a full MHC difference or class II-alloantigen difference only, thus reflecting mainly activation of class IIspecific Lyt-2- T cells, regardless of whether Lyt-2 + T cells were present or absent in the responder cell population (30, 41, 42). Accordingly, no conclusion can be made about the capacity of Lyt-2 + T cells to produce IL-2. Although the Lyt-2 + subset, as defined by marker analysis, is apparently unaffected by anti-i- A treatment, additional experiments are required to determine whether the 5 Early T lymphocytes: Differentiation in vivo of adult intrathymic precursor cells. B.J. Fowlkes, L. Edison, B.J. Mathieson, and T. M. Chused. Manuscript submitted for publication.
14 1042 ABSENCE OF Lyt-2-,L3T4 CELLS IN ANTI-I-A-TREATED MICE frequency of IL-2-producing Lyt-2 + cells is affected. To this end, stimulation with a class I-alloantigen difference only will be used (22, 23). The absence of the lineage of Lyt-2-,L3T4 T cells in the present model was correlated with absence of thymic APC function for class II-specific T cells. Thymic APC are bone marrow-derived (14, 16), and located in the medulla or at the cortico-medullary junction (16, 51). Most of the phenotypically and functionally "mature" thymocytes are located in the medulla (30, 31, 47, 52, 53). Taken together, these studies suggest that generation and/or expansion of the Lyt-2-,L3T4 + subset requires an interaction of an unknown thymocyte precursor cell with I-A-bearing elements, presumably APC, in the thymus medulla. Additional experiments are required to determine whether this interaction requires self-i-a recognition, as might be expected on the basis of experiments by others (54). Furthermore, thymic APC may have a nonspecific role in the expansion of thymocytes through release of factors such as IL-1. Finally, the question whether thymic APC have truly disappeared in anti-i-a-treated mice, or become nonfunctional through loss of Ia-antigens is currently investigated histologically, using a panel of mab with which cortical and medullary thymic stromal cells can be distinguished on tissue sections (55). Notwithstanding the correlation between lack of thymic APC and absence of the Lyt-2-,L3T4 + subset, a contribution of the lack of B cells in anti-i-a-treated mice (17) to the peripheral T cell defects may also be considered. Mice treated neonatally with anti-igm (56-58) express altered Ig idiotype specificities in their T cell repertoire, in addition to lacking B cells. This suggests that idiotypespecific interactions between B cells and T cells contribute to the selection of T cell repertoire specificities. Such effects might also occur in anti-i-a-treated mice. Some differences between anti-igm- and anti-i-a-treated mice, however, should be mentioned: (a) antigen-specific helper T cells are easily generated in anti-igm-treated mice (57, 58), while anti-i-a-treated mice lack T cells of the phenotype commonly associated with helper cell function; (b) although anti-igmtreated mice have impaired antigen-specific T cell proliferative responses, they express normal MLC reactivity (59), contrasting with the strongly impaired MLC reactivity in anti-i-a-treated mice s (this report). It remains to be established whether the absence of the B cell compartment in anti-i-a-treated mice affects the T cell repertoire. We propose that a primary defect is an inability of the Lyt- 2-,L3T4 + subset to develop or expand intrathymically in the absence of functional I-A-bearing APC. Summary In an effort to elucidate the role of intrathymic Ia-bearing antigen-presenting cells (APC) on the development of the class II-restricted T cell repertoire, we examined the effect of neonatal anti-i-a treatment on both intrathymic and splenic APC function; on the generation of Lyt-2-,L3T4 +, Lyt-2+,L3T4 -, and Lyt-2+,L3T4 T cells; and on the development of class I- and class II-specific T cell functions. Both the thymus and the spleen are completely devoid of Lyt- 2-,L3T4 + T cells in young mice treated from birth with anti-i-a, and also lack functions associated with this subset, i.e., alloantigen-specific interleukin 2 production (present report), allo-class II-specific and self-class II-restricted T cell
15 KRUISBEEK ET AL proliferative responses, 3 and helper cell function for the generation of cytotoxic T lymphocyte responses (18). Development of the Lyt-2 +,L3T4- subset proceeds undisturbed in these mice, in accord with the previously reported normal levels of cytotoxic T lymphocyte precursors (18). The thymus contains normal numbers of the immature cortical Lyt-2+,L3T4 + cells, indicating that acquisition of the L3T4 marker, in and of itself, is not influenced by anti-i-a treatment. This striking absence of the lineage of T cells responsible for class II-specific T cell functions is correlated with absence of thymic APC function for class II-restricted T cell clones. When anti-i-a-treated mice are allowed to recover from the antibody treatment, splenic and thymic APC function return to normal in 2-3 wk, and thymic Lyt-2-,L3T4 T cell numbers and functions reappear before such cells are detectable in the spleen. Collectively, these findings suggest that development of the Lyt-2-,L3T4 + lineage of class II-specific T cells is entirely dependent on functional I-A-bearing APC cells in the thymus. In addition, the presence of normal levels of Lyt-2+,L3T4 - T cells argues that generation of the two major subsets of T cells (i.e., Lyt-2+,L3T4 - and Lyt-2-,L3T4 +) occurs through separate events, involving unique sites of interactions between precursor T cells and nonlymphoid major histocompatibility complex-bearing thymus cells. We thank Dr. Thomas R. Malek for his kind contribution of CTLL cells, Dr. Richard A. Miller for his advice in developing the limiting-dilution assay, Doreen Gumbs for her expert technical assistance, Louise Pastuck for excellent secretarial assistance, Drs. Richard J. Hodes and Ronald N. Germain for help in reviewing the manuscript, and Dr. Thomas M. Chused and Linette Edison for their help in running the FACS experiments. Received for publication 19 December 1984 and in revised form 21January References 1. Zinkernagel, R. M., G. N. Calahan,J. Klein, and G. Dennert Cytotoxic T cells learn specificity for self H-2 during differentiation in the thymus. Nature (Lond.). 271: Fink, P.J., and M.J. Bevan H-2 antigens of the thymus determine lymphocyte specificity.j. Exp. Med. 148: Zinkernagel, R. M., and P. C. Doherty MHC-restricted cytotoxic T cells: Studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. lmmunol. 27: Bradley, S. M., A. M. Kruisbeek, and A. Singer Cytotoxic T lymphocyte response in aliogeneic radiation bone marrow chimeras. The chimeric host strictly dictates the self-repertoire of Ia-restricted T cells but not H-2 K/D restricted T cells. J. Exp. Med. 156: Kruisbeek, A. M., S. O. Sharrow, and A. Singer Differences in the MHC restricted self-recognition repertoire of intra-thymic and extra-thymic cytotoxic T lymphocyte precursors. J. Immunol. 130: Wagner, H., C. Hardt, H. Stockinger, K. Pfizenmaier, R. Barlet, and M. Rollinghoff The impact of the thymus on the generation of immunocompetence and diversity of antigen-specific MHC-restricted cytotoxic T-lymphocyte precursors. Immunol. Rev. 58: Doherty, P. C., Korngold, D. H. Schwartz, andj. R. Bennink The development
16 1044 ABSENCE OF Lyt-2-,L3T4 + CELLS IN ANTI-I-A-TREATED MICE and loss of virus-specific thymic competence in bone marrow radiation chimeras and normal mice. Immunol. Rev. 58: Kruisbeek, A. M., S. O. Sharrow, B. J. Mathieson, and A. Singer The H-2 phenotype of the thymus dictates the self-specificity expressed by thymic but not splenic cytotoxic T lymphocyte precursors in thymus-engrafted nude mice. J. Immunol. 127: Singer, A., K. S. Hathcock, and R. J. Hodes Self-recognition in allogeneic thymus chimeras. Self-recognition by T-helper cells from thymus-engrafted nude mice is restricted to the thymic H-2 haplotype. J. Exp. Med. 155: Hedrick, S. M., and J. Watson Genetic control of the immune response to collagen. II. Antibody responses produced in fetal liver restored radiation chimeras and thymus-reconstituted F~ nude mice.j. Exp. Med. 150: Glimcher, L. H., R. H. Schwartz, D. L. Longo, and A. Singer The specificity of the syngeneic mixed lymphocyte response, a primary anti-i-region T cell proliferative response, is determined intrathymically.j. Immunol. 129: Longo, D. L., and R. H. Schwartz Inhibition of antigen-induced T cells from radiation induced bone marrow chimeras by a monoclonal antibody directed against an Ia determinant of the antigen-presenting cell. Proc. Natl. Acad. Sci. USA. 78: Kruisbeek, A. M., M. L. Davis, L. A. Matis, D. L. Longo The self-recognition specificity expressed by T cells from nude mice. Absence of detectable Ia-restricted T cells in nude mice that do exhibit self-k/d restricted T cell responses.j. Exp. Med. 160: Longo, D. L., and R. H. Schwartz T-cell specificity for H-2 and Ir-gene phenotype correlates with the phenotype of thymic antigen-presenting cells. Nature (Lond.). 287: Longo, D. L., and M. L. Davis Early appearance of donor-type antigen presenting cells in the thymuses of 1,200 rad radiation-induced bone marrow chimeras correlates with self-recognition of donor I region gene products. J. Immunol. 130: Barclay, A. N., and G. Mayrhofer Bone marrow origin of Ia-region positive cells in the medulla of rat thymus. J. Exp. Med. 153: Fultz, M.J., I. Scher, F. D. Finkelman, P. Kincade, andj. J. Mond Neonatal suppression with anti-ia antibody. I. Suppression of murine B lymphocyte development. J. Immunol. 129: Kruisbeek, A. M., M.J. Fultz, S. O. Sharrow, A. Singer, andj.j. Mond Early development of the T cell repertoire. In vivo treatment of neonatal mice with anti- Ia antibodies interferes with differentiation of I-restricted T cells but not K/D restricted T cells.j. Exp. Med. 157: Shinohara, N., and D. Sachs Mouse alloantibodies capable of blocking cytotoxic T-cell function. I. Relationship between the antigen reactive with blocking antibodies and the Lyt-2 locus.j. Exp. Med. 150: Nakayma, E., H. Shiku, E. Stockert, H. F. Oetgen, and L.J. Old Cytotoxic T cells: Lyt phenotype and blocking of killing activity by Lyt antisera. Proc. Natl. Acad. Sci. USA. 76: Swain, S., and P. R. Panfili Helper cells activated by allogeneic H-2K or H- 2D differences have an phenotype distinct from those responsive to Ia differences. J. lmmunol. 122: Swain, S. L Significance of Lyt phenotypes: Lyt 2 antibodies block activation of T cells that recognize class I MHC antigens regardless of their function. Proc. Natl. Acad. Sci. USA. 78: Swain, S., D. P. Dialynas, F. W. Fitch, and M. English Monoclonal antibody
17 KRUISBEEK ET AL to L3T4 blocks the function of T cells specific for class II major histocompatibility complex antigens. J. Immunol. 132: Dialynas, D. P., D. B. Wilde, P. Marrack, A. Pierres, K. A. Wall, W. Havran, G. Otten, M. R. Loken, M. Pierres,J. Kappler, and F. W. Fitch Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK 1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Iramunol. Rev. 74: Wilde, D. B., P. Marrack,J. Kappler, D. P. Dialynas, and F. W. Fitch Evidence implicating L3T4 in class II MHC antigen-reactivity: Monoclonal antibody GKI.5 (anti-l3t4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J. Immunol. 131: Marrack, P., R. Endres, R. Shimonkevitz, A. Zlomik, D. Dialynas, F. Fitch, and J. Kappler The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product.j. Exp. Med. 158: Dialynas, D. P., Z. S. Quan, K. A. Wail, A. Pierres, J. Quintans, M. L. Loken, M. Pierres, and F. W. Fitch Characterization of the murine T cell surface molecule designated L3T4, identified by monoclonal antibody GK1.5: Similarity of L3T4 to the human Leu-3/T4 molecule. J. Immunol. 131: Pierres, A., P. Naquet, A. V. Agthoven, F. Bekkhoucha, F. Denizot, Z. Mishal, A.- M. Schmitt-Verhulst, and M. Pierres A rat anti-mouse T4 monoclonal antibody (H ) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4 +, Lyt2,3-, and T4-, Lyt 2,3 +) subsets among anti-ia cytolytic T cell clones.j, lmmunol. 132: MacDonald, H. R., A. L. Glasebrook, andj.-c. Cerottini Clonal heterogeneity in the functional requirement for Lyt-2,3 molecules on cytotoxic T lymphocytes: Analysis by antibody blocking and selective trypsinization. J. Exp. Med. 156: Ceredig, R., D. P. Dialynas, F. W. Fitch, and H. R. MacDonald Precursors of T cell growth factor producing cells in the thymus: Ontogeny, frequency, and quantitative recovery in a subpopulation ofphenotypically mature thymocytes defined by monoclonal antibody GK-1.5.J. Exp. Med. 158: Ceredig, R., A. L. Glasebrook, and H. R. MacDonald Phenotypic and functional properties of murine thymocytes. I. Precursors of cytolytic T lymphocytes and interleukin 2-producing cells are all contained within a subpopulation of "mature" thymocytes, as analyzed by monoclonal antibodies and flow microfluorometry.j. Exp. Med. 155: Janeway, C. A., P.J. Conrad, E. A. Lerner,J. Babick, P. Wettstein, and D. B. Murphy Monoclonal antibodies specific for Ia glycoproteins raised by immunization with activated T cells. Possible role of T cell-bound Ia antigens as target of immunoregulatory cells. J. lmmunol. 132: Click, R. E., L. Benck, and B. J. Alter Immune responses in vitro I. Culture conditions for antibody synthesis. Cell. Immunol. 3: Hammerling, G. J., U. Hammerling, and L. Flaherty Qat-4 and Qat-5, new murine T cell antigens governed by the Tla region, and identified by monoclonal antibodies. J. Exp. Med. 150: Titus, J. A., R. Haughland, S. O. Sharrow, and D. M. Segal Texas Red, a hydrophi}lic, red-emitting fluorophore for use with fluorescein in dual parameter flow micro-fluorometric and fluorescence microscope studies. J. lmmunol. Methods. 50: Lanier, L. L., G. A. Gutman, D. E. Lewis, S. T. Griswold, and N. L. Warner Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1:125.
18 1046 ABSENCE OF Lyt-2-,L3T4 CELLS IN ANTI-I-A-TREATED MICE 37. Ledbetter, J. A., R. V. Crouse, H. S. Micklem, and L. A. Herzenberg T cell subsets defined by expression of Lyt-l,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxic analysis with monoclonal antibodies modifies current views.j. Exp. Med. 152: Monobar, V., E. Brown, W. M. Leiserson, and T. M. Chused Expression of Lyt-1 by a subset of B lymphocytes.j. Immunol. 129: Loken, M. R., and L. A. Herzenberg Analysis of cell populations with a fluorescence activated cell sorter. Ann. N.Y. Acad. Sci. 254: Kristan, A Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell. Biol. 66: Miller, R. A., and O. Stutman Enumeration of IL-2-secreting helper T cells by limiting dilution analysis, and demonstration of unexpectedly high levels of IL-2 production per responding celi.j. Immunol. 128: Kelso, A., and H. R. MacDonald Precursor frequency analysis of lymphokinesecreting alloreactive T lymphocytes. Dissociation of subsets producing interleukin 2, macrophage activating factor, and granulocyte-macrophage colony-stimulating factor on the basis of Lyt-2 pbenotype. J. Exp. Med. 156: Taswell, C Limiting dilution assays for the determination ofimmunocompetent cell frequencies. I. Data analysis. J. Immunol. 126: Kimoto, M., and C. G. Fathman Antigen-reactive T cell clones. I. Transcomplementing hybrid I-A region gene products function effectively in antigen presentation.j. Exp. Med. 152: Fowlkes, B.J., L. Edison, B. Mathieson, and T. M. Chused Differentiation in vitro of an adult precursor thymocyte. In Regulation of the immune system. H. Cantor, L. Chess, and E. Sercarz, editors. Alan Liss, Inc. New York, p Kronke, M., P. Scheurich, K. Pfizenmaier, M. Rollinghoff, and H. Wagner T cell interactions during in vitro cytotoxic T lymphocyte (CTL) responses. V. Precursor frequencies and specificity ofalloreactive helper T cells.j. Exp. Med. 156: Scollay, R., and K. Shortman Thymocyte subpopulations: An experimental review, including flow cytometric cross-correlations between the major murine thymocyte markers. Thymus. 5: Reichert, R. A., W. M. Gallatin, E. C. Butcher, and I. L. Weissman A homing receptor-bearing cortical thymocyte subset: Implications for thymus cell migration and the nature of cortisone-resistant thymocytes. Cell. 38: Mathieson, B. J., B. J. Fowlkes Cell surface antigen expression of thymocytes: Developmental and phenotypical differences of intrathymic subsets. Immunol. Rev. 82: Ceredig, R., R. P. SekaIy, and H. R. McDonald Differentiation in vitro of Lyt- 2 + thymocytes from embryonic Lyt-2- precursors. Nature (Lond.). 303: Duijvestijn, A. M., and E. C. M. Hoefsmit Ultrastructure of the rat thymus: The microenvironment of T lymphocyte maturation. Cell Tissue Res. 218: BIomgren, H., and B. Anderson Characteristics of the immunocompetent cells in the mouse thymus: Cell population changes during cortisone induced atrophy and subsequent regeneration. Cell. Immunol. 1: Van Ewijk, W., P. L. van Soest, and G.J. van den Engh Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2 and T-200.J. lmmunol. 127: Rock, K. L., and B. Benacerraf Thymic T cells are driven to expand upon interaction with self-class II major histocompatibility complex gene products on accessory cells. Proc. Natl. Acad. Sci. USA. 81:1221.
19 KRUISBEEK ET AL Van Vliet, E., M. Melis, and W. V. Ewijk Monoclonal antibodies to stromal cell types of the mouse thymus. Eur. J. lmmunol. 14: Bottomly, K., C. A. Janeway, B. J. Mathieson, and D. E. Mosier Absence of an antigen-specific helper T cell required for the expression of the T 15 idiotype in mice treated with anti-t~ antibody. Eur. J. Imraunol. 10: Sy, M. S., A. Lowy, K. Hayglass, C. A. Janeway, M. Gurish, M. I. Greene, and B. Benacerraf Chronic treatment with rabbit anti-mouse u-chain antibody alters the characteristic immunoglobulin heavy-chain restriction of murine suppressor T- cell factors. Proc. Natl. Acad. Sci. USA. 81: Martinez, A. C., P. Pereira, R. Bernabe, A. Bandeira, E.-L. Larsson, P.-A. Casenave, and A. Coutinho Internal complementarities in the immune system: Regulation of the expression of helper cell idiotypes. Proc. Natl. Aead. Sci. USA. 81: Ron, Y., P. de Baetselier, J. Gordon, M. Feldman, and S. Segal Defective induction of antigen reactive proliferating T cells in B cell-deprived mice. Eur. J. Immunol. 11:964.
Brief Det~nitive Report
 Brief Det~nitive Report THYMIC RECONSTITUTION OF NUDE F MICE WITH ONE OR BOTH PARENTAL THYMUS GRAFTS* BY ROLF M. ZINKERNAGEL, A. ALTHAGE, AND G. CALLAHAN From the Departments of Immunopathology and of
Brief Det~nitive Report THYMIC RECONSTITUTION OF NUDE F MICE WITH ONE OR BOTH PARENTAL THYMUS GRAFTS* BY ROLF M. ZINKERNAGEL, A. ALTHAGE, AND G. CALLAHAN From the Departments of Immunopathology and of
Nature Biotechnology: doi: /nbt.4086
 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
T CELLS FROM NUDE MICE Absence of Detectable Ia-restricted T Cells in Nude Mice That Do Exhibit Self-K/D-restricted T Cell Responses
 SELF-RECOGNITION SPECIFICITY EXPRESSED BY T CELLS FROM NUDE MICE Absence of Detectable Ia-restricted T Cells in Nude Mice That Do Exhibit Self-K/D-restricted T Cell Responses BY ADAM. KRUISBEEK, MARY L.
SELF-RECOGNITION SPECIFICITY EXPRESSED BY T CELLS FROM NUDE MICE Absence of Detectable Ia-restricted T Cells in Nude Mice That Do Exhibit Self-K/D-restricted T Cell Responses BY ADAM. KRUISBEEK, MARY L.
Nature Immunology: doi: /ni.3694
 Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplemental Information Inventory
 Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
IMMUNOGLOBULINS ON THE SURFACE OF LYMPHOCYTES
 IMMUNOGLOBULINS ON THE SURFACE OF LYMPHOCYTES II. THE BONE MARROW AS THE MAIN SOURCE OF LYMPHOCYTES WITH DETECTABLE SURFACE-BouND IMMUNOGLOBULIN* BY EMIL R. UNANUE,{C M.D., HOWARD M. GREY, M.D., ENRIQUE
IMMUNOGLOBULINS ON THE SURFACE OF LYMPHOCYTES II. THE BONE MARROW AS THE MAIN SOURCE OF LYMPHOCYTES WITH DETECTABLE SURFACE-BouND IMMUNOGLOBULIN* BY EMIL R. UNANUE,{C M.D., HOWARD M. GREY, M.D., ENRIQUE
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development
 Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
BD IMag. Streptavidin Particles Plus - DM. Technical Data Sheet. Product Information
 Technical Data Sheet Streptavidin Particles Plus - DM Product Information Material Number: Size: Storage Buffer: 557812 5 ml Aqueous buffered solution containing BSA and 0.09% sodium azide. Description
Technical Data Sheet Streptavidin Particles Plus - DM Product Information Material Number: Size: Storage Buffer: 557812 5 ml Aqueous buffered solution containing BSA and 0.09% sodium azide. Description
OF SOLUBLE HELPER MOLECULES IN T CELL RESPONSES TO ALTERED SELF*
 MAJOR HISTOCOMPATIBILITY COMPLEX RESTRICTION OF SOLUBLE HELPER MOLECULES IN T CELL RESPONSES TO ALTERED SELF* Be JANET M. D. PLATE From the Section of Medical Oncology, Department of Internal Medicine,
MAJOR HISTOCOMPATIBILITY COMPLEX RESTRICTION OF SOLUBLE HELPER MOLECULES IN T CELL RESPONSES TO ALTERED SELF* Be JANET M. D. PLATE From the Section of Medical Oncology, Department of Internal Medicine,
Writing Your Honors Thesis
 Writing Your Honors Thesis Your thesis should be in the common scientific paper format using FIVE separate sections Abstract Introduction Materials and Methods Results Discussion Keep in mind----your thesis
Writing Your Honors Thesis Your thesis should be in the common scientific paper format using FIVE separate sections Abstract Introduction Materials and Methods Results Discussion Keep in mind----your thesis
Chapter 3. Clonal selection
 Chapter 3. Clonal selection I have called this principle, by which each slight variation, if useful, is preserved, by the term of Natural Selection -Charles Darwin, On the Origin of Species, 1859 4 The
Chapter 3. Clonal selection I have called this principle, by which each slight variation, if useful, is preserved, by the term of Natural Selection -Charles Darwin, On the Origin of Species, 1859 4 The
Increased expression of major histocompatibility complex antigens
 Proc. Natl. Acad. Sci. USA Vol. 84, pp. 7624-7628, November 1987 Immunology Increased expression of major histocompatibility complex antigens on lymphocytes from aged mice CHARLES L. SIDMAN*, EDGAR A.
Proc. Natl. Acad. Sci. USA Vol. 84, pp. 7624-7628, November 1987 Immunology Increased expression of major histocompatibility complex antigens on lymphocytes from aged mice CHARLES L. SIDMAN*, EDGAR A.
[GANN, 62, ; April, 1971]
![[GANN, 62, ; April, 1971] [GANN, 62, ; April, 1971]](/thumbs/86/94910685.jpg) [GANN, 62, 139-143; April, 1971] UDC 576.8.097.3:547.854 EVALUATION OF CELL DAMAGE IN IMMUNE REACTIONS BY RELEASE OF RADIOACTIVITY FROM 3H-URIDINE LABELED For quantitative evaluation of cell damage in
[GANN, 62, 139-143; April, 1971] UDC 576.8.097.3:547.854 EVALUATION OF CELL DAMAGE IN IMMUNE REACTIONS BY RELEASE OF RADIOACTIVITY FROM 3H-URIDINE LABELED For quantitative evaluation of cell damage in
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam will have 40 multiple choice questions.
 MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
a. Hypoxanthine was present in the media. MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No.
 MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
isolated from ctr and pictreated mice. Activation of effector CD4 +
 Supplementary Figure 1 Bystander inflammation conditioned T reg cells have normal functional suppressive activity and ex vivo phenotype. WT Balb/c mice were treated with polyi:c (pic) or PBS (ctr) via
Supplementary Figure 1 Bystander inflammation conditioned T reg cells have normal functional suppressive activity and ex vivo phenotype. WT Balb/c mice were treated with polyi:c (pic) or PBS (ctr) via
Antibody used for FC Figure S1. Multimodal characterization of NIR dyes in vitro Figure S2. Ex vivo analysis of HL60 cells homing
 Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Adaptive Immunity: Specific Defenses of the Host
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 17 Adaptive Immunity: Specific Defenses of the Host The Adaptive Immune System Adaptive immunity:
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 17 Adaptive Immunity: Specific Defenses of the Host The Adaptive Immune System Adaptive immunity:
Chapter 14. Second symmetry
 Chapter 14. Second symmetry Tyger Tyger, burning bright In the forests of the night What immortal hand or eye Could frame thy fearful symmetry? -William Blake Songs of Experience (1794) A good general
Chapter 14. Second symmetry Tyger Tyger, burning bright In the forests of the night What immortal hand or eye Could frame thy fearful symmetry? -William Blake Songs of Experience (1794) A good general
colorimetric sandwich ELISA kit datasheet
 colorimetric sandwich ELISA kit datasheet For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity
colorimetric sandwich ELISA kit datasheet For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity
MicroRNAs Modulate Hematopoietic Lineage Differentiation
 Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Supplementary figures
 Mucida et al. Supplementary material Supplementary figures Supplementary Figure 1. Oral administration of OVA suppresses Th2 differentiation, Germinal Center (GC) formation and immunoglobulin class switching
Mucida et al. Supplementary material Supplementary figures Supplementary Figure 1. Oral administration of OVA suppresses Th2 differentiation, Germinal Center (GC) formation and immunoglobulin class switching
Human IgG Antigen ELISA Kit
 Human IgG Antigen ELISA Kit Catalog No: IHUIGGKT Lot No: SAMPLE INTENDED USE This human immunoglobulin G antigen assay is intended for the quantitative determination of total human IgG antigen in serum,
Human IgG Antigen ELISA Kit Catalog No: IHUIGGKT Lot No: SAMPLE INTENDED USE This human immunoglobulin G antigen assay is intended for the quantitative determination of total human IgG antigen in serum,
Cyno Monkey IgG Antigen ELISA Kit
 Cyno Monkey IgG Antigen ELISA Kit Catalog No: ICYIGGKT Lot No: SAMPLE INTENDED USE This cynomolgus macaque (Macaca fascicularis) monkey Immunoglobulin G (IgG) antigen assay is intended for the quantitative
Cyno Monkey IgG Antigen ELISA Kit Catalog No: ICYIGGKT Lot No: SAMPLE INTENDED USE This cynomolgus macaque (Macaca fascicularis) monkey Immunoglobulin G (IgG) antigen assay is intended for the quantitative
BY ISRAEL ZAN-BAR,* ELLEN S. VITETTA, AND SAMUEL STROBER
 THE RELATIONSHIP BETWEEN SURFACE IMMUNOGLOBULIN ISOTYPE AND IMMUNE FUNCTION OF MURINE B LYMPHOCYTES II. Surface Immunoglobulin Isotopes on Unprimed B Cells in the Spleen* BY ISRAEL ZAN-BAR,* ELLEN S. VITETTA,
THE RELATIONSHIP BETWEEN SURFACE IMMUNOGLOBULIN ISOTYPE AND IMMUNE FUNCTION OF MURINE B LYMPHOCYTES II. Surface Immunoglobulin Isotopes on Unprimed B Cells in the Spleen* BY ISRAEL ZAN-BAR,* ELLEN S. VITETTA,
PARAFORMALDEHYDE FIXATION OF HEMATOPOIETIC CELLS FOR QUANTITATIVE FLOW CYTOMETRY (FACS) ANALYSIS 1
 Journal oflmmunological Methods, 47 (1981) 25--30 25 Elsevier/North-Holland Biomedical Press PARAFORMALDEHYDE FIXATION OF HEMATOPOIETIC CELLS FOR QUANTITATIVE FLOW CYTOMETRY (FACS) ANALYSIS 1 L.L. LANIER
Journal oflmmunological Methods, 47 (1981) 25--30 25 Elsevier/North-Holland Biomedical Press PARAFORMALDEHYDE FIXATION OF HEMATOPOIETIC CELLS FOR QUANTITATIVE FLOW CYTOMETRY (FACS) ANALYSIS 1 L.L. LANIER
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang *
 In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
Antibodies to Animal Cells and Serum Proteins
 ANTIBODIES TO ANIMAL CELLS AND SERUM PROTEINS Antibodies to Animal Cells and Serum Proteins Antibodies to Animal Cellular Antigens Antibodies to Mouse Brain Antibodies to Animal Serum Proteins INTERNATIONAL
ANTIBODIES TO ANIMAL CELLS AND SERUM PROTEINS Antibodies to Animal Cells and Serum Proteins Antibodies to Animal Cellular Antigens Antibodies to Mouse Brain Antibodies to Animal Serum Proteins INTERNATIONAL
Bcl-2 family member Bcl-G is not a pro-apoptotic BH3-only protein
 Bcl-2 family member Bcl-G is not a pro-apoptotic BH3-only protein Maybelline Giam 1,2, Toru Okamoto 1,2,3, Justine D. Mintern 1,2,4, Andreas Strasser 1,2 and Philippe Bouillet 1, 2 1 The Walter and Eliza
Bcl-2 family member Bcl-G is not a pro-apoptotic BH3-only protein Maybelline Giam 1,2, Toru Okamoto 1,2,3, Justine D. Mintern 1,2,4, Andreas Strasser 1,2 and Philippe Bouillet 1, 2 1 The Walter and Eliza
CYTOTOXIC T LYMPHOCYTE RESPONSES. Self-Recognition Is Determined Early in T Cell Development BY ADAM. KRUISBEEK,* RICHARD J. HODES, AND ALFRED SINGER
 CYTOTOXIC T LYMPHOCYTE RESPONSES BY CHIMERIC THYMOCYTES Self-Recognition Is Determined Early in T Cell Development BY ADAM. KRUISBEEK,* RICHARD J. HODES, AND ALFRED SINGER From the Immunology Branch, National
CYTOTOXIC T LYMPHOCYTE RESPONSES BY CHIMERIC THYMOCYTES Self-Recognition Is Determined Early in T Cell Development BY ADAM. KRUISBEEK,* RICHARD J. HODES, AND ALFRED SINGER From the Immunology Branch, National
Mouse Factor XII Total ELISA Kit
 Mouse Factor XII Total ELISA Kit Catalog No: IMFXIIKT-TOT Lot No: SAMPLE INTENDED USE This mouse coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor XII
Mouse Factor XII Total ELISA Kit Catalog No: IMFXIIKT-TOT Lot No: SAMPLE INTENDED USE This mouse coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor XII
Interferon Immunosuppression: Mediation by a Suppressor
 INFECTION AND IMMUNITY, Aug. 1980, p. 301-305 0019-9567/80/08-0301/05$02.00/0 Vol. 29, No. 2 Interferon Immunosuppression: Mediation by a Suppressor Factor HOWARD M. JOHNSON* AND J. EDWIN BLALOCK Department
INFECTION AND IMMUNITY, Aug. 1980, p. 301-305 0019-9567/80/08-0301/05$02.00/0 Vol. 29, No. 2 Interferon Immunosuppression: Mediation by a Suppressor Factor HOWARD M. JOHNSON* AND J. EDWIN BLALOCK Department
ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development
 ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development University of Wisconsin-Madison, Production Assistance for Cellular Therapies (PACT) Over the
ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development University of Wisconsin-Madison, Production Assistance for Cellular Therapies (PACT) Over the
Rat Factor XII Total Antigen ELISA Kit
 Rat Factor XII Total Antigen ELISA Kit Catalog No: IRFXIIKT-TOT Lot No: SAMPLE INTENDED USE This rat coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor
Rat Factor XII Total Antigen ELISA Kit Catalog No: IRFXIIKT-TOT Lot No: SAMPLE INTENDED USE This rat coagulation Factor XII antigen assay is intended for the quantitative determination of total Factor
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
A guide to selecting control, diluent and blocking reagents
 Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Specializing in Secondary Antibodies and Conjugates A guide to selecting control, diluent and blocking reagents Optimize your experimental protocols with Jackson ImmunoResearch Secondary antibodies and
Application Note AN001
 Testing hybridoma supernatants with the Spots On Dots Antibody Screening Kit Application Note AN1 Table of Contents Overview... 2 Figure 1. Screening of hybridomas raised against peptide antigens... 3
Testing hybridoma supernatants with the Spots On Dots Antibody Screening Kit Application Note AN1 Table of Contents Overview... 2 Figure 1. Screening of hybridomas raised against peptide antigens... 3
I. Kinetics of Appearance of Thymocytes Using a Direct Intrathymic Adoptive Transfer Assay for Thymocyte Precursors
 STUDIES OF THYMOCYTOPOIESIS IN RATS AND MICE I. Kinetics of Appearance of Thymocytes Using a Direct Intrathymic Adoptive Transfer Assay for Thymocyte Precursors BY IRVING GOLDSCHNEIDER, KRISTIN L. KOMSCHLIES,
STUDIES OF THYMOCYTOPOIESIS IN RATS AND MICE I. Kinetics of Appearance of Thymocytes Using a Direct Intrathymic Adoptive Transfer Assay for Thymocyte Precursors BY IRVING GOLDSCHNEIDER, KRISTIN L. KOMSCHLIES,
Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse
 Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse James W. Tung, Matthew D. Mrazek, Yang Yang, Leonard A. Herzenberg*, and Leonore A. Herzenberg Department
Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse James W. Tung, Matthew D. Mrazek, Yang Yang, Leonard A. Herzenberg*, and Leonore A. Herzenberg Department
Examination in Immunotechnology, 30 May 2011, 8-13
 Examination in Immunotechnology, 30 May 2011, 8-13 1 Each question can give 5p, with a total of 10 questions (i.e. 50 points in total). 2 Write name and personal number on ALL pages (including the cover).
Examination in Immunotechnology, 30 May 2011, 8-13 1 Each question can give 5p, with a total of 10 questions (i.e. 50 points in total). 2 Write name and personal number on ALL pages (including the cover).
BY RICHARD W. DUTTON (From the Department of Biology, University of California, San Diego, La Jolla, California 92037)
 Published Online: 1 December, 1973 Supp Info: http:doi.org10.1084jem.138.6.1496 Downloaded from jem.rupress.org on September 17, 2018 INHIBITORY AND STIMULATORY EFFECTS OF CONCANAVALIN A ON THE RESPONSE
Published Online: 1 December, 1973 Supp Info: http:doi.org10.1084jem.138.6.1496 Downloaded from jem.rupress.org on September 17, 2018 INHIBITORY AND STIMULATORY EFFECTS OF CONCANAVALIN A ON THE RESPONSE
THYMIC CYTOTOXIC T LYMPHOCYTES ARE PRIMED IN VIVO TO MINOR HISTOCOMPATIBILITY ANTIGENS
 THYMIC CYTOTOXIC T LYMPHOCYTES ARE PRIMED IN VIVO TO MINOR HISTOCOMPATIBILITY ANTIGENS BY PAMELA J. FINK,* MICHAEL J. BEVAN, AND IRVING L. WEISSMAN From the Department of Pathology, Stanford University
THYMIC CYTOTOXIC T LYMPHOCYTES ARE PRIMED IN VIVO TO MINOR HISTOCOMPATIBILITY ANTIGENS BY PAMELA J. FINK,* MICHAEL J. BEVAN, AND IRVING L. WEISSMAN From the Department of Pathology, Stanford University
Development of NOG mice
 Development of NOG mice General characteris5cs of NOG mice 1. T and B cell deficient 2. NK cell deficient 3. Reduced macrophage and dendri;c cell func;on 4. Complement ac;vity deficient 5. No incidence
Development of NOG mice General characteris5cs of NOG mice 1. T and B cell deficient 2. NK cell deficient 3. Reduced macrophage and dendri;c cell func;on 4. Complement ac;vity deficient 5. No incidence
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A
 Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
colorimetric sandwich ELISA kit datasheet
 colorimetric sandwich ELISA kit datasheet For the quantitative detection of human IL1-beta in serum, plasma and cell culture supernatants. general information Catalogue Number Product Name Species cross-reactivity
colorimetric sandwich ELISA kit datasheet For the quantitative detection of human IL1-beta in serum, plasma and cell culture supernatants. general information Catalogue Number Product Name Species cross-reactivity
LAMPIRE Hybridoma Project Initiation Form
 1. General Information Company / Institution: Investigator Name: Contact Name: Phone: Fax: 2. Billing / Shipping Information Accounts Payable contact: Company: Dept./Bldg./Room#: Address: Project Name
1. General Information Company / Institution: Investigator Name: Contact Name: Phone: Fax: 2. Billing / Shipping Information Accounts Payable contact: Company: Dept./Bldg./Room#: Address: Project Name
For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine.
 m andw da a For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity Range (calibration
m andw da a For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity Range (calibration
OX40 MARKET LANDSCAPE
 OX40 Agonist OX40 MARKET LANDSCAPE OX40 (a.k.a. CD134) is a costimulatory molecule belonging to the TNF receptor family expressed primarily on activated effector T (T eff) cells and naive regulatory T
OX40 Agonist OX40 MARKET LANDSCAPE OX40 (a.k.a. CD134) is a costimulatory molecule belonging to the TNF receptor family expressed primarily on activated effector T (T eff) cells and naive regulatory T
H-2 ANTIGENS OF THE THYMUS DETERMINE LYMPHOCYTE SPECIFICITY*
 H-2 ANTIGENS OF THE THYMUS DETERMINE LYMPHOCYTE SPECIFICITY* BY PAMELA J. FINK AND MICHAEL J. BEVAN From the Center for Cancer Research and the Department of Biology, Massachusetts Institute of Technology,
H-2 ANTIGENS OF THE THYMUS DETERMINE LYMPHOCYTE SPECIFICITY* BY PAMELA J. FINK AND MICHAEL J. BEVAN From the Center for Cancer Research and the Department of Biology, Massachusetts Institute of Technology,
Monoclonal Antibody Generation. Ivo Lorenz Tri-Institutional Therapeutics Discovery Institute
 Monoclonal Antibody Generation Ivo Lorenz Tri-Institutional Therapeutics Discovery Institute Common Antibody Formats Heavy chain VL VH Light chain Fab CL CH1 VH VL Linker CH2 VH VL Fc CH3 IgG Immunoglobulin
Monoclonal Antibody Generation Ivo Lorenz Tri-Institutional Therapeutics Discovery Institute Common Antibody Formats Heavy chain VL VH Light chain Fab CL CH1 VH VL Linker CH2 VH VL Fc CH3 IgG Immunoglobulin
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION
 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
colorimetric sandwich ELISA kit datasheet
 colorimetric sandwich ELISA kit datasheet For the quantitative detection of mouse Interleukin 6 (IL6) concentrations in serum, plasma and cell culture supernatants. general information Catalogue Number
colorimetric sandwich ELISA kit datasheet For the quantitative detection of mouse Interleukin 6 (IL6) concentrations in serum, plasma and cell culture supernatants. general information Catalogue Number
Immunophenotyping of Peripheral Blood and Bone Marrow Cells by Flow Cytometry *Akanni EO and # Palini A.
 Immunophenotyping of Peripheral Blood and Bone Marrow Cells by Flow Cytometry *Akanni EO and # Palini A. * Department of Haematology & Blood Transfusion,College of Health Science, Ladoke Akintola University
Immunophenotyping of Peripheral Blood and Bone Marrow Cells by Flow Cytometry *Akanni EO and # Palini A. * Department of Haematology & Blood Transfusion,College of Health Science, Ladoke Akintola University
Regulatory B Cell Isolation Kit mouse
 For further information refer to our website www.miltenyibiotec.com For technical questions, please contact your local subsidiary or distributor. Technical Support Team, Germany: E-mail: macstec@miltenyibiotec.de
For further information refer to our website www.miltenyibiotec.com For technical questions, please contact your local subsidiary or distributor. Technical Support Team, Germany: E-mail: macstec@miltenyibiotec.de
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10772 Supplementary Figures: Supplementary Figure 1. Location of CNS1 within of the Foxp3 locus highlighting CNS1 and indicating transcription factor binding motifs downstream of TCR,
doi:10.1038/nature10772 Supplementary Figures: Supplementary Figure 1. Location of CNS1 within of the Foxp3 locus highlighting CNS1 and indicating transcription factor binding motifs downstream of TCR,
Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer
 SUPPLEMENTAL METHODS Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer For Celigo experiments, 0.1 ml containing 5 x 10 4 cells was seeded into 96 well plates for 30 min
SUPPLEMENTAL METHODS Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer For Celigo experiments, 0.1 ml containing 5 x 10 4 cells was seeded into 96 well plates for 30 min
ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity and percent identical sites.
 Identity Human Mouse Supplemental Figure 1. Amino acid sequence alignment of -MyHC. The alignment was created using the ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity
Identity Human Mouse Supplemental Figure 1. Amino acid sequence alignment of -MyHC. The alignment was created using the ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity
Single-cell suspensions of C57BL/6J splenocytes were incubated with CD5 beads
 S S Single-cell suspensions of C57BL/6J splenocytes were incubated with CD5 beads (Miltenyi Biotech) and T cells were positively selected by magnetically activated cell sorting (MACS). 50x10 6 or greater
S S Single-cell suspensions of C57BL/6J splenocytes were incubated with CD5 beads (Miltenyi Biotech) and T cells were positively selected by magnetically activated cell sorting (MACS). 50x10 6 or greater
Human IL-10 ELISA MAX Set Deluxe
 Human IL-10 ELISA MAX Set Deluxe Cat. No. 430604 (5 plates) 430605 (10 plates) 430606 (20 plates) ELISA Set for Accurate Cytokine Quantification from Cell Culture Supernatant, Serum, Plasma or Other Body
Human IL-10 ELISA MAX Set Deluxe Cat. No. 430604 (5 plates) 430605 (10 plates) 430606 (20 plates) ELISA Set for Accurate Cytokine Quantification from Cell Culture Supernatant, Serum, Plasma or Other Body
KLH IgM (Mouse) ELISA Kit
 KLH IgM (Mouse) ELISA Kit Catalog Number KA2461 96 assays Version: 6.1 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3 General
KLH IgM (Mouse) ELISA Kit Catalog Number KA2461 96 assays Version: 6.1 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3 General
TF-1a lymphoblastic leukemia cell line: marking with GFP, phenotyping and sorting
 Supplemental Material Supplemental Methods TF-1a lymphoblastic leukemia cell line: marking with GFP, phenotyping and sorting In order to determine if the multi-parameter FACS approach would be successful
Supplemental Material Supplemental Methods TF-1a lymphoblastic leukemia cell line: marking with GFP, phenotyping and sorting In order to determine if the multi-parameter FACS approach would be successful
Supporting Information
 Supporting Information of Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for Treatment of Metastatic Tumor Lihua Luo, Chunqi Zhu, Hang Yin, Mengshi Jiang, Junlei Zhang, Bing Qin, Zhenyu
Supporting Information of Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for Treatment of Metastatic Tumor Lihua Luo, Chunqi Zhu, Hang Yin, Mengshi Jiang, Junlei Zhang, Bing Qin, Zhenyu
IMMUNE NETWORKS Frequencies of Antibody- and Idiotype-producing B Cell Clones in Various Steady States*
 Brief Definitive Report IMMUNE NETWORKS Frequencies of Antibody- and Idiotype-producing B Cell Clones in Various Steady States* BY ROSA R BERNABE, ANTONIO COUTINHO,t CARLOS MARTINEZ-A, AND PIERRE-ANDRE
Brief Definitive Report IMMUNE NETWORKS Frequencies of Antibody- and Idiotype-producing B Cell Clones in Various Steady States* BY ROSA R BERNABE, ANTONIO COUTINHO,t CARLOS MARTINEZ-A, AND PIERRE-ANDRE
HOST DEFENSE SMALL GROUP PROBLEM SOLVING SESSION CLINICAL IMMUNOLOGIC ASSAYS-II
 HOST DEFENSE SMALL GROUP PROBLEM SOLVING SESSION CLINICAL IMMUNOLOGIC ASSAYS-II Monday, March 24, 2008 2:00 PM 4:00 PM Small Group Classrooms LEARNING GOAL Understanding in vitro assessment of immunologic
HOST DEFENSE SMALL GROUP PROBLEM SOLVING SESSION CLINICAL IMMUNOLOGIC ASSAYS-II Monday, March 24, 2008 2:00 PM 4:00 PM Small Group Classrooms LEARNING GOAL Understanding in vitro assessment of immunologic
7-amino actinomycin D (7ADD) was added to all samples 10 minutes prior to analysis on the flow cytometer in order to gate 7AAD viable cells.
 Antibody staining for Ho uptake analyses For HSC staining, 10 7 BM cells from Ho perfused mice were stained with biotinylated lineage antibodies (CD3, CD5, B220, CD11b, Gr-1, CD41, Ter119), anti Sca-1-PECY7,
Antibody staining for Ho uptake analyses For HSC staining, 10 7 BM cells from Ho perfused mice were stained with biotinylated lineage antibodies (CD3, CD5, B220, CD11b, Gr-1, CD41, Ter119), anti Sca-1-PECY7,
Armenian Hamster IgG Antigen ELISA Kit
 Armenian Hamster IgG Antigen ELISA Kit Catalog No: IAHTIGGKT Lot No: SAMPLE INTENDED USE This Armenian hamster Immunoglobulin G (IgG) antigen assay is intended for the quantitative determination of total
Armenian Hamster IgG Antigen ELISA Kit Catalog No: IAHTIGGKT Lot No: SAMPLE INTENDED USE This Armenian hamster Immunoglobulin G (IgG) antigen assay is intended for the quantitative determination of total
Detection of antibody-stained cell surface and intracellular protein targets with the Agilent 2100 bioanalyzer. Application
 Detection of antibody-stained cell surface and intracellular protein targets with the Agilent 2100 bioanalyzer Application Gerd Luedke and Tobias Preckel Abstract This Application Note describes how the
Detection of antibody-stained cell surface and intracellular protein targets with the Agilent 2100 bioanalyzer Application Gerd Luedke and Tobias Preckel Abstract This Application Note describes how the
BY ALFRED SINGER AND RICHARD J. HODES. From the Immunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20205
 MAJOR HISTOCOMPATIBILITY COMPLEX-RESTRICTED SELF- RECOGNITION IN RESPONSES TO TRINITROPHENYL-FICOLL Adaptive Differentiation and Self-Recognition by B Cells BY ALFRED SINGER AND RICHARD J. HODES From the
MAJOR HISTOCOMPATIBILITY COMPLEX-RESTRICTED SELF- RECOGNITION IN RESPONSES TO TRINITROPHENYL-FICOLL Adaptive Differentiation and Self-Recognition by B Cells BY ALFRED SINGER AND RICHARD J. HODES From the
Mouse TNF-α ELISA MAX Set Deluxe
 Mouse TNF-α ELISA MAX Set Deluxe Cat. No. 430904 (5 plates) 430905 (10 plates) 430906 (20 plates) ELISA Set for Accurate Cytokine Quantification from Cell Culture Supernatant, Serum, Plasma or Other Body
Mouse TNF-α ELISA MAX Set Deluxe Cat. No. 430904 (5 plates) 430905 (10 plates) 430906 (20 plates) ELISA Set for Accurate Cytokine Quantification from Cell Culture Supernatant, Serum, Plasma or Other Body
F4/80, CD11b, Gr-1, NK1.1, CD3, CD4, CD8 and CD19. A-antigen was detected with FITCconjugated
 SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
MHC-RESTRICTED MINIMAL REGULATORY CIRCUIT INITIATED BY A CLASS II-AUTOREACTIVE T CELL CLONE
 Published Online: 1 May, 1987 Supp Info: http://doi.org/10.1084/jem.165.5.1284 Downloaded from jem.rupress.org on July 26, 2018 MHC-RESTRICTED MINIMAL REGULATORY CIRCUIT INITIATED BY A CLASS II-AUTOREACTIVE
Published Online: 1 May, 1987 Supp Info: http://doi.org/10.1084/jem.165.5.1284 Downloaded from jem.rupress.org on July 26, 2018 MHC-RESTRICTED MINIMAL REGULATORY CIRCUIT INITIATED BY A CLASS II-AUTOREACTIVE
High throughput screening: Huh-7 cells were seeded into 96-well plate (2000
 1 SUPPLEMENTARY INFORMATION METHODS 6 7 8 9 1 11 1 1 1 1 16 17 18 19 High throughput screening: Huh-7 cells were seeded into 96-well plate ( cells/well) and infected with MOI of DENV-. One hour post-infection
1 SUPPLEMENTARY INFORMATION METHODS 6 7 8 9 1 11 1 1 1 1 16 17 18 19 High throughput screening: Huh-7 cells were seeded into 96-well plate ( cells/well) and infected with MOI of DENV-. One hour post-infection
Supplementary Figure 1 A green: cytokeratin 8
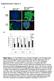 Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Mouse Anti-KLH IgG ELISA Kit
 Mouse Anti-KLH IgG ELISA Kit CATALOG NO: IRKTAH3024 LOT NO: SAMPLE INTENDED USE The mouse anti-klh IgG test kit is based on a solid phase enzyme-linked immunosorbent assay (ELISA). The assay uses KLH for
Mouse Anti-KLH IgG ELISA Kit CATALOG NO: IRKTAH3024 LOT NO: SAMPLE INTENDED USE The mouse anti-klh IgG test kit is based on a solid phase enzyme-linked immunosorbent assay (ELISA). The assay uses KLH for
Nature Immunology: doi: /ni Supplementary Figure 1. Time course of OT-II T reg cell development in thymic slices.
 Supplementary Figure 1 Time course of OT-II T reg cell development in thymic slices. Time course of T reg cell development following addition of OT-II TCR transgenic Rag2 -/- mice (OT-II) thymocytes to
Supplementary Figure 1 Time course of OT-II T reg cell development in thymic slices. Time course of T reg cell development following addition of OT-II TCR transgenic Rag2 -/- mice (OT-II) thymocytes to
IMMUNE NETWORKS Frequencies of Antibody- and Idiotype-producing B Cell Clones in Various Steady States*
 Published Online: 1 August, 1981 Supp Info: http://doiorg/101084/jem1542552 Downloaded from jemrupressorg on October 10, 2018 Brief Definitive Report IMMUNE NETWORKS Frequencies of Antibody- and Idiotype-producing
Published Online: 1 August, 1981 Supp Info: http://doiorg/101084/jem1542552 Downloaded from jemrupressorg on October 10, 2018 Brief Definitive Report IMMUNE NETWORKS Frequencies of Antibody- and Idiotype-producing
DC enriched CD103. CD11b. CD11c. Spleen. DC enriched. CD11c. DC enriched. CD11c MLN
 CD CD11 + CD + CD11 d g CD CD11 + CD + CD11 CD + CD11 + Events (% of max) Events (% of max) CD + CD11 Events (% of max) Spleen CLN MLN Irf fl/fl e Irf fl/fl h Irf fl/fl DC enriched CD CD11 Spleen DC enriched
CD CD11 + CD + CD11 d g CD CD11 + CD + CD11 CD + CD11 + Events (% of max) Events (% of max) CD + CD11 Events (% of max) Spleen CLN MLN Irf fl/fl e Irf fl/fl h Irf fl/fl DC enriched CD CD11 Spleen DC enriched
Custom Antibody Services. Antibodies Designed Just for You. HuCAL Recombinant Monoclonal Antibody Generation Service
 Custom Antibody Services Antibodies Designed Just for You HuCAL Recombinant Monoclonal Antibody Generation Service Antibodies Designed Just for You What if there was a technology that would allow you to
Custom Antibody Services Antibodies Designed Just for You HuCAL Recombinant Monoclonal Antibody Generation Service Antibodies Designed Just for You What if there was a technology that would allow you to
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells
 Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Supplementary Figure 1. Characterization of the POP2 transcriptional and post-transcriptional regulatory elements. (A) POP2 nucleotide sequence
 1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
Titration of Fluorochrome-Conjugated Antibodies for Labeling Cell Surface Markers on Live Cells
 Titration of Fluorochrome-Conjugated Antibodies for Labeling Cell Surface Markers on Live Cells Ruud Hulspas 1 UNIT 6.29 1 Cytonome/ST, Boston, Massachusetts ABSTRACT Nonspecific antibody binding is best
Titration of Fluorochrome-Conjugated Antibodies for Labeling Cell Surface Markers on Live Cells Ruud Hulspas 1 UNIT 6.29 1 Cytonome/ST, Boston, Massachusetts ABSTRACT Nonspecific antibody binding is best
Supplemental Figure 1
 Supplemental Figure 1 A IL-12p7 (pg/ml) 7 6 4 3 2 1 Medium then TLR ligands MDP then TLR ligands Medium then TLR ligands + MDP MDP then TLR ligands + MDP B IL-12p4 (ng/ml) 1.2 1..8.6.4.2. Medium MDP Medium
Supplemental Figure 1 A IL-12p7 (pg/ml) 7 6 4 3 2 1 Medium then TLR ligands MDP then TLR ligands Medium then TLR ligands + MDP MDP then TLR ligands + MDP B IL-12p4 (ng/ml) 1.2 1..8.6.4.2. Medium MDP Medium
SUBCLASSIFICATION OF ACUTE MYELOGENOUS LEUKEMIA PATIENTS BASED ON CHEMOKINE RESPONSIVENESS AND CONSTITUTIVE CHEMOKINE RELEASE BY
 Supplementary Appendix SUBCLASSIFICATION OF ACUTE MYELOGENOUS LEUKEMIA PATIENTS BASED ON CHEMOKINE RESPONSIVENESS AND CONSTITUTIVE CHEMOKINE RELEASE BY THE LEUKEMIA CELLS Øystein Bruserud 1, Anita Ryningen
Supplementary Appendix SUBCLASSIFICATION OF ACUTE MYELOGENOUS LEUKEMIA PATIENTS BASED ON CHEMOKINE RESPONSIVENESS AND CONSTITUTIVE CHEMOKINE RELEASE BY THE LEUKEMIA CELLS Øystein Bruserud 1, Anita Ryningen
BY POLLY MATZINGER AND GALADRIEL MIRKWOOD. (From the Department of Biology, University of California San Diego, La Jolla, California 92093)
 Published Online: 1 July, 1978 Supp nfo: http:doi.org10.1084jem.148.1.84 Downloaded from jem.rupress.org on May 18, 2018 N A FULLY H-2 NCOMPATBLE CHMERA, T CELLS OF DONOR ORGN CAN RESPOND TO MNOR HSTOCOMPATBLTY
Published Online: 1 July, 1978 Supp nfo: http:doi.org10.1084jem.148.1.84 Downloaded from jem.rupress.org on May 18, 2018 N A FULLY H-2 NCOMPATBLE CHMERA, T CELLS OF DONOR ORGN CAN RESPOND TO MNOR HSTOCOMPATBLTY
Brief Definitive Report
 Published Online: 1 September, 1976 Supp Info: http://doi.org/10.1084/jem.144.3.852 Downloaded from jem.rupress.org on October 7, 2018 Brief Definitive Report ANTIGEN-INDUCED CO-CAPPING OF IgM AND IgD-LIKE
Published Online: 1 September, 1976 Supp Info: http://doi.org/10.1084/jem.144.3.852 Downloaded from jem.rupress.org on October 7, 2018 Brief Definitive Report ANTIGEN-INDUCED CO-CAPPING OF IgM AND IgD-LIKE
Quantifying small numbers of antibodies with a near-universal protein-dna chimera
 Quantifying small numbers of antibodies with a near-universal protein-dna chimera Ian Burbulis, Kumiko Yamaguchi, Richard Yu, Orna Resnekov & Roger Brent Supplementary figures and text: Supplementary figure
Quantifying small numbers of antibodies with a near-universal protein-dna chimera Ian Burbulis, Kumiko Yamaguchi, Richard Yu, Orna Resnekov & Roger Brent Supplementary figures and text: Supplementary figure
PHENOTYPE OF PRECURSOR AND AMPLIFIER CELLS
 Published Online: 1 April, 1977 Supp Info: http://doi.org/10.1084/jem.145.4.793 Downloaded from jem.rupress.org on July 15, 2018 T-T INTERACTIONS IN THE INDUCTION OF SUPPRESSOR AND HELPER T CELLS: ANALYSIS
Published Online: 1 April, 1977 Supp Info: http://doi.org/10.1084/jem.145.4.793 Downloaded from jem.rupress.org on July 15, 2018 T-T INTERACTIONS IN THE INDUCTION OF SUPPRESSOR AND HELPER T CELLS: ANALYSIS
Immunogenetics. Immunodeficiency
 4.05.009 Immune response represents a system of recognition of foreign molecules. Immunogenetics Foreign molecules (proteins, glycoproteins, carbohydrates, ssdna, viruses) or parts of foreign molecules
4.05.009 Immune response represents a system of recognition of foreign molecules. Immunogenetics Foreign molecules (proteins, glycoproteins, carbohydrates, ssdna, viruses) or parts of foreign molecules
Supplementary Figure. S1
 Supplementary Figure. S1 Supplementary Figure S1. Correlation of phagocytic ability measured with YG and YO beads. Fresh human monocytes (2 10 6 /ml) were labelled with APC conjugated anti CD14 mab alone
Supplementary Figure. S1 Supplementary Figure S1. Correlation of phagocytic ability measured with YG and YO beads. Fresh human monocytes (2 10 6 /ml) were labelled with APC conjugated anti CD14 mab alone
Immunofluorescent staining and flow cytometric analysis of cells
 UCAN-U 0003 Version 1 November 2010 Page 1 of 7 CMCI (Center for molecular and Cellular Intervention) University Medical Center Utrecht Written By Name Function Date Signature Mark Klein Lab manager Conformation
UCAN-U 0003 Version 1 November 2010 Page 1 of 7 CMCI (Center for molecular and Cellular Intervention) University Medical Center Utrecht Written By Name Function Date Signature Mark Klein Lab manager Conformation
Centre for Innovative Cancer Research, Ottawa Hospital Research Institute, Ottawa, Canada;
 Ex vivo Natural Killer Cell Cytotoxicity Assay Lee-Hwa Tai 1, Christiano Tanese de Souza 1, Andrew P. Makrigiannis 2 and Rebecca Ann C. Auer 3* 1 Centre for Innovative Cancer Research, Ottawa Hospital
Ex vivo Natural Killer Cell Cytotoxicity Assay Lee-Hwa Tai 1, Christiano Tanese de Souza 1, Andrew P. Makrigiannis 2 and Rebecca Ann C. Auer 3* 1 Centre for Innovative Cancer Research, Ottawa Hospital
Rabbit Anti-Mouse/Rat Asialo GM1 Polyclonal Antibody
 Rabbit Anti-Mouse/Rat Asialo GM1 Polyclonal Antibody CL8955 LOT: KQF6371 DESCRIPTION: Cedarlane s Anti-Asialo GM1 polyclonal antibody reacts with mouse and rat Natural Killer (NK) cells. It also exhibits
Rabbit Anti-Mouse/Rat Asialo GM1 Polyclonal Antibody CL8955 LOT: KQF6371 DESCRIPTION: Cedarlane s Anti-Asialo GM1 polyclonal antibody reacts with mouse and rat Natural Killer (NK) cells. It also exhibits
Figure S6. Detection of anti-gfp antibodies in anti-dna and normal plasma without competition DNA--9
 Supplementary Information Ultrasensitive antibody detection by agglutination-pcr (ADAP) Cheng-ting Tsai 1 *, Peter V. Robinson 1 *, Carole A. Spencer 2 and Carolyn R. Bertozzi 3,4ǂ Department of 1 Chemistry,
Supplementary Information Ultrasensitive antibody detection by agglutination-pcr (ADAP) Cheng-ting Tsai 1 *, Peter V. Robinson 1 *, Carole A. Spencer 2 and Carolyn R. Bertozzi 3,4ǂ Department of 1 Chemistry,
SUPPLEMENTARY FIG. S2. Expression of single HLA loci in shns- and shb 2 m-transduced MKs. Expression of HLA class I antigens (HLA-ABC) as well as
 Supplementary Data Supplementary Methods Flow cytometric analysis of HLA class I single locus expression Expression of single HLA loci (HLA-A and HLA-B) by shns- and shb 2 m-transduced megakaryocytes (MKs)
Supplementary Data Supplementary Methods Flow cytometric analysis of HLA class I single locus expression Expression of single HLA loci (HLA-A and HLA-B) by shns- and shb 2 m-transduced megakaryocytes (MKs)
Supporting Information
 Supporting Information Chan et al. 10.1073/pnas.0903849106 SI Text Protein Purification. PCSK9 proteins were expressed either transiently in 2936E cells (1), or stably in HepG2 cells. Conditioned culture
Supporting Information Chan et al. 10.1073/pnas.0903849106 SI Text Protein Purification. PCSK9 proteins were expressed either transiently in 2936E cells (1), or stably in HepG2 cells. Conditioned culture
T-cell response. Taken from NIAID: s.aspx
 T-cell receptor T-cell response 1. Macrophage or dendritic cell digest antigen bacteria, virus 2. Fragments of Ag bind to major histo-compatiblity (MHC) proteins in macrophage. 3. MHC I-Ag fragment expressed
T-cell receptor T-cell response 1. Macrophage or dendritic cell digest antigen bacteria, virus 2. Fragments of Ag bind to major histo-compatiblity (MHC) proteins in macrophage. 3. MHC I-Ag fragment expressed
High Sensitive Rat Leptin ELISA
 High Sensitive Rat Leptin ELISA For the high sensitive quantitative determination of Leptin in rat serum or plasma. Cat. No. KT-379 For Research Use Only. 1 Rev. 091707 PRODUCT INFORMATION High Sensitive
High Sensitive Rat Leptin ELISA For the high sensitive quantitative determination of Leptin in rat serum or plasma. Cat. No. KT-379 For Research Use Only. 1 Rev. 091707 PRODUCT INFORMATION High Sensitive
A Role for Streptococcal Collagen-Like Protein 1 (Scl-1) in M1T1 Group A
 Supplemental Information for: A Role for Streptococcal Collagen-Like Protein 1 (Scl-1) in M1T1 Group A Streptococcus Resistance to Neutrophil Extracellular Traps Simon Döhrmann 1, Sabina Anik 1, Joshua
Supplemental Information for: A Role for Streptococcal Collagen-Like Protein 1 (Scl-1) in M1T1 Group A Streptococcus Resistance to Neutrophil Extracellular Traps Simon Döhrmann 1, Sabina Anik 1, Joshua
Data Sheet ICOS/NFAT Reporter-Jurkat Recombinant Cell Line Catalog #: 79668
 Data Sheet ICOS/NFAT Reporter-Jurkat Recombinant Cell Line Catalog #: 79668 Product Description Recombinant Jurkat T cell expressing firefly luciferase gene under the control of NFAT response elements
Data Sheet ICOS/NFAT Reporter-Jurkat Recombinant Cell Line Catalog #: 79668 Product Description Recombinant Jurkat T cell expressing firefly luciferase gene under the control of NFAT response elements
(From the Division of Immunology, Department of Medicine, Stanford University School of Medicine, Stanford, California 94305)
 MATURATION OF B LYMPHOCYTES IN THE RAT* 2. MIGRATION PATTERN, TISSUE DISTRIBUTION, AND TURNOVER RATE OF UNPRIMED AND PRIMED B LYMPHOCYTES INVOLVED IN THE ADOPTIVE ANTIDINITROPHENYL RESPONSE BY SAMUEL STROBER:~
MATURATION OF B LYMPHOCYTES IN THE RAT* 2. MIGRATION PATTERN, TISSUE DISTRIBUTION, AND TURNOVER RATE OF UNPRIMED AND PRIMED B LYMPHOCYTES INVOLVED IN THE ADOPTIVE ANTIDINITROPHENYL RESPONSE BY SAMUEL STROBER:~
