Multiphoton Microscopy: Seeing deeper and clearer
|
|
|
- MargaretMargaret Sullivan
- 6 years ago
- Views:
Transcription
1 Multiphoton Microscopy: Seeing deeper and clearer Since the invention of simple microscope by Leuwenhoek and Hooke in the 17th century, different types of light microscopy techniques (such as phase contrast, differential interference contrast and fluorescent microscopy) have been developed for biological research over the past few centuries. The main aim of these techniques is to improve the contrast of the microscopic specimen observation, eliminating unwanted background noise. Fluorescent microscopy occupies a unique niche in biological applications; fluorescent objects can be selectively excited and visualized, even in living system. (Lichtman and Conchello, 2005). The sensitivity of fluorescence detection is sufficiently high so that single fluorescent molecules can be detected in the presence of non-fluorescent molecules (e.g., water, amino acids, and lipids) (Eigen and Rigler, 1994). Most of the biological samples need to be studied in their natural habitat. For example, neurons in intact brain tissue are especially challenging for fluorescent microscopy. In wide-field fluorescence microscopy, contrast and resolution are degraded by strong scattering in thick specimen (Denk and Svoboda, 1997). Confocal microscopy can overcome some of the effects of scattering, since the detector pinhole rejects fluorescence from off-focus locations (Conchello and Lichtman, 2005; Denk and Svoboda, 1997). However, scanning a single section excites, and damages the entire specimen. In addition, the pinhole also rejects signal photons emanating from the focus that are scattered on their way out of the tissue. Deep in tissue, confocal microscopy becomes unacceptably wasteful in terms of signal photons (Centonze and White, 1998; Conchello and Lichtman, 2005). Compensating for signal-loss with increase in fluorescence excitation will lead to phototoxicity and photobleaching of specimen. Therefore, wide-field and confocal microscopy are techniques that best for thin specimens applications, such as cultured preparations or the most superficial cell layer in a tissue (<20 mm) (Lichtman et al., 1987). Multiphoton microscopy (MP) has thus evolved as an alternative to conventional single-photon confocal microscopy and has been shown to provide several advantages to overcome the problem mentioned above for thick biological specimen. These include three-dimensionally resolved fluorescence imaging of living cells deep within thick, strongly scattering samples, and reduced phototoxicity, enabling long term imaging of photosensitive biological specimens. 1
2 Principles of Two-Photon Excitation The phenomenon of two-photon excitation arises from the simultaneous absorption of two photons in a single quantitized event. Since the energy of a photon is inversely proportional to its wavelength, the two absorbed photons must have a wavelength about twice that required for one-photon excitation. For example, a fluorophore that normally absorbs ultraviolet light (approximately 350 nanometers wavelength) can also be excited by two photons of near-infrared light (approximately 700 nanometers wavelength) if both reach the fluorophore at the same time (Figure 1). In this case, "the same time" means within an interval of about seconds. Because two-photon excitation depends on simultaneous absorption, the resulting fluorescence emission varies with the square of the excitation intensity. This quadratic relationship between excitation and emission gives rise to many of the significant advantages associated with twophoton excitation microscopy. In order to produce a significant number of two-photon absorption events (in which both photons interact with the fluorophore at the same time), the photon density must be approximately one million times that required to generate the same number of onephoton absorptions. The consequence is that extremely high laser power is required to generate significant two-photon-excited fluorescence. This power level is easily achieved by focusing mode-locked (pulsed) lasers, in which the power during the peak of the pulse is high enough to generate significant two-photon excitation, while the average laser power remains fairly low. In this situation, the resulting two-photon-excited state from which emission occurs is the same singlet state that is populated when carrying out a conventional fluorescence experiment. Thus, fluorescent emission following two-photon excitation is exactly the same as emission generated in normal one-photon excitation. Figure 1 presents a Jablonski diagram illustrating absorption of a single (ultraviolet) photon (Figure 1(a)) and the simultaneous absorption of two near-infrared photons (Figure 1(b)), producing the identical excited state. 2
3 In practice, the narrow localization of two-photon excitation to the illumination focal point ensures that no background fluorescence is produced, so pinhole is not required. This is the significant difference between multiphoton and confocal microscopy. Figure 2 illustrates the photobleaching pattern that arises in the x-z direction from repeated scanning of a single x-y plane (the image plane) in a fluorescein-stained gel. The laser of the confocal system excited fluorophores above and below the focal plane (Figure 2a), contributing to the bleaching observed in these extensive areas. In contrast, two-photon excitation occurs only at the focal plane, and bleaching is therefore confined to this area (Figure 2b). Light Path Boundaries a) b) Coverslip Coverslip X-Y Scanning X-Y Scanning Light Path Boundaries Fluorescent Gel Fluorescent Gel Glass Slide Focus Plane Glass Slide Focus Plane Figure 2. Excitation Photobleaching pattern for a) confocal microscopy; b) Multiphoton microscopy Application of Multiphoton Microscopy Multiphoton microscopy is inherently optical sectioning, which provides deeper penetration (in thick scattering specimen) and reduces photo-toxicity and photo-bleaching (Grace et al., 2005). Therefore, it is ideal for probing sensitive live biological samples that cannot tolerate extrinsic fluorophores staining, or histological sectioning. Below are some examples of advantages of multiphoton applications in long-term time-lapse imaging and dynamic cellular imaging for photosensitive specimen. Long time lapse multiphoton imaging One of the major challenges neurobiologist faced in neuronal morphological study is to capture time-lapse images while minimize the photo-damage of neurons in thick and highly scattering brain specimen. The main reason for photo-damage of neurons in specimen is by the 3
4 process of imaging itself, for example, compensating fluorescent signal loss by high excitation power. Therefore, multiphoton microscopy which is having advantages of deeper optical penetration offer vast potential to capture long term imaging for thick brain specimen. One example of long time lapse multiphoton imaging is reported by Trachtenberg et al (2002) for studying synaptic plasticity in adult mice (Figure 3). They employed a method called open skull technique to gain optical access to the GFP expressing neuron in the mice brain by removing a circular portion of opaque skull. The 8-days repeated in vivo multiphoton imaging studies have suggested that experience-dependent plasticity of cortical receptive fields was accompanied by increased of synapse changes, which might underlie adaptive remodeling of neural circuits. I Figure 3. Long time lapse imaging of the same dendritic segment acquired over 8 day period in the barrel cortex of a mouse. Arrows indicate examples of dendritic spines that stable (yellow) or transient and semi-stable (blue, red). Trachtenberg et al. Nature Vol. 420, (2002) Dynamic cellular imaging Besides the morphological studies of neuron cells that located deep inside the thick brain specimen, their dynamic network activity can also be observed using multiphoton technique with a suitable fluorescent probes. However, imaging the neuron population activity post a significant challenge to present scanning technology because high acquisition rates are needed to measure such population activity simultaneously. One direct example is calcium imaging that probes calcium ions dynamic/oscillation in neuron cells. Calcium ions are important messengers in neuron that are responsible for several key activities such as signaling the release of neurotransmitter, gene expression and facilitate synaptic plasticity. There are two major sources that Ca 2+ ions can enter into cytoplasm of neurons. First route, the Ca 2+ ions enter through voltage operated Ca 2+ channel from extracellular space. When a neuron becomes active, its 4
5 membrane undergoes depolarization and allows Ca 2+ ions entry into cytoplasm. The second route for Ca 2+ entry into neuron is from intracellular endoplasmic reticulum (ER) mediated by receptor operated Ca 2+ channel. The intracellular store of Ca2+ can be released into cytoplasm when inositol triphosphate (IP3) is produced from G-protein coupled receptor on the cell surface by the action of phospholipase C (PLC). IP3 stimulates the receptor operated calcium channel on the ER, which causes the sudden increase of Ca2+ ions in cytoplasm. The application of calcium imaging in Multi-photon was exemplified in Fan et al., (1999), where they utilized the famous protein-based Ca2+ indicators, cameleons for the testing of video-rate scanning Two-photon Excitation Fluorescence Microscopy. Cameleons is a genetically encoded calcium sensor and targetable to specific tissues and intracellular locations (Miyawaki et al., 1997), they have preceding advantages over the small molecule indicators. By utilizing the Fluorescence Resonance Energy Transfer (FRET) principle through cameleons, a ratio-metric imaging recorded using a video-rate scanning two-photon excitation fluorescence microscopy system that provides 30 frame/s of noninterlaced images of 512 X 484 pixels were obtained. With the implementation of video-rate scanning, a ratio imaging of calcium wave in HeLa cells expressing yellow cameleon-2.1 (Figure 4) was obtained. Figure 4. Ratio imaging (535/480 nm) of calcium wave in HeLa cells expressing yellow cameleon-2.1 without targeting signals. The calcium wave (red), which was created with the application of 0.1 mm histamine, which triggers Ca2_ release in the cells, can be seen to propagate across each cell. Images A and F were recorded 1 s before and 6 s after the application of histamine, respectively. Time separation between images B E is 132 ms. Scale bar, 10 µm. Fan et al. Biophysical Journal Vol. 76, (1999) 5
6 Nikon A1R MP Nikon s A1R MP is a unique multiphoton imaging system featuring a high resolution galvanometer scanner and a high speed resonant scanner that is capable of frame rates from 30 fps at 512 X 512 pixels to as fast as 420 fps in band scan mode. New four channel nondescanned multiphoton detectors with higher sensitivity, reduced dark current and broad spectral range allow for real time unmixing of closely spaced probes for deep tissue and accurate, high contrast spectral imaging. This is especially important in multiphoton imaging because of the overlap of emission spectra of probes and autofluorescence, which is often unavoidable when using a single laser line. The Nikon A1R MP also features a newly-developed, one click auto-alignment of the infrared femtosecond Ti:sapphire multiphoton excitation laser, allowing for fast set up, wavelength changes, GVD pre-chirping compensation and total ease of use (Figure 5). This is particularly important because when the multiphoton laser wavelength (or group velocity dispersion precompensation) is changed, the multiphoton laser beam positional pointing at the objective back aperture may also change, resulting in uneven intensity across the image, or a slight misalignment between the IR and visible laser light paths. Verifying the IR laser beam pointing and setting the alignment has traditionally been difficult. This device completely encloses the beam within the instrument from the laser to the objective lens a huge improvement in the operating safety of multiphoton imaging systems as the main danger comes during the laser alignment. Incident Optical Unit Optical Path Detection Figure 5. A1R MP multiphoton laser beam alignment with a single click 6
7 Bright, high resolution imaging is provided by the newly introduced Nikon Lambda S (λs) objective series, featuring the highest numerical apertures (NA) for water immersion objectives yet. The CFI APO LWD 40X WI λs objective with NA 1.15 and a working distance of 610 microns incorporates Nikon s new Nano Crystal Coat technology which the coat particle structure dramatically reduces stray reflection and boosts transmission over wide wavelength range. Ideal for high performance multiphoton imaging, this unique coating provides high transmissions over an expanded correction range from the violet through the near IR range. The latest version of NIS-Elements C Version 3.2 software orchestrates all microscope control and multiphoton acquisition with the capabilities of acquiring data at high speed, control of various motorized devices. This includes fast piezoelectric Z focus positioners and XY translational stages, input/output triggers and flexible experiment design. High speed, high precision unmixing algorithms enable high contrast imaging. References Centonze, V.E., and White, J.G. (1998). Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys. J. 75, Joshua T. Trachtenberg, Brian E. Chen, Graham W. Knott, Guoping Feng, Joshua R. Sanes, Egbert Welker and Karel Svoboda (2002). Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 420, Denk, W., and Svoboda, K. (1997). Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron 18, Eigen, M., and Rigler, R. (1994). Sorting single molecules: applications to diagnostics and evolutionary biotechnology. Proc. Natl. Acad. Sci. USA 91, Grace E. Stutzmann and Ian Parker (2005), Dynamic Multiphoton Imaging: A Live View from Cells to Systems Physiology 20:15-21, 2005 Lichtman, J.W., and Conchello, J.A. (2005). Fluorescence microscopy. Nat. Methods 2, Lichtman, J.W., Magrassi, L., and Purves, D. (1987). Visualization of neuromuscular junctions over periods of several months in living mice. J. Neurosci. 7, Fan G. Y., Fujisaki H, Miyawaki A, Tsay R.K, Tsien Roger Y, and Mark H. Ellisman (1999). Video-Rate Scanning Two-Photon Excitation Fluorescence Microscopy and Ratio Imaging with Cameleons. Biophysical Journal Volume 76 May
Special Techniques 1. Mark Scott FILM Facility
 Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Confocal Microscopes. Evolution of Imaging
 Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
A Brief History of Light Microscopy And How It Transformed Biomedical Research
 A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
Two-Photon Microscopy for Deep Tissue Imaging of Living Specimens
 for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
 Page 1 of 9 Fundamentals and Applications in Multiphoton Excitation Microscopy Two-photon excitation microscopy (also referred to as non-linear, multiphoton, or two-photon laser scanning microscopy) is
Page 1 of 9 Fundamentals and Applications in Multiphoton Excitation Microscopy Two-photon excitation microscopy (also referred to as non-linear, multiphoton, or two-photon laser scanning microscopy) is
CENTER FOR BRAIN EXPERIMENT
 CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Methods of Characterizing Neural Networks
 Methods of Characterizing Neural Networks Ashley Nord University of Minnesota Minneapolis, MN 55414 Advisors: Katsushi Arisaka, Adrian Cheng University of California Los Angeles Los Angeles, CA 90024 September
Methods of Characterizing Neural Networks Ashley Nord University of Minnesota Minneapolis, MN 55414 Advisors: Katsushi Arisaka, Adrian Cheng University of California Los Angeles Los Angeles, CA 90024 September
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Two-photon microscopy in plant research
 Femto2D MULTIPHOTON LASER SCANNING MICROSCOPE Two-photon microscopy in plant research Femtonics Kft. Tűzoltó u. 59. H-1094 Budapest Hungary www.femtonics.eu info@femtonics.eu +36-1-2103349 Advantages of
Femto2D MULTIPHOTON LASER SCANNING MICROSCOPE Two-photon microscopy in plant research Femtonics Kft. Tűzoltó u. 59. H-1094 Budapest Hungary www.femtonics.eu info@femtonics.eu +36-1-2103349 Advantages of
Cellular imaging using Nano- Materials. A Case-Study based approach Arun Murali, Srivats V
 Cellular imaging using Nano- Materials A Case-Study based approach Arun Murali, Srivats V Agenda Discuss a few papers Explain a couple of new imaging techniques and their benefits over conventional imaging
Cellular imaging using Nano- Materials A Case-Study based approach Arun Murali, Srivats V Agenda Discuss a few papers Explain a couple of new imaging techniques and their benefits over conventional imaging
Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers
 Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Confocal Microscopy of Electronic Devices. James Saczuk. Consumer Optical Electronics EE594 02/22/2000
 Confocal Microscopy of Electronic Devices James Saczuk Consumer Optical Electronics EE594 02/22/2000 Introduction! Review of confocal principles! Why is CM used to examine electronics?! Several methods
Confocal Microscopy of Electronic Devices James Saczuk Consumer Optical Electronics EE594 02/22/2000 Introduction! Review of confocal principles! Why is CM used to examine electronics?! Several methods
Absorption of an electromagnetic wave
 In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope
 High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING. Marc Verhaegen
 HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
A simple introduction to multiphoton microscopy
 Journal of Microscopy, Vol. 243, Pt 3 2011, pp. 221 226 Received 29 April 2011; accepted 28 June 2011 doi: 10.1111/j.1365-2818.2011.03532.x A simple introduction to multiphoton microscopy A. USTIONE &
Journal of Microscopy, Vol. 243, Pt 3 2011, pp. 221 226 Received 29 April 2011; accepted 28 June 2011 doi: 10.1111/j.1365-2818.2011.03532.x A simple introduction to multiphoton microscopy A. USTIONE &
Nature Neuroscience: doi: /nn Supplementary Figure 1. Comprehensive opto-mechanical design of the dual-axis microscope.
 Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Optogenetics and Multiphoton Excitation. June 2014
 Optogenetics and Multiphoton Excitation June 2014 Optogenetics and Multiphoton Excitation (MPE) MPE is used in Optogenetics for the usual advantages related to nonlinear excitation: Deeper penetration
Optogenetics and Multiphoton Excitation June 2014 Optogenetics and Multiphoton Excitation (MPE) MPE is used in Optogenetics for the usual advantages related to nonlinear excitation: Deeper penetration
Bi177 - Lecture 13 Microscopy Outside the Box. Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM
 Bi177 - Lecture 13 Microscopy Outside the Box Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM The diffraction limit: Abbe s law The Problem Diffraction limit 100x larger than molecular scale! Green Fluorescent
Bi177 - Lecture 13 Microscopy Outside the Box Fluorescence Nanoscopy TIRF 4-pi STED STORM/PALM The diffraction limit: Abbe s law The Problem Diffraction limit 100x larger than molecular scale! Green Fluorescent
Lab 5: Optical trapping and single molecule fluorescence
 Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Lab 5: Optical trapping and single molecule fluorescence PI: Matt Lang Lab Instructor: Jorge Ferrer Summary Optical tweezers are an excellent experimental tool to study the biophysics of single molecule
Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution
 SUPPLEMENTARY INFORMATION for Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution Yanjie Zhao 1,2, Tingting Yu 1,2, Chao Zhang 1,2, Zhao Li 1,2, Qingming Luo 1,2,
SUPPLEMENTARY INFORMATION for Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution Yanjie Zhao 1,2, Tingting Yu 1,2, Chao Zhang 1,2, Zhao Li 1,2, Qingming Luo 1,2,
Imaging facilities at WUR
 Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Imaging facilities at WUR Advanced light microscopy facilities at Wageningen UR Programme Thursday 13 June 2013 Lunch meeting organized by Cat-Agro Food 12.00 Welcome and sandwich lunch 12.10 Introduction
Spectral Separation of Multifluorescence Labels with the LSM 510 META
 Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
Rice/TCU REU on Computational Neuroscience. Fundamentals of Molecular Imaging
 Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
Supplementary Table 1. Components of an FCS setup (1PE and 2PE)
 Supplementary Table 1. Components of an FCS setup (1PE and 2PE) Component and function Laser source Excitation of fluorophores Microscope with xy-translation stage mounted on vibration isolated optical
Supplementary Table 1. Components of an FCS setup (1PE and 2PE) Component and function Laser source Excitation of fluorophores Microscope with xy-translation stage mounted on vibration isolated optical
Live cell microscopy
 Live cell microscopy 1. Why do live cell microscopy? 2. Maintaining living cells on a microscope stage. 3. Considerations for imaging living cells. 4. Fluorescence labeling of living cells. 5. Imaging
Live cell microscopy 1. Why do live cell microscopy? 2. Maintaining living cells on a microscope stage. 3. Considerations for imaging living cells. 4. Fluorescence labeling of living cells. 5. Imaging
Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng
 Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
D e c N o. 2 8
 D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
D e c. 2 0 0 7 N o. 2 8 CONFOCAL APPLICATION LETTER resolution FRET Acceptor Photobleaching LAS AF Application Wizard FRET with Leica TCS SP5 LAS AF Version 1.7.0 Introduction Fluorescence Resonance Energy
F* techniques: FRAP, FLIP, FRET, FLIM,
 F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
Design for Manufacturability (DFM) in the Life Sciences
 T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
T E C H N I C A L N O T E Design for Manufacturability (DFM) in the Life Sciences Fluorescence Spectroscopy Product Platform Realized with TracePro TM Suite of Opto-Mechanical Design Software Tools Authors:
Imaging of endocrine organs
 Imaging of endocrine organs Helen Christian Department of Physiology, Anatomy & Genetics St Anne s College, University of Oxford Diabetesforum, Stockholm 2017 Islets of Langerhan Pituitary gland Renin
Imaging of endocrine organs Helen Christian Department of Physiology, Anatomy & Genetics St Anne s College, University of Oxford Diabetesforum, Stockholm 2017 Islets of Langerhan Pituitary gland Renin
How to use the SP5 confocal microscope
 How to use the SP5 confocal microscope Mailfert Sébastien (mailfert@ciml.univ-mrs.fr) Tel : 9126 Imaging Immunity (ImagImm) photonic microscopy facility Centre d Immunologie de Marseille-Luminy 2016 /
How to use the SP5 confocal microscope Mailfert Sébastien (mailfert@ciml.univ-mrs.fr) Tel : 9126 Imaging Immunity (ImagImm) photonic microscopy facility Centre d Immunologie de Marseille-Luminy 2016 /
Visualizing Cells Molecular Biology of the Cell - Chapter 9
 Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Visualizing Cells Molecular Biology of the Cell - Chapter 9 Resolution, Detection Magnification Interaction of Light with matter: Absorbtion, Refraction, Reflection, Fluorescence Light Microscopy Absorbtion
Resolution of Microscopes Visible light is nm Dry lens(0.5na), green(530nm light)=0.65µm=650nm for oil lens (1.4NA) UV light (300nm) = 0.13µm f
 Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
Microscopes and Microscopy MCB 380 Good information sources: Alberts-Molecular Biology of the Cell http://micro.magnet.fsu.edu/primer/ http://www.microscopyu.com/ Approaches to Problems in Cell Biology
Biophotonics?? Biophotonics. technology in biomedical engineering. Advantages of the lightwave
 Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Dino-Lite knowledge & education. Fluorescence Microscopes
 Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility
 Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
Super Resolution Microscopy - Breaking the Diffraction Limit Radiological Research Accelerator Facility Sabrina Campelo, Dr. Andrew Harken Outline Motivation Fluorescence Microscopy -Multiphoton Imaging
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
PALM/STORM, BALM, STED
 PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
PALM/STORM, BALM, STED Last class 2-photon Intro to PALM/STORM Cyanine dyes/dronpa This class Finish localization super-res BALM STED Localization microscopy Intensity Bins = pixels xx 2 = ss2 + aa 2 /12
Genetically targeted all-optical electrophysiology with a transgenic Credependent
 Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
Total Internal Reflection Fluorescence Microscopy
 Total Internal Reflection Microscopy Nicole O Neil Indiana University October 24, 2005 Agenda Why use TIRFM? Theory behind TIR Snell s Law Instrumentation Evanescent Wave Excitation of Fluorophores Advantages/Disadvantages
Total Internal Reflection Microscopy Nicole O Neil Indiana University October 24, 2005 Agenda Why use TIRFM? Theory behind TIR Snell s Law Instrumentation Evanescent Wave Excitation of Fluorophores Advantages/Disadvantages
Concept review: Fluorescence
 16 Concept review: Fluorescence Some definitions: Chromophore. The structural feature of a molecule responsible for the absorption of UV or visible light. Fluorophore. A chromophore that remits an absorbed
16 Concept review: Fluorescence Some definitions: Chromophore. The structural feature of a molecule responsible for the absorption of UV or visible light. Fluorophore. A chromophore that remits an absorbed
Fluorescence Microscopy
 Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
cell and tissue imaging by fluorescence microscopy
 cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
cell and tissue imaging by fluorescence microscopy Steven NEDELLEC Plateforme Micropicell SFR Santé François Bonamy Nantes 1 A matter of size Limit of resolution 0.15mm aims: building the image of an object
Contact Details. Dr Alexander Galkin. Office: MBC Room 186. Tel: (028) Frequency and wavelength.
 Contact Details The electromagnetic spectrum Biological Spectroscopy Dr Alexander Galkin Email: a.galkin@qub.ac.uk Dr Alexander Galkin MSc Biomolecular Function - BBC8045 Office: MBC Room 186 Tel: (028)
Contact Details The electromagnetic spectrum Biological Spectroscopy Dr Alexander Galkin Email: a.galkin@qub.ac.uk Dr Alexander Galkin MSc Biomolecular Function - BBC8045 Office: MBC Room 186 Tel: (028)
COPYRIGHTED MATERIAL. Tissue Preparation and Microscopy. General Concepts. Chemical Fixation CHAPTER 1
 CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
CHAPTER 1 Tissue Preparation and Microscopy General Concepts I. Biological tissues must undergo a series of treatments to be observed with light and electron microscopes. The process begins by stabilization
Introduction to Computational Fluorescence Microscopy!
 Introduction to Computational Fluorescence Microscopy! EE367/CS448I: Computational Imaging and Display! stanford.edu/class/ee367! Lecture 13! Gordon Wetzstein! Stanford University! Midterm! Tuesday, Feb
Introduction to Computational Fluorescence Microscopy! EE367/CS448I: Computational Imaging and Display! stanford.edu/class/ee367! Lecture 13! Gordon Wetzstein! Stanford University! Midterm! Tuesday, Feb
The analysis of fluorescence microscopy images for FRET detection
 The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
The most extensively used technique for tissue analysis is light microscopy.
 Fluorescence Theory Quantum yield Wavelength shift Ligand interactions Membrane interactions Using quenchning effects Fluorescence in-vivo Localization Distance measurements FRET The most extensively used
Fluorescence Theory Quantum yield Wavelength shift Ligand interactions Membrane interactions Using quenchning effects Fluorescence in-vivo Localization Distance measurements FRET The most extensively used
[A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal:
![[A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal: [A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal:](/thumbs/93/114465749.jpg) [A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal: Broadcast An entire city should be of a sufficiently
[A complex community of T cells, B cells, NK, DC monocytes,neutrophils, etc.] Lymphocyte Communication Does not Obey at least one Aristotelian Ideal: Broadcast An entire city should be of a sufficiently
Challenges to measuring intracellular Ca 2+ Calmodulin: nature s Ca 2+ sensor
 Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
The new LSM 700 from Carl Zeiss
 The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
The new LSM 00 from Carl Zeiss Olaf Selchow, Bernhard Goetze To cite this version: Olaf Selchow, Bernhard Goetze. The new LSM 00 from Carl Zeiss. Biotechnology Journal, Wiley- VCH Verlag, 0, (), pp.. .
Supporting Information. Two-Photon Luminescence of Single Colloidal Gold NanoRods: Revealing the Origin of Plasmon Relaxation in Small Nanocrystals
 Supporting Information Two-Photon Luminescence of Single Colloidal Gold NanoRods: Revealing the Origin of Plasmon Relaxation in Small Nanocrystals Céline Molinaro 1, Yara El Harfouch 1, Etienne Palleau
Supporting Information Two-Photon Luminescence of Single Colloidal Gold NanoRods: Revealing the Origin of Plasmon Relaxation in Small Nanocrystals Céline Molinaro 1, Yara El Harfouch 1, Etienne Palleau
 Page 1 of 5 Welding plastics with near-ir lasers Improvements in the performance and cost-effectiveness of lasers have led to their wider use for welding thermoplastics. Increasing use of bonded plastics
Page 1 of 5 Welding plastics with near-ir lasers Improvements in the performance and cost-effectiveness of lasers have led to their wider use for welding thermoplastics. Increasing use of bonded plastics
Biosensis P1TM Polar Lipid Tracing Reagent. Catalogue Number: TR-600-P1. For research use only, not for use in clinical and diagnostic procedures.
 Biosensis P1TM Polar Lipid Tracing Reagent Catalogue Number: TR-600-P1 For research use only, not for use in clinical and diagnostic procedures. Version: TR-600-P1/v2/Sep2018 Biosensis Pty Ltd., 51 West
Biosensis P1TM Polar Lipid Tracing Reagent Catalogue Number: TR-600-P1 For research use only, not for use in clinical and diagnostic procedures. Version: TR-600-P1/v2/Sep2018 Biosensis Pty Ltd., 51 West
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
Microscopy from Carl Zeiss. DirectFRAP. News from the Cell. The New Class of Laser Manipulation for the Analysis of Cell Dynamics
 Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
Microscopy from Carl Zeiss DirectFRAP News from the Cell The New Class of Laser Manipulation for the Analysis of Cell Dynamics DirectFRAP. New Insights into Cell Dynamics. Fluorescence breaks new ground:
Directional Surface Plasmon Coupled Emission
 Journal of Fluorescence, Vol. 14, No. 1, January 2004 ( 2004) Fluorescence News Directional Surface Plasmon Coupled Emission KEY WORDS: Surface plasmon coupled emission; high sensitivity detection; reduced
Journal of Fluorescence, Vol. 14, No. 1, January 2004 ( 2004) Fluorescence News Directional Surface Plasmon Coupled Emission KEY WORDS: Surface plasmon coupled emission; high sensitivity detection; reduced
A Thin Layer Imaging with the Total Internal Reflection Fluorescence Microscopy
 Journal of Optoelectronical Nanostructures Islamic Azad University Summer 2017 / Vol. 2, No. 2 A Thin Layer Imaging with the Total Internal Reflection Fluorescence Microscopy Neda Roostaie 1, Elham Sheykhi
Journal of Optoelectronical Nanostructures Islamic Azad University Summer 2017 / Vol. 2, No. 2 A Thin Layer Imaging with the Total Internal Reflection Fluorescence Microscopy Neda Roostaie 1, Elham Sheykhi
Super Resolution Imaging Solution Provider. Imaging Future
 Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
Super Resolution Imaging Solution Provider Imaging Future Imaging Solution More Than Equipment NanoBioImaging(NBI) is the Industrial Partner of HKUST Super Resolution Imaging Center (SRIC). NBI aims to
LiveReceptor AMPAR <Endogenous AMPAR Labeling Reagent>
 9-7 Hongo 2-Chome, Bunkyo-Ku Tokyo 113-0033, Japan LiveReceptor AMPAR Product Background Neurotransmitter receptors including glutamate receptors and GABA receptors
9-7 Hongo 2-Chome, Bunkyo-Ku Tokyo 113-0033, Japan LiveReceptor AMPAR Product Background Neurotransmitter receptors including glutamate receptors and GABA receptors
Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ
 Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ Research at the nanoscale is more effective, when research teams can quickly and easily observe and characterize a wide
Optical Observation - Hyperspectral Characterization of Nano-scale Materials In-situ Research at the nanoscale is more effective, when research teams can quickly and easily observe and characterize a wide
High-throughput three-dimensional (3D) lithographic microfabrication in biomedical applications
 High-throughput three-dimensional (3D) lithographic microfabrication in biomedical applications The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story
High-throughput three-dimensional (3D) lithographic microfabrication in biomedical applications The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story
SUPPLEMENTARY FIGURES
 SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
Femtosecond micromachining in polymers
 Femtosecond micromachining in polymers Prof. Dr Cleber R. Mendonca Daniel S. Corrêa Prakriti Tayalia Dr. Tobias Voss Dr. Tommaso Baldacchini Prof. Dr. Eric Mazur fs-micromachining focus laser beam inside
Femtosecond micromachining in polymers Prof. Dr Cleber R. Mendonca Daniel S. Corrêa Prakriti Tayalia Dr. Tobias Voss Dr. Tommaso Baldacchini Prof. Dr. Eric Mazur fs-micromachining focus laser beam inside
Cell analysis and bioimaging technology illustrated
 Cell analysis and bioimaging technology illustrated The Cell Analysis Center Scientific Bulletin Part 1 Sysmex has been studying and exploring principles of automated haematology analysers, making full
Cell analysis and bioimaging technology illustrated The Cell Analysis Center Scientific Bulletin Part 1 Sysmex has been studying and exploring principles of automated haematology analysers, making full
Sample region with fluorescent labeled molecules
 FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~ λ/2) that can be achieved by conventional
Using a Scientifica Multiphoton SliceScope for Fluorescence Lifetime Imaging
 SciMethods: Applications Using a Scientifica Multiphoton SliceScope for Fluorescence Lifetime Imaging Kim Doré 1, Jonathan Aow 1, Roberto Malinow 1 & Christian Wilms 2 1 : Department of Neuroscience and
SciMethods: Applications Using a Scientifica Multiphoton SliceScope for Fluorescence Lifetime Imaging Kim Doré 1, Jonathan Aow 1, Roberto Malinow 1 & Christian Wilms 2 1 : Department of Neuroscience and
In vivo fast imaging and optogenetic manipulation using genetically-encoded fluorescent indicators and actuators. Serena Bovetti
 In vivo fast imaging and optogenetic manipulation using genetically-encoded fluorescent indicators and actuators Serena Bovetti Istituto Italiano di Tecnologia Genova, Italy Bogliasco, June 6-8 2016 Analyzing
In vivo fast imaging and optogenetic manipulation using genetically-encoded fluorescent indicators and actuators Serena Bovetti Istituto Italiano di Tecnologia Genova, Italy Bogliasco, June 6-8 2016 Analyzing
Confocal Microscopy & Imaging Technology. Yan Wu
 Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Photoacoustic imaging of vascular networks in transgenic mice
 Photoacoustic imaging of vascular networks in transgenic mice J.G. Laufer 1, J.O. Cleary 1,2, E.Z. Zhang 1, M.F. Lythgoe 2, P.C. Beard 1 1. Department of Medical Physics and Bioengineering, University
Photoacoustic imaging of vascular networks in transgenic mice J.G. Laufer 1, J.O. Cleary 1,2, E.Z. Zhang 1, M.F. Lythgoe 2, P.C. Beard 1 1. Department of Medical Physics and Bioengineering, University
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Beyond observation: Microscopy with ultrashort laser pulses to probe and manipulate cortical vasculature
 Prepared for SPIE's newsletter on Optics in Information Systems (Demetri Psaltis and Bahram Javidi, chairs); special issue on Information Processing Applications of Femtosecond Technology (Shaya Fainman,
Prepared for SPIE's newsletter on Optics in Information Systems (Demetri Psaltis and Bahram Javidi, chairs); special issue on Information Processing Applications of Femtosecond Technology (Shaya Fainman,
SI8000 Live Cell Imaging System
 SI8000 Live Cell Imaging System Sony Biotechnology Inc. SI8000 Cell Motion Imaging System The Sony SI8000 Live Cell Imaging System detects and quantifies cellular motion using proprietary video processing
SI8000 Live Cell Imaging System Sony Biotechnology Inc. SI8000 Cell Motion Imaging System The Sony SI8000 Live Cell Imaging System detects and quantifies cellular motion using proprietary video processing
The CQ1 Confocal Quantitative Image Cytometer and its Application to Biological Measurement
 The CQ1 Confocal Quantitative Image Cytometer and its Application to Biological Measurement Hirofumi Sakashita *1 Koji Ohashi *1 Kazuo Ozawa *2 ohei Tsubouchi *1 The CQ1 confocal quantitative image cytometer,
The CQ1 Confocal Quantitative Image Cytometer and its Application to Biological Measurement Hirofumi Sakashita *1 Koji Ohashi *1 Kazuo Ozawa *2 ohei Tsubouchi *1 The CQ1 confocal quantitative image cytometer,
Observation in the GB (Gentle Beam) Capabilities
 A field-emission cathode in the electron gun of a scanning electron microscope provides narrower probing beams at low as well as high electron energy, resulting in both improved spatial resolution and
A field-emission cathode in the electron gun of a scanning electron microscope provides narrower probing beams at low as well as high electron energy, resulting in both improved spatial resolution and
Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone
 Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone Sven Terclavers LSM780 System overview The Scan Module - Core of the LSM 780 1 V/tunable PTC laser ports (405/440, cw/ps;
Imaging & analysis with the LSM780 NLO Discover the secrets beyond the twilight zone Sven Terclavers LSM780 System overview The Scan Module - Core of the LSM 780 1 V/tunable PTC laser ports (405/440, cw/ps;
Welcome! openmicberkeley.wordpress.com. Open Berkeley
 Welcome! openmicberkeley.wordpress.com Agenda Jen Lee: Introduction to FRET Marla Feller: Using FRET sensors to look at time resolved measurements Becky Lamason: Using FRET to determine if a bacterial
Welcome! openmicberkeley.wordpress.com Agenda Jen Lee: Introduction to FRET Marla Feller: Using FRET sensors to look at time resolved measurements Becky Lamason: Using FRET to determine if a bacterial
Meeting the growing needs of developmental biology. Solutions brochure
 Meeting the growing needs of developmental biology Solutions brochure Imaging for developmental biology Outstanding optical quality is at the heart of all Nikon s microscopic imaging systems and this,
Meeting the growing needs of developmental biology Solutions brochure Imaging for developmental biology Outstanding optical quality is at the heart of all Nikon s microscopic imaging systems and this,
Final Exam, 176 points PMB 185: Techniques in Light Microscopy
 Final Exam, 176 points Name PMB 185: Techniques in Light Microscopy Point value is in parentheses at the end of each question. 1) Order the steps in setting up Köhler illumination. It is not necessary
Final Exam, 176 points Name PMB 185: Techniques in Light Microscopy Point value is in parentheses at the end of each question. 1) Order the steps in setting up Köhler illumination. It is not necessary
Giving Color to Life
 Giving Color to Life W i d e f i e l d, C o n f o c a l a n d Two-Photon Microscopy Systems with Exquisite Spectral Resolution Aurora Spectral Technologies is pleased to offer state-of-the-art optical
Giving Color to Life W i d e f i e l d, C o n f o c a l a n d Two-Photon Microscopy Systems with Exquisite Spectral Resolution Aurora Spectral Technologies is pleased to offer state-of-the-art optical
ECE280: Nano-Plasmonics and Its Applications. Week5. Extraordinary Optical Transmission (EOT)
 ECE280: Nano-Plasmonics and Its Applications Week5 Extraordinary Optical Transmission (EOT) Introduction Sub-wavelength apertures in metal films provide light confinement beyond the fundamental diffraction
ECE280: Nano-Plasmonics and Its Applications Week5 Extraordinary Optical Transmission (EOT) Introduction Sub-wavelength apertures in metal films provide light confinement beyond the fundamental diffraction
Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25
 Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25 9:30 9:45 Coffee and Gipfeli 9:45 10:00 Welcome address and introduction B. Hecht Uni Basel H.-J.
Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25 9:30 9:45 Coffee and Gipfeli 9:45 10:00 Welcome address and introduction B. Hecht Uni Basel H.-J.
Plasmonics using Metal Nanoparticles. Tammy K. Lee and Parama Pal ECE 580 Nano-Electro-Opto-Bio
 Plasmonics using Metal Nanoparticles Tammy K. Lee and Parama Pal ECE 580 Nano-Electro-Opto-Bio April 1, 2007 Motivation Why study plasmonics? Miniaturization of optics and photonics to subwavelength scales
Plasmonics using Metal Nanoparticles Tammy K. Lee and Parama Pal ECE 580 Nano-Electro-Opto-Bio April 1, 2007 Motivation Why study plasmonics? Miniaturization of optics and photonics to subwavelength scales
Characterizing Phenotypes of Bacteria by Staining Method
 Experiment 3 Laboratory to Biology III Diversity of Microorganisms / Wintersemester / page 1 Experiment 3 Characterizing Phenotypes of Bacteria by Staining Method Advisor NN Reading Chapters in BBOM 9
Experiment 3 Laboratory to Biology III Diversity of Microorganisms / Wintersemester / page 1 Experiment 3 Characterizing Phenotypes of Bacteria by Staining Method Advisor NN Reading Chapters in BBOM 9
Direct visualization, sizing and concentration measurement of fluorescently labeled nanoparticles using NTA
 Direct visualization, sizing and concentration measurement of fluorescently labeled nanoparticles using NTA NANOSIGHT RANGE Visualize and Measure Nanoparticle Size and Concentration PARTICLE SIZE PARTICLE
Direct visualization, sizing and concentration measurement of fluorescently labeled nanoparticles using NTA NANOSIGHT RANGE Visualize and Measure Nanoparticle Size and Concentration PARTICLE SIZE PARTICLE
Localization Microscopy
 Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Localization Microscopy Theory, Sample Prep & Practical Considerations Patrina Pellett & Ann McEvoy Applications Scientist GE Healthcare, Cell Technologies May 27 th, 2015 Localization Microscopy Talk
Contents. SCHOOL of FLUORESCENCE. For more information, go to lifetechnologies.com/imagingbasics
 MPSF educator packet This packet contains illustrations and figures from the Molecular Probes School of Fluorescence website. They illustrate concepts from the basic physical properties that underlie fluorescence
MPSF educator packet This packet contains illustrations and figures from the Molecular Probes School of Fluorescence website. They illustrate concepts from the basic physical properties that underlie fluorescence
Characterizing Phenotypes of Bacteria by Staining Method
 Experiment 3 Laboratory to Biology III Diversity of Microorganisms / Wintersemester / page 1 Experiment Characterizing Phenotypes of Bacteria by Staining Method Advisor Reading NN Chapters 3.1, 3.7, 3.8,
Experiment 3 Laboratory to Biology III Diversity of Microorganisms / Wintersemester / page 1 Experiment Characterizing Phenotypes of Bacteria by Staining Method Advisor Reading NN Chapters 3.1, 3.7, 3.8,
Widefield Microscopy Bleed-Through
 In widefield microscopy the excitation wavelengths which illuminate the sample, and the emission wavelengths which reach the CCD camera are selected throughout a filter cube. A filter cube consists of
In widefield microscopy the excitation wavelengths which illuminate the sample, and the emission wavelengths which reach the CCD camera are selected throughout a filter cube. A filter cube consists of
Class 7: Methods in Research By: Ray
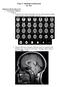 Class 7: Methods in Research By: Ray Method in Brain Research 1. Non-Invasive (Human) o Imaging Computerized (Axial) Tomography (X-rays). Static pictures and high spatial resolution. Horizontal plane only.
Class 7: Methods in Research By: Ray Method in Brain Research 1. Non-Invasive (Human) o Imaging Computerized (Axial) Tomography (X-rays). Static pictures and high spatial resolution. Horizontal plane only.
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Fluorescence Microscopy
 Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
Fluorescence Microscopy Dr. Arne Seitz Swiss Institute of Technology (EPFL) Faculty of Life Sciences Head of BIOIMAGING AND OPTICS BIOP arne.seitz@epfl.ch Fluorescence Microscopy Why do we need fluorescence
SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION
 APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
APPLICATION NOTE SURFACE ENHANCED RAMAN SCATTERING NANOPARTICLES AS AN ALTERNATIVE TO FLUORESCENT PROBES AN EVALUATION Summary: Interest in using nanoparticles specifically, Surface Enhanced Raman Scattering
Chapter One. Construction of a Fluorescent α5 Subunit. Elucidation of the unique contribution of the α5 subunit is complicated by several factors
 4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
4 Chapter One Construction of a Fluorescent α5 Subunit The significance of the α5 containing nachr receptor (α5* receptor) has been a challenging question for researchers since its characterization by
Introduction to Fluorescence Jablonski Diagram
 ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
