SHORT COMMUNICATION PRIMARY CULTURE OF CRUSTACEAN STOMATOGASTRIC GANGLION NEURONES IN A DEFINED MEDIUM
|
|
|
- Basil Garrett
- 6 years ago
- Views:
Transcription
1 J. exp. Biol. 149, (1990) 521 Printed m Great Britain The Company of Biologists Limited 1990 SHORT COMMUNICATION PRIMARY CULTURE OF CRUSTACEAN STOMATOGASTRIC GANGLION NEURONES IN A DEFINED MEDIUM BY ROBERT A. GRAF AND IAN M. COOKE B k6sy Laboratory of Neurobiology and Department of Zoology, University of Hawaii at Manoa, Honolulu, Hawaii 96822, USA Accepted 20 October 1989 The crustacean stomatogastric ganglion, consisting of about 30 identifiable neurones, produces several distinct patterned outputs governing rhythmic movements of the stomach. This system has become a model for addressing cellular properties and synaptic interactions underlying pattern generation in neural networks (see Selverston and Moulins, 1987). Studies of the system have been limited by the need to use acutely isolated intact or semi-intact ganglion preparations. Also, interesting membrane responses and synaptic interactions occur in the neuropile, but recording from sites other than the somata has been difficult using an intact preparation. Primary culture of dissociated neurones from the ganglion may yield new insights on regional membrane properties and longterm (days) responses to neuromodulators of individual neurones that have regenerated neuritic processes. This paper presents our culture methods and some observations of outgrowth from neurones that have been dissociated from the stomatogastric ganglia of two evolutionarily diverse species, the Maine lobster Homarus americanus (Fig. 1) and a tropical semi-terrestrial crab Cardisoma carnifex (Fig. 2). The only other study on stomatogastric neurones in culture of which we are aware used a freshwater crayfish (Pacifastacus leniusculus), in which neurones were plated in media supplemented with serum (Krenz and Fischer, 1988). The culturing procedure is based on that developed for crustacean X-organ neurones (Graf et al. 1988; Cooke et al. 1989) with a number of modifications that optimize conditions for culturing the larger and more delicate stomatogastric neurones. Ganglia were removed from adult animals with 0.5 cm lengths of connective, and submerged for 5min in an antibiotic crab or lobster saline containing loounitsml" 1 penicillin-g, 75 units ml" 1 streptomycin (both from frozen powder) and 0.25/imolml" 1 fungizone (all from Gibco), ph7.6. The dissection and plating were performed in a laminar horizontal flow hood (Baker) using a Wild M5 dissecting microscope equipped with fibre optic illumination. Dissected ganglia were transferred to 35 mm culture dishes containing an Key words: primary culture, defined medium, neurones, invertebrate, stomatogastric ganglion, Crustacea.
2 522 R. A. GRAF AND I. M. COOKE enzymatic saline: 2mgmr 1 collagenase/dispase (Boehringer Mannheim) and Oimgml" 1 elastase I (Sigma) in crab or lobster saline (ph7.6) sterilized by filtration (0.22 ^m pore size). The covered dishes were agitated (22cyclesmin" 1 ) on a shaker for lh at room temperature. Somata with neurites were further separated from their surrounding ganglionic tissues by freeing the loosened connective tissue using a fine jet of enzymatic saline blown from a capillary tube drawn down to an inner tip diameter of 10-20/xm. The dangling somata, held to the ganglion by their neurites, were rinsed of enzyme with two changes of filtered antibiotic saline. Unidentified somata were then individually aspirated using ethanol-sterilized capillary tubes, pulled and fire-polished to yield a smooth tip with an inside diameter of roughly 100/an. The neurones were plated onto 35 mm culture dishes (for substrata, see below) containing 2ml of defined medium; the fluid depth was approximately 2 mm. The defined medium was prepared by mixing sterile Leibowitz-15 (L-15, Gibco) with an equal volume of buffered doublestrength crab or lobster saline (ph7.9, 20mmoll~ 1 Hepes) containing nutrient and antibiotic constituents. At final volume, these were: nommoll" 1 D-glucose, 2mmoll~ 1 L-glutamine (from frozen stock) and O.lmgml" 1 gentamicin. Without adjustments, the final medium ph was usually near the optimal value, 7.7. Medium was usually made just prior to use. The substrata used included uncoated Primaria dishes (Falcon 3801) and Falcon 3001 dishes coated with sterile O.lmgml" 1 poly-l-lysine (Sigma). After neurone dispersal, the dishes were covered and not moved for at least 2 h to aid neuronal adhesion to the substratum. They were then transferred to a sterile, humidified portable incubator chamber (Billups Rothanberg), and placed in the dark at room temperature (20-21 C). Outgrowth generally occurred from sites where membrane was in direct contact with the substratum. If only the soma contacted the substratum, with primary neurite suspended in the medium or severed during dissociation, it sometimes adhered via sparse, thin, membraneous protrusions that clung to the dish after 1 day in culture (not shown). Extensive outgrowth occurred if the severed end of the primary neurite adhered to the substratum. Two forms of outgrowth predominated: a large, thin, veiling (lamellipodial) type (Fig. ID), and a directed branching type (Figs 1A, 2). These forms were not mutually exclusive (Fig. 1B,C) and, when both types were present, the neurone's growth was in transition, usually from veiling to branching. Maximum veil size was typically reached by day 2 and was maintained for a week in static culture. The breadth reached by the veil roughly equalled the soma diameter. The margins of veils were skirted with growth cones that appeared phase dark under phase contrast optics (not shown). Neurones displaying the more directed (linear) growth morphology (Fig. 2) developed neuritic extensions that represented the stabilization of processes following outgrowth from one or a few motile growth cones. The neuritic processes arose from the periphery of a veil within a day of plating (compare day 1 and day 2 of Fig. 2). Well-stabilized regions of the major neurites often appeared to have filamentous structures in parallel array (Fig. 2, day 2). The neurones having well-directed outgrowth continued to show process
3 Stomatogastric neurones in culture 523 Fig. 1. Photomicrographs of outgrowth from isolated Homarus americanus stomatogastric neurones. (A) Low-power phase contrast image of soma with residual axon and lateral processes, 2 days in culture. Arrowhead marks end of residual axon. (B) Higher-power image of the growth cone from A taken at the same time as A. (C) Same growth cone on day 3. Note directed growth extending left from the veil. (D) Composite photographs pieced together from two focal planes (soma and growth cone) to give an example of neuronal surface membrane contour. Note the large veil. Day 2 in culture. (B-D) Hoffman interference modulation contrast microscopy. All cells in defined medium. Calibration bars, 50ym.
4 524 R. A. GRAF AND I. M. COOKE elongation in static culture up to day 7, at which time the cultures were used for other purposes. Typical neurite extension rates may be seen from the development of the leading growth margins of the Cardisoma neurone shown in Fig. 2: from day 1 to day 2 the primary neurite elongated 165 pan, and from day 2 to day 3 the new Day 1 Day 2 Day3 Fig. 2. Hoffmann modulation contrast images of directed outgrowth from a Cardisoma carnifex stomatogastric neurone at different culture ages (as indicated) in static defined medium. At day 1,filopodiaare seen at the peripheral margin of the small veil. By day 2, there has been extensive directed outgrowth from day 1 filopodia. Further extension of several processes occurred by day 3. Note the fibrillar appearance of the stabilized major neurite. Scale bar, 50 fjm.
5 Stomatogastric neurones in culture 525 growth traversed 143 pan. These growth rates varied from cell to cell with a range of ym day" 1. The stomatogastric somata and primary neurites in situ appear from ultrastructural observations to be entirely covered by an unbroken glial sheath (King, 1976), which may explain why it was necessary to change the enzymatic regime from that used for the X-organ neurones. These appear to separate more easily from surrounding glia or other material. Successful outgrowth appears to depend largely on whether conditions are provided that favour neuronal adherence and direct contact with the substratum. Since these neurones grow vigorously in a simple medium that is completely defined, exploration of conditions and factors that modify cell outgrowth and electrical responses should be facilitated. We thank Professor D. K. Hartline for supplying animals for culture experiments, encouragement and suggestions; Dr B. R. Jones for suggestions on the manuscript; and Ms B. A. Tomiyasu for demonstrating the dissection. This research was supported by National Institutes of Health grant NS15453 and National Science Foundation grant BNS to IC, National Institutes of Health grants to the University of Hawaii (Research Centers in Minority Institutions G12 RR03O61, and Biomedical Research Support), and by the University of Hawaii Foundation. References COOKE, I., GRAF, R., GRAU, S., HAYLETT, B., MEYERS, D. AND RUBEN, P. (1989). Crustacean peptidergic neurons in culture show immediate outgrowth in simple medium. Proc. natn. Acad. Sci. U.S.A. 86, GRAF, R. A., GRAU, S., HAYLETT, B. AND COOKE, I. M. (1988). Crustacean peptidergic neurons in primary culture show immediate outgrowth in denned media. Neurosci. Abstr. 14, 869. KING, D. (1976). Organization of crustacean neuropil. II. Distribution of synaptic contacts on identified motor neurons in lobster stomatogastric ganglion. J. Neurocytol. 5, KRENZ, W. D. AND FISCHER, P. (1988). Ionic conductances in crustacean stomatogastric neurons in primary culture. Neurosci. Abstr. 14, 295. SELVERSTON, A. I. AND MOULINS, M. (1987). The Crustacean Stomatogastric System. New York: Springer-Verlag.
6
Section of Morphological and Behavioral Neuroscience Protocol for low density primary neuron cultures
 Institute of Pharmacology and Toxicology Section of Morphological and Behavioral Neuroscience Protocol for low density primary neuron cultures B. Lardi-Studler, C. Sidler, J.-M. Fritschy Revised March
Institute of Pharmacology and Toxicology Section of Morphological and Behavioral Neuroscience Protocol for low density primary neuron cultures B. Lardi-Studler, C. Sidler, J.-M. Fritschy Revised March
Xeno-Free Culture and Differentiation of Neural Stem Cells into Neurons
 Xeno-Free Culture and Differentiation of Neural Stem Cells into Neurons Publication Part Number MAN0007497 Revision 1.0 Introduction As the field of neuroscience moves closer to the reality of neural stem
Xeno-Free Culture and Differentiation of Neural Stem Cells into Neurons Publication Part Number MAN0007497 Revision 1.0 Introduction As the field of neuroscience moves closer to the reality of neural stem
Hippocampal Neuron Dissociation Transfection and Culture in Microfluidics Chambers Yang Geng *
 Hippocampal Neuron Dissociation Transfection and Culture in Microfluidics Chambers Yang Geng * Department of Pediatrics and Bioengineering, Stanford University School of Medicine, Stanford, USA *For correspondence:
Hippocampal Neuron Dissociation Transfection and Culture in Microfluidics Chambers Yang Geng * Department of Pediatrics and Bioengineering, Stanford University School of Medicine, Stanford, USA *For correspondence:
If protein coating is acceptable in the planned experiments, there is another quick and simple way to render the surface hydrophilic.
 1 MEA Handling Warning: Use only liquids or cleaning solutions with a neutral ph (7) on MEAs with a silicon nitride insulation type. Otherwise, the MEAs may be irreversibly damaged. Warning: Do not to
1 MEA Handling Warning: Use only liquids or cleaning solutions with a neutral ph (7) on MEAs with a silicon nitride insulation type. Otherwise, the MEAs may be irreversibly damaged. Warning: Do not to
Amaxa Chicken Neuron Nucleofector Kit
 Amaxa Chicken Neuron Nucleofector Kit For Primary Chicken Hippocampal Neurons Primary dissociated chicken hippocampal neurons, prepared from chicken embryos (E7) as mixed glial cultures. Example for Nucleofection
Amaxa Chicken Neuron Nucleofector Kit For Primary Chicken Hippocampal Neurons Primary dissociated chicken hippocampal neurons, prepared from chicken embryos (E7) as mixed glial cultures. Example for Nucleofection
If protein coating is acceptable in the planned experiments, there is another quick and simple way to render the surface hydrophilic.
 1 MEA Handling Warning: Use only liquids or cleaning solutions with a neutral ph (7) on MEAs with a silicon nitride insulation type. Otherwise, the MEAs may be irreversibly damaged. Warning: Do not to
1 MEA Handling Warning: Use only liquids or cleaning solutions with a neutral ph (7) on MEAs with a silicon nitride insulation type. Otherwise, the MEAs may be irreversibly damaged. Warning: Do not to
Human Skeletal Muscle Myoblast Care Manual: Maintenance and Differentiation from Myoblasts to Myocytes
 Human Skeletal Muscle Myoblast Care Manual: Maintenance and Differentiation from Myoblasts to Myocytes STORAGE CONDITIONS Media: Short Term 4 C 6 months -20 C F or research use only. Not approved for human
Human Skeletal Muscle Myoblast Care Manual: Maintenance and Differentiation from Myoblasts to Myocytes STORAGE CONDITIONS Media: Short Term 4 C 6 months -20 C F or research use only. Not approved for human
Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration?
 Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Supplementary Materials
 Supplementary Materials The potential of Antheraea pernyi silk for spinal cord repair A. Varone 1, D. Knight 2, S. Lesage 2, F. Vollrath 2,3, A.M. Rajnicek 1, W. Huang 1,* 1 Institute of Medical Sciences,
Supplementary Materials The potential of Antheraea pernyi silk for spinal cord repair A. Varone 1, D. Knight 2, S. Lesage 2, F. Vollrath 2,3, A.M. Rajnicek 1, W. Huang 1,* 1 Institute of Medical Sciences,
Supplementary Information:
 Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2015 Supplementary Information: 3D Printed Nervous System on a Chip Blake N. Johnson, a,b Karen
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2015 Supplementary Information: 3D Printed Nervous System on a Chip Blake N. Johnson, a,b Karen
Human Adult Mesothelial Cell Manual
 Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.05 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.05 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Supporting Protocols
 Supporting Protocols This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec ECM Hydrogels. Introduction Cells and organoids may form complex
Supporting Protocols This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec ECM Hydrogels. Introduction Cells and organoids may form complex
3T3-L1 Cell Care Manual Maintenance and Differentiation of 3T3-L1 Preadipocytes to Adipocytes
 3T3-L1 Cell Care Manual Maintenance and Differentiation of 3T3-L1 Preadipocytes to Adipocytes INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0009.02 Must be processed upon shipment receipt. STORAGE CONDITIONS
3T3-L1 Cell Care Manual Maintenance and Differentiation of 3T3-L1 Preadipocytes to Adipocytes INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0009.02 Must be processed upon shipment receipt. STORAGE CONDITIONS
LHCN-M2 cell culture, differentiation treatment, and cross-linking protocol.
 Cell Growth Protocol and Differentiation treatment for the LHCN-M2 Cell Line From: HudsonAlpha/Caltech ENCODE group Date: February 17, 2011 Prepared by: Chun-Hong Zhu and Woodring E. Wright (typed by Brian
Cell Growth Protocol and Differentiation treatment for the LHCN-M2 Cell Line From: HudsonAlpha/Caltech ENCODE group Date: February 17, 2011 Prepared by: Chun-Hong Zhu and Woodring E. Wright (typed by Brian
CARE MANUAL INSTRUCTIONAL MANUAL ZBM SHIPPING CONDITIONS STORAGE CONDITIONS ORDERING INFORMATION AND TECHNICAL SERVICES
 HUMAN ADULT DERMAL FIBROBLAST CARE MANUAL INSTRUCTIONAL MANUAL ZBM0023.01 SHIPPING CONDITIONS Human Adult Dermal Fibroblast Cells Orders are delivered via Federal Express courier. All US and Canada orders
HUMAN ADULT DERMAL FIBROBLAST CARE MANUAL INSTRUCTIONAL MANUAL ZBM0023.01 SHIPPING CONDITIONS Human Adult Dermal Fibroblast Cells Orders are delivered via Federal Express courier. All US and Canada orders
CULTURING OF LEECH NEURONS AND FORMING SYNAPSES IN VITRO. Italy
 CULTURING OF LEECH NEURONS AND FORMING SYNAPSES IN VITRO Josh Titlow 1, Zana R. Majeed 1,2, John G. Nicholls 3 and Robin L. Cooper 1 1 Department of Biology, University of Kentucky, Lexington, KY 40506,
CULTURING OF LEECH NEURONS AND FORMING SYNAPSES IN VITRO Josh Titlow 1, Zana R. Majeed 1,2, John G. Nicholls 3 and Robin L. Cooper 1 1 Department of Biology, University of Kentucky, Lexington, KY 40506,
Barbier. European Synchrotron Radiation Facility (ESRF), Grenoble, France. For correspondence:
 Manganese Cytotoxicity Assay on Hippocampal Neuronal Cell Culture Alexia Daoust 1, 2, Yasmina Saoudi 1, 2, Jacques Brocard 1, 2, Nora Collomb 1, 2, Cécile Batandier 3, Mariano Bisbal 1, 2, Murielle Salomé
Manganese Cytotoxicity Assay on Hippocampal Neuronal Cell Culture Alexia Daoust 1, 2, Yasmina Saoudi 1, 2, Jacques Brocard 1, 2, Nora Collomb 1, 2, Cécile Batandier 3, Mariano Bisbal 1, 2, Murielle Salomé
Seeding, culturing and assaying upcyte and vericyte cells in the Mimetix 3D scaffold
 Seeding, culturing and assaying upcyte and vericyte cells in the Mimetix 3D scaffold Description This note describes how to seed and culture upcyte Hepatocytes as 3D cultures in the Mimetix electrospun
Seeding, culturing and assaying upcyte and vericyte cells in the Mimetix 3D scaffold Description This note describes how to seed and culture upcyte Hepatocytes as 3D cultures in the Mimetix electrospun
ISOLATED IDENTIFIED APLYSIA NEURONS IN CELL CULTURE
 ISOLATED IDENTIFIED APLYSIA NEURONS IN CELL CULTURE DANIEL DAGAN: AND IRWIN B. LEVITAN: Friedrich Miescher-lnstitut, Hnwl, Suitzerlnnd Abstract Methods have been developed for primary culture of large
ISOLATED IDENTIFIED APLYSIA NEURONS IN CELL CULTURE DANIEL DAGAN: AND IRWIN B. LEVITAN: Friedrich Miescher-lnstitut, Hnwl, Suitzerlnnd Abstract Methods have been developed for primary culture of large
Which hydrogel preparation for immunostaining protocol should I use?
 Protocol: Preparation of TissueSpec hydrogels for immunostaining This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec matrix hydrogels.
Protocol: Preparation of TissueSpec hydrogels for immunostaining This protocol may be used prior to immunostaining cells, organoids, or patient-derived xenografts cultured in TissueSpec matrix hydrogels.
Human ipsc-derived Sensory Neuron Progenitors. For the generation of ipsc-derived sensory neurons
 Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Product Information Catalog. No. Product Name Format Stock Conc. Storage on Arrival Thawing Instructions
Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Product Information Catalog. No. Product Name Format Stock Conc. Storage on Arrival Thawing Instructions
Human ipsc-derived Sensory Neuron Progenitors. For the generation of ipsc-derived sensory neurons
 Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Additional Reagents Product Name Supplier Product Code Mitomycin C Sigma-Aldrich M4287 Store at 4 o C for
Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Additional Reagents Product Name Supplier Product Code Mitomycin C Sigma-Aldrich M4287 Store at 4 o C for
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW)
 Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
Human Dermal Fibroblast Manual
 Human Dermal Fibroblast Manual INSTRUCTIONAL MANUAL ZBM0023.04 SHIPPING CONDITIONS Human Adult or Neonatal Dermal Fibroblast Cells Orders are delivered via Federal Express courier. All US and Canada orders
Human Dermal Fibroblast Manual INSTRUCTIONAL MANUAL ZBM0023.04 SHIPPING CONDITIONS Human Adult or Neonatal Dermal Fibroblast Cells Orders are delivered via Federal Express courier. All US and Canada orders
1. Carry the microscope in an upright position with both hands and place the base of the microscope 5cm from the edge of the bench
 The Microscope Operating the compound light microscope 1. Carry the microscope in an upright position with both hands and place the base of the microscope 5cm from the edge of the bench 2. Check that lenses
The Microscope Operating the compound light microscope 1. Carry the microscope in an upright position with both hands and place the base of the microscope 5cm from the edge of the bench 2. Check that lenses
Amaxa Basic Neuron SCN Nucleofector Kit
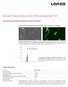 Amaxa Basic Neuron SCN Nucleofector Kit For Primary Mouse Hippocampal Neurons (Small-Cell-Number) Embryonic mouse hippocampal neurons transfected using the Nucleofector Technology A B Approximately 100.000
Amaxa Basic Neuron SCN Nucleofector Kit For Primary Mouse Hippocampal Neurons (Small-Cell-Number) Embryonic mouse hippocampal neurons transfected using the Nucleofector Technology A B Approximately 100.000
Cytotrophoblast Isolation from Term Placenta References:
 Cytotrophoblast Isolation from Term Placenta References: CY Kuo, et al. Placental Basement Membrane Proteins are Required for Effective Cytotrophoblast Invasion in a 3D Bioprinted Placenta Model. Journal
Cytotrophoblast Isolation from Term Placenta References: CY Kuo, et al. Placental Basement Membrane Proteins are Required for Effective Cytotrophoblast Invasion in a 3D Bioprinted Placenta Model. Journal
Protocols for Neural Progenitor Cell Expansion and Dopaminergic Neuron Differentiation
 Protocols for Neural Progenitor Cell Expansion and Dopaminergic Neuron Differentiation In vitro neurological research presents many challenges due to the difficulty in establishing high-yield neuronal
Protocols for Neural Progenitor Cell Expansion and Dopaminergic Neuron Differentiation In vitro neurological research presents many challenges due to the difficulty in establishing high-yield neuronal
axion Protocol Cell Culture on Microelectrode Arrays Cell Type: ArunA Biomedical - mmngfp+ Mouse Motor Neurons GFP+ BioSystems v. 1.
 axion BioSystems Cell Culture on Microelectrode Arrays Cell Type: ArunA Biomedical - mmngfp+ Mouse Motor Neurons GFP+ Protocol v. 1.1 mmngfp+ Mouse Motor Neurons GFP+ from ArunA Biomedical axion BioSystems
axion BioSystems Cell Culture on Microelectrode Arrays Cell Type: ArunA Biomedical - mmngfp+ Mouse Motor Neurons GFP+ Protocol v. 1.1 mmngfp+ Mouse Motor Neurons GFP+ from ArunA Biomedical axion BioSystems
NutriStem V9 XF Medium
 Stem Cells NutriStem V9 XF Medium A defined, xeno-free (XF), serum-free (SF) culture medium for hpsc using vitronectin Instructions for Use Product Description NutriStem V9 XF medium is a defined, xeno-free,
Stem Cells NutriStem V9 XF Medium A defined, xeno-free (XF), serum-free (SF) culture medium for hpsc using vitronectin Instructions for Use Product Description NutriStem V9 XF medium is a defined, xeno-free,
Human Tenocyte Care Manual
 Human Tenocyte Care Manual INSTRUCTION MANUAL SHIPPING CONDITIONS Human Tenocytes ZBM0075.03 Orders are delivered via Federal Express courier. Cryopreserved cells are shipped on dry ice and should be stored
Human Tenocyte Care Manual INSTRUCTION MANUAL SHIPPING CONDITIONS Human Tenocytes ZBM0075.03 Orders are delivered via Federal Express courier. Cryopreserved cells are shipped on dry ice and should be stored
Dissolve it in water and filter it through 0.22µm. (It should dissolve pretty fast in water, just mix it well. Sometimes I let it sit for 30 min)
 CLEANING COVERSLIPS FOR NEURON PLATING (from J. Wesseling) PLUS SUGGESTIONS FOR SUBTRATES Note: Use coverslips from Fisher and do everything using glass material. The cleaning procedure can be substituted
CLEANING COVERSLIPS FOR NEURON PLATING (from J. Wesseling) PLUS SUGGESTIONS FOR SUBTRATES Note: Use coverslips from Fisher and do everything using glass material. The cleaning procedure can be substituted
HUMAN IPSC CULTURE PROTOCOLS
 HUMAN IPSC CULTURE PROTOCOLS FOR TRAINING COURSE IXCELLS BIOTECHNOLOGIES USA, LLC 10340 CAMINO SANTA FE, SUITE C, SAN DIEGO, CA 92121 TABLE OF CONTENTS CHAPTER 1 BEFORE STARTING 2-7 SECTION 1.1 COATING
HUMAN IPSC CULTURE PROTOCOLS FOR TRAINING COURSE IXCELLS BIOTECHNOLOGIES USA, LLC 10340 CAMINO SANTA FE, SUITE C, SAN DIEGO, CA 92121 TABLE OF CONTENTS CHAPTER 1 BEFORE STARTING 2-7 SECTION 1.1 COATING
STANDARD OPERATING PROCEDURE
 STANDARD OPERATING PROCEDURE Culture of the Rat Macrophage Cell Line, NR8383 SOP number: WP1/001 Protocol prepared by: Ewelina Hoffman Developed under NC3R project: NC/C013203/1 INTRODUCTION Procedure
STANDARD OPERATING PROCEDURE Culture of the Rat Macrophage Cell Line, NR8383 SOP number: WP1/001 Protocol prepared by: Ewelina Hoffman Developed under NC3R project: NC/C013203/1 INTRODUCTION Procedure
Peri.4U TM Application Protocol Multiwell-MEA
 Peri.4U TM Application Protocol Multiwell-MEA Measuring Neuronal Electrical Activity of Peri.4U TM (Axiogenesis AG) on the 24-well Multiwell-MEA System (Multi Channel Systems MCS GmbH). Version 1.0: July
Peri.4U TM Application Protocol Multiwell-MEA Measuring Neuronal Electrical Activity of Peri.4U TM (Axiogenesis AG) on the 24-well Multiwell-MEA System (Multi Channel Systems MCS GmbH). Version 1.0: July
User Manual. OriCell TM Mesenchymal Stem Cell Osteogenic Differentiation Medium. Cat. No. GUXMX-90021
 User Manual OriCell TM Mesenchymal Stem Cell Osteogenic Differentiation Cat. No. GUXMX-90021 PRODUCT DESCRIPTION: OriCell TM Mesenchymal Stem Cell Osteogenic Differentiation consists of optimized Mesenchymal
User Manual OriCell TM Mesenchymal Stem Cell Osteogenic Differentiation Cat. No. GUXMX-90021 PRODUCT DESCRIPTION: OriCell TM Mesenchymal Stem Cell Osteogenic Differentiation consists of optimized Mesenchymal
Human Adult Mammary Fibroblast Care Manual
 Human Adult Mammary Fibroblast Care Manual INSTRUCTIONAL MANUAL ZBM0062.05 SHIPPING CONDITIONS Human Adult Mammary Fibroblast Cells Orders are delivered via Federal Express courier. All US and Canada orders
Human Adult Mammary Fibroblast Care Manual INSTRUCTIONAL MANUAL ZBM0062.05 SHIPPING CONDITIONS Human Adult Mammary Fibroblast Cells Orders are delivered via Federal Express courier. All US and Canada orders
µ Slide Membrane ibipore Flow
 The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
Metzger Lab Protocol Book EF May 2003
 I. Preparation for cell isolation: A. Make sure the following items are autoclaved: 1. Glassware* (1) 100 ml beaker (3) wide mouthed 1 liter bottles (1) 100 mm glass petri dish (1) 60 mm sigma coated petri
I. Preparation for cell isolation: A. Make sure the following items are autoclaved: 1. Glassware* (1) 100 ml beaker (3) wide mouthed 1 liter bottles (1) 100 mm glass petri dish (1) 60 mm sigma coated petri
PROTOCOL. Collagen I Thin Gel Coating of Alvetex Scaffold. Introduction. Method. Page 1
 Page 1 Introduction Extra-cellular matrix (ECM) coating of in vitro culture surfaces is commonly used to enhance cellsubstrate adhesion, encourage cell-matrix signalling and to protect shear-sensitive
Page 1 Introduction Extra-cellular matrix (ECM) coating of in vitro culture surfaces is commonly used to enhance cellsubstrate adhesion, encourage cell-matrix signalling and to protect shear-sensitive
MiraCell Endothelial Cells (from ChiPSC12) Kit
 Cat. # Y50055 For Research Use MiraCell Endothelial Cells (from ChiPSC12) Kit Product Manual Table of Contents I. Description...3 II. III. IV. Components...4 Storage...4 Preparation Before Use...4 V. Protocol...5
Cat. # Y50055 For Research Use MiraCell Endothelial Cells (from ChiPSC12) Kit Product Manual Table of Contents I. Description...3 II. III. IV. Components...4 Storage...4 Preparation Before Use...4 V. Protocol...5
Single Molecule FISH on Mouse Tissue Sections
 Single Molecule FISH on Mouse Tissue Sections Shalev Itzkovitz, December 2012 Tissue preparation Solutions and s 10X PBS (Ambion #AM9625) 4% formaldehyde or paraformaldehyde in PBS Cryoprotecting solution:
Single Molecule FISH on Mouse Tissue Sections Shalev Itzkovitz, December 2012 Tissue preparation Solutions and s 10X PBS (Ambion #AM9625) 4% formaldehyde or paraformaldehyde in PBS Cryoprotecting solution:
Well Insert Holder in Deep Petri Dish - AVP015
 Well Insert Holder in Deep Petri Dish - AVP015 Product Information Leaflet Technology for Routine Three Dimensional (3D) Cell Culture t: +44 (0)1740 625266 f: +44 (0)1740 625251 e: info@reinnervate.com
Well Insert Holder in Deep Petri Dish - AVP015 Product Information Leaflet Technology for Routine Three Dimensional (3D) Cell Culture t: +44 (0)1740 625266 f: +44 (0)1740 625251 e: info@reinnervate.com
Axol Guide to Culturing Axol human Neural Progenitor Cells (hnpcs) on Axion BioSystems Microelectrode Arrays. Application Protocol Version 2.
 Axol Guide to Culturing Axol human Neural Progenitor Cells (hnpcs) on Axion BioSystems Microelectrode Arrays Application Protocol Version 2.1 Table of Contents Before You Begin 3 Required Materials 4 Preparing
Axol Guide to Culturing Axol human Neural Progenitor Cells (hnpcs) on Axion BioSystems Microelectrode Arrays Application Protocol Version 2.1 Table of Contents Before You Begin 3 Required Materials 4 Preparing
µ Slide Membrane ibipore Flow
 The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
IncuCyte S3 Neuronal Activity Assay
 IncuCyte S3 Neuronal Activity Assay For the kinetic quantification of neuronal activity and functional connectivity This protocol provides an overview of the methodology which uses the lentiviral based
IncuCyte S3 Neuronal Activity Assay For the kinetic quantification of neuronal activity and functional connectivity This protocol provides an overview of the methodology which uses the lentiviral based
IncuCyte S3 Neuronal Activity Assay
 IncuCyte S3 Neuronal Activity Assay For the kinetic quantification of neuronal activity and functional connectivity This protocol provides an overview of the methodology which uses the lentiviral based
IncuCyte S3 Neuronal Activity Assay For the kinetic quantification of neuronal activity and functional connectivity This protocol provides an overview of the methodology which uses the lentiviral based
All quality control test results are reported on a lot specific Certificate of Analysis which is available at or upon request.
 PRIME-XV NPC Expansion XSFM PRIME-XV NPC Expansion XSFM is a xeno-and serum-free medium optimized for the cultivation and expansion of neural progenitor cells (NPC) that are able to maintain their ability
PRIME-XV NPC Expansion XSFM PRIME-XV NPC Expansion XSFM is a xeno-and serum-free medium optimized for the cultivation and expansion of neural progenitor cells (NPC) that are able to maintain their ability
STANDARD OPERATING PROCEDURE
 STANDARD OPERATING PROCEDURE Culture of the Human Monocyte-Macrophage Cell Line; U937 SOP number: WP1/002 Protocol prepared by: Ewelina Hoffman & Abhinav Kumar Developed under NC3R project: NC/C013203/1
STANDARD OPERATING PROCEDURE Culture of the Human Monocyte-Macrophage Cell Line; U937 SOP number: WP1/002 Protocol prepared by: Ewelina Hoffman & Abhinav Kumar Developed under NC3R project: NC/C013203/1
MimEX TM GI Human Descending Colon Stem Cells
 MimEX TM GI Human Descending Colon Stem Cells Adult Gastrointestinal Stem Cells for Use With MimEX GI Reagents Catalog Number: MIM006 Size: 1 vial, ~ 100,000 cells PRODUCT DESCRIPTION The MimEX GI Tissue
MimEX TM GI Human Descending Colon Stem Cells Adult Gastrointestinal Stem Cells for Use With MimEX GI Reagents Catalog Number: MIM006 Size: 1 vial, ~ 100,000 cells PRODUCT DESCRIPTION The MimEX GI Tissue
TRANSFORMATION OF LEECH MICROGLIAL CELL MORPHOLOGY AND PROPERTIES FOLLOWING CO-CULTURE WITH INJURED CENTRAL NERVOUS SYSTEM TISSUE
 The Journal of Experimental Biology 202, 723 728 (1999) Printed in Great Britain The Company of Biologists Limited 1999 JEB1806 723 TRANSFORMATION OF LEECH MICROGLIAL CELL MORPHOLOGY AND PROPERTIES FOLLOWING
The Journal of Experimental Biology 202, 723 728 (1999) Printed in Great Britain The Company of Biologists Limited 1999 JEB1806 723 TRANSFORMATION OF LEECH MICROGLIAL CELL MORPHOLOGY AND PROPERTIES FOLLOWING
Culture of Human ipsc-derived Neural Stem Cells in a 96-Well Plate Format
 Protocol supplement version 1.0 Culture of Human ipsc-derived Neural Stem Cells in a 96-Well Plate Format Protocol Supplement This protocol supplement is to be used in addition to the Neural Stem Cell
Protocol supplement version 1.0 Culture of Human ipsc-derived Neural Stem Cells in a 96-Well Plate Format Protocol Supplement This protocol supplement is to be used in addition to the Neural Stem Cell
Amaxa Basic Neuron SCN Nucleofector Kit
 Amaxa Basic Neuron SCN Nucleofector Kit For Primary Neural Cells (Small Cell Number) SCN Nucleofector Kits are compatible with Nucleofector ll Devices of serial version S with software version S4 4 or
Amaxa Basic Neuron SCN Nucleofector Kit For Primary Neural Cells (Small Cell Number) SCN Nucleofector Kits are compatible with Nucleofector ll Devices of serial version S with software version S4 4 or
CAROUSEL CELL PASSAGING PROTOCOL OVERVIEW
 CELL MODEL NAME: Carousel Derived from an endometrioid adenocarcinoma of the ovary. Record your lot number here: Product Category: Raphael Ovarian Models Catalog #: AMS.CB010-000001 Please refer to Certificate
CELL MODEL NAME: Carousel Derived from an endometrioid adenocarcinoma of the ovary. Record your lot number here: Product Category: Raphael Ovarian Models Catalog #: AMS.CB010-000001 Please refer to Certificate
WOOD CELL PASSAGING PROTOCOL OVERVIEW
 CELL MODEL NAME: Wood Derived from an infiltrating ductal and lobular carcinoma of the breast. Product Category: Cellini Breast Models Catalog #: CB040-000001 Record your lot number here: Please refer
CELL MODEL NAME: Wood Derived from an infiltrating ductal and lobular carcinoma of the breast. Product Category: Cellini Breast Models Catalog #: CB040-000001 Record your lot number here: Please refer
Transwell Co-culture of Bone Marrow Macrophages with Tumor Cells Mackenzie K. Herroon and Izabela Podgorski *
 Transwell Co-culture of Bone Marrow Macrophages with Tumor Cells Mackenzie K. Herroon and Izabela Podgorski * Department of Pharmacology, Karmanos Cancer Institute, Wayne State University School of Medicine,
Transwell Co-culture of Bone Marrow Macrophages with Tumor Cells Mackenzie K. Herroon and Izabela Podgorski * Department of Pharmacology, Karmanos Cancer Institute, Wayne State University School of Medicine,
Tube Formation Assays in µ-slide Angiogenesis
 Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture. Catalog Number Guidelines for Use
 Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
Neural Cell Culturing Guide
 Neural Cell Culturing Guide Neural Cell Culturing Guide The advent of in vitro culturing of neural cells has been central to driving our understanding of the nervous system. The complexity of neural tissue
Neural Cell Culturing Guide Neural Cell Culturing Guide The advent of in vitro culturing of neural cells has been central to driving our understanding of the nervous system. The complexity of neural tissue
Application Protocol: CD45 CK Immunostaining for patient blood
 REPRODUCTION AND USE This document is protected by copyright and it cannot be used or shared without permission from Vortex Biosciences, Inc. Such permission is given on condition that Vortex Biosciences
REPRODUCTION AND USE This document is protected by copyright and it cannot be used or shared without permission from Vortex Biosciences, Inc. Such permission is given on condition that Vortex Biosciences
DOPA Maturation Media
 Applied StemCell, Inc. (866) 97-180 www.appliedstemcell.com Datasheet Media Product Information Catalog Number Description ASE-933DM Media is serum free media produced using Applied StemCell s proprietary
Applied StemCell, Inc. (866) 97-180 www.appliedstemcell.com Datasheet Media Product Information Catalog Number Description ASE-933DM Media is serum free media produced using Applied StemCell s proprietary
Amaxa 4D-Nucleofector Protocol for Primary Mammalian Neurons For 4D-Nucleofector X Unit Transfection in suspension
 For 4D-Nucleofector X Unit Transfection in suspension Primary mammalian neurons, primary neurons freshly isolated embryonic (E18) or neonatal (P1 2) mammalian neural tissues. Mammalian neurons display
For 4D-Nucleofector X Unit Transfection in suspension Primary mammalian neurons, primary neurons freshly isolated embryonic (E18) or neonatal (P1 2) mammalian neural tissues. Mammalian neurons display
Neural induction - Dual SMAD inhibition
 Neural induction - Dual SMAD inhibition Mark J. Tomishima, SKI Stem Cell Research Facility http://stemcells.mskcc.org, tomishim@mskcc.org This protocol is based on Chambers et al. 2009. [Plating pluripotent
Neural induction - Dual SMAD inhibition Mark J. Tomishima, SKI Stem Cell Research Facility http://stemcells.mskcc.org, tomishim@mskcc.org This protocol is based on Chambers et al. 2009. [Plating pluripotent
Tube Formation Assays in µ-slide Angiogenesis. 1. General Information. Application Note 19
 Tube Formation Assays in µ-slide Angiogenesis Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview... 3 4. Preparation of the Gel and the Slide... 4 4.1. Gel Application... 4 4.2.
Tube Formation Assays in µ-slide Angiogenesis Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview... 3 4. Preparation of the Gel and the Slide... 4 4.1. Gel Application... 4 4.2.
EUCOMM Protocol for ES cells
 EUCOMM Protocol for ES cells 1. SNL Feeder Cells 1.1. Preparing inactivated SNL Feeder Cells 1) Thaw one vial of SNL cells (approximately1.5-2 x 10 6 cells per vial) in a 37 C water bath and dilute into
EUCOMM Protocol for ES cells 1. SNL Feeder Cells 1.1. Preparing inactivated SNL Feeder Cells 1) Thaw one vial of SNL cells (approximately1.5-2 x 10 6 cells per vial) in a 37 C water bath and dilute into
Oligodendrocyte Progenitor Cell Isolation Kit
 Neural Cell Solutions in CNS Research Oligodendrocyte Progenitor Cell Isolation Kit User Manual Cat. No. OPC-K The ultimate procedure for NG2 glia / OPC isolation within two days! Check all kit components
Neural Cell Solutions in CNS Research Oligodendrocyte Progenitor Cell Isolation Kit User Manual Cat. No. OPC-K The ultimate procedure for NG2 glia / OPC isolation within two days! Check all kit components
Isolation of Resting B Lymphocytes from Sixteen Mouse Spleens AfCS Procedure Protocol PP Version 1, 02/19/02
 AfCS Procedure Protocol PP00000016 Version 1, 02/19/02 This procedure describes the isolation of resting B lymphocytes (B cells) from mouse spleens by using negative selection with anti-cd43 and anti-mac-1/cd11b
AfCS Procedure Protocol PP00000016 Version 1, 02/19/02 This procedure describes the isolation of resting B lymphocytes (B cells) from mouse spleens by using negative selection with anti-cd43 and anti-mac-1/cd11b
MARINE INVERTEBRATE TISSUE CULTURE TECHNIQUES AND ITS APPLICATION IN PEARL PRODUCTION
 Technical paper-23 MARINE INVERTEBRATE TISSUE CULTURE TECHNIQUES AND ITS APPLICATION IN PEARL PRODUCTION S. Dharmaraj Central Marine Fisheries Research Institute, Tuticorin Research Centre, Tuticorin Introduction
Technical paper-23 MARINE INVERTEBRATE TISSUE CULTURE TECHNIQUES AND ITS APPLICATION IN PEARL PRODUCTION S. Dharmaraj Central Marine Fisheries Research Institute, Tuticorin Research Centre, Tuticorin Introduction
All quality control test results are reported on a lot specific Certificate of Analysis which is available at or upon request.
 PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
ATCC ANIMAL CELL CULTURE GUIDE
 ATCC ANIMAL CELL CULTURE GUIDE tips and techniques for continuous cell lines The Essentials of Life Science Research Globally Delivered Table of Contents This guide contains general technical information
ATCC ANIMAL CELL CULTURE GUIDE tips and techniques for continuous cell lines The Essentials of Life Science Research Globally Delivered Table of Contents This guide contains general technical information
ETS-Embryo Medium. In Vitro Culture Media. Version 1.0
 ETS-Embryo Medium In Vitro Culture Media Version 1.0 3D Culture Media Product Catalogue No Storage ETS-Embryo Medium, 25 ml (5 x 5 ml) M13-25 -20 C ETS-Embryo Medium, 100 ml M13-100 -20 C Additional Reagents
ETS-Embryo Medium In Vitro Culture Media Version 1.0 3D Culture Media Product Catalogue No Storage ETS-Embryo Medium, 25 ml (5 x 5 ml) M13-25 -20 C ETS-Embryo Medium, 100 ml M13-100 -20 C Additional Reagents
Note that Methylene Blue-stained cultures may require an additional washing step if the second wash is still very blue in appearance.
 Introduction: Cell culture in Alvetex Scaffold allows the formation of multilayered, high-density cell populations which approximate the complexity and structure of in vivo tissues. When viewing an unstained,
Introduction: Cell culture in Alvetex Scaffold allows the formation of multilayered, high-density cell populations which approximate the complexity and structure of in vivo tissues. When viewing an unstained,
3D Endothelial Pericyte Coculturing Kit Product Description Kit Components
 3D Endothelial Pericyte Coculturing Kit (3D-EPC) Cat #8728 Product Description Blood vessels are responsible for transporting blood throughout the body as part of the circulatory system. Their main components
3D Endothelial Pericyte Coculturing Kit (3D-EPC) Cat #8728 Product Description Blood vessels are responsible for transporting blood throughout the body as part of the circulatory system. Their main components
SeedEZ TM PROTOCOLS. METHODS AND PROTOCOLS FOR CELL RECOVERY FROM 3D CELL CULTURES EMBEDDED IN THE SeedEZ TM.
 SeedEZ TM PROTOCOLS Lena Biosciences METHODS AND PROTOCOLS FOR CELL RECOVERY FROM 3D CELL CULTURES EMBEDDED IN THE SeedEZ TM support@lenabio.com FEBRUARY 2013 V2.5 SeedEZ IS A TRADEMARK OF LENA BIOSCIENCES,
SeedEZ TM PROTOCOLS Lena Biosciences METHODS AND PROTOCOLS FOR CELL RECOVERY FROM 3D CELL CULTURES EMBEDDED IN THE SeedEZ TM support@lenabio.com FEBRUARY 2013 V2.5 SeedEZ IS A TRADEMARK OF LENA BIOSCIENCES,
Page 1 of 5. Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR Cy µg (~7.5 nmol) MIR 7901
 Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Adult Neuron Isolation Protocol (R. Kaletsky 9/2014)
 Notes before starting: Adult Neuron Isolation Protocol (R. Kaletsky 9/2014) Begin protocol ~45min before sort time to minimize time between cell harvesting and sorting Prepare pronase solution immediately
Notes before starting: Adult Neuron Isolation Protocol (R. Kaletsky 9/2014) Begin protocol ~45min before sort time to minimize time between cell harvesting and sorting Prepare pronase solution immediately
MyBioSource.com. Human Vitamin K2 ELISA USER INSTRUCTION
 Human Vitamin K2 ELISA USER INSTRUCTION Cat.No MBS163021 Standard Curve Range: 5ng/ml - 1000ng/ml Sensitivity: 2.52ng/ml Size: 96 wells Storage: Store the reagents at 2-8 C. For over 6-month storage refer
Human Vitamin K2 ELISA USER INSTRUCTION Cat.No MBS163021 Standard Curve Range: 5ng/ml - 1000ng/ml Sensitivity: 2.52ng/ml Size: 96 wells Storage: Store the reagents at 2-8 C. For over 6-month storage refer
MyBioSource.com. Human Osteoprotegerin ELISA USER INSTRUCTION
 Human Osteoprotegerin ELISA USER INSTRUCTION Cat.No MBS162588 Standard Curve Range: 0.05ng/ml - 15ng/ml Sensitivity: 0.023ng/ml Size: 96 wells Storage: Store the reagents at 2-8 C. For over 6-month storage
Human Osteoprotegerin ELISA USER INSTRUCTION Cat.No MBS162588 Standard Curve Range: 0.05ng/ml - 15ng/ml Sensitivity: 0.023ng/ml Size: 96 wells Storage: Store the reagents at 2-8 C. For over 6-month storage
Guide to Induced Pluripotent Stem Cell Culture
 Cellular Engineering Technologies, Inc. Guide to Induced Pluripotent Stem Cell Culture User Manual 2500 Crosspark Road E110 Coralville, IA 52241 Phone: 319-665-3000 Email: orders@celleng-tech.com Table
Cellular Engineering Technologies, Inc. Guide to Induced Pluripotent Stem Cell Culture User Manual 2500 Crosspark Road E110 Coralville, IA 52241 Phone: 319-665-3000 Email: orders@celleng-tech.com Table
EXPERIMENT 5 - IDENTIFYING FEATURES OF MUTANT EMBRYO USING NOMARSKI MICROSCOPY (GENE ONE)
 EXPERIMENT 5 - IDENTIFYING FEATURES OF MUTANT EMBRYO USING NOMARSKI MICROSCOPY (GENE ONE) Purpose: To introduce Differential Interference Contrast (DIC) or Nomarski Interference Contrast (NIC) microscopy
EXPERIMENT 5 - IDENTIFYING FEATURES OF MUTANT EMBRYO USING NOMARSKI MICROSCOPY (GENE ONE) Purpose: To introduce Differential Interference Contrast (DIC) or Nomarski Interference Contrast (NIC) microscopy
me239 mechanics of the cell me239 mechanics of the cell 3.1 biopolymers - motivation 3.2 biopolymers - polymerization
 4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
MyBioSource.com. Rat Hydroxyproline ELISA USER INSTRUCTION
 Rat Hydroxyproline ELISA USER INSTRUCTION Cat.No MBS162747 Standard Curve Range: 10ng/L - 4000ng/L Sensitivity: 4.99ng/L Size: 96 wells Storage: Store the reagents at 2-8 C. For over 6-month storage refer
Rat Hydroxyproline ELISA USER INSTRUCTION Cat.No MBS162747 Standard Curve Range: 10ng/L - 4000ng/L Sensitivity: 4.99ng/L Size: 96 wells Storage: Store the reagents at 2-8 C. For over 6-month storage refer
Cell Lineage in the Development of the Leech Nervous System
 Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
SOP 2 Solid Tumor Processing for Cell Culture and Xenografts
 Texas Cancer Cell Repository Cell Culture and Xenograft Repository http://cancer.ttuhsc.edu www.txccr.org www.cogcell.org SOP 2 Solid Tumor Processing for Cell Culture and Xenografts Receiving the Sample:
Texas Cancer Cell Repository Cell Culture and Xenograft Repository http://cancer.ttuhsc.edu www.txccr.org www.cogcell.org SOP 2 Solid Tumor Processing for Cell Culture and Xenografts Receiving the Sample:
Amaxa 4D-Nucleofector Protocol for Rat Hippocampal or Cortical Neurons For 4D-Nucleofector X Unit Transfection in suspension
 For 4D-Nucleofector X Unit Transfection in suspension Isolated from embryonic (E17-18) or neonatal rats (P0-2) and cultured as mixed glial cells Example for of rat cortical neurons Freshly isolated rat
For 4D-Nucleofector X Unit Transfection in suspension Isolated from embryonic (E17-18) or neonatal rats (P0-2) and cultured as mixed glial cells Example for of rat cortical neurons Freshly isolated rat
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
icell Motor Neurons User s Guide Document ID: X1004
 icell Motor Neurons User s Guide Document ID: X1004 Trademarks icell and MyCell are registered trademarks, and Cellular Dynamics and the logo are trademarks of Cellular Dynamics International, Inc. and
icell Motor Neurons User s Guide Document ID: X1004 Trademarks icell and MyCell are registered trademarks, and Cellular Dynamics and the logo are trademarks of Cellular Dynamics International, Inc. and
Protocol version 6.0. Maintenance of Human ipsc-derived Cerebral Cortical Neurons in a 96-Well Plate Format
 Protocol version 6.0 Maintenance of Human ipsc-derived Cerebral Cortical Neurons in a 96-Well Plate Format Protocol version 6.0 Table of Contents What s the Best System for Your Discovery? 2 Product Information
Protocol version 6.0 Maintenance of Human ipsc-derived Cerebral Cortical Neurons in a 96-Well Plate Format Protocol version 6.0 Table of Contents What s the Best System for Your Discovery? 2 Product Information
General Guidelines for Handling Feeder Free Human ipscs. Cells provided were cultured in the presence of Penicillin and Streptomycin.
 General Guidelines for Handling Feeder Free Human ipscs This document provides guidance on how to resuscitate, culture and cryopreserve human induced pluripotent stem cells (ipscs) supplied by the Human
General Guidelines for Handling Feeder Free Human ipscs This document provides guidance on how to resuscitate, culture and cryopreserve human induced pluripotent stem cells (ipscs) supplied by the Human
Atomic Force Microscopy elasticity measurements on living / fixed cells ~ Practical Output
 Atomic Force Microscopy elasticity measurements on living / fixed cells ~ Practical Output Cells Cell type : Adherents carcinoma cells of the cervix (HeLa). Obtained from : ATCC Dish Glass bottom Willco
Atomic Force Microscopy elasticity measurements on living / fixed cells ~ Practical Output Cells Cell type : Adherents carcinoma cells of the cervix (HeLa). Obtained from : ATCC Dish Glass bottom Willco
General Guidelines for Handling Feeder Dependent Human ipscs
 General Guidelines for Handling Feeder Dependent Human ipscs This document provides guidance on how to resuscitate, culture and cryopreserve human induced pluripotent stem cells (ipscs) supplied by the
General Guidelines for Handling Feeder Dependent Human ipscs This document provides guidance on how to resuscitate, culture and cryopreserve human induced pluripotent stem cells (ipscs) supplied by the
Detection of Action Potentials In Vitro by Changes in Refractive Index
 Detection of Action Potentials In Vitro by Changes in Refractive Index David Kleinfeld 1 and Arthur LaPorta 2 1 - Department of Physics, University of California, La Jolla, CA 92093-0319. 2- Laboratory
Detection of Action Potentials In Vitro by Changes in Refractive Index David Kleinfeld 1 and Arthur LaPorta 2 1 - Department of Physics, University of California, La Jolla, CA 92093-0319. 2- Laboratory
Part 1: Human ipsc (hipsc) culture
 Direct imn (dimns) differentiation in monolayer from hipscs * Please refer to tables for media compositions and reagent catalog numbers at the end of the document Part 1: Human ipsc (hipsc) culture 1.1
Direct imn (dimns) differentiation in monolayer from hipscs * Please refer to tables for media compositions and reagent catalog numbers at the end of the document Part 1: Human ipsc (hipsc) culture 1.1
Individual microglia move rapidly and directly to nerve lesions in the leech central nervous system
 Proc. Nati. Acad. Sci. USA Vol. 86, pp. 193-197, February 1989 Neurobiology Individual microglia move rapidly and directly to nerve lesions in the leech central nervous system (nerve iinjury/ceil migration/video
Proc. Nati. Acad. Sci. USA Vol. 86, pp. 193-197, February 1989 Neurobiology Individual microglia move rapidly and directly to nerve lesions in the leech central nervous system (nerve iinjury/ceil migration/video
Supporting Information
 Supporting Information Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers Weibo Guo, 1,2
Supporting Information Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers Weibo Guo, 1,2
IDENTIFICATION OF AGRIN IN ELECTRIC ORGAN EXTRACTS AND LOCALIZATION OF AGRIN-LIKE MOLECULES IN MUSCLE AND CENTRAL NERVOUS SYSTEM
 7. exp. Biol. 132, 223-230 (19S7) 223 Printed in Great Britain The Company of Biologists Limited 1987 IDENTIFICATION OF AGRIN IN ELECTRIC ORGAN EXTRACTS AND LOCALIZATION OF AGRIN-LIKE MOLECULES IN MUSCLE
7. exp. Biol. 132, 223-230 (19S7) 223 Printed in Great Britain The Company of Biologists Limited 1987 IDENTIFICATION OF AGRIN IN ELECTRIC ORGAN EXTRACTS AND LOCALIZATION OF AGRIN-LIKE MOLECULES IN MUSCLE
ANOMALOUS PREFERENCES OF CULTURED MACROPHAGES FOR HYDROPHOB1C AND ROUGHENED SUBSTRATA
 J. Cell Set. 50, 1-7 (1981) Printed in Great Britain Company of Biologists Limited ig8i ANOMALOUS PREFERENCES OF CULTURED MACROPHAGES FOR HYDROPHOB1C AND ROUGHENED SUBSTRATA ABBY RICH* AND ALBERT K. HARRIS
J. Cell Set. 50, 1-7 (1981) Printed in Great Britain Company of Biologists Limited ig8i ANOMALOUS PREFERENCES OF CULTURED MACROPHAGES FOR HYDROPHOB1C AND ROUGHENED SUBSTRATA ABBY RICH* AND ALBERT K. HARRIS
LabCyte CORNEA-MODEL
 LabCyte CORNEA-MODEL Three-dimensional Cultured Human Cornea Epithelial Model User s Manual For Research Use Only Please read this manual before use Updated March, 2017 Table of contents I Characteristics
LabCyte CORNEA-MODEL Three-dimensional Cultured Human Cornea Epithelial Model User s Manual For Research Use Only Please read this manual before use Updated March, 2017 Table of contents I Characteristics
