Guidelines for the Production of Uncooked Comminuted Fermented Meat (UCFM) Products
|
|
|
- Matthew Shelton
- 6 years ago
- Views:
Transcription
1 Guidelines for the Production of Uncooked Comminuted Fermented Meat (UCFM) Products
2 July 2009 Page 2 Prelims Prelims July 2009 Table of Contents 1 Purpose and Scope Definitions Outcomes Microbiological Outcomes ph and Water Activity (a w ) Additive Levels Process Control Measures Raw Materials and Ingredients Storage of Raw Meat Tempering Grinding or Flaking Chopping and Ingredient Addition Filling and Clipping Fermentation Smoking and Maturation (drying) Slicing and Packing Validation Validation Criteria Validation Methods Actions Required when Process is Determined to be Inadequate Implementation, and Monitoring and Verification Procedures Implementation of the Validated Process Monitoring and Verification Requirements Temperature and Time Monitoring ph and Water Activity (a w ) Monitoring E. coli Testing Corrective Action Procedures Records References...29 Appendix 1: Determination of ph...30 Appendix 2: Monitoring Weight Loss...31
3 July 2009 Page 3 Purpose and Scope IMPORTANT DISCLAIMER Every effort has been made to ensure the information in this report is accurate. NZFSA does not accept any responsibility or liability whatsoever for any error of fact, omission, interpretation or opinion that may be present, however it may have occurred. Website A copy of this document can be found at: Review of Guideline This guideline will be reviewed, as necessary, by the New Zealand Food Safety Authority. Suggestions for alterations, deletions or additions to this guideline, should be sent, together with reasons for the change, any relevant data and contact details of the person making the suggestion, to: Assistant Director (Production and Processing) New Zealand Standards Group New Zealand Food Safety Authority PO Box 2835 Wellington Phone: (04) Fax: (04)
4 July 2009 Page 4 Purpose and Scope 1 Purpose and Scope July 2009 These guidelines have been developed to assist manufacturers of Uncooked Comminuted Fermented Meat (UCFM) comply with the Food (Uncooked Comminuted Fermented Meat) Standard It covers the processing requirements and Good Manufacturing Practices (GMP) for the production of safe UCFM products. UCFM means a comminuted fermented meat which has not been heated during production to a core temperature of 65 C for at least 10 minutes, or an equivalent combination of time and higher temperature. Examples of UCFM products commonly produced in New Zealand are salami and pepperoni. The UCFM Standard applies to all manufacturers of UCFM products operating under the Animal Products Act 1999 or the Food Act It came into force on 1 December The Standard focuses mainly on processing requirements for the control of pathogenic bacteria, particularly shiga-like toxin producing Escherichia coli. It was developed based on the expectation that manufacturers have GMP programmes in place covering hygiene and sanitation (e.g. cleaning and sanitation, personnel hygiene, waste management, pest control and calibration), and general process control. The Code of Practice: Manufacture of Processed Meat Products (to be released late 2009) provides guidance on the development and implementation of these GMP programmes. Manufacturers should use the Code of Practice in conjunction with this UCFM guide. In addition to the UCFM Standard requirements, the manufacturer must also meet other relevant requirements of the Food Act 1981 or the Animal Products Act 1999 (i.e. for those operating under a risk management programme), and the Food Standards Code (e.g. use of additives).
5 July 2009 Page 5 Definitions 2 Definitions July 2009 back slopping - using the partially fermented batter from a previous batch to initiate fermentation in a subsequent batch batter mix - all the ingredients in an uncooked comminuted fermented meat recipe that have been combined prior to filling a casing fermentation - in relation to a batter mix, the anaerobic breakdown of sugar into lactic acid within the mix maturation - the process of ageing an uncooked comminuted fermented meat in order to promote drying at a specified temperature and time; also commonly referred to as drying which occurs after the fermentation stage process lethality - the total kill of Escherichia coli resulting from the fermentation and maturation process determined from predictive modelling or challenge studies ph - a measure of acidity or alkalinity; the lower the ph the more acidic the product starter culture - a preparation of microorganisms prepared for the purpose of fermenting meat UCFM - a comminuted fermented meat which has not had its core temperature maintained at 65 C for at least 10 minutes or an equivalent combination of time and higher temperature during production validation the process of collecting evidence by the manufacturing company to confirm that the UCFM manufacturing process meets the requirements of the UCFM Standard water activity - a measure of the water in the food which is available for bacterial growth
6 July 2009 Page 6 Outcomes 3 Outcomes July 2009 The UCFM process must be capable of consistently achieving the outcomes given in this section. The manufacturer must be able to provide evidence that these outcomes can be met. 3.1 Microbiological Outcomes All UCFM products must comply with the microbiological limits specified in Standard of the Australia New Zealand Food Standards Code. Table 1: Microbiological Limits for UCFM Products Microorganism n c m M Coagulase positive ,000 staphylococci/g E.coli /g Salmonella/25 g n = number of samples examined from a lot of food c = maximum number of samples allowed to have results greater than m but less than M m = acceptable microbiological level in a sample M = maximum level which when exceeded in one or more samples would cause the lot to be rejected Manufacturers are not required to routinely test all batches of UCFM products against these criteria, but it is recommended that samples of products are occasionally tested as part of their verification programme.
7 July 2009 Page 7 Outcomes 3.2 ph and Water Activity (a w ) A UCFM product that is intended to be shelf stable (i.e. product can be stored at ambient or room temperature) should have a ph and/or water activity (a w ) which will not allow the growth of any pathogenic or spoilage microorganisms at ambient temperatures. The manufacturer must establish and document these limits for their product(s). It is generally accepted that UCFM products with a combination of ph < 5.2 and a w < 0.95 are shelf stable under ambient conditions (Ross and Shadbolt, 2001). The manufacturer may propose other combinations of ph and a w, but they must be able to scientifically justify the selected parameters, and validate them, if necessary. UCFM products which do not meet the established ph and a w must be refrigerated and stored at 5 C. 3.3 Additive Levels The amount of additives in the final product must comply with the maximum permitted levels specified in Standard of the Food Standards Code. The combined nitrite and nitrate level in UCFM products must not exceed 500 mg/kg.
8 July 2009 Page 8 Process 4 Process July 2009 The process flow diagram shown below describes the key steps for a typical UCFM manufacturing process.
9 July 2009 Page 9 Control Measures 5 Control Measures July 2009 This section provides guidance on control measures that should be implemented by the manufacturer to ensure that GMP is applied and the requirements of the UCFM Standard are met. It is expected that manufacturers will have documented procedures covering these control measures. The UCFM Standard requirements are given in italics and presented in a box. 5.1 Raw Materials and Ingredients Raw Meat Type and quality of meat Pork and beef are the most commonly used meats for UCFM production in New Zealand. Generally, manufacturers use New Zealand beef, but pork can either be produced in New Zealand or imported. New Zealand venison is also used by some manufacturers for venison salami. It is important that only meat of good microbiological quality is used for UCFM production. This is because the process does not include a microbiological kill step, such as cooking, and there are limitations to the numbers of pathogenic bacteria that can be destroyed during fermentation and maturation. Mechanically separated meat or trimmings that are likely to have higher levels of microorganisms should not be used in UCFM products. Veal from young calves is not recommended for use in UCFM products because of the higher prevalence of Salmonella and E. coli O157 in this type of meat compared to beef from adult cattle.
10 July 2009 Page 10 Control Measures Requirement for Escherichia coli count Clause 5(1) An Escherichia coli count for in-going raw meat ingredients used in processing UCFM must be known (to the 98th percentile) and be equivalent to, or below, the process lethality for the validated process. Clause 5(2) The 98th percentile for an Escherichia coli count is determined by using the following: (a) for meat produced in New Zealand: (i) the New Zealand National Microbiological Database Programme; or (ii) data provided by the company supplying the raw meat ingredients; or (iii) data collected by the manufacturer of the UCFM: (b) for meat imported into New Zealand: (i) an overseas data source equivalent to the New Zealand Microbiological Database Programme; or (ii) data provided by the company supplying the raw meat ingredients; or (iii) data collected by the manufacturer of the UCFM Determination of E. coli count in raw meat It is necessary to know the E. coli count in in-going raw meat to determine the process lethality required to produce a safe product. The manufacturer must validate the process and provide evidence to confirm that the process is capable of reducing E. coli in the product to the levels specified in the Food Standards Code. Validation is further discussed in section 6 of this guide. This section discusses the options for determining E. coli count in raw beef, pork and venison. The same approach should be taken for any other type or species of meat used in the production of UCFM products.
11 July 2009 Page 11 Control Measures Beef and venison UCFM manufacturers who use New Zealand beef or venison can use the national profiles for E. coli from the National Microbiological Database (NMD) programme, which is administered by the New Zealand Food Safety Authority (NZFSA). Under the NMD programme, slaughter premises processing cattle, calves, lamb/sheep, deer, goats, chickens and ostriches, test meat for E. coli at least weekly, and their data is recorded in a national database. NZFSA is, therefore, able to provide a national profile for these meats which is likely to reflect most meat produced in New Zealand. Manufacturers of UCFM products can simply use the national profiles and determine the required inactivation of E. coli from these values. Table 2 provides E. coli values for beef and venison from the national profiles as of 30 March NZFSA recognizes that rare contamination events can occur that multiply the usual counts by 1000-fold, and therefore the values have been based on the 98th percentile of national data, not the maximum. Table 2 also shows the required inactivation based on the 98th percentile. A value rounded to the nearest whole log count has also been provided for simplicity. This rounded inactivation number of 2-log includes a safety margin to try to take account of the measurement of uncertainty and sampling. Therefore, the validated UCFM process must be capable of achieving a 2-log reduction in the number of E. coli when New Zealand beef or venison is used as the raw material. Table 2: E. coli Count (98th percentile) and Required Inactivation Species E.coli (log cfu/g) Inactivation number (log) Rounded inactivation number (log) Beef Venison 1.77 * * - calculated from carcass counts. Manufacturers may gain more flexibility for their product or process if they obtain meat from known suppliers and can use the suppliers own NMD data. For instance, individual suppliers may have an E. coli profile with a 98th percentile less than that of the national profile. Therefore, the manufacturer may be able to apply a process with a lower lethality (i.e. less than 2-log reduction). Manufacturers who use imported beef will need to obtain E. coli data from their meat supplier, or do their own tests on their incoming raw materials. Testing should be based on
12 July 2009 Page 12 Control Measures a sampling plan which will provide sufficient data over a certain period so that the manufacturer and regulator can have confidence that the validated process can consistently reduce E. coli to specified limits. The 98th percentile of that data should be used to calculate inactivation requirements. Pork Pork has not been previously included in the NMD programme. The implementation of the NMD for pigs will start in August 2009, therefore, NMD data is not currently available for domestic pork. When sufficient data becomes available, manufacturers of UCFM products can use the national profiles and determine the required inactivation of E. coli from these values, similar to the approach discussed for beef and venison. For imported pork, and in the absence of NMD data for domestic pork, manufacturers may use their meat supplier s own data, when this is available. The 98th percentile of that data should be used to calculate inactivation requirements, as discussed in the previous section. Manufacturers may also do their own tests for their incoming raw materials. Testing should be based on a sampling plan which will provide sufficient data over a certain period so that the manufacturer and regulator can have confidence that the validated process can consistently reduce E. coli to specified limits. A recent survey of domestic and imported pork showed that the 98th percentile of E. coli counts was below 100 cfu/cm 2. Data from Australia and Sweden show similar results. It would therefore be appropriate for manufacturers who do not have sufficient data of their own to target a 2-log E. coli reduction for the initial validation of their UCFM process. However, these manufacturers are expected to do their own tests on their incoming imported pork raw materials and build up sufficient data, or use the NMD national profiles for domestic pork when it becomes available. The UCFM process must be revalidated when results show that the 98th percentile for E. coli counts is greater than 2-log Starter Culture The starter culture selected must be specifically made for the fermentation of meat, and should contain a preparation of microorganisms which: successfully competes with other microorganisms for the nutrients in the batter; produces microbial inhibitors; is microbiologically safe;
13 July 2009 Page 13 Control Measures produces a rapid, controlled reduction of the ph of the batter; and has good stability under the processing conditions. Since individual culture strains may have different temperature optima and tolerances, selection of cultures that perform under the selected processing conditions is critical. Instructions from the supplier of the starter culture regarding the storage and shelf life of the culture should be strictly followed Herbs, Spices and Additives Herbs and spices may be contaminated with pathogenic bacteria such as Salmonella, thus, it is important that these ingredients are purchased from reputable suppliers who are able to meet agreed specifications consistently. Whenever possible, certificates of analysis should be requested from suppliers, and/or compliance to agreed specifications (e.g. microbiological levels) should be verified by the manufacturer. This may be done by taking samples and having them tested. To avoid potential problems with high levels of microbiological contaminants, sterilised spices or extracts of herbs and spices can be used instead. Only food additives permitted by the Food Standards Code can be used in the manufacture of UCFM products. 5.2 Storage of Raw Meat Clause 6(2) Meat and batter mix used in processing UCFM and stored before fermentation must be stored at 5 C or colder. Raw meat for the production of UCFM products must be stored at or below 5 C. This will prevent the growth of pathogenic microorganisms such as E. coli O157:H7, Salmonella, and Staphylococcus aureus. 5.3 Tempering Temperature control during tempering is important for quality and food safety reasons. It should be done in a manner and under conditions that minimise the growth of microorganisms. Frozen meat is usually tempered to about -5 to -2 C to prevent fat from softening and smearing during grinding or chopping.
14 July 2009 Page 14 Control Measures In warm air (10 C or higher), it is difficult to get even tempering of frozen meat to a uniform temperature. The meat surface may be completely thawed and soft while the centre of the meat blocks remains frozen. Tempering to a uniform temperature requires an air temperature either at, or just above, the desired meat temperature (e.g. tempering in a chiller). Under such conditions, tempering of large blocks of cartoned meat may take several days to complete. Procedures should be established to address any problems from entrapment of plastic liners in the meat blocks. 5.4 Grinding or Flaking Meat for UCFM production is usually ground or flaked in the tempered state and the latent heat of melting limits any temperature increase. The ground or flaked meat should be used immediately, or it should be held in a chiller. Grinders and flakers should be checked and maintained regularly to prevent metal contamination from the equipment. 5.5 Chopping and Ingredient Addition The batter is formed by cutting and mixing the ground or flaked meat with the other ingredients in a bowl chopper. The batter must be filled immediately into casings or it must be held in a chiller at or below 5 C Use of Starter Culture and Prohibition of Back Slopping Clause 6(1) Fermentation of UCFM must be initiated by a starter culture. Clause 9. A processor must not use back slopping in processing UCFM. The UCFM manufacturer should follow the instructions provided by the starter culture supplier regarding the preparation and addition of the starter culture. These should include the: reconstitution method (e.g. some freeze-dried cultures have to be dissolved in distilled water for about 30 minutes before adding into the batter), amount of starter culture to be added to any given amount of batter, and
15 July 2009 Page 15 Control Measures type and amount of fermentable sugars to be added. Back slopping (using the partially fermented batter from a previous batch to initiate fermentation in a subsequent batch) is not permitted because this practice can lead to fermentations which are unreliable and difficult to control. Slow or delayed fermentation may permit growth of Staphylococcus aureus, C. botulinum or other bacterial pathogens Additives Food additives must be used at levels permitted under the Food Standards Code. Nitrite is used as a curing agent and antimicrobial agent. It is responsible for the cured meat colour and flavour, and also prevents oxidation. The combined effects of nitrite along with organic acids, low ph and low a w effectively control the growth of Clostridium botulinum during the manufacture and storage of UCFM products. The Food Standards Code allows up to 500mg/kg of nitrite in UCFM products. Nitrite can be toxic to consumers at excessive levels and its addition to the batter should be controlled. The use of pre-blended curing mixtures (i.e. nitrite mixed with salt and other ingredients, and which are usually tinted pink) prevents the addition of excessive amounts of nitrite into the batter. When pure nitrite is used, it is possible that the person weighing the ingredients might confuse the quantity required with that of salt and mistakenly add excessive amounts of nitrite. The manufacturer should therefore have procedures in place for correct weighing and identification of additives and ingredients Sugar, Salt and Other Ingredients The correct type and amount of sugars (e.g. glucose and dextrose), as recommended by the supplier of the starter culture, should be used to optimise the conversion of sugars to acid by the bacteria in the starter culture. This has a direct influence on the rate of ph reduction and the final ph of the product. Salt is an essential ingredient in all types of UCFM products. Typically, 2.5 to 3.0% of salt is added to the batter and this is taken up only by the water in the meat (i.e. actual salt concentrations in the meat end up being higher than 3%). At this level, salt serves several functions, including (1) an initial reduction in water activity, (2) providing a characteristic salty taste, and (3) contributing to increased solubility of myofibrillar proteins (Toldra, 2006). Care should be taken when adding alcohol to the batter as this can inhibit the starter culture and slow the fermentation process.
16 July 2009 Page 16 Control Measures Glucono-δ-lactone (GdL) may be used as an acidulant in combination with a starter culture Reworked Material Clause 10 A processor may only reprocess fully processed compliant product (e.g. salami ends and trim). Only product that has been through the complete validated fermentation and maturation process (e.g. salami ends and off-cuts) may be reworked back into new product. Manufacturers should establish a limit for the amount of rework which can be added to a batch since this can affect its functionality and the additive levels (e.g. nitrite) in the finished product. Manufacturers should also establish a cut-off period for reworking products from one batch to the next to facilitate traceability and recall procedures. For example, some manufacturers have a weekly cut-off (i.e. material produced in a previous week is not reworked into the current week s production). Any UCFM product, whether in stock or returned, which may have been mishandled or exposed to contamination should not be reworked into new UCFM product. 5.6 Filling and Clipping The batter should be hygienically filled into food grade casings. If casings are pre-soaked before filling, they should be soaked in potable water and the water changed regularly. The batter should be kept below 2 C to avoid smearing. Casings should be filled to the correct diameter, because both under- and over- filling can affect the quality of the end product. Diameter size influences the rate of drying and smoking, and ultimately the flavour and texture of the finished product. Procedures should be in place to prevent metal contamination from metal clips during clipping of the sausages. After filling and clipping, the sausages are hung in racks or trolleys. The sausages should be evenly spaced to help air circulation and even drying and/or smoking. The trolleys of sausages are then transferred to a controlled temperature room for fermentation. Procedures should be in place to ensure that products are not contaminated during the transfer of trolleys from one area to another (e.g. splash from wheels).
17 July 2009 Page 17 Control Measures 5.7 Fermentation During fermentation, lactic acid bacteria provided by the starter culture preparation utilises sugar within the meat and sugar added to the batter to produce acid which lowers the ph of the batter and prevents the growth of pathogenic bacteria. During its growth, the starter culture also produces flavour compounds, enzymes, and other compounds which prevent the growth of other bacteria. The rate of acid production and ph fall depends on several factors, including the number and type of lactic-acid bacteria in the starter culture, the fermentation temperature and the amount and type of fermentable sugar. Effective temperature control is critical to the safety of the final product. The validated fermentation temperature must be followed, and it must be monitored since deviations can significantly affect the lethality of the process and E. coli reduction. It is important that the product reaches a ph below 5.2 (or other validated ph) within a specified period to control the growth of pathogenic microorganisms including Staphylococcus aureus and pathogenic E. coli. Slow or delayed fermentation may allow the growth of Staphylococcus aureus, Clostridium botulinum and other bacterial pathogens. This ph reduction also induces protein coagulation and reduction in the meat s water holding capacity which favours water release and facilitates drying (Toldra, 2006). In general, higher fermentation temperatures require shorter fermentation times, and faster ph falls to prevent pathogen growth. For example, at the lower temperature range of 21 C to 24 C, fermentation can take as long as two to three days. At 28 C to 32 C (range commonly used in New Zealand), 16 to 24 hours of fermentation is usually required. In the United States, where faster overall production time are preferred, the fermentation temperature can be as high as 37 C to 40 C for as little as 12 to 18 hours (Hutkins, 2006). To ensure that the ph decreases within the required rate, ph readings must be taken from each batch during the fermentation period. ph monitoring is further discussed in section 7.3 of this guide. 5.8 Smoking and Maturation (drying) Following the fermentation process, the sausages may be smoked or can go directly to maturation.
18 July 2009 Page 18 Control Measures The rate of drying should be properly controlled during maturation. Excessively fast drying should be avoided as this can cause case-hardening, whereas excessively slow drying may result in undesirable microbial growth on the product s surface. The rate and amount of drying during the maturation process depends on factors such as: product ph; temperature; air velocity (rate of airflow over the product); relative humidity; diameter of the sausage; and maturation time. These factors must be established and documented as part of the validation process, and these parameters must be applied by the manufacturer during processing of every batch of UCFM product. Smoking and maturation temperatures must be controlled and monitored as deviations can significantly affect the lethality of the process and E. coli reduction. The rate and extent of drying must also be monitored by measuring the water activity (a w ) or weight loss of the finished product. The monitoring requirements and procedures are discussed in section 7 of this guide. Semi-dry products are typically dried to remove 20 to 30% of the original water and to give final moisture of about 45 to 50% (Hutkins, 2006). The a w of semi-dry products ranges from 0.90 to Dry fermented sausages will lose about 35% water, giving a final moisture of 35% and a w between 0.85 and Slicing and Packing Fermented sausages can be kept whole or sliced before packing into retail packs. Good hygiene is essential during the slicing and packing of UCFM products. Controls should be in place for preventing or minimising contamination of products from equipment and the environment, and cross contamination between raw and UCFM products. Ideally, slicing and packing of UCFM products should be done in dedicated areas (i.e. separate room) which are physically separated from areas where raw materials and products
19 July 2009 Page 19 Control Measures are handled. Slicers, conveyors and packing machines should only be used in this area for UCFM and other ready-to-eat products. When physical separation is not possible, the slicing and packing of raw and UCFM products should be separated by time. For example, some manufacturers pack ready-to-eat products first thing during the day, when there is no raw product in the room and when the equipment is clean. Other controls which should be in place include: procedures for controlling the movement of personnel and equipment from raw to RTE (Ready to eat) areas; effective hygiene routine for personnel before entering RTE areas or handling RTE products; and effective cleaning of equipment and the processing environment.
20 July 2009 Page 20 Validation 6 Validation July 2009 Clause 4(2) The process of manufacturing UCFM must be subject to validation to ensure the number of Escherichia coli organisms in any UCFM at the end of a production process complies with the microbiological limits specified in Standard of the Australia New Zealand Food Standards Code. The UCFM process must be validated to ensure that it is consistently capable of delivering a safe end product. Validation should be done for each type of product, or group of products with the same characteristics. This validation requirement can be met by using a process that has been scientifically validated by a reputable agency or research institute, or published in a scientific journal. This approach can only be applied if the manufacturer complies with all the product and process parameters that the validated process was based on. For most New Zealand manufacturers, it is more likely that they would need to do their own validation. 6.1 Validation Criteria The validated process must comply with all the requirements of the UCFM Standard, as discussed in this guide. The operator must demonstrate that the validated process is: a. capable of producing products that consistently meet: the microbiological limits specified in Standard of the Australia New Zealand Food Standards Code (refer to section 3.1 of this guide), and the established ph and a w appropriate to its required shelf stability; and b. capable of delivering the required process lethality (e.g. 2-log reduction of E. coli). The process must be revalidated every time a change is made in the product (e.g. formulation, sausage diameter, ph and a w ) or process (e.g. fermentation and maturation times and temperatures) which may impact on the capability of the UCFM process to achieve any of the criteria above.
21 July 2009 Page 21 Validation 6.2 Validation Methods There are several methods which can be used for validating the process Challenge Testing Challenge testing involves inoculating a typical batter with known quantities of nonpathogenic acid resistant E. coli and monitoring their numbers throughout the fermentation and maturation stages. Challenge testing is considered to be the ideal method for validation because it gives results that are specific to the product and its characteristics (e.g. formulation, sausage diameter) and the process. However, it can be expensive to perform and it requires a high level of technical competency. For these reasons, it is usually only carried out by large manufacturers. It is recommended that manufacturers who wish to do challenge tests should consult a research agency or laboratory experienced in undertaking such work Predictive Modelling A predictive model can be used to show whether the process is capable of eliminating sufficient numbers of E. coli to meet the established limits, considering the E. coli levels in raw meat used in the manufacture of the UCFM product. There are several models available, but the Meat and Livestock Australia (MLA) model for E. coli inactivation (commonly known in New Zealand as the Tom Ross model) is the one most commonly used in Australia and New Zealand for assessing the inactivation of E. coli in a UCFM product during fermentation and maturation. The model is available from The report "Predicting E. coli inactivation in uncooked comminuted fermented meat products", which is also available from the same website, is recommended reading for manufacturers who intend to use the MLA model. It provides information which will assist manufacturers understand the scientific basis for the model. The report provides an extensive review and analysis of published and unpublished studies regarding the UCFM process and the inactivation of E. coli during processing. From these studies it was found that, once fermentation has begun, the combined effects of temperature and time are responsible for most of the observed death of E. coli. While salt, drying and low ph are essential for setting up conditions in fermented meats so that E.coli are killed, the actual levels do not strongly influence the rate of E. coli inactivation.
22 July 2009 Page 22 Validation Two calculators are provided for the model. The instructions for the calculators should be read carefully before they are used. To use either calculator, the manufacturer needs to know the times and temperatures of the fermentation and maturation (and smoking, if this is applied) processes. The "Quick" calculator is more appropriate for small operations or for those where the fermentation and the maturation temperatures are static (e.g. the entire fermentation period is at 30 C and maturation is at 15 C). The "Advanced" version is more useful for those with variable fermentation and maturation temperatures and who are able to obtain data at regular frequency (e.g. by using a data logger) during the entire fermentation and maturation processes. It uses the same model as the Quick calculator, but allows for more times and temperatures to be specified (such as warm-up times before fermentation, cool-down times after fermentation, or heating steps). This allows for more accurate predictions. Both calculators estimate the average log reduction of E. coli achieved by the particular process. For the process to be valid, the average log reduction must be greater or equal to the E. coli count in the raw meat used. If the manufacturer feels that the outcome of the predictive model is not appropriate for their specific product (e.g. small diameter sausages become overdried when the calculated lethality is applied), a challenge test could be considered to obtain results that are specific to the product and process Use of Historical Data Manufacturers who routinely test their end product may have a lot of data that show that their products consistently meet the microbiological standard for UCFM products. This data would be useful for validation but it cannot be used solely on its own to confirm the effectiveness of the process to destroy the required level of microorganisms because the levels of microorganisms in the raw material used are unknown. For example, the absence of E. coli in the final product would not necessarily mean that the process is effective in killing E. coli when it is possible that this pathogen was not actually present in the raw meat or batter. 6.3 Actions Required when Process is Determined to be Inadequate The manufacturer must take the following actions if validation indicates that the existing process is inadequate and cannot produce UCFM products that will consistently meet the validation criteria given section 6.1:
23 July 2009 Page 23 Validation a. production of any UCFM product using the existing process must cease immediately; b. any product in stock that has been produced using the existing process must not be released for distribution or sale unless the particular batch is exposed to further treatment that is sufficient to destroy any surviving pathogens (e.g. cooking); and c. the manufacturer must make changes in the product or process (e.g. by improving the quality of the raw material and/or the process conditions), and revalidate the process before re-commencing production of UCFM products. There are various ways which may be considered to improve the process so that a bigger reduction in E. coli can be achieved, such as (MLA, 2003): fermenting at a higher temperature; maturing at a higher temperature; extending the maturing time; or having a brief heating step, e.g. bringing the product to 50 C for two minutes. The manufacturer may propose an alternative course of action, but this must first be discussed and agreed to with the evaluator or regulator before it is implemented.
24 July 2009 Page 24 Implementation, and Monitoring and Verification Procedures 7 Implementation, and Monitoring and Verification Procedures July Implementation of the Validated Process UCFM production must be implemented according to the validated process. The process must be revalidated when a change is made in the product or process which may impact on process lethality or the achievement of any of the outcomes discussed in section 3 of this guide (e.g. change in formulation, sausage diameter, ph, a w, fermentation and maturation times and temperatures). 7.2 Monitoring and Verification Requirements Clause 7. Processor to monitor and record certain matters (1) A processor must monitor and record the following matters during the processing of the UCFM for each batch: (a) the temperature and time of fermentation of the UCFM; and (b) the temperature and time of maturation or drying of the UCFM; and (c) the temperature and time of smoking of the UCFM (if applicable). (2) A processor of UCFM must monitor and record the ph and water activity of UCFM during processing. Clause 5. Escherichia coli count for UCFM (3) The number of Escherichia coli organisms in UCFM must be measured and recorded (verification) after the product has finished maturation and when the product is ready for sale at a frequency determined by the operator.
25 July 2009 Page 25 Implementation, and Monitoring and Verification Procedures 7.3 Temperature and Time Monitoring The temperatures of the fermentation and maturation rooms must be controlled so that they consistently comply with the validated temperatures, and they must be monitored for every batch of UCFM. Some plants may have facilities for continuously monitoring room temperature (e.g. data loggers). In other plants, such monitoring will be done manually. Temperatures should be monitored and recorded at least twice daily during the fermentation process, or at a frequency necessary to confirm that the scheduled process is being followed. The manufacturer must establish corrective action procedures for any noncompliance. Thermometers used for monitoring must be properly calibrated. 7.4 ph and Water Activity (a w ) Monitoring The ph of every batch of UCFM should be periodically checked and recorded during fermentation (e.g. within 24 hours) to ensure that the ph reaches 5.2 or lower within the required period. The recommended method for determining ph is given in Appendix 1. The manufacturer must establish corrective action procedures for any product which fails to achieve the required ph within the validated period. For example, the non-compliant product may be cooked. However, non-compliant product cannot be reworked into a UCFM product. ph and water activity of the UCFM product must also be measured after the maturation process (i.e. end product testing). Water activity can be determined using a calibrated water activity meter. Manufacturers who do not have access to such equipment can use product weight loss to estimate the water activity of the product, but it is necessary to establish the correlation between weight loss and a w for the particular product. Establishing the correlation between weight loss and a w for each product produced can be done by having the a w of product samples that have achieved the intended weight loss analysed by a laboratory. Provided that the formulation and processing parameters don t change, the correlation between a w and weight loss should remain constant. Appendix 2 gives a method for determining weight loss. A product should not be released for sale until it has reached its target ph and a w (e.g. combination of ph < 5.2 and a w < 0.95). UCFM products which do not meet the established ph and a w parameters for shelf stability must be refrigerated and stored at 5 C. The label of the product must clearly state that the product requires refrigerated storage.
26 July 2009 Page 26 Implementation, and Monitoring and Verification Procedures 7.5 E. coli Testing The manufacturer must test UCFM products for E. coli as part of ongoing verification of the effectiveness of the process and compliance to the microbiological standards for UCFM. Testing of each batch for E. coli is not required. The number of samples to be tested and the frequency of sampling should be based on a sampling plan which will provide enough data so that the manufacturer and regulator can have confidence that the validated process is consistently achieving the E. coli limits. The level of E. coli in the finished product can be determined using an MPN method of analysis, however, direct plating methods may also be used as long as their lower limit of detection is no greater than 3.6 cfu/g. The manufacturer must establish corrective action procedures for any non-compliance.
27 July 2009 Page 27 Corrective Action Procedures 8 Corrective Action Procedures July 2009 The operator must document corrective action procedures for any non-compliance to regulatory requirements or validated procedures/parameters. In particular, specific corrective actions must be developed for non-compliances to the product and process criteria given in sections 3 and 6.1 of this guide. The corrective actions must address the: restoration of control; identification and disposition of affected product (including initiating a recall, if necessary); and prevention of the re-occurrence of the loss of control. A suitably skilled person should investigate any incidence of non-compliance or process failure, determine the cause of the failure, and determine the appropriate corrective action. A recall may be necessary when non-compliant products have been released for distribution and sale. Guidance on recall procedures is available from
28 July 2009 Page 28 Records 9 Records July 2009 Clause 8. Records Any records generated from the UCFM production process must be kept for four years. All records taken from production must be kept for a minimum of four years from the date of production. [Note: Requirements are different for transitional facilities.] Examples of records which must be kept by the manufacturer are: validation records; fermentation and maturation room time and temperature readings; ph measurements of the product (i.e. during fermentation, and the end product); water activity results for the end product, or weight loss records (with records of establishing the correlation with a w ); equipment calibration records (e.g. thermometers); and traceability and inventory records. Records should include details such as date and time of taking the record, the result, person recording the observation or measurement, the batch number and any action taken following a processing failure or non-compliance to documented procedures.
29 July 2009 Page 29 References 10 References ANZFA Advisory Guidelines for Making Uncooked Fermented Comminuted Meat Products (1996). Hutkins, R.W. (2006) Chapter 6: Meat fermentation. In Microbiology and Technology of Fermented Foods. IFT Press, Blackwell Publishing, USA. MLA (2003) Guidelines for the Safe Manufacture of Smallgoods. Meat and Livestock Australia. Ross, T. and Shadbolt, C.T. (2001) Predicting E. coli Inactivation in Uncooked Comminuted Fermented Meat Products. Meat and Livestock Australia. Toldra, F. (2006) Chapter 28: Biochemistry of fermented meat. In Food Biochemistry and Food Processing. Ed. Hui Y.H. Blackwell Publishing, Iowa, USA.
30 July 2009 Page 30 Appendix 1: Determination of ph Appendix 1: Determination of ph (Based on the ANZFA Advisory Guidelines for Making UCFM Products) 1. Standard method (Food Standards Code Standard 1.6.2) Mince a representative portion of the sample of the UCFM and place that portion in a stoppered bottle with twice its weight of water. Shake at five minute intervals for 30 minutes and determine the ph value of the liquid electrometrically at 20 C. 2. Alternative method ph can also be determined through the use of calibrated, direct-contact ph probes or spear electrodes. They should be standardised against FSC Standard to confirm their accuracy and suitability for determining ph of UCFM products. When testing is done by inserting probes or spear electrodes, it is essential that it is done aseptically so that microorganisms are not introduced into the product. Indicator strips have generally proved to be unsuitable for measuring the ph of fermenting products, as there may be problems establishing adequate contact between the indicator pad and the product. The fat in the product may coat the indicator pad and prevent liquid from the product reacting with the indicator.
31 July 2009 Page 31 Appendix 2: Monitoring Weight Loss Appendix 2: Monitoring Weight Loss (From the MLA Guidelines for the Safe Manufacture of Smallgoods) 1. Make sure your scales are accurate. It is no use putting a 150 g stick on a scale which weighs up to 50 kg the accuracy will not be enough to support your validation. 2. Weigh a representative sample ten sticks from a batch should be sufficient. 3. Tie a label on each stick and write the starting weight and the date on it. 4. Each time you check-weigh the stick, write the new weight and the date on the label. 5. Do not just average the ten weights because that only allows you to say on average my product is shelf stable. 6. Keep each individual weighing this will tell you how variable your process is. If it is so variable that some sticks are too moist to be released you will need to find out why there is uneven drying. 7. Record and retain the results.
Guidance Document. Production of Uncooked Fermented Salami (UCFM) 5 September A guidance document issued by the Ministry for Primary Industries
 Guidance Document Production of Uncooked Fermented Salami (UCFM) 5 September 2017 A guidance document issued by the Ministry for Primary Industries Title Guidance Document: Production of Uncooked Fermented
Guidance Document Production of Uncooked Fermented Salami (UCFM) 5 September 2017 A guidance document issued by the Ministry for Primary Industries Title Guidance Document: Production of Uncooked Fermented
Code of Practice: Processing of Seafood Product. Part 3: HACCP Application, and the Identification of Other Risk Factors and their Controls
 Code of Practice: Processing of Seafood Product Part 3: HACCP Application, and the Identification of Other Risk Factors and their Controls July 2011 Introduction Disclaimer Every effort has been made to
Code of Practice: Processing of Seafood Product Part 3: HACCP Application, and the Identification of Other Risk Factors and their Controls July 2011 Introduction Disclaimer Every effort has been made to
Table of Contents Page
 COMPLIANCE GUIDELINES TO CONTROL LISTERIA MONOCYTOGENES IN POST-LETHALITY EXPOSED READY-TO-EAT MEAT AND POULTRY PRODUCTS Summary of Guidance Material Table of Contents Page 3 A. Introduction 4 B. Control
COMPLIANCE GUIDELINES TO CONTROL LISTERIA MONOCYTOGENES IN POST-LETHALITY EXPOSED READY-TO-EAT MEAT AND POULTRY PRODUCTS Summary of Guidance Material Table of Contents Page 3 A. Introduction 4 B. Control
Utilization of Microbial Data to Improve Food Safety Systems
 Beef Industry Guidance Document Utilization of Microbial Data to Improve Food Safety Systems Prepared by: Dr. Lynn Delmore In conjunction with: North American Meat Association A contractor to the Beef
Beef Industry Guidance Document Utilization of Microbial Data to Improve Food Safety Systems Prepared by: Dr. Lynn Delmore In conjunction with: North American Meat Association A contractor to the Beef
should remain in refrigerated or frozen state.
 Product Description COMMON NAME: Wieners, bologna, kielbasa HOW IS IT TO BE USED? Ready-to-Eat TYPE OF PACKAGE? Vacuum-packaged in plastic film; bulkpacked in plastic bag or plastic bag in cardboard box,
Product Description COMMON NAME: Wieners, bologna, kielbasa HOW IS IT TO BE USED? Ready-to-Eat TYPE OF PACKAGE? Vacuum-packaged in plastic film; bulkpacked in plastic bag or plastic bag in cardboard box,
Code of Practice for Cold and Dry Stores. Part 3: HACCP Application, and the Identification and Control of Other Risk Factors
 Part 3: HACCP Application, and the Identification and December 2006 Page 2 Prelims Prelims December 2006 Table of Contents Prelims...2 Disclaimer...3 Review of Code of Practice...3 Amendment Record...4
Part 3: HACCP Application, and the Identification and December 2006 Page 2 Prelims Prelims December 2006 Table of Contents Prelims...2 Disclaimer...3 Review of Code of Practice...3 Amendment Record...4
Heat Treated, Shelf-Stable
 GENERIC HACCP MODEL FOR Heat Treated, Shelf-Stable Developed: May 29-31, 1996 Chicago, Illinois Submitted to USDA, Food Safety and Inspection Service by the International Meat and Poultry HACCP Alliance
GENERIC HACCP MODEL FOR Heat Treated, Shelf-Stable Developed: May 29-31, 1996 Chicago, Illinois Submitted to USDA, Food Safety and Inspection Service by the International Meat and Poultry HACCP Alliance
Narrative. Description of Process: REVISED JUNE 2017 Commercial Processing Example: Shrimp (Wild), Cooked, Frozen. ABC Shrimp Company, Anywhere, USA
 National Seafood HACCP Alliance for Training and Education REVISED JUNE 2017 Commercial Processing Example: Shrimp (Wild), Cooked, Frozen Example: This is a Special Training Model for illustrative purposes
National Seafood HACCP Alliance for Training and Education REVISED JUNE 2017 Commercial Processing Example: Shrimp (Wild), Cooked, Frozen Example: This is a Special Training Model for illustrative purposes
HPP Safety Validations: a Key Element for the Production of Innovative Dips and Wet Salads. October 17, 2016 Lincoln, NE
 HPP Safety Validations: a Key Element for the Production of Innovative Dips and Wet Salads October 17, 2016 Lincoln, NE PhD. Jessie Usaga Visiting Associate Professor, Cornell University Associate Professor,
HPP Safety Validations: a Key Element for the Production of Innovative Dips and Wet Salads October 17, 2016 Lincoln, NE PhD. Jessie Usaga Visiting Associate Professor, Cornell University Associate Professor,
Interpretation of Microbiological Test Results. Nicola Elviss FW&E Microbiology Network June 2010
 Interpretation of Microbiological Test Results Nicola Elviss FW&E Microbiology Network June 2010 PHLS Guidelines for the microbiological quality of some ready-to-eat foods at the point of sale: 1992, revised
Interpretation of Microbiological Test Results Nicola Elviss FW&E Microbiology Network June 2010 PHLS Guidelines for the microbiological quality of some ready-to-eat foods at the point of sale: 1992, revised
Guidance Document. Meeting Requirements as a Registered Food Importer. A guidance document issued by the Ministry for Primary Industries
 Guidance Document Meeting Requirements as a Registered Food Importer. 23 February 2016 A guidance document issued by the Ministry for Primary Industries Title Meeting Requirements as a Registered Food
Guidance Document Meeting Requirements as a Registered Food Importer. 23 February 2016 A guidance document issued by the Ministry for Primary Industries Title Meeting Requirements as a Registered Food
Attachment 1 Amendment 6 - IS6/IAS6: Section 3.8: Amended New Zealand Standard for Spray Chilling Large Mammals and Large Birds
 Attachment 1 Amendment 6 - IS6/IAS6: Section 3.8: Amended New Zealand Standard for Spray Chilling Large Mammals and Large Birds February 2008 Contents 1 Scope... 2 2 Background... 2 3 Definitions... 3
Attachment 1 Amendment 6 - IS6/IAS6: Section 3.8: Amended New Zealand Standard for Spray Chilling Large Mammals and Large Birds February 2008 Contents 1 Scope... 2 2 Background... 2 3 Definitions... 3
Food establishments that package Time/Temperature Control for Safety (TCS) Food using ROP methods must implement a HACCP plan.
 TULSA HEAL TH Department Food Protection Service 5051 S. 129 th East Ave Tulsa, OK 74134 Phone 918.595.4300 Fax 918.595.4339 www.tulsa-health.org Effective: September 11, 2016 SUBJECT: Reduced Oxygen Packaging
TULSA HEAL TH Department Food Protection Service 5051 S. 129 th East Ave Tulsa, OK 74134 Phone 918.595.4300 Fax 918.595.4339 www.tulsa-health.org Effective: September 11, 2016 SUBJECT: Reduced Oxygen Packaging
ACVM Notice. Agricultural Compounds Exempt from Registration. Requirements for conditions of exemption. 1 August 2017
 ACVM Notice Agricultural Compounds Exempt from Registration Requirements for conditions of exemption Issued under the Agricultural Compounds and Veterinary Medicines Act 1997 TITLE ACVM Notice: Agricultural
ACVM Notice Agricultural Compounds Exempt from Registration Requirements for conditions of exemption Issued under the Agricultural Compounds and Veterinary Medicines Act 1997 TITLE ACVM Notice: Agricultural
The HPA Guidelines for Assessing the Microbiological Safety of Ready-to-eat Foods placed on the market and. Hospital Guidelines
 The HPA Guidelines for Assessing the Microbiological Safety of Ready-to-eat Foods placed on the market and Hospital Guidelines HPA FW&E Microbiology Service Developing Guidelines in Partnership FSA, Defra,
The HPA Guidelines for Assessing the Microbiological Safety of Ready-to-eat Foods placed on the market and Hospital Guidelines HPA FW&E Microbiology Service Developing Guidelines in Partnership FSA, Defra,
GENERIC HACCP MODEL FOR IRRADIATION. Developed: June 5-7, 1996 College Station, TX. Submitted to. USDA, Food Safety and Inspection Service.
 GENERIC HACCP MODEL FOR IRRADIATION Developed: June 5-7, 1996 College Station, TX Submitted to USDA, Food Safety and Inspection Service by the International Meat and Poultry HACCP Alliance on September
GENERIC HACCP MODEL FOR IRRADIATION Developed: June 5-7, 1996 College Station, TX Submitted to USDA, Food Safety and Inspection Service by the International Meat and Poultry HACCP Alliance on September
Generic RMP Models for the Processing of Seafood Product
 Generic RMP Models for the Processing of Seafood Product July 2011 Disclaimer Disclaimer Every effort has been made to ensure the information in this report is accurate. MAF does not accept any responsibility
Generic RMP Models for the Processing of Seafood Product July 2011 Disclaimer Disclaimer Every effort has been made to ensure the information in this report is accurate. MAF does not accept any responsibility
THE BASIC PRINCIPLES OF HACCP
 THE BASIC PRINCIPLES OF HACCP The Meaning of HACCP History of the HACCP System Traditional Inspection HACCP System The Seven Principles of HACCP ١ Meaning of HACCP : Hazard Analysis and Critical Control
THE BASIC PRINCIPLES OF HACCP The Meaning of HACCP History of the HACCP System Traditional Inspection HACCP System The Seven Principles of HACCP ١ Meaning of HACCP : Hazard Analysis and Critical Control
CODE OF HYGIENIC PRACTICE FOR REFRIGERATED PACKAGED FOODS WITH EXTENDED SHELF LIFE CAC/RCP 46-(1999)
 CAC/RCP 46 Page 1 of 20 CODE OF HYGIENIC PRACTICE FOR REFRIGERATED PACKAGED FOODS WITH EXTENDED SHELF LIFE CAC/RCP 46-(1999) TABLE OF CONTENTS INTRODUCTION 3 1 OBJECTIVES 3 2 SCOPE AND USE OF THE DOCUMENT
CAC/RCP 46 Page 1 of 20 CODE OF HYGIENIC PRACTICE FOR REFRIGERATED PACKAGED FOODS WITH EXTENDED SHELF LIFE CAC/RCP 46-(1999) TABLE OF CONTENTS INTRODUCTION 3 1 OBJECTIVES 3 2 SCOPE AND USE OF THE DOCUMENT
Produced by Agriculture and Extension Communications, Virginia Tech
 2005 PUBLICATION 458-501 Safe Processing of Meat and Poultry Jerky Scott Daigle, Meat Laboratory Manager, Department of Food Science and Technology, Virginia Tech Joseph Eifert, Associate Professor and
2005 PUBLICATION 458-501 Safe Processing of Meat and Poultry Jerky Scott Daigle, Meat Laboratory Manager, Department of Food Science and Technology, Virginia Tech Joseph Eifert, Associate Professor and
Risk Management Programme Page: 1 of 8 Date: / / 1. Business Identification. 2. Operator Name, Business Address and Contact Details
 Risk Management Programme Page: 1 of 8 Date: / / 1. Business Identification 1.1 Business ID: 1.2 RMP No.: 1.3 Unique Location Identifier for Dairy Business Operators: 2. Operator Name, Business Address
Risk Management Programme Page: 1 of 8 Date: / / 1. Business Identification 1.1 Business ID: 1.2 RMP No.: 1.3 Unique Location Identifier for Dairy Business Operators: 2. Operator Name, Business Address
Sample Food Safety Plan MEETS BC REGULATORY REQUIREMENTS MIXED GREEN SALAD
 Sample Food Safety Plan MEETS BC REGULATORY REQUIREMENTS MIXED GREEN SALAD Product Description Product Description 1. What is your product name and weight/volume? 2. What type of product is it (e.g., raw,
Sample Food Safety Plan MEETS BC REGULATORY REQUIREMENTS MIXED GREEN SALAD Product Description Product Description 1. What is your product name and weight/volume? 2. What type of product is it (e.g., raw,
Hazard Analysis and Critical Control Points (HACCP) User s Guide
 Hazard Analysis and Critical Control Points (HACCP) User s Guide Sous Vide Reduced Oxygen Packaging (ROP) Template Forward The goal of this Hazard Analysis and Critical Control Points (HACCP) User s Guide
Hazard Analysis and Critical Control Points (HACCP) User s Guide Sous Vide Reduced Oxygen Packaging (ROP) Template Forward The goal of this Hazard Analysis and Critical Control Points (HACCP) User s Guide
INDUSTRY INFORMATION SESSION Agenda
 INDUSTRY INFORMATION SESSION 2012 Agenda Food Safety Enhancement Program (FSEP) update Validation of control measures HACCP system maintenance & reassessment Action plan CFIA verification of FSEP recognized
INDUSTRY INFORMATION SESSION 2012 Agenda Food Safety Enhancement Program (FSEP) update Validation of control measures HACCP system maintenance & reassessment Action plan CFIA verification of FSEP recognized
UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON. D.C. TIME/TEMPERATURE GUIDELINES FOR COOLING HEATED PRODUCTS
 UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON. D.C. I. PURPOSE TIME/TEMPERATURE GUIDELINES FOR COOLING HEATED PRODUCTS This directive clarifies the intent of the
UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON. D.C. I. PURPOSE TIME/TEMPERATURE GUIDELINES FOR COOLING HEATED PRODUCTS This directive clarifies the intent of the
Hazard Analysis and Critical Control Points (HACCP) User s Guide
 Hazard Analysis and Critical Control Points (HACCP) User s Guide Vacuum Packaging Reduced Oxygen Packaging (ROP) Template Forward The goal of this Hazard Analysis and Critical Control Points (HACCP) User
Hazard Analysis and Critical Control Points (HACCP) User s Guide Vacuum Packaging Reduced Oxygen Packaging (ROP) Template Forward The goal of this Hazard Analysis and Critical Control Points (HACCP) User
REPLACEMENT FOR APPENDIX A IN COOKED MEATS. Andrew Milkowski, Ph.D. Adjunct Professor of Meat Science, University of Wisconsin
 REPLACEMENT FOR APPENDIX A IN COOKED MEATS Andrew Milkowski, Ph.D. Adjunct Professor of Meat Science, University of Wisconsin Acknowledgements Many, many people have contributed to the content of this
REPLACEMENT FOR APPENDIX A IN COOKED MEATS Andrew Milkowski, Ph.D. Adjunct Professor of Meat Science, University of Wisconsin Acknowledgements Many, many people have contributed to the content of this
Best Practices for Retailer Operations Producing Raw Ground Beef
 Best Practices for Retailer Operations Producing Raw Ground Beef Developed By: Beef Industry Food Safety Council National Cattlemen s Beef Association Edited by: Kerri B. Harris International HACCP Alliance
Best Practices for Retailer Operations Producing Raw Ground Beef Developed By: Beef Industry Food Safety Council National Cattlemen s Beef Association Edited by: Kerri B. Harris International HACCP Alliance
Guidance Document on E. coli O157:H7 and E. coli O157:NM in Raw Beef
 Guidance Document on E. coli O157:H7 and E. coli O157:NM in Raw Beef February 2014 Bureau of Microbial Hazards, Food Directorate, Health Products and Food Branch 1 Table of Contents 1. Summary... 3 2.
Guidance Document on E. coli O157:H7 and E. coli O157:NM in Raw Beef February 2014 Bureau of Microbial Hazards, Food Directorate, Health Products and Food Branch 1 Table of Contents 1. Summary... 3 2.
Modelling the impact of composition fluctuations and the cold chain on the safe shelf life of Hungarian meat products
 Modelling the impact of composition fluctuations and the cold chain on the safe shelf life of Hungarian meat products Sz. Pércsi, A. Sebők, E. Horváth, Cs. Baár 3rd International Workshop Cold Chain Management
Modelling the impact of composition fluctuations and the cold chain on the safe shelf life of Hungarian meat products Sz. Pércsi, A. Sebők, E. Horváth, Cs. Baár 3rd International Workshop Cold Chain Management
Applying ISO 9001 to making cookies
 Applying ISO 9001 to making cookies How does ISO 9001:2015 apply to a business? Let s say you make cookies First, the standard doesn t consider the actual quality of the cookie, you and your customers
Applying ISO 9001 to making cookies How does ISO 9001:2015 apply to a business? Let s say you make cookies First, the standard doesn t consider the actual quality of the cookie, you and your customers
Chapter 2 Validation of Control Measures 1
 Chapter 2 Validation of Control Measures 1 2.1 Introduction ICMSF previously discussed validation of control measures in the supply chain (Zwietering et al. 2010) and portions of that paper are included
Chapter 2 Validation of Control Measures 1 2.1 Introduction ICMSF previously discussed validation of control measures in the supply chain (Zwietering et al. 2010) and portions of that paper are included
Validation of Industrial Processes with respect to Food Safety. Dubai International food safety conference February
 Validation of Industrial Processes with respect to Food Safety conference February 2010 1 AGENDA 1. HACCP & Validation 2. Validation Concept 3. Validation of Control Measures 4. Validation of Processes
Validation of Industrial Processes with respect to Food Safety conference February 2010 1 AGENDA 1. HACCP & Validation 2. Validation Concept 3. Validation of Control Measures 4. Validation of Processes
Microbiological criteria and shelf-life. ...Current legal situation
 Microbiological criteria and shelf-life...current legal situation Dr. Mary Friel Technical Executive, FSAI Commission Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs Entered into
Microbiological criteria and shelf-life...current legal situation Dr. Mary Friel Technical Executive, FSAI Commission Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs Entered into
Lesson 10: Chapter 4 Module 1 Purchasing and Receiving Foods
 Lesson 10: Chapter 4 Module 1 Purchasing and Receiving Foods Chapter 4: The Flow of Food Safely through your Establishment Module 1: Purchasing and Receiving Foods 59 Module 2: Preparing Foods Safely 69
Lesson 10: Chapter 4 Module 1 Purchasing and Receiving Foods Chapter 4: The Flow of Food Safely through your Establishment Module 1: Purchasing and Receiving Foods 59 Module 2: Preparing Foods Safely 69
Stage IV: Product Commercialization
 Low fat soy yoghurt drink with kiwi flavour Stage IV: Product Commercialization Group B Rong Lu Marianna Christofi Yashorajvardhini Nimbalkar Huiying Shang Zhun Wang Introduction Stage IV: Confirm product
Low fat soy yoghurt drink with kiwi flavour Stage IV: Product Commercialization Group B Rong Lu Marianna Christofi Yashorajvardhini Nimbalkar Huiying Shang Zhun Wang Introduction Stage IV: Confirm product
Processing and packaging of meat and poultry. Your partner for plant engineering and construction.
 PLanning PRODUcTION implementation Processing and packaging of meat and poultry Your partner for plant engineering and construction www.emf.de Our comprehensive range of services: Planning of food processing
PLanning PRODUcTION implementation Processing and packaging of meat and poultry Your partner for plant engineering and construction www.emf.de Our comprehensive range of services: Planning of food processing
TQCSI HACCP Code: 2017
 HACCP Code: 2017 HACCP Code for Food Safety Programs HACCP CODE: 2017 published by: TQCS International Pty Ltd Head Office: 117A Tapleys Hill Road HENDON SA 5014 AUSTRALIA ph: +61 8 8347 0603 fax: +61
HACCP Code: 2017 HACCP Code for Food Safety Programs HACCP CODE: 2017 published by: TQCS International Pty Ltd Head Office: 117A Tapleys Hill Road HENDON SA 5014 AUSTRALIA ph: +61 8 8347 0603 fax: +61
Code of Practice: Processing of Bee Products
 Code of Practice: Processing of Bee Products July 2005 Page 2 Prelims Prelims July 2005 Table of Contents Code of Practice: Processing of Bee Products...1 Prelims...2 Disclaimer...3 Review of Code of Practice...3
Code of Practice: Processing of Bee Products July 2005 Page 2 Prelims Prelims July 2005 Table of Contents Code of Practice: Processing of Bee Products...1 Prelims...2 Disclaimer...3 Review of Code of Practice...3
DRAFT OECD GLP ADVISORY DOCUMENT NO. 19 ON THE MANAGEMENT, CHARACTERISATION AND USE OF TEST ITEMS PREAMBLE
 DRAFT OECD GLP ADVISORY DOCUMENT NO. 19 ON THE MANAGEMENT, CHARACTERISATION AND USE OF TEST ITEMS PREAMBLE 1. This guidance provides clarity for test facilities on the expectations of national Good Laboratory
DRAFT OECD GLP ADVISORY DOCUMENT NO. 19 ON THE MANAGEMENT, CHARACTERISATION AND USE OF TEST ITEMS PREAMBLE 1. This guidance provides clarity for test facilities on the expectations of national Good Laboratory
Quality Assurance Programme - raw meat for further processing plants supplying a fast food chain
 Quality Assurance Programme - raw meat for further processing plants supplying a fast food chain F. EHINGER Röntgenstr. 5, 89312 Günzburg, Germany ehingerf@esca-foodsolutions.de Keywords: quality; meat
Quality Assurance Programme - raw meat for further processing plants supplying a fast food chain F. EHINGER Röntgenstr. 5, 89312 Günzburg, Germany ehingerf@esca-foodsolutions.de Keywords: quality; meat
Achieving FSIS HACCP Validation Compliance. March 15 th and 17 th, 2016
 Achieving FSIS HACCP Validation Compliance March 15 th and 17 th, 2016 Questions? Please use the Chat function on the upper right hand of your screen. Send your questions to Michelle Rossman and she will
Achieving FSIS HACCP Validation Compliance March 15 th and 17 th, 2016 Questions? Please use the Chat function on the upper right hand of your screen. Send your questions to Michelle Rossman and she will
EU Regulation on Microbiological Criteria for Foodstuffs. Mary Howell Hygiene and Microbiology UK Food Standards Agency
 EU Regulation on Microbiological Criteria for Foodstuffs Mary Howell Hygiene and Microbiology UK Food Standards Agency import production processing distribution preparation consumption Importers Agro-chemical
EU Regulation on Microbiological Criteria for Foodstuffs Mary Howell Hygiene and Microbiology UK Food Standards Agency import production processing distribution preparation consumption Importers Agro-chemical
HACCP audit checklist
 Requirement HACCP audit checklist Prerequisite Program Management Commitment 1. Senior management ensures that the responsibilities and authorities are defined and communicated within the company Internal
Requirement HACCP audit checklist Prerequisite Program Management Commitment 1. Senior management ensures that the responsibilities and authorities are defined and communicated within the company Internal
UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC USE OF MICROBIAL PATHOGEN COMPUTER MODELING IN HACCP PLANS
 UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC FSIS NOTICE 25-05 5/4/05 USE OF MICROBIAL PATHOGEN COMPUTER MODELING IN HACCP PLANS I. What is the purpose of this
UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC FSIS NOTICE 25-05 5/4/05 USE OF MICROBIAL PATHOGEN COMPUTER MODELING IN HACCP PLANS I. What is the purpose of this
3M Microbiology. Food safety. maximized. Safety. Processing
 3M Microbiology Food safety Processing maximized Safety Food Safety Maximized The importance of food safety Producing safe and healthy food is critical to the success of one of the largest industries worldwide.
3M Microbiology Food safety Processing maximized Safety Food Safety Maximized The importance of food safety Producing safe and healthy food is critical to the success of one of the largest industries worldwide.
Report #3 Short-term temperature abuse of cooked but not shelf-stable meat and poultry products
 Report #3 Short-term temperature abuse of cooked but not shelf-stable meat and poultry products Laboratory-Based Evidence Supporting Simple Critical Limits for Use with Cured Meat and Poultry Products
Report #3 Short-term temperature abuse of cooked but not shelf-stable meat and poultry products Laboratory-Based Evidence Supporting Simple Critical Limits for Use with Cured Meat and Poultry Products
Microbiology 101 Nina G. Parkinson NGP Consulting November 11, 2014
 Microbiology 101 Nina G. Parkinson NGP Consulting November 11, 2014 Section Summary Microorganisms of importance in foods How they grow? Why are they a problem? How they can be controlled? How they can
Microbiology 101 Nina G. Parkinson NGP Consulting November 11, 2014 Section Summary Microorganisms of importance in foods How they grow? Why are they a problem? How they can be controlled? How they can
Hazard Analysis and Critical Control Point The Almond Board of California. Overview. Definitions
 Hazard Analysis and Critical Control Point The Almond Board of California Overview Hazard Analysis Critical Control Point (HACCP) is the final stage of an integrated, proactive food safety program targeting
Hazard Analysis and Critical Control Point The Almond Board of California Overview Hazard Analysis Critical Control Point (HACCP) is the final stage of an integrated, proactive food safety program targeting
Guidance Document. A guide to HACCP systems in the Meat Industry. Volume March 2017
 Guidance Document A guide to HACCP systems in the Meat Industry Volume 2 This version contains no change in technical content from the version issued in August 2004, but is issued with MPI branding, and
Guidance Document A guide to HACCP systems in the Meat Industry Volume 2 This version contains no change in technical content from the version issued in August 2004, but is issued with MPI branding, and
How to use: Listeria in ready-toeat foods training resource
 Guidance Document How to use: Listeria in ready-toeat foods training resource 7 July 2017 1 Purpose It is a legal requirement under the Animal Products Act (APA) that all staff entering ready-to-eat food
Guidance Document How to use: Listeria in ready-toeat foods training resource 7 July 2017 1 Purpose It is a legal requirement under the Animal Products Act (APA) that all staff entering ready-to-eat food
The Control of Microbial Growth
 11/10/2016 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College CHAPTER 7 The Control of Microbial Growth The Terminology of Microbial Control Sepsis refers to
11/10/2016 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College CHAPTER 7 The Control of Microbial Growth The Terminology of Microbial Control Sepsis refers to
PART III - EI PROCESSING OF ANIMAL PERISHABLE PRODUCTS
 GFSI BENCHMARKING REQUIREMENTS GFSI Guidance Document Version 7 PART III - EI PROCESSING OF ANIMAL PERISHABLE PRODUCTS PART III - EI PROCESSING OF ANIMAL PERISHABLE PRODUCTS Scheme Scope and Key Elements
GFSI BENCHMARKING REQUIREMENTS GFSI Guidance Document Version 7 PART III - EI PROCESSING OF ANIMAL PERISHABLE PRODUCTS PART III - EI PROCESSING OF ANIMAL PERISHABLE PRODUCTS Scheme Scope and Key Elements
The shipment of meat products from the
 The Preparation and Transportation of Meat Products with Particular Emphasis on Chilled Meat Shipments presented by John Bowater at the Intermodal Conference 1996 John Bowater is the founding member of
The Preparation and Transportation of Meat Products with Particular Emphasis on Chilled Meat Shipments presented by John Bowater at the Intermodal Conference 1996 John Bowater is the founding member of
Trotz sorgfältiger inhaltlicher Prüfung übernehmen wir keine Haftung für die Richtigkeit und Aktualität für Unterlagen von Dritten.
 Trotz sorgfältiger inhaltlicher Prüfung übernehmen wir keine Haftung für die Richtigkeit und Aktualität für Unterlagen von Dritten. IFS HPC Issue 2 Joachim Mehnert Technical Director DQS CFS GmbH 23. February
Trotz sorgfältiger inhaltlicher Prüfung übernehmen wir keine Haftung für die Richtigkeit und Aktualität für Unterlagen von Dritten. IFS HPC Issue 2 Joachim Mehnert Technical Director DQS CFS GmbH 23. February
PART III - EIV PROCESSING OF AMBIENT STABLE PRODUCTS
 GFSI BENCHMARKING REQUIREMENTS GFSI Guidance Document Version 7 PART III - EIV PROCESSING OF AMBIENT STABLE PRODUCTS PART III - EIV PROCESSING OF AMBIENT STABLE PRODUCTS Scheme Scope and Key Elements This
GFSI BENCHMARKING REQUIREMENTS GFSI Guidance Document Version 7 PART III - EIV PROCESSING OF AMBIENT STABLE PRODUCTS PART III - EIV PROCESSING OF AMBIENT STABLE PRODUCTS Scheme Scope and Key Elements This
Microbiological Specifications for Foods: Developing Specifications and Testing Options for Out of Specification Results
 Microbiological Specifications for Foods: Developing Specifications and Testing Options for Out of Specification Results Loralyn Ledenbach WAFP Meeting June 13, 2017 Setting micro specs for foods should
Microbiological Specifications for Foods: Developing Specifications and Testing Options for Out of Specification Results Loralyn Ledenbach WAFP Meeting June 13, 2017 Setting micro specs for foods should
ISO 22000:2005 Standard INTERNATIONAL STANDARDS REGISTRATIONS
 ISO 22000:2005 Standard Food Safety Management System INTERNATIONAL STANDARDS REGISTRATIONS 3.1 FOOD SAFETY concept that food will not cause harm to the consumer when it is prepared and/or eaten according
ISO 22000:2005 Standard Food Safety Management System INTERNATIONAL STANDARDS REGISTRATIONS 3.1 FOOD SAFETY concept that food will not cause harm to the consumer when it is prepared and/or eaten according
Guidance Document for Sampling and Lotting of Beef Products and Sample Analysis for Pathogens Developed by the Beef Industry Food Safety Council
 Guidance Document for Sampling and Lotting of Beef Products and Sample Analysis for Pathogens Developed by the Beef Industry Food Safety Council Developed by members of the Beef Industry Food Safety Council
Guidance Document for Sampling and Lotting of Beef Products and Sample Analysis for Pathogens Developed by the Beef Industry Food Safety Council Developed by members of the Beef Industry Food Safety Council
Controlling Listeria What you need to know about USDA s new guidance
 Controlling Listeria What you need to know about USDA s new guidance November 27, 2012 Webinar www.nichemeatprocessing.org CONTROLLING LISTERIA MONOCYTOGENES IN POST-LETHALITY EXPOSED RTE MEAT AND POULTRY
Controlling Listeria What you need to know about USDA s new guidance November 27, 2012 Webinar www.nichemeatprocessing.org CONTROLLING LISTERIA MONOCYTOGENES IN POST-LETHALITY EXPOSED RTE MEAT AND POULTRY
Australian Packaging Covenant Action Plan 1 February February 2015
 ABN: 32 978 420 802 Site Address: 22-26 Buckland Street, Clayton, Vic, 3168 Mailing Address: PO BOX 1096, Clayton South, 3168 Phone No: 03 9541 1500 Australian Packaging Covenant Action Plan 1 February
ABN: 32 978 420 802 Site Address: 22-26 Buckland Street, Clayton, Vic, 3168 Mailing Address: PO BOX 1096, Clayton South, 3168 Phone No: 03 9541 1500 Australian Packaging Covenant Action Plan 1 February
á62ñ MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS
 USP 40 Microbiological Tests / á62ñ Microbiological Examination 1 á62ñ MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS INTRODUCTION The tests described hereafter
USP 40 Microbiological Tests / á62ñ Microbiological Examination 1 á62ñ MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS INTRODUCTION The tests described hereafter
Dried meat Specification
 DRAFT KENYA STANDARD DKS 2723: 2016 ICS 67.120 Dried meat Specification PUBLIC REVIEW DRAFT, MARCH 2017 No copying of this standard without KEBS permission except as permitted by copyright law KEBS 2016
DRAFT KENYA STANDARD DKS 2723: 2016 ICS 67.120 Dried meat Specification PUBLIC REVIEW DRAFT, MARCH 2017 No copying of this standard without KEBS permission except as permitted by copyright law KEBS 2016
Sampling of Cartoned Meat and Preparation for Chemical Lean Determination
 Sampling of Cartoned Meat and Preparation for Chemical Lean Determination To accurately estimate the chemical lean (CL) of a production run of boneless meat from samples taken from the production, it is
Sampling of Cartoned Meat and Preparation for Chemical Lean Determination To accurately estimate the chemical lean (CL) of a production run of boneless meat from samples taken from the production, it is
PRINCIPLES AND GUIDELINES FOR THE CONDUCT OF MICROBIOLOGICAL RISK ASSESSMENT CAC/GL Adopted Amendments 2012, 2014.
 PRINCIPLES AND GUIDELINES FOR THE CONDUCT OF MICROBIOLOGICAL RISK ASSESSMENT CAC/GL 30-1999 Adopted 1999. Amendments 2012, 2014. CAC/GL 30-1999 2 1. INTRODUCTION 2. SCOPE 3. DEFINITIONS 4. GENERAL PRINCIPLES
PRINCIPLES AND GUIDELINES FOR THE CONDUCT OF MICROBIOLOGICAL RISK ASSESSMENT CAC/GL 30-1999 Adopted 1999. Amendments 2012, 2014. CAC/GL 30-1999 2 1. INTRODUCTION 2. SCOPE 3. DEFINITIONS 4. GENERAL PRINCIPLES
Introduction. Seafood HACCP Alliance Training Course 3-1
 Seafood HACCP Alliance Training Course 3-1 Introduction In Module 1 you were introduced to the HACCP concept. In Module 2 you learned about the potential food safety hazards that could be associated with
Seafood HACCP Alliance Training Course 3-1 Introduction In Module 1 you were introduced to the HACCP concept. In Module 2 you learned about the potential food safety hazards that could be associated with
WHY DO THEY PUT MINT IN TOOTHPASTE? WOULD GARLIC BE BETTER?
 Activity 4.22 Student Sheet WHY DO THEY PUT MINT IN TOOTHPASTE? WOULD GARLIC BE BETTER? Purpose To investigate the antibacterial properties of plants. To develop practical skills. YOU NEED Agar plate seeded
Activity 4.22 Student Sheet WHY DO THEY PUT MINT IN TOOTHPASTE? WOULD GARLIC BE BETTER? Purpose To investigate the antibacterial properties of plants. To develop practical skills. YOU NEED Agar plate seeded
QUALITY MANAGEMENT SYSTEM Guidelines & Checklist
 QUALITY MANAGEMENT SYSTEM Guidelines & Checklist 4 th Edition 2017 DISCLAIMER The information provided in this document is intended as guidance only and should not be taken as definitive or exhaustive.
QUALITY MANAGEMENT SYSTEM Guidelines & Checklist 4 th Edition 2017 DISCLAIMER The information provided in this document is intended as guidance only and should not be taken as definitive or exhaustive.
Pack House Management
 U N I T 9 Pack House Management LEARNING / FACILITATING M A T E R I A L S PINEAPPLE PRODUCTION NATIONAL CERTIFICATE I Introduction Welcome to the start of your career in pineapple pack house management.
U N I T 9 Pack House Management LEARNING / FACILITATING M A T E R I A L S PINEAPPLE PRODUCTION NATIONAL CERTIFICATE I Introduction Welcome to the start of your career in pineapple pack house management.
General European OMCL Network (GEON) QUALITY MANAGEMENT DOCUMENT
 General European OMCL Network (GEON) QUALITY MANAGEMENT DOCUMENT PA/PH/OMCL (16) 86 R2 MANAGEMENT OF ENVIRONMENTAL CONDITIONS Full document title and reference Document type Management of Environmental
General European OMCL Network (GEON) QUALITY MANAGEMENT DOCUMENT PA/PH/OMCL (16) 86 R2 MANAGEMENT OF ENVIRONMENTAL CONDITIONS Full document title and reference Document type Management of Environmental
PIC/S GUIDE TO GOOD PRACTICES FOR THE PREPARATION OF MEDICINAL PRODUCTS IN HEALTHCARE ESTABLISHMENTS
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PE 010-3 1 October 2008 PIC/S GUIDE TO GOOD PRACTICES FOR THE PREPARATION OF MEDICINAL PRODUCTS IN HEALTHCARE ESTABLISHMENTS
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PE 010-3 1 October 2008 PIC/S GUIDE TO GOOD PRACTICES FOR THE PREPARATION OF MEDICINAL PRODUCTS IN HEALTHCARE ESTABLISHMENTS
John G. Surak PhD Surak and Associates Clemson, SC
 FSIS POLICIES AND GUIDANCE ON STATISTICAL PROCESS CONTROL PROCEDURES IN SLAUGHTER OPERATIONS FSIS POLICIES AND GUIDANCE ON STATISTICAL PROCESS CONTROL PROCEDURES IN SLAUGHTER OPERATIONS John G. Surak PhD
FSIS POLICIES AND GUIDANCE ON STATISTICAL PROCESS CONTROL PROCEDURES IN SLAUGHTER OPERATIONS FSIS POLICIES AND GUIDANCE ON STATISTICAL PROCESS CONTROL PROCEDURES IN SLAUGHTER OPERATIONS John G. Surak PhD
Cell Growth and DNA Extraction- Technion igem HS
 Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
INSTRUCTIONS FOR FOOD SERVICE FACILITY CHANGE OF OWNERSHIP WITH NO CHANGES TO OPERATION, EQUIPMENT, OR FACILITY
 INSTRUCTIONS FOR FOOD SERVICE FACILITY CHANGE OF OWNERSHIP WITH NO CHANGES TO OPERATION, EQUIPMENT, OR FACILITY Dear Prospective or New Food Service Facility Owner: Pursuant to Code of Maryland Regulation
INSTRUCTIONS FOR FOOD SERVICE FACILITY CHANGE OF OWNERSHIP WITH NO CHANGES TO OPERATION, EQUIPMENT, OR FACILITY Dear Prospective or New Food Service Facility Owner: Pursuant to Code of Maryland Regulation
UNIT TITLE: ORGANISE AND PREPARE FOOD PRODUCTS AND SERVICES NOMINAL HOURS: 35
 UNIT TITLE: ORGANISE AND PREPARE FOOD PRODUCTS AND SERVICES NOMINAL HOURS: 35 UNIT NUMBER: D1.HRS.CL1.10 UNIT DESCRIPTOR: This unit deals with skills and knowledge required to undertake mise-en-place duties
UNIT TITLE: ORGANISE AND PREPARE FOOD PRODUCTS AND SERVICES NOMINAL HOURS: 35 UNIT NUMBER: D1.HRS.CL1.10 UNIT DESCRIPTOR: This unit deals with skills and knowledge required to undertake mise-en-place duties
Standardisation of meat raw material for
 Standardisation of meat raw material for industrial production of food from farmers. directly. 2 Always at a high level with standardised meat Standardised meat is the industrially produced raw material
Standardisation of meat raw material for industrial production of food from farmers. directly. 2 Always at a high level with standardised meat Standardised meat is the industrially produced raw material
How Do I Validate Process Control Measures? Assuring the Credibility of a Pathogen Reduction Strategy. Nancy Rogers Bontempo Mondelēz International
 How Do I Validate Process Control Measures? Assuring the Credibility of a Pathogen Reduction Strategy Nancy Rogers Bontempo Mondelēz International Validation of Process Control Measures The Purpose, and
How Do I Validate Process Control Measures? Assuring the Credibility of a Pathogen Reduction Strategy Nancy Rogers Bontempo Mondelēz International Validation of Process Control Measures The Purpose, and
Establishment Name Date Establishment Address Establishment Number Establishment City, State, Zip Code. Date: Approved by:
 Receiving Carcass / Meat / Poultry From In House contamination and drug residues C, P & B: Product is being received from a HACCP approved plant C: Product Inspection P: Presence of foreign material SOP
Receiving Carcass / Meat / Poultry From In House contamination and drug residues C, P & B: Product is being received from a HACCP approved plant C: Product Inspection P: Presence of foreign material SOP
High Pressure Pasteurization of meat products
 High Pressure Pasteurization of meat products Ahmed Yousef Professor of Food Microbiology The Ohio State University Reciprocal Meat Conference Columbia Missouri June 18, 2003 Novel Processing Technologies
High Pressure Pasteurization of meat products Ahmed Yousef Professor of Food Microbiology The Ohio State University Reciprocal Meat Conference Columbia Missouri June 18, 2003 Novel Processing Technologies
The Control of Microbial Growth
 The Control of Microbial Growth Sepsis refers to microbial contamination. Asepsis is the absence of significant contamination. Aseptic surgery techniques prevent microbial contamination of wounds. Terminology
The Control of Microbial Growth Sepsis refers to microbial contamination. Asepsis is the absence of significant contamination. Aseptic surgery techniques prevent microbial contamination of wounds. Terminology
Discussion Paper: Potential Changes to Provincial Maple Requirements under the Food Safety and Quality Act, 2001
 Discussion Paper: Potential Changes to Provincial Maple Requirements under the Food Safety and Quality Act, 2001 Introduction The maple industry in Ontario is a vital part of the province s heritage and
Discussion Paper: Potential Changes to Provincial Maple Requirements under the Food Safety and Quality Act, 2001 Introduction The maple industry in Ontario is a vital part of the province s heritage and
STANDARD FOR LIVE AND RAW BIVALVE MOLLUSCS CODEX STAN Adopted in Amendment: Revision: 2014 and 2015.
 STANDARD FOR LIVE AND RAW BIVALVE MOLLUSCS CODEX STAN 292-2008 Adopted in 2008. Amendment: 2013. Revision: 2014 and 2015. CODEX STAN 292-2008 2 1. SCOPE This Standard applies to live bivalve molluscs and
STANDARD FOR LIVE AND RAW BIVALVE MOLLUSCS CODEX STAN 292-2008 Adopted in 2008. Amendment: 2013. Revision: 2014 and 2015. CODEX STAN 292-2008 2 1. SCOPE This Standard applies to live bivalve molluscs and
High Pressure Processing
 High Pressure Processing Manufacturing Costs and Facility Design Considerations for Commercial HPP Manufacturing May y6, 2010 0 Introduction Mike Billig VP Marketing, Gridpath Solutions Inc. Gridpath Solutions
High Pressure Processing Manufacturing Costs and Facility Design Considerations for Commercial HPP Manufacturing May y6, 2010 0 Introduction Mike Billig VP Marketing, Gridpath Solutions Inc. Gridpath Solutions
Your guide to Irinox blast chillers and shock freezers
 Your guide to Irinox blast chillers and shock freezers SKOPE PREMIUM RANGE Blast chilling, shock freezing, proofing, thawing and food preservation Multi Fresh Range Irinox technology enables you to cool,
Your guide to Irinox blast chillers and shock freezers SKOPE PREMIUM RANGE Blast chilling, shock freezing, proofing, thawing and food preservation Multi Fresh Range Irinox technology enables you to cool,
Microbial testing. The issue. Why is this important? Things to consider. What to test for
 Microbial testing The issue Microbiological testing of melons is a useful tool to check for contamination of fruit and to test the effectiveness of management practices used to prevent, reduce or eliminate
Microbial testing The issue Microbiological testing of melons is a useful tool to check for contamination of fruit and to test the effectiveness of management practices used to prevent, reduce or eliminate
Food safety can be measured. Check limit values, ensure quality, comply with HACCP with measuring instruments from Testo.
 Food safety can be measured. Check limit values, ensure quality, comply with HACCP with measuring instruments from Testo. Measuring solutions for the food sector Precious goods deserve the greatest care
Food safety can be measured. Check limit values, ensure quality, comply with HACCP with measuring instruments from Testo. Measuring solutions for the food sector Precious goods deserve the greatest care
Listeria Management Plans
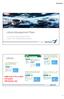 Listeria Management Plans Dr. John Holah, Technical Director, Holchem Honorary Prof., Cardiff Metropolitan University Listeria 2206 cases in Eu in 2015 270 deaths 18% fatality rate 1 Listeria Management
Listeria Management Plans Dr. John Holah, Technical Director, Holchem Honorary Prof., Cardiff Metropolitan University Listeria 2206 cases in Eu in 2015 270 deaths 18% fatality rate 1 Listeria Management
WHO GUIDELINE Stability testing of active pharmaceutical ingredients and finished pharmaceutical products
 WHO GUIDELINE Stability testing of active pharmaceutical ingredients and finished pharmaceutical products 1. Introduction 1.1 Objectives of these guidelines 1.2 Scope of these guidelines 1.3 General principles
WHO GUIDELINE Stability testing of active pharmaceutical ingredients and finished pharmaceutical products 1. Introduction 1.1 Objectives of these guidelines 1.2 Scope of these guidelines 1.3 General principles
Good Agricultural Practices (GAPs)
 Good Agricultural Practices (GAPs) Slide 1: Cover slide Notes to instructor: Welcome participants to this training session. If this session is part of a larger workshop, tell the participants in this next
Good Agricultural Practices (GAPs) Slide 1: Cover slide Notes to instructor: Welcome participants to this training session. If this session is part of a larger workshop, tell the participants in this next
Release of Fish or Fish Product Detained or Recalled for Marine Biotoxin Reasons Guide
 Release of Fish or Fish Product Detained or Recalled for Marine Biotoxin Reasons Guide January 2012 Disclaimer Every effort has been made to ensure the information in this report is accurate. MAF does
Release of Fish or Fish Product Detained or Recalled for Marine Biotoxin Reasons Guide January 2012 Disclaimer Every effort has been made to ensure the information in this report is accurate. MAF does
China s Food Safety System in The Year of The Rooster
 China s Food Safety System in The Year of The Rooster Zhinong Yan, Ph. D. Director, Food Safety, AP 5-25-2017 Agenda I. Food Safety System and Law II. III. IV. Food Safety Standards/Risk Assessment Requirement
China s Food Safety System in The Year of The Rooster Zhinong Yan, Ph. D. Director, Food Safety, AP 5-25-2017 Agenda I. Food Safety System and Law II. III. IV. Food Safety Standards/Risk Assessment Requirement
The IFSQN FSSC Implementation Package
 This is our premiere package for Food Manufacturers looking to achieve certification to FSSC 22000 for Food Safety Management Systems. Food Safety System Certification (FSSC) 22000 is a Global Food Safety
This is our premiere package for Food Manufacturers looking to achieve certification to FSSC 22000 for Food Safety Management Systems. Food Safety System Certification (FSSC) 22000 is a Global Food Safety
FSC36 SAFE FEED/SAFE FOOD GUIDANCE DOCUMENT
 FSC36 SAFE FEED/SAFE FOOD GUIDANCE DOCUMENT FSC36 Safe Feed/Safe Food (www.safefeedsafefood.org) is a facility certification program for the American Feed Industry Association (www.afia.org) Version 7.0
FSC36 SAFE FEED/SAFE FOOD GUIDANCE DOCUMENT FSC36 Safe Feed/Safe Food (www.safefeedsafefood.org) is a facility certification program for the American Feed Industry Association (www.afia.org) Version 7.0
ADVANCED.fst FOOD SAFETY TRAINING IN CANADA PRETEST - ANSWERS
 ADVANCED.fst FOOD SAFETY TRAINING IN CANADA PRETEST - ANSWERS PART 1 THE CHALLENGE TO FOOD SAFETY 1. What is the difference between clean and sanitary? Clean means free of visible soil, food residue and
ADVANCED.fst FOOD SAFETY TRAINING IN CANADA PRETEST - ANSWERS PART 1 THE CHALLENGE TO FOOD SAFETY 1. What is the difference between clean and sanitary? Clean means free of visible soil, food residue and
Risk Management Programme Manual. for Animal Product Processing
 Risk Management Programme Manual October 2009 Page 2 Prelims Prelims October 2009 Table of Contents Prelims... 2 1 Overview of RMP Manual... 7 1.1 RMP Manual 2009... 7 1.2 The New Zealand Food Safety Authority
Risk Management Programme Manual October 2009 Page 2 Prelims Prelims October 2009 Table of Contents Prelims... 2 1 Overview of RMP Manual... 7 1.1 RMP Manual 2009... 7 1.2 The New Zealand Food Safety Authority
Code of Practice. Claim Validation
 Code of Practice Claim Validation Amendments from V1 - V2 1. Introduction & Objective 2. Validation Amended and new points added 3. Claim Integrity New points added 4. Claim Approval Removed 5. Product
Code of Practice Claim Validation Amendments from V1 - V2 1. Introduction & Objective 2. Validation Amended and new points added 3. Claim Integrity New points added 4. Claim Approval Removed 5. Product
Summary of Changes, SQF Code, 7.2. Summary of Change
 Summary of Changes, SQF Code, 7.2 Section/ Page in 7.1 Entire document Summary of Change Clarify the use of the term exempt/exempted 1 Change Change, where appropriate, the term exclude / exclusion and
Summary of Changes, SQF Code, 7.2 Section/ Page in 7.1 Entire document Summary of Change Clarify the use of the term exempt/exempted 1 Change Change, where appropriate, the term exclude / exclusion and
Quality Assurance in Manufacturing Rendered Products
 Quality Assurance in Manufacturing Rendered Products A suppliers perspective! January 29, 2016 Creating sustainable food, feed and fuel ingredients for a growing population Ansen Pond, Ph.D. Product Safety
Quality Assurance in Manufacturing Rendered Products A suppliers perspective! January 29, 2016 Creating sustainable food, feed and fuel ingredients for a growing population Ansen Pond, Ph.D. Product Safety
