P hotoacoustic (PA) tomography is an emerging technology that can produce images of living objects at
|
|
|
- Ilene Richardson
- 6 years ago
- Views:
Transcription
1 OPEN SUBJECT AREAS: OPTICAL IMAGING FLUORESCENT PROTEINS Received 16 September 2013 Accepted 15 January 2014 Published 3 February 2014 Correspondence and requests for materials should be addressed to L.V.W. (lhwang@ biomed.wustl.edu) or V.V.V. (vladislav. verkhusha@einstein. yu.edu) * These authors contributed equally to this work. Multicontrast photoacoustic in vivo imaging using near-infrared fluorescent proteins Arie Krumholz 1 *, Daria M. Shcherbakova 2 *, Jun Xia 1, Lihong V. Wang 1 & Vladislav V. Verkhusha 2 1 Department of Biomedical Engineering, Optical Imaging Laboratory, Washington University in St. Louis, St. Louis, MO 63130, USA, 2 Department of Anatomy and Structural Biology, and Gruss-Lipper Biophotonics Center, Albert Einstein College of Medicine, Bronx, NY 10461, USA. Non-invasive imaging of biological processes in vivo is invaluable in advancing biology. Photoacoustic tomography is a scalable imaging technique that provides higher resolution at greater depths in tissue than achievable by purely optical methods. Here we report the application of two spectrally distinct near-infrared fluorescent proteins, irfp670 and irfp720, engineered from bacterial phytochromes, as photoacoustic contrast agents. irfps provide tissue-specific contrast without the need for delivery of any additional substances. Compared to conventional GFP-like red-shifted fluorescent proteins, irfp670 and irfp720 demonstrate stronger photoacoustic signals at longer wavelengths, and can be spectrally resolved from each other and hemoglobin. We simultaneously visualized two differently labeled tumors, one with irfp670 and the other with irfp720, as well as blood vessels. We acquired images of a mouse as 2D sections of a whole animal, and as localized 3D volumetric images with high contrast and sub-millimeter resolution at depths up to 8 mm. Our results suggest irfps are genetically-encoded probes of choice for simultaneous photoacoustic imaging of several tissues or processes in vivo. P hotoacoustic (PA) tomography is an emerging technology that can produce images of living objects at multiple scales, ranging from organelles to organs 1. In contrast to purely optical methods 2, such as fluorescence imaging and bioluminescence imaging, photoacoustic tomography (PAT) can achieve good spatial resolution at depths of more than 1 mm inside scattering biological tissue 1,3. Based on the photoacoustic effect, PAT detects thermoelastically induced ultrasonic waves as a result of light absorption by biomolecules. Ultrasonic waves are scattered much less than photons in tissue. This enables PAT to be used for high resolution imaging deep in tissue. In PAT, the spatial resolution scales with the imaging depth depending on the application, while still maintaining a high depth-to-resolution ratio 4. Various endogenous and exogenous absorbers can serve as PAT contrast agents, enabling the visualization of vasculature, blood oxygenation levels, biomarkers and gene expression 3. The primary endogenous absorbers are DNA/RNA, hemoglobin, melanin, water, and lipid. Exogenous contrast agents including organic dyes, nanoparticles, and reporter genes, extend the applications of PAT to molecular imaging. The application of genetically encoded contrast agents in PAT is a powerful approach for in vivo deep-tissue monitoring of specific genetically defined cell populations with good spatial resolution 5. Compared with dyes and nanoparticles, genetically encoded probes have several advantages. First, the labeling is specific to the transfected cell population. Second, reporter genes avoid some difficulties associated with targeted delivery, particularly nonspecific accumulation and clearance from the body. Strong light absorbing fluorescent proteins (FPs) 6,7 with low and moderate quantum yields provide excellent contrast for PAT 3. EGFP, DsRed, and mcherry have been applied to imaging of small transparent organisms such as zebrafish and fruit fly pupa 8. For deep-tissue PAT, probes absorbing in the near-infrared (NIR) spectral range are desirable. In the NIR optical window of nm, absorption by hemoglobin and water is low, making probes absorbing within this range particularly useful 9. Although several far-red GFP-like FPs such as mneptune 10, E2-Crimson 11, eqfp670 12, TagRFP and TagRFP have been available, and their utility for in vivo imaging was demonstrated, their absorption was not in the NIR optical window 7. Bacterial phytochromes are promising templates to engineer NIR FPs, NIR reporters 15 and NIR optogenetic tools 16. One NIR FP of this type, called irfp713 (aka irfp) 17, was visualized by using PAT in mice 18. irfp713 was also used as a contrast agent for non-invasive detection of in vivo circulating tumor cells by a combined PA and fluorescence flow cytometry approach 19. SCIENTIFIC REPORTS 4 : 3939 DOI: /srep
2 Imaging multiple contrast agents in PAT can provide essential information about complex physiological and pathogenic processes in living organisms, such as the interaction of a tumor with its microenvironment. At least two different NIR genetically encoded probes are required for imaging more than one specifically defined cell population inside living mammals. Until now, spectrally distinct NIR FPs designed for this purpose have not been available. Additionally, the simultaneous imaging of multiple genetically encoded contrast agents has never before been demonstrated with PAT. Recently developed irfp670 (absorption/emission maxima at 645 nm/670 nm) 20 and irfp720 (absorption/emission maxima at 703 nm/720 nm) 20 FPs, engineered on the basis of two different bacterial phytochromes, are good candidates for multicontrast PAT. These proteins have high affinity for and incorporate endogenous biliverdin, which is abundant in mammalian tissues, as a chromophore. As they do not require any exogenous supplements to be added, these FPs can be used as easily as conventional GFP-like FPs. Expression of these proteins and closely related irfp713 in different types of mammalian cells and tissues, such as various human cells, rat cells, mouse liver and mouse spleen, have been reported 10,13. Both irfp670 and irfp720 proteins have high extinction coefficients and relatively low fluorescence quantum yields (Table 1), which are favorable properties of PA contrast agents, as PA signals are proportional to the product of the molar extinction coefficient and the nonradiative quantum yield 3. Because the two irfps are spectrally distinct and both absorb in the NIR optical window (Figure 1a), we can separate the contribution to the signal of each irfp from that of each other and of hemoglobin. Results Characterization of irfp670 and irfp720 as probes for multicontrast PAT. To verify that irfp670 and irfp720 are indeed the best available candidates for simultaneous deep-tissue imaging in PAT, we compared PA signals of purified irfp670, irfp720, irfp713, and other available far-red shifted fluorescent proteins including mneptune, E2-Crimson and eqfp670 (Table 1). Hemoglobin from lysed blood was used as the reference standard. The purified FPs diluted to equal concentrations were imaged using a PA computed tomography (PACT) setup 21 (Figure 1b) at four different laser wavelengths spanning the spectral region of the FPs absorption (600 nm, 645 nm, 680 nm and 715 nm). The amplitudes of the PACT signals from FPs were normalized to the signal from blood at 600 nm. The values (Figure 1c) show that all FPs engineered from bacterial phytochromes (irfp670, irfp713 and irfp720), with absorption maxima in the NIR optical window, produced signals more than 2-fold higher than blood at wavelengths above 600 nm. These protein s favorable spectra make them more suitable for simultaneous detection with hemoglobin than the other tested farred FPs. Figure 1c also demonstrates that two wavelengths, 645 nm and 715 nm, can be used to separate the signals of irfp670 and irfp720, from each other and from the blood signal. At 645 nm, the signal of irfp670 is nearly twice that of irfp720, whereas at 715 nm, the signal of irfp670 is almost negligible. This corresponds well with the spectral overlay. To estimate the contrast between proteins, we quantified the ratios of the signals of pairs of FPs at two wavelengths. The ratio between irfp670 and irfp720 signals at 645 nm and 715 nm is 11-fold, while for irfp713 and irfp720 at these wavelengths it is 4.3-fold, and for irfp670 and irfp713 at 645 nm and 680 nm it equals to 2.9-fold. These data support the choice of irfp670 and irfp720 for multicontrast PAT. From the above discussion, four wavelengths (645 nm, 715 nm, 750 nm and 800 nm) were selected for multicontrast PA imaging of irfp670 and irfp720, and hemoglobin; the first two wavelengths correspond to the absorption peaks of irfp670 and irfp nm and 800 nm were chosen due to the negligible absorption from the proteins while relatively high hemoglobin absorption was maintained. This combination enabled separate detection of two FPs, and hemoglobin-form lysed blood, not only on the surface but also at depths up to 1.9 cm of chicken breast tissue (Figure 1d). The contribution by each absorber to the signal was estimated using a least squares algorithm. In this method, the signal is modeled as a linear combination of signals from each absorber, and the weight of each absorber to the signal is determined by its molar extinction coefficient. The intrinsic advantage of the PACT s setup to penetrate deep in tissue 1, combined with the NIR-shifted absorption, allowed detection at such a depth. Due to the differences in illumination schemes between the in vitro and in vivo experiments, the noise equivalent concentration (NEC) was not calculated for this system as the sensitivity is correlated with the illumination intensity. However, the in vitro results show that despite strong attenuation of the illumination, the proteins are still separable spectrally from each other and from blood. Next we estimated the applicability of irfp670 and irfp720 in a deep PA macroscopy (deep-pamac) imaging setup 22. Compared with PACT, deep-pamac (which is an acoustic-resolution PAT implementation) images less deeply but can produce a depthresolved top-down cross-section, which can then be raster scanned to produce a volumetric image. Figure 2a is a schematic of the setup. Compared to whole-body PACT, deep-pamac has the additional advantage of allowing the stacking of tissue to simulate depth while maintaining the same geometry. To test the applicability of the irfps for multicontrast deep-pamac, we imaged protein samples of irfp670, irfp720, and whole blood, without and with a tissue overlay of 5.0 mm to simulate depth (Figure 2b). To analyze the sensitivity of the irfps detection deep in tissue, we calculated the NECs. For both proteins the NEC values at the optimal excitation wavelengths (645 nm for irfp670 and 715 nm for irfp720) were more than one order of magnitude lower than for the blood (Figure 2c). We estimated the ratio of signals between irfp670 and irfp720 at 645 nm and 715 nm to be 10-fold, close to what we observed in PACT. Also as in PACT, the proteins (32 mm of each FP) and the blood (2.3 mm concentration of hemoglobin) could be separated using the four wavelengths (Figure 2b). Application of irfp670 and irfp720 to in vivo PAT. After the in vitro experiments, we tested the performance of irfp670 and irfp720 in simultaneous deep-tissue imaging in vivo. We employed a mouse xenograft breast cancer model 23.Ascontrastagents,FPsallowfor Table 1 Optical properties of the far-red and near-infrared fluorescent proteins used in the study FPs Absorption maximum (nm) Excitation maximum (nm) Emission maximum (nm) Molar extinction coefficient (M 21 cm 21 ) Quantum yield of fluorescence Molar extinction coefficient 3 Nonradiative quantum yield (M 21 cm 21 ) mneptune , ,200 E2-Crimson , ,480 eqfp , ,800 irfp , ,120 irfp , ,460 irfp , ,240 SCIENTIFIC REPORTS 4 : 3939 DOI: /srep
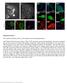
3 Figure 1 Photoacoustic properties of purified irfp670 and irfp720 proteins. (a) Overlay of the molar extinction spectra of irfp670, irfp720, oxygenated hemoglobin (HbO 2 ) and deoxygenated hemoglobin (Hb). (b) Illumination and detection scheme for PACT. (c) Photoacoustic signal amplitudes of equal amounts of purified FPs (mneptune, E2-Crimson, eqrfp670, irfp670, irfp713, irfp720) at the four indicated wavelengths. Lysed bovine blood was used as a reference standard. The values were normalized to blood signal at 600 nm. (d) PACT images of tubes containing lysed blood and equal concentrations of purified irfp670 and irfp720. Total hemoglobin (HbT) (in red), irfp670 (in green) and irfp720 (in blue) signals were detected and unmixed at different depths (0, 0.9 and 1.9 mm) of overlaid tissue. Scale bar, 1 mm. analysis of labeled cells and tissues by conventional fluorescent techniques, such as microscopy, flow cytometry, and whole-body imaging. To select for stably expressing rat adenocarcinoma MTLn3 cells for tumor implantation, preclonal mixtures were selected by fluorescence activated cell sorting (FACS). The selected cells expressing irfp670 and irfp720 were analyzed by microscopy (Figure 3a), and are distinguishable from each other by flow cytometry (Figure 3b). To demonstrate that irfp670 and irfp720 can be used simultaneously and separated from blood signal in vivo, mice were co-injected with two types of MTLn3 cells (expressing either irfp670 or irfp720) causing two tumors to grow spatially close to each other. Three weeks after the injection, the tumors were easily separated from each other by whole-body epifluorescence imaging in two filter channels, using a standard IVIS Spectrum imaging system (Figure 3c). At the same time, we imaged the mice with the two tumors by PACT. The circular-array PACT shown schematically in Figure 1b is designed to accommodate cylindrical objects, such as the mouse trunk 24. The extensive imaging depth of this setup provides 2D cross-sections of a whole mouse body with 0.1 mm in-plane resolution 18. A representative mouse with the two implanted tumors (Figure 4a) was imaged at all four wavelengths (Figure 4b). The combined image with unmixed signals overlaid (Figure 4c) shows both the irfp670 and irfp720 expressing tumors and major blood vessels in a cross-section of the mouse body (see also Supplementary Figures S1 and S2 online). Further, we applied the deep-pamac setup to the same mice to study the tumors in more detail. The deep-pamac, utilized with a 10 MHz center frequency focused transducer, can achieve good resolution (280 mm lateral, 75 mm axial) at depths up to a few millimeters and, due to the setup geometry, gain the ability to simulate depth by overlaying additional biological tissue on the sample 18,25. The results show detailed images of the tumors and surrounding vessels at depths of up to 8 mm inside the mouse body (Figure 5a). Two orthogonal maximum amplitude projection images per each of four wavelengths show different spectral dependence of the signals from the tumors and from the surrounding tissue, thus allowing spectral separation. The spatial locations of the two tumors and blood are detectable even at depths up to 5 mm under overlaid biological tissue (Figure 5b). Although the signal to noise ratio is low, the spectral differences between the absorbers allowed discriminating between regions. The wavelength dependent attenuation of the incident light can lead to inaccuracies in the quantitative values obtained; however, the spectra of the absorbers are different enough to discriminate the locations of the absorbers. This demonstrates the ability to separate image contributions of irfp670, irfp720, and hemoglobin noninvasively in one subject (combined pseudocolor images in Figure 5). The differences in a scale between the proteins could be due to variation in expression. We quantified the PA signals from tumors and blood and estimated the concentration of the irfps to be between mm. Discussion PAT is a promising technique for visualizing biological processes deep inside tissues. In this paper we demonstrated that PAT can be utilized to simultaneously and noninvasively image two differently labeled tumors and blood vessels in vivo. This is the first demonstration of SCIENTIFIC REPORTS 4 : 3939 DOI: /srep
4 Figure 2 Deep-PAMac characterization of irfp670 and irfp720 proteins in phantom. (a) Illumination and detection scheme for deep-pamac. (b) Deep-PAMac cross-sections of the tubes containing whole blood (left, in red), irfp720 (center, in blue) and irfp670 (right, in green) at the four indicated wavelengths, without (left panel) and with (right panel) the overlaid tissue of 5 mm width. Dashed lines indicate the tube boundaries. Spectrally unmixed images of blood (HbT, in red), irfp670 (in green) and irfp720 (in blue) are shown at the bottom. (c) Calculated noise equivalent concentrations (NECs) for each of the purified proteins and total hemoglobin from blood at two depths (0 and 5 mm) mimicked by the tissue overlay. Scale bar, 1 mm. PAT with two NIR genetically encoded probes and hemoglobin as the contrast agents in a living mouse. We showed that, due to the NIR shifted spectra and sufficient spectral difference from each other, irfp670 and irfp720 are more suitable probes for in vivo PAT than conventional far-red GFP-like FPs. Use of GFP-like FPs for PA imaging of mammals is hindered by high hemoglobin absorption at wavelengths below 650 nm. In addition, chromophore formation in GFP-like FPs is oxygen-dependent. Since many tumors and pathogenic tissues are hypoxic, irfp670 and irfp720 are advantageous because the formation of their chromophores does not require oxygen. Furthermore, the NIR FPs exhibit good photoacoustic properties because of their high extinction coefficients and low fluorescence quantum yields, resulting in effective thermal relaxation and strong PA signals. Additionally, irfp670 and irfp720 have shown good photostability, which allows multiple optical exposures without altering the optical properties 13. Genetically encoded irfp670 and irfp720 are produced by living cells and tissues, eliminating the need for delivery of contrast reagents Figure 3 Fluorescence imaging of MTLn3 cells in culture and in xenograft tumors. (a) Wide-field fluorescence microscopy of MTLn3 adenocarcinoma cells stably expressing irfp670 or irfp720. The filter combinations used were: 605/40 nm excitation and 640 long-pass for imaging of irfp670, and 665/ 45 nm excitation and 725/50 nm emission for irfp720. Scale bars, 10 mm. (b) Flow cytometry analysis of MTLn3 cells shown in (a). Cell fluorescence was excited with 635 nm laser and detected with a combination of two indicated emission filters. 10,000 events were analyzed for each cell type. (c) Representative fluorescence images of a living mouse with two tumors each expressing either irfp670 (top tumor) or irfp720 (bottom tumor). The images show the two channels with optimal fluorescence filters for irfp670 (excitation 640/30 nm and emission 680/20 nm) and for irfp720 (excitation 710/30 nm and emission 760/20 nm) and their overlay shown on the right. The images were obtained three weeks after cell injections. The color bars indicate the total fluorescent radiant efficiency in [p/s/cm 2 /sr]/[mw/cm 2 ]. SCIENTIFIC REPORTS 4 : 3939 DOI: /srep
5 Figure 4 PACT imaging of irfp670 and irfp720 in mouse tumor xenograft model. (a) Photograph of the mouse with irfp670 (green circle) and irfp720 (blue circle) expressing tumors growing in the mammary pads, three weeks after cell injection. The yellow line shows the approximate region of the imaged cross-section. The tumor regions are circled in blue and green. (b) PACT images of a mouse cross-section shown in (a) at four indicated wavelengths. (c) Spectrally separated image in pseudocolors (irfp670 in green, irfp720 in blue, total hemoglobin in red). The signals were normalized to the spectrally separated signal for blood. Dashed yellow and black lines show the mouse body borders. Scale bar, 1 mm. Figure 5 Deep-PAMac imaging of irfp670 and irfp720 in mouse tumor xenograft model. Deep-PAMac maximum amplitude projection images of the tumors and surrounding major blood vessels taken with no overlaid tissue (a, b) and with 5 mm overlaid tissue (c, d) three weeks after cell injection. The four left images in panels (a, c) were acquired at the four indicated wavelengths. The pseudocolored images in panels (b, d) correspond to maximum amplitude projections with orthogonal orientations. The volumetric spectrally separated signals (irfp670 in green, irfp720 in blue, total hemoglobin in red) were normalized to the spectrally separated signal for blood. Positions of the tumors are indicated by the gray arrows. SCIENTIFIC REPORTS 4 : 3939 DOI: /srep
6 such as organic dyes or nanoparticles. This property is advantageous for chronic monitoring of processes on a prolonged time scale. Moreover, the NIR FPs can be detected using conventional fluorescence modalities, which are easily accessible and provide additional information. Fluorescence in vivo imaging techniques, such as wholebody planar imaging and fluorescence molecular tomography (FMT) 26, complement PAT because they allow higher detection sensitivity, although at lower resolution than PAT. Lastly, irfp670 and irfp720 fluorescence allows additional postmortem detection of individual cells in histological sections or by using flow cytometry. We took advantage of the scalability of PAT in different implementations and visualized irfp670 and irfp720 expressing tumors and hemoglobin at two scales. First, we imaged whole-mouse 2D sections with PACT, and second, we performed localized volumetric imaging of tumors inside the mouse body with deep-pamac. The deep-pamac approach provided both spectral resolution and maximum amplitude projections in three orthogonal planes. This combination resulted in spectrally resolved volumetric images, even at a depth of 5 mm, as mimicked by the overlaid tissue. Although photoacoustic imaging of single cells expressing FPs was demonstrated in cell culture 27, such a high sensitivity has not been shown in in vivo experiments. Using the presented setup, we were able to detect tumors of a millimeter size and larger inside of a living animal. Further optimization of the system s geometry and imaging setup will allow reaching the higher sensitivity in vivo, where the background absorption from blood and the intervening tissue between the absorbing cells and the surface of the body interfere with the detection. In conclusion, multiple genetically-encoded contrast agents, such as irfp670 and irfp720 separated from hemoglobin, were imaged simultaneously deep in vivo using PAT. The future development and integration of various photoacoustic imaging approaches will advance studies of complex physiological and pathogenic processes for prolonged time periods in living animals at molecular and cellular levels. The presented technique holds great potential for both biological and preclinical studies on model organisms like mice. Although not applicable to humans, current progress in gene transfer technology 23 makes it possible to specifically label a variety of cells and organs in vivo in model animals. As compared to other optical methods, for example epifluorescence imaging, photoacoustic tomography achieves higher absorption-based contrast and visualizes more details because ultrasonic waves are scattered less than photons in tissue. Moreover, it provides additional information due to its ability to detect endogenous contrast agents, such as hemoglobin from blood. Multicontrast PAT, as the technique incorporating distinct NIR FPs demonstrated here, can be further applied to noninvasive detection of several processes including cancer growth, organ development, spread of infection, drug responses and gene activities in a living animal. Methods Protein expression and purification. Bacterial plasmids encoding mneptune, E2- Crimson and eqfp670 with polyhistidine tags were provided by B. Glick (University of Chicago, USA), M. Lin (Stanford University, USA), and K. Lukyanov (Institute of Bioorganic Chemistry, Russia). Recombinant GFP-like FPs were expressed in LMG194 bacterial cells grown in RM medium supplemented with ampicillin, kanamycin, and 0.002% arabinose and then purified using a Ni-NTA agarose (Qiagen) according to the manufacture s protocol. The irfp670, irfp713, and irfp720 with polyhistidine tags on the N-terminus were expressed in LMG194 bacterial cells co-expressing heme oxygenase grown in RM medium supplemented with ampicillin, kanamycin, 0.002% arabinose, and 0.02% rhamnose. The NIR irfps were purified using Ni-NTA agarose and then washed in a buffer where imidazole was substituted for EDTA. All purified FP solutions were diluted to equal concentrations of 32 mm. Photoacoustic studies of purified proteins. To determine the best FP for PA imaging, we constructed two phantoms consisting of approximately 40 mm long, 0.30 mm inner diameter (i.d.) tubing sections (Silastic) filled with one of the FP samples or lysed bovine blood. The tubes were then glued in parallel onto a flat gelatin block and imaged at 600 nm, 645 nm, 680 nm, and 715 nm, with approximately 90 mj incident energy for the three lower wavelengths and 30 mj incident energy at 715 nm. A 512-element ring transducer PACT system with top illumination from either an OPO laser (Vibrant 335I) or a Ti-Sapphite laser (LT-2211A) was used 21. Both lasers were needed to cover the desired wavelength range. The wavelengths were chosen to cover wavelengths near the peak excitation of each of the FPs. The signal amplitude was corrected for laser energy at each wavelength using the average power reading from a power meter for normalization, and the signal from each of the FPs was further normalized to the signal of blood at 600 nm for comparison. The depth analysis was performed on two phantoms consisting of approximately 15 mm long, 1.47 mm inner diameter tubing sections (Silastic) filled with irfp670, irfp720, or blood (lysed or whole) for comparison. The sections were then embedded in chicken breast tissue or glued onto a gelatin block and imaged using either the PACT or deep-pamac setups, with illumination provided by the OPO or Ti-Sapphire laser 28. Additional depths were simulated by stacking sections of chicken breast tissue on the sample surface. Twenty B-mode images were averaged at each depth. The noise equivalent concentration for each sample was calculated by dividing the known concentration of the absorber (32 mm for each FP and 2.3 mm for hemoglobin in the blood) by the signal-to-noise ratio in the region of the sample, and provided an estimate of the expected minimum concentration detectable by each system for this target. Mammalian plasmids and cell culture. The plasmids used for expression of irfp670 and irfp720 in mammalian cells were based on pegfp-n1 backbone (Clontech). The irfp670 and irfp720 genes were PCR-amplified as AgeI-NotI fragments and swapped with the EGFP gene in pegfp-n1 vector, thus generating the pirfp670 and pirfp720 plasmids. A rat adenocarcinoma MTLn3 cell line was grown in amem medium (Life Technologies) containing 5% fetal bovine serum, 0.5% penicillin-streptomycin, and 2 mm glutamine. Plasmid transfections were performed using an Effectene reagent (Qiagen) according to the manufacturer s protocol. Stably expressing preclonal cell mixtures were selected with 700 mg/ml G418 antibiotic. Sorting of fluorescent cells was performed using a MoFlo XDP sorter (Beckman Coulter) equipped with a 676 nm Kr laser and a 700 nm long-pass emission filter. Fluorescence imaging and flow cytometry. For fluorescence microscopy, cells were cultured in 35 mm glass bottom culture dishes with no. 1 cover glasses (MatTek). The MTLn3 cells expressing either irfp670 or irfp720 were imaged using an Olympus IX81 inverted epifluorescence microscope equipped with 200 W Me-Ha arc lamp (Lumen220Pro, Prior), a numerical aperture oil immersion objective lens (UPlanSApo, Olympus), and two filter sets (one a 605/40 nm excitation, 667/30 nm emission and the other a 682/12 nm excitation, 721/42 nm emission). All filter sets were from Chroma. SlideBook v.4.1 software (Intelligent Imaging Innovations) was used to operate Olympus IX81 inverted microscope. Unmixed channels were pseudocolored and overlaid using the SlideBook v.4.1. Discrimination between the two types of MTLn3 cells stably expressing irfp670 and irfp720 (one FP type per cell) was performed using a LSRII cytometer (BD Biosciences) equipped with a 635 nm laser and 660/20 nm and 710/20 nm emission filters. The obtained dot plots were superimposed. Flow cytometry calculations were performed using FlowJo software (Tree Star). To implant xenograph tumors, approximately 10 6 MTLn3 cells stably expressing either irfp670 or irfp720 were injected into the mammary glands of female, 5 7 weeks old, SCID/NCr mice (Taconic), and imaged 3 weeks later using the IVIS Spectrum system (PerkinElmer/Caliper) in epifluorescence mode. Belly fur was removed using a depilatory cream. Mice were fed with AIN-93M Maintenance Purified Diet (TestDiet) to reduce their intrinsic autofluorescence level. All quantitative measurements of fluorescence signals were performed using Living Image v software (PerkinElmer/Caliper). To remove bleed-through of the excitation light into the emission channel, the software encoded adaptive background subtraction was performed. Photoacoustic studies in mouse tumor xenograft model. For both PACT and deep- PAMac, approximately 10 6 MTLn3 cells stably expressing either irfp670 or irfp720 were injected into the mammary glands of four SCID/NCr mice. Three out of the four mice developed tumors which were photoacoustically detected two weeks later using PACT. For PACT, mice were imaged at the tumor region using four wavelengths, 645 nm, 715 nm, 750 nm, and 800 nm. For spectral separation, the images were then processed using a linear least-squares method for the four absorbers 8. The model used is as follows: PA~eC, where e is a matrix of the extinction coefficients of each of the absorbers at each of the wavelengths, PA contains all the measurements per pixel at each wavelength, and C is the concentration of each absorber at each pixel. In this model we assume proper compensation for the incident light; however, for deeper imaging, the incident light attenuation is wavelength dependent and can lead to quantitative errors. We solve for C using a least squares solution: e T {1e e T PA~C The result is in relative values due to the unknown attenuation of light with depth. SCIENTIFIC REPORTS 4 : 3939 DOI: /srep
7 For clear separation from blood, the wavelengths were chosen so that at least one wavelength was near the maximum absorption of each of the irfp proteins, and light at other wavelengths was weakly absorbed. For deep-pamac, a 20 mm by 14 mm area was imaged. Images were taken at the same NIR wavelengths as in the PACT experiment, with depth simulated by stacking approximately 5 mm of chicken breast tissue over the sample. The same least-squares method was performed for spectral separation at both depths. To assess the irfp670 and irfp720 concentrations, we assumed the average total hemoglobin concentration to be similar through the depths imaged, and we normalized the calculated concentrations of irfp670 and irfp720 to the average total hemoglobin concentration at each depth. As noted previously, wavelength dependent attenuation of the incident light can lead to inaccuracies in the estimation of the concentration. Assuming that the illumination is similar per depth for the nearby blood vessels, and that the total hemoglobin concentration is 2.3 mm, we calibrate each of the proteins signal to the total hemoglobin signal at each depth. Consequently, the maximum irfp670 and irfp720 concentrations were estimated to be mm. All animal experiments were performed in AAALAC approved facilities using protocols approved either by the Albert Einstein College of Medicine Animal Usage Committee (fluorescence imaging) or by the Washington University in St. Louis Animal Use Committee (photoacoustic imaging). 1. Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, (2012). 2. Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, (2010). 3. Kim, C., Favazza, C. & Wang, L. V. In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chem Rev 110, (2010). 4. Cai, X. et al. Multi-scale molecular photoacoustic tomography of gene expression. PLoS One 7, e43999 (2012). 5. Li, L., Zemp, R. J., Lungu, G., Stoica, G. & Wang, L. V. Photoacoustic imaging of lacz gene expression in vivo. J Biomed Opt 12, (2007). 6. Piatkevich, K. D. & Verkhusha, V. V. Advances in engineering of fluorescent proteins and photoactivatable proteins with red emission. Current opinion in chemical biology 14, (2010). 7. Shcherbakova, D. M., Subach, O. M. & Verkhusha, V. V. Red fluorescent proteins: advanced imaging applications and future design. Angew Chem Int Ed Engl 51, (2012). 8. Razansky, D. et al. Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo. Nat Photon 3, (2009). 9. Weissleder, R. A clearer vision for in vivo imaging. Nat Biotechnol 19, (2001). 10. Lin, M. Z. et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chemistry & biology 16, (2009). 11. Strack, R. L. et al. A rapidly maturing far-red derivative of DsRed-Express2 for whole-cell labeling. Biochemistry 48, (2009). 12. Shcherbo, D. et al. Near-infrared fluorescent proteins. Nat Methods 7, (2010). 13. Morozova, K. S. et al. Far-red fluorescent protein excitable with red lasers for flow cytometry and superresolution STED nanoscopy. Biophysical journal 99, L13 15 (2010). 14. Piatkevich, K. D. et al. Extended Stokes shift in fluorescent proteins: chromophore-protein interactions in a near-infrared TagRFP675 variant. Scientific reports 3, 1847 (2013). 15. Filonov, G. S. & Verkhusha, V. V. A near-infrared BiFC reporter for in vivo imaging of protein-protein interactions. Chemistry & biology 20, (2013). 16. Piatkevich, K. D., Subach, F. V. & Verkhusha, V. V. Engineering of bacterial phytochromes for near-infrared imaging, sensing, and light-control in mammals. Chem Soc Rev 42, (2013). 17. Filonov, G. S. et al. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat Biotechnol 29, (2011). 18. Filonov, G. S. et al. Deep-tissue photoacoustic tomography of a genetically encoded near-infrared fluorescent probe. Angew Chem Int Ed Engl 51, (2012). 19. Nedosekin, D. A. et al. Synergy of photoacoustic and fluorescence flow cytometry of circulating cells with negative and positive contrasts. J Biophotonics 6, (2013). 20. Shcherbakova, D. M. & Verkhusha, V. V. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nat Methods 10, (2013). 21. Gamelin, J. et al. A real-time photoacoustic tomography system for small animals. Opt Express 17, (2009). 22. Song, K. H. & Wang, L. V. Deep reflection-mode photoacoustic imaging of biological tissue. J Biomed Opt 12, (2007). 23. Kedrin, D. et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods 5, (2008). 24. Xu, Y., Wang, L. V., Ambartsoumian, G. & Kuchment, P. Reconstructions in limited-view thermoacoustic tomography. Med Phys 31, (2004). 25. Zhang, H. F., Maslov, K., Stoica, G. & Wang, L. V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat Biotechnol 24, (2006). 26. Deliolanis, N. C. et al. In vivo tomographic imaging of red-shifted fluorescent proteins. Biomed Opt Express 2, (2011). 27. Galanzha, E. I. et al. Photoacoustic and photothermal cytometry using photoswitchable proteins and nanoparticles with ultrasharp resonances. J Biophotonics 7, in press (2014). doi: /jbio Maslov, K., Zhang, H. F., Hu, S. & Wang, L. V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt Lett 33, (2008). Acknowledgments We thank M. Lin (Stanford University), B. Glick (University of Chicago) and K. Lukyanov (Institute of Bioorganic Chemistry, Russia) for the plasmids encoding the mneptune, E2-Crimson and eqfp670 proteins, respectively. We thank L. Luecking for assistance with maintaining cell cultures and J. Ballard for help with editing the manuscript. This work was supported by the grants GM and CA (to V.V.V.) and EB and Director s Pioneer Award EB (to L.V.W.) from the National Institutes of Health. Author contributions D.M.S. prepared irfp670 and irfp720 expressing cells, characterized irfp670 and irfp720 expression by means of fluorescent imaging. A.K., J.X. and L.V.W. designed and conducted photoacoustic experiments to image irfp670 and irfp720. V.V.V. designed and coordinated the project, and together with D.M.S. and A.K. wrote the manuscript. Additional information Supplementary information accompanies this paper at scientificreports Competing financial interests: L.V.W. has a financial interest in Microphotoacoustics, Inc. and Endra, Inc., which, however, did not support this work. How to cite this article: Krumholz, A., Shcherbakova, D.M., Xia, J., Wang, L.V. & Verkhusha, V.V. Multicontrast photoacoustic in vivo imaging using near-infrared fluorescent proteins. Sci. Rep. 4, 3939; DOI: /srep03939 (2014). This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivs 3.0 Unported license. To view a copy of this license, visit SCIENTIFIC REPORTS 4 : 3939 DOI: /srep
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Genetically targeted all-optical electrophysiology with a transgenic Credependent
 Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
APPLICATION NOTE Rev. 7/2017, v4.0 Fluorescent Nanodiamonds: Bio-applications. Physical and Fluorescence Properties
 APPLICATION NOTE Rev. 7/2017, v4.0 Fluorescent Nanodiamonds: Bio-applications Fluorescent nanodiamonds (FNDs) offer a unique alternative to currently existing fluorescent biomarkers. With exceptional photo
APPLICATION NOTE Rev. 7/2017, v4.0 Fluorescent Nanodiamonds: Bio-applications Fluorescent nanodiamonds (FNDs) offer a unique alternative to currently existing fluorescent biomarkers. With exceptional photo
Qdot nanocrystal. wide range of biological investigations, Qdot nanocrystals are powerful complements
 Feature nanocrystal conjugates for flow cytometry Take the easy route to multicolor flow cytometry. With applications across a wide range of biological investigations, nanocrystals are powerful complements
Feature nanocrystal conjugates for flow cytometry Take the easy route to multicolor flow cytometry. With applications across a wide range of biological investigations, nanocrystals are powerful complements
Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging
 Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging Introduction In this application note, we will discuss the application of quantum dots in fluorescence imaging, both
Quantum Dot applications in Fluorescence Imaging for Calibration and Molecular Imaging Introduction In this application note, we will discuss the application of quantum dots in fluorescence imaging, both
Spectral Separation of Multifluorescence Labels with the LSM 510 META
 Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
Microscopy from Carl Zeiss Spectral Separation of Multifluorescence Labels with the LSM 510 META Indians living in the South American rain forest can distinguish between almost 200 hues of green in their
Toward High-speed Transcranial Photoacoustic Imaging using Compact Near-infrared Pulsed LED Illumination System
 Toward High-speed Transcranial Photoacoustic Imaging using Compact Near-infrared Pulsed LED Illumination System Jeeun Kang a, Haichong Kai Zhang a, Alexis Cheng a, Arman Rahmim a,c, Dean F. Wong a, Jin
Toward High-speed Transcranial Photoacoustic Imaging using Compact Near-infrared Pulsed LED Illumination System Jeeun Kang a, Haichong Kai Zhang a, Alexis Cheng a, Arman Rahmim a,c, Dean F. Wong a, Jin
SUPPLEMENTARY FIGURES
 SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
SYNERGISTIC STRATEGY FOR MULTICOLOR TWO-PHOTON MICROSCOPY: APPLICATION TO THE ANALYSIS OF GERMINAL CENTER REACTIONS IN VIVO ASYLKHAN RAKHYMZHAN, RUTH LEBEN, HANNA ZIMMERMANN, ROBERT GÜNTHER, PEGGY MEX,
TARGETED IMAGING. Maureen Chan and Ruwani Mahathantila
 TARGETED IMAGING Maureen Chan and Ruwani Mahathantila Overview 2 Introduction to fluorescent imaging Fluorescent agents Quantum Dots Physical properties How QDs work In Vivo QD imaging Future Video What
TARGETED IMAGING Maureen Chan and Ruwani Mahathantila Overview 2 Introduction to fluorescent imaging Fluorescent agents Quantum Dots Physical properties How QDs work In Vivo QD imaging Future Video What
Submicron-resolution Photoacoustic Microscopy of Endogenous Light-absorbing Biomolecules
 Washington University in St. Louis Washington University Open Scholarship All Theses and Dissertations (ETDs) Spring 3-26-2014 Submicron-resolution Photoacoustic Microscopy of Endogenous Light-absorbing
Washington University in St. Louis Washington University Open Scholarship All Theses and Dissertations (ETDs) Spring 3-26-2014 Submicron-resolution Photoacoustic Microscopy of Endogenous Light-absorbing
NovoCyte Flow Cytometer
 NovoCyte Flow Cytometer The Flow Cytometer for Everyone 2 Experience the NovoCyte Advantage Focus on advancing your research. Let the flow cytometer do the rest. NovoCyte Flow Cytometer High Performance
NovoCyte Flow Cytometer The Flow Cytometer for Everyone 2 Experience the NovoCyte Advantage Focus on advancing your research. Let the flow cytometer do the rest. NovoCyte Flow Cytometer High Performance
Supporting Information
 Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2017 Supporting Information Supramolecular Conjugated Polymer Materials for Organelle
Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2017 Supporting Information Supramolecular Conjugated Polymer Materials for Organelle
Green Fluorescent Protein (GFP) Purification. Hydrophobic Interaction Chromatography
 Green Fluorescent Protein (GFP) Purification Hydrophobic Interaction Chromatography What is the GFP gene? GFP is a green fluorescent protein that is normally found in jellyfish. It has been engineered
Green Fluorescent Protein (GFP) Purification Hydrophobic Interaction Chromatography What is the GFP gene? GFP is a green fluorescent protein that is normally found in jellyfish. It has been engineered
CENTER FOR BRAIN EXPERIMENT
 CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
DEPArray Technology. Sorting and Recovery of Rare Cells
 DEPArray Technology Sorting and Recovery of Rare Cells Delivering pure, single, viable cells The DEPArray system from Silicon Biosystems is the only automated instrument that can identify, quantify, and
DEPArray Technology Sorting and Recovery of Rare Cells Delivering pure, single, viable cells The DEPArray system from Silicon Biosystems is the only automated instrument that can identify, quantify, and
Monitoring and Optimizing the Lipopolysaccharides-plasmid DNA interaction by FLIM-FRET
 Transactions on Science and Technology Vol. 4, No. 3-3, 342-347, 2017 Monitoring and Optimizing the Lipopolysaccharides-plasmid DNA interaction by FLIM-FRET Nur Syahadatain Abdul Razak 1#, Clarence M.
Transactions on Science and Technology Vol. 4, No. 3-3, 342-347, 2017 Monitoring and Optimizing the Lipopolysaccharides-plasmid DNA interaction by FLIM-FRET Nur Syahadatain Abdul Razak 1#, Clarence M.
Direct visualization, sizing and concentration measurement of fluorescently labeled nanoparticles using NTA
 Direct visualization, sizing and concentration measurement of fluorescently labeled nanoparticles using NTA NANOSIGHT RANGE Visualize and Measure Nanoparticle Size and Concentration PARTICLE SIZE PARTICLE
Direct visualization, sizing and concentration measurement of fluorescently labeled nanoparticles using NTA NANOSIGHT RANGE Visualize and Measure Nanoparticle Size and Concentration PARTICLE SIZE PARTICLE
Roche Molecular Biochemicals Technical Note No. LC 10/2000
 Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Roche Molecular Biochemicals Technical Note No. LC 10/2000 LightCycler Overview of LightCycler Quantification Methods 1. General Introduction Introduction Content Definitions This Technical Note will introduce
Optoacoustic System for 3D Functional and Molecular Imaging in Nude Mice
 Optoacoustic System for 3D Functional and Molecular Imaging in Nude Mice Matthew P. Fronheiser a, Alan Stein a, Don Herzog a, Scott Thompson a, Anton Liopo b, Mohammad Eghtedari b, Massoud Motamedi b,
Optoacoustic System for 3D Functional and Molecular Imaging in Nude Mice Matthew P. Fronheiser a, Alan Stein a, Don Herzog a, Scott Thompson a, Anton Liopo b, Mohammad Eghtedari b, Massoud Motamedi b,
Flow Cytometry - The Essentials
 Flow Cytometry - The Essentials Pocket Guide to Flow Cytometry: 1. Know your Cytometer 2. Understanding Fluorescence and Fluorophores 3. Gating Process 4. Controls 5. Optimization 6. Panel Building 7.
Flow Cytometry - The Essentials Pocket Guide to Flow Cytometry: 1. Know your Cytometer 2. Understanding Fluorescence and Fluorophores 3. Gating Process 4. Controls 5. Optimization 6. Panel Building 7.
Digital ultrasonically encoded (DUE) optical focusing into random media
 Digital ultrasonically encoded (DUE) optical focusing into random media Jian Wei Tay, Puxiang Lai, Yuta Suzuki & Lihong V. Wang* Optical Imaging Laboratory, Department of Biomedical Engineering, Washington
Digital ultrasonically encoded (DUE) optical focusing into random media Jian Wei Tay, Puxiang Lai, Yuta Suzuki & Lihong V. Wang* Optical Imaging Laboratory, Department of Biomedical Engineering, Washington
including, but not limited to:
 *This Section is part of the original Request for Proposal # P08 080. The Contractor should provide the following eligible Scientific Biomedical Research Equipment, Reagents & Supplies including, but not
*This Section is part of the original Request for Proposal # P08 080. The Contractor should provide the following eligible Scientific Biomedical Research Equipment, Reagents & Supplies including, but not
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1: Vector maps of TRMPV and TRMPVIR variants. Many derivatives of TRMPV have been generated and tested. Unless otherwise noted, experiments in this paper use
Supplementary Figure 1 Supplementary Figure 1: Vector maps of TRMPV and TRMPVIR variants. Many derivatives of TRMPV have been generated and tested. Unless otherwise noted, experiments in this paper use
Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers
 Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Time-resolved Measurements Using the Agilent Cary Eclipse Fluorescence Spectrophotometer A Versatile Instrument for Accurate Measurements
 Time-resolved Measurements Using the Agilent Cary Eclipse Fluorescence Spectrophotometer A Versatile Instrument for Accurate Measurements Technical Overview Authors Dr. Fabian Zieschang, Katherine MacNamara,
Time-resolved Measurements Using the Agilent Cary Eclipse Fluorescence Spectrophotometer A Versatile Instrument for Accurate Measurements Technical Overview Authors Dr. Fabian Zieschang, Katherine MacNamara,
Photonic integrated circuits in biochemical food analysis
 Photonic integrated circuits in biochemical food analysis What is Photonics? Page 2 Photonics is the physical science of light generation, detection, and manipulation through e.g. transmission, modulation,
Photonic integrated circuits in biochemical food analysis What is Photonics? Page 2 Photonics is the physical science of light generation, detection, and manipulation through e.g. transmission, modulation,
A nucleic acid-based fluorescent sensor for expeditious detection of pyrophosphate anions at nanomolar concentrations
 Supporting Information for A nucleic acid-based fluorescent sensor for expeditious detection of pyrophosphate anions at nanomolar concentrations Xin Su, Chen Zhang, Xianjin Xiao, Anqin Xu, Zhendong Xu
Supporting Information for A nucleic acid-based fluorescent sensor for expeditious detection of pyrophosphate anions at nanomolar concentrations Xin Su, Chen Zhang, Xianjin Xiao, Anqin Xu, Zhendong Xu
A quantitative protocol for intensity-based live cell FRET imaging.
 A quantitative protocol for intensity-based live cell FRET imaging. Kaminski CF, Rees EJ, Schierle GS. Methods Mol Biol. 2014; 1076:445-454. Department of Chemical Engineering and Biotechnology, Pembroke
A quantitative protocol for intensity-based live cell FRET imaging. Kaminski CF, Rees EJ, Schierle GS. Methods Mol Biol. 2014; 1076:445-454. Department of Chemical Engineering and Biotechnology, Pembroke
Innovations To Meet Your Needs
 Innovations To Meet Your Needs Cooled CCD Camera 1340 x 1037 pixel resolution for greatest image quality 12-bit precision provides 3 orders of linear dynamic range Windows and Power Macintosh Software
Innovations To Meet Your Needs Cooled CCD Camera 1340 x 1037 pixel resolution for greatest image quality 12-bit precision provides 3 orders of linear dynamic range Windows and Power Macintosh Software
GEL IMAGING. The detection and quantitation of specific subsets of posttranslationally
 High Sensitivity Detection and Quantitation of Phosphoproteins in 1D and 2D Gels using the ProXPRESS 2D Proteomic Imager and ProFINDER 2D Image Analysis Software GEL IMAGING A P P L I C A T I O N N O T
High Sensitivity Detection and Quantitation of Phosphoproteins in 1D and 2D Gels using the ProXPRESS 2D Proteomic Imager and ProFINDER 2D Image Analysis Software GEL IMAGING A P P L I C A T I O N N O T
Improved Monitoring of P. aeruginosa on Agar Plates
 Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2015 Improved Monitoring of P. aeruginosa on Agar Plates SUPPLEMENTAL INFORMATION T. A. Webster,
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is The Royal Society of Chemistry 2015 Improved Monitoring of P. aeruginosa on Agar Plates SUPPLEMENTAL INFORMATION T. A. Webster,
PERFORMANCE MADE EASY REAL-TIME PCR
 PERFORMANCE MADE EASY REAL-TIME PCR The MyGo Pro real-time PCR instrument provides unmatched performance in a convenient format. Novel Full Spectrum Optics deliver 120 optical channels of fluorescence
PERFORMANCE MADE EASY REAL-TIME PCR The MyGo Pro real-time PCR instrument provides unmatched performance in a convenient format. Novel Full Spectrum Optics deliver 120 optical channels of fluorescence
Challenges to measuring intracellular Ca 2+ Calmodulin: nature s Ca 2+ sensor
 Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Photon Upconversion Sensitized Nanoprobes for
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Supporting Information Photon Upconversion Sensitized Nanoprobes for Sensing and Imaging of ph
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Supporting Information Photon Upconversion Sensitized Nanoprobes for Sensing and Imaging of ph
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging
 QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
QImaging Camera Application Notes Multicolor Immunofluorescence Imaging In order to image localization of intracellular proteins with high specificity, it is frequently necessary to multiplex antibody
University of Michigan
 University of Michigan Department of Mechanical Engineering Low-cost Non-invasive Diagnosis of Malaria Infected Red Blood Cells Han Yu Undergraduate Student Department of Electrical Engineering and Computer
University of Michigan Department of Mechanical Engineering Low-cost Non-invasive Diagnosis of Malaria Infected Red Blood Cells Han Yu Undergraduate Student Department of Electrical Engineering and Computer
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
nanodsf 2bind: Your service provider for biophysical characterization of proteins Precisely revealing protein folding and stability
 nanodsf Precisely revealing protein folding and stability 2bind: Your service provider for biophysical characterization of proteins This booklet was written and designed by 2bind 08 2015 Any reproduction
nanodsf Precisely revealing protein folding and stability 2bind: Your service provider for biophysical characterization of proteins This booklet was written and designed by 2bind 08 2015 Any reproduction
TECHNICAL BULLETIN. In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits
 In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits Catalog Numbers APPA001 In Vitro Bacterial Split GFP "Fold 'n' Glow" Solubility Assay Kit (Green) APPA008 In Vitro Bacterial
In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits Catalog Numbers APPA001 In Vitro Bacterial Split GFP "Fold 'n' Glow" Solubility Assay Kit (Green) APPA008 In Vitro Bacterial
Noninvasive label-free imaging of microhemodynamics by optical-resolution photoacoustic microscopy
 Noninvasive label-free imaging of microhemodynamics by optical-resolution photoacoustic microscopy Song Hu, Konstantin Maslov, and Lihong V. Wang* Optical Imaging Laboratory, Department of Biomedical Engineering,
Noninvasive label-free imaging of microhemodynamics by optical-resolution photoacoustic microscopy Song Hu, Konstantin Maslov, and Lihong V. Wang* Optical Imaging Laboratory, Department of Biomedical Engineering,
Post-expansion antibody delivery, after epitope-preserving homogenization.
 Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Supplementary Figure 1 Post-expansion antibody delivery, after epitope-preserving homogenization. (a, b) Wide-field fluorescence images of Thy1-YFP-expressing mouse brain hemisphere slice before expansion
Electron microscopy technology of reticulocytes after sorting with
 Electron microscopy technology of reticulocytes after sorting with magnetic beads The Cell Analysis Center Scientific Bulletin Part 2 For efficient analysis of cells, sorting of the target cells is crucial.
Electron microscopy technology of reticulocytes after sorting with magnetic beads The Cell Analysis Center Scientific Bulletin Part 2 For efficient analysis of cells, sorting of the target cells is crucial.
IN VIVO BLOOD OXYGENATION LEVEL MEASUREMENTS USING PHOTOACOUSTIC MICROSCOPY. A Thesis MATHANGI SIVARAMAKRISHNAN
 IN VIVO BLOOD OXYGENATION LEVEL MEASUREMENTS USING PHOTOACOUSTIC MICROSCOPY A Thesis by MATHANGI SIVARAMAKRISHNAN Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment
IN VIVO BLOOD OXYGENATION LEVEL MEASUREMENTS USING PHOTOACOUSTIC MICROSCOPY A Thesis by MATHANGI SIVARAMAKRISHNAN Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment
Plasmid DNA transfection of SW480 human colorectal cancer cells with the Biontex K2 Transfection System
 Plasmid DNA transfection of human colorectal cancer cells with the Biontex K2 Transfection System Stephanie Hehlgans and Franz Rödel, Department of Radiotherapy and Oncology, Goethe- University Frankfurt,
Plasmid DNA transfection of human colorectal cancer cells with the Biontex K2 Transfection System Stephanie Hehlgans and Franz Rödel, Department of Radiotherapy and Oncology, Goethe- University Frankfurt,
Welcome! openmicberkeley.wordpress.com. Open Berkeley
 Welcome! openmicberkeley.wordpress.com Agenda Jen Lee: Introduction to FRET Marla Feller: Using FRET sensors to look at time resolved measurements Becky Lamason: Using FRET to determine if a bacterial
Welcome! openmicberkeley.wordpress.com Agenda Jen Lee: Introduction to FRET Marla Feller: Using FRET sensors to look at time resolved measurements Becky Lamason: Using FRET to determine if a bacterial
Materials and methods. by University of Washington Yeast Resource Center) from several promoters, including
 Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
Supporting online material for Elowitz et al. report Materials and methods Strains and plasmids. Plasmids expressing CFP or YFP (wild-type codons, developed by University of Washington Yeast Resource Center)
pdsipher and pdsipher -GFP shrna Vector User s Guide
 pdsipher and pdsipher -GFP shrna Vector User s Guide NOTE: PLEASE READ THE ENTIRE PROTOCOL CAREFULLY BEFORE USE Page 1. Introduction... 1 2. Vector Overview... 1 3. Vector Maps 2 4. Materials Provided...
pdsipher and pdsipher -GFP shrna Vector User s Guide NOTE: PLEASE READ THE ENTIRE PROTOCOL CAREFULLY BEFORE USE Page 1. Introduction... 1 2. Vector Overview... 1 3. Vector Maps 2 4. Materials Provided...
Time-resolved optical spectroscopy and imaging of breast
 Contributed paper OPTO-ELECTRONICS REVIEW 12(2), 249 253 (2004) Time-resolved optical spectroscopy and imaging of breast P. TARONI *, A. PIFFERI, A. TORRICELLI, and R. CUBEDDU INFM-Dipartimento di Fisica,
Contributed paper OPTO-ELECTRONICS REVIEW 12(2), 249 253 (2004) Time-resolved optical spectroscopy and imaging of breast P. TARONI *, A. PIFFERI, A. TORRICELLI, and R. CUBEDDU INFM-Dipartimento di Fisica,
Ultra-fast photoacoustic flow cytometry with a 0.5 MHz pulse repetition rate nanosecond laser
 Ultra-fast photoacoustic flow cytometry with a 0.5 MHz pulse repetition rate nanosecond laser Dmitry A. Nedosekin, 1 Mustafa Sarimollaoglu, 1 Evgeny V. Shashkov, 1,2 Ekaterina I. Galanzha, 1 Vladimir P.
Ultra-fast photoacoustic flow cytometry with a 0.5 MHz pulse repetition rate nanosecond laser Dmitry A. Nedosekin, 1 Mustafa Sarimollaoglu, 1 Evgeny V. Shashkov, 1,2 Ekaterina I. Galanzha, 1 Vladimir P.
The analysis of fluorescence microscopy images for FRET detection
 The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
The analysis of fluorescence microscopy images for FRET detection Ela Claridge, Dale J. Powner and Michael J.O. Wakelam School of Computer Science, The University of Birmingham B5 2TT Institute for Cancer
See what s really in your sample
 See what s really in your sample Dye-free quantification of the biomolecules that you desire and the contaminants that you fear From the nucleic acid experts! Sample to Insight With QIAxpert Tell DNA from
See what s really in your sample Dye-free quantification of the biomolecules that you desire and the contaminants that you fear From the nucleic acid experts! Sample to Insight With QIAxpert Tell DNA from
Gene Expression Technology
 Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
Gene Expression Technology Bing Zhang Department of Biomedical Informatics Vanderbilt University bing.zhang@vanderbilt.edu Gene expression Gene expression is the process by which information from a gene
HHS Public Access Author manuscript Nat Methods. Author manuscript; available in PMC 2009 November 01.
 Photoconversion in orange and red fluorescent proteins Gert-Jan Kremers 1, Kristin L. Hazelwood 2, Christopher S. Murphy 2, Michael W. Davidson 2, and David W. Piston 1 1 Department of Molecular Physiology
Photoconversion in orange and red fluorescent proteins Gert-Jan Kremers 1, Kristin L. Hazelwood 2, Christopher S. Murphy 2, Michael W. Davidson 2, and David W. Piston 1 1 Department of Molecular Physiology
Melanin Fluorescence Spectra by Step-wise Three Photon Excitation
 Melanin Fluorescence Spectra by Step-wise Three Photon Excitation Zhenhua Lai a,c, Josef Kerimo a,c, Charles A. DiMario a,b,c a Electrical and Computer Engineering Department,Northeastern University, Boston,
Melanin Fluorescence Spectra by Step-wise Three Photon Excitation Zhenhua Lai a,c, Josef Kerimo a,c, Charles A. DiMario a,b,c a Electrical and Computer Engineering Department,Northeastern University, Boston,
Digital resolution enhancement in surface plasmon microscopy
 Digital resolution enhancement in surface plasmon microscopy I.I. Smolyaninov 1) *, J. Elliott 2), G. Wurtz 2), A.V. Zayats 2), C.C. Davis 1) 1) Department of Electrical and Computer Engineering, University
Digital resolution enhancement in surface plasmon microscopy I.I. Smolyaninov 1) *, J. Elliott 2), G. Wurtz 2), A.V. Zayats 2), C.C. Davis 1) 1) Department of Electrical and Computer Engineering, University
Chapter 10: Classification of Microorganisms
 Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy 8 successive taxa
Protein Sequence-Structure-Function Relationship: Testing KE-50 Modification on Recombinant Green Fluorescent Protein (AcGFP)
 The University of Akron IdeaExchange@UAkron Honors Research Projects The Dr. Gary B. and Pamela S. Williams Honors College Spring 2016 Protein Sequence-Structure-Function Relationship: Testing KE-50 Modification
The University of Akron IdeaExchange@UAkron Honors Research Projects The Dr. Gary B. and Pamela S. Williams Honors College Spring 2016 Protein Sequence-Structure-Function Relationship: Testing KE-50 Modification
A simple introduction to multiphoton microscopy
 Journal of Microscopy, Vol. 243, Pt 3 2011, pp. 221 226 Received 29 April 2011; accepted 28 June 2011 doi: 10.1111/j.1365-2818.2011.03532.x A simple introduction to multiphoton microscopy A. USTIONE &
Journal of Microscopy, Vol. 243, Pt 3 2011, pp. 221 226 Received 29 April 2011; accepted 28 June 2011 doi: 10.1111/j.1365-2818.2011.03532.x A simple introduction to multiphoton microscopy A. USTIONE &
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Confocal Microscopes. Evolution of Imaging
 Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Determining the Effect of Delivery Rate on Glucose Uptake by Cancer Cells
 University of Arkansas, Fayetteville ScholarWorks@UARK Biomedical Engineering Undergraduate Honors Theses Biomedical Engineering 5-2016 Determining the Effect of Delivery Rate on Glucose Uptake by Cancer
University of Arkansas, Fayetteville ScholarWorks@UARK Biomedical Engineering Undergraduate Honors Theses Biomedical Engineering 5-2016 Determining the Effect of Delivery Rate on Glucose Uptake by Cancer
In order to do transformation, the gene to be transferred is placed into a plasmid. This is done with the help of restriction enzymes, 7
 Fluorescent Protein Transformation Student Background Genetic transformation occurs when a cell takes up (i.e. takes inside) and expresses a new piece of genetic material DNA. Genetic transformation literally
Fluorescent Protein Transformation Student Background Genetic transformation occurs when a cell takes up (i.e. takes inside) and expresses a new piece of genetic material DNA. Genetic transformation literally
Super-resolution Microscopy
 Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
Semr oc kwhi t epaperser i es : 1. Introduction Super-resolution Microscopy Fluorescence microscopy has revolutionized the study of biological samples. Ever since the invention of fluorescence microscopy
Orflo Application Brief 3 /2017 GFP Transfection Efficiency Monitoring with Orflo s Moxi GO Next Generation Flow Cytometer. Introduction/Background
 Introduction/Background Cell transfection and transduction refer to an array of techniques used to introduce foreign genetic material, or cloning vectors, into cell genomes. The application of these methods
Introduction/Background Cell transfection and transduction refer to an array of techniques used to introduce foreign genetic material, or cloning vectors, into cell genomes. The application of these methods
An automated image processing routine for segmentation of cell cytoplasms in high-resolution autofluorescence images
 An automated image processing routine for segmentation of cell cytoplasms in high-resolution autofluorescence images Alex J. Walsh a, Melissa C. Skala *a a Department of Biomedical Engineering, Vanderbilt
An automated image processing routine for segmentation of cell cytoplasms in high-resolution autofluorescence images Alex J. Walsh a, Melissa C. Skala *a a Department of Biomedical Engineering, Vanderbilt
Introduction to Fluorescence Jablonski Diagram
 ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
TaqPath ProAmp Master Mixes
 PRODUCT BULLETIN es es Applied Biosystems TaqPath ProAmp Master Mixes are versatile master mixes developed for high-throughput genotyping and copy number variation (CNV) analysis protocols that require
PRODUCT BULLETIN es es Applied Biosystems TaqPath ProAmp Master Mixes are versatile master mixes developed for high-throughput genotyping and copy number variation (CNV) analysis protocols that require
Quantitative analysis of recombination in YFP and CFP gene of FRET biosensor induced by lentiviral or retroviral gene transfer.
 Supplementary Information: Quantitative analysis of recombination in and gene of FRET biosensor induced by lentiviral or retroviral gene transfer. Akira T. Komatsubara, Michiyuki Matsuda,, and Kazuhiro
Supplementary Information: Quantitative analysis of recombination in and gene of FRET biosensor induced by lentiviral or retroviral gene transfer. Akira T. Komatsubara, Michiyuki Matsuda,, and Kazuhiro
Cisne Enterprises, Inc. MELANOMA CANCER TEST
 Cisne Enterprises, Inc. MELANOMA CANCER TEST Efficacy Evaluation of Antitumor Activity of Alka Vita - Alkahydroxy in the LOX-GFP Human Melanoma Model Final Report by: Anti-Cancer Lab San Diego California
Cisne Enterprises, Inc. MELANOMA CANCER TEST Efficacy Evaluation of Antitumor Activity of Alka Vita - Alkahydroxy in the LOX-GFP Human Melanoma Model Final Report by: Anti-Cancer Lab San Diego California
Exam MOL3007 Functional Genomics
 Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
Supporting information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information Near Infrared Light-responsive and Injectable Supramolecular Hydrogels for
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information Near Infrared Light-responsive and Injectable Supramolecular Hydrogels for
This Document Contains:
 This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
2.5. Equipment and materials supplied by user PCR based template preparation Influence of temperature on in vitro EGFP synthesis 11
 Manual 15 Reactions LEXSY in vitro Translation Cell-free protein expression kit based on Leishmania tarentolae for PCR-based template generation Cat. No. EGE-2010-15 FOR RESEARCH USE ONLY. NOT INTENDED
Manual 15 Reactions LEXSY in vitro Translation Cell-free protein expression kit based on Leishmania tarentolae for PCR-based template generation Cat. No. EGE-2010-15 FOR RESEARCH USE ONLY. NOT INTENDED
High-throughput three-dimensional (3D) lithographic microfabrication in biomedical applications
 High-throughput three-dimensional (3D) lithographic microfabrication in biomedical applications The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story
High-throughput three-dimensional (3D) lithographic microfabrication in biomedical applications The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story
AnaTag HiLyte Fluor 647 Protein Labeling Kit
 AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
Bioinstrumentation Light Sources Lasers or LEDs?
 Bioinstrumentation Light Sources Lasers or LEDs? A comprehensive analysis of all the factors involved in designing and building life sciences instrumentation reveals that lasers provide superior performance
Bioinstrumentation Light Sources Lasers or LEDs? A comprehensive analysis of all the factors involved in designing and building life sciences instrumentation reveals that lasers provide superior performance
Active targeting of the nucleus using non-peptidic boronate tags
 Supporting Information Active targeting of the nucleus using non-peptidic boronate tags Rui Tang 1, Ming Wang 2, Moumita Ray 1, Ying Jiang 1, Ziwen Jiang 1, Qiaobing Xu 2 *, Vincent M. Rotello 1 * 1 Department
Supporting Information Active targeting of the nucleus using non-peptidic boronate tags Rui Tang 1, Ming Wang 2, Moumita Ray 1, Ying Jiang 1, Ziwen Jiang 1, Qiaobing Xu 2 *, Vincent M. Rotello 1 * 1 Department
In-Cell Western Kits I and II
 Odyssey and Aerius Infrared Imaging Systems In-Cell Western Assay Kits I and II Published November, 2006. The most recent version of this protocol is posted at http://biosupport.licor.com/protocols.jsp
Odyssey and Aerius Infrared Imaging Systems In-Cell Western Assay Kits I and II Published November, 2006. The most recent version of this protocol is posted at http://biosupport.licor.com/protocols.jsp
CHEMOMETRICAL ANALYSIS OF ENDOMETRIAL TISSUE FLUORESCENCE SPECTRA
 CHEMOMETRICAL ANALYSIS OF ENDOMETRIAL TISSUE FLUORESCENCE SPECTRA A.Vaitkuviene a *, E.Auksorius a, D. Fuchs b, V.Gavriushin a a Vilnius University, Institute of Materials Science and Applied Research,
CHEMOMETRICAL ANALYSIS OF ENDOMETRIAL TISSUE FLUORESCENCE SPECTRA A.Vaitkuviene a *, E.Auksorius a, D. Fuchs b, V.Gavriushin a a Vilnius University, Institute of Materials Science and Applied Research,
Adeno-Associated Virus titer and aggregation characterization
 Adeno-Associated Virus titer and aggregation characterization Characterization of gold-labeled Adeno-Associated Virus (AAV) and other small viruses by Nanoparticle Tracking Analysis (NTA) PARTICLE CONCENTRATION
Adeno-Associated Virus titer and aggregation characterization Characterization of gold-labeled Adeno-Associated Virus (AAV) and other small viruses by Nanoparticle Tracking Analysis (NTA) PARTICLE CONCENTRATION
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Materials and Methods Circular dichroism (CD) spectroscopy. Far ultraviolet (UV) CD spectra of apo- and holo- CaM and the CaM mutants were recorded on a Jasco J-715 spectropolarimeter
SUPPLEMENTARY MATERIAL Materials and Methods Circular dichroism (CD) spectroscopy. Far ultraviolet (UV) CD spectra of apo- and holo- CaM and the CaM mutants were recorded on a Jasco J-715 spectropolarimeter
supplementary information
 DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
DOI: 1.138/ncb1839 a b Control 1 2 3 Control 1 2 3 Fbw7 Smad3 1 2 3 4 1 2 3 4 c d IGF-1 IGF-1Rβ IGF-1Rβ-P Control / 1 2 3 4 Real-time RT-PCR Relative quantity (IGF-1/ mrna) 2 1 IGF-1 1 2 3 4 Control /
ab Hypoxic Response Human Flow Cytometry Kit
 ab126585 Hypoxic Response Human Flow Cytometry Kit Instructions for Use For measuring protein levels by flow cytometry: hypoxia-inducible factor 1-alpha (HIF1A) and BCL2/adenovirus E1B 19 kda proteininteracting
ab126585 Hypoxic Response Human Flow Cytometry Kit Instructions for Use For measuring protein levels by flow cytometry: hypoxia-inducible factor 1-alpha (HIF1A) and BCL2/adenovirus E1B 19 kda proteininteracting
Fisher (Fairlawn, NJ) and Sigma-Aldrich (St. Louis, MO) and were used without further. (Promega) and DpnI (New England Biolabs, Beverly, MA).
 175 Appendix III Chapter 4 Methods General. Unless otherwise noted, reagents were purchased from the commercial suppliers Fisher (Fairlawn, NJ) and Sigma-Aldrich (St. Louis, MO) and were used without further
175 Appendix III Chapter 4 Methods General. Unless otherwise noted, reagents were purchased from the commercial suppliers Fisher (Fairlawn, NJ) and Sigma-Aldrich (St. Louis, MO) and were used without further
Supplementary information for. An Ultrasensitive Biosensor for DNA Detection Based on. Hybridization Chain Reaction Coupled with the Efficient
 Supplementary information for An Ultrasensitive Biosensor for DNA Detection Based on Hybridization Chain Reaction Coupled with the Efficient Quenching of Ruthenium Complex to CdTe Quantum Dot Yufei Liu,
Supplementary information for An Ultrasensitive Biosensor for DNA Detection Based on Hybridization Chain Reaction Coupled with the Efficient Quenching of Ruthenium Complex to CdTe Quantum Dot Yufei Liu,
Product: Arrest-In TM Transfection Reagent for RNAi
 Product: Arrest-In TM Transfection Reagent for RNAi Catalog #: ATR1740, ATR1741, ATR1742, ATR1743 Product Description Arrest-In transfection reagent is a proprietary polymeric formulation, developed and
Product: Arrest-In TM Transfection Reagent for RNAi Catalog #: ATR1740, ATR1741, ATR1742, ATR1743 Product Description Arrest-In transfection reagent is a proprietary polymeric formulation, developed and
Lecture #1. Introduction to microarray technology
 Lecture #1 Introduction to microarray technology Outline General purpose Microarray assay concept Basic microarray experimental process cdna/two channel arrays Oligonucleotide arrays Exon arrays Comparing
Lecture #1 Introduction to microarray technology Outline General purpose Microarray assay concept Basic microarray experimental process cdna/two channel arrays Oligonucleotide arrays Exon arrays Comparing
How to perform-control immunostaining experiment - microscopist subjective point of view. Pawel Pasierbek
 How to perform-control immunostaining experiment - microscopist subjective point of view. Pawel Pasierbek Immunolabeling and fluorescent detection became such a standard procedure in the biomedical research
How to perform-control immunostaining experiment - microscopist subjective point of view. Pawel Pasierbek Immunolabeling and fluorescent detection became such a standard procedure in the biomedical research
Genetics Lecture 21 Recombinant DNA
 Genetics Lecture 21 Recombinant DNA Recombinant DNA In 1971, a paper published by Kathleen Danna and Daniel Nathans marked the beginning of the recombinant DNA era. The paper described the isolation of
Genetics Lecture 21 Recombinant DNA Recombinant DNA In 1971, a paper published by Kathleen Danna and Daniel Nathans marked the beginning of the recombinant DNA era. The paper described the isolation of
Nature Structural and Molecular Biology: doi: /nsmb.2937
 Supplementary Figure 1 Multiple sequence alignment of the CtIP N-terminal domain, purified CtIP protein constructs and details of the 2F o F c electron density map of CtIP-NTD. (a) Multiple sequence alignment,
Supplementary Figure 1 Multiple sequence alignment of the CtIP N-terminal domain, purified CtIP protein constructs and details of the 2F o F c electron density map of CtIP-NTD. (a) Multiple sequence alignment,
E N G I N E E R I N G M 1 3 BAC T E R I O P H AGE N I R - I I P L AT F O R M S F O R T U M O R I M A G I N G A P P L I C AT I O N S
 E N G I N E E R I N G M 1 3 BAC T E R I O P H AGE N I R - I I P L AT F O R M S F O R T U M O R I M A G I N G A P P L I C AT I O N S U Y A N G A T S E D E V B I O M O L E C U L A R M A T E R I A L S G R
E N G I N E E R I N G M 1 3 BAC T E R I O P H AGE N I R - I I P L AT F O R M S F O R T U M O R I M A G I N G A P P L I C AT I O N S U Y A N G A T S E D E V B I O M O L E C U L A R M A T E R I A L S G R
Biomedical Imaging & Bioengineering
 A New Federal Institute Focuses on Biomedical Imaging & Bioengineering Donna J. Dean and Brenda J. Korte Innovative projects in fluorescent imaging and other areas of optics and photonics are funded by
A New Federal Institute Focuses on Biomedical Imaging & Bioengineering Donna J. Dean and Brenda J. Korte Innovative projects in fluorescent imaging and other areas of optics and photonics are funded by
CF Dyes Next Generation Fluorescent Dyes Secondary antibody
 CF Dyes Next Generation Fluorescent Dyes Secondary antibody OZYME 10 AVENUE AMPÈRE - CS 30268-78053 ST QUENTIN EN YVELINES CEDEX Tél. : 01 34 60 24 24 - Fax : 01 34 60 92 12 - www.ozyme.fr/info CF Dyes
CF Dyes Next Generation Fluorescent Dyes Secondary antibody OZYME 10 AVENUE AMPÈRE - CS 30268-78053 ST QUENTIN EN YVELINES CEDEX Tél. : 01 34 60 24 24 - Fax : 01 34 60 92 12 - www.ozyme.fr/info CF Dyes
Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit
 Application Note 13 RNA Sample Preparation Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit B. Lam, PhD 1, P. Roberts, MSc 1 Y. Haj-Ahmad, M.Sc., Ph.D 1,2 1 Norgen
Application Note 13 RNA Sample Preparation Non-Organic-Based Isolation of Mammalian microrna using Norgen s microrna Purification Kit B. Lam, PhD 1, P. Roberts, MSc 1 Y. Haj-Ahmad, M.Sc., Ph.D 1,2 1 Norgen
Stabilization of a virus-like particle and its application as a nanoreactor at physiological conditions
 Supporting Information Stabilization of a virus-like particle and its application as a nanoreactor at physiological conditions Lise Schoonen, b Sjors Maassen, b Roeland J. M. Nolte b and Jan C. M. van
Supporting Information Stabilization of a virus-like particle and its application as a nanoreactor at physiological conditions Lise Schoonen, b Sjors Maassen, b Roeland J. M. Nolte b and Jan C. M. van
Developing a real-time fluorescence cell growth monitoring system
 - 65 - Developing a real-time fluorescence cell growth monitoring system Jo-Ting Wang 1, Chun-Han Lu 2, Yao-Nan Wang 2, Ko-Tung Chang 1,* 1 Department of Biological Science and Technology, National Pingtung
- 65 - Developing a real-time fluorescence cell growth monitoring system Jo-Ting Wang 1, Chun-Han Lu 2, Yao-Nan Wang 2, Ko-Tung Chang 1,* 1 Department of Biological Science and Technology, National Pingtung
Photoacoustic assessment of oxygen saturation: effect of red blood cell aggregation
 Photoacoustic assessment of oxygen saturation: effect of red blood cell aggregation Eno Hysi a, Ratan K Saha b and Michael C Kolios a a Department of Physics, Ryerson University, Toronto, Canada b Applied
Photoacoustic assessment of oxygen saturation: effect of red blood cell aggregation Eno Hysi a, Ratan K Saha b and Michael C Kolios a a Department of Physics, Ryerson University, Toronto, Canada b Applied
