DISCOVERY AND VALIDATION OF TARGETS AND BIOMARKERS BY MASS SPECTROMETRY-BASED PROTEOMICS. September, 2011
|
|
|
- Morgan Foster
- 6 years ago
- Views:
Transcription
1 DISCOVERY AND VALIDATION OF TARGETS AND BIOMARKERS BY MASS SPECTROMETRY-BASED PROTEOMICS September,
2 CAPRION PROTEOMICS Leading proteomics-based service provider - Biomarker and target discovery - Multiplexed MRM assays - Biomarker verification and validation Located in: Montreal, Canada Menlo Park, California Founded in employees (42 scientists) Extensive experience - Over 30 industry and government partners - Over 70 large scale studies completed (clinical and pre-clinical) - All major therapeutic areas - Broad range of biological samples, including CSF, plasma, serum, urine, exudate, cells and tissues 2
3 CAPRION PRODUCTS, SERVICES AND INDUSTRY PARTNERS Biomarker Services Biomarker discovery - CellCarta Highly multiplexed assay development Validation assays Drug Targets Oncology antibody therapy Metabolic disease secreted proteins Infectious disease targets In-Vitro diagnostics Infectious disease Diabetes Oncology CNS 3
4 MS-BASED BIOMARKER DISCOVERY PROCESS Biomarker Discovery Label-free, gel-free quantitative mass spectrometry Non-hypothesis based discovery approach Profile 1000 s of proteins in 100 s samples Identify differentially expressed proteins as candidate biomarkers Multiplexed Assays Quantify 1 to 700 proteins in a single MRM assay Proteins from mass spec, literature, transcriptomics, etc. Rapid assay development Ab-free or ab enrichment Synthetic labeled standards for absolute quantification Profile candidate markers in 1,000 s samples 4
5 ANALYSIS OF ANY BIOLOGICAL SAMPLE IS POSSIBLE Plasma, serum, CSF, urine, exudate, etc. Deplete abundant proteins Or antibody enrichment Trypsin digestion to peptides LC-MS analysis FFPE slides Dissolve tissue sections Protein Sample Peptide Sample Enrich proteins or organelles Cells and tissues 5
6 LC-MS ANALYSIS AND MATCHING PEPTIDES ACROSS ALL SAMPLES Peptide samples RP-HPLC and QTOF or Orbitrap Mass Spectrometry Mass / charge ratio Retention time 10,000-20,000 peptides observed, matched and quantified 6
7 EXPRESSION PROFILING AND PROTEIN ID Sequencing by LC-MS/MS Statistical analysis to identify differentially expressed peptides Identification of 500-1,500 proteins 7
8 CASE 1: DOSE RESPONSE STUDY SINGLE PEPTIDE RESULT ACROSS 220 SAMPLES Each data point represents one plasma sample Dose-dependent response. vehicle Increasing dose for each treatment group 8
9 CASE 2: VERTEX PRESS RELEASE: PREDICTIVE BIOMARKERS OF RESPONSE Goal: Predict response to drug before treatment with Telaprevir (personalized medicine) Proteomics analysis of 240 plasma samples collected from HCV patients Pre-dose samples from phase 3 clinical trials Clinical outcome provided Candidate biomarkers identified 34 proteins distinguish responders and non-responders to SOC + Telaprevir 10 proteins distinguish responders and non-responders to SOC alone Significant number of proteins correlated to the underlying severity of liver damage Follow-on study in progress Multiplexed MRM assay developed Verify and validate biomarker candidates in larger population 9
10 MRM-MS Assays Multiplexed Assays for Biomarker Verification and Validation 10
11 MULTIPLEXED MRM ASSAYS: RAPID VALIDATION OF CANDIDATE BIOMARKERS Candidate Biomarker list Literature Proteomics Transcriptomics Multiplexed MRM assay (up to 700 proteins) Biomarker verification funnel Cost and time effective alternative to antibody-based approaches Highly multiplexed Rapid assay development Competitive pricing Quantitative measurements Amenable to GLP analysis Custom assays: Up to 700 proteins per assay Develop ELISA or MRM assay for large scale biomarker validation Off-the-shelf products in development: Preclinical (rat) Tox Panel PCSK9 assay (active/inactive) 11
12 MRM ASSAY DEVELOPMENT STRATEGY 1. Literature 2. Proteomics 3. Transcript Profiling Protein Sequence MSAIQAAWPSGTECIAKYNFHGTAEQD LPFCKGDVLTIVAVTKDPNWYKAKNKV GREGIIPANYVQKREGVKAGTKLSLMP WFHGKITREQAERLLYPPETGLFLVRE STNYPGDYTLCVSCDGKVEHYRIMYHA SKLSIDEEVYFENLKMQLVEHYTSDAD GLCTRLIKPKVMEGTVAAQDEFYRSGW ALNMKELKLLQTIGKGEFGDVMLGDYR GNKVAVKCIKNDATA Candidate markers for MRM assay development can come from proteomics or other sources, including the literature Double selection improves signal/noise and reduces interference 12
13 EXAMPLE OF MRM ASSAY DEVELOPED FROM LIST OF 90 LUNG CANCER Dx CANDIDATES No antibody enrichment Plasma depleted of high abundance proteins 64 of the targeted proteins (71%) successfully detected in un-spiked plasma samples (cancer and controls) 13
14 CANDIDATE CLASSIFIERS IDENTIFIED Normalized Intensity Normalized Intensity CANCER NORMAL CANCER NORMAL Normalized Intensity CANCER Current study: NORMAL Normalized Intensity CANCER NORMAL Larger set of proteins (350 candidate markers) and more samples (~400) from multiple sources 14
15 CUSTOM SOFTWARE FOR PEAK DETECTION AND QUANTIFICATION AUTOMATION OF PROCESS INCREASES QUALITY AND CONFIDENCE IN DATA 15
16 13 C, 15 N-LABELED STANDARDS CAN BE USED Synthetic labeled standards can be used to quantify biomarkers over 4 orders of magnitude 16
17 QUANTIFICATION OF ENDOGENOUS PEPTIDES IN PLASMA Calibration for 3325: y = x (r = ) 2.8e5 2.6e5 2.4e5 2.2e5 2.0e5 1.8e5 1.6e5 1.4e5 1.2e5 1.0e5 100 pg/ml 8.0e4 6.0e4 4.0e4 2.0e4 0.0e Concentration Protein precipitation followed by SPE cleanup of endogenous peptides 500 ul rat plasma LOQ 1-10 pg/ml (CV ~15%) 17
18 ANTIBODIES CAN BE USED TO ENRICH SPECIFIC PROTEINS WHEN NECESSARY Ab s conjugated to magnetic beads Immuno-enrichment followed by trypsin digestion 250 ul plasma LLOQ 100 pg/ml (CV ~15%) 18
19 VERY HIGHLY MULTIPLEXED ASSAYS ARE POSSIBLE BY MRM 1498 peptides in one assay 700 proteins Highly reproducible results # of transitions measured Median Int CV of detected transitions
20 CAPRION MRM ADVANTAGES Over 60 industry and government sponsored studies performed Majority of projects involved multiplexed analysis of over 50 analytes Comprehensive sample preparation capabilities and experience Reproducible high-abundance protein depletion and cell fractionation capabilities Plasma, serum, CSF, urine, exudate, cells and tissues Optimized process for minimal variability Low process variability, including GLP study option Optimized instrumentation (Waters nano-acquity LC/ ABSciex QTRAP 5500 Proprietary software suite for enhanced quality and throughput Automated peptide selection tool for rapid assay development In-house database of experimentally detected peptides Assay development throughput >9000 peptides per week, including collision energy optimization. Signal interference detection and assay quality assessment software Automated data processing through Elucidator Automated bio-statistical analysis 20
21
Proteomic analysis of biological fluids
 Proteomic analysis of biological fluids Anne Gonzalez, CNRS-IPBS, Toulouse Équipe «Analyse Protéomique et Spectrométrie de Masse des Biomolécules» Odile Schiltz-Bernard Monsarrat Workshop «Protéomique
Proteomic analysis of biological fluids Anne Gonzalez, CNRS-IPBS, Toulouse Équipe «Analyse Protéomique et Spectrométrie de Masse des Biomolécules» Odile Schiltz-Bernard Monsarrat Workshop «Protéomique
Application Note. Authors. Abstract
 Automated, High Precision Tryptic Digestion and SISCAPA-MS Quantification of Human Plasma Proteins Using the Agilent Bravo Automated Liquid Handling Platform Application Note Authors Morteza Razavi, N.
Automated, High Precision Tryptic Digestion and SISCAPA-MS Quantification of Human Plasma Proteins Using the Agilent Bravo Automated Liquid Handling Platform Application Note Authors Morteza Razavi, N.
REIMAGINING DRUG DEVELOPMENT:
 Biology Reconstructed REIMAGINING DRUG DEVELOPMENT: Accurate Disease Modeling To Drive Successful Therapies Julia Kirshner, CEO julia@zpredicta.com 1 SUCCESS RATES OF DRUG DEVELOPMENT ARE LOW, " PARTICULARLY
Biology Reconstructed REIMAGINING DRUG DEVELOPMENT: Accurate Disease Modeling To Drive Successful Therapies Julia Kirshner, CEO julia@zpredicta.com 1 SUCCESS RATES OF DRUG DEVELOPMENT ARE LOW, " PARTICULARLY
Pharmacokinetic Analysis of the mab Adalimumab by ELISA and Hybrid LBA/LC/MS: A Comparison Study
 Pharmacokinetic Analysis of the mab Adalimumab by ELISA and Hybrid LBA/LC/MS: A Comparison Study Featuring the SCIEX BioBA Solution Xi Qu 1, Shuyu Hou 1, Meghan McCann 1, Caroline Becker 1, Xun Wang 1,
Pharmacokinetic Analysis of the mab Adalimumab by ELISA and Hybrid LBA/LC/MS: A Comparison Study Featuring the SCIEX BioBA Solution Xi Qu 1, Shuyu Hou 1, Meghan McCann 1, Caroline Becker 1, Xun Wang 1,
Alzheimer's disease biomarkers discovery by high resolution mass spectrometry: a challenging task of the Diatral project
 Franco-British bilateral symposium on clinical research, October 2012 Alzheimer's disease biomarkers discovery by high resolution mass spectrometry: a challenging task of the Diatral project Franck Vandermoere,
Franco-British bilateral symposium on clinical research, October 2012 Alzheimer's disease biomarkers discovery by high resolution mass spectrometry: a challenging task of the Diatral project Franck Vandermoere,
Thermo Scientific Mass Spectrometric Immunoassay (MSIA) Pipette Tips. Next generation immunoaffinity. Robust quantitative platform
 Thermo Scientific Mass Spectrometric Immunoassay (MSIA) Pipette Tips Next generation immunoaffinity Robust quantitative platform Immunoaffinity sample preparation Thermo Scientific Mass Spectrometric Immunoassay
Thermo Scientific Mass Spectrometric Immunoassay (MSIA) Pipette Tips Next generation immunoaffinity Robust quantitative platform Immunoaffinity sample preparation Thermo Scientific Mass Spectrometric Immunoassay
MOSAIQUES DIAGNOSTICS
 MOSAIQUES DIAGNOSTICS CLINICAL PROTEOMICS IN DRUG DEVELOPMENT INFORMATION Clinical Proteomics for early and differential diagnosis FDA Letter of Support Possibilities of a companion test in Drug Development
MOSAIQUES DIAGNOSTICS CLINICAL PROTEOMICS IN DRUG DEVELOPMENT INFORMATION Clinical Proteomics for early and differential diagnosis FDA Letter of Support Possibilities of a companion test in Drug Development
Proteomics. Areas of Application for Proteomics. Most Commonly Used Proteomics Techniques: Limitations: Examples
 Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
A Sub-picogram Quantification Method for Desmopressin in Plasma using the SCIEX Triple Quad 6500 System
 A Sub-picogram Quantification Method for Desmopressin in Plasma using the SCIEX Triple Quad 6500 System A high-throughput method for detecting ultra-low levels (0.5 pg/ml) of a therapeutic peptide in human
A Sub-picogram Quantification Method for Desmopressin in Plasma using the SCIEX Triple Quad 6500 System A high-throughput method for detecting ultra-low levels (0.5 pg/ml) of a therapeutic peptide in human
Ligand Binding Assays: Summary and Consensus from the Bioanalytical Workshop (CC V)
 Ligand Binding Assays: Summary and Consensus from the Bioanalytical Workshop (CC V) Binodh DeSilva Executive Director Immunochemistry and Biomarker Development Bristol-Myers Squibb Contributors Lauren
Ligand Binding Assays: Summary and Consensus from the Bioanalytical Workshop (CC V) Binodh DeSilva Executive Director Immunochemistry and Biomarker Development Bristol-Myers Squibb Contributors Lauren
Thermo Scientific Proteomics & Metabolomics Seminar Tour
 Proteomics & Seminar Tour Ignite your workflows with our with Cutting Edge LC-MS Technology Stop by to learn the latest advances in mass spectrometry workflows, technologies launched at 2012 ASMS and talk
Proteomics & Seminar Tour Ignite your workflows with our with Cutting Edge LC-MS Technology Stop by to learn the latest advances in mass spectrometry workflows, technologies launched at 2012 ASMS and talk
Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge
 Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge G. Scott*, H. Gaus #, B. Rivera*, and M. McGinley* *Phenomenex,
Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge G. Scott*, H. Gaus #, B. Rivera*, and M. McGinley* *Phenomenex,
BIOCRATES Life Sciences AG Short Company Presentation
 BIOCRATES Life Sciences AG Short Company Presentation Dr. Alexandra C. Gruber MBA MMFin Director Business Development / Marketing Austria Showcase Life Science Japan, Tokyo, December 2010 BIOCRATES Life
BIOCRATES Life Sciences AG Short Company Presentation Dr. Alexandra C. Gruber MBA MMFin Director Business Development / Marketing Austria Showcase Life Science Japan, Tokyo, December 2010 BIOCRATES Life
Towards unbiased biomarker discovery
 Towards unbiased biomarker discovery High-throughput molecular profiling technologies are routinely applied for biomarker discovery to make the drug discovery process more efficient and enable personalised
Towards unbiased biomarker discovery High-throughput molecular profiling technologies are routinely applied for biomarker discovery to make the drug discovery process more efficient and enable personalised
A Unique LC-MS Assay for Host Cell Proteins(HCPs) ) in Biologics
 A Unique LC-MS Assay for Host Cell Proteins(HCPs) ) in Biologics Catalin Doneanu,, Ph.D. Biopharmaceutical Sciences, Waters September 16, 2009 Mass Spec 2009 2009 Waters Corporation Host Cell Proteins
A Unique LC-MS Assay for Host Cell Proteins(HCPs) ) in Biologics Catalin Doneanu,, Ph.D. Biopharmaceutical Sciences, Waters September 16, 2009 Mass Spec 2009 2009 Waters Corporation Host Cell Proteins
Proteomics And Cancer Biomarker Discovery. Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar. Overview. Cancer.
 Proteomics And Cancer Biomarker Discovery Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar Overview Proteomics Cancer Aims Tools Data Base search Challenges Summary 1 Overview
Proteomics And Cancer Biomarker Discovery Dr. Zahid Khan Institute of chemical Sciences (ICS) University of Peshawar Overview Proteomics Cancer Aims Tools Data Base search Challenges Summary 1 Overview
leading the way in research & development
 leading the way in research & development people. passion. possibilities. ABBVIE 2 immunology AbbVie Immunology has a demonstrated record of success in identifying and developing both small molecule and
leading the way in research & development people. passion. possibilities. ABBVIE 2 immunology AbbVie Immunology has a demonstrated record of success in identifying and developing both small molecule and
The Five Key Elements of a Successful Metabolomics Study
 The Five Key Elements of a Successful Metabolomics Study Metabolomics: Completing the Biological Picture Metabolomics is offering new insights into systems biology, empowering biomarker discovery, and
The Five Key Elements of a Successful Metabolomics Study Metabolomics: Completing the Biological Picture Metabolomics is offering new insights into systems biology, empowering biomarker discovery, and
Využití cílené proteomiky pro kontrolu falšování potravin: identifikace peptidových markerů v mase pomocí LC- Q Exactive MS/MS
 Využití cílené proteomiky pro kontrolu falšování potravin: identifikace peptidových markerů v mase pomocí LC- Q Exactive MS/MS Michal Godula Ph.D. Thermo Fisher Scientific The world leader in serving science
Využití cílené proteomiky pro kontrolu falšování potravin: identifikace peptidových markerů v mase pomocí LC- Q Exactive MS/MS Michal Godula Ph.D. Thermo Fisher Scientific The world leader in serving science
rapiflex Innovation with Integrity Designed for Molecules that Matter. MALDI TOF/TOF
 rapiflex Designed for Molecules that Matter. Innovation with Integrity MALDI TOF/TOF rapiflex TM The first MALDI-TOF/TOF that adapts to your needs. The rapiflex is the most advanced MALDI TOF/TOF system
rapiflex Designed for Molecules that Matter. Innovation with Integrity MALDI TOF/TOF rapiflex TM The first MALDI-TOF/TOF that adapts to your needs. The rapiflex is the most advanced MALDI TOF/TOF system
TECHNOLOGIES & SERVICES FOR THERAPEUTIC ANTIBODY DEVELOPMENT
 Specialist in tissue analysis by Histology, Immunohistochemistry and In Situ Hybridization TECHNOLOGIES & SERVICES FOR THERAPEUTIC ANTIBODY DEVELOPMENT CONTENTS Histalim: who we are Our areas of expertise
Specialist in tissue analysis by Histology, Immunohistochemistry and In Situ Hybridization TECHNOLOGIES & SERVICES FOR THERAPEUTIC ANTIBODY DEVELOPMENT CONTENTS Histalim: who we are Our areas of expertise
A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation
 A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation A Risk-based Approach for In Vitro Companion Diagnostics
A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation A Risk-based Approach for In Vitro Companion Diagnostics
Development of Multiplex Sensitive Anti-Drug Antibody Assays for CRISPR/Cas9 Gene Therapies
 Development of Multiplex Sensitive Anti-Drug Antibody Assays for CRISPR/Cas9 Gene Therapies September 27, 2017 Junxia Wang editasmedicine.com 1 Overview of the presentation Immunogenicity Introduction
Development of Multiplex Sensitive Anti-Drug Antibody Assays for CRISPR/Cas9 Gene Therapies September 27, 2017 Junxia Wang editasmedicine.com 1 Overview of the presentation Immunogenicity Introduction
Current Best Practices in Commercial Kit Evaluation and Validation for Biomarker Assays
 Current Best Practices in Commercial Kit Evaluation and Validation for Biomarker Assays 10th Workshop on Recent Issues in Bioanalysis, 2016 Paul Rhyne, Ph.D. - Director of Immunoassay Services Copyright
Current Best Practices in Commercial Kit Evaluation and Validation for Biomarker Assays 10th Workshop on Recent Issues in Bioanalysis, 2016 Paul Rhyne, Ph.D. - Director of Immunoassay Services Copyright
Using Mass Spectrometry as a Tool. Emergencies
 United States Department of Health & Human Services Office of the Assistant Secretary for Preparedness and Response Rapid, Sensitive Dose Assessment Using Mass Spectrometry as a Tool to Identify Markers
United States Department of Health & Human Services Office of the Assistant Secretary for Preparedness and Response Rapid, Sensitive Dose Assessment Using Mass Spectrometry as a Tool to Identify Markers
Top-Down, Bottom-Up. The Merging of Two High-Performance Technologies
 As featured in BioRadiations 129 Top-Down, Bottom-Up The Merging of Two High-Performance Technologies Authors: Enrique Dalmasso, Dominic Caseñas, and Shawn Miller Bio-Rad Laboratories, Inc. introduced
As featured in BioRadiations 129 Top-Down, Bottom-Up The Merging of Two High-Performance Technologies Authors: Enrique Dalmasso, Dominic Caseñas, and Shawn Miller Bio-Rad Laboratories, Inc. introduced
PROTEOMICS: TECHNOLOGIES AND GLOBAL MARKETS. BIO034C May John Bergin Project Analyst ISBN:
 PROTEOMICS: TECHNOLOGIES AND GLOBAL MARKETS BIO034C May 2013 John Bergin Project Analyst ISBN: 1-56965-403-4 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 866-285-7215, 781-489-7301 www.bccresearch.com
PROTEOMICS: TECHNOLOGIES AND GLOBAL MARKETS BIO034C May 2013 John Bergin Project Analyst ISBN: 1-56965-403-4 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 866-285-7215, 781-489-7301 www.bccresearch.com
Improving Sensitivity in Bioanalysis using Trap-and-Elute MicroLC-MS
 Improving Sensitivity in Bioanalysis using Trap-and-Elute MicroLC-MS Using the SCIEX M3 MicroLC system for Increased Sensitivity in Antibody Quantitation Remco van Soest and Lei Xiong SCIEX, Redwood City,
Improving Sensitivity in Bioanalysis using Trap-and-Elute MicroLC-MS Using the SCIEX M3 MicroLC system for Increased Sensitivity in Antibody Quantitation Remco van Soest and Lei Xiong SCIEX, Redwood City,
AB SCIEX TripleTOF 5600 SYSTEM. High-resolution quant and qual
 AB SCIEX TripleTOF 5600 SYSTEM High-resolution quant and qual AB SCIEX TripleTOF 5600 SYSTEM AB SCIEX is a global leader in the development of life science analytical technologies that help answer complex
AB SCIEX TripleTOF 5600 SYSTEM High-resolution quant and qual AB SCIEX TripleTOF 5600 SYSTEM AB SCIEX is a global leader in the development of life science analytical technologies that help answer complex
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development
 Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
Lecture 23: Clinical and Biomedical Applications of Proteomics; Proteomics Industry
 Lecture 23: Clinical and Biomedical Applications of Proteomics; Proteomics Industry Clinical proteomics is the application of proteomic approach to the field of medicine. Proteome of an organism changes
Lecture 23: Clinical and Biomedical Applications of Proteomics; Proteomics Industry Clinical proteomics is the application of proteomic approach to the field of medicine. Proteome of an organism changes
Measurement of Hepcidin-25 in Serum Using the Agilent 6490 Triple Quadrupole LC/MS with ifunnel Technology
 Measurement of Hepcidin-25 in Serum Using the Agilent 6490 Triple Quadrupole LC/MS with ifunnel Technology Application Note Clinical Research Authors Christophe Hirtz, Constance Delaby, Pauline Bros, Jérôme
Measurement of Hepcidin-25 in Serum Using the Agilent 6490 Triple Quadrupole LC/MS with ifunnel Technology Application Note Clinical Research Authors Christophe Hirtz, Constance Delaby, Pauline Bros, Jérôme
Investigating Biological Variation of Liver Enzymes in Human Hepatocytes
 Investigating Biological Variation of Liver Enzymes in Human Hepatocytes SWATH Acquisition on the TripleTOF Systems Xu Wang 1, Hui Zhang 2, Christie Hunter 1 1 SCIEX, USA, 2 Pfizer, USA MS/MS ALL with
Investigating Biological Variation of Liver Enzymes in Human Hepatocytes SWATH Acquisition on the TripleTOF Systems Xu Wang 1, Hui Zhang 2, Christie Hunter 1 1 SCIEX, USA, 2 Pfizer, USA MS/MS ALL with
CRYSTAL CITY V REDUX: GUIDANCE FOR INDUSTRY BIOANALYTICAL METHOD VALIDATION DRAFT GUIDANCE (2013) VI. ADDITIONAL ISSUES
 CRYSTAL CITY V REDUX: GUIDANCE FOR INDUSTRY BIOANALYTICAL METHOD VALIDATION DRAFT GUIDANCE (2013) VI. ADDITIONAL ISSUES DELAWARE VALLEY DRUG METABOLISM DISCUSSION GROUP STEVE PICCOLI, BMS RAND JENKINS,
CRYSTAL CITY V REDUX: GUIDANCE FOR INDUSTRY BIOANALYTICAL METHOD VALIDATION DRAFT GUIDANCE (2013) VI. ADDITIONAL ISSUES DELAWARE VALLEY DRUG METABOLISM DISCUSSION GROUP STEVE PICCOLI, BMS RAND JENKINS,
MagSi Beads. Magnetic Silica Beads for Life Science and Biotechnology study
 MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
MagSi Beads Magnetic Silica Beads for Life Science and Biotechnology study MagnaMedics Diagnostics B.V. / Rev. 9.2 / 2012 Wide range of products for numerous applications MagnaMedics separation solutions
Enabling Systems Biology Driven Proteome Wide Quantitation of Mycobacterium Tuberculosis
 Enabling Systems Biology Driven Proteome Wide Quantitation of Mycobacterium Tuberculosis SWATH Acquisition on the TripleTOF 5600+ System Samuel L. Bader, Robert L. Moritz Institute of Systems Biology,
Enabling Systems Biology Driven Proteome Wide Quantitation of Mycobacterium Tuberculosis SWATH Acquisition on the TripleTOF 5600+ System Samuel L. Bader, Robert L. Moritz Institute of Systems Biology,
PXBioVisioN. Protease Inhibition. Analysis of Peptides as Surrogates for Protease Activity to Monitor Protease Inhibition in-vivo
 PXBioVisioN Protease Inhibition Analysis of Peptides as Surrogates for Protease Activity to Monitor Protease Inhibition in-vivo Peptides are Products of Protease Activity Peptidomics is defined as the
PXBioVisioN Protease Inhibition Analysis of Peptides as Surrogates for Protease Activity to Monitor Protease Inhibition in-vivo Peptides are Products of Protease Activity Peptidomics is defined as the
Leading The Way in Life Science Technologies. Planning Calendar
 Leading The Way in Life Science Technologies 2018 Planning Calendar January 1 Reservation: 11/30/17 Material: 12/7/17 l Kinase Assays l Immunotherapy l Stem-Cell Manufacturing Peptalk, January 8 12, San
Leading The Way in Life Science Technologies 2018 Planning Calendar January 1 Reservation: 11/30/17 Material: 12/7/17 l Kinase Assays l Immunotherapy l Stem-Cell Manufacturing Peptalk, January 8 12, San
Bio-Plex suspension array system
 Multiplex Assays Bio-Plex suspension array system tech note 64 Profiling of Human, Canine, and Rat Urine Samples Using Bio-Plex Pro RBM Kidney Toxicity Assays L. Stephen, H. Schnaars, K. Marshall, H. Akey,
Multiplex Assays Bio-Plex suspension array system tech note 64 Profiling of Human, Canine, and Rat Urine Samples Using Bio-Plex Pro RBM Kidney Toxicity Assays L. Stephen, H. Schnaars, K. Marshall, H. Akey,
Quantitative Analysis of Two Cancer Signaling Pathways Using Multiplex-Immunoprecipitation and Targeted Mass Spectrometry
 Quantitative Analysis of Two Cancer Signaling Pathways Using Multiplex-Immunoprecipitation and Targeted Mass Spectrometry John C. Rogers, PhD Senior R&D Manager Thermo Fisher Scientific The world leader
Quantitative Analysis of Two Cancer Signaling Pathways Using Multiplex-Immunoprecipitation and Targeted Mass Spectrometry John C. Rogers, PhD Senior R&D Manager Thermo Fisher Scientific The world leader
Human Cytokine Assay Products from Meso Scale Discovery
 Human Cytokine Assay Products from Meso Scale Discovery Paul F. Grulich, David H. Stewart, Pankaj Oberoi, Rob Calamunci, George Sigal and Jacob N. Wohlstadter In this poster, we present a collection of
Human Cytokine Assay Products from Meso Scale Discovery Paul F. Grulich, David H. Stewart, Pankaj Oberoi, Rob Calamunci, George Sigal and Jacob N. Wohlstadter In this poster, we present a collection of
Recombinant Antibody Production in Therapeutic Antibody Projects. Keshav Vasanthavada Senior Marketing Specialist, GenScript April 7, 2016
 Recombinant Antibody Production in Therapeutic Antibody Projects Keshav Vasanthavada Senior Marketing Specialist, GenScript April 7, 2016 Presentation Outline 1 2 3 4 5 Introduction Recombinant Ab Production
Recombinant Antibody Production in Therapeutic Antibody Projects Keshav Vasanthavada Senior Marketing Specialist, GenScript April 7, 2016 Presentation Outline 1 2 3 4 5 Introduction Recombinant Ab Production
Simultaneous in vivo Quantification and Metabolite Identification of Plasma Samples Using High Resolution QTof and Routine MS E Data Analysis
 Simultaneous in vivo Quantification and Metabolite Identification of Plasma Samples Using High Resolution QTof and Routine MS E Data Analysis Mark Wrona, 1 Paul Rainville, 1 Eric Langlois, 2 Nigel Ewing,
Simultaneous in vivo Quantification and Metabolite Identification of Plasma Samples Using High Resolution QTof and Routine MS E Data Analysis Mark Wrona, 1 Paul Rainville, 1 Eric Langlois, 2 Nigel Ewing,
Biologics Bioanalysis
 Biologics Bioanalysis GUIDE TO INNOVATION Biologics Bioanalysis Guide to Innovation Pharmaceutical companies have leveraged advancements in basic science perhaps more than any other industry. With the
Biologics Bioanalysis GUIDE TO INNOVATION Biologics Bioanalysis Guide to Innovation Pharmaceutical companies have leveraged advancements in basic science perhaps more than any other industry. With the
Integrating an exploratory BM in an early clinical stage in Pharma R&D
 Integrating an exploratory BM in an early clinical stage in Pharma R&D Ulrich Kunz, Translational Medicine and Clinical Pharmacology, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany Content
Integrating an exploratory BM in an early clinical stage in Pharma R&D Ulrich Kunz, Translational Medicine and Clinical Pharmacology, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany Content
Xevo G2-S QTof and TransOmics: A Multi-Omics System for the Differential LC/MS Analysis of Proteins, Metabolites, and Lipids
 Xevo G2-S QTof and TransOmics: A Multi-Omics System for the Differential LC/MS Analysis of Proteins, Metabolites, and Lipids Ian Edwards, Jayne Kirk, and Joanne Williams Waters Corporation, Manchester,
Xevo G2-S QTof and TransOmics: A Multi-Omics System for the Differential LC/MS Analysis of Proteins, Metabolites, and Lipids Ian Edwards, Jayne Kirk, and Joanne Williams Waters Corporation, Manchester,
FDA Perspectives on Biomarkers. Steven Kozlowski, M.D. Office of Biotechnology Products OPS/CDER/FDA
 FDA Perspectives on Biomarkers Steven Kozlowski, M.D. Office of Biotechnology Products OPS/CDER/FDA Importance of Biomarkers Earlier diagnosis Focusing expensive & invasive therapies on the right populations
FDA Perspectives on Biomarkers Steven Kozlowski, M.D. Office of Biotechnology Products OPS/CDER/FDA Importance of Biomarkers Earlier diagnosis Focusing expensive & invasive therapies on the right populations
Proteomics. Proteomics is the study of all proteins within organism. Challenges
 Proteomics Proteomics is the study of all proteins within organism. Challenges 1. The proteome is larger than the genome due to alternative splicing and protein modification. As we have said before we
Proteomics Proteomics is the study of all proteins within organism. Challenges 1. The proteome is larger than the genome due to alternative splicing and protein modification. As we have said before we
Discover New Proteins Using Immunodepletion
 Discover New Proteins Using Immunodepletion Proteomics Peter Mrozinski Application Scientist Agilent Technologies December 11, 2007 Introduction Complexity and dynamic range of protein concentrations present
Discover New Proteins Using Immunodepletion Proteomics Peter Mrozinski Application Scientist Agilent Technologies December 11, 2007 Introduction Complexity and dynamic range of protein concentrations present
Application Note. Kwasi Antwi, Amanda Ribar, Urban A. Kiernan, and Eric E. Niederkofler Thermo Fisher Scientific, Tempe, Arizona
 Analysis of an Antibody Drug Conjugate using MSIA D.A.R.T. S. Technology, an Integral Part of Ligand Binding Mass Spectrometric Immunoassay (LB-MSIA) Workflow Application Note Kwasi Antwi, Amanda Ribar,
Analysis of an Antibody Drug Conjugate using MSIA D.A.R.T. S. Technology, an Integral Part of Ligand Binding Mass Spectrometric Immunoassay (LB-MSIA) Workflow Application Note Kwasi Antwi, Amanda Ribar,
Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y. Report Price Publication date
 F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
Bio-Rad Laboratories BIOPLEX 2200 SYSTEM. BioPlex 2200 ANA Screen with MDSS. The first and only fully-automated, multiplexed ANA Screen
 Bio-Rad Laboratories BIOPLEX 2200 SYSTEM BioPlex 2200 ANA Screen with MDSS The first and only fully-automated, multiplexed ANA Screen Bio-Rad Laboratories BIOPLEX 2200 SYSTEM Like no other The BioPlex
Bio-Rad Laboratories BIOPLEX 2200 SYSTEM BioPlex 2200 ANA Screen with MDSS The first and only fully-automated, multiplexed ANA Screen Bio-Rad Laboratories BIOPLEX 2200 SYSTEM Like no other The BioPlex
Industry Academic Collaboration: A Key to Successful Involvement of Patients Early in Clinical Development
 Industry Academic Collaboration: A Key to Successful Involvement of Patients Early in Clinical Development Aernout van Haarst PhD Director, European Corporate Development Feb 2016 Industry Academic Collaboration
Industry Academic Collaboration: A Key to Successful Involvement of Patients Early in Clinical Development Aernout van Haarst PhD Director, European Corporate Development Feb 2016 Industry Academic Collaboration
PAVING THE WAY FOR ASSESSING IN VIVO DYNAMICS OF MULTIPLE QUALITY ATTRIBUTES FOR PROTEIN THERAPEUTICS
 PAVING THE WAY FOR ASSESSING IN VIVO DYNAMICS OF MULTIPLE QUALITY ATTRIBUTES FOR PROTEIN THERAPEUTICS CASSS Mass Spec September 21, 2017 Haihong Zhou Principal Scientist, Biologics, Vaccines & Bioanalytics
PAVING THE WAY FOR ASSESSING IN VIVO DYNAMICS OF MULTIPLE QUALITY ATTRIBUTES FOR PROTEIN THERAPEUTICS CASSS Mass Spec September 21, 2017 Haihong Zhou Principal Scientist, Biologics, Vaccines & Bioanalytics
For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine.
 m andw da a For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity Range (calibration
m andw da a For the quantitative detection of human IL6 in serum, plasma, cell culture supernatants and urine. general information Catalogue Number Product Name Species cross-reactivity Range (calibration
Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug development
 1 2 3 24 June 2010 EMA/CHMP/641298/2008 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug
1 2 3 24 June 2010 EMA/CHMP/641298/2008 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug
FirePlex mirna Assay. Multiplex microrna profiling from low sample inputs
 FirePlex mirna Assay Multiplex microrna profiling from low sample inputs Abstract We introduce a new assay for multiplex microrna (mirna) discovery and verification that enables simultaneous profiling
FirePlex mirna Assay Multiplex microrna profiling from low sample inputs Abstract We introduce a new assay for multiplex microrna (mirna) discovery and verification that enables simultaneous profiling
Supplementary Figure 1. Utilization of publicly available antibodies in different applications.
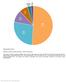 Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
Supplementary Figure 1 Utilization of publicly available antibodies in different applications. The fraction of publicly available antibodies toward human protein targets is shown with data for the following
QIAGEN s NGS Solutions for Biomarkers NGS & Bioinformatics team QIAGEN (Suzhou) Translational Medicine Co.,Ltd
 QIAGEN s NGS Solutions for Biomarkers NGS & Bioinformatics team QIAGEN (Suzhou) Translational Medicine Co.,Ltd 1 Our current NGS & Bioinformatics Platform 2 Our NGS workflow and applications 3 QIAGEN s
QIAGEN s NGS Solutions for Biomarkers NGS & Bioinformatics team QIAGEN (Suzhou) Translational Medicine Co.,Ltd 1 Our current NGS & Bioinformatics Platform 2 Our NGS workflow and applications 3 QIAGEN s
Quantitative analysis of protein-biotherapeutics: Recent advances in LC-MS/MS technology for improved selectivity and sensitivity
 Focus on Therapeutic Oligos & Peptides Quantitative analysis of protein-biotherapeutics: Recent advances in LC-MS/MS technology for improved selectivity and sensitivity With an ever increasing flow of
Focus on Therapeutic Oligos & Peptides Quantitative analysis of protein-biotherapeutics: Recent advances in LC-MS/MS technology for improved selectivity and sensitivity With an ever increasing flow of
Agilent 6430 Triple Quadrupole LC/MS System
 Agilent 6430 Triple Quadrupole LC/MS System Ideal Quantitative LC/MS for UHPLC with Dynamic MRM and Fast Polarity Switching, plus Unsurpassed Sensitivity with Chip LC Summary The Agilent 6430 Triple Quadrupole
Agilent 6430 Triple Quadrupole LC/MS System Ideal Quantitative LC/MS for UHPLC with Dynamic MRM and Fast Polarity Switching, plus Unsurpassed Sensitivity with Chip LC Summary The Agilent 6430 Triple Quadrupole
"Stratification biomarkers in personalised medicine"
 1/12 2/12 SUMMARY REPORT "Stratification biomarkers in personalised medicine" Workshop to clarify the scope for stratification biomarkers and to identify bottlenecks in the discovery and the use of such
1/12 2/12 SUMMARY REPORT "Stratification biomarkers in personalised medicine" Workshop to clarify the scope for stratification biomarkers and to identify bottlenecks in the discovery and the use of such
Pharmaceutical LC/MS Solutions from Agilent Technologies
 Pharmaceutical LC/MS Solutions from Agilent Technologies Application Compendium Overview LC/MS plays a key role in the drug discovery and drug development process. Since the introduction of electrospray
Pharmaceutical LC/MS Solutions from Agilent Technologies Application Compendium Overview LC/MS plays a key role in the drug discovery and drug development process. Since the introduction of electrospray
LC/MS/MS Solutions for Biomarker Discovery QSTAR. Elite Hybrid LC/MS/MS System. More performance, more reliability, more answers
 LC/MS/MS Solutions for Biomarker Discovery QSTAR Elite Hybrid LC/MS/MS System More performance, more reliability, more answers More is better and the QSTAR Elite LC/MS/MS system has more to offer. More
LC/MS/MS Solutions for Biomarker Discovery QSTAR Elite Hybrid LC/MS/MS System More performance, more reliability, more answers More is better and the QSTAR Elite LC/MS/MS system has more to offer. More
Exam MOL3007 Functional Genomics
 Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
Faculty of Medicine Department of Cancer Research and Molecular Medicine Exam MOL3007 Functional Genomics Tuesday May 29 th 9.00-13.00 ECTS credits: 7.5 Number of pages (included front-page): 5 Supporting
European Medicines Agency Evaluation of Medicines for Human Use COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 27 July 2005 EMEA/CHMP/89249/2004 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT Guideline on the Clinical Investigation
European Medicines Agency Evaluation of Medicines for Human Use London, 27 July 2005 EMEA/CHMP/89249/2004 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT Guideline on the Clinical Investigation
Biomarker Measurement- Maximum Information from Limited Volume Barcelona, Spain 16 th November 2011 John Chappell, Immunoassay Services UK
 Biomarker Measurement- Maximum Information from Limited Volume Barcelona, Spain 16 th November 2011 John Chappell, Immunoassay Services UK Biomarker Services Equipment : MSD Sector Imager 2400 (3) Gyros
Biomarker Measurement- Maximum Information from Limited Volume Barcelona, Spain 16 th November 2011 John Chappell, Immunoassay Services UK Biomarker Services Equipment : MSD Sector Imager 2400 (3) Gyros
TECHNICAL NOTE Phosphorylation Site Specific Aptamers for Cancer Biomarker Peptide Enrichment and Detection in Mass Spectrometry Based Proteomics
 TECHNICAL NOTE Phosphorylation Site Specific Aptamers for Cancer Biomarker Peptide Enrichment and Detection in Mass Spectrometry Based Proteomics INTRODUCTION The development of renewable capture reagents
TECHNICAL NOTE Phosphorylation Site Specific Aptamers for Cancer Biomarker Peptide Enrichment and Detection in Mass Spectrometry Based Proteomics INTRODUCTION The development of renewable capture reagents
Peptide libraries: applications, design options and considerations. Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST
 Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Special Order Mouse Biomarker Group 1
 Special Order Mouse Biomarker Group 1 V-PLEX assays: IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-10, IL-12p70, TNF-α Other MSD catalog mouse assays: EPO, GM-CSF, IL-12, IL-12/IL-23p40, IL-13, IL-17,
Special Order Mouse Biomarker Group 1 V-PLEX assays: IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-10, IL-12p70, TNF-α Other MSD catalog mouse assays: EPO, GM-CSF, IL-12, IL-12/IL-23p40, IL-13, IL-17,
of Host changes to respond the early geography. df]
![of Host changes to respond the early geography. df] of Host changes to respond the early geography. df]](/thumbs/77/75743437.jpg) Developing a Routine LC/ /MS/MS Platform for Quantitative Identification of Host Cell Proteins (HCPs) Introduction Unknown HCPs are a Threat to Income Regulators classify residual host cell proteins (HCPs)
Developing a Routine LC/ /MS/MS Platform for Quantitative Identification of Host Cell Proteins (HCPs) Introduction Unknown HCPs are a Threat to Income Regulators classify residual host cell proteins (HCPs)
MultiQuant Software Version 3.0
 MultiQuant Software Version 3.0 Improving Data Quality and Processing Throughput with Better Peak Integration, Quantitative and Qualitative Compound Review for the Analysis of Food, Drinking Water, and
MultiQuant Software Version 3.0 Improving Data Quality and Processing Throughput with Better Peak Integration, Quantitative and Qualitative Compound Review for the Analysis of Food, Drinking Water, and
First Annual Biomarker Symposium Quest Diagnostics Clinical Trials
 First Annual Biomarker Symposium Quest Diagnostics Clinical Trials Terry Robins, Ph.D. Director Biomarker R&D and Scientific Affairs Quest Diagnostics Clinical Trials Key Considerations: Biomarker Development
First Annual Biomarker Symposium Quest Diagnostics Clinical Trials Terry Robins, Ph.D. Director Biomarker R&D and Scientific Affairs Quest Diagnostics Clinical Trials Key Considerations: Biomarker Development
Preclinical Drug Development
 Preclinical Drug Development A guidance prepared by From a 2004 NIH Summit Workshop: A major reason for the tremendous cost of drug development is the high rate of drug candidate failure during clinical
Preclinical Drug Development A guidance prepared by From a 2004 NIH Summit Workshop: A major reason for the tremendous cost of drug development is the high rate of drug candidate failure during clinical
Roche, Roche Molecular Diagnostics and more
 , Molecular and more [Monte Wetzel, PhD] Patients have questions. We provide answers. Group Clear focus on Healthcare Innovation with two strong pillars Pharma Pharma Genentech Chugai Molecular Professional
, Molecular and more [Monte Wetzel, PhD] Patients have questions. We provide answers. Group Clear focus on Healthcare Innovation with two strong pillars Pharma Pharma Genentech Chugai Molecular Professional
HD Regulatory Science Consortium (HD-RSC) A consortium aimed at accelerating treatments for Huntington s disease
 HD Regulatory Science Consortium (HD-RSC) A consortium aimed at accelerating treatments for Huntington s disease Critical Path Institute / CHDI Foundation Huntington s Disease Regulatory Science Consortium
HD Regulatory Science Consortium (HD-RSC) A consortium aimed at accelerating treatments for Huntington s disease Critical Path Institute / CHDI Foundation Huntington s Disease Regulatory Science Consortium
SOMAscan Proteomic Assay Technical White Paper
 SOMAscan Proteomic Assay Technical White Paper Introduction The SOMAscan assay is a highly multiplexed, sensitive, quantitative, and reproducible proteomic tool for discovering previously undetected biomarkers
SOMAscan Proteomic Assay Technical White Paper Introduction The SOMAscan assay is a highly multiplexed, sensitive, quantitative, and reproducible proteomic tool for discovering previously undetected biomarkers
SHORT COURSE. Practical Considerations for Biomarker Bioanalysis: Scientific and Regulatory Perspective SUNDAY, SEPTEMBER 17
 SHORT COURSE SUNDAY, SEPTEMBER 17 OMNI PROVIDENCE HOTEL PROVIDENCE, RI Practical Considerations for Biomarker Bioanalysis: Scientific and Regulatory Perspective SPONSORED BY: Organized by: www.bostonsociety.org
SHORT COURSE SUNDAY, SEPTEMBER 17 OMNI PROVIDENCE HOTEL PROVIDENCE, RI Practical Considerations for Biomarker Bioanalysis: Scientific and Regulatory Perspective SPONSORED BY: Organized by: www.bostonsociety.org
ProteoMiner Protein Enrichment Technology
 Sample Preparation ProteoMiner Protein Enrichment Technology Digging Deeper in the Proteome Detect More Proteins With ProteoMiner Technology Than With Immunodepletion ProteoMiner Technology vs. an Agilent
Sample Preparation ProteoMiner Protein Enrichment Technology Digging Deeper in the Proteome Detect More Proteins With ProteoMiner Technology Than With Immunodepletion ProteoMiner Technology vs. an Agilent
Beth Hutchins, PhD PhRMA ICH Gene Therapy Discussion Group
 ICH Considerations on General Principles to Address the Risk of Inadvertent Germline Integration of Gene Therapy Vectors and Current Topics on Gene Therapy in USA Beth Hutchins, PhD PhRMA ICH Gene Therapy
ICH Considerations on General Principles to Address the Risk of Inadvertent Germline Integration of Gene Therapy Vectors and Current Topics on Gene Therapy in USA Beth Hutchins, PhD PhRMA ICH Gene Therapy
Advances and Challenges in Liquid Chromatography-Mass Spectrometry-based Proteomics Profiling for Clinical Applications*
 Review Advances and Challenges in Liquid Chromatography-Mass Spectrometry-based Proteomics Profiling for Clinical Applications* Wei-Jun Qian, Jon M. Jacobs, Tao Liu, David G. Camp II, and Richard D. Smith
Review Advances and Challenges in Liquid Chromatography-Mass Spectrometry-based Proteomics Profiling for Clinical Applications* Wei-Jun Qian, Jon M. Jacobs, Tao Liu, David G. Camp II, and Richard D. Smith
The TetraQ Difference. Quality Preclinical Drug Development Solutions. Provide advice and tailored solutions, not just a menu of choices
 A leading Australian contract research organisation (CRO) providing integrated preclinical drug development services to the biotech and pharmaceutical industries The TetraQ Difference Located at The University
A leading Australian contract research organisation (CRO) providing integrated preclinical drug development services to the biotech and pharmaceutical industries The TetraQ Difference Located at The University
Nanotechnology: A Brief History and Its Convergence with Medicine. Weston Daniel, PhD Director of Program Management
 Nanotechnology: A Brief History and Its Convergence with Medicine Weston Daniel, PhD Director of Program Management Outline Introduction The Nanoscale Applications Realization of a Vision There s Plenty
Nanotechnology: A Brief History and Its Convergence with Medicine Weston Daniel, PhD Director of Program Management Outline Introduction The Nanoscale Applications Realization of a Vision There s Plenty
MSD MULTI-SPOT Assay System
 MSD MULTI-SPOT Assay System Human MMP 2-Plex Ultra-Sensitive Kit 1-Plate Kit 5-Plate Kit 25-Plate Kit K15033C-1 K15033C-2 K15033C-4 17335-v5-2012Mar 1 MSD Biomarker Assays Human MMP 2-Plex Ultra-Sensitive
MSD MULTI-SPOT Assay System Human MMP 2-Plex Ultra-Sensitive Kit 1-Plate Kit 5-Plate Kit 25-Plate Kit K15033C-1 K15033C-2 K15033C-4 17335-v5-2012Mar 1 MSD Biomarker Assays Human MMP 2-Plex Ultra-Sensitive
Increased Sensitivity through GC- MS/MS
 Introducing InVentiv Health Clinical Important Impact of GLP Certification Dr. Jim Settlage joins our team Success Story & Client Testimonial New Technology for Improved Quantitation and Sensitivity Increased
Introducing InVentiv Health Clinical Important Impact of GLP Certification Dr. Jim Settlage joins our team Success Story & Client Testimonial New Technology for Improved Quantitation and Sensitivity Increased
Why do LC MS and LBA
 Why do LC MS and LBA results differ? A literature evaluation 18 November 2015 Nico van de Merbel An overview In most published BA methods for large molecules, no comparison of platforms (LBA vs LBA, LBA
Why do LC MS and LBA results differ? A literature evaluation 18 November 2015 Nico van de Merbel An overview In most published BA methods for large molecules, no comparison of platforms (LBA vs LBA, LBA
High Resolution Accurate Mass Peptide Quantitation on Thermo Scientific Q Exactive Mass Spectrometers. The world leader in serving science
 High Resolution Accurate Mass Peptide Quantitation on Thermo Scientific Q Exactive Mass Spectrometers The world leader in serving science Goals Explore the capabilities of High Resolution Accurate Mass
High Resolution Accurate Mass Peptide Quantitation on Thermo Scientific Q Exactive Mass Spectrometers The world leader in serving science Goals Explore the capabilities of High Resolution Accurate Mass
Stability of Biological Products
 Stability of Biological Products Dr Jurgen Lindner Principal, BioPharma Consulting & Executive Secretary, APIMAA Biological Products Functional Proteins or Polypeptides (mab s, enzymes & inhibitors, growth
Stability of Biological Products Dr Jurgen Lindner Principal, BioPharma Consulting & Executive Secretary, APIMAA Biological Products Functional Proteins or Polypeptides (mab s, enzymes & inhibitors, growth
Kinase Activity: a total solution
 Kinase Activity: a total solution Protein arrays for multiplex kinetic measurements Robust Instrumentation User friendly software Kinetic data at your fingertips! PamGene s novel platform for profiling
Kinase Activity: a total solution Protein arrays for multiplex kinetic measurements Robust Instrumentation User friendly software Kinetic data at your fingertips! PamGene s novel platform for profiling
The Future of Reference Materials Science and Innovation
 Status and Trends in In-Vitro Diagnostics and Considerations for Reference Materials The Future of Reference Materials Science and Innovation Conference in the frame of the 50th anniversary of the JRC
Status and Trends in In-Vitro Diagnostics and Considerations for Reference Materials The Future of Reference Materials Science and Innovation Conference in the frame of the 50th anniversary of the JRC
2D gel Western blotting using antibodies against ubiquitin, SUMO and acetyl PTM
 2D gel Western blotting using antibodies against ubiquitin, SUMO and acetyl PTM Nancy Kendrick, Jon Johansen & Matt Hoelter, Kendrick Labs Inc www.kendricklabs.com Talk Outline Significance Method description
2D gel Western blotting using antibodies against ubiquitin, SUMO and acetyl PTM Nancy Kendrick, Jon Johansen & Matt Hoelter, Kendrick Labs Inc www.kendricklabs.com Talk Outline Significance Method description
LTQ Orbitrap XL Hybrid FT Mass Spectrometer Unrivaled Performance and Flexibility
 m a s s s p e c t r o m e t r y LTQ Orbitrap XL Hybrid FT Mass Spectrometer Unrivaled Performance and Flexibility Part of Thermo Fisher Scientific LTQ Orbitrap XL OFFERING OUTSTANDING MASS ACCURACY, RESOLVING
m a s s s p e c t r o m e t r y LTQ Orbitrap XL Hybrid FT Mass Spectrometer Unrivaled Performance and Flexibility Part of Thermo Fisher Scientific LTQ Orbitrap XL OFFERING OUTSTANDING MASS ACCURACY, RESOLVING
Utilizing novel technology for the analysis of therapeutic antibodies and host cell protein contamination
 Utilizing novel technology for the analysis of therapeutic antibodies and host cell protein contamination Kelli Jonakin, Ph.D. Senior Field Application Scientist, Seattle, WA Eric Johansen, Ph.D. Senior
Utilizing novel technology for the analysis of therapeutic antibodies and host cell protein contamination Kelli Jonakin, Ph.D. Senior Field Application Scientist, Seattle, WA Eric Johansen, Ph.D. Senior
Current practice and future vision on metabolite profiling and quantification in Drug development
 Current practice and future vision on metabolite profiling and quantification in Drug development Geert Mannens EBF Focus workshop Metabolite Profiling and Quantification Strategies in Drug R&D Sept 25,
Current practice and future vision on metabolite profiling and quantification in Drug development Geert Mannens EBF Focus workshop Metabolite Profiling and Quantification Strategies in Drug R&D Sept 25,
LiquidBiopsy LIQUIDBIOPSY. Automated Rare Template Isolation Platform
 LiquidBiopsy LIQUIDBIOPSY Automated Rare Template Isolation Platform Enabling cancer research with highly multiplexed molecular analysis of serially collected blood samples The LiquidBiopsy Platform simplifies
LiquidBiopsy LIQUIDBIOPSY Automated Rare Template Isolation Platform Enabling cancer research with highly multiplexed molecular analysis of serially collected blood samples The LiquidBiopsy Platform simplifies
Thermo Scientific Solutions for Intact-Protein Analysis. Better, Faster Decisions for. Biotherapeutic Development
 Thermo Scientific Solutions for Intact-Protein Analysis Better, Faster Decisions for Biotherapeutic Development Quickly and Accurately Assess Product Quality and Safety Therapeutic proteins and monoclonal
Thermo Scientific Solutions for Intact-Protein Analysis Better, Faster Decisions for Biotherapeutic Development Quickly and Accurately Assess Product Quality and Safety Therapeutic proteins and monoclonal
Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion
 Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion Robin Thorpe & Meenu Wadhwa Revised Guideline: Differences from original
Revised Immunogenicity Guideline: Assays and methods- Presentation of the draft guideline and introduction of the topics for discussion Robin Thorpe & Meenu Wadhwa Revised Guideline: Differences from original
Workshop and Training Program on Sampling and Detection Methods Applied to Transgenic Crops November 17 19, 2011, NIN, Hyderabad, India
 Workshop and raining Program on Sampling and Detection ethods Applied to ransgenic Crops odified (G) Plants and Food Detection ethods Dr. Clara. Alarcon Pioneer Hi-Bred Hyderabad, 2011 1.Plants 2.Seeds
Workshop and raining Program on Sampling and Detection ethods Applied to ransgenic Crops odified (G) Plants and Food Detection ethods Dr. Clara. Alarcon Pioneer Hi-Bred Hyderabad, 2011 1.Plants 2.Seeds
