Compounding Questions and Answers
|
|
|
- Ophelia Bryan
- 6 years ago
- Views:
Transcription
1 Compounding Questions and Answers JANUARY 10, Question: What is a reliable supplier? Answer: Some examples of reliable suppliers are FDA licensed manufacturers, CA Department of Public Health Food and Drug Branch licensed drug repackagers; CA licensed pharmacies and wholesalers; CA licensed non-resident wholesalers. Prior to making a purchase, it is recommended to check the board s website to verify if the wholesaler or non-resident pharmacy is licensed by the board. If purchasing chemicals from another country, obtain a certificate issued by the FDA authorizing shipment of the product into the U.S. and a certificate of analysis printed in English. As a reminder, any pharmacy purchasing, trading, selling, or transferring drugs to an entity not licensed by the board could be cited and fined up to $5000 per transaction Reference: B&P 4160, 4163, , 4169(a)(1); CCR 1780, 1783, (c) 2. Question: Do cytotoxic agents and other hazardous substances have the same requirements for qualitative and quantitative analysis? Answer: Yes. 3. Question: Is a non-resident pharmacy (NRP) that provides compounded product into CA required to meet the same staffing requirements as CA pharmacies? Answer: No. A non-resident pharmacy (NRP) is a pharmacy located in another state that furnishes dangerous drugs to patients in CA, and is required to be licensed with the board. Part of the licensure requirement is that the NRP be in compliance with pharmacy laws in the state where it is located.
2 The board has no authority to dictate staffing requirements for pharmacies located in states other than CA. The board expects the NRP to be staffed in accordance with requirements where it is located. Reference: Business and Professions Code 4112(a); 4112(d) 4. Question: What constitutes sterile compounding? Answer: First, let s define compounding in general: Compounding means any of the following activities occurring in a licensed pharmacy, by or under the supervision of a licensed pharmacist, pursuant to a prescription: (1) Altering the dosage form or delivery system of a drug (2) Altering the strength of a drug (3) Combining components or active ingredients (4) Preparing a drug product from chemicals or bulk drug substances With the above in mind, sterile compounding is a specific subtype of general compounding whereby there is a requirement for the compounded drug product to be sterile. Sterile compounding almost exclusively involves sterile parenteral compounding for which there are additional requirements. Reference: CCR 1735(a) 1735(d); 1751 et seq Question: Is the adding of 20 meq of potassium chloride to 1000cc of normal saline for intravenous administration considered sterile compounding. Answer: Yes, and this is also considered sterile parenteral compounding. Reference: CCR 1735(a)6. 6. Question: Can a pharmacy mix three liquids (Maalox, Benadryl, and Xylocaine) in equal parts or two creams in equal parts, and would this be considered compounding. Answer: Yes in the examples given, a pharmacy may mix those products in equal parts. And yes, it is considered compounding. Page 2
3 Reference: CCR 1735(a)7. 7. Question: What happens in a situation where an IV is made to be used on a one- time basis for administration within 24 hours for a registered in-patient of a health care facility and the IV product is not used and returned to the pharmacy? Can it be reused? Answer: No. The compounding regulations require specific records for compounded drug products. For each compounded drug product, the pharmacy records shall include: (1) The master formula record. (2) The date the drug product was compounded. (3) The identity of the pharmacy personnel who compounded the drug product. (4) The identity of the pharmacist reviewing the final drug product. (5) The quantity of each component used in compounding the drug product. (6) The manufacturer and lot number of each component. If the manufacturer name is demonstrably unavailable, the name of the supplier may be substituted. Exempt from the requirements of this paragraph are sterile products compounded on a one-time basis for administration within twenty-four hours to an in-patient in a health care facility. (7) The equipment used in compounding the drug product. (8) A pharmacy assigned reference or lot number for the compounded drug product. (9) The expiration date of the final compounded drug product. (10) The quantity or amount of drug product compounded. If all the information is not recorded [as provided by the exemption in (6)] then there is a lack of complete traceability and accountability for the compounded drug product and thus it cannot be reused. Reference: CCR (a)8. Page 3
4 8. Question: Our medical center s policies and procedures have the initial dose of an IV admixture compounded in the pharmacy satellite to assure timely initiation of therapy, with all subsequent doses mixed in the central pharmacy. Is the initial IV admixture compounded in the satellite pharmacy subject to the record keeping requirements? Answer: Yes, with the possible exception of documenting the manufacturer and lot number of each component of the admixture. Reference: CCR (a)(6)9. 9. Question: Is a master formula record equivalent to a recipe card? Answer: Basically, yes. Like a recipe card the master formula record includes the active and inactive ingredients to be used, the process and/or procedure used to prepare the drug, quality reviews required at each step in the preparation of the drug, post-compounding process or procedures required, and the expiration dating requirements. The master formula record must be created prior to compounding the drug product. The prescription document itself may be used as the master formula record If a pharmacy does not routinely compound a particular drug product. Reference: CCR (d) Question: When compounding a product, is it required to have master formula record available and used when the product is compounded? Answer: Yes, the master formula record must be created prior to compounding the drug product and its use will provide guidance for compounding personnel and consistency in the product produced. Reference: CCR (d)11. Page 4
5 11. Question: Is it required to review the master formula record as part of pre-check process? Answer: The law is silent on a pre-check process. However, the master formula record will provide guidance to compounding personnel in what to use and how to compound the particular drug product. So the master formula record could be used in a pre-check process to insure consistency in the compounding process. Reference: CCR Question: What are the requirements for compounding documentation? Answer: The compounding regulations require specific records for compounded drug products. For each compounded drug product, the pharmacy records shall include: (1) The master formula record. (2) The date the drug product was compounded. (3) The identity of the pharmacy personnel who compounded the drug product. (4) The identity of the pharmacist reviewing the final drug product. (5) The quantity of each component used in compounding the drug product. (6) The manufacturer and lot number of each component. If the manufacturer name is demonstrably unavailable, the name of the supplier may be substituted. Exempt from the requirements of this paragraph are sterile products compounded on a one-time basis for administration within twenty-four hours to an in-patient in a health care facility. (7) The equipment used in compounding the drug product. (8) A pharmacy assigned reference or lot number for the compounded drug product. (9) The expiration date of the final compounded drug product. (10) The quantity or amount of drug product compounded. Reference: CCR Page 5
6 13. Question: When using the record-keeping exemption in (a)(6) to compound a one-time Vancomycin IV with a seven-day expiration date and to be used within 24 hours, is the manufacturer and lot number required? Answer: No. The regulations provide for an exemption for sterile products compounded on a one-time basis for administration within twenty-four hours to an in-patient of a health care facility. Reference: CCR (a)(6) Question: When must the manufacturer and lot number be recorded? Answer: This information must be documented if the product is not for a one-time use for a specific patient to be used within 24 hours. Reference: CCR (a)(6) Question: How will the board insure compliance by non-resident pharmacies (NRP s) that provide compounded drug products into CA? Answer: The board does not have the ability to inspect NRPs. However, NRPs are required to be licensed with the board and to maintain compliance with pharmacy regulations of their home state. Also, a NRP performing sterile parenteral compounding as a condition of renewal will be encouraged to submit a completed Compounding Self Assessment Form. Reference: B&P 4112, Question: Is the dilution per the manufacturer s instructions and adding to the IV solution considered compounding? Answer: Yes, if done in a pharmacy. However, statute provides for exemption from sterile compounding licensure if the sterile powder was obtained from a manufacturer and the drug is reconstituted for administration to patients by a health care professional licensed to administer drugs by injection. Reference: CCR 1735(a)(1); B&P (e)17. Page 6
7 17. Question: Are proprietary drug delivery systems such as ADD- Vantage, Mini-Bag Plus, and At-Eas considered compounded products after the vials have been attached to the IV bags? Answer: These types of delivery systems are exempt from the compounding requirements if the sterile powder was obtained from a manufacturer and the drug is reconstituted for administration to patients by a health care professional licensed to administer drugs by injection. Reference: CCR 1735(a)(1); B&P (e) Question: What specifically will be required or what process is acceptable to achieve quality assurance? Answer: Quality assurance, as the term implies, is designed to monitor and ensure the integrity, potency, quality, and labeled strength of compounded products. A quality assurance plan will touch all parts of the compounding process drug product and equipment acquisition/storage; compounding processes; documentation of compounding and related analysis; employee training and monitoring; recall procedure; etc Reference: CCR ; ; ; ; ; 1751 et seq Question: When recycling an IV that was previously compounded by the pharmacy, can the previous lot number of the recycled IV be used as long as the lot number can be traced to all the requirements listed in section (a)? Answer: Yes. Reference: CCR (a) Question: Does every product and/or formulation compounded by a pharmacy have to undergo qualitative and quantitative analysis? If not, can the board provide guidance for selecting products to be analyzed? Answer: The pharmacy, and the pharmacist, are responsible for insuring the compounded product complies quantitatively and qualitatively Page 7
8 with the prescriber s prescription. For compounded product that is compounded on a one-time basis for immediate dispensing, it would not be likely there would be a quantitative or qualitative analysis conducted. For products compounded for on-going therapy it would be expected there would be analysis done initially and on a periodic basis to validate the product and compounding process. The same holds true for sterile injectable drug products too. However, if two or more sterile injectable drug products being compounded from one or more non-sterile ingredients, these end-products shall be quarantined until end-product testing confirms sterility and acceptable levels of pyrogens. Reference: CCR (f); (i); (a); Question: Does CCR section require a pharmacy to test each and every compounded product for integrity, potency, quality, and labeled strength of the compounded product? Answer: No. However, if the compounded product involves a complex process it would seem prudent to have documentation of the final product. This is even more important when the product is compounded on a more routine basis. Compounding involves not just the QA process, but staff training, equipment maintenance, proper documentation and appropriate analysis of products compounded. Reference: CCR ; ; ; ; ; 1751 et seq Question: For the purposes of CCR section (a)(6) and (a), would patients receiving chemotherapy administered in an infusion center that is part of a health care facility be considered in-patients and exempt from the labeling requirements? Answer: If the infusion center is part of the licensed health care facility and the patients receiving care there are registered as hospital inpatients, then yes the exemption provided by CCR 1735(a)(6) would apply. However, the labeling requirements as defined in Page 8
9 CCR would apply and compliance would be expected. Reference: B&P 4027, 4019, 4029; CCR (a)(6), Question: CCR section defines what must be recorded for each compounded drug product. CCR (a)(7) states, The equipment used in compounding the drug product. Does this include tubing sets, spikes, needles, syringes, etc.? Answer: Yes, all equipment used for compounding the drug product must be recorded TPN compounders, homogenizers, scales, syringes, needles, tubing sets, spikes, filters, mortar and pestle, pill making device, infusion devices. If there are more than one of the same device (e.g. - scales, laminar flow hoods) it is recommended to label them in some manner to distinguish which one was used in the process for appropriate completion of the compounding record. Reference: CCR (a)(7) Question: Where would the lot number, manufacturer, and expiration date be recorded? Answer: The law does not specify where or how the information is to be recorded. A pharmacy may develop it own form(s) for the proper documentation. The pharmacy shall maintain the record for three years from the date it was created. Reference: CCR Question: CCR section (d) states, All cytotoxic agents shall bear a special label which states Chemotherapy Dispose of Properly. This appears to give no wiggle room for the text of the message. Answer: There are no exceptions. If a drug is classified as a cytotoxic agent then the special label must be used. Reference: CCR (d)26. Page 9
10 26. Question: Gancyclovir is a cytotoxic agent but is not a chemotherapeutic agent. Does the special label need to be applied? Answer: Yes, the regulation does not provide for exceptions. However, nothing prevents the pharmacist from consulting the patient on the drugs classification and use. Reference: CCR (d) Question: CCR section (b)(1) states, in pertinent part, Cleanroom garb consisting of low-shedding coverall, head cover must be worn inside the designated area at all times. USP 797 does not require the use of a coverall, only a gown. Answer: The board does not enforce USP 797, but expects compliance with board regulations. A coverall is much more encompassing than a gown and would provide better protection during the compounding process. Reference: CCR (b)(1) Question: For a compounded drug product can a pharmacy use an expiration date, or beyond use date, of greater than 180 days? Answer: Yes, if the longer date is supported by stability studies of finished drugs or compounded drug products using the same components and packaging. Reference: CCR (h) Question: If a pharmacy makes a compounded drug product and does the qualitative and quantitative testing that demonstrates it has a stability expiration dating greater than 180 days, can another pharmacy use the same formula, with minor changes, use the same extended expiration date? Answer: No. To use another pharmacy s extended expiration date the formula must use the same components and packaging. Reference: CCR (h)30. Page 10
11 30. Question: Master formulas and compounding records are filed in separate locations, can easily be linked together, and are readily retrievable. Is it an absolute requirement to file these documents together? Answer: No, there is no such requirement for the above records to be maintained together as long as they are readily retrievable and available for inspection. These records may be maintained in a paper or electronic manner. However, qualitative and quantitative analysis reports for compounded drug products shall be retained by the pharmacy and collated (kept together) with the compounding record and master formula. Any records that are maintained electronically shall be maintained so that the pharmacist-in-charge or the pharmacist on duty shall during business hours be able to produce a hard copy and electronic copy. Reference: CCR (c); B&P 4105(d) Question: Is record keeping for compounding just referring to products that are administered intravenously or intraocular (e.g. where sterile preparation is imperative) or does it extend to oral and topical compounding? Answer: The regulations apply to all forms of compounding oral, inhalation, topical, sterile parenteral, etc. The record keeping requirements for sterile compounding are more extensive Reference CCR 1735 et seq & 1751 et seq Question: What is meant by proper acquisition? Answer: Records of proper acquisition of dangerous drugs and dangerous devices would include purchase records that correctly give the date, the names and address of the supplier and the buyer, the drug or device, and its quantity. Also, refer to Question #1 and its answer. Reference: B&P 4059(b) Page 11
4.5 Compounding Compounding in Licensed Pharmacies.
 0 0 0. Compounding. Compounding in Licensed Pharmacies. (a) Compounding means any of the following activities occurring in a licensed pharmacy, by or under the supervision of a licensed pharmacist, pursuant
0 0 0. Compounding. Compounding in Licensed Pharmacies. (a) Compounding means any of the following activities occurring in a licensed pharmacy, by or under the supervision of a licensed pharmacist, pursuant
ENFORCEMENT AND COMPOUNDING COMMITTEE REPORT June 2, 2017
 California State Board of Pharmacy 1625 N. Market Blvd, N219, Sacramento, CA 95834 Phone: (916) 574-7900 Fax: (916) 574-8618 www.pharmacy.ca.gov BUSINESS, CONSUMER SERVICES AND HOUSING AGENCY DEPARTMENT
California State Board of Pharmacy 1625 N. Market Blvd, N219, Sacramento, CA 95834 Phone: (916) 574-7900 Fax: (916) 574-8618 www.pharmacy.ca.gov BUSINESS, CONSUMER SERVICES AND HOUSING AGENCY DEPARTMENT
Compounding Pharmacy Assessment Questionnaire (CPAQ )
 Compounding Pharmacy Assessment Questionnaire (CPAQ ) The International Academy of Compounding Pharmacists represents more than 2,700 pharmacists and other professionals who specialize in the provision
Compounding Pharmacy Assessment Questionnaire (CPAQ ) The International Academy of Compounding Pharmacists represents more than 2,700 pharmacists and other professionals who specialize in the provision
I. Purpose. II. Definitions. Last Approval Date
 Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
Investigational Drugs and Biologics Page 1 of 13 I. Purpose The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics
CGMP Requirements for Investigational Products
 PREP #6 CGMP Requirements for Investigational Products Ji-Eun Kim, RPh, PhD Research Pharmacist Regulatory Affairs Office of Research Compliance December 6, 2016 1 CME Disclosure Statement Northwell Health
PREP #6 CGMP Requirements for Investigational Products Ji-Eun Kim, RPh, PhD Research Pharmacist Regulatory Affairs Office of Research Compliance December 6, 2016 1 CME Disclosure Statement Northwell Health
Microbiology Testing: USP requirements for Sterile and Nonsterile Preparations Webinar Q&A
 USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
USP Antimicrobial Effectiveness Testing Microbiology Testing: Webinar Q&A 1. If a base ingredient is designed for the purpose of compounding a specific type of preparation does this forgo the necessity
BSWH Technician Continuing Education 2016 Part 2: Pharmacy Law - Controlled Substance Security & Drug Supply Chain Security Act (DSCSA) Contact
 BSWH Technician Continuing Education 2016 Part 2: Pharmacy Law - Controlled Substance Security & Drug Supply Chain Security Act (DSCSA) Contact Hours: 1.5 Objectives List some strategies regarding safe
BSWH Technician Continuing Education 2016 Part 2: Pharmacy Law - Controlled Substance Security & Drug Supply Chain Security Act (DSCSA) Contact Hours: 1.5 Objectives List some strategies regarding safe
DSCSA: DRUG SUPPLY CHAIN SECURITY ACT AND IMPLEMENTATION. Chris Smith, R.Ph. Director, Pharmacy Business Intelligence Inmar
 DSCSA: DRUG SUPPLY CHAIN SECURITY ACT AND IMPLEMENTATION Chris Smith, R.Ph Director, Pharmacy Business Intelligence Inmar AGENDA CONTENTS History and key provisions Important concepts Timeline Meaningful
DSCSA: DRUG SUPPLY CHAIN SECURITY ACT AND IMPLEMENTATION Chris Smith, R.Ph Director, Pharmacy Business Intelligence Inmar AGENDA CONTENTS History and key provisions Important concepts Timeline Meaningful
What s the fuss with compounding. Fuss [2] Pharmaceutical Compounding. Pharmaceutical Compounding: Learning Objectives
![What s the fuss with compounding. Fuss [2] Pharmaceutical Compounding. Pharmaceutical Compounding: Learning Objectives What s the fuss with compounding. Fuss [2] Pharmaceutical Compounding. Pharmaceutical Compounding: Learning Objectives](/thumbs/84/89336296.jpg) Pharmaceutical Compounding Legal Requirements and Good Compounding Practices Tom Hazlet Autumn 2005 Pharmaceutical Compounding: Learning Objectives Describe the historical precedents and incidents that
Pharmaceutical Compounding Legal Requirements and Good Compounding Practices Tom Hazlet Autumn 2005 Pharmaceutical Compounding: Learning Objectives Describe the historical precedents and incidents that
GUIDANCE FOR INDUSTRY. Questions and Answers
 GUIDANCE FOR INDUSTRY Prescription Drug Marketing Act (PDMA) Requirements Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
GUIDANCE FOR INDUSTRY Prescription Drug Marketing Act (PDMA) Requirements Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
MINIMUM REQUIREMENTS FOR A VENDOR
 MINIMUM REQUIREMENTS FOR A VENDOR When outsourcing the production of sterile products the first step in vendor evaluation is to see if they meet the minimum requirements. We ve developed a group of questions
MINIMUM REQUIREMENTS FOR A VENDOR When outsourcing the production of sterile products the first step in vendor evaluation is to see if they meet the minimum requirements. We ve developed a group of questions
USP CHAPTER <797> INTRODUCTION RISK LEVELS
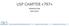 USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE. Fundamentals of Sterile Compounding (8 lessons/8 hours CE)
 Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE CriticalPoint s Sterile Compounding elearning curriculum is written by industry experts and covers both Chapter and
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE CriticalPoint s Sterile Compounding elearning curriculum is written by industry experts and covers both Chapter and
Kansas Pharmacy Act Amendments; Filling and Refilling Prescriptions; Biological Products; Senate Sub. for HB 2055
 Kansas Pharmacy Act Amendments; Filling and Refilling Prescriptions; Biological Products; Senate Sub. for HB 2055 (Act). Senate Sub. for HB 2055 makes several amendments to the Kansas Pharmacy Act The
Kansas Pharmacy Act Amendments; Filling and Refilling Prescriptions; Biological Products; Senate Sub. for HB 2055 (Act). Senate Sub. for HB 2055 makes several amendments to the Kansas Pharmacy Act The
Guidance for Industry Compounding Animal Drugs from Bulk Drug Substances
 #230 Guidance for Industry Compounding Animal Drugs from Bulk Drug Substances DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
#230 Guidance for Industry Compounding Animal Drugs from Bulk Drug Substances DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
j) Direct and Contiguous Compounding Area refers to the specific area where a compound is prepared.
 Modified As a result of May 2011 Board meeting and discussion at Convention 61-02-01-03. Pharmaceutical compounding standards; again on September 13 th, 2011 by Katie Miller and reviewed at the September
Modified As a result of May 2011 Board meeting and discussion at Convention 61-02-01-03. Pharmaceutical compounding standards; again on September 13 th, 2011 by Katie Miller and reviewed at the September
SUPPLEMENTAL NOTE ON HOUSE BILL NO. 2055
 Brief* SESSION OF 2017 SUPPLEMENTAL NOTE ON HOUSE BILL NO. 2055 As Recommended by House Committee on Health and Human Services HB 2055 would amend the Kansas Pharmacy Act (Act) by deleting, adding, and
Brief* SESSION OF 2017 SUPPLEMENTAL NOTE ON HOUSE BILL NO. 2055 As Recommended by House Committee on Health and Human Services HB 2055 would amend the Kansas Pharmacy Act (Act) by deleting, adding, and
Where Quality Meets Flexibility
 Where Quality Meets Flexibility is an industry leading 503B Outsourcing Facility providing sterile and non-sterile compounding services to hospitals, surgery centers, clinics, researchers & patients nationwide.
Where Quality Meets Flexibility is an industry leading 503B Outsourcing Facility providing sterile and non-sterile compounding services to hospitals, surgery centers, clinics, researchers & patients nationwide.
Washington State University INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE
 Washington State University INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE Use of Non-Pharmaceutical Grade Substances and Compounding Drugs in Laboratory Animals Purpose: To prevent contamination, infection
Washington State University INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE Use of Non-Pharmaceutical Grade Substances and Compounding Drugs in Laboratory Animals Purpose: To prevent contamination, infection
GUIDELINES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH MICHIGAN STATE UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY
 GUIDELINES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH MICHIGAN STATE UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY REVISION 4.2018 INTRODUCTION In conducting research with controlled substances, University
GUIDELINES FOR THE USE OF CONTROLLED SUBSTANCES IN RESEARCH MICHIGAN STATE UNIVERSITY ENVIRONMENTAL HEALTH AND SAFETY REVISION 4.2018 INTRODUCTION In conducting research with controlled substances, University
MANUFACTURE OF INVESTIGATIONAL MEDICINAL PRODUCTS
 ANNEX 13 MANUFACTURE OF INVESTIGATIONAL MEDICINAL PRODUCTS Introduction Medicinal products intended for research and development trials are not at present subject either to marketing or manufacturing Community
ANNEX 13 MANUFACTURE OF INVESTIGATIONAL MEDICINAL PRODUCTS Introduction Medicinal products intended for research and development trials are not at present subject either to marketing or manufacturing Community
Chapter WAC Compounding Practices Crosswalk
 Chapter 246-878 WAC Compounding Practices Crosswalk Draft WAC Language WAC 246-878-001 Purpose. 1) The requirements of this chapter apply to any person or facility that possesses a license under 18.64
Chapter 246-878 WAC Compounding Practices Crosswalk Draft WAC Language WAC 246-878-001 Purpose. 1) The requirements of this chapter apply to any person or facility that possesses a license under 18.64
AMENDED Draft Report of the Compounding Workgroup
 AMENDED Draft Report of the Compounding Workgroup This report summarizes the actions taken by a workgroup convened by the Board of Pharmacy pursuant to the enactment clause of HB 1035 passed during the
AMENDED Draft Report of the Compounding Workgroup This report summarizes the actions taken by a workgroup convened by the Board of Pharmacy pursuant to the enactment clause of HB 1035 passed during the
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE. Fundamentals of Sterile Compounding (8 lessons/8 hours CE)
 Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE Fundamentals of Sterile Compounding (8 lessons/8 hours CE) The History of Compounding and USP Sterile Compounding Chapters
Sterile Compounding elearning Course Curriculum 28 lessons with 33 hours of CE Fundamentals of Sterile Compounding (8 lessons/8 hours CE) The History of Compounding and USP Sterile Compounding Chapters
Drug Products, Labeling, and Packaging
 442 Pharmaceutical Industry: Drug Products, Labeling, and Packaging Positions Drug Products, Labeling, and Packaging Ready-to-Administer Packaging for Hazardous Drug Products Intended for Home Use (1711)
442 Pharmaceutical Industry: Drug Products, Labeling, and Packaging Positions Drug Products, Labeling, and Packaging Ready-to-Administer Packaging for Hazardous Drug Products Intended for Home Use (1711)
BRIEFING 1168 Compounding for Phase I Investigational Studies,
 BRIEFING 1168 Compounding for Phase I Investigational Studies, PF 39(5) [Sept. Oct. 2013]. The current proposed chapter in PF 43(3) [May June 2017] is posted online at www.usp.org/usp-nf/notices/compounding-forphase-i-investigational-studies
BRIEFING 1168 Compounding for Phase I Investigational Studies, PF 39(5) [Sept. Oct. 2013]. The current proposed chapter in PF 43(3) [May June 2017] is posted online at www.usp.org/usp-nf/notices/compounding-forphase-i-investigational-studies
TITLE: Research Pharmacy Standard Policy SOP #: INV-100 Page: 1 of 5 Effective Date: 8/31/17
 SOP #: INV-100 Page: 1 of 5 1. POLICY STATEMENT: DF/HCC research pharmacies follow a standard set of policy requirements for clinical trials. 2. BACKGROUND: None 3. RESPONSIBLE PERSONNEL: 3.1. Research
SOP #: INV-100 Page: 1 of 5 1. POLICY STATEMENT: DF/HCC research pharmacies follow a standard set of policy requirements for clinical trials. 2. BACKGROUND: None 3. RESPONSIBLE PERSONNEL: 3.1. Research
U.S. Traceability: the Drug Supply Chain Security Act
 U.S. Traceability: the Drug Supply Chain Security Act Connie Jung, RPh, PhD Associate Director for Policy & Communications (Acting) Office of Drug Security, Integrity and Recalls Office of Compliance U.S.
U.S. Traceability: the Drug Supply Chain Security Act Connie Jung, RPh, PhD Associate Director for Policy & Communications (Acting) Office of Drug Security, Integrity and Recalls Office of Compliance U.S.
Pharmacy. Medication. Checks
 Pharmacy Medication Checks Module 2 Table of Contents Section A... 1 A.1 Clinical Medication Order Check... 1 A.2 Final Product and Computer Order Entry Check... 1 Section B... 2 B.1 Documentation of Pharmacy
Pharmacy Medication Checks Module 2 Table of Contents Section A... 1 A.1 Clinical Medication Order Check... 1 A.2 Final Product and Computer Order Entry Check... 1 Section B... 2 B.1 Documentation of Pharmacy
Community Pharmacy Opening/Renovation Audit
 Community Pharmacy Opening/Renovation Audit Date of Audit: Pharmacy Information Pharmacy Name Address City Postal Code Pharmacy Hours Telephone Mon Tues Wed Thurs Fri Sat Sun Fax e-mail Website Pharmacist(s)
Community Pharmacy Opening/Renovation Audit Date of Audit: Pharmacy Information Pharmacy Name Address City Postal Code Pharmacy Hours Telephone Mon Tues Wed Thurs Fri Sat Sun Fax e-mail Website Pharmacist(s)
HDA Qs and As on the Drug Supply Chain Security Act (DSCSA) VERSION 4.0 June 2016
 (DSCSA) This document, the HDA Qs and As on the Drug Supply Chain Security Act (DSCSA), has been prepared by the Healthcare Distribution Alliance (HDA) in consultation with its Traceability Implementation
(DSCSA) This document, the HDA Qs and As on the Drug Supply Chain Security Act (DSCSA), has been prepared by the Healthcare Distribution Alliance (HDA) in consultation with its Traceability Implementation
Quality is Our Promise.
 Quality is Our Promise. Our goal at KRS Global Biotechnology is to provide the highest quality pharmaceutical preparations. We accomplish this with an unrivaled quality assurance and quality control program
Quality is Our Promise. Our goal at KRS Global Biotechnology is to provide the highest quality pharmaceutical preparations. We accomplish this with an unrivaled quality assurance and quality control program
Learning Objectives. What is a Compounding Pharmacist? Disclosure
 PHARMACY COMPOUNDING: Update on Trends & Regulations Michael Roberge, RPh Certified Compounding Pharmacist Owner, Compounded Solutions in Pharmacy LLC Monroe, CT Learning Objectives Define pharmacy compounding
PHARMACY COMPOUNDING: Update on Trends & Regulations Michael Roberge, RPh Certified Compounding Pharmacist Owner, Compounded Solutions in Pharmacy LLC Monroe, CT Learning Objectives Define pharmacy compounding
Profiles in CSP Insourcing: Scripps Health
 Profiles in CSP Insourcing: Scripps Health Robert Eastin, Pharm.D. Director, Central Pharmacy and Shared Services Scripps Health Hospital Profile Scripps Health is a private, nonprofit integrated health
Profiles in CSP Insourcing: Scripps Health Robert Eastin, Pharm.D. Director, Central Pharmacy and Shared Services Scripps Health Hospital Profile Scripps Health is a private, nonprofit integrated health
Summary of USP* 797 Pharmaceutical Compounding Sterile Preparations
 Summary of USP* 797 Pharmaceutical Compounding Sterile Preparations Source of base information Pharmacopeial Form Vol 29 (4) July Aug. 2003 Effective date January 1, 2004 FDA enforceable Scope The content
Summary of USP* 797 Pharmaceutical Compounding Sterile Preparations Source of base information Pharmacopeial Form Vol 29 (4) July Aug. 2003 Effective date January 1, 2004 FDA enforceable Scope The content
CMS , Medicare Benefit Policy Manual, Chapter 15- Section 50 - Drugs and Biologicals:
 Billing and Coding Guidelines for Drugs and Biologics (Non-chemotherapy) L 34741 Medicare Excerpts: CMS 100-02, Medicare Benefit Policy Manual, Chapter 15- Section 50 - Drugs and Biologicals: 50.2 - Determining
Billing and Coding Guidelines for Drugs and Biologics (Non-chemotherapy) L 34741 Medicare Excerpts: CMS 100-02, Medicare Benefit Policy Manual, Chapter 15- Section 50 - Drugs and Biologicals: 50.2 - Determining
Ghalib Abbasi, RPh, MS, PharmD Pharmacy Technology Consultant Florida, USA
 Ghalib Abbasi, RPh, MS, PharmD Pharmacy Technology Consultant Florida, USA Disclosure Information IV Robotics and Workflow: Design and Integration with other Healthcare and Regulatory Components Ghalib
Ghalib Abbasi, RPh, MS, PharmD Pharmacy Technology Consultant Florida, USA Disclosure Information IV Robotics and Workflow: Design and Integration with other Healthcare and Regulatory Components Ghalib
Regulation Regarding the Packaging and Labeling of Medicinal Products for Human Use
 Regulation Regarding the Packaging and Labeling of Medicinal Products for Human Use, 01.02.2008, ENG The amendments made with the Regulation Amending the Regulation Regarding the Packaging and Labeling
Regulation Regarding the Packaging and Labeling of Medicinal Products for Human Use, 01.02.2008, ENG The amendments made with the Regulation Amending the Regulation Regarding the Packaging and Labeling
Timeline for the Drug Supply Chain and Security Act
 April 2014 Timeline for the Drug Supply Chain and Security Act (Title II, Drug Quality and Security Act, 2013) Overview The Drug Supply Chain and Security Act (Title II, Drug Quality and Security Act,
April 2014 Timeline for the Drug Supply Chain and Security Act (Title II, Drug Quality and Security Act, 2013) Overview The Drug Supply Chain and Security Act (Title II, Drug Quality and Security Act,
Compounding Animal Drugs From Bulk Drug Substances; Draft Guidance for Industry;
 This document is scheduled to be published in the Federal Register on 05/19/2015 and available online at http://federalregister.gov/a/2015-11982, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 05/19/2015 and available online at http://federalregister.gov/a/2015-11982, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Pharmaceutical compounding standards. The minimum standards and technical equipment to be considered as adequate shall include: 1.
 61-02-01-03. Pharmaceutical compounding standards. The minimum standards and technical equipment to be considered as adequate shall include: 1. Definitions. a. "Active chemical or ingredient" refers to
61-02-01-03. Pharmaceutical compounding standards. The minimum standards and technical equipment to be considered as adequate shall include: 1. Definitions. a. "Active chemical or ingredient" refers to
Barcodes on Unit of Use
 Barcodes on Unit of Use 1 What hospitals need Hospitals need medications Ready-to-Administer In Unit-of-Use With Barcode 2 What hospitals need Ready-to-Administer If not, must go through additional pharmacy
Barcodes on Unit of Use 1 What hospitals need Hospitals need medications Ready-to-Administer In Unit-of-Use With Barcode 2 What hospitals need Ready-to-Administer If not, must go through additional pharmacy
9/19/2013 DISCUSSION POINTS COMPOUNDING INDUSTRY TRENDS
 COMPOUNDING PHARMACIES & ASCS Regulatory Update Alison Cherney CEO Ionia Pharmacy cherneyaj@aol.com DISCUSSION POINTS Compounding industry trends Backordered medication trends Federal regulatory statutes
COMPOUNDING PHARMACIES & ASCS Regulatory Update Alison Cherney CEO Ionia Pharmacy cherneyaj@aol.com DISCUSSION POINTS Compounding industry trends Backordered medication trends Federal regulatory statutes
116th Annual Convention
 116th Annual Convention Date: Saturday, October 18, 2014 Time: 10:30 am 12:00 pm Location: Austin Convention Center, Room 16A, Level 4 Title: Activity Type: Speaker: Impetus for Change and Standards of
116th Annual Convention Date: Saturday, October 18, 2014 Time: 10:30 am 12:00 pm Location: Austin Convention Center, Room 16A, Level 4 Title: Activity Type: Speaker: Impetus for Change and Standards of
QUALITY ASSURANCE & AUDIT
 QUALITY ASSURANCE & AUDIT Birinder Kaur Senior Pharmacist Pharmacy Dept National University Of Malaysia Medical Centre Kuala Lumpur birin@ppukm.ukm.edu.my LEARNING OBJECTIVES To define quality assurance
QUALITY ASSURANCE & AUDIT Birinder Kaur Senior Pharmacist Pharmacy Dept National University Of Malaysia Medical Centre Kuala Lumpur birin@ppukm.ukm.edu.my LEARNING OBJECTIVES To define quality assurance
Documenta tion and Records
 Documenta tion and Records Page 1 of 30 Training Outcome of the Module: After completing this module, you will be able to: Recognize the importance of procedures Recognize the importance of record keeping
Documenta tion and Records Page 1 of 30 Training Outcome of the Module: After completing this module, you will be able to: Recognize the importance of procedures Recognize the importance of record keeping
Inspection Form. DPP Information. Inspection Information. Staff Information. Service Information. DPP Designated Member. Street City Postal Code
 Inspection Form DPP Information Name of DPP Owner Name of DPP (if different from owner's name) DPP Designated Member Designated Member Email Designated Member Phone number Street City Postal Code Phone
Inspection Form DPP Information Name of DPP Owner Name of DPP (if different from owner's name) DPP Designated Member Designated Member Email Designated Member Phone number Street City Postal Code Phone
Infection Prevention for Pharmacy Compounding
 Infection Prevention for Pharmacy Compounding Samuel M. Eberwein, PharmD, MS, BCPS November 5, 2018 Infection Prevention Starts with Regulation Compounding Regulation has a Rich History 2019 USP
Infection Prevention for Pharmacy Compounding Samuel M. Eberwein, PharmD, MS, BCPS November 5, 2018 Infection Prevention Starts with Regulation Compounding Regulation has a Rich History 2019 USP
Drug Supply Chain Security Act Implementation
 Drug Supply Chain Security Act (DSCSA) Updates and Actions for Health System Pharmacy Anita Ducca, M.S. Senior Vice President, Regulatory Affairs HDMA Washington, DC Raymond Lake, R.Ph., M.S. Corporate
Drug Supply Chain Security Act (DSCSA) Updates and Actions for Health System Pharmacy Anita Ducca, M.S. Senior Vice President, Regulatory Affairs HDMA Washington, DC Raymond Lake, R.Ph., M.S. Corporate
Ingredient Listing Qty. Unit NDC # Supplier. Sterile Preparation
 3/22/2012; page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Hydromorphone Hydrochloride, USP 0.200 g Citric Acid (Anhydrous), USP 0.04 g Sodium Citrate (Dihydrate), USP 0.04 g Sodium
3/22/2012; page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Hydromorphone Hydrochloride, USP 0.200 g Citric Acid (Anhydrous), USP 0.04 g Sodium Citrate (Dihydrate), USP 0.04 g Sodium
Ingredient Listing Qty. Unit NDC # Supplier. Sterile Preparation
 3/22/2012; page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Morphine Sulfate, USP 0.200 g Sodium Chloride, USP 0.152 g Sterile Water For Injection, USP 15.0 ml Sterile Water For
3/22/2012; page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Morphine Sulfate, USP 0.200 g Sodium Chloride, USP 0.152 g Sterile Water For Injection, USP 15.0 ml Sterile Water For
Compounding Unit-of-Use Kits
 Compounding Unit-of-Use Kits White Paper by Loyd V. Allen PhD, RPh Professor Emeritus, College of Pharmacy, University of Oklahoma Former Chairperson, USP Pharmacy Compounding Expert Committee Editor in
Compounding Unit-of-Use Kits White Paper by Loyd V. Allen PhD, RPh Professor Emeritus, College of Pharmacy, University of Oklahoma Former Chairperson, USP Pharmacy Compounding Expert Committee Editor in
RECOMMENDATIONS FOR DIETARY INGREDIENT PROCESSORS
 RECOMMENDATIONS FOR DIETARY INGREDIENT PROCESSORS Facilities that manufacture 117, pack, or hold dietary supplements are subject to the regulations in 21 CFR Part 111, while those that manufacture, pack,
RECOMMENDATIONS FOR DIETARY INGREDIENT PROCESSORS Facilities that manufacture 117, pack, or hold dietary supplements are subject to the regulations in 21 CFR Part 111, while those that manufacture, pack,
Pharmaceutical Compounding Sterile and Nonsterile Preparations. Jeanne Sun, PharmD
 Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
Pharmaceutical Compounding Sterile and Nonsterile Preparations Jeanne Sun, PharmD Agenda Legal Framework Overview Pharmaceutical Compounding Nonsterile Preparations Pharmaceutical Compounding
The purpose of these guidelines is to describe a standardized approach for the management of
 ASHP Guidelines for the Management of Investigational Drug Products Purpose The purpose of these guidelines is to describe a standardized approach for the management of investigational drug products by
ASHP Guidelines for the Management of Investigational Drug Products Purpose The purpose of these guidelines is to describe a standardized approach for the management of investigational drug products by
The GAP Analysis for Nonsterile Compounding
 VOLUME 17 NUMBER 2 Current & Practical Compounding Information for the Pharmacist provided for by a grant from Perrigo Pharmaceuticals Goal: To provide background information and practical procedures for
VOLUME 17 NUMBER 2 Current & Practical Compounding Information for the Pharmacist provided for by a grant from Perrigo Pharmaceuticals Goal: To provide background information and practical procedures for
Guidance for Industry DRAFT GUIDANCE
 Reprinted from FDA s website by EAS Consulting Group, LLC DSCSA Standards for the Interoperable Exchange of Information for Tracing of Certain Human, Finished, Prescription Drugs: How to Exchange Product
Reprinted from FDA s website by EAS Consulting Group, LLC DSCSA Standards for the Interoperable Exchange of Information for Tracing of Certain Human, Finished, Prescription Drugs: How to Exchange Product
Code of Practice for Holder of Certificate of Registration as an Importer and Exporter of Pharmaceutical Products
 Code of Practice for Holder of Certificate of Registration as an Importer and Exporter of Pharmaceutical Products March 2014 Table of Content INTRODUCTION 3 Section 1: GENERAL RESPONSIBILITIES OF HOLDER
Code of Practice for Holder of Certificate of Registration as an Importer and Exporter of Pharmaceutical Products March 2014 Table of Content INTRODUCTION 3 Section 1: GENERAL RESPONSIBILITIES OF HOLDER
The ABCs and Challenges of GMP The American Experience
 The ABCs and Challenges of GMP The American Experience The Canadian Association of Nuclear Medicine Toronto, Ontario April 22, 2017 Reiko Oyama, R.Ph., B.C.N.P. Washington University School of Medicine
The ABCs and Challenges of GMP The American Experience The Canadian Association of Nuclear Medicine Toronto, Ontario April 22, 2017 Reiko Oyama, R.Ph., B.C.N.P. Washington University School of Medicine
Supply of aseptically - prepared doses of IMPs across legal boundaries. Edition 1. December 2017
 Supply of aseptically - prepared doses of IMPs across legal boundaries Edition 1 December 2017 Endorsed and supported by: NHS Pharmaceutical Quality Assurance Committee 2017 with National Pharmacy Clinical
Supply of aseptically - prepared doses of IMPs across legal boundaries Edition 1 December 2017 Endorsed and supported by: NHS Pharmaceutical Quality Assurance Committee 2017 with National Pharmacy Clinical
Phase Appropriate GMPs for IMPs. Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017
 Phase Appropriate GMPs for IMPs Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017 1 Lets start with References https://mhrainspectorate.blog.gov.uk/2016/0 5/20/manufacture-of-investigationalmedicinal-products-frequently-askedquestions/
Phase Appropriate GMPs for IMPs Presented by: Karen S. Ginsbury For: IFF, October 31, Nov 02, 2017 1 Lets start with References https://mhrainspectorate.blog.gov.uk/2016/0 5/20/manufacture-of-investigationalmedicinal-products-frequently-askedquestions/
Compounding Pharmacy Accreditation Standards. Table of Contents. An Overview of BOC Compounding Pharmacy Accreditation 6/23/2017
 Table of Contents An Overview of BOC Compounding Pharmacy Accreditation 6/23/2017 Overview... 3 Chapter 1: Regulatory Compliance... 3 Section 1.1 Facility Licensure... 3 Section 1.2 External Standards...
Table of Contents An Overview of BOC Compounding Pharmacy Accreditation 6/23/2017 Overview... 3 Chapter 1: Regulatory Compliance... 3 Section 1.1 Facility Licensure... 3 Section 1.2 External Standards...
Ensuring Quality Pharmacy Compounding: Implications for Pharmaceutics Education
 Ensuring Quality Pharmacy Compounding: Implications for Pharmaceutics Education Loyd V. Allen, Jr., Ph.D. Professor & Chair Emeritus University of Oklahoma HSC College of Pharmacy Editor-in in-chief International
Ensuring Quality Pharmacy Compounding: Implications for Pharmaceutics Education Loyd V. Allen, Jr., Ph.D. Professor & Chair Emeritus University of Oklahoma HSC College of Pharmacy Editor-in in-chief International
Guidance for Industry
 Guidance for Industry Handling and Retention of BA and BE Testing Samples U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) May 2004
Guidance for Industry Handling and Retention of BA and BE Testing Samples U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) May 2004
Overview. A brief from
 A brief from Dec 2017 Getty Images What Are Compounded Drugs, and How Can They Be Kept Safe? Drug Quality and Security Act plays key role in important but potentially high-risk aspect of health care Overview
A brief from Dec 2017 Getty Images What Are Compounded Drugs, and How Can They Be Kept Safe? Drug Quality and Security Act plays key role in important but potentially high-risk aspect of health care Overview
This guidance is for immediate implementation.
 Requirements for Transactions with First Responders under Section 582 of the Federal Food, Drug, and Cosmetic Act Compliance Policy Guidance for Industry This guidance is for immediate implementation.
Requirements for Transactions with First Responders under Section 582 of the Federal Food, Drug, and Cosmetic Act Compliance Policy Guidance for Industry This guidance is for immediate implementation.
What To Do or Not To Do? Utility of Medication Traceability to the Patient
 What To Do or Not To Do? Utility of Medication Traceability to the Patient Melsen Kwong, Pharm.D. Associate Director Department of Pharmacy Services Cedars-Sinai Medical Center, Los Angeles, CA, USA Objectives
What To Do or Not To Do? Utility of Medication Traceability to the Patient Melsen Kwong, Pharm.D. Associate Director Department of Pharmacy Services Cedars-Sinai Medical Center, Los Angeles, CA, USA Objectives
Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC
 Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC U.S. House of Representatives Energy and Commerce Committee Subcommittee on Health Subcommittee January 30, 2018
Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC U.S. House of Representatives Energy and Commerce Committee Subcommittee on Health Subcommittee January 30, 2018
HAZARDOUS MATERIALS 7/7/2016 OR THE RIGHT-TO-KNOW HAZARD COMMUNICATION STANDARD (HCS) DISCLAIMER
 HAZARD COMMUNICATION STANDARD (HCS) HAZARDOUS MATERIALS SAFE HANDLING Passed by Congress in 1988 with feedback from National Institute for Occupational Safety and Health (NIOSH) United States Pharmacopeia
HAZARD COMMUNICATION STANDARD (HCS) HAZARDOUS MATERIALS SAFE HANDLING Passed by Congress in 1988 with feedback from National Institute for Occupational Safety and Health (NIOSH) United States Pharmacopeia
SECTION: PATIENT CARE Number:
 SUBJECT: Safe Handling of Hazardous Drugs Page: 1 of 10 INTRODUCTION Purpose: This policy has been developed to promote safe work practices for all employees who prepare or administer hazardous drugs or
SUBJECT: Safe Handling of Hazardous Drugs Page: 1 of 10 INTRODUCTION Purpose: This policy has been developed to promote safe work practices for all employees who prepare or administer hazardous drugs or
INVESTIGATIONAL DRUG MANAGEMENT OVERVIEW
 NORTHWELL HEALTH OFFICE OF RESEARCH COMPLIANCE STUDY FEASIBILITY AND APPROVAL Where can I learn about the investigational drug? Protocol Investigator s brochure Product insert or prescribing information
NORTHWELL HEALTH OFFICE OF RESEARCH COMPLIANCE STUDY FEASIBILITY AND APPROVAL Where can I learn about the investigational drug? Protocol Investigator s brochure Product insert or prescribing information
Cleaning validation of cleanrooms and preparation equipments
 Cleaning validation of cleanrooms and preparation equipments Dr Farshid SADEGHIPOUR Head of production Central Pharmacy, Geneva University Hospitals EAHP Foundation Seminar: Patient Safety; More About
Cleaning validation of cleanrooms and preparation equipments Dr Farshid SADEGHIPOUR Head of production Central Pharmacy, Geneva University Hospitals EAHP Foundation Seminar: Patient Safety; More About
Notice of Rulemaking Hearing
 Department of State Division of Publications 312 Rosa L. Parks, 8th Floor SnodgrassfTN Tower Nashville, TN 37243 Phone: 615.741.2650 Fax: 615.741.5133 Email: register.information@tn.gov For Department
Department of State Division of Publications 312 Rosa L. Parks, 8th Floor SnodgrassfTN Tower Nashville, TN 37243 Phone: 615.741.2650 Fax: 615.741.5133 Email: register.information@tn.gov For Department
BRIEFING. PF 44(4) [July Aug. 2018]. The General Chapters Packaging and. Distribution Expert Committee has canceled the PF 44(4) proposal and is
![BRIEFING. PF 44(4) [July Aug. 2018]. The General Chapters Packaging and. Distribution Expert Committee has canceled the PF 44(4) proposal and is BRIEFING. PF 44(4) [July Aug. 2018]. The General Chapters Packaging and. Distribution Expert Committee has canceled the PF 44(4) proposal and is](/thumbs/95/122852592.jpg) BRIEFING 659 Packaging and Storage Requirements, USP 42 page 6801 and PF 44(4) [July Aug. 2018]. The General Chapters Packaging and Distribution Expert Committee has canceled the PF 44(4) proposal and
BRIEFING 659 Packaging and Storage Requirements, USP 42 page 6801 and PF 44(4) [July Aug. 2018]. The General Chapters Packaging and Distribution Expert Committee has canceled the PF 44(4) proposal and
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act
 Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry U.S. Department of Health and Human Services Food and Drug
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry U.S. Department of Health and Human Services Food and Drug
PHARMACY IV ADMIXTURE
 PHARMACY IV ADMIXTURE Pharmacy 483 January 18, 2007 Kim Donnelly Affiliate Assistant Professor kimdon@u.washington.edu Pharmacist is responsible for ensuring that compounded sterile preparations are properly
PHARMACY IV ADMIXTURE Pharmacy 483 January 18, 2007 Kim Donnelly Affiliate Assistant Professor kimdon@u.washington.edu Pharmacist is responsible for ensuring that compounded sterile preparations are properly
Resolution CM/Res(2016)2 on good reconstitution practices in health care establishments for medicinal products for parenteral use
 Resolution CM/Res(2016)2 on good reconstitution practices in health care establishments for medicinal products for parenteral use (Adopted by the Committee of Ministers on 1 June 2016 at the 1258th meeting
Resolution CM/Res(2016)2 on good reconstitution practices in health care establishments for medicinal products for parenteral use (Adopted by the Committee of Ministers on 1 June 2016 at the 1258th meeting
DRUG ORDERING AND MAINTENANCE
 AND MAINTENANCE Disclaimer: Please note that the instructions provided in this chapter mainly apply to agents provided by the Pharmaceutical Management Branch (PMB), of Cancer Therapy Evaluation Program
AND MAINTENANCE Disclaimer: Please note that the instructions provided in this chapter mainly apply to agents provided by the Pharmaceutical Management Branch (PMB), of Cancer Therapy Evaluation Program
USP Chapter 823 USP 32 (old) vs. USP 35 (new)
 USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
In-State Licensee Newsletter January 2007
 LOUISIANA BOARD OF WHOLESALE DRUG DISTRIBUTORS 12046 Justice Avenue, Suite C Baton Rouge, LA 70816 Phone: 225-295-8567 Fax: 225-295-8568 Website: www.lsbwdd.org email: lsbwdd@bellsouth.net In-State Licensee
LOUISIANA BOARD OF WHOLESALE DRUG DISTRIBUTORS 12046 Justice Avenue, Suite C Baton Rouge, LA 70816 Phone: 225-295-8567 Fax: 225-295-8568 Website: www.lsbwdd.org email: lsbwdd@bellsouth.net In-State Licensee
A Regulatory Update for Home Infusion Clinicians
 A Regulatory Update for Home Infusion Clinicians Kendall Van Pool Vice President of Legislative Affairs NHIA, Alexandria, VA Cynthia Blankenship, Esq. Of Counsel Rose Law Firm, Little Rock, AR Mike Koch,
A Regulatory Update for Home Infusion Clinicians Kendall Van Pool Vice President of Legislative Affairs NHIA, Alexandria, VA Cynthia Blankenship, Esq. Of Counsel Rose Law Firm, Little Rock, AR Mike Koch,
Not for Distribution. The Art, Science, and Technology of Pharmaceutical Compounding
 The Art, Science, and Technology of Pharmaceutical Compounding FIFTH EDITION Loyd V. Allen Jr., PhD EDITOR IN CHIEF International Journal of Pharmaceutical Compounding Edmond, Oklahoma and PROFESSOR EMERITUS
The Art, Science, and Technology of Pharmaceutical Compounding FIFTH EDITION Loyd V. Allen Jr., PhD EDITOR IN CHIEF International Journal of Pharmaceutical Compounding Edmond, Oklahoma and PROFESSOR EMERITUS
CerpassRx Member Handbook
 CerpassRx Member Handbook Welcome CerpassRx is pleased to administer your prescription benefit plan. About Us CerpassRx is an innovative Pharmacy Benefit Administrator that offers access to pharmacies
CerpassRx Member Handbook Welcome CerpassRx is pleased to administer your prescription benefit plan. About Us CerpassRx is an innovative Pharmacy Benefit Administrator that offers access to pharmacies
March 4, Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
GUIDELINES FOR INTRODUCING A LOCALLY MANUFACTURED NEW PHARMACEUTICAL PRODUCT ON THE UGANDA MARKET
 GUIDELINES FOR INTRODUCING A LOCALLY MANUFACTURED NEW PHARMACEUTICAL PRODUCT ON THE UGANDA MARKET National Drug Authority Head Office Rumee Towers Plot 19, Lumumba Avenue P. O. Box 23096 Kampala, Uganda
GUIDELINES FOR INTRODUCING A LOCALLY MANUFACTURED NEW PHARMACEUTICAL PRODUCT ON THE UGANDA MARKET National Drug Authority Head Office Rumee Towers Plot 19, Lumumba Avenue P. O. Box 23096 Kampala, Uganda
Microbiological Quality of Drug Products after Penetration of the Container System for Dose Preparation Prior to Patient Administration
 Microbiological Quality of Drug Products after Penetration of the Container System for Dose Preparation Prior to Patient Administration John W. Metcalfe, Ph.D. Senior Review Microbiologist FDA/CDER/OPS/New
Microbiological Quality of Drug Products after Penetration of the Container System for Dose Preparation Prior to Patient Administration John W. Metcalfe, Ph.D. Senior Review Microbiologist FDA/CDER/OPS/New
INVESTIGATIONAL DRUG SERVICE (IDS) Standard Operating Procedures
 INVESTIGATIONAL DRUG SERVICE (IDS) Standard Operating Procedures The Investigational Drug Service (IDS) strives to meet or exceed the standards of Good Clinical Practice (GCP). 1. UC Davis Health (UCDH)
INVESTIGATIONAL DRUG SERVICE (IDS) Standard Operating Procedures The Investigational Drug Service (IDS) strives to meet or exceed the standards of Good Clinical Practice (GCP). 1. UC Davis Health (UCDH)
PHARMACY IV ADMIXTURE. Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor
 PHARMACY IV ADMIXTURE Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor Pharmacist is responsible for ensuring that compounded sterile preparations are properly prepared, labeled,
PHARMACY IV ADMIXTURE Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor Pharmacist is responsible for ensuring that compounded sterile preparations are properly prepared, labeled,
September 2, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 September 2, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Draft Guidance: Current Good Manufacturing Practice Interim
September 2, 2014 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: FDA Draft Guidance: Current Good Manufacturing Practice Interim
Expiration Dating of Unit-Dose Repackaged Solid Oral Dosage Form Drug Products Guidance for Industry
 Expiration Dating of Unit-Dose Repackaged Solid Oral Dosage Form Drug Products Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Expiration Dating of Unit-Dose Repackaged Solid Oral Dosage Form Drug Products Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Why Do I Test, What Do I Test & When Do I Test It? Ross Caputo, PhD Chief Technical Officer Eagle Analytical Services
 Why Do I Test, What Do I Test & When Do I Test It? Ross Caputo, PhD Chief Technical Officer Eagle Analytical Services Disclosures I, Ross Caputo, declare no conflicts of interest, real or apparent, and
Why Do I Test, What Do I Test & When Do I Test It? Ross Caputo, PhD Chief Technical Officer Eagle Analytical Services Disclosures I, Ross Caputo, declare no conflicts of interest, real or apparent, and
for IND and RDRC Regulated PET Compounding
 Overview of USP Chapter for IND and RDRC Regulated PET Compounding Distributed Manufacturing of PET Radiopharmaceuticals for Multi-Center Clinical Trials SNM, Annual Meeting Toronto, Ontario, Canada
Overview of USP Chapter for IND and RDRC Regulated PET Compounding Distributed Manufacturing of PET Radiopharmaceuticals for Multi-Center Clinical Trials SNM, Annual Meeting Toronto, Ontario, Canada
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503A of the Federal Food, Drug, and Cosmetic Act
 Interim Policy on Compounding Using Bulk Drug Substances Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry U.S. Department of Health and Human Services Food and Drug
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry U.S. Department of Health and Human Services Food and Drug
Prescription Product Supplier Requirements
 Prescription Product Supplier Requirements Prescription Product Suppliers: Prescription product suppliers are those suppliers that manufacture, package, or distribute a prescription pharmaceutical (drug)
Prescription Product Supplier Requirements Prescription Product Suppliers: Prescription product suppliers are those suppliers that manufacture, package, or distribute a prescription pharmaceutical (drug)
INNOVATIVE COMPOUNDING SERVICES
 INNOVATIVE COMPOUNDING SERVICES We are excited to introduce our innovative compounded oral rinses that may offer a safe and effective alternative for the management of side effects associated with chemotherapy
INNOVATIVE COMPOUNDING SERVICES We are excited to introduce our innovative compounded oral rinses that may offer a safe and effective alternative for the management of side effects associated with chemotherapy
We Love Drugs. We Use Drugs
 Where s It Coming From? Counterfeits and Pharmaceutical Pedigree Bryan A. Liang, MD, PhD, JD San Diego Center for Patient Safety University of California, San Diego School of Medicine Institute of Health
Where s It Coming From? Counterfeits and Pharmaceutical Pedigree Bryan A. Liang, MD, PhD, JD San Diego Center for Patient Safety University of California, San Diego School of Medicine Institute of Health
PHARMACY COMPOUNDING 2013!
 PHARMACY COMPOUNDING 2013! Loyd V. Allen, Jr., Ph.D. Professor Emeritus University of Oklahoma HSC College of Pharmacy --- Editor-in-Chief International Journal of Pharmaceutical Compounding and Remington-The
PHARMACY COMPOUNDING 2013! Loyd V. Allen, Jr., Ph.D. Professor Emeritus University of Oklahoma HSC College of Pharmacy --- Editor-in-Chief International Journal of Pharmaceutical Compounding and Remington-The
Being Prepared for Track and Trace: DSCSA 101
 Being Prepared for Track and Trace: DSCSA 101 Susanne Somerville, Founder The LinkLab Eric Garvin, Founder, The LinkLab Brian Daleiden, VP Industry Marketing and Co-Founder, TraceLink Objective of Today
Being Prepared for Track and Trace: DSCSA 101 Susanne Somerville, Founder The LinkLab Eric Garvin, Founder, The LinkLab Brian Daleiden, VP Industry Marketing and Co-Founder, TraceLink Objective of Today
Relevant State and Federal statutory requirements are to be complied with.
 32.0 Pharmacy Unit 32.1 Introduction 32.1.1 Description The purpose of the Pharmacy Unit is to provide all inpatient and outpatient pharmacy services including dispensing, preparation of non-sterile and
32.0 Pharmacy Unit 32.1 Introduction 32.1.1 Description The purpose of the Pharmacy Unit is to provide all inpatient and outpatient pharmacy services including dispensing, preparation of non-sterile and
