Examining the Predictive Accuracy of Sterilizing Mouse Efficacy Models
|
|
|
- Lily Mosley
- 5 years ago
- Views:
Transcription
1 Examining the Predictive Accuracy of Sterilizing Mouse Efficacy Models Eric Nuermberger, MD Center for TB Research, Johns Hopkins University March 20, 2107
2 Current TB regimen development Risk of late-stage attrition 2
3 Pre-Clinical and Clinical Sciences Workgroup (PCS-WG) Mission & Goals Mission Develop and/or validate tools and innovative approaches to address preclinical issues including in vitro and in vivo efficacy, PKPD analyses using appropriate biomarkers, drug safety, metabolism, DDI, etc. These tools may be submitted to regulatory authorities for regulatory review and/or qualification as appropriate. Early goal related to pre-clinical in vitro and in vivo models Evaluate the evidence base and develop criteria for evaluating the utility of various preclinical models to inform and test new drug regimens. Early Evidence Landscape analysis* identified HFS-TB as having an appropriate data inventory to assess predictive accuracy of a preclinical model for clinical outcomes. *Gumbo et al, JID 2015; 211(S3):S83 3
4 Early success EMA qualification opinion on the HFS-TB June 26, 2014 HFS-TB qualified for use in drug development programs as additional and complementary tool HFS-TB can be used in regulatory submissions, esp. for informed design and interpretation of clinical studies HFS-TB is recommended to be useful as follows: To provide preliminary proof of concept for developing a specific drug or combination to treat tuberculosis To select the pharmacodynamic target (e.g. T >MIC, AUC/MIC) To provide data to support PK/PD analyses leading to initial dose selection for non-clinical and clinical studies To assist in confirming dose regimens for later clinical trials taking into account human PK data and exposure-response relationships 4
5 Evaluation of in vivo models Correlations between drug concentration and pathogen survival that are based on in vitro models cannot be expected to reiterate all aspects of in vivo antimycobacterial treatment. Chilukuri et al, CID 2015; 61(S1):S32 Advantages of in vivo models Better reflect the phenotypic heterogeneity in bacterial populations as determined by host-pathogen interactions, including development of tissue pathology Present complexities of drug distribution to, and action at, various sites of infection 5
6 Improving TB regimen development Decreasing risk of attrition 6
7 Mouse model of sterilizing activity PK/Chemical Interaction Single Drug PK in Mouse Combination Efficacy (Mouse Acute Model) Combination Efficacy (Mouse Relapse Model) Confirmation of Efficacy Combination Safety (if needed) Clinical Studies Appropriate Dose Selection in Mice Bactericidal Activity: Initial Screening Sterilizing Activity: Duration of Therapy Secondary Species Infection Model Combination Specific Safety Day -14 d1 Day 0 M2 M3 M4 M5 3 mice mice held for 3-6 months after treatment completion to determine the proportion with microbiological evidence of relapse 7
8 Evaluating the sterilizing mouse model Rationale Past and present role in TB regimen development track record in forecasting treatment-shortening potential of RIF, PZA relapse endpoint considered closest correlate of current phase 3 endpoint Amount of available data on regimens evaluated in clinical trials Does not preclude evaluation of other models 8
9 Evaluating the sterilizing mouse model Rationale Past and present role in TB regimen development track record in forecasting treatment-shortening potential of RIF, PZA relapse endpoint considered closest correlate of current phase 3 endpoint Amount of available data on regimens evaluated in clinical trials Does not preclude evaluation of other models General Aim Quantify the predictive accuracy of mouse TB efficacy models to rank order regimens and estimate the effective treatment duration, by evaluating the proportional and absolute differences in the treatment durations of test and control regimens required to produce the same relapse outcome 9
10 Workplan for evidence-based evaluation of sterilizing mouse model CPTR PCS-WG Mouse Model Sub-team: Dr. Dakshina Chilukuri Dr. Geraint Davies Dr. Geo Derimanov Dr. Nader Fotouhi Dr. Tawanda Gumbo Dr. Debra Hanna Dr. Karen Lacourciere Dr. Barbara Laughon Dr. Anne Lenaerts Dr. Owen McMaster Dr. Nader Fotouhi Dr. Eric Nuermberger Dr. Klaus Romero Dr. Rada Savic Dr. Christine Sizemore Dr. Peter Warner Lindsay Lehmann Gap Analysis, Research Plan (as indicated) Context of Use Sterilizing Mouse Model Statistical Analysis Plan Data Inventory
11 COU Scenario 1: Treatment Duration and Rank-Ordering of Regimens General Description Stage of Drug Development for Use Intended Application Experiments testing drug combinations in mice provide an additional and complementary tool to existing methodology to inform regimen selection, to maximize sterilizing effects. Data produced will support submissions to regulatory agencies throughout the drug development process, to optimize design of clinical trials. Non-clinical PK/PD testing The data from experiments in mice infected with M. tuberculosis, using relapse as the main endpoint, will be used to calculate treatment effect sizes, to then rankorder regimens and estimate clinical treatment duration. 11
12 Data inventory Focus first on mouse strains other than C3HeB/FeJ ( Kramnik ) Inventory identified a variety of relapse-based pre-clinical studies with corresponding clinical trial outcomes data Test regimen intervention Regimen comparison # of expts Combining INH+STR HS vs. H or S monotherapy 1 Shortening duration of INH+STR 6HS vs. 18HS 1 Adding RIF to INH+STR or INH+EMB+PZA HR (or HRS or HREZ) vs. HS (or HEZ) 4 Adding STR to INH+RIF HRS vs. HR 1 Adding PZA to INH+RIF (±STR/EMB) HRZ (or HRSZ or HREZ) vs. HR (or HRS or HRE) 4 Shortening duration of PZA 2HREZ/4RH vs. 6HREZ 1 Increasing dose of RIF High-dose R plus HEZ vs. HREZ 2 Extending dosing interval of 1 st -line Rx HREZ (2/7) vs. HREZ (daily) 1 Replacing EMB with MXF HRMZ vs. HRZ(E) 3 Replacing INH with MXF MRZ(E) vs. HRZ(E) 10 Replacing RIF with RPT HPZ(E) vs. HRZ(E) 7 Replacing RIF+EMB with RPT+MXF HPMZ vs. HRZ 3 Replacing RIF with RPT and extending dosing interval HP(1/7) cont phase vs. HR(2/7) (in continuation phase) 2 Comparing INH+RIF+PZA+EMB with PMD+MXF+PZA PaMZ vs. HRZ(E) 8 12
13 Proposed statistical analysis plan The following analysis approaches are proposed, with the objective of rank ordering specific regimens and estimating their clinical treatment duration: A. Logistic regression to determine predictors of the proportional and absolute change in the treatment duration required to achieve the same probability of relapse, compared between control and test regimens. B. Parametric time-to-event analysis to determine predictors of the timevarying probability of achieving the same proportion of relapses over time, evaluating control and test regimens. C. Non-linear meta-regression analysis to determine specific interpretable parameters for specific proportional and absolute changes in treatment duration necessary to prevent relapses over time, evaluating control and test regimens. 15
14 Summary points An initial step to address the translational gap is to learn what data from what models analyzed in what way best inform key trial design decisions. Evidence-based validation of pre-clinical models is important: to confidently place preclinical models on the critical development path, to increase the efficiency of regulatory interactions, to set a precedent for objective, data-driven processes to apply to other models (e.g., C3HeB/FeJ mouse, marmoset), and to identify gaps in knowledge & in existing tools to drive future research. Evaluation of sterilizing mouse models is the appropriate first step for in vivo models, with other models to follow 16
15 Acknowledgements CPTR PCS-WG Mouse Model Sub-team: Dr. Nicole Ammerman (Johns Hopkins University) Dr. Dakshina Chilukuri (US Food & Drug Administration) Dr. Geraint Davies (University of Liverpool) Dr. Geo Derimanov (Glaxo Smith Kline) Dr. Nader Fotouhi (Global Alliance for TB Drug Development) Dr. Tawanda Gumbo (Baylor University) Dr. Debra Hanna (Critical Path Institute) Dr. Karen Lacourciere Dr. Barbara Laughon (National Institutes of Health) Dr. Anne Lenaerts (Colorado St. University) Dr. Owen McMaster (US Food & Drug Administration) Dr. Khis Mdluli (Global Alliance for TB Drug Development) Dr. Eric Nuermberger (Johns Hopkins University) Dr. Klaus Romero (Critical Path Institute) Dr. Rada Savic (University of California-San Francisco) Dr. Christine Sizemore (National Institutes of Health) Dr. Peter Warner (Bill & Melinda Gates Foundation) Lindsay Lehmann (Critical Path Institute) 17
16 18
17 Estimating treatment duration Effect size may be measured as difference in time to event (e.g., 50% of mice cured). Both absolute and proportional effect sizes will be considered.
18 Development of statistical analysis plan Investigate data sources to determine level of support existing data can provide to accommodate the aim. Define the most appropriate analysis strategy, specific time points to be evaluated. Define the path forward for analytics across the integrated experiment-level database. 20
19 Pre-clinical DDT: CPTR HFS-TB Example HFS-TB System HFS-TB as Actionable Drug Development Tool HFS-TB team Regulatory-endorsed Tool: Context of Use Published HFS-TB Data Sources with Clinical Comparator Data Statistical Evaluation [Enter Presentation Title in Insert Tab > Header & Footer 21
20 Pre-clinical DDT: CPTR HFS-TB Example HFS-TB System HFS-TB as Actionable Drug Development Tool HFS-TB team Regulatory-endorsed Tool: Context of Use Published HFS-TB Data Sources with Clinical Comparator Data Statistical Evaluation [Enter Presentation Title in Insert Tab > Header & Footer 22
21 Context of use Scenario 1: Rank-ordering of regimens and estimation of treatment duration General description: The data from experiments testing drug combinations in mice infected with M. tuberculosis provide an additional and complementary tool to existing methodology to inform regimen selection, to maximize sterilizing effects. These data will support submissions to regulatory agencies throughout the drug development process for an anti-tb regimen, to optimize design of clinical studies. Stage of Drug Development for Use: Non-clinical PKPD testing. Intended Application: The data from experiments using mice infected with M. tuberculosis, using relapse as the main endpoint, will be used to calculate treatment effect magnitudes, to then rank-order regimens and predict clinical treatment duration. 25
22 Data Analysis Methods Analysis 1: Descriptive Correlations Analysis 2: Predictive Accuracy or Forecasting 2a: Correct ranking of PK/PD indices relevant to dose scheduling 2b: Accuracy in generating or refuting hypotheses with relevance to therapeutic strategies 2c: Quantitative accuracy in forecasting PK/PD indices relevant to dose scheduling, dose selection, and breakpoints Weighted by clinical study quality score and number of patients in study 27
23 Studies Identified by Searches Literature Search A: 26 HFS-TB studies (12 combination studies, 10 monotherapy, 4 Monte Carlo simulations) Literature Search B: 17 TB clinical studies, published prior to HFS- TB studies; quality of evidence score of 1 in 15/17 Literature Search C: 20 TB clinical studies, published at least six months after HFS-TB studies; quality of evidence of 1 or 2 in 11/20 Weighting reflected clinical study quality score 28
24 Predictive Accuracy Approach Error (E) was defined as the observed results in a clinical study at time T, minus the predicted value P: E= T-P For a number of trials or experiments i of up to n, this takes the form of the mean absolute percentage error (MAPE), which is given by: MAPE= 1 n n i=1 Accuracy (A) was defined as: A=100%-MAPE Bias (B) was defined as: T i P i T i 100 B= n i=1 (T i P i )/n 29
25 30
26 Summary of Analyses HFS-TB Predicted vs. Clinic Observed Gumbo et al, CID 2015; 61(S1):S25 32
Academia (Consortia) Perspective Topic I: Selection of Agents, Doses and Regimens for Clinical Study
 Academia (Consortia) Perspective Topic I: Selection of Agents, Doses and Regimens for Clinical Study Debra Hanna, Executive Director, Critical Path to TB Drug Regimens 25 November 2016 Outline Consortium
Academia (Consortia) Perspective Topic I: Selection of Agents, Doses and Regimens for Clinical Study Debra Hanna, Executive Director, Critical Path to TB Drug Regimens 25 November 2016 Outline Consortium
CPTR Mission, Structure & Goals for Innovation
 CPTR Mission, Structure & Goals for Innovation Dr. Debra Hanna, PhD Executive Director Critical Path to TB Drug Regimens Event title - DD Month YYYY - Location The Challenge Collaborating for Cures 7 March
CPTR Mission, Structure & Goals for Innovation Dr. Debra Hanna, PhD Executive Director Critical Path to TB Drug Regimens Event title - DD Month YYYY - Location The Challenge Collaborating for Cures 7 March
Evaluating New TB Drugs in Mice: Relevance to Humans, especially with HIV
 Evaluating New TB Drugs in Mice: Relevance to Humans, especially with HIV Eric Nuermberger, M.D. Center for TB Research Johns Hopkins University School of Medicine Baltimore, MD February 27, 2011 Disclosures
Evaluating New TB Drugs in Mice: Relevance to Humans, especially with HIV Eric Nuermberger, M.D. Center for TB Research Johns Hopkins University School of Medicine Baltimore, MD February 27, 2011 Disclosures
CPTR 2013 Workshop September 30 th - October 3 rd. CPTR Roadmap: Accomplishments and Future Direction
 CPTR 2013 Workshop September 30 th - October 3 rd CPTR Roadmap: Accomplishments and Future Direction CPTR Mission & Purpose Accelerate the development of new, safe, and highly effective regimens for TB
CPTR 2013 Workshop September 30 th - October 3 rd CPTR Roadmap: Accomplishments and Future Direction CPTR Mission & Purpose Accelerate the development of new, safe, and highly effective regimens for TB
Qualification opinion
 EMA/CHMP/SAWP/47290/2015 Procedure No.: EMEA/H/SAB/049/1/QO/2014/SME Product Development Scientific Support Department Qualification opinion In-vitro hollow fiber system model of tuberculosis (HSF-TB)
EMA/CHMP/SAWP/47290/2015 Procedure No.: EMEA/H/SAB/049/1/QO/2014/SME Product Development Scientific Support Department Qualification opinion In-vitro hollow fiber system model of tuberculosis (HSF-TB)
Critical Path to TB Drug Regimens Role in Facilitating TB Drug Development
 Critical Path to TB Drug Regimens Role in Facilitating TB Drug Development Debra Hanna, Executive Director, Critical Path to TB Drug Regimens 11 September 2017 CPTR S MISSION AND FOCUS Mission: The Critical
Critical Path to TB Drug Regimens Role in Facilitating TB Drug Development Debra Hanna, Executive Director, Critical Path to TB Drug Regimens 11 September 2017 CPTR S MISSION AND FOCUS Mission: The Critical
Hollow Fiber System-TB 24 February 2014
 Hollow Fiber System Model of Tuberculosis (HFS-TB) as an in vitro preclinical tool for optimization of dose selection and drug regimen in anti-tb drug development Critical Path Institute, on behalf of
Hollow Fiber System Model of Tuberculosis (HFS-TB) as an in vitro preclinical tool for optimization of dose selection and drug regimen in anti-tb drug development Critical Path Institute, on behalf of
Translating TB Therapy Response:
 Translating TB Therapy Response: Application of Mechanistic PKPD Modelling and Pharmacometrics Rada Savic, PhD Associate Professor of Pharmacometrics Dept. of Bioengineering and Therapeutics Sciences Division
Translating TB Therapy Response: Application of Mechanistic PKPD Modelling and Pharmacometrics Rada Savic, PhD Associate Professor of Pharmacometrics Dept. of Bioengineering and Therapeutics Sciences Division
The Critical Path to TB Drug Regimens Initiative
 The Critical Path to TB Drug Regimens Initiative Jan Gheuens, MD, PhD Global Health, Bill & Melinda Gates Foundation Addis Ababa, August 18th, 2010 The new tools that are needed to fight TB are on their
The Critical Path to TB Drug Regimens Initiative Jan Gheuens, MD, PhD Global Health, Bill & Melinda Gates Foundation Addis Ababa, August 18th, 2010 The new tools that are needed to fight TB are on their
Setting Clinical Breakpoints/ECOFFS
 23 rd August 2016 Setting Clinical Breakpoints/ECOFFS Robin A Howe Antimicrobial use in Primary Care An E. coli is grown from blood cultures Cefuroxime MIC 2mg/L Zone around CXM 30ug disc 27mm Is it sensitive?
23 rd August 2016 Setting Clinical Breakpoints/ECOFFS Robin A Howe Antimicrobial use in Primary Care An E. coli is grown from blood cultures Cefuroxime MIC 2mg/L Zone around CXM 30ug disc 27mm Is it sensitive?
Critical Path to TB Drug Regimens (CPTR)
 Critical Path to TB Drug Regimens (CPTR) Why do we need a CPTR initiative? We finally have a pipeline of TB drug candidates Today, we test drugs same way we did 20 years ago Need better tests for drug
Critical Path to TB Drug Regimens (CPTR) Why do we need a CPTR initiative? We finally have a pipeline of TB drug candidates Today, we test drugs same way we did 20 years ago Need better tests for drug
Antifungal PK/PD Made Simple. David Andes, MD University of Wisconsin
 Antifungal PK/PD Made Simple David Andes, MD University of Wisconsin PK/PD Concept Serum Drug Concentration Peak:MIC AUC:MIC Ratio Time Above MIC MIC Time Hypothesis and Concept There is an optimal drug
Antifungal PK/PD Made Simple David Andes, MD University of Wisconsin PK/PD Concept Serum Drug Concentration Peak:MIC AUC:MIC Ratio Time Above MIC MIC Time Hypothesis and Concept There is an optimal drug
Johan W Mouton Canisius-Wilhelmina Hospital Nijmegen, The Netherlands
 Can pk/pd replace clinical trials? Johan W Mouton Canisius-Wilhelmina Hospital Nijmegen, The Netherlands The Traditional Approach Phase Participants Research questions Number Characteristics I 10-50 Usually
Can pk/pd replace clinical trials? Johan W Mouton Canisius-Wilhelmina Hospital Nijmegen, The Netherlands The Traditional Approach Phase Participants Research questions Number Characteristics I 10-50 Usually
8. Clinical Trial Assessment Phase II
 8. Clinical Trial Assessment Phase II Junko Sato, PhD Office of New Drug I, PMDA Disclaimer: The information within this presentation is based on the presenter s expertise and experience, and represents
8. Clinical Trial Assessment Phase II Junko Sato, PhD Office of New Drug I, PMDA Disclaimer: The information within this presentation is based on the presenter s expertise and experience, and represents
Regulatory Pathways for CPTR Drug Development Tools and Methodologies. March 20, 2017
 1 Regulatory Pathways for CPTR Drug Development Tools and Methodologies March 20, 2017 Outline Accelerating the Drug Discovery and Development Timeline Qualification: A formal process of review and acceptance
1 Regulatory Pathways for CPTR Drug Development Tools and Methodologies March 20, 2017 Outline Accelerating the Drug Discovery and Development Timeline Qualification: A formal process of review and acceptance
Exposure-Response Analysis of Lee 1810, a Lead Spectinamide Antibiotic in Mycobacterium tuberculosis Infected Mice
 15 th October 2017 Exposure-Response Analysis of Lee 1810, a Lead Spectinamide Antibiotic in Mycobacterium tuberculosis Infected Mice Santosh Wagh 1, Chetan Rathi 1, Gregory T. Robertson 3, Jiuyu Liu 2,
15 th October 2017 Exposure-Response Analysis of Lee 1810, a Lead Spectinamide Antibiotic in Mycobacterium tuberculosis Infected Mice Santosh Wagh 1, Chetan Rathi 1, Gregory T. Robertson 3, Jiuyu Liu 2,
Models for Computer-Aided Trial & Program Design
 Models for Computer-Aided Trial & Program Design Terrence Blaschke, M.D. VP, Methodology and Science Pharsight Corporation & Professor of Medicine & Molecular Pharmacology Stanford University MODELS What
Models for Computer-Aided Trial & Program Design Terrence Blaschke, M.D. VP, Methodology and Science Pharsight Corporation & Professor of Medicine & Molecular Pharmacology Stanford University MODELS What
PK/PD als Instrument zur Steuerung der klinischen Entwicklung von Antibiotika
 PK/PD als Instrument zur Steuerung der klinischen Entwicklung von Antibiotika Heino Staß Institut für Klinische Pharmakologie Bayer HealthCare AG, Wuppertal, D Role of Clinical Pharmacology in the Development
PK/PD als Instrument zur Steuerung der klinischen Entwicklung von Antibiotika Heino Staß Institut für Klinische Pharmakologie Bayer HealthCare AG, Wuppertal, D Role of Clinical Pharmacology in the Development
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 27 July 2000 CPMP/EWP/2655/99 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 27 July 2000 CPMP/EWP/2655/99 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER
Tuberculosis Drug Accelerator
 Tuberculosis Drug Accelerator Overview of Activities and Portfolio who, why, what, how, by when and where are we now Steve Berthel 1 What is the Tuberculosis Drug Accelerator? The A is a groundbreaking
Tuberculosis Drug Accelerator Overview of Activities and Portfolio who, why, what, how, by when and where are we now Steve Berthel 1 What is the Tuberculosis Drug Accelerator? The A is a groundbreaking
2016 Europe-Nordic-US Symposium New Frontiers in Antibacterial Resistance Research. Pharmacological Approaches to Address AR
 2016 Europe-Nordic-US Symposium New Frontiers in Antibacterial Resistance Research Pharmacological Approaches to Address AR G.L. Drusano, M.D. Professor and Director Institute for Therapeutic Innovation
2016 Europe-Nordic-US Symposium New Frontiers in Antibacterial Resistance Research Pharmacological Approaches to Address AR G.L. Drusano, M.D. Professor and Director Institute for Therapeutic Innovation
2017 National TB Conference National TB Controllers Association 1
 Pharmacokinetics and pharmacodynamics of TB drugs: Prospects for improving TB therapy Eric, M.D. Center for TB Research Johns Hopkins University What s wrong with TB therapy? 6 months is not short course
Pharmacokinetics and pharmacodynamics of TB drugs: Prospects for improving TB therapy Eric, M.D. Center for TB Research Johns Hopkins University What s wrong with TB therapy? 6 months is not short course
Challenges in Antimicrobial Clinical Development. Axel Dalhoff & Heino Staß Institut für Klinische Pharmakologie Bayer AG, Wuppertal, D
 Challenges in Antimicrobial Clinical Development Axel Dalhoff & Heino Staß Institut für Klinische Pharmakologie Bayer AG, Wuppertal, D New antibacterial agents approved in the United States 1983-2002 No.
Challenges in Antimicrobial Clinical Development Axel Dalhoff & Heino Staß Institut für Klinische Pharmakologie Bayer AG, Wuppertal, D New antibacterial agents approved in the United States 1983-2002 No.
Biomarker Utility and Acceptance in Drug Development and Clinical Trials: an FDA Regulatory Perspective
 Biomarker Utility and Acceptance in Drug Development and Clinical Trials: an FDA Regulatory Perspective Chris Leptak, MD/PhD OND Biomarker and Companion Diagnostic Lead CDER/Office of New Drugs, Immediate
Biomarker Utility and Acceptance in Drug Development and Clinical Trials: an FDA Regulatory Perspective Chris Leptak, MD/PhD OND Biomarker and Companion Diagnostic Lead CDER/Office of New Drugs, Immediate
REIMAGINING DRUG DEVELOPMENT:
 Biology Reconstructed REIMAGINING DRUG DEVELOPMENT: Accurate Disease Modeling To Drive Successful Therapies Julia Kirshner, CEO julia@zpredicta.com 1 SUCCESS RATES OF DRUG DEVELOPMENT ARE LOW, " PARTICULARLY
Biology Reconstructed REIMAGINING DRUG DEVELOPMENT: Accurate Disease Modeling To Drive Successful Therapies Julia Kirshner, CEO julia@zpredicta.com 1 SUCCESS RATES OF DRUG DEVELOPMENT ARE LOW, " PARTICULARLY
Identification of the In Vivo Pharmacokinetics and Pharmacodynamic Drivers of Iclaprim
 AAC Accepted Manuscript Posted Online 29 January 2018 Antimicrob. Agents Chemother. doi:10.1128/aac.02550-17 Copyright 2018 American Society for Microbiology. All Rights Reserved. 1 Identification of the
AAC Accepted Manuscript Posted Online 29 January 2018 Antimicrob. Agents Chemother. doi:10.1128/aac.02550-17 Copyright 2018 American Society for Microbiology. All Rights Reserved. 1 Identification of the
Nanomedicine for Improved Efficacy of Tuberculosis Drugs Pharmacokinetic importance
 Nanomedicine for Improved Efficacy of Tuberculosis Drugs Pharmacokinetic importance Emerging Researcher Symposium Dr. Rose Hayeshi 10 October 2012 Outline Challenges in TB treatment Nanomedicine as proposed
Nanomedicine for Improved Efficacy of Tuberculosis Drugs Pharmacokinetic importance Emerging Researcher Symposium Dr. Rose Hayeshi 10 October 2012 Outline Challenges in TB treatment Nanomedicine as proposed
Data Sharing and Models in Pre(Non)- Competitive Space in Addressing Unmet Medical Needs. Critical Path Institute s Alzheimers Drug Development Tool
 Data Sharing and Models in Pre(Non)- Competitive Space in Addressing Unmet Medical Needs Critical Path Institute s Alzheimers Drug Development Tool Vikram Sinha, PhD Director, Division of Pharmacometrics
Data Sharing and Models in Pre(Non)- Competitive Space in Addressing Unmet Medical Needs Critical Path Institute s Alzheimers Drug Development Tool Vikram Sinha, PhD Director, Division of Pharmacometrics
EMA Workshop Non-Clinical Models to Identify PK/PD Indices and PD Targets In Vitro
 EMA Workshop Non-Clinical Models to Identify PK/PD Indices and PD Targets In Vitro G.L. Drusano, M.D. Professor and Director Institute for Therapeutic Innovation University of Florida In Vitro There are
EMA Workshop Non-Clinical Models to Identify PK/PD Indices and PD Targets In Vitro G.L. Drusano, M.D. Professor and Director Institute for Therapeutic Innovation University of Florida In Vitro There are
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL FIXED DOSE COMBINATION PRODUCTS (FDC Products) FOR HIV/AIDS, TUBERCULOSIS, and MALARIA This guideline is intended to provide recommendations to applicants wishing to submit applications
MEDICINES CONTROL COUNCIL FIXED DOSE COMBINATION PRODUCTS (FDC Products) FOR HIV/AIDS, TUBERCULOSIS, and MALARIA This guideline is intended to provide recommendations to applicants wishing to submit applications
Regulatory Perspective on Developing Long Acting ARVs for HIV Treatment/Prevention. FDA Division of Antiviral Products
 Regulatory Perspective on Developing Long Acting ARVs for HIV Treatment/Prevention FDA Division of Antiviral Products None Financial Disclosures FDA Disclaimer The views in this presentation represent
Regulatory Perspective on Developing Long Acting ARVs for HIV Treatment/Prevention FDA Division of Antiviral Products None Financial Disclosures FDA Disclaimer The views in this presentation represent
USING PHARMACOKINETICS TO OPTIMIZE THE DOSING OF ANTITUBERCULOUS DRUGS
 USING PHARMACOKINETICS TO OPTIMIZE THE DOSING OF ANTITUBERCULOUS DRUGS By ABDULLAH SALEH ALSULTAN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF
USING PHARMACOKINETICS TO OPTIMIZE THE DOSING OF ANTITUBERCULOUS DRUGS By ABDULLAH SALEH ALSULTAN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF
RESIST-TB ANNUAL REPORT FOR 2012
 RESIST-TB ANNUAL REPORT FOR 2012 RESIST-TB is an organization of concerned patients, physicians, research scientists and stakeholders. Its mission is to promote and conduct clinical research to cure and
RESIST-TB ANNUAL REPORT FOR 2012 RESIST-TB is an organization of concerned patients, physicians, research scientists and stakeholders. Its mission is to promote and conduct clinical research to cure and
Critical Path Initiative: An Update
 Critical Path Initiative: An Update Raymond Woosley, MD, PhD President and CEO, Critical Path Institute Presented at: FIRST ANNUAL PATIENT-REPORTED OUTCOMES (PRO) CONSORTIUM WORKSHOP March 23, 2010 Bethesda,
Critical Path Initiative: An Update Raymond Woosley, MD, PhD President and CEO, Critical Path Institute Presented at: FIRST ANNUAL PATIENT-REPORTED OUTCOMES (PRO) CONSORTIUM WORKSHOP March 23, 2010 Bethesda,
Agreed with W. Cornell Graduate Program and Tri-I
 Drug Development Course From Molecule to Prescription Weill Cornell Graduate School - Tri-Institutional Therapeutics Discovery Institute ABOUT THIS COURSE This course has been designed in collaboration
Drug Development Course From Molecule to Prescription Weill Cornell Graduate School - Tri-Institutional Therapeutics Discovery Institute ABOUT THIS COURSE This course has been designed in collaboration
Overview of Biomarker Qualification. Marc K Walton MD, PhD Office of Translational Sciences CDER-FDA
 Overview of Biomarker Qualification Marc K Walton MD, PhD Office of Translational Sciences CDER-FDA Categories of Biomarkers Prognostic Predictive Pharmacodynamic Including adverse response Efficacy-response
Overview of Biomarker Qualification Marc K Walton MD, PhD Office of Translational Sciences CDER-FDA Categories of Biomarkers Prognostic Predictive Pharmacodynamic Including adverse response Efficacy-response
Antimycobacterial Drug Development. Ed Cox, MD MPH Office of Antimicrobial Products OND/CDER/FDA
 Antimycobacterial Drug Development Ed Cox, MD MPH Office of Antimicrobial Products OND/CDER/FDA 1 Outline Background Information Clinical Trials Endpoints Data Standards Resources Division Contacts GAIN
Antimycobacterial Drug Development Ed Cox, MD MPH Office of Antimicrobial Products OND/CDER/FDA 1 Outline Background Information Clinical Trials Endpoints Data Standards Resources Division Contacts GAIN
06/03/2009. Overview. Preclinical Support for Exploratory Phase I Clinical Trials. Micro-dosing IND. Pharmacological Active Single Dose IND
 Preclinical Support for Exploratory Phase I Clinical Trials Clive Joseph, DSRD Sandwich Overview Identify the most appropriate development paradigm - traditional vs alternative IND approach Confidence
Preclinical Support for Exploratory Phase I Clinical Trials Clive Joseph, DSRD Sandwich Overview Identify the most appropriate development paradigm - traditional vs alternative IND approach Confidence
FDA Public Hearing: Approval Pathway for Biosimilar. Products. November 2-3, 2010
 FDA Public Hearing: Approval Pathway for Biosimilar and Interchangeable Biological Products November 2-3, 2010 1 The Biotechnology Industry Organization Over 1,100 members, including biotechnology companies,
FDA Public Hearing: Approval Pathway for Biosimilar and Interchangeable Biological Products November 2-3, 2010 1 The Biotechnology Industry Organization Over 1,100 members, including biotechnology companies,
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
Consortia-Based Strategies in Neurodegenerative Diseases: Critical Path Institute s Track Record in Collaborative Efforts
 Consortia-Based Strategies in Neurodegenerative Diseases: Critical Path Institute s Track Record in Collaborative Efforts Martha A. Brumfield, PhD President & CEO Agenda C-Path Model for Collaboration
Consortia-Based Strategies in Neurodegenerative Diseases: Critical Path Institute s Track Record in Collaborative Efforts Martha A. Brumfield, PhD President & CEO Agenda C-Path Model for Collaboration
Research subgroup. Progress reports from the MDRTB WG Subgroups. Tbilisi, 21 September 2007
 Research subgroup Progress reports from the MDRTB WG Subgroups Tbilisi, 21 September 2007 The history of the response to AIDS in Africa can be divided into two phases: 1. fiddling while Rome burns, and
Research subgroup Progress reports from the MDRTB WG Subgroups Tbilisi, 21 September 2007 The history of the response to AIDS in Africa can be divided into two phases: 1. fiddling while Rome burns, and
IDSA is pleased to offer the following comments on specific priority areas identified by FDA:
 October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug development
 1 2 3 24 June 2010 EMA/CHMP/641298/2008 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug
1 2 3 24 June 2010 EMA/CHMP/641298/2008 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug
AN TB IOTIC. This project is funded by the European Union. This project is funded by the European Union.
 TUBERCULOSIS Tuberculosis () today rivals HIV/AIDS as the leading cause of death from infectious diseases. The number of patients has never been higher than today and the growing proportion of drug-resistant
TUBERCULOSIS Tuberculosis () today rivals HIV/AIDS as the leading cause of death from infectious diseases. The number of patients has never been higher than today and the growing proportion of drug-resistant
May 23, :30 PM-5:00 PM - AUDITORIUM A250. May 9, :30 PM -5:00 PM - AUDITORIUM A-950 May 16, :30 PM-5:00 PM - AUDITORIUM A250
 DRUG DEVELOPMENT COURSE - FROM MOLECULE TO PRESCRIPTION WEILL CORNELL GRADUATE SCHOOL - TRI-INSTITUTIONAL THERAPEUTICS DISCOVERY INSTITUTE THURSDAYS FROM 3:00PM - 5:00PM AT 1300 YORK AVE, NEW YORK, N.Y.
DRUG DEVELOPMENT COURSE - FROM MOLECULE TO PRESCRIPTION WEILL CORNELL GRADUATE SCHOOL - TRI-INSTITUTIONAL THERAPEUTICS DISCOVERY INSTITUTE THURSDAYS FROM 3:00PM - 5:00PM AT 1300 YORK AVE, NEW YORK, N.Y.
Chagas Disease Drug Discovery Entering a New Era. Eric Chatelain, PhD Head of Drug Discovery
 Chagas Disease Drug Discovery Entering a New Era Eric Chatelain, PhD Head of Drug Discovery ICOPA Meeting, Mexico, 12 th August 2014 Chagas Disease Effective immune responses provide control of the infection
Chagas Disease Drug Discovery Entering a New Era Eric Chatelain, PhD Head of Drug Discovery ICOPA Meeting, Mexico, 12 th August 2014 Chagas Disease Effective immune responses provide control of the infection
FDA from a Former FDAer: Secrets and insights into regulatory review and drug development
 FDA from a Former FDAer: Secrets and insights into regulatory review and drug development Andrew E. Mulberg, MD, FAAP Vice-President, Global Regulatory Affairs; Former Division Deputy, DGIEP, U.S. FDA
FDA from a Former FDAer: Secrets and insights into regulatory review and drug development Andrew E. Mulberg, MD, FAAP Vice-President, Global Regulatory Affairs; Former Division Deputy, DGIEP, U.S. FDA
Samatasvir (IDX719), a Potent Pan-Genotypic NS5A Inhibitor, for the Treatment of Hepatitis C Virus (HCV) Infection
 Samatasvir (IDX719), a Potent Pan-Genotypic NS5A Inhibitor, for the Treatment of Hepatitis C Virus (HCV) Infection Douglas Mayers, MD December 11, 2013 1 Idenix: Advancing All-Oral, Pan-Genotypic Combination
Samatasvir (IDX719), a Potent Pan-Genotypic NS5A Inhibitor, for the Treatment of Hepatitis C Virus (HCV) Infection Douglas Mayers, MD December 11, 2013 1 Idenix: Advancing All-Oral, Pan-Genotypic Combination
Request for Proposal PRECLINICAL EVALUATION OF NEW DRUG COMBINATIONS AGAINST TUBERCULOSIS (RFP ) INTRODUCTION
 Request for Proposal PRECLINICAL EVALUATION OF NEW DRUG COMBINATIONS AGAINST TUBERCULOSIS (RFP2006.01) The Global Alliance for TB Drug Development (the TB Alliance) is promoting a new paradigm for TB drug
Request for Proposal PRECLINICAL EVALUATION OF NEW DRUG COMBINATIONS AGAINST TUBERCULOSIS (RFP2006.01) The Global Alliance for TB Drug Development (the TB Alliance) is promoting a new paradigm for TB drug
Essential Science: The State of the Art of Pharmacokinetics and Pharmacodynamics for Antimicrobial Drug Development
 Essential Science: The State of the Art of Pharmacokinetics and Pharmacodynamics for Antimicrobial Drug Development 7 September 2018 Nikolas J Onufrak, Pharm.D. Institute for Clinical Pharmacodynamics,
Essential Science: The State of the Art of Pharmacokinetics and Pharmacodynamics for Antimicrobial Drug Development 7 September 2018 Nikolas J Onufrak, Pharm.D. Institute for Clinical Pharmacodynamics,
9th National Conference on the Laboratory Aspects of Tuberculosis
 9th National Conference on the Laboratory Aspects of Tuberculosis Expected discrepancies between molecular and growth-based DST: Which technology is giving the right answer? Edward Desmond, CA Dept. of
9th National Conference on the Laboratory Aspects of Tuberculosis Expected discrepancies between molecular and growth-based DST: Which technology is giving the right answer? Edward Desmond, CA Dept. of
Integrating Biospecimen Collection into Clinical Research
 Integrating Biospecimen Collection into Clinical Research Janet E Dancey, MD, FRCPC Ontario Institute for Cancer Research Program for High Impact Clinical Trials National Cancer Institute of Canada Clinical
Integrating Biospecimen Collection into Clinical Research Janet E Dancey, MD, FRCPC Ontario Institute for Cancer Research Program for High Impact Clinical Trials National Cancer Institute of Canada Clinical
Regulatory Perspective
 Regulatory Perspective Presented by Dr Maria Isaac MASc, MD, PhD, Pharmaceutical Medicine Physician, Psychiatrist Senior Scientific Officer An agency of the European Union Disclaimer The views expressed
Regulatory Perspective Presented by Dr Maria Isaac MASc, MD, PhD, Pharmaceutical Medicine Physician, Psychiatrist Senior Scientific Officer An agency of the European Union Disclaimer The views expressed
Methods in Clinical Cancer Research Workshop Format for Protocol Concept Synopsis Sheet (blank) SYNOPSIS
 B Methods in Clinical Cancer Research Workshop Format for Protocol Concept Synopsis Sheet (blank) elow is the format to follow for the concept sheet for protocols. It is based on the International Committee
B Methods in Clinical Cancer Research Workshop Format for Protocol Concept Synopsis Sheet (blank) elow is the format to follow for the concept sheet for protocols. It is based on the International Committee
Model-Based Qualification of Biomarkers. Klaus Romero, MD MS FCP Director of Clinical Pharmacology and Quantitative Medicine
 Model-Based Qualification of Biomarkers Klaus Romero, MD MS FCP Director of Clinical Pharmacology and Quantitative Medicine Critical Path Initiative (CPI) Critical Path Institute (C-Path) Independent 501(c)3
Model-Based Qualification of Biomarkers Klaus Romero, MD MS FCP Director of Clinical Pharmacology and Quantitative Medicine Critical Path Initiative (CPI) Critical Path Institute (C-Path) Independent 501(c)3
Guideline for the demonstration of efficacy for veterinary medicinal products containing antimicrobial substances
 1 2 3 12 February 2015 EMA/CVMP/261180/2012 Committee for Medicinal Products for Veterinary Use (CVMP) 4 5 6 Guideline for the demonstration of efficacy for veterinary medicinal products containing Draft
1 2 3 12 February 2015 EMA/CVMP/261180/2012 Committee for Medicinal Products for Veterinary Use (CVMP) 4 5 6 Guideline for the demonstration of efficacy for veterinary medicinal products containing Draft
PK-PD TARGET SELECTION It s All About the Goal
 PK-PD TARGET SELECTION It s All About the Goal Paul G. Ambrose, Pharm.D. Chair, USCAST Executive Committee President, Institute for Clinical Pharmacodynamics It s All About the Goal The choice of a rational
PK-PD TARGET SELECTION It s All About the Goal Paul G. Ambrose, Pharm.D. Chair, USCAST Executive Committee President, Institute for Clinical Pharmacodynamics It s All About the Goal The choice of a rational
Voriconazole and Aspergillus spp. Rationale for the EUCAST clinical breakpoints, version May 2012
 Voriconazole and Aspergillus spp. Rationale for the EUCAST clinical breakpoints, version 1.0 20 May 2012 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised
Voriconazole and Aspergillus spp. Rationale for the EUCAST clinical breakpoints, version 1.0 20 May 2012 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised
Extrapolation in Pediatric Product Development: Practical Application of the Principle of Scientific Necessity
 Extrapolation in Pediatric Product Development: Practical Application of the Principle of Scientific Necessity Robert Skip Nelson, MD PhD Deputy Director and Senior Pediatric Ethicist Office of Pediatric
Extrapolation in Pediatric Product Development: Practical Application of the Principle of Scientific Necessity Robert Skip Nelson, MD PhD Deputy Director and Senior Pediatric Ethicist Office of Pediatric
Celerion s Symposia Series: Bridging the Gap from Phase I to Proof-of-Concept. San Francisco, CA Tue 8 th, Apr 2014
 Celerion s Symposia Series: Bridging the Gap from Phase I to Proof-of-Concept San Francisco, CA Tue 8 th, Apr 2014 Mind the Gap: Elements of a Bridging Strategy J. Fred Pritchard, Ph.D. Vice President,
Celerion s Symposia Series: Bridging the Gap from Phase I to Proof-of-Concept San Francisco, CA Tue 8 th, Apr 2014 Mind the Gap: Elements of a Bridging Strategy J. Fred Pritchard, Ph.D. Vice President,
Injectable modified release products
 Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms (EMA/CPMP/EWP/280/96 Corr1) Injectable modified release products Dr Sotiris Michaleas, National Expert for the
Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms (EMA/CPMP/EWP/280/96 Corr1) Injectable modified release products Dr Sotiris Michaleas, National Expert for the
NDA Requirements: Surveillance, Clinical Trial Data, Breakpoints
 NDA Requirements: Surveillance, Clinical Trial Data, Breakpoints Kevin M. Krause Director Microbiology Achaogen, Inc. Presented at the ASM/ESCMID Conference on Drug Development to Meet the Challenge of
NDA Requirements: Surveillance, Clinical Trial Data, Breakpoints Kevin M. Krause Director Microbiology Achaogen, Inc. Presented at the ASM/ESCMID Conference on Drug Development to Meet the Challenge of
Jan Willem van der Laan
 First-in-human studies: Recent experiences in Europe Jan Willem van der Laan Section Pharmacological-Toxicological and Biotechnology Assessment Medicines Evaluation Board, Utrecht First-in-human trials
First-in-human studies: Recent experiences in Europe Jan Willem van der Laan Section Pharmacological-Toxicological and Biotechnology Assessment Medicines Evaluation Board, Utrecht First-in-human trials
The general concept of pharmacodynamics
 Asian PK/PD Educational Workshop The general concept of pharmacodynamics modeling the antibacterial effects relations PK / PD This part uses material from presentations of H. Derendorf (Gainesville, Fla.)
Asian PK/PD Educational Workshop The general concept of pharmacodynamics modeling the antibacterial effects relations PK / PD This part uses material from presentations of H. Derendorf (Gainesville, Fla.)
EU perspective: European procedure for Qualification of novel methodologies for medicine development. Elmer Schabel MD
 EU perspective: European procedure for Qualification of novel methodologies for medicine development Elmer Schabel MD Nothing to disclose See: http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/landing/ex
EU perspective: European procedure for Qualification of novel methodologies for medicine development Elmer Schabel MD Nothing to disclose See: http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/landing/ex
Quantifying Disease Progression for Drug Development. Klaus Romero, MD MS FCP Director of Clinical Pharmacology and Quantitative Medicine
 Quantifying Disease Progression for Drug Development Klaus Romero, MD MS FCP Director of Clinical Pharmacology and Quantitative Medicine C-Path quantitative drug development tools 2 2 Polycystic Kidney
Quantifying Disease Progression for Drug Development Klaus Romero, MD MS FCP Director of Clinical Pharmacology and Quantitative Medicine C-Path quantitative drug development tools 2 2 Polycystic Kidney
DMTC Technology Readiness Levels Guideline
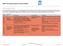 Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Technology Readiness Levels Technology Readiness Levels (TRLs) are used as standardised numerical indicators of the level of maturity of a technology. The standard TRL definitions are given in Table 1.
Guideline for the demonstration of efficacy for veterinary medicinal products containing antimicrobial substances
 1 2 3 16 May 2013 EMA/CVMP/261180/2012 Committee for Medicinal Products for Veterinary Use (CVMP) 4 5 6 Guideline for the demonstration of efficacy for veterinary medicinal products containing Revision
1 2 3 16 May 2013 EMA/CVMP/261180/2012 Committee for Medicinal Products for Veterinary Use (CVMP) 4 5 6 Guideline for the demonstration of efficacy for veterinary medicinal products containing Revision
Bayesian modelling for combination dose-escalation trial that incorporates pharmacokinetic data
 Bayesian modelling for combination dose-escalation trial that incorporates pharmacokinetic data Daniel Lorand (Oncology Early Clinical Biostatistics, Novartis) Basel Biometrics Society Seminar - April
Bayesian modelling for combination dose-escalation trial that incorporates pharmacokinetic data Daniel Lorand (Oncology Early Clinical Biostatistics, Novartis) Basel Biometrics Society Seminar - April
Evidentiary Considerations for Integration of Biomarkers in Drug Development : Statistical Considerations
 Evidentiary Considerations for Integration of Biomarkers in Drug Development : Statistical Considerations August 21. 2015 Aloka Chakravarty, PhD Office of Biostatistics, OTS, CDER U.S. Food and Drug Administration
Evidentiary Considerations for Integration of Biomarkers in Drug Development : Statistical Considerations August 21. 2015 Aloka Chakravarty, PhD Office of Biostatistics, OTS, CDER U.S. Food and Drug Administration
A pre-clinical PKPD framework for biomarker led decision making for prioritising dose and schedules for anti-cancer agents to test in the clinic
 A pre-clinical PKPD framework for biomarker led decision making for prioritising dose and schedules for anti-cancer agents to test in the clinic Rhys D Owen Jones, Oncology imed 1st International Workshop
A pre-clinical PKPD framework for biomarker led decision making for prioritising dose and schedules for anti-cancer agents to test in the clinic Rhys D Owen Jones, Oncology imed 1st International Workshop
ICH CONSIDERATIONS Oncolytic Viruses
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
Session 2 summary Designs & Methods. Pairwise comparisons approach. Dose finding approaches discussed: Guiding principles for good dose selection
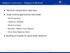 Session 2 summary Designs & Methods Pairwise comparisons approach Dose finding approaches discussed: PK/PD Modeling (Adaptive) MCPMod Model Averaging Bayesian Adaptive Dose Ranging Emax Dose Response Model
Session 2 summary Designs & Methods Pairwise comparisons approach Dose finding approaches discussed: PK/PD Modeling (Adaptive) MCPMod Model Averaging Bayesian Adaptive Dose Ranging Emax Dose Response Model
Comparative Studies Evaluating Mouse Models Used for Efficacy Testing of Experimental Drugs against Mycobacterium tuberculosis
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Mar. 2011, p. 1237 1247 Vol. 55, No. 3 0066-4804/11/$12.00 doi:10.1128/aac.00595-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Comparative
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Mar. 2011, p. 1237 1247 Vol. 55, No. 3 0066-4804/11/$12.00 doi:10.1128/aac.00595-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Comparative
ICH Topic E16 Genomic Biomarkers Related to Drug Response: Context, Structure and Format of Qualification Submissions. Step 3
 European Medicines Agency June 2009 EMEA/CHMP/ICH/380636/2009 ICH Topic E16 Genomic Biomarkers Related to Drug Response: Context, Structure and Format of Qualification Submissions Step 3 NOTE FOR GUIDANCE
European Medicines Agency June 2009 EMEA/CHMP/ICH/380636/2009 ICH Topic E16 Genomic Biomarkers Related to Drug Response: Context, Structure and Format of Qualification Submissions Step 3 NOTE FOR GUIDANCE
Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y. Report Price Publication date
 F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
Workshop on update of TB Guideline - WHO perspective
 Workshop on update of TB Guideline - WHO perspective Christian Lienhardt Research for TB Elimination WHO, Geneva European Medicines Agency, London 25 November 2016 Overview Background From new drugs to
Workshop on update of TB Guideline - WHO perspective Christian Lienhardt Research for TB Elimination WHO, Geneva European Medicines Agency, London 25 November 2016 Overview Background From new drugs to
2013 activity report of the Modelling and Simulation Working Group (MSWG)
 02 June 2014 EMA/303848/2014 2013 activity report of the Modelling and Simulation Working Group (MSWG) Background The Modelling and Simulation Working Group (MSWG) was established in January 2013 to provide
02 June 2014 EMA/303848/2014 2013 activity report of the Modelling and Simulation Working Group (MSWG) Background The Modelling and Simulation Working Group (MSWG) was established in January 2013 to provide
TERMS OF REFERENCE. The form and function of the Stop TB Partnership Working Group on New Drugs, Its Core Group (CG), Subgroups and Secretariat
 TERMS OF REFERENCE The form and function of the Stop TB Partnership Working Group on New Drugs, Its Core Group (CG), Subgroups and Secretariat 1 Rationale for the Stop TB Working Group on New Drugs (hereinafter
TERMS OF REFERENCE The form and function of the Stop TB Partnership Working Group on New Drugs, Its Core Group (CG), Subgroups and Secretariat 1 Rationale for the Stop TB Working Group on New Drugs (hereinafter
Comparative Oncology Program
 The Problem: Non-Integrated Cancer Drug Development A Solution: Integration of Informative Non-Clinical Models of Cancer With Clinical Drug Development Efforts Companion Animal Malignancies as Comparative
The Problem: Non-Integrated Cancer Drug Development A Solution: Integration of Informative Non-Clinical Models of Cancer With Clinical Drug Development Efforts Companion Animal Malignancies as Comparative
Issues in Cancer Drug Development of the Future. Janet Woodcock M.D. Deputy Commissioner/Chief Medical Officer, FDA October 5, 2007
 Issues in Cancer Drug Development of the Future Janet Woodcock M.D. Deputy Commissioner/Chief Medical Officer, FDA October 5, 2007 Agenda: Scientific Issues n Why improve the quality of cancer clinical
Issues in Cancer Drug Development of the Future Janet Woodcock M.D. Deputy Commissioner/Chief Medical Officer, FDA October 5, 2007 Agenda: Scientific Issues n Why improve the quality of cancer clinical
Critical Path Institute:
 Critical Path Institute: Coalition Against Major Diseases (CAMD) Qualification at FDA and CHMP IMI, Barcelona Marc Cantillon, MD Executive Director, CAMD, Critical Path Institute C-Path & FDA MOU October
Critical Path Institute: Coalition Against Major Diseases (CAMD) Qualification at FDA and CHMP IMI, Barcelona Marc Cantillon, MD Executive Director, CAMD, Critical Path Institute C-Path & FDA MOU October
Model-based rationale for drug combinations in tuberculosis
 Model-based rationale for drug combinations in tuberculosis Morris Muliaditan Oscar Della Pasqua Clinical Pharmacology and Therapeutics Group, UCL, London, UK Clinical Pharmacology Modelling & Simulation,
Model-based rationale for drug combinations in tuberculosis Morris Muliaditan Oscar Della Pasqua Clinical Pharmacology and Therapeutics Group, UCL, London, UK Clinical Pharmacology Modelling & Simulation,
Daptomycin: a new-old antibiotic or how did pharmacodynamics bring back to life a disappointing drug?
 Daptomycin: a new-old antibiotic or how did pharmacodynamics bring back to life a disappointing drug? Unité de Pharmacologie cellulaire et moléculaire F. Van Bambeke Origin of daptomycin Daptomycin is
Daptomycin: a new-old antibiotic or how did pharmacodynamics bring back to life a disappointing drug? Unité de Pharmacologie cellulaire et moléculaire F. Van Bambeke Origin of daptomycin Daptomycin is
GSK DDW Tuberculosis DPU portfolio. TB DPU, Tres Cantos GSK DDW
 GSK DDW Tuberculosis DPU portfolio TB DPU, Tres Cantos GSK DDW TB DPU Global portfolio of Projects: GSK+ External Collaborations HTS/MTS Hit2Lead Lead Op Pre-Clincal FTIM Phase IIa Mtb KasA HTS/ELT/FBDD
GSK DDW Tuberculosis DPU portfolio TB DPU, Tres Cantos GSK DDW TB DPU Global portfolio of Projects: GSK+ External Collaborations HTS/MTS Hit2Lead Lead Op Pre-Clincal FTIM Phase IIa Mtb KasA HTS/ELT/FBDD
2401 Gillham Road Kansas City, Missouri Phone (816)
 2401 Gillham Road Kansas City, Missouri 64108 Phone (816) 234-3000 www.childrens-mercy.org Children s Mercy Hospitals and Clinics Division of Clinical Pharmacology and Medical Toxicology CPMT Project.:
2401 Gillham Road Kansas City, Missouri 64108 Phone (816) 234-3000 www.childrens-mercy.org Children s Mercy Hospitals and Clinics Division of Clinical Pharmacology and Medical Toxicology CPMT Project.:
7th Annual Shanghai Symposium on Clinical and Pharmaceutical Solutions through Analysis
 Mechanistic Physiological PhysioPD Models in Drug Development: A Proven Quantitative Systems Pharmacology (QSP) approach Sharan A Pagano SVP, Scientific Alliances at Rosa & Co. LLC 7th Annual Shanghai
Mechanistic Physiological PhysioPD Models in Drug Development: A Proven Quantitative Systems Pharmacology (QSP) approach Sharan A Pagano SVP, Scientific Alliances at Rosa & Co. LLC 7th Annual Shanghai
A Modeling and Simulation Case Study
 A Modeling and Simulation Case Study Impact on an Early Clinical Development Program Ken Kowalski, Steve Olson, Ann Remmers Pfizer Global Research and Development Ann Arbor, MI PAGE June 14-16, 16, 26
A Modeling and Simulation Case Study Impact on an Early Clinical Development Program Ken Kowalski, Steve Olson, Ann Remmers Pfizer Global Research and Development Ann Arbor, MI PAGE June 14-16, 16, 26
Elena BM Breidenstein, PhD 21 April 2018
 Discovery of a Novel Oral Antibiotic, DDS-01 (SMT-571), to Treat Multi-Drug Resistant Neisseria gonorrhoeae Enabled by a Proprietary Transposon Technology Elena BM Breidenstein, PhD 21 April 2018 Forward-Looking
Discovery of a Novel Oral Antibiotic, DDS-01 (SMT-571), to Treat Multi-Drug Resistant Neisseria gonorrhoeae Enabled by a Proprietary Transposon Technology Elena BM Breidenstein, PhD 21 April 2018 Forward-Looking
NINDS/HEAL Biomarker Programs New Biomarker Initiatives at NIH: Neurological Disorders and Pain
 NINDS/HEAL Biomarker Programs New Biomarker Initiatives at NIH: Neurological Disorders and Pain OCTOBER 30, 2018 Mary Ann Pelleymounter, PhD Program Director mary.pelleymounter@nih.gov 1 About the Speaker
NINDS/HEAL Biomarker Programs New Biomarker Initiatives at NIH: Neurological Disorders and Pain OCTOBER 30, 2018 Mary Ann Pelleymounter, PhD Program Director mary.pelleymounter@nih.gov 1 About the Speaker
Current Status: CPTR PBPK Modelling Effort. Lisa Almond Translational Science (DMPK) Simcyp Limited (a Certara Company)
 Current Status: CPTR PBPK Modelling Effort Lisa Almond Translational Science (DMPK) Simcyp Limited (a Certara Company) lisa.almond@certara.com Project Objectives To develop an exploratory PBPK model for
Current Status: CPTR PBPK Modelling Effort Lisa Almond Translational Science (DMPK) Simcyp Limited (a Certara Company) lisa.almond@certara.com Project Objectives To develop an exploratory PBPK model for
Introduction to Quantitative Imaging as a Biomarker in Clinical Trials
 Quantitative Medical Imaging for Clinical Research and Practice Educational Session ACRIN 2009 Introduction to Quantitative Imaging as a Biomarker in Clinical Trials Katarzyna J. Macura, MD, PhD Johns
Quantitative Medical Imaging for Clinical Research and Practice Educational Session ACRIN 2009 Introduction to Quantitative Imaging as a Biomarker in Clinical Trials Katarzyna J. Macura, MD, PhD Johns
Opportunities to Develop Meaningful Biomarkers
 Opportunities to Develop Meaningful Biomarkers Shashi Amur, Ph.D. Scientific Lead Biomarker Qualification Program Office of Translational Sciences Center for Drug Evaluation and Research Food and Drug
Opportunities to Develop Meaningful Biomarkers Shashi Amur, Ph.D. Scientific Lead Biomarker Qualification Program Office of Translational Sciences Center for Drug Evaluation and Research Food and Drug
HD Regulatory Science Consortium (HD-RSC) A consortium aimed at accelerating treatments for Huntington s disease
 HD Regulatory Science Consortium (HD-RSC) A consortium aimed at accelerating treatments for Huntington s disease Critical Path Institute / CHDI Foundation Huntington s Disease Regulatory Science Consortium
HD Regulatory Science Consortium (HD-RSC) A consortium aimed at accelerating treatments for Huntington s disease Critical Path Institute / CHDI Foundation Huntington s Disease Regulatory Science Consortium
Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research
 Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research Drug Development Process by which new chemical entities
Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research Drug Development Process by which new chemical entities
Yodit Belew, MD Senior Medical Officer Division of Antiviral Products OAP/OND/CDER/FDA 1
 Clinical Trials and Research in Children FDA s Perspectives for the development of antiviral products for the treatment of HIV infection in children: Regulatory considerations Pediatric HIV Cure Meeting
Clinical Trials and Research in Children FDA s Perspectives for the development of antiviral products for the treatment of HIV infection in children: Regulatory considerations Pediatric HIV Cure Meeting
Exploratory IND: a study proposal from Novartis
 Exploratory IND: a study proposal from Novartis Rossella Belleli ECD BIOS Novartis 17 Nov 005 Presentation outline What is an exploratory (exp)ind compared to a traditional IND Introduction to traditional
Exploratory IND: a study proposal from Novartis Rossella Belleli ECD BIOS Novartis 17 Nov 005 Presentation outline What is an exploratory (exp)ind compared to a traditional IND Introduction to traditional
Key Activities. Ofer Reizes, Ph.D. Skills Development Director
 Key Activities Ofer Reizes, Ph.D. Skills Development Director 1 Key Activities Core Question: What key activities required for your Value Propositions? Key Partners Key Activities Key Resources Value Proposition
Key Activities Ofer Reizes, Ph.D. Skills Development Director 1 Key Activities Core Question: What key activities required for your Value Propositions? Key Partners Key Activities Key Resources Value Proposition
