A Blueprint for Drug/Diagnostic Co-Development: Next-Generation Sequencing (NGS) in Oncology. September 2014
|
|
|
- Diane Hodge
- 5 years ago
- Views:
Transcription
1 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 1 A Blueprint for Drug/Diagnostic Co-Development: Next-Generation Sequencing (NGS) in Oncology September 2014
2 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 2 TABLE OF CONTENTS Introduction Challenges Glossary of Terms Forum Goals Regulatory considerations and strategies for FDA approval of an NGS platform to be used as a companion diagnostic in oncology Optimal performance characteristics and standardization of markers between different NGS tools and transparent reimbursement strategies The potential for a comprehensive NGS tool capable of querying all known actionable markers and prognostic genetic markers to serve as a companion diagnostic for all cancer patients in order to streamline development, investigation, regulation and clinical application Proposals: Proposal #1: Define a regulatory pathway for cancer panels intended to identify actionable oncogenic alterations that allows flexibility in the appropriate FDA medical device pathway Proposal #2: Approaches for performing validation studies should be based on the types of alterations measured by the assay, rather than every alteration individually Proposal #3: Determine the contents of a cancer panel by classifying potential markers based on current utility in clinical care and clinical trials Proposal #4: Promote the standardization of cancer panels through the use of a common set of samples to ensure reproducibility on each platform Proposal #5: Establish a framework for determining the appropriate reference method rather than relying on any single reference method for all studies 2
3 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 3 INTRODUCTION There has been rapid improvement and implementation of Next Generation Sequencing (NGS) platforms over the last several years. Despite limited progress with reimbursement for NGS-based cancer panels, they are increasingly being utilized to determine treatment options for oncology patients, thereby suggesting the clinical importance of this new approach. Because of this, it is critical that an appropriate regulatory pathway be developed to ensure these panels are reviewed by the US Food and Drug Administration (FDA) and can be amended via an expeditious review process to enable incorporation of the new marker into clinical practice. Use of a broad cancer panel is currently being demonstrated in the Lung-MAP 1 clinical trial, where numerous companies have come together to create a multi-drug, multi-arm, biomarker-driven clinical trial for patients with advanced squamous cell lung cancer. Genomic profiling via a comprehensive NGS panel (Foundation One TM ) and a MET IHC assay is used to match patients to one of several different investigational treatments that are designed to target the genomic alterations found to be driving the growth of their cancer. FDA has supported this trial design with creative and rapid approaches to review both the drugs and diagnostic platforms used in the trial. The Lung-MAP trial is expected to provide data that could support FDA approval of both the drug and associated companion diagnostic. In addition to the Lung-MAP trial, other examples of innovative trial designs include initiatives by the CRUK, the WIN Consortium, and NCI-MATCH. These types of trials serve as a paradigm for the approval of an NGS platform and/or assay as a companion diagnostic for multiple therapeutics. If this new paradigm proves successful, multiple challenges inherent in Companion Diagnostic (CoDx) development today may be overcome, as exemplified in the situations below: Example 1: A pharmaceutical company utilizes a large panel of genomic markers during early stage trials in a molecular subtype of cancer for which there is no approved therapy. These markers are not used for patient selection at this stage. Data is collected for research purposes to evaluate if a marker may be relevant for identifying patients with improved response to the drug. During the analysis, patients with a specific biomarker display a dramatically better response to the drug than patients without the marker. The panel assay that was used during this trial is a laboratory developed test (LDT) and the lab that created the panel test is either not interested or it may not be cost-effective to proceed with a CoDx product. The drug company embarks on a path to develop a simpler PCR based assay to test for the marker. After reviewing multiple proposals from partners, it will take at least 18 months to have an assay ready for the pivotal trial; approximately a 9-month delay compared to the timeline for the drug to be ready. If FDAcleared actionable NGS panels were available, it is possible this type of delay and the need for a switch to a different assay could be avoided. Example 2: Several drug companies are moving forward with products targeted to a specific marker. Each drug company partners with a different diagnostic company to develop an assay. All of the drugs make it through clinical trials, and three separate companion diagnostics with similar, but not congruent, performance characteristics are approved by FDA. Even though each diagnostic product is labeled for use with a specific drug, it is likely that having multiple assays in addition to the already available LDTs will create a confusing situation for both doctors and laboratories. A more standard set of actionable cancer NGS panels may be a way to avoid this situation
4 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 4 Example 3: A pharmaceutical company is required to move forward with an in vitro diagnostic (IVD) CoDx to register their product. Upon successful registration and IVD product launch, multiple LDTs appear on the market combining these same markers into multi-analyte panels. Hospitals and academic centers will use the most cost effective test and have incentives to offer such capabilities internally. Even though a specific drug label might state that the drug must be used with an FDA-approved test, the increased use of NGS panels across centers in the US may lead to use of targeted agents in patients identified with these non-standard tests that may or may not identify the same population for whom the drug product was approved and may not have the same (predictive) clinical utility as the FDA-approved test. Example 4: In the context of increasing adoption of NGS in the community, a patient s tumor may be profiled at any time in the disease course, such as at diagnosis. Future medical need and treatment decisions for that individual at relapse may be called for with existing or novel targeted therapies guided by the presence of a specific biomarker but no current tissue is accessible. Having the past genetic profile based on a FDA-cleared actionable panel would provide higher confidence information for the treating physician. Challenges Currently, all of the marketed oncology panels have been developed by laboratories and validated pursuant to the CLIA regulations as LDTs i.e., no FDA clearance or approval, though moving forward, FDA oversight may be required in a risk-based fashion 2. Given the rapid pace of drug development that relies on accurate identification of molecular markers to determine whether a drug is appropriate for a particular patient, the current one drug/one test paradigm is inefficient and unlikely to be acceptable in the clinic for much longer. The current challenges in this area include: Independent development of each oncology panel by separate organizations with little or no standardization of markers or performance characteristics between the tests leading to heterogeneous patient selection within defined marker sets Different orthogonal reference methods for validation exist for each test making it difficult to draw comparisons even when looking at two sets of validation data side-by-side Unclear process for determining the appropriate reference standards. FDA tends to prefer bidirectional Sanger sequencing as a gold standard (although exceptions exist) but given that new technologies may be more sensitive than bi-directional Sanger sequencing an updated process for selecting reference standards needs to be agreed upon Unpublished validation data Lack of proficiency test guidance for NGS panels Heterogeneity in the quantity and quality of the biopsy material available to support the onetest/one-drug model Accurate determination of the fraction of malignant cells in input DNA over the total of malignant and non-malignant nucleated cells. Currently, this is achieved by a pathology estimate and may be inadequately accounted for in the context of somatic mutation analysis and validation by NGS 2 4
5 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 5 Different underlying platform technologies that have individual strengths and weakness Equal regulatory consideration given to all oncology markers, in that all are assumed to need a PMA when used in the context of determining treatment (applies to predictive and prognostic markers) Reimbursement challenges will remain until standardization of panel content and agreed upon approaches for demonstrating performance are established (i.e. payment for select biomarkers on a panel based on levels of clinical utility) Need for the ability to update panels with newly relevant content A community-wide approach to address these challenges is what brings us together to discuss how to best move towards agreement on regulatory pathways, promoting and implementing standardization of panel content and performance protocols, and reimbursement decisions to ensure patients are receiving accurate, consistent results no matter which oncology panel is utilized. The NGS Working Group, comprised of representatives from the academic, drug development, and government sectors, would like to focus on five proposals that take steps to address these challenges and facilitate development and use of cancer panels. These proposals are related to the following goals to identify existing challenges and opportunities to optimize processes associated with: Forum Goals Regulatory considerations and strategies for FDA approval of an NGS platform to be used as a companion diagnostic in oncology Optimal performance characteristics and standardization of markers between different NGS tools and transparent reimbursement strategies The potential for a comprehensive NGS tool capable of querying all known actionable markers and prognostic genetic markers to serve as a CoDx for all cancer patients in order to streamline development, investigation, regulation and clinical application Glossary of Terms The following key terms were defined by the participants of this working group, in the context of this document: Actionable Marker: Documented alterations with supporting data that allow for a benefit-risk assessment of treatment choice, linking the patient to a FDA approved drug, on- or off-label, a drug in the investigational phase of development, or an established prognostic outcome. Some markers may be specific (e.g., BRAF V600E), while others may represent a functional group (e.g., alterations in Exon 19 of the EGFR gene). For the purposes of this paper, the focus was on targeted sequencing only (i.e., no consideration of whole genome or whole exome sequencing; thus, incidental findings were not considered in this paper). Cancer Panel: Panels are a comprehensive (i.e., point mutations, deletions, amplifications, fusions) selection of multi-marker gene assays of cancer driver alterations as opposed to traditional single-marker assays. 5
6 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 6 Standardization: This working group seeks to define what to measure and not the degree to or performance of the measurements (i.e., level of sensitivity). In other words, standardization in the context of this paper is only agreement on the nature of markers and the performance characteristics of assays to be evaluated on those markers, not a prescribed performance level an assay must attain. The working group has provided suggestions about how to determine appropriate reference standards so that performance may be appropriately assessed. To date, two panels have been developed for actionable genetic panels in other therapeutic areas. The existing panels may serve as useful models in considering the development of an actionable cancer panel: 1) Cystic Fibrosis (CF) panels This approach would be similar to the development of molecular assays currently used in the diagnosis and management of cystic fibrosis (CF products utilize a common set of markers as defined by the American College of Medical Genetics and Genomics and American College of Obstetricians and Gynecologists). Although CF testing may in some cases drive therapeutic choices, the information from genetic testing, stored in the patient s medical record, is used more broadly by physicians when treating a patient. Given the existence of FDA cleared CF panels, drug approval targeted to a specific CF genetic marker (Vertex s Kalydeco) did not require the approval of an additional companion diagnostic. Further, this exemption is relevant in that analytical studies for 510(k) cleared CF genetic panels have included the use of contrived specimens to evaluate performance for rare mutations. Since a similar situation is emerging in oncology (i.e., standard of care including testing for a variety of genetic markers and the need for creative approach to analytical testing) it seems appropriate to consider whether this model could be adapted for oncology, particularly when considering rare tumor types or subpopulations. 2) Microbiology panels Another existing paradigm to consider when discussing regulatory pathways for a molecular oncology panel is microbiology pathogen panels. Non-molecular panels of products for identification of various organisms are currently regulated as Class I exempt products. If information regarding drug resistance of any of these organisms is offered, the classification of the product moves to Class II and requires a 510(k). In addition, microbiology panels based on molecular technology have been cleared via the 510(k) pathway. This model can be extended even to consider which organisms in microbiology need to have more rigorous regulatory pathways. Pathogens that can cause significant disease are regulated as Class III, PMA devices. Class III microbiology targets include HIV, HBV and HCV. It may be possible to extend this existing risk-based approach to oncology targets in that markers for efficacy of drugs may be appropriate to regulate differently than those associated with safety of particular compounds. In addition, more novel approaches such as algorithms designed to choose a particular drug may also be regulated at a higher level on a case-by-case basis. It s interesting to note that at one point microbiology identification panels were regulated as Class III medical devices but were down classified as the technology and use of these panels became standard. In addition to these two models, the working group would like to draw attention to the intended use of the Qiagen therascreen EGFR assay 3. This approval highlights a creative approach for an assay that has shown clinical utility for multiple mutation classes, and has content that has been analytically demonstrated, but importantly has not been demonstrated to have clinical utility for every mutation. This will be an important precedent to consider in the context of the proposals in this paper
7 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 7 The therascreen EGFR RGQ PCR Kit is a real-time PCR test for the qualitative detection of exon 19 deletions and exon 21 (L858R) substitution mutations of the epidermal growth factor receptor (EGFR) gene in DNA derived from formalin-fixed paraffin-embedded (FFPE) nonsmall cell lung cancer (NSCLC) tumor tissue. The test is intended to be used to select patients with NSCLC for whom GILOTRIF (afatinib), an EGFR tyrosine kinase inhibitor (TKI), is indicated. Safety and efficacy of GILOTRIF (afatinib) have not been established in patients whose tumors have L861Q, G719X, S7681, exon 20 insertions, and T790M mutations, which are also detected by the therascreen EGFR RGQ PCR Kit. Proposals 1. Proposal: Define a regulatory pathway for cancer panels intended to identify actionable oncogenic alterations that allows flexibility in the appropriate FDA medical device pathway. Such a pathway could be based on risk classification of different panel components and use the PMA and the 510(k) route depending on the specific marker. Molecular markers are utilized in a variety of ways, not just for directing specific treatments. As noted above, cancer panels are very quickly gaining adoption in the care for oncology patients, especially within the largest academic cancer centers. As a result, it is critical that an appropriate regulatory pathway be made available to ensure FDA reviews these panels. The NGS Working Group encourages discussion on whether pathways other than Class III PMA are possible (i.e., Class II, 510(k) pathway), such that drug approval based on a class II marker could use the 510(k) route, while that based on a class III marker could use the PMA route. Risk determination for individual markers on a panel could be established based on the classification system defined in proposal 3 with Known Actionable markers being classified as class III while Likely Actionable and Investigational and prognostic classified as class II. This approach would enable a platform to be supported by multiple regulatory pathways. Any regulatory proposal also needs to address and mitigate the risk of a wrong test result that does not accurately predict the response to a therapeutic. The NGS Working Group would like to suggest that an appropriate data set that includes a high level of accuracy testing (testing against a reference method, see proposal #5) could adequately address this concern. Of course, certain genomic alterations may require more rigorous review particularly when they are known to be related to safe and effective use of particular treatments. Within this proposal, the working group recognizes there may be exceptions to any broad policy decision on the regulation of genomic oncology panels. For example, if a drug was targeted against a marker not on the panel then a separate companion diagnostic clearance or approval would need to be sought and classification would be decided based on intended use of the marker. The working group proposes that panel content could be driven by an international public body that would have an unbiased approach to determining panel content and have the appropriate scientific expertise. It will be critical to also include pharmaceutical companies in these discussions as much of the biological significance of markers will be based on work performed as part of drug development efforts. Finally, ensuring that payers are included in the discussion will be important to ensure commercial viability of the approach. A proposed intended use for such a product could be as follows: The standardized molecular panel is intended for use in identifying specific molecular alterations known to be involved in cancer development and progression as well as known targets for oncology drug development. These 7
8 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 8 molecular alterations could be used as evidence for clinical study enrollment, if suitable regulatory requirements are met. Molecular alterations that have been cleared or approved to inform specific patient therapeutic treatment can be used for their defined purpose. One final issue to consider in the development of a regulatory pathway for an FDA cleared or approved cancer panel are the ongoing improvements in technology and changes in content. FDAcleared panels may quickly become obsolete based on the rapid evolution and discovery in the oncology field. Identifying a mechanism for ensuring rapid inclusion of new markers, performance data for genomic alterations, and updates to assay performance are also needed. One such approach could be allowing new markers designated for clinical trial use only to be added via the 30-Day Notice PMA Supplement process (would require changes to current guidance) or via a new 510(k) should a Class II risk designation be appropriate. Questions for Discussion for Proposal #1: a. Which type of organization or professional society could make recommendations on an actionable panel? b. Could markers without a specific oncology drug listed in its intended use be considered under lower classification (i.e., Class II, 510(k)) than current companion diagnostics? Is it appropriate to consider higher classifications for some markers based on intended use (i.e., efficacy vs. safety)? What risks need to be mitigated in order to consider a 510(k) pathway? c. Some companion diagnostics have already been approved. Is there a way to bridge the content from the approved CoDx assays onto an NGS panel? Can FDA comment on the nature of the bridging study expectations in the light of it being improbable that complete sets of samples from clinical trials will be available to support bridging studies? Should industry rely on previous approvals for me-too HER2 assays as examples? d. Should a company bridge the me-too PMA/510(k) content to an NGS panel; could the NGS panel that includes those markers be considered the reference standard moving forward? How could such a panel be adapted for clinical practice? e. What would the data set be to support an intended use for a non-drug specific companion diagnostic? Would analytical validation be sufficient or would clinical samples be required? f. What strategies could be considered for handling the mass storage of patient NGS data? How long would/should this data be stored? g. FDA recently issued the draft guidance: Expedited Access for Premarket Approval Medical Devices intended for Unmet Medical Need for Life Threatening or irreversibly Debilitating Disease or Conditions Draft Guidance for Industry and Food and Drug Administration Staff 4. Could this guidance be applied to an Actionable Cancer Panel? Could the following be applied to an application for an actionable cancer panel (from Section (3) of the guidance document): i. Use of banked samples from previously performed prospective studies. ii. Use of literature to support clinical validity? iii. Use of contrived samples in some cases? 4 8
9 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 9 2. Proposal: Approaches for performing validation studies should be based on the types of alterations measured by the assay, rather than every alteration individually. Office of IVDs and Radiological Products (OIR) has been innovative in the approach to analytical studies for multiplex microbiology panels in the hope of decreasing the analytical study requirements while ensuring the safety of multiplex molecular diagnostic panels. The FDA guidance document: Draft Guidance for Industry and Food and Drug Administration Staff - Highly Multiplexed Microbiological/Medical Countermeasure In Vitro Nucleic Acid Based Diagnostic Devices 5 may be an appropriate reference to consider when determining the analytical requirements for a multiplex cancer panel. In addition, it is important to consider the approach taken in the recent Illumina MiSeqDx clearance of comparing to well-characterized composite reference information derived from the combination of multiple sequencing methodologies, publicly available data, and hereditary information. The concept of classifying specific types of genomic alterations and performing analytical studies based on those classifications may also be important to consider, given that reference specimens bearing all possible genomic alterations do not exist. For example, it may be appropriate for a multiplex assay that has numerous point mutations to perform studies for point mutations generally, if the various factors that may affect assay performance (such as sequence context) are addressed in the experimental design. Finally, FDA has historically required some level of validation from the tissue type at which the assay is directed (i.e., lung cancer biopsy if the intended use of the product is for identification of patients for a lung cancer drug) or from blood. While this strategy provides a high degree of assurance of assay performance within each tissue, it may be more important for protein and RNA based assays, rather than for DNA, which is generally less variable. To provide greater flexibility, most NGS validation activities should begin with DNA as the starting specimen rather than the original tissue source, complemented by studies with defined reagents/kits demonstrating reliable DNA extraction across tissue types. Several ongoing or planned studies, including The Cancer Genome Atlas and NCI s MATCH Trial, will add support for reliable DNA retrieval and NGS assay performance from many varieties of tissues. Questions for Discussion for Proposal #2: a. What analytical requirements should be considered for an actionable cancer panel? b. What flexibilities in the level of validation can be adapted for NGS? c. If an interested group were willing to do a study that examined thoroughly the issue of whether the tissue type effects DNA extraction with defined sets of reagents/kits (i.e., are there known interfering substances in some tumor types that require validation from tissue; across various types of tumors - i.e., breast biopsy, lung biopsy, etc.) could FDA utilize this general study to potentially decrease the validation requirements? Or would this sort of study be required for each specific platform? d. The handling of massive datasets and developing tools to check and assure sequence quality, conduct sequence alignment and assembly, and biologically interpret and draw inferences from the data remains increasingly important to the implementation of NGS. In establishing performance validation, what requirements are critical to establish data management standards? e. What requirements should be established specimen pre-analytics (i.e., including tumor cell enrichment)? f. As other technologies (i.e., whole exome sequencing) are adapted for clinical use, can the definition of a cancer panel be adapted to incorporate a broader genetic assessment? 5 9
10 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page Proposal: Determine the contents of a cancer panel by classifying potential markers based on current utility in clinical care and clinical trials and peer-reviewed publications as well as recognized clinical guidelines. Similar to the CF example above, draw upon various sources to determine the recommended marker set for an actionable cancer panel. Rather than attempting to create a specific panel for this paper since there are a variety of groups already working on this issue, the NGS Working Group preparing this paper would like to suggest a process to determining the contents of an actionable cancer panel. The first step is to choose a way to classify various genomic alterations per cancer type. For example, one approach could be to create four buckets: Known Actionable, Likely Actionable, Investigational (i.e., reasonable hypotheses for significance) and Unknown Significance (may be biologically relevant but no known clinical hypothesis at the time of categorization). Note: markers of unknown significance may not be included on an actionable cancer product but are important to consider during the classification process. It is also an important discussion as to whether markers of unknown significance should be included on a panel but not reported until significance has been determined. As noted above in the Qiagen EGFR test, approaches that allow for reporting for many markers, even those without demonstrated clinical utility will be important to provide comprehensive information to physicians. Markers would be placed into the appropriate buckets based on the amount of literature available. Only markers which fall into the Known, Likely Actionable and Investigational buckets could be reported on the panel. As markers moved from the Unknown category into either of the other two categories, they would be eligible to move onto the actionable cancer panel products. The NGS Working Group further proposes that this list should be modified at least annually. The process for reclassifying a marker should be similar to the original classification process. Performance standards for each marker or group of markers will need to be established. Performance for FDA cleared or approved panels will be publically available and the NGS Working Group encourages each CLIA lab to also publish performance data for its LDTs so that comparisons of all cancer panels, regardless of where it was developed, can be generated. The NGS Working Group would also like to encourage reimbursement organizations to require publication of this data as well. Questions for Discussion for Proposal #3: a. Is it desirable to have a single list of markers to measure for the entire industry? b. How should performance standards for each marker or group of markers be established? c. Should markers be stratified/tiered based on level of evidence and tumor type to guide their inclusion in a panel (i.e. availability of clinical data; biologically relevant versus exploratory)? d. Can a panel accommodate complex use of the data? For example, if one wanted to use a gene panel to score tumor mutation burden? 10
11 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page Proposal: Promote the standardization of cancer panels through the development and use of a common set of samples to ensure reproducibility on each platform. For all assays whether they are FDA cleared/approved or LDTs not subject to clearance or approval, we propose that a common set of samples be prepared which test various types of mutations (i.e., insertion/deletions, mutations, rearrangements, etc.) to ensure data is available to show performance of each platform. More specifically, the minimum performance isn t mandated, rather, the NGS Working Group encourages transparency of the data so that decisions on the appropriate product to use can be made based on data. One of the challenging issues with developing panels of standardized materials is determining how they will eventually be replenished. Since a standardization panel, based on well-characterized tissue specimens, would be challenging to prepare and replenish, an approach of creating mock specimens should be considered. Questions for Discussion for Proposal #4: a. Which international organization or group would prepare such samples? How should the work that is currently being performed by NIST and NCI be considered for this proposal? b. How could access to such a sample set be guaranteed? c. Could this type of standardization of assays result in more standard reimbursement? Does having a particular set of data make it easier for the reimbursement bodies to make decisions? Could the results of the proficiency testing be made publicly available? 5. Proposal: Establish a framework for determining the appropriate reference method rather than relying on any single reference method for all studies. Central to all of the discussions in this paper is finding agreement on an appropriate reference method as well as determining what approach should be taken when a reference method is not available. Note: A distinction is made between reference methods and methods that are utilized to resolve discordant results between the original assay result and the reference method. Whether a method can be called a reference method or a discordant resolution method depends on how the analysis is performed. If all samples are tested a priori on two methods then both may be considered reference methods. However, if a third method is utilized but only discordant samples are tested than that method is not considered a reference method. As noted above, each of the current panels have used different orthogonal reference methods in an attempt to ensure truth is reported. OIR has traditionally requested bi-directional Sanger sequencing as the preferred reference method even though it is becoming more often less sensitive than other methods including PCR and NGS. Please see the following table of recent FDA approved CoDx products and the use of reference and discordant resolution methods: 11
12 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 12 Product Name Sample Type Reference Method for Discordant Method Accuracy Study Roche BRAF FFPE Melanoma Sanger sequencing % mutation determined by alternate sequencing method bmx BRAF FFPE Melanoma Sanger sequencing NA Qiagen EGFR FFPE NSCLC Sanger sequencing NA Roche EGFR FFPE NSCLC Sanger sequencing and NA Massively Parallel Sequencing (MPS) Qiagen KRAS FFPE CRC Sanger sequencing NA The following data summaries from the Roche EGFR PMA demonstrate that where methods in addition to bi-directional Sanger sequencing are used it is obvious that more sensitive reference methods are already necessary and as NGS assays continue to improve will become more important. Roche cobas EGFR Mutation Test; PMA Summary P (pages 7 and 8) A total of 406 samples with valid cobas test and Sanger sequencing results were included in the agreement analysis. The agreement analysis between the cobas EGFR Mutation Test results and Sanger sequencing results for the detection of the EGFR mutation demonstrated a PPA of 96.6%, a NPA of 88.3%, and an OPA of 90.6%. The PPA, NPA, and OPA in the detection of exon 19 deletion mutations were all greater than 92%. The PPA, NPA, and OPA in the detection of L858R mutations compared were all greater than 95%. A total of 408 samples with valid cobas test and MPS results were included in the agreement analysis. The agreement analysis between the cobas EGFR Mutation Test results and MPS sequencing results for the detection of the EGFR mutation demonstrated a PPA of 94.0%, a NPA of 97.7%, and an OPA of 96.3%. The PPA, NPA, and OPA in the detection of exon 19 deletion mutations were all greater than 95%. The PPA, NPA, and OPA in the detection of L858R mutations were all greater than 90%. Rather than trying to agree upon a single reference method or simply creating bi-directional Sanger sequencing as a standard, the NGS Working Group proposes examining the following questions for agreeing on an appropriate method: Proposed Reference Method Decision Questions What is the analyte being measured? Assays measuring the same analyte are more attractive reference methods. For example, while FISH as a measure of amplification may be appropriate as a reference method for NGS copy number determination, IHC may be less desirable as only the protein consequences of the alteration are measured. For quantitative analyses, quantitative reference methods such as PCR, FISH, or another comparative array method may be attractive. 12
13 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 13 What is the sensitivity of the NGS assay system relative to proposed reference method? To verify the positive predictive value (PPV) of NGS findings, only reference methods with comparable sensitivity may be used. For example, if the NGS assay has sensitivity down to the 5% minor allele level, bi-directional Sanger sequencing, with sensitivity at a much higher level cannot be used as a confirmatory assay. On the other hand, if in this example the analytical validation experiment seeks to establish NGS detection of variants previously observed using bidirectional sequencing (i.e. establishing the sensitivity of the NGS assay to at least the bi-directional level), the use of bi-directional Sanger sequencing as a reference method may be appropriate. What is the regulatory status of the proposed reference method? If a proposed reference method meeting the criteria above has previously been reviewed and cleared by FDA, this method should be used. However, if no FDA approved reference method is available with the appropriate performance characteristics, other reference methods may be considered, provided that sufficient information is provided to FDA. What if no absolute reference method available? There are examples to draw from in this situation: 1) Use of composite data: a. The Illumina MiSeq clearance to well-characterized composite reference information derived from the combination of multiple sequencing methodologies, publicly available data, and hereditary information. b. Comparison and presentation of data from multiple sources as in the Affymetrix CytoScan clearance. Comparison against a next generation sequencing method is presented in addition to comparison of historical IHC/FISH data and clinical performance data. 2) Reliance on the demonstration of reproducibility and precision (rather than accuracy) as has been done with IHC CoDx assays Questions for Discussion for Proposal #5: a. What principles should be applied in the selection of an appropriate reference method? b. Under what circumstances can composite data serve as the reference method? What properties of composite data are required to serve as a reference method? c. Under what circumstances can the reference method requirement be waived? d. On the issue of sensitivity, what mechanism is appropriate to demonstrate the Limit of Detection (LOD) of NGS panels? 13
14 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 14 Current FDA Device Classification 6 Reference guide: Investigational device exemption (IDE) An IDE allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data required to support a Premarket Approval (PMA) application or a Premarket Notification 510(k) submission to FDA. Clinical studies with devices of significant risk must be approved by FDA and by an Institutional Review Board (IRB) before the study can begin. Studies with devices of non-significant risk must be approved by the IRB only before the study can begin. Devices classified as Class I or II, and if it is not exempt, a 510k will be required for marketing. Classification is risk based, that is, the risk the device poses to the patient and/or the user is a major factor in the class it is assigned. Class I includes devices with the lowest risk and Class III includes those with the greatest risk. Class I devices are deemed to be low risk and are therefore subject to the least regulatory controls. Class II devices are higher risk devices than Class I and require greater regulatory controls to provide reasonable assurance of the device s safety and effectiveness. Class III devices are those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury. Premarket approval (PMA) Products requiring PMAs are Class III devices that pose a significant risk of illness or injury, or devices found not substantially equivalent to Class I and II predicates (although some may go through a de novo process) Premarket notification 510(k) A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device that is not subject to PMA. Same intended use Same technology or new technology that does not raise new questions of safety and effectiveness Data required to support substantial equivalence varies significantly depending on the type of device; it may or may not require clinical trials. CLIA (applicable to IVDs) CLIA refers to the Clinical Laboratory Improvement Amendments of 1988, which establishes quality standards for laboratory testing. CMS assumes primary responsibility for regulation under CLIA
15 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 15 INSIDE BACK COVER BLANK
16 FOCR-NGS-Report-115_1 2/17/15 10:04 AM Page 16 Friends of Cancer Research 1800 M St NW Washington, DC
Regulatory Perspective for Companion Diagnostics
 Regulatory Perspective for Companion Diagnostics The 38 th Annual Midwest Biopharmaceutical Statistical Workshop (MBSW) May 19, 2015 Jennifer Shen, Ph.D., RAC Food and Drug Administration (FDA) Center
Regulatory Perspective for Companion Diagnostics The 38 th Annual Midwest Biopharmaceutical Statistical Workshop (MBSW) May 19, 2015 Jennifer Shen, Ph.D., RAC Food and Drug Administration (FDA) Center
FDA Regulation of Companion Diagnostics
 FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation
 A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation A Risk-based Approach for In Vitro Companion Diagnostics
A Risk-based Approach for In Vitro Companion Diagnostics Device FDA Approval Process Associated with Therapies that have Breakthrough Designation A Risk-based Approach for In Vitro Companion Diagnostics
First Annual Biomarker Symposium Quest Diagnostics Clinical Trials
 First Annual Biomarker Symposium Quest Diagnostics Clinical Trials Terry Robins, Ph.D. Director Biomarker R&D and Scientific Affairs Quest Diagnostics Clinical Trials Key Considerations: Biomarker Development
First Annual Biomarker Symposium Quest Diagnostics Clinical Trials Terry Robins, Ph.D. Director Biomarker R&D and Scientific Affairs Quest Diagnostics Clinical Trials Key Considerations: Biomarker Development
CHARTING THE COURSE FOR PRECISION MEDICINE
 A Friends of Cancer Research White Paper CHARTING THE COURSE FOR PRECISION MEDICINE ADOPTING CONSENSUS ANALYTICAL STANDARDS AND STREAMLINING APPROVAL PATHWAYS FOR POST-MARKET MODIFICATIONS FOR NGS TESTS
A Friends of Cancer Research White Paper CHARTING THE COURSE FOR PRECISION MEDICINE ADOPTING CONSENSUS ANALYTICAL STANDARDS AND STREAMLINING APPROVAL PATHWAYS FOR POST-MARKET MODIFICATIONS FOR NGS TESTS
Medical Devices; Immunology and Microbiology Devices; Classification of the Next Generation
 This document is scheduled to be published in the Federal Register on 06/22/2018 and available online at https://federalregister.gov/d/2018-13406, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 06/22/2018 and available online at https://federalregister.gov/d/2018-13406, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Diagnostics in Oncology Mark Kockx MD, PhD
 HistoGeneX The Real World A Specialized of companion Biomarker & Integrated Pathology Laboratory Diagnostics in Oncology Mark Kockx MD, PhD 1 2 HistoGeneX located in Antwerp, Belgium and Chicago, Illinois
HistoGeneX The Real World A Specialized of companion Biomarker & Integrated Pathology Laboratory Diagnostics in Oncology Mark Kockx MD, PhD 1 2 HistoGeneX located in Antwerp, Belgium and Chicago, Illinois
Association for Molecular Pathology Promoting Clinical Practice, Basic Research, and Education in Molecular Pathology
 Association for Molecular Pathology Promoting Clinical Practice, Basic Research, and Education in Molecular Pathology 9650 Rockville Pike, Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7990 Email:
Association for Molecular Pathology Promoting Clinical Practice, Basic Research, and Education in Molecular Pathology 9650 Rockville Pike, Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7990 Email:
Molecular Diagnosis Challenges & Solutions. Using Molecular Kits or Laboratory Developed Tests (Home Brew), Emphasis on Validation
 Using Molecular Kits or Laboratory Developed Tests (Home Brew), Emphasis on Validation Molecular Diagnosis Challenges & Solutions Behzad Poopak, DCLS PhD Tehran Medical Branch- Islamic Azad University
Using Molecular Kits or Laboratory Developed Tests (Home Brew), Emphasis on Validation Molecular Diagnosis Challenges & Solutions Behzad Poopak, DCLS PhD Tehran Medical Branch- Islamic Azad University
Combination Products Coalition ( CPC ); Points to Consider in Drafting FDA s Co-development Guidance and Other Companion Diagnostic Guidances
 Companion Diagnostics versus Combination Products 1 Under what circumstances is a companion diagnostic a combination product, and when isn t it a combination product? a) If a companion diagnostic is not
Companion Diagnostics versus Combination Products 1 Under what circumstances is a companion diagnostic a combination product, and when isn t it a combination product? a) If a companion diagnostic is not
PMDA Perspectives on Companion Diagnostics Development in Japan. Reiko Yanagihara, Ph.D. Deputy Review Director Office of In Vitro Diagnostics, PMDA
 PMDA Perspectives on Companion Diagnostics Development in Japan Reiko Yanagihara, Ph.D. Deputy Review Director Office of In Vitro Diagnostics, PMDA 1 Disclaimer Content Slide The views and opinions expressed
PMDA Perspectives on Companion Diagnostics Development in Japan Reiko Yanagihara, Ph.D. Deputy Review Director Office of In Vitro Diagnostics, PMDA 1 Disclaimer Content Slide The views and opinions expressed
Personalized Healthcare Diagnostik und Therapie aus einer Hand
 Personalized Healthcare Diagnostik und Therapie aus einer Hand Priv.-Doz. Dr. med. Christian Meisel DVFA Life Science Conference, 17 June 2009 1 The Challenge Personalised healthcare Roche s unique position
Personalized Healthcare Diagnostik und Therapie aus einer Hand Priv.-Doz. Dr. med. Christian Meisel DVFA Life Science Conference, 17 June 2009 1 The Challenge Personalised healthcare Roche s unique position
A new paradigm in testing for NSCLC-targeted therapies
 A new paradigm in testing for NSCLC-targeted therapies Accelerate results, from sample to report, with the first IVD NGS-based test NEW Oncomine Dx Target Test The Ion Torrent Oncomine Dx Target Test is
A new paradigm in testing for NSCLC-targeted therapies Accelerate results, from sample to report, with the first IVD NGS-based test NEW Oncomine Dx Target Test The Ion Torrent Oncomine Dx Target Test is
Disclosures. Laboratory Stakeholders. IVD vs. LDT. FDA Regulation of Laboratory Developed Tests 10/2/2015. FDA Regulation of LDTs
 Disclosures FDA Regulation of Laboratory Developed Tests Beaumont Health System, 24 th Annual Symposium on Molecular Pathology September 16, 2015 Roger D. Klein, MD JD Director, Molecular Pathology Clinical
Disclosures FDA Regulation of Laboratory Developed Tests Beaumont Health System, 24 th Annual Symposium on Molecular Pathology September 16, 2015 Roger D. Klein, MD JD Director, Molecular Pathology Clinical
A New Era of Clinical Diagnostics: How the Business Model is Changing.
 A New Era of Clinical Diagnostics: How the Business Model is Changing. Nancy J Kelley, President and CEO of Nancy J Kelley & Associates Remarks to BTIG Emerging Technologies in Healthcare Diagnostics September
A New Era of Clinical Diagnostics: How the Business Model is Changing. Nancy J Kelley, President and CEO of Nancy J Kelley & Associates Remarks to BTIG Emerging Technologies in Healthcare Diagnostics September
Biomarker Regulation. Regulator s perspective. Jan Müller-Berghaus
 www.pei.de Biomarker Regulation Regulator s perspective Jan Müller-Berghaus The views presented here are my own and do not necessarily reflect the views of the Paul-Ehrlich-Institut or any other regulatory
www.pei.de Biomarker Regulation Regulator s perspective Jan Müller-Berghaus The views presented here are my own and do not necessarily reflect the views of the Paul-Ehrlich-Institut or any other regulatory
Analytical verification methods for the Oncomine Lung cfdna Assay using the Ion S5 XL System
 WHITE PAPER Oncomine Lung cfdna Assay and Ion S5 XL System Analytical verification methods for the Oncomine Lung cfdna Assay using the Ion S5 XL System Key highlights Investigate tumor heterogeneity and
WHITE PAPER Oncomine Lung cfdna Assay and Ion S5 XL System Analytical verification methods for the Oncomine Lung cfdna Assay using the Ion S5 XL System Key highlights Investigate tumor heterogeneity and
Use of Biomarkers in Drug Development. Janet Woodcock M.D. Director, CDER, FDA
 Use of Biomarkers in Drug Development Janet Woodcock M.D. Director, CDER, FDA Biomarkers: Extremely Critical to Drug Development Availability of a pharmacodynamic response marker (e.g., viral load) truly
Use of Biomarkers in Drug Development Janet Woodcock M.D. Director, CDER, FDA Biomarkers: Extremely Critical to Drug Development Availability of a pharmacodynamic response marker (e.g., viral load) truly
Comments on Use of Databases for Establishing the Clinical Relevance of Human Genetic Variants
 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 The American Society of Human Genetics 9650 Rockville Pike Bethesda, MD 20814 24 December
Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 The American Society of Human Genetics 9650 Rockville Pike Bethesda, MD 20814 24 December
Controlling Chaos in Oncology Testing
 HORIZON DISCOVERY Controlling Chaos in Oncology Testing 16 th March 2016 Brian Burke PhD Disclaimer This Presentation does not constitute or form any part of an offer to sell, or invitation to purchase
HORIZON DISCOVERY Controlling Chaos in Oncology Testing 16 th March 2016 Brian Burke PhD Disclaimer This Presentation does not constitute or form any part of an offer to sell, or invitation to purchase
Developing an Accurate and Precise Companion Diagnostic Assay for Targeted Therapies in DLBCL
 Developing an Accurate and Precise Companion Diagnostic Assay for Targeted Therapies in DLBCL James Storhoff, Ph.D. Senior Manager, Diagnostic Test Development World Cdx, Boston, Sep. 10th Molecules That
Developing an Accurate and Precise Companion Diagnostic Assay for Targeted Therapies in DLBCL James Storhoff, Ph.D. Senior Manager, Diagnostic Test Development World Cdx, Boston, Sep. 10th Molecules That
Roche, Roche Molecular Diagnostics and more
 , Molecular and more [Monte Wetzel, PhD] Patients have questions. We provide answers. Group Clear focus on Healthcare Innovation with two strong pillars Pharma Pharma Genentech Chugai Molecular Professional
, Molecular and more [Monte Wetzel, PhD] Patients have questions. We provide answers. Group Clear focus on Healthcare Innovation with two strong pillars Pharma Pharma Genentech Chugai Molecular Professional
Technical Guidance on Development of In Vitro Companion Diagnostics and Corresponding Therapeutic Products
 Administrative Notice December 26, 2013 To: Division of Pharmaceutical Affairs, Prefectural Health Department (Bureau) From: Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry
Administrative Notice December 26, 2013 To: Division of Pharmaceutical Affairs, Prefectural Health Department (Bureau) From: Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry
Functional DNA Quality Analysis Improves the Accuracy of Next Generation Sequencing from Clinical Specimens
 Functional DNA Quality Analysis Improves the Accuracy of Next Generation Sequencing from Clinical Specimens Overview We have developed a novel QC, the SuraSeq DNA Quantitative Functional Index (QFI ).
Functional DNA Quality Analysis Improves the Accuracy of Next Generation Sequencing from Clinical Specimens Overview We have developed a novel QC, the SuraSeq DNA Quantitative Functional Index (QFI ).
In Vitro Companion Diagnostics: Considerations for Regulatory Affairs Professionals
 Allison Nance In Vitro Companion Diagnostics: Considerations for Regulatory Affairs Professionals Executive Director, Regulatory Affairs Celgene Corporation USA TOPRA NJ Event 25 September 2012 What is
Allison Nance In Vitro Companion Diagnostics: Considerations for Regulatory Affairs Professionals Executive Director, Regulatory Affairs Celgene Corporation USA TOPRA NJ Event 25 September 2012 What is
THE WHITE HOUSE Office of the Vice President
 FOR IMMEDIATE RELEASE October 17, 2016 THE WHITE HOUSE Office of the Vice President FACT SHEET: Vice President Biden Delivers Cancer Moonshot Report, Announces Public and Private Sector Actions to Advance
FOR IMMEDIATE RELEASE October 17, 2016 THE WHITE HOUSE Office of the Vice President FACT SHEET: Vice President Biden Delivers Cancer Moonshot Report, Announces Public and Private Sector Actions to Advance
In Vitro Companion Diagnostic Devices
 4 th of Joint Conference of Taiwan and Japan on Medical products regulation In Vitro Companion Diagnostic Devices 2016/12/7 Aoyagi Yumiko Ministry of Health, Labour and Welfare Medical Device Evaluation
4 th of Joint Conference of Taiwan and Japan on Medical products regulation In Vitro Companion Diagnostic Devices 2016/12/7 Aoyagi Yumiko Ministry of Health, Labour and Welfare Medical Device Evaluation
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only. Document issued on: July 14, 2011
 Draft Guidance for Industry and Food and Drug Administration Staff In Vitro Companion Diagnostic Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document issued
Draft Guidance for Industry and Food and Drug Administration Staff In Vitro Companion Diagnostic Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document issued
"Stratification biomarkers in personalised medicine"
 1/12 2/12 SUMMARY REPORT "Stratification biomarkers in personalised medicine" Workshop to clarify the scope for stratification biomarkers and to identify bottlenecks in the discovery and the use of such
1/12 2/12 SUMMARY REPORT "Stratification biomarkers in personalised medicine" Workshop to clarify the scope for stratification biomarkers and to identify bottlenecks in the discovery and the use of such
Docket No. FDA-2011-D-0215 Draft Guidance for Industry and FDA Staff on In Vitro Companion Diagnostic Devices
 VIA Electronic Submission: http://www.regulations.gov Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 RE: Docket No. Draft Guidance
VIA Electronic Submission: http://www.regulations.gov Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 RE: Docket No. Draft Guidance
The Intersection of Genomics Research and the IDE Regulation
 The Intersection of Genomics Research and the IDE Regulation Katherine Donigan, Ph.D. Personalized Medicine Staff FDA/CDRH/OIR October 19, 2017 1 In Vitro Diagnostic (IVD) Regulation Through the 1976 medical
The Intersection of Genomics Research and the IDE Regulation Katherine Donigan, Ph.D. Personalized Medicine Staff FDA/CDRH/OIR October 19, 2017 1 In Vitro Diagnostic (IVD) Regulation Through the 1976 medical
CAPTURE-BASED APPROACH FOR COMPREHENSIVE DETECTION OF IMPORTANT ALTERATIONS
 CAPTURE-BASE APPROACH FOR COMPREHENSIVE ETECTION OF IMPORTANT ALTERATIONS SEQUENCE MUTATIONS MICROSATELLITE INSTABILITY AMPLIFICATIONS GENOMIC REARRANGEMENTS For Research Use Only. Not for iagnostic Purposes.
CAPTURE-BASE APPROACH FOR COMPREHENSIVE ETECTION OF IMPORTANT ALTERATIONS SEQUENCE MUTATIONS MICROSATELLITE INSTABILITY AMPLIFICATIONS GENOMIC REARRANGEMENTS For Research Use Only. Not for iagnostic Purposes.
Recent Trends in Companion Diagnostic Test Development Partnerships
 Recent Trends in Companion Diagnostic Test Development Partnerships Andrew S. Thompson, PhD, Director of Therapy and Analysis, GlobalData Medical, London Tyler Fletcher, Global Head, GlobalData Medical,
Recent Trends in Companion Diagnostic Test Development Partnerships Andrew S. Thompson, PhD, Director of Therapy and Analysis, GlobalData Medical, London Tyler Fletcher, Global Head, GlobalData Medical,
Molecular Diagnostics Regulation Shifting from a Biomarker-Based Approach to an Algorithm-Based Approach
 Molecular Diagnostics Regulation Shifting from a Biomarker-Based Approach to an Algorithm-Based Approach Presented by Kathryn Scheckel Co-authored with Gary Marchant May 27, 2015 Molecular Diagnostics
Molecular Diagnostics Regulation Shifting from a Biomarker-Based Approach to an Algorithm-Based Approach Presented by Kathryn Scheckel Co-authored with Gary Marchant May 27, 2015 Molecular Diagnostics
227 AP and >100 CDx engagements
 Anatomic pathology Q 2 Solutions comprehensive in-house end-to-end anatomic pathology and adjunct molecular services are designed to meet your clinical trial needs. Anatomic pathology is a vital part of
Anatomic pathology Q 2 Solutions comprehensive in-house end-to-end anatomic pathology and adjunct molecular services are designed to meet your clinical trial needs. Anatomic pathology is a vital part of
College of American Pathologists. Statement to the National Institutes of Health on the proposed Genetic Testing Registry.
 College of American Pathologists Statement to the National Institutes of Health on the proposed Genetic Testing Registry July 12, 2010 College of American Pathologists 1350 I Street, NW, Suite 590 Washington,
College of American Pathologists Statement to the National Institutes of Health on the proposed Genetic Testing Registry July 12, 2010 College of American Pathologists 1350 I Street, NW, Suite 590 Washington,
Assay Validation Services
 Overview PierianDx s assay validation services bring clinical genomic tests to market more rapidly through experimental design, sample requirements, analytical pipeline optimization, and criteria tuning.
Overview PierianDx s assay validation services bring clinical genomic tests to market more rapidly through experimental design, sample requirements, analytical pipeline optimization, and criteria tuning.
DNA. Clinical Trials. Research RNA. Custom. Reports CLIA CAP GCP. Tumor Genomic Profiling Services for Clinical Trials
 Tumor Genomic Profiling Services for Clinical Trials Custom Reports DNA RNA Focused Gene Sets Clinical Trials Accuracy and Content Enhanced NGS Sequencing Extended Panel, Exomes, Transcriptomes Research
Tumor Genomic Profiling Services for Clinical Trials Custom Reports DNA RNA Focused Gene Sets Clinical Trials Accuracy and Content Enhanced NGS Sequencing Extended Panel, Exomes, Transcriptomes Research
Molecular Diagnostics
 Molecular Diagnostics Part II: Regulations, Markets & Companies By Prof. K. K. Jain MD, FRACS, FFPM Jain PharmaBiotech Basel, Switzerland May 2018 A Jain PharmaBiotech Report A U T H O R ' S B I O G R
Molecular Diagnostics Part II: Regulations, Markets & Companies By Prof. K. K. Jain MD, FRACS, FFPM Jain PharmaBiotech Basel, Switzerland May 2018 A Jain PharmaBiotech Report A U T H O R ' S B I O G R
May 9, Meeting Summary. Facilitating Antibacterial Drug Development
 May 9, 2012 Meeting Summary Facilitating Antibacterial Drug Development Origins of the Current Public Health Crisis of Antibacterial Resistance Antibacterial drugs play a critical role in the ability to
May 9, 2012 Meeting Summary Facilitating Antibacterial Drug Development Origins of the Current Public Health Crisis of Antibacterial Resistance Antibacterial drugs play a critical role in the ability to
TECHNICAL SHEET IDYLLA BRAF MUTATION TEST
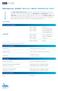 CE IVD TECHNICAL SHEET IDYLLA MUTATION TEST The Idylla, performed on the Biocartis Idylla System, is an in vitro diagnostic Test for the qualitative detection of V600E/ and V600 mutations in codon 600
CE IVD TECHNICAL SHEET IDYLLA MUTATION TEST The Idylla, performed on the Biocartis Idylla System, is an in vitro diagnostic Test for the qualitative detection of V600E/ and V600 mutations in codon 600
American Society of Cytopathology Core Curriculum in Molecular Biology
 American Society of Cytopathology Core Curriculum in Molecular Biology American Society of Cytopathology Core Curriculum in Molecular Biology Chapter 4 Stephanie A. Hamilton, EdD, SCT, MB(ASCP) CM MD Anderson
American Society of Cytopathology Core Curriculum in Molecular Biology American Society of Cytopathology Core Curriculum in Molecular Biology Chapter 4 Stephanie A. Hamilton, EdD, SCT, MB(ASCP) CM MD Anderson
Regulatory Overview of Proposed LDT Framework. FDA Concerns. Background. FDA Proposed Regulatory Approach. By Ben Berg, Meaghan Bailey, RAC
 Regulatory Overview of Proposed LDT Framework By Ben Berg, Meaghan Bailey, RAC On July 31, 2014, the U.S. Food and Drug Administration (FDA or the Agency) notified both the Senate Committee on Health,
Regulatory Overview of Proposed LDT Framework By Ben Berg, Meaghan Bailey, RAC On July 31, 2014, the U.S. Food and Drug Administration (FDA or the Agency) notified both the Senate Committee on Health,
Advancing utility and adoption of clinical genomic diagnostics
 Advancing utility and adoption of clinical genomic diagnostics Laura J. van t Veer Director Applied Genomics, Program Leader Breast Oncology Helen Diller Family Comprehensive Cancer Center University of
Advancing utility and adoption of clinical genomic diagnostics Laura J. van t Veer Director Applied Genomics, Program Leader Breast Oncology Helen Diller Family Comprehensive Cancer Center University of
Sample to Insight. Dr. Bhagyashree S. Birla NGS Field Application Scientist
 Dr. Bhagyashree S. Birla NGS Field Application Scientist bhagyashree.birla@qiagen.com NGS spans a broad range of applications DNA Applications Human ID Liquid biopsy Biomarker discovery Inherited and somatic
Dr. Bhagyashree S. Birla NGS Field Application Scientist bhagyashree.birla@qiagen.com NGS spans a broad range of applications DNA Applications Human ID Liquid biopsy Biomarker discovery Inherited and somatic
Statements on the Regulation of Laboratory Developed Tests
 Statements on the Regulation of Laboratory Developed Tests Current Regulatory Gaps and Perspectives on Oversight of LDTs American Cancer Society Cancer Action Network says Molecular tests, in particular,
Statements on the Regulation of Laboratory Developed Tests Current Regulatory Gaps and Perspectives on Oversight of LDTs American Cancer Society Cancer Action Network says Molecular tests, in particular,
Start With the End in Mind A Diagnostic Company s Perspective on Companion Diagnostic Development. Paul Docherty PhD Relationship Management
 Start With the End in Mind A Diagnostic Company s Perspective on Companion Diagnostic Development Paul Docherty PhD Relationship Management Disclaimer This presentation contains my personal views and research
Start With the End in Mind A Diagnostic Company s Perspective on Companion Diagnostic Development Paul Docherty PhD Relationship Management Disclaimer This presentation contains my personal views and research
Detection of Rare Variants in Degraded FFPE Samples Using the HaloPlex Target Enrichment System
 Detection of Rare Variants in Degraded FFPE Samples Using the HaloPlex Target Enrichment System Application Note Author Linus Forsmark Henrik Johansson Agilent Technologies Inc. Santa Clara, CA USA Abstract
Detection of Rare Variants in Degraded FFPE Samples Using the HaloPlex Target Enrichment System Application Note Author Linus Forsmark Henrik Johansson Agilent Technologies Inc. Santa Clara, CA USA Abstract
Personalized CAR-T Immunotherapy Platform
 GLP, GMP, and CLIA-Certified Lab Personalized CAR-T Immunotherapy Platform Accelerate your cancer research and drug discovery Platform Overview 1500 Existing Hybridomas and Antibody Engineering Custom
GLP, GMP, and CLIA-Certified Lab Personalized CAR-T Immunotherapy Platform Accelerate your cancer research and drug discovery Platform Overview 1500 Existing Hybridomas and Antibody Engineering Custom
GENERAL PAYER CONSIDERATIONS FOR NEW HEALTH TECHNOLOGIES (INCLUDING DIAGNOSTICS)
 FORUM Payer perspectives and actions impacting diagnostics in the US and Europe Eric Faulkner, MPH Director, Quintiles Global Market Access Consulting; Executive Director, Genomics Biotech Emerging Medical
FORUM Payer perspectives and actions impacting diagnostics in the US and Europe Eric Faulkner, MPH Director, Quintiles Global Market Access Consulting; Executive Director, Genomics Biotech Emerging Medical
Recent Initiatives in Precision Medicine PMC Policy Committee Meeting February 20, Laura Koontz, Ph.D. Personalized Medicine Staff CDRH/OIR
 Recent Initiatives in Precision Medicine PMC Policy Committee Meeting February 20, 2018 Laura Koontz, Ph.D. Personalized Medicine Staff CDRH/OIR Agenda Oncopanels 2018 Priorities in Precision Medicine
Recent Initiatives in Precision Medicine PMC Policy Committee Meeting February 20, 2018 Laura Koontz, Ph.D. Personalized Medicine Staff CDRH/OIR Agenda Oncopanels 2018 Priorities in Precision Medicine
Bioresearch Monitoring Inspections in Vitro Diagnostics Devices
 Seite 1 von 7 U.S. Food and Drug Administration Protecting and Promoting Your Health Bioresearch Monitoring Inspections in Vitro Diagnostics Devices TABLE OF CONTENTS Introduction Nature, Scope, & Purpose
Seite 1 von 7 U.S. Food and Drug Administration Protecting and Promoting Your Health Bioresearch Monitoring Inspections in Vitro Diagnostics Devices TABLE OF CONTENTS Introduction Nature, Scope, & Purpose
Specialty Lab Services. Deep science at scale
 Specialty Lab Services Deep science at scale Advancing biomarker research Our broad expertise and global laboratory footprint deliver deep science at scale Specialty assays drive insight into preclinical
Specialty Lab Services Deep science at scale Advancing biomarker research Our broad expertise and global laboratory footprint deliver deep science at scale Specialty assays drive insight into preclinical
Current Topics in NYS Clinical Laboratory Oversight
 June 13, 2018 1 Current Topics in NYS Clinical Laboratory Oversight Derek Symula, Ph.D. and Stephanie Shulman, MPH, MS, MT (ASCP) June 13, 2018 2 (i) Clinical Laboratory Reference System (CLRS) to monitor,
June 13, 2018 1 Current Topics in NYS Clinical Laboratory Oversight Derek Symula, Ph.D. and Stephanie Shulman, MPH, MS, MT (ASCP) June 13, 2018 2 (i) Clinical Laboratory Reference System (CLRS) to monitor,
FDA Drug Approval Process Vicki Seyfert-Margolis, Ph.D.
 Speaker Comparing The Effectiveness Of New Drugs: Should The FDA Be Asking 'Does It Work' Or 'Does It Work Better'? Vicki L. Seyfert-Margolis, PhD Senior Advisor, Science Innovation and Policy U.S. Food
Speaker Comparing The Effectiveness Of New Drugs: Should The FDA Be Asking 'Does It Work' Or 'Does It Work Better'? Vicki L. Seyfert-Margolis, PhD Senior Advisor, Science Innovation and Policy U.S. Food
The EORTC Molecular Screening programme SPECTA
 The EORTC Molecular Screening programme SPECTA February 2016 Denis Lacombe, MD, MSc EORTC, Director General Brussels, Belgium The changing shape of clinical research Phase I RESOURCES Phase III The changing
The EORTC Molecular Screening programme SPECTA February 2016 Denis Lacombe, MD, MSc EORTC, Director General Brussels, Belgium The changing shape of clinical research Phase I RESOURCES Phase III The changing
Perspective: Convergence of CLIA and FDA Requirements A Rational Shift in the Regulatory Paradigm
 Perspective: Convergence of CLIA and FDA Requirements A Rational Shift in the Regulatory Paradigm Planning for Efficiencies of Data, Resources, and Timelines A PRECISION BRIEF Introduction As precision
Perspective: Convergence of CLIA and FDA Requirements A Rational Shift in the Regulatory Paradigm Planning for Efficiencies of Data, Resources, and Timelines A PRECISION BRIEF Introduction As precision
The In Vitro Diagnostic CRO
 The In Vitro Diagnostic CRO Choose Beaufort Because of Our People, Processes and Proven Experience The value of expertise cannot be overstated, especially when it comes to streamlining complicated in vitro
The In Vitro Diagnostic CRO Choose Beaufort Because of Our People, Processes and Proven Experience The value of expertise cannot be overstated, especially when it comes to streamlining complicated in vitro
Re: Comments on FDA s technical assessment of the Diagnostic Accuracy and Innovation Act
 ASSOCIATION FOR MOLECULAR PATHOLOGY Education. Innovation & Improved Patient Care. Advocacy. 9650 Rockville Pike. Suite E205, Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7995 amp@amp.org www.amp.org
ASSOCIATION FOR MOLECULAR PATHOLOGY Education. Innovation & Improved Patient Care. Advocacy. 9650 Rockville Pike. Suite E205, Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7995 amp@amp.org www.amp.org
Challenges in developing Biomarker Assays for patient selection and Companion Diagnostic (CDx) Assays in early and late stage of drug development
 Challenges in developing Biomarker Assays for patient selection and Companion Diagnostic (CDx) Assays in early and late stage of drug development The 9 th JBF Symposium February 8 th, 2018 Kenji Nakamaru
Challenges in developing Biomarker Assays for patient selection and Companion Diagnostic (CDx) Assays in early and late stage of drug development The 9 th JBF Symposium February 8 th, 2018 Kenji Nakamaru
FDA PLANS TO REGULATE TESTS AS DEVICES. August 2010 SPECIAL REPRINT. By Ellen Flannery and Scott Danzis
 August 2010 SPECIAL REPRINT FDA PLANS TO REGULATE LABORATOR ORY DEVELOPED TESTS AS DEVICES By Ellen Flannery and Scott Danzis Reproduced with the kind permission of Global Regulatory Press from the Journal
August 2010 SPECIAL REPRINT FDA PLANS TO REGULATE LABORATOR ORY DEVELOPED TESTS AS DEVICES By Ellen Flannery and Scott Danzis Reproduced with the kind permission of Global Regulatory Press from the Journal
Copyright. Jeremiah J. Kelly (2015). All rights reserved. Further dissemination without express written consent strictly prohibited.
 Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
Statutory Framework for Devices Medical Devices Investigational Use Application IDE (21 CFR 812) Abbreviated IDE Exempt Pre-Market Approval Applications 510(k) Pre-marketing Notification (21 CFR 807(e))
VALUE OF COMPANION DIAGNOSTICS IN PERSONALISED MEDICINE
 VALUE OF COMPANION DIAGNOSTICS IN PERSONALISED MEDICINE Stimulating innovation for improving health through companion diagnostics 5 March 2015 I. Introduction Personalised healthcare provides targeted
VALUE OF COMPANION DIAGNOSTICS IN PERSONALISED MEDICINE Stimulating innovation for improving health through companion diagnostics 5 March 2015 I. Introduction Personalised healthcare provides targeted
January 17, Dear Ms. Jensen,
 Ms. Tamara Syrek Jensen Director, Coverage and Analysis Group Center for Clinical Standards and Quality Centers for Medicare & Medicaid Services Mail Stop #S3-02-01 7500 Security Boulevard Baltimore, Maryland
Ms. Tamara Syrek Jensen Director, Coverage and Analysis Group Center for Clinical Standards and Quality Centers for Medicare & Medicaid Services Mail Stop #S3-02-01 7500 Security Boulevard Baltimore, Maryland
Regulatory Perspectives on NGS-based CDx
 14 th DIA Japan Annual Meeting 2017 November 12-14, 2017 Tokyo Big Sight Ariake Regulatory Perspectives on NGS-based CDx Reiko Yanagihara, Ph.D. Office of In Vitro Diagnostics Deputy Review Director Pharmaceuticals
14 th DIA Japan Annual Meeting 2017 November 12-14, 2017 Tokyo Big Sight Ariake Regulatory Perspectives on NGS-based CDx Reiko Yanagihara, Ph.D. Office of In Vitro Diagnostics Deputy Review Director Pharmaceuticals
Statistical Challenges in Precision Medicine with a Focus on Companion Diagnostics
 Statistical Challenges in Precision Medicine with a Focus on Companion Diagnostics Gregory Campbell, Ph.D. President, GCStat Consulting GCStat@verizon.net Former Director, Division of Biostatistics, Center
Statistical Challenges in Precision Medicine with a Focus on Companion Diagnostics Gregory Campbell, Ph.D. President, GCStat Consulting GCStat@verizon.net Former Director, Division of Biostatistics, Center
Regulatory & Reimbursement Policy Trends Affecting Market Access for Diagnostic Devices Emphasis on Validity and Quality
 Title Date Page Regulatory & Reimbursement Policy Trends Affecting Market Access for Diagnostic Devices Emphasis on Validity and Quality JULY 2014 Page 2 Advances in personalized medicine and diagnostic
Title Date Page Regulatory & Reimbursement Policy Trends Affecting Market Access for Diagnostic Devices Emphasis on Validity and Quality JULY 2014 Page 2 Advances in personalized medicine and diagnostic
Introducing QIAseq. Accelerate your NGS performance through Sample to Insight solutions. Sample to Insight
 Introducing QIAseq Accelerate your NGS performance through Sample to Insight solutions Sample to Insight From Sample to Insight let QIAGEN enhance your NGS-based research High-throughput next-generation
Introducing QIAseq Accelerate your NGS performance through Sample to Insight solutions Sample to Insight From Sample to Insight let QIAGEN enhance your NGS-based research High-throughput next-generation
Corporate Presentation. September 6th, 2018
 Corporate Presentation September 6th, 2018 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than
Corporate Presentation September 6th, 2018 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than
TECHNICAL SHEET IDYLLA KRAS MUTATION TEST
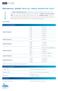 CE IVD TECHNICAL SHEET IDYLLA MUTATION TEST The Test, performed on the Biocartis system, is an in vitro diagnostic Test for the qualitative detection of 21 mutations in codons 12, 13, 59, 61, 117 and 146
CE IVD TECHNICAL SHEET IDYLLA MUTATION TEST The Test, performed on the Biocartis system, is an in vitro diagnostic Test for the qualitative detection of 21 mutations in codons 12, 13, 59, 61, 117 and 146
Agenda. - Introduction Howard Birndorf. - ASR Issues and the Draft ASR FAQ Guidance Patrick Balthrop
 - Introduction Howard Birndorf Agenda - ASR Issues and the Draft ASR FAQ Guidance Patrick Balthrop - IVDMIA Issues and the Draft IVDMIA Guidance Randy Scott - Q&A and Discussion All Who Are We? - Emerging
- Introduction Howard Birndorf Agenda - ASR Issues and the Draft ASR FAQ Guidance Patrick Balthrop - IVDMIA Issues and the Draft IVDMIA Guidance Randy Scott - Q&A and Discussion All Who Are We? - Emerging
Next Generation Oncology Sequencing in your Laboratory. Built by pioneers in cancer genomics and liquid biopsy approaches PROGENEUS
 PROGENEUS Next Generation Oncology Sequencing in your Laboratory Built by pioneers in cancer genomics and liquid biopsy approaches For Research Use Only. Not for iagnostic Purposes. personalgenome.com/progeneus
PROGENEUS Next Generation Oncology Sequencing in your Laboratory Built by pioneers in cancer genomics and liquid biopsy approaches For Research Use Only. Not for iagnostic Purposes. personalgenome.com/progeneus
Oversight of Laboratory Developed Tests
 Oversight of Laboratory Developed Tests APHL Annual Meeting 2015 Indianapolis Alberto Gutierrez, PhD Office of In Vitro Diagnostics and Radiological Health 1 Overview Background IVD regulation Need for
Oversight of Laboratory Developed Tests APHL Annual Meeting 2015 Indianapolis Alberto Gutierrez, PhD Office of In Vitro Diagnostics and Radiological Health 1 Overview Background IVD regulation Need for
Next Generation Molecular Diagnostics in Scotland. Dr Mark Drummond Haematologist, Beatson Cancer Centre
 Next Generation Molecular Diagnostics in Scotland Dr Mark Drummond Haematologist, Beatson Cancer Centre Outline Precision Medicine Regulatory and Funding background in Scotland (briefly) The Gap in the
Next Generation Molecular Diagnostics in Scotland Dr Mark Drummond Haematologist, Beatson Cancer Centre Outline Precision Medicine Regulatory and Funding background in Scotland (briefly) The Gap in the
Re: Docket No. 2006D-0347, Federal Register: July 26, 2007 (Volume 72, Pages )
 1201 Maryland Avenue SW, Ste. 900 Washington, DC 20024 August 27, 2007 BY ELECTRONIC DELIVERY Division of Dockets Management (HFA-305) Food and Drug Administration 56350 Fishers Lane Room 1061 Rockville,
1201 Maryland Avenue SW, Ste. 900 Washington, DC 20024 August 27, 2007 BY ELECTRONIC DELIVERY Division of Dockets Management (HFA-305) Food and Drug Administration 56350 Fishers Lane Room 1061 Rockville,
Corporate Medical Policy
 Corporate Medical Policy Proteogenomic Testing for Patients with Cancer (GPS Cancer Test) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteogenomic_testing_for_patients_with_cancer_gps_cancer_test
Corporate Medical Policy Proteogenomic Testing for Patients with Cancer (GPS Cancer Test) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteogenomic_testing_for_patients_with_cancer_gps_cancer_test
TECHNOLOGIES & SERVICES FOR THERAPEUTIC ANTIBODY DEVELOPMENT
 Specialist in tissue analysis by Histology, Immunohistochemistry and In Situ Hybridization TECHNOLOGIES & SERVICES FOR THERAPEUTIC ANTIBODY DEVELOPMENT CONTENTS Histalim: who we are Our areas of expertise
Specialist in tissue analysis by Histology, Immunohistochemistry and In Situ Hybridization TECHNOLOGIES & SERVICES FOR THERAPEUTIC ANTIBODY DEVELOPMENT CONTENTS Histalim: who we are Our areas of expertise
Molecular Pathology Coding Workgroup Fly-In
 Molecular Pathology Coding Workgroup Fly-In What CPT Is Role of Current Procedural Terminology Nomenclature: Coding CPT is a listing of descriptive terms and identifying codes for reporting medical services
Molecular Pathology Coding Workgroup Fly-In What CPT Is Role of Current Procedural Terminology Nomenclature: Coding CPT is a listing of descriptive terms and identifying codes for reporting medical services
[[Page 63034]] ======================================================================= DEPARTMENT OF HEALTH AND HUMAN SERVICES
![[[Page 63034]] ======================================================================= DEPARTMENT OF HEALTH AND HUMAN SERVICES [[Page 63034]] ======================================================================= DEPARTMENT OF HEALTH AND HUMAN SERVICES](/thumbs/93/114158104.jpg) [Federal Register Volume 79, Number 204 (Wednesday, October 22, 2014)] [Rules and Regulations] [Pages 63034-63036] From the Federal Register Online via the Government Printing Office [www.gpo.gov] [FR
[Federal Register Volume 79, Number 204 (Wednesday, October 22, 2014)] [Rules and Regulations] [Pages 63034-63036] From the Federal Register Online via the Government Printing Office [www.gpo.gov] [FR
What do Cancer Moonshot, PMI, Zika and the CARB National Action Plan All Have in Common? Tuesday, September 20th 3:00 4:00 PM
 What do Cancer Moonshot, PMI, Zika and the CARB National Action Plan All Have in Common? Briefing on the basics of laboratory developed tests (LDTs) and the vital role they play in patient care Tuesday,
What do Cancer Moonshot, PMI, Zika and the CARB National Action Plan All Have in Common? Briefing on the basics of laboratory developed tests (LDTs) and the vital role they play in patient care Tuesday,
Personalized Medicine A new challenge for applied human pharmacology?
 Personalized Medicine A new challenge for applied human pharmacology? Jochen Theis, MD FFPM InHeCon Leipzig 2 nd March 2012 Personalized Medicine: Predicting Variability in Drug Response InHeCon Jochen
Personalized Medicine A new challenge for applied human pharmacology? Jochen Theis, MD FFPM InHeCon Leipzig 2 nd March 2012 Personalized Medicine: Predicting Variability in Drug Response InHeCon Jochen
Corporate Medical Policy
 Corporate Medical Policy Proteogenomic Testing for Patients with Cancer (GPS Cancer Test) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteogenomic_testing_for_patients_with_cancer_gps_cancer_test
Corporate Medical Policy Proteogenomic Testing for Patients with Cancer (GPS Cancer Test) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteogenomic_testing_for_patients_with_cancer_gps_cancer_test
Corporate Medical Policy
 Corporate Medical Policy Proteogenomic Testing for Patients with Cancer (GPS Cancer Test) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteogenomic_testing_for_patients_with_cancer_gps_cancer_test
Corporate Medical Policy Proteogenomic Testing for Patients with Cancer (GPS Cancer Test) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteogenomic_testing_for_patients_with_cancer_gps_cancer_test
Comments from the FDA Working Group on SUBGROUP ANALYSES. Estelle Russek-Cohen, Ph.D. U.S. Food and Drug Administration Center for Biologics
 Comments from the FDA Working Group on SUBGROUP ANALYSES Estelle Russek-Cohen, Ph.D. U.S. Food and Drug Administration Center for Biologics 1 Outline An intro to FDA EMA and FDA on subgroups Companion
Comments from the FDA Working Group on SUBGROUP ANALYSES Estelle Russek-Cohen, Ph.D. U.S. Food and Drug Administration Center for Biologics 1 Outline An intro to FDA EMA and FDA on subgroups Companion
Corporate Presentation. February 2, 2018
 Corporate Presentation February 2, 2018 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements
Corporate Presentation February 2, 2018 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements
Your Best Data: Teaming QIAGEN Chemistry & Bioinformatics to Drive Samples to Insight
 Your Best Data: Teaming QIAGEN Chemistry & Bioinformatics to Drive Samples to Insight Aysel Heckel Director Clinical Solutions Sales Dr. Anne Arens Field Application Scientist Course on Variant Detection
Your Best Data: Teaming QIAGEN Chemistry & Bioinformatics to Drive Samples to Insight Aysel Heckel Director Clinical Solutions Sales Dr. Anne Arens Field Application Scientist Course on Variant Detection
Accessible answers. Targeted sequencing: accelerating and amplifying answers for oncology research
 Accessible answers Targeted sequencing: accelerating and amplifying answers for oncology research Help advance precision medicine Accelerate results with Ion Torrent NGS Life without cancer. This is our
Accessible answers Targeted sequencing: accelerating and amplifying answers for oncology research Help advance precision medicine Accelerate results with Ion Torrent NGS Life without cancer. This is our
Molecular Diagnostics: Market Segmentation and Opportunities
 Molecular Diagnostics: Market Segmentation and Opportunities October 2010 This market report is published by DeciBio, LLC. All rights reserved. Reproduction or redistribution of this report in any form
Molecular Diagnostics: Market Segmentation and Opportunities October 2010 This market report is published by DeciBio, LLC. All rights reserved. Reproduction or redistribution of this report in any form
Ensuring Translational Scientific Understanding Underpins Your Biomarker-Dx Strategy. Thomas Krahn Bayer Pharma AG
 Ensuring Translational Scientific Understanding Underpins Your Biomarker-Dx Strategy Thomas Krahn Bayer Pharma AG Forward-Looking Statements / Disclosures This presentation may contain forward-looking
Ensuring Translational Scientific Understanding Underpins Your Biomarker-Dx Strategy Thomas Krahn Bayer Pharma AG Forward-Looking Statements / Disclosures This presentation may contain forward-looking
ILLUMINA SEQUENCING SYSTEMS
 ILLUMINA SEQUENCING SYSTEMS PROVEN QUALITY. TRUSTED SOLUTIONS. Every day, researchers are using Illumina next-generation sequencing (NGS) systems to better understand human health and disease, as well
ILLUMINA SEQUENCING SYSTEMS PROVEN QUALITY. TRUSTED SOLUTIONS. Every day, researchers are using Illumina next-generation sequencing (NGS) systems to better understand human health and disease, as well
The patient must address the treating physician directly if he/she has any questions or requires information regarding the test results.
 Order Form 1. Ordering of the FoundationOne Service The FoundationOne Service comprises the genetic analysis of tumour tissue and the compilation of a comprehensive report on any mutations found in the
Order Form 1. Ordering of the FoundationOne Service The FoundationOne Service comprises the genetic analysis of tumour tissue and the compilation of a comprehensive report on any mutations found in the
Guidance for Industry and FDA Staff Pharmacogenetic Tests and Genetic Tests for Heritable Markers
 Guidance for Industry and FDA Staff Pharmacogenetic Tests and Genetic Tests for Heritable Markers Document issued on: June 19, 2007 The draft of this guidance was issued on February 9, 2006 U.S. Department
Guidance for Industry and FDA Staff Pharmacogenetic Tests and Genetic Tests for Heritable Markers Document issued on: June 19, 2007 The draft of this guidance was issued on February 9, 2006 U.S. Department
Medical Devices; Exemption From Premarket Notification; Class II Devices; Autosomal
 This document is scheduled to be published in the Federal Register on 11/07/2017 and available online at https://federalregister.gov/d/2017-24162, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/07/2017 and available online at https://federalregister.gov/d/2017-24162, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
FDA Regulatory Hurdles: What is the Status Quo?
 Center for Devices and Radiological Health FDA Regulatory Hurdles: What is the Status Quo? Institute of Medicine Policy Issues in the Development of Personalized Medicine in Oncology Washington, June 8
Center for Devices and Radiological Health FDA Regulatory Hurdles: What is the Status Quo? Institute of Medicine Policy Issues in the Development of Personalized Medicine in Oncology Washington, June 8
Structure and Mandate of FDA
 Structure and Mandate of FDA Leonard Sacks, M.D. Office of Medical Policy Center for Drug Evaluation and Research FDA FDA Clinical Investigator Training Course November 13, 2018 Mission of regulatory agencies
Structure and Mandate of FDA Leonard Sacks, M.D. Office of Medical Policy Center for Drug Evaluation and Research FDA FDA Clinical Investigator Training Course November 13, 2018 Mission of regulatory agencies
October 25, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852
 701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
Preparing For A New Era of Medical Product Development
 Latham & Watkins Health Care & Life Sciences Practice Number 1607 November 7, 2013 Preparing For A New Era of Medical Product Development FDA report demonstrates support for personalized medicine and more
Latham & Watkins Health Care & Life Sciences Practice Number 1607 November 7, 2013 Preparing For A New Era of Medical Product Development FDA report demonstrates support for personalized medicine and more
QIAGEN s NGS Solutions for Biomarkers NGS & Bioinformatics team QIAGEN (Suzhou) Translational Medicine Co.,Ltd
 QIAGEN s NGS Solutions for Biomarkers NGS & Bioinformatics team QIAGEN (Suzhou) Translational Medicine Co.,Ltd 1 Our current NGS & Bioinformatics Platform 2 Our NGS workflow and applications 3 QIAGEN s
QIAGEN s NGS Solutions for Biomarkers NGS & Bioinformatics team QIAGEN (Suzhou) Translational Medicine Co.,Ltd 1 Our current NGS & Bioinformatics Platform 2 Our NGS workflow and applications 3 QIAGEN s
MCW Office of Research Standard Operating Procedure
 MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DRUGS AND BIOLOGICS Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW/FH Faculty
MCW Office of Research Standard Operating Procedure USE AND STORAGE OF INVESTIGATIONAL DRUGS AND BIOLOGICS Unit: Applies to: Human Research Protections Program (HRPP), Office of Research MCW/FH Faculty
Technical Assessment (TA) Summary Form (M00116)
 Technical Assessment (TA) Summary Form (M00116) Please complete the following 2 table summarization of the dossier. Table 1. Summary of Evidence Validation Element For each cell below, please provide the
Technical Assessment (TA) Summary Form (M00116) Please complete the following 2 table summarization of the dossier. Table 1. Summary of Evidence Validation Element For each cell below, please provide the
