Impurity Detection with a New Light Emitting Diode Induced Fluorescence Detector Coupled to the Agilent 7100 Capillary Electrophoresis System
|
|
|
- Louisa Young
- 5 years ago
- Views:
Transcription
1 Impurity Detection with a New Light Emitting Diode Induced Fluorescence Detector Coupled to the Agilent 7100 Capillary Electrophoresis System Application Note Biologics & Biosimilars, Proteomics & Protein Science Author Christian Wenz Agilent Technologies, Inc. Waldbronn, Germany Abstract Light emitting diodes could present alternatives to lasers as light sources for fl uorescence detectors in applications requiring high sensitivity such as impurity detection in purifi ed monoclonal antibody preparations by capillary gel electrophoresis. This Application Note describes the performance of a detector for diode induced fl uorescence from Picometrics coupled to the Agilent 7100 Capillary Electrophoresis System. It shows that a small spiked-in protein impurity in a monoclonal antibody sample derivatized with 3-(2-furoyl)-quinoline-2- carboxaldehyde can be quantifi ed with good accuracy and precision down to a level of 0.0 %. The extrapolated limit of detection was 0.02 %, a value that is close to limits of detection reported for laser-based systems.
2 Introduction Capillary gel electrophoresis (CGE) is an important tool for biopharmaceutical product development and quality control 1. It is used to monitor product purity, detect minor product or process related impurities, and confirm batch-to-batch consistency of therapeutic proteins, especially monoclonal antibodies (mab). In addition to UV detection 2, protein derivatization combined with laser-induced fluorescence (LIF) is often used to enhance the sensitivity of the method. Examples of popular derivatization reagents are -carboxytetramethylrhodamine succinimidyl ester 3 and 3-(2-furoyl)- quinoline-2-carboxaldehyde (FQ) 4,. However, it has been shown that light emitting diodes (LEDs) can be used as alternative light sources for fluorescence detection in CGE. A recently published comparative study of LED and laser-based systems showed a very comparable signal-to-noise (S/N) for an antibody sample derivatized with the above-mentioned reagents, provided that a similar excitation wavelength was used in both cases 6. Potential benefits of LED in comparison to lasers are lower energy consumption, increased baseline stability, and longer lifetime. This Application Note describes the performance of a detector for LED-induced fluorescence (LEDIF) from Picometrics coupled to the Agilent 7100 Capillary Electrophoresis system for the detection of low-level impurities in an mab sample. Impurities were simulated by artificially contaminating a purified mab preparation with small standard proteins. Prior to CGE analysis, samples were derivatized with FQ. Derivatization with this reagent is convenient because it becomes fluorescent only after reaction with a primary amine and, therefore, purification of the derivatized protein is not necessary. Experimental Instrumentation Agilent 7100 Capillary Electrophoresis System (G7100A) Materials The mab used was provided by a biotechnical company. Standard proteins, citrate-phosphate buffer concentrate, N-ethylmaleimide (NEM), and sodium dodecyl sulfate (SDS) were purchased from Sigma Aldrich (St. Louis, MO, USA). Potassium cyanide (KCN) and FQ were purchased from Life Technologies (Paisley, UK). SDS Gel Buffer was purchased from Beckman Coulter (Fullerton, CA, USA). Protein concentrations were measured with the Qubit assay (Life Technologies, Paisley, UK). The Zetalif LED detector equipped with a 480 nm LED and a high pass 10 nm emission filter was obtained from Picometrics (Toulouse, France). All other materials and instrumentation were from Agilent Technologies, Inc. (Waldbronn, Germany). Sample preparation A stock solution of 40 mm FQ in methanol was prepared and stored at 20 C. Immediately before use, an aliquot of this stock solution was diluted with water to 1 mm. NEM solutions were prepared fresh daily. Prior to derivatization, the mab sample was diluted with citrate-phosphate buffer (90 mm sodium citrate, 77 mm sodium phosphate, ph 6.) to a protein concentration of 2 mg/ml. For derivatization with FQ, the following reagents were mixed in 0.-mL microcentrifuge vials: 3 µl of diluted mab 7.0 µl of 10 % SDS 4.0 µl of 100 mm NEM 0. µl of 100 mm KCN 2 µl of 1 mm FQ Final concentrations in the reaction mixture were 1 mg/ml mab, 1 % SDS,.6 mm NEM, 0.7 mm KCN, and 0.3 mm FQ. The solution was incubated for minutes at 7 C and then cooled down on ice. After the addition of 30 µl 10 % SDS, the solution was transferred into 100-µL CE sample vials. For reducing analysis, samples were additionally incubated with 0 mm DTT for 10 minutes at 70 C. CE conditions The LEDIF detector was connected to the CE instrument through the integrated analog-to-digital converter. The range used was 0 /V. The rise time and photomultiplier high voltage were set to 0. s and 610 V, respectively. Fifty µm id bare fused silica capillaries with a total length of 33 cm and an effective length 21 cm were used. Once a day, capillaries were conditioned as follows: 1. High-pressure flush at 2 bar with 0.1 N NaOH for 10 minutes, with 0.1 N HCl for minutes, and with water for 2 minutes 2. High-pressure flush at 4 bar with SDS Gel Buffer for 10 minutes 3. Water dip for both electrodes 4. Voltage equilibration at 16. V for 10 minutes with minutes ramping. Prior to every run, capillaries were conditioned as follows: 1. High-pressure flush at 4 bar with 0.1 N NaOH for 3 minutes, with 0.1 N HCl for 1 minute, with water for 1 minute and with SDS Gel Buffer for 10 minutes 2. Water dip of both electrodes Samples were electrokinetically injected from the outlet vial position by applying kv for 20 seconds and, after a water dip of the outlet electrode, separated by applying 16. kv (00 V/cm) for 30 minutes. Two bar pressure was applied to both inlet and outlet home vials during the run. After use, capillaries were conditioned as follows: 2
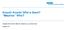
3 1. High-pressure flush at 4 bar with 0.1 N NaOH for 1 minutes 2. High-pressure flush at 3. bar with 0.1 N HCl for minutes, and with water for 10 minutes. Flushes for daily preconditioning and conditioning after use were done in forward direction (that is, pressure was applied to the inlet vial). Prerun flushes were done in backward direction. The capillary temperature was kept at 40 C. For all reagents, 2-mL glass vials were used. The fill volume was 1.2 ml, except for the water dip vials that contained 1.6 ml water and the waste vials that contained 0.6 ml of water. Three separate waste vials were used for the collection of 0.1 N NaOH, 0.1 N HCl/water, and SDS Gel Buffer, respectively. SDS Gel Buffer containing inlet and outlet home vials were exchanged after every sequence of eight runs. Results and Discussion Sensitivity of CGE with LEDIF detection The analysis of a FQ-derivatized mab sample by CGE with LEDIF detection is shown Figure 1. Two detector parameters were optimized in pilot experiments. Firstly, the photomultiplier high voltage was adjusted to a value that resulted in main peak maxima in the range of This was done to take advantage of the full measuring range without risking the rise of the signal beyond the 0 limit. Secondly, the baseline noise reduction parameter rise time was varied between 0 and seconds and then set to a value of 0. seconds that resulted in an acceptable background noise level without affecting the peak shape of small mab impurities (data not shown). The peak present in all runs at approximately 7. minutes might represent a buffer contaminant 4 (Figure 1). The background noise was calculated according to the ASTM over time intervals of 2 minutes around the migration times of the peaks of interest and was between 2 2. m. To estimate the sensitivity of the method, the mab sample was spiked with four standard proteins at a level of 0.2 % (Figure 1). Under nonreducing conditions, where all standard proteins were clearly resolved from mab derived peaks, peak heights of approximately 100 m were observed for three out of four protein spikes, corresponding to an S/N of 40 and an extrapolated limit of detection (LOD) of approximately 0.02 % (S/N > 3). This value is close to LODs reported for protein impurities in FQ-derivatized mab samples obtained with laser-based systems 4, Intact IgG * * * * Light chain Heavy chain * * * * Figure 1. Sensitivity of CGE with LEDIF detection. Samples were analyzed under nonreducing (top) and reducing conditions (bottom). Shown are full-scale electropherograms (left) and an expanded view (right) of the original mab sample (dark blue), the mab sample artificially contaminated with standard proteins at a concentration of 0.2 % (blue) and a control sample without protein (light blue). Molecular weights of standard proteins were 12, 18, 29, and 4 kda, respectively. Standard protein peaks are labeled (*). 3
4 Impurity quantification The performance of the method for low-level impurity quantification was evaluated with mab samples spiked with a single standard protein at concentrations of % (Figure 2). Samples were derivatized and analyzed on two days using different CE instruments. Peak integration was done automatically with the same set of parameters (Table 1). The maximum deviation of the measured standard protein % area from the target value was 12 %, and the average deviation was 7.1 %. A precision of 10 RSD % or better was observed for standard protein concentrations 0.1 %. Only for the lowest concentration was the precision 1 RSD %. An S/N of 8 was calculated for the 0.0 % sample, which confirms the above-mentioned LOD estimation kda LC HC kda Figure 2. Limit of quantification. A 12 kda standard protein was added at concentrations between % to the mab sample and analyzed under reducing conditions. Shown is an overlay of full-scale electropherograms of mab without (bottom) and with 12 kda protein added at concentrations of 0.0, 0.10, 0.1, 0.20, and 0.2 % (top) with a zoom on the 12 kda protein peaks in the inset. Table 1. Accuracy and precision of low level impurity detection by CGE with LEDIF detection. Samples of mab with different amounts of added 12 kda protein (cf. Figure 2) were derivatized and analyzed on two different days with two CE instruments and four runs per instrument (n = 8). Shown is the relative time corrected area (% Area) and the S/N for the 12 kda protein. % Area S/N Target Average SD RSD % %Deviation from target Average SD RSD %
5 Conclusion This work shows that the Agilent 7100 CE instrument equipped with the Picometrics LED-based fluorescence detector is well suited for low-level impurity detection by CGE with FQ-derivatized mab samples. The method allowed the detection of standard proteins spiked into an mab preparation down to a level of 0.02 %, a value that is close to published LODs obtained with laser-based systems. Good accuracy and precision was observed for the quantification of a single protein spike at concentrations in the range of %. Given the similar performance combined with lower energy consumption, increased stability, and longer lifetime of LEDs, the described approach may present an attractive alternative to CE systems with laser-based fluorescence detectors. References 1. A. Guo, et al. Capillary electrophoresis methods for pharmaceutical analysis; Vol. 9 of separation sciences and technology series Ahuja, S. and Jimilar M. I. (Eds.), Academic Press, Chapter 14, (2008). 2. C. Wenz Performance of commercially available gels for protein characterization by capillary gel electrophoresis with UV detection on the Agilent 7100 CE system Agilent Technologies application note, publication number EN. 3. O. Salas-Solano, et al. Optimization and validation of a quantitative capillary electrophoresis sodium dodecyl sulfate method for quality control and stability monitoring of monoclonal antibodies, Anal. Chem. 78, , (2006). 4. D.A. Michels, et al. Fluorescent derivatization method of proteins for characterization by capillary electrophoresis-sodium dodecyl sulfate with laser-induced fluorescence detection Anal. Chem. 79, , (2007).. D.A. Michels, M. Parker, and O. Salas-Solano Quantitative impurity analysis of monoclonal antibody size heterogeneity by CE-LIF: example of development and validation through a quality-by-design framework Electrophoresis 33, 81-26, (2012). 6. A. Rodat-Boutonnet, et al. A comparative study of LED-induced fluorescence and laser-induced fluorescence in SDS-CGE: application to the analysis of antibodies Electrophoresis 33, , (2012).
6 This information is subject to change without notice. Agilent Technologies, Inc., 2014 Published in the USA, March 1, EN
Application Note. Biopharm. Author. Abstract. Christian Wenz Agilent Technologies, Inc. Waldbronn, Germany
 Performance of commercially available gels for protein characterization by capillary gel electrophoresis with UV detection on the Agilent 7100 CE System Application Note Biopharm Author Christian Wenz
Performance of commercially available gels for protein characterization by capillary gel electrophoresis with UV detection on the Agilent 7100 CE System Application Note Biopharm Author Christian Wenz
Comparison of Different Brands of Carrier Ampholytes for Monoclonal Antibody Charge Heterogeneity Analysis by Capillary Isoelectric Focusing
 Comparison of Different Brands of Carrier Ampholytes for Monoclonal Antibody Charge Heterogeneity Analysis by Capillary Isoelectric Focusing Application Note Biotherapeutics & Biosimilars Author Christian
Comparison of Different Brands of Carrier Ampholytes for Monoclonal Antibody Charge Heterogeneity Analysis by Capillary Isoelectric Focusing Application Note Biotherapeutics & Biosimilars Author Christian
Capillary Electrophoresis of Proteins
 Capillary Electrophoresis of Proteins SDS Capillary Gel Electrophoresis SDS-CGE Outline CE-SDS Gel Analysis Description of Technique Method Development Tips PA800 plus kits SDS-MW IgG Purity & Heterogeneity
Capillary Electrophoresis of Proteins SDS Capillary Gel Electrophoresis SDS-CGE Outline CE-SDS Gel Analysis Description of Technique Method Development Tips PA800 plus kits SDS-MW IgG Purity & Heterogeneity
IgG Purity/Heterogeneity and SDS-MW Assays with High- Speed Separation Method and High Throughput Tray Setup
 IgG Purity/Heterogeneity and SDS-MW Assays with High- Speed Separation Method and High Throughput Tray Setup High Throughput Methods to Maximize the Use of the PA 800 Plus system Jose-Luis Gallegos-Perez
IgG Purity/Heterogeneity and SDS-MW Assays with High- Speed Separation Method and High Throughput Tray Setup High Throughput Methods to Maximize the Use of the PA 800 Plus system Jose-Luis Gallegos-Perez
Rediscover the Strengths of Your PA 800 Plus - A Multi-Attribute Protein Analyzer
 Rediscover the Strengths of Your PA 800 Plus - A Multi-Attribute Protein Analyzer Marcia R Santos SCIEX Separations, Brea, CA Introduction Capillary Electrophoresis is a well-accepted analytical separation
Rediscover the Strengths of Your PA 800 Plus - A Multi-Attribute Protein Analyzer Marcia R Santos SCIEX Separations, Brea, CA Introduction Capillary Electrophoresis is a well-accepted analytical separation
Application Note. Abstract. Author. Biopharmaceutical
 Innovator and Biosimilar Monoclonal Antibody-Peptide Map Comparison Using the Agilent 70 Capillary Electrophoresis System and Agilent MatchCompare Software Application Note Biopharmaceutical Author Arunkumar
Innovator and Biosimilar Monoclonal Antibody-Peptide Map Comparison Using the Agilent 70 Capillary Electrophoresis System and Agilent MatchCompare Software Application Note Biopharmaceutical Author Arunkumar
Assay of IgG Purity and Heterogeneity using High-Resolution Sodium Dodecyl Sulfate Capillary Gel Electrophoresis
 Assay of IgG Purity and Heterogeneity using High-Resolution Sodium Dodecyl Sulfate Capillary Gel Electrophoresis Lucy Y. Liu, Chitra Ratnayake, Jeff Chapman, Narasaiah Dontha, Sae Choo, and M. P. Reddy
Assay of IgG Purity and Heterogeneity using High-Resolution Sodium Dodecyl Sulfate Capillary Gel Electrophoresis Lucy Y. Liu, Chitra Ratnayake, Jeff Chapman, Narasaiah Dontha, Sae Choo, and M. P. Reddy
deltadot ANTIBODY ANALYSIS Application Note: Antibodies using LABEL FREE INTRINSIC IMAGING LONDON United Kingdom
 ANTIBODY ANALYSIS using LABEL FREE INTRINSIC IMAGING Application Note: Antibodies Ana l y s i s o f Im m u n o g l o b u l i n G u s i n g deltadot s PEREGRINE I 512-p i x e l La b e l Fr e e Ca p i l
ANTIBODY ANALYSIS using LABEL FREE INTRINSIC IMAGING Application Note: Antibodies Ana l y s i s o f Im m u n o g l o b u l i n G u s i n g deltadot s PEREGRINE I 512-p i x e l La b e l Fr e e Ca p i l
Simplifying CE-SDS Data Processing
 Simplifying CE-SDS Data Processing Approach for Mitigating Product Peak Migration Time Drift Samuel Shepherd 1, KieranKumar Mistry 1, Whitney Lane Smith 2, Nicholas Bond 1, Vivian Lindo 1, Sam Fox 3, Jim
Simplifying CE-SDS Data Processing Approach for Mitigating Product Peak Migration Time Drift Samuel Shepherd 1, KieranKumar Mistry 1, Whitney Lane Smith 2, Nicholas Bond 1, Vivian Lindo 1, Sam Fox 3, Jim
Application Note. Authors. Abstract. Biopharmaceuticals
 Characterization of monoclonal antibodies on the Agilent 126 Infinity Bio-inert Quaternary LC by Size Exclusion Chromatography using the Agilent BioSEC columns Application Note Biopharmaceuticals Authors
Characterization of monoclonal antibodies on the Agilent 126 Infinity Bio-inert Quaternary LC by Size Exclusion Chromatography using the Agilent BioSEC columns Application Note Biopharmaceuticals Authors
High-resolution Analysis of Charge Heterogeneity in Monoclonal Antibodies Using ph-gradient Cation Exchange Chromatography
 High-resolution Analysis of Charge Heterogeneity in Monoclonal Antibodies Using ph-gradient Cation Exchange Chromatography Agilent 1260 Infinity Bio-inert Quaternary LC System with Agilent Bio Columns
High-resolution Analysis of Charge Heterogeneity in Monoclonal Antibodies Using ph-gradient Cation Exchange Chromatography Agilent 1260 Infinity Bio-inert Quaternary LC System with Agilent Bio Columns
Separate and Quantify Rituximab Aggregates and Fragments with High-Resolution SEC
 Separate and Quantify Rituximab Aggregates and Fragments with High-Resolution SEC The Agilent 126 Infinity Bio-Inert Quaternary LC System and the AdvanceBio SEC 3Å, 2.7 µm Column Application Note Biologics
Separate and Quantify Rituximab Aggregates and Fragments with High-Resolution SEC The Agilent 126 Infinity Bio-Inert Quaternary LC System and the AdvanceBio SEC 3Å, 2.7 µm Column Application Note Biologics
LabChip GXII: Antibody Analysis
 WHITE PAPER LabChip GXII: Antibody Analysis Antibody Analysis using microfluidic technology in high throughput Quality by Design Experiments Abstract Current initiatives in Process Analytical Technology
WHITE PAPER LabChip GXII: Antibody Analysis Antibody Analysis using microfluidic technology in high throughput Quality by Design Experiments Abstract Current initiatives in Process Analytical Technology
Disulfide Linkage Analysis of IgG1 using an Agilent 1260 Infinity Bio inert LC System with an Agilent ZORBAX RRHD Diphenyl sub 2 µm Column
 Disulfide Linkage Analysis of IgG1 using an Agilent 126 Infinity Bio inert LC System with an Agilent ZORBAX RRHD Diphenyl sub 2 µm Column Application Note Biotherapeutics & Biosimilars Author M. Sundaram
Disulfide Linkage Analysis of IgG1 using an Agilent 126 Infinity Bio inert LC System with an Agilent ZORBAX RRHD Diphenyl sub 2 µm Column Application Note Biotherapeutics & Biosimilars Author M. Sundaram
Automated and quantitative analysis of biologics
 PA 800 PLUS PHARMACEUTICAL ANALYSIS SYSTEM Automated and quantitative analysis of biologics PA 800 PLUS PHARMACEUTIC AL ANALYSIS SYSTEM Designed for the needs of the biopharmaceutical industry Therapeutic
PA 800 PLUS PHARMACEUTICAL ANALYSIS SYSTEM Automated and quantitative analysis of biologics PA 800 PLUS PHARMACEUTIC AL ANALYSIS SYSTEM Designed for the needs of the biopharmaceutical industry Therapeutic
LIFluor EnhanCE. for use with dsdna 1000 and dsdna 20,000 Kits
 LIFluor EnhanCE for use with dsdna 1000 and dsdna 20,000 Kits For the Separation of Double Stranded DNA by P/ACE Fluorescent Capillary Electrophoresis 725824 AB September 2006 Beckman Coulter, Inc. 4300
LIFluor EnhanCE for use with dsdna 1000 and dsdna 20,000 Kits For the Separation of Double Stranded DNA by P/ACE Fluorescent Capillary Electrophoresis 725824 AB September 2006 Beckman Coulter, Inc. 4300
Characterization of mab aggregation using a Cary 60 UV-Vis Spectrophotometer and the Agilent 1260 Infinity LC system
 Characterization of mab aggregation using a Cary 60 UV-Vis Spectrophotometer and the Agilent 1260 Infinity LC system Application Note Biopharmaceuticals Authors Arunkumar Padmanaban and Sreelakshmy Menon
Characterization of mab aggregation using a Cary 60 UV-Vis Spectrophotometer and the Agilent 1260 Infinity LC system Application Note Biopharmaceuticals Authors Arunkumar Padmanaban and Sreelakshmy Menon
Automated and quantitative
 Automated and quantitative analysis of biologics. PA 800 plus Pharmaceutical Analysis System Genomics Cell Analysis Particle Characterization Capillary Electrophoresis Lab Automation Centrifugation Bioseparation
Automated and quantitative analysis of biologics. PA 800 plus Pharmaceutical Analysis System Genomics Cell Analysis Particle Characterization Capillary Electrophoresis Lab Automation Centrifugation Bioseparation
Charge Heterogeneity Analysis of Rituximab Innovator and Biosimilar mabs
 Charge Heterogeneity Analysis of Rituximab Innovator and Biosimilar mabs Application Note Author Suresh Babu C.V. Agilent Technologies India Pvt. Ltd, Bangalore, India Abstract This Application Note describes
Charge Heterogeneity Analysis of Rituximab Innovator and Biosimilar mabs Application Note Author Suresh Babu C.V. Agilent Technologies India Pvt. Ltd, Bangalore, India Abstract This Application Note describes
TECHNICAL INFORMATION
 T-1860A TECHNICAL INFORMATION Capillary Electrophoresis PRECISION IN CAPILLARY ELECTROPHORESIS Harry Whatley and Jeff Chapman Beckman Coulter, Inc. Introduction Instrumentation for routine chemical analysis
T-1860A TECHNICAL INFORMATION Capillary Electrophoresis PRECISION IN CAPILLARY ELECTROPHORESIS Harry Whatley and Jeff Chapman Beckman Coulter, Inc. Introduction Instrumentation for routine chemical analysis
Unified Workflow for Monoclonal Antibody Charge Heterogeneity, Purity, and Molecular Weight Analysis
 Procedures Unified Workflow for Monoclonal Antibody Charge Heterogeneity, Purity, and Molecular Weight Analysis Separation and Online Detection of Intact mab Variants and Impurities using CESI-MS Bryan
Procedures Unified Workflow for Monoclonal Antibody Charge Heterogeneity, Purity, and Molecular Weight Analysis Separation and Online Detection of Intact mab Variants and Impurities using CESI-MS Bryan
Optimization of cief to Resolve Challenging MAb Samples of Clinical Immunodiagnostic Relevance
 Optimization of cief to Resolve Challenging MAb Samples of Clinical Immunodiagnostic Relevance Sushma Rampal, Sr. Application Scientist, Beckman Coulter Life Sciences, Brea, CA, USA Marcia Santos, Staff
Optimization of cief to Resolve Challenging MAb Samples of Clinical Immunodiagnostic Relevance Sushma Rampal, Sr. Application Scientist, Beckman Coulter Life Sciences, Brea, CA, USA Marcia Santos, Staff
Size Exclusion Chromatography of Biosimilar and Innovator Insulin Using the Agilent AdvanceBio SEC column
 Size Exclusion Chromatography of Biosimilar and Innovator Insulin Using the Agilent AdvanceBio SEC column Application Note Bio-Pharmaceutical Authors M. Sundaram Palaniswamy and Andrew Coffey Agilent Technologies,
Size Exclusion Chromatography of Biosimilar and Innovator Insulin Using the Agilent AdvanceBio SEC column Application Note Bio-Pharmaceutical Authors M. Sundaram Palaniswamy and Andrew Coffey Agilent Technologies,
Capillary Electrophoresis in Quality Control PART II: CE-SDS: Method Development and Robustness
 Capillary Electrophoresis in Quality Control PART II: CE-SDS: Method Development and Robustness Chantal Felten *1 and Oscar Salas Solano 2 1 Alpine Analytical Acadamy, Whistler, British Columbia 2 Seattle
Capillary Electrophoresis in Quality Control PART II: CE-SDS: Method Development and Robustness Chantal Felten *1 and Oscar Salas Solano 2 1 Alpine Analytical Acadamy, Whistler, British Columbia 2 Seattle
Separation of Recombinant Human Erythropoietin (rhepo) using the European Pharmacopoeia Method on the PA 800 plus Pharmaceutical Analysis System
 Separation of Recombinant Human Erythropoietin (rhepo) using the European Pharmacopoeia Method on the PA 800 plus Pharmaceutical Analysis System Marcia R Santos Global Tactical Marketing, Beckman Coulter
Separation of Recombinant Human Erythropoietin (rhepo) using the European Pharmacopoeia Method on the PA 800 plus Pharmaceutical Analysis System Marcia R Santos Global Tactical Marketing, Beckman Coulter
Application Note. Author. Abstract. Biopharmaceuticals. Verified for Agilent 1260 Infinity II LC Bio-inert System. Sonja Schneider
 Combining small-scale purification and analysis of monoclonal antibodies on one instrument Protein purification with high-volume injection using the Agilent 126 Infinity Bio-inert Quaternary LC System
Combining small-scale purification and analysis of monoclonal antibodies on one instrument Protein purification with high-volume injection using the Agilent 126 Infinity Bio-inert Quaternary LC System
AnaTag 5-FAM Protein Labeling Kit
 AnaTag 5-FAM Protein Labeling Kit Catalog # 72053 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate 5-FAM SE (5-carboxyfluorescein) to proteins (e.g., IgG). It provides ample materials
AnaTag 5-FAM Protein Labeling Kit Catalog # 72053 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate 5-FAM SE (5-carboxyfluorescein) to proteins (e.g., IgG). It provides ample materials
E-Coli Cell Lysate By High Performance Capillary Electrophoresis
 E-Coli Cell Lysate By High Performance Capillary Electrophoresis Analysis of E-Coli Lysate Label free analysis using the HPCE-512 ABSTRACT Analytical instruments with the capability of analyzing complex
E-Coli Cell Lysate By High Performance Capillary Electrophoresis Analysis of E-Coli Lysate Label free analysis using the HPCE-512 ABSTRACT Analytical instruments with the capability of analyzing complex
Analysis of Monoclonal Antibody Charge Variants by Capillary Zone Electrophoresis
 Analysis of Monoclonal Antibody Charge Variants by Capillary Zone Electrophoresis Marcia R Santos Global Tactical Marketing, Beckman Coulter Life Sciences, Brea, CA USA INTRODUCTION Charge heterogeneity
Analysis of Monoclonal Antibody Charge Variants by Capillary Zone Electrophoresis Marcia R Santos Global Tactical Marketing, Beckman Coulter Life Sciences, Brea, CA USA INTRODUCTION Charge heterogeneity
Applications. Capillary Electrophoresis. Cell Lysate Analysis. Analysis of E. Coli Lysate. Technology
 Applications Capillary Electrophoresis Cell Lysate Analysis Analysis of E. Coli Lysate withdeltadot Technology Abstract Analytical instruments with the capability of analysing complex mixtures of bio-molecules
Applications Capillary Electrophoresis Cell Lysate Analysis Analysis of E. Coli Lysate withdeltadot Technology Abstract Analytical instruments with the capability of analysing complex mixtures of bio-molecules
High-throughput and Sensitive Size Exclusion Chromatography (SEC) of Biologics Using Agilent AdvanceBio SEC Columns
 High-throughput and Sensitive Size Exclusion Chromatography (SEC) of Biologics Using Agilent AdvanceBio SEC Columns Agilent AdvanceBio SEC 3 Å, 2.7 µm columns Application note Bio-Pharmaceutical Author
High-throughput and Sensitive Size Exclusion Chromatography (SEC) of Biologics Using Agilent AdvanceBio SEC Columns Agilent AdvanceBio SEC 3 Å, 2.7 µm columns Application note Bio-Pharmaceutical Author
CSE Separation of Cancer Cell Lysate
 Application Note: CSE Separation of Cancer Cell Lysate No. 9.200411 Rev. A 2004 Target Discovery, Inc. Target Discovery, Inc. 4015 Fabian Way Palo Alto, CA 94303 Tel: 650-812-8100 www.targetdiscovery.com/eotrol
Application Note: CSE Separation of Cancer Cell Lysate No. 9.200411 Rev. A 2004 Target Discovery, Inc. Target Discovery, Inc. 4015 Fabian Way Palo Alto, CA 94303 Tel: 650-812-8100 www.targetdiscovery.com/eotrol
HT Pico Protein Express LabChip Kit, Frequently Asked Questions
 Protein Detection 1. How does the GXII determine protein concentration? Protein samples contain a single lower marker. Alignment to a single marker does not provide enough constraints to align large proteins,
Protein Detection 1. How does the GXII determine protein concentration? Protein samples contain a single lower marker. Alignment to a single marker does not provide enough constraints to align large proteins,
SDS-CGE Method Development for Purified Protein Vaccine Antigens
 SDS-CGE Method Development for Purified Protein Vaccine Antigens Michael Leach, Elena Newman and Bruce Carpick Sanofi Pasteur Analytical R&D, Biochemistry Overview SDS-CGE method development for purified
SDS-CGE Method Development for Purified Protein Vaccine Antigens Michael Leach, Elena Newman and Bruce Carpick Sanofi Pasteur Analytical R&D, Biochemistry Overview SDS-CGE method development for purified
Molecular Characterization of Biotherapeutics The Agilent 1260 Infi nity Multi-Detector Bio-SEC Solution with Advanced Light Scattering Detection
 Molecular Characterization of Biotherapeutics The Agilent 126 Infi nity Multi-Detector Bio-SEC Solution with Advanced Light Scattering Detection Application Note Biologics and Biosimilars Authors Sonja
Molecular Characterization of Biotherapeutics The Agilent 126 Infi nity Multi-Detector Bio-SEC Solution with Advanced Light Scattering Detection Application Note Biologics and Biosimilars Authors Sonja
AnaTag HiLyte Fluor 750 Microscale Protein Labeling Kit
 AnaTag HiLyte Fluor 750 Microscale Protein Labeling Kit Catalog # 72044 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 750 SE to proteins (e.g., IgG). It provides ample
AnaTag HiLyte Fluor 750 Microscale Protein Labeling Kit Catalog # 72044 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 750 SE to proteins (e.g., IgG). It provides ample
Analysis of Intact and C-terminal Digested IgG1 on an Agilent Bio MAb 5 µm Column
 Analysis of Intact and C-terminal Digested IgG1 on an Agilent Bio MAb µm Column Application Note BioPharma Authors Xiaomi Xu and Phu T Duong Agilent Technologies, Inc. Abstract Nearly all proteins undergo
Analysis of Intact and C-terminal Digested IgG1 on an Agilent Bio MAb µm Column Application Note BioPharma Authors Xiaomi Xu and Phu T Duong Agilent Technologies, Inc. Abstract Nearly all proteins undergo
AnaTag HiLyte Fluor 647 Protein Labeling Kit
 AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
Reducing Cycle Time for Charge Variant Analysis of Monoclonal Antibodies
 Reducing Cycle Time for Charge Variant Analysis of Monoclonal Antibodies Alternating Column Regeneration Using an Agilent 1200 Infinity Series Quick-Change Bio-inert 2-position/10 port Valve Application
Reducing Cycle Time for Charge Variant Analysis of Monoclonal Antibodies Alternating Column Regeneration Using an Agilent 1200 Infinity Series Quick-Change Bio-inert 2-position/10 port Valve Application
AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis
 Application Note Biologics Development AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis Using the Agilent 16 Infinity II Bio-Inert LC Authors Andrew Coffey and Sandeep
Application Note Biologics Development AdvanceBio HIC: a Hydrophobic HPLC Column for Monoclonal Antibody (mab) Variant Analysis Using the Agilent 16 Infinity II Bio-Inert LC Authors Andrew Coffey and Sandeep
Separation of Native Monoclonal Antibodies and Identification of Charge Variants:
 Separation of Native Monoclonal Antibodies and Identification of Charge Variants: Teamwork of the Agilent 31 OFFGEL Fractionator, Agilent 21 Bioanalyzer and Agilent LC/MS Systems Application Note Biosimilar
Separation of Native Monoclonal Antibodies and Identification of Charge Variants: Teamwork of the Agilent 31 OFFGEL Fractionator, Agilent 21 Bioanalyzer and Agilent LC/MS Systems Application Note Biosimilar
HPCE-5 2. Realising the potential of Capillary Electrophoresis. Why is the HPCE-512 so much better?
 HPCE-5 2 Realising the potential of Capillary Electrophoresis Why is the HPCE-512 so much better? A Major Advance in Capillary Electrophoresis The HPCE-512 system based on multi-point detection overcomes
HPCE-5 2 Realising the potential of Capillary Electrophoresis Why is the HPCE-512 so much better? A Major Advance in Capillary Electrophoresis The HPCE-512 system based on multi-point detection overcomes
AnaTag HiLyte Fluor 555 Protein Labeling Kit
 AnaTag HiLyte Fluor 555 Protein Labeling Kit Revision number: 1.3 Last updated: April 2018 Catalog # AS-72045 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 555 SE to
AnaTag HiLyte Fluor 555 Protein Labeling Kit Revision number: 1.3 Last updated: April 2018 Catalog # AS-72045 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 555 SE to
Monoclonal Antibody Charge Variants Identification by Fully Automated Capillary Isoelectric Focusing Mass Spectrometry
 Application Note Pharma & Biopharma Monoclonal Antibody Charge Variants Identification by Fully Automated Capillary Isoelectric Focusing Mass Spectrometry Authors Tony Wang, James Xia, and Rena Wang CMP
Application Note Pharma & Biopharma Monoclonal Antibody Charge Variants Identification by Fully Automated Capillary Isoelectric Focusing Mass Spectrometry Authors Tony Wang, James Xia, and Rena Wang CMP
Improved Sensitivity with the Agilent 1290 Infinity Evaporative Light Scattering Detector (ELSD)
 Improved Sensitivity with the Agilent 1290 Infinity Evaporative Light Scattering Detector (ELSD) Technical Overview Author Edgar Naegele Agilent Technologies, Inc. Waldbronn, Germany Abstract This Technical
Improved Sensitivity with the Agilent 1290 Infinity Evaporative Light Scattering Detector (ELSD) Technical Overview Author Edgar Naegele Agilent Technologies, Inc. Waldbronn, Germany Abstract This Technical
Size Exclusion BioHPLC Columns
 Size Exclusion BioHPLC Columns Size Exclusion Product Families Particle Porosity Functionalities Particle Pore Size Application Sizes Agilent Bio SEC- Silica Fully porous N/A um 00A, 0A, 00A High efficiency
Size Exclusion BioHPLC Columns Size Exclusion Product Families Particle Porosity Functionalities Particle Pore Size Application Sizes Agilent Bio SEC- Silica Fully porous N/A um 00A, 0A, 00A High efficiency
Journal of Chemical and Pharmaceutical Research, 2017, 9(3): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2017, 9(3):390-400 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Determination Of Clips Generated by Acid Hydrolysis
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2017, 9(3):390-400 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Determination Of Clips Generated by Acid Hydrolysis
Microfluidic Assays for High Throughput Screening of Protein Quality in Bioprocess Development
 1 Microfluidic Assays for High Throughput Screening of Protein Quality in Bioprocess Development Bahram Fathollahi Microfluidics R&D CPAC Summer Institute 2009 2 What is LabChip Technology? Miniaturization
1 Microfluidic Assays for High Throughput Screening of Protein Quality in Bioprocess Development Bahram Fathollahi Microfluidics R&D CPAC Summer Institute 2009 2 What is LabChip Technology? Miniaturization
AnaTag HiLyte Fluor 488 Microscale Protein Labeling Kit
 AnaTag HiLyte Fluor 488 Microscale Protein Labeling Kit Revision number: 1.3 Last updated: April 2018 Catalog # AS-72048 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor
AnaTag HiLyte Fluor 488 Microscale Protein Labeling Kit Revision number: 1.3 Last updated: April 2018 Catalog # AS-72048 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor
mab Titer Analysis with the Agilent Bio-Monolith Protein A Column
 mab Titer Analysis with the Agilent Bio-Monolith Protein A Column Application Note Biopharmaceuticals and Biosimilars Authors Emmie Dumont, Isabel Vandenheede, Pat Sandra, and Koen Sandra Research Institute
mab Titer Analysis with the Agilent Bio-Monolith Protein A Column Application Note Biopharmaceuticals and Biosimilars Authors Emmie Dumont, Isabel Vandenheede, Pat Sandra, and Koen Sandra Research Institute
Update on Heparin Contamination: Assessment of Optimized Capillary Electrophoresis, Proton NMR and Ion-Exchange HPLC Methodologies
 Health Canada Health Products and Food Branch Biologics and Genetic Therapies Directorate Update on Heparin Contamination: Assessment of Optimized Capillary Electrophoresis, Proton NMR and Ion-Exchange
Health Canada Health Products and Food Branch Biologics and Genetic Therapies Directorate Update on Heparin Contamination: Assessment of Optimized Capillary Electrophoresis, Proton NMR and Ion-Exchange
Automated and Quantitative Analysis of Biologics
 PA 800 PLUS PHARMACEUTICAL ANALYSIS SYSTEM Automated and Quantitative Analysis of Biologics PA 800 PLUS PHARMACEUTICAL ANALYSIS SYSTEM For Research Use Only. Not for use in diagnostic procedures. Flexibility
PA 800 PLUS PHARMACEUTICAL ANALYSIS SYSTEM Automated and Quantitative Analysis of Biologics PA 800 PLUS PHARMACEUTICAL ANALYSIS SYSTEM For Research Use Only. Not for use in diagnostic procedures. Flexibility
PA 800 plus Catalog. Systems/Software
 PA 800 plus Catalog Built on 20 years of innovation and leadership in the field of capillary electrophoresis, Beckman Coulter s PA 800 plus provides solutions for protein purity analysis, charge heterogeneity
PA 800 plus Catalog Built on 20 years of innovation and leadership in the field of capillary electrophoresis, Beckman Coulter s PA 800 plus provides solutions for protein purity analysis, charge heterogeneity
Clone Selection Using the Agilent 1290 Infinity Online 2D-LC/MS Solution
 Clone Selection Using the Agilent 9 Infinity Online D-LC/MS Solution Application Note Biopharmaceuticals Authors Abstract Ravindra Gudihal and This Application Note describes the Agilent 9 Infinity online
Clone Selection Using the Agilent 9 Infinity Online D-LC/MS Solution Application Note Biopharmaceuticals Authors Abstract Ravindra Gudihal and This Application Note describes the Agilent 9 Infinity online
Rapid UHPLC Analysis of Reduced Monoclonal Antibodies using an Agilent ZORBAX Rapid Resolution High Definition (RRHD) 300SB-C8 Column
 Rapid UHP Analysis of Reduced Monoclonal Antibodies using an Agilent ZORBAX Rapid Resolution High Definition (RRHD) 3SB-C8 Column Application Note BioPharma Authors James Martosella, Phu Duong, Susanne
Rapid UHP Analysis of Reduced Monoclonal Antibodies using an Agilent ZORBAX Rapid Resolution High Definition (RRHD) 3SB-C8 Column Application Note BioPharma Authors James Martosella, Phu Duong, Susanne
APPLICATION INFORMATION
 A-1861A APPLICATION INFORMATION Capillary Electrophoresis USING CIEF TO CHARACTERIZE RECOMBINANT HUMAN MONOCLONAL ANTIBODIES L. C. Santosa, 1 I. S. Krull, 1 and K. L. Grant 2 1 Department of Chemistry,
A-1861A APPLICATION INFORMATION Capillary Electrophoresis USING CIEF TO CHARACTERIZE RECOMBINANT HUMAN MONOCLONAL ANTIBODIES L. C. Santosa, 1 I. S. Krull, 1 and K. L. Grant 2 1 Department of Chemistry,
Performance characteristics of the 1260 Infinity Quaternary LC system
 Performance characteristics of the 1260 Infinity Quaternary LC system The new standard in HPLC Technical Overview Introduction The Agilent 1260 Infinity LC system consists of modular units that operate
Performance characteristics of the 1260 Infinity Quaternary LC system The new standard in HPLC Technical Overview Introduction The Agilent 1260 Infinity LC system consists of modular units that operate
SCIEX Anion Analysis Kit For P/ACE MDQ and P/ACE MDQ plus Capillary Electrophoresis Systems. Instruction Guide
 For P/ACE MDQ and P/ACE MDQ plus Capillary Electrophoresis Systems A49108AE May 2015 AB Sciex Pte. Ltd and its affiliates disclaims all warranties with respect to this document, expressed or implied, including
For P/ACE MDQ and P/ACE MDQ plus Capillary Electrophoresis Systems A49108AE May 2015 AB Sciex Pte. Ltd and its affiliates disclaims all warranties with respect to this document, expressed or implied, including
AnaTag HiLyte Fluor 647 Microscale Protein Labeling Kit
 AnaTag HiLyte Fluor 647 Microscale Protein Labeling Kit Revision number: 1.3 Last updated: May 2018 Catalog # AS-72050 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647
AnaTag HiLyte Fluor 647 Microscale Protein Labeling Kit Revision number: 1.3 Last updated: May 2018 Catalog # AS-72050 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647
Seamless Method Transfer from an Agilent 1260 Infinity Bio-inert LC to an Agilent 1260 Infinity II Bio-inert LC
 Seamless Method Transfer from an Agilent 1 Infinity Bio-inert LC to an Agilent 1 Infinity II Bio-inert LC Charge Variant Analysis of Rituximab Innovator and Biosimilar Application Note Biologics & Biosimilars
Seamless Method Transfer from an Agilent 1 Infinity Bio-inert LC to an Agilent 1 Infinity II Bio-inert LC Charge Variant Analysis of Rituximab Innovator and Biosimilar Application Note Biologics & Biosimilars
"VUPNBUFE BOE RVBOUJUBUJWF
 "VUPNBUFEBOERVBOUJUBUJWF BOBMZTJTPGCJPMPHJDT 1" plus1ibsnbdfvujdbm"obmztjt4ztufn Genomics Cell Analysis Particle Characterization Capillary Electrophoresis Lab Automation Centrifugation Bioseparation Lab
"VUPNBUFEBOERVBOUJUBUJWF BOBMZTJTPGCJPMPHJDT 1" plus1ibsnbdfvujdbm"obmztjt4ztufn Genomics Cell Analysis Particle Characterization Capillary Electrophoresis Lab Automation Centrifugation Bioseparation Lab
Knock! Knock! Who s there? Maurice Who? SungAe Suhr Park, Mee Ko, Eleanor Le, Janice Chen Amgen Inc.
 Knock! Knock! Who s there? Maurice Who? SungAe Suhr Park, Mee Ko, Eleanor Le, Janice Chen Amgen Inc. Abstract SungAe Suhr Park, Mee Ko, Eleanor Le and Janice Chen Amgen Inc. Applications of Capillary Electrophoresis
Knock! Knock! Who s there? Maurice Who? SungAe Suhr Park, Mee Ko, Eleanor Le, Janice Chen Amgen Inc. Abstract SungAe Suhr Park, Mee Ko, Eleanor Le and Janice Chen Amgen Inc. Applications of Capillary Electrophoresis
A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies
 A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies Combining Size Exclusion Chromatography with Method Development and Light Scattering Application Note Biotherapeutics and Biosimilars
A Comprehensive Workflow to Optimize and Execute Protein Aggregate Studies Combining Size Exclusion Chromatography with Method Development and Light Scattering Application Note Biotherapeutics and Biosimilars
Repeatability of C100ht Biologics Analyzer, for High Throughput Glycan Screening
 Repeatability of C100ht Biologics Analyzer, for High Throughput Glycan Screening Marcia Santos, Tingting Li, Mervin Gutierrez, Anna Lou, and Clarence Lew SCIEX Separations, Brea, CA Introduction The ability
Repeatability of C100ht Biologics Analyzer, for High Throughput Glycan Screening Marcia Santos, Tingting Li, Mervin Gutierrez, Anna Lou, and Clarence Lew SCIEX Separations, Brea, CA Introduction The ability
NISTmAb characterization with a high-performance RP chromatography column
 APPLICATION NOTE 21848 NISTmAb characterization with a high-performance RP chromatography column Author Xin Zhang Thermo Fisher Scientific, Sunnyvale, CA, USA Keywords MAbPac RP column, inter-column reproducibility,
APPLICATION NOTE 21848 NISTmAb characterization with a high-performance RP chromatography column Author Xin Zhang Thermo Fisher Scientific, Sunnyvale, CA, USA Keywords MAbPac RP column, inter-column reproducibility,
HPCE-5 2. A Revolution in Capillary Electrophoresis. High Performance Capillary Electrophoresis
 A Revolution in Capillary Electrophoresis High Performance Capillary Electrophoresis Revolutionary Performance Continuous Monitoring of Capillary and Buffer Conditions Sensitive Label-Free 512 Times Better
A Revolution in Capillary Electrophoresis High Performance Capillary Electrophoresis Revolutionary Performance Continuous Monitoring of Capillary and Buffer Conditions Sensitive Label-Free 512 Times Better
Maximizing Chromatographic Resolution of Peptide Maps using UPLC with Tandem Columns
 Maximizing Chromatographic Resolution of Peptide Maps using UPLC with Tandem Columns Hongwei Xie, Martin Gilar, and Jeff Mazzeo Waters Corporation, Milford, MA U.S. APPLICATION BENEFITS The ACQUITY UPLC
Maximizing Chromatographic Resolution of Peptide Maps using UPLC with Tandem Columns Hongwei Xie, Martin Gilar, and Jeff Mazzeo Waters Corporation, Milford, MA U.S. APPLICATION BENEFITS The ACQUITY UPLC
Bio-Monolith Protein G Column - More Options for mab Titer Determination
 Bio-Monolith Protein G Column - More Options for mab Titer Determination Application Note Biologics and Biosimilars Author Phu T. Duong Agilent Technologies, Inc. Introduction In recent years, monoclonal
Bio-Monolith Protein G Column - More Options for mab Titer Determination Application Note Biologics and Biosimilars Author Phu T. Duong Agilent Technologies, Inc. Introduction In recent years, monoclonal
Application Note. Biopharma. Authors. Abstract. James Martosella, Phu Duong Agilent Technologies, Inc Centreville Rd Wilmington, DE 19808
 Reversed-Phase Optimization for Ultra Fast Profiling of Intact and Reduced Monoclonal Antibodies using Agilent ZORBAX Rapid Resolution High Definition 3SB-C3 Column Application Note Biopharma Authors James
Reversed-Phase Optimization for Ultra Fast Profiling of Intact and Reduced Monoclonal Antibodies using Agilent ZORBAX Rapid Resolution High Definition 3SB-C3 Column Application Note Biopharma Authors James
Alternative to 2D gel electrophoresis OFFGEL electrophoresis combined with high-sensitivity on-chip protein detection.
 Alternative to 2D gel electrophoresis OFFGEL electrophoresis combined with high-sensitivity on-chip protein detection Application Note Christian Wenz Andreas Rüfer Abstract Agilent Equipment Agilent 3100
Alternative to 2D gel electrophoresis OFFGEL electrophoresis combined with high-sensitivity on-chip protein detection Application Note Christian Wenz Andreas Rüfer Abstract Agilent Equipment Agilent 3100
CE Oligonucleotide Analysis Kit Instruction Manual
 CE Oligonucleotide Analysis Kit Instruction Manual Catalog Number 148-4140 For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723) Table of Contents Section
CE Oligonucleotide Analysis Kit Instruction Manual Catalog Number 148-4140 For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723) Table of Contents Section
Quantification of genotoxic "Impurity D" in Atenolol by LC/ESI/MS/MS with Agilent 1200 Series RRLC and 6410B Triple Quadrupole LC/MS
 Quantification of genotoxic "Impurity D" in Atenolol by LC/ESI/MS/MS with Agilent 12 Series RRLC and 641B Triple Quadrupole LC/MS Application Note Manufacturing Process Development Author Siji Joseph Agilent
Quantification of genotoxic "Impurity D" in Atenolol by LC/ESI/MS/MS with Agilent 12 Series RRLC and 641B Triple Quadrupole LC/MS Application Note Manufacturing Process Development Author Siji Joseph Agilent
Fast and High Resolution Analysis of Intact and Reduced Therapeutic Monoclonal Antibodies (mabs)
 Fast and High Resolution nalysis of Intact and Reduced Therapeutic Monoclonal ntibodies (mbs) The gilent io-inert L and dvanceio RP-mb olumns pplication Note io-pharmaceutical uthor M. Sundaram Palaniswamy
Fast and High Resolution nalysis of Intact and Reduced Therapeutic Monoclonal ntibodies (mbs) The gilent io-inert L and dvanceio RP-mb olumns pplication Note io-pharmaceutical uthor M. Sundaram Palaniswamy
High-Throughput Glycan Screening to Remove Biopharmaceutical Development Bottlenecks
 High-Throughput Glycan Screening to Remove Biopharmaceutical Development Bottlenecks Mark Lies, Marcia Santos, and Tingting Li SCIEX Separations, Brea, CA Introduction Development of biopharmaceuticals
High-Throughput Glycan Screening to Remove Biopharmaceutical Development Bottlenecks Mark Lies, Marcia Santos, and Tingting Li SCIEX Separations, Brea, CA Introduction Development of biopharmaceuticals
IgG Purity and Heterogeneity Assay Kit For the PA 800 Plus Pharmaceutical Analysis System. Application Guide
 Kit For the PA 800 Plus Pharmaceutical Analysis System RUO-IDV-05-6935-A April 2018 This document is provided to customers who have purchased SCIEX equipment to use in the operation of such SCIEX equipment.
Kit For the PA 800 Plus Pharmaceutical Analysis System RUO-IDV-05-6935-A April 2018 This document is provided to customers who have purchased SCIEX equipment to use in the operation of such SCIEX equipment.
Capillary electrophoresis of heparin and related impurities using highly concentrated buffers in a 25 µm bubble cell capillary
 Capillary electrophoresis of heparin and related impurities using highly concentrated buffers in a µm bubble cell capillary Application Note Drug Testing Authors Robert Weinberger CE Technologies, Inc.
Capillary electrophoresis of heparin and related impurities using highly concentrated buffers in a µm bubble cell capillary Application Note Drug Testing Authors Robert Weinberger CE Technologies, Inc.
Characterize mab Charge Variants by Cation-Exchange Chromatography
 Characterize mab Charge Variants by Cation-Exchange Chromatography Application Note Biologics and Biosimilars Authors Isabel Vandenheede, Emmie Dumont, Pat Sandra, and Koen Sandra Research Institute for
Characterize mab Charge Variants by Cation-Exchange Chromatography Application Note Biologics and Biosimilars Authors Isabel Vandenheede, Emmie Dumont, Pat Sandra, and Koen Sandra Research Institute for
Best Quantification Practices with the Agilent 5200 Fragment Analyzer System
 Application Note Genomics Best Quantification Practices with the Agilent 5 Fragment Analyzer System Authors Chava Pocernich, Jolita Uthe, Whitney Pike, and Kit Sum Wong Agilent Technologies, Inc. Abstract
Application Note Genomics Best Quantification Practices with the Agilent 5 Fragment Analyzer System Authors Chava Pocernich, Jolita Uthe, Whitney Pike, and Kit Sum Wong Agilent Technologies, Inc. Abstract
Developing Robust and Efficient IEX Methods for Charge Variant Analysis of Biotherapeutics Using ACQUITY UPLC H-Class System and Auto Blend Plus
 Developing Robust and Efficient IEX Methods for Charge Variant Analysis of Biotherapeutics Using ACQUITY UPLC H-Class System and Auto Blend Plus Robert Birdsall, Thomas Wheat, and Weibin Chen Waters Corporation,
Developing Robust and Efficient IEX Methods for Charge Variant Analysis of Biotherapeutics Using ACQUITY UPLC H-Class System and Auto Blend Plus Robert Birdsall, Thomas Wheat, and Weibin Chen Waters Corporation,
Fraction Analysis of Cysteine Linked Antibody-Drug Conjugates Using Hydrophobic Interaction. chromatography. Agilent 1260 Infinity II Bio-Inert System
 Application Note Biologics & Biosimilars Fraction Analysis of Cysteine Linked Antibody-Drug Conjugates Using Hydrophobic Interaction Chromatography Agilent 126 Infinity II Bio-Inert System 7 6 5 4 5. 7.5
Application Note Biologics & Biosimilars Fraction Analysis of Cysteine Linked Antibody-Drug Conjugates Using Hydrophobic Interaction Chromatography Agilent 126 Infinity II Bio-Inert System 7 6 5 4 5. 7.5
Reversed-phase Separation of Intact Monoclonal Antibodies Using Agilent ZORBAX Rapid Resolution High Definition 300SB-C8 1.
 Reversed-phase Separation of Intact Monoclonal Antibodies Using Agilent ZORBAX Rapid Resolution High Definition 3SB-C8 1.8 µm Column Application Note Biopharmaceuticals Authors James Martosella and Phu
Reversed-phase Separation of Intact Monoclonal Antibodies Using Agilent ZORBAX Rapid Resolution High Definition 3SB-C8 1.8 µm Column Application Note Biopharmaceuticals Authors James Martosella and Phu
Performance Characteristics of the Agilent 1220 Infinity Gradient LC system
 Performance Characteristics of the Agilent 122 Infinity Gradient LC system An integrated LC system for conventional LC and UHPLC Technical Overview 7 5 4 3 2 1.5 1 1.5 2 2.5 3 Introduction The Agilent
Performance Characteristics of the Agilent 122 Infinity Gradient LC system An integrated LC system for conventional LC and UHPLC Technical Overview 7 5 4 3 2 1.5 1 1.5 2 2.5 3 Introduction The Agilent
Quantitative analysis of PCR fragments with the Agilent 2100 Bioanalyzer. Application Note. Odilo Mueller. Abstract
 Quantitative analysis of PCR fragments with the Agilent 2100 Bioanalyzer Application Note Odilo Mueller Abstract This application note describes how the Agilent Technologies 2100 bioanalyzer can be used
Quantitative analysis of PCR fragments with the Agilent 2100 Bioanalyzer Application Note Odilo Mueller Abstract This application note describes how the Agilent Technologies 2100 bioanalyzer can be used
High Throughput Sub-4 Minute Separation of Antibodies using Size Exclusion Chromatography
 High Throughput Sub-4 Minute Separation of Antibodies using Size Exclusion Chromatography TSKgel APPLICATION NOTE Introduction Gel Filtration Chromatography (GFC) is a powerful analytical tool in the separation
High Throughput Sub-4 Minute Separation of Antibodies using Size Exclusion Chromatography TSKgel APPLICATION NOTE Introduction Gel Filtration Chromatography (GFC) is a powerful analytical tool in the separation
Separating proteins with activated carbon
 Separating proteins with activated carbon Matthew T. Stone and Mikhail Kozlov EMD Millipore Corp. 80 Ashby Road, Bedford, MA 01730, USA Supporting Information Supporting information for Figure 1 Experimental
Separating proteins with activated carbon Matthew T. Stone and Mikhail Kozlov EMD Millipore Corp. 80 Ashby Road, Bedford, MA 01730, USA Supporting Information Supporting information for Figure 1 Experimental
Report. Comparison of magnetic beads coating protocols for immunoaffinity capillary electrophoresis. Natalia Gasilova, Hubert H.
 Report Comparison of magnetic beads coating protocols for immunoaffinity capillary electrophoresis Natalia Gasilova, Hubert H. Girault Laboratoire d Electrochimie Physique et Analytique, Ecole Polytechnique
Report Comparison of magnetic beads coating protocols for immunoaffinity capillary electrophoresis Natalia Gasilova, Hubert H. Girault Laboratoire d Electrochimie Physique et Analytique, Ecole Polytechnique
LavaDigest Protease Monitoring Kit
 LavaDigest Protease Monitoring Kit Ordering LP-031020 LavaDigest protease monitoring kit for up to 2000 assays Order from http://www.fluorotechnics.com.au L.avaDigest Protocol March 2013 Content 2 LavaDigest
LavaDigest Protease Monitoring Kit Ordering LP-031020 LavaDigest protease monitoring kit for up to 2000 assays Order from http://www.fluorotechnics.com.au L.avaDigest Protocol March 2013 Content 2 LavaDigest
Electronic Supplementary Information for The effect of protein concentration on the viscosity of a recombinant albumin solution formulation
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for The effect of protein concentration on the viscosity
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for The effect of protein concentration on the viscosity
Agilent AdvanceBio SEC Columns for Aggregate Analysis: Instrument Compatibility
 Agilent AdvanceBio SEC Columns for Aggregate Analysis: Instrument Compatibility Technical Overview Introduction Agilent AdvanceBio SEC columns are a new family of size exclusion chromatography (SEC) columns
Agilent AdvanceBio SEC Columns for Aggregate Analysis: Instrument Compatibility Technical Overview Introduction Agilent AdvanceBio SEC columns are a new family of size exclusion chromatography (SEC) columns
XactEdit Cas9 Nuclease with NLS User Manual
 XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
One-stop cief and CE-SDS. One-day method dev. Results in a snap. FDA, PDQ.
 One-stop cief and CE-SDS. One-day method dev. Results in a snap. FDA, PDQ. Meet Maurice. He s a real problem solver. Need CE-SDS and cief info on mabs, ADCs and vaccines? Done. He ll even up you one and
One-stop cief and CE-SDS. One-day method dev. Results in a snap. FDA, PDQ. Meet Maurice. He s a real problem solver. Need CE-SDS and cief info on mabs, ADCs and vaccines? Done. He ll even up you one and
Chapter 2 Coated Capillaries for CE-MS of Therapeutic Protein
 Chapter 2 Coated Capillaries for CE-MS of Therapeutic Protein James Q. Xia 2.1 Introduction to Capillary Coating Bare fused-silica capillary is so far the most commonly used type of capillary in CE-UV
Chapter 2 Coated Capillaries for CE-MS of Therapeutic Protein James Q. Xia 2.1 Introduction to Capillary Coating Bare fused-silica capillary is so far the most commonly used type of capillary in CE-UV
Application Note. Author. Abstract. Pharmaceuticals. Detlef Wilhelm ANATOX GmbH & Co. KG. Fuerstenwalde, Germany mau
 Development, validation, and comparison of an HPLC method to analyze paracetamol and related impurities according to the European Pharmacopoeia (EP) and USP using the Agilent 1120 Compact LC and the Agilent
Development, validation, and comparison of an HPLC method to analyze paracetamol and related impurities according to the European Pharmacopoeia (EP) and USP using the Agilent 1120 Compact LC and the Agilent
Quantification of Host Cell Protein Impurities Using the Agilent 1290 Infinity II LC Coupled with the 6495B Triple Quadrupole LC/MS System
 Application Note Biotherapeutics Quantification of Host Cell Protein Impurities Using the Agilent 9 Infinity II LC Coupled with the 6495B Triple Quadrupole LC/MS System Authors Linfeng Wu and Yanan Yang
Application Note Biotherapeutics Quantification of Host Cell Protein Impurities Using the Agilent 9 Infinity II LC Coupled with the 6495B Triple Quadrupole LC/MS System Authors Linfeng Wu and Yanan Yang
Lifetime stability of size exclusion chromatography columns for protein aggregate analysis
 APPLICATION NOTE 72362 Lifetime stability of size exclusion chromatography columns for protein aggregate analysis Authors Amy Farrell 1, Craig Jakes 1, Alexander Ley 2, Mauro De Pra 2, Frank Steiner 2,
APPLICATION NOTE 72362 Lifetime stability of size exclusion chromatography columns for protein aggregate analysis Authors Amy Farrell 1, Craig Jakes 1, Alexander Ley 2, Mauro De Pra 2, Frank Steiner 2,
Analysis of amoxicillin and five impurities on the Agilent 1220 Infinity LC System
 Analysis of amoxicillin and five impurities on the Agilent Infinity LC System LC analysis of impurities down to the.% level with long sub--µm columns, high flow rates and back pressure greater than bar
Analysis of amoxicillin and five impurities on the Agilent Infinity LC System LC analysis of impurities down to the.% level with long sub--µm columns, high flow rates and back pressure greater than bar
Novel instrumentation to gain insight into the aggregation state of proteins by Taylor Dispersion Analysis: a comparison with Dynamic Light Scattering
 Novel instrumentation to gain insight into the aggregation state of proteins by Taylor Dispersion Analysis: a comparison with Dynamic Light Scattering Wendy Louise Hulse *, Rob Forbes, David Goodall, Alex
Novel instrumentation to gain insight into the aggregation state of proteins by Taylor Dispersion Analysis: a comparison with Dynamic Light Scattering Wendy Louise Hulse *, Rob Forbes, David Goodall, Alex
Technical Note. Introduction
 Online HPLC for Biotechnology: Groton Biosystems Automated Reactor Sampling System and Agilent 1200 Series LC System Technical Note Introduction Online analytical instrumentation facilitates productive,
Online HPLC for Biotechnology: Groton Biosystems Automated Reactor Sampling System and Agilent 1200 Series LC System Technical Note Introduction Online analytical instrumentation facilitates productive,
This is not unique to CE. It took for instance about 25 years for HPLC to establish itself properly in the pharmaceutical industry.
 CE Pharm 2017 Troubleshooting Report: CZE and CE-SDS the Discussion Continues Workshop facilitators: Tim Blanc 1, Cari Sänger-van de Griend 2 Scribes: Nathan Lacher 3, David Michels 4, Zoran Sosic 5 1
CE Pharm 2017 Troubleshooting Report: CZE and CE-SDS the Discussion Continues Workshop facilitators: Tim Blanc 1, Cari Sänger-van de Griend 2 Scribes: Nathan Lacher 3, David Michels 4, Zoran Sosic 5 1
CEofix NTMP. Determination of Proteins and Peptides with Capillary Electrophoresis. Introduction. Methods and Materials.
 Application note 130404 bio PHARMACEUTICAL analysis CEofix NTMP Determination of Proteins and Peptides with Capillary Electrophoresis Introduction The CEofix NTMP kit is using Nitrilotri(trimethyl)phosphonic
Application note 130404 bio PHARMACEUTICAL analysis CEofix NTMP Determination of Proteins and Peptides with Capillary Electrophoresis Introduction The CEofix NTMP kit is using Nitrilotri(trimethyl)phosphonic
