Combined Methane Decomposition and Ammonia Formation Cell
|
|
|
- Marcia Pearson
- 6 years ago
- Views:
Transcription
1 University of Central Florida UCF Patents Patent Combined Methane Decomposition and Ammonia Formation Cell John Bradenburg University of Central Florida Find similar works at: University of Central Florida Libraries Recommended Citation Bradenburg, John, "Combined Methane Decomposition and Ammonia Formation Cell" (2006). UCF Patents. Paper This Patent is brought to you for free and open access by the Technology Transfer at STARS. It has been accepted for inclusion in UCF Patents by an authorized administrator of STARS. For more information, please contact
2 I lllll llllllll Ill lllll lllll lllll lllll lllll US B 1 (12) United States Patent Brandenburg (10) Patent No.: US 6,986,870 Bl (45) Date of Patent: Jan.17,2006 (54) COMBINED METHANE DECOMPOSITION AND AMMONIA FORMATION CELL (75) Inventor: John E. Brandenburg, Cape Canaveral, FL (US) (73) Assignee: University of Central Florida Research Foundation, Inc, Orlando, FL (US) ( *) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 U.S.C. 154(b) by 343 days. (21) Appl. No.: 10/646,421 (22) (60) (51) (52) (58) (56) Filed: Aug. 21, 2003 Related U.S. Application Data Provisional application No. 60/409,943, filed on Sep. 11, Int. Cl. COlC 1/02 ( ) COlC 1/04 ( ) U.S. Cl /148; 422/198; 422/211; 422/239; 423/359 Field of Classification Search /148, 422/198, 211, 239; 423/359 See application file for complete search history. 4,142,993 A 4,180,552 A 4,180,553 A 4,390,509 A 4,836,898 A 4,891,202 A References Cited U.S. PATENT DOCUMENTS 3/1979 Elofson et al /447 12/1979 Graham et al /359 12/1979 Null et al /359 6/1983 Weston et al /313 6/1989 Noyes /129 1/1990 Lichtin et al /352 4,938,855 A 4,981,669 A 5,266,175 A 5,411,649 A 6,002,059 A 6,228,341 Bl 6,245,309 Bl 6,293,979 Bl 6,333,014 Bl 6,340,451 Bl 7/1990 Lichtin et al / /1991 Pinto /359 11/1993 Murphy / /1995 Roussy et al / /1999 Hellring et al /500 5/2001 Hebert et al /352 6/2001 Etievant et al /248 9/2001 Choudhary et al / /2001 Filippi /359 1/2002 Pagani et al /359 Primary Examiner-Alexa D. Neckel (74) Attorney, Agent, or Firm-Brian S. Steinberger; Law Offices of Brian S. Steinberger, P.A. (57) ABSTRACT An ammonia synthesis process and apparatus are provided which are energy efficient and minimize greenhouse-gasemission during the processing of natural gas and air. In the process a stream of natural gas is divided into two streams, one of which is mixed with air and ignited to provide heat for the thermal decomposition of natural gas into hydrogen and carbon and also to provide deoxygenated nitrogen for an ammonia synthesis process. The process essentially prepares hydrogen and nitrogen on a low average temperature side of a chemical reactor and then feeds both gases to the high average temperature side of the chemical reactor where they react to form ammonia. The formation of ammonia is exothermic, whereas the thermal decomposition of methane is endothermic and the combustion of methane to remove oxygen is also exothermic; the sum of the heats absorbed and released in these reactions is positive. Catalysts, high temperatures and pressure are used to promote the rapid formation of ammonia, as is standard practice in the chemical industry. Catalysts, and high temperatures are used to promote the thermal decomposition of natural gas and combustion of oxygen that provides hydrogen and nitrogen for ammonia synthesis. 6 Claims, 1 Drawing Sheet NaturaJsu in Combined Process Chemical Reactor
3 U.S. Patent Jan.17,2006 US 6,986,870 Bl - ~ i! 1 Z.5
4 1 COMBINED METHANE DECOMPOSITION AND AMMONIA FORMATION CELL US 6,986,870 Bl This invention claims the benefit of priority to U.S. Provisional Application No. 60/409,943 filed Sep. 11, FIELD OF THE INVENTION This invention relates to the production of ammonia from natural gas and more particularly to an apparatus and its 10 application to a novel method of the production of ammonia from natural gas in concurrent exothermic and endothermic processes that releases no greenhouse gases to the environment. 15 BACKGROUND AND PRIOR ART It is conventional to synthesize ammonia by the Haber Bosch process developed in the mid-twentieth century. The Haber-Bosch ammonia synthesis is accomplished by the 20 reaction of nitrogen and hydrogen at 700 C. and requires 700 atmospheres to achieve good yields. This exothermic reaction releases heat, which must be removed from the reaction facility. Due to low conversion efficiency per pass, hydrogen and nitrogen must be recycled and ammonia 25 removed by condensation at high pressures. Nitrogen is prepared by combustion of the oxygen from air to leave co2 and water vapor; the heat of combustion is used for thermal decomposition of methane. Numerous patents discuss ammonia synthesis from nitro- 30 gen including: U.S. Pat. No. 4,142,993 which deals only with an improved Haber process, using an improved doped carbon catalyst; for ammonia synthesis; U.S. Pat. No. 4,180,552 to Graham, et al. again discloses 3 5 an improved Haber process, which involves hydrogen recovery from ammonia synthesis in which purging streams are recycled to the reaction zone to improve ammonia yields. U.S. Pat. No. 4,180,553 to Null, et al. again also discloses 40 an improved Haber process, which involves hydrogen recovery from ammonia synthesis in which purging streams are recycled to the reaction zone; U.S. Pat. No. 4,390,509 to Weston, et al. in which synthesis gas is used to produce ammonia and digestion 4 5 of phosphate rock and eventually ammonium phosphate; U.S. Pat. No. 4,891,262 to Lichtin, et al. produces ammonia by catalyzed reduction of nitrogen absent photo energy; U.S. Pat. No. 4,938,855 to Lichtin, et al. produces ammonia by photo promoted catalyzed reduction of nitrogen; and, U.S. Pat. No. 4,981,669 to Pinto produces ammonia synthesis gas from natural gas by several reforming 55 steps involving the water reforming shift process by adjusting the temperature of the reforming tubes and the air fed to the secondary reformer stage. Hydrogen can be prepared from natural gas by water reforming or thermal decomposition. Water reforming is 60 most often used because it is exothermic, and thus requires no energy input. Thermal decomposition is not often used because it requires energy input. In thermal decomposition, refractories are heated and natural gas is flowed over them; in the process of the endothermic decomposition the refractories cool and must be reheated. For this reason this process is not often used, as discussed in the McGraw-Hill Ency clopedia of Science and Technology 8'h Ed., 1997, "Carbon Black" p To be cost effective the thermal decomposition process must be combined with some process or processes that release heat. With catalysts the thermal decomposition process can be made to operate at 700 C. This process is sometimes called the Bosch process. Numerous patents discuss hydrogen synthesis in which hydrogen, if desired, can be reacted with nitrogen to produce ammonia including: U.S. Pat. No. 4,836,898 to Noyes discusses high temperature conversion of methane to hydrogen and carbon; U.S. Pat. No. 5,266,175 to Murphy and U.S. Pat. No. 5,411,649 to Roussey, et al. are concerned with conversion of a methane feed to hydrogen by microwave radiation in an electromagnetic field; U.S. Pat. No. 6,002,059 to Hellring, et al. is concerned with the upgrading of natural gas into higher order hydrocarbons; U.S. Pat. No. 6,245,309 Bl to Etievant, et al. discloses a hydrogen generator from fuel gas having a chamber provided with means for maintaining a predetermined temperature and electrical discharges at a reduced current level; and, U.S. Pat. No. 6,293,979 Bl to Choudhary, et al. catalytically converts natural gas to synthesis gas. It is also known to use the exothermic energy from a reaction in one area of a plant to provide energy to facilitate the reaction of endothermic reaction in another area of the plant. Numerous patents discuss such utilization including: U.S. Pat. No. 6,002,059 to Hellring, et al. are concerned with the upgrading of natural gas into higher order hydrocarbons discloses the concept of balancing the heat requirements of the several steps by transferring energy from the exothermic reactions to the endothermic (see col. 7 lines 1-30). U.S. Pat. No. 6,228,341 Bl to Hebert, et al. also involves the transfer of energy from the exothermic reaction area to the reaction streams by indirect heat exchange through heat exchange channels. U.S. Pat. No. 6,245,309 Bl to Etievant, et al. discloses a hydrogen generator from fuel gas having a chamber provided with means for maintaining a predetermined temperature and electrical discharges at a reduced current level. U.S. Pat. No. 6,293,979 Bl to Choudhary, et al. catalytically converts natural gas to synthesis gas by a technique where the exothermic oxidative conversion of the oxygen with the natural gas is carried out in the same environment as the endothermic steam reforming of the natural gas resulting in an enhanced energy efficient environment (see col. 7, lines 15-63). U.S. Pat. No. 6,333,014 Bl to Filippi in a process arrangement for the co-production of ammonia and methanol, transfers energy from a hot stream by indirect heat exchange with another input stream; and, U.S. Pat. No. 6,340,451 B 1 to Pagani, et al. in a plant for the synthesis of ammonia and urea increases its output capacity by using a means for adjusting the various feed streams. The methods disclosed above and their respective apparatuses are deficient in that none suggest carrying out the processing of methane or natural gas into ammonia in a limited environment that prevents the emission of greenhouse gases or utilizing the exothermic energy of one 65 intermediate process enclosed in a reaction vessel to energize the endothermic energy consumption of a second intermediate process in said vessel and adjacent thereto for
5 US 6,986,870 Bl 3 4 improved efficiency of production of ammonia. The importance of ammonia as a safe compound and carrier of hydrogen for fuel cells has been recognized in the new hydrogen fuel economy. Thus, the present invention fills the need for an energy efficient means to produce a safe hydrogen-containing compound, such as ammonia, from natural gas fields without harmful environmental effects. BRIEF SUMMARY OF THE INVENTION It is a primary objective of the invention to develop a novel method and apparatus for the production of ammonia with reduced amounts of carbon dioxide. A second object of the invention is to develop a method and apparatus for the production of ammonia with improved 15 efficiency. A third object of the invention is to develop a method and apparatus for the production of ammonia with improved yield. A fourth object of the invention is to develop a method 20 and apparatus for converting a fossil fuel, such as natural gas, to ammonia without the release of greenhouse gases. A preferred embodiment of the invention is the preparation of ammonia by an apparatus comprising: a reaction chamber with upper and lower reaction cavities; the lower 25 cavity containing a third inner cavity functioning as an oxidation structure for burning of natural gas in air, said upper and lower cavities separated by a hydrogen permeable structure; means for supplying air into said lower inner reaction cavity; an oxidation structure within said lower 30 cavity for burning said air; means for transferring nitrogen from said lower oxidation cavity to the upper reactor cavity for catalytic conversion with hydrogen and means for transferring exothermic energy from said upper reactor cavity to said lower reactor cavity whereby the additional energy need 35 of the lower of said cavities is reduced and the method comprising the steps of: first thermally decomposing natural gas to produce hydrogen endothermically and exothermically burning natural gas in air to produce oxygen-free nitrogen; secondly thereafter scrubbing the nitrogen to 40 remove the water and carbon oxides; thirdly reacting the hydrogen and purified nitrogen in an exothermic reaction to produce ammonia and exothermic energy; fourthly, transferring the excess energy to the endothermic reaction whereby its need for additional energy to be supplied from 45 external sources is reduced. BRIEF DESCRIPTION OF THE FIGURES FIG. 1 shows a Combined Process Chemical (CPC) 50 reactor. DESCRIPTION OF THE PREFERRED EMBODIMENT Before explaining the disclosed embodiment of the present invention in detail, it is to be understood that the invention is not limited in its application to the details of the particular arrangement shown since the invention is capable of other embodiments. Also, the terminology used herein is 60 for the purpose of description and not of limitation. The invention disclosed herein in summary encompasses a device and process for converting natural gas to carbon plus ammonia in a single reactor vessel utilizing a natural gas input flow catalyzed to strip hydrogen and carbon from 65 it in the first process and thereafter utilizing the Haber Boesch process as the second process step in said vessel to provide ammonia in an exothermic step where the resulting heat is recycled to the endothermic first process. The method of the invention is based on the thermal decomposition of natural gas into hydrocarbons, preferably 5 hydrogen, and then by reaction with nitrogen into ammonia in an energy efficient manner that releases no greenhouse gases to the environment. That prevention/reduction in the release of said gases is the chief advantage of this process of this invention over the present process of making ammonia 10 from natural gas which uses the water shift method to make hydrogen feedstock and produces large amounts of gases, primarily carbon dioxide, that are released to the environment. Referring now to the drawing, FIG. 1, wherein characters are used to designate like or corresponding parts throughout the CPC (Combined Process Chemical) reactor which comprises an approximately cylindrical chamber 1 having an upper reaction cavity 2 and a lower reaction cavity 3 divided by a hydrogen permeable membrane 4. The hydrogen permeable membrane 4 can be palladium (Pd) metal. In the lower cavity 3, methane from the natural gas feed line 5 is heated and thermally decomposed on a hot cylinder 6 heated by the internal burning inside 7 of natural gas and air 8. The temperature and pressure of the internal region 7 is approximately one atmosphere of pressure and the combustion temperature is in a range of from about 1500 C. to about 2000 C. A pilot light or electric spark is used to ignite the natural gas and air inside the hot cylinder 6. Air that has been cleansed of oxygen is conducted out of cylinder 6 by pipe 9 to a scrubber 10 to remove carbon oxides and water using technology known to those skilled in the art. Carbon oxides and water and other by products are removed from a scrubber port 11 as limestone or calcium salts. The removal of carbon oxides, water and other by-products by the scrubber leaves a substantially pure nitrogen gas that can be sent by pipe 12 to the upper reaction cavity 2. The upper reaction cavity 2 contains an upper chamber 13 with permeable walls that creates a reservoir for the substantially pure nitrogen gas from the scrubber 10. The upper chamber 13 is conveniently located directly above the hot cylinder 6 of the lower chamber to maximize the heat transfer efficiencies between exothermic and endothermic reactions. Meanwhile, in the lower reaction cavity 3, hydrogen is released by the thermal decomposition of natural gas having methane content in the range of approximately 90 to 98 volume percent. Thus, the terms methane and natural gas are used interchangeably herein. The thermal decomposition of methane occurs in the lower reaction cavity 3 on the hot cylinder and elsewhere within the lower reaction cavity. The decomposition reaction is represented as follows: The hydrogen gas rises and crosses through the membrane 55 4 to react with nitrogen in the upper cavity 2 to form ammonia. Carbon 14 formed in the lower cavity falls near the hot cylinder 6 and is removed by a suitable screw type device that allows the solid carbon residue to exit the chamber. Ammonia is catalytically formed in and about the permeable walls of the upper chamber 13 and is removed from the upper reactor cavity 2 by a pipe 15. The reaction products in the upper reaction cavity 2 include ammonia (NH 3 ) and unreacted nitrogen (N 2 ) and hydrogen (H 2 ), which are separated in a condenser 16. The unreacted nitrogen-hydrogen mixture is a separated fraction that is returned to the stream of nitrogen gas by pipe 17.
6 US 6,986,870 Bl 5 6 As ammonia forms in the upper reaction cavity 2, via known catalytic reaction processes, heat is released as discussed in more detail below. The heat in the upper chamber moves via thermal conduction to the lower reaction cavity 3 because the entire reactor is made of a conductive metal, such as steel. The process of the invention involves first the combustion of natural gas, primarily composed of methane, in air. The combustion of natural gas and air in the inner chamber 7 of the lower cavity 3 of the reaction vessel decomposes the natural gas/methane to release hydrogen and decomposes the air to release nitrogen. The combustion of methane and air is an exothermic reaction, which removes oxygen with the production of heat. Thermodynamically, it is known that the combustion of methane with oxygen produces Kcal/-g mole. To prepare 2 moles N 2 from air which is 78% N 2 plus % (22.4 L/mole of air) CH ~co 2 +2H 2 0 requires 1.28 moles of air per mole N 2, which means 2.56 moles of air for two moles N 2. To burn methane (CH 4 ) in air to get rid of oxygen mole is required per mole of air; 1.28x0.105x212.79=28.6 kcal is released to prepare one mole of purified nitrogen molecules (N 2 ) of which carbon dioxide (C0 2 ) and water (H 2 0) can be removed by a scrubber from gas using conventional techniques. Thus, the C0 2 produced does not 25 enter the atmosphere. The heat released is used to help decompose methane to produce hydrogen and carbon, a reaction, which is endothermic and requires energy of kcal/mole to produce one mole of C and four moles of H. Whereas the synthesis of ammonia is exothermic and 30 releases kcal/mole of ammonia produced. When detailed mass and energy balance is performed we have for heat balance, i.e., 3CH 4 ( kcal)+2n 2 ( ( kcal)+3c Thus the process requires kcal to decompose enough methane to produce 4 moles of ammonia but the production of the purified nitrogen and ammonia itself releases kcal, which even when allowing for heat losses due to hot 40 nitrogen plus carbon oxides and water vapor being cooled as it runs through a scrubber means that enough energy from the exothermic reactions should be available to drive the endothermic reaction. As demonstrated in the present invention, the production of ammonia from natural gas can be a net exothermic process; because, the thermal decomposition of methane is endothermic, the combustion of methane to remove oxygen is exothermic, and the formation of ammonia is exothermic; thus the sum of the heats absorbed and released within a single reaction chamber is positive. 50 The advantages of the present invention are several. The present invention allows the capture of hydrogen from natural gas, which is difficult to liquefy (-164 C.) and store (0.4 g/cc) into ammonia, which has a much higher density (0.8 glee) and is much easier to liquefy (-33 C.) in order to 55 facilitate conversion into a hydrogen economy using ammonia as a hydrogen carrier. In addition, ammonia is readily broken down into hydrogen, which is used in fuel cells to produce water, and nitrogen, both of which can be freely vented into the air without harmful effects on the environ- 60 ment. Since the process utilizes exothermic processes to balance heat requirements of endothermic processes, it uti- lizes energy efficiently thus reduces net energy consumption and in particular, since the only carbon dioxide produced must be scrubbed from the nitrogen feedstock for the Haber process and captured, no carbon dioxide, a known deleteri- 5 ous greenhouse gas, is released into the atmosphere. In summary, the present invention produces ammonia, a valuable hydrogen carrier, in an energy efficient manner and without release of greenhouse gases. The use of catalysts, temperatures and pressures will allow optimization of the 10 process for maximizing synergism between the exothermic processes of combustion and ammonia formation and the endothermic process of thermal decomposition of natural gas so as to achieve maximum ammonia production, optimal heat flows and energy efficiency. While the invention has been described, disclosed, illustrated and shown in various terms of certain embodiments or modifications which it has presumed in practice, the scope of the invention is not intended to be, nor should it be deemed to be, limited thereby and such other modifications 20 or embodiments as may be suggested by the teachings herein are particularly reserved especially as they fall within the breadth and scope of the claims here appended. 35 I claim: 1. An apparatus for the production of ammonia by the reaction of hydrogen and nitrogen comprising: (a) a reaction chamber with both an upper reaction cavity and a lower reaction cavity; (b) said upper and lower cavities separated by a hydrogen permeable structure; (c) a first means for supplying a gaseous hydrocarbon mixture into said lower reaction cavity; ( d) a second means for supplying air into said lower reaction cavity; ( e) an oxidation structure within said lower cavity for burning said air; (f) means for transferring nitrogen from said lower reactor cavity to the upper reactor cavity for catalytic conversion to ammonia in the presence of hydrogen; and, (g) means for transferring exothermic energy from said upper reaction cavity to said lower reaction cavity whereby the additional energy need of the lower of said cavities is reduced. 2. The apparatus according to claim 1 wherein said 45 oxidation structure within the lower cavity for burning air is a hot cylinder heated by the internal burning inside of natural gas and air. 3. The apparatus according to claim 1 wherein the hot cylinder within the lower cavity is used to thermally decompose methane to form hydrogen and carbon. 4. The apparatus according to claim 1 wherein said hydrogen permeable membrane is formed of palladium (Pd) metal. 5. The apparatus according to claim 1 wherein the upper reaction cavity contains a reservoir with permeable walls to receive nitrogen gas that reacts with hydrogen gas from the lower reaction cavity to form ammonia. 6. The apparatus according to claim 5 wherein the ammonia forms during an exothermic energy reaction. * * * * *
Combined Methane Decomposition and Ammonia Formation Cell DIV
 University of Central Florida UCF Patents Patent Combined Methane Decomposition and Ammonia Formation Cell DIV 8-22-2006 John Bradenburg University of Central Florida Find similar works at: http://stars.library.ucf.edu/patents
University of Central Florida UCF Patents Patent Combined Methane Decomposition and Ammonia Formation Cell DIV 8-22-2006 John Bradenburg University of Central Florida Find similar works at: http://stars.library.ucf.edu/patents
DISCLAIMER. Portions of this document may be illegible electronic image products. Images are produced from the best available original document.
 3 rn -I 0 ZLS TL-s DISCLAIMER Portions of this document may be illegible electronic image products. Images are produced from the best available original document. INDIRECT-FIRED GAS TURBINE DUAL FUEL CELL
3 rn -I 0 ZLS TL-s DISCLAIMER Portions of this document may be illegible electronic image products. Images are produced from the best available original document. INDIRECT-FIRED GAS TURBINE DUAL FUEL CELL
Inventors: Paul L. Micheli Mark C. Williams Edward L. Parsons
 S-74,980 Micheli et a1 INDIRECT-FIRED GAS TURBINE BOlTOMED WITH FUEL CELL Inventors: Paul L. Micheli Mark C. Williams Edward L. Parsons DISCLAIMER ' This report was prepared as an account of work sponsored
S-74,980 Micheli et a1 INDIRECT-FIRED GAS TURBINE BOlTOMED WITH FUEL CELL Inventors: Paul L. Micheli Mark C. Williams Edward L. Parsons DISCLAIMER ' This report was prepared as an account of work sponsored
Lecture 4. Ammonia: Production and Storage - Part 1
 Lecture 4 Ammonia: Production and Storage - Part 1 Ammonia or azane is a compound of nitrogen and hydrogen with the formula NH 3. It is a colourless gas with a characteristic pungent smell. Ammonia contributes
Lecture 4 Ammonia: Production and Storage - Part 1 Ammonia or azane is a compound of nitrogen and hydrogen with the formula NH 3. It is a colourless gas with a characteristic pungent smell. Ammonia contributes
CPC field-specific training
 CPC field-specific training C10G, C10L1/00-C10L1/08 José Carlos Pardo Torre Examiner, European Patent Office June 2018 Table of contents Introduction What and where to classify Neighbouring fields Specific
CPC field-specific training C10G, C10L1/00-C10L1/08 José Carlos Pardo Torre Examiner, European Patent Office June 2018 Table of contents Introduction What and where to classify Neighbouring fields Specific
United States Patent (19)
 United States Patent (19) van Dijk et al. (11) 4,407,973 Oct. 4, 1983 54) (75) (73) 21 22 (51) 52) 58) METHANOL FROM COAL AND NATURAL GAS Inventors: Christiaan P. van Dijk, Houston; Aage Solbakken, Montgomery;
United States Patent (19) van Dijk et al. (11) 4,407,973 Oct. 4, 1983 54) (75) (73) 21 22 (51) 52) 58) METHANOL FROM COAL AND NATURAL GAS Inventors: Christiaan P. van Dijk, Houston; Aage Solbakken, Montgomery;
Module 4 : Hydrogen gas. Lecture 29 : Hydrogen gas
 1 P age Module 4 : Hydrogen gas Lecture 29 : Hydrogen gas 2 P age Keywords: Electrolysis, steam reforming, partial oxidation, storage Hydrogen gas is obtained in a very trace amount in atmosphere. It is
1 P age Module 4 : Hydrogen gas Lecture 29 : Hydrogen gas 2 P age Keywords: Electrolysis, steam reforming, partial oxidation, storage Hydrogen gas is obtained in a very trace amount in atmosphere. It is
Fabrication of Nano-Scale Temperature Sensors and Heaters. DIV
 University of Central Florida UCF Patents Patent Fabrication of Nano-Scale Temperature Sensors and eaters. DIV 3-7-2006 Lee Chow University of Central Florida Fred Stevie Lucent Technologies, Inc. Dan
University of Central Florida UCF Patents Patent Fabrication of Nano-Scale Temperature Sensors and eaters. DIV 3-7-2006 Lee Chow University of Central Florida Fred Stevie Lucent Technologies, Inc. Dan
(12) Patent Application Publication (10) Pub. No.: US 2016/ A1
 US 20160083658A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0083658 A1 SANNER (43) Pub. Date: Mar. 24, 2016 (54) METHODS FOR PRODUCTION OF LIQUID Publication Classification
US 20160083658A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0083658 A1 SANNER (43) Pub. Date: Mar. 24, 2016 (54) METHODS FOR PRODUCTION OF LIQUID Publication Classification
Chapter 10 Material Balances for Processes Involving Reaction 10.1 Species Material Balances Processes Involving a Single Reaction
 Chapter 10 Material Balances for Processes Involving Reaction 10.1 Species Material Balances 10.1.1 Processes Involving a Single Reaction The material balance for a species must be augmented to include
Chapter 10 Material Balances for Processes Involving Reaction 10.1 Species Material Balances 10.1.1 Processes Involving a Single Reaction The material balance for a species must be augmented to include
United States Patent (19) Pinto
 United States Patent (19) Pinto 11) Patent Number: Date of Patent: Mar. 21, 1989 54 NITROGEN PRODUCTION (75) Inventor: Alwyn Pinto, Middlesbrough, United Kingdom 73) Assignee: Imperial Chemical Industries
United States Patent (19) Pinto 11) Patent Number: Date of Patent: Mar. 21, 1989 54 NITROGEN PRODUCTION (75) Inventor: Alwyn Pinto, Middlesbrough, United Kingdom 73) Assignee: Imperial Chemical Industries
Fabrication of Sub-Micron Size Thermocouples and Heaters
 University of Central Florida UCF Patents Patent Fabrication of Sub-Micron Size Thermocouples and Heaters 6-14-2005 Lee Chow University of Central Florida Fred Stevie Lucent Technologies, Inc. Dan Zhou
University of Central Florida UCF Patents Patent Fabrication of Sub-Micron Size Thermocouples and Heaters 6-14-2005 Lee Chow University of Central Florida Fred Stevie Lucent Technologies, Inc. Dan Zhou
DISTRIBUTION STATEMENT A Approved for Public Release Distribution Unlimited
 Serial Number Filing Date Inventor 08/95.994 9 September 1997 Eugene E. Nolting Jon Colfield Roy Richard Steven Peterson NOTICE The above identified patent application is available for licensing. Requests
Serial Number Filing Date Inventor 08/95.994 9 September 1997 Eugene E. Nolting Jon Colfield Roy Richard Steven Peterson NOTICE The above identified patent application is available for licensing. Requests
GASOLINE FROM NATURAL GAS BY SULFUR PROCESSING
 GASOLINE FROM NATURAL GAS BY SULFUR PROCESSING BY Erek J. Erekson R. Gopalakrishnan January 1996 Work Performed Under Contract No.: DE-AC22-93PC92114 For U.S. Department of Energy Pittsburgh Energy Technology
GASOLINE FROM NATURAL GAS BY SULFUR PROCESSING BY Erek J. Erekson R. Gopalakrishnan January 1996 Work Performed Under Contract No.: DE-AC22-93PC92114 For U.S. Department of Energy Pittsburgh Energy Technology
US 9,115,913 B1 Aug. 25, 2015
 111111 1111111111111111111111111111111111111111111111111111111111111 US009115913Bl c12) United States Patent Rossi (10) Patent No.: (45) Date of Patent: US 9,115,913 B1 Aug. 25, 2015 (54) FLUID HEATER
111111 1111111111111111111111111111111111111111111111111111111111111 US009115913Bl c12) United States Patent Rossi (10) Patent No.: (45) Date of Patent: US 9,115,913 B1 Aug. 25, 2015 (54) FLUID HEATER
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 2008.0099618A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0099618 A1 ZAKI et al. (43) Pub. Date: May 1, 2008 (54) OXYGEN REMOVAL SYSTEM Publication Classification
US 2008.0099618A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0099618 A1 ZAKI et al. (43) Pub. Date: May 1, 2008 (54) OXYGEN REMOVAL SYSTEM Publication Classification
Module 4: Gaseous Fuel. Lecture 28: Water Gas
 1 P age Module 4: Gaseous Fuel Lecture 28: Water Gas 2 P age Keywords: Coal gasification, steam blast, blast saturation temperature, gas generator 4.3 Water gas Water gas is a gaseous fuel generated in
1 P age Module 4: Gaseous Fuel Lecture 28: Water Gas 2 P age Keywords: Coal gasification, steam blast, blast saturation temperature, gas generator 4.3 Water gas Water gas is a gaseous fuel generated in
IIIIIi. 0 o_l_ll_. ""' Llillg
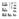 IIIIIi. 0 o_l_ll_ ""' Llillg 11111_ 11111_ DOE Docket S-70,579 u 0 C SPLIT FLOW GASIFIER o Inventor - John S. Halow DISCLAIMER H Thisreportwaspreparedasan accountof worksponsoredby an agencyof the UnitedStates
IIIIIi. 0 o_l_ll_ ""' Llillg 11111_ 11111_ DOE Docket S-70,579 u 0 C SPLIT FLOW GASIFIER o Inventor - John S. Halow DISCLAIMER H Thisreportwaspreparedasan accountof worksponsoredby an agencyof the UnitedStates
Identify three common gaseous pollutants in air and state how each of these pollutants are produced.
 1 Clean dry air contains mainly nitrogen and oxygen. (a) Name two other gases that are in clean dry air.. [2] (b) Air often contains pollutants. Identify three common gaseous pollutants in air and state
1 Clean dry air contains mainly nitrogen and oxygen. (a) Name two other gases that are in clean dry air.. [2] (b) Air often contains pollutants. Identify three common gaseous pollutants in air and state
United States Patent (19) Woodard
 United States Patent (19) Woodard (54) (75) 73 21 22) (51) (52) 58 56) SEMPERMEABLE MEMBRANE DRYER FOR AIR COMPRESSOR SYSTEM Inventor: Michael K. Woodard, Hampton, Va. Assignee: Newport News Shipbuilding
United States Patent (19) Woodard (54) (75) 73 21 22) (51) (52) 58 56) SEMPERMEABLE MEMBRANE DRYER FOR AIR COMPRESSOR SYSTEM Inventor: Michael K. Woodard, Hampton, Va. Assignee: Newport News Shipbuilding
Evonik s Andrussow process expertise for tailor-made solutions
 Evonik s Andrussow process expertise for tailor-made solutions Scope of a standard HCN technology license Evonik Performance Materials GmbH offers plant designs for the production of gaseous and liquid
Evonik s Andrussow process expertise for tailor-made solutions Scope of a standard HCN technology license Evonik Performance Materials GmbH offers plant designs for the production of gaseous and liquid
Questions. Downdraft biomass gasifier. Air. Air. Blower. Air. Syngas line Filter VFD. Gas analyzer(s) (vent)
 Question 1 Questions Biomass gasification is a process where organic matter liberates flammable gases such as hydrogen (H 2 ) and carbon monoxide (CO) when heated to high temperatures. A gasifier is a
Question 1 Questions Biomass gasification is a process where organic matter liberates flammable gases such as hydrogen (H 2 ) and carbon monoxide (CO) when heated to high temperatures. A gasifier is a
1. Process Description:
 1. Process Description: The coal is converted to Raw Syngas in the Gasification Section. The Raw Syngas produced out of the Gasifier would be shifted (water gas shift) to adjust required H2/CO ratio and
1. Process Description: The coal is converted to Raw Syngas in the Gasification Section. The Raw Syngas produced out of the Gasifier would be shifted (water gas shift) to adjust required H2/CO ratio and
The Haber Process 1 of 30 Boardworks Ltd 2012
 The Haber Process 1 of 30 Boardworks Ltd 2012 2 of 30 Boardworks Ltd 2012 What is ammonia? 3 of 30 Boardworks Ltd 2012 Ammonia is an important compound in the manufacture of fertilizer and other chemicals
The Haber Process 1 of 30 Boardworks Ltd 2012 2 of 30 Boardworks Ltd 2012 What is ammonia? 3 of 30 Boardworks Ltd 2012 Ammonia is an important compound in the manufacture of fertilizer and other chemicals
CRYOGENIC SOLVENT ABATEMENT (VOC s )
 CRYOGENIC SOLVENT ABATEMENT (VOC s ) 1. Introduction The technology for removing volatile organic compounds (V.O.C.s) from gas has been developed to meet the emission limits, decreased during the last
CRYOGENIC SOLVENT ABATEMENT (VOC s ) 1. Introduction The technology for removing volatile organic compounds (V.O.C.s) from gas has been developed to meet the emission limits, decreased during the last
Chemical-Looping with Oxygen Uncoupling for capture of carbon dioxide February 2, 2005 (Translation of Swedish patent application)
 Chemical-Looping with Oxygen Uncoupling for capture of carbon dioxide February 2, 2005 (Translation of Swedish patent application) Applicants and inventors Anders Lyngfelt and Tobias Mattisson 1 Chemical-Looping
Chemical-Looping with Oxygen Uncoupling for capture of carbon dioxide February 2, 2005 (Translation of Swedish patent application) Applicants and inventors Anders Lyngfelt and Tobias Mattisson 1 Chemical-Looping
SO 2 Clean for SRU Expansion
 SO 2 Clean for SRU Expansion Jeff Hammerstrom Calabrian Corp jhammerstrom@calabriancorp.com Ron Schendel Process Consultant Ron@RSchendel.com Brimstone Sulfur Symposium Vienna, Austria May 27 31, 2013
SO 2 Clean for SRU Expansion Jeff Hammerstrom Calabrian Corp jhammerstrom@calabriancorp.com Ron Schendel Process Consultant Ron@RSchendel.com Brimstone Sulfur Symposium Vienna, Austria May 27 31, 2013
Green is Seen in Fertilizers Municipal Solid Waste Management. Carrie Farberow Kevin Bailey University of Oklahoma May 1, 2007
 Green is Seen in Fertilizers Municipal Solid Waste Management Carrie Farberow Kevin Bailey University of Oklahoma May 1, 007 MSW Overview EPA 005 Facts and Figures U.S. Waste Produced = 45.7 million ton
Green is Seen in Fertilizers Municipal Solid Waste Management Carrie Farberow Kevin Bailey University of Oklahoma May 1, 007 MSW Overview EPA 005 Facts and Figures U.S. Waste Produced = 45.7 million ton
METHANOL CONVERTER AND SYNLOOP DESIGNS FOR GASIFICATION PLANTS
 METHANOL CONVERTER AND SYNLOOP DESIGNS FOR GASIFICATION PLANTS By E. Filippi, M. Badano METHANOL CASALE S.A. Lugano, Switzerland For presentation at the 2007 WORLD METHANOL CONFERENCE November 27-29 2007,
METHANOL CONVERTER AND SYNLOOP DESIGNS FOR GASIFICATION PLANTS By E. Filippi, M. Badano METHANOL CASALE S.A. Lugano, Switzerland For presentation at the 2007 WORLD METHANOL CONFERENCE November 27-29 2007,
The modern atmosphere
 The modern atmosphere Heat can be transferred from place to place by conduction, convection and radiation. Dark matt surfaces are better at absorbing heat energy than light shiny surfaces. Heat energy
The modern atmosphere Heat can be transferred from place to place by conduction, convection and radiation. Dark matt surfaces are better at absorbing heat energy than light shiny surfaces. Heat energy
HOW PYROLYSIS WASTE TO ENERGY WORKS
 HOW PYROLYSIS WASTE TO ENERGY WORKS The use of pyrolysis in the thermal processing of municipal solid waste is becoming more widespread in application due to the overall flexibility of the pyrolysis process.
HOW PYROLYSIS WASTE TO ENERGY WORKS The use of pyrolysis in the thermal processing of municipal solid waste is becoming more widespread in application due to the overall flexibility of the pyrolysis process.
Biomass gasification plant and syngas clean-up system
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 75 (2015 ) 240 245 The 7 th International Conference on Applied Energy ICAE2015 Biomass gasification plant and syngas clean-up system
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 75 (2015 ) 240 245 The 7 th International Conference on Applied Energy ICAE2015 Biomass gasification plant and syngas clean-up system
*EP A1* EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2001/31
 (19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00112098A1* (11) EP 1 120 98 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 01.08.2001 Bulletin 2001/31
(19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00112098A1* (11) EP 1 120 98 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 01.08.2001 Bulletin 2001/31
1) ABSORPTION The removal of one or more selected components from a gas mixture by absorption is probably the most important operation in the control
 1) ABSORPTION The removal of one or more selected components from a gas mixture by absorption is probably the most important operation in the control of gaseous pollutant emissions. Absorption is a process
1) ABSORPTION The removal of one or more selected components from a gas mixture by absorption is probably the most important operation in the control of gaseous pollutant emissions. Absorption is a process
GTL. and Edited and Revised 2016 by H M Fahmy
 GTL Taken Partly from the Internet and Edited and Revised 2016 by H M Fahmy STEPS TO GET OIL FROM SEA OR EARTH SEISMIC SHOOTING SEISMIC INTERPRETATION ANALYSIS OF SAMPLES PREPARATION OF RIG DRILLING OIL
GTL Taken Partly from the Internet and Edited and Revised 2016 by H M Fahmy STEPS TO GET OIL FROM SEA OR EARTH SEISMIC SHOOTING SEISMIC INTERPRETATION ANALYSIS OF SAMPLES PREPARATION OF RIG DRILLING OIL
The material balance equations, after introducing the known values for the variables, are:
 Specifications: 4 (3 independent) (one is independent, the sum is F in mol) The material balance equations, after introducing the known values for the variables, are: of equations: (e) (b) simultaneously,
Specifications: 4 (3 independent) (one is independent, the sum is F in mol) The material balance equations, after introducing the known values for the variables, are: of equations: (e) (b) simultaneously,
Reforming is an upgrading process in which low octane gasoline is converted to high octane gasoline.
 REFORMING Reforming is an upgrading process in which low octane gasoline is converted to high octane gasoline. Catalytic reforming primarily increases the octane of motor gasoline rather than increasing
REFORMING Reforming is an upgrading process in which low octane gasoline is converted to high octane gasoline. Catalytic reforming primarily increases the octane of motor gasoline rather than increasing
Hydrogen Separation Membrane Applications
 2009 Hydrogen Separation Membrane Applications Eltron Research & Development Inc. 4600 Nautilus Court South Boulder, CO 80301-3241 Doug Jack VP Technology 303.530.0263 x118 djack@eltronresearch.com Carl
2009 Hydrogen Separation Membrane Applications Eltron Research & Development Inc. 4600 Nautilus Court South Boulder, CO 80301-3241 Doug Jack VP Technology 303.530.0263 x118 djack@eltronresearch.com Carl
2011 BRIMSTONE SULFUR SYMPOSIUM. Fundamentals 2011: Ammonia Destruction in SRU Furnaces
 2011 BRIMSTONE SULFUR SYMPOSIUM Fundamentals 2011: Ammonia Destruction in SRU Furnaces Peter Clark, Alberta Sulphur Research Ltd. Paul d Haene, DANA Technical Services Jim Jenkins, Shell Contents: 1. History
2011 BRIMSTONE SULFUR SYMPOSIUM Fundamentals 2011: Ammonia Destruction in SRU Furnaces Peter Clark, Alberta Sulphur Research Ltd. Paul d Haene, DANA Technical Services Jim Jenkins, Shell Contents: 1. History
Carbon To X. Processes
 World CTX Carbon To X Processes Processes and Commercial Operations World CTX: let s Optimize the Use of Carbon Resource Carbon To X Processes Carbon To X technologies are operated in more than 50 plants
World CTX Carbon To X Processes Processes and Commercial Operations World CTX: let s Optimize the Use of Carbon Resource Carbon To X Processes Carbon To X technologies are operated in more than 50 plants
Nuclear Hydrogen for Production of Liquid Hydrocarbon Transport Fuels
 Nuclear Hydrogen for Production of Liquid Hydrocarbon Transport Fuels Charles W. Forsberg Oak Ridge National Laboratory Oak Ridge, Tennessee 37831 Email: forsbergcw@ornl.gov Abstract Liquid fuels (gasoline,
Nuclear Hydrogen for Production of Liquid Hydrocarbon Transport Fuels Charles W. Forsberg Oak Ridge National Laboratory Oak Ridge, Tennessee 37831 Email: forsbergcw@ornl.gov Abstract Liquid fuels (gasoline,
PRISM Membrane Systems for petrochemical applications... tell me more
 PRISM Membrane Systems for petrochemical applications... tell me more Air Products PRISM Membrane Systems are found in petrochemical plants around the world operating efficiently and economically. PRISM
PRISM Membrane Systems for petrochemical applications... tell me more Air Products PRISM Membrane Systems are found in petrochemical plants around the world operating efficiently and economically. PRISM
Pilot Scale Production of Mixed Alcohols from Wood. Supplementary Information
 Pilot Scale Production of Mixed Alcohols from Wood Supplementary Information Richard L. Bain, Kimberly A. Magrini-Bair, Jesse E. Hensley *, Whitney S. Jablonski, Kristin M. Smith, Katherine R. Gaston,
Pilot Scale Production of Mixed Alcohols from Wood Supplementary Information Richard L. Bain, Kimberly A. Magrini-Bair, Jesse E. Hensley *, Whitney S. Jablonski, Kristin M. Smith, Katherine R. Gaston,
PEP Review METHANOL PRODUCTION VIA TOYO PROCESS By Syed N. Naqvi (December 2011) ABSTRACT
 PEP Review 2011-14 METHANOL PRODUCTION VIA TOYO PROCESS By Syed N. Naqvi (December 2011) ABSTRACT This Review updates PEP Review 2001-14 by presenting a techno-economic evaluation of a methanol production
PEP Review 2011-14 METHANOL PRODUCTION VIA TOYO PROCESS By Syed N. Naqvi (December 2011) ABSTRACT This Review updates PEP Review 2001-14 by presenting a techno-economic evaluation of a methanol production
Production of synthesis gas from liquid or gaseous hydrocarbons, and the synthesis gas per se, are covered by group C01B 3/00.
 C10J PRODUCTION OF PRODUCER GAS, WATER-GAS, SYNTHESIS GAS FROM SOLID CARBONACEOUS MATERIAL, OR MIXTURES CONTAINING THESE GASES (synthesis gas from liquid or gaseous hydrocarbons C01B; underground gasification
C10J PRODUCTION OF PRODUCER GAS, WATER-GAS, SYNTHESIS GAS FROM SOLID CARBONACEOUS MATERIAL, OR MIXTURES CONTAINING THESE GASES (synthesis gas from liquid or gaseous hydrocarbons C01B; underground gasification
USOO A United States Patent (19) 11 Patent Number: 6,159,440 Schoubye (45) Date of Patent: Dec. 12, 2000
 USOO61594.40A United States Patent (19) 11 Patent Number: 6,159,440 Schoubye (45) Date of Patent: Dec. 12, 2000 54 PROCESS FOR PRODUCTION OF OTHER PUBLICATIONS AMMONUM THOSULPHATE Thiosulfates, Kirk-Othmer
USOO61594.40A United States Patent (19) 11 Patent Number: 6,159,440 Schoubye (45) Date of Patent: Dec. 12, 2000 54 PROCESS FOR PRODUCTION OF OTHER PUBLICATIONS AMMONUM THOSULPHATE Thiosulfates, Kirk-Othmer
Chemistry Resource Kit
 This is a chapter from the Chemistry Resource Kit The Qenos Chemistry Resource kit has been developed as an information package for secondary students and others who wish to learn about Qenos, plastics
This is a chapter from the Chemistry Resource Kit The Qenos Chemistry Resource kit has been developed as an information package for secondary students and others who wish to learn about Qenos, plastics
United States Patent (19)
 United States Patent (19) Rudolph et al. (54) PROCESS OF GASIFYING SOLID FUELS 75) Inventors: Paul Rudolph, Bad Homburg; Rainer Reimert, Idstein; Osman Turna, Frankfurt am Main, all of Fed. Rep. of Germany
United States Patent (19) Rudolph et al. (54) PROCESS OF GASIFYING SOLID FUELS 75) Inventors: Paul Rudolph, Bad Homburg; Rainer Reimert, Idstein; Osman Turna, Frankfurt am Main, all of Fed. Rep. of Germany
THE PRODUCTION OF SULFURIC ACID
 THE PRODUCTION OF SULFURIC ACID 9.5.3 Present information to describe the steps and chemistry involved in the industrial production of sulfuric acid and analyse the process to predict ways in which the
THE PRODUCTION OF SULFURIC ACID 9.5.3 Present information to describe the steps and chemistry involved in the industrial production of sulfuric acid and analyse the process to predict ways in which the
11. Air and Water. The composition of air is as follows: The fractional distillation of liquid air. The process has 2 fundamental stages:
 The composition of air is as follows: The fractional distillation of liquid air The process has 2 fundamental stages: 1. Air is cooled to a very low temperature so that most of its components liquefy.
The composition of air is as follows: The fractional distillation of liquid air The process has 2 fundamental stages: 1. Air is cooled to a very low temperature so that most of its components liquefy.
United States Patent (19) Winkler et al.
 United States Patent (19) Winkler et al. 54 METHOD AND DEVICE FORSCRUBBING FLUE GASES 75 Inventors: Hermann Winkler, Recklinghausen; Marion Neumann, Linen, both of Germany 73 Assignee: Steag Aktiengesellschaft,
United States Patent (19) Winkler et al. 54 METHOD AND DEVICE FORSCRUBBING FLUE GASES 75 Inventors: Hermann Winkler, Recklinghausen; Marion Neumann, Linen, both of Germany 73 Assignee: Steag Aktiengesellschaft,
Solid State Ammonia Synthesis NHThree LLC
 Solid State Ammonia Synthesis NHThree LLC Jason C. Ganley John H. Holbrook Doug E. McKinley Ammonia - A Sustainable, Emission-Free Fuel October 15, 2007 1 Inside the Black Box: Steam Reforming + Haber-Bosch
Solid State Ammonia Synthesis NHThree LLC Jason C. Ganley John H. Holbrook Doug E. McKinley Ammonia - A Sustainable, Emission-Free Fuel October 15, 2007 1 Inside the Black Box: Steam Reforming + Haber-Bosch
Chapter 13. Thermal Conversion Technologies. Fundamentals of Thermal Processing
 Chapter 13 Thermal Conversion Technologies Fundamentals of Thermal Processing Thermal processing is the conversion of solid wastes into gaseous, liquid and solid conversion products with the concurrent
Chapter 13 Thermal Conversion Technologies Fundamentals of Thermal Processing Thermal processing is the conversion of solid wastes into gaseous, liquid and solid conversion products with the concurrent
PLASMA ARC THE LEADING LIGHT?
 http://www.waste-management-world.com/articles/print/volume-11/issue-6/features/plasma-arc-the-leading-light.html PLASMA ARC THE LEADING LIGHT? 11/01/2010 Various thermal processes are now available for
http://www.waste-management-world.com/articles/print/volume-11/issue-6/features/plasma-arc-the-leading-light.html PLASMA ARC THE LEADING LIGHT? 11/01/2010 Various thermal processes are now available for
Jing Su and Chang-Won Park Dept. of Chemical Engineering, University of Florida, Gainesville, FL 32611
 A Compact Reformer for Portable Fuel Cells Jing Su and Chang-Won Park Dept. of Chemical Engineering, University of Florida, Gainesville, FL 32611 Abstract A compact reformer to generate hydrogen for portable
A Compact Reformer for Portable Fuel Cells Jing Su and Chang-Won Park Dept. of Chemical Engineering, University of Florida, Gainesville, FL 32611 Abstract A compact reformer to generate hydrogen for portable
CO 2 RECOVERY FROM CO 2 REMOVAL UNIT AT GL1Z PLANT
 CO 2 RECOVERY FROM CO 2 REMOVAL UNIT AT GL1Z PLANT Hocine Friha Chemical Engineer Technical Department GL1Z/ Sonatrach Bethioua, Oran, Algeria hfriha@avl.sonatrach.dz ABSTRACT Algeria which has ratified
CO 2 RECOVERY FROM CO 2 REMOVAL UNIT AT GL1Z PLANT Hocine Friha Chemical Engineer Technical Department GL1Z/ Sonatrach Bethioua, Oran, Algeria hfriha@avl.sonatrach.dz ABSTRACT Algeria which has ratified
Fuels. N4 & N5 Homework Questions
 St Peter the Apostle High school Chemistry Department Fuels N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
St Peter the Apostle High school Chemistry Department Fuels N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
HYL III: Status And Trends
 HYL III: Status And Trends by Raúl Quintero, President HYL Technology Division, Hylsa, S.A. de C.V. presented at the Gorham/Intertech Conference on Iron & Steel Scrap, Scrap Substitutes and Direct Steel
HYL III: Status And Trends by Raúl Quintero, President HYL Technology Division, Hylsa, S.A. de C.V. presented at the Gorham/Intertech Conference on Iron & Steel Scrap, Scrap Substitutes and Direct Steel
Simulation of methanol synthesis from syngas obtained through biomass gasification using Aspen Plus
 6th International Conference on Sustainable Solid Waste Management (NAXOS 2018) Simulation of methanol synthesis from syngas biomass gasification using Aspen Plus M. Puig-Gamero, J. Argudo-Santamaria,
6th International Conference on Sustainable Solid Waste Management (NAXOS 2018) Simulation of methanol synthesis from syngas biomass gasification using Aspen Plus M. Puig-Gamero, J. Argudo-Santamaria,
DISCLAIMER. and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
 ER QUICK-SETTING CONCRETE AND A METHOD FOR MAKTNG QUICK-SETTING CONCRETE Arun S. Wagh Dileep S h g h Jose D. Pullockaran Lerry Knox DISCLAIMER This report was prepared as an account of work sponsored by
ER QUICK-SETTING CONCRETE AND A METHOD FOR MAKTNG QUICK-SETTING CONCRETE Arun S. Wagh Dileep S h g h Jose D. Pullockaran Lerry Knox DISCLAIMER This report was prepared as an account of work sponsored by
NON THERMAL PLASMA CONVERSION OF PYROGAS INTO SYNTHESIS GAS
 NON THERMAL PLASMA CONVERSION OF PYROGAS INTO SYNTHESIS GAS Fela Odeyemi, Alexander Rabinovich, and Alexander Fridman Mechanical Engineering and Mechanics Department, Drexel University, Philadelphia PA
NON THERMAL PLASMA CONVERSION OF PYROGAS INTO SYNTHESIS GAS Fela Odeyemi, Alexander Rabinovich, and Alexander Fridman Mechanical Engineering and Mechanics Department, Drexel University, Philadelphia PA
Meyer Steinberg Vice President and Chief Scientist HCE LLC, Melville, NY
 The Highly Efficient Integrated Plasma Fuel Cell (IPFC) Energy Cycle for Conversion of Fossil and Biomass Fuels to Electric Power Generation and Hydrogen and Liquid Transportation Fuel Production with
The Highly Efficient Integrated Plasma Fuel Cell (IPFC) Energy Cycle for Conversion of Fossil and Biomass Fuels to Electric Power Generation and Hydrogen and Liquid Transportation Fuel Production with
II uii IIi
 1111111111111111111111111111111111111111111111111111111111111111111111 II uii IIi (12) United States Patent Magonski et al. (10) Patent No.: US 8,778,550 B2 (45) Date of Patent: Jul. 15, 2014 (54) BATTERY
1111111111111111111111111111111111111111111111111111111111111111111111 II uii IIi (12) United States Patent Magonski et al. (10) Patent No.: US 8,778,550 B2 (45) Date of Patent: Jul. 15, 2014 (54) BATTERY
United States Patent (19) Stokes
 United States Patent (19) Stokes 11) Patent Number: () Date of Patent: Nov. 12, 1985 54 PROCESS FORTREATMENT OF UREA PLANT PROCESS CONDENSATE 75 Inventor: Keith J. Stokes, Weston, Conn. 73 Assignee: James
United States Patent (19) Stokes 11) Patent Number: () Date of Patent: Nov. 12, 1985 54 PROCESS FORTREATMENT OF UREA PLANT PROCESS CONDENSATE 75 Inventor: Keith J. Stokes, Weston, Conn. 73 Assignee: James
Research and Development Initiatives of WRI
 Research and Development Initiatives of WRI Presented at COAL GASIFICATION: WHAT DOES IT MEAN FOR WYOMING? February 28, 2007 www.westernresearch.org Who is WRI? WRI is a 501 (c) 3 research, technology
Research and Development Initiatives of WRI Presented at COAL GASIFICATION: WHAT DOES IT MEAN FOR WYOMING? February 28, 2007 www.westernresearch.org Who is WRI? WRI is a 501 (c) 3 research, technology
Green Chemistry Five ways in which the Chemical industry can become Greener Changing to renewable sources Use of alternatives to hazardous chemicals
 Green Chemistry Green Chemistry refers to the processes in the chemical industry that are being reinvented to make them more sustainable. The term sustain means to keep going. If we use resources faster
Green Chemistry Green Chemistry refers to the processes in the chemical industry that are being reinvented to make them more sustainable. The term sustain means to keep going. If we use resources faster
Pyrometallurgy of iron is still the most important pyrometallurgical process economically.
 1 Pyrometallurgy of iron is still the most important pyrometallurgical process economically. Prehistorically, iron was prepared by simply heating it with charcoal in a fired clay pot. Coke is coal that
1 Pyrometallurgy of iron is still the most important pyrometallurgical process economically. Prehistorically, iron was prepared by simply heating it with charcoal in a fired clay pot. Coke is coal that
Partial Oxidation of Methane to Form Synthesis Gas in a Tubular AC Plasma Reactor
 Partial Oxidation of Methane to Form Synthesis Gas in a Tubular AC Plasma Reactor T.A. Caldwell, H. Le, L.L. Lobban, and R.G. Mallinson Institute for Gas Utilization and Technologies, School of Chemical
Partial Oxidation of Methane to Form Synthesis Gas in a Tubular AC Plasma Reactor T.A. Caldwell, H. Le, L.L. Lobban, and R.G. Mallinson Institute for Gas Utilization and Technologies, School of Chemical
WEATHER MODIFICATION METHOD
 1 of 6 6/16/2012 10:06 PM ( 1 of 1 ) United States Patent 3,613,992 Knollenberg October 19, 1971 WEATHER MODIFICATION METHOD Abstract The present invention provides a method for producing rain or snow
1 of 6 6/16/2012 10:06 PM ( 1 of 1 ) United States Patent 3,613,992 Knollenberg October 19, 1971 WEATHER MODIFICATION METHOD Abstract The present invention provides a method for producing rain or snow
Ammonia plants. Flexible solutions for all feedstocks.
 Ammonia plants. Flexible solutions for all feedstocks. Meeting the challenges of a volatile marketplace. 03 Meeting the challenges of a volatile marketplace. With volatility in worldwide energy prices
Ammonia plants. Flexible solutions for all feedstocks. Meeting the challenges of a volatile marketplace. 03 Meeting the challenges of a volatile marketplace. With volatility in worldwide energy prices
Pathways & industrial approaches for utilization of CO 2
 Pathways & industrial approaches for utilization of CO 2 - by Dr. S. Sakthivel Background: CO 2 is a greenhouse gas and to reduce greenhouse effect, the CO 2 emissions need to be controlled. Large scale
Pathways & industrial approaches for utilization of CO 2 - by Dr. S. Sakthivel Background: CO 2 is a greenhouse gas and to reduce greenhouse effect, the CO 2 emissions need to be controlled. Large scale
The methanol synthesis. Antal Tungler Emeritus professzor MTA Centre for Energy Research 2017
 The methanol synthesis Antal Tungler Emeritus professzor MTA Centre for Energy Research 2017 BME Contents: Introduction History of the methanol and it s uses Properties of the methanol Methanol Production
The methanol synthesis Antal Tungler Emeritus professzor MTA Centre for Energy Research 2017 BME Contents: Introduction History of the methanol and it s uses Properties of the methanol Methanol Production
(52) U.S. Cl. 60/643
 (19) United States 11111 111111 111111 11111 11111 ii!121 111c111159181!!!1111 EH 11111 111111 1111 I111 1111 (12) Patent Application Publication (10) Pub. No.: US 2003/0005698 Al Keller (43) Pub. Date:
(19) United States 11111 111111 111111 11111 11111 ii!121 111c111159181!!!1111 EH 11111 111111 1111 I111 1111 (12) Patent Application Publication (10) Pub. No.: US 2003/0005698 Al Keller (43) Pub. Date:
Crude Oil National 4
 Fuels National 4 A fuel is a chemical which burns to give out energy. When a fuel burns the chemical reaction is known as combustion. When combustion takes place the fuel is reacting with oxygen from the
Fuels National 4 A fuel is a chemical which burns to give out energy. When a fuel burns the chemical reaction is known as combustion. When combustion takes place the fuel is reacting with oxygen from the
HIGH PUITY CARBON MONOXIDE FROM A FEED GAS ARNOLD KELLER AND RONALD SCHENDEL KINETICS TECHNOLOGY INTERNATIONAL CORPORATION MONROVIA, CALIFORNIA
 THE USE OF COSORB R II TO RECOVER HIGH PUITY CARBON MONOXIDE FROM A FEED GAS BY ARNOLD KELLER AND RONALD SCHENDEL KINETICS TECHNOLOGY INTERNATIONAL CORPORATION MONROVIA, CALIFORNIA PRESENTED AT AICHE SUMMER
THE USE OF COSORB R II TO RECOVER HIGH PUITY CARBON MONOXIDE FROM A FEED GAS BY ARNOLD KELLER AND RONALD SCHENDEL KINETICS TECHNOLOGY INTERNATIONAL CORPORATION MONROVIA, CALIFORNIA PRESENTED AT AICHE SUMMER
CO 2 Capture and Storage: Options and Challenges for the Cement Industry
 CO 2 Capture and Storage: Options and Challenges for the Cement Industry Martin Schneider, Düsseldorf, Germany CSI Workshop Beijing, 16 17 November 2008 CO 2 abatement costs will tremendously increase
CO 2 Capture and Storage: Options and Challenges for the Cement Industry Martin Schneider, Düsseldorf, Germany CSI Workshop Beijing, 16 17 November 2008 CO 2 abatement costs will tremendously increase
Chapter 5: Extent of Reaction
 Chapter 5: Extent of Reaction Accumulation = input - output + generation - consumption Steady state system: 0 = input - output + generation - consumption output = input + generation - consumption Input
Chapter 5: Extent of Reaction Accumulation = input - output + generation - consumption Steady state system: 0 = input - output + generation - consumption output = input + generation - consumption Input
DESCRIPTION (OCR text may contain errors)
 DESCRIPTION (OCR text may contain errors) United States Patent lnventor Robert G. Knollenberg Mattoon, Ill. Appl. No. 538,904 Filed Mar. 25, 1966 Patented Oct. 19, 1971 Assignee The United States of America
DESCRIPTION (OCR text may contain errors) United States Patent lnventor Robert G. Knollenberg Mattoon, Ill. Appl. No. 538,904 Filed Mar. 25, 1966 Patented Oct. 19, 1971 Assignee The United States of America
OC22 Show that approximately one fifth of the air is oxygen; show that there is CO 2 and water vapour in air
 Chemistry: 10. Air and Oxygen Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC21 Understand that air is a mixture of gases, and state
Chemistry: 10. Air and Oxygen Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC21 Understand that air is a mixture of gases, and state
Information Centre Nitric Acid Plants. Kittiwake Procal Ltd Page 1 of 6
 Information Centre Kittiwake Procal Ltd Page 1 of 6 Nitric Acid Nitric acid is a strong highly corrosive and toxic acid. Pure nitric acid is colourless but aged solutions can appear yellow due to oxidation.
Information Centre Kittiwake Procal Ltd Page 1 of 6 Nitric Acid Nitric acid is a strong highly corrosive and toxic acid. Pure nitric acid is colourless but aged solutions can appear yellow due to oxidation.
Flue-Gas Treatment by Methane Tri-Reforming Combined with Lime Carbonation and Syngas Production
 High Temperature Solid Looping Cycles Network, Oviedo, Spain, 15-17 Sept. 2009 Flue-Gas Treatment by Methane Tri-Reforming Combined with Lime Carbonation and Syngas Production M. Halmann 1 and A. Steinfeld
High Temperature Solid Looping Cycles Network, Oviedo, Spain, 15-17 Sept. 2009 Flue-Gas Treatment by Methane Tri-Reforming Combined with Lime Carbonation and Syngas Production M. Halmann 1 and A. Steinfeld
REALIZING RENEWABLE ENERGY POTENTIAL
 REALIZING RENEWABLE ENERGY POTENTIAL BY Patrick Hirl, PE Renewable natural gas (RNG) is a universal fuel that enhances energy supply diversity; uses municipal, agricultural and commercial organic waste;
REALIZING RENEWABLE ENERGY POTENTIAL BY Patrick Hirl, PE Renewable natural gas (RNG) is a universal fuel that enhances energy supply diversity; uses municipal, agricultural and commercial organic waste;
EEC 503 Term Project No 2
 Overall Objective EEC 503 Term Project No 2 Spring 2012 In preparing this term paper you are expected to demonstrate: a) resourcefulness in (i) outlining of and focusing on the problem, (ii) using effectively
Overall Objective EEC 503 Term Project No 2 Spring 2012 In preparing this term paper you are expected to demonstrate: a) resourcefulness in (i) outlining of and focusing on the problem, (ii) using effectively
SULPHUR RECOVERY BY TAIL GAS TREATING TECHNOLOGY (MCRC PROCESS) MAXIMUM CLAUS
 International Journal of Science, Environment and Technology, Vol. 3, No 4, 2014, 1609 1613 ISSN 2278-3687 (O) SULPHUR RECOVERY BY TAIL GAS TREATING TECHNOLOGY (MCRC PROCESS) MAXIMUM CLAUS Sulabh Jangra
International Journal of Science, Environment and Technology, Vol. 3, No 4, 2014, 1609 1613 ISSN 2278-3687 (O) SULPHUR RECOVERY BY TAIL GAS TREATING TECHNOLOGY (MCRC PROCESS) MAXIMUM CLAUS Sulabh Jangra
Methanol Production by Gasification of Heavy Residues
 Methanol Production by Gasification of Heavy Residues by C. A. A. Higman Presented at the IChemE Conference "Gasification: An Alternative to Natural Gas" London, 22-23 23 November, 1995 Methanol Production
Methanol Production by Gasification of Heavy Residues by C. A. A. Higman Presented at the IChemE Conference "Gasification: An Alternative to Natural Gas" London, 22-23 23 November, 1995 Methanol Production
COMPREHENSIVE MSW PROCESSING STEPS BIO COKE METHOD
 COMPREHENSIVE MSW PROCESSING STEPS BIO COKE METHOD STEPS BIOCOKE METHOD FOR CONVERSION OF MSW TO COAL AND FUEL OIL STEPS BIOCOKE processing system is designed for converting the MSW which is received on
COMPREHENSIVE MSW PROCESSING STEPS BIO COKE METHOD STEPS BIOCOKE METHOD FOR CONVERSION OF MSW TO COAL AND FUEL OIL STEPS BIOCOKE processing system is designed for converting the MSW which is received on
Synthesis Gas Processes for Synfuels Production
 Synthesis Gas Processes for Synfuels Production Christopher Higman presented at EUROGAS '90 Trondheim, June 1990 Abstract Synthesis Gas Processes for Synfuels Production Christopher Higman Synthetic fuels
Synthesis Gas Processes for Synfuels Production Christopher Higman presented at EUROGAS '90 Trondheim, June 1990 Abstract Synthesis Gas Processes for Synfuels Production Christopher Higman Synthetic fuels
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 2011 O247457A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0247457 A1 Knop et al. (43) Pub. Date: (54) PROCESS FOR PRODUCTION OF DIRECT (52) U.S. Cl.... T5/392 REDUCED
(19) United States US 2011 O247457A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0247457 A1 Knop et al. (43) Pub. Date: (54) PROCESS FOR PRODUCTION OF DIRECT (52) U.S. Cl.... T5/392 REDUCED
Groll (45) Date of Patent: *Jun. 14, (54) MULTI-PLY COOKWARE WITH (56) References Cited
 (1) United States Patent USOO7960034B (10) Patent No.: US 7,960,034 B Groll (45) Date of Patent: *Jun. 14, 011 (54) MULTI-PLY COOKWARE WITH (56) References Cited COPPER-ALUMNUM-STANLESS STEEL U.S. PATENT
(1) United States Patent USOO7960034B (10) Patent No.: US 7,960,034 B Groll (45) Date of Patent: *Jun. 14, 011 (54) MULTI-PLY COOKWARE WITH (56) References Cited COPPER-ALUMNUM-STANLESS STEEL U.S. PATENT
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States US 2013 0123554A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0123554 A1 Ordomskiy et al. (43) Pub. Date: (54) ONE-STEP METHOD FOR BUTADIENE Publication Classification
(19) United States US 2013 0123554A1 (12) Patent Application Publication (10) Pub. No.: US 2013/0123554 A1 Ordomskiy et al. (43) Pub. Date: (54) ONE-STEP METHOD FOR BUTADIENE Publication Classification
Report No. 32 HYDROGEN. December A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE PARK, CALIFORNIA
 Report No. 32 HYDROGEN by RICHARD G. DENNEY December 1967 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 2 3 4 5 6 INTRODUCTION....
Report No. 32 HYDROGEN by RICHARD G. DENNEY December 1967 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I I MENLO PARK, CALIFORNIA CONTENTS 1 2 3 4 5 6 INTRODUCTION....
United States Patent (19) Asano et al.
 United States Patent (19) Asano et al. 54). POROUS MOLD MATERIAL FOR CASTING AND A METHOD OF PRODUCING THE SAME 75 Inventors: Norihiro Asano, Toyohashi; Tatsuhiko Kato, Shinshiro, both of Japan 73 ASSignee:
United States Patent (19) Asano et al. 54). POROUS MOLD MATERIAL FOR CASTING AND A METHOD OF PRODUCING THE SAME 75 Inventors: Norihiro Asano, Toyohashi; Tatsuhiko Kato, Shinshiro, both of Japan 73 ASSignee:
ChE 505 Chapter 1N revised 01/17/13
 ENVIRONMENTAL REACTION ENGINEERING 1.1 Introduction Chemical reactions play a key role in generation of pollutants (e.g. combustion of fossil fuels) as well as in pollution abatement (e.g. automobile exhaust
ENVIRONMENTAL REACTION ENGINEERING 1.1 Introduction Chemical reactions play a key role in generation of pollutants (e.g. combustion of fossil fuels) as well as in pollution abatement (e.g. automobile exhaust
(12) United States Patent
 (12) United States Patent USOO9682534B1 (10) Patent No.: Young et al. (45) Date of Patent: Jun. 20, 2017 (54) CHEMICAL RESISTANT WATERPROOFING (52) U.S. Cl. MEMBRANES CPC... B32B 15/09 (2013.01); B32B5/02
(12) United States Patent USOO9682534B1 (10) Patent No.: Young et al. (45) Date of Patent: Jun. 20, 2017 (54) CHEMICAL RESISTANT WATERPROOFING (52) U.S. Cl. MEMBRANES CPC... B32B 15/09 (2013.01); B32B5/02
Application Note. Hydrogen Purity Analysis by FTIR PROBLEM BACKGROUND
 Hydrogen Purity Analysis by FTIR PROBLEM Historically, hydrogen has been employed in a variety of industrial chemical processes. Typically, the purity requirements for this hydrogen have tolerated contaminant
Hydrogen Purity Analysis by FTIR PROBLEM Historically, hydrogen has been employed in a variety of industrial chemical processes. Typically, the purity requirements for this hydrogen have tolerated contaminant
The Promotional Materials for Guangdong Meixing Fueneng. Technology Co., Ltd.
 The Promotional Materials for Guangdong Meixing Fueneng Technology Co., Ltd. 1: Company Profile Owing to the project of Research and industrialization of key technologies for highefficiency hydrogen fuel
The Promotional Materials for Guangdong Meixing Fueneng Technology Co., Ltd. 1: Company Profile Owing to the project of Research and industrialization of key technologies for highefficiency hydrogen fuel
PRISM Membrane Systems for ammonia plants... tell me more
 PRISM Membrane Systems for ammonia plants... tell me more Air Products PRISM Membrane Systems are found in ammonia synthesis plants around the world operating efficiently and economically. PRISM Membrane
PRISM Membrane Systems for ammonia plants... tell me more Air Products PRISM Membrane Systems are found in ammonia synthesis plants around the world operating efficiently and economically. PRISM Membrane
NEW TECHNOLOGIES IN COAL-FIRED THERMAL POWER PLANTS FOR MORE EFFECTIVE WORK WITH LESS POLLUTION
 UDK 621.311.22:502.174 Dip.el.eng. Igor SEKOVSKI NEW TECHNOLOGIES IN COAL-FIRED THERMAL POWER PLANTS FOR MORE EFFECTIVE WORK WITH LESS POLLUTION Abstract Today people make a lot of analysis, of work of
UDK 621.311.22:502.174 Dip.el.eng. Igor SEKOVSKI NEW TECHNOLOGIES IN COAL-FIRED THERMAL POWER PLANTS FOR MORE EFFECTIVE WORK WITH LESS POLLUTION Abstract Today people make a lot of analysis, of work of
ENERGY. Energy. Power is energy over time. Power. Mechanical Energy. Types of Energy. Ability to do work Unit: Joule (J) J = (kg x m 2 )/s 2
 Energy ENERGY Ability to do work Unit: Joule (J) J = (kg x m 2 )/s 2 Reading: Supplemental Text Materials Chapter 11: pages 225-238 Power Power is energy over time Energy over time Watts (W) 1 W = 1J/s
Energy ENERGY Ability to do work Unit: Joule (J) J = (kg x m 2 )/s 2 Reading: Supplemental Text Materials Chapter 11: pages 225-238 Power Power is energy over time Energy over time Watts (W) 1 W = 1J/s
ENERGY. Reading: Supplemental Text Materials Chapter 11: pages
 ENERGY Reading: Supplemental Text Materials Chapter 11: pages 225-238 Energy Ability to do work Unit: Joule (J) J = (kg x m 2 )/s 2 Power Energy over time Watts (W) 1 W = 1J/s Power is energy over time
ENERGY Reading: Supplemental Text Materials Chapter 11: pages 225-238 Energy Ability to do work Unit: Joule (J) J = (kg x m 2 )/s 2 Power Energy over time Watts (W) 1 W = 1J/s Power is energy over time
