Humidity & Vapour Pressure (1990) (1991)
|
|
|
- Roy Ford
- 6 years ago
- Views:
Transcription
1 Humidity & Vapour Pressure 1(i). A mixture of Ethyl Acetate vapor and air has a relative saturation of 50 percent at 30 C and a total pressure of 100 kn / m2. If the vapor pressure of Ethyl Acetate at 30 C is 16 kn / m3, (a). The percentage of air is. (b). The molal saturation is.. (1990) 2. Fill in the blanks: (i). A wet paper pulp contains 75% water. After 100 kg of water removed in a dryer, it is found that the pulp is now containing 30% water. The weight of the original pulp is (ii). The weather bureau reports a dry bulb temperature of ambient air as 29 C and relative humidity of 80%. The barometer read 750 mm hg. The percentage humidity of ambient air is (Vapor pressure of water at 29 C = 30 mm Hg.). (iii). H 2 S is produced from the reaction, FeS + 2HCL FeCl 2 + H 2 S; 120 kg of FeS react with 150 kg of HCL and 0.5 kmol of H 2 S has been produced. The degree of completion of the reaction and the limiting reactant is (iv). The heat absorbed for isothermal reaction,c 4 H 10 (g) C 2 H 4 (g) + C 2 H 6 (g); At 298 k and 1 atm pressure is Standard heat of combustion, kj /mol: C 4 H 10 (g) = ; C 2 H 4 (g) = ,C 2 H 6 (g) = (1991) 2(e) An evaporator while concentrating an aqueous solution from 10 to 40% solids evaporates kg of water. The amount of solids handled by the system in kgs is (A) 4000, (B) 9000, (C) 4600, (D) 3000, 2(f) 1000 kg of wet solids are to be dried from 60% to 20% moisture (by weight). The mass of moisture removed in kg is (A) 520, (B) 200, (C) 400, (D) 500, 2(g) Assuming that CO 2 obeys perfect gas low, calculate the density of CO 2 (in kg/m 3, at 0 0 C and 2 atm.
2 (A) 1, (B) 2, (C) 3, (D) 4, 2(h) Pure O 2 is mixed with air to produce an enriched air containing 50 volume % of O 2. The ratio of moles of air to O 2 used is (1995) 2.17 In a mixture of benzene vapour and nitrogen gas at a total pressure of 900 mm Hg, if the absolute humidity of benzene is 0.2 Kg benzene/kg nitrogen, the partial pressure of benzene in mm Hg is : a. 180, b. 60.3, c. 720, d. 200, (1996) 7. CaCO3 slurry has to be dried. The drier is designed to remove 100 kg moisture per hour. Air at 20 C and 40% relative humidity, enters the drier and leaves at 650C and 65% relative humidity. What is the weight (in kg) of bone-dry air required per hour? The atmospheric pressure is 103 kpa. If the humidity of the air entering the drier can be varied, what is the minimum amount of dry air required? The constants for Antoine equation for vapour pressure of water in mm Hg may be taken as A = , B = , and C = (1999) 2.11 Fresh orange juice contains 12% (by weight) solids and the rest water. 90% of the fresh juice is sent to an evaporator to remove water and subsequently mixed with the remaining 10% of fresh juice. The resultant product contains 40% solids. The kg of water removed from 1 kg fresh juice is (2002) A) 0.4 B) 0.5 C) 0.6 D) ) Air at a temperature of 20 0 C & 750 mm Hg pressure has a relative humidity of 80%. What is its percentage humidity? Vapour pressure of water at 20 0 C is 17.5 mm Hg. a) b) 80 c) d) (2003) 42. A vessel of volume 1000 m 3 contains air which is saturated with water vapour. The total pressure and temperature are 100 kpa and 20 0 C, respectively. Assuming that the vapour pressure of water at 20 0 C is 2.34 kpa, the amount of water vapour (in kg) in the vessel is approximately (A) 17 (B) 20 (C) 25 (D) 34
3 (2004) 4. A dehumidifier (shown below) is used to completely remove water vapor from air. Which ONE of the following statements is TRUE? (2009) A. Water is the ONLY tie component B. Air is the ONLY tie component, C. BOTH water and air are the components D. There are NO tie components Solutions 5(vi). A binary hydrocarbon liquid mixture of A and B (K = 1.5) containing 60 mole percent A in flash vaporized. The mole fraction of A in the liquid product is (1990) Q.37 The vapor-liquid equilibrium curve of a binary mixture A-B, may be approximated by a linear equation over a narrow range of liquid mole fractions ( 0.2 < xa < 0.3) as follows * ya xa ya is the mole fraction of A in the vapor. 100 moles of a feed ( xa,f = 0.28) is batch distilled to a final residue ( xa,w = 0.2). Using the Rayleigh equation, the number of moles of the residue left behind in the distillation unit, up to 2 digits after the decimal point, is (2013) Stoichiometry, Ideal Gas & Fundamentals 11(i). Pure propane (C5H8) is burnt in an excess of air to give following analysis of combustion products in volume percent: CO2 = 5.0, CO = 3.5, H2 O = 11.4, O2 = 7.0 and N2 = 73.1 Calculate the percentage of excess air used. (1990)
4 12(ii). Limestone mixed with coke is being burnt kiln. An average analysis of the limestone is CaCO 3 : 84.5%, MgCO 3 :11.5% and the rest inert. The coke contains 76% carbon, 21% ash and 3% moisture. The calculation of CaCO 3 is only 95% complete and that of MgCO 3 90%. The carbon in the coke is completely burnt of CO 2. The kiln is fed with 1 kg of coke per 5 kg limestone. Calculate weight percent CaO in the product leaving the kiln. (1991) 2(a). It is desired to make 100 kg of a solution containing 40% salt by mixing solution A containing 25% salt and solution B containing 50% salt. The mass in kg of solution A required is. 2(b). (1992) 1.2 g atoms of carbon and 1.5 g moles of oxygen are reacted to give 1 g mole of carbon dioxide. The limiting reactant is. The percent excess reactant supplied is. 11. The concentration of SO 2 in the flue gases from a boiler was found to be 0.2 kg/m 3 at N.T.P. Determine the concentration of SO 2 in parts per million at N.T.P. Assume that the gases are perfect. (1992) 12(a). The analysis of the gas entering the secondary converter in a contact Sulphuric acids plant 4% SO 2, 13% O 2 and 83% N 2 (volume %). In the converter SO 2 is oxidized to SO 3. The gases leaving converter 0.45% SO 2 on an SO 3 free basis (volume %). Calculate the percent conversion of SO 2. 12(b). Dry methane is burned with dry air. Both are at 25 C initially. The flame temperature is If complete combustion is assumed how much excess air is being used? The reaction is, CH 4 + 2O 2 CO 2 + 2H 2 O Standard heat of reaction = x 10 3 J/g mole of CH 4 reacted. Mean molal specific heat of gases between 25 C and 1300 are in J/(g mole) ( K). CO 2 = 51.88; H 2 O = 40.45; O 2 = 34.01; N 2 = 32.21; (1992)
5 2(a) kg of a solution containing 50% by weight of a salt dissolved in it is cooled. 400 kg of anhydrous salt is separated out. The solubility of the salt at the lower temperature is kg/100 kg of water is, (A). 80; (B). 50; (C). 40; (D). 20 2(b). Methane is completely burned with air. The possible volume percent of carbon dioxide (on dry basis) in the flue gases is, (A) (B) (C) (D) (1993) 12. Iron pyrites (FeS 2 ) is burned with air in 100% excess of that required to oxidize all iron to Fe 2 O 3 and all sulphur to sulphur dioxide. Calculate the composition of exit gases, if 80% of sulphur is oxidized to sulphur trioxide and the rest to sulphur dioxide. All iron is oxidized to Fe 2 O 3 (1993) 11. The Orsat analysis of a flue gas is CO % O 2 7.1% N2 80.2% Determine the percent excess air used in the combustion. The nitrogen present in the flue gas is contributed by air only. (1995) 11. A hydrocarbon is burnt with excess air. The Orsat analysis of the flue gas shows % CO 2, 3.78 % O 2 and % N 2. Calculate the atomic ratio of C : H in the hydrocarbon and the % excess air. (1996) 12. Methanol vapour can be converted into formaldehyde by the following reaction scheme CH 3 OH + ½ O 2 HCHO + H 2 O ; CH 3 OH HCHO + H 2
6 The fresh feed to the process was 0.5 kg mol/ h O 2 and an excess methanol. All of the O 2 reacts in the reactor. Formaldehyde and water are removed from the product stream first, after which H 2 is removed from the recycled methanol. The recycle flow rate of methanol was 1 kg mol/h. The ratio of methanol reacting by decomposition to that by oxidation was 3. Draw the flow diagram and then calculate the per pass conversion of methanol in the reactor and the fresh feed rate of methanol (1996) 2.4 Pure carbon is com,pletelly burnt in oxygen. The flue gas analysis is 70% CO 2, 20% CO and 10% O 2. The percent excess oxygen used is A) 20, B) 12.5, C) 0, D) 10, (1997) 2.7 A sample of well water contains 140 g/m 3 Ca 2+ ions and 345 g/m 3 Na + ions. The hardness of the sample of water, expressed in terms of equivalent CaCO 3 in g/m 3 [assuming atomic masses of Ca : 40, Na : 23, C : 12 and O : 16 ] is A) 350, B) 485, C) 140, D) 345, (1998) kg/h of an aqueous solution of 20% Na 2 CO 3 is cooled gradually to t 0 C, to crystallize out Na 2 CO H 2 O. The solubility of Na 2 CO 3 at t 0 C is 2.1%. Calculate the percentage of Na 2 CO 3 recovered in the form of crystals. (Assume no loss of Na 2 CO 3 through the mother liquor adhering to the crystals and no carry over of crystals with the mother liquor). Draw a neat block diagram showing the inlet and exit compositions and flow rates. [ Molecular weight of Na 2 CO 3 can be assumed to be 106 and that of water to be 18 ]. (1998) 1.5. A solution of specific gravity 1.0 consists of 35% A by weight and the remaining B. If the specific gravity; of A is 0.7, the specific gravity of B is A) 1.25, B) 1.3, C) 1.35, D) 1.2, (1999) 1,7 The molar composition of a gas is 10% H 2, 10% O 2, 30% CO 2 and balance H 2 O. If 50% H 2 O condenses, the final mole percent of H 2 in the gas on a dry basis will be A) 10 %, B) 5 %, C) %, D) 20 %, (2000)
7 1.11 Methane is mixed with stoichiometric proportion of oxygen and completely combusted. The number of additional specifications required to determine the product flow rate and composition is A) 0 B) 1 C) 2 D) 3 (2002) 38) 6 g of carbon is burnt with an amount of air containing 18 g oxygen. The product contain 16.5 g CO 2 and 2.8 g CO besides other constituents. What is the degree of conversion on the basis of disappearance of the limiting reactant? a) 100% b) 95 % c) 75 % d) 20 % (2003) 39) An aqueous solution of 2.45% by weight H 2 SO 4 has a specific gravity of The composition expressed in normality is a) b) c) d) (2003) 42) A sample of natural gas containing 80% Methane (CH 4 ) and the rest Nitrogen (N 2 ) is burnt with 20% excess air. With 80% of the combustibles producing CO 2 and the remainder going to CO the Orsat analysis in volume percent is a) CO 2 : 6.26 CO : 1.56 O 2 : 3.91 H 2 0 : N 2 : b) CO 2 : 7.42 CO : 1.86 O 2 : 4.64 N 2 : c) CO 2 : 6.39 CO : 1.60 O 2 : 3.99 H 2 0 : N 2 : d) CO 2 : 7.60 CO : 1.90 O 2 : 4.75 N 2 : (2003) 6. The weight fraction of methanol in an aqueous solution is The mole fraction of methanol X M satisfies. (A) X M < 0.5 (B) X M = 0.5 (C) 0.5 < X M < 0.64 (D) X M > 0.64 (2004) Statement for Linked Answer Questions 80 & 81 : kg of C 3 H 8 is burnt with 1160 kg of air (Mol. Wt. = 29) to produce 88 kg of CO 2 and 14 kg of CO C 3 H O 2 = 3 CO H 2 O
8 What is the percent excess air used? A) 55 B) 60 C) 65 D) What is the % carbon burnt? A) 63.3 B) 73.3 C) 83.3 D) 93.3 (2007) 31. Air (79 mole % nitrogen and 21 mole % oxygen) is passed over a catalyst at high temperature. Oxygen completely reacts with nitrogen as shown below, 0.5 N 2(g) O 2(g) NO (g) 0.5 N 2(g) + O 2(g) NO 2(g) The molar ratio of NO to NO 2 in the product stream is 2:1. The fractional conversion of nitrogen is A) 0.13 B) 0.20 C) 0.27 D) 0.40 (2008) Common Data for Questions 71, 72 and 73 : Methane and steam are fed to a reactor in molar ratio 1 : 2. The following reactions take place, CH 4(g) + 2H 2 O (g) CO 2(g) + 4H 2(g) CH 4(g) + H 2 O (g) CO (g) + 3H 2(g) where CO 2 is the desired product, CO is the undesired product and H 2 is a byproduct. The exit stream has the following composition Species CH 4 H 2 O CO 2 H 2 CO Mole % The selectivity for desired product relative to undesired product is A) 2.3 B) 3.5 C) 7 D) The fractional yield of CO 2 is (where fractional yield is defined as the ratio of moles of the desired product formed to the moles that would have been formed if there were no side reactions and the limiting reactant had reacted completely) A) 0.7 B) 0.88 C) 1 D) The fractional conversion of methane is A) 0.4 B) 0.5 C) 0.7 D) 0.8 (2008) Q.32 A saturated solution at 30 C contains 5 moles of solute (M.W.=50 kg/kmol) per kg of solvent (M.W.=20 kg/kmol). The solubility at 100 C is 10 moles of the solute per kg of the solvent. If 10 kg of the original solution is heated to 100 C, then the weight of the additional solute that can be dissolved in it, is (A) 0.25 kg (B) 1 kg (C) 2 kg (D) 3.34 kg
9 (2010) Q.33 The products of combustion of methane in atmospheric air (21% O 2 and 79% N 2 ) have the following composition on a dry basis : Products Mole % CO O CO 0.53 N The ratio of the moles of CH 4 to the moles of O 2 in the feed stream is (A) 1.05 (B) 0.60 (C) 0.51 (D) 0.45 (2010) Q. 32 The following combustion reactions occur when methane is burnt. CH O 2 CO H 2 O 2 CH O 2 2 CO + 4 H 2 O 20 % excess air is supplied to the combustor. The conversion of methane is 80 % molar ratio of CO to CO 2 in the flue gas is 1 : 3. Assume air to have 80 mol % N 2 and rest O 2. The O 2 consumed as a PERCENTAGE of O 2 entering the combustor is (A) 20 (B) 62.5 (C) 80 (D) 83.3 (2011) Material Balance 11(ii). For the reaction A B, the process flow diagram is shown in figure.1.the fresh feed of A contents 0.5 % of inert by volume. 80% conversion per pass of A fed to the reactor is obtained. The concentration of inert going into the reactor (after mixing with the recycle stream) must be held at 2% by volume. All streams are ideal gases and the process is at steady state. How many moles need to be recycled per mole of total feed to the reactor at (1). (1990)
10 6. Methanol is produced by the reaction of CO with H 2 CO + H 2 CH 3 OH Only 15% of carbon monoxide entering the reactor is converted to methanol. The methanol formed is condensed and recovered completely. The unreacted CO and H 2 are recycled back to the reactor. The feed will contain H 2 and CO in the ratio of 2 : 1. For 3200 kg/hr of methanol produced, calculate I. Kg mole/hr of fresh feed, II. Kg mole/hr of recycle gas, Mole. Weight of CH 3 OH = 32. (1995) 2.2 The reaction A + B C has been conducted in a reactor as shown
11 2.2 (i) The number of boundaries around which material balances can be written are a. 1, b. 6, c. 3, d. 4, 2.2 (ii) The number of independent balances (material) that can be made around the reactor are a. 1, b. 2, c. 3, d. 4, (1996) 1.5 In the system as shown in Fig. 1.5 each stream contains three components. The maximum number of independent material balances is
12 A) 3, B) 4, C) 6, D) 9, (1997) 2.5 A flow sheet is given in Fig If the single-pass (once-through) conversion of A to B is 20%. Then the rate of recycle R (moles/hr) is A) 300, B) 400, C) 500, D) 600, (1997) 12. Sea water is desalinated by reverse osmosis as shown in Fig. 12.
13 All compositions are on mass basis. Calculate R/E. (1997) 3. Ethylene Oxide is produced by the oxidation of Ethylene over a catalyst. Safety considerations dictate that the gaseous mixture entering the reactor should contain 10 mol Air per mol Ethylene. The conversion per pass is 22%. The Ethylene oxide formed is completely condensed out and the remaining gases recycled. Make up oxygen is added to maintain the requisite oxygen levels. For a plant producing 440 kg/h of Ethylene oxide. (a) Calculate the quantity of pure makeup oxygen to be supplied, in kg/h, in steady; state operation, (b) Draw a neat block diagram showing the major units, flows and compositions, and indicate the envelope / boundary around which the requisite mass balance(s) is/are being made. The relevant reaction is represented by 2 C 2 H 4 + O 2 2 C 2 H 4 O (g) (g) (g) [ Assume atomic masses as : C = 12, 0 = 16, H = 1 ] (5) (1998) 6. It is proposed to produce acetaldehyde by oxidation of ethanol in gas phase C 2 H 5 OH (g) + ½ O 2 (g) CH 3 CHO (g) + H 2 O (g)
14 The ratio of air to ethanol in the fresh feed (before it is mixed with recycle stream) is 10 to 1. The conversion of ethanol on a single pass through the reactor is 25%. The unreacted ethanol is completely separated from the reaction products and recycled. What is the ratio of recycle stream to the fresh feed stream? What is the composition of the outlet stream from the reactor in mass fraction and mole fraction? (1999) 19. The reaction A 2B + C takes place in a catalytic reactor (see diagram below). The reactor effluent is sent to a separator. The overall conversion of A is 95%. The product stream from the separator consists of B, C and 0.5% of A entering the separator, while the recycle stream consists of the remainder of the unreacted A and 1% of B entering the separator. Calculate the single pass conversion of A in the reactor. molar ratio of recycle to feed. (2000) 2.3 A butane isomerization process produces 70 kmol/h of pure isobutane. A purge stream removed continuously contains 85% n butane and 15% impurity (mole %). The feed stream is n- butane containing 1% impurity (mole %). The flow rate of the purge stream will be A) 3 kmol/h B) 4 kmol/h C) 5 kmol/h D) 6 kmol/h (2001)
15 CH-5 The process schematic of a propane dehydrogenation plant is shown below. It is desired to set up a simplified version of the material balance for this plant. Assume that the only reaction is the dehydrogenation of propane to propylene there are no side reactions. The yield of propylene per pass is 30% (i.e., 30% of the propane entering the reactor is converted to propylene). Assume that the amount of carbon formed on the catalyst is negligible. The product flow rate (stream S 5 ) is 50 kmol/h. Calcutta the flow rtes of all the other streams. Notice that all streams except stream S, are pure. (2001) kg of saturated aqueous solution of a highly soluble component A at 60 0 C is cooled to 25 0 C. The solubility limits of A are (0.6 kg A)/(kg water) at 60 0 C and (0.2 kg A)/(kg water) at 25 0 C. The amount, is kgs, of the crystals formed is A) 0.4 B) 0.25 C) 0.2 D) (2002) 41) Na 2 SO 4, 10 H 2 O crystals are formed by cooling 100 kg of 30% by weight aqueous solution of Na 2 SO 4. The final concentration of the solute in the solution is 10%. The weight of crystals is a) 20 b) 32.2 c) d) (2003)
16 5. A distillation column separates 10,000 kg/h of a benzene-toluene mixture as shown in the figure below. In the figure, X F, X D, and X W represent the weight fraction of benzene in the feed, distillate, and residue, respectively. The reflux ratio is (a) 0.5 (B) 0.6 (C) 1.0 (D) 2.0 (2004) kg of Na 2 SO 4 (molecular weight = 142) is present in 330 kg of an aqueous solution. The solution is cooled such that 80 kg of Na 2 SO H 2 O crystals separate out. The weight fraction of Na 2 SO 4 in the remaining solution is (A) 0.00 (B) 0.18 (C) 0.24 (D) 1.00 (2004) 5. A process flow sheet analysis results in the degrees of freedom having a value of 2, which one of the following steps must be next carried out? (a) Identify and add two new independent equations from process model (b) Remove two equations that have been wrongly assumed to be independent (c) Assign values of two variables in the process. (d) Assign value to one variable and remove one equation that was wrongly assumed to be independent. (2005) 41. A metal recovery unit (MRU) of intake capacity 5000 kg/hr treats a liquid product from a plant and recovers 90% of the metal in the metal in the pure form. The unrecovered metal and its associated liquid are sent to a disposal unit along with the untreated product from the plant (see figure below). Find the flow rate (m6) and the weight fraction of the metal (w6). The liquid product flow rate is 7500 higher of composition 0.1 (wt fraction), Assume steady state.
17 (a) m 6 = 7500 kg/hr, w 6 = 0.0 (b) m 6 = 7050 kg/hr, w 6 = (c) m 6 = 4500 kg/hr, w 6 = (d) m 6 = 5600 kg/hr, w 6 = (2005) 42. In the triangular diagram represented below for a batch separation process, a stream F is mixed with a solvent B to produce products R and E. Substance A is the carrier liquid and C is the solute to be extracted. The amounts of B ad E are 1 kg and 1.20 kg respectively. The length FM is 3.1 and length FB is 8.5 units on the figure. The ratio R/E is estimated to be Note : Figure not to scale (a) (b) 2 (c) (d) 2.5 (2005) 43. A feed stream (S1) at 100 kg/hr and containing only A mixes with recycle stream S5 before entering the reactor (see figure below), where the reaction A B takes place. The operation is at steady state. The stream S3 leaving the reactor is separated, without either phase or
18 composition change, into two streams S4 and S5. If the mass fraction of B in S4 is 0.95 and total flow rate of S5 is 10 kg/hr, then the ratio of flow rates of streams (S3/S5), and the flow rate of A in S3 are, respectively. (a) 11 and 110 kg/hr (b) 24 and 240 kg/hr (c) 11 and 5.5 kg/hr (d) 70 and 330 kg/hr (2005) Statement for Linked Answer Questions 76 & 77 : Solvent C is used to extract solute B selectively from, 100 kg/hr feed mixture A+B in a steady state continuous process shown below. The solubility of C in the raffinate and the solubility of A in the extract are negligible. The extract is distilled to recover B in the bottom product. The overhead product is recycled to the extractor. The loss of solvent in the bottoms is compensated by make up solvent S d. The total flow rate of the solvent stream S going to the extractor is 50 kg/hr. The mass fractions (X i s) of some selected streams are indicated in the figure below.
19 76. Distillation bottoms flow rate W and solvent dosing rate S d in kg/hr are (A) W = 50, S d = 50 (B) W = 100, S d = 20 (C) W = 10, S d = 50 (D) W = 50, S d = Feed rate E to the distillation column and overhead product rate T in kg/hr are (A) E = 90, T = 40 (B) E = 80, T = 40 (A) E = 90, T = 50 (B) E = 45, T = 20 (2006) Statement for Linked Answer Questions 78 & 79 : 78. A simplified flowsheet is shown in the figure for production of ethanol from ethylene. The conversion of ethylene in the reactor is 30% and the scrubber following the reactor completely separates ethylene (as top stream) and ethanol and water as bottoms. The last (distillation) column gives an ethanol-water azeotrope (90 mol% ethanol) as the final product and water as waste. The recycle to purge ratio is 34.
20 The reaction is : C 2 H 4 (g) + H 2 O (g) C 2 H 5 OH (g) For an azeotrope product rate of 500 mols/hr, the recycle gas flowrate in mols/hr is A) 30 B) 420 C) 1020 D) For the same process, if fresh H 2 O feed to the reactor is 600 mol/hr and wash water for scrubbing is 20% of the condensables coming out of the reactor, the water flowrate in mols/hr from the distillation column as bottoms is A) 170 B) 220 C) 270 D) 430 (2007) 32. A 35 wt% Na 2 SO 4 solution in water, initially at 50 C, is fed to a crystallizer at 20 C. The product stream contains hydrated crystals Na 2 SO 4.10H 2 O in equilibrium with a 20 wt% Na 2 SO 4 solution. The molecular weights of Na 2 SO 4 and Na 2 SO 4.10H 2 O are 142 and 322, respectively. The feed rate of the 35% solution required to produce 500 kg/hr of hydrated crystals is A) 403 kg/ha B) 603 kg/hr C) 803 kg/hr D) 1103 kg/hr (2008) 34. Carbon black is produced by decomposition of methane : CH 4(g) C (s) + 2H 2 (g) The single pass conversion of methane is 60%. If fresh feed is pure methane and 25% of the methane exiting the reactor is recycled, then the molar ratio of fresh feed stream to recycle stream is A) 0.9 B) 9 C) 10 D) 90 (2008)
21 5. Dehydrogenation of ethane, C 2 H 6 (g) C 2 H 4 (g) + H 2 (g), is carried out in a continuous stirred tank reactor (CSTR). The feed is pure ethane. If the reactor exit stream contains unconverted ethane along with the products, then the number of degrees of freedom for the CSTR is (A) 1, (B) 2, (C) 3, (D) 4 (2009) 28. Pure water (stream W) is to be obtained from a feed containing 5 wt % salt using a desalination unit as shown below: If the overall recovery of pure water (through stream W) is 0.75 kg/kg feed, then the recycle ratio (R/F) is (A) 0.25 (B) 0.5 (C) 0.75 (D) 1.0 (2009) Common Data for Questions 55 and 56 : A flash distillation drum (see figure below) is used to separate a methanol-water mixture. The mole fraction of methanol in the feed is 0.5, and the feed flow rate is 1000 kmol/hr. The feed is preheated in a heater with heat duty Q h and is subsequently flashed in the drum. The flash drum can be assumed to be an equilibrium stage, operating adiabatically. The equilibrium relation between the mole fractions of methanol in the vapor and liquid phases is y* = 4 x. The ratio of distillate to feed flow rate is 0.5.
22 55. The mole fraction of methanol in the distillate is (A) 0.2 (B) 0.7 (C) 0.8 (D) If the enthalpy of the distillate with reference to the feed is 3000 kj/kmol, and the enthalpy of the bottoms with reference to the feed is 1000 kj/kmol, the heat duty of the preheater (Qh in kj/hr) is (A) 2x10 6 (B) 1x10 6 (C) 1x10 6 (D) 2x10 6 (2009) Q. 31 Ammonia is synthesized at 200 bar and 773 K by the reaction N 2 + 3H 2 2NH 3. The yield of ammonia is 0.45 mol/mol of fresh feed. Flow sheet for the process (along with available compositions) is shown below. The single pass conversion for H 2 in the reactor is 20%. The amount of H 2 lost in the purge as a PERCENTAGE of H 2 in fresh feed is (A) 10 (B) 20 (C) 45 (D) 55 (2011) Common Data for Questions 50 and 51 : The reaction A (liq) + B (gas) C (liq) + D (gas), is carried out in a reactor followed by a separator as shown below
23 Notation : Molar flow rate of fresh B is F FB Molar flow rate of A is F A Molar flow rate of recycle gas is F RG Molar fraction of B in recycle gas is Y RB Molar flow rate of purge gas is F PG Molar flow rate of C is F C Here, F FB = 2 mol/s; F A = 1 mol/s; F B /F A = 5 and A is completely converted. Q. 50 If Y RB = 0.3, the ratio of recycle gas to purge gas (F RG /F PG ) is (A) 2 (B) 5 (C) 7 (D) 10 Q. 51 If the ratio of recycle gas to purge gas (F RG /F PG ) is 4 then Y RB is (A) 3/8 (B) 2/5 (C) 1/2 (D) ¾ (2012) Common Data for Questions 48 and 49: A reverse osmosis unit treats feed water (F) containing fluoride and its output consists of a permeate stream (P) and a reject stream (R). Let CF, CP, and CR denote the fluoride concentrations in the feed, permeate, and reject streams, respectively. Under steady state conditions, the volumetric flow rate of the reject is 60 % of the volumetric flow rate of the inlet stream, and CF = 2 mg/l and CP = 0.1 mg/l. Q.48 The value of CR in mg/l, up to one digit after the decimal point, is Q.49 A fraction f of the feed is bypassed and mixed with the permeate to obtain treated water having a fluoride concentration of 1 mg/l. Here also the flow rate of the reject stream is 60% of the flow rate entering the reverse osmosis unit (after the bypass). The value of f, up to 2 digits after the decimal point, is (2013)
24 Energy Balance - Thermophysics 7. The heat reaction at 300 K and at one atmosphere pressure for the following gas phase reaction: A + 3B C; is 50,000 calories per mole of A converted. Data on the molar heat capacity at constant pressure (cal/mol.k) of the various components are: C p for A = x 10-3 T, T in K C p for B = 7 C p for C = 26 Calculate the heat of reaction at 500 K and at one atmosphere pressure (1994) 13. Bituminous coal with a calorific value of KJ/Kg is used for generating steam in a boiler. How much coal has to be burnt to generate 1 MW of energy. Efficiency of combustion is How much air is needed if 50% excess air is to be used. Assume that coal contains 87% carbon and 33% ash. (1995) 13. A feed at 1298 K, consisting of flue gas (CO 2, O 2 and N 2 ) and air, is passed through a bed of pure carbon. The two reactions that occur both go to completion. CO 2 (g) + C(s) 2CO (g), H 0 R at 298 K = 170 kj/mol O 2 (g) + 2C(s) 2CO (g), H 0 R at 298 K = kj/mol The combustor is adiabatic and the product gases exit at 1298 K. Calculate the required moles of CO 2 per mol of O 2 in the feed stream, so that the net heat generated is zero and the bed temperature remains constant at 1298 K. Data : Mean Molar Heat Capacities, Cpm Substance Cpm, kj/(mol)(k) C 0.02 O CO 0.03 CO (1997) 1.5 For the case of a fuel gas undergoing combustion with air, if the air/fuel ratio is increased, the adiabatic flame temperature will A) increase B) decrease C) increase or decrease depending on the fuel type D) not change (2001)
25 2.22 A rigid vessel, containing three moles of nitrogen gas at 30 0 C, is heated to C. Assume the average heat capacities of nitrogen to be Cp = 29.1 J/mol 0 C and Cv = 20.8 J/mol 0 C. The heat required, neglecting the heat capacity of the vessel, is A) J B) J C) 4576 J D) J (2002) 43) Heat capacity of air can be approximately expresses as Cp = x 10-3 T where Cp is in J/(mol)(K) and T is in K. The heat given off by 1 mole of air when cooled at 1 atmospheric pressure from C to C is a) kj b) kj c) kj d) kj (2003) Q are based on the data supplied in the paragraph below One mole of methane undergoes complete combustion in a stoichiometric amount of air. The reaction proceeds as CH 4 + 2O 2 CO 2 + 2H 2 O. Both the reactants and the products are in gas phase. H = kj/mol of methane. 40. Mole fraction of water vapour in the product gases is about (A) 0.19 (B) 0.33 (C) 0.40 (D) If the average specific heat of all the gases/vapour is 40 J/(mol k), the maximum temperature rise of the exhaust gses in C would be approximately equal to (A) 1225 (B) 1335 (C) 1525 (D) 1735 (2004, Both Stoichiometry and Thermophysics) kg/hr of saturated steam at 1 bar (enthalpy kj/kg) is mixed adiabatically with superheated steam at 450 C and 1 bar (enthalpy kj/kg). The product is superheated steam at 350 C and 1 bar (enthalpy kj/kg). The flow rate of the product is A) 711 kg/ha B) 1111 kg/hr C) 1451 kg/hr D) 2051 kg/hr (2008) Common Data for Questions 55 and 56 : A flash distillation drum (see figure below) is used to separate a methanol-water mixture. The mole fraction of methanol in the feed is 0.5, and the feed flow rate is 1000 kmol/hr. The feed is preheated in a heater with heat duty Q h and is subsequently flashed in the drum. The flash
26 drum can be assumed to be an equilibrium stage, operating adiabatically. The equilibrium relation between the mole fractions of methanol in the vapor and liquid phases is y* = 4 x. The ratio of distillate to feed flow rate is The mole fraction of methanol in the distillate is (A) 0.2 (B) 0.7 (C) 0.8 (D) If the enthalpy of the distillate with reference to the feed is 3000 kj/kmol, and the enthalpy of the bottoms with reference to the feed is 1000 kj/kmol, the heat duty of the preheater (Qh in kj/hr) is (A) 2x10 6 (B) 1x10 6 (C) 1x10 6 (D) 2x10 6 (2009, ALSO IN MATERIAL BALANCE) Energy Balance - Thermochemistry 1(ii). The following data on heats of combustion at 25 C are given Compound Heat of combustion at 25 C. n-heptane C7H10 (g) kj / mol Ethyl Alcohol C2H5OH (g) kj / mol Heats of formation of CO2 (g) and H2O (l) are -380 kj / mol and 280 kj / mol respectively. (a). The heat of formation of gaseous n-heptane at 25 C is (b). The heat of formation of gaseous Ethyl Alcohol at 25 C is.. (1990) 12. Pure CO is mixed with 100% excess air and burnt. Only 80% of CO burns. The reactants are at 1000C and the products are at 3000C. Calculate the amount of heat added or removed per Kg mole of CO fed to the reactor.
27 Data : Mean molal specific heats between 25 0 C and T 0 C (given below) in KJ Kg.mole.K are Gas T = C T = C CO CO O N Standard heat of formation at 250C in KJ/Kg mole are CO CO (1995) Q.35 Calculate the heat required (in kj, up to 1 digit after the decimal point) to raise the temperature of 1 mole of a solid material from 100 C to 1000 C. The specific heat (Cp) of the material (in J/mol-K) is expressed as Cp = T, where T is in K. Assume no phase change. (2013)
GATE Solution 2000 to 2015 GATE SOLUTION to Detailed solution of each question CHEMICAL ENGINEERING GATE SOLUTION
 SAMPLE STUDY MATERIAL GATE SOLUTION 000 to 015 Detailed solution of each question CHEMICAL ENGINEERING GATE SOLUTION Subject-wise reducing year CONTENTS GATE Solution 1. Process Calculations 1-19. Thermodynamics
SAMPLE STUDY MATERIAL GATE SOLUTION 000 to 015 Detailed solution of each question CHEMICAL ENGINEERING GATE SOLUTION Subject-wise reducing year CONTENTS GATE Solution 1. Process Calculations 1-19. Thermodynamics
S.E. (Chemical) (First Semester) EXAMINATION, 2012 PROCESS CALCULATIONS (2008 PATTERN) Time : Three Hours Maximum Marks : 100
 Total No. of Questions 12] [Total No. of Printed Pages 8 Seat No. [4162]-185 S.E. (Chemical) (First Semester) EXAMINATION, 2012 PROCESS CALCULATIONS (2008 PATTERN) Time : Three Hours Maximum Marks : 100
Total No. of Questions 12] [Total No. of Printed Pages 8 Seat No. [4162]-185 S.E. (Chemical) (First Semester) EXAMINATION, 2012 PROCESS CALCULATIONS (2008 PATTERN) Time : Three Hours Maximum Marks : 100
Furnace. 1. (10 points) mol/s 53.5% H2O CO2
 MEEBAL Exam 3 December 2012 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
MEEBAL Exam 3 December 2012 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
Problems in chapter 9 CB Thermodynamics
 Problems in chapter 9 CB Thermodynamics 9-82 Air is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet
Problems in chapter 9 CB Thermodynamics 9-82 Air is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet
Chapter 10 Material Balances for Processes Involving Reaction 10.1 Species Material Balances Processes Involving a Single Reaction
 Chapter 10 Material Balances for Processes Involving Reaction 10.1 Species Material Balances 10.1.1 Processes Involving a Single Reaction The material balance for a species must be augmented to include
Chapter 10 Material Balances for Processes Involving Reaction 10.1 Species Material Balances 10.1.1 Processes Involving a Single Reaction The material balance for a species must be augmented to include
CHEN-2120, Fall 2009 Exam 3 Open Book. Please print your name on this cover page and sign your name on this cover sheet as. Name.
 CHEN-2120, Fall 2009 Exam 3 Open Book Please print your name on every page! When you turn the exam in, you will separate it into individual problems and turn them in separately at the front table. You
CHEN-2120, Fall 2009 Exam 3 Open Book Please print your name on every page! When you turn the exam in, you will separate it into individual problems and turn them in separately at the front table. You
Chemical Engineering Principles-I Dr.Ali H.Abbar Answers: 2.6 Material Balance Problems Involving Multiple Units process flowsheet (flowchart)
 5. Methane burns with O 2 to produce a gaseous product that contains CH 4, O 2, CO 2, CO, H 2 O, and H 2. How many independent element balances can you write for this system? 6. Solve the problems (1,
5. Methane burns with O 2 to produce a gaseous product that contains CH 4, O 2, CO 2, CO, H 2 O, and H 2. How many independent element balances can you write for this system? 6. Solve the problems (1,
UNIVERSITY OF TORONTO FA CUL TY OF APPLIED SCIENCE AND ENGINEERING . DEPARTMENT OF CHEMICAL ENGINEERING AND APPLIED CHEMISTRY
 NAME: STUDENT NUMBER: ----------- UNIVERSITY OF TORONTO FA CUL TY OF APPLIED SCIENCE AND ENGINEERING. DEPARTMENT OF CHEMICAL ENGINEERING AND APPLIED CHEMISTRY CHE 208 Process Engineering, Fall-2017 EXAMINER:
NAME: STUDENT NUMBER: ----------- UNIVERSITY OF TORONTO FA CUL TY OF APPLIED SCIENCE AND ENGINEERING. DEPARTMENT OF CHEMICAL ENGINEERING AND APPLIED CHEMISTRY CHE 208 Process Engineering, Fall-2017 EXAMINER:
INDUSTRIAL STOICHIOMETRY II
 INDUSTRIAL STOICHIOMETRY II 3rd Semester, B.Sc. Chemical Engineering Session 2011 Delivered by: Mr. Usman Ali Department of Chemical Engineering University of Engineering & Technology, Lahore Units and
INDUSTRIAL STOICHIOMETRY II 3rd Semester, B.Sc. Chemical Engineering Session 2011 Delivered by: Mr. Usman Ali Department of Chemical Engineering University of Engineering & Technology, Lahore Units and
THE UNIVERSITY OF JORDAN
 THE UNIVERSITY OF JORDAN The University of Jordan Faculty of Engineering & Technology Chemical Engineering Department Fuel and Energy Material Balance Part 3: Combustion Reactions Dr.-Ing. Zayed Al-Hamamre
THE UNIVERSITY OF JORDAN The University of Jordan Faculty of Engineering & Technology Chemical Engineering Department Fuel and Energy Material Balance Part 3: Combustion Reactions Dr.-Ing. Zayed Al-Hamamre
Chapter 6. Multiphase Systems. Dr. M. A. A. Shoukat Choudhury Website:
 Chapter 6 Multiphase Systems Dr. M. A. A. Shoukat Choudhury Email: shoukat@buet.ac.bd Website: http://teacher.buet.ac.bd/shoukat/ Multiphase Systems Why Study? - Phase change operations such as freezing,
Chapter 6 Multiphase Systems Dr. M. A. A. Shoukat Choudhury Email: shoukat@buet.ac.bd Website: http://teacher.buet.ac.bd/shoukat/ Multiphase Systems Why Study? - Phase change operations such as freezing,
Michigan State University DEPARTMENT OF CHEMICAL ENGINEERING AND MATERIALS SCIENCE. ChE 321: Thermodynamics Spring 2017
 Michigan State University Name PID DEPARTMENT OF CHEMICAL ENGINEERING AND MATERIALS SCIENCE ChE 321: Thermodynamics Spring 2017 February 22, 2017, CLOSED NOTES Ver A. General Instructions Submit all problems
Michigan State University Name PID DEPARTMENT OF CHEMICAL ENGINEERING AND MATERIALS SCIENCE ChE 321: Thermodynamics Spring 2017 February 22, 2017, CLOSED NOTES Ver A. General Instructions Submit all problems
PLANT and PROCESS PRINCIPLES COMBUSTION PROCESSES
 PLANT and PROCESS PRINCIPLES COMBUSTION PROCESSES This work covers Outcome 4 of the syllabus for the Edexcel HNC/D module Plant Process Principles 21725P and part of the Engineering Council Certificate
PLANT and PROCESS PRINCIPLES COMBUSTION PROCESSES This work covers Outcome 4 of the syllabus for the Edexcel HNC/D module Plant Process Principles 21725P and part of the Engineering Council Certificate
The material balance equations, after introducing the known values for the variables, are:
 Specifications: 4 (3 independent) (one is independent, the sum is F in mol) The material balance equations, after introducing the known values for the variables, are: of equations: (e) (b) simultaneously,
Specifications: 4 (3 independent) (one is independent, the sum is F in mol) The material balance equations, after introducing the known values for the variables, are: of equations: (e) (b) simultaneously,
Chapter 5: Extent of Reaction
 Chapter 5: Extent of Reaction Accumulation = input - output + generation - consumption Steady state system: 0 = input - output + generation - consumption output = input + generation - consumption Input
Chapter 5: Extent of Reaction Accumulation = input - output + generation - consumption Steady state system: 0 = input - output + generation - consumption output = input + generation - consumption Input
Energy Balances and Numerical Methods Design Project. Production of Maleic Anhydride
 Energy Balances and Numerical Methods Design Project Production of Maleic Anhydride Maleic anhydride is a chemical intermediate that is used to produce resins, surface coatings, lubricant additives, and
Energy Balances and Numerical Methods Design Project Production of Maleic Anhydride Maleic anhydride is a chemical intermediate that is used to produce resins, surface coatings, lubricant additives, and
EDEXCEL NATIONAL CERTIFICATE/DIPLOMA. PRINCIPLES AND APPLICATIONS of THERMODYNAMICS NQF LEVEL 3 OUTCOME 3 -COMBUSTION
 EDEXCEL NATIONAL CERTIFICATE/DIPLOMA PRINCIPLES AND APPLICATIONS of THERMODYNAMICS NQF LEVEL 3 OUTCOME 3 -COMBUSTION CONTENT Know about combustion processes and the calorific value of fuels Combustion
EDEXCEL NATIONAL CERTIFICATE/DIPLOMA PRINCIPLES AND APPLICATIONS of THERMODYNAMICS NQF LEVEL 3 OUTCOME 3 -COMBUSTION CONTENT Know about combustion processes and the calorific value of fuels Combustion
1. Process Description:
 1. Process Description: The coal is converted to Raw Syngas in the Gasification Section. The Raw Syngas produced out of the Gasifier would be shifted (water gas shift) to adjust required H2/CO ratio and
1. Process Description: The coal is converted to Raw Syngas in the Gasification Section. The Raw Syngas produced out of the Gasifier would be shifted (water gas shift) to adjust required H2/CO ratio and
Lecture 4. Ammonia: Production and Storage - Part 1
 Lecture 4 Ammonia: Production and Storage - Part 1 Ammonia or azane is a compound of nitrogen and hydrogen with the formula NH 3. It is a colourless gas with a characteristic pungent smell. Ammonia contributes
Lecture 4 Ammonia: Production and Storage - Part 1 Ammonia or azane is a compound of nitrogen and hydrogen with the formula NH 3. It is a colourless gas with a characteristic pungent smell. Ammonia contributes
S.E. (Mechanical) (First Semester) EXAMINATION, 2012 APPLIED THERMODYNAMICS (2008 PATTERN) Time : Three Hours Maximum Marks : 100
 Total No. of Questions 12] [Total No. of Printed Pages 8 Seat No. [4162]-111 S.E. (Mechanical) (First Semester) EXAMINATION, 2012 APPLIED THERMODYNAMICS (2008 PATTERN) Time : Three Hours Maximum Marks
Total No. of Questions 12] [Total No. of Printed Pages 8 Seat No. [4162]-111 S.E. (Mechanical) (First Semester) EXAMINATION, 2012 APPLIED THERMODYNAMICS (2008 PATTERN) Time : Three Hours Maximum Marks
Methanol Production by Gasification of Heavy Residues
 Methanol Production by Gasification of Heavy Residues by C. A. A. Higman Presented at the IChemE Conference "Gasification: An Alternative to Natural Gas" London, 22-23 23 November, 1995 Methanol Production
Methanol Production by Gasification of Heavy Residues by C. A. A. Higman Presented at the IChemE Conference "Gasification: An Alternative to Natural Gas" London, 22-23 23 November, 1995 Methanol Production
Design Project Energy Balances and Numerical Methods Styrene Manufacture
 Design Project Energy Balances and Numerical Methods Styrene Manufacture Styrene is the monomer used to make polystyrene, which has many uses [1]. In the current process, styrene is produced by the dehydrogenation
Design Project Energy Balances and Numerical Methods Styrene Manufacture Styrene is the monomer used to make polystyrene, which has many uses [1]. In the current process, styrene is produced by the dehydrogenation
Chapter 13. Thermal Conversion Technologies. Fundamentals of Thermal Processing
 Chapter 13 Thermal Conversion Technologies Fundamentals of Thermal Processing Thermal processing is the conversion of solid wastes into gaseous, liquid and solid conversion products with the concurrent
Chapter 13 Thermal Conversion Technologies Fundamentals of Thermal Processing Thermal processing is the conversion of solid wastes into gaseous, liquid and solid conversion products with the concurrent
ME ENGINEERING THERMODYNAMICS UNIT III QUESTION BANK SVCET
 1. A vessel of volume 0.04m 3 contains a mixture of saturated water and steam at a temperature of 250 0 C. The mass of the liquid present is 9 kg. Find the pressure, mass, specific volume, enthalpy, entropy
1. A vessel of volume 0.04m 3 contains a mixture of saturated water and steam at a temperature of 250 0 C. The mass of the liquid present is 9 kg. Find the pressure, mass, specific volume, enthalpy, entropy
COVENANT UNIVERSITY NIGERIA TUTORIAL KIT OMEGA SEMESTER PROGRAMME: CHEMICAL ENGINEERING
 OVENANT UNIVERSITY NIGERIA TUTORIAL KIT OMEGA SEMESTER PROGRAMME: HEMIAL ENGINEERING OURSE: HE 30 DISLAIMER The contents of this document are intended for practice and leaning purposes at the undergraduate
OVENANT UNIVERSITY NIGERIA TUTORIAL KIT OMEGA SEMESTER PROGRAMME: HEMIAL ENGINEERING OURSE: HE 30 DISLAIMER The contents of this document are intended for practice and leaning purposes at the undergraduate
SHRI RAMSWAROOP MEMORIAL COLLEGE OF ENGG. & MANAGEMENT B.Tech. [SEM IV (ME-41, 42,43 & 44)] QUIZ TEST-1 (Session: )
![SHRI RAMSWAROOP MEMORIAL COLLEGE OF ENGG. & MANAGEMENT B.Tech. [SEM IV (ME-41, 42,43 & 44)] QUIZ TEST-1 (Session: ) SHRI RAMSWAROOP MEMORIAL COLLEGE OF ENGG. & MANAGEMENT B.Tech. [SEM IV (ME-41, 42,43 & 44)] QUIZ TEST-1 (Session: )](/thumbs/72/67555697.jpg) QUIZ TEST-1 Q.1. In a stage of an impulse turbine provided with a single row wheel, the mean diameter of the blade ring is 80cm and the speed of the rotation is 3000rpm. The steam issues from the nozzle
QUIZ TEST-1 Q.1. In a stage of an impulse turbine provided with a single row wheel, the mean diameter of the blade ring is 80cm and the speed of the rotation is 3000rpm. The steam issues from the nozzle
HYSYS WORKBOOK By: Eng. Ahmed Deyab Fares.
 HYSYS WORKBOOK 2013 By: Eng. Ahmed Deyab Fares eng.a.deab@gmail.com adeyab@adeyab.com Mobile: 002-01227549943 - Email: adeyab@adeyab.com 1 Flash Separation We have a stream containing 15% ethane, 20% propane,
HYSYS WORKBOOK 2013 By: Eng. Ahmed Deyab Fares eng.a.deab@gmail.com adeyab@adeyab.com Mobile: 002-01227549943 - Email: adeyab@adeyab.com 1 Flash Separation We have a stream containing 15% ethane, 20% propane,
R13. II B. Tech I Semester Regular/Supplementary Examinations, Oct/Nov THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max.
 SET - 1 1. a) Discuss about PMM I and PMM II b) Explain about Quasi static process. c) Show that the COP of a heat pump is greater than the COP of a refrigerator by unity. d) What is steam quality? What
SET - 1 1. a) Discuss about PMM I and PMM II b) Explain about Quasi static process. c) Show that the COP of a heat pump is greater than the COP of a refrigerator by unity. d) What is steam quality? What
Faculty of Petroleum & Renewable Energy Engineering. Engineering. Sem 2 (2013/14) Faculty of Petroleum & Renewable Energy Engineering.
 FACULTY OF PETROLEUM & RENEWABLE ENERGY ENGINEERING Topic Learning Outcomes CHAPTER 3.5 3.6 At the end of this course students will be able to Material Balances on Multiple-Unit Processes with Recycle
FACULTY OF PETROLEUM & RENEWABLE ENERGY ENGINEERING Topic Learning Outcomes CHAPTER 3.5 3.6 At the end of this course students will be able to Material Balances on Multiple-Unit Processes with Recycle
Problems at the Cumene Production Facility, Unit 800
 Problems at the Cumene Production Facility, Unit 800 Background Cumene (isopropyl benzene) is produced by reacting propylene with benzene. During World War II, cumene was used as an octane enhancer for
Problems at the Cumene Production Facility, Unit 800 Background Cumene (isopropyl benzene) is produced by reacting propylene with benzene. During World War II, cumene was used as an octane enhancer for
The project goal is to design a process capable of converting methane, obtained from remote
 Introduction The project goal is to design a process capable of converting methane, obtained from remote sites, to a more easily transportable fuel, methanol. The design should focus on minimizing the
Introduction The project goal is to design a process capable of converting methane, obtained from remote sites, to a more easily transportable fuel, methanol. The design should focus on minimizing the
Cyclohexane Production with Aspen Plus V8.0
 Cyclohexane Production with Aspen Plus V8.0 1. Lesson Objectives Construct an Aspen Plus flowsheet simulation of the production of cyclohexane via benzene hydrogenation Become familiar with user interface
Cyclohexane Production with Aspen Plus V8.0 1. Lesson Objectives Construct an Aspen Plus flowsheet simulation of the production of cyclohexane via benzene hydrogenation Become familiar with user interface
Energy Balances and Numerical Methods Spring Design Project. Production of Ethylene Oxide
 Process Description Energy Balances and Numerical Methods Spring 00 Design Project Production of Ethylene Oxide Figure 1 is a preliminary process flow diagram (PFD) for the ethylene oxide production process.
Process Description Energy Balances and Numerical Methods Spring 00 Design Project Production of Ethylene Oxide Figure 1 is a preliminary process flow diagram (PFD) for the ethylene oxide production process.
Exercise 5. Simulation of HDA plant in UniSim
 Process Systems Engineering Prof. Davide Manca Politecnico di Milano Exercise 5 Simulation of HDA plant in UniSim Lab assistant: Adriana Savoca Davide Manca Process Systems Engineering Politecnico di Milano
Process Systems Engineering Prof. Davide Manca Politecnico di Milano Exercise 5 Simulation of HDA plant in UniSim Lab assistant: Adriana Savoca Davide Manca Process Systems Engineering Politecnico di Milano
Energy Balances and Numerical Methods Design Project. Production of Methyl Tertiary-Butyl Ether
 Energy Balances and Numerical Methods Design Project Production of Methyl Tertiary-Butyl Ether Methyl Tertiary-Butyl Ether () is a gasoline additive used to increase octane number that is produced from
Energy Balances and Numerical Methods Design Project Production of Methyl Tertiary-Butyl Ether Methyl Tertiary-Butyl Ether () is a gasoline additive used to increase octane number that is produced from
R13 SET - 1 '' ''' '' ' '''' Code No: RT31035
 R13 SET - 1 III B. Tech I Semester Regular/Supplementary Examinations, October/November - 2016 THERMAL ENGINEERING II (Mechanical Engineering) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists
R13 SET - 1 III B. Tech I Semester Regular/Supplementary Examinations, October/November - 2016 THERMAL ENGINEERING II (Mechanical Engineering) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists
ChE 455 Fall 2001 Major 1. Ethylene Oxide Production
 10/19/01 ChE 455 Fall 2001 Major 1 Ethylene Oxide Production Ethylene oxide is a chemical used to make ethylene glycol (the primary ingredient in antifreeze). It is also used to make poly(ethylene oxide),
10/19/01 ChE 455 Fall 2001 Major 1 Ethylene Oxide Production Ethylene oxide is a chemical used to make ethylene glycol (the primary ingredient in antifreeze). It is also used to make poly(ethylene oxide),
9/12/2018. Course Objectives MSE 353 PYROMETALLURGY. Prerequisite. Course Outcomes. Forms of Assessment. Course Outline
 Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 353 PYROMETALLURGY Course Objectives Understand the fundamental concepts of pyrometallurgy Understand the concepts of materials and energy
Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 353 PYROMETALLURGY Course Objectives Understand the fundamental concepts of pyrometallurgy Understand the concepts of materials and energy
CHAPTER 2 COMBUSTION PROPERTIES
 CHAPTER 2 COMBUSTION PROPERTIES Combustion Properties 1. Calorific value (CV) 2. Specific Gravity (SG) 3. Wobbe Number 4. Heat of Combustion 5. Flammability Limit 6. Flame Speed 7. Ignition Temperature
CHAPTER 2 COMBUSTION PROPERTIES Combustion Properties 1. Calorific value (CV) 2. Specific Gravity (SG) 3. Wobbe Number 4. Heat of Combustion 5. Flammability Limit 6. Flame Speed 7. Ignition Temperature
Thermodynamic Data. CO (g, 0 C, 1 atm) CO (g,100 C, 1 atm):
 Thermodynamic Data It is not possible to know the absolute value of Uˆ or Ĥ for a pure substance, but you can determine the change in U ˆ ( U ˆ ) or H ˆ ( Hˆ ) corresponding to a specified change of state
Thermodynamic Data It is not possible to know the absolute value of Uˆ or Ĥ for a pure substance, but you can determine the change in U ˆ ( U ˆ ) or H ˆ ( Hˆ ) corresponding to a specified change of state
Fluid Mechanics, Heat Transfer, and Thermodynamics Fall Design Project. Production of Dimethyl Ether
 Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2001 Design Project Production of Dimethyl Ether We are investigating the feasibility of constructing a new, grass-roots, 50,000 tonne/y, (1 tonne
Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2001 Design Project Production of Dimethyl Ether We are investigating the feasibility of constructing a new, grass-roots, 50,000 tonne/y, (1 tonne
Enthalpy Calculations. Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions.
 Enthalpy Calculations Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions. Enthalpy Change as a Result of Temperature Sensible heat is the
Enthalpy Calculations Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions. Enthalpy Change as a Result of Temperature Sensible heat is the
Chemistry Resource Kit
 This is a chapter from the Chemistry Resource Kit The Qenos Chemistry Resource kit has been developed as an information package for secondary students and others who wish to learn about Qenos, plastics
This is a chapter from the Chemistry Resource Kit The Qenos Chemistry Resource kit has been developed as an information package for secondary students and others who wish to learn about Qenos, plastics
Lecture No.1. Vapour Power Cycles
 Lecture No.1 1.1 INTRODUCTION Thermodynamic cycles can be primarily classified based on their utility such as for power generation, refrigeration etc. Based on this thermodynamic cycles can be categorized
Lecture No.1 1.1 INTRODUCTION Thermodynamic cycles can be primarily classified based on their utility such as for power generation, refrigeration etc. Based on this thermodynamic cycles can be categorized
SSRG International Journal of Chemical Engineering Research ( SSRG IJCER ) Volume 5 Issue 2 May to Aug 2018
 Simulation and Energy Optimization of Ammonia Synthesis Loop Ashutosh Desai#1, Shreyans Shah#2, Sanchit Goyal#3 Under the guidance of, Prof. Arvind Prasad Department of chemical engineering, Dwarkadas.J.Sanghavi
Simulation and Energy Optimization of Ammonia Synthesis Loop Ashutosh Desai#1, Shreyans Shah#2, Sanchit Goyal#3 Under the guidance of, Prof. Arvind Prasad Department of chemical engineering, Dwarkadas.J.Sanghavi
Chemistry of Petrochemical Processes
 Chemistry of Petrochemical Processes ChE 464 Instructor: Dr. Ahmed Arafat, PhD Office: building 45 room 106 E-mail: akhamis@kau.edu.sa www.kau.edu.sa.akhamis files Book Chemistry of Petrochemical Processes
Chemistry of Petrochemical Processes ChE 464 Instructor: Dr. Ahmed Arafat, PhD Office: building 45 room 106 E-mail: akhamis@kau.edu.sa www.kau.edu.sa.akhamis files Book Chemistry of Petrochemical Processes
Reduction of Sulphur in Superior Kerosene
 Reduction of Sulphur in Superior Kerosene S.Karthick #1, S.Theepa Priya #1 Department of Chemical Engineering, Vel Tech High Tech Dr.Rangarajan Dr.Sakunthala Engineering College, 1 karthicks151196@gmail.com
Reduction of Sulphur in Superior Kerosene S.Karthick #1, S.Theepa Priya #1 Department of Chemical Engineering, Vel Tech High Tech Dr.Rangarajan Dr.Sakunthala Engineering College, 1 karthicks151196@gmail.com
Thomas G. Braga Manager, Research and Development. SulfaTreat, a Business Unit of M I L.L.C. A Smith/Schlumberger Company
 Thomas G. Braga Manager, Research and Development SulfaTreat, a Business Unit of M I L.L.C. A Smith/Schlumberger Company Who is SulfaTreat? A World Leader in H 2 S Removal for More than a Decade Today
Thomas G. Braga Manager, Research and Development SulfaTreat, a Business Unit of M I L.L.C. A Smith/Schlumberger Company Who is SulfaTreat? A World Leader in H 2 S Removal for More than a Decade Today
Thermal Oxidation plants February 2016
 Thermal Oxidation plants February 2016 Thermal Oxidation of gaseous waste Typical Processed Stream: Contaminated Air by Hydrocarbons Contaminated Air by Solvents Contaminated Air by Stripping/Scrubbing
Thermal Oxidation plants February 2016 Thermal Oxidation of gaseous waste Typical Processed Stream: Contaminated Air by Hydrocarbons Contaminated Air by Solvents Contaminated Air by Stripping/Scrubbing
CHAPTER 1 BASIC CONCEPTS
 GTU Paper Analysis CHAPTER 1 BASIC CONCEPTS Sr. No. Questions Jan 15 Jun 15 Dec 15 May 16 Jan 17 Jun 17 Nov 17 May 18 Differentiate between the followings; 1) Intensive properties and extensive properties,
GTU Paper Analysis CHAPTER 1 BASIC CONCEPTS Sr. No. Questions Jan 15 Jun 15 Dec 15 May 16 Jan 17 Jun 17 Nov 17 May 18 Differentiate between the followings; 1) Intensive properties and extensive properties,
WSA-DC NEXT GENERATION TOPSØE WSA TECHNOLOGY FOR STRONGER SO 2 GASES AND VERY HIGH CONVERSION. Helge Rosenberg Haldor Topsoe
 WSA-DC NEXT GENERATION TOPSØE WSA TECHNOLOGY FOR STRONGER SO 2 GASES AND VERY HIGH CONVERSION Helge Rosenberg Haldor Topsoe Up to now, Topsøe WSA (Wet gas Sulphuric Acid) plants have been in operation
WSA-DC NEXT GENERATION TOPSØE WSA TECHNOLOGY FOR STRONGER SO 2 GASES AND VERY HIGH CONVERSION Helge Rosenberg Haldor Topsoe Up to now, Topsøe WSA (Wet gas Sulphuric Acid) plants have been in operation
Improvement of distillation column efficiency by integration with organic Rankine power generation cycle. Introduction
 Improvement of distillation column efficiency by integration with organic Rankine power generation cycle Dmitriy A. Sladkovskiy, St.Petersburg State Institute of Technology (technical university), Saint-
Improvement of distillation column efficiency by integration with organic Rankine power generation cycle Dmitriy A. Sladkovskiy, St.Petersburg State Institute of Technology (technical university), Saint-
Heat Balance in Pyrometallurgical Processes
 Heat Balance in Pyrometallurgical Processes The heat balance shows the important sources of heat energy and their relative contribution to the total energy usage in a process The heat balance in general
Heat Balance in Pyrometallurgical Processes The heat balance shows the important sources of heat energy and their relative contribution to the total energy usage in a process The heat balance in general
Pilot Scale Production of Mixed Alcohols from Wood. Supplementary Information
 Pilot Scale Production of Mixed Alcohols from Wood Supplementary Information Richard L. Bain, Kimberly A. Magrini-Bair, Jesse E. Hensley *, Whitney S. Jablonski, Kristin M. Smith, Katherine R. Gaston,
Pilot Scale Production of Mixed Alcohols from Wood Supplementary Information Richard L. Bain, Kimberly A. Magrini-Bair, Jesse E. Hensley *, Whitney S. Jablonski, Kristin M. Smith, Katherine R. Gaston,
Supercritical Water Coal Conversion with Aquifer-Based Sequestration of CO 2
 Supercritical Water Coal Conversion with Aquifer-Based Sequestration of CO 2 Profs. Reginald Mitchell, 1 Christopher Edwards 1 and Scott Fendorf 2 1 Mechanical Engineering Department 2 Department of Geological
Supercritical Water Coal Conversion with Aquifer-Based Sequestration of CO 2 Profs. Reginald Mitchell, 1 Christopher Edwards 1 and Scott Fendorf 2 1 Mechanical Engineering Department 2 Department of Geological
MTBE Production. Process Description. Possibility of Changing Process Feed Conditions
 Production You work in a facility that produces about 100,000 tonne/y of methyl tertiary butyl ether (). is an oxygenated fuel additive that is blended with gasoline to promote CO 2 formation over CO formation
Production You work in a facility that produces about 100,000 tonne/y of methyl tertiary butyl ether (). is an oxygenated fuel additive that is blended with gasoline to promote CO 2 formation over CO formation
Design of Extraction Column Methanol Recovery System for the TAME Reactive Distillation Process
 Design of Extraction Column Methanol Recovery System for the TAME Reactive Distillation Process Muhammad A. Al-Arfaj * Chemical Engineering Department King Fahd University of Petroleum and Minerals, Dhahran,
Design of Extraction Column Methanol Recovery System for the TAME Reactive Distillation Process Muhammad A. Al-Arfaj * Chemical Engineering Department King Fahd University of Petroleum and Minerals, Dhahran,
CEMENT PLANT ENVIRONMENTAL TECHNOLOGY FOR ACHIEVING HIGH SO 2 REMOVAL
 CEMENT PLANT ENVIRONMENTAL TECHNOLOGY FOR ACHIEVING HIGH SO 2 REMOVAL Sara Safariyeganeh sara.safariyeganeh@mecsglobal.com Cary Joe Lovett, PE joe.lovett@holcim.com Abstract - The EPA has adopted revised
CEMENT PLANT ENVIRONMENTAL TECHNOLOGY FOR ACHIEVING HIGH SO 2 REMOVAL Sara Safariyeganeh sara.safariyeganeh@mecsglobal.com Cary Joe Lovett, PE joe.lovett@holcim.com Abstract - The EPA has adopted revised
Correctly Modeling and Calculating Combustion Efficiencies In Fired Equipment
 Correctly Modeling and Calculating Combustion Efficiencies In Fired Equipment David Schmitt, President Increase Performance, Inc. Tulsa, Oklahoma Fired equipment includes furnaces, fired heaters, fired
Correctly Modeling and Calculating Combustion Efficiencies In Fired Equipment David Schmitt, President Increase Performance, Inc. Tulsa, Oklahoma Fired equipment includes furnaces, fired heaters, fired
Q1. Which of the following diatomic species is paramagnetic?
 Q1. Which of the following diatomic species is paramagnetic? A) O 2 B) N 2 C) F 2 D) CO E) NO + Q2. Which of the following statements about the CO 3 2 ion is false? A) One C O bond is shorter than the
Q1. Which of the following diatomic species is paramagnetic? A) O 2 B) N 2 C) F 2 D) CO E) NO + Q2. Which of the following statements about the CO 3 2 ion is false? A) One C O bond is shorter than the
Optimization of Single and Multiple Unit Chemical Processes: A. case study on the optimization of a styrene plant design
 Optimization of Single and Multiple Unit Chemical Processes: A case study on the optimization of a styrene plant design by Piero Bracamonte A thesis submitted to the faculty of The University of Mississippi
Optimization of Single and Multiple Unit Chemical Processes: A case study on the optimization of a styrene plant design by Piero Bracamonte A thesis submitted to the faculty of The University of Mississippi
MECHANICAL ENGINEERING DEPARTMENT, OITM
 Sem.:4 th Subject: Energy Conversion Paper: ME-201E UNIT-1 Q1. Explain the seismometer with its working principle. (Important Question) (20) Q2. Classify the fuels and define calorific value of fuels.
Sem.:4 th Subject: Energy Conversion Paper: ME-201E UNIT-1 Q1. Explain the seismometer with its working principle. (Important Question) (20) Q2. Classify the fuels and define calorific value of fuels.
Austro Energy Systems Int. AG. Gas reformer AES3000
 Austro Energy Systems Int. AG Gas reformer AES3000 1 Gas reformer AES3000 Purification of associated gas from the hydrogen sulfide and high hydrocarbons and conversion of its qualities into similar properties
Austro Energy Systems Int. AG Gas reformer AES3000 1 Gas reformer AES3000 Purification of associated gas from the hydrogen sulfide and high hydrocarbons and conversion of its qualities into similar properties
MIE 517 Final Celebration of Learning Wednesday, April 19, 2017, 2-4:30pm
 MIE 517 Final Celebration of Learning Wednesday, April 19, 2017, 2-4:30pm Instructions: Answer all questions and show all work for which you wish to receive credit in the answer booklets. You may use one
MIE 517 Final Celebration of Learning Wednesday, April 19, 2017, 2-4:30pm Instructions: Answer all questions and show all work for which you wish to receive credit in the answer booklets. You may use one
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project. Production of Allyl Chloride
 Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Allyl Chloride We are investigating the feasibility of constructing a new, grass-roots, 20,000 metric tons/year, allyl chloride
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Allyl Chloride We are investigating the feasibility of constructing a new, grass-roots, 20,000 metric tons/year, allyl chloride
LECTURE 11 Principles of combustion III
 LECTURE 11 Principles of combustion III Exercise 1 Exercise 2 Exercise 3 Keywords: Combustion, Excess air, Stoichiometric air, Furnace, Blast furnace Exercise 1 1) A furnace is heated by combusting a gaseous
LECTURE 11 Principles of combustion III Exercise 1 Exercise 2 Exercise 3 Keywords: Combustion, Excess air, Stoichiometric air, Furnace, Blast furnace Exercise 1 1) A furnace is heated by combusting a gaseous
CRYSTALLIZATION SOLUBILITY CURVE
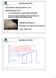 CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
Chapter 1 Basic Concepts
 Jan 15 Jun 15 Chapter 1 Basic Concepts GTU Paper Analysis (New Syllabus) Sr. No. Questions Differentiate between the followings; 1) Intensive properties and extensive properties, 2) Point function and
Jan 15 Jun 15 Chapter 1 Basic Concepts GTU Paper Analysis (New Syllabus) Sr. No. Questions Differentiate between the followings; 1) Intensive properties and extensive properties, 2) Point function and
Energy Balances and Numerical Methods Design Project. Ammonia Production
 Energy Balances and Numerical Methods Design Project Ammonia Production Your assignment is to continue to evaluate the feasibility of a process to produce 50,000 tonne/y of ammonia from syngas. A suggested
Energy Balances and Numerical Methods Design Project Ammonia Production Your assignment is to continue to evaluate the feasibility of a process to produce 50,000 tonne/y of ammonia from syngas. A suggested
Energy Balances and Numerical Methods Design Project. Production of Cumene
 Process Description Energy Balances and Numerical Methods Design Project Production of Cumene Figure 1 is a preliminary process flow diagram (PFD) for the cumene production process. The raw materials are
Process Description Energy Balances and Numerical Methods Design Project Production of Cumene Figure 1 is a preliminary process flow diagram (PFD) for the cumene production process. The raw materials are
Techno-Economic Analysis for Ethylene and Oxygenates Products from the Oxidative Coupling of Methane Process
 Techno-Economic Analysis for Ethylene and Oxygenates Products from the Oxidative Coupling of Methane Process Daniel Salerno, Harvey Arellano-Garcia, Günter Wozny Berlin Institute of Technology Chair of
Techno-Economic Analysis for Ethylene and Oxygenates Products from the Oxidative Coupling of Methane Process Daniel Salerno, Harvey Arellano-Garcia, Günter Wozny Berlin Institute of Technology Chair of
Exercise 5. Simulation of HDA plant in UniSim
 Process Systems Engineering Prof. Davide Manca Politecnico di Milano Exercise 5 Simulation of HDA plant in UniSim Lab assistants: Roberto Totaro Salman Nazir Davide Manca Process Systems Engineering Politecnico
Process Systems Engineering Prof. Davide Manca Politecnico di Milano Exercise 5 Simulation of HDA plant in UniSim Lab assistants: Roberto Totaro Salman Nazir Davide Manca Process Systems Engineering Politecnico
Fluid Mechanics, Heat Transfer, and Thermodynamics. Design Project. Production of Acetone
 Fluid Mechanics, Heat Transfer, and Thermodynamics Design Project Production of Acetone We are investigating the feasibility of constructing a new, grass-roots, 15,000 metric tons/year, acetone plant.
Fluid Mechanics, Heat Transfer, and Thermodynamics Design Project Production of Acetone We are investigating the feasibility of constructing a new, grass-roots, 15,000 metric tons/year, acetone plant.
Distillation DEPARTMENT OF CHEMICAL ENGINEERING
 Distillation DEPARTMENT OF CHEMICAL ENGINEERING 2 3 Weeping in distillation column 4 Distillation. Introduction Unit operation Separation process A feed mixture of two or more components is separated into
Distillation DEPARTMENT OF CHEMICAL ENGINEERING 2 3 Weeping in distillation column 4 Distillation. Introduction Unit operation Separation process A feed mixture of two or more components is separated into
w w w. s t u p i d s i d. c o m
 'l'olal No. ol Questions-121 [Tol,al No. of Printed Pages-8+4 t37621-174 S.E. (Chem.) (First Semester) EXAMINATION, 20f0 CH EMICAL PROCESS CALCUI"ATION (2008 coilrse) Tirne : Three Hours Maximum Marks
'l'olal No. ol Questions-121 [Tol,al No. of Printed Pages-8+4 t37621-174 S.E. (Chem.) (First Semester) EXAMINATION, 20f0 CH EMICAL PROCESS CALCUI"ATION (2008 coilrse) Tirne : Three Hours Maximum Marks
Material Balances Design Project Manufacture of Diethyl Ether
 Material Balances Design Project Manufacture of Diethyl Ether Diethyl ether (DEE) is a colorless, highly volatile, flammable liquid with a characteristic odor. It is an important solvent in the production
Material Balances Design Project Manufacture of Diethyl Ether Diethyl ether (DEE) is a colorless, highly volatile, flammable liquid with a characteristic odor. It is an important solvent in the production
Reg. No. : Question Paper Code : B.E./B.Tech. DEGREE EXAMINATION, NOVEMBER/DECEMBER Second Semester
 ws15 Reg. No. : Question Paper Code : 27425 B.E./B.Tech. DEGREE EXAMINATION, NOVEMBER/DECEMBER 2015. Time : Three hours Second Semester Marine Engineering MV 6201 MARINE ENGINEERING THERMODYNAMICS (Regulations
ws15 Reg. No. : Question Paper Code : 27425 B.E./B.Tech. DEGREE EXAMINATION, NOVEMBER/DECEMBER 2015. Time : Three hours Second Semester Marine Engineering MV 6201 MARINE ENGINEERING THERMODYNAMICS (Regulations
CHAPTER 1 FUNDAMENTAL OF COMBUSTION
 CHAPTER 1 FUNDAMENTAL OF COMBUSTION Definition Rapid oxidation of a fuel accompanied by the release of heat and/or light together with the formation of combustion products Fuel + oxygen Heat/light + combustion
CHAPTER 1 FUNDAMENTAL OF COMBUSTION Definition Rapid oxidation of a fuel accompanied by the release of heat and/or light together with the formation of combustion products Fuel + oxygen Heat/light + combustion
W. D. WANG *, C. Y. ZHOU
 Oil Shale, 2009, Vol. 26, No. 2, pp. 108 113 ISSN 0208-189X doi: 10.3176/oil.2009.2.03 2009 Estonian Academy Publishers RETORTING OF PULVERIZED OIL SHALE IN FLUIDIZED-BED PILOT PLANT W. D. WANG *, C. Y.
Oil Shale, 2009, Vol. 26, No. 2, pp. 108 113 ISSN 0208-189X doi: 10.3176/oil.2009.2.03 2009 Estonian Academy Publishers RETORTING OF PULVERIZED OIL SHALE IN FLUIDIZED-BED PILOT PLANT W. D. WANG *, C. Y.
Separations and Reactors. Acrylic Acid Production via the Catalytic Partial Oxidation of Propylene
 Separations and Reactors Acrylic Acid Production via the Catalytic Partial Oxidation of Propylene Process Information Background Acrylic acid (AA) is used as a precursor for a wide variety of chemicals
Separations and Reactors Acrylic Acid Production via the Catalytic Partial Oxidation of Propylene Process Information Background Acrylic acid (AA) is used as a precursor for a wide variety of chemicals
R.K.Yadav/Automobile Engg Dept/New Polytechnic Kolhapur. Page 1
 6.1 Types of fuels 4 Marks Definition, classification, properties, Calorific value of fuels. Ultimate analysis and proximate analysis of solid fuels. Liquid fuels- Comparative information about composition,
6.1 Types of fuels 4 Marks Definition, classification, properties, Calorific value of fuels. Ultimate analysis and proximate analysis of solid fuels. Liquid fuels- Comparative information about composition,
Separations and Reaction Engineering Spring Design Project. Production of Acetone
 Process Objective Function Separations and Reaction Engineering Spring 2000 Design Project Production of Acetone We would like to complete our investigation of the economic feasibility of producing 15,000
Process Objective Function Separations and Reaction Engineering Spring 2000 Design Project Production of Acetone We would like to complete our investigation of the economic feasibility of producing 15,000
Written Exam 02 March Problems - Time: 2 hours PLEASE NOTICE
 Politecnico di Milano Department of Energy - School of Industrial Engineering Course Energy Systems LM prof. S. Consonni, E. Martelli, M. Romano - Academic Year 2014/15 Written Exam 02 March 2015 - Problems
Politecnico di Milano Department of Energy - School of Industrial Engineering Course Energy Systems LM prof. S. Consonni, E. Martelli, M. Romano - Academic Year 2014/15 Written Exam 02 March 2015 - Problems
Fluid Mechanics, Heat Transfer, Fluid Mechanics Design Project. Production of Ethanol
 Fluid Mechanics, Heat Transfer, Fluid Mechanics Design Project Production of Ethanol Your assignment is to continue evaluating the details of a process to produce 30,000 tonne/y of ethanol from ethylene.
Fluid Mechanics, Heat Transfer, Fluid Mechanics Design Project Production of Ethanol Your assignment is to continue evaluating the details of a process to produce 30,000 tonne/y of ethanol from ethylene.
DESCRIPTION OF A SYSTEM THAT TRANSFORM S ORGANIC MATER IALS INTO SYNTETIC FUEL S
 DESCRIPTION OF A SYSTEM THAT TRANSFORM S ORGANIC MATER IALS INTO SYNTETIC FUEL S 1 INDEX 2 DESCRIPTION OF THE SISTEM.. 4 3 USABLE MATERIALS..6 2 4 CARACTERISTICS OF THE MATERIAL..7 5 CARACTERISTICS OF
DESCRIPTION OF A SYSTEM THAT TRANSFORM S ORGANIC MATER IALS INTO SYNTETIC FUEL S 1 INDEX 2 DESCRIPTION OF THE SISTEM.. 4 3 USABLE MATERIALS..6 2 4 CARACTERISTICS OF THE MATERIAL..7 5 CARACTERISTICS OF
Example SPC-2: Effect of Increasing Column P on a C3 splitter
 Example SPC-2: Effect of Increasing Column P on a C3 splitter Consider the separation of a mixture of 50 mol/hr of propane C 3 H 8 (1) and 50 mol/hr propene, C 3 H 6 (2) at a pressure of 1.1 bar and a
Example SPC-2: Effect of Increasing Column P on a C3 splitter Consider the separation of a mixture of 50 mol/hr of propane C 3 H 8 (1) and 50 mol/hr propene, C 3 H 6 (2) at a pressure of 1.1 bar and a
Chapter 13 Coal Boiler Flue Gas Scrubber
 Chapter 13 Coal Boiler Flue Gas Scrubber The Application The following case study is a limestone flue gas scrubber. Coal-fired boiler flue gas is quenched and sent to the scrubber where it mixes with wet
Chapter 13 Coal Boiler Flue Gas Scrubber The Application The following case study is a limestone flue gas scrubber. Coal-fired boiler flue gas is quenched and sent to the scrubber where it mixes with wet
EBTAX: The Conversion of Ethane to Aromatics via Catalytic Conversion
 University of Wyoming Wyoming Scholars Repository Honors Theses AY 15/16 Undergraduate Honors Theses 2016 EBTAX: The Conversion of Ethane to Aromatics via Catalytic Conversion Emily Schwichtenberg University
University of Wyoming Wyoming Scholars Repository Honors Theses AY 15/16 Undergraduate Honors Theses 2016 EBTAX: The Conversion of Ethane to Aromatics via Catalytic Conversion Emily Schwichtenberg University
CO 2 Capture and Storage: Options and Challenges for the Cement Industry
 CO 2 Capture and Storage: Options and Challenges for the Cement Industry Martin Schneider, Düsseldorf, Germany CSI Workshop Beijing, 16 17 November 2008 CO 2 abatement costs will tremendously increase
CO 2 Capture and Storage: Options and Challenges for the Cement Industry Martin Schneider, Düsseldorf, Germany CSI Workshop Beijing, 16 17 November 2008 CO 2 abatement costs will tremendously increase
1. Introduction. 2. Base Case Design
 21st European Symposium on Computer Aided Process Engineering ESCAPE 21 E.N. Pistikopoulos, M.C. Georgiadis and A.C. Kokossis (Editors) 2011 Elsevier B.V. All rights reserved. Plantwide Control of a Cumene
21st European Symposium on Computer Aided Process Engineering ESCAPE 21 E.N. Pistikopoulos, M.C. Georgiadis and A.C. Kokossis (Editors) 2011 Elsevier B.V. All rights reserved. Plantwide Control of a Cumene
ECONOMIC OPTIMIZATION OF AN ETHYLBENZENE PROCESS. by Erin Leigh Dyer. Oxford May 2015
 ECONOMIC OPTIMIZATION OF AN ETHYLBENZENE PROCESS by Erin Leigh Dyer A thesis submitted to the faculty of The University of Mississippi in partial fulfillment of the requirements of the Sally McDonnell
ECONOMIC OPTIMIZATION OF AN ETHYLBENZENE PROCESS by Erin Leigh Dyer A thesis submitted to the faculty of The University of Mississippi in partial fulfillment of the requirements of the Sally McDonnell
Process description The Johnson Matthey/BP fixed-bed FT technology comprises a series of reaction vessels charged with a proprietary BP catalyst.
 Flowsheet: Fischer Tropsch (FT) Process description The Johnson Matthey/BP fixed-bed FT technology comprises a series of reaction vessels charged with a proprietary BP catalyst. Syngas derived from a variety
Flowsheet: Fischer Tropsch (FT) Process description The Johnson Matthey/BP fixed-bed FT technology comprises a series of reaction vessels charged with a proprietary BP catalyst. Syngas derived from a variety
Lecture 5. Exercises on stoichiometry
 Lecture 5 Contents Exercise i Exercise ii Do yourself Exercise iii Exercise iv Exercise V Exercise Vi Do yourself Exercise Vii Exercises on stoichiometry Exercise i A pyritic ore is reduced with hydrogen
Lecture 5 Contents Exercise i Exercise ii Do yourself Exercise iii Exercise iv Exercise V Exercise Vi Do yourself Exercise Vii Exercises on stoichiometry Exercise i A pyritic ore is reduced with hydrogen
Fluid Mechanics, Heat Transfer, and Thermodynamics Fall Design Project. Production of Drying Oil
 Introduction Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2003 Design Project Production of Drying Oil Drying oils are additives to paints and varnishes to aid in the drying process when these
Introduction Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2003 Design Project Production of Drying Oil Drying oils are additives to paints and varnishes to aid in the drying process when these
Keywords: Eutecic freeze crystallization, Solid-liquid equilibrium, Recovery of acetic acid, Heat of sublimation.
 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY Removal of Organic Acids from Effluent via Freeze Crystallization Tarak C. Padhiyar *1, Prof. Suchen B. Thakore 2 *1,2 L.D. College
IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY Removal of Organic Acids from Effluent via Freeze Crystallization Tarak C. Padhiyar *1, Prof. Suchen B. Thakore 2 *1,2 L.D. College
PROCESS DESIGN AND OPTIMIZATION: ANALYSIS OF AN ETHYLBENZENE PRODUCTION PLANT. by Butler VanVeckhoven
 PROCESS DESIGN AND OPTIMIZATION: ANALYSIS OF AN ETHYLBENZENE PRODUCTION PLANT by Butler VanVeckhoven A thesis submitted to the faculty of The University of Mississippi in partial fulfillment of the requirements
PROCESS DESIGN AND OPTIMIZATION: ANALYSIS OF AN ETHYLBENZENE PRODUCTION PLANT by Butler VanVeckhoven A thesis submitted to the faculty of The University of Mississippi in partial fulfillment of the requirements
1) ABSORPTION The removal of one or more selected components from a gas mixture by absorption is probably the most important operation in the control
 1) ABSORPTION The removal of one or more selected components from a gas mixture by absorption is probably the most important operation in the control of gaseous pollutant emissions. Absorption is a process
1) ABSORPTION The removal of one or more selected components from a gas mixture by absorption is probably the most important operation in the control of gaseous pollutant emissions. Absorption is a process
Item Hydrogen Gas Plant
 Item 6530. Hydrogen Gas Plant Hydro-Chem Hydrogen Generating Plant 90,000 scfh @ 200 psig. Purity 99.99% Hydrogen generating plant engineered by Hydro-Chem built in 1980. Design capacity is 90,000 scfh
Item 6530. Hydrogen Gas Plant Hydro-Chem Hydrogen Generating Plant 90,000 scfh @ 200 psig. Purity 99.99% Hydrogen generating plant engineered by Hydro-Chem built in 1980. Design capacity is 90,000 scfh
Annexure-I List of Products
 Annexure-I List of Products Sr. No. Name of Products Quantity (MT/Month) 1. Mono Chloro Acetic Acid (MCA) 300 2. Chloro Acetyl Chloride (CAC) 150 3. Tri Chloro Acetyl Chloride (TCAC) 150 4. Aluminum Chloroide
Annexure-I List of Products Sr. No. Name of Products Quantity (MT/Month) 1. Mono Chloro Acetic Acid (MCA) 300 2. Chloro Acetyl Chloride (CAC) 150 3. Tri Chloro Acetyl Chloride (TCAC) 150 4. Aluminum Chloroide
