EXPERIMENT 5 Chemistry 110 COMPOSITION OF A MIXTURE
|
|
|
- Allan Jacobs
- 6 years ago
- Views:
Transcription
1 EXPERIMENT 5 Chemistry 110 PURPOSE: The purpose of this experiment is to determine the percent composition of a mixture. COMPOSITION OF A MIXTURE Most matter is a mixture of many substances. For example, crude oil is a mixture. Most of the foods we eat are mixtures. Often it is a mixture of many different compounds of varying molecular mass. It is separated into its component parts such as gasoline and lubricant oil by utilizing the differing physical properties, in this case boiling point. Most mixtures can be separated by physical change In today s experiment you will determine the percent composition by mass of a sand (SiO 2 ) and Copper (II) sulfate (CuSO4)mixture. It will require separation by physical means. You will use the differences in water solubility to separate the two substances. Sand (SiO 2 ) is insoluble in water whereas copper (II) sulfate is water soluble. When water is added to the mixture, the sand will not dissolve, but the copper (II) sulfate will. Then, the sand will be dried. In this way, you will separate sand from the original mixture. Safety goggles must be worn at all times PROCEDURE 1. Weigh a clean dry (empty) evaporating dish to the nearest 0.01 of a gram. Record the mass below. 2. Obtain an unknown from your instructor. Weigh the unknown mixture in the evaporating dish. Record the exact mass to the nearest 0.01 g. Make some visual observations about your mixture: DATA Mass, grams Evaporating Dish Evaporating Dish plus Unknown mixture 3. Carefully, transfer the entire unknown sample into a 400 ml beaker. DO NOT PUT ANY EXCESS CHEMICALS BACK INTO THE REAGENT BOTTLES. 4. Obtain approximately 200 ml of de-ionized water. Pour, with stirring, the water into the beaker. If after stirring for a few minutes there is still solid CuSO4, add 25 ml more water. Make sure all of the copper (II) sulfate is dissolved. 5. Allow the sand to settle, then carefully decant the liquid into a 250 ml beaker. See "How to decant " on the next page. This can be done at your bench. Discard the liquid in the beaker if there was no sand loss. 6. Add about 50 ml of deionized water with stirring to wash the sand. Allow the sand to settle and then decant the wash water into the 150 ml beaker. 1 1/9/2018
2 HOW TO DECANT glass stirring rod Decanting means to let the solid settle to the bottom of the beaker. Then, carefully pour off the top liquid layer. Hold a glass stirring rod on top of the beaker to guide the stream of liquid down into another beaker. 7. Repeat step 6 with another 50 ml portion of deionized water. (This is to remove the CuSO4). What color should the liquid be if all of the CuSO4has been removed? 8. What should you do if you think that some CuSO4 still remains in the sand? 8. Transfer all the sand to the evaporating dish with a rubber policeman. Use a stream of deionized water to transfer the last particles of sand. Decant the water used in transferring. Use a medicine dropper to draw off as much of the remaining water as possible without removing any of the sand. 9. Prepare a steam bath using your 250 ml beaker. Set-up a ring stand with an iron ring. Place your wire gauze on top of the iron ring. Add tap water to a 250 ml beaker until it is about 2/3 full and then warm the water using a Bunsen burner. Dry the sand by heating the wet sand in the evaporating dish over the steam bath. evaporating dish Wire gauze Iron ring evaporating dish tongs NOTE: Use the evaporating dish tongs (located in the long vertical locker at your lab bench) to handle the hot evaporating dish. 2 1/9/2018
3 10. Stir the sand when it looks dry to check for dampness. If the sand still appears dry, remove it, dry the bottom of the dish, and allow it to cool on the base of your ring stand. Weigh the evaporating dish and sand. Record to the mass to g. 11. Reheat the sand for another 5 minutes. Cool and weigh as above. Repeat this process until you have Dried to Constant Mass (see below). Two weighings must be within grams. DRY TO CONSTANT MASS You cannot tell by looking at the sand whether or not the sand is really dry. You must heat the sand, weigh it, reheat, and then, reweigh the sand. The heating and weighing process is continued until two consecutive weighings are fairly close. This process is called Drying to Constant Mass. This is the only way to know if all of the water has been driven off! DATA Use the lowest value obtained to calculate the mass of your sand. 1st Weighing Mass of Evaporating Dish plus Sand 2nd Weighing 3rd Weighing 12. After you have calculated your percent sand and obtained the correct value, dispose of your sand. (If your experimental value is too high your instructor will ask you to reheat and reweigh your sand). * DISPOSAL: Dispose the sand in the waste container labeled "Waste Sand CALCULATIONS 1. Calculate the mass of unknown sample. 2. Calculate the mass of sand. 3. Calculate the mass of copper (II) sulfate, CuSO4. 3 1/9/2018
4 4. Calculate the percentage (by weight) of sand in the sample. Write this on your report sheet. Then, ask your instructor for the correct sand. (Do this after you calculate your % Sand). Accepted value from instructor 5. Calculate the percent error. 6. Calculate the percentage (by weight) of copper (II) sulfate, CuSO4, in the sample. % Error 4 1/9/2018
5 Chem. 110 Lab Report Name Lab Section EXPERIMENT 5 Date Initials COMPOSITION OF A MIXTURE CALCULATIONS: Show all set-ups. 1. Calculate the mass of unknown sample. 2. Calculate the mass of sand. 3. Calculate the mass of copper (II) sulfate, CuSO4. 4. Calculate the percentage (by weight) of sand in the sample. Correct % Sand (From Instructor) 5. Ask your instructor for the correct percent sand (take your report sheet). Calculate the percent error. 6. Calculate the percentage (by weight) of copper (II) sulfate, CuSO4, in the sample. % Error QUESTIONS AND PROBLEMS 1. Give a reason why (other than calculations) your percentage of sand might be lower than the correct value. 5 1/9/2018
6 2. Give a reason why (other than calculations) your percentage of sand might be higher than the correct value. 3. A mixture of KCl and SiO 2 weighed 4.23 g. The KCl was dissolved in water and then the solution was decanted and dried leaving only 2.76 g of dry sand. Calculate the percent sand in the sample. Set-up: 4. What are 2 ways that a mixture of sand and CuSO4 is different from a compound like K 2 CO 3 1) 2) 6 1/9/2018
Copper Odyssey. Chemical Reactions of Copper
 Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
CHEM 1215 LAB NOTES EXPT #2: PHYSICAL AND CHEMICAL CHANGES 1
 CHEM 1215 LAB NOTES EXPT #2: PHYSICAL AND CHEMICAL CHANGES 1 TECHNIQUES: chemical and physical changes, reactions, observations READING: PHYSICAL AND CHEMICAL CHANGES e.g. Tro chapter 1 SAFETY: Safety
CHEM 1215 LAB NOTES EXPT #2: PHYSICAL AND CHEMICAL CHANGES 1 TECHNIQUES: chemical and physical changes, reactions, observations READING: PHYSICAL AND CHEMICAL CHANGES e.g. Tro chapter 1 SAFETY: Safety
DETERMINATION of the EMPIRICAL FORMULA
 DETERMINATION of the EMPIRICAL FORMULA One of the fundamental statements of the atomic theory is that elements combine in simple whole number ratios. This observation gives support to the theory of atoms,
DETERMINATION of the EMPIRICAL FORMULA One of the fundamental statements of the atomic theory is that elements combine in simple whole number ratios. This observation gives support to the theory of atoms,
To identify and classify various types of chemical reactions.
 Cycle of Copper Reactions Minneapolis Community and Technical College v.11.17 Objectives: To observe and document copper s chemical changes in five different reactions and verify that copper is conserved
Cycle of Copper Reactions Minneapolis Community and Technical College v.11.17 Objectives: To observe and document copper s chemical changes in five different reactions and verify that copper is conserved
Skills in Science. Lab equipment. (Always draw 2D) Drawings below are NOT to scale. Beaker - A general purpose container with a pouring lip.
 Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
EMPIRICAL FORMULA OF MAGNESIUM OXIDE
 EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
EMPIRICAL FORMULA OF MAGNESIUM OXIDE
 EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
Preparation of copper(ii) sulfate from copper(ii) nitrate
 Student s Name: Date: Background Preparation of copper(ii) sulfate from copper(ii) nitrate The purpose of this laboratory activity is to prepare copper(ii) sulfate from copper(ii) nitrate. This is done
Student s Name: Date: Background Preparation of copper(ii) sulfate from copper(ii) nitrate The purpose of this laboratory activity is to prepare copper(ii) sulfate from copper(ii) nitrate. This is done
TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION
 EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
IDENTIFYING UNKNOWN SUBSTANCES
 IDENTIFYING UNKNOWN SUBSTANCES LAB 15 EXPERIMENT STUDENT BOOK Chapter 1, page 25 TOOLBOX Page 4 and 36 Goal Identify unknown substances with the help of different tests. 1. What is the independent variable
IDENTIFYING UNKNOWN SUBSTANCES LAB 15 EXPERIMENT STUDENT BOOK Chapter 1, page 25 TOOLBOX Page 4 and 36 Goal Identify unknown substances with the help of different tests. 1. What is the independent variable
Gravimetric Analysis: Determination of % Sulfur in Fertilizer
 Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
Experiment. Molar Mass of an Unknown Sulfate Salt by Gravimetric Techniques 1
 Experiment. Molar Mass of an Unknown Sulfate Salt by Gravimetric Techniques 1 This lab is to reacquaint you with some basic laboratory techniques and serves as a warm-up to the experiments in this course.
Experiment. Molar Mass of an Unknown Sulfate Salt by Gravimetric Techniques 1 This lab is to reacquaint you with some basic laboratory techniques and serves as a warm-up to the experiments in this course.
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION
 GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
Chapter 8. Gravimetric Analysis
 Chapter 8 Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a :
Chapter 8 Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a :
solvent diffusion dissolving soluble
 What do we call it when a liquid changes into a solid? What do we call it when a liquid turns into a gas? What do we call it when a gas turns into a liquid? What do we call the solid that dissolves in
What do we call it when a liquid changes into a solid? What do we call it when a liquid turns into a gas? What do we call it when a gas turns into a liquid? What do we call the solid that dissolves in
Recrystallization with a Single Solvent
 Experiment: Recrystallization Part II: Purification of Solids In Part I of the recrystallization experiment, you learned about the factors which make a good recrystallization solvent, and you learned how
Experiment: Recrystallization Part II: Purification of Solids In Part I of the recrystallization experiment, you learned about the factors which make a good recrystallization solvent, and you learned how
2. Crystallization. A. Background
 2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
EMP I RICAL FORMULA OF MAGNESI U M OXIDE
 Experiment 6 Name: 53 EMP I RICAL FORMULA OF MAGNESI U M OXIDE In this experiment, you will synthesize oxide via the reaction pathways summarized in Figure 1. Note that [1] is the main reaction and [2]
Experiment 6 Name: 53 EMP I RICAL FORMULA OF MAGNESI U M OXIDE In this experiment, you will synthesize oxide via the reaction pathways summarized in Figure 1. Note that [1] is the main reaction and [2]
H N 2. Decolorizing carbon O. O Acetanilide
 Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
Copper Smelting by an Ancient Method
 Copper Smelting by an Ancient Method EXPERIMENT ## Prepared by Paul C. Smithson, Berea College, based on Yee et al., 004 Using beads of a copper-containing mineral, students will produce beads of nearly
Copper Smelting by an Ancient Method EXPERIMENT ## Prepared by Paul C. Smithson, Berea College, based on Yee et al., 004 Using beads of a copper-containing mineral, students will produce beads of nearly
Pre-Lab Exercises Lab 8: Biochemistry
 Pre-Lab Exercises Lab 8: Biochemistry Name Date Section 1. List the 3 basic components of a DNA nucleotide, and draw a simple picture to show how they interact. 2. Consider the amine bases in DNA. List
Pre-Lab Exercises Lab 8: Biochemistry Name Date Section 1. List the 3 basic components of a DNA nucleotide, and draw a simple picture to show how they interact. 2. Consider the amine bases in DNA. List
EXPERIMENT 3: Identification of a Substance by Physical Properties
 EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
Elemental Mass Percent and Empirical Formula From Decomposition of a Copper Oxide
 EXPERIMENT Elemental Mass Percent and Empirical Formula From Decomposition 6 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College The student will heat
EXPERIMENT Elemental Mass Percent and Empirical Formula From Decomposition 6 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College The student will heat
Experiment 13: Determination of Molecular Weight by Freezing Point Depression
 1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
(aq) + 5e - Mn 2+ (aq) + 4H 2
 EXPERIMENT 20 Titrimetric Determination of Iron INTRODUCTION Potassium permanganate is widely used as an oxidizing agent in titrimetric analysis. In acidic solution, a permanganate ion undergoes reduction
EXPERIMENT 20 Titrimetric Determination of Iron INTRODUCTION Potassium permanganate is widely used as an oxidizing agent in titrimetric analysis. In acidic solution, a permanganate ion undergoes reduction
TITANIUM DIOXIDE. SYNONYMS Titania; CI Pigment white 6; CI (1975) No ; INS No. 171 DEFINITION DESCRIPTION FUNCTIONAL USES CHARACTERISTICS
 TITANIUM DIOXIDE Prepared at the 71 st JECFA (2009) and published in FAO JECFA Monographs 7 (2009), superseding specifications prepared at the 67 th JECFA (2006) and published in FAO JECFA Monographs 3
TITANIUM DIOXIDE Prepared at the 71 st JECFA (2009) and published in FAO JECFA Monographs 7 (2009), superseding specifications prepared at the 67 th JECFA (2006) and published in FAO JECFA Monographs 3
An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+
 An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+ LAB ADV COMP 8 From Advanced Chemistry with Vernier, Vernier Software & Technology, 2004 INTRODUCTION A titration, as you recall, is a
An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+ LAB ADV COMP 8 From Advanced Chemistry with Vernier, Vernier Software & Technology, 2004 INTRODUCTION A titration, as you recall, is a
Total Dissolved Solids
 Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Salinity in Seawater
 Salinity in Seawater Objective To familiarize students with the different methods used for measuring salinity of water. Introduction: Salinity exerts profound impacts on the marine environment. It controls
Salinity in Seawater Objective To familiarize students with the different methods used for measuring salinity of water. Introduction: Salinity exerts profound impacts on the marine environment. It controls
Determination of the Empirical Formula of Magnesium Oxide
 Determination of the Empirical Formula of Magnesium Oxide The quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. Magnesium
Determination of the Empirical Formula of Magnesium Oxide The quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. Magnesium
Mon. Tues. Wed. Thurs. Fri. AM or PM B
 Name: (cf. Honesty Declaration Statement on page 20) Laboratory Day (circle) Lab Room Locker Lab. Session (circle) Lab. Section Mon. Tues. Wed. Thurs. Fri. AM or PM B Date experiment is performed MARK:
Name: (cf. Honesty Declaration Statement on page 20) Laboratory Day (circle) Lab Room Locker Lab. Session (circle) Lab. Section Mon. Tues. Wed. Thurs. Fri. AM or PM B Date experiment is performed MARK:
Periodic Properties: Analysis of Group I, II and Transition Metals
 Periodic Properties: Analysis of Group I, II and Transition Metals Procedure All solutions should be mixed thoroughly after any additions. Clearly record all observations (indicating what you did), even
Periodic Properties: Analysis of Group I, II and Transition Metals Procedure All solutions should be mixed thoroughly after any additions. Clearly record all observations (indicating what you did), even
Analysis of Calcium Carbonate Tablets
 Experiment 9 Analysis of Calcium Carbonate Tablets Prepared by Ross S. Nord, Eastern Michigan University PURPOSE To perform a gravimetric exercise to determine weight percent of active ingredient in a
Experiment 9 Analysis of Calcium Carbonate Tablets Prepared by Ross S. Nord, Eastern Michigan University PURPOSE To perform a gravimetric exercise to determine weight percent of active ingredient in a
Name Honors Chemistry / /
 Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Evaluation copy. Total Dissolved Solids. Computer INTRODUCTION
 Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Episode 608: Latent heat
 Episode 608: Latent heat Energy is involved in changes of phase, even though there is no change of temperature. Summary Discussion: Defining specific latent heat. (10 minutes) Demonstration: Boiling water.
Episode 608: Latent heat Energy is involved in changes of phase, even though there is no change of temperature. Summary Discussion: Defining specific latent heat. (10 minutes) Demonstration: Boiling water.
Experiment 2: Preparation of the Artificial Sweetener Dulcin
 Experiment 2: Preparation of the Artificial Sweetener Dulcin Organic compounds known as sugars are carbohydrates that occur widely in nature. For example, sucrose (aka table sugar) is found in sugar can,
Experiment 2: Preparation of the Artificial Sweetener Dulcin Organic compounds known as sugars are carbohydrates that occur widely in nature. For example, sucrose (aka table sugar) is found in sugar can,
TESTING THE WATERS HOW GOOD IS THAT BOTTLED WATER AND HOW EFFECTIVE IS YOUR WATER FILTER
 TESTING THE WATERS HOW GOOD IS THAT BOTTLED WATER AND HOW EFFECTIVE IS YOUR WATER FILTER TEACHER NOTES This experiment is designed for students working singly or in groups of two. One run through the series
TESTING THE WATERS HOW GOOD IS THAT BOTTLED WATER AND HOW EFFECTIVE IS YOUR WATER FILTER TEACHER NOTES This experiment is designed for students working singly or in groups of two. One run through the series
The Copper Group. The Separation of the Copper Group:
 5/6/09 :06 PM The Copper Group The Separation of the Copper Group: Facts: The SULFIDES of Lead, Mercury (II), Bismuth, Copper, Cadmium, Arsenic, Antimony, and Tin are insoluble in dilute HCI The sulfides
5/6/09 :06 PM The Copper Group The Separation of the Copper Group: Facts: The SULFIDES of Lead, Mercury (II), Bismuth, Copper, Cadmium, Arsenic, Antimony, and Tin are insoluble in dilute HCI The sulfides
Partner: Cathy 22 March Separation and Qualitative Determination of Cations and Anions
 Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017)
 CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017) SEPARATION AND RECOVERY OF ORGANIC COMPOUNDS, THIN LAYER CHROMATOGRAPHY, COLUMN CHROMATOGRAPHY, CRYSTALLIZATION, AND MELTING POINTS Overview
CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017) SEPARATION AND RECOVERY OF ORGANIC COMPOUNDS, THIN LAYER CHROMATOGRAPHY, COLUMN CHROMATOGRAPHY, CRYSTALLIZATION, AND MELTING POINTS Overview
Teknik Bioseparasi. Dina Wahyu. Genap/ Maret 2014
 4. Teknik Bioseparasi Dina Wahyu Genap/ Maret 2014 Outline Chemical Reaction Engineering 1 2 3 4 5 6 7 Pendahuluan mempelajari ruang lingkup teknik bioseparasi dan teknik cel disruption Teknik Pemisahan
4. Teknik Bioseparasi Dina Wahyu Genap/ Maret 2014 Outline Chemical Reaction Engineering 1 2 3 4 5 6 7 Pendahuluan mempelajari ruang lingkup teknik bioseparasi dan teknik cel disruption Teknik Pemisahan
LAD B3 (pg! 1 of 6! ) Analysis by Redox Titration Name Per
 LAD B3 (pg! 1 of 6! ) Name Per Introduction As you know, one common type of reaction in chemistry is oxidation-reduction. It involves the transfer of electrons from one species to another. Atoms undergo
LAD B3 (pg! 1 of 6! ) Name Per Introduction As you know, one common type of reaction in chemistry is oxidation-reduction. It involves the transfer of electrons from one species to another. Atoms undergo
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard.
 1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
1. Marie mixed 5 g of carbon with 5 g of lead oxide. She heated the mixture strongly for 15 minutes in a fume cupboard. After 15 minutes, Marie found some shiny beads in the mixture. a. (i) Marie collected
The Crystal Forest Favorite Holiday Demonstrations
 The Crystal Forest Favorite Holiday Demonstrations SCIENTIFIC Introduction Put a new twist on crystal growing. In this class participation demonstration, students cut out and assemble miniature trees and
The Crystal Forest Favorite Holiday Demonstrations SCIENTIFIC Introduction Put a new twist on crystal growing. In this class participation demonstration, students cut out and assemble miniature trees and
Nickel Electroplating
 Nickel Electroplating In a galvanic or voltaic electrochemical cell, the spontaneous reaction occurs and electrons flow from the anode (oxidation) to the cathode (reduction). In an electrolytic cell, a
Nickel Electroplating In a galvanic or voltaic electrochemical cell, the spontaneous reaction occurs and electrons flow from the anode (oxidation) to the cathode (reduction). In an electrolytic cell, a
Experiment: Preparation of Adipic Acid by Oxidative Cleavage of Cyclohexene
 Experiment: Preparation of Adipic Acid by xidative Cleavage of Cyclohexene Under mild conditions, only the pi bond of the alkene is cleaved to form 1,2-diols or epoxides. Under more rigorous oxidation
Experiment: Preparation of Adipic Acid by xidative Cleavage of Cyclohexene Under mild conditions, only the pi bond of the alkene is cleaved to form 1,2-diols or epoxides. Under more rigorous oxidation
Lab #3: Law of Definite Proportions
 Name Lab #3: Law of Definite Proportions Sept. 21, 2016 Purpose To find the percent composition and therefore definite ratio of the elements in magnesium oxide. Background When magnesium and oxygen are
Name Lab #3: Law of Definite Proportions Sept. 21, 2016 Purpose To find the percent composition and therefore definite ratio of the elements in magnesium oxide. Background When magnesium and oxygen are
Experiment 2: The Chromatography of Organic Compounds
 Experiment 2: The Chromatography of Organic Compounds INTRODUCTION When performing an organic reaction, it is very common to observe the formation of other compounds in addition to your desired product;
Experiment 2: The Chromatography of Organic Compounds INTRODUCTION When performing an organic reaction, it is very common to observe the formation of other compounds in addition to your desired product;
» Talc is a native, hydrous magnesium silicate, sometimes containing a small proportion of aluminum silicate.
 Change to read: Talc» Talc is a native, hydrous magnesium silicate, sometimes containing a small proportion of aluminum silicate. Packaging and storage Preserve in well closed containers. Identification
Change to read: Talc» Talc is a native, hydrous magnesium silicate, sometimes containing a small proportion of aluminum silicate. Packaging and storage Preserve in well closed containers. Identification
Forensics with TI-Nspire Technology
 Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com Science Objectives Identify characteristics of different soils to demonstrate that a suspect has been at a scene.
Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com Science Objectives Identify characteristics of different soils to demonstrate that a suspect has been at a scene.
Core Lab: Energy Changes During Melting and Evaporation
 Science 1206 Name: Core Lab: Energy Changes During Melting and Evaporation Background: When a substance melts, it changes from a solid to a liquid. When a substance evaporates (or vaporizes), it changes
Science 1206 Name: Core Lab: Energy Changes During Melting and Evaporation Background: When a substance melts, it changes from a solid to a liquid. When a substance evaporates (or vaporizes), it changes
Part A (Level 1) A Matching (4 marks, 1 mark each) B Multiple-choice questions (20 marks, 2 marks each) Name: ( ) Time and Marks Class: Date:
 Unit 5 S1 Science Test The wonderful solvent - water Name: ( ) Time and Marks Class: Date: Part A: 35 min / 100 marks Parts A & B: 45 min / 120 marks Note: 1 Attempt ALL questions. 2 Write your answers
Unit 5 S1 Science Test The wonderful solvent - water Name: ( ) Time and Marks Class: Date: Part A: 35 min / 100 marks Parts A & B: 45 min / 120 marks Note: 1 Attempt ALL questions. 2 Write your answers
Experiment 3: The Chromatography of Organic Compounds
 Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
Lab 2: Determination of the empirical formula of the product of magnesium heating
 Chemistry 140 Please have parts 1, 2, 3 and 4 (tables only) ready before class on Wednesday, October 11. Write an abstract and paper-clip it to the front of your individual writeup. The abstract and the
Chemistry 140 Please have parts 1, 2, 3 and 4 (tables only) ready before class on Wednesday, October 11. Write an abstract and paper-clip it to the front of your individual writeup. The abstract and the
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Group IV and V Qualitative Analysis
 Group IV/V Analysis Page 1 Illinois Central College CHEMISTRY 132 Laboratory Section: Group IV and V Qualitative Analysis Name: Equipment 1-tray of dropper bottles 2-micro spatulas 2-wooden test tube blocks
Group IV/V Analysis Page 1 Illinois Central College CHEMISTRY 132 Laboratory Section: Group IV and V Qualitative Analysis Name: Equipment 1-tray of dropper bottles 2-micro spatulas 2-wooden test tube blocks
Principles and Practice of Agarose Gel Electrophoresis
 Edvo-Kit #101 Principles and Practice of Agarose Gel Electrophoresis Experiment Objective: The objective of this experiment is to develop a basic understanding of electrophoretic theory, and to gain "hands-on"
Edvo-Kit #101 Principles and Practice of Agarose Gel Electrophoresis Experiment Objective: The objective of this experiment is to develop a basic understanding of electrophoretic theory, and to gain "hands-on"
EXPERIMENT 6. Determination of the Ideal Gas Law Constant - R. Magnesium metal reacts with hydrochloric acid according to the following reaction,
 EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
Chem 2115 Experiment #9. Consumer Chemistry: Determining the Iron Content in Supplements
 Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
CONSERVATION OF MATTER AND CHEMICAL PROPERTIES
 1 CONSERVATION OF MATTER AND CHEMICAL PROPERTIES I. OBJECTIVES AND BACKGROUND The object of this experiment is to demonstrate the conservation of matter- or more particularly, the conservation of "atoms"
1 CONSERVATION OF MATTER AND CHEMICAL PROPERTIES I. OBJECTIVES AND BACKGROUND The object of this experiment is to demonstrate the conservation of matter- or more particularly, the conservation of "atoms"
Principles of Gel Filtration Chromatography
 EDVOTEK P.O. Box 1232 West Bethesda, MD 20827-1232 The Biotechnology Principles of Gel Filtration Chromatography 108 EDVO-Kit # Storage: Store entire experiment at room temperature. Experiment Objective:
EDVOTEK P.O. Box 1232 West Bethesda, MD 20827-1232 The Biotechnology Principles of Gel Filtration Chromatography 108 EDVO-Kit # Storage: Store entire experiment at room temperature. Experiment Objective:
Lab 4B: Moles of Iron & Copper
 Lab 4B: Moles of Iron & Copper Name: Block: Group Members: Date: / /2018 Due Date: Drop Date: Criteria Objective: Clearly states the purpose of the experiment, written in your own words and briefly outlines
Lab 4B: Moles of Iron & Copper Name: Block: Group Members: Date: / /2018 Due Date: Drop Date: Criteria Objective: Clearly states the purpose of the experiment, written in your own words and briefly outlines
Principles of Gel Filtration Chromatography
 Edvo-Kit #108 Principles of Gel Filtration Chromatography Experiment Objective: The objective of this experiment is to introduce the principles of gel fi ltration chromatography as a method that separates
Edvo-Kit #108 Principles of Gel Filtration Chromatography Experiment Objective: The objective of this experiment is to introduce the principles of gel fi ltration chromatography as a method that separates
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS
 EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
7. Determination of Melting Points
 7. Determination of Melting Points This experiment consists of three parts. In the first part, you will determine the melting point range of three known compounds. This part is mostly for practice, to
7. Determination of Melting Points This experiment consists of three parts. In the first part, you will determine the melting point range of three known compounds. This part is mostly for practice, to
Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost
 Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost by Page 1/21 Contents What is the effect of compost on soil properties?... 3 Introduction:...
Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost by Page 1/21 Contents What is the effect of compost on soil properties?... 3 Introduction:...
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley)
 Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Measuring Manganese Concentration Using Spectrophotometry
 Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
Tex-239-F, Asphalt Release Agent
 Contents: Section 1 Overview...2 Section 2 Part I, 7-day Asphalt Stripping Test...3 Section 3 Part II, Mixture Slide Test...5 Section 4 Part III, Asphalt Performance Test...7 Section 5 Part IV, Release
Contents: Section 1 Overview...2 Section 2 Part I, 7-day Asphalt Stripping Test...3 Section 3 Part II, Mixture Slide Test...5 Section 4 Part III, Asphalt Performance Test...7 Section 5 Part IV, Release
Analysis of Alum, AlK(SO 4 ) 2 12H 2 O AP Chemistry Laboratory #2
 Analysis of Alum, AlK(SO 4 ) 2 12H 2 O AP Chemistry Laboratory #2 Catalo No. AP6354 Publication No. 6354A Introduction When a compound is synthesized, tests are carried out to confirm whether the compound
Analysis of Alum, AlK(SO 4 ) 2 12H 2 O AP Chemistry Laboratory #2 Catalo No. AP6354 Publication No. 6354A Introduction When a compound is synthesized, tests are carried out to confirm whether the compound
USEPA Membrane Filtration Method Method m-tec. Scope and Application: For potable water, nonpotable water, recreation water and wastewater.
 , MF, m-tec, 8367 DOC316.53.001210 USEPA Membrane Filtration Method Method 8367 1 m-tec Scope and Application: For potable water, nonpotable water, recreation water and wastewater. 1 USEPA accepted. Test
, MF, m-tec, 8367 DOC316.53.001210 USEPA Membrane Filtration Method Method 8367 1 m-tec Scope and Application: For potable water, nonpotable water, recreation water and wastewater. 1 USEPA accepted. Test
Assistant Lecturer. Sahar Mohammed Shakir Assistant Lecturer. Abdul Hafeedh Hameed Assistant Lecturer. Ali Basim
 Assistant Lecturer Sahar Mohammed Shakir Assistant Lecturer Abdul Hafeedh Hameed Assistant Lecturer Ali Basim Solid organic cpd.s when isolated from organic reaction are impure; they are contaminated with
Assistant Lecturer Sahar Mohammed Shakir Assistant Lecturer Abdul Hafeedh Hameed Assistant Lecturer Ali Basim Solid organic cpd.s when isolated from organic reaction are impure; they are contaminated with
Eutrophication Using Up Oxygen In Water
 Eutrophication Using Up Oxygen In Water Topic Water pollution causing oxygen depletion in water by living organisms Introduction Farming is a major cause of freshwater pollution. Sewage and farm animal
Eutrophication Using Up Oxygen In Water Topic Water pollution causing oxygen depletion in water by living organisms Introduction Farming is a major cause of freshwater pollution. Sewage and farm animal
TECHNICAL GRADE MOLYBDENUM OXIDE
 Procedure for the Assaying of TECHNICAL GRADE MOLYBDENUM OXIDE GUIDELINES FROM R INTRODUCTION This Guideline on good practice in relation to the Assaying of Molybdenite Concentrates is one of a six part
Procedure for the Assaying of TECHNICAL GRADE MOLYBDENUM OXIDE GUIDELINES FROM R INTRODUCTION This Guideline on good practice in relation to the Assaying of Molybdenite Concentrates is one of a six part
M. It is expressed in units such a g/cc,
 Experiment 2 Density Part I: Density of a solid Density is defined as mass per unit volume, D = V M. It is expressed in units such a g/cc, g/ml, lb/ft 3, etc. Determination of mass is done by weighing
Experiment 2 Density Part I: Density of a solid Density is defined as mass per unit volume, D = V M. It is expressed in units such a g/cc, g/ml, lb/ft 3, etc. Determination of mass is done by weighing
crystallization melting individual molecules crystal lattice
 ... modular publisher: H. A. Neidig 3 TECH laboratory program in chemistry organic editor: Joe Jeffers 703. i8.. Purifying Acetanilide by Recrystallization prepared by Carl Wigal, Lebanon Valley College
... modular publisher: H. A. Neidig 3 TECH laboratory program in chemistry organic editor: Joe Jeffers 703. i8.. Purifying Acetanilide by Recrystallization prepared by Carl Wigal, Lebanon Valley College
ENGI Environmental Laboratory. Lab #2. Solids Determination. Faculty of Engineering & Applied Science
 ENGI 9628 Environmental Laboratory Lab #2 Solids Determination Faculty of Engineering & Applied Science SOLIDS DETERMINATION PURPOSE Using Standard Methods for solids determination. THEORY Total solids
ENGI 9628 Environmental Laboratory Lab #2 Solids Determination Faculty of Engineering & Applied Science SOLIDS DETERMINATION PURPOSE Using Standard Methods for solids determination. THEORY Total solids
The determination of copper in brass
 The determination of copper in brass Objective - To determine the amount of copper in a brass sample Background Brass is an alloy made of copper and zinc. Most brass contains about 60% copper. The proportions
The determination of copper in brass Objective - To determine the amount of copper in a brass sample Background Brass is an alloy made of copper and zinc. Most brass contains about 60% copper. The proportions
Experimental techniques
 Unit 1 Experimental techniques In this unit you will come to understand the general principles behind various experimental techniques. Experimental investigation is a very important aspect of Physical
Unit 1 Experimental techniques In this unit you will come to understand the general principles behind various experimental techniques. Experimental investigation is a very important aspect of Physical
Name Lab Section Date. Sediment Lab
 Name Lab Section Date. Investigating Stokes Law Sediment Lab ds = density of solid, g/cm dw = density of water, g/cm g = gravity, 980 cm/second 2 D = particle diameter in centimeters μ = molecular viscosity,
Name Lab Section Date. Investigating Stokes Law Sediment Lab ds = density of solid, g/cm dw = density of water, g/cm g = gravity, 980 cm/second 2 D = particle diameter in centimeters μ = molecular viscosity,
Laser ACB 50 Product Code: Revised Date: 03/17/2009. Laser ACB 50
 DESCRIPTION Laser ACB 50 Laser ACB 50 is a peroxide/sulfuric acid system that replaces bichromate, chromic acid, or nitricsulfuric acid pickles commonly used for pickling copper, brass and bronze alloys.
DESCRIPTION Laser ACB 50 Laser ACB 50 is a peroxide/sulfuric acid system that replaces bichromate, chromic acid, or nitricsulfuric acid pickles commonly used for pickling copper, brass and bronze alloys.
Human DNA Alu Amplification by Polymerase Chain Reaction (PCR)* Laboratory Procedure
 Human DNA Alu Amplification by Polymerase Chain Reaction (PCR)* Laboratory Procedure *Polymerase Chain Reaction is covered by patents owned by Hoffmann-La Roche, Inc. This experiment was adapted from Laboratory
Human DNA Alu Amplification by Polymerase Chain Reaction (PCR)* Laboratory Procedure *Polymerase Chain Reaction is covered by patents owned by Hoffmann-La Roche, Inc. This experiment was adapted from Laboratory
The 2017 Wisconsin Crystal Growing Competition Handbook
 Molecular Structure Laboratory Chemistry Department University of Wisconsin-Madison The 2017 Wisconsin Crystal Growing Competition Handbook September 9, 2016 http://xray.chem.wisc.edu/wicgc_2017.html Official
Molecular Structure Laboratory Chemistry Department University of Wisconsin-Madison The 2017 Wisconsin Crystal Growing Competition Handbook September 9, 2016 http://xray.chem.wisc.edu/wicgc_2017.html Official
Research Paper On The Comparison Of The LDH Isoenzymes From Scuds From Two Different Geographic Locations
 Research Paper On The Comparison Of The LDH Isoenzymes From Scuds From Two Different Geographic Locations Abstract Two different populations of Gammarus fasciatus were studied to see if their LDH isoenzymes
Research Paper On The Comparison Of The LDH Isoenzymes From Scuds From Two Different Geographic Locations Abstract Two different populations of Gammarus fasciatus were studied to see if their LDH isoenzymes
PHASE CHANGES. Time Temperature Observations. Name(s)
 3 5 PHASE CHANGES PHASE CHANGES Name(s) The activities presented here focus on the energy changes that occur in substances undergoing a phase change. The first activity will take the most time to complete.
3 5 PHASE CHANGES PHASE CHANGES Name(s) The activities presented here focus on the energy changes that occur in substances undergoing a phase change. The first activity will take the most time to complete.
Year 7 Chemistry HW Questions
 Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Soil Particle Density Protocol
 Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
EXPERIMENT 5. Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline
 EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
YEAR 7 SCIENCE EXAMINATION. Semester MULTIPLE CHOICE QUESTION BOOK 1 MATERIAL REQUIRED / RECOMMENDED FOR THIS PAPER:
 YEAR 7 SCIENCE EXAMINATION Semester 2 2016 MULTIPLE CHOICE QUESTION BOOK 1 STUDENT NAME: TEACHER: DATE: Time allowed for this exam: (Book 1 and Book 2 Combined) Reading time before commencing work: Working
YEAR 7 SCIENCE EXAMINATION Semester 2 2016 MULTIPLE CHOICE QUESTION BOOK 1 STUDENT NAME: TEACHER: DATE: Time allowed for this exam: (Book 1 and Book 2 Combined) Reading time before commencing work: Working
The Aluminum Group. Separation of the Aluminum Group from all others
 5/18/09 :6 PM The Aluminum Group Separation of the Aluminum Group from all others Facts: Addition of NHCl, NH40H, and (NH 4) 2S to a solution containing all the cations not precipitated in the preceding
5/18/09 :6 PM The Aluminum Group Separation of the Aluminum Group from all others Facts: Addition of NHCl, NH40H, and (NH 4) 2S to a solution containing all the cations not precipitated in the preceding
ALTERNATIVE TRANSPORTATION FUELS LAB
 ALTERNATIVE TRANSPORTATION FUELS LAB Purpose: To examine the energy content of various alternative fuels which could be used for powering our vehicles in the future. As gasoline becomes increasingly expensive,
ALTERNATIVE TRANSPORTATION FUELS LAB Purpose: To examine the energy content of various alternative fuels which could be used for powering our vehicles in the future. As gasoline becomes increasingly expensive,
Experiment 30A ENERGY CONTENT OF FUELS
 Experiment 30A ENERGY CONTENT OF FUELS FV 12/10/2012 MATERIALS: 12-oz. aluminum beverage can with top cut out and holes on side, thermometer, 100 ml graduated cylinder, 800 ml beaker, long-stem lighter,
Experiment 30A ENERGY CONTENT OF FUELS FV 12/10/2012 MATERIALS: 12-oz. aluminum beverage can with top cut out and holes on side, thermometer, 100 ml graduated cylinder, 800 ml beaker, long-stem lighter,
The table below shows some of the properties of the elements cobalt and nickel.
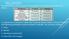 BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
, to form the products magnesium oxide, MgO(s), and magnesium nitride, Mg 3
 Active Metals in the Periodic Table Periodic Trends and the Properties of the Elements SCIENTIFIC Introduction Like good experiments, good demonstrations often lead to other demonstrations. What follows
Active Metals in the Periodic Table Periodic Trends and the Properties of the Elements SCIENTIFIC Introduction Like good experiments, good demonstrations often lead to other demonstrations. What follows
SPECTROPHOTOMETRIC DETERMINATION OF IRON
 SPECTROPHOTOMETRIC DETERMINATION OF IRON In this experiment you will determine trace amounts of iron using spectrophotometric methods. BACKGROUND In solution ferrous iron combines with 2,2 bipyridyl to
SPECTROPHOTOMETRIC DETERMINATION OF IRON In this experiment you will determine trace amounts of iron using spectrophotometric methods. BACKGROUND In solution ferrous iron combines with 2,2 bipyridyl to
By Authority Of THE UNITED STATES OF AMERICA Legally Binding Document
 By Authority Of THE UNITED STATES OF AMERICA Legally Binding Document By the Authority Vested By Part 5 of the United States Code 552(a) and Part 1 of the Code of Regulations 51 the attached document has
By Authority Of THE UNITED STATES OF AMERICA Legally Binding Document By the Authority Vested By Part 5 of the United States Code 552(a) and Part 1 of the Code of Regulations 51 the attached document has
Pre-Lab 5: Magnesium and Magnesium Oxide
 Name: Pre-Lab 5: Magnesium and Magnesium Oxide Section: Answer the following questions after reading the background information at the beginning of the lab. This should be completed before coming to lab.
Name: Pre-Lab 5: Magnesium and Magnesium Oxide Section: Answer the following questions after reading the background information at the beginning of the lab. This should be completed before coming to lab.
Spectrophotometry of DNA and RNA
 Spectrophotometry of DNA and RNA Many of the techniques used to study cells are focused on characterization of the molecules that make up cells. Since the molecules are invisible, they are studied using
Spectrophotometry of DNA and RNA Many of the techniques used to study cells are focused on characterization of the molecules that make up cells. Since the molecules are invisible, they are studied using
