Copyright 2004 The Pharmaceutical & Healthcare Sciences Society
|
|
|
- Eleanore Johns
- 6 years ago
- Views:
Transcription
1 Whyte, W., and Eaton, T. (2004) Microbial risk assessment in pharmaceutical cleanrooms. European Journal of Parenteral and Pharmaceutical Sciences, 9 (1). pp ISSN Copyright 2004 The Pharmaceutical & Healthcare Sciences Society A copy can be downloaded for personal non-commercial research or study, without prior permission or charge Content must not be changed in any way or reproduced in any format or medium without the formal permission of the copyright holder(s) When referring to this work, full bibliographic details must be given Deposited on: 26 September 2013 Enlighten Research publications by members of the University of Glasgow
2 European Journal of Parenteral & Pharmaceutical Sciences 2004; 9(1): Parenteral Society Microbial risk assessment in pharmaceutical cleanrooms W Whyte 1 and T Eaton 2 1 James Watt Building, University of Glasgow, Scotland, UK. 2 AstraZeneca, Macclesfield, UK. The microbial risk to aseptically manufactured products in pharmaceutical cleanrooms can be assessed by the use of fundamental equations that model the dispersion, transfer and deposition of microbial contamination, and the use of numerical values or risk descriptors. This can be done in two-stages, with the first stage used to assess the transfer of contamination from all of the sources within the cleanroom suite and the second stage used to assess both air and surface contact contamination within critical production areas. These two methods can be used to assess and reduce microbial risk at the preliminary design stage of the cleanroom and associated manufacturing process or, retrospectively, for an established manufacturing operation. Introduction A number of systems exist for managing risk during manufacturing. These include Fault Tree Analysis (FTA) 1, Failure Mode and Effect Analysis (FMEA) 2, Hazard and Operational Studies (HAZOP) 3, and Hazard Analysis and Critical Control Point (HACCP) 4. The HACCP system, having been developed for the food industry, appears to be the most suitable risk management system for the methodical assessment, control and monitoring of microbial risk in the pharmaceutical industry 5. An important part of a risk management system is the risk assessment process, where the importance or degree of risk associated with each identified hazard is assessed. Each hazard is assigned a numerical value or descriptor of risk. Those hazards with a high degree of risk can then be considered further in order to reduce the risk to a more acceptable level. A useful risk assessment method is the FMECA criticality method outlined in International Electronic Commission report on the procedure for Failure Mode and Effect Analysis (FMEA) 2. The FMECA method is based on the following equation: Equation 1 Risk = criticality of the occurrence frequency of occurrence This risk equation was considered in a previously published paper 6, and shown to be more correct than a similar equation that includes a third variable of detection. It was also established that criticality, when considering microbial contamination, reflects the importance of a hazard in terms of the number of microbes from the hazard i.e. source, that are deposited onto the product; this should be expressed in terms of the concentration of microbes on, or in, a source, and their Corresponding author: W Whyte, James Watt Building, University of Glasgow, Scotland, UK. w.whyte@mech.gla.ac.uk likelihood of dispersion, transfer, and deposition. The frequency variable should be expressed as either the time the product is exposed, or the frequency of contamination incidents. If this is accepted, then the criticality risk assessment Equation 1 is the same as the fundamental equations described below, and its scientific basis vindicated. To accurately assess microbial risk, the basic models of risk must be known so that the fundamental variables that predict risk and their importance in relation to the other variables can be determined. The overall equation that applies to the risk of microbial contamination of a product has been derived 6 and is as follows: Equation 2 No. of microbes deposited on a product = C S P d P a A T where: C = concentration of microbial contamination on, or in, a source (number/cm 2 for a surface, or number/cm 3 for air); S = the quantity of surface material, or air, that is dispersed, from a source in a given time (cm 2 /s for surfaces, and cm 3 /s for air dispersion); this can also be expressed as the quantity dispersed per frequency of occurrence; P d = proportion of microorganisms dispersed from a source that are transferred to the area adjacent to the product; P a = proportion of microorganisms in the adjacent area that are deposited per unit area of the product (/cm 2 ); A = area of surface onto which microbes are deposit (cm 2 ); T = time, during which transfers occur(s); this can also be expressed as frequency of occurrence. Two simplified versions of Equation 2 were derived 6 to model surface contact and airborne deposition of microbes into, or onto, a product. Equation 3 models surface contact contamination: Equation 3 Number of microorganisms deposited by surface contact over 16
3 MICROBIAL RISK ASSESSMENT IN PHARMACEUTICAL CLEANROOMS 17 a given time (no.) = microbes on contaminating surface (no./cm 2 ) x transfer coefficient x area of product that is contacted (cm 2 ) frequency of contact where: the transfer coefficient is the proportion of microorganisms on a contaminating surface (such as a glove) that are transferred to the product. Equation 4 models airborne deposition onto a product and is as follows: Equation 4 Number of airborne microorganisms deposited onto the product in a given time (no.) = deposition rate of microbe carrying particles (no./(cm 2.s)) x area of product exposed (cm 2 ) x time of exposure (s) Most microbes in the cleanroom air are rafted on particles of skin, their average size being between about 8µm and 20µm and these microbe carrying particles will deposit into the product, mainly by gravity 7. The deposition rate of microbe carrying particles into, or onto, a product can be ascertained from settle plates exposed adjacent to the exposed product 8. To obtain the most accurate estimate of the amount of microbial contamination by airborne deposition or surface contact, numerical values should be substituted into the above equations. However, in pharmaceutical production, much of this numerical information is unknown and it is therefore necessary to employ risk assessment methods using descriptors that act as surrogates for the required numerical data. To obtain the highest degree of accuracy from the use of descriptors, those that best describe the variables in the above equations should be chosen. They should be combined as indicated in the equations, so that the relative importance of the variables is retained. This can be done using the FMECA method as described in the following sections of this paper. Overall deposition model It is useful to start a microbial risk assessment of a pharmaceutical production area by considering all of the microbial hazards in the various clean areas in the manufacturing suite; a detailed consideration of critical areas will be carried out later. In this paper, all microbial sources are considered to be potential hazards. To carry out an overall risk assessment, the analysis should be based on Equation 2. The concentration of microbes in the air, or on surfaces, is one of the required variables and numerical values should be available, and used. However, numerical values are likely to be unavailable for other variables in the equation. The time duration, or frequency of microbial contamination in cleanroom, can be continuous or unknown. For example, the rate of deposition of microbes from cleanroom air is continuous, the rate not varying significantly during production. Also, the frequency of incidents of surface contamination, such as when personnel touch the product, is normally unknown, personnel often being unaware of its occurrence. Thus, for the purpose of an overall risk assessment, the variable of time (T) is not used. Numerical information is also rarely available about the dispersion, transfer and deposition of micro-organisms. Consequently, a simplified version of the equation using surrogate descriptors for these unattainable variables is shown as Equation 5 and should be used for the analysis. Equation 5 Risk from microbial contamination (risk rating) = A B C D where: A = microbial contamination on, or in, a source; B = ease of dispersion and transfer; C = proximity of source from critical area; D = effectiveness of control method. The risk rating of each source is determined by assigning risk scores to risk factors A to D. Given in Table 1 is an example of risk scores that can be used. It should be noted that the chance of transfer of contamination (risk factor C) is assessed from the distance the source is from the product. This is a reasonable approach, but may need modification in some circumstances e.g. a person upstream of the product in a unidirectional air flow will have a different chance of contaminating the product than someone standing the same distance downstream. Other risk factors may need similar modification. A comprehensive identification of all the sources of microbial risk, is fundamental to the success of this approach. A method to identify and group sources of microbial contamination has been discussed by Whyte 5. Shown in Figure 1, in Mind Map format, are the groupings of microbial sources that should be considered. During an actual assessment, a more extensive identification of sources is expected. Also shown in Figure 1 are the connections between people and other sources, emphasising the fact that people are the primary source of microbes in a cleanroom. Table 1: Scores for risk factors used for assessing hazards Risk factor (A) Risk factor (B) Risk factor (C) Risk factor (D) Amount of microbial Ease of dispersion, Proximity (location) Effectiveness contamination or transfer, of of source from of control on, or in, a source microorganisms critical area method 0 = nil 0 = nil 0 = remote 0 = full barrier control* 0.5 = very low 0.5 = very low 0.5 = in outside 0.5 = very good control corridor, air lock 1 = low 1 = low 1 = periphery of 1 = good control cleanroom 1.5 = medium 1.5 = medium 1.5 = general 1.5 = some control area of cleanroom 2 = high 2 = high 2 = critical area 2 = no control *Complete physical barrier between source and critical area
4 18 W WHYTE AND T EATON Figure 1. Microbial sources in a cleanroom. Table 2. Calculation of the risk rating for an identified source. Group and Source 1. Areas adjacent to production cleanroom 1.a Air outside production cleanroom (corridor) Risk factor A. Risk factor B. Risk factor C. Risk factor D. Risk rating Conc. of microbes Ease of movement to Proximity to product Effectiveness of product control (method given) It is now possible to obtain a risk rating by assessing each identified source and assigning risk scores to the four risk factors. Shown in Table 2 is the first line of such a resultant assessment with a set of assigned scores and the risk rating obtained. From the risk rating values, the importance of each source can be assigned a risk rating of high, medium or low. For example, hazards that have a risk rating of: (a) 4 and over may be considered as high ; (b) below 4, and over or equal to 2, as medium ; and (c) less than 2 as low. The designation of high to a source does not mean that it has a high risk to the product, but within a list of possible hazards it is likely to be one with a higher degree of risk. Hazards with a high or medium risk can then be further considered with a view to reducing their risk. This can be (positive outflow from the cleanroom and air dilution in corridors) 0.75 done by reducing the risk scores of one or more of factors A to D. The list of sources, and their risk ratings, can then be used in subsequent parts of a risk management analysis based on HACCP, to determine whether adequate control methods are used, and if the risks or their control methods are adequately monitored. This is can be done as indicated in Table 3, where the sources assessed previously are further considered. The HACCP system can then be further utilised to set monitoring schedules, appropriate action and alert limits, verify that the system works, prepare documentation and implement training5. Critical area risk assessment method The method of assessing risk described in the previous section is used to carry out an overall assessment of all microbial risks in all areas of the pharmaceutical Table 3. List of sources with risk rating and consideration of a suitable control and valid monitoring methods Source to be controlled Risk rating Suitable control method Valid monitoring methods 1.a. Air outside production cleanroom (corridor) 0.75 Adequate air ventilation conditions 1. air supply volumes; 2. air pressurisation; 3. airborne particle conc.; 4. settle plates and airborne microbial sampling. 1. Areas adjacent to critical area
5 MICROBIAL RISK ASSESSMENT IN PHARMACEUTICAL CLEANROOMS 19 production cleanroom suite. However, it will normally be found that the highest risk occurs in the critical area where the product is open to contamination. A more detailed risk assessment of the critical area may then be required to assess: (1) microbial contamination coming from contaminating surfaces, such as gloves or clothing, touching the product; and (2) the deposition of microbe carrying particles from the air. It is necessary to assess these two routes separately, as the numerical values or descriptors used are basically different, and when steps are taken to minimise risks, the methods used will also be different. Surface contact contamination Surface contact contamination can occur during the following stages of manufacture: 1. Transfers into the critical area; 2. Setting-up of production machinery; 3. Normal production; 4. Interventions. The overall risk to the product by surface contact can be assessed by adding the risk from each of these stages, as shown in Equation 6. Equation 6 Risk by surface contact = risk from critical transfers + risk from setting up + risk during normal production + risk during interventions When developing risk equations it is important to decide whether the risk factors should be multiplied, or added, as this has a substantial effect on the accuracy of the risk assessment. If risk factors are independent of each other, then they should be added together; this is the situation in Equation 6 where the risk of contamination in one stage of production has no direct influence on any other stage. However, if the risk factors are dependent on each other, then they should be multiplied. This is the case with the variables in the fundamental Equations 2, 3 and 4, as each variable (risk factor) is dependant on the others to determine the overall magnitude of the risk of contamination. One example of a dependent risk factor is time, as the time the product is exposed to contamination directly influences, or gears up, the degree of risk of the other risk factors. A practical way of deciding whether to add or multiply risk factors is to allocate zero to a risk factor, and find out if the result is the one that is anticipated It is now necessary to model the risk of each of the four stages given in Equation 6. It is not sufficient to list all of the factors thought to cause microbial risk during production, and multiply or add their risk scores together. If this is done, inaccuracies will emerge. For example, if two or more descriptors are used to describe the risk caused by the same fundamental variable, then the importance of that variable will be exaggerated, especially if the risk scores are multiplied together. Numerical values, or descriptors, should therefore be chosen to measure or describe the variables in the surface contact contamination Equation 3, and combined as indicated in Equation 6. The first variable in Equation 3 is microbial contamination on contacting surfaces. This variable is not used here, because many surfaces are not sampled for microbial contamination. If they are, they will be often found to be sterile, or any counts may be inaccurate because of the introduction of microorganisms during the sampling. However, if reliable surface concentration results are available then these can be included to improve the risk estimate. The second variable in Equation 3 is the transfer coefficient relating to the proportion of surface contamination that is transferred from the contaminating surface to the product. It is highly unlikely that any numerical values will be available for this variable, and descriptors should be used. The third variable is the area of the product that is contacted. If this varies between different stages of the process, then this variable should be incorporated to improve the risk analysis. However, it will be more common that the area will remain constant, and there is no advantage in including this variable. The variable of time, or frequency, which was not used as a risk variable in the overall risk assessment discussed above, is used here, as it is likely to be available, or can be determined. Time can be expressed either as the time the product is exposed to contamination, or the frequency of a contamination incident occurring. Utilising these assumptions, a simple risk equation that combines the surface contact contamination from the four stages of manufacture is given in Equation 7. Equation 7 Risk by surface contact = α [no. of critical transfers] + β [setting up complexity] + χ [operator involvement complexity of critical area no. of manipulations] + δ [no. of interventions] Where α, β, χ and δ are weighting coefficients It can be seen in this equation that the risk during normal production variable indicated in Equation 6 now comprises of three risk descriptors. As these three descriptors are relatively dependent on each other, they need to be multiplied together. However, this combined risk score will be much greater than any of the other three stages. In addition, some stages are more likely to contribute more surface contamination e.g. interventions than other stages. These imbalances in the risk equation can be corrected by assigning weighting coefficients. These were shown in Equation 7 as α, β, χ and δ, and examples of values that can be assigned are shown in Equation 8: Equation 8 Risk by surface contact = 1 [no. of critical transfers] [setting up complexity] x [body involvement complexity of critical area no. of manipulations] + 2 [no. of interventions]
6 20 W WHYTE AND T EATON Table 4. Risk factors and scores used to assess contamination contact surface risks per production batch Manufacturing Stage Risk factor Risk score 0 Risk score 1 Risk score 2 Risk score 3 = high = nil = low = medium Transfers into the Number of critical None No more than 10 No more than 30 More than 30 Critical Areas transfers Setting up Production Setting up complexity No set up activities Not complex. More complex. Complex. Time to set Machinery Time to set up no Time to set up no up more than 30 min more than 10 min more than 30 min Normal Production Operator involvement None Hands Hands and arms Body Complexity of critical Not Applicable Simple Some complexity Complex area Number of manipulations None No more than No more than More than 50% 5% of time 50% of time of time Interventions Number of interventions None No more than 5 No more than 10 More than 10 It is now necessary to allocate risk scores to each risk factor in Equation 8. Risk scores are usually assigned by an aggregation of opinion from an expert committee set up to carry out this task. Some scoring systems use only numerical values, and these may range as high as from 0 to 10. However, it is unrealistic to expect that risk can be allocated in such fine divisions, and a range of no more than six levels is reasonable. It is also easier for a person to describe risk by one or two simple words that can be allocated a number e.g. not possible = 0, very unlikely = 1, unlikely = 2, possible = 3, likely = 4, very likely = 5, definite = 6. It is difficult to find a scoring system that is free of criticism but a simple 4-level system is used here i.e. nil - 0, low-1, medium-2 and high-3. Given in Table 4 is an example of surface contact risk factors and associated scores which could be applied to a manufacturing process. This should be modified to fit the process being investigated. It should also be noted that when constructing a risk score table the normal range of occurrence should be evenly spread to match the range of the risk scores. This is especially important if different stages, or phases, of production have to be compared. The scores allocated are then used with Equation 8 to obtain a risk rating. Risk ratings can now be used to see where improvements are best made to reduce the risk. An example is given. Example: A cleanroom has a filling machine used for aseptic filling of vials that is located within a unidirectional flow workstation. Vials are fed to the pointof-fill through a hot-air depyrogenation tunnel. Product solution, stored in a sterilized holding tank, is fed to the filling machine through sterilizing grade filters and filled into the vials. Lyophilisation stoppers, transferred into the filling area, are partially seated onto the vials, which are accumulated into a mobile isolator, transferred into a sterilized freeze drier, and dried. The stoppers are then fully seated before being removed from the freeze drier for capping and crimping. The risk scores (bold) for each risk factor, and the risk rating, as calculated by Equation 8 for the four stages, are given in Table 5. Table 5 indicates that the filling and stoppering stage had the highest risk assessment and the operations of this stage should be targeted to reduce the contact surface risk. Risk factors were addressed in the following manner: Table 5. Risk scores and risk ratings for contact surface risk for all manufacturing stages Manufacturing Transfers into Setting up Normal Normal Normal Interventions Risk rating* phase the Critical Production Production Production Production Areas Machinery Number of Body Complexity of manipulations involvement critical area (% of total time) (A) (B) (C1) (C2) (C3) (D) Vial washing and None None 1% None Simple 1 sterilisation score=0 score=0 score=1 score=0 score=1 score=1 2.0 Solution storage 3 5 min 1% None Simple None score=1 score=1 score=1 score=0 score=1 score=0 2.5 Filling and stopper min 3% None Simple 38 placement score=3 score=3 score=1 score=0 score=1 score= Freeze drying and min One None Simple None 4.5 capping score=3 score=1 manipulation score=0 score=1 score=0 score=0.5 * Risk rating = [1 x A] + [1.5 x B] + 0.5[C1 x C2 x C3] + [2 x D]
7 MICROBIAL RISK ASSESSMENT IN PHARMACEUTICAL CLEANROOMS The number of critical transfers: The vial stoppers were supplied in bags of 500. With a single batch size of up to 25,000 units, up to 50 critical transfers were required. As the stopper feed bowl had adequate capacity, the stoppers were supplied in bags of 1000, reducing the number of transfers by a factor of 50%. 2. Filling machine set up complexity and time: Control of filling volume was achieved via a time-pressure system with a high level of automated control. However, this required a complex aseptic assembly of machine parts that took on average 35 minutes to complete. Modifications to the pipework were undertaken, whilst maintaining its cleaning and sterilisation functionality. The resultant assembly operation utilised less parts, was less complex, and had an average assembly time of 9 minutes. 3. The number of interventions: Stopper-to-stopper adhesion, a consequence of the sterilisation process, resulted in feedbowl blockage, which then required manual removal with sterilised forceps. Modifications to the feed bowl, to automatically reject adhered stoppers, reduced the average number of interventions from 38 to 4. The above modifications reduced the risk rating of the filling and stoppering stage from 10.5 to 5.5. The risk occurring during an individual manufacturing stage can, if required, be investigated in more detail. For example, the setting-up stage may be complex, with a large number of steps. The FMECA Equation 1 given in the introduction of this paper can be used: Risk = criticality of the occurrence x frequency of occurrence The stage to be investigated should be broken down into individual steps. The risk scoring system should be set and scores allocated to the criticality and time for each step, these being multiplied together to obtain a risk rating. For illustration purposes, and using an arbitrary scoring system, a single step is shown in Table 4. Criticality expresses the importance of a source, and it has been shown in Section 1 that it should be expressed in terms of the microbial concentration of the contaminating surface and the likelihood that these microbes are dispersed, transferred, and then deposited onto the product. As shown in Table 6, microbial concentration of the contaminating surface can be scored and an overall score used for the component of dispersion, transfer and deposition (these three components can also be scored individually if required). Time may be scored in relation to the time the product is exposed to contamination, or as contamination incidence frequency. Table 6. Calculation of risk rating for an individual step Criticality Time = Risk rating Score of concentration Score of dispersion Score of time, C x D.T.D x T of microbes (C) x transportation or frequency (T) x deposition (D.T.P) 1 x 3 x 5 = 15 Risk assessment of airborne deposition Airborne deposition using sampling results Equation 4 is the fundamental equation used to calculate airborne dispersion onto or into, pharmaceutical products. This equation has been shown to predict airborne contamination over a wide range of conditions during pharmaceutical production 8. The variables required for solution of the equation are: Microbial deposition rate: This can be obtained from the counts on settle plates exposed adjacent to the product. The results from settle plates are commonly reported as: number of colony forming units (cfus) settling onto a settle plate of a given area exposed over a given period of time. This is a deposition rate, but for our purposes must be presented in a more scientific way: cfus/cm 2 /hour is the most convenient. To achieve an accurate simulation of contamination, settle plates must be positioned adjacent to the exposed product and open only when contamination occurs; this can be done by opening and closing the plates when production starts and stops. However, the plate should remain open if production stops and an unplanned intervention is necessary when the product is still exposed. Settle plates 9cm in diameter (64 cm 2 in area) are normally exposed for four hours, although larger 14 cm diameter plates (154cm 2 area), exposed for the same period, will give more accurate counts. Settle plate counts taken in cleanrooms often give a series of zero results, with an occasional positive. This lack of sensitivity can be improved by using the larger plates during a period of high sampling intensity, or combining results obtained from routine sampling results, and averaging them over a one or two year period. It is necessary to check that dehydration of the microbiological medium in the settle plates, caused by air movement, does not reduce the count. Experience shows that as long as the plate is well filled with agar medium, and its exposure in unidirectional flow is not much over four hours, the resultant count is not significantly affected 9. However, it is necessary to confirm this on an individual basis. Surface area: The surface area of the product exposed to airborne contamination is required. As it is known that gravitational settling mainly governs the deposition of microbe carrying particles during pharmaceutical manufacturing 7, 10, only the horizontal area of the exposed product is required. This might be the area of the inner neck of a container, or the upwards-facing area of a solid product. Time product exposed: The time the product is exposed to airborne microbial contamination is required. The total manufacturing time should not be used, but the average time a single product is exposed to the airborne environment. For example, the first product through a filling line may be exposed for only a few seconds, but the last one will be exposed for the total time of the process; the average exposure time is therefore 50% of the total process time. If a holding area e.g. turntable or hopper is loaded in batches
8 22 W WHYTE AND T EATON then the 50% time should used for each batch rather that the total time. Any time the product is covered or protected from airborne deposition should also be taken into consideration. Using these variables as indicated in Equation 4, the number of microorganisms deposited during a manufacturing operation can be calculated as shown in the following example of high airborne contamination: Example: Containers are filled within a unidirectional flow workstation. Several settle plates are exposed for four hours in the area adjacent to the open containers. The average microbial count from the 14 cm diameter Petri dishes (154 cm 2 area) was found to be 1. The deposition rate (no./(cm 2.hr)) of the microorganisms can be calculated from the settle plate data i.e. Deposition rate (no./(cm 2.hr)) = average count on settle plate [area of Petri dish (cm 2 ) time plate exposed (hr)] = 1 [154 4] = The container has an inner neck area of 2 cm 2 and is open to contamination during filling for an average of 10 minutes. The number of microbes that would contaminate the product by depositing through the neck area is then calculated from Equation 4, although the units used are hours instead of seconds i.e. No of airborne microbes deposited into product in a given time = deposition rate (no./(cm 2.hr)) exposed product surface area (cm 2 ) time of product exposure(hr) = [10 60] = It is a reasonable assumption that microbe-carrying particles will be deposited randomly throughout production 11 and therefore 5.4 containers in 10,000 will be contaminated. Clearly, a contamination rate of about 1 in 2000 is unacceptable and must be improved. If the time the product is exposed, its surface area, or the airborne microbial concentration is reduced, then the contamination rate can be reduced; this can be calculated. If, for example, the filling time is reduced to 6 seconds, the airborne contamination of containers will be reduced 100 times to 5.4 containers in a million. Reduction of the risk of microbial airborne deposition The previous section demonstrates that the risk of airborne contamination is dependent on the microbial deposition rate, the exposure time, and the deposition area, and can be readily calculated. It may be possible, especially at the design stage, to reduce the exposure time and deposition area by redesigning the process. However, the possibility of reducing microbial deposition onto the product requires the assistance of risk assessment. Pharmaceutical products open to microbial contamination will be located in a EU Grade A environment, as found within a separative air device such as a unidirectional airflow workstation or cabinet, and this will be located in a turbulently-ventilated EU Grade B or C background cleanroom area. Airborne contamination will then occur from two sources and routes. These are: 1. Air transferred from a Grade B or C cleanroom into the separative air device. The amount of undesirable transfer depends on the effectiveness of the separative device in preventing the entry of airborne contamination. 2. Microorganisms dispersed from personnel into the air within the critical area. The fundamental equations predict that this depends on how much of their body is within the critical area, the personnel s microbial dispersion rate, the effectiveness of their cleanroom clothing in reducing dispersion, and the time personnel is within the area. A reduction in airborne contamination risk is best achieved by separate consideration of these two sources. Air transfer into the critical area. Ljungqvist and Reinmuller 12 have described a method that can be used to determine the penetration of cleanroom air into an enhanced air device. They suggest, firstly, that the airflow round the area is investigated by smoke visualisation techniques to gain an insight into any problems. The area outside the enhanced air device is then seeded with a known concentration of small airborne particles, and the number of particles that penetrate into the critical area during simulated production, is measured. The ratio of particles found at the critical area to those seeded outside, is then calculated and is used as an indication of risk. The effectiveness of the enhanced air device in preventing penetration of outside contamination can then be investigated and its performance improved if required. Reduction in airborne microbial contaminants dispersed within the critical area: It is necessary to return to the fundamental Equation 2 to determine the best risk equation that can be used to assess risk. This is likely to be similar to Equation 9. Equation 9 Airborne risk = [amount of personnel s body within area] [effectiveness of clothing] [proportion of time personnel are within critical zone during production] The accuracy of the equation may be increased by assigning weighting coefficients to balance the contribution of risk, in the manner similar to that undertaken for Equation 8. The effectiveness of clothing is an important factor. This can be ascertained by the use of a dispersal chamber 13, 14, but this apparatus is generally not available outside the research laboratory. It may therefore be necessary to assess clothing in terms of the following descriptors: (a) how well the clothing envelopes the body; (b) the effectiveness of the clothing fabric in filtering the body s emissions [use of the pore diameter of interstices of the fabric, and the particle penetration through the fabric 13, 14 ]; and (c) the prevention of body debris being pumped out from insufficiently sealed openings at the neck, face, cuffs and ankles.
9 MICROBIAL RISK ASSESSMENT IN PHARMACEUTICAL CLEANROOMS 23 A risk assessment should then be carried out by a method similar to that described in Section 3 and steps taken, where possible, to reduce the degree of risk by: (1) reducing the amount of a persons body within the critical area; (2) improving the effectiveness of cleanroom clothing and; (3) reducing the time within the critical area. The effectiveness of the reduction of risk by use of one or both of these methods described above can be determined by settle plate sampling. Discussion The use of risk management systems is well establish in the electrical and mechanical industries, but seldom used in managing microbial risk in aseptic pharmaceutical manufacturing. Various risk management methods are available but the HACCP system, being devised for the food industry, is probably the best system to use with microbial contamination in pharmaceutical cleanrooms. A major component of risk management is risk assessment, where the degree of risk for each hazard is assessed. Described within this paper are risk assessment systems based on fundamental models of microbial contamination that allow the most effective risk assessment methods to be utilised and support the established FMECA method of assessing risk. A method of risk assessment has been developed, firstly, to provide an overall assessment of contamination from all areas of the cleanroom. Secondly, a risk assessment method has been developed that focuses on the manufacturing process in the critical area and examines separately the surface contact and airborne deposition routes of contamination. For surface contact contamination, using risk factors that have either numerical values or descriptors of risk, a risk rating can be calculated for each critical stage of a manufacturing operation or a multiphase manufacturing operation. By appropriate use of the values allocated to the risk factors, the step, stage or phase of manufacture with the highest risk can be identified and the risks addressed accordingly. For airborne contamination, the fundamental equation used to calculate airborne contamination is well established and hence the associated contamination rate in the critical area can be readily calculated. Reductions in product exposure time and deposition area can, if necessary, be effected to reduce the microbial risk and overall contamination rate. However, a further risk assessment method will be required to assess and reduce the risk from airborne contamination. Manufacturing stages, or steps, with the highest risk ratings can, if deemed unacceptable, be modified or amended to reduce the microbial risk. At some stage, however, the question of what is an acceptable level of risk will be encountered. This may arise because further reductions in the risk rating are not possible, or significant capital expenditure and investment in new items of equipment is required. At this stage, agreement between manufacturing, engineering and quality assurance personnel, utilising the risk data and other relevant supporting information (e.g. ongoing environmental microbiological results, sterility test results, and product simulation test data) will be required, as well as consideration of the potential microbial risk to the patient. Utilisation of a risk management system will assist in this task. References 1. IEC , Fault tree analysis (FTA). International Electrotechnical Commission, Geneva, Switzerland IEC , Analysis techniques for system reliability - Procedure for failure mode and effect analysis (FMEA). International Electronic Commission Geneva, Switzerland, Kletz T. Hazop and Hazan, Institute of Chemical Engineers, Rugby, UK Hazard Analysis Critical Control Point (HACCP) system and guidelines for its application. Codex Alimentarius Commission. Alinorm 97/13. Annex to Appendix II. Joint FAO/WHO Food Standards Programme, Rome: Food and Agricultural Organization of the United Nations, Whyte W. A cleanroom contamination control system. European Journal of Parenteral and Pharmaceutical Sciences 2002; 7(2): Whyte W and Eaton T. Microbiological contamination models for use in risk assessment during pharmaceutical production. European Journal of Parenteral and Pharmaceutical Sciences 2004; 9(1): Whyte W. Sterility Assurance and models for assessing airborne bacterial contamination. Journal of Parenteral Science and Technology 1986; 40: Whyte W. In support of settle plates. Journal of Pharmaceutical Science and Technology 1996; 50: Whyte W and Nivon L. Airborne bacterial sampling: the effect of dehydration and sampling time. Journal of Parenteral Science and Technology 1986; 40: Whyte W. Settling and impaction of particles into containers in manufacturing pharmacies. Journal of Parenteral Science and Technology 1981; 35: Whyte W. Matheis W. Dean-Netcher M and Edwards A. Airborne contamination during blow-fill-seal pharmaceutical production. PDA Journal of Pharmaceutical Science & Technology 1998; 52: Ljungqvist B and Reinmüller B. Hazard analysis of airborne contamination in cleanrooms - application of a method of limitation of risks. PDA Journal of Pharmaceutical Science and Technology 1995; 49: Whyte W and Bailey PV. Reduction of microbial dispersion by clothing. Journal of Parenteral Science and Technology 1985; 39: IEST-RP-CC003.3, Garment system considerations for cleanrooms and other controlled environments. Institute of Environmental Sciences and Technology, Rolling Meadows, Ill, USA
Copyright 2004 The Pharmaceutical & Healthcare Sciences Society
 Whyte, W., and Eaton, T. (2004) Microbiological contamination models for use in risk assessment during pharmaceutical production.european Journal of Parenteral and Pharmaceutical Sciences, 9 (1). pp. 11-15.
Whyte, W., and Eaton, T. (2004) Microbiological contamination models for use in risk assessment during pharmaceutical production.european Journal of Parenteral and Pharmaceutical Sciences, 9 (1). pp. 11-15.
ISO INTERNATIONAL STANDARD. Cleanrooms and associated controlled environments Biocontamination control Part 1: General principles and methods
 INTERNATIONAL STANDARD ISO 14698-1 First edition 2003-09-01 Cleanrooms and associated controlled environments Biocontamination control Part 1: General principles and methods Salles propres et environnements
INTERNATIONAL STANDARD ISO 14698-1 First edition 2003-09-01 Cleanrooms and associated controlled environments Biocontamination control Part 1: General principles and methods Salles propres et environnements
Science and Technology Feature Microbial transfer by surface contact in cleanrooms
 European Journal of Parenteral & Pharmaceutical Sciences 2015; 20(4): 127-131 2015 Pharmaceutical and Healthcare Sciences Society Science and Technology Feature Microbial transfer by surface contact in
European Journal of Parenteral & Pharmaceutical Sciences 2015; 20(4): 127-131 2015 Pharmaceutical and Healthcare Sciences Society Science and Technology Feature Microbial transfer by surface contact in
Overview and Introduction Annex 1 Revision
 Overview and Introduction Annex 1 Revision Paul Moody, Inspector Pharmig Current Hot Topics in Pharmaceutical Microbiology, Dublin May 30 th, 2018 Scope History Process of Revision Rationale for Revision
Overview and Introduction Annex 1 Revision Paul Moody, Inspector Pharmig Current Hot Topics in Pharmaceutical Microbiology, Dublin May 30 th, 2018 Scope History Process of Revision Rationale for Revision
Environmental Control Requirements. Gordon Farquharson July 2017
 Environmental Control Requirements Gordon Farquharson July 2017 Learning Objectives Learn how contamination control standards and guidance sets requirements for Critical Parameters ISO-14644-1 Classification
Environmental Control Requirements Gordon Farquharson July 2017 Learning Objectives Learn how contamination control standards and guidance sets requirements for Critical Parameters ISO-14644-1 Classification
ANNEX 1: COMMENT SUBMISSION DOCUMENT
 Submission Comments on: GMP Annex 1: Proposal for amendments to the environmental classification table for particles and associated text, amendment to section 42 concerning acceptance criteria for media
Submission Comments on: GMP Annex 1: Proposal for amendments to the environmental classification table for particles and associated text, amendment to section 42 concerning acceptance criteria for media
On behalf of the PHSS Pharmaceutical and Healthcare Sciences Society (UK).
 31 st March 2015 Submission of comments on ' Concept paper on the revision of annex 1 of the guidelines on good manufacturing practice manufacture of sterile medicinal products EMA/INS/GMP/735037/2014
31 st March 2015 Submission of comments on ' Concept paper on the revision of annex 1 of the guidelines on good manufacturing practice manufacture of sterile medicinal products EMA/INS/GMP/735037/2014
Review Validation of aseptic processes for pharmaceuticals
 OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
Managing Aseptic Interventions
 Managing Aseptic Interventions James Agalloco This article outlines a comprehensive approach for organizing a firm s aseptic operations, including planning for routine and nonroutine interventions, establishing
Managing Aseptic Interventions James Agalloco This article outlines a comprehensive approach for organizing a firm s aseptic operations, including planning for routine and nonroutine interventions, establishing
FDA s Guidance for Industry
 Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM
 Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM DCVMN Workshop, Hyderabad, 4-8 April 2016 G. Somasundaram Associate Director - Technology Management Overview Key Regulatory
Sterilizing and Bioburden Filter Risk Assessment in Vaccine Processes as part of QRM DCVMN Workshop, Hyderabad, 4-8 April 2016 G. Somasundaram Associate Director - Technology Management Overview Key Regulatory
Compounding Aseptic Isolators (CAI) James T Wagner
 Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
Aseptic Processing Practices and Process Validation of Aseptic Operators
 Aseptic Processing Practices and Process Validation of Aseptic Operators CBE Pty Ltd This training program is copyright to CBE Pty Ltd and may not be modified, reproduced, sold, loaned, hired or traded
Aseptic Processing Practices and Process Validation of Aseptic Operators CBE Pty Ltd This training program is copyright to CBE Pty Ltd and may not be modified, reproduced, sold, loaned, hired or traded
Microbial contamination is a
 B I O P R O C E S S TECHNICAL Barrier Vial Technology A Global Approach to the Aseptic Filling Process Diego López-Alvarez, Sergi Roura, and J.A. Garcia Microbial contamination is a concern and a constant
B I O P R O C E S S TECHNICAL Barrier Vial Technology A Global Approach to the Aseptic Filling Process Diego López-Alvarez, Sergi Roura, and J.A. Garcia Microbial contamination is a concern and a constant
Aseptic Process Validation
 Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
HVAC and Risk Management RACI Meeting 7 th December Agenda. Design. Project Management. Context. Qualification. Construction
 HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
Biological contamination events in isolators: What lessons can be learned?
 WHITE PAPER SCIENCE DRIVEN BIO-DECONTAMINATION LS001-MKT-008 Rev 1US Biological contamination events in isolators: What lessons can be learned? James Drinkwater, Bioquell UK Process & Compliance Director.
WHITE PAPER SCIENCE DRIVEN BIO-DECONTAMINATION LS001-MKT-008 Rev 1US Biological contamination events in isolators: What lessons can be learned? James Drinkwater, Bioquell UK Process & Compliance Director.
How to Handle Excursions in Environmental Monitoring (EM) and Personnel Monitoring (PM) in an Aseptic Processing Plant
 How to Handle Excursions in Environmental Monitoring (EM) and Personnel Monitoring (PM) in an Aseptic Processing Plant Randy Hutt, Ph.D. Associate Director Sterility Assurance and Microbiology Luitpold
How to Handle Excursions in Environmental Monitoring (EM) and Personnel Monitoring (PM) in an Aseptic Processing Plant Randy Hutt, Ph.D. Associate Director Sterility Assurance and Microbiology Luitpold
Environmental Monitoring of Aseptic Processing Areas - 2
 Environmental Monitoring of Aseptic Processing Areas - 2 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Active Air Monitoring (Viables) Air Sieve Samplers Draw air
Environmental Monitoring of Aseptic Processing Areas - 2 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Active Air Monitoring (Viables) Air Sieve Samplers Draw air
This compilation of the complex
 Manufacturing in a Global Marketplace Reference Matrices on Regulations for Classified (Environmentally Controlled) Areas Betty Seawell, William Miele, and Jean Huxsoll The complex world of international
Manufacturing in a Global Marketplace Reference Matrices on Regulations for Classified (Environmentally Controlled) Areas Betty Seawell, William Miele, and Jean Huxsoll The complex world of international
Overview of a sterility assurance program for PET drugs
 Coalition for PET Drug Approval Radiopharmaceutical Sciences Council Overview of a sterility assurance program for PET drugs Eric Webster, PETNET Solutions Disclosures Employee of PETNET Solutions, a Siemens
Coalition for PET Drug Approval Radiopharmaceutical Sciences Council Overview of a sterility assurance program for PET drugs Eric Webster, PETNET Solutions Disclosures Employee of PETNET Solutions, a Siemens
Complaints Investigations Root Cause Analysis
 Complaints Investigations Root Cause Analysis Dr. Ademola Daramola International Relations Specialist Drugs US FDA India Office New Delhi February 22nd 2018 Information presented in this presentation does
Complaints Investigations Root Cause Analysis Dr. Ademola Daramola International Relations Specialist Drugs US FDA India Office New Delhi February 22nd 2018 Information presented in this presentation does
PHCT 401 Aseptic Processes & Techniques
 PHCT 401 Aseptic Processes & Techniques Principles of Aseptic Techniques Aseptic Processing Sterility Testing Laminar flow air cleaning Quality Control Tests Personnel Principles of Aseptic Techniques
PHCT 401 Aseptic Processes & Techniques Principles of Aseptic Techniques Aseptic Processing Sterility Testing Laminar flow air cleaning Quality Control Tests Personnel Principles of Aseptic Techniques
Getting it Right Microbial Quality Risk Management
 Programme Linda Musker Quality Control North West Mark Oldcorne All Wales Pharmaceutical Quality Assurance Getting it Right Microbial Quality Risk Management Strategic Background (MAO) The Problem (LM)
Programme Linda Musker Quality Control North West Mark Oldcorne All Wales Pharmaceutical Quality Assurance Getting it Right Microbial Quality Risk Management Strategic Background (MAO) The Problem (LM)
INSPECTOR S OBSERVATIONS AND WARNING LETTER
 Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
Environmental Monitoring of Aseptic Processing Areas - 1
 Environmental Monitoring of Aseptic Processing Areas - 1 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Training Course Agenda Overview of Environmental Monitoring
Environmental Monitoring of Aseptic Processing Areas - 1 A war against an invisible enemy DCVMN - Víctor Maqueda - May 30, 31, June 1 2016 1 Training Course Agenda Overview of Environmental Monitoring
ISO INTERNATIONAL STANDARD. Cleanrooms and associated controlled environments Biocontamination control Part 1: General principles and methods
 INTERNATIONAL STANDARD ISO 14698-1 First edition 2003-09-01 Cleanrooms and associated controlled environments Biocontamination control Part 1: General principles and methods Salles propres et environnements
INTERNATIONAL STANDARD ISO 14698-1 First edition 2003-09-01 Cleanrooms and associated controlled environments Biocontamination control Part 1: General principles and methods Salles propres et environnements
 CONTENTS 1 INTRODUCTION The Regulatory Focus on Quality Risk Management Objectives of Risk Assessment and Risk Management: The Key Concepts Key Terms in Relation to Risk and Risk Assessment Structure of
CONTENTS 1 INTRODUCTION The Regulatory Focus on Quality Risk Management Objectives of Risk Assessment and Risk Management: The Key Concepts Key Terms in Relation to Risk and Risk Assessment Structure of
Update to the Manufacturing Principles for Medicinal Products
 Update to the Manufacturing Principles for Medicinal Products Michel Lok, Head of Office Robyn Oatey, GMP Auditor, Medicines Office of Manufacturing Quality Therapeutic Goods Administration Introduction
Update to the Manufacturing Principles for Medicinal Products Michel Lok, Head of Office Robyn Oatey, GMP Auditor, Medicines Office of Manufacturing Quality Therapeutic Goods Administration Introduction
USP Chapter 823 USP 32 (old) vs. USP 35 (new)
 USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective
 Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
GB/T Translated English of Chinese Standard: GBT NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA
 Translated English of Chinese Standard: GBT16294-2010 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA ICS 13.040.30
Translated English of Chinese Standard: GBT16294-2010 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA ICS 13.040.30
Bio-contamination control PHSS Technical Monograph No. 20. Gordon Farquharson, July 2016
 Bio-contamination control PHSS Technical Monograph No. 20 Gordon Farquharson, July 2016 The UK PHSS (Pahramaceuical and Health Care Sciences Socierty prepared Monograph No. 20 in September 2014. This presentation
Bio-contamination control PHSS Technical Monograph No. 20 Gordon Farquharson, July 2016 The UK PHSS (Pahramaceuical and Health Care Sciences Socierty prepared Monograph No. 20 in September 2014. This presentation
Annex A2. Guidance on Process Validation Scheme for Aseptically Processed Products
 Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
Webinar. The New EU-GMP Annex 1 draft Dupont. GOP-Innovations your Partner for Practical Training and e-learning. 8 June 2018
 Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
For sterile products manufacturing, Lyoseal is an innovative technology. presented by Mr. Tony Bouzerar from BioCorp offers us detailed
 For sterile products manufacturing, Lyoseal is an innovative technology to improve quality problems with final drug product due to crimping issues and inadequate stopper seating. It eliminates aluminum
For sterile products manufacturing, Lyoseal is an innovative technology to improve quality problems with final drug product due to crimping issues and inadequate stopper seating. It eliminates aluminum
Why change is inevitable in aseptic manufacturing?
 Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Packaging Operations Technical Subcommittee
 and Sterile About the Presenter John Derek Thompson Lead Sterile Engineer, DePuy Synthes Certified Professional with the IOPP Member of the Medical Device Technical Committee Chairman of the Member of
and Sterile About the Presenter John Derek Thompson Lead Sterile Engineer, DePuy Synthes Certified Professional with the IOPP Member of the Medical Device Technical Committee Chairman of the Member of
ISO INTERNATIONAL STANDARD. Cleanrooms and associated controlled environments Part 4: Design, construction and start-up
 INTERNATIONAL STANDARD ISO 14644-4 First edition 2001-04-01 Cleanrooms and associated controlled environments Part 4: Design, construction and start-up Salles propres et environnements maîtrisés apparentés
INTERNATIONAL STANDARD ISO 14644-4 First edition 2001-04-01 Cleanrooms and associated controlled environments Part 4: Design, construction and start-up Salles propres et environnements maîtrisés apparentés
Risk Analysis: myths, confusions and real sense
 Risk Analysis: myths, confusions and real sense Dr. Alexander Fedotov, Director of Invar-project Company, Moscow, Russia fedotov@invar-project.ru www.invar-project.ru 1. What is risk analysis? 2. GMP EU
Risk Analysis: myths, confusions and real sense Dr. Alexander Fedotov, Director of Invar-project Company, Moscow, Russia fedotov@invar-project.ru www.invar-project.ru 1. What is risk analysis? 2. GMP EU
Identification and Control of Critical Process Points Using Complementary Risk Assessments (corrected)
 Identification and Control of Critical Process Points Using Complementary Risk Assessments (corrected) Karen McCullough MMI Associates (908) 534-6463 PDA Metro Chapter Day June 2, 2009 RISK is a four letter
Identification and Control of Critical Process Points Using Complementary Risk Assessments (corrected) Karen McCullough MMI Associates (908) 534-6463 PDA Metro Chapter Day June 2, 2009 RISK is a four letter
PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D.
 Basic GMP Requirement PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D. Topic Process validation What and Why? Principle of process validation Manufacturing process validation Aseptic process validation
Basic GMP Requirement PROCESS VALIDATION ROCHAPON WACHAROTAYANKUN, PH.D. Topic Process validation What and Why? Principle of process validation Manufacturing process validation Aseptic process validation
PHARMACY IV ADMIXTURE. Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor
 PHARMACY IV ADMIXTURE Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor Pharmacist is responsible for ensuring that compounded sterile preparations are properly prepared, labeled,
PHARMACY IV ADMIXTURE Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor Pharmacist is responsible for ensuring that compounded sterile preparations are properly prepared, labeled,
Aseptic Processing Current Issues & Trends
 Aseptic Processing Current Issues & Trends 1 Introduction Richard M. Johnson Member, PDA for 20+ years President & CEO since 2009 Ladies and Gentlemen, I am happy to be here with you. Senhoras e Senhores,
Aseptic Processing Current Issues & Trends 1 Introduction Richard M. Johnson Member, PDA for 20+ years President & CEO since 2009 Ladies and Gentlemen, I am happy to be here with you. Senhoras e Senhores,
PHARMACY IV ADMIXTURE
 PHARMACY IV ADMIXTURE Pharmacy 483 January 18, 2007 Kim Donnelly Affiliate Assistant Professor kimdon@u.washington.edu Pharmacist is responsible for ensuring that compounded sterile preparations are properly
PHARMACY IV ADMIXTURE Pharmacy 483 January 18, 2007 Kim Donnelly Affiliate Assistant Professor kimdon@u.washington.edu Pharmacist is responsible for ensuring that compounded sterile preparations are properly
Product/patient Oriented Contamination Control. Content
 Product/patient Oriented Contamination Control An alternative look at contamination control Koos Agricola Content Product/patient Contamination Contamination mechanisms Contamination control solutions
Product/patient Oriented Contamination Control An alternative look at contamination control Koos Agricola Content Product/patient Contamination Contamination mechanisms Contamination control solutions
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 18593 First edition 2004-06-01 Microbiology of food and animal feeding stuffs Horizontal methods for sampling techniques from surfaces using contact plates and swabs Microbiologie
INTERNATIONAL STANDARD ISO 18593 First edition 2004-06-01 Microbiology of food and animal feeding stuffs Horizontal methods for sampling techniques from surfaces using contact plates and swabs Microbiologie
Sterility Assurance Level and Aseptic Manufacturing Process in Pharmaceuticals
 Review Article ISSN 2277-3657 Available online at www.ijpras.com Volume 3, Issue 4 (2014),10-15 International Journal of Pharmaceutical Research & Allied Sciences Sterility Assurance Level and Aseptic
Review Article ISSN 2277-3657 Available online at www.ijpras.com Volume 3, Issue 4 (2014),10-15 International Journal of Pharmaceutical Research & Allied Sciences Sterility Assurance Level and Aseptic
Biocontamination control in pharmaceutical production
 White Paper Data Sheet Biocontamination control in pharmaceutical production and compliance to ISO 14698 with validated active microbial air samplers Tim Sandle, Ph.D., Head of Microbiology at Bio Products
White Paper Data Sheet Biocontamination control in pharmaceutical production and compliance to ISO 14698 with validated active microbial air samplers Tim Sandle, Ph.D., Head of Microbiology at Bio Products
Supplementary Training Modules on Good Manufacturing Practice. Validation. WHO Technical Report Series, No. 937, Annex 4.
 Supplementary Training Modules on Good Manufacturing Practice Validation WHO Technical Report Series, No. 937, 2006. Annex 4. Validation Slide 1 of 48 August 2006 Validation! Part 1. General overview on
Supplementary Training Modules on Good Manufacturing Practice Validation WHO Technical Report Series, No. 937, 2006. Annex 4. Validation Slide 1 of 48 August 2006 Validation! Part 1. General overview on
Whitley Automated Spiral Platers
 Whitley Automated Spiral Platers 01 An Introduction to Spiral Plating Even though spiral plating was invented over 40 years ago, some microbiologists are yet to experience the advantages of spiral plating
Whitley Automated Spiral Platers 01 An Introduction to Spiral Plating Even though spiral plating was invented over 40 years ago, some microbiologists are yet to experience the advantages of spiral plating
EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT
 1 EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT UNDERSTAND THE PROGRESSION OF REGULATORY THINKING AND HOW TO SUCCESSFULLY IMPLEMENT THE PROPOSED CHANGES BALTIMORE, MD MAY 6TH AND 7TH, 2019 UNIVERSITY
1 EU GMP ANNEX 1 CHANGES CLARIFICATION AND IMPACT UNDERSTAND THE PROGRESSION OF REGULATORY THINKING AND HOW TO SUCCESSFULLY IMPLEMENT THE PROPOSED CHANGES BALTIMORE, MD MAY 6TH AND 7TH, 2019 UNIVERSITY
Media Fill A Process Simution. Presented By Shikha Chauhan
 Media Fill A Process Simution Presented By Shikha Chauhan 21 CFR 211.113 Validation of Aseptic Processing and Sterilization Process Simulation / Media Fill Filtration Efficacy Sterilization of Equipment,
Media Fill A Process Simution Presented By Shikha Chauhan 21 CFR 211.113 Validation of Aseptic Processing and Sterilization Process Simulation / Media Fill Filtration Efficacy Sterilization of Equipment,
BIO & PHARMA ANALYTICAL TECHNIQUES
 BIO & PHARMA ANALYTICAL TECHNIQUES Chapter 11 by Dr. Siti Umairah Mokhtar Faculty of Engineering Technology umairah@ump.edu.my Chapter Description Aims Discuss theory, principles and application of analytical
BIO & PHARMA ANALYTICAL TECHNIQUES Chapter 11 by Dr. Siti Umairah Mokhtar Faculty of Engineering Technology umairah@ump.edu.my Chapter Description Aims Discuss theory, principles and application of analytical
Chapter 6-1: Failure Modes Effect Analysis (FMCEA)
 Chapter 6-1: Failure Modes Effect Analysis (FMCEA) Learning Outcomes: After careful studying this lecture You should be able: To Define FMEA To understand the use of Failure Modes Effect Analysis (FMEA)
Chapter 6-1: Failure Modes Effect Analysis (FMCEA) Learning Outcomes: After careful studying this lecture You should be able: To Define FMEA To understand the use of Failure Modes Effect Analysis (FMEA)
Whitley Automated Spiral Platers
 Whitley Automated Spiral Platers 01 An Introduction to Spiral Plating Even though spiral plating was invented over 40 years ago, some microbiologists are yet to experience the advantages of spiral plating
Whitley Automated Spiral Platers 01 An Introduction to Spiral Plating Even though spiral plating was invented over 40 years ago, some microbiologists are yet to experience the advantages of spiral plating
PhEn-602 Notes # 4 J. Manfredi
 PhEn-602 Notes # 4 J. Manfredi Spring 2009 1 Basic definitions Clean Room: A room in which the concentration of airborne particles is controlled and contains one or more clean zones Clean Zone: A defined
PhEn-602 Notes # 4 J. Manfredi Spring 2009 1 Basic definitions Clean Room: A room in which the concentration of airborne particles is controlled and contains one or more clean zones Clean Zone: A defined
Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119
 Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119 February 28, 2018 Manufacture of sterile drugs (GUI-0119) Author: Health Canada Date issued: February 28, 2018 Implementation
Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119 February 28, 2018 Manufacture of sterile drugs (GUI-0119) Author: Health Canada Date issued: February 28, 2018 Implementation
Testo Expert Knowledge. The GxP Dictionary 1st edition. Definitions relating to GxP and Quality Assurance
 Testo Expert Knowledge The GxP Dictionary 1st edition Definitions relating to GxP and Quality Assurance Note: Some of the information contained in this GxP Dictionary does not apply equally to all countries.
Testo Expert Knowledge The GxP Dictionary 1st edition Definitions relating to GxP and Quality Assurance Note: Some of the information contained in this GxP Dictionary does not apply equally to all countries.
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 16170 First edition 2016-07-01 Corrected version 2017-04 In situ test methods for high efficiency filter systems in industrial facilities Méthodes d essai in situ pour les systèmes
INTERNATIONAL STANDARD ISO 16170 First edition 2016-07-01 Corrected version 2017-04 In situ test methods for high efficiency filter systems in industrial facilities Méthodes d essai in situ pour les systèmes
BIOTRAK REAL-TIME VIABLE PARTICLE COUNTER ROOT CAUSE INVESTIGATION GUIDANCE
 BIOTRAK REAL-TIME VIABLE PARTICLE COUNTER ROOT CAUSE INVESTIGATION GUIDANCE APPLICATION NOTE CC-123 (US) Introduction This guidance provides recommendations for how the BioTrak Real-Time Viable Particle
BIOTRAK REAL-TIME VIABLE PARTICLE COUNTER ROOT CAUSE INVESTIGATION GUIDANCE APPLICATION NOTE CC-123 (US) Introduction This guidance provides recommendations for how the BioTrak Real-Time Viable Particle
PDA: A Global. Association. Contamination Control: Particles, Bio-contamination, Aseptic manufacturing. PDA Parenteral 2014 Munich Conference
 PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
MicroFunnel Filter Funnels
 MicroFunnel Filter Funnels Description Increase laboratory efficiency with a newly designed, disposable filter funnel and Petri dish all in one. Certified. Each lot is certified for microbiological analysis
MicroFunnel Filter Funnels Description Increase laboratory efficiency with a newly designed, disposable filter funnel and Petri dish all in one. Certified. Each lot is certified for microbiological analysis
ISO INTERNATIONAL STANDARD. Road construction and maintenance equipment Asphalt mixing plants Terminology and commercial specifications
 INTERNATIONAL STANDARD ISO 15642 First edition 2003-05-15 Road construction and maintenance equipment Asphalt mixing plants Terminology and commercial specifications Équipement pour la construction et
INTERNATIONAL STANDARD ISO 15642 First edition 2003-05-15 Road construction and maintenance equipment Asphalt mixing plants Terminology and commercial specifications Équipement pour la construction et
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 13408-2 Second edition 2018-01 Aseptic processing of health care products Part 2: Sterilizing filtration Traitement aseptique des produits de santé Partie 2: Filtration stérilisante
INTERNATIONAL STANDARD ISO 13408-2 Second edition 2018-01 Aseptic processing of health care products Part 2: Sterilizing filtration Traitement aseptique des produits de santé Partie 2: Filtration stérilisante
Tests to Support Sterility Claim. Imtiaz Ahmed
 Tests to Support Sterility Claim Imtiaz Ahmed Sterile Product As per TGO 77, a sterile product must comply with the requirements of the following tests: Sterility Test Bacterial Endotoxins Test Appendix
Tests to Support Sterility Claim Imtiaz Ahmed Sterile Product As per TGO 77, a sterile product must comply with the requirements of the following tests: Sterility Test Bacterial Endotoxins Test Appendix
GUIDELINES ON GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEXES
 GUIDELINES ON GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEXES National Drug Authority Plot 46-48, Lumumba Avenue, P. O. Box 23096, Kampala, Uganda. Tel: +256-0414 - 255665/347391/2 Fax: +256-0414
GUIDELINES ON GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEXES National Drug Authority Plot 46-48, Lumumba Avenue, P. O. Box 23096, Kampala, Uganda. Tel: +256-0414 - 255665/347391/2 Fax: +256-0414
Systematic Risk Management: An Overview of ICH Q-9
 Systematic Risk Management: An Overview of ICH Q-9 March 12, 2014 12014 ParagonRx International LLC Today s Session Systematic Risk Management: An Overview of ICH Q-9 Speakers: Jeff Fetterman, President,
Systematic Risk Management: An Overview of ICH Q-9 March 12, 2014 12014 ParagonRx International LLC Today s Session Systematic Risk Management: An Overview of ICH Q-9 Speakers: Jeff Fetterman, President,
Quality Risk Management
 Quality Risk Management Audit Expectations and Observations Matthew Davis Lead Auditor Office of Manufacturing Quality, TGA CAPSIG 4 th May 2011 www.tga.gov.au 2 QRM - TGA Expectations and Observations
Quality Risk Management Audit Expectations and Observations Matthew Davis Lead Auditor Office of Manufacturing Quality, TGA CAPSIG 4 th May 2011 www.tga.gov.au 2 QRM - TGA Expectations and Observations
Culturing microorganisms may be hazardous
 Practical 8 - S(d) The Effect of Penicillin on Bacterial Growth In this practical focuses on the practical skills of: Planning defining the problem You will be developing other assessed skills throughout
Practical 8 - S(d) The Effect of Penicillin on Bacterial Growth In this practical focuses on the practical skills of: Planning defining the problem You will be developing other assessed skills throughout
GOOD MANUFACTURING PRACTICE
 GOOD MANUFACTURING PRACTICE Introduction The first WHO draft text on good manufacturing practices (GMP) was prepared in 1967 by a group of consultants at the request of the Twentieth World Health Assembly
GOOD MANUFACTURING PRACTICE Introduction The first WHO draft text on good manufacturing practices (GMP) was prepared in 1967 by a group of consultants at the request of the Twentieth World Health Assembly
Allergy Laboratories, Inc. 10/4/13
 Allergy Laboratories, Inc. 10/4/13 SEP 04, 2013 Department of Health and Human Services Public Health Service Food and Drug Administration Office of Regulatory Affairs 12420 Parklawn Drive ELEM-2152 Rockville,
Allergy Laboratories, Inc. 10/4/13 SEP 04, 2013 Department of Health and Human Services Public Health Service Food and Drug Administration Office of Regulatory Affairs 12420 Parklawn Drive ELEM-2152 Rockville,
ISPE Aseptic Conference February 2014 Washington, D.C.
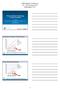 Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Industry Perspective on PET Manufacturing Comparison of EU and US
 Standards for Imaging Endpoints and Manufacturing of PET Radiopharmaceuticals Industry Perspective on PET Manufacturing Comparison of EU and US Guidances and associated Regulations Natcher Conference Center
Standards for Imaging Endpoints and Manufacturing of PET Radiopharmaceuticals Industry Perspective on PET Manufacturing Comparison of EU and US Guidances and associated Regulations Natcher Conference Center
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids
 Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
Monday 8 th July Symposium 1 - Environmental Monitoring of Production Areas
 Monday 8 th July 2013 Symposium 1 - Environmental Monitoring of Production Areas Dealing with Environmental Monitoring Out of Specification Results and Changes to ISO 14644 David Buckley Rev 2 1 Kaoru
Monday 8 th July 2013 Symposium 1 - Environmental Monitoring of Production Areas Dealing with Environmental Monitoring Out of Specification Results and Changes to ISO 14644 David Buckley Rev 2 1 Kaoru
A copy can be downloaded for personal non-commercial research or study, without prior permission or charge
 Whyte, W., Whyte, W.M., Blake, S., and Green, G. (2013) ispersion of microbes from floors when walking in ventilated rooms. International Journal of Ventilation, 12 (3). pp. 271-284. ISSN 1473-3315 Copyright
Whyte, W., Whyte, W.M., Blake, S., and Green, G. (2013) ispersion of microbes from floors when walking in ventilated rooms. International Journal of Ventilation, 12 (3). pp. 271-284. ISSN 1473-3315 Copyright
Transfusion equipment for medical use. Part 5: Transfusion sets for single use with pressure infusion apparatus
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 1135-5 First edition 2015-12-01 Transfusion equipment for medical use Part 5: Transfusion sets for single use with pressure infusion apparatus
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 1135-5 First edition 2015-12-01 Transfusion equipment for medical use Part 5: Transfusion sets for single use with pressure infusion apparatus
The ABCs and Challenges of GMP The American Experience
 The ABCs and Challenges of GMP The American Experience The Canadian Association of Nuclear Medicine Toronto, Ontario April 22, 2017 Reiko Oyama, R.Ph., B.C.N.P. Washington University School of Medicine
The ABCs and Challenges of GMP The American Experience The Canadian Association of Nuclear Medicine Toronto, Ontario April 22, 2017 Reiko Oyama, R.Ph., B.C.N.P. Washington University School of Medicine
Microbiological Methods
 Microbiological Methods Making Media Pouring Culture Plates Sterile Technique Inoculating Plates and Culture Tubes Use of a Plate Counter to Estimate Microbial Population Densities Culturing Microorganisms
Microbiological Methods Making Media Pouring Culture Plates Sterile Technique Inoculating Plates and Culture Tubes Use of a Plate Counter to Estimate Microbial Population Densities Culturing Microorganisms
Product and Package Stability Studies The Application of FDA Guidance
 Product and Package Stability Studies The Application of FDA Guidance Introduction In February 2008, the FDA issued Guidance to Industry dealing with physical tests for packaging for sterile medical devices
Product and Package Stability Studies The Application of FDA Guidance Introduction In February 2008, the FDA issued Guidance to Industry dealing with physical tests for packaging for sterile medical devices
ISO/TC 238. Secretariat: SIS Solid biofuels Determination of particle size distribution for uncompressed fuels
 DRAFT INTERNATIONAL STANDARD ISO/DIS 17827-2 Solid biofuels Determination of particle size distribution for uncompressed fuels Part 2: Vibrating screen method using sieves with aperture of 3,15 mm and
DRAFT INTERNATIONAL STANDARD ISO/DIS 17827-2 Solid biofuels Determination of particle size distribution for uncompressed fuels Part 2: Vibrating screen method using sieves with aperture of 3,15 mm and
Cell Culture Flasks DATA SHEET
 DATA SHEET Cell Culture Flasks In research cell culture flasks are used as a matter of routine for the cultivation of eukaryotic cells. They are optimal products for adherent cells as well as for suspension
DATA SHEET Cell Culture Flasks In research cell culture flasks are used as a matter of routine for the cultivation of eukaryotic cells. They are optimal products for adherent cells as well as for suspension
SITE MASTER FILE For MHRA
 ABC CO., LTD. For MHRA Prepared by Date Approved by Date Verified by Date Document No.: SMF, Version No: 01, Effective Date: 09/05/06 Document No.: SMF Version No.: 01 Effective Date: 09/05/06 Page 2 of
ABC CO., LTD. For MHRA Prepared by Date Approved by Date Verified by Date Document No.: SMF, Version No: 01, Effective Date: 09/05/06 Document No.: SMF Version No.: 01 Effective Date: 09/05/06 Page 2 of
A copy can be downloaded for personal non-commercial research or study, without prior permission or charge
 Whyte, W., Green, G., and Albisu, A. (2007) Collection efficiency and design of microbial air samplers. Journal of Aerosol Science, 38 (1). pp. 97-110. ISSN 0021-8502 Copyright 2006 Elsevier Ltd. A copy
Whyte, W., Green, G., and Albisu, A. (2007) Collection efficiency and design of microbial air samplers. Journal of Aerosol Science, 38 (1). pp. 97-110. ISSN 0021-8502 Copyright 2006 Elsevier Ltd. A copy
John F. Koerner, MPH, CIH
 Critical Elements of Containment and Ventilation Practice for Healthcare Microbial Remediation John F. Koerner, MPH, CIH Vice President, Environmental Sciences Compliance Environmental International, Inc.
Critical Elements of Containment and Ventilation Practice for Healthcare Microbial Remediation John F. Koerner, MPH, CIH Vice President, Environmental Sciences Compliance Environmental International, Inc.
LUNCH AND LEARN. September 11, CE Activity Information & Accreditation
 LUNCH AND LEARN Environmental Monitoring and Contamination Control September 11, 2015 Featured Speaker: Scott Sutton, Ph.D. The Microbiology Network N. Chili, New York 1 CE Activity Information & Accreditation
LUNCH AND LEARN Environmental Monitoring and Contamination Control September 11, 2015 Featured Speaker: Scott Sutton, Ph.D. The Microbiology Network N. Chili, New York 1 CE Activity Information & Accreditation
Guidance for Industry
 Guidance for Industry for the Submission Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry for the Submission Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products Center for Drug Evaluation and Research (CDER) Center
Biocidal Surface Test - Clinell Wipes ~ Project Report Prepared for GAMA Healthcare Ltd ~ Huddersfield Microbiology Services Oct 06
 Biocidal Surface Test - Clinell Wipes ~ Project Report Prepared for GAMA Healthcare Ltd ~ Huddersfield Microbiology Services Oct 06 Summary The ability of Clinell wipes to disinfect contaminated steel
Biocidal Surface Test - Clinell Wipes ~ Project Report Prepared for GAMA Healthcare Ltd ~ Huddersfield Microbiology Services Oct 06 Summary The ability of Clinell wipes to disinfect contaminated steel
HAZARD ANALYSIS AND CRITICAL CONTROL POINTS FOR WATER SUPPLIES. Kevin Hellier. Melbourne Water Corporation
 HAZARD ANALYSIS AND CRITICAL CONTROL POINTS FOR WATER SUPPLIES Paper Presented by : Kevin Hellier Authors: Kevin Hellier, Water Quality Engineer Melbourne Water Corporation 63 rd Annual Water Industry
HAZARD ANALYSIS AND CRITICAL CONTROL POINTS FOR WATER SUPPLIES Paper Presented by : Kevin Hellier Authors: Kevin Hellier, Water Quality Engineer Melbourne Water Corporation 63 rd Annual Water Industry
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL. EudraLex The Rules Governing Medicinal Products in the European Union
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 25 November 2008 (rev.) EudraLex The Rules Governing Medicinal Products in the European Union Volume
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 25 November 2008 (rev.) EudraLex The Rules Governing Medicinal Products in the European Union Volume
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 14644-2 First edition 2000-09-15 Cleanrooms and associated controlled environments Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1
INTERNATIONAL STANDARD ISO 14644-2 First edition 2000-09-15 Cleanrooms and associated controlled environments Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1
UNDER SECRETARY FOR HEALTH S INFORMATION LETTER MICROBIOLOGICAL ASSESSMENT OF PHARMACEUTICAL CLEANROOMS
 DEPARTMENT OF VETERANS AFFAIRS Veterans Health Administration Washington DC 20420 IL 10-2006-008 In Reply Refer To: 10NB UNDER SECRETARY FOR HEALTH S INFORMATION LETTER MICROBIOLOGICAL ASSESSMENT OF PHARMACEUTICAL
DEPARTMENT OF VETERANS AFFAIRS Veterans Health Administration Washington DC 20420 IL 10-2006-008 In Reply Refer To: 10NB UNDER SECRETARY FOR HEALTH S INFORMATION LETTER MICROBIOLOGICAL ASSESSMENT OF PHARMACEUTICAL
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE
 XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
Study Summary. Results: All tested media-filled vials were negative for growth of any microorganisms.
 A 7 Days Microbial Ingress Test of Single Use Vials Utilizing the EQUASHIELD Closed System Drug Transfer Device Study by Nelson Laboratories (Salt Lake City, UT) 2013, updated in 2014* Study Summary Abstract:
A 7 Days Microbial Ingress Test of Single Use Vials Utilizing the EQUASHIELD Closed System Drug Transfer Device Study by Nelson Laboratories (Salt Lake City, UT) 2013, updated in 2014* Study Summary Abstract:
Sterilization of health care products Microbiological methods. Part 1: Determination of a population of microorganisms on products
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11737-1 Third edition 2018-01 Sterilization of health care products Microbiological methods Part 1: Determination of a population of microorganisms
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11737-1 Third edition 2018-01 Sterilization of health care products Microbiological methods Part 1: Determination of a population of microorganisms
M I C R O B I O L O G I C A L T O O L S F O R Q U A L I T Y A S S U R A N C E I N H A T C H E R Y : Sampling Procedures
 Issue No.28 / January 2010 M I C R O B I O L O G I C A L T O O L S F O R Q U A L I T Y A S S U R A N C E I N H A T C H E R Y : Sampling Procedures by Dr Vincent TURBLIN Deputy Regional Market Manager Poultry
Issue No.28 / January 2010 M I C R O B I O L O G I C A L T O O L S F O R Q U A L I T Y A S S U R A N C E I N H A T C H E R Y : Sampling Procedures by Dr Vincent TURBLIN Deputy Regional Market Manager Poultry
Part 2: Sterilizing filtration
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 13408-2 Second edition 2018-01 Aseptic processing of health care products Part 2: Sterilizing filtration Traitement aseptique des produits de santé
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 13408-2 Second edition 2018-01 Aseptic processing of health care products Part 2: Sterilizing filtration Traitement aseptique des produits de santé
Special aspects of European Ordinances regarding Cleaning and Disinfection in Food Premises
 Module 6 Special aspects of European Ordinances regarding Cleaning and Disinfection in Food Premises This project has been funded with support from the European Commission. This publication [communication]
Module 6 Special aspects of European Ordinances regarding Cleaning and Disinfection in Food Premises This project has been funded with support from the European Commission. This publication [communication]
In-Use Stability of Biological Parenteral Products:
 In-Use Stability of Biological Parenteral Products: Aspects of physicochemical and microbiological stability after container penetration, reconstitution, dilution and hold time University Medical Center,
In-Use Stability of Biological Parenteral Products: Aspects of physicochemical and microbiological stability after container penetration, reconstitution, dilution and hold time University Medical Center,
