|
|
|
- Jonathan Washington
- 5 years ago
- Views:
Transcription
1 Vapour Absorption Refrigeration Systems Based On Water- Lithium Bromide Pair Nagendra M CBM Engineer, Hindusthan Zink.Ltd The objectives of this lesson are to: 1. Introduce vapour absorption refrigeration systems based on water-lithium bromide. 2. Discuss properties of water-lithium bromide solution and describe pressure temperature- concentration (p-t-ξ) and enthalpy temperature-concentration (h-t-ξ) charts. 3. Present steady-flow analysis of a single stage, water-lithium bromide system. 4. Discuss practical problems in actual water-lithium bromide systems. 5. Describe commercial water-lithium bromide systems. 6. Discuss heat sources for water-lithium bromide systems. 7. Discuss typical application data for water-lithium bromide systems. 8. Discuss briefly the methods of capacity control in water-lithium bromide systems Introduction Vapour absorption refrigeration systems utilizing water-lithium bromide pair are broadly utilized as a part of substantial limit aerating and cooling systems. In these systems water is utilized as refrigerant and an answer of lithium bromide in water is utilized as permeable. Since water is utilized as refrigerant, utilizing these systems it is impractical to give refrigeration at below zero temperatures. Subsequently it is utilized just as a part of applications obliging refrigeration at temperatures over 0 o C. Hence these systems are utilized for aerating and cooling applications. The investigation of this system is moderately simple as the vapour produced in the generator is very nearly immaculate refrigerant (water), dissimilar to ammonia water systems where both smelling salts and water vapour are created in the generator Properties of water-lithium bromide solutions Composition: The composition of water-lithium bromide solutions can be expressed either in mass fraction (ξ) or mole fraction (x). For water-lithium bromide solutions, the mass fraction ξ is defined as the ratio of mass of anhydrous lithium bromide to the total mass of solution, i.e.,
2 where m L and m W are the mass of anhydrous lithium bromide and water in solution, respectively. The composition can also be expressed in terms of mole fraction of lithium bromide as: where n L and n W are the number of moles of anhydrous lithium bromide and water in solution, respectively. The number moles of lithium bromide and water can easily be obtained from their respective masses in solution and molecular pressures, thus; where ML (= 86.8 kg/kmol) and MW (= 18.0 kg/kmol) are the molecular pressures of anhydrous lithium bromide and water respectively Vapour pressure of water-lithium bromide solutions Applying Raoult s law, the vapour pressure of water-lithium bromide solution with the vapour pressure exerted by lithium bromide being negligibly small is given by: P = (1 x) P W where P W is the immersion pressure of unadulterated water at the same temperature as that of the arrangement and x is the mole part of lithium bromide in arrangement. It is observed that Raoult's law is just give or take right for exceptionally dilute solutions of water lithium bromide (i.e., as x 0). Solid aqueous solutions of water-lithium bromide are found to go astray unequivocally from Raoult's law in a negative way. Case in point, at 50 percent mass fraction of lithium bromide and 25 o C, Raoult's law predicts a vapour pressure of 26.2 mbar, though real estimations demonstrate that it is just 8.5 mbar. The degree of real vapour pressure to that anticipated by Raoult's law is known as movement coefficient. For the above case, the action coefficient is The vapour pressure information of water-lithium bromide solutions can be helpfully spoken to in a Dühring plot. In a Dühring plot, the temperature of the arrangement is plotted as abscissa on a direct scale, the immersion temperature of unadulterated water is
3 plotted as ordinate on the right hand side (straight scale) and the pressure on a logarithmic scale is plotted as ordinate on the left hand side. The plot shows the pressure temperature values for different steady focus lines (isosters), which are straight on Dühring plot. Figures 1.1 shows the Dühring plot. The Dühring plot can be utilized for discovering the vapour pressure information furthermore for plotting the working cycle. Figure 1.2 shows the water-lithium bromide built absorption refrigeration system with respect to Dühring plot. Different sorts of charts indicating vapour pressure information for water-lithium bromide systems are likewise accessible in writing. Figure 1.3 shows another outline wherein the mass portion of lithium bromide is plotted on abscissa, while immersion temperature of immaculate water and vapour pressure are plotted as ordinates. Likewise indicated are lines of steady arrangement temperature on the outline. Pressure - temperature composition information is additionally accessible as exact equation. Fig.1.1. A typical Dühring plot
4 Fig 1.2 H2O-LiBr system with a solution heat exchanger on Dühring plot Fig.1.3: Pressure-Temperature-Concentration diagram for H2O-LiBr solution Enthalpy of water-lithium bromide solutions Since strong water-lithium bromide solution deviates from ideal solution behaviour, it is observed that when water and anhydrous lithium bromide at same temperature are mixed adiabatically, the temperature of the solution increases considerably. This indicates that the mixing is an exothermic process with a negative heat of mixing. Hence the specific enthalpy of the solution is given by: h = ξ.h L + (1 ξ) h W + Δh mix where hl and hw are the specific enthalpies of pure lithium bromide and water, respectively at the same temperature. Figure 1.4 shows a chart giving the specific enthalpy-temperature-mass fraction data for water-lithium bromide solutions. The chart is drawn by taking reference enthalpy of 0 kj/kg for liquid water at 0 o C and solid anhydrous lithium bromide salt at 25 o C.
5 Fig.1.4: Enthalpy Temperature - Concentration diagram for H2O-LiBr solution Enthalpy values for pure water (liquid and superheated vapour) The enthalpy of pure water vapour and liquid at different temperatures and pressures can be obtained from pure water property data. For all practical purposes, liquid water enthalpy, hw,liquid at any temperature T can be obtained from the equation: hw,liquid = 4.19 (T Tref ) kj / kg where Tref is the reference temperature, 0 o C The water vapour generated in the generator of water-lithium bromide system is in super heated condition as the generator temperature is much higher than the saturation water temperature at that pressure. The enthalpy of superheated water vapour, hw,sup at low pressures and temperature T can be obtained approximately by the equation: hw,sup = (T Tref ) Crystallization The pressure-temperature-mass part and enthalpy-temperature-mass portion charts (Figs. 1.3 and 1.4) show lines stamped as crystallization in the lower right segment. The area to one side and below these crystallization lines indicates solidification of LiBr salt. In the crystallization area a two-phase mixture (slush) of water-lithium bromide arrangement and crystals of
6 immaculate LiBr exist in harmony. The water-lithium bromide system ought to work far from the crystallization district as the arrangement of solid crystals can hinder the funnels and valves. Crystallization can happen when the hot arrangement rich in LiBr salt is cooled in the arrangement heat exchanger to low temperatures. To maintain a strategic distance from this condenser pressure lessening below a certain worth because of say, low cooling water temperature in the condenser ought to be evaded. Thus in business systems, the condenser pressure is falsely looked after high despite the fact that the temperature of the accessible warmth sink is low. This really decreases the execution of the system, yet is vital for fitting operation of the system. It ought to be noted from the property charts that the whole water-lithium bromide system works under vacuum Steady flow analysis of Water-Lithium Bromide Systems Figure 1.5 shows the schematic of the system indicating various state points. A steady flow analysis of the system is carried out with the following assumptions: i. Steady state and steady flow. ii. Changes in potential and kinetic energies across each component are negligible. iii. No pressure drops due to friction. iv. Only pure refrigerant boils in the generator. The nomenclature followed is:.m = mass flow rate of refrigerant, kg/s.m ss = mass flow rate of strong solution (rich in LiBr), kg/s.m ws = mass flow rate of weak solution (weak in LiBr), kg/s
7 Fig.1.5: Schematic of a H2O-LiBr system A: Absorber; C: Condenser; E: Evaporator; G: Generator; P: Solution Pump SHX: Solution HX; ER: Refrigerant Expansion valve ES: Solution Expansion valve The circulation ratio (λ) is defined as the ratio of strong solution flow rate to refrigerant flow rate. It is given by:. The analysis is carried out by applying mass and energy balance across each component.
8 The first term in the above equation m(h4 - h5 ) represents the enthalpy change of water as changes its state from vapour at state 4 to liquid at state 5. The second term m (h10 - h5 ) represents the sensible heat transferred as solution at state 10 is cooled to solution at state 5. where vsol is the specific volume of the solution which can be taken to be approximately equal to m3/kg. Even though the solution pump work is small it is still required in the selection of suitable pump.
9 in the above equation the 1st term on the RHS m(h1 - h7 ) represents energy required to generate water vapour at state 1 from solution at state 7 and the 2 nd term m (h8- h7 ) represents the sensible heat required to heat the solution from state 7 to state 8. In order to find the steady-state performance of the system from the above set of equations, one needs to know the operating temperatures, weak and strong solution concentrations, effectiveness of solution heat exchanger and the refrigeration capacity. It is generally assumed that the solution at the exit of absorber and generator is at equilibrium so that the equilibrium P-T-ξ and h- T-ξ charts can be used for evaluating solution property data. The effectiveness of solution heat exchanger, εhx is given by:
10 From the above equation the temperature of the weak solution entering the generator (T7) can be obtained since T6 is almost equal to T5 and T8 is equal to the generator temperature Tg. The temperature of superheated water vapour at state 1 may be assumed to be equal to the strong solution temperature T Practical problems in water-lithium bromide systems Practical problems typical to water-lithium bromide systems are: 1. Crystallization 2. Air leakage, and 3. Pressure drops As mentioned before to prevent crystallization the condenser pressure has to be maintained at certain level, irrespective of cooling water temperature. This can be done by regulating the flow rate of cooling water to the condenser. Additives are also added in practical systems to inhibit crystallization. Since the entire system operates under vacuum, outside air leaks into the system. Hence an air purging system is used in practical systems. Normally a two-stage ejector type purging system is used to remove air from the system. Since the operating pressures are very small and specific volume of vapour is very high, pressure drops due to friction should be minimized. This is done by using twin- and single-drum arrangements in commercial systems Commercial systems Commercial water-lithium bromide systems can be: 1. Single stage or single-effect systems, and 2. Multi stage or multi-effect systems Single stage systems operate under two pressures one corresponding to the condenser-generator (high pressure side) and the other corresponding to evaporator absorber. Single stage systems can be either: 1. Twin drum type, or 2. Single drum type
11 Since evaporator and absorber operate at the same pressure they can be housed in a single vessel, similarly generator and condenser can be placed in another vessel as these two components operate under a single pressure. Thus a twin drum system consists of two vessels operating at high and low pressures. Figure 1.6 shows a commercial, single stage, twin drum system. Fig.1.6: A commercial, twin-drum type, water-lithium bromide system As indicated in the figure, the cooling water (which goes about as heat sink) flows first to absorber, concentrates heat from absorber and after that flows to the condenser for condenser heat extraction. This is known as arrangement course of action. This game plan is worthwhile as the obliged cooling water flow rate will be little furthermore by sending the cooling water first to the absorber, the condenser can be operated at a higher pressure to anticipate crystallization. It is likewise conceivable to have cooling water flowing parallels to condenser and absorber; on the other hand, the cooling water necessity for this situation will be high. A refrigerant pump circulates fluid water in evaporator and the water is spread onto evaporator tubes for good heat and mass exchange. Heater tubes (steam or high temp water or hot oil) are inundated in the solid arrangement pool of generator for vapour generation. Pressure drops in the middle of evaporator and absorber and in the middle of generator and condenser are
12 minimized, huge measured vapour lines are eliminated and air spillages can likewise be decreased because of less number of joints. Figure 15.7 shows a solitary stage arrangement of single drum sort in which all the four segments are housed in the same vessel. The vessel is separated into high and low pressure sides by utilizing a diaphragm. Fig.1.7: A commercial, single-drum type, water-lithium bromide system In multi-effect systems a progression of generators operating at continuously decreasing pressures are utilized. Heat is supplied to the highest stage generator operating at the highest pressure. The enthalpy of the steam generated from this generator is utilized to generate some more refrigerant vapour in the lower stage generator et cetera. In this way the heat info to the system is utilized effectively by generating more refrigerant vapour prompting higher COPs. Notwithstanding, these systems are more mind boggling in development and oblige a much higher heat source temperatures in the highest stage generator. Figures 1.8 and 1.9 show business double-effect systems. Figure 1.10 shows the double effect cycle on Dühring plot.
13 Fig.1.8: A commercial, double-effect, water-lithium bromide system Fig.1.9: A commercial, double-effect, water-lithium bromide system
14 Fig.1.10: Double affect VARS on Dühring plot 1.6. Heat sources for water-lithium bromide systems Water-lithium bromide systems can be driven utilizing a wide mixed bag of heat sources. Substantial limit systems are generally determined by steam or boiling hot water. Little limit systems are generally determined straightforwardly by oil or gas. A common single effect system obliges a heat source at a temperature of around 120oC to create chilled water at 7oC when the condenser operates at around 46oC and the absorber operates at around 40oC. The COPs acquired aor in the scope of 0.6 to 0.8 for single effect systems while it can be as high as 1.2 to 1.4 for multi-effect systems Minimum heat source temperatures for LiBr-Water systems Application data for a single-stage water-lithium bromide vapour absorption system with an output chilled water temperature of 6.7 o C (for air conditioning applications) is shown in Table 1.1. Table 1.1. Application data for a single-stage water-lithium bromide system The above values are simulated values, which were validated on actual commercial systems with very efficient heat and mass transfer design. If the heat and mass transfer is not very efficient, then the actual required heat source temperatures will be higher than the reported values. For given cooling water
15 temperature, if the heat source temperature drops below the minimum temperature given above, then the COP drops significantly. For a given cooling water temperature, if the heat source temperature drops below a certain temperature (minimum generation temperature), then the system will not function. Minimum generation temperature is typically 10 to 15oC lower than the minimum heat source temperature. If air cooled condensers and absorbers are used, then the required minimum heat source temperatures will be much higher ( 150oC). The COP of the system can be increased significantly by multi effect (or multi-stage) systems. However, addition of each stage increases the required heat source temperature by approximately 50oC. 1.8 Capacity control Capacity control means capacity reduction depending upon load as the capacity will be Limit control implies limit lessening relying on burden as the limit will be greatest with no control. Regularly under both full and additionally part stacks the outlet temperature of chilled water is kept up at a close steady esteem. The refrigeration limit is then regulated by either: 1. Regulating the flow rate of feeble arrangement pumped to the generator through the arrangement pump 2. Decreasing the generator temperature by throttling the supply steam, or by diminishing the flow rate of heated water 3. Expanding the condenser temperature by bypassing a percentage of the cooling water supplied to the condenser System 1 does not influence the COP altogether as the obliged heat data lessens with diminishment in powerless arrangement flow rate, notwithstanding, since this may prompt the issue of crystallization, numerous a period a combination of the over three techniques are utilized as a part of business systems to control the capacity
R13. II B. Tech I Semester Regular/Supplementary Examinations, Oct/Nov THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max.
 SET - 1 1. a) Discuss about PMM I and PMM II b) Explain about Quasi static process. c) Show that the COP of a heat pump is greater than the COP of a refrigerator by unity. d) What is steam quality? What
SET - 1 1. a) Discuss about PMM I and PMM II b) Explain about Quasi static process. c) Show that the COP of a heat pump is greater than the COP of a refrigerator by unity. d) What is steam quality? What
 Air Cycle Refrigeration Systems Nagendra M CBM Engineer, Hindusthan Zink.Ltd The specific objectives of the lesson This lesson discusses various gas cycle refrigeration systems based on air, namely: 1.
Air Cycle Refrigeration Systems Nagendra M CBM Engineer, Hindusthan Zink.Ltd The specific objectives of the lesson This lesson discusses various gas cycle refrigeration systems based on air, namely: 1.
THERMAL ENERGY ANALYSIS OF SOLAR POWERED VAPOUR ABSORPTION COOLING SYSTEM
 International Journal of Mechanical Engineering (IJME) ISSN(P): 2319-2240 ; ISSN(E): 2319-2259 Vol. 5, Issue 1, Dec-Jan 2016, 131-142 IASET THERMAL ENERGY ANALYSIS OF SOLAR POWERED VAPOUR ABSORPTION COOLING
International Journal of Mechanical Engineering (IJME) ISSN(P): 2319-2240 ; ISSN(E): 2319-2259 Vol. 5, Issue 1, Dec-Jan 2016, 131-142 IASET THERMAL ENERGY ANALYSIS OF SOLAR POWERED VAPOUR ABSORPTION COOLING
Conceptual Design of Nuclear CCHP Using Absorption Cycle
 Conceptual Design of Nuclear CCHP Using Absorption Cycle International Conference on Opportunities and Challenges for Water Cooled Reactors in the 21 st Century Vienna, Austria, October 27-30, 2009 Gyunyoung
Conceptual Design of Nuclear CCHP Using Absorption Cycle International Conference on Opportunities and Challenges for Water Cooled Reactors in the 21 st Century Vienna, Austria, October 27-30, 2009 Gyunyoung
II. SYSTEM DESCRIPTION AND MATHEMATICAL MODELING
 Mathematical Modeling and Analysis of Absorption Refrigeration System Using Waste Heat of Diesel Genset Yashvir Singh 1*, Deepak Kumar 2, Ajay Kumar 3, Amneesh Singla 4 1,2,3,4 Mechanical Engineering,
Mathematical Modeling and Analysis of Absorption Refrigeration System Using Waste Heat of Diesel Genset Yashvir Singh 1*, Deepak Kumar 2, Ajay Kumar 3, Amneesh Singla 4 1,2,3,4 Mechanical Engineering,
Parametric Study of a Double Effect Absorption Refrigeration System
 Parametric Study of a Double Effect Absorption Refrigeration System 1 Abbas Alpaslan KOCER, 2 Murat OZTURK 1 Uluborlu Selahattin Karasoy Vocational School, Suleyman Demirel University, 32260, Isparta Turkey,
Parametric Study of a Double Effect Absorption Refrigeration System 1 Abbas Alpaslan KOCER, 2 Murat OZTURK 1 Uluborlu Selahattin Karasoy Vocational School, Suleyman Demirel University, 32260, Isparta Turkey,
ME ENGINEERING THERMODYNAMICS UNIT III QUESTION BANK SVCET
 1. A vessel of volume 0.04m 3 contains a mixture of saturated water and steam at a temperature of 250 0 C. The mass of the liquid present is 9 kg. Find the pressure, mass, specific volume, enthalpy, entropy
1. A vessel of volume 0.04m 3 contains a mixture of saturated water and steam at a temperature of 250 0 C. The mass of the liquid present is 9 kg. Find the pressure, mass, specific volume, enthalpy, entropy
Performance Assessment of Large Vapor Absorption System
 Performance Assessment of Large Vapor Absorption System Loveneesh Talwar 1, Mukesh Padha 2 Assistant Professor, Department of Electrical Engineering, YCET, Jammu, India 1 Lecturer, Department of Electrical
Performance Assessment of Large Vapor Absorption System Loveneesh Talwar 1, Mukesh Padha 2 Assistant Professor, Department of Electrical Engineering, YCET, Jammu, India 1 Lecturer, Department of Electrical
Pool Boiling Heat Transfer to Water/Lithium Bromide Mixture
 International Conference on Challenges and Opportunities in Mechanical Engineering, Industrial Engineering and Management Studies 548 Pool Boiling Heat Transfer to Water/Lithium Bromide Mixture A. Sathyabhama
International Conference on Challenges and Opportunities in Mechanical Engineering, Industrial Engineering and Management Studies 548 Pool Boiling Heat Transfer to Water/Lithium Bromide Mixture A. Sathyabhama
OUTCOME 2 TUTORIAL 2 STEADY FLOW PLANT
 UNIT 47: Engineering Plant Technology Unit code: F/601/1433 QCF level: 5 Credit value: 15 OUTCOME 2 TUTORIAL 2 STEADY FLOW PLANT 2 Be able to apply the steady flow energy equation (SFEE) to plant and equipment
UNIT 47: Engineering Plant Technology Unit code: F/601/1433 QCF level: 5 Credit value: 15 OUTCOME 2 TUTORIAL 2 STEADY FLOW PLANT 2 Be able to apply the steady flow energy equation (SFEE) to plant and equipment
CHAPTER 1 BASIC CONCEPTS
 GTU Paper Analysis CHAPTER 1 BASIC CONCEPTS Sr. No. Questions Jan 15 Jun 15 Dec 15 May 16 Jan 17 Jun 17 Nov 17 May 18 Differentiate between the followings; 1) Intensive properties and extensive properties,
GTU Paper Analysis CHAPTER 1 BASIC CONCEPTS Sr. No. Questions Jan 15 Jun 15 Dec 15 May 16 Jan 17 Jun 17 Nov 17 May 18 Differentiate between the followings; 1) Intensive properties and extensive properties,
Department of Mechanical and Materials Engineering. Solar Energy Research Institute Faculty of Engineering and Built environment.
 Ranj Sirwan 1, Yusoff Ali 1, and K. Sopian 2 1 Department of Mechanical and Materials Engineering 2 Solar Energy Research Institute Faculty of Engineering and Built environment National University of Malaysia
Ranj Sirwan 1, Yusoff Ali 1, and K. Sopian 2 1 Department of Mechanical and Materials Engineering 2 Solar Energy Research Institute Faculty of Engineering and Built environment National University of Malaysia
2. The data at inlet and exit of the turbine, running under steady flow, is given below.
 3 rd week quiz 1. Identify the correct path of fluid flow in a steam power plant. a) Steam turbine-pump-boiler-condenser. b) Economizer- evaporator- superheater. c) Pump-turbine-condenser-evaporator. d)
3 rd week quiz 1. Identify the correct path of fluid flow in a steam power plant. a) Steam turbine-pump-boiler-condenser. b) Economizer- evaporator- superheater. c) Pump-turbine-condenser-evaporator. d)
Investigation of Separator Parameters in Kalina Cycle Systems
 Research Article International Journal of Current Engineering and Technology E-ISSN 2277 46, P-ISSN 2347-56 24 INPRESSCO, All Rights Reserved Available at http://inpressco.com/category/ijcet Investigation
Research Article International Journal of Current Engineering and Technology E-ISSN 2277 46, P-ISSN 2347-56 24 INPRESSCO, All Rights Reserved Available at http://inpressco.com/category/ijcet Investigation
ABSTRACT. Christopher Somers, Masters of Science, Dr. Reinhard Radermacher, Mechanical Engineering
 ABSTRACT Title of Document: SIMULATION OF ABSORPTION CYCLES FOR INTEGRATION INTO REFINING PROCESSES Christopher Somers, Masters of Science, 2009 Directed By: Dr. Reinhard Radermacher, Mechanical Engineering
ABSTRACT Title of Document: SIMULATION OF ABSORPTION CYCLES FOR INTEGRATION INTO REFINING PROCESSES Christopher Somers, Masters of Science, 2009 Directed By: Dr. Reinhard Radermacher, Mechanical Engineering
Conceptual Design of Nuclear CCHP Using Absorption Cycle
 IAEA-CN-164-5S04 Conceptual Design of Nuclear CCHP Using Absorption Cycle Hyeonmin Kim, Gyunyoung Heo Department of Nuclear Engineering, Kyung Hee University, 1 Seocheon-dong, Giheung-gu, Yongin-si, Gyeonggi-do,
IAEA-CN-164-5S04 Conceptual Design of Nuclear CCHP Using Absorption Cycle Hyeonmin Kim, Gyunyoung Heo Department of Nuclear Engineering, Kyung Hee University, 1 Seocheon-dong, Giheung-gu, Yongin-si, Gyeonggi-do,
Chapter 5: Thermodynamic Processes and Cycles
 Chapter 5: Thermodynamic Processes and Cycles 5-6) This problem examines the Rankine heat engine introduced in Figure 5-5. Saturated steam at T = 250 C enters the turbine and the condenser operates at
Chapter 5: Thermodynamic Processes and Cycles 5-6) This problem examines the Rankine heat engine introduced in Figure 5-5. Saturated steam at T = 250 C enters the turbine and the condenser operates at
Solution Crystallization Detection for double-effect LiBr-H 2 O steam absorption chiller
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 75 (2015 ) 1522 1528 The 7 th International Conference on Applied Energy ICAE2015 Solution Crystallization Detection for double-effect
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 75 (2015 ) 1522 1528 The 7 th International Conference on Applied Energy ICAE2015 Solution Crystallization Detection for double-effect
CRISTALIZATION UNIT YUSRON SUGIARTO
 CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
Problems in chapter 9 CB Thermodynamics
 Problems in chapter 9 CB Thermodynamics 9-82 Air is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet
Problems in chapter 9 CB Thermodynamics 9-82 Air is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet
Revue des Energies Renouvelables Spécial ICT3-MENA Bou Ismail (2015) Numerical study of a single effect ejector-absorption cooling system
 Revue des Energies Renouvelables Spécial ICT3-MENA Bou Ismail (2015) 71-77 Numerical study of a single effect ejector-absorption cooling system D. Sioud 1*, M. Bourouis 2 et A. Bellagi 1 1 Unité de Recherche
Revue des Energies Renouvelables Spécial ICT3-MENA Bou Ismail (2015) 71-77 Numerical study of a single effect ejector-absorption cooling system D. Sioud 1*, M. Bourouis 2 et A. Bellagi 1 1 Unité de Recherche
Review Questions for the FE Examination
 110 THE FIRST LAW OF THERMODYNAMICS [CHAP. 4 4.1FE Review Questions for the FE Examination Select a correct statement of the first law if kinetic and potential energy changes are negligible. (A) Heat transfer
110 THE FIRST LAW OF THERMODYNAMICS [CHAP. 4 4.1FE Review Questions for the FE Examination Select a correct statement of the first law if kinetic and potential energy changes are negligible. (A) Heat transfer
Design Features of Combined Cycle Systems
 Design Features of Combined Cycle Systems 1.0 Introduction As we have discussed in class, one of the largest irreversibilities associated with simple gas turbine cycles is the high temperature exhaust.
Design Features of Combined Cycle Systems 1.0 Introduction As we have discussed in class, one of the largest irreversibilities associated with simple gas turbine cycles is the high temperature exhaust.
- 2 - SME Q1. (a) Briefly explain how the following methods used in a gas-turbine power plant increase the thermal efficiency:
 - 2 - Q1. (a) Briefly explain how the following methods used in a gas-turbine power plant increase the thermal efficiency: i) regenerator ii) intercooling between compressors (6 marks) (b) Air enters a
- 2 - Q1. (a) Briefly explain how the following methods used in a gas-turbine power plant increase the thermal efficiency: i) regenerator ii) intercooling between compressors (6 marks) (b) Air enters a
and Exergy Analysis of a Typical LiBr/H 2 O VAR
 CHAPTER - 3 Design and Evaluation of a 3 TR VAR System and Exergy Analysis of a Typical LiBr/H 2 O VAR System 3.1 Introduction The continuous increase in the cost and demand for energy has led to more
CHAPTER - 3 Design and Evaluation of a 3 TR VAR System and Exergy Analysis of a Typical LiBr/H 2 O VAR System 3.1 Introduction The continuous increase in the cost and demand for energy has led to more
AREN 2110: Thermodynamics Spring 2010 Homework 7: Due Friday, March 12, 6 PM
 AREN 2110: Thermodynamics Spring 2010 Homework 7: Due Friday, March 12, 6 PM 1. Answer the following by circling the BEST answer. 1) The boundary work associated with a constant volume process is always
AREN 2110: Thermodynamics Spring 2010 Homework 7: Due Friday, March 12, 6 PM 1. Answer the following by circling the BEST answer. 1) The boundary work associated with a constant volume process is always
The Performance of Compression-absorption Heat Pump System Using Low-temperature Geothermal Tail Water
 Proceedings World Geothermal Congress 01 Melbourne, Australia, 19- April 01 The Performance of Compression-absorption Heat Pump System Using Low-temperature Geothermal Tail Water Yulie Gong, Chao Luo,
Proceedings World Geothermal Congress 01 Melbourne, Australia, 19- April 01 The Performance of Compression-absorption Heat Pump System Using Low-temperature Geothermal Tail Water Yulie Gong, Chao Luo,
S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering
![S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering](/thumbs/95/122495961.jpg) S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering Time: 3 Hrs. Prelim Question Paper Solution [Marks : 70 Q.1 Attempt any FIVE of the following. [10] Q.1(a) Explain difference between Thermodynamic
S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering Time: 3 Hrs. Prelim Question Paper Solution [Marks : 70 Q.1 Attempt any FIVE of the following. [10] Q.1(a) Explain difference between Thermodynamic
SIMPACK - MODEL DEVELOPMENT PACKAGE FOR POWER PLANTS
 SIMPACK - MODEL DEVELOPMENT PACKAGE FOR POWER PLANTS 1.0 OVERVIEW SIMPACK is a totally integrated set of simulation software development modules for power plants. It is template based modeling tool and
SIMPACK - MODEL DEVELOPMENT PACKAGE FOR POWER PLANTS 1.0 OVERVIEW SIMPACK is a totally integrated set of simulation software development modules for power plants. It is template based modeling tool and
SUMMER 13 EXAMINATION
 Important Instructions to examiners: 1) The answers should be examined by key words and not as word-to-word as given in the model answer scheme. 2) The model answer and the answer written by candidate
Important Instructions to examiners: 1) The answers should be examined by key words and not as word-to-word as given in the model answer scheme. 2) The model answer and the answer written by candidate
Myth Busters Absorption Cooling Technology. Rajesh Dixit
 Myth Busters Absorption Cooling Technology Rajesh Dixit 1 Acknowledgements Hitachi-Johnson Controls A/C Japan Shuichiro Uchida Takashi Nishiyama Shigehiro Doi 2 Learning Objectives Busting Myths About
Myth Busters Absorption Cooling Technology Rajesh Dixit 1 Acknowledgements Hitachi-Johnson Controls A/C Japan Shuichiro Uchida Takashi Nishiyama Shigehiro Doi 2 Learning Objectives Busting Myths About
Advanced heat driven cooling cycles for low-temperature waste heat recovery
 CDTI-NEDO Joint Workshop on Energy Saving Engineering - Effective Use of Thermal Energy Advanced heat driven cooling cycles for low-temperature waste heat recovery 02/13/2018 Tatsuo Fujii Research & Development
CDTI-NEDO Joint Workshop on Energy Saving Engineering - Effective Use of Thermal Energy Advanced heat driven cooling cycles for low-temperature waste heat recovery 02/13/2018 Tatsuo Fujii Research & Development
Thermodynamic Analysis of Combined Power and Cooling Cycle Using Process Heat from a Passout Turbine as a Heating Source
 Thermodynamic Analysis of Combined Power and Cooling Cycle Using Process Heat from a Passout Turbine as a Heating Source *Ram Darash Patel, **Priti Shukla, ***Satyashree Ghodke *Research Scholar,Department
Thermodynamic Analysis of Combined Power and Cooling Cycle Using Process Heat from a Passout Turbine as a Heating Source *Ram Darash Patel, **Priti Shukla, ***Satyashree Ghodke *Research Scholar,Department
Optimization of Irreversibilities in Water-Lithium Bromide Absorption Refrigeration System
 August 016 IJIRT Volume 3 Issue 3 ISSN: 349-600 Optimization of Irreversibilities in Water-Lithium Bromide Absorption Refrigeration System P.Tharun Sai, Dr.K.Appa Rao Lakkireddy Balireddy College of Engineering
August 016 IJIRT Volume 3 Issue 3 ISSN: 349-600 Optimization of Irreversibilities in Water-Lithium Bromide Absorption Refrigeration System P.Tharun Sai, Dr.K.Appa Rao Lakkireddy Balireddy College of Engineering
Feedwater Heaters (FWH)
 Feedwater Heaters (FWH) A practical Regeneration process in steam power plants is accomplished by extracting or bleeding, steam from the turbine at various points. This steam, which could have produced
Feedwater Heaters (FWH) A practical Regeneration process in steam power plants is accomplished by extracting or bleeding, steam from the turbine at various points. This steam, which could have produced
MCG THERMODYNAMICS II. 22 April 2008 Page 1 of 7 Prof. W. Hallett
 Faculté de génie Génie mécanique Faculty of Engineering Mechanical Engineering MCG2131 - THERMODYNAMICS II 22 April 2008 Page 1 of 7 Prof. W. Hallett Closed book. Non-programmable calculators only allowed.
Faculté de génie Génie mécanique Faculty of Engineering Mechanical Engineering MCG2131 - THERMODYNAMICS II 22 April 2008 Page 1 of 7 Prof. W. Hallett Closed book. Non-programmable calculators only allowed.
Experimental investigation of solar-driven double ejector refrigeration system
 Experimental investigation of solar-driven double ejector refrigeration system Jedsada Visedmanee a *, Anan Pongtornkulpanich a, Sakda Somkun a and Ananchai U-kaew b a Thermal Energy Research Unit, School
Experimental investigation of solar-driven double ejector refrigeration system Jedsada Visedmanee a *, Anan Pongtornkulpanich a, Sakda Somkun a and Ananchai U-kaew b a Thermal Energy Research Unit, School
DESIGN OF VAPOUR ABSORPTION REFRIGERATION SYSTEM OF 1.5TR CAPACITY BY WASTE HEAT RECOVERY PROCESS
 Volume 115 No. 7 2017, 613-619 ISSN: 1311-8080 (printed version); ISSN: 1314-3395 (on-line version) url: http://www.ijpam.eu ijpam.eu DESIGN OF VAPOUR ABSORPTION REFRIGERATION SYSTEM OF 1.5TR CAPACITY
Volume 115 No. 7 2017, 613-619 ISSN: 1311-8080 (printed version); ISSN: 1314-3395 (on-line version) url: http://www.ijpam.eu ijpam.eu DESIGN OF VAPOUR ABSORPTION REFRIGERATION SYSTEM OF 1.5TR CAPACITY
Combined Heat and Power
 Lecture 12 Combined Heat and Power Combustion Turbines and Co-generation Combustion Turbines and Combined Heat and Power (CHP) Systems See B. K. Hodge, Chapter 5 and Chapter 11. ISBN: 978-0-470-14250-9
Lecture 12 Combined Heat and Power Combustion Turbines and Co-generation Combustion Turbines and Combined Heat and Power (CHP) Systems See B. K. Hodge, Chapter 5 and Chapter 11. ISBN: 978-0-470-14250-9
Improvement of the Performance for an Absorption Refrigerating System with. Lithium bromide-water as Refrigerant by Increasing Absorption Pressure 1
 Improvement of the Performance for an Absorption Refrigerating System with Lithium bromide-water as Refrigerant by Increasing Absorption Pressure 1 Guozhen Xie Guogang Sheng Guang Li Shuyuan Pan Professor
Improvement of the Performance for an Absorption Refrigerating System with Lithium bromide-water as Refrigerant by Increasing Absorption Pressure 1 Guozhen Xie Guogang Sheng Guang Li Shuyuan Pan Professor
Distillation DEPARTMENT OF CHEMICAL ENGINEERING
 Distillation DEPARTMENT OF CHEMICAL ENGINEERING 2 3 Weeping in distillation column 4 Distillation. Introduction Unit operation Separation process A feed mixture of two or more components is separated into
Distillation DEPARTMENT OF CHEMICAL ENGINEERING 2 3 Weeping in distillation column 4 Distillation. Introduction Unit operation Separation process A feed mixture of two or more components is separated into
ENHANCEMENT OF REFRIGERATION EFFECT USING FLUE GASES FROM CHIMNEY
 International Journal of Mechanical Engineering and Technology (IJMET) Volume 9, Issue 4, April 2018, pp. 364 372, Article ID: IJMET_09_04_041 Available online at http://www.iaeme.com/ijmet/issues.asp?jtype=ijmet&vtype=9&itype=4
International Journal of Mechanical Engineering and Technology (IJMET) Volume 9, Issue 4, April 2018, pp. 364 372, Article ID: IJMET_09_04_041 Available online at http://www.iaeme.com/ijmet/issues.asp?jtype=ijmet&vtype=9&itype=4
Supersonic Nozzle Flow in the Two-Phase Ejector as Water Refrigeration System by Using Waste Heat
 HEFAT2012 9 th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics 16 18 July 2012 Malta Supersonic Nozzle Flow in the Two-Phase Ejector as Water Refrigeration System by Using
HEFAT2012 9 th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics 16 18 July 2012 Malta Supersonic Nozzle Flow in the Two-Phase Ejector as Water Refrigeration System by Using
EXTRA CREDIT OPPORTUNITY: Due end of day, Thursday, Dec. 14
 EXRA CREDI OPPORUNIY: Due end of day, hursday, Dec. 4 his extra credit set of questions is an opportunity to improve your test scores (including an insurance policy for your final exam grade). here are
EXRA CREDI OPPORUNIY: Due end of day, hursday, Dec. 4 his extra credit set of questions is an opportunity to improve your test scores (including an insurance policy for your final exam grade). here are
International Journal of Advance Engineering and Research Development
 Scientific Journal of Impact Factor (SJIF): 4.72 International Journal of Advance Engineering and Research Development Volume 4, Issue 9, September -2017 Review of Thermal Characteristics of Diesel Fired
Scientific Journal of Impact Factor (SJIF): 4.72 International Journal of Advance Engineering and Research Development Volume 4, Issue 9, September -2017 Review of Thermal Characteristics of Diesel Fired
Enhancement of CO2 Refrigeration Cycle Using an Ejector: 1D Analysis
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2006 Enhancement of CO2 Refrigeration Cycle Using an Ejector: 1D Analysis Elias
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2006 Enhancement of CO2 Refrigeration Cycle Using an Ejector: 1D Analysis Elias
Reg. No. : Question Paper Code : B.E./B.Tech. DEGREE EXAMINATION, NOVEMBER/DECEMBER Second Semester
 ws15 Reg. No. : Question Paper Code : 27425 B.E./B.Tech. DEGREE EXAMINATION, NOVEMBER/DECEMBER 2015. Time : Three hours Second Semester Marine Engineering MV 6201 MARINE ENGINEERING THERMODYNAMICS (Regulations
ws15 Reg. No. : Question Paper Code : 27425 B.E./B.Tech. DEGREE EXAMINATION, NOVEMBER/DECEMBER 2015. Time : Three hours Second Semester Marine Engineering MV 6201 MARINE ENGINEERING THERMODYNAMICS (Regulations
Available from Deakin Research Online:
 This is the published version: Yilmaz, Ferdi, Hessami, Mir Akbar, Kouzani, Abbas and Hu, Eric 00, Incorporation of a hybrid cooling system in water cooled power stations to reduce water and energy consumptions,
This is the published version: Yilmaz, Ferdi, Hessami, Mir Akbar, Kouzani, Abbas and Hu, Eric 00, Incorporation of a hybrid cooling system in water cooled power stations to reduce water and energy consumptions,
Thermodynamic Modelling of a Vapor Absorption Cogeneration Cycle
 Research Journal of Engineering Sciences ISSN 2278 9472 Thermodynamic Modelling of a Vapor Absorption Cogeneration Cycle Abstract Agarwal Priyank CO 2 Research and Green Technologies Centre, VIT University,
Research Journal of Engineering Sciences ISSN 2278 9472 Thermodynamic Modelling of a Vapor Absorption Cogeneration Cycle Abstract Agarwal Priyank CO 2 Research and Green Technologies Centre, VIT University,
Chapter 1 STEAM CYCLES
 Chapter 1 STEAM CYCLES Assoc. Prof. Dr. Mazlan Abdul Wahid Faculty of Mechanical Engineering Universiti Teknologi Malaysia www.fkm.utm.my/~mazlan 1 Chapter 1 STEAM CYCLES 1 Chapter Objectives To carry
Chapter 1 STEAM CYCLES Assoc. Prof. Dr. Mazlan Abdul Wahid Faculty of Mechanical Engineering Universiti Teknologi Malaysia www.fkm.utm.my/~mazlan 1 Chapter 1 STEAM CYCLES 1 Chapter Objectives To carry
Solar Powered Vapour Absorption Refrigeration (SPVAR) System as a rural microenterprise
 Solar Powered Vapour Absorption Refrigeration (SPVAR) System as a rural microenterprise Anurag Mudgal, Pandit Deendayal Petroleum University, India Jatin Kumar Patel, Pandit Deendayal Petroleum University,
Solar Powered Vapour Absorption Refrigeration (SPVAR) System as a rural microenterprise Anurag Mudgal, Pandit Deendayal Petroleum University, India Jatin Kumar Patel, Pandit Deendayal Petroleum University,
UNIVERSITY OF TORONTO FA CUL TY OF APPLIED SCIENCE AND ENGINEERING . DEPARTMENT OF CHEMICAL ENGINEERING AND APPLIED CHEMISTRY
 NAME: STUDENT NUMBER: ----------- UNIVERSITY OF TORONTO FA CUL TY OF APPLIED SCIENCE AND ENGINEERING. DEPARTMENT OF CHEMICAL ENGINEERING AND APPLIED CHEMISTRY CHE 208 Process Engineering, Fall-2017 EXAMINER:
NAME: STUDENT NUMBER: ----------- UNIVERSITY OF TORONTO FA CUL TY OF APPLIED SCIENCE AND ENGINEERING. DEPARTMENT OF CHEMICAL ENGINEERING AND APPLIED CHEMISTRY CHE 208 Process Engineering, Fall-2017 EXAMINER:
UNIVERSITY OF TORONTO FACULTY OF APPLIED SCIENCE AND ENGINEERING FINAL EXAMINATION, DECEMBER 2008 MIE 411H1 F - THERMAL ENERGY CONVERSION
 UNIVERSITY OF TORONTO FACULTY OF APPLIED SCIENCE AND ENGINEERING FINAL EXAMINATION, DECEMBER 2008 MIE 411H1 F - THERMAL ENERGY CONVERSION Exam Type: X Examiner: J.S. Wallace You may use your copy of the
UNIVERSITY OF TORONTO FACULTY OF APPLIED SCIENCE AND ENGINEERING FINAL EXAMINATION, DECEMBER 2008 MIE 411H1 F - THERMAL ENERGY CONVERSION Exam Type: X Examiner: J.S. Wallace You may use your copy of the
ENERGY AND EXERGY ANALYSIS OF HEAT PUMP USING R744/R32 REFRIGERANT MIXTURE
 THERMAL SCIENCE, Year 2014, Vol. 18, No. 5, pp. 1649-1654 1649 ENERGY AND EXERGY ANALYSIS OF HEAT PUMP USING R744/R32 REFRIGERANT MIXTURE by Fang WANG, Xiao-Wei FAN, Jie CHEN, and Zhi-Wei LIAN School of
THERMAL SCIENCE, Year 2014, Vol. 18, No. 5, pp. 1649-1654 1649 ENERGY AND EXERGY ANALYSIS OF HEAT PUMP USING R744/R32 REFRIGERANT MIXTURE by Fang WANG, Xiao-Wei FAN, Jie CHEN, and Zhi-Wei LIAN School of
Theoretical analysis of an integrated thermoelectric-absorption cooling system
 Theoretical analysis of an integrated thermoelectric-absorption cooling system R. Boukhanouf (corresponding author) and A. Supasuteekul School of the Built Environment University of Nottingham, Nottingham,
Theoretical analysis of an integrated thermoelectric-absorption cooling system R. Boukhanouf (corresponding author) and A. Supasuteekul School of the Built Environment University of Nottingham, Nottingham,
PI Heat and Thermodynamics - Course PI 25 CRITERION TEST. of each of the following a. it
 Heat and Thermodynamics - Course PI 25 CRITERION TESTS PI 25-1 - 1. Define: heat temperature (c) enthalpy 2. State the applies to meaning water: of each of the following a. it saturation temperature subcooled
Heat and Thermodynamics - Course PI 25 CRITERION TESTS PI 25-1 - 1. Define: heat temperature (c) enthalpy 2. State the applies to meaning water: of each of the following a. it saturation temperature subcooled
Energy and Exergy Analysis of Combined Ejector-Absorption Refrigeration System
 Energy and Exergy Analysis of Combined Ejector- Seyed Reza Fakheri 1, Hadi Kargar Sharif Abad 2, Hossein Sakhaii Nia 3 1 M.A Student, Azad University of Science and Research of Semnan, Mechanics College,
Energy and Exergy Analysis of Combined Ejector- Seyed Reza Fakheri 1, Hadi Kargar Sharif Abad 2, Hossein Sakhaii Nia 3 1 M.A Student, Azad University of Science and Research of Semnan, Mechanics College,
Reading Problems , 11.36, 11.43, 11.47, 11.52, 11.55, 11.58, 11.74
 Rankine Cycle Reading Problems 11.1 11.7 11.29, 11.36, 11.43, 11.47, 11.52, 11.55, 11.58, 11.74 Definitions working fluid is alternately vaporized and condensed as it recirculates in a closed cycle the
Rankine Cycle Reading Problems 11.1 11.7 11.29, 11.36, 11.43, 11.47, 11.52, 11.55, 11.58, 11.74 Definitions working fluid is alternately vaporized and condensed as it recirculates in a closed cycle the
System Analysis on Absorption Chiller Utilizing Intermediate Wasted Heat from Micro Gas Turbine and Solid Oxide Fuel Cell Hybrid System
 System Analysis on Absorption Chiller Utilizing Intermediate Wasted Heat from Micro Gas Turbine and Solid Oxide Fuel Cell Hybrid System *Hiroshi Suzuki, *Miki Yamada, **Yoshiyuki Komoda and **Hiromoto
System Analysis on Absorption Chiller Utilizing Intermediate Wasted Heat from Micro Gas Turbine and Solid Oxide Fuel Cell Hybrid System *Hiroshi Suzuki, *Miki Yamada, **Yoshiyuki Komoda and **Hiromoto
A. the temperature of the steam at the turbine exhaust increases. B. additional moisture is removed from the steam entering the turbine.
 P77 Overall nuclear power plant thermal efficiency will decrease if... A. the temperature of the steam at the turbine exhaust increases. B. additional moisture is removed from the steam entering the turbine.
P77 Overall nuclear power plant thermal efficiency will decrease if... A. the temperature of the steam at the turbine exhaust increases. B. additional moisture is removed from the steam entering the turbine.
Energy Mix Opportunity of Vapor Absorption Refrigeration System Integrated With Solar Collectors: A Case Study of Hotel Shangri-La
 Proceedings of IOE Graduate Conference, 2016 pp. 349 354 Energy Mix Opportunity of Vapor Absorption Refrigeration System Integrated With Solar Collectors: Prashant Raj Neupane 1, Laxman Poudel 1 1 Department
Proceedings of IOE Graduate Conference, 2016 pp. 349 354 Energy Mix Opportunity of Vapor Absorption Refrigeration System Integrated With Solar Collectors: Prashant Raj Neupane 1, Laxman Poudel 1 1 Department
APPLICATION OF CUSTOMIZED ABSORPTION HEAT PUMPS WITH HEATING CAPACITIES ABOVE 500 kw PROJECT: ACKERMANNBOGEN, MUNICH
 APPLICATION OF CUSTOMIZED ABSORPTION HEAT PUMPS WITH HEATING CAPACITIES ABOVE 500 kw PROJECT: ACKERMANNBOGEN, MUNICH Michael Radspieler, Peter Zachmeier, Christian Schweigler, Bavarian Center for Applied
APPLICATION OF CUSTOMIZED ABSORPTION HEAT PUMPS WITH HEATING CAPACITIES ABOVE 500 kw PROJECT: ACKERMANNBOGEN, MUNICH Michael Radspieler, Peter Zachmeier, Christian Schweigler, Bavarian Center for Applied
Waste Heat Recovery of IC Engine Using VAR System
 Waste Heat Recovery of IC Engine Using VAR System #1 Ketan Bhore, #2 Prof. Sharad Bhosale 1 Heat Power Engineering, Savitribai Phule Pune University, TCOER, Pune, India 2 Heat Power Engineering, Savitribai
Waste Heat Recovery of IC Engine Using VAR System #1 Ketan Bhore, #2 Prof. Sharad Bhosale 1 Heat Power Engineering, Savitribai Phule Pune University, TCOER, Pune, India 2 Heat Power Engineering, Savitribai
LECTURE-14. Air Refrigeration Cycles. Coefficient of Performance of a Refrigerator:
 Lecturer: -Dr. Esam Mejbil Abid Subject: Air Conditioning and Refrigeration Year: Fourth B.Sc. Babylon University College of Engineering Department of Mechanical Engineering LECTURE-14 Air Refrigeration
Lecturer: -Dr. Esam Mejbil Abid Subject: Air Conditioning and Refrigeration Year: Fourth B.Sc. Babylon University College of Engineering Department of Mechanical Engineering LECTURE-14 Air Refrigeration
Chapter 10 POWER CYCLES. Department of Mechanical Engineering
 Chapter 10 VAPOR AND COMBINED POWER CYCLES Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University it 2 Objectives Analyze vapor power cycles in which h the working fluid is alternately
Chapter 10 VAPOR AND COMBINED POWER CYCLES Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University it 2 Objectives Analyze vapor power cycles in which h the working fluid is alternately
Minimization of Irreversibilities in Vapour Absorption system by placing Organic Rankine Cycle at Condenser
 International Research Journal of Engineering and Technology (IRJET e-issn: 395-0056 Volume: 03 Issue: 08 Aug-016 www.irjet.net p-issn: 395-007 Minimization of Irreversibilities in Vapour Absorption system
International Research Journal of Engineering and Technology (IRJET e-issn: 395-0056 Volume: 03 Issue: 08 Aug-016 www.irjet.net p-issn: 395-007 Minimization of Irreversibilities in Vapour Absorption system
Div. 5 7:30 am Div. 2 10:30 am Div. 4 12:30 am Prof. Naik Prof. Braun Prof. Bae
 ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW: Div. 5 7:30 am Div. 2 10:30 am Div. 4 12:30 am Prof. Naik Prof. Braun Prof. Bae Div. 3 2:30 pm Div. 1 4:30 pm Div. 6 4:30 pm Prof. Chen Prof.
ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW: Div. 5 7:30 am Div. 2 10:30 am Div. 4 12:30 am Prof. Naik Prof. Braun Prof. Bae Div. 3 2:30 pm Div. 1 4:30 pm Div. 6 4:30 pm Prof. Chen Prof.
CHAPTER 2 STUDY OF 210 MW BOILER SYSTEM 2.1 DESCRIPTION OF 210 MW BOILER
 8 CHAPTER 2 STUDY OF 210 MW BOILER SYSTEM 2.1 DESCRIPTION OF 210 MW BOILER Detailed study has been carried out on a 210 MW boiler system with regard to model development, control and optimization. Figure
8 CHAPTER 2 STUDY OF 210 MW BOILER SYSTEM 2.1 DESCRIPTION OF 210 MW BOILER Detailed study has been carried out on a 210 MW boiler system with regard to model development, control and optimization. Figure
Thermodynamic Data. CO (g, 0 C, 1 atm) CO (g,100 C, 1 atm):
 Thermodynamic Data It is not possible to know the absolute value of Uˆ or Ĥ for a pure substance, but you can determine the change in U ˆ ( U ˆ ) or H ˆ ( Hˆ ) corresponding to a specified change of state
Thermodynamic Data It is not possible to know the absolute value of Uˆ or Ĥ for a pure substance, but you can determine the change in U ˆ ( U ˆ ) or H ˆ ( Hˆ ) corresponding to a specified change of state
Exergy Analysis of a Single-Effect Water-Lithium Bromide Absorption Chiller Powered by Waste Energy Source for Different Cooling Capacities
 Energy and Power 2013, 3(6): 106-118 DOI: 10.5923/j.ep.20130306.02 Exergy Analysis of a Single-Effect Water-Lithium Bromide Absorption Chiller Powered by Waste Energy Source for Different Cooling Capacities
Energy and Power 2013, 3(6): 106-118 DOI: 10.5923/j.ep.20130306.02 Exergy Analysis of a Single-Effect Water-Lithium Bromide Absorption Chiller Powered by Waste Energy Source for Different Cooling Capacities
R13 SET - 1 '' ''' '' ' '''' Code No: RT31035
 R13 SET - 1 III B. Tech I Semester Regular/Supplementary Examinations, October/November - 2016 THERMAL ENGINEERING II (Mechanical Engineering) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists
R13 SET - 1 III B. Tech I Semester Regular/Supplementary Examinations, October/November - 2016 THERMAL ENGINEERING II (Mechanical Engineering) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists
Available online at ScienceDirect. Energy Procedia 49 (2014 ) SolarPACES 2013
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 49 (2014 ) 993 1002 SolarPACES 2013 Thermal storage concept for solar thermal power plants with direct steam generation M. Seitz
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 49 (2014 ) 993 1002 SolarPACES 2013 Thermal storage concept for solar thermal power plants with direct steam generation M. Seitz
Low-Grade Waste Heat Recovery for Power Production using an Absorption-Rankine Cycle
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2010 Low-Grade Waste Heat Recovery for Power Production using an Absorption-Rankine
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2010 Low-Grade Waste Heat Recovery for Power Production using an Absorption-Rankine
Secondary Systems: Steam System
 Secondary Systems: Steam System K.S. Rajan Professor, School of Chemical & Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 10 Table of Contents 1 SECONDARY SYSTEM
Secondary Systems: Steam System K.S. Rajan Professor, School of Chemical & Biotechnology SASTRA University Joint Initiative of IITs and IISc Funded by MHRD Page 1 of 10 Table of Contents 1 SECONDARY SYSTEM
THERMODYNAMIC ANALYSIS OF A SMALL-CAPACITY AMMONIA / WATER ABSORPTION HEAT PUMP FOR HEATING AND COOLING
 - - THERMODYNAMIC ANALYSIS OF A SMALL-CAPACITY AMMONIA / WATER ABSORPTION HEAT PUMP FOR HEATING AND COOLING H. MOSER, Assistant lecturer R. RIEBERER, Associate Professor D. DORNSTÄDTER, Alumni Institute
- - THERMODYNAMIC ANALYSIS OF A SMALL-CAPACITY AMMONIA / WATER ABSORPTION HEAT PUMP FOR HEATING AND COOLING H. MOSER, Assistant lecturer R. RIEBERER, Associate Professor D. DORNSTÄDTER, Alumni Institute
Experimental testing of an innovative Lithium-Bromide water absorption refrigeration cycle coupled with ice storage
 Experimental testing of an innovative Lithium-Bromide water absorption refrigeration cycle coupled with ice storage Jorge A. J. Caeiro The Bartlett School of Graduate Studies, Faculty of the Built Environment,
Experimental testing of an innovative Lithium-Bromide water absorption refrigeration cycle coupled with ice storage Jorge A. J. Caeiro The Bartlett School of Graduate Studies, Faculty of the Built Environment,
Chapters 5, 6, and 7. Use T 0 = 20 C and p 0 = 100 kpa and constant specific heats unless otherwise noted. Note also that 1 bar = 100 kpa.
 Chapters 5, 6, and 7 Use T 0 = 20 C and p 0 = 100 kpa and constant specific heats unless otherwise noted. Note also that 1 bar = 100 kpa. 5-1. Steam enters a steady-flow device at 16 MPa and 560 C with
Chapters 5, 6, and 7 Use T 0 = 20 C and p 0 = 100 kpa and constant specific heats unless otherwise noted. Note also that 1 bar = 100 kpa. 5-1. Steam enters a steady-flow device at 16 MPa and 560 C with
Heat Transfer Theory. Jennie Borgström
 Heat Transfer Theory Jennie Borgström Modes of heat transfer Law of physics Heat = Energy If you take a hot spot and a cold spot the heat will always be transferred from the hot to the cold Three ways
Heat Transfer Theory Jennie Borgström Modes of heat transfer Law of physics Heat = Energy If you take a hot spot and a cold spot the heat will always be transferred from the hot to the cold Three ways
W t. Strategy: We need to step through the equipments and figure out the inlets and outlets to allow us to calculate the work and heat terms.
 Problem et G 10.1 olution pring 00 YT ) A schematic of the Rankine cycle and representation on a T- diagram: 1 Boiler P 0 W p pump 0 P 0 Condenser turbine 0 4 C 475 o C Depends on your last name. The information
Problem et G 10.1 olution pring 00 YT ) A schematic of the Rankine cycle and representation on a T- diagram: 1 Boiler P 0 W p pump 0 P 0 Condenser turbine 0 4 C 475 o C Depends on your last name. The information
Chapter 1 Basic Concepts
 Jan 15 Jun 15 Chapter 1 Basic Concepts GTU Paper Analysis (New Syllabus) Sr. No. Questions Differentiate between the followings; 1) Intensive properties and extensive properties, 2) Point function and
Jan 15 Jun 15 Chapter 1 Basic Concepts GTU Paper Analysis (New Syllabus) Sr. No. Questions Differentiate between the followings; 1) Intensive properties and extensive properties, 2) Point function and
COEFFICIENT OF PERFORMANCE OF TWO PHASE CONDENSING EJECTOR REFRIGERATION SYSTEM WITH R-22
 INTERNATIONAL JOURNAL OF RESEARCH IN COMPUTER APPLICATIONS AND ROBOTICS ISSN 2320-7345 COEFFICIENT OF PERFORMANCE OF TWO PHASE CONDENSING EJECTOR REFRIGERATION SYSTEM WITH R-22 Kuldip Kumar 1, Anjani Kumar
INTERNATIONAL JOURNAL OF RESEARCH IN COMPUTER APPLICATIONS AND ROBOTICS ISSN 2320-7345 COEFFICIENT OF PERFORMANCE OF TWO PHASE CONDENSING EJECTOR REFRIGERATION SYSTEM WITH R-22 Kuldip Kumar 1, Anjani Kumar
Lecture 4. Ammonia: Production and Storage - Part 1
 Lecture 4 Ammonia: Production and Storage - Part 1 Ammonia or azane is a compound of nitrogen and hydrogen with the formula NH 3. It is a colourless gas with a characteristic pungent smell. Ammonia contributes
Lecture 4 Ammonia: Production and Storage - Part 1 Ammonia or azane is a compound of nitrogen and hydrogen with the formula NH 3. It is a colourless gas with a characteristic pungent smell. Ammonia contributes
Second Law of Thermodynamics
 Second Law of Thermodynamics Content Heat engine and its efficiency. Reversible and irreversible processes. The Carnot machine. Kelvin Planck Statement. Refrigerator and Coefficient of Performance. Statement
Second Law of Thermodynamics Content Heat engine and its efficiency. Reversible and irreversible processes. The Carnot machine. Kelvin Planck Statement. Refrigerator and Coefficient of Performance. Statement
Pinch Analysis for Power Plant: A Novel Approach for Increase in Efficiency
 Pinch Analysis for Power Plant: A Novel Approach for Increase in Efficiency S. R. Sunasara 1, J. J. Makadia 2 * 1,2 Mechanical Engineering Department, RK University Kasturbadham, Rajkot-Bhavngar highway,
Pinch Analysis for Power Plant: A Novel Approach for Increase in Efficiency S. R. Sunasara 1, J. J. Makadia 2 * 1,2 Mechanical Engineering Department, RK University Kasturbadham, Rajkot-Bhavngar highway,
CRYSTALLIZATION SOLUBILITY CURVE
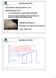 CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
Comfort Cooling Application Using Fixed Focus Solar Parabolic Dish Concentrator Integrated with Double Effect Vapor Absorption Machine
 Comfort Cooling Application Using Fixed Focus Solar Parabolic Dish Concentrator Integrated with Double Effect Vapor Absorption Machine Anagha Pathak 1*, Kiran Deshpande 1, Niranjan Kurhe 1, Pravin Baste
Comfort Cooling Application Using Fixed Focus Solar Parabolic Dish Concentrator Integrated with Double Effect Vapor Absorption Machine Anagha Pathak 1*, Kiran Deshpande 1, Niranjan Kurhe 1, Pravin Baste
Enthalpy Calculations. Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions.
 Enthalpy Calculations Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions. Enthalpy Change as a Result of Temperature Sensible heat is the
Enthalpy Calculations Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions. Enthalpy Change as a Result of Temperature Sensible heat is the
THERMODYNAMIC ANALYSIS OF R134A DMAC VAPOR ABSORPTION REFRIGERATION (VAR) SYSTEM
 THERMODYNAMIC ANALYSIS OF R134A DMAC VAPOR ABSORPTION REFRIGERATION (VAR) SYSTEM 1 V. Mariappan, 2 M. Udayakumar, 3 Pratisthit Lal Shrestha, 4 S. Suresh 1 Assistant Professor, 2 Professor, 3 M.Tech. Scholar,
THERMODYNAMIC ANALYSIS OF R134A DMAC VAPOR ABSORPTION REFRIGERATION (VAR) SYSTEM 1 V. Mariappan, 2 M. Udayakumar, 3 Pratisthit Lal Shrestha, 4 S. Suresh 1 Assistant Professor, 2 Professor, 3 M.Tech. Scholar,
DOUBLE-EFFECT SOLAR ABSORPTION THERMAL ENERGY STORAGE
 Jurnal Mekanikal December 2012, No.35, 38-53 DOUBLE-EFFECT SOLAR ABSORPTION THERMAL ENERGY STORAGE R.A. Rasih 1 and F.N. Ani *2 1 Faculty of Mechanical Engineering, Universiti Teknologi Mara Pulau Pinang,
Jurnal Mekanikal December 2012, No.35, 38-53 DOUBLE-EFFECT SOLAR ABSORPTION THERMAL ENERGY STORAGE R.A. Rasih 1 and F.N. Ani *2 1 Faculty of Mechanical Engineering, Universiti Teknologi Mara Pulau Pinang,
Eng Thermodynamics I: Sample Final Exam Questions 1
 Eng3901 - Thermodynamics I: Sample Final Exam Questions 1 The final exam in Eng3901 - Thermodynamics I consists of four questions: (1) 1st Law analysis of a steam power cycle, or a vapour compression refrigeration
Eng3901 - Thermodynamics I: Sample Final Exam Questions 1 The final exam in Eng3901 - Thermodynamics I consists of four questions: (1) 1st Law analysis of a steam power cycle, or a vapour compression refrigeration
Lecture No.3. The Ideal Reheat Rankine Cycle
 Lecture No.3 The Ideal Reheat Rankine Cycle 3.1 Introduction We noted in the last section that increasing the boiler pressure increases the thermal efficiency of the Rankine cycle, but it also increases
Lecture No.3 The Ideal Reheat Rankine Cycle 3.1 Introduction We noted in the last section that increasing the boiler pressure increases the thermal efficiency of the Rankine cycle, but it also increases
EXPERIMENTAL ANALYSIS OF TRIPLE FLUID VAPOUR ABSORPTION REFRIGERATION SYSTEM DRIVEN BY ELECTRICAL ENERGY AND ENGINE WASTE HEAT. Kancheepuram, India
 EXPERIMENTAL ANALYSIS OF TRIPLE FLUID VAPOUR ABSORPTION REFRIGERATION SYSTEM DRIVEN BY ELECTRICAL ENERGY AND ENGINE WASTE HEAT Balasubramanian ANDI 1*, Venkatesan JAYARAMAN 2, Suresh SIVAN 3, Mariappan
EXPERIMENTAL ANALYSIS OF TRIPLE FLUID VAPOUR ABSORPTION REFRIGERATION SYSTEM DRIVEN BY ELECTRICAL ENERGY AND ENGINE WASTE HEAT Balasubramanian ANDI 1*, Venkatesan JAYARAMAN 2, Suresh SIVAN 3, Mariappan
ANALYSIS OF DIFFERENT TYPES OF REGULATION AND ITS EFFICIENCY IN STEAM POWER CYCLES MASTER THESIS
 ANALYSIS OF DIFFERENT TYPES OF REGULATION AND ITS EFFICIENCY IN STEAM POWER CYCLES MASTER THESIS Author: Ricardo Sánchez Pereiro Advisor: Piotr Krzyslak Poznan University of Technology 11/06/2012 INDEX
ANALYSIS OF DIFFERENT TYPES OF REGULATION AND ITS EFFICIENCY IN STEAM POWER CYCLES MASTER THESIS Author: Ricardo Sánchez Pereiro Advisor: Piotr Krzyslak Poznan University of Technology 11/06/2012 INDEX
Module 05 Lecture 35 : Low temperature process design Key words: Refrigeration system
 Refrigeration System Module 05 Lecture 35 : Low temperature process design Key words: Refrigeration system A refrigerator is simply a heat pump where heat is rejected to atmosphere( the sink). Fig.41.3(lecture
Refrigeration System Module 05 Lecture 35 : Low temperature process design Key words: Refrigeration system A refrigerator is simply a heat pump where heat is rejected to atmosphere( the sink). Fig.41.3(lecture
Lecture No.1. Vapour Power Cycles
 Lecture No.1 1.1 INTRODUCTION Thermodynamic cycles can be primarily classified based on their utility such as for power generation, refrigeration etc. Based on this thermodynamic cycles can be categorized
Lecture No.1 1.1 INTRODUCTION Thermodynamic cycles can be primarily classified based on their utility such as for power generation, refrigeration etc. Based on this thermodynamic cycles can be categorized
DEVELOPMENT OF TRIPLE-EFFECT ABSORPTION CHILLER-HEATER
 DEVELOPMENT OF TRIPLE-EFFECT ABSORPTION CHILLER-HEATER Kiyoyuki Mori, Japan Gas Association Masahiro Oka, Japan Gas Association Toshikuni Ohhashi, Japan Gas Association 1. INTRODUCTION Working at the request
DEVELOPMENT OF TRIPLE-EFFECT ABSORPTION CHILLER-HEATER Kiyoyuki Mori, Japan Gas Association Masahiro Oka, Japan Gas Association Toshikuni Ohhashi, Japan Gas Association 1. INTRODUCTION Working at the request
S.E. (Mechanical) (First Semester) EXAMINATION, 2012 APPLIED THERMODYNAMICS (2008 PATTERN) Time : Three Hours Maximum Marks : 100
 Total No. of Questions 12] [Total No. of Printed Pages 8 Seat No. [4162]-111 S.E. (Mechanical) (First Semester) EXAMINATION, 2012 APPLIED THERMODYNAMICS (2008 PATTERN) Time : Three Hours Maximum Marks
Total No. of Questions 12] [Total No. of Printed Pages 8 Seat No. [4162]-111 S.E. (Mechanical) (First Semester) EXAMINATION, 2012 APPLIED THERMODYNAMICS (2008 PATTERN) Time : Three Hours Maximum Marks
COMPARATIVE ANALYSES OF TWO IMPROVED CO 2 COMBINED COOLING, HEATING, AND POWER SYSTEMS DRIVEN BY SOLAR ENERGY
 S93 Introduction COMPARATIVE ANALYSES OF TWO IMPROVED CO 2 COMBINED COOLING, HEATING, AND POWER SYSTEMS DRIVEN BY SOLAR ENERGY by Wanjin BAI a* and Xiaoxiao XU b a School of Mechanical and Vehicle Engineering,
S93 Introduction COMPARATIVE ANALYSES OF TWO IMPROVED CO 2 COMBINED COOLING, HEATING, AND POWER SYSTEMS DRIVEN BY SOLAR ENERGY by Wanjin BAI a* and Xiaoxiao XU b a School of Mechanical and Vehicle Engineering,
MUZAFFARPUR INSTITUTE OF TECHNOLOGY COURSE FILE OF THERMODYNAMICS FACULTY NAME: AMIT KUMAR ASSISTANT PROFESSOR DEPARTMENT OF MECHANICAL ENGINEERING
 MUZAFFARPUR INSTITUTE OF TECHNOLOGY COURSE FILE OF THERMODYNAMICS FACULTY NAME: AMIT KUMAR ASSISTANT PROFESSOR DEPARTMENT OF MECHANICAL ENGINEERING CONTENTS 1. Cover Page& Content 2. Vision of the Department
MUZAFFARPUR INSTITUTE OF TECHNOLOGY COURSE FILE OF THERMODYNAMICS FACULTY NAME: AMIT KUMAR ASSISTANT PROFESSOR DEPARTMENT OF MECHANICAL ENGINEERING CONTENTS 1. Cover Page& Content 2. Vision of the Department
