ISPE Annual Meeting 29 October 1 November 2017 San Diego, CA. Need systems approach to pharmaceutical manufacturing
|
|
|
- Elmer Spencer
- 6 years ago
- Views:
Transcription
1 Aligning Quality Excellence with ICH(Quality Systems Management) Prabir K Basu Need systems approach to pharmaceutical manufacturing Pharmaceutical manufacturing needs to be approached as a system that regards the organization as comprising of three purposefully designed parts that are interconnected viz. input, process and output. A systems approach makes an organization to function as a unit despite being a complex entity that's subject to changes from within and outside. This approach allows analysis of problems in a systematic fashion identifying the factors affecting the system and its various components thus allowing the design of proactive solutions to overcome problems and reduce risks to the operation and product quality. Pharmaceutical manufacturing is a system which has a set of parts that interact and affect each other, thereby creating a larger whole of a complex thing. Pharmaceutical manufacturing is complex and is growing more complex everyday with globalization globalization often brings uncontrollable risks to supply as well as quality. The old ways are inadequate for our dynamic world. Systems thinking will allow a model of decision making that will help pharma manufacturing effectively deal with changes and adapt. A maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high quality drug products without extensive regulatory oversight Dr. Woodcock, 2005 Prabir K Basu 1
2 Lack of a systems approach to CGMP makes it difficult to comply GMPs provide guidance on the manufacture and control of pharmaceutical product, but GMPs do not address the systems needed to bring a quality product to market The guidance provided by GMPs is mostly on how to address problems after the fact, such as CAPA, change control, etc. but do not profess proactive continual improvement GMPs, as it is, provide a discrete set of instructions that involve many actors and many independent systems it is difficult to see the big picture without a proper systems approach. GMPs do not drive a lifecycle approach to quality and there is no concept of continuous improvement in GMPs Issues where an action impacts (or is affected by) the environment surrounding the issue (including people, equipment, culture, etc.), cannot be solved without a systems approach. This is particularly important in this global environment. GMPs only touch on management responsibility and employee participation in quality. There is no specific reference in the GMPs on the necessity of a robust quality culture in an organization Without a systems approach, problems reoccur and are made worse by past attempts to fix them. Systems thinking would allow people to make their understanding of GMPs more explicit in a manner similar to how engineers use engineering principles to analyze and improve their understanding of mechanical systems. Prabir K Basu 3 FDA Industry effort to implement a Quality Systems Approach to cgmp Regulation since early 2000 GMPs originated in 1970s and have been only incrementally added to. The ISO Quality Management thinking was not embedded in the GMPs. In 2002, FDA issued a report titled Pharmaceutical CGMPs for the 21st century a Risk Based Approach In 2006, the US Food and Drug Administration issued a document entitled, Guidance for Industry: Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations. This document represented FDA s efforts to encourage the pharmaceutical and biotechnology industry to adopt...modern quality systems and risk management approaches... to management of GMP compliance. The idea was that a system of management controls can serve to support and maintain a company s GMP compliance status. The establishment of management controls is a concept that is embodied in the FDA guidance and has also been advanced by FDA in other contexts in recent years. Since then, there has been multiple FDA Industry efforts to implement Quality Systems approach to pharma manufacturing 2
3 Lawrence Yu, CDER Update: Quality Metrics, Quality Culture, and other Initiatives, 15th ANNUAL PIA PR REGULATORY CONFERENCE Innovation and Compliance as an Enabler of Quality System Robustness, JUNE 22, 2017, Ritz Carlton Hotel San Juan PR My goal today is to show that CDER s Quality Journey is synergistic with the evolution of the OPEX model at St. Gallen s Around , the OPEX team in Switzerland, along with its industry collaborators independently embarked upon developing a systems approach to manufacturing and quality in pharma St.Gallen Operational Excellence Model provides analysis of pharma manufacturing based on a systems approach 3
4 The St. Gallen OPEX Model is a Total Quality Management System OPEX model comprises of sub systems that are critical and they reinforce each other TPM represents equipment effectiveness and overall efficiency of a comprehensive productive maintenance system TQM represents the rigorous problem solving process which should be in an operation where Total Quality and Continuous Improvement are key objectives. Six Sigma and Lean approaches are very much aligned to this concept. JIT represents optimization of manufacturing operations and ultimate customer satisfaction. It is one of the key elements of Total Quality Management and is possible only when good processes, systems and a culture of continuous improvement is in place. Management System incorporates other key elements of Total Quality Management such as management commitment, employee participation, continuous improvement and training. St. Gallen OPEX framework contains different blocks How efficiently fixed assets are being managed while effectively using new process technology? The TQM System is a measure of quality performance (e.g. reduce variability, scrap rates, complaint rates etc.). TPM TQM JIT Foundation The main objective of the foundation elements is to support the deployment of TQM, TPM and JIT principles. Elements Effective Management System Enablers Prabir K Basu The main objective of JIT is the reduction of working capital (incl. inventories) by simultaneously increasing service levels. The main objective of an effective Management System is a learning organization that is managed by clear objectives. 8 4
5 St. Gallen OPEX Framework enables continuous improvement Preventive maintenance Stabilize the Underlying System TPM Process management TQM Customer integration Cross funct. Effective Supplier Planning Housekeeping product Layoutoptimization technology quality adherence development usage management Standardized Standardized Standardized equipment processes replenishment Stable running machines Stable processes Low inventory Self directed High continuous Low abseentism teams improvement rates & fluctuation Effective Management System Set up time reductions Low absenteeism & fluctuation Direction setting Management commitment and company culture Employee involvement & continuous improvement Functional integration & qualification Manage the Flow & Reduction of Waste JIT Pull system Flexible workforce Operational Performance Continuous Improvement Management & Leadership Source: University of St.Gallen Operational Excellence (OPEX) and Quality management have similar objectives The ultimate objectives of both OPEX and Quality are to reduce variation, streamline operations, minimize chances of errors and thus achieve a State of Control and manage the risks of noncompliance and wasteful operations. Risk management (ICH Q9) can be defined as the identification, assessment, and prioritization of risks to minimize uncertainty in processes, operations and ultimately quality. Project prioritization activity should be both cost and risk driven both are really the same in fact, risk to quality should have a priority over cost to prevent quality deterioration. In order to implement a risk informed decision making approach, risk management activities should be embedded into organizational structures and processes at both the operational and strategic levels. Prabir K Basu 10 5
6 Similarities between OPEX and Quality Management programs System based approach with focus on establishing effective systems Both attempt to standardize systems to increase reliability. Both aim to develop closed loop processes that enable continuous improvement. Proactive risk management that goes beyond compliance. Generating actionable data from key performance indicators (KPIs). Performance measurement is key to success in both programs. Collaboration among cross functional teams. Management commitment and employee participation is key in both programs. Requires documentation and knowledge management OPEX approach can be a measure of a robust Quality System Operational Excellence (OPEX) efforts are generally aimed as business goals which seek to reduce inefficiencies and increase quality. They have all the elements of an effective quality system built in. OPEX is a process that involves taking care of customers' needs, keeping the employees motivated and empowered, and continually improving the current activities at the workplace. The important components of a true operational excellence program (OPEX) are striving for stable operations, minimizing variations, continuous improvement, management commitment, and employee participation. These are also key components of any successful Quality Management program. Therefore, one should be able to use similar metrics for OPEX implementation and Quality in a pharmaceutical plant Prabir K Basu 12 6
7 The OPEX framework incorporates and has the ability to measure the core elements of Quality Management Quality Management Requires: Systems and Techniques formal system of operations and quality management should be in place Processes Analysis of processes and continuous improvement is a focus. Look after the process and the product looks after itself. (Hutchins, 1990) Management This is a way of life for a company and management must be committed and lead the effort People ongoing education and training supports drive to quality and efficiency. Employees must be encouraged to take responsibility and communicate effectively Teamwork everyone works individually and as a team Culture a radical change in culture and way of work in an organization Metrics built into the model allowing actionable responses OPEX captures performance relative to a standard, allows and encourages comparison, and supports a Quality Operations Excellence strategy. Prabir K Basu 13 Common Architecture for Quality Management & Operational Excellence Operational/Quality Management/Excellence (Quality & Cost) Stable Operations Continuous Improvement Facilities & Equipment Quality Systems Personnel Development Knowledge Management Commitment Employee Participation Technology Prabir K Basu 14 7
8 These are also the chapters of an important document issued by the FDA in the last decade Chapter 1: Pharmaceutical Quality System Chapter 2: Management Responsibilities Chapter 3: Continual Improvement of Process Performance and Product Quality Chapter 4: Continual Improvement of the Pharmaceutical Quality System Chapter 5 : Glossary Which is this document? Prabir K Basu 15 Guidance for Industry Q10 Pharmaceutical Quality System Additional copies are available from: Office of Communication Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration New Hampshire Ave.,Bldg. 51, Room 2201 Silver Spring, MD (Tel) Office of Communication, Outreach and Development, HFM-40 Center for Biologics Evaluation and Research Food and Drug Administration 1401 Rockville Pike, Rockville, MD (Tel) or U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) April 2009 ICH 8
9 Vision of ICH Q10 Quality Systems Develop a harmonized pharmaceutical quality system applicable across the lifecycle of the product emphasizing an integrated approach to quality risk management and science. (Brussels July 2003) ICH Q10 describes one comprehensive model for an effective pharmaceutical quality system. (ICH Q10) Prabir K Basu 17 Key Elements of Q10 Q10 emphasizes systems approach to Quality Management by establishing and maintaining a state of control and continuous improvement as key elements as is in any systems approach to process/operations management Q10 is based on a set of stated Objectives, supported by specified Enablers which support continual improvement of product quality and the Quality System itself. It relies heavily on the concept of Management Responsibility and Employee Participation for the Quality Systems 9
10 Pharmaceutical Quality System(ICH Q 10) Achieve Product Realization Establish & Maintain State of Control Facilitate Continual Improvement Stable Operations Stable Processes Quality Assurance Management Committment (Effective Management System) Knowledge Management (Prevention not Reaction) Quality Risk Management (Prioritization based not just on cost but also on risk) Prabir K Basu 10
11 ICH Q10 and Pharmaceutical Quality Systems were designed to address the gap in systems approach to GMPs Regulatory agencies and the pharmaceutical industry recognized this deficiency in approach to cgmps and thus in the recent years have proposed a systems approach to the cgmps. The overarching philosophy articulated in both the CGMP regulations and in robust modern quality systems is: Quality should be built into the product, and testing alone cannot be relied on to ensure product quality. Quality Systems Approach to Pharmaceutical CGMP Regulations, FDA, 2006 ICH Q10 aims to promote a paradigm shift from discrete GMP compliance procedures at each stage of the product lifecycle to a comprehensive quality systems approach over the lifecycle of the product Neil Wilkinson, ICH Q10 Pharmaceutical Quality System WCC PDA Dinner Meeting, Jan 2012 What is missing? Measuring Quality or Metrics A systems approach cannot be effective without an appropriate way to measure performance and continually improve Prabir K Basu 21 We need an integrated OPEX, Quality Systems Management, and EHS program Most companies use different teams and programs for OPEX, Quality, Quality Systems, EHS etc. Each group is trying to implement their own systems and measure performance in separate efforts These programs should be integrated into one and there should be a unified framework or system for all these programs. In addition to cost savings, quality and safety goals also can be achieved by an overall systems management and overall performance tracking and management system. FDA and industry are debating Quality Metrics. Why duplicate efforts? Determine how to measure the overall pharmaceutical Operations system and that will provide metrics for quality and safety also With minor adjustments to the work that St. Gallen is doing, we can make it s model acceptable to both the regulatory agencies and the industry. All the basic elements are already there in the OPEX model, and Quality Metrics is embedded in the already existing Operational Excellence programs of many pharmaceutical companiesaround theworld. Prabir K Basu 22 11
12 Pharmaceutical Production System Model The new Pharmaceutical Production System Model (PPSM) proposed by St. Gallen combines both OPEX and Quality Management goals. It was developed specifically for the FDA Quality Metrics Project. The model enables a structured analysis of the components of a Pharmaceutical Quality system. The St.Gallen OPEX Model as Basis for the PPSM A System Approach to Pharmaceutical Manufacturing TPM: Total Preventive Maintenance; TQM: Total Quality Management; JIT: Just in Time; PQS: Pharmaceutical Quality System 24 12
13 PPSM is a successful attempt to represent suitable metrics for the Pharmaceutical Quality System Human beings adjust behavior based on the metrics they're held against. Anything you measure will impel a person to optimize his score on that metric. What you measure is what you'll get. 1 Perhaps what you measure is what you get. More likely, what you measure is all you ll get. What you don t (or can t) measure is lost H. Thomas Johnson Thus the discrete metrics recommended in revised FDA Draft Guidance for Quality Metrics (2016) has obvious limitations. These are also inherently lagging indicators and do not reflect the effectiveness and efficiency of the quality systems. An effective performance measurement system should provide timely, accurate feedback on the efficiency and effectiveness of operations. 2 A Pharmaceutical Quality System is successful when it assures an ongoing state of control. 3 PPSM provides a structured and holistic depiction of the relevant data from the St. Gallen OPEX database which truly reflects the efficiency and the efficiency of the pharmaceutical operations/quality system. In addition, it also positions the three metrics, suggested in the revised FDA Draft Guidance (2016) within the broader context of holistic understanding. 3 Food & Drug Administration, Quality System (Drugs), you are what you measure 2 Kaplan, R., & Norton, D. (1993, September/October ). Putting the balanced scorecard to work. Harvard Business Review, pp Summary Pursuing operational excellence is both a challenge and a commitment. But for operations, willing to take it on, the result is improved performance across a range of outcomes, including product quality, safety, and ultimately, financial performance. Operational excellence is a journey, not a destination. It s also an iterative, cross functionalprocess bynature,involvingmultipledepartments,processes and stakeholders. Creating silos of quality, EHS, operations management creates roadblocks to achieving operational excellence, while an integrated system streamlines the path forward. ThesameistrueforFDA sapproaches. Individual approaches such as Quality by Design, PAT, ICH, Quality Metrics, etc. create more confusion among companies. They sometimes worry, what s next? Perhaps, if we wait long enough a new approach will be there? Operational excellence provides an unique opportunity and format to combine all of these diverse efforts into one and make it very effective and easy to adopt. 13
Quality Metrics: A Regulatory Perspective
 Quality Metrics: A Regulatory Perspective By Tara Gooen Bizjak, MBSci CDER Office of Policy for Pharmaceutical Quality Presented By Dr. Ademola Daramola US FDA Office of International Programs Indian Pharmaceutical
Quality Metrics: A Regulatory Perspective By Tara Gooen Bizjak, MBSci CDER Office of Policy for Pharmaceutical Quality Presented By Dr. Ademola Daramola US FDA Office of International Programs Indian Pharmaceutical
Future of Pharmaceutical Quality and the Path to Get There
 Future of Pharmaceutical Quality and the Path to Get There Lawrence Yu, Ph.D. Deputy Director, Office of Pharmaceutical Quality FDA Center for Drug Evaluation and Research 3rd PQRI/FDA Conference on Advancing
Future of Pharmaceutical Quality and the Path to Get There Lawrence Yu, Ph.D. Deputy Director, Office of Pharmaceutical Quality FDA Center for Drug Evaluation and Research 3rd PQRI/FDA Conference on Advancing
IVT Laboratory Week Jerry Lanese. Ph.D. The Lanese Group, Inc The Lanese Group, Inc.
 IVT Laboratory Week-2015 Jerry Lanese Ph.D. The Lanese Group, Inc. 1 Name Job What brought you here. 2 FDA interest in Quality Metrics What are Quality Metrics Quality metrics FDA will request The impact
IVT Laboratory Week-2015 Jerry Lanese Ph.D. The Lanese Group, Inc. 1 Name Job What brought you here. 2 FDA interest in Quality Metrics What are Quality Metrics Quality metrics FDA will request The impact
Guidance for Industry Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations
 Guidance for Industry Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations DRAFT GUIDANCE This draft guidance document is being distributed for comment purposes only.
Guidance for Industry Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations DRAFT GUIDANCE This draft guidance document is being distributed for comment purposes only.
Guidance for Industry
 Guidance for Industry Q8, Q9, and Q10 Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics
Guidance for Industry Q8, Q9, and Q10 Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics
The Impact of Quality Culture on Quality Risk Management. FDA Perspective on Quality Culture; how it Impacts Risk Management
 The Impact of Quality Culture on Quality Risk Management FDA Perspective on Quality Culture; how it Impacts Risk Management Teresa Gorecki Practice Lead Compliance Architects Agenda The WHAT Definitions
The Impact of Quality Culture on Quality Risk Management FDA Perspective on Quality Culture; how it Impacts Risk Management Teresa Gorecki Practice Lead Compliance Architects Agenda The WHAT Definitions
The Future of Pharmaceutical Quality
 The Future of Pharmaceutical Quality First SQA/BIRS Center Meeting Purdue University Polytechnic Institute Louis W. Yu, Ph.D. Chief Quality Officer Unique Challenges of the Pharmaceutical Industry a. The
The Future of Pharmaceutical Quality First SQA/BIRS Center Meeting Purdue University Polytechnic Institute Louis W. Yu, Ph.D. Chief Quality Officer Unique Challenges of the Pharmaceutical Industry a. The
Q10 PHARMACEUTICAL QUALITY SYSTEM
 Q10 PHARMACEUTICAL QUALITY SYSTEM This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights
Q10 PHARMACEUTICAL QUALITY SYSTEM This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights
FDA Quality Metrics, Data Integrity and Application of Statistics Throughout Process Validation in a Global Economy
 CBI Statistics in Validation FDA Quality Metrics, Data Integrity and Application of Statistics Throughout Process Validation in a Global Economy Jerry Lanese Ph.D. The Lanese Group, Inc. 2017 The Lanese
CBI Statistics in Validation FDA Quality Metrics, Data Integrity and Application of Statistics Throughout Process Validation in a Global Economy Jerry Lanese Ph.D. The Lanese Group, Inc. 2017 The Lanese
Effective Risk Management A Catalyst For Quality Performance
 Page 1 of 6 Guest Column June 8, 2016 Effective Risk Management: A Catalyst For Quality Performance By Bikash Chatterjee, President and Chief Science Officer, Pharmatech Associates When we think of risk
Page 1 of 6 Guest Column June 8, 2016 Effective Risk Management: A Catalyst For Quality Performance By Bikash Chatterjee, President and Chief Science Officer, Pharmatech Associates When we think of risk
Lean Enterprise Achieving efficiency in a world demanding effectiveness
 Lean Enterprise Achieving efficiency in a world demanding effectiveness by Ron Sutter and Rick Nieves finding new answers in business. 2012 CGN Global Lean Enterprise Achieving efficiency in a world demanding
Lean Enterprise Achieving efficiency in a world demanding effectiveness by Ron Sutter and Rick Nieves finding new answers in business. 2012 CGN Global Lean Enterprise Achieving efficiency in a world demanding
Largest Independent Pharmaceutical Benchmarking Survey
 Largest Independent Pharmaceutical Benchmarking Survey Operational Excellence in the Pharmaceutical Industry II Operational Excellence in the Pharmaceutical Industry Largest Independent Pharmaceutical
Largest Independent Pharmaceutical Benchmarking Survey Operational Excellence in the Pharmaceutical Industry II Operational Excellence in the Pharmaceutical Industry Largest Independent Pharmaceutical
Analysis of the Implementation of Total Productive Maintenance, Total Quality Management, and Just-In-Time in Pharmaceutical Manufacturing
 J Pharm Innov (2010) 5:181 192 DOI 10.1007/s12247-010-9095-x CASE REPORT Analysis of the Implementation of Total Productive Maintenance, Total Quality Management, and Just-In-Time in Pharmaceutical Manufacturing
J Pharm Innov (2010) 5:181 192 DOI 10.1007/s12247-010-9095-x CASE REPORT Analysis of the Implementation of Total Productive Maintenance, Total Quality Management, and Just-In-Time in Pharmaceutical Manufacturing
Enabling Improvement through the Product Lifecycle: Change Management within a PQS
 Enabling Improvement through the Product Lifecycle: Change Management within a PQS Denise DiGiulio, Senior Advisor OPQ Office of Process and Facilities PDA Chapter Meeting Melbourne, Australia April 3,
Enabling Improvement through the Product Lifecycle: Change Management within a PQS Denise DiGiulio, Senior Advisor OPQ Office of Process and Facilities PDA Chapter Meeting Melbourne, Australia April 3,
Performance Management, Balanced Scorecards and Business Intelligence: Alignment for Business Results
 Performance Management, Balanced Scorecards and Business Intelligence: Alignment for Business Results Introduction The concept of performance management 1 is not a new one, though modern management constructs
Performance Management, Balanced Scorecards and Business Intelligence: Alignment for Business Results Introduction The concept of performance management 1 is not a new one, though modern management constructs
Extending Lean Initiatives Across the Organization:
 A White Paper by Exact Software Extending Lean Initiatives Across the Organization: How greater efficiencies can extend beyond the manufacturing floor and back-office Business Unified Executive Overview
A White Paper by Exact Software Extending Lean Initiatives Across the Organization: How greater efficiencies can extend beyond the manufacturing floor and back-office Business Unified Executive Overview
USING OPERATIONAL EXCELLENCE (OPEX) AND LEAN MANAGEMENT TO IMPROVE ORGANIZATIONAL INTELLIGENCE
 PHARMATALK 11 TH MAY 2015 BERLIN USING OPERATIONAL EXCELLENCE (OPEX) AND LEAN MANAGEMENT TO IMPROVE ORGANIZATIONAL INTELLIGENCE Dr. Joerg Tautrim Strategic Lean consultant, Director Lean Institute Germany
PHARMATALK 11 TH MAY 2015 BERLIN USING OPERATIONAL EXCELLENCE (OPEX) AND LEAN MANAGEMENT TO IMPROVE ORGANIZATIONAL INTELLIGENCE Dr. Joerg Tautrim Strategic Lean consultant, Director Lean Institute Germany
More than 2000 organizations use our ERM solution
 5 STEPS TOWARDS AN ACTIONABLE RISK APPETITE Contents New Defining Pressures Risk Appetite and Risk Tolerance Benefits The 5 Best of Practices Risk Assessments Benefits of an Actionable Risk Appetite More
5 STEPS TOWARDS AN ACTIONABLE RISK APPETITE Contents New Defining Pressures Risk Appetite and Risk Tolerance Benefits The 5 Best of Practices Risk Assessments Benefits of an Actionable Risk Appetite More
Culture Change. Sustaining a Lean Enterprise Transformation? Presented by Industrial Solutions, Inc
 Culture Change Sustaining a Lean Enterprise Transformation? Presented by Quick Diagnostic Answer the questions on the sheet in front of you. Calculate your score 10 minutes Why is Change So Hard? We focused
Culture Change Sustaining a Lean Enterprise Transformation? Presented by Quick Diagnostic Answer the questions on the sheet in front of you. Calculate your score 10 minutes Why is Change So Hard? We focused
ICH Q8/Q8(R)
 Pharmaceutical Quality for the 21 st Century Temple University May 06, 2008 Joseph Famulare, Deputy Director FDA CDER Office of Compliance CDER Office of Compliance and the Critical Path Initiative Since
Pharmaceutical Quality for the 21 st Century Temple University May 06, 2008 Joseph Famulare, Deputy Director FDA CDER Office of Compliance CDER Office of Compliance and the Critical Path Initiative Since
QUALITY MANAGEMENT SYSTEM 8/30/2017
 QUALITY MANAGEMENT SYSTEM 1 MEDICINAL PRODUCT IS FIT FOR ITS PURPOSE ONLY WHEN It is the right product It is properly sealed in its container and protected against damage and contamination It is the right
QUALITY MANAGEMENT SYSTEM 1 MEDICINAL PRODUCT IS FIT FOR ITS PURPOSE ONLY WHEN It is the right product It is properly sealed in its container and protected against damage and contamination It is the right
A Guide to the. Incorporating the Essential Elements of Strategy Within Your Organization. Empower
 A Guide to the Balanced Scorecard Incorporating the Essential Elements of Strategy Within Your Organization This guide covers Create Keeping strategy creation practical, focused and agile Empower Empowering
A Guide to the Balanced Scorecard Incorporating the Essential Elements of Strategy Within Your Organization This guide covers Create Keeping strategy creation practical, focused and agile Empower Empowering
Visionary Leadership. Systems Perspective. Student-Centered Excellence
 Core Values and Concepts These beliefs and behaviors are embedded in high-performing organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
Core Values and Concepts These beliefs and behaviors are embedded in high-performing organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
Service Differentiation: Your 3-Step Plan. Differentiation, Service DNA, and Continuous Improvement in Field Service
 Service Differentiation: Your 3-Step Plan Differentiation, Service DNA, and Continuous Improvement in Field Service Introduction This white paper discusses service differentiation: doing more with less,
Service Differentiation: Your 3-Step Plan Differentiation, Service DNA, and Continuous Improvement in Field Service Introduction This white paper discusses service differentiation: doing more with less,
Outsourcing - managing risks and opportunities over which you now have less control
 Outsourcing - managing risks and opportunities over which you now have less control Kevin O Donnell, PhD PDA Meeting, Dublin 22 nd November 2018 Slide 2 Typical Examples The key GMP Requirements & Guidance
Outsourcing - managing risks and opportunities over which you now have less control Kevin O Donnell, PhD PDA Meeting, Dublin 22 nd November 2018 Slide 2 Typical Examples The key GMP Requirements & Guidance
6 Steps to Effective Supplier Quality Audits. A Guide For Medical Device Companies
 6 Steps to Effective Supplier Quality Audits A Guide For Medical Device Companies 1 Table of Contents 3 Why Supplier Quality Audits Matter 4 Manual vs. Automated Supplier Audits 5 Six Steps to Effective
6 Steps to Effective Supplier Quality Audits A Guide For Medical Device Companies 1 Table of Contents 3 Why Supplier Quality Audits Matter 4 Manual vs. Automated Supplier Audits 5 Six Steps to Effective
Lean Gold Certification Blueprint
 The Lean Certification Blueprint provides additional useful information beyond the Body of Knowledge. The Body of Knowledge specifies the competencies, topics, and subtopics required by different types
The Lean Certification Blueprint provides additional useful information beyond the Body of Knowledge. The Body of Knowledge specifies the competencies, topics, and subtopics required by different types
CORE VALUES AND CONCEPTS
 CORE VALUES AND CONCEPTS The Criteria are built on the following set of interrelated core values and concepts: visionary leadership customer-driven excellence organizational and personal learning valuing
CORE VALUES AND CONCEPTS The Criteria are built on the following set of interrelated core values and concepts: visionary leadership customer-driven excellence organizational and personal learning valuing
Quadrant I. Module 25: Balanced Scorecard
 Quadrant I Module 25: Balanced Scorecard 1. Learning Outcomes 2. Introduction 3. Balanced Scorecard Framework 4. Balanced Scorecard 5. Organisational Effectiveness 6. Balanced Scorecard & Organisational
Quadrant I Module 25: Balanced Scorecard 1. Learning Outcomes 2. Introduction 3. Balanced Scorecard Framework 4. Balanced Scorecard 5. Organisational Effectiveness 6. Balanced Scorecard & Organisational
Textvergleich. Verglichene Dokumente MEDIA3917.pdf. ICH_Q10_Step4.pdf
 Textvergleich Verglichene Dokumente MEDIA3917.pdf ICH_Q10_Step4.pdf Übersicht 2270 Wort/Wörter hinzugefügt 1338 Wort/Wörter gelöscht 3558 Wort/Wörter übereinstimmend 157 Block/Blöcke übereinstimmend Blättern
Textvergleich Verglichene Dokumente MEDIA3917.pdf ICH_Q10_Step4.pdf Übersicht 2270 Wort/Wörter hinzugefügt 1338 Wort/Wörter gelöscht 3558 Wort/Wörter übereinstimmend 157 Block/Blöcke übereinstimmend Blättern
Banishing Waste and Delivering Value in Your BCM Program
 Lean Times Require Lean Thinking Banishing Waste and Delivering Value in Your BCM Program Session B19: Milen Kutev, MBCP, SCPM, PMP British Columbia Automobile Association / BCAA Why am I talking today?
Lean Times Require Lean Thinking Banishing Waste and Delivering Value in Your BCM Program Session B19: Milen Kutev, MBCP, SCPM, PMP British Columbia Automobile Association / BCAA Why am I talking today?
added Fine-tune your balanced scorecard and make it a machine geared toward continuous improvement by Alex Fedotowsky
 added by Alex Fedotowsky In 50 Words Or Less A balanced scorecard (BSC) can be used as a cyclic, continuous improvement tool to create synergy within an organization. Incorporating lean, Six Sigma, theory
added by Alex Fedotowsky In 50 Words Or Less A balanced scorecard (BSC) can be used as a cyclic, continuous improvement tool to create synergy within an organization. Incorporating lean, Six Sigma, theory
2. Risk-based compliance was also a key component in the FDA's new approach for dealing with electronic records and signatures: 21 CFR Part 11.
 LITERATURE REVIEW: Risk management is well known and practiced in many industries and several industry task forces have developed guidance documents that facilitate risk management. Risk management for
LITERATURE REVIEW: Risk management is well known and practiced in many industries and several industry task forces have developed guidance documents that facilitate risk management. Risk management for
Quality Systems Frameworks. RIT Software Engineering
 Quality Systems Frameworks Some Major Quality Frameworks ISO 9000 family of standards A general international standard for organizational quality systems Oriented towards assessment and certification Malcolm-Baldrige
Quality Systems Frameworks Some Major Quality Frameworks ISO 9000 family of standards A general international standard for organizational quality systems Oriented towards assessment and certification Malcolm-Baldrige
Can Quality Solve the Equation?
 Pricing Authority Expectations Supply Chain Complexity Counterfeiting Can Quality Solve the Equation? Quality Drugs to Patients & An Engaged Workforce Jennifer Magnani & Anders Vinther Article in PDA Journal
Pricing Authority Expectations Supply Chain Complexity Counterfeiting Can Quality Solve the Equation? Quality Drugs to Patients & An Engaged Workforce Jennifer Magnani & Anders Vinther Article in PDA Journal
POSITION DESCRIPTION Property Operations Manager
 POSITION DESCRIPTION Property Operations Manager The BlueCross Vision A dynamic organisation, BlueCross is supported by a team of great staff, who are willing to challenge traditions. With a long history
POSITION DESCRIPTION Property Operations Manager The BlueCross Vision A dynamic organisation, BlueCross is supported by a team of great staff, who are willing to challenge traditions. With a long history
Guidance for Industry
 Guidance for Industry Process Validation: General Principles and Practices U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry Process Validation: General Principles and Practices U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
GMP Challenges to Global Pharma Companies
 GMP Challenges to Global Pharma Companies Muralidhara B. Gavini, Ph.D. Senior Assistant Country Director India Mumbai Office, Office of International Programs Office of the Commissioner Food & Drug Administration
GMP Challenges to Global Pharma Companies Muralidhara B. Gavini, Ph.D. Senior Assistant Country Director India Mumbai Office, Office of International Programs Office of the Commissioner Food & Drug Administration
Agile Projects 7. Agile Project Management 21
 Contents Contents 1 2 3 4 Agile Projects 7 Introduction 8 About the Book 9 The Problems 10 The Agile Manifesto 12 Agile Approach 14 The Benefits 16 Project Components 18 Summary 20 Agile Project Management
Contents Contents 1 2 3 4 Agile Projects 7 Introduction 8 About the Book 9 The Problems 10 The Agile Manifesto 12 Agile Approach 14 The Benefits 16 Project Components 18 Summary 20 Agile Project Management
Pharmaceutical Quality for 21 st Century Initiative
 Quality Metrics Alicia Mozzachio, RPh, MPH Senior Advisor for International Activities Office of Policy for Pharmaceutical Quality (OPPQ) Center for Drug Evaluation and Research U.S. Food and Drug Administration
Quality Metrics Alicia Mozzachio, RPh, MPH Senior Advisor for International Activities Office of Policy for Pharmaceutical Quality (OPPQ) Center for Drug Evaluation and Research U.S. Food and Drug Administration
B Y D A N D E N E H Y
 The General B Y D A N D E N E H Y The general manager embodies the heart and soul of a private club. General managers (GM) work hand in hand with the board to carry out the club s mission and vision and
The General B Y D A N D E N E H Y The general manager embodies the heart and soul of a private club. General managers (GM) work hand in hand with the board to carry out the club s mission and vision and
Quality in Global Sourcing
 Quality in Global Sourcing Steve Greer Global Compliance Leader P&G Beauty & Grooming Current Challenges 1 Supplier Related Incidents High profile material contamination incidents have highlighted risks
Quality in Global Sourcing Steve Greer Global Compliance Leader P&G Beauty & Grooming Current Challenges 1 Supplier Related Incidents High profile material contamination incidents have highlighted risks
New Development Bank Information Technology Policy
 New Development Bank Information Technology Policy Owner: IT Department Version: 2016 V2 Date: [16] March 2016 Corporate Procurement Policy All rights reserved. Any unauthorized use, duplication or disclosure
New Development Bank Information Technology Policy Owner: IT Department Version: 2016 V2 Date: [16] March 2016 Corporate Procurement Policy All rights reserved. Any unauthorized use, duplication or disclosure
An Introduction to Strategic Planning for Service Organizations
 A Jolt Consulting Group White Paper An Introduction to Strategic Planning for Service Organizations April 2011 PO BOX 1217, SARATOGA SPRINGS, NY 12866 PAGE 1 of 9 Table of Contents Strategic Planning Challenges...
A Jolt Consulting Group White Paper An Introduction to Strategic Planning for Service Organizations April 2011 PO BOX 1217, SARATOGA SPRINGS, NY 12866 PAGE 1 of 9 Table of Contents Strategic Planning Challenges...
Oursourcing and Supplier Qualification from the perspective of the pharmaceutical Industry
 Oursourcing and Supplier Qualification from the perspective of the pharmaceutical Industry 1 Presentation Outline Pharmaceutical Quality Management Partner Selection and Qualification Shipping Qualification
Oursourcing and Supplier Qualification from the perspective of the pharmaceutical Industry 1 Presentation Outline Pharmaceutical Quality Management Partner Selection and Qualification Shipping Qualification
FOSTERING A CULTURE OF QUALITY
 PROPRIETARY FOSTERING A CULTURE OF QUALITY Ron Lear and Kevin Schaaff Collaboration space, Alexandria, VA LSSSIG MEETING MARCH 29, 2017 AGENDA INTRODUCTION BOOZ ALLEN S APPROACH TO FOSTERING A CULTURE
PROPRIETARY FOSTERING A CULTURE OF QUALITY Ron Lear and Kevin Schaaff Collaboration space, Alexandria, VA LSSSIG MEETING MARCH 29, 2017 AGENDA INTRODUCTION BOOZ ALLEN S APPROACH TO FOSTERING A CULTURE
When cost cutting alone isn t enough
 Consumer products Of special interest to Consumer products executives Insights for 5executives When cost cutting alone isn t enough Sustainable cost reduction means knowing your culture EY s Global Consumer
Consumer products Of special interest to Consumer products executives Insights for 5executives When cost cutting alone isn t enough Sustainable cost reduction means knowing your culture EY s Global Consumer
Achieving Operational Excellence in Pharma & Biotech Adapting best practice to meet the unique characteristics of the industry
 Achieving Operational Excellence in Pharma & Biotech Adapting best practice to meet the unique characteristics of the industry Executive Summary With the pharmaceutical industry currently going through
Achieving Operational Excellence in Pharma & Biotech Adapting best practice to meet the unique characteristics of the industry Executive Summary With the pharmaceutical industry currently going through
Core Values and Concepts
 Core Values and Concepts These beliefs and behaviors are embedded in high-performing organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
Core Values and Concepts These beliefs and behaviors are embedded in high-performing organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
Is your operational risk management helping execute your strategy?
 Is your operational risk management helping execute your strategy? Five imperatives to effectively integrate operational risk management into business processes to help achieve desired business performance.
Is your operational risk management helping execute your strategy? Five imperatives to effectively integrate operational risk management into business processes to help achieve desired business performance.
Historical Phases of Production
 Lean 101 Overview Lean Background Taiichi Ohno and Shigeo Shingo developed Lean Manufacturing at Toyota over a period of 20-30 years. Their intention was not to develop some sort of unified field theory
Lean 101 Overview Lean Background Taiichi Ohno and Shigeo Shingo developed Lean Manufacturing at Toyota over a period of 20-30 years. Their intention was not to develop some sort of unified field theory
Practice at UVa. & Chief Financial Officer
 Management Methodologies in Practice at UVa Balanced Scorecard Gary Nimax, Office of the Vice President & Chief Financial Officer Six Sigma Priscilla Shuler, Quality Performance & Improvement, Medical
Management Methodologies in Practice at UVa Balanced Scorecard Gary Nimax, Office of the Vice President & Chief Financial Officer Six Sigma Priscilla Shuler, Quality Performance & Improvement, Medical
PART 1: INTRODUCTION. Purpose of the BIZBOK Guide. What is Business Architecture?
 PART 1: INTRODUCTION Purpose of the BIZBOK Guide A Guide to the Business Architecture Body of Knowledge (the BIZBOK Guide) provides a practical guide for business architecture practitioners and individuals
PART 1: INTRODUCTION Purpose of the BIZBOK Guide A Guide to the Business Architecture Body of Knowledge (the BIZBOK Guide) provides a practical guide for business architecture practitioners and individuals
RESULTS. SAMPLE OF AN ACTUAL REPORT (PART II) Name of site left off. CERTIFIED: No Yes
 MC WORKFORCE DEVELOPMENT BOARD Technical Assistance Report For XXX Center or Organization From the Certification Subcommittee Of the Quality Assurance Committee DATE SAMPLE OF AN ACTUAL REPORT (PART II)
MC WORKFORCE DEVELOPMENT BOARD Technical Assistance Report For XXX Center or Organization From the Certification Subcommittee Of the Quality Assurance Committee DATE SAMPLE OF AN ACTUAL REPORT (PART II)
Modernizing Pharmaceutical Quality Systems; Studying Quality Metrics and Quality Culture;
 This document is scheduled to be published in the Federal Register on 06/29/2018 and available online at https://federalregister.gov/d/2018-14005, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 06/29/2018 and available online at https://federalregister.gov/d/2018-14005, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Agile Essentials Track: Business Services
 Agile Essentials Track: Business Services Presenter: Mark Thomas Synopsis Are you a victim of building the wrong solutions slowly? If so, you re not alone, and considering an Agile approach may be the
Agile Essentials Track: Business Services Presenter: Mark Thomas Synopsis Are you a victim of building the wrong solutions slowly? If so, you re not alone, and considering an Agile approach may be the
ICH Q10/WHO TRS A Regulatory Perspective
 Presentation by: Dr. A. Ramkishan Asst. Drugs Controller (India) Govt. of India ICH Q10/WHO TRS A Regulatory Perspective Contents of Presentation Introduction History of WHO GMP TRS guidelines A Unique
Presentation by: Dr. A. Ramkishan Asst. Drugs Controller (India) Govt. of India ICH Q10/WHO TRS A Regulatory Perspective Contents of Presentation Introduction History of WHO GMP TRS guidelines A Unique
Performance Measurement for Organizational Improvement
 12-ICIT 9-11/4/07 in RoC Going for Gold ~ Kaizen & Org. Development Paper #: 05-04 Page- 1 /6 Performance Measurement for Organizational Improvement Puah Kok Yong Lecturer, Ngee Ann Polytechnic, Singapore
12-ICIT 9-11/4/07 in RoC Going for Gold ~ Kaizen & Org. Development Paper #: 05-04 Page- 1 /6 Performance Measurement for Organizational Improvement Puah Kok Yong Lecturer, Ngee Ann Polytechnic, Singapore
Project Management Assessment. Apply an In-Depth Approach to Project Management to Achieve Systematic Success
 Management Assessment Apply an In-Depth Approach to Management to Achieve Systematic Success Your Journey Starts Here. Understand Your PM Strengths & Weaknesses Evaluate Performance and Required Next Steps
Management Assessment Apply an In-Depth Approach to Management to Achieve Systematic Success Your Journey Starts Here. Understand Your PM Strengths & Weaknesses Evaluate Performance and Required Next Steps
THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture
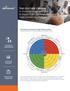 denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
CHAPTER 4 PRODUCT DEVELOPMENT LIFE CYCLE
 CHAPTER 4 PRODUCT DEVELOPMENT LIFE CYCLE 1 Learning Objectives Review the Systems Development Life Cycle (SDLC). Examine the problems and alternatives with SDLC. Know the key issues in ERP implementation
CHAPTER 4 PRODUCT DEVELOPMENT LIFE CYCLE 1 Learning Objectives Review the Systems Development Life Cycle (SDLC). Examine the problems and alternatives with SDLC. Know the key issues in ERP implementation
FDA s Role in Vaccine Supply
 IOM Committee on Review of Priorities of the National Vaccine Plan Stakeholder Workshop #1 24 July 2008 FDA s Role in Vaccine Supply Norman W. Baylor, Ph.D., Director Office of Vaccines Research and Review
IOM Committee on Review of Priorities of the National Vaccine Plan Stakeholder Workshop #1 24 July 2008 FDA s Role in Vaccine Supply Norman W. Baylor, Ph.D., Director Office of Vaccines Research and Review
Welcome to Coral Springs. From Bean Field to Baldrige: Lessons Learned From One City s Journey
 From Bean Field to Baldrige: Lessons Learned From One City s Journey Robert Goehrig Director, Budget, Strategy, and Communication City of Coral Springs, FL rgoehrig@coralsprings.org 1 Welcome to Coral
From Bean Field to Baldrige: Lessons Learned From One City s Journey Robert Goehrig Director, Budget, Strategy, and Communication City of Coral Springs, FL rgoehrig@coralsprings.org 1 Welcome to Coral
SKF Manufacturing Excellence. Kent Viitanen Director, Industrial Division Manufacturing & Supply
 SKF Manufacturing Excellence Kent Viitanen Director, Industrial Division Manufacturing & Supply Capital Markets day 2010 What Manufacturing Excellence means for SKF? Our continuous strive to be World Class
SKF Manufacturing Excellence Kent Viitanen Director, Industrial Division Manufacturing & Supply Capital Markets day 2010 What Manufacturing Excellence means for SKF? Our continuous strive to be World Class
Asset management Overview, principles and terminology
 ISO 2012 All rights reserved ISO/PC 251/N183 Date: 2012-02-26 ISO/CD 55000.2 ISO/TC 251/WG 1 Secretariat: BSI Asset management Overview, principles and terminology Gestion d'actifs Vue d'ensemble, les
ISO 2012 All rights reserved ISO/PC 251/N183 Date: 2012-02-26 ISO/CD 55000.2 ISO/TC 251/WG 1 Secretariat: BSI Asset management Overview, principles and terminology Gestion d'actifs Vue d'ensemble, les
Insights into the St.Gallen OPEX and Quality Metrics Research
 Insights into the St.Gallen OPEX and Quality Metrics Research GSIA SSPI New Year Event & New Member Welcome Drink Prof. Dr. Thomas Friedli Nicolas Ponce January 23, 2018 Agenda 1 2 3 4 4 Introduction St.Gallen
Insights into the St.Gallen OPEX and Quality Metrics Research GSIA SSPI New Year Event & New Member Welcome Drink Prof. Dr. Thomas Friedli Nicolas Ponce January 23, 2018 Agenda 1 2 3 4 4 Introduction St.Gallen
A Holistic Framework for Business Excellence
 Tutorials, J. Roberts Research Note 9 June 2003 A Holistic Framework for Business Excellence Most enterprises seek continuous improvement in the quality of their products, services and management. A holistic
Tutorials, J. Roberts Research Note 9 June 2003 A Holistic Framework for Business Excellence Most enterprises seek continuous improvement in the quality of their products, services and management. A holistic
Core Values and Concepts
 Core Values and Concepts These beliefs and behaviors are embedded in highperforming organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
Core Values and Concepts These beliefs and behaviors are embedded in highperforming organizations. They are the foundation for integrating key performance and operational requirements within a results-oriented
The Strategic Management Maturity Model. Eight Dimensions of Strategic Management
 The Strategic Management Maturity Model Many of our clients ask a similar question as they work to improve their strategic management at their organizations: where do we stand compared with other high
The Strategic Management Maturity Model Many of our clients ask a similar question as they work to improve their strategic management at their organizations: where do we stand compared with other high
Driving effective quality governance using Quality Metrics
 Driving effective quality governance using Quality Metrics IPA Advanced GMP Workshop November 208 McKinsey & Company Contents Perspectives on best practices in Quality Metrics Group exercise 2 Regulatory
Driving effective quality governance using Quality Metrics IPA Advanced GMP Workshop November 208 McKinsey & Company Contents Perspectives on best practices in Quality Metrics Group exercise 2 Regulatory
The Strategic Management Maturity Model. Eight Dimensions of Strategic Management
 The Strategic Management Maturity Model Many Institute clients ask a similar question as they work to improve their strategic management at their organizations: where do we stand compared with other high
The Strategic Management Maturity Model Many Institute clients ask a similar question as they work to improve their strategic management at their organizations: where do we stand compared with other high
CENTRAL PROCESS REPOSITORY
 CENTRAL PROCESS REPOSITORY IMPRIVA Inc. 101A Clay St. #196 San Francisco, CA 94111 877.838.9714 www.impriva.com U Over a seven week period, IMPRIVA identified and discussed the most common business process
CENTRAL PROCESS REPOSITORY IMPRIVA Inc. 101A Clay St. #196 San Francisco, CA 94111 877.838.9714 www.impriva.com U Over a seven week period, IMPRIVA identified and discussed the most common business process
INTEGRITY MANAGEMENT CONTINUOUS IMPROVEMENT. Foundation for an Effective Safety Culture
 INTEGRITY MANAGEMENT CONTINUOUS IMPROVEMENT Foundation for an Effective Safety Culture June 2011 Foundation for an Effective Safety Culture describes the key elements of organizational culture and business
INTEGRITY MANAGEMENT CONTINUOUS IMPROVEMENT Foundation for an Effective Safety Culture June 2011 Foundation for an Effective Safety Culture describes the key elements of organizational culture and business
ORGANIZATIONAL STRATEGY AND ALIGNMENT FOR CUSTOMER EXPERIENCE MANAGEMENT
 ORGANIZATIONAL STRATEGY AND ALIGNMENT FOR CUSTOMER EXPERIENCE MANAGEMENT By: Richard English, Director, Strategic Consulting, Avaya Professional Services C ustomer experience maturity is instrumental in
ORGANIZATIONAL STRATEGY AND ALIGNMENT FOR CUSTOMER EXPERIENCE MANAGEMENT By: Richard English, Director, Strategic Consulting, Avaya Professional Services C ustomer experience maturity is instrumental in
The Drug Industry at a Crossroad: PAT s Role
 The Drug Industry at a Crossroad: PAT s Role The old paradigm must make way for the new; here s how it can happen. By Efraim Shek, Ph.D., Senior Vice President, Pharmaceutical Development, NeuroMolecular
The Drug Industry at a Crossroad: PAT s Role The old paradigm must make way for the new; here s how it can happen. By Efraim Shek, Ph.D., Senior Vice President, Pharmaceutical Development, NeuroMolecular
GLP Compliance Assurance vs. Quality Assurance. Quality Beyond GLP Compliance
 GLP Compliance Assurance vs. Quality Assurance Quality Beyond GLP Compliance Jennifer Pangborn Quality Assurance Specialist 1 Outline Why QAU is here? Quality Assurance beyond GLP Compliance Expanding
GLP Compliance Assurance vs. Quality Assurance Quality Beyond GLP Compliance Jennifer Pangborn Quality Assurance Specialist 1 Outline Why QAU is here? Quality Assurance beyond GLP Compliance Expanding
Breakout Session A. Asset Management Best Practices and What Trends You Should Know (ISO 55000: Asset Management)
 Breakout Session A Asset Management Best Practices and What Trends You Should Know (ISO 55000: Asset Management) Life Science Leader magazine February 2014 Where is the time/energy of FDA? Inspections
Breakout Session A Asset Management Best Practices and What Trends You Should Know (ISO 55000: Asset Management) Life Science Leader magazine February 2014 Where is the time/energy of FDA? Inspections
A conversation about the framework for performance excellence PERFORM LIKE A ROCK STAR!
 A conversation about the framework for performance excellence PERFORM LIKE A ROCK STAR! Like Olympic athletes, pursing performance excellence in our work implies that we optimize every factor that goes
A conversation about the framework for performance excellence PERFORM LIKE A ROCK STAR! Like Olympic athletes, pursing performance excellence in our work implies that we optimize every factor that goes
PHMSA Update Safety Management Systems
 PHMSA Update Safety Management Systems Oklahoma Pipeline Safety Seminar Tulsa, Ok Wednesday November 19, 2014 9:30-10:30 AM Chris McLaren - 1 - Today s Agenda Importance of Management Systems Safety Culture
PHMSA Update Safety Management Systems Oklahoma Pipeline Safety Seminar Tulsa, Ok Wednesday November 19, 2014 9:30-10:30 AM Chris McLaren - 1 - Today s Agenda Importance of Management Systems Safety Culture
Learning Objectives. System-Based Thinking as Related to Systems-Based Inspections and Audits
 SYSTEMS-BASED THINKING, RISK MANAGEMENT, AND CORPORATE CULTURE: Perspectives on 21 st Century Compliance Dennis J. Runser, Ph.D., RAC TMI Regulatory Compliance Consulting Learning Objectives 1) System-Based
SYSTEMS-BASED THINKING, RISK MANAGEMENT, AND CORPORATE CULTURE: Perspectives on 21 st Century Compliance Dennis J. Runser, Ph.D., RAC TMI Regulatory Compliance Consulting Learning Objectives 1) System-Based
Business Process Management Introduction. Marek Zborowski PhD.
 Business Process Management Introduction Marek Zborowski PhD. BUSINESS PROCESS MANAGEMENT We will learn in this area: What is Business Process Management (BPM) Why BPM is required How the discipline of
Business Process Management Introduction Marek Zborowski PhD. BUSINESS PROCESS MANAGEMENT We will learn in this area: What is Business Process Management (BPM) Why BPM is required How the discipline of
Request for Quality Metrics Guidance for Industry
 Request for Quality Metrics Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be
Request for Quality Metrics Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be
Ensuring BD Success With Metrics-Based Management
 Ensuring BD Success With Metrics-Based Management Presented by Vicki Griesinger, Director of Operations 16th Annual APMP Conference and Exhibits June 9, 2005 Capability Maturity Model and CMM are registered
Ensuring BD Success With Metrics-Based Management Presented by Vicki Griesinger, Director of Operations 16th Annual APMP Conference and Exhibits June 9, 2005 Capability Maturity Model and CMM are registered
The COSO Risk Framework: A reference for internal control? Transition from COSO I to COSO II
 The COSO Risk Framework: A reference for internal control? Transition from COSO I to COSO II S P E A K E R : D O T T. FA B I O A C C A R D I C O U R S E O F B U S I N E S S A U D I T I N G U N I V E R
The COSO Risk Framework: A reference for internal control? Transition from COSO I to COSO II S P E A K E R : D O T T. FA B I O A C C A R D I C O U R S E O F B U S I N E S S A U D I T I N G U N I V E R
Strategy Maps and the Balanced Scorecard. Don Breckenridge Jr. President
 Strategy Maps and the Balanced Scorecard Don Breckenridge Jr. President The Typical Owner Owners are typically the Rainmakers Rainmakers tend to work FOR the business instead of ON the business. Most don
Strategy Maps and the Balanced Scorecard Don Breckenridge Jr. President The Typical Owner Owners are typically the Rainmakers Rainmakers tend to work FOR the business instead of ON the business. Most don
QUALITY MANAGEMENT IN THE BOARD ROOM
 Building the Executive Business Case for EQMS CONNECT: lnsresearch.com Building the Executive Business Case for EQMS Executive Overview... 3 Section 1: Research Demographics... 5 Section 2: Quality Maturity
Building the Executive Business Case for EQMS CONNECT: lnsresearch.com Building the Executive Business Case for EQMS Executive Overview... 3 Section 1: Research Demographics... 5 Section 2: Quality Maturity
The Economics of CMMI
 NDIA CMMI Working Group NDIA Systems Engineering Division NDIA Systems Engineering Conference October 28, 2009 CMMI is registered in the U.S. Patent and Trademark Office by Carnegie Mellon University.
NDIA CMMI Working Group NDIA Systems Engineering Division NDIA Systems Engineering Conference October 28, 2009 CMMI is registered in the U.S. Patent and Trademark Office by Carnegie Mellon University.
IMPACT MEASUREMENT METHODOLOGY
 IMPACT MEASUREMENT METHODOLOGY 1 TABLE OF CONTENTS 1. Introduction 3 2. Impact investing idea 3 3. Simpact s theory of change 5 4. Purpose of impact measurement 8 5. Simpact s approach for measuring impact
IMPACT MEASUREMENT METHODOLOGY 1 TABLE OF CONTENTS 1. Introduction 3 2. Impact investing idea 3 3. Simpact s theory of change 5 4. Purpose of impact measurement 8 5. Simpact s approach for measuring impact
Proposed International Standard on Auditing 315 (Revised)
 Exposure Draft July 2018 Comments due: November 2, 2018 International Standard on Auditing Proposed International Standard on Auditing 315 (Revised) Identifying and Assessing the Risks of Material Misstatement
Exposure Draft July 2018 Comments due: November 2, 2018 International Standard on Auditing Proposed International Standard on Auditing 315 (Revised) Identifying and Assessing the Risks of Material Misstatement
What is ITIL 4. Contents
 What is ITIL 4 Contents What is ITIL and why did ITIL need to evolve?... 1 Key Concepts of Service Management... 1 The Nature of Value... 2 How Value Creation Is Enabled Through Services... 2 Key Concepts
What is ITIL 4 Contents What is ITIL and why did ITIL need to evolve?... 1 Key Concepts of Service Management... 1 The Nature of Value... 2 How Value Creation Is Enabled Through Services... 2 Key Concepts
ETA Sector Strategies Technical Assistance Initiative Self-Assessment Pilot Tool 2.0
 Sector Strategies Organizational Is your organization demand-driven and sector-focused? ABOUT THIS TOOL This self-assessment tool is designed to help local and regional workforce entities (and current
Sector Strategies Organizational Is your organization demand-driven and sector-focused? ABOUT THIS TOOL This self-assessment tool is designed to help local and regional workforce entities (and current
Risk Based Validation. Why, How and with what tools?
 Risk Based Validation Why, How and with what tools? Tech Talk Agenda History of FDA GMP initiative for the 21 st Century. Industry response to FDA initiative. Harmonisation through ICH. ASTM Standard on
Risk Based Validation Why, How and with what tools? Tech Talk Agenda History of FDA GMP initiative for the 21 st Century. Industry response to FDA initiative. Harmonisation through ICH. ASTM Standard on
Risk Management in the 21 st Century Ameren Business Risk Management
 Management in the 21 st Century Ameren Business Management Charles A. Bremer V.P. Ameren Service Center/Information Technology Ameren Services Co. November, 2007 Ameren s History 2 Ameren Today Electric
Management in the 21 st Century Ameren Business Management Charles A. Bremer V.P. Ameren Service Center/Information Technology Ameren Services Co. November, 2007 Ameren s History 2 Ameren Today Electric
Ensuring An Effective Quality System. M. Kowolenko, Ph.D. SVP, Biopharmaceutical Operations & Technology
 Ensuring An Effective Quality System M. Kowolenko, Ph.D. SVP, Biopharmaceutical Operations & Technology Role of the Quality Unit Compliance with all relevant regulations and commitments made in license
Ensuring An Effective Quality System M. Kowolenko, Ph.D. SVP, Biopharmaceutical Operations & Technology Role of the Quality Unit Compliance with all relevant regulations and commitments made in license
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program?
 Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
PDA Comments ICH Q10; Pharmaceutical Quality System October Suggested Revision
 PDA s ICH Q10; Pharmaceutical Quality System October 2007 Section Line 1.1 54 The inspectional impact of the optional nature of Q10 beyond what is required by current GMP can be further clarified. 1.1
PDA s ICH Q10; Pharmaceutical Quality System October 2007 Section Line 1.1 54 The inspectional impact of the optional nature of Q10 beyond what is required by current GMP can be further clarified. 1.1
TOTAL PERFORMANCE SCORECARD
 Anca ȘERBAN Oana DUMITRAȘCU Department of Management, Marketing and Business Administration Faculty of Economics, "Lucian Blaga" University Sibiu, Romania TOTAL PERFORMANCE SCORECARD Keywords Balanced
Anca ȘERBAN Oana DUMITRAȘCU Department of Management, Marketing and Business Administration Faculty of Economics, "Lucian Blaga" University Sibiu, Romania TOTAL PERFORMANCE SCORECARD Keywords Balanced
The Business Case for a Project and Portfolio Management Platform. Craig McQueen Agora Consulting Partners Inc.
 The Business Case for a Project and Portfolio Management Platform Craig McQueen Agora Consulting Partners Inc. July 2010 The Business Case for a Project and Portfolio Management Platform This page intentionally
The Business Case for a Project and Portfolio Management Platform Craig McQueen Agora Consulting Partners Inc. July 2010 The Business Case for a Project and Portfolio Management Platform This page intentionally
While the recognition
 Designing the Perfect Change Control System Change control systems today are expected to be designed in a way that provides a system to not only document and approve changes, but also to anticipate change
Designing the Perfect Change Control System Change control systems today are expected to be designed in a way that provides a system to not only document and approve changes, but also to anticipate change
