PDA: A Global Association
|
|
|
- Roxanne Audra Jones
- 6 years ago
- Views:
Transcription
1 Quality Culture Metrics? PDA s Seeking Answers PDA: A Global Association Steven Mendivil PDA Quality Metric Task Force Leader 1
2 FDA is Interested in Quality Metrics FDASIA (7/12) - Section 706 Opportunity to help with Drug Shortages while also assessing quality and compliance risk FDA is exploring Quality Metrics as an input into their Inspectional Risk Model PDA Commented on FDA s Drug Shortage Strategic Plan 3/13/13 One of PDA s Strength is their work on Quality Systems 2 2
3 Quality Metric Proposals FDA requested input on potential Quality Metrics by 12/20/13. At least four Quality Metric proposals submitted to FDA And a special meeting 3
4 Highlights from Brookings Proposed Consensus Metrics Lot acceptance rate Product Compliance rate Confirmed Out of Spec rate Recall rate Consensus set of metrics are somewhat rudimentary, and provide limited information about the culture of quality at a given organization. Many remarked that a strong quality culture is a critical component in driving the system and processes that underpin the quality control and assurance infrastructure at an organization However, Quality culture is also difficult to capture through metrics. PDA believes the next step is focusing on Objective Quality Culture Metrics 4
5 What is Quality Culture and how can you measure it True Quality Culture an environment in which employees not only follow quality guidelines but also consistently see others taking quality-focused actions, hear others talking about quality, and feel quality all around them. From Harvard Business Review April 2014: Creating a Culture of Quality. Financial incentives don t reduce errors. Employees must be passionate about eliminating mistakes. Ashwin Srinivasan and Bryan Kurey of CEB Financial incentives don t reduce errors People must be passionate about eliminating mistakes 5
6 Quantifying Quality Culture December 2013 PDA Quality Metric Conference breakout session identified key factors for Quality Culture 1. Communication & Transparency 2. Commitment & Engagement 3. Technical Excellence 4. Standardization of Criteria or Requirements 5. Cross Functional Vision PDA will focus on Quality Culture at Quality Metric Workshop in Dec
7 Objective Quality Culture Metrics PDA has launched two Quality Culture Surveys 1. Help identify attributes of Quality Culture and Quality System Maturity 2. Correlate Quality Culture behavior scoring (subjective) to Quality System maturity scoring (objective) 3. Can Quality System Maturity scoring differentiate sites? Objective quality culture metrics must be verifiable 7
8 PDA s Quality Culture Survey Three Survey Sections: 1. Demographic 2. Observed Quality Culture behavior (management and co-workers) Subjective assessment at the work site 3. Quality System Maturity scoring Objective attributes Quality System Maturity as Surrogate for Quality Culture 8
9 PDA s Quality Culture Survey Section 2: Quality Culture Behavior Scoring Assessment of behaviors observed for co-workers and management over last 6-18 months for: 1. Communication & Transparency 2. Commitment & Engagement 3. Technical Excellence 4. Standardization of Criteria or Requirements 5. Cross Functional Vision 6. Rewards and Recognition 7. Speak Up for Quality 9
10 PDA s Quality Culture Survey Section 3: Quality System Maturity Scoring Objective scoring of attributes in 6 areas 1. Prevention Programs 2. Quality Management and Issue Escalation 3. Training and Personnel Development 4. Quality Management Systems 5. People and Communication 6. Continuous Improvement Survey Questions Score the Maturity of these Quality System Attributes 10
11 Survey Results to Seed Discussion Survey results to be presented at PDA 2014 Quality Metric Workshop Plenary Presentations on Quality Culture Three Breakout Sessions Planned 1. Quality Culture Attributes in Pharma Industry 2. Measuring the Maturity of Quality Systems 3. Objective Measures for Quality Culture and Quality System Maturity Use of Voting Clickers to Determine Participants Input 11
12 Final Thoughts No perfect quality metrics; determining trade offs. First, carefully consider unintended consequences Balanced Meaningful Metric Scorecard including Quality Culture Quality Culture / Quality System Maturity metrics will go beyond cgmp requirements Data trending is more valuable than direct comparison Can globally applicable metrics be developed? FDA s use of Quality Metrics can be a game changer 12
13 PDA Quality Metric Task Support Steven Mendivil (Amgen) Task Force Leader Anders Vinther (Sanofi Pasteur) Glenn Wright (Lilly) Gabriele Gori (Novartis) Ian Elvins (Consultant) Joyce Bloomfield (Merck) Marty Nealey (Hospira) Anil Sawant (J&J) Sue Schniepp (Allergy Labs) Veronique Davoust (Pfizer) Cylia Chen-Ooi (Amgen) Pritesh Patel (Allergan) Edwin Rivera Martinez (Sanofi) Robert Keiffer (Consultant) If you didn t get an contact PDA at sci_reg@pda.org or go to 13
14 Thank You 14
Aspiring to Measure Quality Culture
 Aspiring to Measure Quality Culture Denyse Baker PDA Director of Science and Regulatory Affairs Pharmaceutical Quality Congress The Unintended Consequences of Quality June 14-16, 2017 - Bethesda, MD 1
Aspiring to Measure Quality Culture Denyse Baker PDA Director of Science and Regulatory Affairs Pharmaceutical Quality Congress The Unintended Consequences of Quality June 14-16, 2017 - Bethesda, MD 1
PDA Quality Culture Program Overview
 PDA Quality Culture Program Overview Are you ready to assess your site s quality culture? Cylia Chen, PDA Quality Culture team PDA SoCal Chapter Nov. 2016 1 What is FDA (and industry) Striving for? A maximally
PDA Quality Culture Program Overview Are you ready to assess your site s quality culture? Cylia Chen, PDA Quality Culture team PDA SoCal Chapter Nov. 2016 1 What is FDA (and industry) Striving for? A maximally
Tuesday, February 21, :15 a.m. 5:30 p.m. Registration Open. 8:15 a.m. 8:30 a.m. Continental Breakfast
 2017 PDA Pharmaceutical Quality Metrics and Quality Culture Conference February 21 22, 2017 Bethesda North Marriott Hotel & Conference Center Bethesda, MD As of January 30, 2017 Tuesday, February 21, 2017
2017 PDA Pharmaceutical Quality Metrics and Quality Culture Conference February 21 22, 2017 Bethesda North Marriott Hotel & Conference Center Bethesda, MD As of January 30, 2017 Tuesday, February 21, 2017
Parenteral Drug Association 2020 Strategic Plan
 Parenteral Drug Association 2020 Strategic Plan November 2015 Connecting People, Science and Regulation Table of Contents Introduction...3 Strategic Planning Committee...6 Mission, Vision, Values and Motto...7
Parenteral Drug Association 2020 Strategic Plan November 2015 Connecting People, Science and Regulation Table of Contents Introduction...3 Strategic Planning Committee...6 Mission, Vision, Values and Motto...7
AGENDA. 12:00 p.m. 1:00 p.m. Registration 1:00 p.m. 4:00 p.m. Pre-Conference Workshop: Data Integrity Problems on the Rise
 AGENDA Pre-Conference Workshop, June 14 12:00 p.m. 1:00 p.m. Registration 1:00 p.m. 4:00 p.m. Pre-Conference Workshop: Data Integrity Problems on the Rise Data integrity issues have become a common and
AGENDA Pre-Conference Workshop, June 14 12:00 p.m. 1:00 p.m. Registration 1:00 p.m. 4:00 p.m. Pre-Conference Workshop: Data Integrity Problems on the Rise Data integrity issues have become a common and
CC: Richard Levy, PDA; Denyse Baker, PDA
 Bethesda Towers 4350 East West Highway Suite 150 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org OFFICERS Chair Harold Baseman ValSource Chair-Elect Martin VanTrieste Amgen
Bethesda Towers 4350 East West Highway Suite 150 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org OFFICERS Chair Harold Baseman ValSource Chair-Elect Martin VanTrieste Amgen
Regulatory Affairs & Quality Advisory Board. A Parenteral Drug Association (PDA) Advisory Board
 Regulatory Affairs & Quality Advisory Board A Parenteral Drug Association (PDA) Advisory Board Purpose of Presentation RAQAB has a goal of improving communication of RAQAB activities to the membership
Regulatory Affairs & Quality Advisory Board A Parenteral Drug Association (PDA) Advisory Board Purpose of Presentation RAQAB has a goal of improving communication of RAQAB activities to the membership
Regulatory Affairs and Quality Advisory Board. A Parenteral Drug Association (PDA) Advisory Board
 Regulatory Affairs and Quality Advisory Board A Parenteral Drug Association (PDA) Advisory Board Introduction Ladies and Gentlemen, I am happy to be here with you tonight NE Chapter Member since 1986 Work
Regulatory Affairs and Quality Advisory Board A Parenteral Drug Association (PDA) Advisory Board Introduction Ladies and Gentlemen, I am happy to be here with you tonight NE Chapter Member since 1986 Work
Future of Pharmaceutical Quality and the Path to Get There
 Future of Pharmaceutical Quality and the Path to Get There Lawrence Yu, Ph.D. Deputy Director, Office of Pharmaceutical Quality FDA Center for Drug Evaluation and Research 3rd PQRI/FDA Conference on Advancing
Future of Pharmaceutical Quality and the Path to Get There Lawrence Yu, Ph.D. Deputy Director, Office of Pharmaceutical Quality FDA Center for Drug Evaluation and Research 3rd PQRI/FDA Conference on Advancing
Division of Docket Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852
 Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway Suite 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway Suite 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
Connecting People, Science and Regulation
 Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway, Ste 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway, Ste 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
European Medicines Agency Quality Working Party, London
 Europe ggmbh Adalbertstraße 9 16548 Glienicke/Berlin Germany Tel: +49 (33056) 2377-10 Fax : +49 (33056) 2377-77 Georg Roessling, PhD General Manager Senior Vice President PDA Europe http://europe.pda.org
Europe ggmbh Adalbertstraße 9 16548 Glienicke/Berlin Germany Tel: +49 (33056) 2377-10 Fax : +49 (33056) 2377-77 Georg Roessling, PhD General Manager Senior Vice President PDA Europe http://europe.pda.org
April 9, Dear Sir/Madam,
 Bethesda Towers 4350 East West Highway Suite 150 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org OFFICERS Chair Harold Baseman ValSource Chair-Elect Martin VanTrieste Amgen
Bethesda Towers 4350 East West Highway Suite 150 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org OFFICERS Chair Harold Baseman ValSource Chair-Elect Martin VanTrieste Amgen
FDA s Draft Guidance Request for Quality Metrics: What All Drug and Biologics Manufacturers Need to Know June 2017
 FDA s Draft Guidance Request for Quality Metrics: What All Drug and Biologics Manufacturers Need to Know June 2017 Discussion Topics Quality Concepts The Journey The Guidance Details Industry Responses
FDA s Draft Guidance Request for Quality Metrics: What All Drug and Biologics Manufacturers Need to Know June 2017 Discussion Topics Quality Concepts The Journey The Guidance Details Industry Responses
Pharmaceutical Companies and Social Media: Developing New Strategies
 Pharmaceutical Companies and Social Media: Developing New Strategies Jena Cutie jena@saaraams.com Karthika Karindalam karthika@saaraams.com 1 2 3 Executive Summary Best Practices and Strategies Pharma
Pharmaceutical Companies and Social Media: Developing New Strategies Jena Cutie jena@saaraams.com Karthika Karindalam karthika@saaraams.com 1 2 3 Executive Summary Best Practices and Strategies Pharma
Culture & the QP. Frank Hallinan Annual QP Forum 16 April 2015.
 Culture & the QP. Frank Hallinan. 2015 Annual QP Forum 16 April 2015. The Big Picture: NOT about Handel, Heaney or, Hurling! What is responsibility of QP? Certify individual batches of a medicine. Assure
Culture & the QP. Frank Hallinan. 2015 Annual QP Forum 16 April 2015. The Big Picture: NOT about Handel, Heaney or, Hurling! What is responsibility of QP? Certify individual batches of a medicine. Assure
Metrics in Microbiology Monitoring Practices
 Metrics in Microbiology Monitoring Practices Crystal Booth, M.M. Masters of Microbiology from North Carolina State University Former Associate Director of Microbiology at Novartis Metrics in Microbiology
Metrics in Microbiology Monitoring Practices Crystal Booth, M.M. Masters of Microbiology from North Carolina State University Former Associate Director of Microbiology at Novartis Metrics in Microbiology
Paradigm Change in Manufacturing Operations
 Technical Report No. 56 (Revised 2016) Application of Phase-Appropriate Quality System and cgmp to the Development of Therapeutic Protein Drug Substance (API or Biological Active Substance) Paradigm Change
Technical Report No. 56 (Revised 2016) Application of Phase-Appropriate Quality System and cgmp to the Development of Therapeutic Protein Drug Substance (API or Biological Active Substance) Paradigm Change
France Pharma RepTrak 2017
 France Pharma RepTrak 2017 The World s Most Reputable Pharmaceutical Companies in France Olivier Forlini, Director Reputation Institute France June 2017 1 Why Measure Reputation? The success of your company
France Pharma RepTrak 2017 The World s Most Reputable Pharmaceutical Companies in France Olivier Forlini, Director Reputation Institute France June 2017 1 Why Measure Reputation? The success of your company
Quality in Global Sourcing
 Quality in Global Sourcing Steve Greer Global Compliance Leader P&G Beauty & Grooming Current Challenges 1 Supplier Related Incidents High profile material contamination incidents have highlighted risks
Quality in Global Sourcing Steve Greer Global Compliance Leader P&G Beauty & Grooming Current Challenges 1 Supplier Related Incidents High profile material contamination incidents have highlighted risks
Comments from Cross-Industry Quality Metrics Collaboration Group regarding Docket FDA D-2537: Request for Quality Metrics.
 November 25, 2015 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane Room 1061 Rockville, MD 20852 Comments from Cross-Industry
November 25, 2015 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane Room 1061 Rockville, MD 20852 Comments from Cross-Industry
March 27, Food and Drug. Dear Sir/Madam: another public. from one plant. manufacturing the risk based. enclosed. contact me.
 Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway, Ste. 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
Connecting People, Science and Regulation Bethesda Towers 4350 East West Highway, Ste. 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe ggmbh Am Borsigturm
CDER /OPQ Office of Surveillance Quality Metrics in Surveillance
 CDER /OPQ Office of Surveillance Quality Metrics in Surveillance Russell Wesdyk Acting Director Office of Surveillance Office of Surveillance Goals Builds from the shared vision A maximally efficient,
CDER /OPQ Office of Surveillance Quality Metrics in Surveillance Russell Wesdyk Acting Director Office of Surveillance Office of Surveillance Goals Builds from the shared vision A maximally efficient,
ISPE Quality Metrics Program An Update
 ISPE Quality Metrics Program An Update Diane Hagerty, Genentech / Roche Vice President, Global Quality Systems & Processes 17 September 2014 ISPE Quality Metrics Program Overview Quality Metrics Industry
ISPE Quality Metrics Program An Update Diane Hagerty, Genentech / Roche Vice President, Global Quality Systems & Processes 17 September 2014 ISPE Quality Metrics Program Overview Quality Metrics Industry
Changes without Prior Approval
 Changes without Prior Approval Breakout Session Summary Rick Smith Aventis Pasteur, Inc. Issues Discussed Risk Analysis for Post Approval Changes Comparability Protocols Development Reports Other Risk-Based
Changes without Prior Approval Breakout Session Summary Rick Smith Aventis Pasteur, Inc. Issues Discussed Risk Analysis for Post Approval Changes Comparability Protocols Development Reports Other Risk-Based
Updates on Quality Metrics - Industry Perspectives Dan Snider Vice President, Research and Development Mylan Inc.
 GPhA/FDA FALL TECHNICAL CONFERENCE Updates on Quality Metrics - Industry Perspectives Dan Snider Vice President, Research and Development Mylan Inc. Disclaimer This presentation contains a summary of the
GPhA/FDA FALL TECHNICAL CONFERENCE Updates on Quality Metrics - Industry Perspectives Dan Snider Vice President, Research and Development Mylan Inc. Disclaimer This presentation contains a summary of the
Points to Consider for Aseptic Processing Part 2 May 2016
 Points to Consider for Aseptic Processing Part 2 May 2016 PDA Points to Consider for Aseptic Processing Part 2 Task Force Authors and Contributors Harold Baseman, Valsource, LLC (Co-Chair) Gabriele Gori,
Points to Consider for Aseptic Processing Part 2 May 2016 PDA Points to Consider for Aseptic Processing Part 2 Task Force Authors and Contributors Harold Baseman, Valsource, LLC (Co-Chair) Gabriele Gori,
11/19/2014. Welcome. Engaging Feedback Mechanisms Creating a Balanced Scorecard. Housekeeping. Agenda 8:00 9:15 9:15 11:30 11:30 12:00.
 Welcome Engaging Feedback Mechanisms Creating a Balanced Scorecard Housekeeping Agenda 8:00 9:15 Icebreaker 9:15 11:30 Engaging Feedback Mechanisms Creating a Balanced Scorecard 11:30 12:00 Assignments
Welcome Engaging Feedback Mechanisms Creating a Balanced Scorecard Housekeeping Agenda 8:00 9:15 Icebreaker 9:15 11:30 Engaging Feedback Mechanisms Creating a Balanced Scorecard 11:30 12:00 Assignments
Building Quality Culture and Capabilities
 Building Quality Culture and Capabilities IPA Advanced GMP Workshop November 2018 McKinsey & Company 1 The typical questions we face on quality culture : 1 2 How much does it matter? Can you measure it?
Building Quality Culture and Capabilities IPA Advanced GMP Workshop November 2018 McKinsey & Company 1 The typical questions we face on quality culture : 1 2 How much does it matter? Can you measure it?
Creating Valuable Values
 orion-partners.com Creating Valuable Values Helping Shop Direct develop a new set of values ORION CASE STUDY Helping Shop Direct develop a new set of values So often the fate of new corporate values is
orion-partners.com Creating Valuable Values Helping Shop Direct develop a new set of values ORION CASE STUDY Helping Shop Direct develop a new set of values So often the fate of new corporate values is
Quality Metrics and Data Driven Decisions. Disclaimer
 Quality Metrics and Data Driven Decisions PDA Pharmaceutical Quality Metrics and Quality Culture Conference February 21, 2017 CDR Tara Gooen Bizjak Senior Science Policy Advisor Div. of Regulations, Guidance,
Quality Metrics and Data Driven Decisions PDA Pharmaceutical Quality Metrics and Quality Culture Conference February 21, 2017 CDR Tara Gooen Bizjak Senior Science Policy Advisor Div. of Regulations, Guidance,
Division of Docket Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, rm Rockville, MD 20852
 October 3, 2008 PDA Global Headquarters Bethesda Towers 4350 East West Highway Suite 200 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org OFFICERS Chair: John Shabushnig,
October 3, 2008 PDA Global Headquarters Bethesda Towers 4350 East West Highway Suite 200 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org OFFICERS Chair: John Shabushnig,
The Patient-Driven Value Chain: Charting the Course to Excellence
 The Patient-Driven Value Chain: Charting the Course to Excellence Wayne McDonnell October 26, 2010 Notes accompany this presentation. Please select Notes Page view. These materials can be reproduced only
The Patient-Driven Value Chain: Charting the Course to Excellence Wayne McDonnell October 26, 2010 Notes accompany this presentation. Please select Notes Page view. These materials can be reproduced only
Connecting People, Science and Regulation
 Connecting People, Science and Regulation October 13th, 2016 Bethesda Towers 4350 East West Highway, Ste 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe
Connecting People, Science and Regulation October 13th, 2016 Bethesda Towers 4350 East West Highway, Ste 600 Bethesda, MD 20814 USA Tel: +1 (301) 656-5900 Fax: +1 (301) 986-0296 www.pda.org PDA Europe
The Journey From Good to Great: Transforming the Pharmaceutical Regulatory System
 The Journey From Good to Great: Transforming the Pharmaceutical Regulatory System 2 nd Annual FDA/PQRI Conference Advancing Product Quality October 6, 2015 Martin VanTrieste, R.Ph. Senior Vice President,
The Journey From Good to Great: Transforming the Pharmaceutical Regulatory System 2 nd Annual FDA/PQRI Conference Advancing Product Quality October 6, 2015 Martin VanTrieste, R.Ph. Senior Vice President,
Tech-Clarity Insight: Improving Portfolio Decision Making. Marrying PPM Best Practice Processes and Technology to Drive ROI
 Tech-Clarity Insight: Improving Portfolio Decision Making Marrying PPM Best Practice Processes and Technology to Drive ROI Tech-Clarity, Inc. 2011 Table of Contents Table of Contents... 2 Executive Overview...
Tech-Clarity Insight: Improving Portfolio Decision Making Marrying PPM Best Practice Processes and Technology to Drive ROI Tech-Clarity, Inc. 2011 Table of Contents Table of Contents... 2 Executive Overview...
ICH Q12 Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management
 ICH Q12 Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management Keith O. Webber, Ph.D. Sr. Director, Global Regulator Affairs Rx Perrigo Company, plc Q U A L I T Y A F F
ICH Q12 Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management Keith O. Webber, Ph.D. Sr. Director, Global Regulator Affairs Rx Perrigo Company, plc Q U A L I T Y A F F
Working together to achieve shared goals in product packaging and labeling
 Working together to achieve shared goals in product packaging and labeling Following on from our 10th anniversary edition Pharma Packaging and Labeling will be returning to Philadelphia for its 11th edition.
Working together to achieve shared goals in product packaging and labeling Following on from our 10th anniversary edition Pharma Packaging and Labeling will be returning to Philadelphia for its 11th edition.
The Four Stages of Cultural Transformation
 WHITE PAPER 9403 PAGE 1 800.535.1559 The Four Stages of Cultural Transformation A White Paper from AchieveIt Harvard Business School has reported 90 percent of strategies fail due to poor execution. Fortune
WHITE PAPER 9403 PAGE 1 800.535.1559 The Four Stages of Cultural Transformation A White Paper from AchieveIt Harvard Business School has reported 90 percent of strategies fail due to poor execution. Fortune
Essential Service 8: Assure a Competent Public Health and Personal Healthcare Workforce
 Essential Service 8: Assure a Competent Public Health and Personal Healthcare Workforce Breakout Session D: 9:05AM-11:00AM Facilitators: Melissa Howard, PhD, MPH, MCHES Florence Greer, MPH, MPA Do we have
Essential Service 8: Assure a Competent Public Health and Personal Healthcare Workforce Breakout Session D: 9:05AM-11:00AM Facilitators: Melissa Howard, PhD, MPH, MCHES Florence Greer, MPH, MPA Do we have
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program?
 Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
Bridging the Strategy Execution Divide
 Bridging the Strategy Execution Divide Presented by: Chrissy Coley, SunGard Higher Education March 21, 2011 Session Rules of Etiquette Please turn off your cell phone/pager If you must leave the session
Bridging the Strategy Execution Divide Presented by: Chrissy Coley, SunGard Higher Education March 21, 2011 Session Rules of Etiquette Please turn off your cell phone/pager If you must leave the session
Working together to achieve shared goals in product packaging and labeling
 Working together to achieve shared goals in product packaging and labeling Following on from our 10th anniversary edition Pharma Packaging and Labeling will be returning to Philadelphia for its 11th edition.
Working together to achieve shared goals in product packaging and labeling Following on from our 10th anniversary edition Pharma Packaging and Labeling will be returning to Philadelphia for its 11th edition.
MISO ELMP. Goals & Experience. Jeff Bladen Executive Director, Market Development
 MISO ELMP Goals & Experience Jeff Bladen Executive Director, Market Development HARVARD ELECTRICITY POLICY GROUP EIGHTY-NINTH PLENARY SESSION JANUARY 25-26, 2018 1 MISO saw gaps with legacy LMP market
MISO ELMP Goals & Experience Jeff Bladen Executive Director, Market Development HARVARD ELECTRICITY POLICY GROUP EIGHTY-NINTH PLENARY SESSION JANUARY 25-26, 2018 1 MISO saw gaps with legacy LMP market
AIRMIC ERM Forum 2017
 AIRMIC ERM Forum 2017 Information systems and analytics: The risk manager s essential toolkit How can data management and analytics be used to make smarter risk and risk financing decisions, and understand
AIRMIC ERM Forum 2017 Information systems and analytics: The risk manager s essential toolkit How can data management and analytics be used to make smarter risk and risk financing decisions, and understand
An Industry Perspective: Reducing the Complexity and Impact of Regulatory Changes in Latin America
 An Industry Perspective: Reducing the Complexity and Impact of Regulatory Changes in Latin America SPEAKERS: Janett Mugaburu-Richards, M.S. Pfizer Inc. Kavita Ramalingam Iyer, Ph.D Merck & Co., Inc. Contents
An Industry Perspective: Reducing the Complexity and Impact of Regulatory Changes in Latin America SPEAKERS: Janett Mugaburu-Richards, M.S. Pfizer Inc. Kavita Ramalingam Iyer, Ph.D Merck & Co., Inc. Contents
Click to edit Master title style
 Measuring Performance, Driving Excellence Click to edit Master title style ISPE Nordic, Stockholm WTC November 26 th 2015 Dr. Nuala Calnan, Research Fellow, Dublin Institute of Technology 11/26/2015 1
Measuring Performance, Driving Excellence Click to edit Master title style ISPE Nordic, Stockholm WTC November 26 th 2015 Dr. Nuala Calnan, Research Fellow, Dublin Institute of Technology 11/26/2015 1
2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges Thursday, May 9 Registration Open Continental Breakfast
 2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges End-to-End Product Development Manufacturing, Supply, and Quality May 9-10, 2019 Hilton Long Beach Long Beach, CA As
2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges End-to-End Product Development Manufacturing, Supply, and Quality May 9-10, 2019 Hilton Long Beach Long Beach, CA As
2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges Thursday, May 9 Registration Open Continental Breakfast
 2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges End-to-End Product Development Manufacturing, Supply, and Quality May 9-10, 2019 Hilton Long Beach Long Beach, CA As
2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges End-to-End Product Development Manufacturing, Supply, and Quality May 9-10, 2019 Hilton Long Beach Long Beach, CA As
The Future of Pharmaceutical Quality
 The Future of Pharmaceutical Quality First SQA/BIRS Center Meeting Purdue University Polytechnic Institute Louis W. Yu, Ph.D. Chief Quality Officer Unique Challenges of the Pharmaceutical Industry a. The
The Future of Pharmaceutical Quality First SQA/BIRS Center Meeting Purdue University Polytechnic Institute Louis W. Yu, Ph.D. Chief Quality Officer Unique Challenges of the Pharmaceutical Industry a. The
ISPE s Process Capability Team
 4 September 7 INDUSTRY MATURITY IN THE ASSESSMENT AND USE OF PROCESS CAPABILITY Arne Zilian Head MS&T Processes & Standards Novartis Pharma AG Process Validation Statistics Conference 5 September 7 ISPE
4 September 7 INDUSTRY MATURITY IN THE ASSESSMENT AND USE OF PROCESS CAPABILITY Arne Zilian Head MS&T Processes & Standards Novartis Pharma AG Process Validation Statistics Conference 5 September 7 ISPE
Balancing Your Safety Metrics Why Leading Metrics May Be Misleading
 Session No. 574 Balancing Your Safety Metrics Why Leading Metrics May Be Misleading Paul Esposito, CSP, CIH President, STAR Consultants, Inc. Introduction Using data collected from leading companies worldwide,
Session No. 574 Balancing Your Safety Metrics Why Leading Metrics May Be Misleading Paul Esposito, CSP, CIH President, STAR Consultants, Inc. Introduction Using data collected from leading companies worldwide,
Perspectives on FDA s Secure Supply Chain Pilot Program
 Perspectives on FDA s Secure Supply Chain Pilot Program PDA Annual Meeting, Las Vegas, March 16-18, 2015 Facts around US imports Did you know that the US imports: 40% of finished dosage drugs 80% of Active
Perspectives on FDA s Secure Supply Chain Pilot Program PDA Annual Meeting, Las Vegas, March 16-18, 2015 Facts around US imports Did you know that the US imports: 40% of finished dosage drugs 80% of Active
Wise Governance: Enhancing Long Term Care
 Wise Governance: Enhancing Long Term Care Effective Practices for the Boards of Non-profit Organizations Engaged In Senior Living and Long Term Care Services Steven Chies Lead Consultant, Care Paradigms
Wise Governance: Enhancing Long Term Care Effective Practices for the Boards of Non-profit Organizations Engaged In Senior Living and Long Term Care Services Steven Chies Lead Consultant, Care Paradigms
Patients are Counting on Us: It s Time to Act
 Patients are Counting on Us: It s Time to Act Brian Johnson Chairman Rx-360 Senior Director Supply Chain Security Pfizer, Inc. 24 th Global GS1 Healthcare Conference October 2, 2013 THE WALL STREET JOURNAL
Patients are Counting on Us: It s Time to Act Brian Johnson Chairman Rx-360 Senior Director Supply Chain Security Pfizer, Inc. 24 th Global GS1 Healthcare Conference October 2, 2013 THE WALL STREET JOURNAL
Employee Engagement: Helping Your Staff Go Above and Beyond. Shannon Bennett, Quality Management Supervisor. Disclosures
 Continual Improvement Forum Employee Engagement: Helping Your Staff Go Above and Beyond Shannon Bennett, Quality Management Supervisor June 13 th, 2016 Disclosures Relevant Financial Relationship(s): Nothing
Continual Improvement Forum Employee Engagement: Helping Your Staff Go Above and Beyond Shannon Bennett, Quality Management Supervisor June 13 th, 2016 Disclosures Relevant Financial Relationship(s): Nothing
THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture
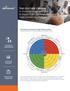 denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
Achieving Performance Excellence
 Achieving Performance Excellence Baldrige 101 Workshop ASQ Professional Development Summit Today s Discussion Introduce the Baldrige framework Value and Benefits of Baldrige Regional Program MN, ND & SD
Achieving Performance Excellence Baldrige 101 Workshop ASQ Professional Development Summit Today s Discussion Introduce the Baldrige framework Value and Benefits of Baldrige Regional Program MN, ND & SD
PhRMA REPS MARY OATES AND MOHEB NASR (RAPPORTEUR) ON ICH Q12
 PhRMA REPS MARY OATES AND MOHEB NASR (RAPPORTEUR) ON ICH Q12 At the ISPE Quality Manufacturing Conference in early June, Pfizer Quality Global Operations, Environmental Health and Safety VP Mary Oates,
PhRMA REPS MARY OATES AND MOHEB NASR (RAPPORTEUR) ON ICH Q12 At the ISPE Quality Manufacturing Conference in early June, Pfizer Quality Global Operations, Environmental Health and Safety VP Mary Oates,
Executive Summary. The State of Employee Engagement pg. 2
 the STATE of EMPLOYEE ENGAGEMENt 2018 v Executive Summary 2018 has been named The Year of Employee Experience by Forbes; highlighting an increasing need for employers to offer engaging experiences to retain
the STATE of EMPLOYEE ENGAGEMENt 2018 v Executive Summary 2018 has been named The Year of Employee Experience by Forbes; highlighting an increasing need for employers to offer engaging experiences to retain
Pharmaceutical Quality System (ICH Q10) Conference
 This conference is supported by The Parenteral Drug Association Presents: Pharmaceutical Quality System (ICH Q10) Conference November 5-6, 2012 Keio Plaza Hotel Tokyo, Japan Program Planning Committee
This conference is supported by The Parenteral Drug Association Presents: Pharmaceutical Quality System (ICH Q10) Conference November 5-6, 2012 Keio Plaza Hotel Tokyo, Japan Program Planning Committee
Andy Wright Group Director, The New York Times Job Market
 MARKET INTELLIGENCE: Investing in the Future of the Pharmaceutical Industry Dear Colleagues, Our region s pharmaceutical industry has long been recognized as an important engine of the area job market.
MARKET INTELLIGENCE: Investing in the Future of the Pharmaceutical Industry Dear Colleagues, Our region s pharmaceutical industry has long been recognized as an important engine of the area job market.
Patients are Counting on Us: A Little Less Conversation It s Time to Act
 Patients are Counting on Us: A Little Less Conversation It s Time to Act Martin VanTrieste SVP Quality Amgen, Inc. PDA Missouri Valley Chapter Supply Chain Security in the Pharmaceutical Industry Thousand
Patients are Counting on Us: A Little Less Conversation It s Time to Act Martin VanTrieste SVP Quality Amgen, Inc. PDA Missouri Valley Chapter Supply Chain Security in the Pharmaceutical Industry Thousand
Analyzing which software needs to be utilized to ensure a timely and more automated process
 Clinical Data Integration & Management March 20 th Program Day One 07:30 Registration and refreshments 08:15 Chair s opening remarks Terry Katz, Director, Head of DM and Statistics, Merck Animal Health
Clinical Data Integration & Management March 20 th Program Day One 07:30 Registration and refreshments 08:15 Chair s opening remarks Terry Katz, Director, Head of DM and Statistics, Merck Animal Health
Embedding High-Performance Culture through New Approaches to Performance Management and Behavior Change
 Embedding High-Performance Culture through New Approaches to Performance Management and Behavior Change Elaine D. Pulakos, PDRI a CEB Company Alan Colquitt, Eli Lilly and Company Sharon Arad, Cargill Session
Embedding High-Performance Culture through New Approaches to Performance Management and Behavior Change Elaine D. Pulakos, PDRI a CEB Company Alan Colquitt, Eli Lilly and Company Sharon Arad, Cargill Session
Understanding GxP (GMP, GCP & GLP) for FDA Regulated Industries
 Microrite, Inc. brings you this unique learning experience in Understanding GxP (GMP, GCP & GLP) for FDA Regulated Industries; Part of Microrite s step-by-step webinar series. Understanding GxP (GMP, GCP
Microrite, Inc. brings you this unique learning experience in Understanding GxP (GMP, GCP & GLP) for FDA Regulated Industries; Part of Microrite s step-by-step webinar series. Understanding GxP (GMP, GCP
Balanced Scorecard. MA. DESIREE D. BELDAD, Ph.D. FEBRUARY 9, 2012
 Balanced Scorecard MA. DESIREE D. BELDAD, Ph.D. FEBRUARY 9, 2012 IMAGINE ENTERING THE COCKPIT of a modern jet airplane and seeing only a single instrument there. How do you feel about boarding the plane
Balanced Scorecard MA. DESIREE D. BELDAD, Ph.D. FEBRUARY 9, 2012 IMAGINE ENTERING THE COCKPIT of a modern jet airplane and seeing only a single instrument there. How do you feel about boarding the plane
An Introduction to Double Dragon Consulting
 An Introduction to Double Dragon Consulting www.doubledragonconsulting.com About DDC Our Experience: - All dosage forms, aseptic processes, API manufacture, OTC - Remediation of 483 observations, Warning
An Introduction to Double Dragon Consulting www.doubledragonconsulting.com About DDC Our Experience: - All dosage forms, aseptic processes, API manufacture, OTC - Remediation of 483 observations, Warning
Quality Metrics: A Regulatory Perspective
 Quality Metrics: A Regulatory Perspective By Tara Gooen Bizjak, MBSci CDER Office of Policy for Pharmaceutical Quality Presented By Dr. Ademola Daramola US FDA Office of International Programs Indian Pharmaceutical
Quality Metrics: A Regulatory Perspective By Tara Gooen Bizjak, MBSci CDER Office of Policy for Pharmaceutical Quality Presented By Dr. Ademola Daramola US FDA Office of International Programs Indian Pharmaceutical
CMC Activities for Commercialization: PAI, QMS, and BLA/NDA. NEPDA Dinner Meeting Cambridge, MA 16 May 2018
 CMC Activities for Commercialization: PAI, QMS, and BLA/NDA Advancing from Clinical to Commercial The Commercialization Roadmap NEPDA Dinner Meeting Cambridge, MA 16 May 2018 Standards Certification Education
CMC Activities for Commercialization: PAI, QMS, and BLA/NDA Advancing from Clinical to Commercial The Commercialization Roadmap NEPDA Dinner Meeting Cambridge, MA 16 May 2018 Standards Certification Education
Making a start on engagement with the Engagement Bridge. Workshop Pack 1.3
 Making a start on engagement with the Engagement Bridge Workshop Pack 1.3 Key points of the Engagement Bridge Model Every organization is different. The model gives you the areas to look at, ideas and
Making a start on engagement with the Engagement Bridge Workshop Pack 1.3 Key points of the Engagement Bridge Model Every organization is different. The model gives you the areas to look at, ideas and
10 RULES OF EMPLOYEE ENGAGEMENT. Thomas G. Cornillie, SPHR Director of Human Resources Cincinnati Incorporated April 24, 2013
 10 RULES OF EMPLOYEE ENGAGEMENT Thomas G. Cornillie, SPHR Director of Human Resources Cincinnati Incorporated April 24, 2013 Employee Engagement To win in the marketplace you must first win in the workplace.
10 RULES OF EMPLOYEE ENGAGEMENT Thomas G. Cornillie, SPHR Director of Human Resources Cincinnati Incorporated April 24, 2013 Employee Engagement To win in the marketplace you must first win in the workplace.
SM&CR Culture Measurement Toolkit
 SM&CR Culture Measurement Toolkit November 2018 V 1.1 Contents 1 Introduction... 3 2 Scope... 4 3 Cultural Drivers... 5 4 Culture Matrix... 7 5 Staff Survey... 10 6 Self Assessment... 10 7 Performance
SM&CR Culture Measurement Toolkit November 2018 V 1.1 Contents 1 Introduction... 3 2 Scope... 4 3 Cultural Drivers... 5 4 Culture Matrix... 7 5 Staff Survey... 10 6 Self Assessment... 10 7 Performance
Using PSP/TSP in a Performance Review. Nicole Flohr Larry Whitford November 4, 2014
 Using PSP/TSP in a Performance Review Nicole Flohr Larry Whitford November 4, 2014 Who We Are Beckman Coulter: Beckman Coulter: Chemistry & Immunoassay Hematology & Urinalysis Molecular Diagnostics Workflow
Using PSP/TSP in a Performance Review Nicole Flohr Larry Whitford November 4, 2014 Who We Are Beckman Coulter: Beckman Coulter: Chemistry & Immunoassay Hematology & Urinalysis Molecular Diagnostics Workflow
Chicken Farmers of Ontario New Entrant Chicken Processors Program Stakeholder Engagement Workshop
 Chicken Farmers of Ontario New Entrant Chicken Processors Program Stakeholder Engagement Workshop May 26, 2017 Mississauga, Ontario 2 Table of Contents Page Executive Summary 3 Purpose of Session 4 Current
Chicken Farmers of Ontario New Entrant Chicken Processors Program Stakeholder Engagement Workshop May 26, 2017 Mississauga, Ontario 2 Table of Contents Page Executive Summary 3 Purpose of Session 4 Current
Competing for Image Leadership
 Competing for Image Leadership By Peter Carlin, Ellen Gordon, Ruchika Kapur, and Shannon Clancy Image leadership in oncology is taking on increased importance as performance, customer satisfaction and
Competing for Image Leadership By Peter Carlin, Ellen Gordon, Ruchika Kapur, and Shannon Clancy Image leadership in oncology is taking on increased importance as performance, customer satisfaction and
FDA Quality Metrics Guidance
 FDA Quality Metrics Guidance Marlène García Swider, Ph.D., CQM, CSSBB Institute of Validation Technology Quality Metrics Conference February 22 24, 2016 Coronado Island, San Diego, CA Personal Claim This
FDA Quality Metrics Guidance Marlène García Swider, Ph.D., CQM, CSSBB Institute of Validation Technology Quality Metrics Conference February 22 24, 2016 Coronado Island, San Diego, CA Personal Claim This
Developing Enterprise-Wide Measures for Tracking Performance of Acquisition Organizations
 Pittsburgh, PA 15213-3890 Developing Enterprise-Wide Measures for Tracking Performance of Acquisition Organizations Wolfhart Goethert Carnegie Mellon University Pittsburgh, PA 15213-3890 Sponsored by the
Pittsburgh, PA 15213-3890 Developing Enterprise-Wide Measures for Tracking Performance of Acquisition Organizations Wolfhart Goethert Carnegie Mellon University Pittsburgh, PA 15213-3890 Sponsored by the
2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges Thursday, May 9 Registration Open Continental Breakfast
 2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges End-to-End Product Development Manufacturing, Supply, and Quality May 9-10, 2019 Hilton Long Beach Long Beach, CA As
2019 PDA Biosimilars and Vaccines Conference: Lifecycle Similarities and Challenges End-to-End Product Development Manufacturing, Supply, and Quality May 9-10, 2019 Hilton Long Beach Long Beach, CA As
CONFERENCE AGENDA USER CONFERENCE 2016 USER CONFERENCE 2016
 DAY 1: Monday, April 25, 2016 Welcome to Fort Lauderdale Join Us For Some Cocktails & Horderves at the Ritz Carlton Bar Starting at 5:30 PM Come and Relax After Your Trip, Network with EHS Professionals
DAY 1: Monday, April 25, 2016 Welcome to Fort Lauderdale Join Us For Some Cocktails & Horderves at the Ritz Carlton Bar Starting at 5:30 PM Come and Relax After Your Trip, Network with EHS Professionals
Request for Quality Metrics Guidance for Industry
 Request for Quality Metrics Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be
Request for Quality Metrics Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be
Quality Metrics Journey to Excellence. Steve Greer Corp QA External Engagement Leader Procter & Gamble
 Quality Metrics Journey to Excellence Steve Greer Corp QA External Engagement Leader Procter & Gamble Acknowledgements Tara Gooen Bizjak FDA, CDER/OPQ/OPPQ Ashley Boam FDA, CDER/OPQ/OPPQ Alex Viehman FDA,
Quality Metrics Journey to Excellence Steve Greer Corp QA External Engagement Leader Procter & Gamble Acknowledgements Tara Gooen Bizjak FDA, CDER/OPQ/OPPQ Ashley Boam FDA, CDER/OPQ/OPPQ Alex Viehman FDA,
Process Capability: Practical Challenges to Implementation
 Process Capability: Practical Challenges to Implementation [in pharmaceutical manufacturing] 33rd Quality & Productivity Research Conference Tempe, AZ June 16, 2016 Julia O Neill julia.oneill@tunnellconsulting.com
Process Capability: Practical Challenges to Implementation [in pharmaceutical manufacturing] 33rd Quality & Productivity Research Conference Tempe, AZ June 16, 2016 Julia O Neill julia.oneill@tunnellconsulting.com
PQRI-FDA Workshop on Process Drift: Detection, Measurement, and Control in the Manufacture of Pharmaceuticals. December 1-3, 2010 Bethesda, Maryland
 PQRI-FDA Workshop on Process Drift: Detection, Measurement, and Control in the Manufacture of Pharmaceuticals December 1-3, 2010 Bethesda, Maryland Please note start time on December 1 has changed. SCOPE
PQRI-FDA Workshop on Process Drift: Detection, Measurement, and Control in the Manufacture of Pharmaceuticals December 1-3, 2010 Bethesda, Maryland Please note start time on December 1 has changed. SCOPE
Immunotoxicology Technical Committee (ITC) HESI Assembly of Members January 19, 2009
 Immunotoxicology Technical Committee (ITC) HESI Assembly of Members January 19, 2009 Committee Leadership Dr. Ellen Evans (Schering-Plough Research Institute), Chair Dr. Tom Kawabata (Pfizer Inc.), Vice
Immunotoxicology Technical Committee (ITC) HESI Assembly of Members January 19, 2009 Committee Leadership Dr. Ellen Evans (Schering-Plough Research Institute), Chair Dr. Tom Kawabata (Pfizer Inc.), Vice
Supplier Oversight PQRI. September Steven Lynn, MS, CMQ/OE Vice President Global Quality Compliance Mylan
 Supplier Oversight September 2014 PQRI Steven Lynn, MS, CMQ/OE Vice President Global Quality Compliance Mylan Agenda Mylan Background Setting the Stage with a Scenario Current State Thinking Supplier Qualification
Supplier Oversight September 2014 PQRI Steven Lynn, MS, CMQ/OE Vice President Global Quality Compliance Mylan Agenda Mylan Background Setting the Stage with a Scenario Current State Thinking Supplier Qualification
Singapore Clinical Research Professional (CRP) /Clinical Research Coordinator Society (CRCS) Forum
 Singapore Clinical Research Professional (CRP) /Clinical Research Coordinator Society (CRCS) Forum 25 August 2017 TOPIC: Issue Management / Quality Risk Management Implications with ICH GCP E6 (R2) and
Singapore Clinical Research Professional (CRP) /Clinical Research Coordinator Society (CRCS) Forum 25 August 2017 TOPIC: Issue Management / Quality Risk Management Implications with ICH GCP E6 (R2) and
TRAINING TITLE: Quality Risk Management Certification (CERT-004)
 TRAINING TITLE: Quality Risk Management Certification (CERT-004) OVERVIEW: The importance of quality systems has been recognized in the life sciences industry and it is becoming evident that quality risk
TRAINING TITLE: Quality Risk Management Certification (CERT-004) OVERVIEW: The importance of quality systems has been recognized in the life sciences industry and it is becoming evident that quality risk
GLOBAL MARKETS AND MANUFACTURING TECHNOLOGIES FOR PROTEIN DRUGS
 GLOBAL MARKETS AND MANUFACTURING TECHNOLOGIES FOR PROTEIN DRUGS BIO021E May 2016 Shalini Shahani Dewan Project Analyst ISBN: 1-62296-288-5 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
GLOBAL MARKETS AND MANUFACTURING TECHNOLOGIES FOR PROTEIN DRUGS BIO021E May 2016 Shalini Shahani Dewan Project Analyst ISBN: 1-62296-288-5 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA
46 Statistics. Every HR Professional Should Know. Global Attitudes Toward Work Qualtrics
 46 Statistics Every HR Professional Should Know Global Attitudes Toward Work Qualtrics In all countries, people turn to friends first when looking for a new job more than job sites, family, a former employer
46 Statistics Every HR Professional Should Know Global Attitudes Toward Work Qualtrics In all countries, people turn to friends first when looking for a new job more than job sites, family, a former employer
proving value through data
 1 GUIDE proving value through data Go Beyond Cost-Based ROI Metrics 2017 Freeman. All Rights Reserved. 2 gauging the value of events With the unique ability to facilitate interpersonal engagement and deeper
1 GUIDE proving value through data Go Beyond Cost-Based ROI Metrics 2017 Freeman. All Rights Reserved. 2 gauging the value of events With the unique ability to facilitate interpersonal engagement and deeper
HR certification: basic course
 HR certification: basic course What makes the program unique: It is a modular program covering all major areas of the integrated talent There are trainings for different levels of HR professionals (basic
HR certification: basic course What makes the program unique: It is a modular program covering all major areas of the integrated talent There are trainings for different levels of HR professionals (basic
Ensuring Quality & Regulatory Compliance when Collaborating with a Service Provider
 DPT Thought Leadership Issue 2 Ensuring Quality & Regulatory Compliance when Collaborating with a Service Provider I N T R O D U C T I O N In recent years, the pharmaceutical industry has given greater
DPT Thought Leadership Issue 2 Ensuring Quality & Regulatory Compliance when Collaborating with a Service Provider I N T R O D U C T I O N In recent years, the pharmaceutical industry has given greater
LIFE TESTING FOR DEVICE COMBINATION PRODUCTS
 LIFE TESTING FOR DEVICE COMBINATION PRODUCTS APPROACHES AND CHALLENGES FOR INTEGRATING DEVICES INTO A COMPREHENSIVE STABILITY PROGRAM CLINT JUDD PRINCIPAL QUALITY ENGINEER INTRODUCTION What is shelf life
LIFE TESTING FOR DEVICE COMBINATION PRODUCTS APPROACHES AND CHALLENGES FOR INTEGRATING DEVICES INTO A COMPREHENSIVE STABILITY PROGRAM CLINT JUDD PRINCIPAL QUALITY ENGINEER INTRODUCTION What is shelf life
SMART PLANT Workflow for Business Process Automation in a Regulated Environment
 SMART PLANT Workflow for Business Process Automation in a Regulated Environment Mary Grow, VP ET & Business Processes, JHP Pharmaceuticals Ty Chasey MES Consultant, Gray Matter Systems 17 19 August 2011
SMART PLANT Workflow for Business Process Automation in a Regulated Environment Mary Grow, VP ET & Business Processes, JHP Pharmaceuticals Ty Chasey MES Consultant, Gray Matter Systems 17 19 August 2011
PDA Comments ICH Q10; Pharmaceutical Quality System October Suggested Revision
 PDA s ICH Q10; Pharmaceutical Quality System October 2007 Section Line 1.1 54 The inspectional impact of the optional nature of Q10 beyond what is required by current GMP can be further clarified. 1.1
PDA s ICH Q10; Pharmaceutical Quality System October 2007 Section Line 1.1 54 The inspectional impact of the optional nature of Q10 beyond what is required by current GMP can be further clarified. 1.1
Amgen Supply Chain Segmentation The Journey to October 23, 2014
 Amgen Supply Chain Segmentation The Journey to 2022 October 23, 2014 Introduction Rayne Waller VP Global Supply Chain at Amgen Responsible for: Corporate and Regional Supply Chain Functions Contract Manufacturing
Amgen Supply Chain Segmentation The Journey to 2022 October 23, 2014 Introduction Rayne Waller VP Global Supply Chain at Amgen Responsible for: Corporate and Regional Supply Chain Functions Contract Manufacturing
SMALL MOLECULE API CONTRACT MANUFACTURER QUALITY BENCHMARKING
 SMALL MOLECULE API CONTRACT MANUFACTURER QUALITY BENCHMARKING (2ND EDITION) JANUARY, 2017 INTRODUCTION Table of Contents COPYRIGHT AND USAGE GUIDELINES...5 INTRODUCTION...6 METHODOLOGY...7 RESPONDENT DEMOGRAPHICS
SMALL MOLECULE API CONTRACT MANUFACTURER QUALITY BENCHMARKING (2ND EDITION) JANUARY, 2017 INTRODUCTION Table of Contents COPYRIGHT AND USAGE GUIDELINES...5 INTRODUCTION...6 METHODOLOGY...7 RESPONDENT DEMOGRAPHICS
How to Hit the Easy Button for a FSMA-Compliant Food Safety Plan
 How to Hit the Easy Button for a FSMA-Compliant Food Safety Plan Bryan Armentrout, CEO The Food Leadership Group And Gary Nowacki, CEO TraceGains, Inc. Bryan Armentrout CEO The Food Leadership Group Gary
How to Hit the Easy Button for a FSMA-Compliant Food Safety Plan Bryan Armentrout, CEO The Food Leadership Group And Gary Nowacki, CEO TraceGains, Inc. Bryan Armentrout CEO The Food Leadership Group Gary
10/30/2014. Definitions. Definitions. Leadership s role in enabling a Quality Culture CULTURE LEADERSHIP
 Leadership s role in enabling a V E R ONICA C R UZ, P H D E X E C U T IV E V P/ C O O Quality Workshop - October 29, 2014 Elizabeth, NJ 1 Definitions Dictionary: Leader: A person who guides a group Culture:
Leadership s role in enabling a V E R ONICA C R UZ, P H D E X E C U T IV E V P/ C O O Quality Workshop - October 29, 2014 Elizabeth, NJ 1 Definitions Dictionary: Leader: A person who guides a group Culture:
