Environmental Management System Audit
|
|
|
- Robert Hoover
- 6 years ago
- Views:
Transcription
1 Auditing Your EMS
2 Environmental Management System Audit A systematic and documented verification process of objectively obtaining and evaluating evidence to determine whether an organization s environmental management system conforms to the environmental management system audit criteria set by the organization, and for communication of the results of this process to management.
3 What is Required by EO 13423? EMS shall be reviewed and updated annually or more frequently, as appropriate, For the purpose of conformance to E.O , an EMS shall be considered fully implemented when (1) it has been the subject of a formal audit by a qualified party outside the control or scope of the EMS Once conformance has been declared, the EMS shall then be audited by a qualified party outside of the control or scope of the EMS at least every three years from the date of the initial declaration. Conformance declaration shall be renewed as appropriate based on agency guidance.
4 EMS Audit Addresses all of EMS (documentation and implementation) Not a performance audit or a compliance audit Does the system conform to set criteria? Has EMS been properly implemented, maintained? Provides information to management and organization to allow continual improvement Performed by those with EMS auditing training/experience Provides periodic snap-shot assessment to verify system
5 What do you need to do? Develop audit procedure(s) and programs that describe the when, how, who, and where of how the EMS auditing will be done Conduct audits Keep proper records Feed audit information into rest of EMS
6 Internal vs. External Audits Internal, first party audits are conducted by participants of the EMS being audited (can be employees, or consultants as agents of the organization). External, second party audits are conducted by auditors from outside the scope of the EMS in question (but can be from same organization). External, third party audits are conducted by independent, ISO registrars. In all cases, audit should cover same elements!!!!
7 Audit Criteria Policies, practices, procedures or requirements against which the auditor compares collected audit evidence about the subject matter. Requirements may include but are not limited to standards, guidelines, specified organizational requirements, and legislative or regulatory requirements.
8 Audit Evidence Verifiable information, records or statements of fact. Audit evidence, which can be qualitative or quantitative, is used by the auditor to determine whether audit criteria are met. Audit evidence is typically based on interviews, examination of documents, observation of activities and conditions, existing results of measurements and tests or other means within the scope of the audit.
9 Audit Findings Results of the evaluation of the collected audit evidence compared with the agreed audit criteria. The audit findings provide the basis for the audit report.
10 Audit Conclusion Professional judgement or opinion expressed by an auditor about the subject matter of the audit, based on and limited to reasoning the auditor has applied to audit findings.
11 AUDITING TOOLS AND TECHNIQUES
12 The Three C s of Auditing EMS Conformance Meets the requirements (implements the shalls ) Consistency Various elements inter-related (e.g., significant aspects reflected in emergency planning) Continual Improvement Mechanisms in place to improve (including fixing non-conformances and improving performance)
13 Basic Rules of Auditing Be objective - i.e., do not keep an audit going until you find a problem Be fair and consistent Stay focused on the criteria Stay within the scope and plan Remember, the audit is there to improve the EMS
14 Audit Planning Addresses the specifics of who, what, where, when, and how Identifies auditors Identifies when the audit(s) will occur Identifies what format, checklists, etc will be used Establishes specific agendas Must align with site procedure for auditing
15 Audit Approach Can do either all at one time, or divided up throughout the year. If divided, must decide on whether to do one element of the standard across the plant, or all elements in parts of the facility Keep in mind the issue of consistency!
16 Audit Approach (cont.) Audits typically include: Interviews Site Reconnaissance Records review In any type of audit, the auditor will compare evidence to criteria, and develop findings
17 Specific Evidence to Look For Consistency of identified aspects and observed aspects How activities match procedures Employee awareness of relevant EMS elements Addressing non-conformances through the non-conformance process Is the system effective and adequate? Is top management committed to the EMS?
18 How to Interview Attitude of the auditor Purpose of the interview Types of questions; open, closed, leading, antagonistic, vague
19 Typical Questions of any Employee 1. What does the EMS policy say and what does it mean to you? 2. What is your familiarity with the EMS program? 3. What do you do in case of a procedural nonconformance? 4. What do you do in case of a emergency? 5. What kind of training have you received? 6. How do you communicate environmental concerns or ideas? 7. What do you do if you receive environmentallyrelated communication from external parties?
20 Typical Questions of Critical Operations 1. What are the significant aspects and impacts associated with your function? 2. How do you know what to do? (Ask for procedures and operating criteria). 3. What specific training have you received? 4. What are the objectives and targets associated with your function? 5. What is your responsibility for monitoring and measurement activities? 6. What records do you keep? 7. (Any other specific questions prompted by answers 1-6 and/or specific circumstances?)
21 Types of Interviewees - Follow the Significant Aspect afacility management and management representatives adepartment managers aemp responsible parties adocument control and record departments a Engineering aoperations employees (plant, administration) ahuman resources and training acontractor management and purchasing asecurity
22 What Auditors Look for in Terms of Documentation Does your documented system respond to the standard? Do the procedures describe what s happening? Is the documentation controlled? Are all employees informed? Are the procedures followed, by everyone, all the time? Is there objective evidence that the procedures are being followed?
23 Typical Documents and Records to Review (by Element) Aspects Procedures Aspects list Significant determination information Significant aspects/impacts list Legal and Other Requirements Listings of applicable legal and other requirements Appropriate instructions for compliance Permits, manifests, etc.
24 Objectives and Targets and Environmental Management Programs rminutes/notes of objectives and target development rlist of objectives and targets rrelated action plans Structure and Responsibility rjob descriptions rorganizational charts
25 Training Awareness and Competence Training needs listings/matrix Manuals, course materials Sign-in sheets Test records, certificate copies, etc. Communication Specific work instructions Records of communication and correspondence
26 Document Control * Documents, procedures, and manuals Operational Control * Critical operations/aspects listing/matrix * Specific work instructions * Environmental issues and instructions within other work instructions * Contractor policies, work orders, etc. * Supplier requirements
27 Emergency Preparedness and Response Emergency plans and protocols Practice and drill results Monitoring and Measurement Objectives and target action plans Specific procedures and work instructions Records of monitoring and measurement data collected
28 Nonconformance, Corrective and Preventive Action Corrective actions reports Evidence of discussion and follow-up (meeting notes, etc.) Records Records EMS Audit Specific audit procedures, checklists, forms EMS audit notes and working documents EMS audit reports
29 Management Review 4Meeting agendas and attendance 4Meeting minutes and action items 4Evidence of follow-up actions, reports, etc.
30 Summary Ensure good auditing skills Remember the three C s -Conformance, Consistency, Continual Improvement Let the standard be your guide Remember the goal is to improve the EMS
31 Resources Interviewing Techniques for Auditors USDA course AUDT7012G Auditing guide on OFEE website: AL_AUDITOR_TRAINING2.pdf
AUDITING CONCEPTS. July 2008 Page 1 of 7
 AUDITING CONCEPTS 1. BACKGROUND Each of the twenty aspects in SAP Sections B and C has been separated into components that need to be addressed individually. As well as addressing the specific SAP requirement,
AUDITING CONCEPTS 1. BACKGROUND Each of the twenty aspects in SAP Sections B and C has been separated into components that need to be addressed individually. As well as addressing the specific SAP requirement,
Internal Auditing and Control of Nonconforming Work
 Internal Auditing and Control of Nonconforming Work ISO/IEC 17025, clauses 4.14 and 4.9 www.aphl.org What will be covered in this training? Internal Auditing What is it? Who performs it? What are the possible
Internal Auditing and Control of Nonconforming Work ISO/IEC 17025, clauses 4.14 and 4.9 www.aphl.org What will be covered in this training? Internal Auditing What is it? Who performs it? What are the possible
ISO 14001:2015 Gap Analysis Check Sheet
 ? CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context The organization shall determine external and internal issues that are relevant to its purpose and that affect its ability
? CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context The organization shall determine external and internal issues that are relevant to its purpose and that affect its ability
Robin Costas / Lynda Podhorniak EMS Co-coordinators
 U.S. Environmental Protection Agency ENVIRONMENTAL SCIENCE CENTER, FORT MEADE, MARYLAND ESC EP17.05 Environmental Management System Procedure for: EMS Audits: Internal, External, and Compliance Effective
U.S. Environmental Protection Agency ENVIRONMENTAL SCIENCE CENTER, FORT MEADE, MARYLAND ESC EP17.05 Environmental Management System Procedure for: EMS Audits: Internal, External, and Compliance Effective
Humantech Environmental Management System Manual
 Humantech Management System Version 1.0 March 2014 Humantech, Inc. Humantech Management System Revision No.: 1 Date : 03-10-14 Prepared by: Approved by: (EMR) President Revision History Revision Date Description
Humantech Management System Version 1.0 March 2014 Humantech, Inc. Humantech Management System Revision No.: 1 Date : 03-10-14 Prepared by: Approved by: (EMR) President Revision History Revision Date Description
Utah Army National Guard Transforming EMS Compliance at the Utah National Guard with an Automated Tool
 Utah Army National Guard Transforming EMS Compliance at the Utah National Guard with an Automated Tool Environment, Energy & Sustainability Symposium May 2009 ISO 14001 Compliance Challenges > Questions
Utah Army National Guard Transforming EMS Compliance at the Utah National Guard with an Automated Tool Environment, Energy & Sustainability Symposium May 2009 ISO 14001 Compliance Challenges > Questions
ISO 9001: 2015 Quality Management System Certification. Awareness Training
 ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
FO-5 PR-1, FO-1,2 PR-1 EM-6 PR-1, FE-1, NA-1 FO-10 FO-7, EM-2 EM-2. ISO Environmental Management Systems - Specification Yes.
 ISO 14001 Environmental Management Systems - Specification Yes Minor No Comments/Questions 4.1 GENERAL The organization shall establish and maintain an environmental management system, the requirements
ISO 14001 Environmental Management Systems - Specification Yes Minor No Comments/Questions 4.1 GENERAL The organization shall establish and maintain an environmental management system, the requirements
ISO 14001: 2004 Standard Review. Review of the ISO 14001:2004 Standard
 Review of the ISO 14001:2004 Standard Achieving Continual Improvement with ISO 14001: 2004 Continual Improvement Management Review Environmental Policy Checking Implementation & Operation Planning Sections
Review of the ISO 14001:2004 Standard Achieving Continual Improvement with ISO 14001: 2004 Continual Improvement Management Review Environmental Policy Checking Implementation & Operation Planning Sections
Best Practices: Safety Culture. Aria Behrouzi PD Committee Member NAYGN
 Best Practices: Safety Culture Aria Behrouzi PD Committee Member NAYGN Safety Culture Dr. G. Kenneth Koves 2013 May 12 Grand Hyatt Hotel Washington DC, USA Agenda What is safety culture? Why is it important?
Best Practices: Safety Culture Aria Behrouzi PD Committee Member NAYGN Safety Culture Dr. G. Kenneth Koves 2013 May 12 Grand Hyatt Hotel Washington DC, USA Agenda What is safety culture? Why is it important?
Management System Manual International Compliance Group
 Granting, refusing, maintaining, renewing, suspending, restoring or withdrawing certification. Page 1-1 Initial certification audit General - ICG s auditing work is conducted in two stages: Stage 1 and
Granting, refusing, maintaining, renewing, suspending, restoring or withdrawing certification. Page 1-1 Initial certification audit General - ICG s auditing work is conducted in two stages: Stage 1 and
Audit Standards 6/23/2017. Outline. Let s Refresh. Changes to the IIA Standards
 Audit Standards Let s Refresh Outline Changes in the Standards Changes in the Yellowbook Standards Attribute/General Standards Performance/Fieldwork Standards Reporting Standards Key Differences Changes
Audit Standards Let s Refresh Outline Changes in the Standards Changes in the Yellowbook Standards Attribute/General Standards Performance/Fieldwork Standards Reporting Standards Key Differences Changes
THE COMPLETE GUIDE TO ISO14001
 THE COMPLETE GUIDE TO ISO14001 1. Introduction... 3 Plan Do Check Act... 5 2. Requirements... 7 Environmental Policy... 7 Environmental Aspects... 7 Legal and Other Requirements... 8 Objectives & Targets...
THE COMPLETE GUIDE TO ISO14001 1. Introduction... 3 Plan Do Check Act... 5 2. Requirements... 7 Environmental Policy... 7 Environmental Aspects... 7 Legal and Other Requirements... 8 Objectives & Targets...
version 1 / 96 R Green Stars Hotel Environmental Management System
 Environmental Management Manual for Hotels in Hong Kong version 1 / 96 R Green Stars Hotel Environmental Management System 2002 Acknowledgements: This document is a revised version of the manual published
Environmental Management Manual for Hotels in Hong Kong version 1 / 96 R Green Stars Hotel Environmental Management System 2002 Acknowledgements: This document is a revised version of the manual published
Environmental Management System Guidance
 Environmental Management System Guidance Table of Contents Environmental Management System Guidance 1 Introduction 3 2 Gap Analysis 4 3 Implementation & Development 6 4 About Your Organization 7 4.1 Organizational
Environmental Management System Guidance Table of Contents Environmental Management System Guidance 1 Introduction 3 2 Gap Analysis 4 3 Implementation & Development 6 4 About Your Organization 7 4.1 Organizational
Laboratory Quality Assurance Manager & Laboratory Assessor RULES & HANDBOOK
 EOQ Personnel Registration Scheme Laboratory Quality Assurance Manager & RULES & HANDBOOK Prepared by: Dr. Eugenia Soboleva, Quality Austria In accordance with the working group on EOQ product development
EOQ Personnel Registration Scheme Laboratory Quality Assurance Manager & RULES & HANDBOOK Prepared by: Dr. Eugenia Soboleva, Quality Austria In accordance with the working group on EOQ product development
Pre Audit Transition Gap Analysis QMS and EMS
 Pre Audit Transition Gap Analysis QMS and EMS Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 and ISO 14001:2015 standards
Pre Audit Transition Gap Analysis QMS and EMS Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 and ISO 14001:2015 standards
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT
 INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
Correlation matrices between ISO 9001:2008 and ISO 9001:2015
 Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 19011 Second edition 2011-11-15 Guidelines for auditing management systems Lignes directrices pour l audit des systèmes de management Reference number ISO 19011:2011(E) ISO 2011
INTERNATIONAL STANDARD ISO 19011 Second edition 2011-11-15 Guidelines for auditing management systems Lignes directrices pour l audit des systèmes de management Reference number ISO 19011:2011(E) ISO 2011
Making the Transition to ISO 14001:2015 ISO EMS Support Tools
 Making the Transition to ISO 14001:2015 ISO EMS Support Tools Friday 15 th September 2017 12:30-13:30 BST (GMT +1) For more information, view the SC1 website: https://committee.iso.org/home/tc207sc1 Speakers
Making the Transition to ISO 14001:2015 ISO EMS Support Tools Friday 15 th September 2017 12:30-13:30 BST (GMT +1) For more information, view the SC1 website: https://committee.iso.org/home/tc207sc1 Speakers
ISO & ISO TRAINING DAY 4 : Certifying ISO 37001
 ISO 19600 & ISO 37001 TRAINING DAY 4 : Certifying ISO 37001 2017 SLIDE 1 DAY 4 Program Part 1 : Audit rules 1. Audit principles 2. Types of findings Part 2 : Audit process 3. The steps of an audit 4. Audit
ISO 19600 & ISO 37001 TRAINING DAY 4 : Certifying ISO 37001 2017 SLIDE 1 DAY 4 Program Part 1 : Audit rules 1. Audit principles 2. Types of findings Part 2 : Audit process 3. The steps of an audit 4. Audit
ISO Monitoring and Measurement Nonconformance and Corrective and Preventative Action
 ISO 14001 4.5.1 Monitoring and Measurement 4.5.2 Nonconformance and Corrective and Preventative Action 4.5.1 Monitoring and Measurement The organization shall establish and maintain documented procedures
ISO 14001 4.5.1 Monitoring and Measurement 4.5.2 Nonconformance and Corrective and Preventative Action 4.5.1 Monitoring and Measurement The organization shall establish and maintain documented procedures
Technical Services Document #: TS-0007 Internal Audit Procedure Version #: 01
 1. Purpose The purpose of this procedure is to define the process used to manage the Internal Audits of the Quality Management System for Technical Services. 2. Scope This procedure applies to all Internal
1. Purpose The purpose of this procedure is to define the process used to manage the Internal Audits of the Quality Management System for Technical Services. 2. Scope This procedure applies to all Internal
Process Mapping and Process- Based Internal Audits
 Process Mapping and Process- Based Internal Audits Presented by Shannon Craddock of Perry Johnson Registrars, Inc. February 5, 2014 Today s Topics Why Are We Doing This? Process Terminology Process Mapping
Process Mapping and Process- Based Internal Audits Presented by Shannon Craddock of Perry Johnson Registrars, Inc. February 5, 2014 Today s Topics Why Are We Doing This? Process Terminology Process Mapping
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru
 FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
FDIS ISO Overview of Changes
 FDIS ISO 45001 Overview of Changes Austin Matthews EHS Assistant Program Manager Welcome from PJR Headquarters: 755 W. Big Beaver Rd, Suite 1340 Troy, MI 48084 Phone: 1-800-800-7910 Introduction of speaker
FDIS ISO 45001 Overview of Changes Austin Matthews EHS Assistant Program Manager Welcome from PJR Headquarters: 755 W. Big Beaver Rd, Suite 1340 Troy, MI 48084 Phone: 1-800-800-7910 Introduction of speaker
Procedures: QP 4 through QP 8, QP 16, QP 17, and QP 19
 SRI Quality System Registrar Procedures: QP 4 through QP 8, QP 16, QP 17, and QP 19 Booklet Version 171122 Revision Date QP 4.0 Pre-Audit Registration Procedures 15 11/07/15 QP 5.0 On-Site Audit Procedure
SRI Quality System Registrar Procedures: QP 4 through QP 8, QP 16, QP 17, and QP 19 Booklet Version 171122 Revision Date QP 4.0 Pre-Audit Registration Procedures 15 11/07/15 QP 5.0 On-Site Audit Procedure
Procedure 14 Internal Audits
 Procedure 14 Internal Audits Table of Contents 1 Introduction... 2 2 Audit Planning... 2 2.1 Head Office... 2 2.2 Critical Locations... 3 3 Conducting the Audit... 3 4 Non-conformances... 3 5 Client file
Procedure 14 Internal Audits Table of Contents 1 Introduction... 2 2 Audit Planning... 2 2.1 Head Office... 2 2.2 Critical Locations... 3 3 Conducting the Audit... 3 4 Non-conformances... 3 5 Client file
PG&E Gas Operations. Gas Safety Excellence API 1173
 PG&E Gas Operations Gas Safety Excellence API 1173 Gas Safety Excellence Framework PG&E Confidential 2 Our Strategic Drivers Why did PG&E Gas Operations launch and sustain Gas Safety Excellence? Provide
PG&E Gas Operations Gas Safety Excellence API 1173 Gas Safety Excellence Framework PG&E Confidential 2 Our Strategic Drivers Why did PG&E Gas Operations launch and sustain Gas Safety Excellence? Provide
Internal Audit Quality Analysis Evaluation against the Standards International Standards for the Professional Practice of Internal Auditing (2017)
 Internal Audit Quality Analysis Evaluation against the Standards International Standards for the Professional Practice of Internal Auditing (2017) Assessor 1: Assessor 2: Date: Date: Legend: Generally
Internal Audit Quality Analysis Evaluation against the Standards International Standards for the Professional Practice of Internal Auditing (2017) Assessor 1: Assessor 2: Date: Date: Legend: Generally
Continual Improvement
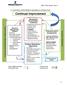 15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
CONDUCT MODERATIONS FOR DUMMIES
 CONDUCT MODERATIONS FOR DUMMIES TWO TYPES OF MODERATION INTERNAL (For the Training Provider) Internal moderation ensures that assessments conducted in a single learning provider, are consistent, accurate
CONDUCT MODERATIONS FOR DUMMIES TWO TYPES OF MODERATION INTERNAL (For the Training Provider) Internal moderation ensures that assessments conducted in a single learning provider, are consistent, accurate
Quality Management System Guidance. ISO 9001:2015 Clause-by-clause Interpretation
 Quality Management System Guidance ISO 9001:2015 Clause-by-clause Interpretation Table of Contents 1 INTRODUCTION... 4 1.1 IMPLEMENTATION & DEVELOPMENT... 5 1.2 MANAGING THE CHANGE... 5 1.3 TOP MANAGEMENT
Quality Management System Guidance ISO 9001:2015 Clause-by-clause Interpretation Table of Contents 1 INTRODUCTION... 4 1.1 IMPLEMENTATION & DEVELOPMENT... 5 1.2 MANAGING THE CHANGE... 5 1.3 TOP MANAGEMENT
QUICK START GUIDE. for FSSC Implementation. Copyright 2016 Vinca, LLC.
 QUICK START GUIDE for FSSC 22000 Implementation www.22000-tools.com Copyright 2016 Vinca, LLC. CONTENTS WHAT IS FSSC 22000? BENEFITS OF FSSC22000 IMPLEMENTING FSSC 22000 AND PREPARING FOR CERTIFICATION
QUICK START GUIDE for FSSC 22000 Implementation www.22000-tools.com Copyright 2016 Vinca, LLC. CONTENTS WHAT IS FSSC 22000? BENEFITS OF FSSC22000 IMPLEMENTING FSSC 22000 AND PREPARING FOR CERTIFICATION
ISO 14001:2015. EMS Manual.
 www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This EMS manual is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This EMS manual is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
Desk Audit of. Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT
 Desk Audit of Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT-90-5001-02.1 Reviewed by: Element Requirements Applicable 1. Is a quality policy defined
Desk Audit of Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT-90-5001-02.1 Reviewed by: Element Requirements Applicable 1. Is a quality policy defined
QUICK START GUIDE. SQF Implementation. for.
 QUICK START GUIDE for SQF Implementation www.22000-tools.com CONTENTS WHAT IS SQF? BENEFITS OF SQF SQF LEVELS SQF MODULES IMPLEMENTING SQF AND PREPARING FOR CERTIFICATION ASSIGN YOUR PEOPLE RESOURCES
QUICK START GUIDE for SQF Implementation www.22000-tools.com CONTENTS WHAT IS SQF? BENEFITS OF SQF SQF LEVELS SQF MODULES IMPLEMENTING SQF AND PREPARING FOR CERTIFICATION ASSIGN YOUR PEOPLE RESOURCES
Software Auditor Skills Training Course Offered by The Westfall Team
 Software Auditor Skills Training Course Software Auditor Skills is a 2-day course that is a subset of the Software Auditing course. This course is designed to provide a knowledge base and practical skills
Software Auditor Skills Training Course Software Auditor Skills is a 2-day course that is a subset of the Software Auditing course. This course is designed to provide a knowledge base and practical skills
HOW TO CONDUCT THE INTERNAL AUDITS EFFECTIVELY
 HOW TO CONDUCT THE INTERNAL AUDITS EFFECTIVELY As per ISO 9001 2015 & ISO 19011 PMI, PMP, PMBOK and the PMI Registered Education Provider logo are registered marks of the Project Management Institute,
HOW TO CONDUCT THE INTERNAL AUDITS EFFECTIVELY As per ISO 9001 2015 & ISO 19011 PMI, PMP, PMBOK and the PMI Registered Education Provider logo are registered marks of the Project Management Institute,
ISO 9001:2015 ISO 14001:2015
 ISO 9001:2015 ISO 14001:2015 Integrated Management Systems Manual [Preview] [Company Name] ADDRESS Phone: Phone: Fax: Fax: The holder of this manual is cautioned that the information contained herein must
ISO 9001:2015 ISO 14001:2015 Integrated Management Systems Manual [Preview] [Company Name] ADDRESS Phone: Phone: Fax: Fax: The holder of this manual is cautioned that the information contained herein must
Energy Management System (EnMS) White Paper
 Energy Management System (EnMS) White Paper ISO 50001 / BS EN 16001:2009 Lakshy Management Consultant Pvt Ltd www.lakshy.com aiming excellence WHAT ISISO 50001 / BS EN 16001:2009STANDARD? Standard for
Energy Management System (EnMS) White Paper ISO 50001 / BS EN 16001:2009 Lakshy Management Consultant Pvt Ltd www.lakshy.com aiming excellence WHAT ISISO 50001 / BS EN 16001:2009STANDARD? Standard for
The National Heavy Vehicle Accreditation Scheme (NHVAS)
 The National Heavy Vehicle Accreditation Scheme (NHVAS) Mass Management Guide April 2009 0 of 21 NHVAS Maintenance Management Guide April 2009 The National Heavy Vehicle Accreditation Scheme (NHVAS) Mass
The National Heavy Vehicle Accreditation Scheme (NHVAS) Mass Management Guide April 2009 0 of 21 NHVAS Maintenance Management Guide April 2009 The National Heavy Vehicle Accreditation Scheme (NHVAS) Mass
Pre Audit Transition Gap Analysis EMS (ISO 14001:2015 Only)
 Pre Audit Transition Gap Analysis EMS (ISO 14001:2015 Only) Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 14001:2015 standards
Pre Audit Transition Gap Analysis EMS (ISO 14001:2015 Only) Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 14001:2015 standards
Vendor Qualification Survey
 1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
ISO 9001:2015 Readiness Review
 ISO 9001:2015 Readiness Review Company Name Address Certification No. Contact Name Job Title Telephone Email BSI is committed to ensuring a smooth assessment for all clients wishing to certify to ISO 9001:2015,
ISO 9001:2015 Readiness Review Company Name Address Certification No. Contact Name Job Title Telephone Email BSI is committed to ensuring a smooth assessment for all clients wishing to certify to ISO 9001:2015,
RBA Auditor Guidebook
 2017 RBA Auditor Guidebook Page1 Table of Contents 1. Auditors*... 3 Auditor Responsibilities... 3 Audit Team Members... 4 2. Audit Firm Information... 4 3. Auditor Competencies... 5 General Competencies
2017 RBA Auditor Guidebook Page1 Table of Contents 1. Auditors*... 3 Auditor Responsibilities... 3 Audit Team Members... 4 2. Audit Firm Information... 4 3. Auditor Competencies... 5 General Competencies
BROOKHAVEN NATIONAL LABORATORY SBMS Interim Procedure
 BROOKHAVEN NATIONAL LABORATORY SBMS Interim Procedure Interim Procedure Number: 2004-18001-005 Revision: 12 on 1-26-07 Title: 18001 Audit Checklist Point of Contact: Pat Williams Management System: Occupational
BROOKHAVEN NATIONAL LABORATORY SBMS Interim Procedure Interim Procedure Number: 2004-18001-005 Revision: 12 on 1-26-07 Title: 18001 Audit Checklist Point of Contact: Pat Williams Management System: Occupational
ISO 14001:2004 Environmental Management System -The Gap Analysis Checklist
 ISO 14001:2004 Environmental Management System -The Gap Analysis Checklist GUIDELINES FOR USE OF THE GAP ANALYSIS CHECKLIST This gap analysis checklist is prepared for use in evaluating your Environmental
ISO 14001:2004 Environmental Management System -The Gap Analysis Checklist GUIDELINES FOR USE OF THE GAP ANALYSIS CHECKLIST This gap analysis checklist is prepared for use in evaluating your Environmental
Clause-byclause. Interpretation. Transitioning to ISO 9001:2015
 We re committed to helping you and your organization understand the updated requirements. This guidance document identifies the steps you should take to achieve compliance to ISO 9001:2015, and more importantly;
We re committed to helping you and your organization understand the updated requirements. This guidance document identifies the steps you should take to achieve compliance to ISO 9001:2015, and more importantly;
This procedure is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without prior written consent.
 www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This procedure is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This procedure is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
INTRODUCTION TO ISO 14001
 INTRODUCTION TO ISO 14001 An environmental management system, such as ISO 14001, is a powerful tool that can be utilized by an organization. The obvious beneficiaries are those organizations that have
INTRODUCTION TO ISO 14001 An environmental management system, such as ISO 14001, is a powerful tool that can be utilized by an organization. The obvious beneficiaries are those organizations that have
ISO 9001:2008 Quality Management System QMS Manual
 2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
Supplier Audit Procedure /20/17
 1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
Performing Audits. Def An assessment of the methods and procedures used. Philip Randall
 Performing Audits Def An assessment of the methods and procedures used Philip Randall pcubed@mweb.co.za Master Control Much of the material has been taken from information available on the internet and/or
Performing Audits Def An assessment of the methods and procedures used Philip Randall pcubed@mweb.co.za Master Control Much of the material has been taken from information available on the internet and/or
10/5/2016. Quality Assessment Review. Agenda. What s the purpose of a QAR? Internal Audit Manager Training October 3-4, 2016
 Quality Assessment Review Internal Audit Manager Training October 3-4, 2016 Lori Clark CIGA, CCEP, CGAP Compliance & Audit Specialist State University System of Florida Agenda What s the purpose of a QAR?
Quality Assessment Review Internal Audit Manager Training October 3-4, 2016 Lori Clark CIGA, CCEP, CGAP Compliance & Audit Specialist State University System of Florida Agenda What s the purpose of a QAR?
DECISION TO ACCREDIT
 DECISION TO ACCREDIT Determine the requirements of the regulator, legislation and customer s needs prior to deciding on the standard to implement. Process is based on the following: Top management s decision
DECISION TO ACCREDIT Determine the requirements of the regulator, legislation and customer s needs prior to deciding on the standard to implement. Process is based on the following: Top management s decision
ASQ Madison, WI Section Using your ISO 9001:2008 QMS to Plan for the 2015 Changes
 ASQ Madison, WI Section 1217 Using your ISO 9001:2008 QMS to Plan for the 2015 Changes SESSION AGENDA High Level Review of Requirements Historical Perspective Basic Requirements Change It s a Process Inputs,
ASQ Madison, WI Section 1217 Using your ISO 9001:2008 QMS to Plan for the 2015 Changes SESSION AGENDA High Level Review of Requirements Historical Perspective Basic Requirements Change It s a Process Inputs,
Harmonization day 2015 FSSC audit reporting
 Harmonization day 2015 FSSC 22000 audit reporting Today s topics 1. Basics audit reporting 2. Definition audit report 3. Audit report requirements 4. Conclusions 1. Basics audit reporting Starting point
Harmonization day 2015 FSSC 22000 audit reporting Today s topics 1. Basics audit reporting 2. Definition audit report 3. Audit report requirements 4. Conclusions 1. Basics audit reporting Starting point
ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015. Joan Richter ASQ Baltimore (Section 502) June 2016
 ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015 Joan Richter ASQ Baltimore (Section 502) June 2016 HISTORY OF ISO 9001 Standard (28 Years of ISO) 1987 Procedures
ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015 Joan Richter ASQ Baltimore (Section 502) June 2016 HISTORY OF ISO 9001 Standard (28 Years of ISO) 1987 Procedures
American Association for Laboratory Accreditation
 Page 1 of 18 The following pages present the requirements from R104 General s: Accreditation of Field Testing and Field Calibration Laboratories in a checklist format. The policies, procedures and activities
Page 1 of 18 The following pages present the requirements from R104 General s: Accreditation of Field Testing and Field Calibration Laboratories in a checklist format. The policies, procedures and activities
HACCPEUROPA PUBLICATIONS ISO 22000:2005 FOOD SAFETY QUALITY MANUAL. ISO 22000:2005 Quality Manual
 HACCPEUROPA PUBLICATIONS ISO 22000:2005 FOOD SAFETY QUALITY MANUAL ISO 22000:2005 Quality Manual QUALITY MANUAL ISO 22000:2005 Food Safety Management HACCPEuropa Publications 2012 Table of Contents Introduction...
HACCPEUROPA PUBLICATIONS ISO 22000:2005 FOOD SAFETY QUALITY MANUAL ISO 22000:2005 Quality Manual QUALITY MANUAL ISO 22000:2005 Food Safety Management HACCPEuropa Publications 2012 Table of Contents Introduction...
QUALITY SYSTEM PROCEDURES
 1/5 1.0 Purpose In accordance to ISO/TS 16949, American Mitsuba Corporation is responsible for the evaluation and selection of suppliers on their ability to supply products in accordance to customer requirements,
1/5 1.0 Purpose In accordance to ISO/TS 16949, American Mitsuba Corporation is responsible for the evaluation and selection of suppliers on their ability to supply products in accordance to customer requirements,
SQF 2000 Guidance. Guidance for Food Sector Category 4 Fresh Produce Pack House Operations. 1st Edition
 SQF 2000 for Food Sector Category 4 Fresh Produce Pack House Operations 1st Edition SEPTEMBER 2010 Safe Quality Food Institute 2345 Crystal Drive, Suite 800 Arlington, VA 22202 USA 202-220-0635 www.sqfi.com
SQF 2000 for Food Sector Category 4 Fresh Produce Pack House Operations 1st Edition SEPTEMBER 2010 Safe Quality Food Institute 2345 Crystal Drive, Suite 800 Arlington, VA 22202 USA 202-220-0635 www.sqfi.com
exit Introduction back home next
 begin Introduction The learning objectives of this training are for students to: Gain a general understanding of environmental management systems (EMS) Understand the Air Force s environmental management
begin Introduction The learning objectives of this training are for students to: Gain a general understanding of environmental management systems (EMS) Understand the Air Force s environmental management
Moving from ISO 14001:2004 to ISO 14001:2015 Transition Guide
 ISO Revisions Final Standard Moving from ISO 14001:2004 to ISO 14001:2015 Transition Guide ISO 14001 - Environmental Management System - Transition Guide Successful businesses understand that it is the
ISO Revisions Final Standard Moving from ISO 14001:2004 to ISO 14001:2015 Transition Guide ISO 14001 - Environmental Management System - Transition Guide Successful businesses understand that it is the
2017 Archaeology Audit Program Procedure Manual. April 2017
 2017 Archaeology Audit Program Procedure Manual April 2017 Table of Contents Contents Table of Contents... 2 1.0 Introduction and Scope... 3 2.0 Audit Objectives... 3 3.0 Audit Procedures... 4 3.1 Audit
2017 Archaeology Audit Program Procedure Manual April 2017 Table of Contents Contents Table of Contents... 2 1.0 Introduction and Scope... 3 2.0 Audit Objectives... 3 3.0 Audit Procedures... 4 3.1 Audit
Correlation Matrix & Change Summary
 The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
3410N Assurance engagements relating to sustainability reports
 3410N Assurance engagements relating to sustainability reports Royal NIVRA 3410N ASSURANCE ENGAGEMENTS RELATING TO SUSTAINABILITY REPORTS Introduction Scope of this Standard ( T1 and T2) 1. This Standard
3410N Assurance engagements relating to sustainability reports Royal NIVRA 3410N ASSURANCE ENGAGEMENTS RELATING TO SUSTAINABILITY REPORTS Introduction Scope of this Standard ( T1 and T2) 1. This Standard
Supplier Audit Procedure /2/17
 1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
INTERNAL AUDIT IN CORPORATE GOVERNANCE
 INTERNAL AUDIT IN CORPORATE GOVERNANCE PhD. Candidate Felicia Gabriela UNGUREANU Academy of Economic Studies, Bucharest Abstract Internal Audit, compared with verification of transactions and compliance
INTERNAL AUDIT IN CORPORATE GOVERNANCE PhD. Candidate Felicia Gabriela UNGUREANU Academy of Economic Studies, Bucharest Abstract Internal Audit, compared with verification of transactions and compliance
Quality management systems
 L E C T U R E 9 Quality management systems LECTURE 9 - OVERVIEW Quality management system based on ISO 9000 WHAT IS QMS (QUALITY MANAGEMENT SYSTEM) Goal: Meet customer needs Quality management system includes
L E C T U R E 9 Quality management systems LECTURE 9 - OVERVIEW Quality management system based on ISO 9000 WHAT IS QMS (QUALITY MANAGEMENT SYSTEM) Goal: Meet customer needs Quality management system includes
Quality Manual ISO 9001:2000
 Quality Manual ISO 9001:2000 Page 2 of 23 TABLE OF CONTENTS COVER PAGE...1 TABLE OF CONTENTS...2 SIGNATURES...3 QUALITY POLICY...4 INTRODUCTION...5 CORPORATE PROFILE...9 4.O QUALITY MANAGEMENT SYSTEM...10
Quality Manual ISO 9001:2000 Page 2 of 23 TABLE OF CONTENTS COVER PAGE...1 TABLE OF CONTENTS...2 SIGNATURES...3 QUALITY POLICY...4 INTRODUCTION...5 CORPORATE PROFILE...9 4.O QUALITY MANAGEMENT SYSTEM...10
INTLCO Training Guide. We Train Professionals TM INTLCO All Rights Reserved
 INTLCO Training Guide We Train Professionals TM 2017 INTLCO All Rights Reserved Welcome Contents At INTLCO, we believe that the purpose of training is to bring about change and improvement, whether that
INTLCO Training Guide We Train Professionals TM 2017 INTLCO All Rights Reserved Welcome Contents At INTLCO, we believe that the purpose of training is to bring about change and improvement, whether that
 DQS AUDITOR Auditing software with meaningfull reports http://www.dqs.co.za/dqsauditorsoftware Flexible Solutions for your internal and second party Audits Download Demo Easy to Install Browse to the installation
DQS AUDITOR Auditing software with meaningfull reports http://www.dqs.co.za/dqsauditorsoftware Flexible Solutions for your internal and second party Audits Download Demo Easy to Install Browse to the installation
On the Path to ISO Accreditation
 On the Path to ISO 17025 Accreditation What We Wish We d Known Before We Started And Some Definitions: Language of ISO 17025 Version: 2013-08-29 1 Susan Humphries, QA Officer Bureau of Food Laboratories,
On the Path to ISO 17025 Accreditation What We Wish We d Known Before We Started And Some Definitions: Language of ISO 17025 Version: 2013-08-29 1 Susan Humphries, QA Officer Bureau of Food Laboratories,
Pre Audit Transition Gap Analysis QMS (ISO 9001 Only)
 Pre Audit Transition Gap Analysis QMS (ISO 9001 Only) Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 standard and
Pre Audit Transition Gap Analysis QMS (ISO 9001 Only) Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 standard and
Leveraging ISO s Quality Management System to Achieve 24/7 Inspection Readiness. Randy Querry Accreditation Manager Clinical
 Leveraging ISO 15189 s Quality Management System to Achieve 24/7 Inspection Readiness Randy Querry Accreditation Manager Clinical Goals Provide brief background on A2LA Differentiate between CLIA and ISO
Leveraging ISO 15189 s Quality Management System to Achieve 24/7 Inspection Readiness Randy Querry Accreditation Manager Clinical Goals Provide brief background on A2LA Differentiate between CLIA and ISO
IS-BAO Internal Audit Manual
 IS-BAO Internal Audit Manual Suite 16.33, 999 University Street Montreal, Quebec, H3C 5J9, Canada Tel 1-514-954-6198 Fax: 1-514-954-6161 www.ibac.org IS-BAO Internal Audit Manual Copyright All rights reserved
IS-BAO Internal Audit Manual Suite 16.33, 999 University Street Montreal, Quebec, H3C 5J9, Canada Tel 1-514-954-6198 Fax: 1-514-954-6161 www.ibac.org IS-BAO Internal Audit Manual Copyright All rights reserved
Welding Performance Qualification Test Administrators GUIDE FOR APPLICANTS
 BRITISH COLUMBIA SAFETY AUTHORITY Welding Performance Qualification Test Administrators GUIDE FOR APPLICANTS Prepared by: Ed Hurd P.Eng. Provincial Safety Manager, Boilers Date: March 2, 2012 Controlled
BRITISH COLUMBIA SAFETY AUTHORITY Welding Performance Qualification Test Administrators GUIDE FOR APPLICANTS Prepared by: Ed Hurd P.Eng. Provincial Safety Manager, Boilers Date: March 2, 2012 Controlled
25 D.L. Martin Drive Mercersburg, PA (717)
 EMS MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 13 January 2017 Kip Heefner Environmental Management Representative Daniel J. Fisher President & CEO D.L.
EMS MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 13 January 2017 Kip Heefner Environmental Management Representative Daniel J. Fisher President & CEO D.L.
Quality Management Evaluation & Audit Policy
 Title Quality Management Evaluation & Audit policy Document ID Director Mark Reynolds Status Final Owner Neil McCrirrick Version 1.1 Author Mark Reynolds Version Date 07/11/2011 Quality Management Evaluation
Title Quality Management Evaluation & Audit policy Document ID Director Mark Reynolds Status Final Owner Neil McCrirrick Version 1.1 Author Mark Reynolds Version Date 07/11/2011 Quality Management Evaluation
INTEGRATED MANAGEMENT SYSTEM INTERNAL AUDITS
 1 of 6 uncontrolled. This copy is only valid only at the time of printing. Unauthorized reproduction of this document is prohibited. 2 of 6 CONTENTS 1.0 PURPOSE... 3 2.0 SCOPE... 3 3.0 RESPONSIBILITY...
1 of 6 uncontrolled. This copy is only valid only at the time of printing. Unauthorized reproduction of this document is prohibited. 2 of 6 CONTENTS 1.0 PURPOSE... 3 2.0 SCOPE... 3 3.0 RESPONSIBILITY...
Virginia Department of Environmental Quality EMS Manual
 The Virginia Department of Environmental Quality (DEQ) has implemented an environmental management system (EMS) based on DEQ E 3 /ISO 14001, the International and American National Environmental Management
The Virginia Department of Environmental Quality (DEQ) has implemented an environmental management system (EMS) based on DEQ E 3 /ISO 14001, the International and American National Environmental Management
"Welder Certification"
 "Welder Certification" The purpose of this presentation is to introduce you to the various aspects of welder certification. This is not intended to cover all of the requirements for certification but only
"Welder Certification" The purpose of this presentation is to introduce you to the various aspects of welder certification. This is not intended to cover all of the requirements for certification but only
DALAIR LIMITED QUALITY MANUAL
 DALAIR LIMITED QUALITY MANUAL BS EN ISO 001:2015 Issue No.: Issue Date: 5//1 Page 2 of In consideration of BS EN ISO 001:2015 Dalair Limited by way of this document and its management systems seek to demonstrate
DALAIR LIMITED QUALITY MANUAL BS EN ISO 001:2015 Issue No.: Issue Date: 5//1 Page 2 of In consideration of BS EN ISO 001:2015 Dalair Limited by way of this document and its management systems seek to demonstrate
GUIDEBOOK CODE OF CONDUCT MANAGEMENT SYSTEMS
 GUIDEBOOK CODE OF CONDUCT MANAGEMENT SYSTEMS 2005 Levi Strauss & Co. Page 1 of 57 Table of content SECTION I: INTRODUCTION... 3 1. Introduction... 3 2. How to use this Guidebook... 3 SECTION II: THE MANAGEMENT
GUIDEBOOK CODE OF CONDUCT MANAGEMENT SYSTEMS 2005 Levi Strauss & Co. Page 1 of 57 Table of content SECTION I: INTRODUCTION... 3 1. Introduction... 3 2. How to use this Guidebook... 3 SECTION II: THE MANAGEMENT
Gap analysis for transition from OHSAS to ISO Clauses of ISO Clauses of OHSAS Evidence required
 4 Context of the organisation 4.1 Understanding your organization and its context New requirement! Have the OH&S-related internal and external factors and conditions been identified that could affect,
4 Context of the organisation 4.1 Understanding your organization and its context New requirement! Have the OH&S-related internal and external factors and conditions been identified that could affect,
ASIS Standards: Auditing for. Improvement. Security, Risk and Resilience. Auditing. Value Added. Auditing
 Opportunities for Improvement ANSI/ASIS SPC.1 2009 Planning an Audit Value Added Auditing Evaluating Effectiveness Implementing a Successful Audit ASIS Standards: Auditing for Improvement Security, Risk
Opportunities for Improvement ANSI/ASIS SPC.1 2009 Planning an Audit Value Added Auditing Evaluating Effectiveness Implementing a Successful Audit ASIS Standards: Auditing for Improvement Security, Risk
General requirements for the competence of testing and calibration laboratories. In this presentation:
 General requirements for the competence of testing and calibration laboratories ISO/IEC 17025:2017 In this presentation: Explanation New requirement Interpretation / Examples / Questions Agenda Welcome
General requirements for the competence of testing and calibration laboratories ISO/IEC 17025:2017 In this presentation: Explanation New requirement Interpretation / Examples / Questions Agenda Welcome
ISO/IEC INTERNATIONAL STANDARD. Information technology Security techniques Guidelines for information security management systems auditing
 INTERNATIONAL STANDARD ISO/IEC 27007 First edition 2011-11-15 Information technology Security techniques Guidelines for information security management systems auditing Technologies de l'information Techniques
INTERNATIONAL STANDARD ISO/IEC 27007 First edition 2011-11-15 Information technology Security techniques Guidelines for information security management systems auditing Technologies de l'information Techniques
ISCC 204 AUDIT REQUIREMENTS AND RISK MANAGEMENT. Version 3.0
 ISCC 204 AUDIT REQUIREMENTS AND RISK MANAGEMENT Version 3.0 II Copyright notice 2016 ISCC System GmbH This ISCC document is protected by copyright. It is freely available from the ISCC website or upon
ISCC 204 AUDIT REQUIREMENTS AND RISK MANAGEMENT Version 3.0 II Copyright notice 2016 ISCC System GmbH This ISCC document is protected by copyright. It is freely available from the ISCC website or upon
ISO 14001:2015 Updates and Key Themes
 ISO 14001:2015 Updates and Key Themes November 10, 2016 Alex Lowry Agenda Overview of changes in ISO 14001:2015 standard Discussion of key ISO 14001:2015 themes Context of the organization Internal and
ISO 14001:2015 Updates and Key Themes November 10, 2016 Alex Lowry Agenda Overview of changes in ISO 14001:2015 standard Discussion of key ISO 14001:2015 themes Context of the organization Internal and
Quality Management System
 Quality Management System ZYQ Testing Laboratory 11111 Testing Avenue Testing Town, Alaska 22222 (555) 555-5555 Date Prepared: January 19, 2009 1 Please note that this Quality Management System (QM) was
Quality Management System ZYQ Testing Laboratory 11111 Testing Avenue Testing Town, Alaska 22222 (555) 555-5555 Date Prepared: January 19, 2009 1 Please note that this Quality Management System (QM) was
ISO/TC 176/SC 2 Document N1224, July 2014
 ISO/TC 176/SC 2 Document N1224, July 2014 Correlation matrices between ISO 9001:2008 and ISO/DIS 9001 This document gives correlation matrices from ISO 9001:2008 to the current Draft International Standard
ISO/TC 176/SC 2 Document N1224, July 2014 Correlation matrices between ISO 9001:2008 and ISO/DIS 9001 This document gives correlation matrices from ISO 9001:2008 to the current Draft International Standard
9110 Correlation matrices
 9110 Correlation matrices 9110:2016 to 9110:2012 9110:2012 to 9110:2016 This document provides correlation matrices from 9110:2016 to 9110:2012 and 9110:2012 to 9110:2016. This document can be used to
9110 Correlation matrices 9110:2016 to 9110:2012 9110:2012 to 9110:2016 This document provides correlation matrices from 9110:2016 to 9110:2012 and 9110:2012 to 9110:2016. This document can be used to
MR Credits 2.1 and 2.2 for both NC and CI: (Construction Waste Management)
 Siena for LEED CI and NC Materials & Resources (MR): MR Credits 2.1 and 2.2 for both NC and CI: (Construction Waste Management) In this credit, you have to remove construction or demolition debris and
Siena for LEED CI and NC Materials & Resources (MR): MR Credits 2.1 and 2.2 for both NC and CI: (Construction Waste Management) In this credit, you have to remove construction or demolition debris and
EHQMS Manual & Policy Document
 Quality management input comprises the standard requirements from ISO 9001:2015 which are strategically deployed by our organization to achieve customer satisfaction through process control. Environmental
Quality management input comprises the standard requirements from ISO 9001:2015 which are strategically deployed by our organization to achieve customer satisfaction through process control. Environmental
Quality and Medical Devices: ISO 13485:2003
 Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
To support organizations in making a successful transition to IATF Quality Partner has developed several documents to help:
 IATF 16949: 2016 has arrived! Hurry, time to make the transition is short Quality Partner Newsletter November 2016 For More Information Visit www.qualitypartner.co.uk Author: Paul Hardiman Welcome to the
IATF 16949: 2016 has arrived! Hurry, time to make the transition is short Quality Partner Newsletter November 2016 For More Information Visit www.qualitypartner.co.uk Author: Paul Hardiman Welcome to the
