Redistribution of the UK centrally authorised product portfolio
|
|
|
- Elfreda Newman
- 5 years ago
- Views:
Transcription
1 11 April 2018 EMA/17176/2018 Redistribution of the UK centrally authorised product portfolio EMA working groups on committees operational preparedness 1. Executive Summary The United Kingdom submitted on 29 March 2017 the notification of its intention to withdraw from the Union pursuant to Article 50 of the Treaty on European Union. This means that unless a ratified withdrawal agreement establishes another date, all Union primary and secondary law ceases to apply to the United Kingdom from 30 March 2019, 00:00h (CET). The United Kingdom will then become a 'third country'. As a consequence, the MHRA and VMD will no longer be able to engage in centralised regulatory procedures, as (Co)-Rapporteurs, which are expected to finalise after 30 March The redistribution of the UK centrally authorised product (CAP) portfolio involves the reallocation of UK rapporteurs and co-rapporteurs from EMA s Committee for Medicinal Products for Human Use (CHMP), Pharmacovigilance Risk Assessment Committee (PRAC), Committee for Advanced Therapies (CAT) and Committee for Medicinal Products for Veterinary Use (CVMP). The redistribution follows a multifaceted approach and takes into account the diverse expertise in the European medicines regulatory network and the workload associated with each medicine. It allows Member States to participate in EMA activities according to their individual capacity, as indicated in the feedback received from the capacity-building surveys distributed to the network. The methodology is flexible, easy to implement, and can be applied equally to human and veterinary medicines. The methodology involves redistribution based on current expertise with a class of medicines. It also foresees building on existing knowledge, for example, by allocating medicines to the current corapporteur for a particular product, or to the peer reviewer involved in the initial marketing authorisation application. It takes into account the legal basis of the product, for example, generic medicines will be allocated to newer National Competent Authorities (NCAs) who have indicated in the survey that they would like to increase their involvement with such medicines. Clusters of products with the same international non-proprietary name (INN) and/or belonging to the same marketing authorisation holder (MAH) will be allocated to a single rapporteur in order to facilitate review of post-authorisation procedures and ultimately improve efficiency within the Network. 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44 (0) Facsimile +44 (0) Send a question via our website An agency of the European Union European Medicines Agency, Reproduction is authorised provided the source is acknowledged.
2 The redistribution of the UK centrally authorised product portfolio started before 30 March 2019 to ensure timely preparedness of the European medicines regulatory Network. In addition, EMA will facilitate the transfer of knowledge from the UK to the new (Co)-Rapporteurs once marketing authorisation holders have been informed of the changes. The new (Co)-Rapporteurs will only take full responsibility for the re-allocated products as of 30 March 2019, when the UK withdraws from the European Union and becomes a third country. 2. Background The United Kingdom submitted on 29 March 2017 the notification of its intention to withdraw from the Union pursuant to Article 50 of the Treaty on European Union. This means that unless a ratified withdrawal agreement establishes another date, all Union primary and secondary law ceases to apply to the United Kingdom from 30 March 2019, 00:00h (CET). The United Kingdom will then become a 'third country'. As a consequence, the MHRA and VMD will no longer be able to engage in centralised regulatory procedures, as (Co)-Rapporteurs, which are expected to finalise after 30 March The EMA working groups on Committees Operational Preparedness for human and veterinary medicines were given a mandate to explore options for a robust allocation of the workload across the European medicines regulatory network. At the December 2017 Management Board meeting, the methodology for the redistribution of the work currently carried out post authorisation for CAPs by the MHRA and the VMD was endorsed. 3. General principles The general principles, adopted by the EMA Management Board, which should guide the redistribution of the UK product portfolio were to: ensure business continuity; ensure knowledge retention, either building on existing knowledge, or through knowledge transfer; allow compliance with the legally required timelines and to maintain the quality of the output; be as easy as possible to implement and, in addition, to be sustainable; strive to allow all NCAs to participate in EMA activities, as per the capacity and capability of each NCA, so as to ensure an optimised and robust allocation of the workload across the Network; The proposal for redistribution of the workload should also take into consideration the outcome of the surveys on capacity building in the Network. EMA/17176/2018 Page 2/6
3 4. Methodology - Human Medicines The redistribution of the UK centrally authorised product portfolio of human medicines comprises the reallocation of UK rapporteurs and co-rapporteurs from CHMP, PRAC and CAT. It follows a multifaceted approach and takes into consideration the results from the surveys on capacity. The methodology also takes into account the scientific expertise and knowledge within the Network and the workload associated with each medicine. A maximum number of products (ceiling) is allocated per NCA to ensure that all NCAs are allowed to participate in EMA activities as per each NCAs capacity. The ceiling is adjusted for each role (CHMP Rap, CHMP Co-Rap or PRAC Rap) based on the number of products to be allocated. UK CHMP Rapporteur The UK CHMP Rapporteurships to be allocated also include the role of PRAC Rapporteur for medicinal products that were authorised before 2012 and the PRAC Co-Rapporteurships for newer products (see figure 1). Figure 1. UK CHMP and PRAC roles to be redistributed. UK CHMP Rapporteurships for generic and hybrid medicinal products are allocated to newer MSs who have indicated in the surveys the wish to increase their involvement in these types of products. In addition, generic medicinal products are associated with a low workload in the post authorisation phase, therefore not requiring a substantial increase in resources for these NCAs. UK CHMP Rapporteurships for all other products (i.e. full applications and biosimilars) are allocated to the current Co-Rapporteur to a maximum of 10 medicinal products per NCA. The ceiling of 10 products per NCA ensures an optimised and robust allocation of the workload across the Network. Clusters of products with the same INN and belonging to the same MAH are allocated to a single rapporteur in order to facilitate review of post-authorisation procedures and ultimately improve efficiency within the network. The remaining medicinal products are allocated based on the current expertise in the Network (current expertise in the Network as CHMP Rapporteur, based on ATC code distribution) and the workload associated to each medicine in post authorisation, as CHMP Rapporteur. The methodology for the redistribution of UK CHMP Rapporteurships will ensure business continuity since it provides continuity in the lifecycle of the medicinal product, with the NCA involved in initial review also being involved in the post authorisation phase, hence facilitating knowledge transfer. In situations where the new NCA was not previously involved in the lifecycle of the product, the quality of EMA/17176/2018 Page 3/6
4 the output can be maintained due to the NCA s previous involvement in the lifecycle of similar products (i.e. same INN or same ATC code). UK CHMP Co-Rapporteur UK CHMP Co-Rapporteurships are allocated to the Peer Reviewer involved in the initial application for marketing authorisation to a maximum of 15 medicinal products per NCA. The redistribution to the Peer Reviewer will facilitate knowledge transfer. When a Peer Reviewer has not been appointed, medicinal products are allocated based on the current expertise within the network (current expertise in the Network as CHMP Co-Rapporteur, based on ATC code distribution) and the workload associated with each medicine in post authorisation (for procedures that require Co-Rapporteur s involvement). For products redistributed based on expertise, knowledge transfer will be facilitated, even if the new Co-Rapporteur was not previously involved in the lifecycle of the product, due to previous involvement with similar products (same therapeutic indication) in post authorisation as Co-Rapporteur, thus maintaining the quality of the output. Redistribution of products based on workload will lead to an increased number of CHMP Co- Rapporteurships being allocated to newer NCAs, providing them with an opportunity to build capacity. The CHMP Co-Rapporteurships that become available due to the allocation of UK CHMP Rapporteurships to the current Co-Rapporteur (i.e. vacant CHMP Co-Rapporteurships) will be allocated following the same methodology. UK PRAC Rapporteur The UK PRAC Rapporteurships to be allocated following the methodology described below comprise only medicinal products which have been approved after 2012 and for which the UK is PRAC Rapporteur only, i.e. the UK does not hold any other roles within the assessment team for a given medicinal product (see figure 2). Figure 2. UK PRAC Rapporteur roles to be redistributed. The UK PRAC Rapporteurships for generic medicinal products are automatically allocated to the PRAC Rapporteur of the reference medicinal product. For all other types of medicinal products allocation is based on clusters of products with the same INN, current expertise in the Network (current expertise in the Network at the level of the PRAC, based on ATC code distribution) and the workload associated to each medicine in post authorisation, as PRAC Rapporteur. The Peer Reviewer of the initial application for marketing authorisation was also taken into consideration when the delegation of the Peer Reviewer also had expertise (at the level of the PRAC) in the ATC of the product to be redistributed. EMA/17176/2018 Page 4/6
5 Each NCA is allocated a maximum of 5 medicinal products as PRAC Rapporteur. The methodology for the redistribution of UK PRAC Rapporteurships will facilitate the review of periodic safety update report single assessments (PSUSAs) and improves efficiency within the Network since clusters of products with the same INN are redistributed to the same PRAC Rapporteur. The redistribution of medicinal products based on the expertise in the Network (i.e. ATC code distribution) facilitates knowledge transfer due to current experience in the evaluation of similar products at the PRAC, thus maintaining the quality of the output. Inclusivity and sustainability of the Network was addressed by spreading allocation of UK PRAC Rapporteurships to NCAs which currently have the lowest number of products within a given ATC code. 5. Process for assignment of new CHMP/CAT/PRAC (Co)- Rapporteurs - Human Medicines The process for assignment of new CHMP/PRAC/CAT (Co)-Rapporteurs is based on the aforementioned general principles, using a multifaceted approach for allocation of the UK product portfolio within the Network following the agreed criteria, taking into consideration the survey results and applying a ceiling per NCA, as described above. Any medicinal products which ultimately were not retained by NCAs using this process of assignment were subject to an open bidding. 6. Methodology - Veterinary Medicines The redistribution of the UK centrally authorised product portfolio of veterinary medicines comprises the reallocation of UK Rapporteurs and Co-Rapporteurs from CVMP. It follows a multifaceted approach and takes into consideration the results from the survey on capacity. By establishing NCAs planned increased involvement in the centralised procedure it is possible to allow all NCAs to participate in EMA activities, as per the capacity of each NCA. The methodology also takes into account the scientific expertise and knowledge within the Network and balanced workload distribution. UK CVMP Rapporteur The UK CVMP Rapporteurships are primarily allocated to the current CVMP Co-Rapporteur ensuring a balanced workload distribution. Although the principle of re-allocating the UK Rapporteurship to the Co-Rapporteur is a straight forward concept, in a small number of cases additional considerations were taken into account such as the surveys results indicating no capacity of the concerned NCA to undertake new Rapporteurships and maintaining, whenever possible, the same Rapporteur for similar products (e.g. range of vaccines with same MAH). Similarly to the approach taken for human medicines, the methodology for the redistribution of the UK portfolio for veterinary medicines ensures continuity in the lifecycle approach since the NCA involved in the initial review will also be involved in post-authorisation, and therefore knowledge transfer will be facilitated. Similar products (e.g. range of vaccines) are, as far as possible, redistributed to the same Rapporteur which facilitates review of post-authorisation procedures and improves efficiency within the Network. EMA/17176/2018 Page 5/6
6 UK CVMP Co-Rapporteur UK CVMP Co-Rapporteurships are allocated to the Peer Reviewer involved in the initial application for marketing authorisation. The redistribution to the Peer Reviewer will facilitate knowledge transfer. In the absence of peer reviewers, redistribution of products is based on workload which allows less involved NCAs to participate in EMA activities as Co-Rapporteur, which will provide an opportunity to build capacity. The methodology also aims at maintaining the same Co-Rapporteur for similar products (e.g. vaccines range) which are allocated to the same Co-Rapporteur. However, knowledge transfer will be facilitated even if the new Co-Rapporteur was not previously involved in the lifecycle of the product due to previous involvement with similar products (e.g. same active substance) in post-authorisation, thus maintaining the quality of the output. The CVMP Co-Rapporteurships that become available due to the allocation of UK CVMP Rapporteurships to the current Co-Rapporteur (i.e. vacant CVMP Co-Rapporteurships) will be allocated following the same methodology. 7. Process for assignment of new CVMP (Co)-Rapporteurs - Veterinary Medicines The process for assignment of new CVMP (Co)-Rapporteurs is based on the aforementioned general principles, using a multifaceted approach for allocation of the UK product portfolio within the Network following the agreed criteria, taking into consideration the survey results and applying a ceiling per NCA, as described above. Any medicinal products which ultimately were not retained by NCAs using this process of assignment were subject to an open bidding. 8. Conclusions The methodology for the redistribution of the UK product portfolio for CHMP/CVMP/CAT/PRAC (Co)- Rapporteurships for human and veterinary products was endorsed by the Management Board at its December 2017 meeting. The methodology is flexible, easy to explain and implement, and can be applied equally to human and veterinary medicines. It involves a multifaceted approach for allocation of the UK product portfolio within the Network, which takes into consideration the results of the surveys, the expertise within the Network and workload for each medicinal product. The proposal ensures an optimised and robust allocation of the workload across the Network and guarantees efficiency within the Network, making it sustainable. In addition, it allows NCAs to participate in EMA activities, as per the capacity of each NCA. EMA/17176/2018 Page 6/6
EMA Working Groups on Committees' Operational Preparedness
 EMA Working Groups on Committees' Operational Preparedness Mandate and objectives Industry Stakeholder Meeting on Brexit Presented by Monica Dias and Anthony Humphreys An agency of the European Union Background
EMA Working Groups on Committees' Operational Preparedness Mandate and objectives Industry Stakeholder Meeting on Brexit Presented by Monica Dias and Anthony Humphreys An agency of the European Union Background
Update on EMA Brexit preparedness
 Update on EMA Brexit preparedness SME Office Info Day on Supporting innovative medicines development and early access 17 November 2017 Presented by Anthony Humphreys An agency of the European Union EMA
Update on EMA Brexit preparedness SME Office Info Day on Supporting innovative medicines development and early access 17 November 2017 Presented by Anthony Humphreys An agency of the European Union EMA
Applying for EU marketing authorisation. For medicinal products for human use. An agency of the European Union
 Applying for EU marketing authorisation For medicinal products for human use An agency of the European Union Medicines can be authorised throughout the EU by means of a single application procedure. The
Applying for EU marketing authorisation For medicinal products for human use An agency of the European Union Medicines can be authorised throughout the EU by means of a single application procedure. The
Report from EMA industry survey on Brexit preparedness
 11 July 2018 EMA/450315/2018 Corr. 1 * * Date correction on page 4. Space removed on page 5. Space removed on page 6. 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44 (0)20 3660
11 July 2018 EMA/450315/2018 Corr. 1 * * Date correction on page 4. Space removed on page 5. Space removed on page 6. 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44 (0)20 3660
Guidance on preparing for Brexit in the centralised procedure
 Guidance on preparing for Brexit in the centralised procedure SME info day: Regulatory toolbox for medicines and combined devices developers Presented by Leonor Enes on 26 October 2018 SME Office, Stakeholders
Guidance on preparing for Brexit in the centralised procedure SME info day: Regulatory toolbox for medicines and combined devices developers Presented by Leonor Enes on 26 October 2018 SME Office, Stakeholders
Marketing Authorisation Routes in the EU
 Marketing Authorisation Routes in the EU The EU medicines regulatory system and the European Medicines Agency: an introduction for international regulators and non-governmental organisations 18 September
Marketing Authorisation Routes in the EU The EU medicines regulatory system and the European Medicines Agency: an introduction for international regulators and non-governmental organisations 18 September
Monthly statistics report: November 2016
 7 December 2016 EMA/819205/2016 Information Management Division Medicinal products for human use (cumulative figures for the year to date) This document provides current information related to the volume
7 December 2016 EMA/819205/2016 Information Management Division Medicinal products for human use (cumulative figures for the year to date) This document provides current information related to the volume
Monthly statistics report: December 2017
 19 January 2018 EMA/39932/2018 Information Management Division Medicinal products for human use (cumulative figures for the year to date) This document provides current information related to the volume
19 January 2018 EMA/39932/2018 Information Management Division Medicinal products for human use (cumulative figures for the year to date) This document provides current information related to the volume
New pharmacovigilance systems and services
 New pharmacovigilance systems and services 17 September 2015, PCWP/HCPWP joint meeting Presented by Peter Arlett, Head of Pharmacovigilance department An agency of the European Union Background The new
New pharmacovigilance systems and services 17 September 2015, PCWP/HCPWP joint meeting Presented by Peter Arlett, Head of Pharmacovigilance department An agency of the European Union Background The new
Pharmacovigilance. An agency of the European Union
 Pharmacovigilance An agency of the European Union Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse reactions and other medicine-related
Pharmacovigilance An agency of the European Union Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse reactions and other medicine-related
Signal Management in the EU and future extended access to EudraVigilance for industry
 Signal Management in the EU and future extended access to EudraVigilance for industry 6 th Industry Stakeholder Platform Meeting, 18 th December 2015 Presented by Georgy Genov 7 December 2015 Head of Signal
Signal Management in the EU and future extended access to EudraVigilance for industry 6 th Industry Stakeholder Platform Meeting, 18 th December 2015 Presented by Georgy Genov 7 December 2015 Head of Signal
Pharmacovigilance: Information systems and services
 Pharmacovigilance: Information systems and services Supporting business activities of the revised pharmacovigilance legislation through better information systems An agency of the European Union To deliver
Pharmacovigilance: Information systems and services Supporting business activities of the revised pharmacovigilance legislation through better information systems An agency of the European Union To deliver
Pilot of MAH signal detection in EudraVigilance
 Pilot of MAH signal detection in EudraVigilance 13 th Industry Stakeholder Platform operation of EU pharmacovigilance Presented by Julie Durand Signal & Incident Management Service - Pharmacovigilance
Pilot of MAH signal detection in EudraVigilance 13 th Industry Stakeholder Platform operation of EU pharmacovigilance Presented by Julie Durand Signal & Incident Management Service - Pharmacovigilance
Pharmacovigilance: Information systems and Services
 Pharmacovigilance: Information systems and Services 3 rd Industry stakeholder platform operation of EU Pharmacovigilance legislation Presented by Peter Arlett Head of Pharmacovigilance Department An agency
Pharmacovigilance: Information systems and Services 3 rd Industry stakeholder platform operation of EU Pharmacovigilance legislation Presented by Peter Arlett Head of Pharmacovigilance Department An agency
Introductory cover note to the List of European Union reference dates and frequency of submission of Periodic Safety Update Reports
 24 October 2016 EMA/606369/2012 Rev.15 Procedure Management and Committees Support Introductory cover note to the List of European Union reference dates and frequency of submission of Periodic Safety Update
24 October 2016 EMA/606369/2012 Rev.15 Procedure Management and Committees Support Introductory cover note to the List of European Union reference dates and frequency of submission of Periodic Safety Update
The role of patients at the EMA
 The role of patients at the EMA Nathalie Bere Patient relations coordinator / European Medicines Agency An agency of the European Union What is the European Medicines Agency (EMA) The EMA is the EU regulatory
The role of patients at the EMA Nathalie Bere Patient relations coordinator / European Medicines Agency An agency of the European Union What is the European Medicines Agency (EMA) The EMA is the EU regulatory
Draft document circulated to Committees drafting group members 20 October Committees consultation November Adoption January 2015
 28 January 2015 EMA/636600/2014 Human Medicines Research and Development Support Division Guidance on meetings with applicants on the responses to questions received from European Medicines Agency Scientific
28 January 2015 EMA/636600/2014 Human Medicines Research and Development Support Division Guidance on meetings with applicants on the responses to questions received from European Medicines Agency Scientific
Mandate, objectives and rules of procedure for the CVMP Ad Hoc Expert Group on Veterinary Novel Therapies (ADVENT)
 6 October 2016 EMA/CVMP/ADVENT/630299/2014 Rev.2 Committee for Medicinal Products for Veterinary Use (CVMP) Mandate, objectives and rules of procedure for the CVMP Ad Hoc Expert Group on Veterinary Novel
6 October 2016 EMA/CVMP/ADVENT/630299/2014 Rev.2 Committee for Medicinal Products for Veterinary Use (CVMP) Mandate, objectives and rules of procedure for the CVMP Ad Hoc Expert Group on Veterinary Novel
Optimising early access tools: Revision of the guidelines on Accelerated Assessment and Conditional Marketing Authorisation
 Optimising early access tools: Revision of the guidelines on Accelerated Assessment and Conditional Marketing Authorisation High-level overview of comments received during the public consultation Presented
Optimising early access tools: Revision of the guidelines on Accelerated Assessment and Conditional Marketing Authorisation High-level overview of comments received during the public consultation Presented
PRIority MEdicines (PRIME)
 PRIority MEdicines (PRIME) Support to development of priority medicines for unmet medical needs. EuropaBIO information day, 15 October 2015 Jordi Llinares, Head of product development scientific support.
PRIority MEdicines (PRIME) Support to development of priority medicines for unmet medical needs. EuropaBIO information day, 15 October 2015 Jordi Llinares, Head of product development scientific support.
Priority Medicines (PRIME) scheme
 Priority Medicines (PRIME) scheme DGRA Congress, Bonn, 15 June 2016 Presented by Christelle Bouygues Regulatory Affairs Officer An agency of the European Union Outline Why Prime? What is PRIME? PRIME Eligibility
Priority Medicines (PRIME) scheme DGRA Congress, Bonn, 15 June 2016 Presented by Christelle Bouygues Regulatory Affairs Officer An agency of the European Union Outline Why Prime? What is PRIME? PRIME Eligibility
Agreed by the ERAWP April Adopted by the CVMP for release for consultation 4 June 2015
 1 2 3 4 June 2015 EMA/CVMP/ERA/698394/2014 Committee for Medicinal Products for Veterinary Use 4 5 6 7 8 Concept paper on the testing strategy and risk assessment for plants in the Phase II of the environmental
1 2 3 4 June 2015 EMA/CVMP/ERA/698394/2014 Committee for Medicinal Products for Veterinary Use 4 5 6 7 8 Concept paper on the testing strategy and risk assessment for plants in the Phase II of the environmental
Functioning of the PRAC
 Functioning of the PRAC Sixth Stakeholders forum on the implementation of the new Pharmacovigilance legislation, November 8 th 2012 Presented by: Almath Spooner Vice Chair, Pharmacovigilance Risk Assessment
Functioning of the PRAC Sixth Stakeholders forum on the implementation of the new Pharmacovigilance legislation, November 8 th 2012 Presented by: Almath Spooner Vice Chair, Pharmacovigilance Risk Assessment
SPOR data management services - high level changes
 6 July 2017 EMA/551661/2016 version 3 Information Management Division Table of contents 1. Introduction... 2 2. New solutions for access and use of SPOR data... 2 2.1. RMS backward compatibility... 2 2.2.
6 July 2017 EMA/551661/2016 version 3 Information Management Division Table of contents 1. Introduction... 2 2. New solutions for access and use of SPOR data... 2 2.1. RMS backward compatibility... 2 2.2.
EU scientific regulatory support mechanisms and initiatives for innovation in drug development: the EMA perspective
 EU scientific regulatory support mechanisms and initiatives for innovation in drug development: the EMA perspective FAMHP Workshop, Brussels, 2 nd May 2016 Presented by Zahra Hanaizi Product Development
EU scientific regulatory support mechanisms and initiatives for innovation in drug development: the EMA perspective FAMHP Workshop, Brussels, 2 nd May 2016 Presented by Zahra Hanaizi Product Development
Explanatory note on fees payable to the European Medicines Agency
 30 March 2015 EMA/167155/2014 Executive Director Explanatory note on s payable to the European Medicines Agency The s, exemptions and definitions described in this Explanatory Note apply as of 1 April
30 March 2015 EMA/167155/2014 Executive Director Explanatory note on s payable to the European Medicines Agency The s, exemptions and definitions described in this Explanatory Note apply as of 1 April
Issues identified by stakeholders: follow-up from EMA s ATMP workshop
 2 February 2017 EMA/48099/2017 Human Medicines Research and Development Support Division On 27 May 2016 EMA hosted a workshop 1 aimed to foster ATMP development and enable expanded patient access in the
2 February 2017 EMA/48099/2017 Human Medicines Research and Development Support Division On 27 May 2016 EMA hosted a workshop 1 aimed to foster ATMP development and enable expanded patient access in the
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Overview of the Agency s role, activities and priorities for An agency of the European Union
 Overview of the Agency s role, activities and priorities for 2015 An agency of the European Union The Agency's prioroties for 2015 In light of the above influences and other business-environment factors,
Overview of the Agency s role, activities and priorities for 2015 An agency of the European Union The Agency's prioroties for 2015 In light of the above influences and other business-environment factors,
Joint CVMP/CHMP Working group on the Application of the 3Rs in Regulatory Testing of Medical Products
 Joint CVMP/CHMP Working group on the Application of the 3Rs in Regulatory Testing of Medical Products Biennial report 2016/2017 Executive summary This report is aimed at informing pharmaceutical companies
Joint CVMP/CHMP Working group on the Application of the 3Rs in Regulatory Testing of Medical Products Biennial report 2016/2017 Executive summary This report is aimed at informing pharmaceutical companies
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on. 02/10/2018 SmPC, Labelling and PL. 30/04/2018 SmPC
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Scientific advice and its impact on marketing authorisation application reviews
 Scientific advice and its impact on marketing authorisation application reviews SME info day: Tools to support innovative medicines development and early access Presented by Jan Regnström, MD, PhD Scientific
Scientific advice and its impact on marketing authorisation application reviews SME info day: Tools to support innovative medicines development and early access Presented by Jan Regnström, MD, PhD Scientific
EudraVigilance auditable requirement project: ADRreports.eu portal update
 EudraVigilance auditable requirement project: ADRreports.eu portal update Patients and Consumers Working Party 30 th November 2016 Francois Domergue, Business project manager An agency of the European
EudraVigilance auditable requirement project: ADRreports.eu portal update Patients and Consumers Working Party 30 th November 2016 Francois Domergue, Business project manager An agency of the European
The new EudraVigilance System Public Communications Plan for EMA and National Competent Authorities in the EEA
 22 November 2017 /413091/2017 The new EudraVigilance System Public Communications Plan for and National Competent Authorities in the EEA An overview of the planned public communication milestones 30 Churchill
22 November 2017 /413091/2017 The new EudraVigilance System Public Communications Plan for and National Competent Authorities in the EEA An overview of the planned public communication milestones 30 Churchill
Work plan for the CVMP Pharmacovigilance Working Party (PhVWP-V) 2018
 30 January 2018 EMA/CVMP/PhVWP/384293/2017 Committee for Medicinal Products for Veterinary Use (CVMP) Work plan for the CVMP Pharmacovigilance Working Party (PhVWP-V) 2018 Chairpersons Chair: E. Dewaele
30 January 2018 EMA/CVMP/PhVWP/384293/2017 Committee for Medicinal Products for Veterinary Use (CVMP) Work plan for the CVMP Pharmacovigilance Working Party (PhVWP-V) 2018 Chairpersons Chair: E. Dewaele
Preparing for the withdrawal is therefore not just a matter for EU and national authorities, but also for private parties.
 19 June 2018 CMDh/361/2017, Rev.2 Questions and Answers related to the United Kingdom's withdrawal from the European Union with regard to national authorised medicinal products for human use Introduction
19 June 2018 CMDh/361/2017, Rev.2 Questions and Answers related to the United Kingdom's withdrawal from the European Union with regard to national authorised medicinal products for human use Introduction
ACG Public Forum. Join ACG, the FDA, and EMA for a discussion on biosimilars and IBD. Monday, 12:45 pm 2:15 pm
 ACG Public Forum Join ACG, the FDA, and EMA for a discussion on biosimilars and IBD Monday, 12:45 pm 2:15 pm ACG 2017: FDA-EMA workshop on biosimilars Joachim Musaus EMA Product Lead Gastroenterology Human
ACG Public Forum Join ACG, the FDA, and EMA for a discussion on biosimilars and IBD Monday, 12:45 pm 2:15 pm ACG 2017: FDA-EMA workshop on biosimilars Joachim Musaus EMA Product Lead Gastroenterology Human
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on. 04/06/2018 Annex II and Labelling
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Content. Introduction: Approval of ATMPs. Support to ATMP developers. Role of EMA and National Competent Authorities
 Content Introduction: Role of EMA and National Competent Authorities Approval of ATMPs What are ATMPs? Centralised procedure Support to ATMP developers 1 Introductory statements This talk is on marketing
Content Introduction: Role of EMA and National Competent Authorities Approval of ATMPs What are ATMPs? Centralised procedure Support to ATMP developers 1 Introductory statements This talk is on marketing
PRIME. 7 th STAMP meeting Brussels, 27 th June Presented by Sonia Ribeiro Head of Regulatory Affairs Office, Human Medicines Evaluation Division
 PRIME 7 th STAMP meeting Brussels, 27 th June 2017 Presented by Sonia Ribeiro Head of Regulatory Affairs Office, Human Medicines Evaluation Division An agency of the European Union Overview of one-year
PRIME 7 th STAMP meeting Brussels, 27 th June 2017 Presented by Sonia Ribeiro Head of Regulatory Affairs Office, Human Medicines Evaluation Division An agency of the European Union Overview of one-year
Guidelines on good pharmacovigilance practices (GVP)
 15 September 2014 EMA/475236/2014 Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with revision 1 of module III on pharmacovigilance inspections and of module
15 September 2014 EMA/475236/2014 Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with revision 1 of module III on pharmacovigilance inspections and of module
Publication of Risk Management Plan (RMP) summaries:
 Publication of Risk Management Plan (RMP) summaries: Analysis of the experience of the 1-year pilot phase Presented by Juan Garcia Burgos and Caroline Voltz An agency of the European Union Outline Background
Publication of Risk Management Plan (RMP) summaries: Analysis of the experience of the 1-year pilot phase Presented by Juan Garcia Burgos and Caroline Voltz An agency of the European Union Outline Background
ENCePP Code of Conduct Revision 4
 ENCePP Code of Conduct Revision 4 WG2 proposal - agenda item 6 16 th ENCePP Plenary, 21 November 2017 Presented by Rosa Gini, Agenzia Regionale di Sanità (ARS) della Toscana, and Thomas Goedecke, Pharmacovigilance
ENCePP Code of Conduct Revision 4 WG2 proposal - agenda item 6 16 th ENCePP Plenary, 21 November 2017 Presented by Rosa Gini, Agenzia Regionale di Sanità (ARS) della Toscana, and Thomas Goedecke, Pharmacovigilance
Commission. Product. Decision. Information issued on. Issued 2 / affected 3 amended on. 28/09/2017 SmPC, Labelling and PL
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification 1 Decision Information issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification 1 Decision Information issued on Issued 2 / affected
Orphan Medicines Figures
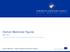 Orphan Medicines Figures 2000-207 Orphan Medicines - Product Development Scientific Support An agency of the European Union Applications for orphan medicinal product designation 2000 2006 20 206 207 Total
Orphan Medicines Figures 2000-207 Orphan Medicines - Product Development Scientific Support An agency of the European Union Applications for orphan medicinal product designation 2000 2006 20 206 207 Total
PRAC recommendations on signals
 26 January 2017 EMA/PRAC/5653/2017 Pharmacovigilance Risk Assessment Committee (PRAC) Adopted at the PRAC meeting of 9-12 January 2017 This document provides an overview of the recommendations adopted
26 January 2017 EMA/PRAC/5653/2017 Pharmacovigilance Risk Assessment Committee (PRAC) Adopted at the PRAC meeting of 9-12 January 2017 This document provides an overview of the recommendations adopted
Conditional marketing authorisation
 Conditional marketing authorisation Report on ten years of experience at the EMA Presented by Zigmars Sebris on 27 June 2017 Regulatory Affairs Office, Human Medicines Evaluation Division An agency of
Conditional marketing authorisation Report on ten years of experience at the EMA Presented by Zigmars Sebris on 27 June 2017 Regulatory Affairs Office, Human Medicines Evaluation Division An agency of
Overview of comments received on 'Draft Guideline on the chemistry of active substances (veterinary) (EMA/CVMP/QWP/49477/2017)
 23 October 2017 EMA/CVMP/QWP/502315/2017 Committee for Medicinal Products for Veterinary Use (CVMP) Overview of comments received on 'Draft Guideline on the chemistry of active substances (veterinary)
23 October 2017 EMA/CVMP/QWP/502315/2017 Committee for Medicinal Products for Veterinary Use (CVMP) Overview of comments received on 'Draft Guideline on the chemistry of active substances (veterinary)
Medicinal product no longer authorised
 Procedural steps taken and scientific information after the authorisation Application number IAIN/0022 Scope B.V.a.1.d - PMF - Inclusion of a new, updated or Opinion/ Notification 1 issued on Commission
Procedural steps taken and scientific information after the authorisation Application number IAIN/0022 Scope B.V.a.1.d - PMF - Inclusion of a new, updated or Opinion/ Notification 1 issued on Commission
Engagement with stakeholders
 Engagement with stakeholders 2 nd International Awareness Session - The EU medicines regulatory system and the European Medicines Agency Presented by Juan Garcia Burgos and Marie-Helene Pinheiro on 8 March
Engagement with stakeholders 2 nd International Awareness Session - The EU medicines regulatory system and the European Medicines Agency Presented by Juan Garcia Burgos and Marie-Helene Pinheiro on 8 March
GVP Module X - additional monitoring of medicines
 GVP Module X - additional monitoring of medicines 6 th Stakeholders forum on the implementation of the new pharmacovigilance legislation Mick Foy, Group Manager - MHRA An agency of the European Union Contents
GVP Module X - additional monitoring of medicines 6 th Stakeholders forum on the implementation of the new pharmacovigilance legislation Mick Foy, Group Manager - MHRA An agency of the European Union Contents
Considerations on regulatory aspects
 Considerations on regulatory aspects Regulatory framework for medicinal products in the context of therapeutic use of bacteriophages EMA Workshop on 8 June Presented by Zigmars Sebris on 8 June 2015 Regulatory
Considerations on regulatory aspects Regulatory framework for medicinal products in the context of therapeutic use of bacteriophages EMA Workshop on 8 June Presented by Zigmars Sebris on 8 June 2015 Regulatory
EudraVigilance Registration Updates
 EudraVigilance Registration Updates Context, Challenges, Next steps 14th industry stakeholder platform operation of EU pharmacovigilance Presented by Oana Agheorghiesei (Project Manager) An agency of the
EudraVigilance Registration Updates Context, Challenges, Next steps 14th industry stakeholder platform operation of EU pharmacovigilance Presented by Oana Agheorghiesei (Project Manager) An agency of the
The European Medicines Agency: A well-established Agency of the EU protecting human and animal health for all EU citizens
 14 July 2016 EMA/457243/2016 The European Medicines Agency: A well-established Agency of the EU protecting human and animal health for all EU citizens 1. Who we are The European Medicines Agency (EMA)
14 July 2016 EMA/457243/2016 The European Medicines Agency: A well-established Agency of the EU protecting human and animal health for all EU citizens 1. Who we are The European Medicines Agency (EMA)
Opinion/ Commission. Notification. Decision. Issued 2 / affected 3 amended on. 22/12/2016 n/a. 09/12/2016 n/a. 07/12/2016 SmPC
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Reflection paper on the use of heat treatment to inactivate endogenous retroviruses in live immunological veterinary medicinal products
 10 September 2015 EMA/CVMP/IWP/37924/2014 Committee for Medicinal Products for Veterinary Use (CVMP) Reflection paper on the use of heat treatment to inactivate endogenous retroviruses in live immunological
10 September 2015 EMA/CVMP/IWP/37924/2014 Committee for Medicinal Products for Veterinary Use (CVMP) Reflection paper on the use of heat treatment to inactivate endogenous retroviruses in live immunological
The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines
 The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines Health care uncertainty assessment workshop Session 3: The challenges of health
The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines Health care uncertainty assessment workshop Session 3: The challenges of health
Guidance for individual laboratories for transfer of quality control methods validated in collaborative trials with a view to implementing 3Rs
 9 November 2017 EMA/CHMP/CVMP/3Rs/94436/2014 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) Guidance for individual laboratories for transfer
9 November 2017 EMA/CHMP/CVMP/3Rs/94436/2014 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) Guidance for individual laboratories for transfer
Mandate and objectives for the EMA Working Party on Quality Review of Documents (QRD)
 1 July 2014 EMA/403106/2014 Mandate and objectives for the EMA Working Party on Quality Review of Documents 1. General considerations The European Medicines Agency (EMA) created an ad hoc Working Group
1 July 2014 EMA/403106/2014 Mandate and objectives for the EMA Working Party on Quality Review of Documents 1. General considerations The European Medicines Agency (EMA) created an ad hoc Working Group
Explanatory note on fees payable to the European Medicines Agency
 09 December 2013 EMA/458574/2013 Executive Director Explanatory note on s payable to the European Medicines Agency The s, exemptions and definitions described in this Explanatory Note apply as of 1 January
09 December 2013 EMA/458574/2013 Executive Director Explanatory note on s payable to the European Medicines Agency The s, exemptions and definitions described in this Explanatory Note apply as of 1 January
1. Aim. 2. Who needs to complete an e-doi?
 24 April 2015 EMA/627294/2014, Rev. 1 European Medicines Agency Procedural guidance on inclusion of declared interests in the European Medicines Agency s electronic declaration of interests form (for scientific
24 April 2015 EMA/627294/2014, Rev. 1 European Medicines Agency Procedural guidance on inclusion of declared interests in the European Medicines Agency s electronic declaration of interests form (for scientific
Pre-accession product information linguistic review process (PALC III)
 28 July 2011 EMA/48630/2011 rev. 1 Patient Health Protection Pre-accession product information linguistic review process (PALC III) Product information linguistic review process Extension of Commission
28 July 2011 EMA/48630/2011 rev. 1 Patient Health Protection Pre-accession product information linguistic review process (PALC III) Product information linguistic review process Extension of Commission
Q&A - Pharmacovigilance Legislation
 CMDh/257/2012, Rev.18 July 2017 Regulation (EU) No 1235/2010 and Directive 2010/84/EU Table of contents 1. a. As of 21 July 2012, new requirements for marketing authorisation applications have been introduced
CMDh/257/2012, Rev.18 July 2017 Regulation (EU) No 1235/2010 and Directive 2010/84/EU Table of contents 1. a. As of 21 July 2012, new requirements for marketing authorisation applications have been introduced
Guidelines on good pharmacovigilance practices (GVP)
 12 April 2013 EMA/131811/2013 Patient Health Protection Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with revision of module II, launch of public consultation
12 April 2013 EMA/131811/2013 Patient Health Protection Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with revision of module II, launch of public consultation
EMA role in GMP Manufacturing and Quality Compliance
 EMA role in GMP Manufacturing and Quality Compliance III all Russian GMP Conference Kazan 2018, Russia Presented by Roberto Conocchia Manufacturing and Quality Compliance European Medicines Agency An agency
EMA role in GMP Manufacturing and Quality Compliance III all Russian GMP Conference Kazan 2018, Russia Presented by Roberto Conocchia Manufacturing and Quality Compliance European Medicines Agency An agency
Furthermore, the level of information and the subsequent assessment will vary on a case by case basis.
 17 March 2016 EMA/352178/2013 Committee for Medicinal Products for Human Use Committee for Medicinal Products for Veterinary Use Points to consider for the overall assessment of a supply shortage of a
17 March 2016 EMA/352178/2013 Committee for Medicinal Products for Human Use Committee for Medicinal Products for Veterinary Use Points to consider for the overall assessment of a supply shortage of a
Guidance on the classification of veterinary medicinal products indicated for minor use minor species (MUMS) / limited market
 18 December 2014 EMA/CVMP/388694/2014 Committee for Medicinal Products for Veterinary Use (CVMP) Guidance on the classification of veterinary medicinal products indicated for minor use minor species (MUMS)
18 December 2014 EMA/CVMP/388694/2014 Committee for Medicinal Products for Veterinary Use (CVMP) Guidance on the classification of veterinary medicinal products indicated for minor use minor species (MUMS)
Summary of Product Characteristics Advisory Group (SmPC AG) activity report
 14 March 2016 Scientific and Regulatory Management Department Summary of Product Characteristics Advisory Group (SmPC AG) 2010-2015 activity report Quality assurance of SmPCs 1. Introduction During the
14 March 2016 Scientific and Regulatory Management Department Summary of Product Characteristics Advisory Group (SmPC AG) 2010-2015 activity report Quality assurance of SmPCs 1. Introduction During the
Roles and responsibilities of members and alternates, rapporteur and peer reviewers, experts and observers of the Paediatric Committee (PDCO)
 Last revision: 16 September 2010 EMA/537415/2008 Human Medicines Development and Evaluation Roles and responsibilities of members and alternates, rapporteur and peer reviewers, experts and observers of
Last revision: 16 September 2010 EMA/537415/2008 Human Medicines Development and Evaluation Roles and responsibilities of members and alternates, rapporteur and peer reviewers, experts and observers of
Procedure management of variations
 Procedure management of variations Process changes made and current challenges Presented by Alberto Ganan and Iordanis Gravanis on 24 April 2015 Procedure Management Department An agency of the European
Procedure management of variations Process changes made and current challenges Presented by Alberto Ganan and Iordanis Gravanis on 24 April 2015 Procedure Management Department An agency of the European
Title: Processing pharmacovigilance data in renewal procedures for centrally authorised medicinal products for veterinary use
 Work instructions Title: Processing pharmacovigilance data in renewal procedures for centrally authorised medicinal products for veterinary use Applies to: Veterinary Medicines (APH and VROS) Status: PUBLIC
Work instructions Title: Processing pharmacovigilance data in renewal procedures for centrally authorised medicinal products for veterinary use Applies to: Veterinary Medicines (APH and VROS) Status: PUBLIC
Work plan for the GMP/GDP Inspectors Working Group for 2017
 13 January 2017 EMA/INS/GMP/584202/2016 Work plan for the GMP/GDP Inspectors Working Group for 2017 Chairperson: David Cockburn Adopted: December 2016 1. Meetings scheduled for 2017 Face-to-face meetings
13 January 2017 EMA/INS/GMP/584202/2016 Work plan for the GMP/GDP Inspectors Working Group for 2017 Chairperson: David Cockburn Adopted: December 2016 1. Meetings scheduled for 2017 Face-to-face meetings
Orphan Medicines Figures
 Orphan Medicines Figures 000-05 Orphan Medicines - Product Development Scientific Support An agency of the European Union Status of Orphan Applications 000 005 006 00 0 0 03 04 05 Total Applications submitted
Orphan Medicines Figures 000-05 Orphan Medicines - Product Development Scientific Support An agency of the European Union Status of Orphan Applications 000 005 006 00 0 0 03 04 05 Total Applications submitted
Commission. Product. Notification 1. Decision. Information issued on. Issued 2 / affected 3 amended on 30/01/2018 PL
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification 1 Decision Information issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification 1 Decision Information issued on Issued 2 / affected
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on. 16/10/2018 Annex II and PL
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
EudraVigilance auditable requirement project
 22 November 2017 EMA/835422/2016 Information Management Division EudraVigilance training plan (version 5) Project Maintenance Group 1 consultation 11 December 2015 Eudravigilance Expert Working Group consultation
22 November 2017 EMA/835422/2016 Information Management Division EudraVigilance training plan (version 5) Project Maintenance Group 1 consultation 11 December 2015 Eudravigilance Expert Working Group consultation
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on 14/06/ /07/2018 PL
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Minutes of the ninth meeting of the EMA Human Scientific Committees Working Party with Patients' and Consumers' Organisations (PCWP)
 17 March 2010 EMA/625710/2009 Minutes of the ninth meeting of the EMA Human Scientific Committees Working Party with Patients' and Consumers' Organisations (PCWP) 30 September 2009 Role Chairpersons: Present:
17 March 2010 EMA/625710/2009 Minutes of the ninth meeting of the EMA Human Scientific Committees Working Party with Patients' and Consumers' Organisations (PCWP) 30 September 2009 Role Chairpersons: Present:
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on. 03/08/2018 n/a. 25/07/2018 Labelling
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Implementation strategy of ICH Q3D guideline
 1 2 3 1 July 2016 EMA/404489/2016 Committee for Medicinal Products for Human use (CHMP) 4 5 Draft Draft agreed by QWP and BWP June 2016 Adopted by CHMP for release for consultation June 2016 Start of public
1 2 3 1 July 2016 EMA/404489/2016 Committee for Medicinal Products for Human use (CHMP) 4 5 Draft Draft agreed by QWP and BWP June 2016 Adopted by CHMP for release for consultation June 2016 Start of public
Work plan for the GMP/GDP Inspectors Working Group for 2018
 30 November 2017 EMA/INS/GMP/504401/2017 Inspections, Human Medicines Pharmacovigilance & Committees Division Work plan for the GMP/GDP Inspectors Working Group for 2018 Chairperson: Brendan Cuddy Adopted:
30 November 2017 EMA/INS/GMP/504401/2017 Inspections, Human Medicines Pharmacovigilance & Committees Division Work plan for the GMP/GDP Inspectors Working Group for 2018 Chairperson: Brendan Cuddy Adopted:
EMA pharmacovigilance system manual
 13 October 2016 EMA/623550/2013 Inspections, Human Medicines Pharmacovigilance & Committees Division Version 1.2 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44 (0)20 3660 6000
13 October 2016 EMA/623550/2013 Inspections, Human Medicines Pharmacovigilance & Committees Division Version 1.2 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44 (0)20 3660 6000
Overview of recent changes in the centralised procedure
 Overview of recent changes in the centralised procedure 2 nd Industry stakeholder platform, 9 November 2015 Presented by: Michael Berntgen Head of Scientific & Regulatory Management Department An agency
Overview of recent changes in the centralised procedure 2 nd Industry stakeholder platform, 9 November 2015 Presented by: Michael Berntgen Head of Scientific & Regulatory Management Department An agency
Comments from: Name of organisation or individual. Merck Sharp & Dohme (MSD)
 5 November 2015 EMA/733775/2015 Product Development Scientific Support Department Overview of comments on Total Kidney Volume (TKV) as a prognostic biomarker for use in clinical trials evaluating patients
5 November 2015 EMA/733775/2015 Product Development Scientific Support Department Overview of comments on Total Kidney Volume (TKV) as a prognostic biomarker for use in clinical trials evaluating patients
Guidelines on good pharmacovigilance practices (GVP)
 7 June 2013 EMA/321625/2013 Patient Health Protection Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with launch of public consultation of module VI revision
7 June 2013 EMA/321625/2013 Patient Health Protection Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with launch of public consultation of module VI revision
Recent update of the guidance for Parallel EMA/FDA scientific advice
 Recent update of the guidance for Parallel EMA/FDA scientific advice Industry stakeholder platform on research and development support, 15.11.2017 Presented by Thorsten Vetter An agency of the European
Recent update of the guidance for Parallel EMA/FDA scientific advice Industry stakeholder platform on research and development support, 15.11.2017 Presented by Thorsten Vetter An agency of the European
Questions and answers on preparation, management and assessment of periodic safety update reports (PSURs)
 21 June 2018 EMA/CVMP/PhVWP/126661/2009-Rev.4-corr. Committee for Medicinal Products for Veterinary Use Questions and answers on preparation, management and assessment of periodic safety update reports
21 June 2018 EMA/CVMP/PhVWP/126661/2009-Rev.4-corr. Committee for Medicinal Products for Veterinary Use Questions and answers on preparation, management and assessment of periodic safety update reports
How are medicines evaluated at the EMA
 How are medicines evaluated at the EMA Presented by: Nathalie Bere Patient interaction / Stakeholders and communication Division An agency of the European Union The European System Centralised Procedure
How are medicines evaluated at the EMA Presented by: Nathalie Bere Patient interaction / Stakeholders and communication Division An agency of the European Union The European System Centralised Procedure
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on. 17/01/2019 SmPC and PL
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Explanatory Note to GVP Module VII
 31 October 2017 EMA/670256/2017 Human Medicines Evaluation Division Explanatory Note to GVP Module VII Since the start of the Periodic Safety Update Report (PSUR) single assessment (PSUSA), the procedure
31 October 2017 EMA/670256/2017 Human Medicines Evaluation Division Explanatory Note to GVP Module VII Since the start of the Periodic Safety Update Report (PSUR) single assessment (PSUSA), the procedure
European Medicines Agency policy on changes in scope of paediatric investigation plan (PIP) decisions
 3 May 2013 EMA/472551/2012 Rev.1 Human Medicines Development and Evaluation European Medicines Agency policy on changes in scope of paediatric investigation plan Background Regulation (EC) No 1901/2006
3 May 2013 EMA/472551/2012 Rev.1 Human Medicines Development and Evaluation European Medicines Agency policy on changes in scope of paediatric investigation plan Background Regulation (EC) No 1901/2006
Concept paper on the development of a guideline on quality and equivalence of topical products
 1 2 3 4 5 6 7 8 2 December 2014 EMA/CHMP/QWP/558185/2014 Committee for Medicinal Products for Human use (CHMP) Concept paper on the development of a guideline on quality and equivalence of topical Draft
1 2 3 4 5 6 7 8 2 December 2014 EMA/CHMP/QWP/558185/2014 Committee for Medicinal Products for Human use (CHMP) Concept paper on the development of a guideline on quality and equivalence of topical Draft
EMA/CAT support to ATMP developers
 EMA/CAT support to ATMP developers CAT-ISCT Workshop: Challenge and opportunities for the successful development and approval of Advanced Therapy Medicinal Products. Presented by Patrick Celis on 25 September
EMA/CAT support to ATMP developers CAT-ISCT Workshop: Challenge and opportunities for the successful development and approval of Advanced Therapy Medicinal Products. Presented by Patrick Celis on 25 September
We appreciate the opportunity to submit these comments for your consideration.
 February 28, 2018 European Medicines Agency 30 Churchill Place Canary Wharf London E14 5EU United Kingdom via email to rp-stats-qa@ema.europa.eu Dear Sir or Madam: The International Society for Pharmaceutical
February 28, 2018 European Medicines Agency 30 Churchill Place Canary Wharf London E14 5EU United Kingdom via email to rp-stats-qa@ema.europa.eu Dear Sir or Madam: The International Society for Pharmaceutical
Commission. Product. Notification 1. Decision. Information issued on. Issued 2 / affected 3 amended on. 04/04/2018 n/a
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification 1 Decision Information issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification 1 Decision Information issued on Issued 2 / affected
European Medicines Agency decision
 EMA/643705/2014 European Medicines Agency decision P/0306/2014 of 24 November 2014 on the granting of a product specific waiver for purified adenylate cyclase recombinant protein carrying subfragments
EMA/643705/2014 European Medicines Agency decision P/0306/2014 of 24 November 2014 on the granting of a product specific waiver for purified adenylate cyclase recombinant protein carrying subfragments
Recommendations on eligibility to PRIME scheme
 1 June 2016 EMA/345382/2016 Press office Adopted at the CHMP meeting of 23-26 May 2016 During its May 2016 meeting, the CHMP reviewed 18 recommendations for eligibility to PRIME: 4 were granted and 14
1 June 2016 EMA/345382/2016 Press office Adopted at the CHMP meeting of 23-26 May 2016 During its May 2016 meeting, the CHMP reviewed 18 recommendations for eligibility to PRIME: 4 were granted and 14
Commission. Product. Notification. Decision. Issued 2 / affected 3 amended on. 30/11/2018 n/a 21/11/2018 PL. 14/11/2018 n/a
 Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
Procedural steps taken and scientific information after the authorisation Application Scope Opinion/ Commission Product Summary number Notification Decision Information 1 issued on Issued 2 / affected
