PQS performance specification
|
|
|
- Brittany Green
- 6 years ago
- Views:
Transcription
1 PQS performance specification WHO/PQS/E10/SB01.1 Original: English Distribution: General TITLE: Safety box for the disposal of used sharps Specification reference: E10/SB01.1 Product verification protocol: E10/SB01-VP.1 Date of origin: Date of last revision: Replaces PIS specification E10/IC.1 and IC.2, rev Contents: 1. Scope Normative references: Terms and definitions: Requirements: General: Performance: Functionality: Shipping and storage volume before use: Nominal capacity: Maximum capacity: Sharps aperture: Handles: Colour: Bio-hazard marking: Fill line: Resistance to penetration: Resistance to damage during drops from height: Stability and spillage: Environmental requirements: Temperature resistance: Water resistance: Physical characteristics: Overall dimensions: Minimum dimensions: Sharps aperture dimensions: Weight: Interface requirements: Human factors: Sharps aperture marking: Tamper-proofing: Handling: Materials: Warranty:... 6 PQS-E10-SB01.doc 1 of 9 12/10/2007
2 4.9 Servicing provision: Disposal and recycling: Instructions: Training: Verification: Packaging: On-site installation : Product dossier : On-site maintenance: Change notification: Defect reporting: Annex 1 International bio-hazard symbol Scope This specification describes the performance requirements for sharps safety boxes, constructed of cardboard or other materials, intended safely and efficiently to contain, store, transport and dispose of used sharps. 2. Normative references: AS 4031:1992: Non-reusable containers for the collection of sharp medical items in health care areas. BS 7320:1990: Specification for sharps containers. DHHS (NIOSH) Publication No : Selecting, Evaluating and Using Sharps Disposal Containers. 3. Terms and definitions: Auto-disable or AD syringe: A syringe and needle assembly, of any capacity, complying with ISO 7886 part 3: 2005, or later edition. Reuse prevention feature syringe: A syringe and needle assembly, of any capacity, complying with ISO : 2006, or later edition. Disposable syringe: A syringe and needle assembly, of any capacity, complying with ISO standard 7886 part 1: 1993 or later edition. In writing: means communication by letter, fax or . Legal Manufacturer: The natural or legal person with responsibility for the design, manufacture, packaging and labelling of a product or device before it is placed on the market under his own name, regardless of whether these operations are carried out by that person himself or on his behalf by a third party. Maximum capacity: The number of 0.5ml AD syringes that a sharps box can contain without syringes projecting above the fill line printed on the outside of the container. Montreal Protocol: Montreal Protocol on Substances that Deplete the Ozone Layer. Reseller: A commercial entity, licensed to act on behalf of a Legal Manufacturer, and which carries product liability and warranty responsibilities no less onerous than those carried by the Legal Manufacturer. PQS-E10-SB01.doc 2 of 9 12/10/2007
3 Syringe: An auto-disable, syringe with reuse prevention feature or disposable syringe and needle assembly. Sharps safety box: A container intended to safely hold used sharps. Sharps: In this context: syringes and needles; sharps syringes and needles, phlebotomy devices, IV insertion needles, including butterflies, lancets, scalpels and suture needles, which, through direct contact with health workers, waste handling/processing personnel, or the public at large, may penetrate the skin if brought into direct contact with it. 4. Requirements: Poorly managed sharps waste exposes health workers and the general community to injuries, infection and environmental pollution. The efficient, safe and environmentally acceptable management and disposal of such waste ensures that sharps are contained and that the risks of needle-stick injury, air and ground water pollution are minimized. 4.1 General: Sharps safety box of , 2.5, 5, 10, 15 or 20 litre nominal storage capacity intended safely and efficiently to contain, transport and store used sharps until final destruction, safe disposal or recycling. 4.2 Performance: Functionality: The safety box must safely contain contaminated sharps: at the point of use; during temporary storage; during handling and transport to the point of treatment and final disposal Shipping and storage volume before use: Boxes must be supplied flat-packed or nested to minimize shipping and storage volume Nominal capacity: Boxes must accept no less than 20 nbr. 0.5ml AD syringes per nominal litre of storage capacity. This capacity is to be achieved when syringes are dropped in randomly, needle first, with 25mm unsheathed non-retractable needles attached and plungers fully depressed. No syringe must protrude from the container or above the fill line and the box must be capable of being correctly and permanently closed without any risk of needle-stick injury Maximum capacity: The maximum capacity is allowed to exceed the nominal capacity of 20 syringes per nominal litre provided all the conditions of clause still apply. 1 The 1.25 litre sharps safety box is intended primarily or use in settings where community health workers give small numbers of routine injections outside health facilities. Typically the boxes will be used to dispose of standard AD immunization syringes or compact pre-filled injection devices. PQS-E10-SB01.doc 3 of 9 12/10/2007
4 4.2.5 Sharps aperture: Boxes must be fitted with a sharps aperture, capable of receiving syringes and needle assemblies of all standard sizes up to and including 20 ml, together with other sharps. It must be possible to close and seal the aperture at any time between empty and full to maximum capacity. The closure mechanism must pass the test for security of attachment of aperture closure devices described in BS 7320:1990, Appendix B Handles: Boxes must be supplied with a handle or other lifting device which allows the container to be carried safely with one hand. The lifting device must be positioned above the fill line, must not obstruct access to the sharps aperture, and must be sufficiently robust to ensure that it does not to break during use and during transport to the disposal site. It must remain attached to the box when the box is filled with sharps to its maximum capacity and tested in accordance with BS 7320:1990, Appendix A Colour: Boxes can be the colour of unbleached sulphate board, or non-chlorine bleached white, or yellow Bio-hazard marking: Boxes must be clearly marked with the international bio-hazard warning not less than 50mm diameter, printed in black or red on each of the front and back faces of the box. Refer to Annex Fill line: The maximum recommended fill line must be clearly marked on all vertical faces of the box, in black or red Resistance to penetration: The average of forces needed to penetrate samples taken from each position must not be less than 15 N, and the minimum force required to penetrate any sample taken from any position must not be less than 12.5 N Resistance to damage during drops from height: Boxes must pass the drop test described in E10/SB01-VP. After 100 drops, no syringe should have fallen out of the container; the box should not be seriously damaged, and no more than one needle should have penetrated the container walls Stability and spillage: Boxes must not tip over when placed on a 15 degree non-slip plane with its short axis parallel to the line of tilt in general accordance with the test method in AS 4031:1992, Appendix D. If overturning occurs, the arrangement of the sharps aperture should minimize the risk that sharps are spilled. 4.3 Environmental requirements: Temperature resistance: Cardboard boxes, filled to their maximum capacity, must be able to resist temperatures of up to 170 C for periods up to 30 minutes without spilling any part of the load. PQS-E10-SB01.doc 4 of 9 12/10/2007
5 4.3.2 Water resistance: Boxes, filled to their maximum capacity, must be able to withstand 48 hours at 43 C and 90% relative humidity in 5 mm of water, without spilling any part of the load. 4.4 Physical characteristics: Overall dimensions: Assembled box dimensions should be selected to accommodate the full range of sharps, as defined in clause 3, and to allow effective filling of the box Minimum dimensions: The minimum height from the bottom of the container to the fill line must be no less than 150mm for 2.5 litre boxes and 230mm for other sizes Sharps aperture dimensions: 38 mm diameter, or 38mm width and breadth. Larger apertures are allowed, but must be fitted with an engineered protective feature for example a flange on a plastic safety box Weight: No specific restriction, consistent with keeping shipping weight to a minimum. 4.5 Interface requirements: External dimensions should be chosen to allow the box to fit within the treatment loading mechanisms. 4.6 Human factors: Sharps aperture marking: The aperture must be clearly visible against the colour of the container Tamper-proofing: To reduce the risk of needlestick injury, the lowest point of the sharps aperture must be at least 50 mm above the maximum recommended fill line marked on the exterior of the box Handling: It must be possible to carry the box in one hand without spillage of contents and without risk of needle stick injury, both before and after final closure of the sharps aperture. 4.7 Materials: The following materials are permitted: Bio-degradable cardboard-based materials post-consumer recycled material is preferred; Other bio-degradable board materials. Non-toxic inks, glues and dyes. Hard recyclable plastic (plastic containers should not be incinerated). Metal. If incineration is the final treatment option, the following materials are not permitted: Materials which are not bio-degradable. PQS-E10-SB01.doc 5 of 9 12/10/2007
6 Materials which emit ozone depleting substances as defined in the Montreal Protocol; Materials which generate toxic emissions during incineration at any temperature between 650 C and 1,200 C; Materials which release gases with a high global warming potential. 4.8 Warranty: 100% of boxes are to remain physically intact and satisfactory for use when used in compliance with this performance specification. 4.9 Servicing provision: The product is a consumable item with no maintenance requirement Disposal and recycling: Boxes are disposed of after a single use cycle if made of cardboard. If made of plastic or metal, they are typically taken to a treatment site to be reused, recycled or disposed of Instructions: In addition to the international bio-hazard symbol, clear pictorial instructions without writing are to be printed on two sides of the container showing: How to assemble the box. How to use the box as a container for contaminated sharps; Syringe disposal direction (needle down). How to close the sharps aperture when the box is full Training: Training on the assembly, use and disposal of safety boxes will be provided by the health care programme when the boxes are first introduced, and subsequently during supervisory visits 4.13 Verification: In accordance with PQS Verification Protocol E10/SB01-VP 5. Packaging: Recyclable cardboard is to be used. 6. On-site installation : Not applicable. 7. Product dossier : The legal manufacturer or reseller is to provide WHO with a pre-qualification dossier containing the following: Dossier examination fee in US dollars. General information about the legal manufacturer, including name and address. Unique identification reference for the product type. PQS-E10-SB01.doc 6 of 9 12/10/2007
7 Full specifications of the product being offered, covering all the requirements set out in this document, including details of product marking and traceability. Certified photocopies of all type-approvals obtained for the product, including CE marking and the like. Certified photocopies of the legal manufacturer s ISO quality system certification. Where available, laboratory test report(s) proving conformity with the product specifications. One sample of each size and type of box. One sample of the instruction insert. Indicative cost of the product per 100, 1,000 and 10,000 units, EXW (Incoterms 2000). 8. On-site maintenance: None required. 9. Change notification: The legal manufacturer or reseller is to advise WHO in writing of any changes which adversely affect the performance of the product after PQS pre-qualification has taken place. 10. Defect reporting: The legal manufacturer or reseller is to advise WHO and the UN purchasing agencies in writing in the event of safety-related product recalls, component defects and other similar events. PQS-E10-SB01.doc 7 of 9 12/10/2007
8 11. Annex 1 International bio-hazard symbol 2 Colour red or black 50mm minimum diameter 2 Source: Laboratory biosafety manual (Third edition) World Health Organization, Geneva PQS-E10-SB01.doc 8 of 9 12/10/2007
9 Revision history: Date Change summary Reason for change Approved Changes in response to WHO internal review. UK Further changes in response to WHO internal review. UK 2. AS 4031 added : 1.25 litre option added. Request from the field. Footnote added. UK : wording changed. Clarification : material clarified : four omitted : dimension changed. 4.7: glue added. 4.11: in writing added. Manufacturers review comments UK 4.2.1: Sharps deleted from first bullet point : Title changed. or yellow added. Biohazard marking Incorporation of PATH comments reference removed. to extent agreed during internal 4.2.8: New biohazard marking review clause added. Subsequent clauses re-numbered : Card omitted. Cardboard added. UK PQS-E10-SB01.doc 9 of 9 12/10/2007
PQS performance specification
 PQS performance specification WHO/PQS/E06/IN02.1 Original: English Distribution: General TITLE: Cold Chain Monitor Specification reference: E06/IN02.1 Product verification protocol: E06/IN02.VP.1 Date
PQS performance specification WHO/PQS/E06/IN02.1 Original: English Distribution: General TITLE: Cold Chain Monitor Specification reference: E06/IN02.1 Product verification protocol: E06/IN02.VP.1 Date
PQS performance specification
 PQS performance specification WHO/PQS/E04/CB01.1 Original: English Distribution: General TITLE: Vaccine cold box Specification reference: E04/CB01.1 Product verification protocol: E04/CB01-VP.1 Date of
PQS performance specification WHO/PQS/E04/CB01.1 Original: English Distribution: General TITLE: Vaccine cold box Specification reference: E04/CB01.1 Product verification protocol: E04/CB01-VP.1 Date of
PQS performance specification. TITLE: Vaccine Vial Monitor Specification reference: E006/IN05.3
 PQS performance specification WHO/PQS/E006/IN05.3 Original: English Distribution: General TITLE: Vaccine Vial Monitor Specification reference: E006/IN05.3 Product verification: E006/IN05.VP.3 Issue date:
PQS performance specification WHO/PQS/E006/IN05.3 Original: English Distribution: General TITLE: Vaccine Vial Monitor Specification reference: E006/IN05.3 Product verification: E006/IN05.VP.3 Issue date:
PQS performance specification
 PQS performance specification WHO/PQS/E04/CB01.1 Original: English Distribution: General TITLE: Vaccine cold box Specification reference: E04/CB01.1 Product verification protocol: E04/CB01-VP.1 Date of
PQS performance specification WHO/PQS/E04/CB01.1 Original: English Distribution: General TITLE: Vaccine cold box Specification reference: E04/CB01.1 Product verification protocol: E04/CB01-VP.1 Date of
PQS performance specification
 PQS performance specification WHO/PQS/E004/VC01.2 Original: English Distribution: General TITLE: Vaccine carrier Specification reference: E004/VC01.2 Product verification protocol: E004/VC01-VP.2 Issue
PQS performance specification WHO/PQS/E004/VC01.2 Original: English Distribution: General TITLE: Vaccine carrier Specification reference: E004/VC01.2 Product verification protocol: E004/VC01-VP.2 Issue
PQS Independent type-testing protocol
 PQS Independent type-testing protocol WHO/PQS/E06/TH03.VP.1 Original: English Distribution: General TITLE: Portable alcohol stem thermometer Product verification protocol: E06/TH03.VP.1 Applies to specification
PQS Independent type-testing protocol WHO/PQS/E06/TH03.VP.1 Original: English Distribution: General TITLE: Portable alcohol stem thermometer Product verification protocol: E06/TH03.VP.1 Applies to specification
PQS Type-examination protocol
 PQS Type-examination protocol WHO/PQS/E03/PV01-VP1.1 Original: English Distribution: General TITLE: Solar power system for compression-cycle vaccine refrigerator or combined refrigerator-icepack freezer.
PQS Type-examination protocol WHO/PQS/E03/PV01-VP1.1 Original: English Distribution: General TITLE: Solar power system for compression-cycle vaccine refrigerator or combined refrigerator-icepack freezer.
PQS Independent type-testing protocol
 PQS Independent type-testing protocol WHO/PQS/E004/VC01-VP.2 Original: English Distribution: General TITLE: Vaccine carrier Product verification protocol: E004/VC01-VP.2 Applies to specification ref(s):
PQS Independent type-testing protocol WHO/PQS/E004/VC01-VP.2 Original: English Distribution: General TITLE: Vaccine carrier Product verification protocol: E004/VC01-VP.2 Applies to specification ref(s):
PQS Independent type-testing protocol
 PQS Independent type-testing protocol WHO/PQS/E006/IN05-VP.3 Original: English Distribution: General TITLE: Vaccine Vial Monitor Product verification protocol: E006/IN05.VP.3 Specification reference: E006/IN05.3
PQS Independent type-testing protocol WHO/PQS/E006/IN05-VP.3 Original: English Distribution: General TITLE: Vaccine Vial Monitor Product verification protocol: E006/IN05.VP.3 Specification reference: E006/IN05.3
Pre-qualification of single-use injection devices under the PQS system: A guideline for manufacturers
 Pre-qualification of single-use injection devices under the PQS system: A guideline for manufacturers June 2005 Pre-qualification of single-use injection devices under the PQS system: A guideline for manufacturers
Pre-qualification of single-use injection devices under the PQS system: A guideline for manufacturers June 2005 Pre-qualification of single-use injection devices under the PQS system: A guideline for manufacturers
Plastics collapsible containers for human blood and blood components. Part 1: Conventional containers
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 3826-1 Second edition 2013-06-01 Plastics collapsible containers for human blood and blood components Part 1: Conventional containers Poches en
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 3826-1 Second edition 2013-06-01 Plastics collapsible containers for human blood and blood components Part 1: Conventional containers Poches en
WHO PQS Prequalification System
 WHO PQS Prequalification System for Injection Devices and sharps safety disposal Dr. Isaac Gobina WHO/HIS/EMP/RHT/PQT UNICEF Supply Division 29 March 2017 1 Outline 1. Why PQS? 2. Scope of PQS 3. PQS process
WHO PQS Prequalification System for Injection Devices and sharps safety disposal Dr. Isaac Gobina WHO/HIS/EMP/RHT/PQT UNICEF Supply Division 29 March 2017 1 Outline 1. Why PQS? 2. Scope of PQS 3. PQS process
Biological and Regulated Medical Waste Plan
 Biological and Regulated Medical Waste Plan TUFTS ENVIRONMENTAL HEALTH AND SAFETY OCTOBER 2016 Table of Contents I. Purpose... 3 II. Responsibilities... 3 III. Sink and sewer discharge of specific medical
Biological and Regulated Medical Waste Plan TUFTS ENVIRONMENTAL HEALTH AND SAFETY OCTOBER 2016 Table of Contents I. Purpose... 3 II. Responsibilities... 3 III. Sink and sewer discharge of specific medical
HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT ONTARIO
 1. Introduction HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT ONTARIO This Collection Location standards agreement ( Agreement ) applies to the receiving, handling and
1. Introduction HEALTH PRODUCTS STEWARDSHIP ASSOCIATION COLLECTION LOCATION STANDARDS AGREEMENT ONTARIO This Collection Location standards agreement ( Agreement ) applies to the receiving, handling and
Medical Waste Disposal
 Medical Waste Disposal Hazardous Healthcare / Medical Waste Flowchart INFECTIOUS WASTE SHARPS PATHOLOGICAL WASTE LANDFILL AREA Chemical Waste Flowchart CHEMICAL STREAM ORGANIC WASTE FLAMABLE INORGANIC
Medical Waste Disposal Hazardous Healthcare / Medical Waste Flowchart INFECTIOUS WASTE SHARPS PATHOLOGICAL WASTE LANDFILL AREA Chemical Waste Flowchart CHEMICAL STREAM ORGANIC WASTE FLAMABLE INORGANIC
December 2009 CDC-NIH
 December 00 CDC-NIH Guidelines for Biosafety Laboratory Competency, CDC and the Assocation of Public Health Laboratories, CDC MMWR Supplement/Vol. 60 April, 0 PI: BSL BMBL th Edition CDC-NIH Dec. 00 N/A
December 00 CDC-NIH Guidelines for Biosafety Laboratory Competency, CDC and the Assocation of Public Health Laboratories, CDC MMWR Supplement/Vol. 60 April, 0 PI: BSL BMBL th Edition CDC-NIH Dec. 00 N/A
Bufab document Page 0/11 Date Version 1.1 BUFAB SUPPLIER MANUAL
 Bufab document Page 0/11 Version 1.1 BUFAB SUPPLIER MANUAL Bufab document Page 1/11 Contents Inquiry/offer... 3 Order... 3 Sales confirmation... 3 Transport... 3 Invoice... 3 Delivery delays... 4 Packaging
Bufab document Page 0/11 Version 1.1 BUFAB SUPPLIER MANUAL Bufab document Page 1/11 Contents Inquiry/offer... 3 Order... 3 Sales confirmation... 3 Transport... 3 Invoice... 3 Delivery delays... 4 Packaging
PQS performance specification
 PQS performance specification WHO/PQS/E004/CB02.1 Original: English Distribution: General TITLE: Large capacity vaccine cold box Specification reference: E004/CB02.1 Product verification protocol: E004/CB02-VP.1
PQS performance specification WHO/PQS/E004/CB02.1 Original: English Distribution: General TITLE: Large capacity vaccine cold box Specification reference: E004/CB02.1 Product verification protocol: E004/CB02-VP.1
Clinical Waste. Policy. Responsibilities
 Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
AAMI Quality Systems White Paper: Comparison of 21 CFR Part 820 to ISO 13485:2016 1
 AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward, Owner, Ward Sciences
AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward, Owner, Ward Sciences
Part 4: Prefilled syringes
 INTERNATIONAL STANDARD ISO 11040-4 Third edition 2015-04-01 Prefilled syringes Part 4: Glass barrels for injectables and sterilized subassembled syringes ready for filling Seringues préremplies Partie
INTERNATIONAL STANDARD ISO 11040-4 Third edition 2015-04-01 Prefilled syringes Part 4: Glass barrels for injectables and sterilized subassembled syringes ready for filling Seringues préremplies Partie
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 11140-3 Second edition 2007-03-15 Sterilization of health care products Chemical indicators Part 3: Class 2 indicator systems for use in the Bowie and Dick-type steam penetration
INTERNATIONAL STANDARD ISO 11140-3 Second edition 2007-03-15 Sterilization of health care products Chemical indicators Part 3: Class 2 indicator systems for use in the Bowie and Dick-type steam penetration
Product development version
 PQS Type-testing protocol WHO/PQS/E004/CB04/VP.1 Original: English Distribution: General TITLE: Vaccine cold box long-term storage 10 days Product verification protocol: E004/CB04-VP.1 Applies to specification
PQS Type-testing protocol WHO/PQS/E004/CB04/VP.1 Original: English Distribution: General TITLE: Vaccine cold box long-term storage 10 days Product verification protocol: E004/CB04-VP.1 Applies to specification
Planning for Safe Syringe Disposal
 Planning for Safe Syringe Disposal Making Medical Injections Safer Version 2 July 14, 2004 Table of Contents The safe syringe disposal planning process...2 Step 1: Review existing legislation and policy....3
Planning for Safe Syringe Disposal Making Medical Injections Safer Version 2 July 14, 2004 Table of Contents The safe syringe disposal planning process...2 Step 1: Review existing legislation and policy....3
WHO NEEDS TO KNOW THIS PROGRAM All NYU employees that generate, handle or transport RMW should be familiar with this written program.
 Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Plastics Blow-moulded polypropylene containers for packaging of liquid foodstuffs
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 13106 First edition 2014-05-15 Plastics Blow-moulded polypropylene containers for packaging of liquid foodstuffs Plastiques Récipients en polypropylène
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 13106 First edition 2014-05-15 Plastics Blow-moulded polypropylene containers for packaging of liquid foodstuffs Plastiques Récipients en polypropylène
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 8535-1 Fourth edition 2006-08-01 Diesel engines Steel tubes for highpressure fuel injection pipes Part 1: Requirements for seamless cold-drawn single-wall tubes Moteurs diesels
INTERNATIONAL STANDARD ISO 8535-1 Fourth edition 2006-08-01 Diesel engines Steel tubes for highpressure fuel injection pipes Part 1: Requirements for seamless cold-drawn single-wall tubes Moteurs diesels
Lenovo Global Labeling Guide
 Overview of Symbols and Labels Page 1 of 15 Date: Dec 18, 2008 Lenovo Global Labeling Guide Volume 8 - Overview of Symbols and Special Labels Lenovo Part Number 41U3004 Current Edition: 2.0 Release Date:
Overview of Symbols and Labels Page 1 of 15 Date: Dec 18, 2008 Lenovo Global Labeling Guide Volume 8 - Overview of Symbols and Special Labels Lenovo Part Number 41U3004 Current Edition: 2.0 Release Date:
AAMI Quality Systems White Paper
 AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017, Updated February 2018 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward,
AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017, Updated February 2018 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward,
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB
 Translated English of Chinese Standard: GB18671-2009 www.chinesestandard.net Sales@ChineseStandard.net ICS 11.040.20 NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB C 31 Replacing GB 18671-2002
Translated English of Chinese Standard: GB18671-2009 www.chinesestandard.net Sales@ChineseStandard.net ICS 11.040.20 NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB C 31 Replacing GB 18671-2002
ISO INTERNATIONAL STANDARD. Medical gas pipeline systems Part 2: Anaesthetic gas scavenging disposal systems
 INTERNATIONAL STANDARD ISO 7396-2 Second edition 2007-04-01 Medical gas pipeline systems Part 2: Anaesthetic gas scavenging disposal systems Réseaux de distribution de gaz médicaux Partie 2: Réseaux d'évacuation
INTERNATIONAL STANDARD ISO 7396-2 Second edition 2007-04-01 Medical gas pipeline systems Part 2: Anaesthetic gas scavenging disposal systems Réseaux de distribution de gaz médicaux Partie 2: Réseaux d'évacuation
INSTRUMENT RETURN PROCEDURES
 INSTRUMENT RETURN PROCEDURES An instrument problem may be reported by calling, faxing, e-mailing, or mailing BioFire Defense. The contact numbers are located below. You will also need the following documents:
INSTRUMENT RETURN PROCEDURES An instrument problem may be reported by calling, faxing, e-mailing, or mailing BioFire Defense. The contact numbers are located below. You will also need the following documents:
Biosafety Checklist. 07-BiosafetyChecklist-LTC-SOP-v2.0-17Feb of 6
 Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
The Edge Protection Federation Containment Systems Product Specification, Test Methods
 The Edge Protection Federation Containment Systems Product Specification, Test Methods Revision 2 August 2016 This Standard defines how the Edge Protection Federation defines Containment. This document
The Edge Protection Federation Containment Systems Product Specification, Test Methods Revision 2 August 2016 This Standard defines how the Edge Protection Federation defines Containment. This document
DRAFT. Module 6. HEALTHCARE WASTE MANAGEMENT
 Module 6. HEALTHCARE WASTE MANAGEMENT Existing situation Learning Objectives To explain types of healthcare waste and associated risks To outline policy & guidelines on Healthcare Waste Management (HCWM)
Module 6. HEALTHCARE WASTE MANAGEMENT Existing situation Learning Objectives To explain types of healthcare waste and associated risks To outline policy & guidelines on Healthcare Waste Management (HCWM)
TABLE OF CONTENTS FOREWORD... 2 INTRODUCTION SCOPE NORMATIVE REFERENCES DEFINITIONS AND ABBREVIATIONS REQUIREMENTS...
 REFERENCE REV TITLE SPECIFICATION FOR HARD HATS DATE: OCTOBER 2018 PAGE: 1 OF 9 TABLE OF CONTENTS Page FOREWORD... 2 INTRODUCTION... 3 1 SCOPE... 3 2 NORMATIVE REFERENCES... 3 3 DEFINITIONS AND ABBREVIATIONS...
REFERENCE REV TITLE SPECIFICATION FOR HARD HATS DATE: OCTOBER 2018 PAGE: 1 OF 9 TABLE OF CONTENTS Page FOREWORD... 2 INTRODUCTION... 3 1 SCOPE... 3 2 NORMATIVE REFERENCES... 3 3 DEFINITIONS AND ABBREVIATIONS...
POSITION PAPER Moving from the MDD to the MDR
 A summary of Key Changes regarding Sterile Packaging and considerations on recommended changes to standards Introduction EN ISO 11607 specifies the requirements and test methods for materials, preformed
A summary of Key Changes regarding Sterile Packaging and considerations on recommended changes to standards Introduction EN ISO 11607 specifies the requirements and test methods for materials, preformed
TÜV SÜD BABT Production Quality Certification Scheme
 TÜV SÜD BABT Production Quality Certification Scheme The Production Quality Certification Scheme for Manufacturers A Certification Body of Copyright TÜV SÜD BABT 2014 Page 1 of 38 CONTENTS Page AMENDMENT
TÜV SÜD BABT Production Quality Certification Scheme The Production Quality Certification Scheme for Manufacturers A Certification Body of Copyright TÜV SÜD BABT 2014 Page 1 of 38 CONTENTS Page AMENDMENT
Requirements for the construction and testing of packagings for class 6.2 infectious substances of category A
 Chapter 6.3 6.3.1 General Requirements for the construction and testing of packagings for class 6. infectious substances of category A NOTE: The requirements of this Chapter don't apply to packagings used
Chapter 6.3 6.3.1 General Requirements for the construction and testing of packagings for class 6. infectious substances of category A NOTE: The requirements of this Chapter don't apply to packagings used
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
ISO 3008 INTERNATIONAL STANDARD. Fire-resistance tests Door and shutter assemblies. Essais de résistance au feu Assemblages porte et volet
 INTERNATIONAL STANDARD ISO 3008 Third edition 2007-09-15 Fire-resistance tests Door and shutter assemblies Essais de résistance au feu Assemblages porte et volet Reference number ISO 2007 Provläsningsexemplar
INTERNATIONAL STANDARD ISO 3008 Third edition 2007-09-15 Fire-resistance tests Door and shutter assemblies Essais de résistance au feu Assemblages porte et volet Reference number ISO 2007 Provläsningsexemplar
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
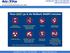 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
UN 3291 Clinical and Related Wastes Services Specification
 SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
ISO Packaging Plastics drums Non-removable head (tight head) drums with a nominal capacity of 208,2 l and 220 l
 INTERNATIONAL STANDARD ISO 20848-2 First edition 2006-09-15 Packaging Plastics drums Part 2: Non-removable head (tight head) drums with a nominal capacity of 208,2 l and 220 l Emballages Fûts en matière
INTERNATIONAL STANDARD ISO 20848-2 First edition 2006-09-15 Packaging Plastics drums Part 2: Non-removable head (tight head) drums with a nominal capacity of 208,2 l and 220 l Emballages Fûts en matière
MortarNet (updated to include Insect Barrier) Master Specification
 326 Melton Rd. Burns Harbor, IN 46304 Tel: 800-664-6638 Fax: 219-787-5088 Web: www.mortarnet.com MortarNet (updated to include Insect Barrier) Master Specification MASONRY ACCESSORIES 04 05 23 This MANU-SPEC
326 Melton Rd. Burns Harbor, IN 46304 Tel: 800-664-6638 Fax: 219-787-5088 Web: www.mortarnet.com MortarNet (updated to include Insect Barrier) Master Specification MASONRY ACCESSORIES 04 05 23 This MANU-SPEC
UNITED NATIONS / DOT PERFORMANCE CERTIFICATION 4G DESIGN QUALIFICATION
 UNITED NATIONS / DOT PERFORMANCE CERTIFICATION 4G DESIGN QUALIFICATION 6 x 2.5 Liter Plastic Bottle Packaging (PT 04003) with Two Case Sealing Mechanisms: #1) Taped Top and Bottom Flaps #2) Taped Top and
UNITED NATIONS / DOT PERFORMANCE CERTIFICATION 4G DESIGN QUALIFICATION 6 x 2.5 Liter Plastic Bottle Packaging (PT 04003) with Two Case Sealing Mechanisms: #1) Taped Top and Bottom Flaps #2) Taped Top and
MATERIAL SAFETY DATA SHEET. for AEROFOAM NBR
 MATERIAL SAFETY DATA SHEET for AEROFOAM NBR Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND OF THE COMPANY PRODUCT NAME: Aerofoam NBR USE OF THE PRODUCT: Insulation and protection for
MATERIAL SAFETY DATA SHEET for AEROFOAM NBR Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND OF THE COMPANY PRODUCT NAME: Aerofoam NBR USE OF THE PRODUCT: Insulation and protection for
TRAPEZOIDAL SHEET METAL RAIL LIFT/VARIO for trapezoidal sheet metal roofs
 Photovoltaic mounting systems Assembly Instructions TRAPEZOIDAL SHEET METAL RAIL LIFT/VARIO for trapezoidal sheet metal roofs 1 Table of contents 1 Introduction 1.1 Intended use 3 1.2 About the document
Photovoltaic mounting systems Assembly Instructions TRAPEZOIDAL SHEET METAL RAIL LIFT/VARIO for trapezoidal sheet metal roofs 1 Table of contents 1 Introduction 1.1 Intended use 3 1.2 About the document
TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION. SPECIFICATION NO. TxDOT DATED: JUNE 2007 MES Class Code:
 TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION SPECIFICATION NO. MES Class Code: 050000 THERMAL PROPERTY ANALYZER PUBLICATION This specification is a product of the Texas Department of Transportation
TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION SPECIFICATION NO. MES Class Code: 050000 THERMAL PROPERTY ANALYZER PUBLICATION This specification is a product of the Texas Department of Transportation
Medical Devices. The technical requirements also apply when the submitter is not the legal manufacturer 1 (i.e: a distribution company).
 UNFPA Technical requirements for medical devices 1. Introduction The following document provides UNFPA s technical requirements in the procurement of medical devices (medical equipment, renewable medical
UNFPA Technical requirements for medical devices 1. Introduction The following document provides UNFPA s technical requirements in the procurement of medical devices (medical equipment, renewable medical
BUFAB Supplier Manual 2.0
 Page 1/11 BUFAB Supplier Manual 2.0 Page 2/11 Table of contents Flowchart order handling... 2 Order handling... 3 Packing instructions... 4 General... 4 Labels... 5 Pallets... 5-6 Packing pallets... 6
Page 1/11 BUFAB Supplier Manual 2.0 Page 2/11 Table of contents Flowchart order handling... 2 Order handling... 3 Packing instructions... 4 General... 4 Labels... 5 Pallets... 5-6 Packing pallets... 6
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea
 How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
CHECKLIST FOR USE WITH THE R2 PRACTICES
 CHECKLIST FOR USE WITH THE R2 PRACTICES I. FACILITY INFORMATION Include facility name, address, contact information, essential descriptive information about the facility operations. II. R2 PROVISIONS Provision
CHECKLIST FOR USE WITH THE R2 PRACTICES I. FACILITY INFORMATION Include facility name, address, contact information, essential descriptive information about the facility operations. II. R2 PROVISIONS Provision
PI s Name Date Bldg./Rm#
 PI s Name Date Bldg./Rm# Animal Biosafety Level 3 (ABSL-3) Yes No 1. Is access to the animal facility limited or restricted only to those persons authorized for program or support purposes? Yes No 2. Does
PI s Name Date Bldg./Rm# Animal Biosafety Level 3 (ABSL-3) Yes No 1. Is access to the animal facility limited or restricted only to those persons authorized for program or support purposes? Yes No 2. Does
Forest Stewardship Council
 Forest Stewardship Council January 2018 Requirements for use of the FSC trademarks by non-certificate holders crosswalk V1-0 and V2-0 This crosswalk has been created to help facilitate the public consultation
Forest Stewardship Council January 2018 Requirements for use of the FSC trademarks by non-certificate holders crosswalk V1-0 and V2-0 This crosswalk has been created to help facilitate the public consultation
BUREAU OF INDIAN STANDARDS. DRAFT FOR COMMENTS ONLY (Not to be reproduced without the permission of BIS or used as an Indian Standard)
 For BIS Use Only Doc No. CED 50(7474) BUREAU OF INDIAN STANDARDS DRAFT FOR COMMENTS ONLY (Not to be reproduced without the permission of BIS or used as an Indian Standard) POLYETHYLENE FITTINGS FOR USE
For BIS Use Only Doc No. CED 50(7474) BUREAU OF INDIAN STANDARDS DRAFT FOR COMMENTS ONLY (Not to be reproduced without the permission of BIS or used as an Indian Standard) POLYETHYLENE FITTINGS FOR USE
Container Owners Association. Code of practice, for a single-use flexitank system.
 Container Owners Association Code of practice, for a single-use flexitank system. COA Code of practice, for a single use flexitank system: Version 5 rev. September 2016 www.containerownersassociation.org
Container Owners Association Code of practice, for a single-use flexitank system. COA Code of practice, for a single use flexitank system: Version 5 rev. September 2016 www.containerownersassociation.org
The Bowie and Dick Type Test Does size and penetration really matter?
 The Bowie and Dick Type Test Does size and penetration really matter? Presented by Terry McAuley Sterilisation and Infection Control Consultant Discuss the purpose of the Bowie Dick type test Examine the
The Bowie and Dick Type Test Does size and penetration really matter? Presented by Terry McAuley Sterilisation and Infection Control Consultant Discuss the purpose of the Bowie Dick type test Examine the
Biosafety Level 2 Criteria Based on Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition
 Biosafety Level 2 Criteria Based on Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition Biosafety Level 2 (BSL-2): Biosafety Level 2 builds upon BSL-1. BSL-2 is suitable for work
Biosafety Level 2 Criteria Based on Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition Biosafety Level 2 (BSL-2): Biosafety Level 2 builds upon BSL-1. BSL-2 is suitable for work
Technical Specification for. Insulators. Part 1. Insulators for High and Extra-High Voltage Overhead Lines
 Page: 1 / 18 for Insulators Part 1 Insulators for High and Extra-High Voltage Overhead Lines 1.2 Cap-and-pin insulators made of pre-stressed glass This technical specification is valid for the business
Page: 1 / 18 for Insulators Part 1 Insulators for High and Extra-High Voltage Overhead Lines 1.2 Cap-and-pin insulators made of pre-stressed glass This technical specification is valid for the business
INSTRUMENT RETURN PROCEDURES
 INSTRUMENT RETURN PROCEDURES An instrument problem may be reported by calling, e-mailing, faxing or mailing BioFire Defense. The contact numbers are located below. You will also need the following documents:
INSTRUMENT RETURN PROCEDURES An instrument problem may be reported by calling, e-mailing, faxing or mailing BioFire Defense. The contact numbers are located below. You will also need the following documents:
RapidPort EZ Port Applier DIRECTIONS FOR USE (DFU)
 RapidPort EZ Port Applier DIRECTIONS FOR USE (DFU) RapidPort EZ Port Applier Ref. No. C-20390 RapidPort EZ Port Applier INTRODUCTION The RapidPort EZ Port Applier is an optional accessory for the LAP-BAND
RapidPort EZ Port Applier DIRECTIONS FOR USE (DFU) RapidPort EZ Port Applier Ref. No. C-20390 RapidPort EZ Port Applier INTRODUCTION The RapidPort EZ Port Applier is an optional accessory for the LAP-BAND
UNITED NATIONS / DOT PERFORMANCE CERTIFICATION 4G DESIGN QUALIFICATION
 UNITED NATIONS / DOT PERFORMANCE CERTIFICATION 4G DESIGN QUALIFICATION 6 x 1 Liter Square Plastic Bottle with Two Neck Finish Options: #1) 38-439 Neck and #2) 45mm Neck TEST REPORT #: 15-CA2077 u n 4G
UNITED NATIONS / DOT PERFORMANCE CERTIFICATION 4G DESIGN QUALIFICATION 6 x 1 Liter Square Plastic Bottle with Two Neck Finish Options: #1) 38-439 Neck and #2) 45mm Neck TEST REPORT #: 15-CA2077 u n 4G
Lab Biosafety Self-Audit Form (Applies to all microbial work.)
 Principal Investigator: Lab Biosafety Self-Audit Form (Applies to all microbial work.) Office Phone#: Lab Location: Lab Phone #: Person Completing Audit: Date: Type Of Biological Material Used Human samples
Principal Investigator: Lab Biosafety Self-Audit Form (Applies to all microbial work.) Office Phone#: Lab Location: Lab Phone #: Person Completing Audit: Date: Type Of Biological Material Used Human samples
Classification and packaging for infectious waste of Category A. Transmitted by the experts from Canada and the United Kingdom *
 United Nations Secretariat ST/SG/AC.10/C.3/2017/25 Distr.: General 13 April 2017 Original: English Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification
United Nations Secretariat ST/SG/AC.10/C.3/2017/25 Distr.: General 13 April 2017 Original: English Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification
CCD-104 / GS-41. Hand Cleaners / Hand Soaps
 CCD-104 / GS-41 Hand Cleaners / Hand Soaps Introduction Green Seal and the Environmental Choice Program M are designed to support a continuing effort to improve and maintain environmental quality by reducing
CCD-104 / GS-41 Hand Cleaners / Hand Soaps Introduction Green Seal and the Environmental Choice Program M are designed to support a continuing effort to improve and maintain environmental quality by reducing
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 6670 Second edition 2002-08-01 Instant coffee Sampling method for bulk units with liners Café soluble Méthode d'échantillonnage pour emballages en vrac avec doublure Reference
INTERNATIONAL STANDARD ISO 6670 Second edition 2002-08-01 Instant coffee Sampling method for bulk units with liners Café soluble Méthode d'échantillonnage pour emballages en vrac avec doublure Reference
Standard and Directives on Medical Devices
 Standard and Directives on Medical Devices Definition A Medical Device is identified by means of its INTENDED PURPOSE Intended to treat, prevent or control physiological characteristics of a living being
Standard and Directives on Medical Devices Definition A Medical Device is identified by means of its INTENDED PURPOSE Intended to treat, prevent or control physiological characteristics of a living being
Changes in the EXCiPACT 2017 Edition Requirements from the 2012 Edition
 Changes in the EXCiPACT 2017 Edition Requirements from the 2012 Edition Blue text indicates changes to both GMP and GDP requirements Orange text indicates specific changes to the GDP requirements Items
Changes in the EXCiPACT 2017 Edition Requirements from the 2012 Edition Blue text indicates changes to both GMP and GDP requirements Orange text indicates specific changes to the GDP requirements Items
SAFETY DATA SHEET. The mixture does not meet the criteria for classification.
 SAFETY DATA SHEET 1. Identification Product identifier Other means of identification None. Recommended use Research use only. Recommended restrictions None known. Manufacturer/Importer/Supplier/Distributor
SAFETY DATA SHEET 1. Identification Product identifier Other means of identification None. Recommended use Research use only. Recommended restrictions None known. Manufacturer/Importer/Supplier/Distributor
TECHNICAL REQUIREMENTS (CLAUSE 5)
 LECTURE 4 THE ELEMENTS OF ISO/IEC 17025 - TECHNICAL REQUIREMENTS (CLAUSE 5) Slide 1 ISO 17025 - RELATIONSHIP OF EACH REQUIREMENTS Management System Organization Quality system Continual Improvement Management
LECTURE 4 THE ELEMENTS OF ISO/IEC 17025 - TECHNICAL REQUIREMENTS (CLAUSE 5) Slide 1 ISO 17025 - RELATIONSHIP OF EACH REQUIREMENTS Management System Organization Quality system Continual Improvement Management
Procurement Specifications Painted, Plated and Pad Printed Parts PS002
 1.0 Purpose The purpose of this document is to ensure that painted, plated and printed parts meet both Kamek and end-customer requirements, and, when applicable, regulatory authorities requirements. 2.0
1.0 Purpose The purpose of this document is to ensure that painted, plated and printed parts meet both Kamek and end-customer requirements, and, when applicable, regulatory authorities requirements. 2.0
TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION
 TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION MES Class Code No. 335000 STAND-ALONE EIGHT-PAGE BUCKLE FOLDER WITH DOCUMENT FEEDER PUBLICATION This specification is a product of the Texas
TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION MES Class Code No. 335000 STAND-ALONE EIGHT-PAGE BUCKLE FOLDER WITH DOCUMENT FEEDER PUBLICATION This specification is a product of the Texas
SPECIFICATION NO. TxDOT DATED: JUNE 2005
 TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION CABINET, CORROSION TESTING PUBLICATION This specification is a product of the Texas Department of Transportation (TxDOT). It is the practice
TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION CABINET, CORROSION TESTING PUBLICATION This specification is a product of the Texas Department of Transportation (TxDOT). It is the practice
This document is a preview generated by EVS
 CONSOLIDATED VERSION IEC 62304 Edition 1.1 2015-06 colour inside Medical device software Software life cycle processes IEC 62304:2006-05+AMD1:2015-06 CSV(en) THIS PUBLICATION IS COPYRIGHT PROTECTED Copyright
CONSOLIDATED VERSION IEC 62304 Edition 1.1 2015-06 colour inside Medical device software Software life cycle processes IEC 62304:2006-05+AMD1:2015-06 CSV(en) THIS PUBLICATION IS COPYRIGHT PROTECTED Copyright
ThePeople s RepublicofChina
 ThePeople s RepublicofChina EDICTOFGOVERNMENT± Inordertopromotepubliceducationandpublicsafety,equaljusticefor al,abeterinformedcitizenry,theruleoflaw,worldtradeandworld peace,thislegaldocumentisherebymadeavailableonanoncommercial
ThePeople s RepublicofChina EDICTOFGOVERNMENT± Inordertopromotepubliceducationandpublicsafety,equaljusticefor al,abeterinformedcitizenry,theruleoflaw,worldtradeandworld peace,thislegaldocumentisherebymadeavailableonanoncommercial
TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION
 TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION SPECIFICATION NO. * PRINTING CERTIFICATE OF TITLE ENVELOPE PUBLICATION This specification is a product of the Texas Department of Transportation
TEXAS DEPARTMENT OF TRANSPORTATION GENERAL SERVICES DIVISION SPECIFICATION NO. * PRINTING CERTIFICATE OF TITLE ENVELOPE PUBLICATION This specification is a product of the Texas Department of Transportation
COMPILED BY FUNCTIONAL RESP. APPROVED BY AUTHORIZED BY TABLE OF CONTENTS FOREWORD... 2 INTRODUCTION SCOPE NORMATIVE REFERENCES...
 TITLE SPECIFICATION FOR PVC ADHESIVE DATE: JUNE 2017 PAGE: 1 OF 19 COMPILED BY FUNCTIONAL RESP. APPROVED BY AUTHORIZED BY. TLADI CONSULTING.. Z NGQWALA TECHNICIAN UNDERGROUND. M MAGEMBA GENERAL MANAGER:(A)
TITLE SPECIFICATION FOR PVC ADHESIVE DATE: JUNE 2017 PAGE: 1 OF 19 COMPILED BY FUNCTIONAL RESP. APPROVED BY AUTHORIZED BY. TLADI CONSULTING.. Z NGQWALA TECHNICIAN UNDERGROUND. M MAGEMBA GENERAL MANAGER:(A)
(4) Production inspection is the inspection that must initially be conducted on each newly manufactured IBC.
 Subpart O Testing of IBCs 178.800 Purpose and scope. This subpart prescribes certain testing requirements for IBCs identified in subpart N of this part. [Amdt. 178 103, 59 FR 38074, July 26, 1994, as amended
Subpart O Testing of IBCs 178.800 Purpose and scope. This subpart prescribes certain testing requirements for IBCs identified in subpart N of this part. [Amdt. 178 103, 59 FR 38074, July 26, 1994, as amended
PRODUCT HSE: Clauses HSE Technological purchase
 REFERENCE SYSTEM PRODUCT HSE: Clauses HSE Technological purchase Reference : 83570258-HSE-ITA-EN Revision : 001 Date of revision : 05 Nov 2012 Group revision Page 1 / 13 APPLICABILITY COUNTRY - SITE COMPANY
REFERENCE SYSTEM PRODUCT HSE: Clauses HSE Technological purchase Reference : 83570258-HSE-ITA-EN Revision : 001 Date of revision : 05 Nov 2012 Group revision Page 1 / 13 APPLICABILITY COUNTRY - SITE COMPANY
Wilson-Hurd ATF Quality Manual
 Wilson-Hurd ATF Quality Manual Wilson-Hurd Manufacturing Company Advanced Technical Facility (ATF) 311 Winton Street Wausau, WI 54403 Rev. Date: 04 May 2017 Pete Dehne Vice President, Operations Table
Wilson-Hurd ATF Quality Manual Wilson-Hurd Manufacturing Company Advanced Technical Facility (ATF) 311 Winton Street Wausau, WI 54403 Rev. Date: 04 May 2017 Pete Dehne Vice President, Operations Table
/ Clinical Waste & Offensive Waste Disposal Procedures
 / Clinical Waste & Offensive Waste Disposal Procedures Document Control Document Created by Shane McAteer Ver. 1 26/01/2011 Gary Stratmann Ver. 3.1 08/01/2016 Last Updated by 1 Introduction This clinical
/ Clinical Waste & Offensive Waste Disposal Procedures Document Control Document Created by Shane McAteer Ver. 1 26/01/2011 Gary Stratmann Ver. 3.1 08/01/2016 Last Updated by 1 Introduction This clinical
Dŵr Cymru Welsh Water s Self-lay Policy for Water Mains and Services July 2015
 Dŵr Cymru Welsh Water s Self-lay Policy for Water Mains and Services July 2015 Introduction This Self-Lay Policy applies when a developer decides to self-lay water mains and services (ie. construct water
Dŵr Cymru Welsh Water s Self-lay Policy for Water Mains and Services July 2015 Introduction This Self-Lay Policy applies when a developer decides to self-lay water mains and services (ie. construct water
ISO INTERNATIONAL STANDARD. Non-destructive testing of welds Visual testing of fusion-welded joints
 INTERNATIONAL STANDARD ISO 17637 First edition 2003-07-15 Non-destructive testing of welds Visual testing of fusion-welded joints Contrôle non destructif des assemblages soudés Contrôle visuel des assemblages
INTERNATIONAL STANDARD ISO 17637 First edition 2003-07-15 Non-destructive testing of welds Visual testing of fusion-welded joints Contrôle non destructif des assemblages soudés Contrôle visuel des assemblages
SHIPPER S RESPONSIBILITIES
 International Civil Aviation Organization 29/8/07 WORKING PAPER DANGEROUS GOODS PANEL (DGP) TWENTY-FIRST MEETING Montréal, 5 to 16 November 2007 Agenda Item 2: Development of recommendations for amendments
International Civil Aviation Organization 29/8/07 WORKING PAPER DANGEROUS GOODS PANEL (DGP) TWENTY-FIRST MEETING Montréal, 5 to 16 November 2007 Agenda Item 2: Development of recommendations for amendments
Environmental Management Chapter ALABAMA DEPARTMENT OF ENVIRONMENTAL MANAGEMENT LAND DIVISION MEDICAL WASTE PROGRAM ADMINISTRATIVE CODE
 ALABAMA DEPARTMENT OF ENVIRONMENTAL MANAGEMENT LAND DIVISION MEDICAL WASTE PROGRAM ADMINISTRATIVE CODE CHAPTER 335-17-3 COLLECTION OF MEDICAL WASTE TABLE OF CONTENTS 335-17-3-.01 Collections Of Untreated
ALABAMA DEPARTMENT OF ENVIRONMENTAL MANAGEMENT LAND DIVISION MEDICAL WASTE PROGRAM ADMINISTRATIVE CODE CHAPTER 335-17-3 COLLECTION OF MEDICAL WASTE TABLE OF CONTENTS 335-17-3-.01 Collections Of Untreated
INSTARMAC PERMANENT COLD LAY SURFACING MATERIALS ULTRACRETE INSTANT ROAD REPAIR 6 MM
 H A P A S Instarmac Group plc Danny Morson Way Birch Coppice Business Park Dordon, Tamworth Staffordshire B78 1SE Tel: 01827 872244 Fax: 01827 874466 HAPAS Certificate e-mail: enquiries@instarmac.co.uk
H A P A S Instarmac Group plc Danny Morson Way Birch Coppice Business Park Dordon, Tamworth Staffordshire B78 1SE Tel: 01827 872244 Fax: 01827 874466 HAPAS Certificate e-mail: enquiries@instarmac.co.uk
Electronics Disposition
 Environmental Partner Performance Standard Page 1 of 9 1.0 Purpose The purpose of this Standard is to define minimum requirements for electronics disposition programs. 2.0 Applicability These requirements
Environmental Partner Performance Standard Page 1 of 9 1.0 Purpose The purpose of this Standard is to define minimum requirements for electronics disposition programs. 2.0 Applicability These requirements
ISO INTERNATIONAL STANDARD. Metallic materials Tensile testing Part 2: Method of test at elevated temperature
 INTERNATIONAL STANDARD ISO 6892-2 First edition 2011-02-15 Metallic materials Tensile testing Part 2: Method of test at elevated temperature Matériaux métalliques Essai de traction Partie 2: Méthode d'essai
INTERNATIONAL STANDARD ISO 6892-2 First edition 2011-02-15 Metallic materials Tensile testing Part 2: Method of test at elevated temperature Matériaux métalliques Essai de traction Partie 2: Méthode d'essai
GS-10 GREEN SEAL STANDARD FOR COATED PRINTING PAPER. EDITION 2.1 July 12, 2013
 GS-10 GREEN SEAL STANDARD FOR COATED PRINTING PAPER EDITION 2.1 July 12, 2013 Green Seal, Inc. 1001 Connecticut Ave. NW, Ste 827 Washington, DC USA 20036-5525 (202) 872-6400 FAX (202) 872-4324 www.greenseal.org
GS-10 GREEN SEAL STANDARD FOR COATED PRINTING PAPER EDITION 2.1 July 12, 2013 Green Seal, Inc. 1001 Connecticut Ave. NW, Ste 827 Washington, DC USA 20036-5525 (202) 872-6400 FAX (202) 872-4324 www.greenseal.org
PMA 31-G. English. Printed: Doc-Nr: PUB / / 000 / 00
 PMA 31-G English 1 Information about the documentation 1.1 About this documentation Read this documentation before initial operation or use. This is a prerequisite for safe, trouble-free handling and
PMA 31-G English 1 Information about the documentation 1.1 About this documentation Read this documentation before initial operation or use. This is a prerequisite for safe, trouble-free handling and
Environmental Management System
 Environmental Management System Moreton Bay Research Station (MBRS) Clinical and Related Waste 1. Scope This policy applies to all clinical and related waste (refer to definitions in Section 6) from the
Environmental Management System Moreton Bay Research Station (MBRS) Clinical and Related Waste 1. Scope This policy applies to all clinical and related waste (refer to definitions in Section 6) from the
VOLTAGE MULTIPLIERS, INC. QUALITY SUPPLEMENT TO PURCHASE ORDERS
 The requirements of this supplement apply to the purchase order to which it is attached / referenced, to the extent applicable. Acceptance of the purchase order by the vendor constitutes acceptance of
The requirements of this supplement apply to the purchase order to which it is attached / referenced, to the extent applicable. Acceptance of the purchase order by the vendor constitutes acceptance of
Medical devices Quality management systems Requirements for regulatory purposes
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 13485 Third edition 2016-03-01 Medical devices Quality management systems Requirements for regulatory purposes Dispositifs médicaux Systèmes de
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 13485 Third edition 2016-03-01 Medical devices Quality management systems Requirements for regulatory purposes Dispositifs médicaux Systèmes de
Health Care Waste Management - To Reduce the Burden of Disease, Health- Care Waste Needs Sound Management, Including Alternatives to Incineration
 Health Care Waste Management - To Reduce the Burden of Disease, Health- Care Waste Needs Sound Management, Including Alternatives to Incineration Fact Sheet No. 281 August 2004 In the last few years there
Health Care Waste Management - To Reduce the Burden of Disease, Health- Care Waste Needs Sound Management, Including Alternatives to Incineration Fact Sheet No. 281 August 2004 In the last few years there
DEMOLITION TRADE SPECIFICATION
 DEMOLITION TRADE SPECIFICATION GENERAL a) BDW Trading Limited Barratt Homes and David Wilson Homes are all trading names of BDW Trading Limited the Company. b) Site Condition The Contractor is to examine
DEMOLITION TRADE SPECIFICATION GENERAL a) BDW Trading Limited Barratt Homes and David Wilson Homes are all trading names of BDW Trading Limited the Company. b) Site Condition The Contractor is to examine
LABORATORY WASTE DISPOSAL GUIDE
 LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
3. Definitions Refer to Doc 525: Diesel-fueled Incinerator Operation, Maintenance and Monitoring Guidance for definitions.
 Audit Checklist: Incinerator Operation Document Number: 209 1. Purpose This document provides a checklist to guide managers who must audit incinerator operation to ensure that incinerators are being operated
Audit Checklist: Incinerator Operation Document Number: 209 1. Purpose This document provides a checklist to guide managers who must audit incinerator operation to ensure that incinerators are being operated
