Copyright 2017 Aegis Sciences Corporation All Rights Reserved
|
|
|
- Susanna Quinn
- 6 years ago
- Views:
Transcription
1 Oral Fluid Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms (CCFs), leak-resistant plastic bags, absorbent material, shipping containers, and disposable gloves). 2. Ensure that there is a means for washing hands, suitable clean surface for the collector to use as a work area, and a secure temporary storage area for maintaining specimens until they are transferred to the laboratory. 3. Ensure access to collection supplies is restricted only to the collector(s)/donor(s) and other authorized personnel. Oral Fluid Collection 1. Obtain and complete the applicable Laboratory Request Form (instructions are included in Appendix 1). For electronic requisitions see Appendix 2. a. Write the donor s name and date of birth on both the adhesive label and the Requisition Form b. Enter remaining requisition data c. Verify with the donor their name and date of birth 2. Check the Quantisal packaging to ensure the expiration date has not been exceeded. a. Do not use expired devices i. Ask the practitioner if an alternative specimen may be collected ii. Discard expired devices iii. Order more supplies 3. Ask the donor to swish and spit with water at least 10 minutes prior to the specimen a. The donor must then refrain from consumption of food (including gum, candy, etc.) or beverage (including water, coffee, soda, etc.) until after sample 4. Instruct the donor to wash their hands thoroughly and not touch or handle anything prior to opening the collection kit. 5. Ask the donor to accumulate saliva in his/her mouth prior to starting 6. Open Quantisal package. Ask the donor to remove the collection device components (transport tube and sealed collector pad) from its package. Set the transport tube aside for later use. 7. Ask the donor to peel open the sealed package and remove the collector pad. Keep the pointed end of the device pointed down once opened. Do not handle the pad or set it on a counter. 8. Instruct donor to position the pad under the tongue, close their mouth as they would with a thermometer, and keep their head down to allow gravity to help with saliva
2 NOTE: Advise the donor to avoid chewing or sucking the pad 9. The collection device should remain under the tongue until the stem indicator turns completely blue (see successful collection photo). The collection technician must not remove the collection device at the first sign of blue in the indicator window, as illustrated in the failed attempt photo. 10. Prior to removing collection pad from the donor s mouth, the collection technician must put on clean medical gloves. 11. Remove collection device as soon as the indicator turns completely blue. 12. If the indicator has not turned blue in 10 minutes, remove the device and discard. The donor may try drinking water. After drinking water and waiting 10 minutes, try to recollect with another device. 13. If donor cannot provide an adequate oral fluid specimen, the physician may request an alternative specimen type (blood or urine). The alternative specimen should be collected and submitted for testing instead of the OF kit to ensure accuracy of test results. DO NOT send in the oral fluid specimen, as the blue color will continue to develop during transport and the laboratory will be unaware of the insufficient volume. Discard the specimen. 14. Hold the Quantisal transport tube in an upright position and uncap by pushing up the red cap. 15. It is critical not to spill or empty the proprietary blue buffer liquid (3mL) from the tube. 16. With clean gloved hands from step 10, the collection technician should insert the device into the uncapped transport tube and carefully replace the cap. Ensure you hear a definite snap when closing the transport tube. NOTE: Do not ingest blue buffer solution or place device in mouth after it has made contact with the buffer solution *In order to avoid contamination, the swab must only be handled with clean donor hands or clean gloved hands 17. Remove the label from the top of the Laboratory Request Form and place the label length-wise on the side of the tube DO NOT wrap the label around the tube otherwise the barcode cannot be read. 18. Place the closed transport tube into the specimen bag with the absorbent pad. a. Fold and place the completed Laboratory Request Form along with a copy of the donor s insurance card in the back pouch of the Aegis specimen bag. b. If the patient does not have an insurance card, you must obtain and verify their social security number and document it in the demographics section of the Laboratory Request Form. 19. It is important that the specimen be in one compartment and the paperwork in the other to avoid contamination of the requisition in the event of specimen leakage. 20. Seal the bag using the zip-top closure. 21. Immediately place the sealed specimen bag into the proper shipping container. 22. Oral Fluid specimens may be shipped in the same package as other specimens collected that day.
3 23. If no urine specimens are collected on the same day you collect an oral fluid sample, place the FedEx Billable Stamp on the Small Clinical Box and proceed with shipping. The Small Clinical Box may be shipped along with urine specimens in the FedEx Clinical Pak if space permits. If oral fluid specimens are being shipped along with urine in the Large Clinical Box, no other outer packaging is needed. 24. Remove gloves and discard into the Hazardous waste container. 25. Wash and dry your hands. 26. Ship to the laboratory within 24 hours of collection using the preprinted FedEx labels provided by Aegis. NOTE: If the specimen(s) cannot be shipped within 24 hours of collection, refrigeration is recommended. If the specimen(s) cannot be shipped within 48 hours of collection, freezing is recommended. Laboratory Request Form Completion Diagnosis Code(s) Appendix 1 NOTE: All requisitions must have a valid ICD-10 code provided by the physician to support the medical necessity of the order. Diagnosis codes are 3-7 characters (e.g., M79.604). The first digit is alpha, 2 nd and 3 rd are numeric and 4-7 can be alpha and/or numeric. 1. Codes beginning with a numeric digit are not valid. All diagnosis codes must begin with a letter. 2. V58.69 is not a valid code Billing/Insurance Obtain insurance information and ALWAYS validate it with the patient. Mark the appropriate check box on the Laboratory Request Form. If Worker s Comp, Letter of Protection (LOP), or Auto: Completely fill out the Aegis Insurance Information Form and make a copy of the applicable Letter of Protection (LOP), the front and back of the auto insurance card and the patient s health insurance card and send in with the specimen. Patient Information Fill in patients complete Social Security Number, First Name, Middle Initial, Last Name, Sex, Date of Birth, Address, City, State, Zip Code and Phone Number Patient Signature Ask the patient to verify their information and get their signature
4 Collector s Initials Legibly write your initials in the box. Date Collected This is the Date of Service (DOS). Requesting Provider Select the appropriate requesting provider (Choose only one). Sample Label With the patient present and after completing the patient information, remove the label and place it on the top of the specimen device. Test Selected Select the test(s) requested by the provider. Prescribed Medication(s) Mark the drugs prescribed and the appropriate usage either Daily or PRN. Provider Signature Obtain the ordering provider s signature. Appendix 2 Contact Aegis Sciences Corporation at (800) for options to utilize an Electronic Laboratory Requisition Packaging and shipping samples Appendix 3 At the end of the business day package all secured specimens for shipment. Select the appropriate container based on the number of specimens. Place the FedEx Express Paid Shipping label on the package for pickup. Schedule pickup by calling GoFedEx ( ) or go to fedex.com. Small white Aegis urine specimen box ( white box ) up to 6 specimens
5 Small and Large Aegis Clinical Boxes ( Aegis box ) up to 20 Clinical pack bag
Specimen labeling and shipping protocol.
 Specimen labeling and shipping protocol. SENDING YOUR URINE OR ORAL FLUID SPECIMENS TO THE LABORATORY Preparing specimens for the laboratory 2 Specimen labeling and shipping We ask that you refer to the
Specimen labeling and shipping protocol. SENDING YOUR URINE OR ORAL FLUID SPECIMENS TO THE LABORATORY Preparing specimens for the laboratory 2 Specimen labeling and shipping We ask that you refer to the
RNAPro SAL ORAL SPECIMEN COLLECTION SYSTEM. Catalog Number RPSAL-701. Transparent Plastic Compression Tube. Compression Seal
 RNAPro SAL ORAL SPECIMEN COLLECTION SYSTEM Catalog Number RPSAL-701 Transparent Plastic Compression Tube Splitting Unit Equipped with Variable Filtration Media Compression Seal Ergonomically Correct Handle
RNAPro SAL ORAL SPECIMEN COLLECTION SYSTEM Catalog Number RPSAL-701 Transparent Plastic Compression Tube Splitting Unit Equipped with Variable Filtration Media Compression Seal Ergonomically Correct Handle
Unless otherwise directed, collect the following specimens from each person who may have been exposed:
 Shipping Instructions for Specimens Collected from People Who May Have Been Exposed to Chemical-Terrorism Agents Section One: Collecting and Labeling Specimens Required Specimens Unless otherwise directed,
Shipping Instructions for Specimens Collected from People Who May Have Been Exposed to Chemical-Terrorism Agents Section One: Collecting and Labeling Specimens Required Specimens Unless otherwise directed,
PLATELET-ORIENTED INHIBITION IN NEW TIA
 PLATELET-ORIENTED INHIBITION IN NEW TIA AND MINOR ISCHEMIC STROKE ANCILLARY BIOMARKERS STUDY BLOOD SPECIMEN PROCEDURE MANUAL US Sites DNA Only DNA and Plasma CONTENTS I. DNA ONLY SPECIMEN PROCEDURES...
PLATELET-ORIENTED INHIBITION IN NEW TIA AND MINOR ISCHEMIC STROKE ANCILLARY BIOMARKERS STUDY BLOOD SPECIMEN PROCEDURE MANUAL US Sites DNA Only DNA and Plasma CONTENTS I. DNA ONLY SPECIMEN PROCEDURES...
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples
 Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS
 ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube
 Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube Tiger Top/SST 5.0 ml Screw Cap Tube Apply barcode label starting at the top of the sample tube (the end closest to the cap). The barcode
Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube Tiger Top/SST 5.0 ml Screw Cap Tube Apply barcode label starting at the top of the sample tube (the end closest to the cap). The barcode
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS
 ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
Manual of Procedures Version 2.1 May, 2017
 Specimen Collection, Processing and Internet-based Conversational Engagement Clinical Trial (I-CONECT) in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Manual of Procedures
Specimen Collection, Processing and Internet-based Conversational Engagement Clinical Trial (I-CONECT) in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Manual of Procedures
Morris Animal. FOUNDATION Golden Retriever Lifetime Study
 Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Annual Veterinary Sample Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you
Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Annual Veterinary Sample Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you
Standard Operating Procedure. Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples
 Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea
 How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
Standard Operating Procedure
 A tool to monitor your environmental exposure to common antineoplastic agents used in preparation and administration areas. Standard Operating Procedure Page 1 1. INTRODUCTION 3 2. WHAT TO DO AFTER RECEIVING
A tool to monitor your environmental exposure to common antineoplastic agents used in preparation and administration areas. Standard Operating Procedure Page 1 1. INTRODUCTION 3 2. WHAT TO DO AFTER RECEIVING
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS
 UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
CLIA Complexity: Waived for Finger-stick Whole Blood. Instructional video available online at quidel.com/sofia2lyme.
 Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity: Waived for Finger-stick Whole Blood Instructional
Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package Insert. CLIA Complexity: Waived for Finger-stick Whole Blood Instructional
MAY Research Blood and Tissue Kit Instructions
 Kit Inventory Instruction sheet Requisition Form Tissue Collection Supplies (2) Ambient Transport Bag (2) 4mL orange cap cryovials (2) 6mL Formalin (2) specimen labels ( 4 per sheet) (1) graduated pipet
Kit Inventory Instruction sheet Requisition Form Tissue Collection Supplies (2) Ambient Transport Bag (2) 4mL orange cap cryovials (2) 6mL Formalin (2) specimen labels ( 4 per sheet) (1) graduated pipet
Select a topic below or click here to be guided through the process.
 Select a topic below or click here to be guided through the process. 1. After Collection Is Complete 2. Making Forensic Corrections 3. Completing Chain of Custody 4. Making Copies of Chain of Custody Document
Select a topic below or click here to be guided through the process. 1. After Collection Is Complete 2. Making Forensic Corrections 3. Completing Chain of Custody 4. Making Copies of Chain of Custody Document
35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING
 35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING 35.1 Purpose Participants who enroll in the PVDOMICS study with be asked to provide biological specimens (blood and urine). The
35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING 35.1 Purpose Participants who enroll in the PVDOMICS study with be asked to provide biological specimens (blood and urine). The
TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015
 TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015 The TJEMS Drug Box Program Best Practices relate to the use of the TJEMS Drug Box. These best practices sever to provide guidance
TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015 The TJEMS Drug Box Program Best Practices relate to the use of the TJEMS Drug Box. These best practices sever to provide guidance
Preservation, Custody, and Shipment
 Watershed Monitoring Section Sampler Training Preservation, Custody, and Shipment Liz Miller February 28, 2018 Most recent custody sheet versions: Surface Water = January 2018 Ground Water = October 2017
Watershed Monitoring Section Sampler Training Preservation, Custody, and Shipment Liz Miller February 28, 2018 Most recent custody sheet versions: Surface Water = January 2018 Ground Water = October 2017
trucollect -plus - Stabilization and Transport Kit (10)
 trucollect -plus - Stabilization and Transport Kit (10) Whole blood specimen dry stabilization, transport, and storage For Research Use Only Not for use in diagnostic procedures PN 520254 Patents Granted
trucollect -plus - Stabilization and Transport Kit (10) Whole blood specimen dry stabilization, transport, and storage For Research Use Only Not for use in diagnostic procedures PN 520254 Patents Granted
Laboratory (LAB) prepares sample kits prior to Epidemiologist Investigator (EPI) arrival.
 Standard Operating Procedure for Sample Collection of Heavy Metals (As, Hg, U, Cd, Mn, Se), Creatinine, Phthalate Metabolites, 2,4-DCP, 2,5-DCP and Pyrethroids for Biomonitoring A. Scope and Purpose The
Standard Operating Procedure for Sample Collection of Heavy Metals (As, Hg, U, Cd, Mn, Se), Creatinine, Phthalate Metabolites, 2,4-DCP, 2,5-DCP and Pyrethroids for Biomonitoring A. Scope and Purpose The
transport of biological materials
 UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO STANDARD OPERATING PROCEDURE transport of biological materials Prepared by Date Adopted Procedure # IBC Approved EHS B001 May 13, 2014 ENVIRONMENTAL
UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO STANDARD OPERATING PROCEDURE transport of biological materials Prepared by Date Adopted Procedure # IBC Approved EHS B001 May 13, 2014 ENVIRONMENTAL
Guidance Note H1402 Packaging and Transport of waste from suspect
 uid Guidance Note H1402 Packaging and Transport of waste from suspect and confirmed cases of the Ebola Virus Revision: 1.0 Date: 21/10/2014 Contents 1 Purpose... 3 2 Introduction... 3 3 Regulations...
uid Guidance Note H1402 Packaging and Transport of waste from suspect and confirmed cases of the Ebola Virus Revision: 1.0 Date: 21/10/2014 Contents 1 Purpose... 3 2 Introduction... 3 3 Regulations...
Title: Engineering Services - Pneumatic Tube System Operational - Guidelines
 Document Owner: Timothy Markijohn Content Expert: Bobby Schnetzler Last Approved Date: 05/30/2015 Printed copies are for reference only. Please refer to the electronic copy for the latest version. I. Purpose
Document Owner: Timothy Markijohn Content Expert: Bobby Schnetzler Last Approved Date: 05/30/2015 Printed copies are for reference only. Please refer to the electronic copy for the latest version. I. Purpose
Transporting Biological Materials Training UCCBB
 Transporting Biological Materials Training UCCBB Histocompatibility Clinical Immunology University of Colorado Cord Blood Bank Stem Cell Laboratory Flow Cytometry Objectives: In this course you will learn:
Transporting Biological Materials Training UCCBB Histocompatibility Clinical Immunology University of Colorado Cord Blood Bank Stem Cell Laboratory Flow Cytometry Objectives: In this course you will learn:
GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS
 GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS Table of Contents General Information Regulations Definitions Recommended packing procedure Universal precautions
GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS Table of Contents General Information Regulations Definitions Recommended packing procedure Universal precautions
GENERAL SPECIMEN COLLECTION AND HANDLING HANDBOOK
 GENERAL SPECIMEN COLLECTION AND HANDLING HANDBOOK Building 27, Ibn Sina Building, Block B, Ground floor, Dubai Healthcare City, U.A.E. ABOUT US After working 20 years in USA, Dow Healthcare Management
GENERAL SPECIMEN COLLECTION AND HANDLING HANDBOOK Building 27, Ibn Sina Building, Block B, Ground floor, Dubai Healthcare City, U.A.E. ABOUT US After working 20 years in USA, Dow Healthcare Management
Zika Testing: Specimen Collection, Packaging and Shipping
 August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
Retinoblastoma Genetic Test Submission Guide
 Retinoblastoma Genetic Test Submission Guide Forms Samples Forms 1c, 1d AS APPLICABLE Form 1b REQUISITION FORM Form 1a INFORMED CONSENT Ship Results Impact Genetics 1100 Bennett Road - Unit 4 Bowmanville,
Retinoblastoma Genetic Test Submission Guide Forms Samples Forms 1c, 1d AS APPLICABLE Form 1b REQUISITION FORM Form 1a INFORMED CONSENT Ship Results Impact Genetics 1100 Bennett Road - Unit 4 Bowmanville,
Last Revised: 1/2018 Prior Version: 12/2014 SOP NUMBER: SC-402 Page 1 of 9
 SOP NUMBER: SC-402 Page 1 of 9 1. PURPOSE: To standardize and describe the process for safely transporting human research participant specimens within, to or from University Hospitals (UH) facilities.
SOP NUMBER: SC-402 Page 1 of 9 1. PURPOSE: To standardize and describe the process for safely transporting human research participant specimens within, to or from University Hospitals (UH) facilities.
i-stat iccnet CHSA Edition 1.2 Page 1 of 7 i-stat Rosy Tirimacco / Paul Simpson
 Page 1 of 7 i-stat Edition No: 1.2 Author: Rosy Tirimacco / Paul Simpson Date: January, 2012 Operative Date: January, 2012 Review Date: January, 2013 Location: Country Health SA Integrated Cardiovascular
Page 1 of 7 i-stat Edition No: 1.2 Author: Rosy Tirimacco / Paul Simpson Date: January, 2012 Operative Date: January, 2012 Review Date: January, 2013 Location: Country Health SA Integrated Cardiovascular
SAMPLE PRESERVATION Liz Miller February 21, 2017
 SAMPLE PRESERVATION Liz Miller February 21, 2017 Most recent custody sheet versions: Surface Water = January 2017 Ground Water = October 2016 Can be found at https://fldeploc.dep.state.fl.us/status/ Pay
SAMPLE PRESERVATION Liz Miller February 21, 2017 Most recent custody sheet versions: Surface Water = January 2017 Ground Water = October 2016 Can be found at https://fldeploc.dep.state.fl.us/status/ Pay
WILDLIFE DNA SAMPLING GUIDE. Instructions for the Wildlife DNA Sampling Kit
 WILDLIFE DNA SAMPLING GUIDE Instructions for the Wildlife DNA Sampling Kit WILDLIFE DNA SAMPLING GUIDE Instructions for the Wildlife DNA Sampling Kit This guide is designed to accompany the Wildlife DNA
WILDLIFE DNA SAMPLING GUIDE Instructions for the Wildlife DNA Sampling Kit WILDLIFE DNA SAMPLING GUIDE Instructions for the Wildlife DNA Sampling Kit This guide is designed to accompany the Wildlife DNA
Training Presentation for Packing & Shipping Samples to Creative Testing Solutions (CTS)
 Training Presentation for Packing & Shipping Samples to Creative Testing Solutions (CTS) Packing, Shipping and Transportation of Donor Samples To Creative Testing Solutions Materials Segregation of Samples
Training Presentation for Packing & Shipping Samples to Creative Testing Solutions (CTS) Packing, Shipping and Transportation of Donor Samples To Creative Testing Solutions Materials Segregation of Samples
I-STAT POCT System PRINCIPLE:
 PRINCIPLE: I-STAT POCT System The i-stat system uses a single disposable cartridge. The cartridge, which has been filled with fresh blood, is placed into a hand-held analyzer for analysis. The results
PRINCIPLE: I-STAT POCT System The i-stat system uses a single disposable cartridge. The cartridge, which has been filled with fresh blood, is placed into a hand-held analyzer for analysis. The results
SITE PROCEDURES NASAL SWAB
 SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
Rapid-VIDITEST. RSV Card. One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual
 Rapid-VIDITEST RSV Card One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42
Rapid-VIDITEST RSV Card One step RSV Card test for the detection of Respiratory Syncytial Virus from nasal specimens. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42
Microscope Slides and Paraffin Block Storage and Handling
 Title: Microscope Slides and Paraffin Block Storage and Handling Origination Date: 30 November 2017 Total Pages: 8 Effective Date: 22 February 2018 SOP Number: LTC-SOP-075 v1.0 Written By: ACTG/IMPAACT
Title: Microscope Slides and Paraffin Block Storage and Handling Origination Date: 30 November 2017 Total Pages: 8 Effective Date: 22 February 2018 SOP Number: LTC-SOP-075 v1.0 Written By: ACTG/IMPAACT
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. LBD Biospecimen Collection Protocol 4. Study Visit
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. LBD Biospecimen Collection Protocol 4. Study Visit
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
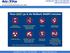 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
Tamper Evident Anti-Doping Equipment from Versapak
 Phone: +44 (0)20 8333 5300 Email: info@versapak-anti-doping.com www.versapak-anti-doping.com Tamper Evident Anti-Doping Equipment from Versapak PRODUCT GUIDE Versapak Doping Control Limited has been manufacturing
Phone: +44 (0)20 8333 5300 Email: info@versapak-anti-doping.com www.versapak-anti-doping.com Tamper Evident Anti-Doping Equipment from Versapak PRODUCT GUIDE Versapak Doping Control Limited has been manufacturing
RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease
 RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease RISK LAB MANUAL 4/22/2011 Risk Stratification Study LAB MANUAL
RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease RISK LAB MANUAL 4/22/2011 Risk Stratification Study LAB MANUAL
Instructions for Use: Duodenoscope Sampling Kit
 Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
NPHL Packaging & Shipping Revisions
 NPHL Packaging & Shipping Revisions Karen Stiles, SM(ASCP) CM State Training Coordinator Assistant Chemical Terrorism Laboratory Coordinator Nebraska Public Health Laboratory 402-559-3590 kstiles@unmc.edu
NPHL Packaging & Shipping Revisions Karen Stiles, SM(ASCP) CM State Training Coordinator Assistant Chemical Terrorism Laboratory Coordinator Nebraska Public Health Laboratory 402-559-3590 kstiles@unmc.edu
Special Specimen Collection Procedures for Coagulation Testing
 Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Both you and the responsible veterinary staff must sign the form affirming a match of the dog with the PERMANENT ID# AND THE BLOOD SAMPLE.
 Veterinary Appointment Blood can be collected by your veterinarian or a licensed veterinary technician and must be processed according to the instructions below. OptiGen does not supply a sampling kit,
Veterinary Appointment Blood can be collected by your veterinarian or a licensed veterinary technician and must be processed according to the instructions below. OptiGen does not supply a sampling kit,
COMBINE FGF23 and R01 Kit. Procedure Manual (Draft)
 UW Medicine SCHOOL OF MEDICINE COMBINE FGF23 and R01 Kit Procedure Manual (Draft) Clinical Research Laboratory Study Coordinator Kim van Leuven, MT(ASCP) kvleuven@u.washington.edu (206) 543-9032 Laboratory
UW Medicine SCHOOL OF MEDICINE COMBINE FGF23 and R01 Kit Procedure Manual (Draft) Clinical Research Laboratory Study Coordinator Kim van Leuven, MT(ASCP) kvleuven@u.washington.edu (206) 543-9032 Laboratory
Cytotoxic Waste Sorting, Handling and Disposal
 Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
INTERNATIONAL SHIPPING GUIDE. Send testing to Mayo Clinic from around the world
 INTERNATIONAL SHIPPING GUIDE Send testing to Mayo Clinic from around the world Contents Ship to us from around the world 3 - Prior to shipping your first order, follow these steps 3 Step 1. Pre-shipping
INTERNATIONAL SHIPPING GUIDE Send testing to Mayo Clinic from around the world Contents Ship to us from around the world 3 - Prior to shipping your first order, follow these steps 3 Step 1. Pre-shipping
WATER SAMPLING INSTRUCTIONS READ CAREFULLY AND COMPLETELY BEFORE SAMPLE COLLECTION Call the laboratory if you have questions.
 WATER SAMPLING INSTRUCTIONS READ CAREFULLY AND COMPLETELY BEFORE SAMPLE COLLECTION Call the laboratory if you have questions. GENERAL PROCEDURES AND INFORMATION Relevant to your request for water analysis,
WATER SAMPLING INSTRUCTIONS READ CAREFULLY AND COMPLETELY BEFORE SAMPLE COLLECTION Call the laboratory if you have questions. GENERAL PROCEDURES AND INFORMATION Relevant to your request for water analysis,
Radiation Safety Program Hunter College of the City University of New York
 Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
Laboratory Sample Manual
 Laboratory Sample Manual RCI BMT 13-TLEC/PBMTC LTE1401 Natural History and Biology of Late Effects Following Hematopoietic Cell Transplant for Childhood Hematologic Malignancies Page 1 I. Study Contact
Laboratory Sample Manual RCI BMT 13-TLEC/PBMTC LTE1401 Natural History and Biology of Late Effects Following Hematopoietic Cell Transplant for Childhood Hematologic Malignancies Page 1 I. Study Contact
LRA by ELISA/ACT. Patient Test Preparation Instructions RESTORE YOUR HEALTH WITH. Tests & Treatment Plans. Dear Patient,
 Dear Patient, RESTORE YOUR HEALTH WITH LRA by ELISA/ACT Tests & Treatment Plans Your healthcare practitioner has recommended the LRA by ELISA/ACT tests to identify items that are overburdening your immune
Dear Patient, RESTORE YOUR HEALTH WITH LRA by ELISA/ACT Tests & Treatment Plans Your healthcare practitioner has recommended the LRA by ELISA/ACT tests to identify items that are overburdening your immune
Biofluids Collection Training Slides
 North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
Biofluids Collection Training Slides
 North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
Saliva DNA Collection, Preservation and Isolation Kit 50 Individual Devices
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Saliva DNA Collection, Preservation and Isolation Kit 50 Individual
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Saliva DNA Collection, Preservation and Isolation Kit 50 Individual
UN3373 Sample Transport Are You at Risk?
 UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015
 Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015 NCRAD Leaders Tatiana Foroud, Principal Investigator Kelley Faber, Project
Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015 NCRAD Leaders Tatiana Foroud, Principal Investigator Kelley Faber, Project
Illumina Clinical Services Laboratory
 Illumina Clinical Services Laboratory CLIA Certificate No.: 05D1092911 Illumina Clinical Services Laboratory TruGenome Technical Sequence Data Test Requisition Form The Illumina Clinical Services Laboratory
Illumina Clinical Services Laboratory CLIA Certificate No.: 05D1092911 Illumina Clinical Services Laboratory TruGenome Technical Sequence Data Test Requisition Form The Illumina Clinical Services Laboratory
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Staff Tatiana Foroud Colleen Mitchell Scott Kaiser BioSEND Training Webinar Overview 1. Study Reminders 2.
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Staff Tatiana Foroud Colleen Mitchell Scott Kaiser BioSEND Training Webinar Overview 1. Study Reminders 2.
UN3373 Sample Transport Are You at Risk?
 UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
Urine Specimen Collection Guidelines. United States Department of Transportation. Office of Drug and Alcohol Policy and Compliance
 Urine Specimen Collection Guidelines United States Department of Transportation Office of Drug and Alcohol Policy and Compliance Revised - Effective August 25, 2008 DOT Urine Specimen Collection Guidelines
Urine Specimen Collection Guidelines United States Department of Transportation Office of Drug and Alcohol Policy and Compliance Revised - Effective August 25, 2008 DOT Urine Specimen Collection Guidelines
Storage Cap. EPA Method 325 Fenceline Monitoring. Arrow Points Up. Sampling Protocol. Screen Cap
 EPA Method 325 Fenceline Monitoring Sampling Protocol Ar Arrow Points Up Screen Cap Equipment List EPA 325 Sampling Kits (Two kits are in the field at a given time, identified as Kit A and Kit B)*. Contents
EPA Method 325 Fenceline Monitoring Sampling Protocol Ar Arrow Points Up Screen Cap Equipment List EPA 325 Sampling Kits (Two kits are in the field at a given time, identified as Kit A and Kit B)*. Contents
Human IL-1 alpha ELISA Kit
 Human IL-1 alpha ELISA Kit Catalog No: CDK025B Quantity: 2 x 96 tests SPECIFICITY : RANGE : SENSITIVITY : INCUBATION : SAMPLE TYPES : Recognizes both natural and recombinant human IL-1 alpha 31.2 pg /
Human IL-1 alpha ELISA Kit Catalog No: CDK025B Quantity: 2 x 96 tests SPECIFICITY : RANGE : SENSITIVITY : INCUBATION : SAMPLE TYPES : Recognizes both natural and recombinant human IL-1 alpha 31.2 pg /
Processing, Aliquoting, Recording and Storing of C1 Biospecimens
 Processing, Aliquoting, Recording and Storing of C1 Biospecimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
Processing, Aliquoting, Recording and Storing of C1 Biospecimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
Phlebotomist Instructions
 Phlebotomist Instructions July 2016 This guide is offered to our vendors and contracted companies to help them safely and legally package and ship medical specimens to Mayo Medical Laboratories for testing.
Phlebotomist Instructions July 2016 This guide is offered to our vendors and contracted companies to help them safely and legally package and ship medical specimens to Mayo Medical Laboratories for testing.
Urine Protein Concentration, Preservation and Isolation Kit 25 Individual Devices Product Insert Product #: 38200
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Urine Protein Concentration, Preservation and Isolation Kit 25
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Urine Protein Concentration, Preservation and Isolation Kit 25
BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. PBS Biospecimen Collection
BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. PBS Biospecimen Collection
Department of Laboratories St. Louis, MO ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE:
 Page 1 of 7 PROCEDURE: LEAD ESA LEADCARE II / HEALTHY KIDS EXPRESS ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE: The LEADCARE II System relies on electrochemistry and
Page 1 of 7 PROCEDURE: LEAD ESA LEADCARE II / HEALTHY KIDS EXPRESS ISSUE DATE: June 2007 REVISION DATE: May 2012 REVIEWED DATE: May 2018 PRINCIPLE: The LEADCARE II System relies on electrochemistry and
Caris Molecular Profiling Shipper Kit Instructions
 Caris Molecular Profiling Shipper Kit Instructions Preparing the Shipper Kit Step by Step procedure You need a Caris Paraffin Block and Slide Shipper Kit. If you don t have shipper kits stored at your
Caris Molecular Profiling Shipper Kit Instructions Preparing the Shipper Kit Step by Step procedure You need a Caris Paraffin Block and Slide Shipper Kit. If you don t have shipper kits stored at your
The Children s Hospital of Philadelphia Department of Pathology and Laboratory Medicine
 TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
TheChildren shospitalofphiladelphia DepartmentofPathologyandLaboratoryMedicine Muscle Biopsy - General Instructions The Division of Neuropathology, Department of Pathology and Laboratory Medicine, Children
GUIDE THE GUIDE TO GETTING AN ONCOTYPE DX TEST
 STEP BY STEP GUIDE STEP BY STEP THE GUIDE TO GETTING AN ONCOTYPE DX TEST ONCOTYPE DX IS A TEST THAT MAY HELP BREAST CANCER PATIENTS DETERMINE IF THEY WILL BENEFIT FROM CHEMOTHERAPY Choosing the optimal
STEP BY STEP GUIDE STEP BY STEP THE GUIDE TO GETTING AN ONCOTYPE DX TEST ONCOTYPE DX IS A TEST THAT MAY HELP BREAST CANCER PATIENTS DETERMINE IF THEY WILL BENEFIT FROM CHEMOTHERAPY Choosing the optimal
Human CRP Precoated ELISA kit
 Absorption 450 nm CL76153K-48/CL76153K-96 Human CRP Precoated ELISA kit Product Description: 2.4 The Human CRP ELISA kit is to be used for the in-vitro quantitative determination of CRP in human serum,
Absorption 450 nm CL76153K-48/CL76153K-96 Human CRP Precoated ELISA kit Product Description: 2.4 The Human CRP ELISA kit is to be used for the in-vitro quantitative determination of CRP in human serum,
WELCOME FROM YOUR PROJECT TEAM!
 WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
Drinking Water Quality Sampling. The Why, Where, When, and How
 Drinking Water Quality Sampling The Why, Where, When, and How 2010 PNWS AWWA Water Works Conference Tacoma, WA May 14th Lynn Kirby, Water Quality Engineer, Seattle Public Utilities What s Covered Today?
Drinking Water Quality Sampling The Why, Where, When, and How 2010 PNWS AWWA Water Works Conference Tacoma, WA May 14th Lynn Kirby, Water Quality Engineer, Seattle Public Utilities What s Covered Today?
Please consult the Product Package Insert before running the test
 Please consult the Product Package Insert before running the test Sample Collection, Preparation, Storage and Volume Q. What type of blood sample should be used with the PIFA Heparin/PF4 Rapid Assay? A.
Please consult the Product Package Insert before running the test Sample Collection, Preparation, Storage and Volume Q. What type of blood sample should be used with the PIFA Heparin/PF4 Rapid Assay? A.
Reagent Kit Selection Guide
 Reagent Kit Selection Guide Introduction The BioChain Nucleic Acid Preparation Technology Introduction BioChain Institute Inc. specializes in developing advanced, efficient and reliable technologies in
Reagent Kit Selection Guide Introduction The BioChain Nucleic Acid Preparation Technology Introduction BioChain Institute Inc. specializes in developing advanced, efficient and reliable technologies in
Table of Content. 1. Introduction. 2. Temperature Controlled Shipping. 3. Packaging, Labeling and Marking. 4. Shipment Documentation
 March 2017 Table of Content 1. Introduction 2. Temperature Controlled Shipping 3. Packaging, Labeling and Marking 3.1 Exempt Specimen 3.2 Category B 3.3 Category A 4. Shipment Documentation 5. Health
March 2017 Table of Content 1. Introduction 2. Temperature Controlled Shipping 3. Packaging, Labeling and Marking 3.1 Exempt Specimen 3.2 Category B 3.3 Category A 4. Shipment Documentation 5. Health
ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN
 ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login. After signing up, please
ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login. After signing up, please
BioSpecimen Exchange for Neurological Disorders, BioSEND
 NINDS Biorepository BioSpecimen Exchange for Neurological Disorders, BioSEND Training Webinar Presented by: Scott Kaiser Webinar Overview 1. Site Equipment 2. BioSEND Kit Contents and Ordering Sample Labelling
NINDS Biorepository BioSpecimen Exchange for Neurological Disorders, BioSEND Training Webinar Presented by: Scott Kaiser Webinar Overview 1. Site Equipment 2. BioSEND Kit Contents and Ordering Sample Labelling
DiviTum V2. User s Manual IVD. This User s Manual is intended for DiviTum V2. For use in the United States and Japan only for research purposes
 DiviTum V2 User s Manual This User s Manual is intended for DiviTum V2 For use in the United States and Japan only for research purposes IVD 40 Ref. 933 rev. EN 2 1 Contents DIVITUM ASSAY FOR MEASURING
DiviTum V2 User s Manual This User s Manual is intended for DiviTum V2 For use in the United States and Japan only for research purposes IVD 40 Ref. 933 rev. EN 2 1 Contents DIVITUM ASSAY FOR MEASURING
Contamination Prevention and Decontamination
 Contamination Prevention and Decontamination Introduction FilmArray is an automated in vitro diagnostic (IVD) system that utilizes nested multiplex PCR (nmpcr) and high-resolution melting analysis to detect
Contamination Prevention and Decontamination Introduction FilmArray is an automated in vitro diagnostic (IVD) system that utilizes nested multiplex PCR (nmpcr) and high-resolution melting analysis to detect
Maxwell RSC Stabilized Saliva DNA Kit
 TECHNICAL MANUAL Maxwell RSC Stabilized Saliva DNA Kit Instructions for Use of Product AS1630 Note: To use the Maxwell RSC Stabilized Saliva DNA Kit, you must have the Stabilized Saliva DNA method loaded
TECHNICAL MANUAL Maxwell RSC Stabilized Saliva DNA Kit Instructions for Use of Product AS1630 Note: To use the Maxwell RSC Stabilized Saliva DNA Kit, you must have the Stabilized Saliva DNA method loaded
Department Of Pathology Point of Care Testing POC Quick Vue In-Line Strep A Version#4
 Department Of Pathology Point of Care Testing POC.014.04 Quick Vue In-Line Strep A Version#4 Printed copies are for reference only. Please refer to the electronic copy for the latest version. A. Purpose:
Department Of Pathology Point of Care Testing POC.014.04 Quick Vue In-Line Strep A Version#4 Printed copies are for reference only. Please refer to the electronic copy for the latest version. A. Purpose:
Urine Specimen Collection Guidelines. United States Department of Transportation. Office of Drug and Alcohol Policy and Compliance
 Urine Specimen Collection Guidelines United States Department of Transportation Office of Drug and Alcohol Policy and Compliance Revised January 2018 DOT Urine Specimen Collection Guidelines 2 DOT Urine
Urine Specimen Collection Guidelines United States Department of Transportation Office of Drug and Alcohol Policy and Compliance Revised January 2018 DOT Urine Specimen Collection Guidelines 2 DOT Urine
Urine Specimen Collection Guidelines. United States Department of Transportation. Office of Drug and Alcohol Policy and Compliance
 Urine Specimen Collection Guidelines United States Department of Transportation Office of Drug and Alcohol Policy and Compliance Revised July 3, 2014 DOT Urine Specimen Collection Guidelines 2 DOT Urine
Urine Specimen Collection Guidelines United States Department of Transportation Office of Drug and Alcohol Policy and Compliance Revised July 3, 2014 DOT Urine Specimen Collection Guidelines 2 DOT Urine
Maxwell RSC Buffy Coat DNA Kit
 TECHNICAL MANUAL Maxwell RSC Buffy Coat DNA Kit Instructions for Use of Product AS1540 Note: To use the Maxwell RSC Buffy Coat DNA Kit, you must have the Buffy Coat DNA method loaded on the Maxwell RSC
TECHNICAL MANUAL Maxwell RSC Buffy Coat DNA Kit Instructions for Use of Product AS1540 Note: To use the Maxwell RSC Buffy Coat DNA Kit, you must have the Buffy Coat DNA method loaded on the Maxwell RSC
Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol. Non-human animal blood and CSF Protocol
 Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol Thank you for your interest and concerns regarding Rat Lungworm in Hawaii. Our facility has a species specific biological
Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol Thank you for your interest and concerns regarding Rat Lungworm in Hawaii. Our facility has a species specific biological
Infectious Waste Disposal Procedure 25 PA Code 284 Ursinus College Collegeville, PA 19426
 The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
Transporting Biological Materials
 Transporting Biological Materials Researchers at the University of Arkansas often collaborate with researchers from institutions throughout the state and surrounding states as well. The Office of Environmental
Transporting Biological Materials Researchers at the University of Arkansas often collaborate with researchers from institutions throughout the state and surrounding states as well. The Office of Environmental
Centre d expertise en analyse environnementale du Québec
 Centre d expertise en analyse environnementale du Québec Methods for taking, preserving and analyzing samples to monitor the water quality of pools and other artificial reservoirs DR-09-05 Edition 2007-06-20
Centre d expertise en analyse environnementale du Québec Methods for taking, preserving and analyzing samples to monitor the water quality of pools and other artificial reservoirs DR-09-05 Edition 2007-06-20
ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN
 ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login.
ADAPTIVE BIOTECHNOLOGIES DIAGNOSTIC PORTAL: ACCOUNT LOGIN 1. Create an account. To set up your account for our online order entry and reporting system, please go to: https://diagnostics.adaptivebiotech.com/account/login.
TG AUTOMOTIVE SEALING KENTUCKY, LLC (TGASK) LABEL REQUIREMENTS
 TG AUTOMOTIVE SEALING KENTUCKY, LLC (TGASK) LABEL REQUIREMENTS INTRODUCTION: This standard document provides guidelines for the printing and placement of Shipping / Parts Identification Labels. The intent
TG AUTOMOTIVE SEALING KENTUCKY, LLC (TGASK) LABEL REQUIREMENTS INTRODUCTION: This standard document provides guidelines for the printing and placement of Shipping / Parts Identification Labels. The intent
Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date:
 STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
Porcine Interleukin 1 Beta(IL-1β) ELISA Kit
 Porcine Interleukin 1 Beta(IL-1β) ELISA Kit Cat.No: DEIA-T6104 Lot. No. (See product label) Size 96T Intended Use The Porcine Interleukin 1 Beta(IL-1β) ELISA Kit is to be used for the vitro quantitative
Porcine Interleukin 1 Beta(IL-1β) ELISA Kit Cat.No: DEIA-T6104 Lot. No. (See product label) Size 96T Intended Use The Porcine Interleukin 1 Beta(IL-1β) ELISA Kit is to be used for the vitro quantitative
Welcome to the Wisconsin Solo & Small Firm Conference!
 Dear Exhibitor: Welcome to the Wisconsin Solo & Small Firm Conference! Your show will be held at the Kalahari Resort in Wisconsin Dells, WI on October 25-26, 2018. As your official service contractor our
Dear Exhibitor: Welcome to the Wisconsin Solo & Small Firm Conference! Your show will be held at the Kalahari Resort in Wisconsin Dells, WI on October 25-26, 2018. As your official service contractor our
trucollect -ADK (Application Development Kit) PN
 PROTOCOL trucollect -ADK (Application Development Kit) PN 520245 Biological Specimen Collection, Dry-Stabilization, Transport and Storage; AFA Extraction Tube (130 µl) For Research Use Only Not for use
PROTOCOL trucollect -ADK (Application Development Kit) PN 520245 Biological Specimen Collection, Dry-Stabilization, Transport and Storage; AFA Extraction Tube (130 µl) For Research Use Only Not for use
