APPENDIX D1. BLOOD SPECIMEN PROCEDURES
|
|
|
- Regina Gibson
- 6 years ago
- Views:
Transcription
1 APPENDIX D1. BLOOD SPECIMEN PROCEDURES Blood Specimen Collection & Storage Supplies: Red-top vacutainer tubes Lavender vacutainer tube 10mL white-top cryogenic vial 10mL blue-top cryogenic vial WIND Study labels for red-top vacutainer tubes WIND Study labels for white-top cryogenic vial WIND Study labels for blue-top cryogenic vial 4ºC Refrigerator (standard refrigerator) -80 C freezer (freezer must NOT be warmer than -70 C) Biohazard bag or box 1. At the discretion of the clinician and parent/legal guardian, EMLA cream (lidocaine 2.5% and prilocaine 2.5%) or other topical anesthetic (e.g., LMX4 [lidocaine 4%]) will be applied to the skin to prevent pain associated with needle insertion. 2. In order to maintain vein patency and optimal blood flow, blood may be collected first into several syringes, followed by transfer to the appropriate vacutainer tubes for processing. Phlebotomists should defer to their local institutional procedures for specific directives on pediatric blood collection methods. 3. A phlebotomist will collect 0.5mL of blood in a lavender vacutainer tube, invert 8 to 10 times immediately after collection, and send it out for real-time analysis of complete blood count (CBC) with differential at their local hematology laboratory, following their usual procedures. Exam sites without a designated local hematology laboratory should contact Project Coordinator Ashley Sullivan (phone: ; afsullivan@partners.org) the EMNet Coordinating Center prior to the exam to discuss how the CBC with differential will be performed. 4. The phlebotomist will attempt to collect 4.5mL to 14.5mL of blood in red-top vacutainer tubes, and invert 5 times immediately after collection. If the minimum redtop blood volume (4.5mL ml) is not acquired through the blood draw, another blood draw may be re-attempted or one may schedule another blood draw. 5. The red-top tubes will be labeled with the WIND Study tube labels including: sample type (blood), 6-digit study ID (xxxxxx), date (mm/dd/yyyy) and time (hh:mm). 6. The red-top blood specimen should be centrifuged at 3000 RPM at room temperature until the serum is fully separated from the pellet (approximately 10 minutes.) 7. The serum supernatant will be siphoned off and placed into a cryogenic vial with a white cap and the remaining pellet precipitate will be transferred to a separate cryogenic vial with a blue cap. Please do NOT siphon too close to the top of the pellet when extracting the serum as the thin layer of white blood cells contains the DNA for testing. 8. The blue top and white top cryogenic vials will be labeled with the WIND Study labels including: sample type (serum or pellet), 6-digit study ID (xxxxxx), date (mm/dd/yyyy) and time (hh:mm). 9. A strip of clear cryogenic tape should be wrapped around each cryogenic vial to prevent the label from falling off. 10. Details about the collection, including volume, number of tubes, sample type, 6-digit study ID (xxxxxx), date (mm/dd/yyyy) and time (hh:mm) will be entered in REDCap. 11. Once the samples have been transferred to the cryogenic vials, if you plan to store samples in biohazard bags, please place each sample into its own separate
2 specimen biohazard bag (i.e., the serum sample and the pellet sample will each be placed into their own separate biohazard bags). 12. Place the specimen on ice for transport to an -80 C freezer. For all participants, both the serum and pellet will be stored. For participants who did not agree to the genetics portion of the study, the pellet will NOT undergo genetics analysis, but will be used for non-genetics testing. Both the serum and the pellet tubes will be stored at -70 o C or -80 o C. Storage at 4 o C (in standard refrigerator) for up to 24 hours prior to freezing at -70 o C or -80 o C is acceptable if necessary. If -70 or -80 o C storage is not available, arrangements will be made with the EMNet Coordinating Center prior to the exam to discuss storage options. 13. Each specimen is stored in the -80 C freezer until samples are ready to be shipped. The EMNet Coordinating Center (the WIND Study Team) will notify sites when to ship out samples, so please keep samples on-site and DO NOT ship out until instructed to do so by the EMNet Coordinating Center.
3 Blood Collection Flow Diagram Kit The WIND Study 42-month Exam Blood Kit contains supplies for the blood draw Collection Complete Blood Count with Differential for real-time analysis at your local lab. Collect blood in a lavender-top vacutainer tube. Label according to institutional procedures for send-out to your local hematology laboratory. Various Assays (e.g., IgE) for centralized analysis by WIND Study Team. Collect blood in red top vacutainer tubes. Label each tube with WIND Study labels ONLY. Each WIND Study label includes: sample type (red top tube) participant study ID collection date collection time Invert tube 5 times immediately after collection. Centrifuge blood at 3000RPM for 10min at room temperature. Processing Invert tube 8 to 10 times immediately after collection. Send out for real-time analysis at your local hematology laboratory, following your institutional guidelines. Aliquot serum into white cap cryogenic vial. Aliquot pellet into blue cap cryogenic vial. Storage Not applicable Label each cryogenic vial with WIND Study labels ONLY. Each label includes: sample type (serum or pellet) participant study ID collection date collection time Store both the serum (white cap) and the pellet (blue cap) in -80C freezer. Do not ship samples until instructed to do so by the EMNet Coordinating Center.
4 Shipping of Serum and Pellet to Massachusetts General Hospital (MGH) Note: Only lab personnel with IATA certification may package and ship the serum and pellet specimens on dry ice. Site PIs/lead clinicians should be present during the specimen mailing process to ensure that all specimens are included in the mailing, to give the necessary supplies to the person preparing the package, and to include the completed Blood Serum and Pellet shipment list in the mailing. Specimens will be shipped once designated by the EMNet Coordinating Center. Mindful of holidays, specimens should be sent only on Mondays, Tuesdays, or Wednesdays so that the specimens do not arrive on a Saturday, Sunday, or holiday. Prior to shipment, the shipment list should be matched against data in REDCap to ensure that all specimens are accounted for. Supplies: Sterile gloves Biohazard bag(s) Absorbent material WIND Study 42-Month Exam Shipment List 5 lbs (2.27 kgs) dry ice (small pellets rather than large blocks) Sample box Styrofoam box (to hold dry ice and sample box) Cardboard shipping box (to hold Styrofoam box) Biohazard sticker or biohazard bag (for top of Styrofoam box lid) Exempt Human Specimen label Dry Ice shipment label 1. The EMNet Coordinating Center (the WIND Study Team) will notify sites when to ship out samples, so please keep samples on-site and DO NOT ship out until instructed to do so by the EMNet Coordinating Center. The Coordinating Center will provide the shipping address at the time shipping schedules are made. 2. Verify that your lab has received notification from the EMNet Coordinating Center that it is okay to ship out samples. 3. Put on sterile gloves and remove samples from freezer. 4. If each sample is not already in a biohazard bag, place the blood serum and pellet sample in a biohazard bag (only ONE sample per bag) with absorbent material, while noting on the Shipment List their inclusion. Place the biohazard bag(s) in sample box(es) 5. Add bubble wrap or foam padding around the bags for cushioning and put the lid on the sample box. 6. Place the sample box(es) in the Styrofoam box. 7. Completely surround the box with samples with the 5 lbs (2.27 kgs) of dry ice. 8. Insert padding around the sample box(es) and dry ice if there is additional space in the Styrofoam box. 9. Put lid on Styrofoam box and place into cardboard box. 10. Affix an orange biohazard sticker (or a bag that is pre-labeled with the universal biohazard symbol) to the Styrofoam box lid if one is not already present. 11. Put completed Shipment List in an envelope and place on top of Styrofoam lid. 12. Keep a copy of the Shipment List for your files and a copy to Project Coordinator Ashley Sullivan (afsullivan@partners.org). 13. Seal cardboard box and affix the completed Dry Ice label, which is required to be on the box and also notes the weight of the dry ice.
5 14. On the FedEx label, be sure to check off Dry Ice and note the weight of dry ice. 15. Attach Exempt Human Specimen label near the Dry Ice label. Ship samples (overnight) to the address provided by the EMNet Coordinating Center. FedEx is the preferred carrier for blood shipments. Please use the FedEx Priority Overnight service. On the day that the specimens are sent, the site PI/lead clinician should both Project Coordinator Ashley Sullivan and Principal Investigator Carlos Camargo the FedEx tracking number for the mailing.
6 WIND STUDY/MARC-35 Exam Serum and Pellet Shipment List WIND Study Site Number: Directions: Use this form to keep track of all samples for the WIND Study during storage and include a copy of this form when shipping. Use one line for each study ID. Study ID (X X X X X X) Serum included in shipment? Pellet included in shipment? Collection Date (mm/dd/yyyy) Collection Time (hh:mm)
7 Laboratory Evaluations/Assays Blood specimens: Allergen-Specific IgE, Total IgE, Genetic testing CBC with Differential The complete blood count with differential will be performed in real-time at the local laboratory of each site/clinician office, following their local guidelines. Results will be entered into REDCap. Allergen Specific Immunoglobulin E (ASIgE) The selection of items parallels the modified Asthma Predictive Index (mapi) allergy testing as much as possible (1). Because the mapi was performed on older children, skin prick testing was utilized. With our younger cohort, we will use ASIgE to help evaluate for major aeroallergens and food allergens; an approach agreed upon by the first author of the mapi manuscript (personal communication with Dr. Theresa Guilbert, October 2008). This test will be performed using a Fluorescence Enzyme Immunoassay. (Package insert: ImmunoCAP System Specific IgE FEIA, Uppsala, Sweden Rev February 2005), Phadia Immunology Reference Laboratory (PiRL). Total IgE This test will also be performed using a Fluorescence Enzyme Immunoassay. (Package insert: Phadia CAP System IgE FEIA. issued August 2000, revised March 2006) Phadia Immunology Reference Laboratory (PiRL) Genetic Testing In accordance with the consent form, pellet specimens, which remain after centrifuging the blood and extracting the serum, are being collected for the purpose of establishing a DNA biorepository to examine the possible genetic causes of severe bronchiolitis, recurrent wheezing, asthma and related concepts. For those participants who have declined participation in the optional genetic sub-study, the pellet will be used to perform non-genetic testing covered under the consent. 1. Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006 May 11;354(19):
SITE PROCEDURES NASAL SWAB
 SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING
 35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING 35.1 Purpose Participants who enroll in the PVDOMICS study with be asked to provide biological specimens (blood and urine). The
35. BIOREPOSITORY CORE URINE SPECIMENS COLLECTION, PROCESSING AND SHIPPING 35.1 Purpose Participants who enroll in the PVDOMICS study with be asked to provide biological specimens (blood and urine). The
PLATELET-ORIENTED INHIBITION IN NEW TIA
 PLATELET-ORIENTED INHIBITION IN NEW TIA AND MINOR ISCHEMIC STROKE ANCILLARY BIOMARKERS STUDY BLOOD SPECIMEN PROCEDURE MANUAL US Sites DNA Only DNA and Plasma CONTENTS I. DNA ONLY SPECIMEN PROCEDURES...
PLATELET-ORIENTED INHIBITION IN NEW TIA AND MINOR ISCHEMIC STROKE ANCILLARY BIOMARKERS STUDY BLOOD SPECIMEN PROCEDURE MANUAL US Sites DNA Only DNA and Plasma CONTENTS I. DNA ONLY SPECIMEN PROCEDURES...
BioSpecimen Exchange for Neurological Disorders, BioSEND
 NINDS Biorepository BioSpecimen Exchange for Neurological Disorders, BioSEND Training Webinar Presented by: Scott Kaiser Webinar Overview 1. Site Equipment 2. BioSEND Kit Contents and Ordering Sample Labelling
NINDS Biorepository BioSpecimen Exchange for Neurological Disorders, BioSEND Training Webinar Presented by: Scott Kaiser Webinar Overview 1. Site Equipment 2. BioSEND Kit Contents and Ordering Sample Labelling
BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. PBS Biospecimen Collection
BioSpecimen Exchange for Neurological Disorders (BioSEND) Parkinsonism Biomarker Subtypes Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. PBS Biospecimen Collection
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. LBD Biospecimen Collection Protocol 4. Study Visit
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Webinar Overview 1. Study Reminders 2. Site Equipment 3. LBD Biospecimen Collection Protocol 4. Study Visit
Biofluids Collection Training Slides
 Longitudinal Early-onset Alzheimer s Disease Study in collaboration with The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact
Longitudinal Early-onset Alzheimer s Disease Study in collaboration with The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar
 BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Staff Tatiana Foroud Colleen Mitchell Scott Kaiser BioSEND Training Webinar Overview 1. Study Reminders 2.
BioSpecimen Exchange for Neurological Disorders (BioSEND) LBD Training Webinar BioSEND Training Staff Tatiana Foroud Colleen Mitchell Scott Kaiser BioSEND Training Webinar Overview 1. Study Reminders 2.
34. PVDOMICS BIOSPECIMEN BLOOD PROCESSING AND SHIPPING. Chapter 35 describes collection, processing and shipment of urine specimens.
 34. PVDOMICS BIOSPECIMEN BLOOD PROCESSING AND SHIPPING 34.1 Purpose and Background Participants who enroll in the PVDOMICS study will be asked to provide biological samples (blood and urine) for OMICS
34. PVDOMICS BIOSPECIMEN BLOOD PROCESSING AND SHIPPING 34.1 Purpose and Background Participants who enroll in the PVDOMICS study will be asked to provide biological samples (blood and urine) for OMICS
Special Specimen Collection Procedures for Coagulation Testing
 Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease
 RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease RISK LAB MANUAL 4/22/2011 Risk Stratification Study LAB MANUAL
RISK Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn s Disease RISK LAB MANUAL 4/22/2011 Risk Stratification Study LAB MANUAL
Blood-Based Biospecimen Training Slides
 Neurodegeneration in Aging Down Syndrome: A Longitudinal Study of Cognition and Biomarkers of Alzheimer's Disease in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Blood-Based
Neurodegeneration in Aging Down Syndrome: A Longitudinal Study of Cognition and Biomarkers of Alzheimer's Disease in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Blood-Based
Biofluids Collection Training Slides
 North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
Biofluids Collection Training Slides
 North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
North American Prodromal Synucleinopathy (NAPS) & The National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) Biofluids Collection Training Slides Contact Information Questions?
Biofluids Collection Training Slides
 A Phase 2 Randomized Double-Blind Placebo-Controlled Trial to Evaluate the Efficacy and Safety of BHV-4157 in Patients with Mild to Moderate Alzheimer s Disease (T2 PROTECT AD) & The National Centralized
A Phase 2 Randomized Double-Blind Placebo-Controlled Trial to Evaluate the Efficacy and Safety of BHV-4157 in Patients with Mild to Moderate Alzheimer s Disease (T2 PROTECT AD) & The National Centralized
MAY Research Blood and Tissue Kit Instructions
 Kit Inventory Instruction sheet Requisition Form Tissue Collection Supplies (2) Ambient Transport Bag (2) 4mL orange cap cryovials (2) 6mL Formalin (2) specimen labels ( 4 per sheet) (1) graduated pipet
Kit Inventory Instruction sheet Requisition Form Tissue Collection Supplies (2) Ambient Transport Bag (2) 4mL orange cap cryovials (2) 6mL Formalin (2) specimen labels ( 4 per sheet) (1) graduated pipet
Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015
 Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015 NCRAD Leaders Tatiana Foroud, Principal Investigator Kelley Faber, Project
Alzheimer s Disease Center (ADC) New Site Training for the National Cell Repository for Alzheimer s Disease (NCRAD) Summer 2015 NCRAD Leaders Tatiana Foroud, Principal Investigator Kelley Faber, Project
Phlebotomist Instructions
 Phlebotomist Instructions July 2016 This guide is offered to our vendors and contracted companies to help them safely and legally package and ship medical specimens to Mayo Medical Laboratories for testing.
Phlebotomist Instructions July 2016 This guide is offered to our vendors and contracted companies to help them safely and legally package and ship medical specimens to Mayo Medical Laboratories for testing.
Processing, Aliquoting, Recording and Storing of C1 Biospecimens
 Processing, Aliquoting, Recording and Storing of C1 Biospecimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
Processing, Aliquoting, Recording and Storing of C1 Biospecimens NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
Zika Testing: Specimen Collection, Packaging and Shipping
 August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11
 Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11 A Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole
Pathology and Correlative Science Instructions for NSABP Foundation Protocol FB-11 A Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole
Laboratory Sample Manual
 Laboratory Sample Manual RCI BMT 13-TLEC/PBMTC LTE1401 Natural History and Biology of Late Effects Following Hematopoietic Cell Transplant for Childhood Hematologic Malignancies Page 1 I. Study Contact
Laboratory Sample Manual RCI BMT 13-TLEC/PBMTC LTE1401 Natural History and Biology of Late Effects Following Hematopoietic Cell Transplant for Childhood Hematologic Malignancies Page 1 I. Study Contact
COMBINE FGF23 and R01 Kit. Procedure Manual (Draft)
 UW Medicine SCHOOL OF MEDICINE COMBINE FGF23 and R01 Kit Procedure Manual (Draft) Clinical Research Laboratory Study Coordinator Kim van Leuven, MT(ASCP) kvleuven@u.washington.edu (206) 543-9032 Laboratory
UW Medicine SCHOOL OF MEDICINE COMBINE FGF23 and R01 Kit Procedure Manual (Draft) Clinical Research Laboratory Study Coordinator Kim van Leuven, MT(ASCP) kvleuven@u.washington.edu (206) 543-9032 Laboratory
Both you and the responsible veterinary staff must sign the form affirming a match of the dog with the PERMANENT ID# AND THE BLOOD SAMPLE.
 Veterinary Appointment Blood can be collected by your veterinarian or a licensed veterinary technician and must be processed according to the instructions below. OptiGen does not supply a sampling kit,
Veterinary Appointment Blood can be collected by your veterinarian or a licensed veterinary technician and must be processed according to the instructions below. OptiGen does not supply a sampling kit,
SAMPLE PROCESSING SOP. Purpose: To standardize a processing procedure for sample aliquoting in the ACRL.
 SAMPLE PROCESSING SOP Purpose: To standardize a processing procedure for sample aliquoting in the ACRL. Procedure: 1. Processing: a. One unit (or vial) of the sample type is thawed overnight in the walk-in
SAMPLE PROCESSING SOP Purpose: To standardize a processing procedure for sample aliquoting in the ACRL. Procedure: 1. Processing: a. One unit (or vial) of the sample type is thawed overnight in the walk-in
Pathology and Correlative Science Instructions for NRG Oncology Trials: San Francisco Bank Kits Version
 Pathology and Correlative Science Instructions for NRG Oncology Trials: San Francisco Bank Kits Version Note: In an effort to go Green and reduce paper the NRGBB-SF will no longer include a full instruction
Pathology and Correlative Science Instructions for NRG Oncology Trials: San Francisco Bank Kits Version Note: In an effort to go Green and reduce paper the NRGBB-SF will no longer include a full instruction
Standard Operating Procedure. Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples
 Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
ESETT PHARMACOKINETIC PHARMACODYNAMIC (PK/PD) STUDY TRAINING
 ESETT PHARMACOKINETIC PHARMACODYNAMIC (PK/PD) STUDY TRAINING ESETT VIRTUAL INVESTIGATOR MEETING OCTOBER 4, 2017 Lisa Coles, MS, PhD Research Assistant Professor Dept of Experimental and Clinical Pharmacology
ESETT PHARMACOKINETIC PHARMACODYNAMIC (PK/PD) STUDY TRAINING ESETT VIRTUAL INVESTIGATOR MEETING OCTOBER 4, 2017 Lisa Coles, MS, PhD Research Assistant Professor Dept of Experimental and Clinical Pharmacology
1. Introduction. 2. Responsibilities. 3. Equipment. 4. Method. Blood tube ID and sample preparation No: 009D
 Blood tube ID and sample preparation No: 009D 1. Introduction Accurate identification and preparation of samples is vital to ensure success of the study. It is essential that each sample is individually
Blood tube ID and sample preparation No: 009D 1. Introduction Accurate identification and preparation of samples is vital to ensure success of the study. It is essential that each sample is individually
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
INTERNATIONAL SHIPPING GUIDE. Send testing to Mayo Clinic from around the world
 INTERNATIONAL SHIPPING GUIDE Send testing to Mayo Clinic from around the world Contents Ship to us from around the world 3 - Prior to shipping your first order, follow these steps 3 Step 1. Pre-shipping
INTERNATIONAL SHIPPING GUIDE Send testing to Mayo Clinic from around the world Contents Ship to us from around the world 3 - Prior to shipping your first order, follow these steps 3 Step 1. Pre-shipping
I-SPOT Laboratory Manual Log of Changes from Version 3 to Version 4
 Log of Changes from Version 3 to Version 4 Throughout document changed Footer from July 2013 Version 3 to November 2013 Version 4 Throughout document changed aliquot to cryovial Page 2 Updated Page numbers
Log of Changes from Version 3 to Version 4 Throughout document changed Footer from July 2013 Version 3 to November 2013 Version 4 Throughout document changed aliquot to cryovial Page 2 Updated Page numbers
WELCOME FROM YOUR PROJECT TEAM!
 WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
Systemic Synuclein Sampling Study. Biospecimen Collection, Processing, and Shipment Manual
 S4 Biospecimen Collection, Processing, and Shipment Manual 25 February 2016 1 Table of Contents 1.0 Biorepository Information 5 1.1 Biorepository Contacts 1.2 Hours of Operation 1.3 Holiday Schedules and
S4 Biospecimen Collection, Processing, and Shipment Manual 25 February 2016 1 Table of Contents 1.0 Biorepository Information 5 1.1 Biorepository Contacts 1.2 Hours of Operation 1.3 Holiday Schedules and
Sample Submission Guide
 Molecular Biology Department Iranian Biological Resource Center Sample Submission Guide DNA Extraction Service - Blood Samples Blood Samples Collection Instructions: 1. Collect 2-5 ml of blood from the
Molecular Biology Department Iranian Biological Resource Center Sample Submission Guide DNA Extraction Service - Blood Samples Blood Samples Collection Instructions: 1. Collect 2-5 ml of blood from the
INSTRUCTION MANUAL FOR LABORATORY AND SPECIMEN PROCEDURES
 INSTRUCTION MANUAL FOR LABORATORY AND SPECIMEN PROCEDURES CARDIOVASCULAR INFLAMMATION REDUCTION TRIAL (CIRT) Sponsor: Brigham and Women s Hospital All individuals connected with the clinical phase of this
INSTRUCTION MANUAL FOR LABORATORY AND SPECIMEN PROCEDURES CARDIOVASCULAR INFLAMMATION REDUCTION TRIAL (CIRT) Sponsor: Brigham and Women s Hospital All individuals connected with the clinical phase of this
Systemic Synuclein Sampling Study. Biospecimen Collection, Processing, and Shipment Manual
 Systemic Synuclein Sampling Study S4 Biospecimen Collection, Processing, and Shipment Manual Version 2.0 of the S4 Biologics Manual is to be used in conjunction with Version 2.0 of the S4 Protocol 21 March
Systemic Synuclein Sampling Study S4 Biospecimen Collection, Processing, and Shipment Manual Version 2.0 of the S4 Biologics Manual is to be used in conjunction with Version 2.0 of the S4 Protocol 21 March
SAMPLE SUBMISSION INSTRUCTIONS
 ISOFLUX System SAMPLE SUBMISSION INSTRUCTIONS Checklist for completion of sample submission: Pre-collection Signed agreement in place to process samples (Purchase Order, Research Agreement, etc.) As much
ISOFLUX System SAMPLE SUBMISSION INSTRUCTIONS Checklist for completion of sample submission: Pre-collection Signed agreement in place to process samples (Purchase Order, Research Agreement, etc.) As much
Laboratory Manual. Insights on Selected Procoagulation markers and Outcomes in stroke Trial. I-SPOT Laboratory Manual
 Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) November 2013December 2015 Version 54 1 Table of
Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) November 2013December 2015 Version 54 1 Table of
Laboratory Manual. Insights on Selected Procoagulation markers and Outcomes in stroke Trial. I-SPOT Laboratory Manual. December 2015 Version 5 1
 Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) December 2015 Version 5 1 Table of Contents Title
Laboratory Manual Insights on Selected Procoagulation markers and Outcomes in stroke Trial (Response to Insulin Administration and Blood Glucose Control) December 2015 Version 5 1 Table of Contents Title
Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol. Non-human animal blood and CSF Protocol
 Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol Thank you for your interest and concerns regarding Rat Lungworm in Hawaii. Our facility has a species specific biological
Hawaii Island Rat Lungworm Working Group Jarvi Lab Veterinary Sample Submission Protocol Thank you for your interest and concerns regarding Rat Lungworm in Hawaii. Our facility has a species specific biological
Manual of Procedures. National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD)
 Manual of Procedures National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) North American Prodromal Synucleinopathy (NAPS) Biospecimen Collection, Processing, and Version
Manual of Procedures National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) North American Prodromal Synucleinopathy (NAPS) Biospecimen Collection, Processing, and Version
Unless otherwise directed, collect the following specimens from each person who may have been exposed:
 Shipping Instructions for Specimens Collected from People Who May Have Been Exposed to Chemical-Terrorism Agents Section One: Collecting and Labeling Specimens Required Specimens Unless otherwise directed,
Shipping Instructions for Specimens Collected from People Who May Have Been Exposed to Chemical-Terrorism Agents Section One: Collecting and Labeling Specimens Required Specimens Unless otherwise directed,
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
Morris Animal. FOUNDATION Golden Retriever Lifetime Study
 Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Annual Veterinary Sample Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you
Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Annual Veterinary Sample Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you
National Institute of Neurological Disorders and Stroke Biorepository:
 National Institute of Neurological Disorders and Stroke Biorepository: BioSpecimen Exchange for Neurological Disorders, BioSEND Biospecimen Collection, Processing, and Shipment Manual for Exosome LRRK2
National Institute of Neurological Disorders and Stroke Biorepository: BioSpecimen Exchange for Neurological Disorders, BioSEND Biospecimen Collection, Processing, and Shipment Manual for Exosome LRRK2
Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube
 Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube Tiger Top/SST 5.0 ml Screw Cap Tube Apply barcode label starting at the top of the sample tube (the end closest to the cap). The barcode
Proper Labeling of Sample Tubes: Tiger Top/SST 5.0 ml Screw Cap Tube Tiger Top/SST 5.0 ml Screw Cap Tube Apply barcode label starting at the top of the sample tube (the end closest to the cap). The barcode
Collecting Blood and Isolating PBMCs
 MyCell Products Application Protocol Collecting Blood and Isolating PBMCs Introduction: Blood Collection and Infectious Disease Testing A trained phlebotomist must collect blood samples using Vacutainer
MyCell Products Application Protocol Collecting Blood and Isolating PBMCs Introduction: Blood Collection and Infectious Disease Testing A trained phlebotomist must collect blood samples using Vacutainer
Instructions for Use: Duodenoscope Sampling Kit
 Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
Instructions for Use: Duodenoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Duodenoscope Sampling Kit Endoscope sampling and culturing kit CK-250,
BioFIND. (Fox Investigation for New Discovery of Biomarkers) Research Biomarkers Laboratory Manual
 BF THE FOX INVESTIGATION FOR NEW DISCOVERY OF BIOMARKERS Play a Part in Parkinson s Research BioFIND (Fox Investigation for New Discovery of Biomarkers) Research Biomarkers Laboratory Manual Protocol #:
BF THE FOX INVESTIGATION FOR NEW DISCOVERY OF BIOMARKERS Play a Part in Parkinson s Research BioFIND (Fox Investigation for New Discovery of Biomarkers) Research Biomarkers Laboratory Manual Protocol #:
WELCOME FROM YOUR PROJECT TEAM!
 WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
WELCOME FROM YOUR PROJECT TEAM! LabCorp Clinical Trials has been selected by University of California San Francisco to perform the DNA Extraction for Protocol POINT NCT00991029. As such, we have the pleasure
Instructions for Shipping Material from Outside the United States
 Instructions for Shipping Material from Outside the United States Last Updated: 2/18/2013 Contents Shipping Address... 3 Shipping Agencies... 3 Recommended Standard Shippers:... 3 Full service carriers:...
Instructions for Shipping Material from Outside the United States Last Updated: 2/18/2013 Contents Shipping Address... 3 Shipping Agencies... 3 Recommended Standard Shippers:... 3 Full service carriers:...
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea
 How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
transport of biological materials
 UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO STANDARD OPERATING PROCEDURE transport of biological materials Prepared by Date Adopted Procedure # IBC Approved EHS B001 May 13, 2014 ENVIRONMENTAL
UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO STANDARD OPERATING PROCEDURE transport of biological materials Prepared by Date Adopted Procedure # IBC Approved EHS B001 May 13, 2014 ENVIRONMENTAL
ESETT PHARMACOKINETIC PHARMACODYNAMIC (PK/PD) STUDY. Lisa Coles, MS, PhD University of Minnesota
 ESETT PHARMACOKINETIC PHARMACODYNAMIC (PK/PD) STUDY Lisa Coles, MS, PhD University of Minnesota Outline Study progress Overview Procedures Questions Study Progress Contracts Training Site initiation Subject
ESETT PHARMACOKINETIC PHARMACODYNAMIC (PK/PD) STUDY Lisa Coles, MS, PhD University of Minnesota Outline Study progress Overview Procedures Questions Study Progress Contracts Training Site initiation Subject
Training Presentation for Packing & Shipping Samples to Creative Testing Solutions (CTS)
 Training Presentation for Packing & Shipping Samples to Creative Testing Solutions (CTS) Packing, Shipping and Transportation of Donor Samples To Creative Testing Solutions Materials Segregation of Samples
Training Presentation for Packing & Shipping Samples to Creative Testing Solutions (CTS) Packing, Shipping and Transportation of Donor Samples To Creative Testing Solutions Materials Segregation of Samples
Manual of Procedures. TRACK TBI Biorepository
 TRACK TBI BioRepository MOP BR v3 Effective: 10/1/2015 Manual of Procedures TRACK TBI Biorepository 1 October 2015 MOP #: 002 Version #: 3 10/01/2015 TABLE OF CONTENTS 1 Abbreviations... 5 2 Purpose...
TRACK TBI BioRepository MOP BR v3 Effective: 10/1/2015 Manual of Procedures TRACK TBI Biorepository 1 October 2015 MOP #: 002 Version #: 3 10/01/2015 TABLE OF CONTENTS 1 Abbreviations... 5 2 Purpose...
Collecting Blood for ips Cell Reprogramming Europe
 MyCell Products Application Protocol Collecting Blood for ips Cell Reprogramming Europe Introduction: Blood Collection and Infectious Disease Testing All persons collecting and handling any blood samples
MyCell Products Application Protocol Collecting Blood for ips Cell Reprogramming Europe Introduction: Blood Collection and Infectious Disease Testing All persons collecting and handling any blood samples
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples
 Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Protocol TN01 Pathway to Prevention Henry Rodriguez, M.D.
 Protocol TN01 Pathway to Prevention Henry Rodriguez, M.D. Laboratory Manual of Operations Version 2.0 LABORATORY MANUAL OF OPERATIONS TN01 Pathway to Prevention Study Laboratory Manual of Operations -Table
Protocol TN01 Pathway to Prevention Henry Rodriguez, M.D. Laboratory Manual of Operations Version 2.0 LABORATORY MANUAL OF OPERATIONS TN01 Pathway to Prevention Study Laboratory Manual of Operations -Table
CT Laboratory Response Network. CT Preparedness Nebraska. Supplies. Supplies cont. CT Preparedness for the Sentinel Hospital.
 CT Laboratory Response Network CDC Level Three Chemical Terrorism Hospital Preparedness Training Best Practices Karen Stiles, MT(ASCP) SM State Training Coordinator Nebraska Public Health Laboratory Phone
CT Laboratory Response Network CDC Level Three Chemical Terrorism Hospital Preparedness Training Best Practices Karen Stiles, MT(ASCP) SM State Training Coordinator Nebraska Public Health Laboratory Phone
Environmental Health and Safety Dry Ice Shipping Training Post-Test
 Name: Department: Work (BLDG/RM): Environmental Health and Safety Dry Ice Shipping Training Post-Test Title: PI/Supervisor: Email: *NOTE* By completing this test, you are confirming that you have reviewed
Name: Department: Work (BLDG/RM): Environmental Health and Safety Dry Ice Shipping Training Post-Test Title: PI/Supervisor: Email: *NOTE* By completing this test, you are confirming that you have reviewed
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS
 UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
UNIVERSITY OF TOLEDO
 UNIVERSITY OF TOLEDO SUBJECT: SHIPPING, PACKAGING AND RECEIPT OF Procedure No: HM-08-031 HAZARDOUS MATERIALS PROCEDURE Employees of the University of Toledo attempting to transport hazardous materials,
UNIVERSITY OF TOLEDO SUBJECT: SHIPPING, PACKAGING AND RECEIPT OF Procedure No: HM-08-031 HAZARDOUS MATERIALS PROCEDURE Employees of the University of Toledo attempting to transport hazardous materials,
Specimen labeling and shipping protocol.
 Specimen labeling and shipping protocol. SENDING YOUR URINE OR ORAL FLUID SPECIMENS TO THE LABORATORY Preparing specimens for the laboratory 2 Specimen labeling and shipping We ask that you refer to the
Specimen labeling and shipping protocol. SENDING YOUR URINE OR ORAL FLUID SPECIMENS TO THE LABORATORY Preparing specimens for the laboratory 2 Specimen labeling and shipping We ask that you refer to the
SHIPMENT OF CLINICAL SPECIMENS TO THE IMPAACT SPECIMEN REPOSITORY
 SOP Number: SHP-001.01.02 Title: Shipment of Clinical Specimens to the NICHD Specimen Repository Version: 2.0 Effective Date: September 12, 2012 Approved By: Rohan Hazra, NICHD Last Review Date: September
SOP Number: SHP-001.01.02 Title: Shipment of Clinical Specimens to the NICHD Specimen Repository Version: 2.0 Effective Date: September 12, 2012 Approved By: Rohan Hazra, NICHD Last Review Date: September
The laboratory offers several types of forensics tests. The most common tests that we perform are: Ignitable liquid analysis for its presence in fire
 The laboratory offers several types of forensics tests. The most common tests that we perform are: Ignitable liquid analysis for its presence in fire debris. Identification of ignitable liquids Unidentified
The laboratory offers several types of forensics tests. The most common tests that we perform are: Ignitable liquid analysis for its presence in fire debris. Identification of ignitable liquids Unidentified
Caris Molecular Profiling Shipper Kit Instructions
 Caris Molecular Profiling Shipper Kit Instructions Preparing the Shipper Kit Step by Step procedure You need a Caris Paraffin Block and Slide Shipper Kit. If you don t have shipper kits stored at your
Caris Molecular Profiling Shipper Kit Instructions Preparing the Shipper Kit Step by Step procedure You need a Caris Paraffin Block and Slide Shipper Kit. If you don t have shipper kits stored at your
Supplementary File 3: DNA and RNA isolation
 Supplementary File 3: DNA and RNA isolation Q-CROC-02 Biopsy protocol For the purposes of this protocol, four needle core biopsies (NCBs) of lymph node tissue are isolated from each patient using a 16G
Supplementary File 3: DNA and RNA isolation Q-CROC-02 Biopsy protocol For the purposes of this protocol, four needle core biopsies (NCBs) of lymph node tissue are isolated from each patient using a 16G
BIOSPECIMEN COLLECTION SOP Phenotype, Genotype, and Biomarkers (PGB) in ALS and Related Disorders
 The purpose of this Standard Operating Procedure (SOP) is to describe the instructions for collection, processing, and storage of collected specimens for the PGB Study. 1. Definitions 1. EDTA An EDTA tube
The purpose of this Standard Operating Procedure (SOP) is to describe the instructions for collection, processing, and storage of collected specimens for the PGB Study. 1. Definitions 1. EDTA An EDTA tube
Guide to preparation of liquid biopsies for nucleic acid extraction
 Guide to preparation of liquid biopsies for nucleic acid extraction CRI 002 version 8, April 2017 Table of Contents 1 Guidelines on experiment design... 2 2 Collecting and preserving liquid biopsies for
Guide to preparation of liquid biopsies for nucleic acid extraction CRI 002 version 8, April 2017 Table of Contents 1 Guidelines on experiment design... 2 2 Collecting and preserving liquid biopsies for
UNH Guide to Shipping with Dry Ice
 UNH Guide to Shipping with Dry Ice Created by Andy Glode University of New Hampshire Office of Environmental Health and Safety 2003-2016. University of New Hampshire Office of Environmental Health and
UNH Guide to Shipping with Dry Ice Created by Andy Glode University of New Hampshire Office of Environmental Health and Safety 2003-2016. University of New Hampshire Office of Environmental Health and
Dear Colleague, We look forward to working with you and helping you to reach your study goals. Best Regards, Your Illumina Genomic Services Team
 Dear Colleague, Thank you for using Illumina FastTrack Services. We are committed to your success. Project success will be determined by DNA preparation, sample shipping and good communication between
Dear Colleague, Thank you for using Illumina FastTrack Services. We are committed to your success. Project success will be determined by DNA preparation, sample shipping and good communication between
Refer to the VCH Intranet to make sure you are using the most updated protocol
 Updated 0 April 05 Refer to the VCH Intranet to make sure you are using the most updated protocol General Guidelines:. Phlebotomy should be done by experienced personnel.. Specimen collection must not
Updated 0 April 05 Refer to the VCH Intranet to make sure you are using the most updated protocol General Guidelines:. Phlebotomy should be done by experienced personnel.. Specimen collection must not
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
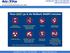 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
ACTG Laboratory Technologist Committee Revised Version 1.0 ACTG Lab Man ACTG Guidelines for Shipping Category A Infectious Substances 04 March 2008
 AIDS CLINICAL TRIALS NETWORK* (ACTN) GUIDELINES FOR SHIPMENT AND RECEIPT OF CATEGORY A INFECTIOUS SPECIMENS AND OTHER DANGEROUS GOODS ACTN-Specific Instructions and Information 1.0 General information
AIDS CLINICAL TRIALS NETWORK* (ACTN) GUIDELINES FOR SHIPMENT AND RECEIPT OF CATEGORY A INFECTIOUS SPECIMENS AND OTHER DANGEROUS GOODS ACTN-Specific Instructions and Information 1.0 General information
Manual of Procedures. National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD)
 Manual of Procedures National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) A Phase 2 Randomized Double-Blind Placebo-Controlled Trial to Evaluate the Efficacy and Safety
Manual of Procedures National Centralized Repository for Alzheimer s Disease and Related Dementias (NCRAD) A Phase 2 Randomized Double-Blind Placebo-Controlled Trial to Evaluate the Efficacy and Safety
National Institute of Neurological Disorders and Stroke Biorepository:
 - LBD National Institute of Neurological Disorders and Stroke Biorepository: BioSpecimen Exchange for Neurological Disorders, BioSEND Biospecimen Collection, Processing, and for Lewy Body Dementia (LBD)
- LBD National Institute of Neurological Disorders and Stroke Biorepository: BioSpecimen Exchange for Neurological Disorders, BioSEND Biospecimen Collection, Processing, and for Lewy Body Dementia (LBD)
Instructions for Use: Flexible Endoscope Sampling Kit
 Instructions for Use: Flexible Endoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Flexible Endoscope Sampling Kit Endoscope Culture Sample Kit CK-250,
Instructions for Use: Flexible Endoscope Sampling Kit Brand Name of Product Generic Name of Product Product Code Number(s) Intended Use Flexible Endoscope Sampling Kit Endoscope Culture Sample Kit CK-250,
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS
 ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS
 ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
ARMED FORCES REPOSITORY OF SPECIMEN SAMPLES FOR THE IDENTIFICATION OF REMAINS COLLECTION INSTRUCTIONS 1. Purpose The following DNA collection instructions are designed to give specific direction to installations/sites
For certification, manufacturers must perform a sample comparison with a Secondary Reference Laboratory (SRL) following the NGSP protocol.
 NGSP Manufacturer Information Packet MANUFACTURER CERTIFICATION General Instructions: If a manufacturer is interested in certifying a method as traceable to the Diabetes Control and Complications Trial
NGSP Manufacturer Information Packet MANUFACTURER CERTIFICATION General Instructions: If a manufacturer is interested in certifying a method as traceable to the Diabetes Control and Complications Trial
Working Instruction (WI) Summary Box
 Working Instruction (WI) Summary Box Study Name PD-CRAFT WI Title Site lab procedures WI Index Number WI 01 Version 1.0 Approval Date 27 November 2017 Effective Date 28 November 2017 Review Date 28 May
Working Instruction (WI) Summary Box Study Name PD-CRAFT WI Title Site lab procedures WI Index Number WI 01 Version 1.0 Approval Date 27 November 2017 Effective Date 28 November 2017 Review Date 28 May
Morris Animal. FOUNDATION Golden Retriever Lifetime Study. Golden Retreiver Lifetime Study Biopsy Kit: Collection & Shipping Instructions
 Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Biopsy Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you for your participation
Morris Animal FOUNDATION Golden Retriever Lifetime Study Golden Retreiver Lifetime Study Biopsy Kit: Collection & Shipping Instructions Tips for Participating Veterinarians Thank you for your participation
Agarose Gel Electrophoresis Lab
 Agarose Gel Electrophoresis ACTIVITY AT A GLANCE Goal: This lab will determine the presence or absence of PCR products and uantify the size (length of the DNA molecule) of the products. Learning Objectives:
Agarose Gel Electrophoresis ACTIVITY AT A GLANCE Goal: This lab will determine the presence or absence of PCR products and uantify the size (length of the DNA molecule) of the products. Learning Objectives:
Laboratory (LAB) prepares sample kits prior to Epidemiologist Investigator (EPI) arrival.
 Standard Operating Procedure for Sample Collection of Heavy Metals (As, Hg, U, Cd, Mn, Se), Creatinine, Phthalate Metabolites, 2,4-DCP, 2,5-DCP and Pyrethroids for Biomonitoring A. Scope and Purpose The
Standard Operating Procedure for Sample Collection of Heavy Metals (As, Hg, U, Cd, Mn, Se), Creatinine, Phthalate Metabolites, 2,4-DCP, 2,5-DCP and Pyrethroids for Biomonitoring A. Scope and Purpose The
UCSF Guide to Shipping with Dry Ice
 UCSF Guide to Shipping with Dry Ice This guide has been adapted from University of New Hampshire - Office of Environmental Health and Safety; our appreciation to Andy Glode for providing us with this reference
UCSF Guide to Shipping with Dry Ice This guide has been adapted from University of New Hampshire - Office of Environmental Health and Safety; our appreciation to Andy Glode for providing us with this reference
Submitting Rabies Specimens to the DSHS Lab
 Mosaic from Louis Pasteurís' crypt representing the shepherd Jean- Baptiste Jupille (second person ever to be vaccinated against rabies) struggling against a rabid dog. From the CDC Public Health Image
Mosaic from Louis Pasteurís' crypt representing the shepherd Jean- Baptiste Jupille (second person ever to be vaccinated against rabies) struggling against a rabid dog. From the CDC Public Health Image
Guideline for Shipping Items 1 on Dry Ice That are Not Dangerous Goods
 Guideline for Shipping Items 1 on Dry Ice That are Not Dangerous Goods INTRODUCTION Dry ice is a hazardous material; therefore the U.S. Department of Transportation (DOT) and the International Air Transport
Guideline for Shipping Items 1 on Dry Ice That are Not Dangerous Goods INTRODUCTION Dry ice is a hazardous material; therefore the U.S. Department of Transportation (DOT) and the International Air Transport
All data should be sent by from the laboratory and from the SRL by directly to the NGSP Network Coordinator (above address).
 NGSP Level I Laboratory Information Packet LEVEL I LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in directly certifying their method as traceable to the Diabetes Control
NGSP Level I Laboratory Information Packet LEVEL I LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in directly certifying their method as traceable to the Diabetes Control
Table of Content. 1. Introduction. 2. Temperature Controlled Shipping. 3. Packaging, Labeling and Marking. 4. Shipment Documentation
 March 2017 Table of Content 1. Introduction 2. Temperature Controlled Shipping 3. Packaging, Labeling and Marking 3.1 Exempt Specimen 3.2 Category B 3.3 Category A 4. Shipment Documentation 5. Health
March 2017 Table of Content 1. Introduction 2. Temperature Controlled Shipping 3. Packaging, Labeling and Marking 3.1 Exempt Specimen 3.2 Category B 3.3 Category A 4. Shipment Documentation 5. Health
Protocol for amplification of measles sequencing window (N-450)
 Annex 7.1 Protocol for amplification of measles sequencing window (N-450) NOTE: This document is intended to provide basic test method details and is not an SOP. Laboratories need to develop their own
Annex 7.1 Protocol for amplification of measles sequencing window (N-450) NOTE: This document is intended to provide basic test method details and is not an SOP. Laboratories need to develop their own
HOW TO ORDER Revised
 HOW TO ORDER Revised 2015 9 29 How to Order Animal Health Testing Our Mission We have been working hard to get the word out about the risks of zoonotic infection to the veterinary and medical communities
HOW TO ORDER Revised 2015 9 29 How to Order Animal Health Testing Our Mission We have been working hard to get the word out about the risks of zoonotic infection to the veterinary and medical communities
foodproof Sample Preparation Kit II
 For food testing purposes FOR IN VITRO USE ONLY foodproof Sample Preparation Kit II Version 1, October 2007 For isolation of bacterial DNA from enrichment cultures of food samples prior to PCR analysis
For food testing purposes FOR IN VITRO USE ONLY foodproof Sample Preparation Kit II Version 1, October 2007 For isolation of bacterial DNA from enrichment cultures of food samples prior to PCR analysis
Manual of Procedures Version 2.1 May, 2017
 Specimen Collection, Processing and Internet-based Conversational Engagement Clinical Trial (I-CONECT) in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Manual of Procedures
Specimen Collection, Processing and Internet-based Conversational Engagement Clinical Trial (I-CONECT) in collaboration with The National Cell Repository for Alzheimer s Disease (NCRAD) Manual of Procedures
General Information and Guidelines
 General Information and Guidelines Please refer to the test information on the website for specific guidelines on type of specimen submitted. The following is intended to serve as a guideline. We do request
General Information and Guidelines Please refer to the test information on the website for specific guidelines on type of specimen submitted. The following is intended to serve as a guideline. We do request
NGSP Level II Laboratory Information Packet
 NGSP Level II Laboratory Information Packet LEVEL II LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in certifying their method as traceable to the Diabetes Control and Complications
NGSP Level II Laboratory Information Packet LEVEL II LABORATORY CERTIFICATION General Instructions: If a laboratory is interested in certifying their method as traceable to the Diabetes Control and Complications
National Plant Diagnostic Network. Standard Operating Procedure for Plant Diagnostic Laboratories. Disease Common Name Pathogen Scientific Name
 National Plant Diagnostic Network Standard Operating Procedure for Plant Diagnostic Laboratories Disease Common Name Pathogen Scientific Name VERSION X.X Month/Day/Year Table of Contents Background...#
National Plant Diagnostic Network Standard Operating Procedure for Plant Diagnostic Laboratories Disease Common Name Pathogen Scientific Name VERSION X.X Month/Day/Year Table of Contents Background...#
trucollect -plus - Stabilization and Transport Kit (10)
 trucollect -plus - Stabilization and Transport Kit (10) Whole blood specimen dry stabilization, transport, and storage For Research Use Only Not for use in diagnostic procedures PN 520254 Patents Granted
trucollect -plus - Stabilization and Transport Kit (10) Whole blood specimen dry stabilization, transport, and storage For Research Use Only Not for use in diagnostic procedures PN 520254 Patents Granted
DiviTum V2. User s Manual IVD. This User s Manual is intended for DiviTum V2. For use in the United States and Japan only for research purposes
 DiviTum V2 User s Manual This User s Manual is intended for DiviTum V2 For use in the United States and Japan only for research purposes IVD 40 Ref. 933 rev. EN 2 1 Contents DIVITUM ASSAY FOR MEASURING
DiviTum V2 User s Manual This User s Manual is intended for DiviTum V2 For use in the United States and Japan only for research purposes IVD 40 Ref. 933 rev. EN 2 1 Contents DIVITUM ASSAY FOR MEASURING
