Complaint Handling and Trending At Stryker Orthopaedics
|
|
|
- Joshua Beasley
- 6 years ago
- Views:
Transcription
1 Complaint Handling and Trending At Stryker Orthopaedics John Lynch Reliability Engineer Stryker Orthopaedics Richard Campos Senior Reliability Engineer Stryker Orthopaedics March 8 10, 2011 Hyatt Regency Cincinnati, OH
2 Our Company Stryker A broad based global leader in medical technologies Founded 1941 by Dr. Homer Stryker Company went public in ,000 employees $7.3B sales in 89 countries for 2010 $4.3B worldwide sales of orthopaedic implants in 2010 Diversified businesses Joint reconstruction of knees, hips, shoulders Operating room equipment Medical beds and stretchers Craniomaxillofacial Endoscopy Trauma Spine
3 Broad Product Range
4 Joint Replacement The hip joint is a ball-and-socket joint, formed by the femoral head at the upper end of the thigh bone, and the rounded socket, or acetabulum, in the pelvis. An artificial joint is a medical option available to relieve pain and restore quality of life to patients with joint degeneration or arthritis. JOHNNY BENCH
5 System Overview Data Triggers Analysis Integrated data handling, risk management, and analytics
6 Data Complaint Handling Triggers Analysis
7 Why do we investigate complaints We want to improve our products for our users and patients Regulations
8 What is a complaint? Any written, oral, or expression of dissatisfaction relative to the identity, quality, Voice durability, of Customer reliability, - A customer safety, has effectiveness, had an unsatisfactory performance, experience or appearance with our product
9 Where do we monitor complaints?
10 Intake Tools Required Best practices SOPs Forms Hotline Expedited device returns
11 Data Management Tools Required Validated database 3 rd party solution Company specific solution Key Attributes Time Bases Event types Root Cause types
12 Voices of Customer (VOC) Damaged blister Discrepancy noted in blister Contents rattling in box Dent on inner blister Blister does not look right Stem moved in packaging Packaging damaged Failed pre-op inspection
13 Translating VOC to Event Types Damaged Shipping damage blister Discrepancy Shipping noted damage in blister Contents Shipping rattling damage in box Dent Shipping on inner damage blister Blister Shipping does not damage look right Stem Shipping moved in packaging damage Packaging User mishandling damaged Failed User pre-op mishandling inspection
14 Categorization of complaints Shipping damage Shipping damage Shipping damage Shipping damage Shipping damage Shipping damage User mishandling User mishandling
15 Data Risk Management and Trending Triggers Analysis
16 Value Stream
17 Risk Management Process ANALYSIS PRODUCT 1 Hazard & Harm lists EVALUATION Acceptability criteria Plan Act Do Check CONTROL Effective Measures Plan Do REPORT Controlled Documents Act Check POST / PRODUCTION PRODUCT 2 Surveillance
18 Product Complaint Trending 1 2 Scope the Complaints 3 Scope Sales Estimate the complaint rate 4 Compare to acceptable rate What is the valid statistical rate? 5 Communicate to Stakeholders What was the risk assessment at product launch? Corrective Action How are the findings effectively communicated? How many patient exposures? What is the issue?
19 Scoping Complaints Pareto the issue 1 2 By supplier, tool, batch, distributor, shipper By product line, brand, family, specific item By region, country, facility, user, patient By failure mode, hazard, harm (severity) Timebase the issue Choice of date affects sales association (event, surgery, production ) Choice of binning affects sample size (monthly, quarterly ) Examples: For implantable devices, timebase on the implantation date For corrective action effectivity, timebase on the production date
20 Scoping Sales Product Association Determine optimal product association 1 Example: Reusable Medical Instrument Medical Uses Pareto the issue By bulk complaint to sales # units shipped # implants sold Timebase reminder Match timebase chosen for complaint scoping
21 Complaint Rate Estimation Valid Statistical Technique Linear regression Y = cumulative complaints X = cumulative sales Speed = Rise over Run c1+c2 The Speed at the second data position is Cumulative Complaints c1 s1 s1+s2 (c1+c2) - c1 (s1+s2) - s1 c2 = = Complaint Rate s2 Cumulative Sales
22 Compare to Acceptable Rate Review Product Risk Management files Risk Tables, Fault Trees, FMEAs Determine risk ratings for subject issue Compare rate and severity to risk ratings Is this a new risk? If known, is there residual risk? Example of documented Risk Assessment High O5 Acceptable occurrence Moderate Low Remote Negligible O4 O3 O2 O1 S0 S1 S2 S3 S4 S5 None Limited Temporary or Reversible Intervention Required Permanent Impairment Death Acceptable Severity
23 Communicate to Stakeholders Executive Issue Summary Issue description Associated products Harms and Hazards Supply chain scope Statistical method Statistical results Risk reassessment Action request
24 Let s drive an example
25 Hip Stem Packaging Damage PETG double blister package, sealed with Tyvek lids and retaining foam
26 Causal associations Double blister break: Inner blister break: Readily detected and replaced Sterility not compromised
27 Product Complaint Trending Scope the Complaints 2 1 VOC Damaged Hip stem PETG Package Blisters Database 95 % Coded events as Damaged Package Timebase Binning by Production Date Pareto 98% complaints from 1 Product Family Harms Minor delay in surgery
28 Product Complaint Trending Scope the Sales 2 1 Association Hip stem sales Timebase Binning by Production Date Pareto 76 % from Region1 (worst case distribution path)
29 Product Complaint Trending Estimate the Complaint Rate 2 1 Technique Linear regression for Region 1 Fit quality Residuals 99% adjusted R-square Normal, independent, homogeneous Cumulative Complaints Cumulative Sales
30 Product Complaint Trending Risk Re-assessment and Communication Action is Needed! Occurrence: Trend rate Severity: Event details
31 So How did We Do? The Package was redesigned to secure the stem for the most stringent distribution path The Risk reduction was Effective Cumulative Complaints Complaints level off Cumulative Sales
32 Conclusion Our integrated processes effectively reduce patient and user risk Pillars Complaint Handling Risk Management Trending Supporting pillars Corrective Action system Decision makers
33 Thank You for your Attention Any Questions?
HydroSet. Injectable HA Bone Substitute
 HydroSet Injectable HA Bone Substitute The Latest Advancement in Bone Substitute Technology Fast Setting Injectable or Manual Implantation Osteoconductive Excellent Wet-field Characteristics Isothermic
HydroSet Injectable HA Bone Substitute The Latest Advancement in Bone Substitute Technology Fast Setting Injectable or Manual Implantation Osteoconductive Excellent Wet-field Characteristics Isothermic
Incorporating Risk Management into Complaint and Service Experience. Presented by: Richard J. DeRisio, M.S. Kinetic Concepts, Inc.
 Incorporating Risk Management into Complaint and Service Experience Presented by: Richard J. DeRisio, M.S. Kinetic Concepts, Inc. 1 Management of Complaint and Service Experience A Compliance Headache?
Incorporating Risk Management into Complaint and Service Experience Presented by: Richard J. DeRisio, M.S. Kinetic Concepts, Inc. 1 Management of Complaint and Service Experience A Compliance Headache?
FMEA Failure Mode Effects Analysis. ASQ/APICS Joint Meeting May 10, 2017
 FMEA Failure Mode Effects Analysis ASQ/APICS Joint Meeting May 10, 2017 FMEA (Failure Mode and Effects Analysis) Failure Mode and Effects Analysis Agenda What is it? Motivation FMEA Methods Examples What
FMEA Failure Mode Effects Analysis ASQ/APICS Joint Meeting May 10, 2017 FMEA (Failure Mode and Effects Analysis) Failure Mode and Effects Analysis Agenda What is it? Motivation FMEA Methods Examples What
TERMS AND DEFINITIONS
 TERMS AND DEFINITIONS Advisory notice A notice issued by the organization, subsequent to delivery of the medical device, to provide supplementary information and/or to advise what action should be taken
TERMS AND DEFINITIONS Advisory notice A notice issued by the organization, subsequent to delivery of the medical device, to provide supplementary information and/or to advise what action should be taken
MEDICAL PACKAGING SPECIALISTS
 MEDICAL PACKAGING SPECIALISTS We have multiple locations and clean rooms to serve you: Elkhart, IN 48,000 sq. ft Plymouth, MN 74,000 sq. ft Madison, WI 350,000 sq. ft Class 8 clean rooms in all manufacturing
MEDICAL PACKAGING SPECIALISTS We have multiple locations and clean rooms to serve you: Elkhart, IN 48,000 sq. ft Plymouth, MN 74,000 sq. ft Madison, WI 350,000 sq. ft Class 8 clean rooms in all manufacturing
FSC36 SAFE FEED/SAFE FOOD GUIDANCE DOCUMENT
 FSC36 SAFE FEED/SAFE FOOD GUIDANCE DOCUMENT FSC36 Safe Feed/Safe Food (www.safefeedsafefood.org) is a facility certification program for the American Feed Industry Association (www.afia.org) Version 7.0
FSC36 SAFE FEED/SAFE FOOD GUIDANCE DOCUMENT FSC36 Safe Feed/Safe Food (www.safefeedsafefood.org) is a facility certification program for the American Feed Industry Association (www.afia.org) Version 7.0
Policy and Procedure Manual
 Policy and Procedure Manual Finance and Accounting Procurement Services F-PS-01.00 SUBJECT/TITLE: PURPOSE: DEFINITION: STATEMENT OF VENDOR OWNED INVENTORY (CONSIGNMENT) POLICY This policy will cover all
Policy and Procedure Manual Finance and Accounting Procurement Services F-PS-01.00 SUBJECT/TITLE: PURPOSE: DEFINITION: STATEMENT OF VENDOR OWNED INVENTORY (CONSIGNMENT) POLICY This policy will cover all
Power Digital Imaging
 OfficePACS Power Digital Imaging Power Unleashed Organize According to Workflow PowerGroup allows physicians to organize studies by key fields and customize the PatientFinder to the way they work. Find
OfficePACS Power Digital Imaging Power Unleashed Organize According to Workflow PowerGroup allows physicians to organize studies by key fields and customize the PatientFinder to the way they work. Find
GENERAL SURGERY DEVICES
 GENERAL SURGERY DEVICES LAPAROSCOPIC INSTRUMENTS SUTURE PASSERS TROCARS ULTRASONIC SCALPELS REPROCESSED SURGICAL DEVICES STRYKER S PORTFOLIO OF REPROCESSED SINGLE-USE DEVICES Laparoscopic devices reprocessed
GENERAL SURGERY DEVICES LAPAROSCOPIC INSTRUMENTS SUTURE PASSERS TROCARS ULTRASONIC SCALPELS REPROCESSED SURGICAL DEVICES STRYKER S PORTFOLIO OF REPROCESSED SINGLE-USE DEVICES Laparoscopic devices reprocessed
IQCP for Commercially Prepared CLSI-Exempt Media
 Please note that some references to protocol, publications, performance data etc. are fictitious in this EXAMPLE. Please use your own DATA for your IQCP. The following represents one example of how you
Please note that some references to protocol, publications, performance data etc. are fictitious in this EXAMPLE. Please use your own DATA for your IQCP. The following represents one example of how you
True North at Zimmer. Made as if intended for my family
 True North at Zimmer History of Zimmer US Orthopaedic Multinational founded in 1927: Justin O. Zimmer 1972: Acquired by Bristol-Myers Squibb 2001:Spin-off Zimmer from BMS and NYSE (ZMH) Justin O. Zimmer
True North at Zimmer History of Zimmer US Orthopaedic Multinational founded in 1927: Justin O. Zimmer 1972: Acquired by Bristol-Myers Squibb 2001:Spin-off Zimmer from BMS and NYSE (ZMH) Justin O. Zimmer
Standards and Regulatory Considerations for Orthopaedic Implants
 Standards and Regulatory Considerations for Orthopaedic Implants Constituencies Patients Payers (Insurance) Physicians Manufacturers Research Community Government Legal Community Professional Organizations
Standards and Regulatory Considerations for Orthopaedic Implants Constituencies Patients Payers (Insurance) Physicians Manufacturers Research Community Government Legal Community Professional Organizations
USP <797> Compliance Common Challenges and Potential Solutions
 USP Compliance Common Challenges and Potential Solutions Angela Yaniv, Pharm.D Assistant Director - Sterile Products May 2, 2017 - OSHP Annual Meeting Angela Yaniv has no actual or potential conflict
USP Compliance Common Challenges and Potential Solutions Angela Yaniv, Pharm.D Assistant Director - Sterile Products May 2, 2017 - OSHP Annual Meeting Angela Yaniv has no actual or potential conflict
So, How Will You Audit a Risk Assessment in ISO 9001:2015?
 So, How Will You Audit a Risk Assessment in ISO 9001:2015? Bob Deysher Senior Consultant Quality Support Group, Inc. bob.deysher@qualitysupportgroup.com 2017 QSG, Inc. Inc. Questions? Does ISO 9001:2015
So, How Will You Audit a Risk Assessment in ISO 9001:2015? Bob Deysher Senior Consultant Quality Support Group, Inc. bob.deysher@qualitysupportgroup.com 2017 QSG, Inc. Inc. Questions? Does ISO 9001:2015
TECHNICAL CONTENT PROPOSED STEEP SLOPE REGULATION
 1 TECHNICAL CONTENT PROPOSED STEEP SLOPE REGULATION Draft 2/08/10 (Revised 02-14-11,Revised 02-21-11, Revised 03-03-11, Revised 04-19-11, Revised 04-20-11, Revised 05-05-11, Revised 05-09-11). DEFINITIONS
1 TECHNICAL CONTENT PROPOSED STEEP SLOPE REGULATION Draft 2/08/10 (Revised 02-14-11,Revised 02-21-11, Revised 03-03-11, Revised 04-19-11, Revised 04-20-11, Revised 05-05-11, Revised 05-09-11). DEFINITIONS
Continuous Improvement Toolkit. Risk Analysis. Continuous Improvement Toolkit.
 Continuous Improvement Toolkit Risk Analysis The Continuous Improvement Map Managing Risk FMEA Understanding Performance Check Sheets Data Collection PDPC RAID Log* Risk Analysis* Fault Tree Analysis Traffic
Continuous Improvement Toolkit Risk Analysis The Continuous Improvement Map Managing Risk FMEA Understanding Performance Check Sheets Data Collection PDPC RAID Log* Risk Analysis* Fault Tree Analysis Traffic
Best Practices: Integration of Risk Management and Corrective and Preventive Action
 Best Practices: Integration of Risk Management and Corrective and Preventive Action Presented by: Norman L. Collazo Worldwide Director of Strategic Quality Cordis Corporation, a Johnson & Johnson company
Best Practices: Integration of Risk Management and Corrective and Preventive Action Presented by: Norman L. Collazo Worldwide Director of Strategic Quality Cordis Corporation, a Johnson & Johnson company
Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS
 Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS Key Topics Definitions Essential Principles Classification Conformity Assessment Framework License to Manufacture, Import,
Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS Key Topics Definitions Essential Principles Classification Conformity Assessment Framework License to Manufacture, Import,
Managing Your Audit. Effective Preparation to Successful Certification. John Kukoly BRC Global Standards. BRC Global Standards. Trust in Quality.
 Managing Your Audit Effective Preparation to Successful Certification John Kukoly BRC Global Standards Agenda Preparing for the audit Managing the audit Non-conformities Corrective Action Root Cause Analysis
Managing Your Audit Effective Preparation to Successful Certification John Kukoly BRC Global Standards Agenda Preparing for the audit Managing the audit Non-conformities Corrective Action Root Cause Analysis
PRESENTATION OVERVIEW
 Conformance and Interoperability (C&I) Validation Workshop for EAC Region Laico Regency Hotel, Nairobi, Kenya 21st 23rd October 2015 PART 2 Requirements for Accreditation Bodies and Testing Laboratories
Conformance and Interoperability (C&I) Validation Workshop for EAC Region Laico Regency Hotel, Nairobi, Kenya 21st 23rd October 2015 PART 2 Requirements for Accreditation Bodies and Testing Laboratories
MEASURE FOR MEASURE: QUALITY METRICS
 MEASURE FOR MEASURE: QUALITY METRICS PDA Midwest Chapter Dinner Meeting, Northbrook, IL-9 November 2017 Felicia Ford-Rice, Director, Strategic Compliance 2017 PAREXEL INTERNATIONAL CORP. AGENDA Robust
MEASURE FOR MEASURE: QUALITY METRICS PDA Midwest Chapter Dinner Meeting, Northbrook, IL-9 November 2017 Felicia Ford-Rice, Director, Strategic Compliance 2017 PAREXEL INTERNATIONAL CORP. AGENDA Robust
Continuous Improvement Toolkit. Pareto Analysis. Continuous Improvement Toolkit.
 Continuous Improvement Toolkit Pareto Analysis The Continuous Improvement Map Managing Risk FMEA Understanding Performance Check Sheets Data Collection PDPC RAID Log* Risk Assessment* Fault Tree Analysis
Continuous Improvement Toolkit Pareto Analysis The Continuous Improvement Map Managing Risk FMEA Understanding Performance Check Sheets Data Collection PDPC RAID Log* Risk Assessment* Fault Tree Analysis
Continuous Improvement Toolkit. QFD (Quality Function Deployment) Continuous Improvement Toolkit.
 Continuous Improvement Toolkit QFD (Quality Function Deployment) The Continuous Improvement Map Managing Risk FMEA Understanding Performance Check Sheets Data Collection PDPC RAID Log* Risk Assessment*
Continuous Improvement Toolkit QFD (Quality Function Deployment) The Continuous Improvement Map Managing Risk FMEA Understanding Performance Check Sheets Data Collection PDPC RAID Log* Risk Assessment*
We ve built a STRONG CASE. for changing the way you sterilize. Reusable Sterilization Container System
 We ve built a STRONG CASE for changing the way you sterilize. Reusable Sterilization Container System Reusable Sterilization Container System Introducing the DePuy Synthes REUSABLE STERILIZATION CONTAINER
We ve built a STRONG CASE for changing the way you sterilize. Reusable Sterilization Container System Reusable Sterilization Container System Introducing the DePuy Synthes REUSABLE STERILIZATION CONTAINER
Inspection Hot Topics
 Inspection Hot Topics QP Forum, Trinity College Ciara Turley, Inspector, HPRA 16 th April 2015 Content Hot topics = common deficiencies Data based on 100 inspections conducted in 2014 Inspections of non-sterile
Inspection Hot Topics QP Forum, Trinity College Ciara Turley, Inspector, HPRA 16 th April 2015 Content Hot topics = common deficiencies Data based on 100 inspections conducted in 2014 Inspections of non-sterile
HACCP audit checklist
 Requirement HACCP audit checklist Prerequisite Program Management Commitment 1. Senior management ensures that the responsibilities and authorities are defined and communicated within the company Internal
Requirement HACCP audit checklist Prerequisite Program Management Commitment 1. Senior management ensures that the responsibilities and authorities are defined and communicated within the company Internal
QAA1, Appendix 2. APQP Elements. Customer order. 1.1 Customer specifications
 QAA1, Appendix 2 APQP Elements The objectives, expectations and requirements for documenting individual elements of the APQP Status Reports (see QSR-1, Appendix 3) are described as follows: Customer order
QAA1, Appendix 2 APQP Elements The objectives, expectations and requirements for documenting individual elements of the APQP Status Reports (see QSR-1, Appendix 3) are described as follows: Customer order
A guide for business professionals to help improve the effectiveness of their POS display design and manufacturing journey.
 A guide for business professionals to help improve the effectiveness of their design and manufacturing journey. How to turn your project into a success In the business world, presentation is everything.
A guide for business professionals to help improve the effectiveness of their design and manufacturing journey. How to turn your project into a success In the business world, presentation is everything.
Systematic Risk Management: An Overview of ICH Q-9
 Systematic Risk Management: An Overview of ICH Q-9 March 12, 2014 12014 ParagonRx International LLC Today s Session Systematic Risk Management: An Overview of ICH Q-9 Speakers: Jeff Fetterman, President,
Systematic Risk Management: An Overview of ICH Q-9 March 12, 2014 12014 ParagonRx International LLC Today s Session Systematic Risk Management: An Overview of ICH Q-9 Speakers: Jeff Fetterman, President,
Listen-Only Mode All attendees are in listen-only mode.
 Listen-Only Mode All attendees are in listen-only mode. Please keep your phones on mute to improve everyone s experience. -You can use *1 to unmute your line -And then to mute, press *1 again Grievance
Listen-Only Mode All attendees are in listen-only mode. Please keep your phones on mute to improve everyone s experience. -You can use *1 to unmute your line -And then to mute, press *1 again Grievance
VideojetConnect TM Remote Service. For select Ethernet-enabled Videojet printers
 VideojetConnect TM Remote Service For select Ethernet-enabled Videojet printers Use the power of data and connectivity to drive productivity With immediate access to your printer data, you can now experience
VideojetConnect TM Remote Service For select Ethernet-enabled Videojet printers Use the power of data and connectivity to drive productivity With immediate access to your printer data, you can now experience
Supplier Assurance Program. CBE Pty Ltd
 Supplier Assurance Program CBE Pty Ltd This training program is copyright to CBE Pty Ltd and may not be modified, reproduced, sold, loaned, hired or traded in any form without its express written permission.
Supplier Assurance Program CBE Pty Ltd This training program is copyright to CBE Pty Ltd and may not be modified, reproduced, sold, loaned, hired or traded in any form without its express written permission.
Audit Report No: QE /12 - S. Hooks Industrial, Inc. Contact Person: Mr. John Valentine Phone: « »
 Audit Report No: QE - 43699-2/12 - S Company Name: Hooks Industrial, Inc. Contact Person: Mr. John Valentine Phone: «281 251-9551» Report Date: 2013-10-22 Audit Dates: 2013-10-22-2013-10-22 Audit Duration:
Audit Report No: QE - 43699-2/12 - S Company Name: Hooks Industrial, Inc. Contact Person: Mr. John Valentine Phone: «281 251-9551» Report Date: 2013-10-22 Audit Dates: 2013-10-22-2013-10-22 Audit Duration:
Getting Started with Risk in ISO 9001:2015
 Getting Started with Risk in ISO 9001:2015 Executive Summary The ISO 9001:2015 standard places a great deal of emphasis on using risk to drive processes and make decisions. The old mindset of using corrective
Getting Started with Risk in ISO 9001:2015 Executive Summary The ISO 9001:2015 standard places a great deal of emphasis on using risk to drive processes and make decisions. The old mindset of using corrective
PROPYLUX HS rods are exclusively produced by Westlake Plastics.
 PROPYLUX HS rods are exclusively produced by Westlake Plastics. Launched in 2004 by Westlake Plastics Europe, PROPYLUX HS has gained wide acceptance by orthopedic device manufacturers in Europe. PROPYLUX
PROPYLUX HS rods are exclusively produced by Westlake Plastics. Launched in 2004 by Westlake Plastics Europe, PROPYLUX HS has gained wide acceptance by orthopedic device manufacturers in Europe. PROPYLUX
ISO Part 1 and Part 2 Compliance Requirements. Cathriona O Neill
 ISO 11607 Part 1 and Part 2 Compliance Requirements Cathriona O Neill I.S. EN 11607 Introduction ISO 11607 is the principal guidance document. Packaging for terminally sterilised medical devices - Part
ISO 11607 Part 1 and Part 2 Compliance Requirements Cathriona O Neill I.S. EN 11607 Introduction ISO 11607 is the principal guidance document. Packaging for terminally sterilised medical devices - Part
AAMI Quality Systems White Paper: Comparison of 21 CFR Part 820 to ISO 13485:2016 1
 AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward, Owner, Ward Sciences
AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward, Owner, Ward Sciences
GLOBALIZATION: CHALLENGES AND SOLUTIONS
 GLOBALIZATION: CHALLENGES AND SOLUTIONS Kaiser J. Aziz, Ph.D. Senior Clinical & Regulatory Affairs Consultant The FDA Group, LLC THIS PRESENTATION WILL ADDRESS THE FOLLOWING TOPICS: Overview of Globalization
GLOBALIZATION: CHALLENGES AND SOLUTIONS Kaiser J. Aziz, Ph.D. Senior Clinical & Regulatory Affairs Consultant The FDA Group, LLC THIS PRESENTATION WILL ADDRESS THE FOLLOWING TOPICS: Overview of Globalization
LAMINAR FLOW BENCH GUIDELINES
 LAMINAR FLOW BENCH GUIDELINES 4.2.6 Personnel should not be a source of contamination. 4.2.7 Directional airflow within production or packing areas should assist in preventing contamination. Airflows should
LAMINAR FLOW BENCH GUIDELINES 4.2.6 Personnel should not be a source of contamination. 4.2.7 Directional airflow within production or packing areas should assist in preventing contamination. Airflows should
Engineering Intern. Quality-
 Who we want Challengers. People who seek out the hard projects and work to find just the right solutions. Teammates. Partners who listen to ideas, share thoughts and work together to move the business
Who we want Challengers. People who seek out the hard projects and work to find just the right solutions. Teammates. Partners who listen to ideas, share thoughts and work together to move the business
The Risk Management + Design Controls Connection: What Device Makers Need to Know
 !!! The Risk Management + Design Controls Connection: What Device Makers Need to Know Jon Speer Founder & VP of QA/RA greenlight.guru Table of Contents 1 Intended Use & User Needs 6 Verification, Validation,
!!! The Risk Management + Design Controls Connection: What Device Makers Need to Know Jon Speer Founder & VP of QA/RA greenlight.guru Table of Contents 1 Intended Use & User Needs 6 Verification, Validation,
Medi-Therm Precise. Easy. Versatile.
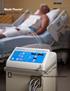 Medi-Therm Precise. Easy. Versatile. Medi-Therm Hyper/Hypothermia System Patient warming and cooling are often critical factors in achieving positive outcomes. The Stryker Medi-Therm system can be used
Medi-Therm Precise. Easy. Versatile. Medi-Therm Hyper/Hypothermia System Patient warming and cooling are often critical factors in achieving positive outcomes. The Stryker Medi-Therm system can be used
Transporting Ebola Contaminated Items: Category A Infectious Substance
 Transporting Ebola Contaminated Items: Category A Infectious Substance Scope: The U.S. Department of Transportation (DOT s) Hazardous Materials Regulations (HMR; 49 C.F.R., Parts 171-180) govern the transportation
Transporting Ebola Contaminated Items: Category A Infectious Substance Scope: The U.S. Department of Transportation (DOT s) Hazardous Materials Regulations (HMR; 49 C.F.R., Parts 171-180) govern the transportation
Captor Containment DOUBLE WALL TANK SYSTEMS.
 Captor Containment DOUBLE WALL TANK SYSTEMS Captor Containment System Protects Bulk Storage Profits Without Jeopardizing Safety or the Environment Flanged Outlets and other fitting designs can be securely
Captor Containment DOUBLE WALL TANK SYSTEMS Captor Containment System Protects Bulk Storage Profits Without Jeopardizing Safety or the Environment Flanged Outlets and other fitting designs can be securely
Process Risk through Application of FMEA. A Case Study. INTERPHEX March 17, 2009
 Managing Process Risk through Application of FMEA to Batch Records A Case Study INTERPHEX March 17, 2009 Jon Hardy Lonza Head of Operations Hopkinton, MA Fred Greulich Maxiom Consulting Group, Inc. Director
Managing Process Risk through Application of FMEA to Batch Records A Case Study INTERPHEX March 17, 2009 Jon Hardy Lonza Head of Operations Hopkinton, MA Fred Greulich Maxiom Consulting Group, Inc. Director
Pharmaceutical Quality and Risk Assessment: A Case study
 Research Article Pharmaceutical Quality and Risk Assessment: A Case study Keerthana M.D, N. Vishal Gupta* Department of Pharmaceutics, JSS College of Pharmacy, Sri Shivarathreeswara Nagara, Mysore, Jagadguru
Research Article Pharmaceutical Quality and Risk Assessment: A Case study Keerthana M.D, N. Vishal Gupta* Department of Pharmaceutics, JSS College of Pharmacy, Sri Shivarathreeswara Nagara, Mysore, Jagadguru
About Us. A young organisation, but with deep roots.
 About Us Adler Ortho is an Italian Company totally devoted to development, manufacturing and marketing of surgical medical devices for Orthopaedics. Innovation is the foundation of our Company, pursued
About Us Adler Ortho is an Italian Company totally devoted to development, manufacturing and marketing of surgical medical devices for Orthopaedics. Innovation is the foundation of our Company, pursued
Importance of the CAPA System
 Passion for Quality Importance of the CAPA System PIA 06/20/2013 Pepe Rodríguez-Pérez, PhD Business Excellence Consulting Inc www.capapr.com...+ www.calidadpr.com Purpose and Importance of the CAPA System
Passion for Quality Importance of the CAPA System PIA 06/20/2013 Pepe Rodríguez-Pérez, PhD Business Excellence Consulting Inc www.capapr.com...+ www.calidadpr.com Purpose and Importance of the CAPA System
InVivo Therapeutics. Developing Innovative Products for Spinal Cord Injury
 1 Developing Innovative Products for Spinal Cord Injury 2 Forward-Looking Statements Before we begin, we would like to remind everyone that during our presentation, we will be making forward-looking statements
1 Developing Innovative Products for Spinal Cord Injury 2 Forward-Looking Statements Before we begin, we would like to remind everyone that during our presentation, we will be making forward-looking statements
Finite Element Analysis of Medical Devices: WS Atkins Perspective
 Industry Sector RTD Thematic Area Date Biomedical 13 th Nov 2001 Finite Element Analysis of Medical Devices: WS Atkins Perspective Stuart Kelly WS Atkins Consultants Ltd, Bristol, UK Summary: This presentation
Industry Sector RTD Thematic Area Date Biomedical 13 th Nov 2001 Finite Element Analysis of Medical Devices: WS Atkins Perspective Stuart Kelly WS Atkins Consultants Ltd, Bristol, UK Summary: This presentation
Audit of Departmental Security
 Audit of Departmental Security Office of the Chief Audit and Evaluation Executive Audit and Assurance Services Directorate October 2013 Cette publication est également disponible en français. This publication
Audit of Departmental Security Office of the Chief Audit and Evaluation Executive Audit and Assurance Services Directorate October 2013 Cette publication est également disponible en français. This publication
Product safety and conformity in the automotive supply chain in the case of product nonconformities 1 st Edition, February 2018 Online-Download-Docume
 Quality Management in the Automotive Industry Product safety and conformity in the automotive supply chain in the case of Product nonconformities 1 st Edition, February 2018 Online-Download-Document Product
Quality Management in the Automotive Industry Product safety and conformity in the automotive supply chain in the case of Product nonconformities 1 st Edition, February 2018 Online-Download-Document Product
 Personal Check Money Order Credit Card Visa MC Discover Amex LYMPHOCYTE TRANSFORMATION TESTING (LTT) AND/OR METAL ION TESTING SERVICE (ALL FIELDS REQUIRED) Patient Last, First Name, M.I. Mail or e-mail
Personal Check Money Order Credit Card Visa MC Discover Amex LYMPHOCYTE TRANSFORMATION TESTING (LTT) AND/OR METAL ION TESTING SERVICE (ALL FIELDS REQUIRED) Patient Last, First Name, M.I. Mail or e-mail
R.Raffaelli Bologna 3 maggio 2017
 R.Raffaelli Bologna 3 maggio 2017 The data are the end of pipe of a process based on security (data not numbers: accreditation and professional competence) traceability, repeatability and comparison (ex
R.Raffaelli Bologna 3 maggio 2017 The data are the end of pipe of a process based on security (data not numbers: accreditation and professional competence) traceability, repeatability and comparison (ex
Managing for Daily Improvement
 Managing for Daily Improvement Standard Work and Tools For Management to Drive Continuous Improvement Front Line Leadership Development System Module Part 1 of 12 MDI Workshop Agenda 2 Day Monday Tuesday
Managing for Daily Improvement Standard Work and Tools For Management to Drive Continuous Improvement Front Line Leadership Development System Module Part 1 of 12 MDI Workshop Agenda 2 Day Monday Tuesday
Linking Forecasting with Operations and Finance. Bill Tonetti November 15, 2017 IIF Foresight Practitioner Conference
 Linking Forecasting with Operations and Finance Bill Tonetti November 15, 2017 IIF Foresight Practitioner Conference About the Speaker Bill Tonetti Founding Member, Foresight Practitioner Advisory Board
Linking Forecasting with Operations and Finance Bill Tonetti November 15, 2017 IIF Foresight Practitioner Conference About the Speaker Bill Tonetti Founding Member, Foresight Practitioner Advisory Board
Materion AMTS Supplier Quality Manual
 Advanced Materials Technologies and Services Inc. Materion AMTS Supplier Quality Manual Supplier Name: Supplier Address: Suppliers shall review the attached Supplier Quality Manual and acknowledge receipt
Advanced Materials Technologies and Services Inc. Materion AMTS Supplier Quality Manual Supplier Name: Supplier Address: Suppliers shall review the attached Supplier Quality Manual and acknowledge receipt
Functional Assessment and Clinical Outcome
 Tissue Engineering Functional Assessment and Clinical Outcome STEVEN A. GOLDSTEIN Orthopaedic Research Laboratories, Department of Orthopaedic Surgery, University of Michigan, Ann Arbor, Michigan 48109,
Tissue Engineering Functional Assessment and Clinical Outcome STEVEN A. GOLDSTEIN Orthopaedic Research Laboratories, Department of Orthopaedic Surgery, University of Michigan, Ann Arbor, Michigan 48109,
Idaho Panhandle National Forests
 United States Department of Agriculture Forest Service Idaho Panhandle National Forests Sandpoint Ranger District 1602 Ontario Road Sandpoint, ID 83864-9509 (208)263-5111 File Code: 1950 Date: July 14,
United States Department of Agriculture Forest Service Idaho Panhandle National Forests Sandpoint Ranger District 1602 Ontario Road Sandpoint, ID 83864-9509 (208)263-5111 File Code: 1950 Date: July 14,
Where ideas take shape. MEDICAL
 Where ideas take shape. MEDICAL Persistently achieving For more than 50 years, medical companies have turned to Pexco for premium injection molding, medical tubing, CNC machining and custom medical plastic
Where ideas take shape. MEDICAL Persistently achieving For more than 50 years, medical companies have turned to Pexco for premium injection molding, medical tubing, CNC machining and custom medical plastic
Pharmacovigilance and the Generic Industry
 Pharmacovigilance and the Generic Industry Presented by Joan Janulis, RAC Vice President Lachman Consultant Services Inc. 2015 Lachman Consultant Services, Inc. All rights reserved. Legal Notice The information
Pharmacovigilance and the Generic Industry Presented by Joan Janulis, RAC Vice President Lachman Consultant Services Inc. 2015 Lachman Consultant Services, Inc. All rights reserved. Legal Notice The information
Project Summary Evaluation of Pollution Prevention Opportunities for Mold Release Agents
 EPA United States National Risk Management Environmental Protection Research Laboratory Agency Research Triangle Park, NC 27711 Research and Development EPA/600/SR-96/075 July 1996 Project Summary Evaluation
EPA United States National Risk Management Environmental Protection Research Laboratory Agency Research Triangle Park, NC 27711 Research and Development EPA/600/SR-96/075 July 1996 Project Summary Evaluation
SUPPLIER GUIDELINES MANUAL
 1.0 INTRODUCTION 1.1 This guidelines manual defines the minimum requirements for Supreme Machined Products Co.; Inc. LTD (to be referenced as Supreme throughout the document) Supplier s and does not supersede
1.0 INTRODUCTION 1.1 This guidelines manual defines the minimum requirements for Supreme Machined Products Co.; Inc. LTD (to be referenced as Supreme throughout the document) Supplier s and does not supersede
FEC QUALITY MANUAL. Federal Equipment Company River Rd. Cincinnati, Ohio
 QMS-20 2016 FEC QUALITY MANUAL Federal Equipment Company 5298 River Rd Cincinnati, Ohio 45233 www.federalequipment.com www.fecheliports.com www.usdrillhead.com www.orionseals.com www.tkf.com REVISION:
QMS-20 2016 FEC QUALITY MANUAL Federal Equipment Company 5298 River Rd Cincinnati, Ohio 45233 www.federalequipment.com www.fecheliports.com www.usdrillhead.com www.orionseals.com www.tkf.com REVISION:
Laboratory OOS Investigations The Missing Link
 Laboratory OOS Investigations The Missing Link India Pharmaceutical Alliance Carmelo Rosa, Psy.D Director Division of Drug Quality I November 6-17, 2017 Indian Pharmaceutical Alliance DISCLAIMER: The views
Laboratory OOS Investigations The Missing Link India Pharmaceutical Alliance Carmelo Rosa, Psy.D Director Division of Drug Quality I November 6-17, 2017 Indian Pharmaceutical Alliance DISCLAIMER: The views
FDA Update on Compounding
 FDA Update on Compounding Julie Dohm, JD, PhD Senior Science Advisor for Compounding, Center for Drug Evaluation and Research; Agency Lead on Compounding, FDA Compounding A Snapshot Compounded drugs: Are
FDA Update on Compounding Julie Dohm, JD, PhD Senior Science Advisor for Compounding, Center for Drug Evaluation and Research; Agency Lead on Compounding, FDA Compounding A Snapshot Compounded drugs: Are
TRELLIS COLLAGEN RIBBON
 TRELLIS COLLAGEN RIBBON 147321-1 English (en) The following languages are included in this packet: M Wright Medical Technology, Inc. 5677 Airline Rd. Arlington, TN 38002 USA www.wmt.com August 2012 Printed
TRELLIS COLLAGEN RIBBON 147321-1 English (en) The following languages are included in this packet: M Wright Medical Technology, Inc. 5677 Airline Rd. Arlington, TN 38002 USA www.wmt.com August 2012 Printed
R.H. SHEPPARD CO., INC. HANOVER, PA QUALITY BY DESIGN SUPPLIER QUALITY MANUAL NINTH EDITION
 R.H. SHEPPARD CO., INC. HANOVER, PA. 17331 QUALITY BY DESIGN NINTH EDITION TABLE OF CONTENTS TITLE PAGE 1. SUPPLIER QUALITY SYSTEM REQUIREMENTS 1 2. PURCHASE ORDERS, SPECIFICATIONS AND PRINTS 1 3. REQUESTS
R.H. SHEPPARD CO., INC. HANOVER, PA. 17331 QUALITY BY DESIGN NINTH EDITION TABLE OF CONTENTS TITLE PAGE 1. SUPPLIER QUALITY SYSTEM REQUIREMENTS 1 2. PURCHASE ORDERS, SPECIFICATIONS AND PRINTS 1 3. REQUESTS
2 Year Operating Plan Summary
 2 Year Operating Plan 2017 2019 Summary December 2016 Introduction This document presents a summary of the Kingston Hospital NHS Foundation Trust s 2 year Operating Plan for 2017/19 which was submitted
2 Year Operating Plan 2017 2019 Summary December 2016 Introduction This document presents a summary of the Kingston Hospital NHS Foundation Trust s 2 year Operating Plan for 2017/19 which was submitted
Presentation of Capabilities Reverse Logistics
 Presentation of Capabilities Reverse Logistics Woodfield Distribution, LLC v081617 Reverse Logistics About Us Description of Services Reverse Distribution Channels DSCSA/Sustainability About Us WDSrx Woodfield
Presentation of Capabilities Reverse Logistics Woodfield Distribution, LLC v081617 Reverse Logistics About Us Description of Services Reverse Distribution Channels DSCSA/Sustainability About Us WDSrx Woodfield
MEDICAL DEVICE GUIDANCE
 Effective from 1 January 2013 MEDICAL DEVICE GUIDANCE GN-15: Guidance on Medical Device Product Registration Revision 5 CONTENTS 1. INTRODUCTION... 4 1.1. Scope... 5 1.2. Definitions... 5 2. RISK CLASSIFICATION
Effective from 1 January 2013 MEDICAL DEVICE GUIDANCE GN-15: Guidance on Medical Device Product Registration Revision 5 CONTENTS 1. INTRODUCTION... 4 1.1. Scope... 5 1.2. Definitions... 5 2. RISK CLASSIFICATION
Guidelines on procedures and data requirements for changes to approved biotherapeutic products. Proposed guidelines
 0 0 0 0 WHO/PAC for BTPs_DRAFT/ Oct 0 ENGLISH ONLY Guidelines on procedures and data requirements for changes to approved biotherapeutic products Proposed guidelines NOTE: This document has been prepared
0 0 0 0 WHO/PAC for BTPs_DRAFT/ Oct 0 ENGLISH ONLY Guidelines on procedures and data requirements for changes to approved biotherapeutic products Proposed guidelines NOTE: This document has been prepared
Price and Availability
 Price and Availability Outline Introduction Relative cost of materials Example MECH 321 Mech. Eng. Dept. - Concordia University lecture 21/1 Current Prices on the web (a) : - Short term trends: fluctuations
Price and Availability Outline Introduction Relative cost of materials Example MECH 321 Mech. Eng. Dept. - Concordia University lecture 21/1 Current Prices on the web (a) : - Short term trends: fluctuations
Process Capability and relationship to Quality management
 Process Capability and relationship to Quality management Oct. 6, 2015 Barbara Allen, Ph.D. Global Quality Systems Eli Lilly and Company Core Objective Safely & reliably manufacture quality medicines for
Process Capability and relationship to Quality management Oct. 6, 2015 Barbara Allen, Ph.D. Global Quality Systems Eli Lilly and Company Core Objective Safely & reliably manufacture quality medicines for
ROTEK. IIInnInstI Instrument Corp. ISO 9001 Quality System Manual
 ROTEK IIInnInstI Instrument Corp. ISO 9001 Quality System Manual QSM900100-01 REV O MANUAL NO: 10 ISSUED TO: Rotek Web Site DATE OF ISSUE: 17 July 2002 APPROVALS: ORIGINATOR: Lawrence E. Weissbach Lawrence
ROTEK IIInnInstI Instrument Corp. ISO 9001 Quality System Manual QSM900100-01 REV O MANUAL NO: 10 ISSUED TO: Rotek Web Site DATE OF ISSUE: 17 July 2002 APPROVALS: ORIGINATOR: Lawrence E. Weissbach Lawrence
Process. Developing and Managing the Risk Management Corrective Action Plan. Process. Session No Page 1 WELCOME. Agenda.
 Developing and Managing the Risk Corrective Action Plan WELCOME Developing and Managing the Risk Corrective Action Plan Welcome to Session No. 1134 Developing and Managing the Risk Corrective Action Plan
Developing and Managing the Risk Corrective Action Plan WELCOME Developing and Managing the Risk Corrective Action Plan Welcome to Session No. 1134 Developing and Managing the Risk Corrective Action Plan
Progressive Metal Forming, inc Hall Road Hamburg, MI 48139
 SUPPLIER QUALITY MANUAL Progressive Metal Forming, inc. 10850 Hall Road Hamburg, MI 48139 www.pmfdraw.com Rev. 1/30/17 Page 1 of 11 TABLE OF CONTENTS 1.0 INTRODUCTION. 3 2.0 SUPPLIER QUALITY GUIDELINES...3
SUPPLIER QUALITY MANUAL Progressive Metal Forming, inc. 10850 Hall Road Hamburg, MI 48139 www.pmfdraw.com Rev. 1/30/17 Page 1 of 11 TABLE OF CONTENTS 1.0 INTRODUCTION. 3 2.0 SUPPLIER QUALITY GUIDELINES...3
PSMF in Practice Your Questions Answered! GPvP Symposium, 14 March 2014 Jonathan Rowell, Senior GPvP Inspector
 PSMF in Practice Your Questions Answered! GPvP Symposium, 14 March 2014 Jonathan Rowell, Senior GPvP Inspector Content Answer industry questions related to the PSMF MHRA inspector s preparation: How we
PSMF in Practice Your Questions Answered! GPvP Symposium, 14 March 2014 Jonathan Rowell, Senior GPvP Inspector Content Answer industry questions related to the PSMF MHRA inspector s preparation: How we
a solution for reducing the
 Outsourcing the precision cleaning, a solution for reducing the environmental impact Trade fair Hanover 2015 Heiko ZSCHIEDRICH Hanover April 15, 2015 Summary 1. Introduction and presentation of ECP 2.
Outsourcing the precision cleaning, a solution for reducing the environmental impact Trade fair Hanover 2015 Heiko ZSCHIEDRICH Hanover April 15, 2015 Summary 1. Introduction and presentation of ECP 2.
ALTA Precision Inc. Quality Procedure
 ALTA Precision Inc. Quality Procedure Procedure QAP 7.4.2 Issue 7 Date September 28, 2015 Page 1 of 5 ALTA Quality Requirements for Suppliers Prepared and issued by Marc Dumouchel Approved by Sonia Alonso
ALTA Precision Inc. Quality Procedure Procedure QAP 7.4.2 Issue 7 Date September 28, 2015 Page 1 of 5 ALTA Quality Requirements for Suppliers Prepared and issued by Marc Dumouchel Approved by Sonia Alonso
Precision in fixation
 For more information, visit Medartis at www.medartis.com or contact us directly: Medartis AG Hochbergerstrasse 60E CH-4057 Basel P +41 61 633 34 34 F +41 61 633 34 00 www.medartis.com Precision in fixation
For more information, visit Medartis at www.medartis.com or contact us directly: Medartis AG Hochbergerstrasse 60E CH-4057 Basel P +41 61 633 34 34 F +41 61 633 34 00 www.medartis.com Precision in fixation
Visteon Corporation. Customer Specific Requirements Fifth Edition. For Use with ISO/TS 16949:2009
 Visteon Corporation Customer Specific Requirements Fifth Edition For Use with ISO/TS 16949:2009 SP-WW-SG-1007 Page 1 of 9 Printed Copies of this I. Introduction Quality professionals from Visteon collaboratively
Visteon Corporation Customer Specific Requirements Fifth Edition For Use with ISO/TS 16949:2009 SP-WW-SG-1007 Page 1 of 9 Printed Copies of this I. Introduction Quality professionals from Visteon collaboratively
GLOBAL HEALTH SUPPLY CHAIN QUALITY ASSURANCE
 GLOBAL HEALTH SUPPLY CHAIN QUALITY ASSURANCE Medical Device Product Questionnaire This questionnaire is used to collect information from vendors with regards to medical devices that fall in any of the
GLOBAL HEALTH SUPPLY CHAIN QUALITY ASSURANCE Medical Device Product Questionnaire This questionnaire is used to collect information from vendors with regards to medical devices that fall in any of the
Hazard Analysis and Critical Control Point The Almond Board of California. Overview. Definitions
 Hazard Analysis and Critical Control Point The Almond Board of California Overview Hazard Analysis Critical Control Point (HACCP) is the final stage of an integrated, proactive food safety program targeting
Hazard Analysis and Critical Control Point The Almond Board of California Overview Hazard Analysis Critical Control Point (HACCP) is the final stage of an integrated, proactive food safety program targeting
Delivering Customer Value
 Profit-Ability LLC, Value Delivery Delivering Customer Value Summary The heartbeat to our delivery practice integrates the past 20 years of teachings from the late Paul Barringer who passed on 12/21/2016.
Profit-Ability LLC, Value Delivery Delivering Customer Value Summary The heartbeat to our delivery practice integrates the past 20 years of teachings from the late Paul Barringer who passed on 12/21/2016.
Panel Discussion: European Medical Device Regulations Preparing for the Storm Moderator: Lenita Y. Sims Spears, Senior Quality Consultant/Senior
 Panel Discussion: European Medical Device Regulations Preparing for the Storm Moderator: Lenita Y. Sims Spears, Senior Quality Consultant/Senior Regulatory and Compliance Counsel, BioTeknica Panelists:
Panel Discussion: European Medical Device Regulations Preparing for the Storm Moderator: Lenita Y. Sims Spears, Senior Quality Consultant/Senior Regulatory and Compliance Counsel, BioTeknica Panelists:
Association of American Railroads Quality Assurance System Evaluation (QASE) Checklist Rev. 1/12/2017
 Company: Prepared By: Date: Changes from previous version highlighted in yellow. Paragraph Element Objective Evidence 2.1 Objective of Quality Assurance Program 2.2 Applicability and Scope 2.3 QA Program
Company: Prepared By: Date: Changes from previous version highlighted in yellow. Paragraph Element Objective Evidence 2.1 Objective of Quality Assurance Program 2.2 Applicability and Scope 2.3 QA Program
HSE Integrated Risk Management Policy. Part 3. Managing and Monitoring Risk Registers Guidance for Managers
 HSE Integrated Management Policy Part 3 Managing and Monitoring Registers Guidance for Managers HSE Integrated Management Policy Part 3 Managing and Monitoring Registers Guidance for Managers Identify
HSE Integrated Management Policy Part 3 Managing and Monitoring Registers Guidance for Managers HSE Integrated Management Policy Part 3 Managing and Monitoring Registers Guidance for Managers Identify
Ergonomic Kneeling Chair
 Ergonomic Kneeling Chair Model: SC-205B / SC-205L / SC-205P Sierra Comfort is a leading provider of products suited for massage, relaxation, holistic health and ergonomics. While staying committed to quality
Ergonomic Kneeling Chair Model: SC-205B / SC-205L / SC-205P Sierra Comfort is a leading provider of products suited for massage, relaxation, holistic health and ergonomics. While staying committed to quality
The Value of Continuous Accounting for Business. White Paper. Establishing the Foundation for a Strategic Finance Organization.
 The Value of Continuous Accounting for Business Establishing the Foundation for a Strategic Finance Organization White Paper Sponsored by 1 Ventana Research 2016 Table of Contents A New Approach to Managing
The Value of Continuous Accounting for Business Establishing the Foundation for a Strategic Finance Organization White Paper Sponsored by 1 Ventana Research 2016 Table of Contents A New Approach to Managing
MDSAP AUDIT PROCESS. A Manufacturer s Perspective. Connie Hoy EVP Regulatory Affairs Cynosure, Inc.
 MDSAP AUDIT PROCESS A Manufacturer s Perspective Connie Hoy EVP Regulatory Affairs Cynosure, Inc. Cynosure Located in Westford, MA Largest manufacturer of Medical Lasers Second location in Hicksville,
MDSAP AUDIT PROCESS A Manufacturer s Perspective Connie Hoy EVP Regulatory Affairs Cynosure, Inc. Cynosure Located in Westford, MA Largest manufacturer of Medical Lasers Second location in Hicksville,
26 PROCESS SAFETY MANAGEMENT
 26 PROCESS SAFETY MANAGEMENT QUIZ 1 (20 POINTS) True/False (5 points) 1. SARA Title III required companies to develop emergency preparedness plans; recognition, knowledge, and inventories of hazardous
26 PROCESS SAFETY MANAGEMENT QUIZ 1 (20 POINTS) True/False (5 points) 1. SARA Title III required companies to develop emergency preparedness plans; recognition, knowledge, and inventories of hazardous
Title: ISO 9001 Quality Manual Doc. No: CAP-1004 Page 1 of 22
 Doc. No: CAP-1004 Page 1 of 22 Doc. No: CAP-1004 Page 2 of 22 Table of Contents 1. ABOUT THE ORGANIZATION... 4 1.1. ORGANIZATIONAL STRUCTURE... 4 2. PURPOSE, SCOPE AND USERS... 5 3. TERMS AND DEFINITIONS...
Doc. No: CAP-1004 Page 1 of 22 Doc. No: CAP-1004 Page 2 of 22 Table of Contents 1. ABOUT THE ORGANIZATION... 4 1.1. ORGANIZATIONAL STRUCTURE... 4 2. PURPOSE, SCOPE AND USERS... 5 3. TERMS AND DEFINITIONS...
TST Medical Devices. company profile
 TST Medical Devices company profile WE ARE LOOKING FORWARD TO THE FUTURE, ANTICIPATING CUSTOMERS NEEDS AND COMING UP WITH SOLUTIONS. We are offering quality and value. We strive for continuous improvment.
TST Medical Devices company profile WE ARE LOOKING FORWARD TO THE FUTURE, ANTICIPATING CUSTOMERS NEEDS AND COMING UP WITH SOLUTIONS. We are offering quality and value. We strive for continuous improvment.
FAIRFIELD GLOBAL SUPPLIER QUALITY PROGRAM
 FAIRFIELD GLOBAL SUPPLIER QUALITY PROGRAM 1 QA.101 rev. 3-2012 PURPOSE OF THIS STANDARD Fairfield is committed to continuous product quality improvement. Our management team is convinced that only with
FAIRFIELD GLOBAL SUPPLIER QUALITY PROGRAM 1 QA.101 rev. 3-2012 PURPOSE OF THIS STANDARD Fairfield is committed to continuous product quality improvement. Our management team is convinced that only with
A Systems Approach to Risk Management Through Leading Indicators
 A Systems Approach to Risk Management Through Leading Indicators Nancy Leveson MIT Goal To identify potential for an accident before it occurs Underlying assumption: Major accidents not due to a unique
A Systems Approach to Risk Management Through Leading Indicators Nancy Leveson MIT Goal To identify potential for an accident before it occurs Underlying assumption: Major accidents not due to a unique
