Crystallization kinetics of amorphous lactose, whey-permeate and whey powders
|
|
|
- Lynn Black
- 6 years ago
- Views:
Transcription
1 Carbohydrate Research 342 (2007) Crystallization kinetics of amorphous lactose, whey-permeate and whey powders Alexander Ibach * and Matthias Kind Institut für Thermische Verfahrenstechnik, Universität Karlsruhe (TH), Postfach 6980, D Karlsruhe, Germany Received 2 January 2007; received in revised form 24 February 2007; accepted 2 March 2007 Available online 6 March 2007 Abstract Amorphous lactose, whey-permeate and whey powders have been converted to their crystalline forms by exposure to air at various temperatures and relative humidities. The total time required for sorption, induction and crystallization of these powders was observed by following the time-dependent mass change of the powders during treatment. These experiments have shown that higher temperatures and relative humidities lead to shorter crystallization times. Lactose crystallizes within 1 min at an air temperature of 100 C and relative air humidity of 80%, whereas whey-permeate and whey powders requires up to 5 min at the same set of conditions. Thus, as previously described, the presence of proteins and salts in the whey-permeate and whey powders reduces the crystallization rate. The rate constants and activation energies have been determined over a range of temperatures and humidities to enable the calculation of crystallization times for the design of an industrial process that crystallizes whey and whey-permeate powders. Finally, the crystallization rates found in this work are sufficiently fast to be applicable in an industrial process that crystallizes whey and whey-permeate powders. Ó 2007 Elsevier Ltd. All rights reserved. Keywords: Crystallization; Amorphous powder; Lactose; Whey-permeate; Whey 1. Introduction The handling of whey powder products is often difficult due to the lactose component in the powder. Lactose solidifies in an amorphous state during rapid drying in a spray dryer. 1 Amorphous lactose is thermodynamically unstable and hygroscopic, absorbing moisture from the surrounding and subsequently plasticizing. This causes the individual whey powder particles to become sticky. 2 The temperature at which this transition from an amorphous solid-state to a viscous rubbery state occurs is known as the glass transition temperature. 3 The glass transition temperature can fall below ambient temperature when the powder absorbs sufficient moisture during storage, leading to caking of the powder. This presents a problem during subsequent handling processes, such as pneumatic conveying. A further decrease in the glass * Corresponding author. Tel.: ; a.ibach@ gmx.de transition temperature is accompanied by a higher molecular mobility of the lactose. At this point, the lactose begins to crystallize after an initial induction period. 4 The crystalline modification is thermodynamically stable and significantly less hygroscopic. The aim of this work is to develop a practical method for reducing the caking tendency of lactose in whey powder by introducing a controlled crystallization step following the spray-drying process. The resulting powder should thus be more free-flowing and easier to handle, 5 even when it is exposed to humid conditions. 6 Knowledge of the effect of various parameters, such as temperature and humidity, on the rate of crystallization is therefore necessary. The focus of the current investigation is the determination of the kinetic parameters associated with the crystallization of amorphous lactose in pure lactose, whey-permeate and whey powders. Various authors have investigated the crystallization kinetics of lactose at low temperatures. 7 9 These workers have found that amorphous and crystalline lactose have different sorption isotherms; the amorphous form has a /$ - see front matter Ó 2007 Elsevier Ltd. All rights reserved. doi: /j.carres
2 1358 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) much higher moisture content at a given humidity than the crystalline form. During crystallization, the amorphous lactose will initially absorb moisture from the surroundings due to its hygroscopic nature, and subsequently release moisture as it crystallizes. The crystallization kinetics can be determined from the mass change of the powder. Jouppila and Roos 10 have investigated the crystallization of amorphous lactose in milk powders and have shown that lactose crystallization in milk powder is delayed compared with the crystallization of pure lactose powders. Haque and Roos 11 have suggested that the crystallization of lactose in spraydried lactose protein mixtures at room temperature is delayed by the presence of whey-protein isolate, Na-caseinate, albumin and gelatine. A reduced form of the Johnson Mehl Avrami (JMA) theory can be used for modelling the lactose crystallization kinetics. This theory describes many solid-state reactions that occur through a process of nucleation and growth, and is given by the following equations: 16,17 h ¼ 1 expf ðk ðt t Ind ÞÞ n g ð1þ kðt Þ¼k 0 exp E A ð2þ ~R T h is the fraction crystallized, k is the crystallization rate constant, t is the time, t Ind is the induction time, n is the reaction order, k 0 is a the pre-exponential factor, E A the activation energy of the crystallization and er is the ideal gas constant. These parameters are determined experimentally in this work so that Eqs. 1 and 2 can be used in future calculations when designing a whey powder crystallization process. 2. Results and discussion Figure 1 shows the change in moisture content with time of pure lactose samples exposed to 60 C and various relative humidities. All measurements were conducted twice to check for reproducibility. Initially, the moisture content of the samples increases to about 6%. After a short induction time at this moisture content, the amorphous lactose begins to crystallize, and some of the moisture is released to the surrounding air. Thus, sorption, induction and crystallization can be tracked online by observing the mass change of the sample. The crystallization times determined in this work can be used for designing an industrial process that produces stable crystalline lactose powders. Assuming that the lactose crystallizes completely as a- lactose-monohydrate, the final moisture content of the sample must be at least 5% to account for the bound hydrate water. However, Figure 1 shows that the moisture content of the lactose at the end of a 60 C conversion is between 1% and 1.5%. This suggests that the amorphous lactose crystallizes not only to a hydrate, but also to different anhydrous forms, including possibly b-anhydrous lactose, a-anhydrous lactose and mixed anhydrous forms of a- and b-lactose in molar ratios of 5:3, 3:2 and 4:1. 18 The types of crystals that form depend on the relative humidity and temperature of crystallization, the powder composition, and the time of exposure. 19,20 The experiments show that less a-lactosemonohydrate forms at higher crystallization temperatures. The compositions of the powders produced in this work have been investigated by Nijdam and co-workers. 21 Figure 2 shows measurements of the overall-crystallization times required for amorphous lactose powder at different temperatures and relative humidities. Higher temperatures and relative humidities result in shorter crystallization times. At the highest temperature and relative humidity tested, the time for crystallization is only 1 min. In this case, diffusion of water into the particles is likely to limit the crystallization process. Figure 3 shows the change in moisture content of whey-permeate samples exposed to 60 C and various relative humidities with time. The moisture content increases initially in the same manner as for lactose. Figure 1. Sorption- and crystallization behaviour of amorphous lactose at 60 C.
3 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) Figure 2. Overall times for sorption, induction and crystallization of amorphous lactose powder at various temperatures and relative humidities. Figure 3. Sorption- and crystallization behaviour of amorphous whey-permeate powder at 60 C. However, in this case, the moisture content increases to a much higher value. Clearly, the salts and proteins present in the whey-permeate powder contribute to the hygroscopicity of the powder. At relative humidities between 40% and 60%, the moisture content decreases after a brief induction period. This decrease in moisture content is caused by the crystallization of the amorphous lactose. At relative humidities between 70% and 80%, the moisture content increases up to a certain value, remains at this level for a certain time, and then increases further towards a higher equilibrium value. Under these conditions the crystallization is so fast that the release of water through the crystallization overlaps the absorption process. Therefore, the crystallization is already complete before the sorption equilibrium moisture content is reached. Note that the higher moisture content at these conditions for whey-permeate in comparison to lactose powder may be affected by a solubilization process of substances like salts or proteins. The end of crystallization corresponds to a point just after the shoulder in the moisture content profile. Figure 4 shows measurements of the overall-crystallization times required for amorphous whey-permeate powder at different temperatures and relative humidities. The crystallization time of amorphous whey-permeate powder is longer than that of pure lactose. As reported in literature, 11,22 24 the crystallization is delayed by the presence of salts and proteins, which effectively lowers the concentration of lactose, and hence lengthens the mean path length required for lactose molecules to migrate in order to bond with other lactose molecules. Figure 5 shows that amorphous whey powder crystallizes in a similar manner to amorphous whey-permeate powder. At 80% humidity, a similar shoulder appears in the moisture content profile. Figure 6 shows measurements of the overall-crystallization times required for amorphous whey powder at different temperatures and relative humidities. Once
4 1360 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) Figure 4. Overall times for sorption, induction and crystallization of amorphous whey-permeate powder at various temperatures and relative humidities. Figure 5. Sorption- and crystallization behaviour of amorphous whey powder at 50 C. Figure 6. Overall times for sorption, induction and crystallization of amorphous whey powder at various temperatures and relative humidities. again, this figure shows that amorphous whey behaves similarly to amorphous whey-permeate powder. The required residence times for the crystallization of amorphous whey and whey-permeate powders are of the
5 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) same order of magnitude, although the concentration of proteins in whey is slightly higher, or equivalently, the concentration of lactose is slightly lower. The raw data obtained can be transformed into a crystalline fraction h versus (t t Ind ), as follows: h ¼ w max w ð3þ w max w final h is the fraction of the lactose that has been crystallized. w max and w final are the maximum moisture content at time t Ind and the moisture content at the end of the crystallization, respectively. Eq. 1 can be rearranged as follows: ln½ lnð1 hþš ¼ n lnðt t Ind ÞþnlnðkÞ ð4þ A plot of ln[ ln(1 h)] versus ln(t t Ind ) gives a straight line with a slope of n and an intercept of n Æ ln(k). Figure 7 shows such a plot for lactose crystallization at 50 C and various relative humidities. The crystallization rate constants k for lactose, whey-permeate and whey powder, calculated using the slope and intercept in Eq. 4, are shown in Figures In the case of whey-permeate and whey powder, the reaction order n and the crystallization rate constants k could only be determined properly at relative humidities less than 70%, because the form of the curve differs from Eq. 4 at higher relative humidities. Values for the reaction order n at various temperatures and relative humidities are given in Tables 1 3. Figures 8 10 show that the rate constant increases markedly with increasing relative humidity and temperature, which indicates that the mass change of the sample is controlled by crystallization rather than mass transfer of moisture within the particles or at the surfaces of the particles. However, at a high temperature of 110 C and a relative humidity of 70%, the rate Figure 7. Transformed crystallization time for lactose powder at 50 C and diverse relative humidities. Figure 8. Crystallization rate constants k of lactose powder at various temperatures and relative humidities.
6 1362 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) Figure 9. Crystallization rate constants k of whey-permeate powder at various temperatures and relative humidities. Figure 10. Crystallization rate constants k of whey powder at various temperatures and relative humidities. Table 1. Reaction order n for the crystallization of amorphous lactose powder u ( ) n ( ) T =50 C T =60 C T =70 C T =80 C T =90 C T = 100 C T = 110 C constant decreases. An XRD analysis of the samples has shown that, at these conditions, lactose powder crystallizes solely as b-lactose, which could explain this break in the trend. Figures 8 10 confirm that the
7 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) Table 2. Reaction order n for the crystallization of amorphous whey-permeate powder u ( ) n ( ) T =50 C T =60 C T =70 C T =80 C T =90 C T = 100 C Table 3. Reaction order n for the crystallization of amorphous whey powder u ( ) n ( ) T =50 C T =60 C T =70 C T =80 C T =90 C T = 100 C crystallization of whey-permeate and whey powder is slower than that of pure lactose powder, which we have explained above is due to the presence of salts and proteins. Eq. 2 can be rearranged to give the following equation: lnðkþ ¼lnðk 0 Þ E A ~R 1 ð5þ T A plot of ln(k) versus 1 results in a straight line with T slope E A and intercept ln(k 0 ). Figure 11 shows such er a plot for lactose powder crystallization at various relative humidities. The pre-exponential factors k 0 of the Arrhenius-equation are given in Table 4. Table 4. Pre-exponential factors of the Arrhenius-equation for the crystallization of lactose, whey-permeate and whey powder at various relative humidities u ( ) Lactose powder Whey-permeate powder Whey powder k 0 (1/s) k 0 (1/s) k 0 (1/s) E E E E E E E E E E E E E E E+20 Figure 11. Arrhenius-plots JMA rate constants for lactose powder determined gravimetrically at various relative humidities.
8 1364 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) Figure 12. Activation energies for the crystallization of amorphous lactose, whey-permeate and whey powder at various relative humidities. Figure 12 shows that the activation energies for the crystallization of amorphous lactose, whey-permeate and whey powders are dependant on the relative humidity. This can be seen most clearly in the case of lactose, for which the activation energy decreases from 164 kj/ mol to approximately 40 kj/mol as the relative humidity is increased from 20% to 50%. At higher relative humidities, the activation energy of crystallization assumes a constant value. The activation energy for the whey powders could not be measured at lower relative humidities, due to prohibitively long crystallization times, which prevented measurements at these relative humidities. Note that the rate constants k, induction times t Ind, reaction order n, pre-exponential factors k 0 and the activation energies E A determined in this work can be used in future calculations for designing a whey powder crystallization process Experimental equipment The crystallization kinetics were determined gravimetrically at various temperatures and humidities using a balance to follow the mass change of the amorphous powders, while they absorbed and desorbed moisture from the surroundings (Fig. 13). The sample was exposed to defined conditions in a custom made wind tunnel, where hot humid air with a velocity of 0.2 m/s flowed over the sample. The tunnel had an external heating jacket with recirculating oil, whose temperature was controlled at the dry-bulb temperature of the airflow in order to avoid any condensation on the walls. 3. Experimental 3.1. Materials Amorphous lactose, whey-permeate and whey powders were provided by the German producers Uelzena, Meggle and Lactoprot. The compositions of the wheypermeate and whey powders are given in Table 5. The amorphous nature of these products was confirmed by X-ray powder diffraction (XRPD) and differential scanning calorimetry (DSC). Table 5. Composition of amorphous lactose, whey-permeate and whey powders Lactose (wt %) Protein (wt %) Salt (wt %) Lactose powder 99.7 n.a. n.a. Whey-permeate powder Whey powder Figure 13. Layout of the balance and the cylindrical wind tunnel (inner diameter d i =210 mm).
9 A. Ibach, M. Kind / Carbohydrate Research 342 (2007) The sample was dispersed on a specimen holder, which consisted of four dishes, one above the other, each with a diameter of 62 mm. The holder was inserted into the tunnel through a port at the bottom, and attached to the balance (Type E 2000 D, Sartorius, Göttingen) via a magnetic-coupling device. A slight overpressure was set in the box containing the balance, so that hot, humid air could not rise upwards towards the balance. The resultant airflow into the wind tunnel was negligible compared with the flow of hot humid air within the wind tunnel. The coupling devices and connections were heated electrically to prevent water condensation on these parts. The mass change of a sample was continually recorded to track the crystallization process Sample preparation The lactose and whey powder samples were sieved onto the dishes using a mesh in order to form a mono-layered particle bed with particles of 150 lm or smaller. The powders were then dried under vacuum at 40 C for 10 h. Before starting the experiment, the sample was heated for 1 h in an oven at a temperature that was 10 C higher than the intended process temperature within the wind tunnel. Thus, moisture condensation onto the sample when it was inserted in the wind tunnel was prevented. To ensure that no lactose has crystallized while heating in the oven, samples were analyzed with differential scanning calorimeter and X-ray diffraction (XRD) measurements in preexaminations. The dry weight of each lactose sample was determined after the crystallization process by drying a portion of the sample for 24 h under vacuum at 105 C. At these conditions, the bound hydration water in a-lactosemonohydrate was released completely. The dry weights of the whey-permeate and whey powders could not be determined in this manner, because Maillard reactions, due to the presence of the proteins, would have resulted in a progressive mass loss of the sample as the lactose and protein reacted to form water. Therefore, these samples were dried for 10 h under vacuum at 70 C. These samples were subsequently put into a desiccator with P 2 O 5 as the drying agent, and the air within the desiccator was purged with N 2. The samples were then allowed to cool down to ambient temperature, after which the dry weights were determined. 4. Conclusions Time-dependent crystallization of amorphous lactose in pure lactose, whey-permeate and whey powders was investigated. Higher temperatures and relative humidities result in faster lactose crystallization. At the crystallization conditions used in this work, the required crystallization time for pure amorphous lactose powder ranged from 1 to 100 min. Whey-permeate and whey powders required more time to crystallize than pure lactose powder; the quickest crystallization for these powders could be achieved in only 5 min. Clearly, the presence of proteins and salts in the whey-permeate and whey powders hinder the crystallization rate. The rate constants and activation energies have been determined over a range of temperatures and humidities to enable the calculation of crystallization times for the design of an industrial process that crystallizes whey and whey-permeate powders. Finally, the crystallization rates are sufficiently fast so that an industrial process that crystallizes whey and whey-permeate powders in the range of minutes is feasible. Acknowledgement This work was supported by the FEI (Forschungskreis der Ernährungsindustrie e.v., Bonn), the AiF and the Ministry of Economics and Technology. Project No.: AiF-FV N. References 1. Vuataz, G. Lait 2002, 82, Palzer, S.; Zürcher, U. Chem. Ing. Tech. 2004, 76, Roos, Y.; Karel, M. J. Food Sci. 1992, 57, Vuataz, G. Food Preservation by Moisture Control; Elsevier: Amsterdam, Aguilera, J. M.; Del Valle, J. M.; Karel, M. Trends Food Sci. Technol. 1995, 6, Saito, Z. Food Microstruct. 1988, 7, Elamin, A. A.; Sebhatu, T.; Ahlneck, C. Int. J. Pharm. 1995, 119, Buckton, G.; Darcy, P. Int. J. Pharm. 1996, 136, Stubberud, L.; Forbes, R. T. Int. J. Pharm. 1998, 163, Jouppila, K.; Roos, Y. H. J. Dairy Sci. 1994, 77, Haque, M. K.; Roos, Y. H. J. Food Sci. 2004, 69, Johnson, W. A.; Mehl, R. F. Trans. Am. Inst. Min. Metall. Eng. 1939, 135, Avrami, M. J. Chem. Phys. 1939, 7, Avrami, M. J. Chem. Phys. 1940, 8, Avrami, M. J. Chem. Phys. 1941, 9, Schmitt, E. A.; Law, D.; Zhang, G. G. Z. J. Pharm. Sci. 1998, 88, Maffezzoli, A.; Kenny, J. M.; Torre, L. Thermochim. Acta 1995, 269/270, Drapier-Beche, N.; Fanni, J.; Parmentier, M.; Vilasi, M. J. Dairy Sci. 1997, 80, Jouppila, K.; Kansikas, J.; Roos, Y. Biotechnol. Prog. 1998, 14, Haque, M. K.; Roos, Y. H. Carbohydr. Res. 2005, 340, Nijdam, J.; Ibach, A.; Eichhorn, K.; Kind, M. Carbohydr. Res., submitted for publication. 22. Ibach, A.; Kind, M. Chem. Ing. Tech. 2007, 79, Chen, N.; Morikawa, J.; Hashimoto, T. Thermochim. Acta 2005, 431, Longinotti, M. P.; Mazzobre, M. F.; Buera, M. P.; Corti, H. R. Phys. Chem. Chem. Phys. 2002, 4,
Water in Food, Lausanne 2004
 Water in Food, Lausanne 2004 Characterising the Amorphous State in a Pharmaceutical Powder Using Moisture and Organic Vapour Sorption Frank Thielmann,* Daniel Burnett,** Jürgen Adolphs,*** *Surface Measurement
Water in Food, Lausanne 2004 Characterising the Amorphous State in a Pharmaceutical Powder Using Moisture and Organic Vapour Sorption Frank Thielmann,* Daniel Burnett,** Jürgen Adolphs,*** *Surface Measurement
A study of the crystallization kinetics in Se 68 Ge 22 Pb 10 chalcogenide glass
 Indian Journal of Engineering & Materials Sciences Vol. 11, December 2004, pp. 511-515 A study of the crystallization kinetics in Se 68 Ge 22 Pb 10 chalcogenide glass N Mehta a, P Agarwal b & A Kumar a
Indian Journal of Engineering & Materials Sciences Vol. 11, December 2004, pp. 511-515 A study of the crystallization kinetics in Se 68 Ge 22 Pb 10 chalcogenide glass N Mehta a, P Agarwal b & A Kumar a
Keywords. Aluminium-based amorphous alloys; melt spinning; crystallization behaviour; microhardness.
 PRAMANA c Indian Academy of Sciences Vol. 65, No. 4 journal of October 2005 physics pp. 745 751 Effect of rare-earth elements on nanophase evolution, crystallization behaviour and mechanical properties
PRAMANA c Indian Academy of Sciences Vol. 65, No. 4 journal of October 2005 physics pp. 745 751 Effect of rare-earth elements on nanophase evolution, crystallization behaviour and mechanical properties
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Effect of Cu and P on the Crystallization Behavior of Fe-Rich Hetero-Amorphous FeSiB Alloy
 Materials Transactions, Vol. 50, No. 11 (2009) pp. 2515 to 2520 #2009 The Japan Institute of Metals Effect of Cu and P on the Crystallization Behavior of Fe-Rich Hetero-Amorphous FeSiB Alloy Liying Cui
Materials Transactions, Vol. 50, No. 11 (2009) pp. 2515 to 2520 #2009 The Japan Institute of Metals Effect of Cu and P on the Crystallization Behavior of Fe-Rich Hetero-Amorphous FeSiB Alloy Liying Cui
Study on crystallization behaviour of co-polyamide 66 containing triaryl phosphine oxide
 Bull. Mater. Sci., Vol. 35, No. 2, April 212, pp. 233 242. c Indian Academy of Sciences. Study on crystallization behaviour of co-polyamide 66 containing triaryl phosphine oxide YANG XIAO FENG, LI QIAO
Bull. Mater. Sci., Vol. 35, No. 2, April 212, pp. 233 242. c Indian Academy of Sciences. Study on crystallization behaviour of co-polyamide 66 containing triaryl phosphine oxide YANG XIAO FENG, LI QIAO
New Developments in Spray-Dried Lactose
 New Developments in Spray-Dried Lactose Gerad Bolhuis, Klaas Kussendrager, and John Langridge* Recent advances in spraydrying technology have led to the production of new directly compressible lactose
New Developments in Spray-Dried Lactose Gerad Bolhuis, Klaas Kussendrager, and John Langridge* Recent advances in spraydrying technology have led to the production of new directly compressible lactose
Thermal Analysis. Dr. Lidia Tajber School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin
 Thermal Analysis Dr. Lidia Tajber School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin Characterisation for Pharma Active pharmaceutical ingredients (API, drugs) Organic molecules, peptides,
Thermal Analysis Dr. Lidia Tajber School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin Characterisation for Pharma Active pharmaceutical ingredients (API, drugs) Organic molecules, peptides,
DETERMINATION OF AVRAMI PARAMETER IN THE CASE OF NON-ISOTHERMAL SURFACE CRYSTALLIZATION OF POWDERED GLASSES
 1 Proceedings of 16 -th International conference of Glass and Ceramics, Varna, Bulgaria, 26-30,09,Varna 2008 DETERMINATION OF AVRAMI PARAMETER IN THE CASE OF NON-ISOTHERMAL SURFACE CRYSTALLIZATION OF POWDERED
1 Proceedings of 16 -th International conference of Glass and Ceramics, Varna, Bulgaria, 26-30,09,Varna 2008 DETERMINATION OF AVRAMI PARAMETER IN THE CASE OF NON-ISOTHERMAL SURFACE CRYSTALLIZATION OF POWDERED
Crystallization behavior of the Zr 63 Al 7.5 Cu 17.5 Ni 10 B 2 amorphous alloy during isothermal annealing
 Intermetallics 13 (2005) 907 911 www.elsevier.com/locate/intermet Short communication Crystallization behavior of the Zr 63 Al 7.5 Cu 17.5 Ni 10 B 2 amorphous alloy during isothermal annealing J.S.C. Jang
Intermetallics 13 (2005) 907 911 www.elsevier.com/locate/intermet Short communication Crystallization behavior of the Zr 63 Al 7.5 Cu 17.5 Ni 10 B 2 amorphous alloy during isothermal annealing J.S.C. Jang
C.J. KEDWARD, W. MACNAUGHTAN, AND J.R. MITCHELL ABSTRACT:
 JFS: Crystallization Kinetics of Amorphous Lactose as a Function of Moisture Content Using Isothermal Differential Scanning Calorimetry C.J. KEDWARD, W. MACNAUGHTAN, AND J.R. MITCHELL ABSTRACT: Isothermal
JFS: Crystallization Kinetics of Amorphous Lactose as a Function of Moisture Content Using Isothermal Differential Scanning Calorimetry C.J. KEDWARD, W. MACNAUGHTAN, AND J.R. MITCHELL ABSTRACT: Isothermal
Quantitation of amorphous content
 13 Quantitation of amorphous content Amorphous fractions can significantly change the physicochemical properties of active pharmaceutical ingredients and drug products. Solvias offers a variety of analytical
13 Quantitation of amorphous content Amorphous fractions can significantly change the physicochemical properties of active pharmaceutical ingredients and drug products. Solvias offers a variety of analytical
PHARMACEUTICAL PRINCIPLES OF SOLID DOSAGE FORMS
 PHARMACEUTICAL PRINCIPLES OF SOLID DOSAGE FORMS J. T. Carstensen, Ph.D. Professor of Pharmacy University of Wisconsin Madison, Wisconsin TECHNOMIC ^PUBLISHING CO.. INC J 1.ANCASTER BASET, TABLE OF CONTENTS
PHARMACEUTICAL PRINCIPLES OF SOLID DOSAGE FORMS J. T. Carstensen, Ph.D. Professor of Pharmacy University of Wisconsin Madison, Wisconsin TECHNOMIC ^PUBLISHING CO.. INC J 1.ANCASTER BASET, TABLE OF CONTENTS
Crystallization kinetics of PHB and its blends 3.1 Introduction
 3 3.1 Introduction The crystallization process is a transition from liquid phase (melts) into solid phase after cooling. The crystallization kinetics of PHB and its blends is investigated by using differential
3 3.1 Introduction The crystallization process is a transition from liquid phase (melts) into solid phase after cooling. The crystallization kinetics of PHB and its blends is investigated by using differential
New Cu-based Bulk Metallic Glasses with High Strength of 2000 MPa
 Materials Science Forum Online: 2004-03-15 ISSN: 1662-9752, Vols. 449-452, pp 945-948 doi:10.4028/www.scientific.net/msf.449-452.945 2004 Trans Tech Publications, Switzerland New Cu-based Bulk Metallic
Materials Science Forum Online: 2004-03-15 ISSN: 1662-9752, Vols. 449-452, pp 945-948 doi:10.4028/www.scientific.net/msf.449-452.945 2004 Trans Tech Publications, Switzerland New Cu-based Bulk Metallic
Solid and Liquid States of Lactose
 2 Solid and Liquid States of Lactose Y.H. Roos Lactose in dairy systems can exist in various crystalline and non-crystalline forms. These forms affect lactose behaviour, particularly in processing and
2 Solid and Liquid States of Lactose Y.H. Roos Lactose in dairy systems can exist in various crystalline and non-crystalline forms. These forms affect lactose behaviour, particularly in processing and
Weibing Xu, 1,3 Guodong Liang, 1 Wei Wang, 1 Shupei Tang, 1 Pingsheng He, 2 Wei-Ping Pan 3. Hefei , Anhui, China. Bowling Green, Kentucky 42101
 Poly(propylene) Poly(propylene)-Grafted Maleic Anhydride Organic Montmorillonite (PP PP-g-MAH Org- MMT) Nanocomposites. II. Nonisothermal Crystallization Kinetics Weibing Xu, 1,3 Guodong Liang, 1 Wei Wang,
Poly(propylene) Poly(propylene)-Grafted Maleic Anhydride Organic Montmorillonite (PP PP-g-MAH Org- MMT) Nanocomposites. II. Nonisothermal Crystallization Kinetics Weibing Xu, 1,3 Guodong Liang, 1 Wei Wang,
Polymorph Screening Technology by Controlling Crystallization
 A publication of 2221 CHEMICAL ENGINEERING TRANSACTIONS VOL. 32, 213 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 213, AIDIC Servizi S.r.l., ISBN 978-88-9568-23-5; ISSN 1974-9791 The Italian
A publication of 2221 CHEMICAL ENGINEERING TRANSACTIONS VOL. 32, 213 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 213, AIDIC Servizi S.r.l., ISBN 978-88-9568-23-5; ISSN 1974-9791 The Italian
Effect of melt temperature on the oxidation behavior of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres
 available at www.sciencedirect.com www.elsevier.com/locate/matchar Effect of melt temperature on the oxidation behavior of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres Hukui Chen
available at www.sciencedirect.com www.elsevier.com/locate/matchar Effect of melt temperature on the oxidation behavior of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres Hukui Chen
Grain Growth Control of BaTiO 3 Ceramics with CuO/BaO=2.5 Mixture Addition
 Grain Growth Control of BaTiO 3 Ceramics with CuO/BaO=2.5 Mixture Addition Cheng-Fu Yang 1, Chien-Chen Diao 2, and Chien-Min Cheng 3 Department of Chemical and Material Eng., N.U.K., Kaohsiung, Taiwan,
Grain Growth Control of BaTiO 3 Ceramics with CuO/BaO=2.5 Mixture Addition Cheng-Fu Yang 1, Chien-Chen Diao 2, and Chien-Min Cheng 3 Department of Chemical and Material Eng., N.U.K., Kaohsiung, Taiwan,
Calorimetric Study of the Energetics and Kinetics of Interdiffusion in Cu/Cu 6. Film Diffusion Couples. Department of Physics
 Calorimetric Study of the Energetics and Kinetics of Interdiffusion in Cu/Cu 6 Thin Film Diffusion Couples K. F. Dreyer, W. K. Niels, R. R. Chromik, D. Grosman, and E. J. Cotts Department of Physics Binghamton
Calorimetric Study of the Energetics and Kinetics of Interdiffusion in Cu/Cu 6 Thin Film Diffusion Couples K. F. Dreyer, W. K. Niels, R. R. Chromik, D. Grosman, and E. J. Cotts Department of Physics Binghamton
Water Activity Themodynamic and Kinetic Consequenses on state changes in foods
 Water Activity Themodynamic and Kinetic Consequenses on state changes in foods Reims, France May 26-27 202 Dr Ted P. Labuza Department of Food Science and Nutrition University of Minnesota St Paul 55108
Water Activity Themodynamic and Kinetic Consequenses on state changes in foods Reims, France May 26-27 202 Dr Ted P. Labuza Department of Food Science and Nutrition University of Minnesota St Paul 55108
Detection and Quantification of Amorphous Content in Pharmaceutical Materials
 Detection and Quantification of Amorphous Content in Pharmaceutical Materials Steven R. Aubuchon, Ph.D. TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA and Leonard C. Thomas DSC Solutions,
Detection and Quantification of Amorphous Content in Pharmaceutical Materials Steven R. Aubuchon, Ph.D. TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA and Leonard C. Thomas DSC Solutions,
PHASE TRANSFORMATIONS IN PLASMA SPRAYED HYDROXYAPATITE COATINGS
 Scripta mater. 42 (2000) 103 109 www.elsevier.com/locate/scriptamat PHASE TRANSFORMATIONS IN PLASMA SPRAYED HYDROXYAPATITE COATINGS C.F. Feng, K.A. Khor, E.J. Liu and P. Cheang* School of Mechanical and
Scripta mater. 42 (2000) 103 109 www.elsevier.com/locate/scriptamat PHASE TRANSFORMATIONS IN PLASMA SPRAYED HYDROXYAPATITE COATINGS C.F. Feng, K.A. Khor, E.J. Liu and P. Cheang* School of Mechanical and
Hydrogen isotopes permeation in a fluoride molten salt for nuclear fusion blanket
 J. Plasma Fusion Res. SERIES, Vol. (5) Hydrogen isotopes permeation in a fluoride molten salt for nuclear fusion blanket Akira Nakamura, Satoshi Fukada and Ryosuke Nishiumi Department of Advanced Energy
J. Plasma Fusion Res. SERIES, Vol. (5) Hydrogen isotopes permeation in a fluoride molten salt for nuclear fusion blanket Akira Nakamura, Satoshi Fukada and Ryosuke Nishiumi Department of Advanced Energy
Compression stress induced flow temperature reduction in a bulk Zr 41:2 Ti 13:8 Cu 12:5 Ni 10:0 Be 22:5 metallic glass
 Scripta Materialia 47 (2002) 787 791 www.actamat-journals.com Compression stress induced flow temperature reduction in a bulk Zr 41:2 Ti 13:8 Cu 12:5 Ni 10:0 Be 22:5 metallic glass H.J. Jin, X.J. Gu, F.
Scripta Materialia 47 (2002) 787 791 www.actamat-journals.com Compression stress induced flow temperature reduction in a bulk Zr 41:2 Ti 13:8 Cu 12:5 Ni 10:0 Be 22:5 metallic glass H.J. Jin, X.J. Gu, F.
PHYSICAL STABILITY OF SPRAY DRIED SOLID DISPERSIONS OF AMORPHOUS TOLFENAMIC ACID AND POLYVINYLPYRROLIDONE K-30
 PHYSICAL STABILITY OF SPRAY DRIED SOLID DISPERSIONS OF AMORPHOUS TOLFENAMIC ACID AND POLYVINYLPYRROLIDONE K-30 Copenhagen, Denmark Outline of Today s Presentation Introduction Spray drying method Amorphous
PHYSICAL STABILITY OF SPRAY DRIED SOLID DISPERSIONS OF AMORPHOUS TOLFENAMIC ACID AND POLYVINYLPYRROLIDONE K-30 Copenhagen, Denmark Outline of Today s Presentation Introduction Spray drying method Amorphous
G16 - THERMAL ANALYSIS
 001-1012PDG.pdf 1 1 G16 - THERMAL ANALYSIS 2 3 4 5 6 7 8 9 Thermal analysis is a group of techniques in which the variation of a physical property of a substance is measured as a function of temperature.
001-1012PDG.pdf 1 1 G16 - THERMAL ANALYSIS 2 3 4 5 6 7 8 9 Thermal analysis is a group of techniques in which the variation of a physical property of a substance is measured as a function of temperature.
Combustion of Al Al 2 O 3 mixtures in air
 Journal of the European Ceramic Society 25 (2005) 1575 1579 Combustion of Al Al 2 O 3 mixtures in air Alexander Gromov, Alexander Ilyin, Alexander Ditts, Vladimir Vereshchagin Chemical Department, Tomsk
Journal of the European Ceramic Society 25 (2005) 1575 1579 Combustion of Al Al 2 O 3 mixtures in air Alexander Gromov, Alexander Ilyin, Alexander Ditts, Vladimir Vereshchagin Chemical Department, Tomsk
Physical pharmacy. dr basam al zayady
 Physical pharmacy Lec 5 dr basam al zayady Liquefaction of Gases: When a gas is cooled, it loses some of its kinetic energy in the form of heat, and the velocity of the molecules decreases. If pressure
Physical pharmacy Lec 5 dr basam al zayady Liquefaction of Gases: When a gas is cooled, it loses some of its kinetic energy in the form of heat, and the velocity of the molecules decreases. If pressure
EFFECT OF BOTH TALC FINENESS AND TALC LOADING ON HETEROGENEOUS NUCLEATION OF BLOCK COPOLYMER POLYPROPYLENE
 EFFECT OF BOTH TALC FINENESS AND TALC LOADING ON HETEROGENEOUS NUCLEATION OF BLOCK COPOLYMER POLYPROPYLENE Piergiovanni Ercoli Malacari, IMIFabi Spa, Milano, Italy Abstract The heterogeneous nucleation
EFFECT OF BOTH TALC FINENESS AND TALC LOADING ON HETEROGENEOUS NUCLEATION OF BLOCK COPOLYMER POLYPROPYLENE Piergiovanni Ercoli Malacari, IMIFabi Spa, Milano, Italy Abstract The heterogeneous nucleation
Feasibility study of using agriculture waste as desiccant for air conditioning system
 Renewable Energy 28 (2003) 1617 1628 www.elsevier.com/locate/renene Feasibility study of using agriculture waste as desiccant for air conditioning system J. Khedari a,, R. Rawangkul a, W. Chimchavee b,
Renewable Energy 28 (2003) 1617 1628 www.elsevier.com/locate/renene Feasibility study of using agriculture waste as desiccant for air conditioning system J. Khedari a,, R. Rawangkul a, W. Chimchavee b,
CRYSTALLIZATION AND DYNAMIC MECHANICAL BEHAVIOUR OF NR-EVA BLENDS.
 CHAPTER IV - MISCIBILITY. CRYSTALLIZATION AND DYNAMIC MECHANICAL BEHAVIOUR OF NR-EVA BLENDS. The results of the above study have been communicated for publicaticln in POLYMER. The basic issue confronting
CHAPTER IV - MISCIBILITY. CRYSTALLIZATION AND DYNAMIC MECHANICAL BEHAVIOUR OF NR-EVA BLENDS. The results of the above study have been communicated for publicaticln in POLYMER. The basic issue confronting
Hopping Conduction Mechanism in Amorphous Cuo- Bi 2 o 3 Semiconducting Pellets
 Hopping Conduction Mechanism in Amorphous Cuo- Bi 2 o 3 Semiconducting Pellets W. J. Gawande 1*, S. S. Yawale 2 and S. P. Yawale 1 1 P.G. Department of Physics, Government Vidarbha Institute of Science
Hopping Conduction Mechanism in Amorphous Cuo- Bi 2 o 3 Semiconducting Pellets W. J. Gawande 1*, S. S. Yawale 2 and S. P. Yawale 1 1 P.G. Department of Physics, Government Vidarbha Institute of Science
XRD, DTA AND DENSITY STUDIES OF LITHIUM BORATE GLASSES CONTAINING COPPER A. A. Soliman
 , pp. 188 197 XRD, DTA AND DENSITY STUDIES OF LITHIUM BORATE GLASSES CONTAINING COPPER A. A. Soliman Physics Department, Faculty of Girls, Ain Shams University, Heliopolis, Egypt Received 4 November, 2008
, pp. 188 197 XRD, DTA AND DENSITY STUDIES OF LITHIUM BORATE GLASSES CONTAINING COPPER A. A. Soliman Physics Department, Faculty of Girls, Ain Shams University, Heliopolis, Egypt Received 4 November, 2008
PHASE TRANSFORMATION AND CRYSTALLIZATION KINETICS OF Se 90 In 8 Sb 2 CHALCOGENIDE GLASS
 Chalcogenide Letters Vol. 5, No. 6, July 2008, p. 126 136 PHASE TRANSFORMATION AND CRYSTALLIZATION KINETICS OF Se 90 In 8 Sb 2 CHALCOGENIDE GLASS Praveen K. Jain *, Deepika, K.S.Rathore, N.S.Saxena Semi-conductor
Chalcogenide Letters Vol. 5, No. 6, July 2008, p. 126 136 PHASE TRANSFORMATION AND CRYSTALLIZATION KINETICS OF Se 90 In 8 Sb 2 CHALCOGENIDE GLASS Praveen K. Jain *, Deepika, K.S.Rathore, N.S.Saxena Semi-conductor
Crystallization study of Te Bi Se glasses
 Bull. Mater. Sci., Vol. 26, No. 5, August 2003, pp. 547 551. Indian Academy of Sciences. Crystallization study of Te Bi Se glasses MANISH SAXENA* and P K BHATNAGAR Department of Sciences and Humanities,
Bull. Mater. Sci., Vol. 26, No. 5, August 2003, pp. 547 551. Indian Academy of Sciences. Crystallization study of Te Bi Se glasses MANISH SAXENA* and P K BHATNAGAR Department of Sciences and Humanities,
Porous Bulk Metallic Glass Fabricated by Powder Consolidation
 Journal of Minerals & Materials Characterization & Engineering, Vol. 7, No.2, pp 97-104, 2008 jmmce.org Printed in the USA. All rights reserved Porous Bulk Metallic Glass Fabricated by Powder Consolidation
Journal of Minerals & Materials Characterization & Engineering, Vol. 7, No.2, pp 97-104, 2008 jmmce.org Printed in the USA. All rights reserved Porous Bulk Metallic Glass Fabricated by Powder Consolidation
Corrosion Behavior of Cu Zr Ti Nb Bulk Glassy Alloys
 Materials Transactions, Vol. 44, No. 4 (3 pp. 749 to 753 #3 The Japan Institute of Metals Corrosion Behavior of Cu Bulk Glassy Alloys Chunling Qin 1; *, Katsuhiko Asami 1, Tao Zhang 1, Wei Zhang 2 and
Materials Transactions, Vol. 44, No. 4 (3 pp. 749 to 753 #3 The Japan Institute of Metals Corrosion Behavior of Cu Bulk Glassy Alloys Chunling Qin 1; *, Katsuhiko Asami 1, Tao Zhang 1, Wei Zhang 2 and
SUB-MICROMETER ORDER CORROSION OF SILVER BY SULFUR VAPOR IN AIR STUDIED BY MEANS OF QUARTZ CRYSTAL MICROBALANCE
 16th International Corrosion Congress September 19-24,, Beijing, China SUB-MICROMETER ORDER CORROSION OF SILVER BY SULFUR VAPOR IN AIR STUDIED BY MEANS OF QUARTZ CRYSTAL MICROBALANCE *Jun ichi SAKAI 1,
16th International Corrosion Congress September 19-24,, Beijing, China SUB-MICROMETER ORDER CORROSION OF SILVER BY SULFUR VAPOR IN AIR STUDIED BY MEANS OF QUARTZ CRYSTAL MICROBALANCE *Jun ichi SAKAI 1,
CRISTALIZATION UNIT YUSRON SUGIARTO
 CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
Physical Aging and Fragility of Amorphous Sucrose by DSC
 Physical Aging and Fragility of Amorphous Sucrose by DSC R. Bruce Cassel, Ph.D. TA Instruments, 109 Lukens Drive, New Castle DE 19720, USA ABSTRACT A method for quantifying physical aging and fragility
Physical Aging and Fragility of Amorphous Sucrose by DSC R. Bruce Cassel, Ph.D. TA Instruments, 109 Lukens Drive, New Castle DE 19720, USA ABSTRACT A method for quantifying physical aging and fragility
Generation of high drug loading amorphous solid dispersions by Spray Drying
 IDS 2018 21st International Drying Symposium València, Spain, 11-14 September 2018 DOI: http://dx.doi.org/10.4995/ids2018.2018.7871 Generation of high drug loading amorphous solid dispersions by Spray
IDS 2018 21st International Drying Symposium València, Spain, 11-14 September 2018 DOI: http://dx.doi.org/10.4995/ids2018.2018.7871 Generation of high drug loading amorphous solid dispersions by Spray
Comparison of coal reactivityduring conversion into different oxidizing medium
 Journal of Physics: Conference Series PAPER OPEN ACCESS Comparison of coal reactivityduring conversion into different oxidizing medium To cite this article: A G Korotkikh et al 2016 J. Phys.: Conf. Ser.
Journal of Physics: Conference Series PAPER OPEN ACCESS Comparison of coal reactivityduring conversion into different oxidizing medium To cite this article: A G Korotkikh et al 2016 J. Phys.: Conf. Ser.
Densification and grain growth of TiO 2 -doped ZnO
 Materials Science-Poland, Vol. 25, No. 4, 2007 Densification and grain growth of TiO 2 -doped ZnO K. YILDIZ *, N. KARAKU, N. TOPLAN, H. Ö. TOPLAN Sakarya University, Metallurgy and Materials Engineering,
Materials Science-Poland, Vol. 25, No. 4, 2007 Densification and grain growth of TiO 2 -doped ZnO K. YILDIZ *, N. KARAKU, N. TOPLAN, H. Ö. TOPLAN Sakarya University, Metallurgy and Materials Engineering,
K Vinodhini, K Srinivasan
 Morphological Changes of α-lactose Monohydrate (α-lm) Single Crystals under Different Crystallization Conditions Using Polar Protic and Aprotic Solvents K Vinodhini, K Srinivasan To cite this version:
Morphological Changes of α-lactose Monohydrate (α-lm) Single Crystals under Different Crystallization Conditions Using Polar Protic and Aprotic Solvents K Vinodhini, K Srinivasan To cite this version:
Nonisothermal Phase Transformation of the Glassy Composition TeSe 20
 Egypt. J. Sol., Vol. (23), No. (2), (2000) 259 Nonisothermal Phase Transformation of the Glassy Composition TeSe 20 M.B. El-Den Physics Department, Faculty of Science, Ain Shams University Cairo, Egypt
Egypt. J. Sol., Vol. (23), No. (2), (2000) 259 Nonisothermal Phase Transformation of the Glassy Composition TeSe 20 M.B. El-Den Physics Department, Faculty of Science, Ain Shams University Cairo, Egypt
LYOPHILIZATION. Introduction to Freeze-Drying Third of Four Lectures. Freezing, Annealing, Primary, and Secondary Drying. J. Jeff Schwegman, Ph.D.
 LYOPHILIZATION Introduction to Freeze-Drying Third of Four Lectures Freezing, Annealing, Primary, and Secondary Drying J. Jeff Schwegman, Ph.D. AB BioTechnologies P.O. Box 1430 Bloomington, Indiana, 47402
LYOPHILIZATION Introduction to Freeze-Drying Third of Four Lectures Freezing, Annealing, Primary, and Secondary Drying J. Jeff Schwegman, Ph.D. AB BioTechnologies P.O. Box 1430 Bloomington, Indiana, 47402
Periodic structural fluctuations during the solidification of aluminum alloys studied by neutron diffraction
 Materials Science and Engineering A 367 (4) 8 88 Periodic structural fluctuations during the solidification of aluminum alloys studied by neutron diffraction N. Iqbal a,, N.H. van Dijk a, V.W.J. Verhoeven
Materials Science and Engineering A 367 (4) 8 88 Periodic structural fluctuations during the solidification of aluminum alloys studied by neutron diffraction N. Iqbal a,, N.H. van Dijk a, V.W.J. Verhoeven
Phase transformation kinetics and microstructure of NiTi shape memory alloy: effect of hydrostatic pressure
 Bull. Mater. Sci., Vol., No. 4, August 2017, pp. 799 803 DOI.07/s12034-017-1413-1 Indian Academy of Sciences Phase transformation kinetics and microstructure of NiTi shape memory alloy: effect of hydrostatic
Bull. Mater. Sci., Vol., No. 4, August 2017, pp. 799 803 DOI.07/s12034-017-1413-1 Indian Academy of Sciences Phase transformation kinetics and microstructure of NiTi shape memory alloy: effect of hydrostatic
SUCCIPACK Development of active, intelligent and sustainable food PACKaging using Polybutylenesuccinate
 Page 1 / 13 SUCCIPACK Development of active, intelligent and sustainable food PACKaging using Polybutylenesuccinate Project co-funded by the European Commission within the Seventh Framework Programme (2007-2013)
Page 1 / 13 SUCCIPACK Development of active, intelligent and sustainable food PACKaging using Polybutylenesuccinate Project co-funded by the European Commission within the Seventh Framework Programme (2007-2013)
Detailed study of the crystallization behaviour of the metallic glass Fe 75 Si 9 B 16
 Journal of Alloys and Compounds 386 (2005) 165 173 Detailed study of the crystallization behaviour of the metallic glass Fe 75 Si 9 B 16 K. Chrissafis, M.I. Maragakis, K.G. Efthimiadis, E.K. Polychroniadis
Journal of Alloys and Compounds 386 (2005) 165 173 Detailed study of the crystallization behaviour of the metallic glass Fe 75 Si 9 B 16 K. Chrissafis, M.I. Maragakis, K.G. Efthimiadis, E.K. Polychroniadis
Effects of the Sb Te crystallization-induced layer on crystallization behaviors and properties of phase change optical disk
 Surface and Coatings Technology 177 178 (2004) 79 799 Effects of the Sb Te crystallization-induced layer on crystallization behaviors and properties of phase change optical disk Wei Hsiang Wang, Li Chun
Surface and Coatings Technology 177 178 (2004) 79 799 Effects of the Sb Te crystallization-induced layer on crystallization behaviors and properties of phase change optical disk Wei Hsiang Wang, Li Chun
Physical Aging and Fragility of Amorphous Polyethylene Terephthalate
 Physical Aging and Fragility of Amorphous Polyethylene Terephthalate R. Bruce Cassel, Ph.D. TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA ABSTRACT Physical aging of amorphous polyethylene
Physical Aging and Fragility of Amorphous Polyethylene Terephthalate R. Bruce Cassel, Ph.D. TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA ABSTRACT Physical aging of amorphous polyethylene
Chapter 45 The Characterization of Ammonium Nitrate Mini-Prills
 Chapter 45 The Characterization of Ammonium Nitrate Mini-Prills Erica Lotspeich and Vilem Petr Abstract Ammonium nitrate (AN) is commonly used as a fertilizer and is the fundamental ingredient of industrial
Chapter 45 The Characterization of Ammonium Nitrate Mini-Prills Erica Lotspeich and Vilem Petr Abstract Ammonium nitrate (AN) is commonly used as a fertilizer and is the fundamental ingredient of industrial
Technical brochure FlowLac
 L W TABLETING DIRECT COMPRESSION SPRAY-DRIED LACTOSE AC Technical brochure FlowLac MEGGLE spray-dried lactose grades for direct compression: FlowLac General information Direct compression (DC) tablet manufacture
L W TABLETING DIRECT COMPRESSION SPRAY-DRIED LACTOSE AC Technical brochure FlowLac MEGGLE spray-dried lactose grades for direct compression: FlowLac General information Direct compression (DC) tablet manufacture
New Methods for Preparing Cyclodextrin Inclusion Compounds. I. Heating in a Sealed Container
 No. 11 4609 New Methods for Preparing Cyclodextrin Inclusion Compounds. I. Heating in a Sealed Container YOSHINOBU NAKAI,* KEIJI YAMAMOTO, KATSUHIDE TERADA, and DAIICHI WATANABE Faculty of Pharmaceutical
No. 11 4609 New Methods for Preparing Cyclodextrin Inclusion Compounds. I. Heating in a Sealed Container YOSHINOBU NAKAI,* KEIJI YAMAMOTO, KATSUHIDE TERADA, and DAIICHI WATANABE Faculty of Pharmaceutical
Crystallization behaviour of biodegradable poly(ethylene succinate) from the amorphous state
 Polymer 44 (2003) 5429 5437 www.elsevier.com/locate/polymer Crystallization behaviour of biodegradable poly(ethylene succinate) from the amorphous state Zhaobin Qiu a,b, *, Takayuki Ikehara a,c, Toshio
Polymer 44 (2003) 5429 5437 www.elsevier.com/locate/polymer Crystallization behaviour of biodegradable poly(ethylene succinate) from the amorphous state Zhaobin Qiu a,b, *, Takayuki Ikehara a,c, Toshio
Decomposition of Li 2 CO 3 in existence of SiO 2 in mould flux of steel casting
 KIM, J-W., LEE, Y-D., KANG, Y-D and LEE, H-G. Decomposition of Li 2 CO 3 in existence of SiO 2 in mould flux of steel casting. VII International Conference on Molten Slags Fluxes and Salts, The South African
KIM, J-W., LEE, Y-D., KANG, Y-D and LEE, H-G. Decomposition of Li 2 CO 3 in existence of SiO 2 in mould flux of steel casting. VII International Conference on Molten Slags Fluxes and Salts, The South African
acta physica slovaca vol. 50 No. 4, August 2000 HEATING RATE LIMITED KINETICS OF CRYSTALLIZATION OF FINEMET+NI AMORPHOUS RIBBONS
 acta physica slovaca vol. 50 No. 4, 525 532 August 2000 HEATING RATE LIMITED KINETICS OF CRYSTALLIZATION OF FINEMET+NI AMORPHOUS RIBBONS E. Illeková, P. Duhaj Institute of Physics, Slovak Academy of Sciences,
acta physica slovaca vol. 50 No. 4, 525 532 August 2000 HEATING RATE LIMITED KINETICS OF CRYSTALLIZATION OF FINEMET+NI AMORPHOUS RIBBONS E. Illeková, P. Duhaj Institute of Physics, Slovak Academy of Sciences,
Study of Calcination-Carbonation of Calcium Carbonate in Different Fluidizing Mediums for Chemical Looping Gasification in Circulating Fluidized Beds
 Engineering Conferences International ECI Digital Archives 10th International Conference on Circulating Fluidized Beds and Fluidization Technology - CFB-10 Refereed Proceedings Spring 5-2-2011 Study of
Engineering Conferences International ECI Digital Archives 10th International Conference on Circulating Fluidized Beds and Fluidization Technology - CFB-10 Refereed Proceedings Spring 5-2-2011 Study of
Hot Deformation Behavior of High Strength Low Alloy Steel by Thermo Mechanical Simulator and Finite Element Method
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Hot Deformation Behavior of High Strength Low Alloy Steel by Thermo Mechanical Simulator and Finite Element Method To cite this
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Hot Deformation Behavior of High Strength Low Alloy Steel by Thermo Mechanical Simulator and Finite Element Method To cite this
Properties of Ceramic Materials
 1-5 Superplasticity: There are two basic types of superplasticity, termed transformation and structural superplasticity respectively. (A third type of superplasticity, termed temperature-cycling superplasticity,
1-5 Superplasticity: There are two basic types of superplasticity, termed transformation and structural superplasticity respectively. (A third type of superplasticity, termed temperature-cycling superplasticity,
Viscous flow behavior and thermal properties of bulk amorphous Mg 58 Cu 31 Y 11 alloy
 Intermetallics 15 (2007) 1303e1308 www.elsevier.com/locate/intermet Viscous flow behavior and thermal properties of bulk amorphous Mg 58 Cu 31 Y 11 alloy Y.C. Chang a, T.H. Hung a, H.M. Chen a, J.C. Huang
Intermetallics 15 (2007) 1303e1308 www.elsevier.com/locate/intermet Viscous flow behavior and thermal properties of bulk amorphous Mg 58 Cu 31 Y 11 alloy Y.C. Chang a, T.H. Hung a, H.M. Chen a, J.C. Huang
The Glass Transition in Polymers
 The Glass Transition in Polymers Introduction : Collections of molecules can exist in three possible physical states: solid, liquid and gas. In polymeric materials, things are not so straightforward. For
The Glass Transition in Polymers Introduction : Collections of molecules can exist in three possible physical states: solid, liquid and gas. In polymeric materials, things are not so straightforward. For
Coupling Hollow Fe 3 O 4 -Fe Nanoparticles with Graphene Sheets for High-performance Electromagnetic Wave Absorbing Material
 Supporting Information Coupling Hollow Fe 3 O 4 -Fe Nanoparticles with Graphene Sheets for High-performance Electromagnetic Wave Absorbing Material Bin Qu,,#,, Chunling Zhu, *, Chunyan Li, Xitian Zhang,
Supporting Information Coupling Hollow Fe 3 O 4 -Fe Nanoparticles with Graphene Sheets for High-performance Electromagnetic Wave Absorbing Material Bin Qu,,#,, Chunling Zhu, *, Chunyan Li, Xitian Zhang,
PAT for freeze drying: cycle optimisation in the laboratory
 PAT for freeze drying: cycle optimisation in the laboratory Dr. Henning Gieseler, PhD., Department of Pharmaceutics, University of Erlangen-Nuremberg Freeze drying is generally known to be a time consuming
PAT for freeze drying: cycle optimisation in the laboratory Dr. Henning Gieseler, PhD., Department of Pharmaceutics, University of Erlangen-Nuremberg Freeze drying is generally known to be a time consuming
Assessing the Effects of Process Temperature on Crystallization Kinetics of Polyphenylene Sulfide Utilizing Differential Scanning Calorimetry (DSC)
 Assessing the Effects of Process Temperature on Crystallization Kinetics of Polyphenylene Sulfide Utilizing Differential Scanning Calorimetry (DSC) Keywords: polyphenylene sulfide, non-isothermal kinetics,
Assessing the Effects of Process Temperature on Crystallization Kinetics of Polyphenylene Sulfide Utilizing Differential Scanning Calorimetry (DSC) Keywords: polyphenylene sulfide, non-isothermal kinetics,
Relationship between Hydrate-Free Hold Time and Subcooling for a Natural Gas System Containing Kinetic Hydrate Inhibitors
 Relationship between Hydrate-Free Hold Time and Subcooling for a Natural Gas System Containing Kinetic Hydrate Inhibitors This study observed simple chemical kinetics behavior for the first appearance
Relationship between Hydrate-Free Hold Time and Subcooling for a Natural Gas System Containing Kinetic Hydrate Inhibitors This study observed simple chemical kinetics behavior for the first appearance
Effect of Amorphous Transformation on Electrochemical Capacities of Rare Earth Mg Based Alloys
 Z. Phys. Chem. 220 (2006) 631 639 / DOI 10.1524/zpch.2006.220.5.631 by Oldenbourg Wissenschaftsverlag, München Effect of Amorphous Transformation on Electrochemical Capacities of Rare Earth Mg Based Alloys
Z. Phys. Chem. 220 (2006) 631 639 / DOI 10.1524/zpch.2006.220.5.631 by Oldenbourg Wissenschaftsverlag, München Effect of Amorphous Transformation on Electrochemical Capacities of Rare Earth Mg Based Alloys
Part III : Nucleation and growth. Module 4 : Growth of precipitates and kinetics of nucleation and growth. 4.1 Motivating question/phenomenon
 Part III : Nucleation and growth Module 4 : Growth of precipitates and kinetics of nucleation and growth 4.1 Motivating question/phenomenon In Figure. 20 we show, schematically, a morphology of precipitates
Part III : Nucleation and growth Module 4 : Growth of precipitates and kinetics of nucleation and growth 4.1 Motivating question/phenomenon In Figure. 20 we show, schematically, a morphology of precipitates
Best Practices in Formulation and Lyophilization Development Proteins, mabs and ADCs
 Best Practices in Formulation and Lyophilization Development Proteins, mabs and ADCs Best Practices in Formulation and Lyophilization Development The ultimate goal of formulation development is a stable
Best Practices in Formulation and Lyophilization Development Proteins, mabs and ADCs Best Practices in Formulation and Lyophilization Development The ultimate goal of formulation development is a stable
Garth J. Simpson Department of Chemistry Purdue University
 ULATRAFAST LASERS FOR THE DETECTION AND QUANTITATION OF TRACE CRYSTALLINITY IN AMORPHOUS FORMULATIONS BY SONICC Garth J. Simpson Department of Chemistry Purdue University 1 This document was presented
ULATRAFAST LASERS FOR THE DETECTION AND QUANTITATION OF TRACE CRYSTALLINITY IN AMORPHOUS FORMULATIONS BY SONICC Garth J. Simpson Department of Chemistry Purdue University 1 This document was presented
LEACHING OF ILMENITE AND PRE-OXIDIZED ILMENITE IN HYDROCHLORIC ACID TO OBTAIN HIGH GRADE TITANIUM DIOXIDE R. Vásquez, A. Molina
 LEACHING OF ILMENITE AND PRE-OXIDIZED ILMENITE IN HYDROCHLORIC ACID TO OBTAIN HIGH GRADE TITANIUM DIOXIDE R. Vásquez, A. Molina Material Science Department. Simon Bolivar University Caracas, Venezuela
LEACHING OF ILMENITE AND PRE-OXIDIZED ILMENITE IN HYDROCHLORIC ACID TO OBTAIN HIGH GRADE TITANIUM DIOXIDE R. Vásquez, A. Molina Material Science Department. Simon Bolivar University Caracas, Venezuela
PXRD INVESTIGATION OF CHANGES IN DEHYDRATION BEHAVIOR OF GSK HYDRATE AFTER MICRONIZATION
 PXRD INVESTIGATION OF CHANGES IN DEHYDRATION BEHAVIOR OF GSK241572 HYDRATE AFTER MICRONIZATION Feirong Kang Physical Properties Group - Pharmaceutical Development GlaxoSmithKline King of Prussia, PA This
PXRD INVESTIGATION OF CHANGES IN DEHYDRATION BEHAVIOR OF GSK241572 HYDRATE AFTER MICRONIZATION Feirong Kang Physical Properties Group - Pharmaceutical Development GlaxoSmithKline King of Prussia, PA This
EFFECT OFTHE TYPE OFCARBON MATERIALON THE REDUCTION KINETICS OF BARIUM SULFATE
 EFFECT OFTHE TYPE OFCARBON MATERIALON THE REDUCTION KINETICS OF BARIUM SULFATE M. Sh. Bafghi 1,*, A. Yarahmadi 2, A. Ahmadi 3 and H. Mehrjoo 3 * msbafghi@iust.ac.ir Received: April 2011 Accepted: July
EFFECT OFTHE TYPE OFCARBON MATERIALON THE REDUCTION KINETICS OF BARIUM SULFATE M. Sh. Bafghi 1,*, A. Yarahmadi 2, A. Ahmadi 3 and H. Mehrjoo 3 * msbafghi@iust.ac.ir Received: April 2011 Accepted: July
Effect of Ammonium Salt on Structural, Conductivity and Optical Properties of PVDF Thin Film Electrolytes
 International Journal of TechnoChem Research ISSN:2395-4248 www.technochemsai.com Vol.02, No.03, pp 239-243, 2016 Effect of Ammonium Salt on Structural, Conductivity and Optical Properties of PVDF Thin
International Journal of TechnoChem Research ISSN:2395-4248 www.technochemsai.com Vol.02, No.03, pp 239-243, 2016 Effect of Ammonium Salt on Structural, Conductivity and Optical Properties of PVDF Thin
Determination and use of moisture diffusion coefficient to characterize drying of northern red oak (Quercus rubra) *
 Wood Sci. Technol. 27:409-420 (1993) Wood Science and Technology Springer-Verlag 1993 Determination and use of moisture diffusion coefficient to characterize drying of northern red oak (Quercus rubra)
Wood Sci. Technol. 27:409-420 (1993) Wood Science and Technology Springer-Verlag 1993 Determination and use of moisture diffusion coefficient to characterize drying of northern red oak (Quercus rubra)
CHAPTER SEVEN EXPERIMENTAL RESULTS EFFECT OF THE STEEL S COMPOSITION 7.1 EFFECT OF ANNEALING TREATMENT ON STEEL B
 CHAPTER SEVEN EXPERIMENTAL RESULTS EFFECT OF THE STEEL S COMPOSITION 7 7.1 EFFECT OF ANNEALING TREATMENT ON STEEL B In order to understand the precipitation behaviour of the Laves phase more precisely,
CHAPTER SEVEN EXPERIMENTAL RESULTS EFFECT OF THE STEEL S COMPOSITION 7 7.1 EFFECT OF ANNEALING TREATMENT ON STEEL B In order to understand the precipitation behaviour of the Laves phase more precisely,
Hypoeutectoid Carbon Steels. Hypereutectoid Carbon Steels
 Hypoeutectoid Carbon Steels Another example: Amount of carbon? 1035 Steel: white regions are proeutectoid ferrite grains By the end of this lecture you should be able to predict the amount of carbon in
Hypoeutectoid Carbon Steels Another example: Amount of carbon? 1035 Steel: white regions are proeutectoid ferrite grains By the end of this lecture you should be able to predict the amount of carbon in
Crystallization Behavior of Polyamide-6 Microcellular Nanocomposites*
 Crystallization Behavior of Polyamide-6 Microcellular Nanocomposites* MINGJUN YUAN, LIH-SHENG TURNG, SHAOQIN GONG AND ANDREAS WINARDI Polymer Engineering Center Department of Mechanical Engineering University
Crystallization Behavior of Polyamide-6 Microcellular Nanocomposites* MINGJUN YUAN, LIH-SHENG TURNG, SHAOQIN GONG AND ANDREAS WINARDI Polymer Engineering Center Department of Mechanical Engineering University
CHAPTER 7 MICRO STRUCTURAL PROPERTIES OF CONCRETE WITH MANUFACTURED SAND
 99 CHAPTER 7 MICRO STRUCTURAL PROPERTIES OF CONCRETE WITH MANUFACTURED SAND 7.1 GENERAL Characterizing the mineralogy of the samples can be done in several ways. The SEM identifies the morphology of the
99 CHAPTER 7 MICRO STRUCTURAL PROPERTIES OF CONCRETE WITH MANUFACTURED SAND 7.1 GENERAL Characterizing the mineralogy of the samples can be done in several ways. The SEM identifies the morphology of the
Effect of biobased and biodegradable nucleating agent on the isothermal crystallization of poly(lactic acid)
 Korean J. Chem. Eng., 25(3), 599-608 (2008) SHORT COMMUNICATION Effect of biobased and biodegradable nucleating agent on the isothermal crystallization of poly(lactic acid) Kyung Su Kang, Sang Il Lee*,
Korean J. Chem. Eng., 25(3), 599-608 (2008) SHORT COMMUNICATION Effect of biobased and biodegradable nucleating agent on the isothermal crystallization of poly(lactic acid) Kyung Su Kang, Sang Il Lee*,
Annealing of Amorphous Sm 5 Fe 17 Melt-Spun Ribbon
 Materials Transactions, Vol. 49, No. 6 (2008) pp. 1446 to 1450 #2008 The Japan Institute of Metals Annealing of Amorphous Sm 5 Fe 17 Melt-Spun Ribbon Tetsuji Saito Department of Mechanical Science and
Materials Transactions, Vol. 49, No. 6 (2008) pp. 1446 to 1450 #2008 The Japan Institute of Metals Annealing of Amorphous Sm 5 Fe 17 Melt-Spun Ribbon Tetsuji Saito Department of Mechanical Science and
CHAPTER 4. SYNTHESIS OF ALUMINIUM SELENIDE (Al 2 Se 3 ) NANO PARTICLES, DEPOSITION AND CHARACTERIZATION
 40 CHAPTER 4 SYNTHESIS OF ALUMINIUM SELENIDE (Al 2 Se 3 ) NANO PARTICLES, DEPOSITION AND CHARACTERIZATION 4.1 INTRODUCTION Aluminium selenide is the chemical compound Al 2 Se 3 and has been used as a precursor
40 CHAPTER 4 SYNTHESIS OF ALUMINIUM SELENIDE (Al 2 Se 3 ) NANO PARTICLES, DEPOSITION AND CHARACTERIZATION 4.1 INTRODUCTION Aluminium selenide is the chemical compound Al 2 Se 3 and has been used as a precursor
CRYSTALLIZATION SOLUBILITY CURVE
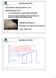 CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
A comparison of a novel single-pan calorimeter with a conventional heat-flux differential scanning calorimeter
 High Temperatures ^ High Pressures, 2, volume 32, pages 311 ^ 319 15 ECTP Proceedings pages 297 ^ 35 DOI:1.168/htwu26 A comparison of a novel single-pan calorimeter with a conventional heat-flux differential
High Temperatures ^ High Pressures, 2, volume 32, pages 311 ^ 319 15 ECTP Proceedings pages 297 ^ 35 DOI:1.168/htwu26 A comparison of a novel single-pan calorimeter with a conventional heat-flux differential
Analysis of Solute Distribution in Ice Formed in Progressive Freeze-concentration
 Food Sci. Technol. Res., 19 (3), 369 374, 2013 Analysis of Solute Distribution in Ice Formed in Progressive Freeze-concentration Mihiri Gunathilake, Kiyomi Shimmura and Osato Miyawaki * Department of Food
Food Sci. Technol. Res., 19 (3), 369 374, 2013 Analysis of Solute Distribution in Ice Formed in Progressive Freeze-concentration Mihiri Gunathilake, Kiyomi Shimmura and Osato Miyawaki * Department of Food
Polymorph Screening Strategies and a Concomitant Polymorph Case Study
 Polymorph Screening Strategies and a Concomitant Polymorph Case Study Aniruddh Andy Singh, Ph.D. Mahmoud Mirmehrabi, Ph.D., P.Eng. Johnson Matthey Pharma Services Chemical and Crystallization Research
Polymorph Screening Strategies and a Concomitant Polymorph Case Study Aniruddh Andy Singh, Ph.D. Mahmoud Mirmehrabi, Ph.D., P.Eng. Johnson Matthey Pharma Services Chemical and Crystallization Research
6. In this temperature time graph for the heating of H 2O at a constant rate, the segment DE represents the
 1. Which of the following contains particles with the least freedom of motion? A) CO 2( ) B) HCl(aq) C) F 2(g) D) MgBr 2(s) E) C 6H 12O 6(aq) 2. During boiling, the temperature of a pure liquid substance
1. Which of the following contains particles with the least freedom of motion? A) CO 2( ) B) HCl(aq) C) F 2(g) D) MgBr 2(s) E) C 6H 12O 6(aq) 2. During boiling, the temperature of a pure liquid substance
Membrane Technologies for Tritium Recovering in the Fusion Fuel Cycle
 Membrane Technologies for Tritium Recovering in the Fusion Fuel Cycle S. Tosti 1), L. Bettinali 1), C. Rizzello 2), V. Violante 1) 1) Euratom-ENEA Fusion Association, C. R. ENEA Frascati, 00044 Frascati
Membrane Technologies for Tritium Recovering in the Fusion Fuel Cycle S. Tosti 1), L. Bettinali 1), C. Rizzello 2), V. Violante 1) 1) Euratom-ENEA Fusion Association, C. R. ENEA Frascati, 00044 Frascati
Effect of phase separation on crystallization of glasses in the BaO TiO 2 SiO 2 system
 Paper Effect of phase separation on crystallization of glasses in the BaO TiO 2 SiO 2 system Hiroyuki HIJIYA, Tetsuo KISHI and Atsuo YASUMORI Department of Materials Science and Technology, Tokyo University
Paper Effect of phase separation on crystallization of glasses in the BaO TiO 2 SiO 2 system Hiroyuki HIJIYA, Tetsuo KISHI and Atsuo YASUMORI Department of Materials Science and Technology, Tokyo University
Laser Annealing of Amorphous Ni-Ti Shape Memory Alloy Thin Films
 Laser Annealing of Amorphous Ni-Ti Shape Memory Alloy Thin Films Xi Wang, Zhenyu Xue, Joost J. Vlassak Division of Engineering and Applied Sciences, Harvard University, Cambridge, MA, U.S.A. Yves Bellouard
Laser Annealing of Amorphous Ni-Ti Shape Memory Alloy Thin Films Xi Wang, Zhenyu Xue, Joost J. Vlassak Division of Engineering and Applied Sciences, Harvard University, Cambridge, MA, U.S.A. Yves Bellouard
Formation and Soft Magnetic Properties of Co Fe Si B Nb Bulk Glassy Alloys
 Materials Transactions, Vol. 43, No. 5 (2002) pp. 1230 to 1234 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Formation and Soft Magnetic Properties of Co Fe Si B Nb Bulk Glassy Alloys Akihisa
Materials Transactions, Vol. 43, No. 5 (2002) pp. 1230 to 1234 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Formation and Soft Magnetic Properties of Co Fe Si B Nb Bulk Glassy Alloys Akihisa
Soft Magnetic Properties of Nanocystalline Fe Si B Nb Cu Rod Alloys Obtained by Crystallization of Cast Amorphous Phase
 Materials Transactions, Vol. 43, No. 9 (2002) pp. 2337 to 2341 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Soft Magnetic Properties of Nanocystalline Fe Si B Nb Cu Rod Alloys Obtained
Materials Transactions, Vol. 43, No. 9 (2002) pp. 2337 to 2341 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Soft Magnetic Properties of Nanocystalline Fe Si B Nb Cu Rod Alloys Obtained
Effect Of Heat Treatments In The Silicon Eutectic Crystal Evolution In Al-Si Alloys A.Forn 1, Mª T. Baile 2, E. Martin 3 and E.
 Materials Science Forum Vols. 480-481 (2005) pp. 367-372 online at http://www.scientific.net 2005 Trans Tech Publications, Switzerland Effect Of Heat Treatments In The Silicon Eutectic Crystal Evolution
Materials Science Forum Vols. 480-481 (2005) pp. 367-372 online at http://www.scientific.net 2005 Trans Tech Publications, Switzerland Effect Of Heat Treatments In The Silicon Eutectic Crystal Evolution
Can Poly(ε-Caprolactone) crystals nucleate glassy Polylactide?
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2017 Supporting Information Can Poly(ε-Caprolactone) crystals nucleate glassy Polylactide? Matteo
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2017 Supporting Information Can Poly(ε-Caprolactone) crystals nucleate glassy Polylactide? Matteo
THE METHODS MATUSITA, KISSINGER AND OZAWA IN THE STUDY OF THE CRYSTALLIZATION OF GLASSES. THE CASE OF Ge-Sb-Te ALLOYS
 Chalcogenide Letters Vol. 4, No. 2, February 2007, p. 23-33 THE METHODS MATUSITA, KISSINGER AND OZAWA IN THE STUDY OF THE CRYSTALLIZATION OF GLASSES. THE CASE OF Ge-Sb-Te ALLOYS L. Heireche, M. Belhadji
Chalcogenide Letters Vol. 4, No. 2, February 2007, p. 23-33 THE METHODS MATUSITA, KISSINGER AND OZAWA IN THE STUDY OF THE CRYSTALLIZATION OF GLASSES. THE CASE OF Ge-Sb-Te ALLOYS L. Heireche, M. Belhadji
Equilibrium Relationships between Oxide Compounds in MgO Ti 2 O 3 Al 2 O 3 with Iron at K and Variations in Stable Oxides with Temperature
 , pp. 2012 2018 Equilibrium Relationships between xide Compounds in 2 3 2 3 with Iron at 1 873 K and Variations in Stable xides with Temperature Hideki N 1) and Toshio IBUTA 2) 1) Division of Materials
, pp. 2012 2018 Equilibrium Relationships between xide Compounds in 2 3 2 3 with Iron at 1 873 K and Variations in Stable xides with Temperature Hideki N 1) and Toshio IBUTA 2) 1) Division of Materials
Phase Transition Behavior of Nylon-66, Nylon-48, and Blends
 Polymer Journal, Vol. 35, No. 2, pp 173 177 (2003) Phase Transition Behavior of Nylon-66, Nylon-48, and Blends Gongzheng ZHANG,Takafumi WATANABE, Hirohisa YOSHIDA, and Tadashi KAWAI Graduate School of
Polymer Journal, Vol. 35, No. 2, pp 173 177 (2003) Phase Transition Behavior of Nylon-66, Nylon-48, and Blends Gongzheng ZHANG,Takafumi WATANABE, Hirohisa YOSHIDA, and Tadashi KAWAI Graduate School of
