SIGNIFICANT FIGURES WORKSHEET
|
|
|
- Marjory O’Brien’
- 6 years ago
- Views:
Transcription
1 SIGNIFICANT FIGURES WORKSHEET PART 1 - Determine the number of significant figures in the following numbers. 1.) ) ) ) ) 5,000 6.) 5, ) 6, ) ) ) 10,001 PART 2 Rewrite/round each of the following numbers so that it has 3 significant figures. 1.) ) x ) ) ) 10,800, ) ) ) 90,185 5.) x ) ROUNDING & SIGNIFICANT FIGURES WORKSHEET Perform the following operations expressing the answer with the correct number of significant figures. 1.) 1.35 m x m = 2.) 1,035 m 2 = 42 m 3.) cm x 3.2 cm x cm = 4.) 150 L 3 = 4 L 5.) mm x mm x mm = 6.) x 10 3 m 2 = x 10 2 m 7.) g g = 8.) ml ml + 6 ml = 9.) 0.15 cm cm cm = 10.) 505 kg kg = ============================================================= DENSITY PROBLEMS WORKSHEET (round your answers to the correct # of SFs) 1.) Determine the density of a rectangular piece of concrete that measures 3.7 cm by 2.1 cm by 5.8 cm and has a mass of 43.8 grams. 2.) Determine the density of a piece of granite that measures 5.02 cm by 1.35 cm by 2.78 cm and has a mass of grams. 3.) Determine the density of a brick in which grams occupies 4.01 cm 3.
2 4.) Compare the densities of the concrete (from #1), the granite (from #2), and the brick (from #3). Arrange them from lightest to heaviest. 5.) Gold has a density of g/cm 3. Find the mass of 6.39 cm 3 of gold. 6.) Determine the volume of 6.37 grams of magnesium if its density is 1.29 g/cm 3. 7.) Determine the volume of grams of iron if its density is 2.27 g/cm 3. 8.) A graduated cylinder contains 30.0 ml of water. An object is placed in the cylinder and the water level moves to 46.7 ml. Find the density if the mass of the object is grams. 9.) A ball has a mass of 6.03 kilograms and a volume of 1.57 cm 3. Find the density of the ball (in g/cm 3 ). 10.) A piece of wood has a mass of 5.75 grams and a volume of 0.95 cm 3. Find its density. ============================================================== UNIT CONVERSIONS WORKSHEET 1.) 360 g to μg 11.) m to Mm 2.) cg to g 12.) 3.80 dl to L 3.) cm 3 to ml 13.) x 10 7 m to km 4.) g to cg 14.) 30.2 μl to L 5.) 2.85 x 10 4 L to dm 3 15.) 4.06 x nm to m 6.) 41.5 ml to L 16.) 1.05 dm 3 to cm 3 7.) 281 cm 3 to L 17.) Mm to m 8.) L to dl 18.) 4.32 L to cm 3 9.) 61.2 ml to dm 3 19.) x 10-5 km to m 10.) L to ml 20.) 6.58 m to nm ============================================================= UNIT 1 REVIEW WORKSHEET Part 1 - Unit Conversions 1.) kg to g 2.) 2830 mm to m 3.) 19.3 L to cl 4.) 3.4 g to Mg 5.) 6.75 x 10 5 cm 3 to dm 3
3 Part 2 - Tell the number of significant figures in each of the following measurements. 6.) 48 cm 7.) g 8.) m 9.) K 10.) 3700 mm 11.) 400. cm 3 12.) g 13.) g Part 3 - Perform each of the following calculations, expressing the answer to the correct number of significant figures. 14.) cm cm cm = 15.) 8.3 m x 4.0 m x m = 16.) 4.93 mm 2 = mm 17.) ml - 32 ml = Part 4 - Percent Error 18.) Experimental value = 1.24 g, Accepted value = 1.30 g 19.) Experimental value = 22.2 L, Accepted value = 22.4 L 20.) A person attempting to lose weight on a diet weighed 175 lb on a bathroom scale at home. An hour later at the doctor's office, on a more accurate scale, this person's weight is recorded as 178 lb. Assuming that there was no real weight change in that hour, what is the percent error between these readings? Part 5 Density 21.) What is the mass of a sample of material that has a volume of 55.1 cm 3 and a density of 6.72 g/cm 3? 22.) A sample of a substance that has a density of g/ml has a mass of g. Calculate the volume of the sample.
4 INTRODUCTION Thickness of Aluminum Foil The density of a material is given by the formula D = m/v (Density = mass divided by Volume). From Geometry, it is known that the volume of a rectangular solid is length times width times height (V = l. w. h). It can be assumed that the thickness of the aluminum foil is the height. The density of aluminum can be looked up in a table. Therefore, using substitution and then rearranging the equation, one can solve for the height. PROCEDURE 1. Select four (4) rectangular pieces of aluminum foil. Each piece should be a different size. 2. Determine the mass of each piece of foil (in grams). Record these measurements in the data table below. 3. Carefully measure the length and the width of each piece of aluminum foil. (Make sure you use the metric side of the ruler!) Record these measurements in the data table also. 4. The accepted thickness of aluminum foil is cm for heavy duty aluminum foil. The density of aluminum is g/cm 3. DATA TABLE Trial Mass (g) Length (cm) Width (cm) Thickness (cm) Error (cm) Percent Error Average CALCULATIONS You must show sample calculations (in detail - for only one of your trials) for (A) thickness, (B) error, and (C) percent error. NOTE: error = accepted value experimental value ANALYSIS Under your sample calculations, write out one potential source of error* in the lab. * Errors in the measuring device or the reading of the measuring device are not acceptable. For example, "we read the ruler incorrectly." would NOT be an acceptable source of error. Your source of error should come from the lab procedure that you followed or assumptions that were made about the materials used. THIS LAB IS DUE ON: **CRITERIA IN ORDER TO RECEIVE CREDIT FOR THIS ASSIGNMENT: * All numbers are clear & legible. * All numbers are labeled with their correct units. * Calculations are shown in detail. * Calculations are shown in a neat and logical order. * No messy cross-outs or eraser marks. * Failure to follow these criteria will result in your having to re-submit your lab. It will be considered a late grade when you re-submit.
5 UNCERTAINTY IN MEASUREMENT REMINDERS: Always CLEARLY show all of your work (even if that means that you use another sheet of paper) & always include units and significant figures. * These measurements MUST be made IN CLASS! STATION #1 Determine the volume of one (1) penny using the water displacement method. Show your work. STATION #2 Determine the volume of the object by the overflow method. For precision, do the reading three different times for an average. * Trial 1 Average volume = * Trial 2 * Trial 3 STATION #3 Determine the density of copper. Use water displacement to determine the volume of the copper. Use the accepted value for the density of copper and calculate your percent error. * Volume of copper Determining % error: * Mass of copper Experimental density Density of copper Accepted density 8.92 g/ml (experimental) Error Percent Error - STATION #4 Determine the density of the wood. * Length * Mass * Width Volume * Height Density - STATION #5 Mass 50.0 ml of water and determine the percent error. * Mass of empty beaker Determining % error: * Mass of beaker ml water Experimental mass Mass of 50.0 ml of water Accepted mass Error Remember that 1.0 ml of water = 1.0 gram of water Percent Error
6 STATION #6 Determine the volume of a cylinder using the formula V = πr 2 h. Use this value as the "accepted volume". Then, fill the cylinder with water and measure its contents as accurately as possible. Use this value of the "experimental volume" to determine the percent error. Accepted Volume Experimental Volume * Diameter of cylinder * Volume of cylinder Radius of cylinder (r) Radius squared (r 2 ) * Height of cylinder (h) Volume of cylinder Remember that 1 ml = 1 cm 3. Percent Error Experimental volume Accepted volume Error Percent Error STATION #7 Mass the evaporating dish and watch glass to the correct number of significant figures. * Mass of evaporating dish and watch glass STATION #8 Mass one (1) level teaspoon of salt (NaCl) indirectly in an evaporating dish. * Mass of empty evaporating dish * Mass of dish + salt Mass of salt STATION #9 Compute the mass of the brick, given that the density of the brick is 1.92 g/cm 3. Brick Holes (Think... what shape are these holes?) * Length of brick * Height of holes * Width of brick * Diameter of holes * Height of brick Radius of holes Volume of brick Volume of holes without holes Actual volume of the brick Mass of brick - JUST TO EMPHASIZE MY POINT AGAIN... make sure that you show all of your work (even if it is adding or subtracting), always include units with your answer, and make sure your measurements and answers have the correct number of significant figures.
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter.
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter.
Density. The purpose of this experiment is to investigate the topic of density by determining the densities of some materials.
 Density Goals The purpose of this experiment is to investigate the topic of density by determining the densities of some materials. Introduction There is a lesson in the old bromide: Which is heavier,
Density Goals The purpose of this experiment is to investigate the topic of density by determining the densities of some materials. Introduction There is a lesson in the old bromide: Which is heavier,
Directions: Complete the following prior to class. Be prepared to show your work on the board.
 Worksheet L4X Student s Name Directions: Complete the following prior to class. Be prepared to show your work on the board. 1) Convert 30 ml to oz, qt, L, gal. 2) Convert 16 oz to kg, lb, tons, g. Page
Worksheet L4X Student s Name Directions: Complete the following prior to class. Be prepared to show your work on the board. 1) Convert 30 ml to oz, qt, L, gal. 2) Convert 16 oz to kg, lb, tons, g. Page
CHAPTER 2 HW PRACTICE SOLUTIONS
 CHAPTER 2 HW PRACTICE SOLUTIONS SIGNIFICANT FIGURES 1.) How many significant figures are shown in each of the following numbers? a. 0.0320 3 SF c. 503.10 5 SF e. 0.00702 3 SF b. 8000 1 SF d. 91,000,000
CHAPTER 2 HW PRACTICE SOLUTIONS SIGNIFICANT FIGURES 1.) How many significant figures are shown in each of the following numbers? a. 0.0320 3 SF c. 503.10 5 SF e. 0.00702 3 SF b. 8000 1 SF d. 91,000,000
Experiment: Measurements
 Experiment: Measurements I. INTRODUCTION Measurements are essential to experimental sciences such as chemistry, physics, biology, and geology. The measurements are usually made using the metric system
Experiment: Measurements I. INTRODUCTION Measurements are essential to experimental sciences such as chemistry, physics, biology, and geology. The measurements are usually made using the metric system
Density (d) is a property of a substance equal to the ratio of its mass (m) to its volume (V): d = m V
 Experiment 2 Name: DEN 14 Si TY In this experiment, you will learn about density and practice the proper way to report measurements and calculated quantities. Properties Properties of a substance can be
Experiment 2 Name: DEN 14 Si TY In this experiment, you will learn about density and practice the proper way to report measurements and calculated quantities. Properties Properties of a substance can be
Determining the Density of Unknown Substances
 Determining the Density of Unknown Substances Introduction: Most of the elements on the periodic table are metals and solids. These elements have observable properties that make it possible to identify
Determining the Density of Unknown Substances Introduction: Most of the elements on the periodic table are metals and solids. These elements have observable properties that make it possible to identify
M. It is expressed in units such a g/cc,
 Experiment 2 Density Part I: Density of a solid Density is defined as mass per unit volume, D = V M. It is expressed in units such a g/cc, g/ml, lb/ft 3, etc. Determination of mass is done by weighing
Experiment 2 Density Part I: Density of a solid Density is defined as mass per unit volume, D = V M. It is expressed in units such a g/cc, g/ml, lb/ft 3, etc. Determination of mass is done by weighing
Lab 0.4: Density and Thickness of Aluminum Foil
 Name Block Lab 0.4: Density and Thickness of Aluminum Foil Student will be able to: Correctly use measuring instruments with accuracy Correctly apply the principles of significant figures in measurements
Name Block Lab 0.4: Density and Thickness of Aluminum Foil Student will be able to: Correctly use measuring instruments with accuracy Correctly apply the principles of significant figures in measurements
Density What is density? How do you measure density?
 Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
Density Measurements Background
 Density Measurements Background The density (ρ) of a substance or an object is defined by its mass (m) divided by its volume (V):!"#$%&' =!"##!"#$%&!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! =!! The units used
Density Measurements Background The density (ρ) of a substance or an object is defined by its mass (m) divided by its volume (V):!"#$%&' =!"##!"#$%&!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! =!! The units used
Lab Section. Experiment 2 Density. Introduction
 Experiment 2 Density Introduction Density (given the symbol d or ρ depending upon which book you read) is an intrinsic property of materials. The term intrinsic means that it is independent upon the amount
Experiment 2 Density Introduction Density (given the symbol d or ρ depending upon which book you read) is an intrinsic property of materials. The term intrinsic means that it is independent upon the amount
DENSITY of Solids and Liquids
 Regents Earth Science Name: Date: Lab # DENSITY of Solids and Liquids Introduction: Density is the term used to describe the relationship between the mass of an object and its volume. Under given conditions
Regents Earth Science Name: Date: Lab # DENSITY of Solids and Liquids Introduction: Density is the term used to describe the relationship between the mass of an object and its volume. Under given conditions
T. Trimpe Lesson 1: Length
 T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
Copyright 2014 Science Doodles
 Copyright 2014 Science Doodles If a scientist comes across an unknown element, could she use density to identify the element? A. Yes, because each element has a specific density. B. No, because density
Copyright 2014 Science Doodles If a scientist comes across an unknown element, could she use density to identify the element? A. Yes, because each element has a specific density. B. No, because density
Topic 1 Review Questions Set 1
 Topic 1 Review Questions Set 1 Base your answers to questions 1-5 on your knowledge of Earth Science, the Earth Science Reference Tables, and the diagrams below. The diagrams represent three samples of
Topic 1 Review Questions Set 1 Base your answers to questions 1-5 on your knowledge of Earth Science, the Earth Science Reference Tables, and the diagrams below. The diagrams represent three samples of
Introduction to Density
 Introduction to Density INTRODUCTION Which is heavier, a pound of aluminum or a pound of lead? The answer, of course, is neither, but many people confuse the words "heavy" and "dense". "Heavy" refers to
Introduction to Density INTRODUCTION Which is heavier, a pound of aluminum or a pound of lead? The answer, of course, is neither, but many people confuse the words "heavy" and "dense". "Heavy" refers to
SOLIDS: Mass, Volume and Density Measurements
 CHEM 1411 Lab Solids: Mass, Volume, and Density 23 SOLIDS: Mass, Volume and Density Measurements A. Overview Review Sections 1.4-1.6 in your textbook (Chemistry: The Central Science, 9th Ed., Brown, LeMay,
CHEM 1411 Lab Solids: Mass, Volume, and Density 23 SOLIDS: Mass, Volume and Density Measurements A. Overview Review Sections 1.4-1.6 in your textbook (Chemistry: The Central Science, 9th Ed., Brown, LeMay,
Measurement and Density - Experiment 1
 Measurement and Density - Experiment 1 Purpose: The purpose of this experiment is to acquaint you with some common metric units of length, volume, and mass. You will also measure the density of an unknown
Measurement and Density - Experiment 1 Purpose: The purpose of this experiment is to acquaint you with some common metric units of length, volume, and mass. You will also measure the density of an unknown
T. Trimpe Lesson 1: Length
 T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
v = 5.00cm x 3.00cm x 2.00cm 2. What is the volume of a marble that has a mass of 3.0 g and a density of 2.7 g/ml? Formula (density) Substitution
 Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
Name Honors Chemistry / /
 Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Practice with Properties of Matter. Practice with Physical and Chemical Changes. Practice with Mixtures and Pure Substances
 Chapter 1 Practice Problems Practice with Properties of Matter Indicate whether each of the following statements describes a physical or a chemical property. a) the normal color of elemental bromine is
Chapter 1 Practice Problems Practice with Properties of Matter Indicate whether each of the following statements describes a physical or a chemical property. a) the normal color of elemental bromine is
2.3 Density and Density Calculations
 2.3 Density and Density Calculations When people say that lead is heavier than wood, they do not mean that a pea sized piece of lead weighs more than a truckload of pine logs. What they mean is that a
2.3 Density and Density Calculations When people say that lead is heavier than wood, they do not mean that a pea sized piece of lead weighs more than a truckload of pine logs. What they mean is that a
Experiment #3. Density
 Experiment #3. Density Goals 1. To measure and calculate the density of various substances. 2. To use significant figures correctly in calculations. Background Density is the mass per unit volume of a
Experiment #3. Density Goals 1. To measure and calculate the density of various substances. 2. To use significant figures correctly in calculations. Background Density is the mass per unit volume of a
Density. How tightly the atoms are packed together in an object
 Density How tightly the atoms are packed together in an object Mass vs Weight Mass is the amount of matter in an object. (it doesn t change) Weight is determined based on the amount of gravity pulling
Density How tightly the atoms are packed together in an object Mass vs Weight Mass is the amount of matter in an object. (it doesn t change) Weight is determined based on the amount of gravity pulling
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
DETERMINATION OF DENSITY
 DETERMINATION OF DENSITY Dr. Barbara B. Bunn. Adapted from Experiments in General Chemistry ; Wiley 1996J.G. Wardeska, T.T-S. Huang, R.W. Kopp; used by permission. Tested by Ms. Janice Orr s Chemistry
DETERMINATION OF DENSITY Dr. Barbara B. Bunn. Adapted from Experiments in General Chemistry ; Wiley 1996J.G. Wardeska, T.T-S. Huang, R.W. Kopp; used by permission. Tested by Ms. Janice Orr s Chemistry
This slide show has been prepared under fair use exemption of the U.S. Copyright Law and are restricted from further use.
 This slide show has been prepared under fair use exemption of the U.S. Copyright Law and are restricted from further use. Density & Dimensional Analysis Density - the volume which is occupied by a specific
This slide show has been prepared under fair use exemption of the U.S. Copyright Law and are restricted from further use. Density & Dimensional Analysis Density - the volume which is occupied by a specific
Experiment #3. Density and Specific Gravity.
 Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
EXPERIMENT 6. Determination of the Ideal Gas Law Constant - R. Magnesium metal reacts with hydrochloric acid according to the following reaction,
 EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
ES 106 Laboratory # 1 PROPERTIES OF WATER
 ES 106 Laboratory # 1 PROPERTIES OF WATER 1-1 Introduction What are the physical and chemical properties of water that make it so unique and necessary for living things? When you look at water, taste and
ES 106 Laboratory # 1 PROPERTIES OF WATER 1-1 Introduction What are the physical and chemical properties of water that make it so unique and necessary for living things? When you look at water, taste and
Practice Problems; Chapter#1
 Practice Problems; Chapter#1 #1. Determine the density of an object that has a mass of 149.8 g and displaces 12.1 ml of water when placed in a graduated cylinder. A) 8.08 g/ml B) 1.38 g/ml C) 12.4 g/ml
Practice Problems; Chapter#1 #1. Determine the density of an object that has a mass of 149.8 g and displaces 12.1 ml of water when placed in a graduated cylinder. A) 8.08 g/ml B) 1.38 g/ml C) 12.4 g/ml
Practice Problems; Chapter#1
 Practice Problems; Chapter#1 #1. Determine the density of an object that has a mass of 149.8 g and displaces 12.1 ml of water when placed in a graduated cylinder. A) 8.08 g/ml B) 1.38 g/ml C) 12.4 g/ml
Practice Problems; Chapter#1 #1. Determine the density of an object that has a mass of 149.8 g and displaces 12.1 ml of water when placed in a graduated cylinder. A) 8.08 g/ml B) 1.38 g/ml C) 12.4 g/ml
CHEMISTRY - CLUTCH CH.1 - INTRO TO GENERAL CHEMISTRY.
 !! www.clutchprep.com CONCEPT: MATTER Chemistry is the study of matter and the changes it undergoes, with the being its basic functional unit. When two or more of these elements chemically bond together
!! www.clutchprep.com CONCEPT: MATTER Chemistry is the study of matter and the changes it undergoes, with the being its basic functional unit. When two or more of these elements chemically bond together
CHEMISTRY - MCQUARRIE 4E CH.1 - CHEMISTRY & THE SCIENTIFIC METHOD.
 !! www.clutchprep.com CONCEPT: MATTER Chemistry is the study of matter and the changes it undergoes, with the being its basic functional unit. When two or more of these elements chemically bond together
!! www.clutchprep.com CONCEPT: MATTER Chemistry is the study of matter and the changes it undergoes, with the being its basic functional unit. When two or more of these elements chemically bond together
Quest Chapter 18. If you don t already know, check out the materials online, or read #4.
 1 (part 1 of 2) In everyday use, the word dense is often used interchangeably with the word hard. In physics, density and hardness have completely different meanings. Which object is the densest? 1. lead
1 (part 1 of 2) In everyday use, the word dense is often used interchangeably with the word hard. In physics, density and hardness have completely different meanings. Which object is the densest? 1. lead
Creation Settings. can be separated into two or more pure substances by distillation. can be separated into two or more substances by chromatography.
 Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
Page 1 of 10 TEST BANK (ACCT3321_201_1220) > CONTROL PANEL > POOL MANAGER > POOL CANVAS Pool Canvas Add, modify, and remove questions. Select a question type from the Add drop-down list and click Go to
S2i Sunday, September 8, 2012
 Agenda: 1. How to study for this course 2. Review of Significant Figures 3. Practice Problems S2i Sunday, September 8, 2012 How to Study for CHEM 212 DO THE HOMEWORK o Start it early in case you are having
Agenda: 1. How to study for this course 2. Review of Significant Figures 3. Practice Problems S2i Sunday, September 8, 2012 How to Study for CHEM 212 DO THE HOMEWORK o Start it early in case you are having
Appearance Conductivity test result Acid test result. of bubbles malleable
 ST Lab Pretest A- Metals, Metalloids, Non Metals 1. Complete the table. SUBSTANCE METAL METALLOID Appearance Conductivity test result Acid test result Light blinks constant stream Lustrous, of bubbles
ST Lab Pretest A- Metals, Metalloids, Non Metals 1. Complete the table. SUBSTANCE METAL METALLOID Appearance Conductivity test result Acid test result Light blinks constant stream Lustrous, of bubbles
ADDITIONAL PRACTICE WITH WRITING CONVERSION FACTORS
 ADDITIONAL PRACTICE WITH WRITING CONVERSION FACTORS Note: This worksheet is for additional practice. It is not due for a grade. Note: Unless specifically told otherwise, assume that percentage-by-mass
ADDITIONAL PRACTICE WITH WRITING CONVERSION FACTORS Note: This worksheet is for additional practice. It is not due for a grade. Note: Unless specifically told otherwise, assume that percentage-by-mass
Science 8. Unit 1. Booklet
 Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = grams]
![1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = grams] 1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = grams]](/thumbs/89/98796796.jpg) CHEMISTRY 51 SPRING 2016 NAME: Exam 1 Multiple Choice (2 pts each) 1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = 453.6 grams] a) 34.3 pounds b) 167 pounds c) 343 pounds d) 16.7 pounds
CHEMISTRY 51 SPRING 2016 NAME: Exam 1 Multiple Choice (2 pts each) 1) An object weighs 75.7 kg. What is this weight in lbs? [1 lb = 453.6 grams] a) 34.3 pounds b) 167 pounds c) 343 pounds d) 16.7 pounds
Cu (s) Cu 2+ (aq) Cu(OH) 2 (s) CuO (s) Cu 2+ (aq) Cu (s)
 Cycle of Copper Reactions Lab Exercise The following is a protocol for the multi-step transformation of copper metal based upon the following chemical transformations: Cu (s) Cu 2+ (aq) Cu(OH) 2 (s) CuO
Cycle of Copper Reactions Lab Exercise The following is a protocol for the multi-step transformation of copper metal based upon the following chemical transformations: Cu (s) Cu 2+ (aq) Cu(OH) 2 (s) CuO
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example,
 SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
There are 2 different types of numbers Exact -Measured
 Significant Figures When measuring or using our calculators we must determine the correct answer; our calculators are mindless drones and don t know the correct answer. There are 2 different types of numbers
Significant Figures When measuring or using our calculators we must determine the correct answer; our calculators are mindless drones and don t know the correct answer. There are 2 different types of numbers
Calculating Ballast By Troy Averill
 Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
Density Answers to the End of module questions
 IDS 101 Density Answers to the End of module questions 1. You are experimenting with the mysterious substance X. You carefully measure the mass of a uniform 2 ml sample of pure substance X. You slice it
IDS 101 Density Answers to the End of module questions 1. You are experimenting with the mysterious substance X. You carefully measure the mass of a uniform 2 ml sample of pure substance X. You slice it
NOTE: If substances have the SAME VOLUME, they can have DIFFERENT MASSES.
 Grade 8 SCIENCE Chapter 8 questions Page 306 1. When particles are CLOSER TOGETHER, DENSITY is GREATER. 2. Particles in SOLIDS have FEWER SPACES between them. The ATTRACTIVE FORCES between the particles
Grade 8 SCIENCE Chapter 8 questions Page 306 1. When particles are CLOSER TOGETHER, DENSITY is GREATER. 2. Particles in SOLIDS have FEWER SPACES between them. The ATTRACTIVE FORCES between the particles
to water. You add 1 drop of phenolphthalein and the solution turns pink. This observation tells you Na 2
 1A End of Lab Questions Notes 9/11/18 Lab 4 1 You add 100 g of to water You add 1 drop of phenolphthalein and the solution turns pink This observation tells you is a base 2 You add vinegar (09 M acetic
1A End of Lab Questions Notes 9/11/18 Lab 4 1 You add 100 g of to water You add 1 drop of phenolphthalein and the solution turns pink This observation tells you is a base 2 You add vinegar (09 M acetic
Unit-2. Properties of Matter. Solutions 2.1 Matter & Density page V 1 =4.3.6=72 cm 3. Volume of the removed triangular prism (V 2 )
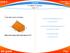 2.1 & Density page - 61 1. A solid object is given in the figure. Volume of the rectangular prism (V 1 ) V 1 =4.3.6=72 cm 3 Volume of the removed triangular prism (V 2 ) V 2 =((2.3)/2).6=18 cm 3 What is
2.1 & Density page - 61 1. A solid object is given in the figure. Volume of the rectangular prism (V 1 ) V 1 =4.3.6=72 cm 3 Volume of the removed triangular prism (V 2 ) V 2 =((2.3)/2).6=18 cm 3 What is
Chapter 01 Conversion factors and Algebra Worksheet (KEY)
 Chapter 01 Conversion factors and Algebra Worksheet (KEY) 1. Perform the following calculations. a. 29.875 g (3.675 cm 3 11.5 g cm 3) (9.223 g cm 3 11.5 g = cm 3) a. b. b. 14.23 ml (19.30 g ml 11.74 g
Chapter 01 Conversion factors and Algebra Worksheet (KEY) 1. Perform the following calculations. a. 29.875 g (3.675 cm 3 11.5 g cm 3) (9.223 g cm 3 11.5 g = cm 3) a. b. b. 14.23 ml (19.30 g ml 11.74 g
Chemistry Attitudes, Skills, & Knowledge Survey (CASKS) Form 3
 Chemistry Attitudes, Skills, & Knowledge Survey (CASKS) Form 3 Directions to Students: Do not open this booklet until you are told to do so. Please respond to the following items by marking the best answer
Chemistry Attitudes, Skills, & Knowledge Survey (CASKS) Form 3 Directions to Students: Do not open this booklet until you are told to do so. Please respond to the following items by marking the best answer
Chemistry: The Central Science, 12e (Brown et al.) Chapter 1 Introduction: Matter and Measurement. 1.1 Multiple-Choice Questions
 Chemistry: The Central Science, 12e (Brown et al.) Chapter 1 Introduction: Matter and Measurement 1.1 Multiple-Choice Questions 1) In the following list, only is not an example of matter. A) planets B)
Chemistry: The Central Science, 12e (Brown et al.) Chapter 1 Introduction: Matter and Measurement 1.1 Multiple-Choice Questions 1) In the following list, only is not an example of matter. A) planets B)
Geometric formulas for volume include: Where r is the radius of the cylinder and h is the height. Volume of a rectangular prism = LxWxH
 Supplement Density The density of an object is defined as its mass per unit of volume. The formula below may be used to calculate the density of an object: Density = mass/volume Volume may be measured
Supplement Density The density of an object is defined as its mass per unit of volume. The formula below may be used to calculate the density of an object: Density = mass/volume Volume may be measured
Objective: Classify metals, nonmetals, and metalloids based on their properties.
 Do Now Date: September 2, 2014 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Do Now Date: September 2, 2014 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Total Grade /150 Checked by
 FIRST LETTER OF YOUR LAST NAME CHEMISTRY 1127 EXAM I NAME (PRINT) SECTION SIGNATURE TA PLEASE READ THE FOLLOWING INSTRUCTIONS Do NOT begin the exam until asked to do so. There are 8 numbered pages, a useful
FIRST LETTER OF YOUR LAST NAME CHEMISTRY 1127 EXAM I NAME (PRINT) SECTION SIGNATURE TA PLEASE READ THE FOLLOWING INSTRUCTIONS Do NOT begin the exam until asked to do so. There are 8 numbered pages, a useful
MATHEMATICS Y4 Measuring 4602 Units to estimate or measure length, mass or capacity. Equipment MathsGoGoGo
 MATHEMATICS Y4 Measuring 4602 Units to estimate or measure length, mass or capacity. Equipment Paper, pencil, ruler Various measuring instruments, balance scales Maths Go Go Go 4602 Units to estimate and
MATHEMATICS Y4 Measuring 4602 Units to estimate or measure length, mass or capacity. Equipment Paper, pencil, ruler Various measuring instruments, balance scales Maths Go Go Go 4602 Units to estimate and
CHEM 10113, Quiz 1 August 31, All answers must use the correct number of significant figures, and must show units!
 CHEM 10113, Quiz 1 August 31, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! 1. (2 points) SHOW ALL WORK. A 34.6 g bar of aluminum occupies
CHEM 10113, Quiz 1 August 31, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! 1. (2 points) SHOW ALL WORK. A 34.6 g bar of aluminum occupies
CHAPTER 1. Conversions. General Plan for Converting Measurements. State the relationship between the unit given and the unit sought as an equality.
 CHAPTER 1 Conversions One of the aims of chemistry is to describe changes to tell what changed, how it changed, and what it changed into. Another aim of chemistry is to look at matter and its changes and
CHAPTER 1 Conversions One of the aims of chemistry is to describe changes to tell what changed, how it changed, and what it changed into. Another aim of chemistry is to look at matter and its changes and
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley)
 Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
The following are the completed but unbalanced equations. Each equation is numbered to match each step of the cycle:
 REACTIONS OF COPPER Copper will undergo many types of reactions. In this experiment you will observe a sequence of copper reactions. The sequence begins with copper metal and ends with copper metal, so
REACTIONS OF COPPER Copper will undergo many types of reactions. In this experiment you will observe a sequence of copper reactions. The sequence begins with copper metal and ends with copper metal, so
Objective: Classify metals, nonmetals, and metalloids based on their properties.
 Do Now Date: September 6, 2016 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday, September
Do Now Date: September 6, 2016 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday, September
Experiment 3: Determination of an Empirical Formula
 Background Information The composition of a compound is defined by its chemical formula, which gives the number ratio of the different elements in the compound. For example, water has a fixed composition
Background Information The composition of a compound is defined by its chemical formula, which gives the number ratio of the different elements in the compound. For example, water has a fixed composition
GEL Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Grade: /25
 GEL 4250 - Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Name: Section: Grade: /25 COMPLETE & TURN IN ONLY PAGES THAT HAVE A FIELD FOR YOUR NAME. ALL OTHER PAGES
GEL 4250 - Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Name: Section: Grade: /25 COMPLETE & TURN IN ONLY PAGES THAT HAVE A FIELD FOR YOUR NAME. ALL OTHER PAGES
Activity of metals SCIENTIFIC. Demonstration and Inquiry. Introduction. Concepts. Background. Inquiry Approach. Demonstration Questions
 Activity of Metals Demonstration and Inquiry SCIENTIFIC Introduction Chemical reactions are not formulas on a piece of paper they are dynamic and exciting events! The demonstration of aluminum with copper(ii)
Activity of Metals Demonstration and Inquiry SCIENTIFIC Introduction Chemical reactions are not formulas on a piece of paper they are dynamic and exciting events! The demonstration of aluminum with copper(ii)
Chem 5, Spring 2016 Exam 1 (Chapter 1 and 2)
 Chem 5, Spring 2016 Exam 1 (Chapter 1 and 2) NAME 95 pt Mark the answers for Questions 1-41 on your Scantron. Each Question is worth 2 pt. In some cases you will be asked to mark more than one answer.
Chem 5, Spring 2016 Exam 1 (Chapter 1 and 2) NAME 95 pt Mark the answers for Questions 1-41 on your Scantron. Each Question is worth 2 pt. In some cases you will be asked to mark more than one answer.
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
Name Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS located
CHM101 Lab - Energy Grading Rubric
 Name Team Name CHM101 Lab - Energy Grading Rubric To participate in this lab you must have splash- proof goggles, proper shoes and attire. Criteria Points possible Points earned Lab Performance Printed
Name Team Name CHM101 Lab - Energy Grading Rubric To participate in this lab you must have splash- proof goggles, proper shoes and attire. Criteria Points possible Points earned Lab Performance Printed
Objective: Classify metals, nonmetals, and metalloids based on their properties.
 Do Now Date: September 8, 2015 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Do Now Date: September 8, 2015 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
D =? D = M = 10 g = 5 g/cm 3 M = 10 g V 2 cm 3 V = 2 cm 3 5 g/cm 3
 Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Chapters 1&2 Practice Problems
 Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Name Chapters 1&2 Practice Problems Multiple Choice No Calculators Allowed! 1. Which physical state of matter exhibits the greatest change in volume with changes in temperature or pressure? a) solid b)
Name # Date Period! FINDING&VOLUME&AND&DENSITY& !!! !!!!!!!
 Name # Date Period FINDING&VOLUME&AND&DENSITY& TherectangularshapedboxinFigure1isfilledwithdrysand.Findthe volumeanddensityofthesand. 1.Firstfindthevolumeofthebox.Thisisalsothevolumeofthesand. Usethediagramtofindthedimensionsofthebox.
Name # Date Period FINDING&VOLUME&AND&DENSITY& TherectangularshapedboxinFigure1isfilledwithdrysand.Findthe volumeanddensityofthesand. 1.Firstfindthevolumeofthebox.Thisisalsothevolumeofthesand. Usethediagramtofindthedimensionsofthebox.
EXPERIMENT 5 Chemistry 110 COMPOSITION OF A MIXTURE
 EXPERIMENT 5 Chemistry 110 PURPOSE: The purpose of this experiment is to determine the percent composition of a mixture. COMPOSITION OF A MIXTURE Most matter is a mixture of many substances. For example,
EXPERIMENT 5 Chemistry 110 PURPOSE: The purpose of this experiment is to determine the percent composition of a mixture. COMPOSITION OF A MIXTURE Most matter is a mixture of many substances. For example,
Forensics with TI-Nspire Technology
 Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com Science Objectives Identify characteristics of different soils to demonstrate that a suspect has been at a scene.
Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com Science Objectives Identify characteristics of different soils to demonstrate that a suspect has been at a scene.
Lesson 26: Volume and Surface Area
 NYS COMMON CORE MATHEMATICS CURRICULUM Lesson 6 7 3 Student Outcomes Students solve real-world and mathematical problems involving volume and surface areas of threedimensional objects composed of cubes
NYS COMMON CORE MATHEMATICS CURRICULUM Lesson 6 7 3 Student Outcomes Students solve real-world and mathematical problems involving volume and surface areas of threedimensional objects composed of cubes
Science Physical Science Grades 6 and 8
 Science Physical Science Grades 6 and 8 Subject: Physical Science: Thermal Energy Level: Grade 6 and 8 The Hot in the Cold Abstract: Students will conduct an experiment and collect data on the exchange
Science Physical Science Grades 6 and 8 Subject: Physical Science: Thermal Energy Level: Grade 6 and 8 The Hot in the Cold Abstract: Students will conduct an experiment and collect data on the exchange
Answer the pre-lab questions that appear at the end of this lab exercise.
 Experiment 3 Enery and Heat Capacity Pre-lab Assinment Before comin to lab: Read the lab thorouhly. Answer the pre-lab questions that appear at the end of this lab exercise. Backround The oal of this lab
Experiment 3 Enery and Heat Capacity Pre-lab Assinment Before comin to lab: Read the lab thorouhly. Answer the pre-lab questions that appear at the end of this lab exercise. Backround The oal of this lab
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter.
 1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
Overview. Learning Objectives: This module provides step-by-step instructions in how to do the Bulk Density Protocol.
 Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
Assignment 01 (A) 5- How many µg are in g? a) 1.34 x 10 4 b) c) 1.34 d) e) (1 gram = micrograms.
 Assignment 01 (A) 1- Which of the following is a pure substance? a) air b) nitrogen c) blue-cheese salad dressing d) concrete (It contains only a single element or substance.) 2- Passing an electric current
Assignment 01 (A) 1- Which of the following is a pure substance? a) air b) nitrogen c) blue-cheese salad dressing d) concrete (It contains only a single element or substance.) 2- Passing an electric current
LESSON hat is density? 24
 LESSON hat is density? 24 Rocks are heavy. A small box of rocks has a large. Yet a box of the same size containing corks has a small. How can objects that are the same size have different es? Scientists
LESSON hat is density? 24 Rocks are heavy. A small box of rocks has a large. Yet a box of the same size containing corks has a small. How can objects that are the same size have different es? Scientists
Duncan. UNIT 8 - Chemical Equations BALANCING EQUATIONS PRACTICE WORKSHEET 14.) C2H6 + O2 CO2 + H2O. 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.
 BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
DRIP, DRIP, DRIP OR THE CASE OF
 DRIP, DRIP, DRIP OR THE CASE OF THE LEAKY FAUCET Outcome (lesson objective) Students will gather scientific data and be able to utilize the data to generalize the results over a long period of time. Student/Class
DRIP, DRIP, DRIP OR THE CASE OF THE LEAKY FAUCET Outcome (lesson objective) Students will gather scientific data and be able to utilize the data to generalize the results over a long period of time. Student/Class
Recycling Paper. Prepared By: Şenay Purzer, Venkatesh Merwade, Brad Harriger, David Eichinger, and Erin Doherty
 Recycling Paper Grade Level: 5 Total Time Required: approximately five 45-minute sessions Prepared By: Şenay Purzer, Venkatesh Merwade, Brad Harriger, David Eichinger, and Erin Doherty Lesson Objectives:
Recycling Paper Grade Level: 5 Total Time Required: approximately five 45-minute sessions Prepared By: Şenay Purzer, Venkatesh Merwade, Brad Harriger, David Eichinger, and Erin Doherty Lesson Objectives:
ENV 4001: ENVIRONMENTAL SYSTEMS ENGINEERING. University of South Florida Civil & Environmental Eng.
 ENV 4001: ENVIRONMENTAL SYSTEMS ENGINEERING Fall 2017 Quiz #1 Wednesday, September 27 University of South Florida Civil & Environmental Eng. Prof. J.A. Cunningham Instructions: 1. You may read these instructions,
ENV 4001: ENVIRONMENTAL SYSTEMS ENGINEERING Fall 2017 Quiz #1 Wednesday, September 27 University of South Florida Civil & Environmental Eng. Prof. J.A. Cunningham Instructions: 1. You may read these instructions,
Specific Heat. q = csm T (1)
 INTRODUCTION Just as people can be identified by their appearance and their behavior, substances are described and identified by their physical (appearance) and chemical (behavior) properties. Physical
INTRODUCTION Just as people can be identified by their appearance and their behavior, substances are described and identified by their physical (appearance) and chemical (behavior) properties. Physical
Density Determi nations
 f EXPERIMENT 3 Density Determi nations OBJECTIVE Density is an important characteristic property of matter, and may be used as a method of identification. In this experiment, you will determine the densities
f EXPERIMENT 3 Density Determi nations OBJECTIVE Density is an important characteristic property of matter, and may be used as a method of identification. In this experiment, you will determine the densities
Copper Odyssey. Chemical Reactions of Copper
 Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
TEST I REVIEW. 1. All of the following are properties of antimony. Which one is not a
 TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
Part II. [24 pts] Problems [6 pts] How many ml of soda are there in a bottle that contains 16 liq oz?
![Part II. [24 pts] Problems [6 pts] How many ml of soda are there in a bottle that contains 16 liq oz? Part II. [24 pts] Problems [6 pts] How many ml of soda are there in a bottle that contains 16 liq oz?](/thumbs/96/129255969.jpg) CHM 1030 Examination 1A January 30, 2002 SOME GENERAL INFORMATION 1 in = 2.54 cm 1 mile = 5280 ft 16 oz = 1 lb = 453.6 g 32 liq oz = 1 qt = 0.25 gal = 0.946 L speed of light (c) = 2.998 10 8 m/s 1 cal
CHM 1030 Examination 1A January 30, 2002 SOME GENERAL INFORMATION 1 in = 2.54 cm 1 mile = 5280 ft 16 oz = 1 lb = 453.6 g 32 liq oz = 1 qt = 0.25 gal = 0.946 L speed of light (c) = 2.998 10 8 m/s 1 cal
The Varying Density of Sea Water Based on
 Based on http://www.maine.gov/doc/nrimc/mgs/education/lessons/act37.htm Focus on Inquiry The student will collect and analyze data to determine the effects of temperature and dissolved on sea water. Lesson
Based on http://www.maine.gov/doc/nrimc/mgs/education/lessons/act37.htm Focus on Inquiry The student will collect and analyze data to determine the effects of temperature and dissolved on sea water. Lesson
PHARMACOPOEIAL DISCUSSION GROUP BULK AND TAPPED DENSITY OF SOLIDS
 PHARMACOPOEIAL DISCUSSION GROUP BULK AND TAPPED DENSITY OF SOLIDS It is understood that sign-off covers the technical content of the draft and each party will adapt it as necessary to conform to the usual
PHARMACOPOEIAL DISCUSSION GROUP BULK AND TAPPED DENSITY OF SOLIDS It is understood that sign-off covers the technical content of the draft and each party will adapt it as necessary to conform to the usual
Refer to the Appendices at the back of your lab manual to review laboratory techniques, significant figures and statistical treatment of data.
 LABORATORY EXPERIMENT A: Volume of a Solid Cylinder PRELABORATORY ASSIGNMENT: Refer to the Appendices at the back of your lab manual to review laboratory techniques, sinificant fiures and statistical treatment
LABORATORY EXPERIMENT A: Volume of a Solid Cylinder PRELABORATORY ASSIGNMENT: Refer to the Appendices at the back of your lab manual to review laboratory techniques, sinificant fiures and statistical treatment
Basic Measuring and Calculations. Formulas, Workings Hints and Tips
 Basic Measuring and Calculations Formulas, Workings Hints and Tips Start by writing down the formula When working out mathematics Ensure you show all working out (Marks will be awarded for working out)
Basic Measuring and Calculations Formulas, Workings Hints and Tips Start by writing down the formula When working out mathematics Ensure you show all working out (Marks will be awarded for working out)
Total Dissolved Solids
 Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids LabQuest 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Math for Water Operators
 Math for Water Operators 1 Remember the Three Rules for Conquering Math Always look up the proper formula This means disregarding all the numbers and recognizing the type of problem. Write it down Always
Math for Water Operators 1 Remember the Three Rules for Conquering Math Always look up the proper formula This means disregarding all the numbers and recognizing the type of problem. Write it down Always
LAB II CRYSTAL STRUCTURE AND CRYSTAL GROWTH PART 1: CRYSTAL GROWTH. I. Introduction
 LAB II CRYSTAL STRUCTURE AND CRYSTAL GROWTH This lab will be divided into two parts. In the first part, you will be growing crystals from a seed crystal in a very visual demonstration of heterogeneous
LAB II CRYSTAL STRUCTURE AND CRYSTAL GROWTH This lab will be divided into two parts. In the first part, you will be growing crystals from a seed crystal in a very visual demonstration of heterogeneous
Lab 1: Tools for Biologists
 Biology 107 General Biology Lab 1: Tools for Biologists Scientists use a variety of tools to carry out laboratory experiments and to understand and manipulate data. Students need to develop familiarity
Biology 107 General Biology Lab 1: Tools for Biologists Scientists use a variety of tools to carry out laboratory experiments and to understand and manipulate data. Students need to develop familiarity
