Melting Range 3. Melting Range
|
|
|
- Josephine Barber
- 6 years ago
- Views:
Transcription
1 Melting Range 3 Melting Range Background Information The melting range of a pure solid organic is the temperature range at which the solid is in equilibrium with its liquid. As heat is added to a solid, the solid eventually changes to a liquid. This occurs as molecules acquire enough energy to overcome the intermolecular forces previously binding them together in an orderly crystalline lattice. Melting does not occur instantaneously, because molecules must absorb the energy and then physically break the binding forces. Typically the outside of a crystal will melt faster than the inside, because it takes time for heat to penetrate. (Imagine an ice cube melting from the outside in, and not doing so instantly ) The melting range of a compound is one of the characteristic properties of a pure solid. The melting range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid. Most pure organics melt over a narrow temperature range of 1-2ºC, if heated slowly enough. Impure samples will normally have melting ranges that are both larger (>1ºC) and begin lower. Taking the melting range of a sample is useful for two reasons: 1. Identification of an unknown sample (compare it s observed melting range with that of known compounds) 2. Assessment of sample purity for a known substance. By comparing observed range for an actual sample to the known range for a pure sample, you can tell whether your actual sample is pure or contaminated (the range is depressed and broadened) The presence of impurity has two effects on a substance s melting range: 1. Melting range depression (lower end of the range drops) 2. Melting range broadening (the range simply increases. Often the low end drops a lot, the high end less so or sometimes not much at all.) A melting range of 5º or more indicates that a compound is impure. Why? The reason for this depression/broadening is that contaminants disrupt the consistency and organization of the crystal lattice at the molecular level. Contaminants don t fit correctly into what would be the normal pure lattice. How does this manifest itself? 1. The disruption weakens the lattice, so that the lattice can be broken down more easily; the weakened structure melts more easily at reduced temperature (depression). 2. Disruption of the lattice makes it non-uniform. At the molecular level, the molecules closest to the impurities melt fastest. Further away from the impurities, the crystal lattice is relatively undisturbed and therefore melts at or nearer the normal temperature. Miscellaneous notes on melting range depression/broadening: 1. Only soluble impurities, which are incorporated into the crystal structure at the molecular level, cause depression and broadening. An insoluble piece of metal or wood ionic salt crystal has negligible effect, because only a few organic molecules will be in contact and will be affected. 2. At the chemical level, it is impossible to raise the melting point of an already pure substance. It s melting point can be depressed by contamination, but not raised. Practical: If the melting point for an unknown sample is observed to be in between that of two candidates whose pure mp s are known, the unknown can t actually be equal to the lower-melting candidate. (Short of the rapid-heating effect, see later.) Most likely the unknown sample is an impure version of the higher melting candidate. For example:
2 Melting Range 4 suppose an unknown sample X melts at º, and is thought to be either candidate A (known range is º) or B (known range is º). Sample X cannot be candidate A, but it can be an impure and thus depressed version of candidate B. 3. Often contaminated solids are purified by recrystallization. If the resulting melting range is unchanged, the original sample probably was pure to begin with. But if the resulting melting point gets higher, the original sample was obviously impure. 4. When crystals are isolated by filtration from a solvent, it is important to allow complete drying/evaporation of the solvent in order to get a good melting range. Residual solvent functions as a contaminant and will depress/broaden the melting range for a crystal. 5. When two chemicals are mixed, the resulting melting point is not the average of the two mp s. It is always depressed from the melting point of the major component in the mixture. This is true even if the impurity is higher melting (when pure) than the major component. For example, if a chemical that normally melts at 130º is contaminated by a small amount of material that when pure melts at 200º, the resulting mixture will not melt between 130º and 200º. Rather, the melting point of the major component will be depressed, and the observed melting range will begin lower than 130º. 6. Even when two chemicals with exactly the same melting point when pure are mixed, the resulting melting point is depressed. Mixed Melting Points That mixtures have depressed melting points, even when both components have comparable melting points when each is pure, provides a useful laboratory technique. Consider the following situation and flow chart. If an unknown candidate X melts at a temperature close to that of two potential candidates A and B, you can identify it by taking X+A mixed melting point, and X+B mixed melting point. If X is equal to either candidate, one of these mixed melting points will not be depressed. If the mixture with X+A is not depressed, X = A. if the mixture with X+B is not depressed, X = B. If both mixtures are depressed, then X A or B. unknown X: mp = Candidate A=benzoin mp = Candidate B = cinnamic acid mp = Does X = A, or does X = B, or is neither correct? mix X with A, and take resulting melting point Observed mp = Conclusion: X = benzoin Observed mp < 133 Conclusion: X benzoin mix X with B, and take resulting melting point Observed mp = Conclusion: X = cinnamic acid Observed mp < 133 Conclusion: X cinnamic acid
3 Melting Range 5 The Rate of Heating, and Some Practical Tips It takes time for a crystal to absorb heat and to melt, from the outside in. Just as when you place an ice-cube into a liquid that is >0º, it doesn t melt instantly. To get maximal accuracy in taking a melting range, heating should proceed at only 1º/minute! This is the standard heating rate when publishing melting ranges in scientific journals. This is also inconveniently slow, especially if you don t know where your sample is likely to melt (as when examining an unknown). Q: What happens if I heat too fast? A: Your melting range will be too broad, but this time you will see inflation on the high end! If a sample should melt at º, but you are heating fast, it will still probable begin to melt at about 130º, but the full sample won t have time to absorb heat and finish melting by 131º. Instead, the heating device may have warmed well above 131º before the interior liquefies, so the observed range may appear to be º. Both the magnitude of the range and the high end of the range may be misleading. For doing routine samples, it is appropriate to be warming at 5 degrees per minute around the temperature at which melting occurs. This broadens the range somewhat, but not badly. And it keeps the melting point experiment from taking forever. Practical tip 1: If the approximate temperature at which your sample should melt is known, the sample can be quickly heated to within 10-15º of its melting point. Then the heating rate can be slowed to 2-4º per minute until the sample melts. For example, if you know your material should melt around 180º, but you are just trying to check the purity, you can heat rapidly until you are up to 165º or so, and only when you are getting close turn the heating rate down. Practical tip 2: If you have no clue where your sample will melt, it s common to heat rapidly to get a ballpark estimate of where melting will occur. 60º? 140º? 240º? If it turns out to be 240º and you heated only cautiously from the beginning, it will take a looooong time to get to the measurement. By heating rapidly, you can get an orientation melting point quickly, and then repeat with more care for a more precise reading. Often you don t even need to prepare a fresh sample, because after cooling the melted sample often recrystallizes. Practical tip 3: Course versus finely ground. Heat transfer problems are minimized if the sample is ground finely. If the particles are too coarse, they do not pack as well, causing air pockets that slow heat transfer. Because the thermometer keeps heating while the sample is melting rather slowly, the high end of your range will be inflated. Practical tip 4: Loading too much sample makes it harder for the interior to get heated and melted. Because the thermometer keeps heating while the sample is melting rather slowly, the high end of your range will be inflated. Sagging Sometimes slight changes, such as shrinking and sagging, occur in the crystalline structure of the sample before melting occurs. The temperature at the bottom end of the melting range corresponds to the first appearance of liquid within the sample mixture; if the crystals are changing their appearance, but you don t yet see any actual liquid, you should not record this as the lower end of the melting range yet.
4 Melting Range 6 The Experiment: (Work alone or with One Partner) Overview, if working with a partner: You will run three samples. 1. One will be either pure urea (mp = ) or pure cinnamic acid (mp = ). Whichever you run should be the opposite of what your partner runs. Record your range, and share your observed results with your partner 2. The second will be mixture of the two, either 4:1 cinnamic acid:urea or 1:4 cinnamic acid:urea. Whichever mixture you run should be the opposite of the mixture that your partner runs. Record your range, and share your observed results with your partner. 3. The third will be an unknown. Record your range and identify which of the unknown candidates is really yours. (You and partner must run different unknowns. You do not need to share this result with your partner.) Unknown Candidates Acetanilide Benzoic Acid Cinnamic acid Salicylic acid Sulfanilamide Succinic acid Learning goals: Learn how to run a melting point device and measure melting range By comparing results for the two mixtures, see how not all mixtures depress/broaden to the same extent. Identify your unknown from the list shown below. If working alone: You will run five samples. 1. Run both pure urea (mp = ) and pure cinnamic acid (mp = ). 2. Run both the 4:1 cinnamic acid:urea and the 1:4 cinnamic acid:urea mixtures. 3. Run one unknown. (You and partner must run different unknowns.) Lab Report Requirements No introduction or procedure write-up is required. Fill in the data section on the report hand in (next page in manual), and answer the questions.
5 Melting Range 7 Melting Point Lab Report. Chem 355 Name: Partner s Name (if you shared data with a partner: Experimental Data melting range My Known: (U or C or both) My mixture: (4:1 C:U or 4:1 U:C or both) Partner s mixture (4:1 C:U or 4:1 U:C) My Unknown: (1, 2, 3, or 4 ) Which compound is your unknown? (from the list on page 4) Any doubts, discussion, or logic on your identification of unknown. (Not necessary, but if you have a tricky one or one that for whatever reason you get wrong, if your discussion shows some reasonable analysis or logic, it may help you get partial credit! J) Discussion questions: 1. Compare the ranges observed with the two mixtures. a. Did they depress and broaden about the same, or different? b. What does that say about the degree of depression and broadening that occurs when mixtures are used? Do all impurities depress to the same degree, or by some predictable formula? Or do you think it s more of a case-by-case deal? 2. Strictly speaking, why is it incorrect to speak of a melting point? 3. How will your melting range be perturbed if you haven t completely dried your sample? (For example, after you ve filtered crystals away from a solvent, and/or have washed the crystals with solvent )
6 Melting Range 8 4. What s the advantage of a finely powdered sample over a coarser sample? How will your melting range be perturbed with coarse sample? 5. What s the advantage of putting in a relatively small amount of sample as opposed to putting in lots and lots of sample? How will your melting range be perturbed with huge sample? 6. Why is it desirable to heat the sample relatively slowly? How will your melting range be perturbed by heating too fast? 7. You have a sample that you are sure is Jaspersium, which has a list melting range of Suppose you run your sample and observe a melting range of Is your sample impure, or did you heat too fast? Suppose you run your sample and observe a melting range of Is your sample impure, or did you heat too fast? 8. You have isolated an unknown compound that shows an observed melting range of Which is it more likely to be, candidate X (list mp 97-98) or candidate Y (list mp 86-87). Why might your sample not have the same melting range as either of the known compounds, given that it must be one of them? 9. Three test tubes labeled A, B, and C contain substances with approximately the same melting points. How could you prove the test tubes contained three different chemical compounds?
Appendix 4: Melting Points
 Appendix 4: Melting Points Melting points of Pure Substances The melting point of a substance is the temperature at which the solid phase of the substance is at equilibrium with the liquid phase. Consider
Appendix 4: Melting Points Melting points of Pure Substances The melting point of a substance is the temperature at which the solid phase of the substance is at equilibrium with the liquid phase. Consider
Recrystallization II 23
 Recrystallization II 23 Chem 355 Jasperse RECRYSTALLIZATIN-Week 2 1. Mixed Recrystallization of Acetanilide 2. Mixed Recrystallization of Dibenzylacetone 3. Recrystallization of an Unknown Background Review:
Recrystallization II 23 Chem 355 Jasperse RECRYSTALLIZATIN-Week 2 1. Mixed Recrystallization of Acetanilide 2. Mixed Recrystallization of Dibenzylacetone 3. Recrystallization of an Unknown Background Review:
Melting Point 1. Figure 2. A close-up of the "business end" of the Mel-Temp apparatus. Figure 1. The mel-temp device.
 Melting Point 1 The temperature at which a solid melts is known as the melting point (MP) of that substance. The melting point is a physical property of a solid and can be used to help identify a substance.
Melting Point 1 The temperature at which a solid melts is known as the melting point (MP) of that substance. The melting point is a physical property of a solid and can be used to help identify a substance.
Kinetic vs. Thermodynamic Control
 Experiment: Kinetic vs. Thermodynamic Control of rganic Reactions Kinetic vs. Thermodynamic Control During your study of reactions this year you have examined many mechanisms. You have used these mechanisms
Experiment: Kinetic vs. Thermodynamic Control of rganic Reactions Kinetic vs. Thermodynamic Control During your study of reactions this year you have examined many mechanisms. You have used these mechanisms
Experiment 4 MELTING POINTS OF ORGANIC COMPOUNDS 1
 Experiment 4 MELTING POINTS OF ORGANIC COMPOUNDS 1 Background: The melting point of an organic solid is probably the most widely used physical constant. If you want a quick, easy, and cheap way to characterize
Experiment 4 MELTING POINTS OF ORGANIC COMPOUNDS 1 Background: The melting point of an organic solid is probably the most widely used physical constant. If you want a quick, easy, and cheap way to characterize
ORGANIC CHEMISTRY I CHEMISTRY 261. MACEWAN UNIVERSITY Winter 2016
 ORGANIC CHEMISTRY I CHEMISTRY 261 MACEWAN UNIVERSITY Winter 2016 LABORATORY INFORMATION SAFETY a. PERSONAL PROTECTION b. LABORATORY ACTIVITIES USING CHEMICALS a. HANDLING/DISPENSING b. SPILLING c. DISPOSING
ORGANIC CHEMISTRY I CHEMISTRY 261 MACEWAN UNIVERSITY Winter 2016 LABORATORY INFORMATION SAFETY a. PERSONAL PROTECTION b. LABORATORY ACTIVITIES USING CHEMICALS a. HANDLING/DISPENSING b. SPILLING c. DISPOSING
Chapter 4: Recrystallization & Melting Point
 Chapter 4: Recrystallization & Melting Point Recrystallization A purification technique for impure solid compounds A several-step process Can be on on a microscale or macroscale Melting Point Verifies
Chapter 4: Recrystallization & Melting Point Recrystallization A purification technique for impure solid compounds A several-step process Can be on on a microscale or macroscale Melting Point Verifies
Purification Of A Solid By Recrystallization AND Identification By Melting Point Determination
 Purification Of A Solid By Recrystallization AND Identification By Melting Point Determination Refer back to your recrystallization and melting point experiments. In this experiment you must purify your
Purification Of A Solid By Recrystallization AND Identification By Melting Point Determination Refer back to your recrystallization and melting point experiments. In this experiment you must purify your
CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017)
 CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017) SEPARATION AND RECOVERY OF ORGANIC COMPOUNDS, THIN LAYER CHROMATOGRAPHY, COLUMN CHROMATOGRAPHY, CRYSTALLIZATION, AND MELTING POINTS Overview
CH241 Experiment #1 (Weeks of September 11, 18, and 25, 2017) SEPARATION AND RECOVERY OF ORGANIC COMPOUNDS, THIN LAYER CHROMATOGRAPHY, COLUMN CHROMATOGRAPHY, CRYSTALLIZATION, AND MELTING POINTS Overview
7. Determination of Melting Points
 7. Determination of Melting Points This experiment consists of three parts. In the first part, you will determine the melting point range of three known compounds. This part is mostly for practice, to
7. Determination of Melting Points This experiment consists of three parts. In the first part, you will determine the melting point range of three known compounds. This part is mostly for practice, to
The Chemical Importance of Purity Acquired Through Recrystalization and Analyzed by Melting Point
 The Chemical Importance of Purity Acquired Through Recrystalization and Analyzed by Melting Point Codi Gail Hefner 1 and Brenton Matulka 1 1 University of Northwest Missouri State University Abstract:
The Chemical Importance of Purity Acquired Through Recrystalization and Analyzed by Melting Point Codi Gail Hefner 1 and Brenton Matulka 1 1 University of Northwest Missouri State University Abstract:
Recrystallization with a Single Solvent
 Experiment: Recrystallization Part II: Purification of Solids In Part I of the recrystallization experiment, you learned about the factors which make a good recrystallization solvent, and you learned how
Experiment: Recrystallization Part II: Purification of Solids In Part I of the recrystallization experiment, you learned about the factors which make a good recrystallization solvent, and you learned how
2. Crystallization. A. Background
 2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
The Recrystallization of Benzoic Acid and Determination of its Melting Point
 Doreen Foy Organic Chemistry 1 Laboratory The Recrystallization of Benzoic Acid and Determination of its Melting Point Hannah Loch 10/01/2012 Abstract The goal of the experiment was to obtain pure benzoic
Doreen Foy Organic Chemistry 1 Laboratory The Recrystallization of Benzoic Acid and Determination of its Melting Point Hannah Loch 10/01/2012 Abstract The goal of the experiment was to obtain pure benzoic
AQA Chemistry A-level
 AQA Chemistry A-level Required Practical 10 Preparation of a pure organic solid, test of its purity, and preparation of a pure organic liquid Reflux Reflux: continuous boiling and condensing of a reaction
AQA Chemistry A-level Required Practical 10 Preparation of a pure organic solid, test of its purity, and preparation of a pure organic liquid Reflux Reflux: continuous boiling and condensing of a reaction
Lab 4: Recrystallization
 Lab 4: Recrystallization Pre Lab Question: (Answer submitted in a separate piece of paper at the beginning of lab) 1. Calculate how much 95% ethanol will be required to dissolve 0.8 g of sulfanilamide
Lab 4: Recrystallization Pre Lab Question: (Answer submitted in a separate piece of paper at the beginning of lab) 1. Calculate how much 95% ethanol will be required to dissolve 0.8 g of sulfanilamide
Physical pharmacy. dr basam al zayady
 Physical pharmacy Lec 5 dr basam al zayady Liquefaction of Gases: When a gas is cooled, it loses some of its kinetic energy in the form of heat, and the velocity of the molecules decreases. If pressure
Physical pharmacy Lec 5 dr basam al zayady Liquefaction of Gases: When a gas is cooled, it loses some of its kinetic energy in the form of heat, and the velocity of the molecules decreases. If pressure
2. Crystallization. A. Background
 2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
Lab 4: Recrystallization
 Lab 4: Recrystallization Objectives: - Purify an impure sample of an antibiotic. - Practice the crystallization technique. Introduction: The purpose of this experiment is to introduce the technique of
Lab 4: Recrystallization Objectives: - Purify an impure sample of an antibiotic. - Practice the crystallization technique. Introduction: The purpose of this experiment is to introduce the technique of
E24 PURIFICATION OF ORGANIC COMPOUNDS Distillation, recrystallisation, melting and boiling point determination
 E24 PURIFICATION OF ORGANIC COMPOUNDS Distillation, recrystallisation, melting and boiling point determination THE TASK To learn the main techniques of purifying organic compounds. THE SKILLS By the end
E24 PURIFICATION OF ORGANIC COMPOUNDS Distillation, recrystallisation, melting and boiling point determination THE TASK To learn the main techniques of purifying organic compounds. THE SKILLS By the end
Experiment 2. Recrystallization: The Purification of Crystalline Organic Compounds
 Experiment 2 Recrystallization: The Purification of Crystalline Organic Compounds The method selected for purifying a given organic compound depends on a number of factors such as the physical state of
Experiment 2 Recrystallization: The Purification of Crystalline Organic Compounds The method selected for purifying a given organic compound depends on a number of factors such as the physical state of
Lab 2 Guide: Recrystallization (Aug 31 Sept 4)
 Lab 2 Guide: Recrystallization (Aug 31 Sept 4) How Recrystallization Works- The Basic Idea Recrystallization is a purification technique; it allows us to remove impurities in a sample. The idea is you
Lab 2 Guide: Recrystallization (Aug 31 Sept 4) How Recrystallization Works- The Basic Idea Recrystallization is a purification technique; it allows us to remove impurities in a sample. The idea is you
Chem 355 Jasperse DISTILLATION
 Chem 355 Jasperse DISTILLATION 1 Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize.
Chem 355 Jasperse DISTILLATION 1 Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize.
Changes for Organic Chemistry 2521 Labs
 Changes for Organic Chemistry 2521 Labs Chapter 3 Crystallization Part 1 (Starts on page 56) Test the solubility of three compounds with three solvents. There are four compounds to choose from: 1. Resorcinol
Changes for Organic Chemistry 2521 Labs Chapter 3 Crystallization Part 1 (Starts on page 56) Test the solubility of three compounds with three solvents. There are four compounds to choose from: 1. Resorcinol
H N 2. Decolorizing carbon O. O Acetanilide
 Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
Determination of the Molar Mass of a Compound by Freezing Point Depression
 Determination of the Molar Mass of a Compound by Freezing Point Depression Objective: The objective of this experiment is to determine the molar mass of an unknown solute by measuring the freezing point
Determination of the Molar Mass of a Compound by Freezing Point Depression Objective: The objective of this experiment is to determine the molar mass of an unknown solute by measuring the freezing point
CH361/361H Week 1 Lecture. Laboratory Notebook Keeping, Melting Point Determination, Recrystallization, & Yields. Identify unknown.
 sodium salt of unknown aromatic carboxylic acid + soluble impurities + insoluble impurities total mass:? g insoluble impurities mass:? g colored dye(s) mass: ~ 20 mg dissolve in water, filter aq. sol n
sodium salt of unknown aromatic carboxylic acid + soluble impurities + insoluble impurities total mass:? g insoluble impurities mass:? g colored dye(s) mass: ~ 20 mg dissolve in water, filter aq. sol n
Experiment 1 MOLAR MASS DETERMINATION BY FREEZING POINT DEPRESSION
 1 Experiment 1 MOLAR MASS DETERMINATION BY FREEZING POINT DEPRESSION Whenever a substance is dissolved in a solvent, the vapor pressure of the solvent is lowered. As a result of the decrease in the vapor
1 Experiment 1 MOLAR MASS DETERMINATION BY FREEZING POINT DEPRESSION Whenever a substance is dissolved in a solvent, the vapor pressure of the solvent is lowered. As a result of the decrease in the vapor
EXPERIMENT 7A. Chemical Separation by Filtration and Recrystallization INTRODUCTION
 EXPERIMENT 7A Chemical Separation by Filtration and Recrystallization INTRODUCTION The solubilities of solid substances in different kinds of liquid solvents vary widely. Substances that we call salts
EXPERIMENT 7A Chemical Separation by Filtration and Recrystallization INTRODUCTION The solubilities of solid substances in different kinds of liquid solvents vary widely. Substances that we call salts
Assistant Lecturer. Sahar Mohammed Shakir Assistant Lecturer. Abdul Hafeedh Hameed Assistant Lecturer. Ali Basim
 Assistant Lecturer Sahar Mohammed Shakir Assistant Lecturer Abdul Hafeedh Hameed Assistant Lecturer Ali Basim Solid organic cpd.s when isolated from organic reaction are impure; they are contaminated with
Assistant Lecturer Sahar Mohammed Shakir Assistant Lecturer Abdul Hafeedh Hameed Assistant Lecturer Ali Basim Solid organic cpd.s when isolated from organic reaction are impure; they are contaminated with
Experiment 3 MELTING POINTS AND RANGES. New Technique. Discussion. Melting points and ranges.
 E3-1 Experiment 3 Fig. 3-1 MELTING POINTS AND RANGES New Technique Antoine Lavoisier (1743-1794) One of first scientists to make chemistry quantitative. http://www.homepages.hetnet.nl/~b1beukema/wohler.html
E3-1 Experiment 3 Fig. 3-1 MELTING POINTS AND RANGES New Technique Antoine Lavoisier (1743-1794) One of first scientists to make chemistry quantitative. http://www.homepages.hetnet.nl/~b1beukema/wohler.html
COMPUTER SIMULATION OF ENZYME KINETICS
 COMPUTER SIMULATION OF ENZYME KINETICS I. Introduction. Enzymes are biological catalysts. A catalyst alters the speed at which a chemical reaction reaches its completion or equilibrium point. It does not
COMPUTER SIMULATION OF ENZYME KINETICS I. Introduction. Enzymes are biological catalysts. A catalyst alters the speed at which a chemical reaction reaches its completion or equilibrium point. It does not
Chem2211L. Lab Report. Recrystallization
 Chem2211L Lab Report Recrystallization September 27, 2016 Experiment #3: Recrystallization Experiment Date: September 20, 2016 Lab Partners: Judienne Archer Tonja Bryant Alexis Dunn Objective: To separate
Chem2211L Lab Report Recrystallization September 27, 2016 Experiment #3: Recrystallization Experiment Date: September 20, 2016 Lab Partners: Judienne Archer Tonja Bryant Alexis Dunn Objective: To separate
EXPERIMENT 15 FREEZING POINT: A COLLIGATIVE PROPERTY OF SOLUTIONS
 EXPERIMENT 15 FREEZING POINT: A COLLIGATIVE PROPERTY OF SOLUTIONS INTRODUCTION Scientists seldom work alone today. Instead they work in teams on projects, sharing the labor of carrying out the experiments
EXPERIMENT 15 FREEZING POINT: A COLLIGATIVE PROPERTY OF SOLUTIONS INTRODUCTION Scientists seldom work alone today. Instead they work in teams on projects, sharing the labor of carrying out the experiments
PHASE CHANGES. Time Temperature Observations. Name(s)
 3 5 PHASE CHANGES PHASE CHANGES Name(s) The activities presented here focus on the energy changes that occur in substances undergoing a phase change. The first activity will take the most time to complete.
3 5 PHASE CHANGES PHASE CHANGES Name(s) The activities presented here focus on the energy changes that occur in substances undergoing a phase change. The first activity will take the most time to complete.
THE MOLECULAR WEIGHT OF A SOLUTE*
 Name Per THE MOLECULAR WEIGHT OF A SOLUTE* One of the most helpful things that can be known about an element or compound is its molecular weight (molecular mass, gram molecular mass, atomic mass, or gram
Name Per THE MOLECULAR WEIGHT OF A SOLUTE* One of the most helpful things that can be known about an element or compound is its molecular weight (molecular mass, gram molecular mass, atomic mass, or gram
Experiment 3: The Chromatography of Organic Compounds
 Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
ALE 20. Crystalline Solids, Unit Cells, Liquids and the Uniqueness of Water
 Name Chem 162, Section: Group Number: ALE 20. Crystalline Solids, Unit Cells, Liquids and the Uniqueness of Water (Reference: pp. 463 473 of Sec. 12.6 Silberberg 5 th edition) How are the particles within
Name Chem 162, Section: Group Number: ALE 20. Crystalline Solids, Unit Cells, Liquids and the Uniqueness of Water (Reference: pp. 463 473 of Sec. 12.6 Silberberg 5 th edition) How are the particles within
Melting Point Determination Application Note #1
 Application Note #1 Introduction A few basic guidelines must be carefully followed to avoid errors during melting point determinations with OptiMelt. The way in which the sample is prepared and the instrument
Application Note #1 Introduction A few basic guidelines must be carefully followed to avoid errors during melting point determinations with OptiMelt. The way in which the sample is prepared and the instrument
Year 7 Chemistry HW Questions
 Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
CHMA2000 EXPT 2: Recrystallization
 CHMA2000 EXPT 2: Recrystallization Experimental Objectives: At the end of this experiment you should be able to: 1. Perform a recrystallization 2. Determine the best solvent for the recrystallization of
CHMA2000 EXPT 2: Recrystallization Experimental Objectives: At the end of this experiment you should be able to: 1. Perform a recrystallization 2. Determine the best solvent for the recrystallization of
Experiment 2: Preparation of the Artificial Sweetener Dulcin
 Experiment 2: Preparation of the Artificial Sweetener Dulcin Organic compounds known as sugars are carbohydrates that occur widely in nature. For example, sucrose (aka table sugar) is found in sugar can,
Experiment 2: Preparation of the Artificial Sweetener Dulcin Organic compounds known as sugars are carbohydrates that occur widely in nature. For example, sucrose (aka table sugar) is found in sugar can,
Experiment 13: Determination of Molecular Weight by Freezing Point Depression
 1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
Chemical Industry. School experiments. Melting Point. Helper Booklet. learn easily. for Melting Point Determination
 Chemical Industry School experiments Melting Point Helper Booklet Natural science 8 laws Tips experience and Tricks live learn easily for Melting Point Determination Dear Reader First of all, thank you
Chemical Industry School experiments Melting Point Helper Booklet Natural science 8 laws Tips experience and Tricks live learn easily for Melting Point Determination Dear Reader First of all, thank you
White Paper. Crystallization in Process Chemistry Applying Simple PAT Tools. Author: Des O'Grady PhD, METTLER TOLEDO
 Crystallization in Process Chemistry Applying Simple PAT Tools Author: Des O'Grady PhD, Crystallization is a common step used during the synthesis of organic compounds to isolate and purify the desired
Crystallization in Process Chemistry Applying Simple PAT Tools Author: Des O'Grady PhD, Crystallization is a common step used during the synthesis of organic compounds to isolate and purify the desired
Colligative Properties of Solutions: Freezing Point Depression
 E1 Colligative Properties of Solutions: Freezing Point Depression PURPOSE The experiment to be performed is divided into three sections: (a) In part 1, the FP of the pure solvent, cyclohexane, is determined
E1 Colligative Properties of Solutions: Freezing Point Depression PURPOSE The experiment to be performed is divided into three sections: (a) In part 1, the FP of the pure solvent, cyclohexane, is determined
RECRYSTALLIZATION, FILTRATION AND MELTING POINT
 RECRYSTALLIZATIN, FILTRATIN AND MELTING PINT Recrystallization is one of the main purification methods for solid compounds on virtually any scale, from mg to kg to tons. From the experimental point of
RECRYSTALLIZATIN, FILTRATIN AND MELTING PINT Recrystallization is one of the main purification methods for solid compounds on virtually any scale, from mg to kg to tons. From the experimental point of
Lab 3: Calibration of a Melting Point Apparatus
 Written by Danielle M. Solano & Jesse Bergkamp Department of Chemistry & Biochemistry California State University, Bakersfield Objectives By the end of this laboratory, you should have developed the skills
Written by Danielle M. Solano & Jesse Bergkamp Department of Chemistry & Biochemistry California State University, Bakersfield Objectives By the end of this laboratory, you should have developed the skills
UNKNOWN UNKNOWNS Requirements: classify your functional group
 UNKNOWN UNKNOWNS Candidates: ALCOHOLS Table 70.2, p. 766, 767 CARBOXYLIC ACIDS Table 70.1, p 764,765 ALDEHYDES Table 70.3, p. 767, 768 AMINES Table 70.5, p. 769, 770 KETONES Tabl e 70.14, p. 776 Requirements:
UNKNOWN UNKNOWNS Candidates: ALCOHOLS Table 70.2, p. 766, 767 CARBOXYLIC ACIDS Table 70.1, p 764,765 ALDEHYDES Table 70.3, p. 767, 768 AMINES Table 70.5, p. 769, 770 KETONES Tabl e 70.14, p. 776 Requirements:
EXPERIMENT 3: Identification of a Substance by Physical Properties
 EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
Colligative Properties of Solutions: Freezing Point Depression
 E1 Colligative Properties of Solutions: Freezing Point Depression PURPOSE The experiment to be performed is divided into three sections: (a) In part 1, the FP of the pure solvent, cyclohexane, is determined
E1 Colligative Properties of Solutions: Freezing Point Depression PURPOSE The experiment to be performed is divided into three sections: (a) In part 1, the FP of the pure solvent, cyclohexane, is determined
Solids SECTION Critical Thinking
 SECTION 10.3 Solids A gas has neither a definite volume nor a definite shape. A liquid has a definite volume, but not a definite shape. A solid, the third state, has a definite volume and a definite shape.
SECTION 10.3 Solids A gas has neither a definite volume nor a definite shape. A liquid has a definite volume, but not a definite shape. A solid, the third state, has a definite volume and a definite shape.
Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay
 Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay Lecture - 31 Standard Costing - Material, Labor and Overhead Variances Dear students,
Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay Lecture - 31 Standard Costing - Material, Labor and Overhead Variances Dear students,
CH 338M - Final Exam Lab day:
 C 338M - Final Exam Name Lab day: (141 points) 1. (20)You have just accepted a job in an industrial laboratory where the director of the lab asks you to solve certain problems, indicated below. Describe
C 338M - Final Exam Name Lab day: (141 points) 1. (20)You have just accepted a job in an industrial laboratory where the director of the lab asks you to solve certain problems, indicated below. Describe
Science Physical Science Grades 6 and 8
 Science Physical Science Grades 6 and 8 Subject: Physical Science: Thermal Energy Level: Grade 6 and 8 The Hot in the Cold Abstract: Students will conduct an experiment and collect data on the exchange
Science Physical Science Grades 6 and 8 Subject: Physical Science: Thermal Energy Level: Grade 6 and 8 The Hot in the Cold Abstract: Students will conduct an experiment and collect data on the exchange
Chemical reactions and electrolysis
 Chemical reactions and electrolysis Higher Revision Questions Name: Class: Date: Time: 95 minutes Marks: 95 marks Comments: Page of 29 (a) Magnesium metal is shaped to make magnesium ribbon. Explain why
Chemical reactions and electrolysis Higher Revision Questions Name: Class: Date: Time: 95 minutes Marks: 95 marks Comments: Page of 29 (a) Magnesium metal is shaped to make magnesium ribbon. Explain why
Revised Molar Mass Measurement Lab Purpose: Background:
 Revised Molar Mass Measurement Lab (Based on prior Ch b experiment, incorporating revision suggestions from Tsze Tsang) Purpose: To use the colligative properties of cyclohexane solutions to determine
Revised Molar Mass Measurement Lab (Based on prior Ch b experiment, incorporating revision suggestions from Tsze Tsang) Purpose: To use the colligative properties of cyclohexane solutions to determine
Phase Diagrams Revised: 1/27/16 PHASE DIAGRAMS. Adapted from Bill Ponder, Collin College & MIT OpenCourseWare INTRODUCTION
 PHASE DIAGRAMS Adapted from Bill Ponder, Collin College & MIT OpenCourseWare INTRODUCTION A phase diagram is a graphical representation of the physical states of a substance as they relate to temperature
PHASE DIAGRAMS Adapted from Bill Ponder, Collin College & MIT OpenCourseWare INTRODUCTION A phase diagram is a graphical representation of the physical states of a substance as they relate to temperature
IGCSE(A*-G) Edexcel - Chemistry
 IGCSE(A*-G) Edexcel - Chemistry Principles of Chemistry States of Matter NOTES 1.1 Understand the arrangement, movement and energy of the particles in each of the three states of matter: solid, liquid
IGCSE(A*-G) Edexcel - Chemistry Principles of Chemistry States of Matter NOTES 1.1 Understand the arrangement, movement and energy of the particles in each of the three states of matter: solid, liquid
Determining Melting Temperature. Sample
 Determining Melting Temperature Experiment 1 The melting temperature of a compound is the temperature at which it changes from a solid to a liquid. This is a physical property often used to help identify
Determining Melting Temperature Experiment 1 The melting temperature of a compound is the temperature at which it changes from a solid to a liquid. This is a physical property often used to help identify
Experiment 2: The Chromatography of Organic Compounds
 Experiment 2: The Chromatography of Organic Compounds INTRODUCTION When performing an organic reaction, it is very common to observe the formation of other compounds in addition to your desired product;
Experiment 2: The Chromatography of Organic Compounds INTRODUCTION When performing an organic reaction, it is very common to observe the formation of other compounds in addition to your desired product;
Chapter 25 Separating Mixtures
 Chapter 25 Separating Mixtures A solution is formed when a solid dissolves in a liquid The solid is referred to as the solute. The liquid is referred to as the solvent. A dilute solution is one where there
Chapter 25 Separating Mixtures A solution is formed when a solid dissolves in a liquid The solid is referred to as the solute. The liquid is referred to as the solvent. A dilute solution is one where there
Crystallization & Filtration
 Crystallization & Filtration Purification of Salicyclic Acid and Sodium Chloride Separation processes Liquid-liquid extraction Adsorption Filtration Solid-liquid extraction (leaching) Elution chromatography
Crystallization & Filtration Purification of Salicyclic Acid and Sodium Chloride Separation processes Liquid-liquid extraction Adsorption Filtration Solid-liquid extraction (leaching) Elution chromatography
Chapter 12 Metals. crystalline, in which particles are in highly ordered arrangement. (Have MP.)
 Chapter 12 Metals 12.1 Classification of Solids Covalent Ionic Molecular Metallic Solids Solids Solids Solids Molecular consist of molecules held next to each other by IMF s. Relatively low to moderate
Chapter 12 Metals 12.1 Classification of Solids Covalent Ionic Molecular Metallic Solids Solids Solids Solids Molecular consist of molecules held next to each other by IMF s. Relatively low to moderate
 Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY LONG QUESTIONS Analytical Chemistry: The science of chemical characterization is called analytical chemistry. OR The branch of chemistry which deals with
Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY LONG QUESTIONS Analytical Chemistry: The science of chemical characterization is called analytical chemistry. OR The branch of chemistry which deals with
9. PHOSPHORUS 563. Formula weight Very gradually evolves free I a with KI.
 9. PHOSPHORUS 563 A solution of 302.2 g. of KH 3 PO 4, 198 g. of KOH, 120 g. of KF and 0.355 g. of K 3 CrO 4 in one liter of water is prepared. A 215-ml. portion of this solution is electrolyzed in a large
9. PHOSPHORUS 563 A solution of 302.2 g. of KH 3 PO 4, 198 g. of KOH, 120 g. of KF and 0.355 g. of K 3 CrO 4 in one liter of water is prepared. A 215-ml. portion of this solution is electrolyzed in a large
Precipitation Reactions and Gravimetric Analysis
 Precipitation Reactions and Gravimetric Analysis Titrations where the titrant forms a precipitate with the analyte. Not always so straightforward a number of requirements need to be met. Precipitation
Precipitation Reactions and Gravimetric Analysis Titrations where the titrant forms a precipitate with the analyte. Not always so straightforward a number of requirements need to be met. Precipitation
19. The preparation and purification of methyl-3-nitrobenzoate Student Sheet
 19. The preparation and purification of methyl-3-nitrobenzoate Student Sheet In this experiment you will learn or develop skills in preparative organic chemistry by making and purifying a sample of an
19. The preparation and purification of methyl-3-nitrobenzoate Student Sheet In this experiment you will learn or develop skills in preparative organic chemistry by making and purifying a sample of an
Let s Move It! Gel Electrophoresis Using Food Dye Student Guide
 Let s Move It! Gel Electrophoresis Using Food Dye Student Guide Purpose This lab explores the principle of electrophoresis, an important technique used in biochemistry and molecular biology. You will:
Let s Move It! Gel Electrophoresis Using Food Dye Student Guide Purpose This lab explores the principle of electrophoresis, an important technique used in biochemistry and molecular biology. You will:
Specifying A Moisture Analyser
 Moisture Control & Measurement Ltd. Thorp Arch Trading Estate Wetherby West Yorkshire LS23 7BJ United Kingdom tel: fax: email: web: +44 (0)1937 844000 +44 (0)1937 849424 sales@mcm-moisture.com www.mcm-moisture.com
Moisture Control & Measurement Ltd. Thorp Arch Trading Estate Wetherby West Yorkshire LS23 7BJ United Kingdom tel: fax: email: web: +44 (0)1937 844000 +44 (0)1937 849424 sales@mcm-moisture.com www.mcm-moisture.com
BASIC ELECTROPHORESIS
 Ref. ELECBASICA (4 practices) 1. EXPERIMENT OBJETIVE BASIC ELECTROPHORESIS The aim of this experiment is to introduce students to the knowledge of electrophoretic theory and to familiarize themselves with
Ref. ELECBASICA (4 practices) 1. EXPERIMENT OBJETIVE BASIC ELECTROPHORESIS The aim of this experiment is to introduce students to the knowledge of electrophoretic theory and to familiarize themselves with
Chapter 4. Crystallization
 Chapter 4 Crystallization Topic Crystallization Principle of Crystallization Purity and Yields Solubility and Solubility Curves ass and nergy Balances in Crystallization earning utcome t is expected that
Chapter 4 Crystallization Topic Crystallization Principle of Crystallization Purity and Yields Solubility and Solubility Curves ass and nergy Balances in Crystallization earning utcome t is expected that
Quiz 2 practice Quiz
 Name: ate: 1. The following data were recorded while determining the solubility of a certain salt. Temp. ( C) 10 20 30 40 50 Grams Solute/ 100 g H 2 O 30 33 36 39 42 Which graph best represents the solubility
Name: ate: 1. The following data were recorded while determining the solubility of a certain salt. Temp. ( C) 10 20 30 40 50 Grams Solute/ 100 g H 2 O 30 33 36 39 42 Which graph best represents the solubility
Solubility Equilibrium
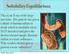 Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
hydroxynitrile ester dihaloalkane alkane alkene haloalkane alcohol amine nitrile ketone HCN + KCN Nucleophilic addition carboxylic acid
 6.2.5 Synthesis dihaloalkane poly(alkene) high pressure atalyst polymerization Br 2, l 2 Electrophilic addition alkene alkane 2, Nickel atalyst addition/reduction Br 2, l 2 UV light Free radical Substitution
6.2.5 Synthesis dihaloalkane poly(alkene) high pressure atalyst polymerization Br 2, l 2 Electrophilic addition alkene alkane 2, Nickel atalyst addition/reduction Br 2, l 2 UV light Free radical Substitution
CH 112 Special Assignment #4 Chemistry to Dye for: Part A
 CH 112 Special Assignment #4 Chemistry to Dye for: Part A PRE-LAB ASSIGNMENT: Make sure that you read this handout and bring the essentials to lab with you. Here are the pre-lab questions for this week.
CH 112 Special Assignment #4 Chemistry to Dye for: Part A PRE-LAB ASSIGNMENT: Make sure that you read this handout and bring the essentials to lab with you. Here are the pre-lab questions for this week.
ADVANCED ELECTROPHORESIS
 Ref. ELECAVANZADA (4 practices) 1. EXPERIMENT OBJETIVE ADVANCED ELECTROPHORESIS The aim of this experiment is to introduce students to the knowledge of electrophoretic theory and to familiarize themselves
Ref. ELECAVANZADA (4 practices) 1. EXPERIMENT OBJETIVE ADVANCED ELECTROPHORESIS The aim of this experiment is to introduce students to the knowledge of electrophoretic theory and to familiarize themselves
How Do You Choose Cookware?
 Cookin' Chem Activity 6 How Do You Choose Cookware? GOALS In this activity you will: Explore the concept of specific heat capacity. Experimentally determine the specific heat capacity of various substances.
Cookin' Chem Activity 6 How Do You Choose Cookware? GOALS In this activity you will: Explore the concept of specific heat capacity. Experimentally determine the specific heat capacity of various substances.
Class 4J Spring Term Unit 4D Solids, liquids and how they can be separated Adapted from QCA Science Unit 4D
 Class 4J Spring Term 2003 Science Unit 4D Solids, liquids and how they can be separated Adapted from QCA Science Unit 4D ABOUT THE UNIT In this unit children learn about the differences between solids
Class 4J Spring Term 2003 Science Unit 4D Solids, liquids and how they can be separated Adapted from QCA Science Unit 4D ABOUT THE UNIT In this unit children learn about the differences between solids
Mike Fenner. Shipping solder paste. Paste vehicle and powder. Once made. Shipping and storing solder paste. Shipping and storing solder paste
 Shipping and storing solder Shipping and storing solder Mike Fenner Technical Manager Indium Europe August 2006 Slide 1 Slide 2 We ship world wide Shipping and storing solder In Europe we make several
Shipping and storing solder Shipping and storing solder Mike Fenner Technical Manager Indium Europe August 2006 Slide 1 Slide 2 We ship world wide Shipping and storing solder In Europe we make several
Temperature and KE Lab 8th th Grade PSI Science Score /23. Part I: Kinetic Energy
 Temperature and KE Lab 8th th Grade PSI Science Lab Name Score /23 Part I: Kinetic Energy Objective: Investigate how the motion and spacing of water molecules differ in hot water versus cold water. Predict:
Temperature and KE Lab 8th th Grade PSI Science Lab Name Score /23 Part I: Kinetic Energy Objective: Investigate how the motion and spacing of water molecules differ in hot water versus cold water. Predict:
Chapter 15 Fundamentals of Metal Forming. Materials Processing. Deformation Processes. MET Manufacturing Processes
 MET 33800 Manufacturing Processes Chapter 15 Fundamentals of Metal Forming Before you begin: Turn on the sound on your computer. There is audio to accompany this presentation. Materials Processing Chapters
MET 33800 Manufacturing Processes Chapter 15 Fundamentals of Metal Forming Before you begin: Turn on the sound on your computer. There is audio to accompany this presentation. Materials Processing Chapters
COPPER CYCLE EXPERIMENT 3
 COPPER CYCLE EXPERIMENT 3 INTRODUCTION One simple way to state the aim of chemistry is: The study of matter and its transformations. In this experiment, a copper sample will appear in five different forms
COPPER CYCLE EXPERIMENT 3 INTRODUCTION One simple way to state the aim of chemistry is: The study of matter and its transformations. In this experiment, a copper sample will appear in five different forms
Thermochemistry/phase changes review Station 1
 Thermochemistry/phase changes review Station 1 (Show all work) Answers are posted below to check yourself 1. Calculate the heat necessary to raise the temperature of 40.0 g of aluminum from 20.0 o C to
Thermochemistry/phase changes review Station 1 (Show all work) Answers are posted below to check yourself 1. Calculate the heat necessary to raise the temperature of 40.0 g of aluminum from 20.0 o C to
CHAPTER 19 HEAT ENGINES AND REFRIGERATORS
 CHAPTER 19 HEAT ENGINES AND REFRIGERATORS In this chapter, we will examine devices that turn heat into work. I swear, I haven t confused you people for Mechanical Engineering majors! Biological processes
CHAPTER 19 HEAT ENGINES AND REFRIGERATORS In this chapter, we will examine devices that turn heat into work. I swear, I haven t confused you people for Mechanical Engineering majors! Biological processes
A Software Metrics Primer
 C12625211.fm Page 153 Monday, July 9, 2007 11:28 AM Chapter 12 A Software Metrics Primer 1 In this chapter: Why Measure Software.................................................153 What to Measure......................................................154
C12625211.fm Page 153 Monday, July 9, 2007 11:28 AM Chapter 12 A Software Metrics Primer 1 In this chapter: Why Measure Software.................................................153 What to Measure......................................................154
Marketing Automation: One Step at a Time
 Marketing Automation: One Step at a Time 345 Millwood Road Chappaqua, NY 10514 www.raabassociatesinc.com Imagine a wall. Your small business is on one side. A pot of gold is on the other. The gold is the
Marketing Automation: One Step at a Time 345 Millwood Road Chappaqua, NY 10514 www.raabassociatesinc.com Imagine a wall. Your small business is on one side. A pot of gold is on the other. The gold is the
Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay
 Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay Lecture - 32 Standard Costing, Mix, Yield, Sales and Fixed Overhead Variances The
Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay Lecture - 32 Standard Costing, Mix, Yield, Sales and Fixed Overhead Variances The
PHEN 612 SPRING 2008 WEEK 13 LAURENT SIMON
 PHEN 612 SPRING 2008 WEEK 13 LAURENT SIMON Crystallization Crystallization is a common separation process in Commodity inorganic industry (e.g., salts) Food industry (e.g., sugars) Pharmaceutical manufacturing
PHEN 612 SPRING 2008 WEEK 13 LAURENT SIMON Crystallization Crystallization is a common separation process in Commodity inorganic industry (e.g., salts) Food industry (e.g., sugars) Pharmaceutical manufacturing
PHASE EQUILIBRIA AND THE PHASE RULE
 PHASE EQUILIBRIA AND THE PHASE RULE Introduction The three primary phases of matter are often defined individually under different conditions. In practice we encounter states of matter in coexistence.
PHASE EQUILIBRIA AND THE PHASE RULE Introduction The three primary phases of matter are often defined individually under different conditions. In practice we encounter states of matter in coexistence.
6. In this temperature time graph for the heating of H 2O at a constant rate, the segment DE represents the
 1. Which of the following contains particles with the least freedom of motion? A) CO 2( ) B) HCl(aq) C) F 2(g) D) MgBr 2(s) E) C 6H 12O 6(aq) 2. During boiling, the temperature of a pure liquid substance
1. Which of the following contains particles with the least freedom of motion? A) CO 2( ) B) HCl(aq) C) F 2(g) D) MgBr 2(s) E) C 6H 12O 6(aq) 2. During boiling, the temperature of a pure liquid substance
Class XII Chapter 1 The Solid State Chemistry
 Class XII Chapter 1 The Solid State Chemistry Question 1.1: Define the term 'amorphous'. Give a few examples of amorphous solids. Amorphous solids are the solids whose constituent particles are of irregular
Class XII Chapter 1 The Solid State Chemistry Question 1.1: Define the term 'amorphous'. Give a few examples of amorphous solids. Amorphous solids are the solids whose constituent particles are of irregular
Equilibrium phase diagram of metallic alloy
 Equilibrium phase diagram of metallic alloy Motivation New structure, concentration (mixing level) (at what temperature? for how long? ) Phase Diagrams - Introduction. Many materials systems can exist
Equilibrium phase diagram of metallic alloy Motivation New structure, concentration (mixing level) (at what temperature? for how long? ) Phase Diagrams - Introduction. Many materials systems can exist
Deeper. Deeper. Deeper. Deeper. Deeper. Deeper. What is the advantage of breaking a project down into tasks and sub-tasks?
 A 1 A 2 What is the advantage of breaking a project down into tasks and sub-tasks? Tasks are natural units of work, estimation, and assignment. What is the advantage of breaking down goals, over breaking
A 1 A 2 What is the advantage of breaking a project down into tasks and sub-tasks? Tasks are natural units of work, estimation, and assignment. What is the advantage of breaking down goals, over breaking
A. Description of the solid state according to the kinetic-molecular theory (KMT):
 A. Description of the solid state according to the kinetic-molecular theory (KMT): Have a definite shape and volume and a slow average kinetic energy Particles of a SOLID appear to vibrate around fixed
A. Description of the solid state according to the kinetic-molecular theory (KMT): Have a definite shape and volume and a slow average kinetic energy Particles of a SOLID appear to vibrate around fixed
W8 Wednesday, November 3, :15 PM
 Presentation Notes Paper Bio Return to Main Menu PRESENTATION W8 Wednesday, November 3, 1999 2:15 PM USING THE ICED T MODEL TO TEST SUBJECTIVE SOFTWARE QUALITIES Andy Roth Rational Software INTERNATIONAL
Presentation Notes Paper Bio Return to Main Menu PRESENTATION W8 Wednesday, November 3, 1999 2:15 PM USING THE ICED T MODEL TO TEST SUBJECTIVE SOFTWARE QUALITIES Andy Roth Rational Software INTERNATIONAL
Skills in Science. Lab equipment. (Always draw 2D) Drawings below are NOT to scale. Beaker - A general purpose container with a pouring lip.
 Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
WET ANALYSIS OF GOLD-SILVER ALLOYS OF HIGH GOLD CONTENT 1
 WET ANALYSIS OF GOLD-SILVER ALLOYS OF HIGH GOLD CONTENT EARLE R. CALEY AND LOWELL W. SHANK Department of Chemistry, The Ohio State University, Columbus, Ohio ABSTRACT -silver alloys dissolve completely
WET ANALYSIS OF GOLD-SILVER ALLOYS OF HIGH GOLD CONTENT EARLE R. CALEY AND LOWELL W. SHANK Department of Chemistry, The Ohio State University, Columbus, Ohio ABSTRACT -silver alloys dissolve completely
The internal structure of a material plays an important part on its mechanical properties.!
 Phase Diagrams The internal structure of a material plays an important part on its mechanical properties.! There is a strong correlation between micro structure and mechanical properties. Definitions Component!
Phase Diagrams The internal structure of a material plays an important part on its mechanical properties.! There is a strong correlation between micro structure and mechanical properties. Definitions Component!
Let s Move It! Gel Electrophoresis Using Food Dye Student Guide
 Let s Move It! Gel Electrophoresis Using Food Dye Student Guide Purpose This lab explores the principle of electrophoresis, an important technique used in biochemistry and molecular biology. You will:
Let s Move It! Gel Electrophoresis Using Food Dye Student Guide Purpose This lab explores the principle of electrophoresis, an important technique used in biochemistry and molecular biology. You will:
