Electrochemical impedance study of initial lithium ion intercalation into graphite powders
|
|
|
- Ira Blake
- 6 years ago
- Views:
Transcription
1 Electrochimica Acta 46 (2001) Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby a, Frank E. Little b a Center for Electrochemical Systems and Hydrogen Research, Texas Engineering Experiment Station, Texas A&M Uni ersity, College Station, TX , USA b Center for Space Power, Texas Engineering Experiment Station, Texas A&M Uni ersity, College Station, TX , USA Received 5 July 2000; received in revised form 4 December 2000 Abstract A Johnson Matthey 287 (JM 287) graphite powder disks sandwiched between two nickel screens was used as working electrodes in 1.0 M lithium hexafluorophosphate in mixed carbonate ester electrolyte. Their intrinsic impedance and lithium intercalation kinetics were determined using several different electrochemical impedance spectroscopy (EIS) protocols. The thermal stability of the electrolyte was similarly studied using a floating palladium wire working electrode. Studies at 25 C and 65 C show that a first high frequency depressed semicircle, a second depressed semicircle, and a sloping line in the low frequency range are respectively related to the initial formation of a solid electrolyte interface (SEI) film, the charge-transfer reaction, and lithium diffusion in graphite. The intrinsic resistance is in series with the reaction impedance and effectively increases the latter. The charge-transfer semicircle at the open-circuit potential moves to the low frequency range, whereas the semicircle associated with the freshly formed SEI film moves to high frequency range as lithium insertion proceeds. The passivating SEI film formed at 25 C slows down co-insertion of the electrolyte into graphite when the electrode is cycled at 65 C, which increases the cycle life of graphite at elevated temperature Elsevier Science Ltd. All rights reserved. Keywords: Electrochemical impedance spectroscopy; Solid electrolyte interface; Electrochemical reaction kinetics; Intrinsic resistance; Cycle stability 1. Introduction Graphite is a most commonly used intercalation anode for lithium-ion batteries because of its high capacity (more than 300 mah/g), flat potential profile, and reasonable cost. However the capacity stability of graphite is sensitive to electrode operating temperature. The cycle life of graphite at room temperature has been shown to improve by pre-cycling it at a lower temperature [1], and the cycle life of graphite becomes reduced * Corresponding author. Tel.: ; fax: address: cswang@pop.tamu.edu (C. Wang). when it is cycled at elevated temperature [2]. The temperature of formation of the complex passivating film known as the solid electrolyte interface (SEI) on the graphite surface must therefore play an important role in helping to stabilize the intercalation electrode capacity as a function of temperature [3]. The less passivating SEI film formed at elevated temperature may decrease the conductivity of the graphite agglomerate, and reduce the reversible capacity [4] by decreasing the amount of available active materials and by slowing the kinetics of electrochemical lithium insertion extraction. A quantitative study of differences in the formation of the passive layer as a function of temperature should therefore help in understanding the mechanism of capacity degradation.
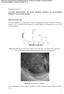
2 1794 C. Wang et al. / Electrochimica Acta 46 (2001) Electrochemical impedance spectroscopy (EIS) is a very sensitive detector of interfacial change, and is therefore commonly used to investigate the kinetics of growth and other properties of SEI films formed at room temperature [5 7]. While published EIS Nyquist plots as a function of potential for graphite intercalation electrodes generally resemble each other, explanations for the first depressed high frequency semicircle differ. It has generally been believed that this semicircle is related to the SEI film [5,6]. However this does not explain how it appears before the electrode is initially charged to 0.8 V versus Li, before the SEI film is believed to form. In an EIS study of SEI growth rather than its initial formation, Barsoukov et al. [5] investigated initial Li intercalation below the potential plateau for SEI film formation at room temperature and at 20 C. Recently, Chang and Sohn attributed the first depressed high frequency semicircle to contact resistance between electrode particles and the current collector [7]. The special EIS protocols given here show that the first high-frequency depressed semicircle results from the SEI film, although it is also influenced by the electrode contact impedance [8]. However, a reasonable explanation as to why this semicircle appears before initially charging to 0.8 V versus Li is required. The formation and growth of the SEI film on graphite at room temperature and at 65 C is investigated by in-situ EIS for the first time in the present work. Special EIS protocols were used to study on the electrochemical reaction resistance and intrinsic (i.e. physical) resistance change during initial Li insertion into graphite. 2. Experimental 2.1. Electrode and cell preparation JM 287 graphite powder (Johnson Matthey, Inc.), with a particle size of about 15 microns and a BET surface area of 13.5 m 2 /g was used as the intercalation anode material. Composite electrodes were prepared from a mixture of 92 wt% JM 287 (ca. 50 mg) with 8 wt% polyvinylidene fluoride between two nickel screen current collectors using 1-methyl-2-pyrrolidinone as the solvent. After drying overnight at 120 C, the electrode was pressed into a sandwich structure with a geometric surface area of 2.0 cm 2 (ca. 0.8 mm). The configuration of a typical electrode is shown in Fig. 1 [9]. Electrochemical measurements were conducted in a special four-electrode PTFE cell. Two lithium foils were used as both counter and reference electrodes, and a floating palladium wire was used as an another working electrode to monitor the stability of the electrolyte at elevated temperature. All potentials given are versus the Li/Li + reference electrode in the experimental electrolyte, which was 1.0 M lithium hexafluorophosphate (LiPF 6 ) in a 1:1 by volume ethylene carbonate (EC)- dimethylcarbonate (DMC) mixture (High Purity Lithium Battery Grade, Mitsubishi Chemical Company) unless otherwise specified in Fig. 5. Cells were assembled in an argon-filled glove box. Charge (lithium intercalation) and discharge (lithium extraction) characteristics were measured between +0.0 and +1.5Vata constant current using an Arbin (College Station, TX) automatic battery cycler. The graphite sandwich electrode was charged/discharged on one side only, and the voltage across its two sides was used to monitor its overall internal contact resistance. During initial Li insertion at 65 C, a current of 5 ma DC current was passed through the electrode to obtain the intrinsic resistance of electrode. To avoid confusion concerning the various electrode resistances discussed here, the expression intrinsic impedance is used below to refer to those physical impedance elements of resistance present within the thickness of the electrode, excluding any electrochemical (faradaic) elements. They include the electronic impedance of the series-parallel array of graphite particles, the transmission lines of contact impedances between the particles (including ionic contacts and electronic contacts with and within the binder), and the electronic impedances of the current collectors and contact resistances between them and the neighboring graphite particles. This intrinsic impedance can be evaluated by an in situ EIS method EIS measurement Fig. 1. Schematic diagram of graphite electrode configuration. This was measured over the frequency range 65 khz to 1.0 mhz at a potentiostatic signal amplitude of 5 mv, using a frequency response analyzer (Solartron, FRA 1250) and an electrochemical interface (Solartron, model 1286). EIS measurements were performed after 4 h of galvanostatic charging of freshly prepared JM 287 graphite electrodes, following a rest period of 10 h. Charging was at the 72 h full charge rate, based on an assumed maximum charge of 372 mah/g. The potential
3 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 2. Schematic diagram of cell and special Solatron electrochemical interface terminal-to-electrode connections. The suggested equivalent circuit for Li insertion extraction into graphite electrode is also shown. R G, R RG, and R CG : electronic graphite resistance, reference electrode to anode ionic resistance, and reference electrode to counter electrode ionic resistance. R pc, R pp, R SEI and R ct : graphite particles-to-current collector, particles-to-particles, SEI film, and charge-transfer resistances. C pc and C pp : particles-to-current collector and particles-to-particles contact capacitances. Q SEI and Q dl : constant-phase elements for the SEI film and for the double-layer respectively. Z GFW : generalized finite Warburg element for lithium diffusion in graphite. was recorded before each galvanostatic current interruption. For intrinsic resistance measurement, three intrinsic EIS scans were made using different methods for connecting the Solatron 1286 electrochemical interface terminals to the electrode. These were as follows: 1. between the two sides of the graphite electrode, connecting the RE 2 and WE terminals of the interface together to one of the sides, and RE 1 and CE to the other (called Protocol A in Section 3); 2. as (i), with RE 2 and WE connected to one side and CE to the other, with a lithium foil reference electrode connected to RE 1 (Protocol B); 3. with RE 2 and WE to one side and RE 1 to the other, with a lithium foil connected to CE as a counter electrode (Protocol C). EIS measurements for the determination of electrochemical reaction kinetics were obtained using the following protocols: 1. with terminals RE 2 and WE connected to both sides of the electrode (Protocol D); 2. with terminals RE 2 and WE connected to one side only (Protocol E); 3. with terminal RE 2 to one side and terminal WE to the other (Connection F). A schematic diagram of the cell and the different interface terminal-to-electrode connections, including the geometrical arrangement of the counter and reference electrodes for each protocol, is shown in Fig. 2. The differences between the three EIS scans thus obtained allow determination of the influence of contact resistance on electrochemical reaction kinetics. The manner in which the information is derived is described in a later section. Fitting of impedance spectra to the proposed equivalent circuit was performed by using the code Z view (Version 1.4, Scribner Associates, Inc.). 3. Results and discussion 3.1. Capacity stability of JM 287 graphite cycled at 65 C Aurbach et al. have reported that pre-cycling of graphite intercalation electrodes at low temperature improved their cycle life at room temperature [1]. They attributed this to a more advantageous SEI film formed at low temperature. To identify the effect of the SEI film first formed at room temperature on the cycling stability of graphite at elevated temperature, JM 287 graphite was cycled at 65 C with and without pre-cycling at 25 C, as shown in Fig. 3. The capacity of JM 287 graphite declined quickly to less than 40 mah/g when it was cycled at 65 C. However, only a slow capacity decline at 65 C was observed in Fig. 3b when JM 287 was pre-cycled 17 times at 25 C. This result
4 1796 C. Wang et al. / Electrochimica Acta 46 (2001) shows that the SEI film preformed at room temperature can enhance the capacity stability of graphite at elevated temperature Initial charge discharge beha ior of JM 287 graphite at 25 C and 65 C To investigate the influence of temperature on the formation of the SEI film, JM 287 electrodes were cycled at 25 C and 65 C. Charge discharge curves for the first cycle are given in Fig. 4. When the charge had reached 200 mah/g at 65 C, it was necessary to interrupt the 5 ma current passed through the electrode for intrinsic resistance measurement because the electrode conductivity became so low that a significant voltage difference was observed across the electrode. Under these conditions, the voltage across the electrode on charging/discharging on one side only was used to monitor the contact resistance. The results shown in Fig. 3 may be summarized as follows: 1. Cycling at elevated temperature causes an increase in irreversible capacity and in the potential for SEI film formation. Fig. 3. Charge discharge capacity versus cycle number for JM 287 graphite anodes. (a) Cycles at 65 C with cycling current of 6.5 ma/g. (b) After pre-cycling at 25 C for 17 cycles with further cycling at 65 C, with charge current of 5.1 ma/g.
5 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 4. Initial charge discharge profiles for JM 287 graphite with current of 5 ma/g at 25 C and 65 C. In some cases, the JM 287 graphite anode was compressed between two PTFE holders with small holes to allow penetration of electrolyte. 2. During the initial insertion of Li into JM 287 at 65 C, the potential first decreased to 1.08 V and then increased to 1.12 V, showing that the rate of electrolyte reduction was faster than the rate of charging. The high rate of electrolyte reduction may be caused by co-insertion of the electrolyte into graphite, giving an increase in voltage between the two sides of the electrode because of the contact resistance on charging on one side only. When the electrode was operated uncompressed, the voltage between the two sides of electrode could be as high as 1.0 V at the end of charge, while no potential plateau for Li insertion into graphite was observed. Finally the electrode broke up, resulting in electronic disconnection of graphite particles from the bulk. It was observed that much of the graphite was stripped from the Ni mesh substrate and fell to the bottom of the cell after the first cycle. Failure of graphite anodes due to disintegration with electronic disconnection has also been reported during initial Li insertion into graphite in PC [10] and PC-DMC (1:1.86) [11] solutions at room temperature. Thus, the performance of graphite electrodes in EC-DMC (1:1) at 65 C is similar to that in these electrolytes at room temperature. Compression of each side of the graphite electrode between two PTFE holders avoided the mechanical breakdown of the electrode, and gave a voltage difference of only 60 mv across the electrode at the end of charge at 0 V. Compression allows a greater quantity of graphite to become active, therefore to become involved in SEI film formation and in the co-intercalation of electrolyte. This increases the overall apparent charge capacity at potentials in excess of 0.2 V to more than 1100 mah/g. Further charging to 0.2 V resulted in an additional 450 mah/g, but only 10% of this was reversible. A probable explanation for this low reversible capacity is that continued SEI film growth (or electrolyte co-intercalation) occurs below 0.2 V because the film formed at 65 C is less passive [8]. Fig. 3 shows that the temperature at which initial SEI film formation occurs is critical in determining the cycling stability of graphite, and that EIS studies of the initial charging of JM 287 anodes at different temperatures are a convenient means of studying the phenomena involved Analysis of the first EIS (high-frequency) depressed semicircle A correct interpretation of the Nyquist plots obtained is imperative if EIS is to be used as a diagnostic tool. The special EIS protocols were applied to JM 287 graphite anodes to determine if the first depressed semicircle (i.e. that at the highest frequency) is related
6 1798 C. Wang et al. / Electrochimica Acta 46 (2001) to the SEI film or to contact impedances between both graphite particles and the current collectors as Chang et al. [7] suggest. In Fig. 2, R G, R RG, and R CG are the electronic graphite resistance, the reference electrode to anode ionic resistance, and the reference electrode to counter electrode ionic resistance. R pc, R pp, R SEI and R ct are the graphite particles-to-current collector, particles-toparticles, SEI film, and charge-transfer resistances. C pc and C pp are the particles-to-current collector and particles-to-particles contact capacitances. Q SEI and Q dl are constant-phase elements for the SEI film and for the double-layer respectively, and Z GFW is the generalized finite Warburg element for lithium diffusion in graphite. Here R SEI /Q SEI can be considered as three R/C circuits in series due to the SEI film of a multilayer structure, c.f., the similar equivalent circuit given by Levi and Aurbach [10]. From the arrangements in Fig. 2, it is clear that EIS obtained using Protocols A and D reflect the intrinsic graphite impedance and the graphite to electrolyte reaction impedance, respectively. The intrinsic impedance from Protocol A includes the electronic resistance of the series-parallel array of graphite particles, the transmission lines for the various contact resistances, viz. the current collector-to-particle impedances on one side, the particle-to-particle impedances through the electrode, and the particle-to-current collector impedances on the other side. The real parts of the arrays of electronic impedances for the graphite particles, for the particles-to-current collectors, and for particle-to-particle contact may be obtained from Protocol A EIS measurements by the intersections of the high-frequency line, and of the first and second semicircles, with the real axis, respectively [8,9]. The current corresponding to the electrochemical reaction of Li + using Protocol D must also go through the particle-to-particle, and particles-to-current collector arrays. Thus, the intrinsic impedance is in series with the reaction impedance, as is shown in the equivalent circuit of Fig. 2. The electrochemical impedance for Li insertion/extraction kinetics corresponds to two depressed semicircles with a low-frequency line for Li diffusion. The two depressed semicircles may be attributed to the impedance of the SEI film and to the charge-transfer reaction. If the electrochemical interface terminal WE is disconnected from the G 1 side of the electrode, the EIS using Protocol D becomes that for Protocol E. The graphite particle to current collector distance in Protocol E is effectively double that of Protocol D whereas the particle-current collector area is halved. The contact impedance in series with the reaction kinetic EIS in Protocol E should then be twice that for Protocol D. If terminal CE is moved from lithium foil counter-electrode to G 2 side of electrode in Protocol E, the EIS will become that for Protocol B. The transelectrode Li charge discharge current in Protocol E is replaced by an electronic transelectrode current in Protocol B. Hence, the EIS using Protocol B will be that for the intrinsic impedance involved in the kinetic EIS using Protocol E, minus the electrochemical kinetic impedance. Similarly, the EIS using Protocol E will becomes that using Protocol C if terminal RE 1 is moved from the Li reference electrode to the G 2 side of the graphite electrode. In protocol C, the transelectrode Li charge discharge AC current is induced an AC voltage of 5 mv amplitude. Therefore the EIS using Protocol C also reflects the intrinsic impedance involved in the EIS obtained using Protocol E. The intrinsic impedance in series with the reaction kinetic EIS in Protocol E, which can be measured by EIS using Protocol B and C, should be half of that measured using Protocol A. This may be explained as follows. In Protocol E, the electrochemical reaction current generated from graphite particles at the closer (G 1 ) side must go through the interposed graphite particles and through one particles-to-current collector boundary. However, the electrochemical current generated from the particles on the G 2 side must pass through the electrode thickness before being collected through the particles-to-current collector contact. Therefore the average intrinsic impedance series with the reaction kinetic EIS in Protocol E is half of the overall electronic impedance of the series-parallel array of graphite particles, the particle-to-particle contact resistance transmission line, plus the particles-to-current collector contact impedance. As stated earlier, the intrinsic resistance measured from Protocol A includes the entire electronic resistance of the series-parallel array of graphite particles, with that of the transmission line for the various contact impedances, i.e. the current collector-to-particles on one side, particle-to-particle, and the particlesto-current collector on the other side. If the particle-to-current collector impedances are similar for each side, then the intrinsic impedances measured using Protocols B and C should be equal to half of that using Protocol A. Finally, the intrinsic resistance should not be present in Protocol F (see Fig. 2) because it automatically eliminates the intrinsic resistance of the graphite electrode. The following results can therefore be expected from the six EIS protocols: 1. The intrinsic resistance using Protocol B should equal to that using Protocol C, but half of that using Protocol A. 2. The intrinsic resistance for Protocol F is zero, whereas that for Protocol D should be half of that for Protocol E. Therefore the EIS spectra for Protocol D should lie between those for Protocol E and F, since the intrinsic impedance is in series with reaction EIS (Fig. 2).
7 C. Wang et al. / Electrochimica Acta 46 (2001) The impedance using Protocol E should be equal to the sum of the impedances using Protocols F and B (or C). Fig. 5 shows the results of the six protocols for intrinsic and kinetic impedance measurements at 80 mv vs. Li/Li + on two JM 287 graphite electrodes. As expected, results on these two samples show that: 1. The impedance measured using Protocol D lies midway between those obtained using Protocols E and F. 2. The impedance measured using Protocol E is equal to the sum of the impedances using Protocols F and B (or C). 3. The intrinsic resistance measured using Protocol B is almost equal to that using Protocol C, 40 50% of that using Protocol A, the disparity being attributable to differences in contact impedance between the two sides of the electrode. The results in Fig. 5 strongly suggest that: 1. The intrinsic impedance associated with the reaction kinetic EIS can be measured using Protocols B and C. 2. About the half of total intrinsic resistance is in series with the reaction kinetic EIS. 3. The first depressed semicircle is associated with the SEI film, although its value is also influenced by the electrode contact impedance. The reason for this is as follows. If the first EIS depressed semicircle in kinetic impedance measurement is to graphite particle-to-current collector contact, the diameter of the first semicircle using Protocol E should be twice that for Protocol D (Fig. 2). This follows because the graphite particlecurrent collector area is doubled in the latter. In addition, the first semicircle should then not be present in Protocol F, since it automatically eliminates the intrinsic resistance of the electrode. However, the kinetic impedance results in Fig. 5 show that the first depressed semicircle is present in the Protocol F EIS, and is smaller than that for Protocol D. The first semicircle for Protocol D lies mid-way between those for F and E, but is not half of the size of that for Protocol E, as would be expected from Ref. [7]. To investigate SEI film formation and co-insertion of the electrolyte into graphite as a function of temperature, EIS of JM 287 electrodes was conducted during the first intercalation half-cycle at 25 C and 65 C. Electrode compression is important in determining the irreversible capacity in the first cycle at 65 C (Fig. 3), and commercial Li-ion electrodes are compressed. The JM 287 graphite anode used for EIS measurement was therefore compressed on each side by two PTFE holders containing small holes to allow penetration of electrolyte EIS measurements on JM 287 anodes during the initial charge at 25 C Fig. 6 shows the potential measured at the end of each charge current pulse (4 h, corresponding to 6.5 mah/g) and the apparent characteristic frequency as a function of charge capacity. The apparent characteristic frequency is defined by the frequency at the highest imaginary point of each semicircle. After 10 h relaxation time for each current pulse, EIS protocols A and D were used for intrinsic impedance and lithium insertion extraction kinetic measurement, respectively. Fig. 7a shows the reaction kinetics impedance measurement at different Li content. Fig. 7b and c show selected Nyquist plots for reaction kinetic and intrinsic impedances at the Li insertion levels specified in Fig. 6. The change in potential for JM 287 using the pulsed charging currents shown in Fig. 6 was similar to the potential change using continuous galvanostatic charging at 25 C shown in Fig. 4. However, the charge capacity in the former case was higher than that in the latter. This is in agreement with previous results, i.e. the use of a low charging current increases irreversible capacity [9]. Fig. 7a and b show that the shape of the Nyquist plot for kinetic Li insertion extraction at the open-circuit potential (OCP, point A in the Figs.) was a depressed semicircle in the high frequency region and a sloping line of variable gradient in the low frequency region. On Li intercalation, the dimensions of the depressed semicircles decrease, and a new depressed semicircle appears in the middle frequency region below 0.5 V (point D). Fig. 6c shows that the overall structure of the Nyquist plot for intrinsic impedance consists of a depressed semicircle with a reactance line. The resistance determined from the intersection of the high-frequency line with the real axis is the electronic resistance of the graphite, and the small semicircle corresponds to the particle-to-current collector contact impedance [9]. The high-frequency inductive reactance in the EIS used for intrinsic resistance measurement is an instrumental artifact which disappears when the measured resistance is high [9]. The contact resistance in the electrode decreases with Li content due to the volume expansion on lithiation. On initial Li insertion into JM 287, electronic resistance initially decreases as the potential falls from the OCP to 0.94 V (point B in Fig. 6c), then it begins to increase, reaching a maximum value at 0.63 V in the potential region where the SEI film is first formed. The initial decrease in the graphite electronic resistance may be attributed to removing impurities from the graphite surface, whereas the following increase may be due to co-insertion of electrolyte into the graphite during the process of SEI formation. Below 0.6 V (point C) the electronic resistance of the graphite again decreased because of the high conductivity of lithiated graphite [9]. The use of the special EIS proto-
8 Fig. 5. Nyquist plots for kinetics of lithium insertion extraction (a and c) and intrinsic impedance (b and d) of JM 287 graphite in 1.0 M LiPF 6, PC:EC:DMC (1:1:3) solution at 80 mv using different Solatron electrochemical interface terminal-electrode connections. (a) and (b) after 12 galvanostatic (5 ma/g) charge discharge cycles, (c) and (d) after five potentiostic charge discharge cycles at 1.5 and 0 V, following by 40 galvanostatic (5 ma/g) charge discharge cycles. Each potentiostic charge discharge step was initiated only when the current became less than 1 A C. Wang et al. / Electrochimica Acta 46 (2001)
9 C. Wang et al. / Electrochimica Acta 46 (2001) cols in the previous section shows that the first depressed semicircle consisted of a combination of contact and SEI film impedance. However, the large semicircle in Fig. 7b at the OCP cannot be explained in this manner since the SEI film had not yet formed and the contact resistance was very small (Fig. 7c) at the OCP (point A). Furthermore, the dimensions of the first semicircle in the high frequency region decrease with the amount of Li inserted in the potential region where SEI film formation occurs ( V). The only explanation accounting for these observations is that the second semicircle is related to that for the charge-transfer reaction, which initially relates to the impedance corresponding to the first semicircle at the OCP. On insertion of lithium, the first semicircle moves to the high-frequency region, while the second moves to the low-frequency region, leading to complete separation of the two at low potentials. This explanation is verified by the variation of the apparent characteristic frequency as a function of the amount of lithium inserted (Fig. 6), and by numerical analysis of EIS in Fig. 7a given in a later section. During further charge discharge cycling, the apparent characteristic frequencies of the two depressed semicircles were relatively stable, and both semicircles were affected by SEI film formation. Analysis of EIS data has the possibility of giving multiple interpretations of the reaction mechanism. However, if the data are analyzed logically, it is possible to minimize uncertainties. The logic used in this and previous work was to propose an impedance model based on experimental results obtained in this laboratory and elsewhere [6,7,10,12,13]. The common feature of the EIS for Li insertion extraction to and from graphite is the presence of two depressed semicircles, with a Warburg-like line corresponding to the finite diffusion of lithium in graphite particles. The results show that the first depressed semicircle is largely due to the SEI film impedance, increased somewhat by the contact resistance within the electrode. An equivalentcircuit model consisting of four or five parallel RC circuits was used to simulate the SEI film [6,10]. This describes a multilayer SEI film with a compact structure in the innermost layer and a porous structure in the Fig. 6. Potential and apparent characteristic frequency of two semicircles dependence of JM 287 anode as a function of charge capacity during initial charging at 25 C. The potentials were measured at the end of current pulses (6.5 ma/g for 4 h). After 10 h relaxation time at each point, EIS measurements for intrinsic and reaction resistance were obtained.
10 1802 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 7. Nyquist plots for (a) and (b), reaction kinetics and (c), intrinsic impedance measured at different degrees of intercalation levels during initial Li insertion into a JM 287 anode at 25 C. (b) is the selected EIS from (a) as specified in Fig. 6. (c) is the selected intrinsic impedance as specified in Fig. 6. EIS Protocol D was used for lithium insertion extraction kinetic measurement and Protocol A was used for intrinsic impedance measurement. The solid lines in (b) are the fitting results from the equivalent circuit
11 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 8. Equivalent circuit used for analysis of impedance spectra for initial Li intercalation into JM 287 graphite at 25 C and 65 C. R e and R G : ionic electrolyte and electronic graphite resistances. R 1 R 3 : resistances for Li + migration through each of the three model SEI film layers (R 1 includes the SEI graphite contact resistance). C 1 C 3 : the corresponding SEI layer capacitances (C 1 includes the contact impedance). R ct and Q dl : charge-transfer resistance and constant phase element related to double-layer capacitance. Z GFW : generalized finite Warburg impedance for solid-state Li diffusion in the bulk electrode. outer layer. This will result in a number of fitting parameters, which may give multiple results. Peled et al. suggested the use of a model consisting of three different layers (three parallel RC circuits) to fit their EIS data [12]. A series of three parallel RC circuits was therefore used in this work to attempt to fit the first depressed semicircle. An individual parallel RC to simulate contact impedance was not used, due to its very small value ( 0.01 in Fig. 7c), which increases the impedance of SEI layer to a small extent only in the highest frequency range. Since the intrinsic impedance is in series with the reaction kinetic impedance, any change in the electronic resistance of the graphite will affect the electrolyte resistance measured from the intersection of the high-frequency line with the real axis. The second semicircle in Fig. 7a and b is not a real parallel RC circuit, but a depressed semicircle. Hence, a constant phase element (CPE, symbol Q dl ) is used for simulating the EIS for the charge-transfer reaction [13]. The expression for the admittance response of the Q dl is: 1/Q dl =C dl n cos(n /2)+jC dl n sin(n /2) (1) where is the angular frequency, j= 1, C dl is considered to be a pseudo double layer capacitance (pseudo-c dl ) where n is not equal to 1, but lies between 0and1. The generalized finite Warburg element (GFW) was used to fit the lithium diffusion in graphit and may be expressed as follows: Z GFW =R tanh[( j T) ]/( j T) (2) where T is a time constant, R is resistance, and is an exponent. Fig. 8 depicts the equivalent circuit used to fit the impedance of the first Li insertion extraction cycle of JM 287 graphite. A step by step, double-check fitting method was used in the numerical analysis of the impedance spectra during the first charge process. In this, the fitted parameter values in the previous step (with lower lithium content) were used as initial values for the following step, and then the fitted parameters in the following step were used as initial values to fit the spectra of the previous step. The two processes were repeated until the values of the parameters fitted became the same by fitting in both the forward and backward directions. The relative standard deviation for all parameters fitted did not exceed 15%. Some simulated impedance spectra for the first charge at room temperature are compared with experiment in Fig. 7b. The proposed model describes the experimental data satisfactorily for different degrees of intercalation. The parameters obtained from fitting the room-temperature data for the first charge process are shown in Fig. 9. The resistances of all three SEI layers have a similar value around However, the capacitances of the three SEI layers, which are inversely proportional to their thicknesses, are quite different. The small capacitance (C 1 ) may be attributed to that of the compact inner layer (in contact with graphite) and the larger capacitance (C 3 ) to that of the outer porous layer (in contact with the electrolytic solution), because
12 1804 C. Wang et al. / Electrochimica Acta 46 (2001) the latter has the larger surface area [6]. The very small value of the three resistances (R 1 R 3 ) and the larger values of the three capacitances (C 1 C 3 )attheocp indicate that the semicircle represents the impedance of the charge-transfer reaction. On charging, the capacitances of the three SEI layers rapidly decrease on going from the OCP to 0.8 V. The rate of decrease becomes slower during further charge to 0.6 V. The changes in Fig. 9. Parameter values obtained from fitting the experimental impedance spectra of JM 287 graphite during initial charge (Li insertion) at 25 C. The double-layer capacitance C dl is considered to be a pseudo-c dl because the parameter n is around 0.5, not 1.
13 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 9. (Continued). the capacitance values indicate that the SEI film forms between OCP and 0.8 V, and then further growth occurs during charge to 0.6 V. SEI formation can also be inferred from the increase in resistances R 1 R 3 in the potential range from OCP to 0.8 V. On further charging, R 1 continues to increase, but R 2 and R 3 begin to decrease. The decrease in R 2 and R 3 may be associated with electrolyte co-insertion into graphite [15],
14 1806 C. Wang et al. / Electrochimica Acta 46 (2001) giving a volume expansion resulting in a decrease in the resistivity and in the thickness of the less compact middle and outer layers of the SEI film. Compression resulting from electrolyte co-insertion in the potential range V decreases the thickness of the outer porous layer, giving an increase in C 3. When lithium insertion begins at less than 0.2V, the thicknesses of the three layers become relatively stable, with some scatter for C 3. The influence of the contact impedance of graphite on the SEI impedance is very small because the contact resistance change during first Li insertion process ( 0.01 in Fig. 6c) is much smaller than that for R 1, which lies between 0.2 and 1.5. The above analysis of the formation and growth of the SEI film on graphite using EIS is in agreement with results observed by atomic force microscopy [16] and SEM [17]. SEI film formation also affects the charge transfer reaction at the graphite-sei film interface. In Fig. 9 the double layer capacitance C dl is considered to be a pseudo capacitance because n is not equal to 1. During the first charge, the pseudo-c dl increases somewhat in the SEI film formation potential range ( V). Then increases is more rapidly at lithium insertion potentials around 0.2 V, and then remains essentially stable (with some scattered points) on further Li insertion. The charge transfer resistance initially increases during SEI formation, and then decreases from 0.6 to 0.2 V, stabilizing at below 0.2 V. The small increase in pseudo-c dl but rapid decreases in R ct as potential goes from 0.6 to 0.2 V may be explained by an increase in the contact area between graphite and the SEI film as a result of electrolyte co-intercalation into graphite. Electrolyte co-intercalation begins at the edge of the graphite planes [15], opening them and increasing the contact area between the graphite and the SEI film. The results shown in Fig. 9 also confirm that the decrease in capacitance of each SEI layer and the increase in the pseudo-c dl value during initial lithium insertion into graphite are associated with a depressed SEI semicircle. The latter moves to the high frequency region, while the depressed charge-transfer semicircle moves to the low frequency region. Both finally become separated below 0.5 V. Lithium insertion into graphite decreases the graphite electronic (R G ) resistance, but by-product contamination of the electrolyte due to its reduction during SEI formation [14] will increase the contact resistance (R e ). This results in a change in R e +R G during SEI formation and in the Li insertion region EIS measurements on JM 287 anodes during initial charging at 65 C Another identical JM 287 electrode was pulse charged at 65 C. Fig. 9 shows that the measured potential and the apparent characteristic frequency at the end of each charge current pulse (4 h, 6.5 ma/g) at 65 C as a function of charge capacity. After 10 h relaxation time for each current pulse, EIS Protocols A and C were used for lithium insertion extraction kinetic and intrinsic impedance measurement, respectively. Fig. 11a shows the reaction kinetics impedance measurement at different Li content. Fig. 11b e show selected Nyquist plots to determine the reaction kinetics and intrinsic resistances at the Li insertion levels specified in Fig. 10. Experimental data are given as dots, and fitted results are solid lines. The reaction kinetics and intrinsic impedance after 1 day on stand at 25 C (point B in Fig. 11b) were almost the same as those at the OCP in Fig. 7b (point A), confirming that the two JM 287 electrodes were identical. To investigate the influence of SEI film formation at 25 C on behavior at 65 C, the JM 287 electrode was first pulsed charge at 65 C to 350 mah/g, and then was cooled to 25 C. It was then further charged at this temperature to 90 mv versus Li/Li +, where the SEI film was completely formed. The cell was then heated to 65 C, and further charged with same pulsed current. The negative values in Figs. 10 and 11a correspond to the relaxation time imposed after the electrode was assembled in the glove box at 25 C. Over 1 day of relaxation at 25 C, the potential of the JM 287 potential gradually increased from 2.8 to 2.95 V (Fig. 10). However, the apparent characteristic frequency was stable at 516 Hz, and the reaction resistance increased slightly (Fig. 11b). The intrinsic resistance decreased, with a change in its impedance plot from two semicircles to one small semicircle (Fig. 11c). The two intrinsic impedance semicircles are associated with particle-tocurrent collector and particle-to-particle contact impedances [9]. The decrease in intrinsic resistance with time may result from electrolyte impregnation into the electrode porosity, and perhaps to reaction with impurities. When the electrode was charged with a pulsed current at 65 C, the potential rapidly decreased, then stabilized at 1.1 V (Fig. 10). The apparent characteristic frequency of the first semicircle in Fig. 10 increased during the change in potential from 2.95 to 1.1 V, and then began to decrease on further charging at 1.1 V. This behavior is quite different from that at 25 C (Fig. 6). The existence of a potential plateau at 1.1 V indicates that no passive SEI film formed during this period. The passive SEI film formed after charging at 25 C, covered now by new film, was not useful in initiating passive SEI film formation during further charging at 65 C. This is clear because the potential was still at ca. 1.1 V after the sample was charged again at 65 C. However, the film did inhibit electrolyte co-insertion into graphite, as the numerical analysis in the following section shows. This did improve the cycling capacity stability of JM 287 at 65 C, as Fig. 3 shows. The reaction impedance during charge at 65 C (Fig.
15 C. Wang et al. / Electrochimica Acta 46 (2001) d) shows an increase in resistance of the semicircles during the initial charge at 65 C, followed by a small decrease during the second charge. The intrinsic resistance of the graphite showed an increase during initial charge cycles at 65 C, following by a decrease and then increase again during the second charge (Fig. 11e). The increase in intrinsic and reaction resistances during the first charge at 65 C may be related to electrolyte co-insertion into graphite. The decrease in the same resistances during the second charge may be explained by the conversion of SEI film from a metastable to a stable phase [8]. A continued increase in intrinsic resistance was observed during charge at 25 C after an initial charge of 350 mah/g at 65 C, indicating different behavior from that shown in Fig. 7c. Fig. 10. Potential and apparent characteristic frequency of semicircle dependence of a JM 287 anode on charging capacity during the initial charge process at 65 C. The potentials were measured at the end of current pulses (6.5 ma/g for 4 h). After 10 h relaxation time at each point, EIS measurements for intrinsic and reaction resistance were obtained. After pulsed charging at 65 C to 350 mah/g, the electrode was cooled to 25 C and further charged to 90 mv, at which the SEI film was completely formed. The graphite was then heated to 65 C and further charged at the same pulse current. The negative time corresponds to the time the graphite electrode was assembled in the glove box at 25 C.
16 1808 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 11. Nyquist plots for (a), (b), and (d) reaction kinetics and (c) and (e) intrinsic impedance measured at different degrees of intercalation levels during initial Li insertion into a JM 287 anode at 25 C and 65 C. (b) and (d) are the selected EIS from (a), which is specified in Fig. 10. (c) and (e) are the selected intrinsic impedance specified in Fig. 10. EIS Protocol D was used for measurement of lithium insertion extraction kinetics and Protocol A was used for intrinsic impedance measurement. The solid lines in (b) are the
17 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 11. (Continued). To gain insight into the changes in JM 287 impedance in Fig. 11a, numerical analysis were performed using the equivalent circuit in Fig. 8. The change of parameters as determined from analysis of impedance spectra in Fig. 10a is shown in Fig. 12. During the first charge from the OCP to 1.1 V at 65 C, the three SEI capacitances rapidly decreased, but the corresponding resistances were almost constant except for an initial increase from the OCP to 2.38 V. This indicates that an SEI film with a very thin inner layer and a porous outer layer was formed on the graphite surface. As in charging at 25 C, the pseudo-c dl increased in this potential range. Further charging at the 1.1 V plateau initially produced a further decrease in the three SEI capacitances, followed by an increase, with a continuous decrease in the pseudo-c dl throughout the potential region. This change can be explained by co-insertion of electrolyte. The capacitance of each layer SEI can be approximately calculated using the following equation: C i = o A/d i (3) where d i is the thickness of the ith layer of the SEI film, o is the vacuum permittivity, is the general permittivity, and A is the electrode area. The increase in capacitance of the innermost layer may be explained by the increase in SEI film thickness, along with further electrolyte co-insertion into graphite. The latter exfoliates the graphite planes, giving graphite SEI film mixtures or graphite dust [10] with porous inner layer structures of increased area and capacitance. As at 25 C (Fig. 9), C 3 will also increase in the potential range for electrolyte co-intercalation at 65 C due to the resulting volume expansion. Initial electrolyte co-insertion into the graphite planes at 65 C forms a zigzag interface between the SEI film and graphite [15] (as at 25 C), resulting in an increase in the contact area between the SEI and graphite. This leads to an initial increase in C dl but a decrease in R ct.in contrast, further electrolyte co-insertion into the graphite planes at 65 C exfoliates the graphite layers, giving a reduced contact area between the active bulk graphite and SEI, which results in the increase in R ct and the associated decrease in pseudo-c dl, as Fig. 12 shows. Co-insertion of electrolyte also results in a large
18 1810 C. Wang et al. / Electrochimica Acta 46 (2001) increase in intrinsic resistance, as is shown at points I, J, and K in Fig. 11e. Other evidence for electrolyte co-intercalation is that the discharge capacity of the graphite on its first discharge under these conditions is only about 80 mah/g, compared to 300 mah/g for graphite charged at 25 C. The low discharge capacity is not due to mechanical breakdown of the graphite electrode because the contact resistance is still about 6 during the first charge (Fig. 11e). Some graphite powder was found at the bottom of the cell after the first cycle, which may result from the co-intercalation of electrolyte into graphite. The increase in the characteristic frequency of the charge-transfer process is associated with in an decrease in pseudo-c dl, which results in the mingling of the charge transfer and SEI film semicircles, again very different behavior from that at 25 C. In addition, the amount of co-insertion of electrolyte is small at 25 C, giving a small reduction in C 3, associated with decreasing resistances in the SEI film layers and in R ct and increasing pseudo-c dl in the co-insertion potential region. The passive SEI layer at 25 C was considered to be completely formed after charging to a potential of 90 mv. The values R 2, R 3 and R ct at 65 C were at least five times higher than at 25 C (Fig. 9). Thus, the layer produced by pre-charging at 65 C to 350 mah/g, followed by charging at 25 C was at least five times thicker than that formed at 25 C. A comparison of the C 3 values at 90 mv in Figs. 9 and 12 shows that the thickness of porous layer formed after pre-charging at Fig. 12. Parameter values obtained from fitting the experimental impedance spectra in Fig. 11a during the initial charge at 25 C and 65 C using the equivalent circuit of Fig. 8 and the procedure in Fig. 7.
19 C. Wang et al. / Electrochimica Acta 46 (2001) Fig. 12. (Continued). 65 C was three times greater than that produced at 25 C. The fact that charging at 25 C following initial charging at 65 C limited electrolyte co-insertion during a following charge at 65 C is shown by the decrease in C 1, with R ct,, C 3, and the intrinsic resistance remaining stable during the second charge cycle at 65 C (Fig. 11e). A large decrease in C 1 C 3 but an increase in R 1 R 3 and R ct, which resulted from the growth of SEI film, were observed during the following 30 charge discharge cycles. The unstable SEI film also increased the intrinsic resistance, leading to a separation of graphite particles. The intrinsic impedance was not included in the equivalent circuit in Fig. 8 for the reasons already discussed. The contact resistances during the first charge at 65 C and after charging at 25 C were within 0.5 (Fig. 11c and e), and only 25% of this value ( 0.13 ) will affect the reaction resistance as measured by Protocol D [8]. The total resistance of the first SEI film semicircle was higher than 4 (Fig. 12). Hence, the relative error in the analysis of a SEI film using the equivalent circuit in Fig. 8 was smaller than 4%, increasing to 10% during the second charge cycle at 65 C. Finally, the thermal stability of the electrolyte at 65 C was monitored in situ by measuring the impedance of a floating Pd wire electrode. Selected Nyquist plots for this electrode in the high frequency
In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li 0.35 TiO 3 Ceramic at Different Li Insertion Levels
 A1196 Journal of The Electrochemical Society, 151 8 A1196-A1201 2004 0013-4651/2004/151 8 /A1196/6/$7.00 The Electrochemical Society, Inc. In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li
A1196 Journal of The Electrochemical Society, 151 8 A1196-A1201 2004 0013-4651/2004/151 8 /A1196/6/$7.00 The Electrochemical Society, Inc. In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li
In situ investigation of electrochemical lithium intercalation into graphite powder
 www.elsevier.nl/locate/jelechem Journal of Electroanalytical Chemistry 489 (2000) 55 67 In situ investigation of electrochemical lithium intercalation into graphite powder Chunsheng Wang a, *, Imran Kakwan
www.elsevier.nl/locate/jelechem Journal of Electroanalytical Chemistry 489 (2000) 55 67 In situ investigation of electrochemical lithium intercalation into graphite powder Chunsheng Wang a, *, Imran Kakwan
Supplemental Information for:
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplemental Information for: A Novel Lithium-sulfur Battery Cathode from Butadiene Rubber-caged
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplemental Information for: A Novel Lithium-sulfur Battery Cathode from Butadiene Rubber-caged
Capacity fade study of lithium-ion batteries cycled at high discharge rates
 Journal of Power Sources 117 (2003) 160 169 Capacity fade study of lithium-ion batteries cycled at high discharge rates Gang Ning, Bala Haran, Branko N. Popov * Department of Chemical Engineering, University
Journal of Power Sources 117 (2003) 160 169 Capacity fade study of lithium-ion batteries cycled at high discharge rates Gang Ning, Bala Haran, Branko N. Popov * Department of Chemical Engineering, University
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES
 PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES A. Concheso, R. Santamaría, R. Menéndez, R. Alcántara #, P. Lavela #, J.L. Tirado # Instituto Nacional del Carbón (CSIC), Apdo.
PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES A. Concheso, R. Santamaría, R. Menéndez, R. Alcántara #, P. Lavela #, J.L. Tirado # Instituto Nacional del Carbón (CSIC), Apdo.
Dynamic and Galvanic Stability of Stretchable Supercapacitors
 Supporting Information for Dynamic and Galvanic Stability of Stretchable Supercapacitors By Xin Li, Taoli Gu and Bingqing Wei* Department of Mechanical Engineering, University of Delaware, Newark, DE 19716
Supporting Information for Dynamic and Galvanic Stability of Stretchable Supercapacitors By Xin Li, Taoli Gu and Bingqing Wei* Department of Mechanical Engineering, University of Delaware, Newark, DE 19716
Supplementary Figure 1:
 b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from
 Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from polyimide used to seal Titanium (Ti) current collectors
Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from polyimide used to seal Titanium (Ti) current collectors
School of Materials Science and Engineering, South China University of Technology,
 Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
Chapter 7. Evaluation of Electrode Performance by. Electrochemical Impedance
 Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Improving cyclic performance of Si anode for lithium-ion batteries by forming an intermetallic skin
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Re-building Daniell Cell with a Li-Ion exchange Film
 Supplementary Information Re-building Daniell Cell with a Li-Ion exchange Film Xiaoli Dong, Yonggang Wang*, Yongyao Xia Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative
Supplementary Information Re-building Daniell Cell with a Li-Ion exchange Film Xiaoli Dong, Yonggang Wang*, Yongyao Xia Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative
High-Performance All-Solid-State Lithium-Sulfur. Battery Enabled by a Mixed-Conductive Li 2 S
 Supporting information High-Performance All-Solid-State Lithium-Sulfur Battery Enabled by a Mixed-Conductive Li 2 S Nanocomposite Fudong Han, Jie Yue, Xiulin Fan, Tao Gao, Chao Luo, Zhaohui Ma, Liumin
Supporting information High-Performance All-Solid-State Lithium-Sulfur Battery Enabled by a Mixed-Conductive Li 2 S Nanocomposite Fudong Han, Jie Yue, Xiulin Fan, Tao Gao, Chao Luo, Zhaohui Ma, Liumin
EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY
 EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY Weiming Lu and D.D.L. Chung Composite Materials Research Laboratory University at Buffalo The State
EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY Weiming Lu and D.D.L. Chung Composite Materials Research Laboratory University at Buffalo The State
How initial nucleation influences discharge capacities of Li-O 2 cells
 How initial nucleation influences discharge capacities of Li-O 2 cells Ali Rinaldi 1, Olivia Wijaya 1, Denis Yu 2, Harry.E. Hoster 1 1TUM CREATE Centre for Electromobility #10-02 CREATE Tower, Singapore
How initial nucleation influences discharge capacities of Li-O 2 cells Ali Rinaldi 1, Olivia Wijaya 1, Denis Yu 2, Harry.E. Hoster 1 1TUM CREATE Centre for Electromobility #10-02 CREATE Tower, Singapore
Cycle life performance of lithium-ion pouch cells
 Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Supporting information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting information An amorphous material with sponge-like structure as anode for Liion and
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting information An amorphous material with sponge-like structure as anode for Liion and
Electronic supplementary information. Efficient energy storage capabilities promoted by hierarchically MnCo 2 O 4
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral
 Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries
Supporting Information
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information High performance All-Solid-State Li-Se Batteries induced
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information High performance All-Solid-State Li-Se Batteries induced
FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE POROSITY EVOLUTION MODEL
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016)
 6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
Novel Materials for Lithium-Ion Batteries
 Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells
 Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Final Report for AOARD Grant FA Lithium-air Battery Research. December 2009
 Final Report for AOARD Grant FA 4869-7-1-49 Lithium-air Battery Research December 29 Name of Principal Investigators: Prof. N. Munichandraiah - e-mail address : muni@ipc.iisc.ernet.in - Institution : Indian
Final Report for AOARD Grant FA 4869-7-1-49 Lithium-air Battery Research December 29 Name of Principal Investigators: Prof. N. Munichandraiah - e-mail address : muni@ipc.iisc.ernet.in - Institution : Indian
The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning
 2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
Application Note. Application Note. Electrochemical Impedance Spectroscopy - A Battery Monitoring and Fault Diagnostic Tool Introduction
 Application Note Electrochemical Impedance Spectroscopy - A Battery Monitoring and Fault Diagnostic Tool Introduction Application Note Integration of new battery technologies for portable devices and automotive
Application Note Electrochemical Impedance Spectroscopy - A Battery Monitoring and Fault Diagnostic Tool Introduction Application Note Integration of new battery technologies for portable devices and automotive
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Surface graphited carbon scaffold enables simple
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Surface graphited carbon scaffold enables simple
Supporting Information
 Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Supplementary Information
 Supplementary Information Facile growth of hierarchical hematite ( -Fe 2 O 3 ) nanopetals on FTO by pulse reverse electrodeposition for photoelectrochemical water splitting Pravin S. Shinde, Geun Ho Go
Supplementary Information Facile growth of hierarchical hematite ( -Fe 2 O 3 ) nanopetals on FTO by pulse reverse electrodeposition for photoelectrochemical water splitting Pravin S. Shinde, Geun Ho Go
A design of Solid-State Li-S cell with evaporated Lithium anode to eliminate shuttle effects
 Supporting Information A design of Solid-State Li-S cell with evaporated Lithium anode to eliminate shuttle effects Yujie Hao, a Sheng Wang, a Feng Xu, a Yijie Liu, a Ningning Feng, a Ping He, *a Haoshen
Supporting Information A design of Solid-State Li-S cell with evaporated Lithium anode to eliminate shuttle effects Yujie Hao, a Sheng Wang, a Feng Xu, a Yijie Liu, a Ningning Feng, a Ping He, *a Haoshen
In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries
 Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
All-solid-state Batteries with Thick Electrode Configurations
 All-solid-state Batteries with Thick Electrode Configurations Yuki Kato, * Shinya Shiotani, Keisuke Morita, Kota Suzuki, Masaaki Hirayama, Ryoji Kanno Toyota Motor Europe NV/SA, Hoge Wei 33, 1930 Zaventem,
All-solid-state Batteries with Thick Electrode Configurations Yuki Kato, * Shinya Shiotani, Keisuke Morita, Kota Suzuki, Masaaki Hirayama, Ryoji Kanno Toyota Motor Europe NV/SA, Hoge Wei 33, 1930 Zaventem,
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201704947 Bioinspired, Spine-Like, Flexible, Rechargeable Lithium-Ion
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201704947 Bioinspired, Spine-Like, Flexible, Rechargeable Lithium-Ion
High energy all-solid-state lithium batteries with
 Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
Rechargeable Sodium-ion Batteries with a Cl-doped
 Supplementary Information: Room-Temperature All-solidstate Rechargeable Sodium-ion Batteries with a Cl-doped Na 3 PS 4 Superionic Conductor Iek-Heng Chu a, Christopher Kompella a, Han Nguyen a, Zhuoying
Supplementary Information: Room-Temperature All-solidstate Rechargeable Sodium-ion Batteries with a Cl-doped Na 3 PS 4 Superionic Conductor Iek-Heng Chu a, Christopher Kompella a, Han Nguyen a, Zhuoying
Supporting Information. Amorphous Red Phosphorus Embedded in Highly Ordered. Mesoporous Carbon with Superior Lithium and Sodium Storage.
 Supporting Information Amorphous Red Phosphorus Embedded in Highly Ordered Mesoporous Carbon with Superior Lithium and Sodium Storage Capacity Weihan Li, Zhenzhong Yang, Minsi Li, Yu Jiang, Xiang Wei,
Supporting Information Amorphous Red Phosphorus Embedded in Highly Ordered Mesoporous Carbon with Superior Lithium and Sodium Storage Capacity Weihan Li, Zhenzhong Yang, Minsi Li, Yu Jiang, Xiang Wei,
SUPPORTING INFORMATION. A Rechargeable Aluminum-Ion Battery Based on MoS 2. Microsphere Cathode
 SUPPORTING INFORMATION A Rechargeable Aluminum-Ion Battery Based on MoS 2 Microsphere Cathode Zhanyu Li a, Bangbang Niu a, Jian Liu a, Jianling Li a* Feiyu Kang b a School of Metallurgical and Ecological
SUPPORTING INFORMATION A Rechargeable Aluminum-Ion Battery Based on MoS 2 Microsphere Cathode Zhanyu Li a, Bangbang Niu a, Jian Liu a, Jianling Li a* Feiyu Kang b a School of Metallurgical and Ecological
ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency
 ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency Elmira Memarzadeh Lotfabad a, Peter Kalisvaart a,*, Kai Cui b, Alireza Kohandehghan
ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency Elmira Memarzadeh Lotfabad a, Peter Kalisvaart a,*, Kai Cui b, Alireza Kohandehghan
Factors Governing Life of High-Energy Lithium-Ion Cells
 Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Factors Governing Life of High-Energy Lithium-Ion Cells D.P. Abraham IBA 2013 March 11, 2013 Barcelona, Spain Research sponsors are both Government and Private Sector 2 Diagnostics Overview Use of characterization
Study of lithium glassy solid electrolyte/electrode interface by impedance analysis
 Bull. Mater. Sci., Vol. 23, No. 3, June 2, pp. 179 183. Indian Academy of Sciences. Study of lithium glassy solid electrolyte/electrode interface by impedance analysis A KARTHIKEYAN, P VINATIER and A LEVASSEUR*
Bull. Mater. Sci., Vol. 23, No. 3, June 2, pp. 179 183. Indian Academy of Sciences. Study of lithium glassy solid electrolyte/electrode interface by impedance analysis A KARTHIKEYAN, P VINATIER and A LEVASSEUR*
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Deterioration mechanism of LiNi 0.8 Co 0.15 Al 0.05
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Deterioration mechanism of LiNi 0.8 Co 0.15 Al 0.05
Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries
 /8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
/8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
Supporting Information
 Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
SUPPLEMENTARY INFORMATION. Elucidating the Alkaline Oxygen Evolution Reaction Mechanism on Platinum
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 SUPPLEMENTARY INFORMATION Elucidating the Alkaline Oxygen Evolution Reaction
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 SUPPLEMENTARY INFORMATION Elucidating the Alkaline Oxygen Evolution Reaction
MXene-Bonded Activated Carbon as a Flexible. Electrode for High-Performance Supercapacitors
 Supporting information MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors Lanyong Yu, Longfeng Hu, Babak Anasori, Yi-Tao Liu, Qizhen Zhu, Peng Zhang, Yury Gogotsi,
Supporting information MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors Lanyong Yu, Longfeng Hu, Babak Anasori, Yi-Tao Liu, Qizhen Zhu, Peng Zhang, Yury Gogotsi,
Final Report for AOARD Grant AOARD Carbon-coated current collectors for high-power Li-ion secondary batteries /20
 Final Report for AOARD Grant AOARD-10-4155 Carbon-coated current collectors for high-power Li-ion secondary batteries 2011.9/20 Name of Principal Investigators: - e-mail address : nlw001@ntu.edu.tw - Institution
Final Report for AOARD Grant AOARD-10-4155 Carbon-coated current collectors for high-power Li-ion secondary batteries 2011.9/20 Name of Principal Investigators: - e-mail address : nlw001@ntu.edu.tw - Institution
Three-dimensional graphene-based hierarchically porous carbon. composites prepared by a dual-template strategy for capacitive
 Electronic Supplementary Information (ESI) Three-dimensional graphene-based hierarchically porous carbon composites prepared by a dual-template strategy for capacitive deionization Xiaoru Wen, a Dengsong
Electronic Supplementary Information (ESI) Three-dimensional graphene-based hierarchically porous carbon composites prepared by a dual-template strategy for capacitive deionization Xiaoru Wen, a Dengsong
Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes
 Manuscript# IMLB-070, LBNL-52142 Revised to Journal of Power Sources (Dec 13, 2002) Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes Seung-Wan Song, a Kathryn A. Striebel, a,z Xiangyun Song a
Manuscript# IMLB-070, LBNL-52142 Revised to Journal of Power Sources (Dec 13, 2002) Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes Seung-Wan Song, a Kathryn A. Striebel, a,z Xiangyun Song a
Topic: Electrochemical Application of Carbon Materials MILD-EXFOLIATED GRAPHITE AS AN ANODE MATERIAL FOR LITHIUM ION BATTERY.
 Paper ID: 373 Topic: Electrochemical Application of Carbon Materials MILD-EXFOLIATED GRAPHITE AS AN ANODE MATERIAL FOR LITHIUM ION BATTERY Lin Zou, Yong-Ping Zheng, Feiyu Kang, Wanci Shen, Can Xu Laboratory
Paper ID: 373 Topic: Electrochemical Application of Carbon Materials MILD-EXFOLIATED GRAPHITE AS AN ANODE MATERIAL FOR LITHIUM ION BATTERY Lin Zou, Yong-Ping Zheng, Feiyu Kang, Wanci Shen, Can Xu Laboratory
Hierarchical 3D ZnCo 2 O 4 Nanowire Arrays/Carbon Cloth Anodes for A Novel Class of High-Performance Flexible Lithium-ion Batteries
 Supporting Information Hierarchical 3D ZnCo 2 O 4 Nanowire Arrays/Carbon Cloth Anodes for A Novel Class of High-Performance Flexible Lithium-ion Batteries Bin Liu, Jun Zhang, Xianfu Wang, Gui Chen, Di
Supporting Information Hierarchical 3D ZnCo 2 O 4 Nanowire Arrays/Carbon Cloth Anodes for A Novel Class of High-Performance Flexible Lithium-ion Batteries Bin Liu, Jun Zhang, Xianfu Wang, Gui Chen, Di
Supporting Information
 Supporting Information Earth Abundant Fe/Mn-Based Layered Oxide Interconnected Nanowires for Advanced K-Ion Full Batteries Xuanpeng Wang, Xiaoming Xu, Chaojiang Niu*, Jiashen Meng, Meng Huang, Xiong Liu,
Supporting Information Earth Abundant Fe/Mn-Based Layered Oxide Interconnected Nanowires for Advanced K-Ion Full Batteries Xuanpeng Wang, Xiaoming Xu, Chaojiang Niu*, Jiashen Meng, Meng Huang, Xiong Liu,
SUPPLEMENTARY INFORMATION
 Electrochemical stiffness in lithium-ion batteries Hadi Tavassol 1#, Elizabeth M. C. Jones 2,4#, Nancy R. Sottos 3,4*, and Andrew A. Gewirth 1* 1 Department of Chemistry, 2 Department of Mechanical Science
Electrochemical stiffness in lithium-ion batteries Hadi Tavassol 1#, Elizabeth M. C. Jones 2,4#, Nancy R. Sottos 3,4*, and Andrew A. Gewirth 1* 1 Department of Chemistry, 2 Department of Mechanical Science
Corrosion Rate Measurement on C-Steel
 Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Nitrogen-Doped Graphdiyne Applied for Lithium-
 Supporting Information for Nitrogen-Doped Graphdiyne Applied for Lithium- Ion Storage Shengliang Zhang,, Huiping Du,, Jianjiang He,, Changshui Huang,*, Huibiao Liu, Guanglei Cui and Yuliang Li Qingdao
Supporting Information for Nitrogen-Doped Graphdiyne Applied for Lithium- Ion Storage Shengliang Zhang,, Huiping Du,, Jianjiang He,, Changshui Huang,*, Huibiao Liu, Guanglei Cui and Yuliang Li Qingdao
Supporting Information
 Supporting Information Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO 3 Yifan Dong, Tyler Slade, Matthew J. Stolt, Linsen Li, Steven N. Girard, Liqiang
Supporting Information Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO 3 Yifan Dong, Tyler Slade, Matthew J. Stolt, Linsen Li, Steven N. Girard, Liqiang
Kinetic Characteristics of Different Materials used for Bolting Applications
 Kinetic Characteristics of Different Materials used for Bolting Applications Report Kinetic Characteristics of Different Materials used for Bolting Applications Overview One of the most common problems
Kinetic Characteristics of Different Materials used for Bolting Applications Report Kinetic Characteristics of Different Materials used for Bolting Applications Overview One of the most common problems
Carbon Nanofiber Modified Graphite Electrode. Performance for Lithium Ion Secondary Battery
 Carbon Nanofiber Modified Graphite Electrode Performance for Lithium Ion Secondary Battery Seung-Hwan Moon, Myung-Soo Kim Department of Chemical Engineering, Myoungji University, Korea Corresponding author
Carbon Nanofiber Modified Graphite Electrode Performance for Lithium Ion Secondary Battery Seung-Hwan Moon, Myung-Soo Kim Department of Chemical Engineering, Myoungji University, Korea Corresponding author
Unlocking the Potential of Amorphous Red Phosphorus Films as Long-term Stable. Negative Electrode for the Lithium Battery
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Unlocking the Potential of Amorphous Red Phosphorus
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Unlocking the Potential of Amorphous Red Phosphorus
Impedance Behavior of LSCF/YDC/LSCF Symmetrical Half Cell Prepared by Plasma Spray
 Impedance Behavior of /YDC/ Symmetrical Half Cell Prepared by Plasma Spray Z. Stoynov 1, D. Vladikova 1, G. Raikova 1*, D. Soysal 2, Z. Ilhan 2, S. Ansar 2 1 Institute of Electrochemistry and Energy Systems
Impedance Behavior of /YDC/ Symmetrical Half Cell Prepared by Plasma Spray Z. Stoynov 1, D. Vladikova 1, G. Raikova 1*, D. Soysal 2, Z. Ilhan 2, S. Ansar 2 1 Institute of Electrochemistry and Energy Systems
Supplementary Information
 Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2017 Supplementary Information Self-Standing Bi 2 O 3 Nanoparticles/Carbon Nanofiber
Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2017 Supplementary Information Self-Standing Bi 2 O 3 Nanoparticles/Carbon Nanofiber
Supplementary Information. Capacity fade in high energy silicon-graphite electrodes for lithium-ion batteries
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Information Capacity fade in high energy silicon-graphite electrodes for lithium-ion
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Information Capacity fade in high energy silicon-graphite electrodes for lithium-ion
Lithium Batteries with Nearly Maximum Metal. Storage Supporting Information
 Lithium Batteries with Nearly Maximum Metal Storage Supporting Information Abdul-Rahman O. Raji,, Rodrigo Villegas Salvatierra,, Nam Dong Kim, Xiujun Fan, Yilun Li, Gladys A. L. Silva, Junwei Sha and James
Lithium Batteries with Nearly Maximum Metal Storage Supporting Information Abdul-Rahman O. Raji,, Rodrigo Villegas Salvatierra,, Nam Dong Kim, Xiujun Fan, Yilun Li, Gladys A. L. Silva, Junwei Sha and James
Electronic Supporting Information. Synthesis of single crystalline hexagonal nanobricks of
 Electronic Supporting Information Synthesis of single crystalline hexagonal nanobricks of LiNi 1/3 Co 1/3 Mn 1/3 O 2 with high percentage of exposed {010} active facets as high rate performance cathode
Electronic Supporting Information Synthesis of single crystalline hexagonal nanobricks of LiNi 1/3 Co 1/3 Mn 1/3 O 2 with high percentage of exposed {010} active facets as high rate performance cathode
Final Report for FA Carbon-coated current collectors for high-power Li-ion secondary batteries /29
 Final Report for FA2386-11-1-4100 Carbon-coated current collectors for high-power Li-ion secondary batteries 2012.8/29 Name of Principal Investigators: - e-mail address : nlw001@ntu.edu.tw - Institution
Final Report for FA2386-11-1-4100 Carbon-coated current collectors for high-power Li-ion secondary batteries 2012.8/29 Name of Principal Investigators: - e-mail address : nlw001@ntu.edu.tw - Institution
De-ionized water. Nickel target. Supplementary Figure S1. A schematic illustration of the experimental setup.
 Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
A hyperbranched conjugated Schiff base polymer network: a. potential negative electrode for flexible thin film batteries
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 A hyperbranched conjugated Schiff base polymer network: a potential negative electrode for flexible
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 A hyperbranched conjugated Schiff base polymer network: a potential negative electrode for flexible
Effects of Fluoroethylene Carbonate (FEC) on Anode and Cathode Interfaces at Elevated Temperatures
 Journal of The Electrochemical Society, 162 (9) A1683-A1692 (2015) 0013-4651/2015/162(9)/A1683/10/$33.00 The Electrochemical Society Effects of Fluoroethylene Carbonate (FEC) on Anode and Cathode Interfaces
Journal of The Electrochemical Society, 162 (9) A1683-A1692 (2015) 0013-4651/2015/162(9)/A1683/10/$33.00 The Electrochemical Society Effects of Fluoroethylene Carbonate (FEC) on Anode and Cathode Interfaces
Supporting Information for
 Supporting Information for Self-stabilized solid electrolyte interface on host-free Li metal anode towards high areal capacity and rate utilization Zhenglin Hu 1,3, Shu Zhang 1, Shanmu Dong*,1, Quan Li
Supporting Information for Self-stabilized solid electrolyte interface on host-free Li metal anode towards high areal capacity and rate utilization Zhenglin Hu 1,3, Shu Zhang 1, Shanmu Dong*,1, Quan Li
The Li-O 2 Battery with a Dimethylformamide Electrolyte
 The Li-O 2 Battery with a Dimethylformamide Electrolyte Yuhui Chen, Stefan A. Freunberger, Zhangquan Peng, Fanny Bardé and Peter G. Bruce* School of Chemistry, University of St. Andrews, North Haugh, St.
The Li-O 2 Battery with a Dimethylformamide Electrolyte Yuhui Chen, Stefan A. Freunberger, Zhangquan Peng, Fanny Bardé and Peter G. Bruce* School of Chemistry, University of St. Andrews, North Haugh, St.
A membrane-free ferrocene-based high-rate semiliquid
 Supporting Information A membrane-free ferrocene-based high-rate semiliquid battery Yu Ding,, Yu Zhao,, and Guihua Yu, * Materials Science and Engineering Program and Department of Mechanical Engineering,
Supporting Information A membrane-free ferrocene-based high-rate semiliquid battery Yu Ding,, Yu Zhao,, and Guihua Yu, * Materials Science and Engineering Program and Department of Mechanical Engineering,
CARBON CONDUCTIVE ADDITIVES FOR ELECTRODES IN ELECTROCHEMICAL ENERGY STORAGE DEVICES
 CARBON CONDUCTIVE ADDITIVES FOR ELECTRODES IN ELECTROCHEMICAL ENERGY STORAGE DEVICES 15.03.2013 Flavio F. C. Mornaghini, Dario Cericola, Pirmin Ulmann, Thomas Hucke and Michael E. Spahr CARBON CONDUCTIVE
CARBON CONDUCTIVE ADDITIVES FOR ELECTRODES IN ELECTROCHEMICAL ENERGY STORAGE DEVICES 15.03.2013 Flavio F. C. Mornaghini, Dario Cericola, Pirmin Ulmann, Thomas Hucke and Michael E. Spahr CARBON CONDUCTIVE
Electrochemical study on magnesium anodes in NaCl and CaSO 4 Mg(OH) 2 aqueous solutions
 Electrochimica Acta xxx (2005) xxx xxx Electrochemical study on magnesium anodes in NaCl and CaSO 4 Mg(OH) 2 aqueous solutions Fidel Guadarrama-Muñoz a, Juan Mendoza-Flores a, Ruben Duran-Romero a, J.
Electrochimica Acta xxx (2005) xxx xxx Electrochemical study on magnesium anodes in NaCl and CaSO 4 Mg(OH) 2 aqueous solutions Fidel Guadarrama-Muñoz a, Juan Mendoza-Flores a, Ruben Duran-Romero a, J.
Supporting Information
 Supporting Information Garnet electrolyte with an ultra-low interfacial resistance for Li-metal batteries Yutao Li, Xi Chen, Andrei Dolocan, Zhiming Cui, Sen Xin, Leigang Xue, Henghui Xu, Kyusung Park,
Supporting Information Garnet electrolyte with an ultra-low interfacial resistance for Li-metal batteries Yutao Li, Xi Chen, Andrei Dolocan, Zhiming Cui, Sen Xin, Leigang Xue, Henghui Xu, Kyusung Park,
Li 2 OHCl Crystalline Electrolyte for Stable Metallic Lithium Anodes
 Supporting Information Li 2 OHCl Crystalline Electrolyte for Stable Metallic Lithium Anodes Zachary D. Hood, 1,2, Hui Wang, 1, Amaresh Samuthira Pandian, 1 Jong Kahk Keum 1,3 and Chengdu Liang 1,* 1 Center
Supporting Information Li 2 OHCl Crystalline Electrolyte for Stable Metallic Lithium Anodes Zachary D. Hood, 1,2, Hui Wang, 1, Amaresh Samuthira Pandian, 1 Jong Kahk Keum 1,3 and Chengdu Liang 1,* 1 Center
Supporting information
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Supporting information A Low Temperature Molten Salt Process for Aluminothermic
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Supporting information A Low Temperature Molten Salt Process for Aluminothermic
Electronic Supplementary Information
 Electronic Supplementary Information New insight into working mechanism of Lithium/Sulfur batteries: in situ and operando X-ray diffraction characterization Sylwia Waluś,* a,b Céline Barchasz, a Jean-François
Electronic Supplementary Information New insight into working mechanism of Lithium/Sulfur batteries: in situ and operando X-ray diffraction characterization Sylwia Waluś,* a,b Céline Barchasz, a Jean-François
Electronic Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2013
 Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
High Rate and Durable, Binder Free Anode Based on Silicon Loaded MoO 3 Nanoplatelets
 Supplementary Information High Rate and Durable, Binder Free Anode Based on Silicon Loaded O 3 Nanoplatelets Alejandro Martinez-Garcia, Arjun Kumar Thapa,Ruvini Dharmadasa,, Tu Q. Nguyen, Jacek Jasinski,
Supplementary Information High Rate and Durable, Binder Free Anode Based on Silicon Loaded O 3 Nanoplatelets Alejandro Martinez-Garcia, Arjun Kumar Thapa,Ruvini Dharmadasa,, Tu Q. Nguyen, Jacek Jasinski,
measurements. The spectra show electrolyte in contact with the carbon fibre based gas diffusion layer
 Supplementary Figure 1. 7 Li NMR spectra of electrolyte in contact with potential substrates for in-situ NMR measurements. The spectra show electrolyte in contact with the carbon fibre based gas diffusion
Supplementary Figure 1. 7 Li NMR spectra of electrolyte in contact with potential substrates for in-situ NMR measurements. The spectra show electrolyte in contact with the carbon fibre based gas diffusion
Application in High-Performance Lithium-
 Solution Ionic Strength Engineering as a Generic Strategy to Coat Graphene Oxide (GO) on Various Functional Particles and Its Application in High-Performance Lithium- Sulfur (Li-S) Batteries Jiepeng Rong,Mingyuan
Solution Ionic Strength Engineering as a Generic Strategy to Coat Graphene Oxide (GO) on Various Functional Particles and Its Application in High-Performance Lithium- Sulfur (Li-S) Batteries Jiepeng Rong,Mingyuan
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED
 FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface
 Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface Xiaobiao Wu a, Sihui Wang a, Xiaochen Lin a, Guiming Zhong a, Zhengliang Gong
Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface Xiaobiao Wu a, Sihui Wang a, Xiaochen Lin a, Guiming Zhong a, Zhengliang Gong
Supporting Information
 Supporting Information Hydrogenation Driven Conductive Na 2 Nanoarrays as Robust Binder-Free Anodes for Sodium-Ion Batteries Shidong Fu, Jiangfeng Ni, Yong Xu, Qiao Zhang*, and Liang Li*, College of Physics,
Supporting Information Hydrogenation Driven Conductive Na 2 Nanoarrays as Robust Binder-Free Anodes for Sodium-Ion Batteries Shidong Fu, Jiangfeng Ni, Yong Xu, Qiao Zhang*, and Liang Li*, College of Physics,
Transition from Super-lithiophobicity to Super-lithiophilicity of Garnet Solid-State Electrolyte
 Supporting Information Transition from Super-lithiophobicity to Super-lithiophilicity of Garnet Solid-State Electrolyte Wei Luo, 1,2, Yunhui Gong, 1,3, Yizhou Zhu, 1,3 Kun (Kelvin) Fu, 1,3 Jiaqi Dai, 1,3
Supporting Information Transition from Super-lithiophobicity to Super-lithiophilicity of Garnet Solid-State Electrolyte Wei Luo, 1,2, Yunhui Gong, 1,3, Yizhou Zhu, 1,3 Kun (Kelvin) Fu, 1,3 Jiaqi Dai, 1,3
Supplementary Information for
 Supplementary Information for An elastic and Li-ion-percolating hybrid membrane stabilizes Li metal plating Quan Pang, Laidong Zhou, Linda F. Nazar* Department of Chemistry and the Waterloo Institute for
Supplementary Information for An elastic and Li-ion-percolating hybrid membrane stabilizes Li metal plating Quan Pang, Laidong Zhou, Linda F. Nazar* Department of Chemistry and the Waterloo Institute for
Synthesis, characterization and cycling performance of novel chromium oxide cathode materials for lithium batteries
 Journal of Power Sources 1 (003) 1 1 Synthesis, characterization and cycling performance of novel chromium oxide cathode materials for lithium batteries Ramaraja P. Ramasamy, P. Ramadass, Bala S. Haran,
Journal of Power Sources 1 (003) 1 1 Synthesis, characterization and cycling performance of novel chromium oxide cathode materials for lithium batteries Ramaraja P. Ramasamy, P. Ramadass, Bala S. Haran,
Toward the Design of High Voltage Magnesium-Lithium Hybrid Batteries using Dual-Salt Electrolytes
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Supplemental Information Toward the Design of High Voltage Magnesium-Lithium Hybrid Batteries using
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Supplemental Information Toward the Design of High Voltage Magnesium-Lithium Hybrid Batteries using
Spray Drying Method for Large-Scale and High. Performance Silicon Negative Electrodes in Li-ion. Batteries
 SUPPORTING INFORMATION Spray Drying Method for Large-Scale and High Performance Silicon Negative Electrodes in Li-ion Batteries Dae Soo Jung, Tae Hoon Hwang, Seung Bin Park, and Jang Wook Choi,,* Graduate
SUPPORTING INFORMATION Spray Drying Method for Large-Scale and High Performance Silicon Negative Electrodes in Li-ion Batteries Dae Soo Jung, Tae Hoon Hwang, Seung Bin Park, and Jang Wook Choi,,* Graduate
Supplementary Information
 Supplementary Information Low Temperature Plasma Synthesis of Mesoporous Fe 3 O 4 Nanorods Grafted on Reduced Graphene Oxide for High Performance Lithium Storage Quan Zhou, a Zongbin Zhao,* a Zhiyu Wang,
Supplementary Information Low Temperature Plasma Synthesis of Mesoporous Fe 3 O 4 Nanorods Grafted on Reduced Graphene Oxide for High Performance Lithium Storage Quan Zhou, a Zongbin Zhao,* a Zhiyu Wang,
SUPPORTING INFORMATION. Graduate School of EEWS (WCU), Korea Advanced Institute of Science and Technology, 373-1
 SUPPORTING INFORMATION Electrospun Core-Shell Fibers for Robust Silicon Nanoparticle Based Lithium Ion Battery Anodes Tae Hoon Hwang, Yong Min Lee, Byung Seon Kong, Jin-Seok Seo, and Jang Wook Choi,,*
SUPPORTING INFORMATION Electrospun Core-Shell Fibers for Robust Silicon Nanoparticle Based Lithium Ion Battery Anodes Tae Hoon Hwang, Yong Min Lee, Byung Seon Kong, Jin-Seok Seo, and Jang Wook Choi,,*
Nanostructured Li 2 S-C Composites as Cathode Material for High Energy Lithium/Sulfur Batteries
 Supplementary Information Nanostructured Li 2 S-C Composites as Cathode Material for High Energy Lithium/Sulfur Batteries Kunpeng Cai 1,, Min-Kyu Song 1,, Elton J. Cairns 2,3, and Yuegang Zhang 1,,* 1
Supplementary Information Nanostructured Li 2 S-C Composites as Cathode Material for High Energy Lithium/Sulfur Batteries Kunpeng Cai 1,, Min-Kyu Song 1,, Elton J. Cairns 2,3, and Yuegang Zhang 1,,* 1
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information In situ electrochemical activation of Ni-based colloids from NiCl 2 electrode
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information In situ electrochemical activation of Ni-based colloids from NiCl 2 electrode
Kuang-Che Hsiao. Supervisor: Prof. Tony West
 New Potential Cathode Materials for Lithium-ion ion Battery - Synthesis and characterization of Li 1+x FePO 4-x N x cathode - Kuang-Che Hsiao Supervisor: Prof. Tony West 08/06/2010 E-mail: dtp09kh@sheffield.ac.uk
New Potential Cathode Materials for Lithium-ion ion Battery - Synthesis and characterization of Li 1+x FePO 4-x N x cathode - Kuang-Che Hsiao Supervisor: Prof. Tony West 08/06/2010 E-mail: dtp09kh@sheffield.ac.uk
A novel rechargeable battery with magnesium anode, titanium dioxide cathode, and magnesim borohydride/tetraglyme electrolyte
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 A novel rechargeable battery with magnesium anode, titanium dioxide cathode, and magnesim borohydride/tetraglyme
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 A novel rechargeable battery with magnesium anode, titanium dioxide cathode, and magnesim borohydride/tetraglyme
An Anode-Free Sodium Battery through In-Situ Plating of Sodium Metal
 Supporting Information An Anode-Free Sodium Battery through In-Situ Plating of Sodium Metal Adam P. Cohn 1, Nitin Muralidharan 2, Rachel Carter 1, Keith Share 2, and Cary L. Pint 1,2 * 1 Department of
Supporting Information An Anode-Free Sodium Battery through In-Situ Plating of Sodium Metal Adam P. Cohn 1, Nitin Muralidharan 2, Rachel Carter 1, Keith Share 2, and Cary L. Pint 1,2 * 1 Department of
ScienceDirect. Growth of Carbon Nano Tubes on Copper Substrate Suitable for Lithium Ion Battery Anode
 Available online at www.sciencedirect.com ScienceDirect Procedia Materials Science 11 (2015 ) 634 638 5th International Biennial Conference on Ultrafine Grained and Nanostructured Materials, UFGNSM15 Growth
Available online at www.sciencedirect.com ScienceDirect Procedia Materials Science 11 (2015 ) 634 638 5th International Biennial Conference on Ultrafine Grained and Nanostructured Materials, UFGNSM15 Growth
