ADVANCED AP PLACEMENT CHEMISTRY. Activity Series. Introduction. Objective. Chemicals and Equipment
|
|
|
- Thomas Watts
- 6 years ago
- Views:
Transcription
1 ADVANCED AP PLACEMENT CHEMISTRY Introduction Activity Series An activity series of metals is a table of metals arranged in the order of their decreasing chemical activity or the ease at which the metal will give up one or more electrons to form positive ions. This table is similar to the electrochemical series of elements. For example if you take the group of metals magnesium, mercury and nickel, magnesium is the most reactive and mercury the least. To empirically determine which of these metals is more reactive, place a piece of the metal in a salt solution of the other. The more reactive metal will replace the less reactive metal and the less reactive will appear in the solid form. The reactive metal has been oxidized, the less reactive metal has been reduced. A similar series can be constructed from nonmetallic halogens. The most active halogen is the most easily reduced, accepting electrons from the less active species and replacing it in the halide. Oxidation and reduction reactions occur concurrently therefore the number of electrons lost must equal the number of electrons gained. A good reducing agent easily gives up it electrons and is oxidized. Likewise, a good oxidizing agent has a strong tendency to acquire electrons and is reduced. Objective In these experiments, an activity series for a group of metals and a group of non-metals will be determined empirically. Chemicals and Equipment Materials included in this kit: 15 strips Magnesium metal 15 strips Copper metal
2 15 strips Lead metal 60 pieces Zinc metal 3 50mL Copper Nitrate, 0.1M 3 50mL Magnesium Nitrate, 0.1M 3 50mL Zinc Nitrate, 0.1M 3 50mL Lead Nitrate, 0.1M 1 100mL Bromine Water 1 100mL Chlorine Water 1 100mL Iodine Water 2 50mL Sodium Bromide, 0.1M 2 50mL Sodium Chloride, 0.1M 2 50mL Potassium Iodide, 0.1M 2 100ml Mineral Oil 1 set Student study and analysis copy masters 1 Teacher Guide Materials required but not included: 15 each 24 well microtiter plates 180 each test tubes with stoppers Safety equipment required: Rubber gloves Apron Safety goggles Safety Note: Lead compounds are very poisonous. Chlorine water, Bromine water and Iodine water give off irritating fumes and are poisonous by ingestion. Use extreme care when handling these compounds. Wash your hand thoroughly. Procedure Activity Series of Selected Metals 1.) Set up a 4 4 analysis matrix in the microtiter plate by placing 1 ml (15 drops) of each of the nitrate solutions on the following wells: Copper nitrate - wells 2-4 of column 1 Lead nitrate - wells 1, 3 & 4 of column 2 Zinc nitrate - wells 1,2 & 4 of column 3 Magnesium Nitrate - wells 1, 2 & 3 of column 4 2.) Place a small piece of copper in each of the wells of the first row containing a solution. Add a lead piece to the solutions of the second row, zinc pieces to the third row and magnesium pieces to the forth row.
3 Cu Pb Zn Mg Cu 2+ Pb 2+ Zn 2+ Mg 2+ Analysis Matrix 3.) Allow the plate to stand at least 5 minutes. Determine if a reaction has occurred in the wells by looking for a chemical deposit on the metal or a precipitate in the bottom of the well. Record your data and determine for each well which metal was more reactive. Activity series of selected halogens. 1.) Halogens, Cl 2, Br 2 and I 2 are soluble in nonpolar solvents such as mineral oil. The presence of a halogen will be determined by the color it exhibits in mineral oil. To determine the colors, place 1mL (15 drops) of Chlorine water, Bromine water and Iodine water in 3 separate test tubes. Add 1mL of mineral oil to each test tube stopper and shake for 15 seconds. Place the test tubes in a test tube rack and allow the mineral oil and water to separate. Record the color of the mineral oil layer (upper layer) for each halogen.
4 2.) Determine if the halide ions impart color to mineral oil by repeating step 1.) with 1mL each of the solutions of NaCl, KI and NaBr. Record your results. 3.) Set up a 3 3 analysis matrix similar to the one used is the first experiment using test tubes. Place 1mL of the solution of NaCl in to each of two test tubes. Do the same for the solutions of KI and NaBr. To the NaCl test tubes add 1mL of Bromine water in one and 1mL of Iodine water in the other. To the KI test tubes add Chlorine water and Bromine water and to the NaBr test tubes add Iodine water and Chlorine water. Add 1mL of mineral to each test tube, stopper all of the test tubes and shake for 15 seconds. Allow the layers to separate and record the colors for the mineral oil layer in each test tube. NaCl NaBr KI Cl 2 water Br 2 water I 2 water Analysis Matrix
5 Chemical disposal: Collect the solutions for each experiment and keep separate. Dispose of these solutions in accordance with local regulations. Discussion and Laboratory Report (1) In your lab report include balanced net ionic equations for all reactions that occurred with the metals. List the metals in decreasing ease of oxidation (activity series) and compare to an activity series found in your textbook. (2) Write reduction half reactions for each metal ion and arrange the list in order of decreasing ease of reduction. Compare your list to a table of reduction potentials found in your textbook or the CRC Handbook of Chemistry and Physics. (3) Describe what occurs when halogen and halide ions are extracted by a nonpolar solvent such as mineral oil. (4) Write balanced net ionic equations for the reactions which occurred with the halogens. List the halogens in decreasing order of reactivity. Compare your list to an activity series in your textbook. (5) Write reduction half reactions for each of the halogens. Arrange in order of decreasing ease of reduction. Compare the list to the table of reduction potentials as in step (2) above.
OXIDATION-REDUCTION EXPERIMENT
 Chem 112 OXIDATION-REDUCTION EXPERIMENT INTRODUCTION An oxidation-reduction (redox) reaction involves the movement of electrons from one reactant to another. Many reactions that you have already studied
Chem 112 OXIDATION-REDUCTION EXPERIMENT INTRODUCTION An oxidation-reduction (redox) reaction involves the movement of electrons from one reactant to another. Many reactions that you have already studied
Which of these is the formula for disulfur heptoxide? A. S 2 O 7 B. S 7 O 2 C. SO 2 D. N 2 O
 Which of these is the formula for disulfur heptoxide? A. S 2 O 7 B. S 7 O 2 C. SO 2 D. N 2 O Which of these is the correct chemical formula for a molecule of oxygen? A. O B. O -2 C. O +2 D. O 2 Which of
Which of these is the formula for disulfur heptoxide? A. S 2 O 7 B. S 7 O 2 C. SO 2 D. N 2 O Which of these is the correct chemical formula for a molecule of oxygen? A. O B. O -2 C. O +2 D. O 2 Which of
Periodic Trends and the Properties of Elements
 Page 1 - The Alkaline Earth Metals Introduction The periodic table is the most recognized symbol of chemistry across the world. It is a valuable tool that allows scientists not only to classify the elements
Page 1 - The Alkaline Earth Metals Introduction The periodic table is the most recognized symbol of chemistry across the world. It is a valuable tool that allows scientists not only to classify the elements
Activity of metals SCIENTIFIC. Demonstration and Inquiry. Introduction. Concepts. Background. Inquiry Approach. Demonstration Questions
 Activity of Metals Demonstration and Inquiry SCIENTIFIC Introduction Chemical reactions are not formulas on a piece of paper they are dynamic and exciting events! The demonstration of aluminum with copper(ii)
Activity of Metals Demonstration and Inquiry SCIENTIFIC Introduction Chemical reactions are not formulas on a piece of paper they are dynamic and exciting events! The demonstration of aluminum with copper(ii)
Periodic Trends and the Properties of Elements The Alkaline Earth Metals
 Introduction Periodic Trends and the Properties of Elements The Alkaline Earth Metals The periodic table is the most recognized symbol of chemistry across the world. It is a valuable tool that allows scientists
Introduction Periodic Trends and the Properties of Elements The Alkaline Earth Metals The periodic table is the most recognized symbol of chemistry across the world. It is a valuable tool that allows scientists
[ Cl ] - [[Mg 2+ ] ] Experiment 7: Oxidation-Reduction Reactions. transfer e -
![[ Cl ] - [[Mg 2+ ] ] Experiment 7: Oxidation-Reduction Reactions. transfer e - [ Cl ] - [[Mg 2+ ] ] Experiment 7: Oxidation-Reduction Reactions. transfer e -](/thumbs/74/70175814.jpg) Experiment 7: OxidationReduction Reactions PURPOSE Become familiar with the concepts of oxidation and reduction and how these reactions occur. Carry out several such reactions and learn to recognize when
Experiment 7: OxidationReduction Reactions PURPOSE Become familiar with the concepts of oxidation and reduction and how these reactions occur. Carry out several such reactions and learn to recognize when
EXPERIMENT 5. The Periodic Table INTRODUCTION
 EXPERIMENT 5 The Periodic Table INTRODUCTION The modern periodic law states that when the chemical elements are arranged in order of increasing atomic number, chemical and physical properties repeat periodically
EXPERIMENT 5 The Periodic Table INTRODUCTION The modern periodic law states that when the chemical elements are arranged in order of increasing atomic number, chemical and physical properties repeat periodically
Single/Double Displacement Lab
 Pre-lab Answer this question at your first lab station only. Table 1: 1. Take a few pellets, one piece or tweezer full of each metal and put them each in a separate well in the well plate. Place well plate
Pre-lab Answer this question at your first lab station only. Table 1: 1. Take a few pellets, one piece or tweezer full of each metal and put them each in a separate well in the well plate. Place well plate
S1 Building Blocks Summary Notes
 S1 Building Blocks Summary Notes Atoms & Molecules 1 We are developing our understanding of atoms and molecules. Atoms are the simplest building blocks of every substance in the universe. There are just
S1 Building Blocks Summary Notes Atoms & Molecules 1 We are developing our understanding of atoms and molecules. Atoms are the simplest building blocks of every substance in the universe. There are just
Oxidation and Reduction
 Oxidation and Reduction An oxidation reaction is one in which oxygen is added to a substance. Example: Methane is oxidised when it burns in air. Oxygen is added to the carbon in methane, forming carbon
Oxidation and Reduction An oxidation reaction is one in which oxygen is added to a substance. Example: Methane is oxidised when it burns in air. Oxygen is added to the carbon in methane, forming carbon
Electricity and Chemistry
 Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Electricity and Chemistry Electrochemistry: It is a branch of chemistry that deals with the reactions involving the conversion of chemical energy into electrical energy and vice-versa. Electrochemical
Learn Chemistry. Starter for Ten 9. Redox. Registered Charity Number
 Learn Chemistry Starter for Ten 9. Redox Developed by Dr Kristy Turner, RSC School Teacher Fellow 2011-2012 at the University of Manchester, and Dr Catherine Smith, RSC School Teacher Fellow 2011-2012
Learn Chemistry Starter for Ten 9. Redox Developed by Dr Kristy Turner, RSC School Teacher Fellow 2011-2012 at the University of Manchester, and Dr Catherine Smith, RSC School Teacher Fellow 2011-2012
Equation Writing and Predicting Products Chemistry I Acc
 Introduction: Equation Writing and Predicting Products Chemistry I Acc If you examine your bicycle after it has been left out in the rain a number of times you will find that it has begun to rust. Rust
Introduction: Equation Writing and Predicting Products Chemistry I Acc If you examine your bicycle after it has been left out in the rain a number of times you will find that it has begun to rust. Rust
Partner: Cathy 22 March Separation and Qualitative Determination of Cations and Anions
 Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
Double Award Science: Chemistry
 New Specification Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Higher Tier ML [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING
New Specification Centre Number Candidate Number General Certificate of Secondary Education 2014 2015 Double Award Science: Chemistry Unit C1 Higher Tier ML [GSD22] WEDNESDAY 25 FEBRUARY 2015, MORNING
Will a displacement reaction occur?
 Will a displacement reaction occur? Setting the scene The reactivity series lists metals in order of how reactive they are. More reactive metals are able to displace less reactive metals from their compounds.
Will a displacement reaction occur? Setting the scene The reactivity series lists metals in order of how reactive they are. More reactive metals are able to displace less reactive metals from their compounds.
I. PHYSICAL PROPERTIES. PROPERTY METALS NON-METALS 1.Lustre Metals have shining surface. They do not have shining surface.
 Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
IGCSE Chemistry: Electrochemistry and Redox Whole Unit Overview
 IGCSE Chemistry: Electrochemistry and Redox Whole Unit Overview (Please note: (S) denotes material in the Supplement (Extended syllabus) only) Learning Outcomes Suggested Teaching Activities Resources
IGCSE Chemistry: Electrochemistry and Redox Whole Unit Overview (Please note: (S) denotes material in the Supplement (Extended syllabus) only) Learning Outcomes Suggested Teaching Activities Resources
Duncan. UNIT 8 - Chemical Equations BALANCING EQUATIONS PRACTICE WORKSHEET 14.) C2H6 + O2 CO2 + H2O. 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.
 BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
N5 Chemistry Unit 3: Chemistry in Society Homework 3.2
 N5 hemistry Unit 3: hemistry in Society Homework 3.2 Name Teacher 1. Which of the following metals can be extracted from its oxide by heat alone? 5. Which of the following is a carboxylic acid? luminium
N5 hemistry Unit 3: hemistry in Society Homework 3.2 Name Teacher 1. Which of the following metals can be extracted from its oxide by heat alone? 5. Which of the following is a carboxylic acid? luminium
GraspIT AQA GCSE Chemical changes
 A. Reactivity of metals The reactivity series, metal oxides and extractions 1. Three metals, X, Y and Z were put into water. The reactions are shown below: a) Use the diagrams to put metals X, Y and Z
A. Reactivity of metals The reactivity series, metal oxides and extractions 1. Three metals, X, Y and Z were put into water. The reactions are shown below: a) Use the diagrams to put metals X, Y and Z
TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION
 EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
Metals. N4 & N5 Homework Questions
 St Peter the Apostle High School Chemistry Department Metals N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
St Peter the Apostle High School Chemistry Department Metals N4 & N5 Homework Questions Answer questions as directed by your teacher. National 4 level questions are first followed by National 5 level questions.
Summer Assignment Coversheet
 Summer Assignment Coversheet Course: A.P. Chemistry Teachers Names: Mary Engels Assignment Title: Summer Assignment A Review Assignment Summary/Purpose: To review the Rules for Solubility, Oxidation Numbers,
Summer Assignment Coversheet Course: A.P. Chemistry Teachers Names: Mary Engels Assignment Title: Summer Assignment A Review Assignment Summary/Purpose: To review the Rules for Solubility, Oxidation Numbers,
Set 3 Marking Scheme : Electrochemistry Na +, H + -, NO 3, OH -, OH - Na +, H + OH - Its lower than in electrochemical series
 8. Write the formula of all ions present in the electrolyte. Write the formula of ion/ions which is/are attracted to anode and cathode. Which is selectively discharged? Give a reason. Write the half equation
8. Write the formula of all ions present in the electrolyte. Write the formula of ion/ions which is/are attracted to anode and cathode. Which is selectively discharged? Give a reason. Write the half equation
Compounds & Reactions Week 1. Writing Formulas & Balancing Equations. Write the chemical formula for each molecular (covalent) compound.
 Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Sn 2+ (aq) + 2 Ag + (aq) Sn 4+ (aq) + 2 Ag(s),
 1. Which change in oxidation number represents oxidation? A) Sn 2+ (aq) Sn 4+ (aq) B) Sn 2+ (aq) Sn(s) C) Sn 4+ (aq) Sn 2+ (aq) D) Sn 4+ (aq) Sn(s) E) Sn(s) Sn 2 (aq) 2. In the reaction Sn 2+ (aq) + 2
1. Which change in oxidation number represents oxidation? A) Sn 2+ (aq) Sn 4+ (aq) B) Sn 2+ (aq) Sn(s) C) Sn 4+ (aq) Sn 2+ (aq) D) Sn 4+ (aq) Sn(s) E) Sn(s) Sn 2 (aq) 2. In the reaction Sn 2+ (aq) + 2
Topic Reacting masses Level GCSE Outcomes 1. To calculate reacting masses 2. To set out mole calculations in a grid format
 Topic Reacting masses Level GCSE Outcomes 1. To calculate reacting masses 2. To set out mole calculations in a grid format Problems on Reacting Masses of Solids Section 1 1. What is the mass of magnesium
Topic Reacting masses Level GCSE Outcomes 1. To calculate reacting masses 2. To set out mole calculations in a grid format Problems on Reacting Masses of Solids Section 1 1. What is the mass of magnesium
Nomenclature. A systematic method of writing chemical formulas and naming compounds
 Nomenclature A systematic method of writing chemical formulas and naming compounds Chemical symbols Symbols are used to represent elements Either one capital letter, or a capital letter with a lower case
Nomenclature A systematic method of writing chemical formulas and naming compounds Chemical symbols Symbols are used to represent elements Either one capital letter, or a capital letter with a lower case
OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/02. TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Higher Tier)
 OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/02 TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Higher Tier) TUESDAY 10 JUNE 2014: Afternoon DURATION: 1 hour plus your
OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/02 TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Higher Tier) TUESDAY 10 JUNE 2014: Afternoon DURATION: 1 hour plus your
Alchemy: A Cross-Curricular Activity Copper, Silver, and Gold Redox Reactions
 Alchemy: A Cross-Curricular Activity Copper, Silver, and Gold Redox Reactions SCIENTIFIC Introduction Turn an ordinary copper penny into silver and then into gold! Get rich quick by demonstrating this
Alchemy: A Cross-Curricular Activity Copper, Silver, and Gold Redox Reactions SCIENTIFIC Introduction Turn an ordinary copper penny into silver and then into gold! Get rich quick by demonstrating this
Look at the measuring cylinders. What happened to the volume of the water and the wax after freezing? the volume of water... the volume of wax...
 1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
1. Meera poured 7 cm 3 of water into a measuring cylinder. She poured 7 cm 3 of melted wax into another measuring cylinder. She put both measuring cylinders into a freezer for 24 hours. water before freezing
The Crystal Forest Favorite Holiday Demonstrations
 The Crystal Forest Favorite Holiday Demonstrations SCIENTIFIC Introduction Put a new twist on crystal growing. In this class participation demonstration, students cut out and assemble miniature trees and
The Crystal Forest Favorite Holiday Demonstrations SCIENTIFIC Introduction Put a new twist on crystal growing. In this class participation demonstration, students cut out and assemble miniature trees and
Chemistry Themed MATERIALS Part 2 Reactivity of Metals and Redox
 Chemistry Themed MATERIALS Part 2 Reactivity of Metals and Redox 2016-2017 1 2 Chemistry in the Community-2016-2017 Materials: Reactivity of Metals and Redox W 10/5 Balancing Quiz Demo AgNO 3 + Cu and
Chemistry Themed MATERIALS Part 2 Reactivity of Metals and Redox 2016-2017 1 2 Chemistry in the Community-2016-2017 Materials: Reactivity of Metals and Redox W 10/5 Balancing Quiz Demo AgNO 3 + Cu and
Ionic Naming Quiz #1. Ionic Naming Quiz #1. March 26, Day 05 Ionic Quiz, SDR Note, Exit Card.notebook
 Ionic Naming Quiz #1 Name the following ionic compounds: 1) NaBr sodium bromide 2) Sc(OH) 3 scandium hydroxide 3) V 2 (SO 4 ) 3 vanadium (III) sulfate 4) NH 4 F ammonium fluoride 5) CaCO 3 calcium carbonate
Ionic Naming Quiz #1 Name the following ionic compounds: 1) NaBr sodium bromide 2) Sc(OH) 3 scandium hydroxide 3) V 2 (SO 4 ) 3 vanadium (III) sulfate 4) NH 4 F ammonium fluoride 5) CaCO 3 calcium carbonate
Predicting Reaction Products
 Predicting Reaction Products Once you classify a chemical reaction, write the products and balance the reaction. Review Diatomic Molecules! Elements that exist only as compounds in the natural world: Iodine
Predicting Reaction Products Once you classify a chemical reaction, write the products and balance the reaction. Review Diatomic Molecules! Elements that exist only as compounds in the natural world: Iodine
I. PHYSICAL PROPERTIES PROPERTY METALS NON-METALS
 Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Elements can be classified as metals and non-metals on the basis of their properties. Example of some metals are : Iron (Fe), Aluminium (Al), Silver (Ag), Copper (Cu) Examples of some non-metals are :
Chemical Formulas. In a chemical formula, the element with the positive charge is always written first.
 Bonding Part 2 Chemical Formulas Chemical formulas a group of chemical symbols and numbers that represent the number of atoms of each element that make up a compound. ( H O) 2 In a chemical formula, the
Bonding Part 2 Chemical Formulas Chemical formulas a group of chemical symbols and numbers that represent the number of atoms of each element that make up a compound. ( H O) 2 In a chemical formula, the
Ionic Naming Quiz #1. Ionic Naming Quiz #2 Write the formulas for the following ionic compounds:
 Ionic Naming Quiz #1 Name the following ionic compounds: 1) NaBr sodium bromide 2) Sc(OH) 3 scandium hydroxide 3) V 2 (SO 4 ) 3 vanadium (III) sulfate 4) NH 4 F ammonium fluoride 5) CaCO 3 calcium carbonate
Ionic Naming Quiz #1 Name the following ionic compounds: 1) NaBr sodium bromide 2) Sc(OH) 3 scandium hydroxide 3) V 2 (SO 4 ) 3 vanadium (III) sulfate 4) NH 4 F ammonium fluoride 5) CaCO 3 calcium carbonate
Copper Odyssey. Chemical Reactions of Copper
 Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Our country, our future S2 CHEMISTRY DURATION: 2 HOUR
 Our country, our future S2 CHEMISTRY Exam 1 DURATION: 2 HOUR INSTRUCTIONS: This paper consists of two sections A and B, Attempt all questions in section A and B For section A, circle the most correct alternative
Our country, our future S2 CHEMISTRY Exam 1 DURATION: 2 HOUR INSTRUCTIONS: This paper consists of two sections A and B, Attempt all questions in section A and B For section A, circle the most correct alternative
GraspIT AQA GCSE Chemical changes
 A. Reactivity of metals The reactivity series, metal oxides and extractions 1. Three metals, X, Y and Z were put into water. The reactions are shown below: a) Use the diagrams to put metals X, Y and Z
A. Reactivity of metals The reactivity series, metal oxides and extractions 1. Three metals, X, Y and Z were put into water. The reactions are shown below: a) Use the diagrams to put metals X, Y and Z
GCSE Chemistry B. Mark Scheme for June Unit B742/01: Modules C4, C5, C6 (Foundation Tier) General Certificate of Secondary Education
 GCSE Chemistry B Unit B742/01: Modules C4, C5, C6 (Foundation Tier) General Certificate of Secondary Education Mark Scheme for June 2017 Oxford Cambridge and RSA Examinations OCR (Oxford Cambridge and
GCSE Chemistry B Unit B742/01: Modules C4, C5, C6 (Foundation Tier) General Certificate of Secondary Education Mark Scheme for June 2017 Oxford Cambridge and RSA Examinations OCR (Oxford Cambridge and
INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES. Introduction. Electrochemistry Revised 4/28/14
 INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES Introduction Electrochemical Cells In this part of the experiment, four half cells are created by immersing metal strips of zinc, copper,
INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES Introduction Electrochemical Cells In this part of the experiment, four half cells are created by immersing metal strips of zinc, copper,
Voltaic Cells. An Energizing Experience. Using the energy of a spontaneous redox reaction to do work. aka Galvanic Cells.
 Voltaic Cells aka Galvanic Cells Using the energy of a spontaneous redox reaction to do work. Chapter 20 An Energizing Experience 1 A piece of copper is dropped into an aqueous solution of zinc nitrate,
Voltaic Cells aka Galvanic Cells Using the energy of a spontaneous redox reaction to do work. Chapter 20 An Energizing Experience 1 A piece of copper is dropped into an aqueous solution of zinc nitrate,
Angel International School - Manipay 3 rd Term Examination July, 2015
 Grade 08 Angel International School - Manipay 3 rd Term Examination July, 2015 Chemistry Duration: 2 Hours Index No:- Part I Choose the correct answer and underline it. 1. Which of the following correctly
Grade 08 Angel International School - Manipay 3 rd Term Examination July, 2015 Chemistry Duration: 2 Hours Index No:- Part I Choose the correct answer and underline it. 1. Which of the following correctly
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS
 EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
Name: Date: Period: Page: Predicting Products. Predict the products, and balance each reaction. Use the reaction type as a hint.
 Name: Date: Period: Page: Predicting Products Predict the products, and balance each reaction. Use the reaction type as a hint. For reactions with a transition metal, the most likely charge on the metal
Name: Date: Period: Page: Predicting Products Predict the products, and balance each reaction. Use the reaction type as a hint. For reactions with a transition metal, the most likely charge on the metal
21. sodium nitrite 31. potassium carbonate. 23. aluminum hydroxide 33. nickel (II) carbonate. 24. ammonium hydroxide 34.
 N.E. Packet 1 Nomenclature WS 1 (Ionic Compounds) Name: Date: Per: Write the name for each of the following compounds. 1. CaCO 3 11. BaSO 4 2. FeO 12. Zn(NO 3 ) 2 3. K 2 SO 3 13. CuSO 4 4. AgCl 14. AlCl
N.E. Packet 1 Nomenclature WS 1 (Ionic Compounds) Name: Date: Per: Write the name for each of the following compounds. 1. CaCO 3 11. BaSO 4 2. FeO 12. Zn(NO 3 ) 2 3. K 2 SO 3 13. CuSO 4 4. AgCl 14. AlCl
1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is.. The precipitate which forms first is
 CHEMISTRY 12 UNIT 3 - REVIEW Ksp Part A - Multiple Choice 1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is A. Fe 2 (SO 4 ) 3 (s) 3 Fe 2+ (aq) + 2 SO 4 3 (aq)
CHEMISTRY 12 UNIT 3 - REVIEW Ksp Part A - Multiple Choice 1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is A. Fe 2 (SO 4 ) 3 (s) 3 Fe 2+ (aq) + 2 SO 4 3 (aq)
Chapter 12 Reactivity of Metals 12.1 Different Reactivities of Metals Recall an experiment performed in F.3
 Chapter 12 Reactivity of Metals 12.1 Different Reactivities of Metals Recall an experiment performed in F.3 p.1/9 When freshly cut, potassium has a shiny surface and it reacts vigorously with water, giving
Chapter 12 Reactivity of Metals 12.1 Different Reactivities of Metals Recall an experiment performed in F.3 p.1/9 When freshly cut, potassium has a shiny surface and it reacts vigorously with water, giving
Experiment 3: Determination of an Empirical Formula
 Background Information The composition of a compound is defined by its chemical formula, which gives the number ratio of the different elements in the compound. For example, water has a fixed composition
Background Information The composition of a compound is defined by its chemical formula, which gives the number ratio of the different elements in the compound. For example, water has a fixed composition
INDIAN SCHOOL MUSCAT
 INDIAN SCHOOL MUSCAT DEPARTMENT OF CHEMISTRY QUESTION BANK CLASS X CHEMICAL REACTIONS AND EQUATIONS One mark questions 1. What change in color is observed when white silver chloride is left exposed to
INDIAN SCHOOL MUSCAT DEPARTMENT OF CHEMISTRY QUESTION BANK CLASS X CHEMICAL REACTIONS AND EQUATIONS One mark questions 1. What change in color is observed when white silver chloride is left exposed to
GCSE 0236/02 SCIENCE HIGHER TIER CHEMISTRY 1
 Surname Other Names Centre Number 0 Candidate Number GCSE 0236/02 SCIENCE IGER TIER CEMISTRY 1 A.M. TUESDAY, 12 June 2012 45 minutes For s use Question Maximum Mark 1. 7 Mark Awarded 2. 8 ADDITIONAL MATERIALS
Surname Other Names Centre Number 0 Candidate Number GCSE 0236/02 SCIENCE IGER TIER CEMISTRY 1 A.M. TUESDAY, 12 June 2012 45 minutes For s use Question Maximum Mark 1. 7 Mark Awarded 2. 8 ADDITIONAL MATERIALS
ADDITIONAL SCIENCE/CHEMISTRY
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 S16-4472-02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER A.M. THURSDAY, 19 May 2016 1 hour For s use Question Maximum Mark Mark
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 S16-4472-02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER A.M. THURSDAY, 19 May 2016 1 hour For s use Question Maximum Mark Mark
KULLEĠĠ SAN BENEDITTU Boys Secondary, Kirkop
 KULLEĠĠ SAN BENEDITTU Boys Secondary, Kirkop Mark HALF-YEARLY EXAMINATION 2013/2014 Junior Lyceum Programme FORM 4 CHEMISTRY TIME: 1h 30min Question 1 2 3 4 5 6 7 Global Mark Max. Mark 10 16 15 11 8 20
KULLEĠĠ SAN BENEDITTU Boys Secondary, Kirkop Mark HALF-YEARLY EXAMINATION 2013/2014 Junior Lyceum Programme FORM 4 CHEMISTRY TIME: 1h 30min Question 1 2 3 4 5 6 7 Global Mark Max. Mark 10 16 15 11 8 20
EXPERIMENT 3: Identification of a Substance by Physical Properties
 EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
EXPERIMENT. The Reaction of Magnesium with Hydrochloric Acid; The Molar Volume of Hydrogen
 EXPERIMENT The Reaction of Magnesium with Hydrochloric Acid; The Molar Volume of Hydrogen PURPOSE In this experiment you will determine the volume of the hydrogen gas which is produced when a sample of
EXPERIMENT The Reaction of Magnesium with Hydrochloric Acid; The Molar Volume of Hydrogen PURPOSE In this experiment you will determine the volume of the hydrogen gas which is produced when a sample of
EPA Primary. (mg/l as CaCO3) (mg/l as CaCO3)
 NORTH TEXAS MUNICIPAL WATER DISTRICT - Wylie Water Analysis Jan-2018 Mineral Analysis Raw Treated Standards Residue on Evaporation 412 456 500 1000 Silica (SiO2) 3.63 3.41 Iron (Fe) 0.378 0.259 0.3 0.3
NORTH TEXAS MUNICIPAL WATER DISTRICT - Wylie Water Analysis Jan-2018 Mineral Analysis Raw Treated Standards Residue on Evaporation 412 456 500 1000 Silica (SiO2) 3.63 3.41 Iron (Fe) 0.378 0.259 0.3 0.3
SNC 2D0-01 Chemistry Review Chapters 5 and 6
 SNC 2D0-01 Chemistry Review Chapters 5 and 6 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. A chemical change is distinguished from a physical
SNC 2D0-01 Chemistry Review Chapters 5 and 6 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. A chemical change is distinguished from a physical
Unit - 04 Metals. Fe Fe e Cu Cu e Cu e Cu Fe e Fe Score (2) Time (2 minute)
 Chemistry Standard - X Unit - 4 Metals Concept: Displacement reactions of metals 1. Copper gets deposited on the surface of the iron nail if the nail is kept immersed in copper sulphate. Choose the reactions
Chemistry Standard - X Unit - 4 Metals Concept: Displacement reactions of metals 1. Copper gets deposited on the surface of the iron nail if the nail is kept immersed in copper sulphate. Choose the reactions
3. [7 points] How many significant figures should there be in the answer to the following problem?
![3. [7 points] How many significant figures should there be in the answer to the following problem? 3. [7 points] How many significant figures should there be in the answer to the following problem?](/thumbs/83/87492512.jpg) 1 of 6 10/20/2009 3:52 AM 1. [7 points] What is the correct name for ZnF 2? (a) zinc fluorine (b) zinc (II) fluoride (c) zinc fluoride (d) fluoride zinc 2. [7 points] What is the correct name for P 2 O
1 of 6 10/20/2009 3:52 AM 1. [7 points] What is the correct name for ZnF 2? (a) zinc fluorine (b) zinc (II) fluoride (c) zinc fluoride (d) fluoride zinc 2. [7 points] What is the correct name for P 2 O
1. Which of the given statements about the reaction below are incorrect?
 1. Which of the given statements about the reaction below are incorrect? 2PbO(s) + C(s) 2Pb(s) + CO 2 (g) a. Lead is getting reduced b. Carbon dioxide is getting oxidised c. Carbon is getting oxidised
1. Which of the given statements about the reaction below are incorrect? 2PbO(s) + C(s) 2Pb(s) + CO 2 (g) a. Lead is getting reduced b. Carbon dioxide is getting oxidised c. Carbon is getting oxidised
look down at cross on paper paper cross on paper
 1 The equation for the reaction between sodium thiosulfate and hydrochloric acid is given below. Na 2 S 2 O 3 (aq) + 2HCl (aq) 2NaCl (aq) + S(s) + SO 2 (g) + H 2 O(l) The speed of this reaction was investigated
1 The equation for the reaction between sodium thiosulfate and hydrochloric acid is given below. Na 2 S 2 O 3 (aq) + 2HCl (aq) 2NaCl (aq) + S(s) + SO 2 (g) + H 2 O(l) The speed of this reaction was investigated
Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons?
 Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons? b) Electrons? c) Neutrons? 1 CH3OS Warm Up (Sept 13) 1. What is an isotope? 2. Neon has two major isotopes, Neon 20 and
Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons? b) Electrons? c) Neutrons? 1 CH3OS Warm Up (Sept 13) 1. What is an isotope? 2. Neon has two major isotopes, Neon 20 and
Experiment 1 Hard-Soft Acids and Bases: Altering the Cu + /Cu 2+ Equilibrium with Nitrogen, Oxygen, or Halide Ligands
 Experiment 1 Hard-Soft Acids and Bases: Altering the Cu + /Cu 2+ Equilibrium with Nitrogen, Oxygen, or Halide Ligands *Parts of the experiment are based on C. E. Ophardt & G. Wollaston, J. Chem. Ed. 1991,
Experiment 1 Hard-Soft Acids and Bases: Altering the Cu + /Cu 2+ Equilibrium with Nitrogen, Oxygen, or Halide Ligands *Parts of the experiment are based on C. E. Ophardt & G. Wollaston, J. Chem. Ed. 1991,
Solubility Equilibrium
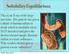 Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
ADDITIONAL SCIENCE/CHEMISTRY. A.M. TUESDAY, 14 January hour
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
ADDITIONAL SCIENCE/CHEMISTRY. A.M. TUESDAY, 14 January hour
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CEMISTRY CEMISTRY 2 IGER TIER A.M. TUESDAY, 14 January 2014 1 hour For s use Question Maximum Mark Mark Awarded 1. 8
236/02 SCIENCE. HIGHER TIER (Grades D-A*) CHEMISTRY 1. P.M. FRIDAY, 19 January (45 minutes)
 Candidate Name Centre Number Candidate Number WELSH JOINT EDUCATION COMMITTEE General Certificate of Secondary Education CYD-BWYLLGOR ADDYSG CYMRU Tystysgrif Gyffredinol Addysg Uwchradd 236/02 SCIENCE
Candidate Name Centre Number Candidate Number WELSH JOINT EDUCATION COMMITTEE General Certificate of Secondary Education CYD-BWYLLGOR ADDYSG CYMRU Tystysgrif Gyffredinol Addysg Uwchradd 236/02 SCIENCE
Formula & Equation Writing
 Formula & Equation Writing Book column Be Na Mg column Ca lithium Ionic Equations Ionic Formulae Balanced Equations Formula Equations Word Equations carbonate Transition Metals C 3 valency valency Using
Formula & Equation Writing Book column Be Na Mg column Ca lithium Ionic Equations Ionic Formulae Balanced Equations Formula Equations Word Equations carbonate Transition Metals C 3 valency valency Using
MATERIAL SAFETY DATA SHEET B2AL-1
 UNITED STATES METAL POWDERS, INC. HMIS RATING: 408 U.S. HIGHWAY 202 HEALTH: 1 FLEMINGTON, NJ 08822 FLAMMABILITY: 0 REACTIVITY: 0 TELEPHONE: 908-782-5454 PERS. PROTECTION: E SECTION I. MATERIAL IDENTIFICATION
UNITED STATES METAL POWDERS, INC. HMIS RATING: 408 U.S. HIGHWAY 202 HEALTH: 1 FLEMINGTON, NJ 08822 FLAMMABILITY: 0 REACTIVITY: 0 TELEPHONE: 908-782-5454 PERS. PROTECTION: E SECTION I. MATERIAL IDENTIFICATION
Chemistry. TOPIC : Redox Reactions. products are PH3 and NaH2PO2. This reaction is an examplee of. (b) Reduction (d) Neutralization
 TOPIC : Redox Reactions Date : Marks : 1 mks Time : ½ hr 1. When P reacts with caustic soda, the products are PH and NaHPO. This reaction is an examplee of (a) Oxidation (c) Oxidation and reduction (redox)
TOPIC : Redox Reactions Date : Marks : 1 mks Time : ½ hr 1. When P reacts with caustic soda, the products are PH and NaHPO. This reaction is an examplee of (a) Oxidation (c) Oxidation and reduction (redox)
JSUNIL TUTORIAL, SAMASTIPUR
 Chapter 1 Chemical Reactions and Equations Q 1. Why should a magnesium ribbon be cleaned before burning in air? Ans. Before burning in air, the magnesium ribbon is cleaned by rubbing with a sandpaper.
Chapter 1 Chemical Reactions and Equations Q 1. Why should a magnesium ribbon be cleaned before burning in air? Ans. Before burning in air, the magnesium ribbon is cleaned by rubbing with a sandpaper.
P.M. FRIDAY, 12 June hour For Examiner s use only
 Surname Centre Number Candidate Number Other Names 0 GCSE 4462/02 S15-4462-02 SCIENCE A/CHEMISTRY CHEMISTRY 1 HIGHER TIER P.M. FRIDAY, 12 June 2015 1 hour For s use Question Maximum Mark Mark Awarded 1.
Surname Centre Number Candidate Number Other Names 0 GCSE 4462/02 S15-4462-02 SCIENCE A/CHEMISTRY CHEMISTRY 1 HIGHER TIER P.M. FRIDAY, 12 June 2015 1 hour For s use Question Maximum Mark Mark Awarded 1.
GCSE Mark Scheme Chemistry 1 Foundation only questions Summer 2010
 GCSE Mark Scheme Chemistry 1 Foundation only questions Summer 2010 1 (i) 3 yes B (1) no C (1) metal D (1) (ii) 1 conducts heat / malleable / ductile high boiling point conducts sonorous high density 1
GCSE Mark Scheme Chemistry 1 Foundation only questions Summer 2010 1 (i) 3 yes B (1) no C (1) metal D (1) (ii) 1 conducts heat / malleable / ductile high boiling point conducts sonorous high density 1
Please write the balanced net ionic reaction for each one. Then answer the accompanying question.
 AP Chemistry Net Ionic Rx Practice Test A CLASS SET PLEASE RETURN!! Please write the balanced net ionic reaction for each one. Then answer the accompanying question. 1) A piece of potassium is dropped
AP Chemistry Net Ionic Rx Practice Test A CLASS SET PLEASE RETURN!! Please write the balanced net ionic reaction for each one. Then answer the accompanying question. 1) A piece of potassium is dropped
Solution Concentrations
 Next Generation Science Standards NGSS Science and Engineering Practices: Asking questions and defining problems Developing and using models Planning and carrying out investigations Analyzing and interpreting
Next Generation Science Standards NGSS Science and Engineering Practices: Asking questions and defining problems Developing and using models Planning and carrying out investigations Analyzing and interpreting
COPPER CYCLE EXPERIMENT 3
 COPPER CYCLE EXPERIMENT 3 INTRODUCTION One simple way to state the aim of chemistry is: The study of matter and its transformations. In this experiment, a copper sample will appear in five different forms
COPPER CYCLE EXPERIMENT 3 INTRODUCTION One simple way to state the aim of chemistry is: The study of matter and its transformations. In this experiment, a copper sample will appear in five different forms
Boiling point in C. Colour in aqueous solution. Fluorine 188 colourless. Chlorine 35 pale green. Bromine X orange.
 Q1.This question is about halogens and their compounds. The table below shows the boiling points and properties of some of the elements in Group 7 of the periodic table. Element Boiling point in C Colour
Q1.This question is about halogens and their compounds. The table below shows the boiling points and properties of some of the elements in Group 7 of the periodic table. Element Boiling point in C Colour
GCSE 4472/02 CHEMISTRY 2 HIGHER TIER ADDITIONAL SCIENCE/CHEMISTRY. P.M. MONDAY, 20 May hour For Examiner s use only Question Maximum Mark 1.
 Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER P.M. MONDAY, 20 May 2013 1 hour For s use Question Maximum Mark 1. 8 Mark Awarded
Surname Centre Number Candidate Number Other Names 0 GCSE 4472/02 ADDITIONAL SCIENCE/CHEMISTRY CHEMISTRY 2 HIGHER TIER P.M. MONDAY, 20 May 2013 1 hour For s use Question Maximum Mark 1. 8 Mark Awarded
Reactivity Series. Question Paper. Cambridge International Examinations. Score: /39. Percentage: /100
 Reactivity Series Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International Examinations Metals Reactivity Series Question Paper Time llowed: 47 minutes Score:
Reactivity Series Question Paper Level Subject Exam oard Topic Sub-Topic ooklet O Level hemistry ambridge International Examinations Metals Reactivity Series Question Paper Time llowed: 47 minutes Score:
The Synthesis of Copper Metal
 CHEM 109 Introduction to Chemistry Revision 1.0 The Synthesis of Copper Metal To learn about Oxidation-Reduction reactions. To learn about Half-Reactions and Half-Cells. To learn about the Activity of
CHEM 109 Introduction to Chemistry Revision 1.0 The Synthesis of Copper Metal To learn about Oxidation-Reduction reactions. To learn about Half-Reactions and Half-Cells. To learn about the Activity of
OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/01. TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Foundation Tier)
 OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/01 TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Foundation Tier) TUESDAY 10 JUNE 2014: Afternoon DURATION: 1 hour plus
OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/01 TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Foundation Tier) TUESDAY 10 JUNE 2014: Afternoon DURATION: 1 hour plus
CLASSI ICATION OF MAT R AND HOMOGENEOUS AND HETEROGENEOUS MIXTURES
 Experiment 3 Name: CLASSI ICATION OF MAT R AND HOMOGENEOUS AND HETEROGENEOUS MIXTURES Classification of Matter A pure substance is matter with definite and constant composition with distinct chemical properties.
Experiment 3 Name: CLASSI ICATION OF MAT R AND HOMOGENEOUS AND HETEROGENEOUS MIXTURES Classification of Matter A pure substance is matter with definite and constant composition with distinct chemical properties.
TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY. Unit 2 Metals
 TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY Unit 2 Metals Part 2 Metals differ in their reactivity with other chemicals and this influences their uses. Describe observable changes when metals react
TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY Unit 2 Metals Part 2 Metals differ in their reactivity with other chemicals and this influences their uses. Describe observable changes when metals react
1. Nickel has a face-centered-cubic unit cell. The density of nickel is 6.84 g/cm 3. Calculate the atomic radius of nickel.
 ADDITIONAL PRACTICE ON UNIT CELLS 1. Nickel has a face-centered-cubic unit cell. The density of nickel is 6.84 g/cm 3. Calculate the atomic radius of nickel. 2. Tungsten metal exists in a body-centered-cubic
ADDITIONAL PRACTICE ON UNIT CELLS 1. Nickel has a face-centered-cubic unit cell. The density of nickel is 6.84 g/cm 3. Calculate the atomic radius of nickel. 2. Tungsten metal exists in a body-centered-cubic
(Cell) (Fax)
 (Cell) +27 79 620 9375 (Fax) +27 86 605 1503 info@mylab.co.za www.mylab.co.za Mylab Grade 10 to 12 Kit, Apparatus and Chemical content list. Item Quantity/Box Apron 1 Back Plate 1 Bamboo Sticks 3 Base
(Cell) +27 79 620 9375 (Fax) +27 86 605 1503 info@mylab.co.za www.mylab.co.za Mylab Grade 10 to 12 Kit, Apparatus and Chemical content list. Item Quantity/Box Apron 1 Back Plate 1 Bamboo Sticks 3 Base
Experiment 3 * Thermochromism in the Ionic Conductor, Cu 2 HgI 4
 Experiment 3 * Thermochromism in the Ionic Conductor, Cu 2 HgI 4 *This lab taken from Teaching General Chemistry; A Materials Science Companion, Eds. Ellis, A.B.; Geselbracht, M.J.; Johnson, B.J.; Lisensky,
Experiment 3 * Thermochromism in the Ionic Conductor, Cu 2 HgI 4 *This lab taken from Teaching General Chemistry; A Materials Science Companion, Eds. Ellis, A.B.; Geselbracht, M.J.; Johnson, B.J.; Lisensky,
UNIVERSAL METHOD OF DETERMINING BROMIDES IN RAW MATERIALS AND PRODUCTS FORMED IN MAGNESIUM PRODUCTION
 Metallurgist, Vol. 53, Nos. 3 4, 2009 UNIVERSAL METHOD OF DETERMINING BROMIDES IN RAW MATERIALS AND PRODUCTS FORMED IN MAGNESIUM PRODUCTION A. P. Nechitailov, A. G. Suss, A. V. Panov, T. G. Golovanova,
Metallurgist, Vol. 53, Nos. 3 4, 2009 UNIVERSAL METHOD OF DETERMINING BROMIDES IN RAW MATERIALS AND PRODUCTS FORMED IN MAGNESIUM PRODUCTION A. P. Nechitailov, A. G. Suss, A. V. Panov, T. G. Golovanova,
Analysis of dissolved nitrite (NO 2- )
 Analysis of dissolved nitrite (NO 2 ) Table of content 1. PURPOSE... 2 2. PRINCIPLE... 2 3. REQUIREMENTS... 3 3.1. EQUIPMENT AND MATERIALS... 3 3.2. REAGENTS... 3 4. PROCEDURE... 3 4.1. SAMPLE PREPARATION...
Analysis of dissolved nitrite (NO 2 ) Table of content 1. PURPOSE... 2 2. PRINCIPLE... 2 3. REQUIREMENTS... 3 3.1. EQUIPMENT AND MATERIALS... 3 3.2. REAGENTS... 3 4. PROCEDURE... 3 4.1. SAMPLE PREPARATION...
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET
 INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
Method (0.1 to 8.0 mg/l Cu) TNTplus 860
 Chlorine, Total, Bulk Powder, 8167 DOC316.53.01255 Bathocuproine Method 1 Method 10238 (0.1 to 8.0 mg/l Cu) TNTplus 860 Scope and Application: For water, wastewater and process water. Digestion may be
Chlorine, Total, Bulk Powder, 8167 DOC316.53.01255 Bathocuproine Method 1 Method 10238 (0.1 to 8.0 mg/l Cu) TNTplus 860 Scope and Application: For water, wastewater and process water. Digestion may be
The final oxidation product, iron (III), then combines with oxygen and water to form iron (III) oxide, or "rust".
 EXPERIMENT 19 Corrosion and Electrolytic Cells CORROSION OF IRON Corrosion is a naturally occurring redox process that oxidizes metals to their oxides and/or sulfides. In Part A we will be focusing primarily
EXPERIMENT 19 Corrosion and Electrolytic Cells CORROSION OF IRON Corrosion is a naturally occurring redox process that oxidizes metals to their oxides and/or sulfides. In Part A we will be focusing primarily
Formula & Equation Writing
 Formula & Equation Writing Book 2 H H Al Al H Al(H) 3 H Ionic Equations Ionic Formulae Balanced Equations Formula Equations Word Equations Transition Metals Using Brackets Awkward Customers More than 2
Formula & Equation Writing Book 2 H H Al Al H Al(H) 3 H Ionic Equations Ionic Formulae Balanced Equations Formula Equations Word Equations Transition Metals Using Brackets Awkward Customers More than 2
Name: Mods: Date Agenda Homework
 Name: Mods: UNIT 6 FORMULA WRITING, FORMULA WEIGHTS, and PERCENT COMPOSITION Date Agenda Homework Wed Dec 3 Thurs Dec 4 Fri Dec 5 Mon Dec 8 Tues Dec 9 Wed Dec 10 (1/2 Day) Thurs Dec 11 Fri Dec 12 Mon Dec
Name: Mods: UNIT 6 FORMULA WRITING, FORMULA WEIGHTS, and PERCENT COMPOSITION Date Agenda Homework Wed Dec 3 Thurs Dec 4 Fri Dec 5 Mon Dec 8 Tues Dec 9 Wed Dec 10 (1/2 Day) Thurs Dec 11 Fri Dec 12 Mon Dec
 CHAPTER 3 METALS AND NON-METALS About 118 elements are known today. There are more than 90 metals, 22 non metals and a few metalloids. Sodium (Na), potassium (K), magnesium(mg), aluminium(al), calcium(ca),
CHAPTER 3 METALS AND NON-METALS About 118 elements are known today. There are more than 90 metals, 22 non metals and a few metalloids. Sodium (Na), potassium (K), magnesium(mg), aluminium(al), calcium(ca),
3.3 Minerals. Describe the characteristics that define minerals.
 3.3 Minerals Describe the characteristics that define minerals. Are you a mineral? There used to be a TV commercial that said "you are what you eat." If that s true - and to some extent it is - then you
3.3 Minerals Describe the characteristics that define minerals. Are you a mineral? There used to be a TV commercial that said "you are what you eat." If that s true - and to some extent it is - then you
1. What volume of water is required to make a 4.65 M solution from 5.2 g of NaBr (MM = g/mol)?
 CHEMISTRY 110 EXAM II Answer Key March 22, 2013 1. What volume of water is required to make a 4.65 M solution from 5.2 g of NaBr (MM = 102.894 g/mol)? A. 4.65 ml B. 10.9 ml C. 11 ml D. 235 ml E. 240 ml
CHEMISTRY 110 EXAM II Answer Key March 22, 2013 1. What volume of water is required to make a 4.65 M solution from 5.2 g of NaBr (MM = 102.894 g/mol)? A. 4.65 ml B. 10.9 ml C. 11 ml D. 235 ml E. 240 ml
