A great many properties of crystals are determined by imperfections.
|
|
|
- Natalie Skinner
- 5 years ago
- Views:
Transcription
1 Defect in ceramics A great many properties of crystals are determined by imperfections. Electrical conductivity Diffusion transport imperfection Optical properties Rate of kinetic process Precipitation Kinetic processes Densification Grain coarsening High temperature creep deformation Point defects : defined as deviations from the perfect atomic arrangement. Missing ions Substituted ions Interstitial ions Associated valence electrons Point defects occur in all crystalline materials 101
2 Point defect : ionic defects electric defects Ionic defects vacancies interstitial substitutional solutes Electric defects formed when valence electrons are excited into higher orbital energy levels. Such an excitation may create an electron in the conduction band and/or an electron hole in the valence band of the crystal. Point defects in ceramic can be formed by thermal excitation solutes (impurities) oxidation or reduction In the solid-state analogy, the perfect crystal may be regarded as a neutral medium into which the charged defects are dissolved. Solid- state defect interaction (defect chemistry) similar solution-chemical interaction 102
3 Point defect types concentration Depend on density melting point electrical conductivity diffusion optical absorb strong clues defect behavior The formation of atomic defects requires the breaking of interatomic bonds The melting temperature (T m ) of a compound scale with the strength of its interatomic bonding. scale to T/ T m (homologous temperature) defect properties diffusion 103
4 Ex : At equal temperature, the conc. of defects in MgO is many orders of magnitude lower than NaCl. MgO (T m = 2825 ), NaCl (T m < ) At equal homologous temperature, the defect conc. are rather similar. Defect sties in the crystal structure A close packed sublattice of ions does not easily accommodate interstitial defect. Ex : rocksalt structure anion vacancies Anion interstitials > cation vacancies (high energy) cation interstitials The location of solutes and impurities in the lattice often depends on the compatibility in size with host ions. valence Solutes that differ in valence from the host (aliovalent) must be compensated by additional charged defects in order to maintain overall electrical neutrality in the crystal. 104
5 Nonstoichiometric compounds are those in which the metal/anion ratio deviates from the ideal value on which the structure is based, due to the existence of multiple ion valence state. Transition metal oxides (NiO, FeO, TiO 2...) are often highly defective. Ex : Fe 1-x O Cation-deficient due to the presence of a significant fraction (at least 5%) of the iron being in the Fe 3+ state. 105
6 Intrinsic ionic disorder Crystalline defects in ionic materials Frenkel defects Schottky Intrinsic defects, they can be thermally generated in perfect crystal. Extrinsic defects, which are formed only by the addition of impurities or solute. A Frenkel defect is formed when an atom is displaced from its normal site onto an interstitial site forming a defect pair a vacancy an interstitial In ionic materials, cation / anion can undergo this kind of displacement. In metals covalent compounds, Frenkel defects can also form. They differ from those in ionic compounds only in that the defect need not be electrically charged. 106
7 The Schottky defect is unique to ionic compound and is represent by the simultaneous creation of both cation vacancies. anion The vacancies must be formed in the stoichiometric ratio in order to preserve the electrical neutrality of the crystal. Ex: NaCl MgO a schottky pair TiO 2 the Schottky defect consist + Al 2 O 3 the Schottky defect consist + 107
8 Consider the change in free energy of a perfect crystal with initial free energy G 0, upon forming n Frenkel defect pairs at an energy of per pair. The free energy of the crystal becomes: The change in free energy ( : configurational entropy of the crystal) (Ω is the number of distinct ways in which the defect can be arranged) (1) If these are arranged on a total of N lattice site, the vacancies can be arranged in Ω V ways. ( h v : number of vacancies n i : number of interstitial) (2) If these are arranged on a total of N lattice site, the interstitial can be arranged in Ω i ways. 108
9 The total number of configuration: (stirling s approximation ) The total free energy change: To find the equilibrium conc. of defects. (for dilute conc. of defect ) For the conc. of defects: 109
10 In table 2.1, defect conc. are shown as a function of Δh and temperature, assuming that. 110
11 Intrinsic V.S. Extrinsic Behavior The concentration of intrinsic defect can be small in highly refractory ceramics of large defect formation energy. Intrinsic defect T Extrinsic defect solute (especially aliovalent solutes) oxidation process reduction process process Intrinsic defect concentrations increase with temperature. Extrinsic defects with the exception of nonstoichiometry, concentration is constant. Ex. Rocksalt structure type Nacl and MgO In both, the dominant intrinsic defect is the Schottky defect. The concentration of defects Δh : Energy of formation Δg : Formation free energy k : Boltzman constant High Δh, with the stronger bonding and higher T m. 111
12 At 700, n v /N (MgO) has many orders of magnitude fewer than n v /N (NaCl). But at =1, There is a great difference in the purity level with zone-refining procedures, NaCl ~ 1ppm(impurities) MgO ~ 50ppm(impurities) aliovalent cations. ( Because of the much higher processing temperatures necessary, contamination from crucibles is harder to avoid.) MgO, the impurity concentration, is much greater than the intrinsic defect concentration. NaCl, intrinsic defect can in practice be achieved rather easily. 112
13 Units for defect concentration : mole fraction, # fraction of defect n, relative to # of possible site N. n : number per unit volume ( no./cm 3 or cm -3 ) N : density of atoms in solid ( ~10 23 cm -3 ) Na (Avogadro s no.) = /mole 113
14 Kroger -Vink Notation 缺陷總類 電荷 位置 (,) : negative charge (.) : positive charge Cluster defects or defect associates The concentration of defects is denoted by [ ] ex: square brackets 114
15 Defect chemical reactions From a statistical thermodynamic viewpoint, how defect concentration depend on formation energy (Δh) temperature (T) An equivalent way to view the formation of defects is as a chemical reaction, for which there is an equilibrium constant which is governed by the law of mass action. Ex. Schottky reaction for NaCl and MgO (indicates the creation of defects from a perfect lattice.) mass-action equilibrium constant When only the intrinsic defects are present, ( [ ] : mole fraction ( ) at fixed T, vacancies conc. is a constant ) ( Δg s : the formation free energy for Schottky pair.) 115
16 Defect chemical reaction must obey (quasichemical reaction) (chemical reaction) mass mass site balance charge balance charge mass balance : a chemical reaction cannot create or loss mass. site balance : the ratio of cation to anion site of the crystal must be preserved, although the total no. of sites can be increased or decreased. Charge balance : total effective charge is balanced. (cation and anion vacancies must be formed in the stoichiometric ratios, the effective charges are automatically balanced.) Ex: Schottky reaction for Al 2 O 3 and BaTiO 3 Ex: Frenkel reaction for AgCl +(V i ) (site balance is maintained here since the formation of interstitials dose not create new crystal sites.) 116
17 In addition to the formation of intrinsic defect, reaction of particular interest include : The incorporation of solutes Formation of intrinsic electron defects Oxidation/reduction Defect association and precipitation Solute Incorporation Solute may enter solid solution in crystal as either substitutional or interstitial species. Isovalent substitution, NiO in MgO (MgO) Aliovalent substitution, -greater or lesser in valence than the host, must be charge compensated in solid solution. (1)formation of additional ionic defect (2)liberating electrons and holes ionic compensation electronic compensation 117
18 Ionic compensation mechanisms : Ex1: The dissolution of Al 2 O 3 in MgO Based on, we may presume that Al 3+ will substitute for Mg 2+,the O ions are likely to occupy additional O lattice sites. Ex2: The incorporation of MgO into Al 2 O 3 Mg ion may enter the solid solution substitutionally. interstitially. (1) If it is substitutional: (2) If it is interstitial: (3) Self-compensating (from both interstitial/substitutional defect) If, no single one will be the dominant mechanism of incorporation. ( simultaneously in equilibrium) 118
Mechanisms of Diffusion II. Ionic Crystals L5 11/3/03-1-
 Mechanisms of Diffusion II. Ionic Crystals 3.05 L5 11/3/03-1- Charges on point imperfections Point imperfections in ionic crystals are generally electrically charged. 3.05 L5 11/3/03 - (a) Unit cell in
Mechanisms of Diffusion II. Ionic Crystals 3.05 L5 11/3/03-1- Charges on point imperfections Point imperfections in ionic crystals are generally electrically charged. 3.05 L5 11/3/03 - (a) Unit cell in
TOPIC 2. STRUCTURE OF MATERIALS III
 Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Imperfections, Defects and Diffusion
 Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Crystal Defects. Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K)
 Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities)
 Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities) 1 Structural Imperfections A perfect crystal has the lowest internal energy E Above absolute zero
Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities) 1 Structural Imperfections A perfect crystal has the lowest internal energy E Above absolute zero
Chapter 12: Structures & Properties of Ceramics
 Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Review of structures for ceramics How are impurities accommodated in the ceramic lattice? In what ways are ceramic phase diagrams similar
Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Review of structures for ceramics How are impurities accommodated in the ceramic lattice? In what ways are ceramic phase diagrams similar
Lecture 3: Description crystal structures / Defects
 Lecture 3: Description crystal structures / Defects Coordination Close packed structures Cubic close packing Hexagonal close packing Metallic structures Ionic structures with interstitial sites Important
Lecture 3: Description crystal structures / Defects Coordination Close packed structures Cubic close packing Hexagonal close packing Metallic structures Ionic structures with interstitial sites Important
Learning Objectives. Chapter Outline. Solidification of Metals. Solidification of Metals
 Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Defects and Diffusion
 Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Defect in crystals. Primer in Materials Science Spring
 Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
Physical Ceramics. Principles for Ceramic Science and Engineering. Yet-Ming Chiang Massachusetts Institute of Technology Cambridge, Massachusetts
 Physical Ceramics Principles for Ceramic Science and Engineering Yet-Ming Chiang Massachusetts Institute of Technology Cambridge, Massachusetts Dunbar P. Birnie, III University of Arizona Tucson, Arizona
Physical Ceramics Principles for Ceramic Science and Engineering Yet-Ming Chiang Massachusetts Institute of Technology Cambridge, Massachusetts Dunbar P. Birnie, III University of Arizona Tucson, Arizona
Unit-1 THE SOLID STATE QUESTIONS VSA QUESTIONS (1 - MARK QUESTIONS)
 Unit-1 THE SOLID STATE QUESTIONS VSA QUESTIONS (1 - MARK QUESTIONS) 1. What are anistropic substances. 2. Why are amorphous solids isotropic in nature?. Why glass is regarded as an amorphous solid? 4.
Unit-1 THE SOLID STATE QUESTIONS VSA QUESTIONS (1 - MARK QUESTIONS) 1. What are anistropic substances. 2. Why are amorphous solids isotropic in nature?. Why glass is regarded as an amorphous solid? 4.
POINT DEFECTS IN CRYSTALS
 POINT DEFECTS IN CRYSTALS Overview Vacancies & their Clusters Interstitials Defects in Ionic Crytals Frenkel defect Shottky defect Part of MATERIALS SCIENCE & ENGINEERING A Learner s Guide AN INTRODUCTORY
POINT DEFECTS IN CRYSTALS Overview Vacancies & their Clusters Interstitials Defects in Ionic Crytals Frenkel defect Shottky defect Part of MATERIALS SCIENCE & ENGINEERING A Learner s Guide AN INTRODUCTORY
 Defects in solids http://www.bath.ac.uk/podcast/powerpoint/inaugural_lecture_250407.pdf http://www.materials.ac.uk/elearning/matter/crystallography/indexingdirectionsandplanes/indexing-of-hexagonal-systems.html
Defects in solids http://www.bath.ac.uk/podcast/powerpoint/inaugural_lecture_250407.pdf http://www.materials.ac.uk/elearning/matter/crystallography/indexingdirectionsandplanes/indexing-of-hexagonal-systems.html
NPTEL COURSE ADVANCED CERAMICS FOR STRATEGIC APPLICATIONS QUESTIONS AND ANSWERS
 NPTEL COURSE ADVANCED CERAMICS FOR STRATEGIC APPLICATIONS QUESTIONS AND ANSWERS Q1: What do you understand by Ceramics? Ans: Ceramics are a group of chemical compounds, either simple (consisting of only
NPTEL COURSE ADVANCED CERAMICS FOR STRATEGIC APPLICATIONS QUESTIONS AND ANSWERS Q1: What do you understand by Ceramics? Ans: Ceramics are a group of chemical compounds, either simple (consisting of only
Physics of Transition Metal Oxides
 Physics of Transition Metal Oxides Lecture 11 Defects in oxides Defects in oxides: We have looked at a variety of defects already. Today we discuss structural defects, giving rise to distinct phases impurity
Physics of Transition Metal Oxides Lecture 11 Defects in oxides Defects in oxides: We have looked at a variety of defects already. Today we discuss structural defects, giving rise to distinct phases impurity
MSE 351 Engineering Ceramics I
 Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 351 Engineering Ceramics I Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 351 Engineering Ceramics I Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
IMPERFECTIONSFOR BENEFIT. Sub-topics. Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface
 IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
Intrinsic Defects and Defect
 Chapter 4 Intrinsic Defects and Defect Concentrations in MgAl 2 O 4 Spinel 4.1 Introduction MgAl 2 O 4 spinel is an important industrial ceramic with a range of applications that take advantage of its
Chapter 4 Intrinsic Defects and Defect Concentrations in MgAl 2 O 4 Spinel 4.1 Introduction MgAl 2 O 4 spinel is an important industrial ceramic with a range of applications that take advantage of its
Module 10. Crystal Defects in Metals I. Lecture 10. Crystal Defects in Metals I
 Module 10 Crystal Defects in Metals I Lecture 10 Crystal Defects in Metals I 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Introduction Keywords:
Module 10 Crystal Defects in Metals I Lecture 10 Crystal Defects in Metals I 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Introduction Keywords:
Order in materials. Making Solid Stuff. Primary Bonds Summary. How do they arrange themselves? Results from atomic bonding. What are they?
 Making Solid Stuff Primary Bonds Summary What are they? Results from atomic bonding So the atoms bond together! Order in materials No long range order to atoms Gases little or no interaction between components
Making Solid Stuff Primary Bonds Summary What are they? Results from atomic bonding So the atoms bond together! Order in materials No long range order to atoms Gases little or no interaction between components
From sand to silicon wafer
 From sand to silicon wafer 25% of Earth surface is silicon Metallurgical grade silicon (MGS) Electronic grade silicon (EGS) Polycrystalline silicon (polysilicon) Single crystal Czochralski drawing Single
From sand to silicon wafer 25% of Earth surface is silicon Metallurgical grade silicon (MGS) Electronic grade silicon (EGS) Polycrystalline silicon (polysilicon) Single crystal Czochralski drawing Single
Materials Science. Imperfections in Solids CHAPTER 5: IMPERFECTIONS IN SOLIDS. Types of Imperfections
 In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
(a) Would you expect the element P to be a donor or an acceptor defect in Si?
 MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
much research (in physics, chemistry, material science, etc.) have been done to understand the difference in materials properties.
 1.1: Introduction Material science and engineering Classify common features of structure and properties of different materials in a well-known manner (chemical or biological): * bonding in solids are classified
1.1: Introduction Material science and engineering Classify common features of structure and properties of different materials in a well-known manner (chemical or biological): * bonding in solids are classified
General Characteristics of Solid State
 General Characteristics of Solid State (i) They have definite mass, volume and shape. (ii) Intermolecular distances are short. (iii) Intermolecular forces are strong. (iv) Their constituent particles (atoms,
General Characteristics of Solid State (i) They have definite mass, volume and shape. (ii) Intermolecular distances are short. (iii) Intermolecular forces are strong. (iv) Their constituent particles (atoms,
Bulk Diffusion in Alumina: Solving the Corundum Conundrum
 Bulk Diffusion in Alumina: Solving the Corundum Conundrum Nicholas D.M. Hine 1,2,3 K. Frensch 3, W.M.C Foulkes 1,2, M.W. Finnis 2,3, A. H. Heuer 3,4 1 Theory of Condensed Matter Group, Cavendish Laboratory,
Bulk Diffusion in Alumina: Solving the Corundum Conundrum Nicholas D.M. Hine 1,2,3 K. Frensch 3, W.M.C Foulkes 1,2, M.W. Finnis 2,3, A. H. Heuer 3,4 1 Theory of Condensed Matter Group, Cavendish Laboratory,
Development of Microstructure in Eutectic Alloys
 CHAPTER 10 PHASE DIAGRAMS PROBLEM SOLUTIONS Development of Microstructure in Eutectic Alloys 10.16 Briefly explain why, upon solidification, an alloy of eutectic composition forms a microstructure consisting
CHAPTER 10 PHASE DIAGRAMS PROBLEM SOLUTIONS Development of Microstructure in Eutectic Alloys 10.16 Briefly explain why, upon solidification, an alloy of eutectic composition forms a microstructure consisting
Dept.of BME Materials Science Dr.Jenan S.Kashan 1st semester 2nd level. Imperfections in Solids
 Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
Chapter 12 Metals. crystalline, in which particles are in highly ordered arrangement. (Have MP.)
 Chapter 12 Metals 12.1 Classification of Solids Covalent Ionic Molecular Metallic Solids Solids Solids Solids Molecular consist of molecules held next to each other by IMF s. Relatively low to moderate
Chapter 12 Metals 12.1 Classification of Solids Covalent Ionic Molecular Metallic Solids Solids Solids Solids Molecular consist of molecules held next to each other by IMF s. Relatively low to moderate
1. Use the Ellingham Diagram (reproduced here as Figure 0.1) to answer the following.
 315 Problems 1. Use the Ellingham Diagram (reproduced here as Figure 0.1) to answer the following. (a) Find the temperature and partial pressure of O 2 where Ni(s), Ni(l), and NiO(s) are in equilibrium.
315 Problems 1. Use the Ellingham Diagram (reproduced here as Figure 0.1) to answer the following. (a) Find the temperature and partial pressure of O 2 where Ni(s), Ni(l), and NiO(s) are in equilibrium.
Materials Science and Engineering: An Introduction
 Materials Science and Engineering: An Introduction Callister, William D. ISBN-13: 9780470419977 Table of Contents List of Symbols. 1 Introduction. 1.1 Historical Perspective. 1.2 Materials Science and
Materials Science and Engineering: An Introduction Callister, William D. ISBN-13: 9780470419977 Table of Contents List of Symbols. 1 Introduction. 1.1 Historical Perspective. 1.2 Materials Science and
Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number:
 Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number: Instructions: Answer all questions and show your work. You will not receive partial credit unless you show your work.
Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number: Instructions: Answer all questions and show your work. You will not receive partial credit unless you show your work.
Stacking Oranges. Packing atoms together Long Range Order. What controls the nearest number of atoms? Hard Sphere Model. Hard Sphere Model.
 { Stacking atoms together Crystal Structure Stacking Oranges Packing atoms together Long Range Order Crystalline materials... atoms pack in periodic, 3D arrays typical of: -metals -many ceramics -some
{ Stacking atoms together Crystal Structure Stacking Oranges Packing atoms together Long Range Order Crystalline materials... atoms pack in periodic, 3D arrays typical of: -metals -many ceramics -some
Imperfections in the Atomic and Ionic Arrangements
 Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
Point Defects LATTICE VACANCIES 585. DIFFUSION 588 Metals 591. COLOR CENTERS 592 F centers 592 Other centers in alkali halides 593 PROBLEMS 595
 ch20.qxd 9/22/04 5:27 PM Page 583 20 Point Defects LATTICE VACANCIES 585 DIFFUSION 588 Metals 591 COLOR CENTERS 592 F centers 592 Other centers in alkali halides 593 PROBLEMS 595 1. Frenkel defects 595
ch20.qxd 9/22/04 5:27 PM Page 583 20 Point Defects LATTICE VACANCIES 585 DIFFUSION 588 Metals 591 COLOR CENTERS 592 F centers 592 Other centers in alkali halides 593 PROBLEMS 595 1. Frenkel defects 595
Chapter 2 Crystal Growth and Wafer Preparation
 Chapter 2 Crystal Growth and Wafer Preparation Professor Paul K. Chu Advantages of Si over Ge Si has a larger bandgap (1.1 ev for Si versus 0.66 ev for Ge) Si devices can operate at a higher temperature
Chapter 2 Crystal Growth and Wafer Preparation Professor Paul K. Chu Advantages of Si over Ge Si has a larger bandgap (1.1 ev for Si versus 0.66 ev for Ge) Si devices can operate at a higher temperature
Chapter 5. Imperfections in Solids
 Chapter 5 Imperfections in Solids Chapter 5 2D Defects and Introduction to Diffusion Imperfections in Solids Issues to Address... What types of defects arise in solids? Can the number and type of defects
Chapter 5 Imperfections in Solids Chapter 5 2D Defects and Introduction to Diffusion Imperfections in Solids Issues to Address... What types of defects arise in solids? Can the number and type of defects
Electrical conduction in ceramics
 Laurea Magistrale in Scienza dei Materiali Materiali Inorganici Funzionali Electrical conduction in ceramics Prof. Antonella Glisenti - Dip. Scienze Chimiche - Università degli Studi di Padova Conductivity
Laurea Magistrale in Scienza dei Materiali Materiali Inorganici Funzionali Electrical conduction in ceramics Prof. Antonella Glisenti - Dip. Scienze Chimiche - Università degli Studi di Padova Conductivity
Electrical Properties of Polymers, Ceramics, Dielectrics, and Amorphous Materials. Dae Yong JEONG Inha University
 Electrical Properties of Polymers, Ceramics, Dielectrics, and Amorphous Materials Dae Yong JEONG Inha University Review & Introduction We learned about the electronic transfer in Metal and Semiconductor.
Electrical Properties of Polymers, Ceramics, Dielectrics, and Amorphous Materials Dae Yong JEONG Inha University Review & Introduction We learned about the electronic transfer in Metal and Semiconductor.
E45 Midterm 01 Fall 2007! By the 0.2% offset method (shown on plot), YS = 500 MPa
 1.!Mechanical Properties (20 points) Refer to the following stress-strain plot derived from a standard uniaxial tensile test of a high performance titanium alloy to answer the following questions. Show
1.!Mechanical Properties (20 points) Refer to the following stress-strain plot derived from a standard uniaxial tensile test of a high performance titanium alloy to answer the following questions. Show
Halbleiter Prof. Yong Lei Prof. Thomas Hannappel
 Halbleiter Prof. Yong Lei Prof. Thomas Hannappel yong.lei@tu-ilmenau.de thomas.hannappel@tu-ilmenau.de http://www.tu-ilmenau.de/nanostruk/ Solid State Structure of Semiconductor Semiconductor manufacturing
Halbleiter Prof. Yong Lei Prof. Thomas Hannappel yong.lei@tu-ilmenau.de thomas.hannappel@tu-ilmenau.de http://www.tu-ilmenau.de/nanostruk/ Solid State Structure of Semiconductor Semiconductor manufacturing
CHAPTER 5: DIFFUSION IN SOLIDS
 CHAPTER 5: DIFFUSION IN SOLIDS ISSUES TO ADDRESS... How does diffusion occur? Why is it an important part of processing? How can the rate of diffusion be predicted for some simple cases? How does diffusion
CHAPTER 5: DIFFUSION IN SOLIDS ISSUES TO ADDRESS... How does diffusion occur? Why is it an important part of processing? How can the rate of diffusion be predicted for some simple cases? How does diffusion
Point Defects. Vacancies are the most important form. Vacancies Self-interstitials
 Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
Point Defects in Metals
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Point Defects in Metals 5.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327 C (600 K). Assume an energy
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Point Defects in Metals 5.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327 C (600 K). Assume an energy
Hei Wong.
 Defects and Disorders in Hafnium Oxide and at Hafnium Oxide/Silicon Interface Hei Wong City University of Hong Kong Email: heiwong@ieee.org Tokyo MQ2012 1 Outline 1. Introduction, disorders and defects
Defects and Disorders in Hafnium Oxide and at Hafnium Oxide/Silicon Interface Hei Wong City University of Hong Kong Email: heiwong@ieee.org Tokyo MQ2012 1 Outline 1. Introduction, disorders and defects
Defects and transport in Ce-doped La 27 W 5 O 55.5
 Defects and transport in Ce-doped La 27 W 5 O 55.5 Nadezda Alyeshkina Master Thesis in Materials, Energy and Nanotechnology UNIVERSITY OF OSLO DEPARTMENT OF CHEMISTRY June 2013 ii Preface This thesis represents
Defects and transport in Ce-doped La 27 W 5 O 55.5 Nadezda Alyeshkina Master Thesis in Materials, Energy and Nanotechnology UNIVERSITY OF OSLO DEPARTMENT OF CHEMISTRY June 2013 ii Preface This thesis represents
Single vs Polycrystals
 WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 6 THE IMPERFECT SOLID STATE
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
Material Science. Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore India
 Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 5. Diffusion Learning objectives: - To know the
Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 5. Diffusion Learning objectives: - To know the
Al2O3-MgO system: magnesia and spinel Magnesia
 Al 2 O 3 -MgO system: magnesia and spinel 1-1.2. Magnesia Magnesium oxide (MgO, magnesia) occurs naturally as the mineral periclase; a metamorphic mineral formed by the breakdown of dolomite, CaMg (CO
Al 2 O 3 -MgO system: magnesia and spinel 1-1.2. Magnesia Magnesium oxide (MgO, magnesia) occurs naturally as the mineral periclase; a metamorphic mineral formed by the breakdown of dolomite, CaMg (CO
Solid. Imperfection in solids. Examples of Imperfections #2. Examples of Imperfections #1. Solid
 Solid Imperfection in solids By E-mail: Patama.V@chula.ac.th Solid State of materials Rigid Strong bonding ionic, van der aals, metal bonding normally has crystal structure Examples of Imperfections #
Solid Imperfection in solids By E-mail: Patama.V@chula.ac.th Solid State of materials Rigid Strong bonding ionic, van der aals, metal bonding normally has crystal structure Examples of Imperfections #
Introduction to Point Defects. Version 2.1. Andrew Green, MATTER John Humphreys, UMIST/University of Manchester Ross Mackenzie, Open University
 Introduction to Point Defects Version 2.1 Andrew Green, MATTER John Humphreys, UMIST/University of Manchester Ross Mackenzie, Open University October 1997 Assumed Pre-knowledge This module has been developed
Introduction to Point Defects Version 2.1 Andrew Green, MATTER John Humphreys, UMIST/University of Manchester Ross Mackenzie, Open University October 1997 Assumed Pre-knowledge This module has been developed
SOLID STATE
 SOLID STATE Short Answer Questions: 1. Derive Bragg s equation? Ans. Bragg s equation: W.H. Bragg has proposed an equation to explain the relation between inter planar distance (d) and wave length ( λ
SOLID STATE Short Answer Questions: 1. Derive Bragg s equation? Ans. Bragg s equation: W.H. Bragg has proposed an equation to explain the relation between inter planar distance (d) and wave length ( λ
Now, let s examine how atoms are affected as liquids transform into solids.
 Now, let s examine how atoms are affected as liquids transform into solids. 1 Before we deal with PROPERTIES of materials, it s beneficial to remember where we have come from, and where we are going. Later,
Now, let s examine how atoms are affected as liquids transform into solids. 1 Before we deal with PROPERTIES of materials, it s beneficial to remember where we have come from, and where we are going. Later,
Chapter Outline Dislocations and Strengthening Mechanisms. Introduction
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Definition and description of different diffusion terms
 Definition and description of different diffusion terms efore proceeding further, it is necessary to introduce different terms frequently used in diffusion studies. Many terms will be introduced, which
Definition and description of different diffusion terms efore proceeding further, it is necessary to introduce different terms frequently used in diffusion studies. Many terms will be introduced, which
CHAPTER THE SOLID STATE
 133 CHAPTER THE SOLID STATE 1. The ability of a substances to assume two or more crystalline structures is called [1990] Isomerism Polymorphism Isomorphism Amorphism 2. Most crystals show good cleavage
133 CHAPTER THE SOLID STATE 1. The ability of a substances to assume two or more crystalline structures is called [1990] Isomerism Polymorphism Isomorphism Amorphism 2. Most crystals show good cleavage
MSE 6020: Defects and Microstructure in Materials Spring 2015, Tuesday and Thursday, 3:30-4:45 pm, Rice Hall 032
 MSE 6020: Defects and Microstructure in Materials Spring 2015, Tuesday and Thursday, 3:30-4:45 pm, Rice Hall 032 Contact Information: Instructor: Leonid Zhigilei Office: Wilsdorf Hall 303D Office Hours:
MSE 6020: Defects and Microstructure in Materials Spring 2015, Tuesday and Thursday, 3:30-4:45 pm, Rice Hall 032 Contact Information: Instructor: Leonid Zhigilei Office: Wilsdorf Hall 303D Office Hours:
Introduction to Materials Science
 EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
Free Electron Model What kind of interactions hold metal atoms together? How does this explain high electrical and thermal conductivity?
 Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Mechanical Lustrous
Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Mechanical Lustrous
Chapter 18: Electrical Properties
 Chapter 18: Electrical Properties What are the physical phenomena that distinguish conductors, semiconductors, and insulators? For metals, how is conductivity affected by imperfections, T, and deformation?
Chapter 18: Electrical Properties What are the physical phenomena that distinguish conductors, semiconductors, and insulators? For metals, how is conductivity affected by imperfections, T, and deformation?
Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties
 Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties A perfect crystal, with every atom of the same type in the correct position, does not exist. There always exist crystalline defects,
Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties A perfect crystal, with every atom of the same type in the correct position, does not exist. There always exist crystalline defects,
Unit 1 The Solid State
 Points to Remember Amorphous and Crystalline Solids Unit 1 The Solid State Amorphous- short range order, Irregular shape eg-glass Crystalline Solids- long range order, regular shape eg : NaCl Molecular
Points to Remember Amorphous and Crystalline Solids Unit 1 The Solid State Amorphous- short range order, Irregular shape eg-glass Crystalline Solids- long range order, regular shape eg : NaCl Molecular
C h a p t e r 4 : D e f e c t s i n C r y s t a l s
 C h a p t e r 4 : D e f e c t s i n C r y s t a l s...perfection's a gift of The gods, few can boast they possess it - and most Of you, my dears, don't. - Ovid, The Art of Love Chapter 4: Defects in Crystals...
C h a p t e r 4 : D e f e c t s i n C r y s t a l s...perfection's a gift of The gods, few can boast they possess it - and most Of you, my dears, don't. - Ovid, The Art of Love Chapter 4: Defects in Crystals...
Lab IV: Electrical Properties
 Lab IV: Electrical Properties Study Questions 1. How would the electrical conductivity of the following vary with temperature: (a) ionic solids; (b) semiconductors; (c) metals? Briefly explain your answer.
Lab IV: Electrical Properties Study Questions 1. How would the electrical conductivity of the following vary with temperature: (a) ionic solids; (b) semiconductors; (c) metals? Briefly explain your answer.
The Nernst-Einstein equation indicates that the ratio β /D for a given material varies only with temperature. Calculate β/d for oxygen ions in Zr 0.
 The Nernst-Einstein equation indicates that the ratio β /D for a given material varies only with temperature. Calculate β/d for oxygen ions in 0.8 Y 0.2 1.9 at 800 C. 1 The Nernst-Einstein equation indicates
The Nernst-Einstein equation indicates that the ratio β /D for a given material varies only with temperature. Calculate β/d for oxygen ions in 0.8 Y 0.2 1.9 at 800 C. 1 The Nernst-Einstein equation indicates
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
Point coordinates. x z
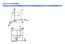 Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 6 THE IMPERFECT SOLID STATE
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
Metals I. Anne Mertens
 "MECA0139-1: Techniques "MECA0462-2 additives : et Materials 3D printing", Selection", ULg, 19/09/2017 25/10/2016 Metals I Anne Mertens Introduction Outline Metallic materials Materials Selection: case
"MECA0139-1: Techniques "MECA0462-2 additives : et Materials 3D printing", Selection", ULg, 19/09/2017 25/10/2016 Metals I Anne Mertens Introduction Outline Metallic materials Materials Selection: case
1.10 Close packed structures cubic and hexagonal close packing
 1.9 Description of crystal structures The most common way for describing crystal structure is to refer the structure to the unit cell. The structure is given by the size and shape of the cell and the position
1.9 Description of crystal structures The most common way for describing crystal structure is to refer the structure to the unit cell. The structure is given by the size and shape of the cell and the position
The Structure of Materials
 The Structure of Materials Samuel M. Allen Edwin L. Thomas Massachusetts Institute of Technology Cambridge, Massachusetts / John Wiley & Sons, Inc. New York Chichester Weinheim Brisbane Singapore Toronto
The Structure of Materials Samuel M. Allen Edwin L. Thomas Massachusetts Institute of Technology Cambridge, Massachusetts / John Wiley & Sons, Inc. New York Chichester Weinheim Brisbane Singapore Toronto
3. Solidification & Crystalline Imperfections
 3. Solidification & Crystalline Imperfections solidification (casting process) of metals divided into two steps (1) nucleation formation of stable nuclei in the melt (2) growth of nuclei into crystals
3. Solidification & Crystalline Imperfections solidification (casting process) of metals divided into two steps (1) nucleation formation of stable nuclei in the melt (2) growth of nuclei into crystals
Strengthening Mechanisms
 ME 254: Materials Engineering Chapter 7: Dislocations and Strengthening Mechanisms 1 st Semester 1435-1436 (Fall 2014) Dr. Hamad F. Alharbi, harbihf@ksu.edu.sa November 18, 2014 Outline DISLOCATIONS AND
ME 254: Materials Engineering Chapter 7: Dislocations and Strengthening Mechanisms 1 st Semester 1435-1436 (Fall 2014) Dr. Hamad F. Alharbi, harbihf@ksu.edu.sa November 18, 2014 Outline DISLOCATIONS AND
Materials Aspects of GaAs and InP Based Structures
 AT&T Materials Aspects of GaAs and InP Based Structures V. Swaminathan AT&T Belt Laboratories Breinigsvil/e, Pennsylvania A. T. Macrander Argonne National Laboratory Argonne, Illinois m Prentice Hall,
AT&T Materials Aspects of GaAs and InP Based Structures V. Swaminathan AT&T Belt Laboratories Breinigsvil/e, Pennsylvania A. T. Macrander Argonne National Laboratory Argonne, Illinois m Prentice Hall,
Phosphorous Problem. AkMB Rashid Professor, Department of MME BUET, Dhaka
 09 Phosphorous Problem AkMB Rashid Professor, Department of MME BUET, Dhaka Today s Topics Behaviour of phosphorous in metal and slag Oxidation of phosphorous Effect of temperature Effect of metal and
09 Phosphorous Problem AkMB Rashid Professor, Department of MME BUET, Dhaka Today s Topics Behaviour of phosphorous in metal and slag Oxidation of phosphorous Effect of temperature Effect of metal and
Solid State Device Fundamentals
 Solid State Device Fundamentals ENS 345 Lecture Course by Alexander M. Zaitsev alexander.zaitsev@csi.cuny.edu Tel: 718 982 2812 Office 4N101b 1 Interatomic bonding Bonding Forces and Energies Equilibrium
Solid State Device Fundamentals ENS 345 Lecture Course by Alexander M. Zaitsev alexander.zaitsev@csi.cuny.edu Tel: 718 982 2812 Office 4N101b 1 Interatomic bonding Bonding Forces and Energies Equilibrium
CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS
 MODULE ONE CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS CRYSTAL STRUCTURE Metallic crystal structures; BCC, FCC and HCP Coordination number and Atomic Packing Factor (APF) Crystal imperfections:
MODULE ONE CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS CRYSTAL STRUCTURE Metallic crystal structures; BCC, FCC and HCP Coordination number and Atomic Packing Factor (APF) Crystal imperfections:
MSE 352 Engineering Ceramics II
 Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 352 Engineering Ceramics II 3 Credit Hours Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical
Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 352 Engineering Ceramics II 3 Credit Hours Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical
MSE 170 Midterm review
 MSE 170 Midterm review Exam date: 11/2/2008 Mon, lecture time Place: Here! Close book, notes and no collaborations A sheet of letter-sized paper with double-sided notes is allowed Material on the exam
MSE 170 Midterm review Exam date: 11/2/2008 Mon, lecture time Place: Here! Close book, notes and no collaborations A sheet of letter-sized paper with double-sided notes is allowed Material on the exam
Chapter 2. Ans: e (<100nm size materials are called nanomaterials)
 Chapter 2 1. Materials science and engineering include (s) the study of: (a) metals (b) polymers (c) ceramics (d) composites (e) nanomaterials (f) all of the above Ans: f 2. Which one of the following
Chapter 2 1. Materials science and engineering include (s) the study of: (a) metals (b) polymers (c) ceramics (d) composites (e) nanomaterials (f) all of the above Ans: f 2. Which one of the following
Agenda o Semiconductor Materials o Crystal Growth o Intrinsic Semiconductors o Extrinsic Semiconductors
 E84 Lecture 3/6/14 K. Candler Agenda o Semiconductor Materials o Crystal Growth o Intrinsic Semiconductors o Extrinsic Semiconductors Introduction A semiconductor is a material that has electrical conductivity
E84 Lecture 3/6/14 K. Candler Agenda o Semiconductor Materials o Crystal Growth o Intrinsic Semiconductors o Extrinsic Semiconductors Introduction A semiconductor is a material that has electrical conductivity
ECE440 Nanoelectronics. Lecture 08 Review of Solid State Physics
 ECE440 Nanoelectronics Lecture 08 Review of Solid State Physics A Brief review of Solid State Physics Crystal lattice, reciprocal lattice, symmetry Crystal directions and planes Energy bands, bandgap Direct
ECE440 Nanoelectronics Lecture 08 Review of Solid State Physics A Brief review of Solid State Physics Crystal lattice, reciprocal lattice, symmetry Crystal directions and planes Energy bands, bandgap Direct
EGN 3365 Review on Metals, Ceramics, & Polymers, and Composites by Zhe Cheng
 EGN 3365 Review on Metals, Ceramics, & Polymers, and Composites 2017 by Zhe Cheng Expectations on Chapter 11 Chapter 11 Understand metals are generally categorized as ferrous alloys and non-ferrous alloys
EGN 3365 Review on Metals, Ceramics, & Polymers, and Composites 2017 by Zhe Cheng Expectations on Chapter 11 Chapter 11 Understand metals are generally categorized as ferrous alloys and non-ferrous alloys
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS ev /atom = exp. kt ( =
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
MAKING SOLID SOLUTIONS WITH ALKALI HALIDES (AND BREAKING THEM)
 MAKING SOLID SOLUTIONS WITH ALKALI HALIDES (AND BREAKING THEM) John B. Brady Department of Geology Smith College Northampton, MA 01063 jbrady@science.smith.edu INTRODUCTION When two cations have the same
MAKING SOLID SOLUTIONS WITH ALKALI HALIDES (AND BREAKING THEM) John B. Brady Department of Geology Smith College Northampton, MA 01063 jbrady@science.smith.edu INTRODUCTION When two cations have the same
Chapter: The d and f Block Elements
 Chapter: The d and f Block Elements Introduction to d block elements Question 1 In Tc ( Z = 43) and Tb( Z = 65) which one is inner transition metal and which one is transition metal and why? The outer
Chapter: The d and f Block Elements Introduction to d block elements Question 1 In Tc ( Z = 43) and Tb( Z = 65) which one is inner transition metal and which one is transition metal and why? The outer
Problem 1 (10 points): Mark True (T) or False (F) for the following statements.
 MSE 170 Final 03/19/09 166pts. total Exam is closed book, closed notes, closed neighbors Instruction: 1. Write your name and student I on top of page. 2. Write legibly. 3. Show work as needed to justify
MSE 170 Final 03/19/09 166pts. total Exam is closed book, closed notes, closed neighbors Instruction: 1. Write your name and student I on top of page. 2. Write legibly. 3. Show work as needed to justify
Defects and crystal growth
 CRYSTL GROWTH CRYSTL GROWTH steps and questions 1. Reactants in molten form Si 2. Transport to S/L interface 3. dsorbtion: entropy decreases -TDS increases DH decreases (exo) 4. Critical nucleus size DS
CRYSTL GROWTH CRYSTL GROWTH steps and questions 1. Reactants in molten form Si 2. Transport to S/L interface 3. dsorbtion: entropy decreases -TDS increases DH decreases (exo) 4. Critical nucleus size DS
Chapter 2 Structure of Materials
 Chapter 2 Structure of Materials In this chapter, we will briefly go through the hierarchical structure of materials. First, the atomic bonding and crystal structures are covered briefly. Then, the emphasis
Chapter 2 Structure of Materials In this chapter, we will briefly go through the hierarchical structure of materials. First, the atomic bonding and crystal structures are covered briefly. Then, the emphasis
Overall Conclusions and Future Projections OVERALL CONCLUSIONS
 OVERALL CONCLUSIONS This article brings the thesis to a close by presenting the conclusions drawn from the outcome of the radiation effects on the structural and optical properties of heavy metal oxide
OVERALL CONCLUSIONS This article brings the thesis to a close by presenting the conclusions drawn from the outcome of the radiation effects on the structural and optical properties of heavy metal oxide
Free Electron Model What kind of interactions hold metal atoms together? How does this explain high electrical and thermal conductivity?
 Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Free Electron
Electrical Good conductors of heat & electricity Create semiconductors Oxides are basic ionic solids Aqueous cations (positive charge, Lewis acids) Reactivity increases downwards in family Free Electron
Chapter 7 Dislocations and Strengthening Mechanisms. Dr. Feras Fraige
 Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
METALLIC CRYSTALS. tend to be densely packed. have several reasons for dense packing: have the simplest crystal structures.
 METALLIC CRYSTALS tend to be densely packed. have several reasons for dense packing: -Typically, only one element is present, so all atomic radii are the same. -Metallic bonding is not directional. -Nearest
METALLIC CRYSTALS tend to be densely packed. have several reasons for dense packing: -Typically, only one element is present, so all atomic radii are the same. -Metallic bonding is not directional. -Nearest
Atomic structure, arrangement, and movement Introduction to Materials Introduction Types of Materials Structure-Property-Processing Relationship
 Atomic structure, arrangement, and movement to Materials Types of Materials Structure-Property-Processing Relationship Environmental Effects on Material Behavior Materials Design and Selection Atomic Structure
Atomic structure, arrangement, and movement to Materials Types of Materials Structure-Property-Processing Relationship Environmental Effects on Material Behavior Materials Design and Selection Atomic Structure
Dislocations and Plastic Deformation
 Dislocations and Plastic Deformation Edge and screw are the two fundamental dislocation types. In an edge dislocation, localized lattice distortion exists along the end of an extra half-plane of atoms,
Dislocations and Plastic Deformation Edge and screw are the two fundamental dislocation types. In an edge dislocation, localized lattice distortion exists along the end of an extra half-plane of atoms,
Chapter 3: Atomic and Ionic Arrangements. Chapter 3: Atomic and Ionic Arrangements Cengage Learning Engineering. All Rights Reserved.
 Chapter 3: Atomic and Ionic Arrangements 3-1 Learning Objectives 1. 2. 3. 4. 5. 6. 7. 8. Short-range order versus long-range order Amorphous materials Lattice, basis, unit cells, and crystal structures
Chapter 3: Atomic and Ionic Arrangements 3-1 Learning Objectives 1. 2. 3. 4. 5. 6. 7. 8. Short-range order versus long-range order Amorphous materials Lattice, basis, unit cells, and crystal structures
VLSI Technology Dr. Nandita Dasgupta Department of Electrical Engineering Indian Institute of Technology, Madras
 VLSI Technology Dr. Nandita Dasgupta Department of Electrical Engineering Indian Institute of Technology, Madras Lecture - 5 Crystal Structure contd So far, we have discussed about the crystal structure
VLSI Technology Dr. Nandita Dasgupta Department of Electrical Engineering Indian Institute of Technology, Madras Lecture - 5 Crystal Structure contd So far, we have discussed about the crystal structure
