DENSITY of Solids and Liquids
|
|
|
- Shanna Green
- 5 years ago
- Views:
Transcription
1 Regents Earth Science Name: Date: Lab # DENSITY of Solids and Liquids Introduction: Density is the term used to describe the relationship between the mass of an object and its volume. Under given conditions of temperature and pressure, the density of a material is constant. The density of any earth material can be determined by measuring its mass and volume and using the equation: Density = Mass Volume The density of fluids in either the liquid or gaseous state is very important when considering earth science topics such as ocean current flow, the turnover of lakes, wind patterns in the atmosphere and movement of the plastic asthenosphere. In this lab we will determine the density of three different liquids and then plot a graph to illustrate the differences in density. Objective: You will be able to calculate the densities of different materials and recognize that density is one of the most important properties of matter. 100 ml graduated cylinder Glycerol Water Alcohol Electronic Balance Triple beam balance Solid objects Ruler Calculator Procedures: Part A - Solids 1. Measure the mass of each object using a triple beam balance. Your answer should be recorded in grams (g) to the nearest tenth (0.1) on Report Sheet Find the volume of each object using a metric ruler and the equation in the above or by using the water displacement method. Your answer will be in cm 3 or ml. 3. Calculate the density of each object by dividing the volume into the mass. 4. Record your data on Report Sheet 1. Density Solids and Fluids 9/12/2011 1
2 REPORT SHEET 1 Material Mass (g) Volume (cm 3 ) Density (g/cm 3 ) Procedure: Part B: Liquids 1. Determine the mass of a clean, dry graduated cylinder on the electronic balance. Record its mass below. This will be used in future calculations. 2. Accurately measure 10 ml of water into the graduated cylinder. Record the mass of the cylinder and 10 ml of water in the data table. 3. Add successive amounts of 5 ml each into the graduate to equal 15 ml, 20 ml, 25 ml and 30 ml. Mass the graduate and each amount of water and record it in the data table. 4. Repeat procedures 2 & 3 with the alcohol. Be sure to record all measurements. 5. Repeat procedures 2 & 3 with the glycerol. Record all measurements. Density Solids and Fluids 9/12/2011 2
3 REPORT SHEET 2 Mass of Empty, DRY cylinder = Volume of Liquid (ml) Mass Cylinder + liquid (g) Mass of Liquid (g) Density of Liquid (D= m/v) Average Density (g/ml) 10 ml water 15 ml water 20 ml water 25 ml water 30 ml water 10 ml alcohol 15 ml alcohol 20 ml alcohol 25 ml alcohol 30 ml alcohol 10 ml glycerol 15 ml glycerol 20 ml glycerol 25 ml glycerol 30 ml glycerol Graphing: 1. Subtract the mass of the clean, dry graduated cylinder from each total to determine the mass of the liquid ALONE. 2. Using this value for the mass of the liquid and the volume from the graduated cylinder calculate the density of each liquid and find the average. 3. On the graph paper provided, place VOLUME on the horizontal (X) axis and MASS on the vertical (Y) axis. Label each axis and include units! Density Solids and Fluids 9/12/2011 3
4 Density of Liquids (Mass vs. Volume) Density Solids and Fluids 9/12/2011 4
5 Questions: 1. If one of the original solid materials in this lab were cut in half, the density of each half would be A) less than the original sample B) the same as the original sample C) greater than the original sample 2. In which phase (state) do most Earth materials have their greatest density? A) Gas B) Solid C) Liquid 3. What would be the expected change in density as the temperature of a material increases? Hint: think about a hot air balloon 4. What would be the expected change in density of a material as pressure increases? Hint: think about a propane or SCUBA tank.. 5. Water is an unusual earth material because it is most dense in which phase? 6. What is the density of a rock which has a mass of 35 grams and a volume of 7.0 cubic centimeters? A) 42 g/cm3 B) 0.20 g/cm3 C) 28 g/cm3 D) 5.0 g/cm3 7. Which liquid in this experiment has the least density? What was its average calculated density? Density Solids and Fluids 9/12/2011 5
6 8. If all three (3) liquids were poured into one (1) container, how would the mixture settle? Draw a picture of what it would look like; be sure to label each liquid. 10. The diagram below shows a glass jar containing a clear liquid and a floating rock. Which conclusion about the relative density of the rock and the liquid is true? A) The rock and the liquid have the same density. B) The rock is less dense than the liquid. C) The rock is more dense than the liquid. Density Solids and Fluids 9/12/2011 6
7 11. The diagram below shows equal masses of four different earth materials at different temperatures. Which material has the greatest density? A) granite B) iron C) dry air D) water 12. A student calculates the densities of five different pieces of aluminum, each having a different volume. Which graph best represents this relationship? Density Solids and Fluids 9/12/2011 7
Topic 1 Review Questions Set 1
 Topic 1 Review Questions Set 1 Base your answers to questions 1-5 on your knowledge of Earth Science, the Earth Science Reference Tables, and the diagrams below. The diagrams represent three samples of
Topic 1 Review Questions Set 1 Base your answers to questions 1-5 on your knowledge of Earth Science, the Earth Science Reference Tables, and the diagrams below. The diagrams represent three samples of
Copyright 2014 Science Doodles
 Copyright 2014 Science Doodles If a scientist comes across an unknown element, could she use density to identify the element? A. Yes, because each element has a specific density. B. No, because density
Copyright 2014 Science Doodles If a scientist comes across an unknown element, could she use density to identify the element? A. Yes, because each element has a specific density. B. No, because density
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter.
 1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
1. The diagram below represents a solid object with a density of 3 grams per cubic centimeter. What is the mass of this object? A) 0.5 g B) 2 g C) 18 g D) 36 g Base your answers to questions 2 through
Determining the Density of Unknown Substances
 Determining the Density of Unknown Substances Introduction: Most of the elements on the periodic table are metals and solids. These elements have observable properties that make it possible to identify
Determining the Density of Unknown Substances Introduction: Most of the elements on the periodic table are metals and solids. These elements have observable properties that make it possible to identify
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter. Density
v = 5.00cm x 3.00cm x 2.00cm 2. What is the volume of a marble that has a mass of 3.0 g and a density of 2.7 g/ml? Formula (density) Substitution
 Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
Name: STUDY GUIDE: MEASUREMENT, DENSITY, MASS, VOLUME, AND DIMENSIONAL ANALYSIS (2016) 1. A solid block is 5.00 cm high, 3.00 cm wide, and 2.00 cm long. It has a mass of 129 g. What is the density? Formula
T. Trimpe Lesson 1: Length
 T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
SIGNIFICANT FIGURES WORKSHEET
 SIGNIFICANT FIGURES WORKSHEET PART 1 - Determine the number of significant figures in the following numbers. 1.) 0.02 2.) 0.020 3.) 501 4.) 501.0 5.) 5,000 6.) 5,000. 7.) 6,051.00 8.) 0.0005 9.) 0.1020
SIGNIFICANT FIGURES WORKSHEET PART 1 - Determine the number of significant figures in the following numbers. 1.) 0.02 2.) 0.020 3.) 501 4.) 501.0 5.) 5,000 6.) 5,000. 7.) 6,051.00 8.) 0.0005 9.) 0.1020
Experiment #3. Density
 Experiment #3. Density Goals 1. To measure and calculate the density of various substances. 2. To use significant figures correctly in calculations. Background Density is the mass per unit volume of a
Experiment #3. Density Goals 1. To measure and calculate the density of various substances. 2. To use significant figures correctly in calculations. Background Density is the mass per unit volume of a
Density. How tightly the atoms are packed together in an object
 Density How tightly the atoms are packed together in an object Mass vs Weight Mass is the amount of matter in an object. (it doesn t change) Weight is determined based on the amount of gravity pulling
Density How tightly the atoms are packed together in an object Mass vs Weight Mass is the amount of matter in an object. (it doesn t change) Weight is determined based on the amount of gravity pulling
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet
 DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter.
DETERMINING THE DENSITY OF LIQUIDS & SOLIDS - worksheet 17 Density, like color, odor, melting point, and boiling point, is a physical property of matter. Therefore, density may be used in identifying matter.
Density Measurements Background
 Density Measurements Background The density (ρ) of a substance or an object is defined by its mass (m) divided by its volume (V):!"#$%&' =!"##!"#$%&!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! =!! The units used
Density Measurements Background The density (ρ) of a substance or an object is defined by its mass (m) divided by its volume (V):!"#$%&' =!"##!"#$%&!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! =!! The units used
Measurement and Density - Experiment 1
 Measurement and Density - Experiment 1 Purpose: The purpose of this experiment is to acquaint you with some common metric units of length, volume, and mass. You will also measure the density of an unknown
Measurement and Density - Experiment 1 Purpose: The purpose of this experiment is to acquaint you with some common metric units of length, volume, and mass. You will also measure the density of an unknown
Experiment #3. Density and Specific Gravity.
 Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
Experiment #3. Density and Specific Gravity. Goals 1. To measure and calculate the density and specific gravity of various substances. 2. To use significant figures correctly in calculations. Background
T. Trimpe Lesson 1: Length
 T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
T. Trimpe 2008 http://sciencespot.net/ Lesson 1: Length Metric Units The basic unit of length in the metric system is the meter and is represented by a lowercase m. Metric Units 1 Kilometer (km) = 1000
Experiment: Measurements
 Experiment: Measurements I. INTRODUCTION Measurements are essential to experimental sciences such as chemistry, physics, biology, and geology. The measurements are usually made using the metric system
Experiment: Measurements I. INTRODUCTION Measurements are essential to experimental sciences such as chemistry, physics, biology, and geology. The measurements are usually made using the metric system
STUDENT NAME DATE ID PERIOD GRADE 5 SCIENCE
 STUDENT NAME DATE ID PERIOD GRADE 5 SCIENCE Administered October 2006 DIRECTIONS Read each question and choose the best answer. Be sure to mark all of your answers. SAMPLE A Objects That Conduct Heat
STUDENT NAME DATE ID PERIOD GRADE 5 SCIENCE Administered October 2006 DIRECTIONS Read each question and choose the best answer. Be sure to mark all of your answers. SAMPLE A Objects That Conduct Heat
Lab 0.4: Density and Thickness of Aluminum Foil
 Name Block Lab 0.4: Density and Thickness of Aluminum Foil Student will be able to: Correctly use measuring instruments with accuracy Correctly apply the principles of significant figures in measurements
Name Block Lab 0.4: Density and Thickness of Aluminum Foil Student will be able to: Correctly use measuring instruments with accuracy Correctly apply the principles of significant figures in measurements
CHAPTER 2 HW PRACTICE SOLUTIONS
 CHAPTER 2 HW PRACTICE SOLUTIONS SIGNIFICANT FIGURES 1.) How many significant figures are shown in each of the following numbers? a. 0.0320 3 SF c. 503.10 5 SF e. 0.00702 3 SF b. 8000 1 SF d. 91,000,000
CHAPTER 2 HW PRACTICE SOLUTIONS SIGNIFICANT FIGURES 1.) How many significant figures are shown in each of the following numbers? a. 0.0320 3 SF c. 503.10 5 SF e. 0.00702 3 SF b. 8000 1 SF d. 91,000,000
Density. The purpose of this experiment is to investigate the topic of density by determining the densities of some materials.
 Density Goals The purpose of this experiment is to investigate the topic of density by determining the densities of some materials. Introduction There is a lesson in the old bromide: Which is heavier,
Density Goals The purpose of this experiment is to investigate the topic of density by determining the densities of some materials. Introduction There is a lesson in the old bromide: Which is heavier,
Item 36 has not been slated for public release in 2011.
 S Use the power-plant diagrams and information below to answer questions 35 37. Power Plants 35. Which describes a chemical change and a physical change that take place during the production of electricity
S Use the power-plant diagrams and information below to answer questions 35 37. Power Plants 35. Which describes a chemical change and a physical change that take place during the production of electricity
The table below shows some of the properties of the elements cobalt and nickel.
 BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
BELL RINGER The table below shows some of the properties of the elements cobalt and nickel. A scientist has a sample of metal that could be either cobalt or nickel. Which of the following properties could
Density What is density? How do you measure density?
 Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
Density What is density? How do you measure density? What is density? Density describes how much mass is in a given volume of a material. Mass: the amount of matter in an object. Volume: the amount of
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley)
 Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Thermal Conduction and Surface Area
 Chapter 16 Thermal Energy and Heat Investigation 16A Thermal Conduction and Surface Area Background Information The quantity of energy transferred by heat from a body depends on a number of physical properties
Chapter 16 Thermal Energy and Heat Investigation 16A Thermal Conduction and Surface Area Background Information The quantity of energy transferred by heat from a body depends on a number of physical properties
Oceanography Page 1 of 6 Lab: Ocean Salinity and Density M.Sewell rm #70
 Oceanography Page 1 of 6 Salty Water! Description: This lab is designed to demonstrate the formation of the world s oceans and why the oceans are salty, as well as the changes that take place in density
Oceanography Page 1 of 6 Salty Water! Description: This lab is designed to demonstrate the formation of the world s oceans and why the oceans are salty, as well as the changes that take place in density
Name Honors Chemistry / /
 Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Name Honors Chemistry / / SOL Questions Chapter 1 Each of the following questions below appeared on an SOL Chemistry Exam. For each of the following bubble in the correct answer on your scantron. 1. The
Bulk Density Protocol
 Bulk Density Protocol Purpose To measure the bulk density of each horizon in a soil profile Overview In the field, students collect three soil samples from each horizon in a soil profile using a container
Bulk Density Protocol Purpose To measure the bulk density of each horizon in a soil profile Overview In the field, students collect three soil samples from each horizon in a soil profile using a container
Science 8. Unit 1. Booklet
 Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
Science 8 Unit 1 Mixture and Flow of Matter Booklet Name: Class: 1 TOPIC 1 REINFORCEMENT The Particle Model Goal Demonstrate your understanding of the particle model and changes of state. BLM 1-1 Answer
ES 106 Laboratory # 1 PROPERTIES OF WATER
 ES 106 Laboratory # 1 PROPERTIES OF WATER 1-1 Introduction What are the physical and chemical properties of water that make it so unique and necessary for living things? When you look at water, taste and
ES 106 Laboratory # 1 PROPERTIES OF WATER 1-1 Introduction What are the physical and chemical properties of water that make it so unique and necessary for living things? When you look at water, taste and
CHM101 Lab - Energy Grading Rubric
 Name Team Name CHM101 Lab - Energy Grading Rubric To participate in this lab you must have splash- proof goggles, proper shoes and attire. Criteria Points possible Points earned Lab Performance Printed
Name Team Name CHM101 Lab - Energy Grading Rubric To participate in this lab you must have splash- proof goggles, proper shoes and attire. Criteria Points possible Points earned Lab Performance Printed
DENSITY OF MATTER. To experimentally determine the densities of given solids and liquids.
 DENSITY OF MATTER PURPOSE: To experimentally determine the densities of iven solids and liquids. EQUIPMENT: Triple-beam balance, vernier caliper, raduated, lass block, metal, metal sphere, wooden sphere,
DENSITY OF MATTER PURPOSE: To experimentally determine the densities of iven solids and liquids. EQUIPMENT: Triple-beam balance, vernier caliper, raduated, lass block, metal, metal sphere, wooden sphere,
THE MOLECULAR WEIGHT OF A SOLUTE*
 Name Per THE MOLECULAR WEIGHT OF A SOLUTE* One of the most helpful things that can be known about an element or compound is its molecular weight (molecular mass, gram molecular mass, atomic mass, or gram
Name Per THE MOLECULAR WEIGHT OF A SOLUTE* One of the most helpful things that can be known about an element or compound is its molecular weight (molecular mass, gram molecular mass, atomic mass, or gram
D =? D = M = 10 g = 5 g/cm 3 M = 10 g V 2 cm 3 V = 2 cm 3 5 g/cm 3
 Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Using the formula for density, answer the following calculation problems. M D = (g/ml or g/cm 3 ) Density = Mass D = M M = (g) Volume V D V V = (ml or cm 3 ) For all of your problems, please complete them
Density Answers to the End of module questions
 IDS 101 Density Answers to the End of module questions 1. You are experimenting with the mysterious substance X. You carefully measure the mass of a uniform 2 ml sample of pure substance X. You slice it
IDS 101 Density Answers to the End of module questions 1. You are experimenting with the mysterious substance X. You carefully measure the mass of a uniform 2 ml sample of pure substance X. You slice it
Name Lab Section Date. Sediment Lab
 Name Lab Section Date. Investigating Stokes Law Sediment Lab ds = density of solid, g/cm dw = density of water, g/cm g = gravity, 980 cm/second 2 D = particle diameter in centimeters μ = molecular viscosity,
Name Lab Section Date. Investigating Stokes Law Sediment Lab ds = density of solid, g/cm dw = density of water, g/cm g = gravity, 980 cm/second 2 D = particle diameter in centimeters μ = molecular viscosity,
2.3 Density and Density Calculations
 2.3 Density and Density Calculations When people say that lead is heavier than wood, they do not mean that a pea sized piece of lead weighs more than a truckload of pine logs. What they mean is that a
2.3 Density and Density Calculations When people say that lead is heavier than wood, they do not mean that a pea sized piece of lead weighs more than a truckload of pine logs. What they mean is that a
M. It is expressed in units such a g/cc,
 Experiment 2 Density Part I: Density of a solid Density is defined as mass per unit volume, D = V M. It is expressed in units such a g/cc, g/ml, lb/ft 3, etc. Determination of mass is done by weighing
Experiment 2 Density Part I: Density of a solid Density is defined as mass per unit volume, D = V M. It is expressed in units such a g/cc, g/ml, lb/ft 3, etc. Determination of mass is done by weighing
Calculating Ballast By Troy Averill
 Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
Calculating Ballast By Troy Averill Lesson Overview: Students use the dimensions of the cargo hold of a ship s hull to calculate the volume of material that can be contained. Students will then use the
A Hydrogen Powered Bottle Rocket
 A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
Triple Beam Balance: add the three together: 700g + 20g + 2.9g = 722.9g Metric base unit for mass is gram.
 6 th Grade 2 nd Nine Week CSA Study Guide 2015-16 SOL 6.1b: Make precise and consistent measurements and estimations. Graduated Cylinder: read from the lowest part of the water line curve. Reading: 56ml
6 th Grade 2 nd Nine Week CSA Study Guide 2015-16 SOL 6.1b: Make precise and consistent measurements and estimations. Graduated Cylinder: read from the lowest part of the water line curve. Reading: 56ml
Bulk Density Protocol
 Bulk Density Protocol Purpose To measure the soil bulk density of each horizon in your soil profile. Overview Students obtain a soil sample in the field using a container with a measured volume. The soil
Bulk Density Protocol Purpose To measure the soil bulk density of each horizon in your soil profile. Overview Students obtain a soil sample in the field using a container with a measured volume. The soil
PHYSICAL SCIENCE 0652/06
 www.xtremepapers.com Centre Number Candidate Number Name CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education PHYSICAL SCIENCE 0652/06 Paper 6 Alternative to Practical
www.xtremepapers.com Centre Number Candidate Number Name CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education PHYSICAL SCIENCE 0652/06 Paper 6 Alternative to Practical
COMPOSITION OF SEAWATER
 COMPOSITION OF SEAWATER Ocean water is a combination of freshwater and a variety of dissolved substances. Salinity is a measure of the amount of dissolved salts in seawater, measured in parts per thousand
COMPOSITION OF SEAWATER Ocean water is a combination of freshwater and a variety of dissolved substances. Salinity is a measure of the amount of dissolved salts in seawater, measured in parts per thousand
Directions: Complete the following prior to class. Be prepared to show your work on the board.
 Worksheet L4X Student s Name Directions: Complete the following prior to class. Be prepared to show your work on the board. 1) Convert 30 ml to oz, qt, L, gal. 2) Convert 16 oz to kg, lb, tons, g. Page
Worksheet L4X Student s Name Directions: Complete the following prior to class. Be prepared to show your work on the board. 1) Convert 30 ml to oz, qt, L, gal. 2) Convert 16 oz to kg, lb, tons, g. Page
Effective January 2008 All indicators in Standard / 11
 Scientific Inquiry 6-1 The student will demonstrate an understanding of technological design and scientific inquiry, including the process skills, mathematical thinking, controlled investigative design
Scientific Inquiry 6-1 The student will demonstrate an understanding of technological design and scientific inquiry, including the process skills, mathematical thinking, controlled investigative design
TEST I REVIEW. 1. All of the following are properties of antimony. Which one is not a
 TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
TEST I REVIEW I Identify the letter of the choice that best completes the statement or answers the question. 1. All of the following are properties of antimony. Which one is not a physical property? a.
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example,
 SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility,
7. The diagram below represents cross sections of equal-size beakers A, B, and C filled with beads.
 Base your answers to questions 1 and 2 on the diagram below and on your knowledge of Earth science. The diagram represents four tubes, labeled A, B, C, and D, each containing 150 ml of sediments. Tubes
Base your answers to questions 1 and 2 on the diagram below and on your knowledge of Earth science. The diagram represents four tubes, labeled A, B, C, and D, each containing 150 ml of sediments. Tubes
Density (d) is a property of a substance equal to the ratio of its mass (m) to its volume (V): d = m V
 Experiment 2 Name: DEN 14 Si TY In this experiment, you will learn about density and practice the proper way to report measurements and calculated quantities. Properties Properties of a substance can be
Experiment 2 Name: DEN 14 Si TY In this experiment, you will learn about density and practice the proper way to report measurements and calculated quantities. Properties Properties of a substance can be
A Hydrogen Powered Bottle Rocket
 A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
A Hydrogen Powered Bottle Rocket Rockets are made by filling plastic water bottles with a mixture of hydrogen gas and oxygen gas. The rocket is launched by igniting the mixture with a flame. The bottle
GEL Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Grade: /25
 GEL 4250 - Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Name: Section: Grade: /25 COMPLETE & TURN IN ONLY PAGES THAT HAVE A FIELD FOR YOUR NAME. ALL OTHER PAGES
GEL 4250 - Hydrogeology (Groundwater) LAB 2: POROSITY & HYDRAULIC CONDUCTIVITY - Porosity Segment - Name: Section: Grade: /25 COMPLETE & TURN IN ONLY PAGES THAT HAVE A FIELD FOR YOUR NAME. ALL OTHER PAGES
NOTE: If substances have the SAME VOLUME, they can have DIFFERENT MASSES.
 Grade 8 SCIENCE Chapter 8 questions Page 306 1. When particles are CLOSER TOGETHER, DENSITY is GREATER. 2. Particles in SOLIDS have FEWER SPACES between them. The ATTRACTIVE FORCES between the particles
Grade 8 SCIENCE Chapter 8 questions Page 306 1. When particles are CLOSER TOGETHER, DENSITY is GREATER. 2. Particles in SOLIDS have FEWER SPACES between them. The ATTRACTIVE FORCES between the particles
LESSON hat is density? 24
 LESSON hat is density? 24 Rocks are heavy. A small box of rocks has a large. Yet a box of the same size containing corks has a small. How can objects that are the same size have different es? Scientists
LESSON hat is density? 24 Rocks are heavy. A small box of rocks has a large. Yet a box of the same size containing corks has a small. How can objects that are the same size have different es? Scientists
LAB 9A: TEMPERATURE AND HEAT, PART A
 Name: Period: LAB 9A: TEMPERATURE AND HEAT, PART A Hot and cold are familiar sensations. What happens when something hot comes in contact with something cold? Think about putting some ice cubes in a drink.
Name: Period: LAB 9A: TEMPERATURE AND HEAT, PART A Hot and cold are familiar sensations. What happens when something hot comes in contact with something cold? Think about putting some ice cubes in a drink.
STAAR Vocabulary noticing something about the world around you. using clues to find the answer. everything everywhere. to sort into groups
 Observation noticing something about the world around you Inference using clues to find the answer Matter everything everywhere Classify to sort into groups Physical Property something you observe with
Observation noticing something about the world around you Inference using clues to find the answer Matter everything everywhere Classify to sort into groups Physical Property something you observe with
Investigating the Sun s Influence on the Water Cycle
 Chapter Water on Earth Chapter Science Investigation Investigating the Sun s Influence on the Water Cycle Find Out Do this activity to find out how the sun s angle can affect the evaporation of water.
Chapter Water on Earth Chapter Science Investigation Investigating the Sun s Influence on the Water Cycle Find Out Do this activity to find out how the sun s angle can affect the evaporation of water.
UCS Mi-STAR Science 7 Semester 1 Midterm Exam Review Guide
 Name Date Hour 2018-2019 UCS Mi-STAR Science 7 Semester 1 Midterm Exam Review Guide Question How does cold air compare to warm air? Cold air weighs more than hot air. When a 9-centimeter balloon is filled
Name Date Hour 2018-2019 UCS Mi-STAR Science 7 Semester 1 Midterm Exam Review Guide Question How does cold air compare to warm air? Cold air weighs more than hot air. When a 9-centimeter balloon is filled
EOC 7th Grade District Common Assessment
 EOC 7th Grade District Common Assessment 1. Todd needs to measure 50 grams of table salt for an experiment. Which measuring device will most accurately measure 50 grams? 2. What is the length (in millimeters)
EOC 7th Grade District Common Assessment 1. Todd needs to measure 50 grams of table salt for an experiment. Which measuring device will most accurately measure 50 grams? 2. What is the length (in millimeters)
Specific Heat Activity
 Title of Lesson Subject Area Age or Grade Level Specific Heat Activity Understanding energy transfers within the lense of specific heat 7 th grade This lab is designed to be an easy way to teach students
Title of Lesson Subject Area Age or Grade Level Specific Heat Activity Understanding energy transfers within the lense of specific heat 7 th grade This lab is designed to be an easy way to teach students
Introduction to Density
 Introduction to Density INTRODUCTION Which is heavier, a pound of aluminum or a pound of lead? The answer, of course, is neither, but many people confuse the words "heavy" and "dense". "Heavy" refers to
Introduction to Density INTRODUCTION Which is heavier, a pound of aluminum or a pound of lead? The answer, of course, is neither, but many people confuse the words "heavy" and "dense". "Heavy" refers to
Laboratory Demonstration No. 1
 Laboratory Demonstration No. 1 Purpose: 1. to investigate the role of volume and flow rate in the cleansing of a contaminated water body 2. to determine the residence time 3. to apply the residence time
Laboratory Demonstration No. 1 Purpose: 1. to investigate the role of volume and flow rate in the cleansing of a contaminated water body 2. to determine the residence time 3. to apply the residence time
ALTERNATIVE TRANSPORTATION FUELS LAB
 ALTERNATIVE TRANSPORTATION FUELS LAB Purpose: To examine the energy content of various alternative fuels which could be used for powering our vehicles in the future. As gasoline becomes increasingly expensive,
ALTERNATIVE TRANSPORTATION FUELS LAB Purpose: To examine the energy content of various alternative fuels which could be used for powering our vehicles in the future. As gasoline becomes increasingly expensive,
EXPERIMENT 3: Identification of a Substance by Physical Properties
 EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
The Varying Density of Sea Water Based on
 Based on http://www.maine.gov/doc/nrimc/mgs/education/lessons/act37.htm Focus on Inquiry The student will collect and analyze data to determine the effects of temperature and dissolved on sea water. Lesson
Based on http://www.maine.gov/doc/nrimc/mgs/education/lessons/act37.htm Focus on Inquiry The student will collect and analyze data to determine the effects of temperature and dissolved on sea water. Lesson
OBJECTIVES INTRODUCTION
 Initial and Final Heights of Thin Films Developed by: Mike Evangelista, Nathan Haden, Alex Jannini, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater and Mariano Savelski,
Initial and Final Heights of Thin Films Developed by: Mike Evangelista, Nathan Haden, Alex Jannini, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater and Mariano Savelski,
An Insulated Cola Bottle
 An Insulated Cola Bottle Computer 12 Insulation slows the flow of heat. Glass, plastics such as Styrofoam, wool, fiber glass, aluminum foil, air, and a vacuum are some of the many materials used for heat
An Insulated Cola Bottle Computer 12 Insulation slows the flow of heat. Glass, plastics such as Styrofoam, wool, fiber glass, aluminum foil, air, and a vacuum are some of the many materials used for heat
Two variables in this investigation are location of trap and size of jar.
 An eighth-grade class wants to identify a representative sample of the crawling and flying insects living in the schoolyard. The students build and set five traps like the one shown below. They place the
An eighth-grade class wants to identify a representative sample of the crawling and flying insects living in the schoolyard. The students build and set five traps like the one shown below. They place the
Evaluation copy. Energy Content of Fuels. computer OBJECTIVES MATERIALS
 Energy Content of Fuels Computer 9 Energy content is an important property of fuels. This property helps scientists and engineers determine the usefulness of a fuel. Energy content is the amount of heat
Energy Content of Fuels Computer 9 Energy content is an important property of fuels. This property helps scientists and engineers determine the usefulness of a fuel. Energy content is the amount of heat
LAB National Science Teachers Association. Lab Handout. Introduction
 Lab Handout Lab 16. Surface Materials and Temperature Change: How Does the Nature of the Surface Material Covering a Specific Location Affect Heating and Cooling Rates at That Location? Introduction Inner
Lab Handout Lab 16. Surface Materials and Temperature Change: How Does the Nature of the Surface Material Covering a Specific Location Affect Heating and Cooling Rates at That Location? Introduction Inner
CHAPTER 1. Conversions. General Plan for Converting Measurements. State the relationship between the unit given and the unit sought as an equality.
 CHAPTER 1 Conversions One of the aims of chemistry is to describe changes to tell what changed, how it changed, and what it changed into. Another aim of chemistry is to look at matter and its changes and
CHAPTER 1 Conversions One of the aims of chemistry is to describe changes to tell what changed, how it changed, and what it changed into. Another aim of chemistry is to look at matter and its changes and
PHARMACOPOEIAL DISCUSSION GROUP BULK AND TAPPED DENSITY OF SOLIDS
 PHARMACOPOEIAL DISCUSSION GROUP BULK AND TAPPED DENSITY OF SOLIDS It is understood that sign-off covers the technical content of the draft and each party will adapt it as necessary to conform to the usual
PHARMACOPOEIAL DISCUSSION GROUP BULK AND TAPPED DENSITY OF SOLIDS It is understood that sign-off covers the technical content of the draft and each party will adapt it as necessary to conform to the usual
Lab Section. Experiment 2 Density. Introduction
 Experiment 2 Density Introduction Density (given the symbol d or ρ depending upon which book you read) is an intrinsic property of materials. The term intrinsic means that it is independent upon the amount
Experiment 2 Density Introduction Density (given the symbol d or ρ depending upon which book you read) is an intrinsic property of materials. The term intrinsic means that it is independent upon the amount
London Examinations IGCSE
 Centre No. Paper Reference Surname Initial(s) Candidate No. Signature Paper Reference(s) 4335/03 4437/08 London Examinations IGCSE Chemistry 4335 Paper 3 Science (Double Award) 4437 Paper 8 Foundation
Centre No. Paper Reference Surname Initial(s) Candidate No. Signature Paper Reference(s) 4335/03 4437/08 London Examinations IGCSE Chemistry 4335 Paper 3 Science (Double Award) 4437 Paper 8 Foundation
Common Core State Standards. Next Generation Science Standards
 EVAPORATION INVESTIGATION Description Students conduct an experiment to investigate the effects of various factors on the rate of evaporation. Grade Level 6 12 Objectives Students will: Make a prediction
EVAPORATION INVESTIGATION Description Students conduct an experiment to investigate the effects of various factors on the rate of evaporation. Grade Level 6 12 Objectives Students will: Make a prediction
Modeling Your Water Balance
 Modeling Your Water Balance Purpose To model a soil s water storage over a year Overview Students create a physical model illustrating the soil water balance using glasses to represent the soil column.
Modeling Your Water Balance Purpose To model a soil s water storage over a year Overview Students create a physical model illustrating the soil water balance using glasses to represent the soil column.
Overview. Learning Objectives: This module provides step-by-step instructions in how to do the Bulk Density Protocol.
 Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
Overview This module provides step-by-step instructions in how to do the Bulk Density Protocol. Learning Objectives: After completing this module, you will be able to: Explain why bulk density is worth
BACKGROUND: Porosity permeability. PART I Materials Procedure EMPTY the tube.
 Name: Water Movement (adapted from Wards) LAB BACKGROUND: While some precipitation falls to the earth and runs off into streams and river, another portion seeps slowly through the soil into the upper layers
Name: Water Movement (adapted from Wards) LAB BACKGROUND: While some precipitation falls to the earth and runs off into streams and river, another portion seeps slowly through the soil into the upper layers
Comprehending Currents II Density-Driven Ocean Currents
 II Density-Driven Ocean Currents Adapted from NASA s visit to an Ocean Planet Curriculum http://topex-www.jpl.nasa.gov/education/activities.html Currents in the ocean are important because they transport
II Density-Driven Ocean Currents Adapted from NASA s visit to an Ocean Planet Curriculum http://topex-www.jpl.nasa.gov/education/activities.html Currents in the ocean are important because they transport
Copper Odyssey. Chemical Reactions of Copper
 Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Unit-2. Properties of Matter. Solutions 2.1 Matter & Density page V 1 =4.3.6=72 cm 3. Volume of the removed triangular prism (V 2 )
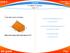 2.1 & Density page - 61 1. A solid object is given in the figure. Volume of the rectangular prism (V 1 ) V 1 =4.3.6=72 cm 3 Volume of the removed triangular prism (V 2 ) V 2 =((2.3)/2).6=18 cm 3 What is
2.1 & Density page - 61 1. A solid object is given in the figure. Volume of the rectangular prism (V 1 ) V 1 =4.3.6=72 cm 3 Volume of the removed triangular prism (V 2 ) V 2 =((2.3)/2).6=18 cm 3 What is
Do not write on the question paper, do not turn in the question paper. Put your answers on a new paper. Unless told otherwise.
 APES Unit 2 Packet Due Sept 10 at the beginning of class Instructions for all homework and labs: Put your name and date on top right corner of all your papers. Put the name of the problem or lab at the
APES Unit 2 Packet Due Sept 10 at the beginning of class Instructions for all homework and labs: Put your name and date on top right corner of all your papers. Put the name of the problem or lab at the
Practice with Properties of Matter. Practice with Physical and Chemical Changes. Practice with Mixtures and Pure Substances
 Chapter 1 Practice Problems Practice with Properties of Matter Indicate whether each of the following statements describes a physical or a chemical property. a) the normal color of elemental bromine is
Chapter 1 Practice Problems Practice with Properties of Matter Indicate whether each of the following statements describes a physical or a chemical property. a) the normal color of elemental bromine is
European Pharmacopoeia
 i PHARMACOPOEIAL DISCUSSION GROUP REV 1 - CORR 1 G02 Bulk density and tapped density of powders It is understood that sign-off covers the technical content of the draft and each party will adapt it as
i PHARMACOPOEIAL DISCUSSION GROUP REV 1 - CORR 1 G02 Bulk density and tapped density of powders It is understood that sign-off covers the technical content of the draft and each party will adapt it as
Building a Thermometer
 Building a Thermometer Purpose To build an instrument that can be used to measure water temperature Overview Students will construct a soda-bottle thermometer, which is similar to the thermometer used
Building a Thermometer Purpose To build an instrument that can be used to measure water temperature Overview Students will construct a soda-bottle thermometer, which is similar to the thermometer used
DETERMINATION OF DENSITY
 DETERMINATION OF DENSITY Dr. Barbara B. Bunn. Adapted from Experiments in General Chemistry ; Wiley 1996J.G. Wardeska, T.T-S. Huang, R.W. Kopp; used by permission. Tested by Ms. Janice Orr s Chemistry
DETERMINATION OF DENSITY Dr. Barbara B. Bunn. Adapted from Experiments in General Chemistry ; Wiley 1996J.G. Wardeska, T.T-S. Huang, R.W. Kopp; used by permission. Tested by Ms. Janice Orr s Chemistry
KEY CONCEPTS AND PROCESS SKILLS
 Soil Columns 40- to 2-3 50-minute sessions ACTIVITY OVERVIEW 4 L A B O R AT O R Y Students investigate the composition of two different types of soil by separating the soils with water. By creating and
Soil Columns 40- to 2-3 50-minute sessions ACTIVITY OVERVIEW 4 L A B O R AT O R Y Students investigate the composition of two different types of soil by separating the soils with water. By creating and
Objective: Classify metals, nonmetals, and metalloids based on their properties.
 Do Now Date: September 2, 2014 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Do Now Date: September 2, 2014 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Evaluation copy. Energy Content of Fuels. computer OBJECTIVES MATERIALS
 Energy Content of Fuels Computer 24 is an important property of fuels. This property helps scientists and engineers determine the usefulness of a fuel. is the amount of heat produced by the burning of
Energy Content of Fuels Computer 24 is an important property of fuels. This property helps scientists and engineers determine the usefulness of a fuel. is the amount of heat produced by the burning of
1 Elements. TAKE A LOOK 2. Identify Look at the illustration and identify one source of iron that comes to Earth from somewhere else.
 CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
CHAPTER 4 1 Elements SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: What is an element? How do elements differ from
Evaluation copy. Energy Content of Fuels. computer OBJECTIVES MATERIALS
 Energy Content of Fuels Computer 24 is an important property of fuels. This property helps scientists and engineers determine the usefulness of a fuel. is the amount of heat produced by the burning of
Energy Content of Fuels Computer 24 is an important property of fuels. This property helps scientists and engineers determine the usefulness of a fuel. is the amount of heat produced by the burning of
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5349025480* CO-ORDINATED SCIENCES 0654/63 Paper 6 Alternative to Practical October/November 2015
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5349025480* CO-ORDINATED SCIENCES 0654/63 Paper 6 Alternative to Practical October/November 2015
Salinity in Seawater
 Salinity in Seawater Objective To familiarize students with the different methods used for measuring salinity of water. Introduction: Salinity exerts profound impacts on the marine environment. It controls
Salinity in Seawater Objective To familiarize students with the different methods used for measuring salinity of water. Introduction: Salinity exerts profound impacts on the marine environment. It controls
Objective: Classify metals, nonmetals, and metalloids based on their properties.
 Do Now Date: September 8, 2015 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Do Now Date: September 8, 2015 Objective: Classify metals, nonmetals, and metalloids based on their properties. Draw a table listing the properties of metals, nonmetals, and metalloids. Tuesday September
Density Determi nations
 f EXPERIMENT 3 Density Determi nations OBJECTIVE Density is an important characteristic property of matter, and may be used as a method of identification. In this experiment, you will determine the densities
f EXPERIMENT 3 Density Determi nations OBJECTIVE Density is an important characteristic property of matter, and may be used as a method of identification. In this experiment, you will determine the densities
Bio EOC Topics for Living Things, Metric Measurement, Microscope and The Scientific Method
 Bio EOC Topics for Living Things, Metric Measurement, Microscope and The Scientific Method Laboratory Equipment o Identification of laboratory equipment; for example beaker, triple beam balance, microscope,
Bio EOC Topics for Living Things, Metric Measurement, Microscope and The Scientific Method Laboratory Equipment o Identification of laboratory equipment; for example beaker, triple beam balance, microscope,
metric 1.2 (Compare/contrast characteristics in a given set) beaker hotplate stopwatch hand lens rain gauge greatest least fewest closest slowest
 Observe/Measure 1.1 (Observe and measure using SI units) mass length time appropriate type of unit, appropriate type of tool volume temperature International System of Units grams milligrams meters millimeters
Observe/Measure 1.1 (Observe and measure using SI units) mass length time appropriate type of unit, appropriate type of tool volume temperature International System of Units grams milligrams meters millimeters
Global Warming and Sea Level Rise
 MATERIALS SUITABLE FOR GRADES 1-8 This activity will show how increased temperatures will hasten the melting of ice in the environment, contributing to a rise in sea level and subsequent flooding of coastal
MATERIALS SUITABLE FOR GRADES 1-8 This activity will show how increased temperatures will hasten the melting of ice in the environment, contributing to a rise in sea level and subsequent flooding of coastal
Underwater Avalanche: Turbidity Currents Investigating Density Currents
 Underwater Avalanche: Turbidity Currents Investigating Density Currents OBJECTIVE Students will investigate how changes in density affect the rate at which slurry moves down a slope and what effect changes
Underwater Avalanche: Turbidity Currents Investigating Density Currents OBJECTIVE Students will investigate how changes in density affect the rate at which slurry moves down a slope and what effect changes
