Oxidation Reactions. This oxide will from only if thermodynamics favour a reaction of the form: M + O 2 = MO 2. Which must form rapidly (favourable(
|
|
|
- Mercy Montgomery
- 5 years ago
- Views:
Transcription
1 Oxidation of s Oxidation is a general term used to define the reaction between a metal or alloy and its environment. s or alloys are oxidised when heated to elevated temperatures es in air or highly oxidised atmosphere with excess air or oxygen Oxidation can also take place in reducing environments (low oxygen potential) In industrial practice, metals and alloys are rarely exposed to pure oxygen. The atmosphere may contain oxidising species in addition to oxygen. The corresponding atmospheres consist mainly of, N 2, H 2 O, C, S 2, HCl, Cl 2, NH 3, S, etc.. Thus, H 2 O and C may play an important role in affecting the oxidation behaviour of a metal. Understanding oxidation of metals and alloys has been behind the successful development of alloys that resist environmental attack k at high temperatures Oxidation resistance of these alloys is due to the presence of reactive r alloying elements, such as Cr, Al and Si, which can form stable, protective external oxide s on the metal surfaces and so prevent further corrosion Oxidation Reactions During the initial stages of oxidation reaction, a gas- adsorbed film will form and develop by nucleation and growth to form a thicker oxide. (g) o o o o o 1 Adsorption (g) Oxide nucleation & growth Oxide dissolution This oxide will from only if thermodynamics favour a reaction of the form: M + = M Which must form rapidly (favourable( kinetics) - e - 3 Mn+ n+ Scale growth Internal oxidation 2 (g) M n+ Growth of porosity and microcracks 4
2 A metal can also be oxidised by either water vapour (H 2 O) or C. M + H 2 O = MO + H 2 Thermodynamics of oxidation Thermodynamics predict the relative stability of an oxide with respect to its metal, but does not predict how protective an oxide will be and M + C = MO + CO An oxide will form on a metal surface when the oxygen potential in the environment is greater than the oxygen partial pressure in equilibrium with the oxide Kinetics of Oxidation (Oxidation Rates) The basis for developing high temperature oxidation- resistant alloys is the formation of a protective oxide layer between the metal and the environment. Reaction kinetics are controlled by the rates of diffusion of metal ions and / or across the barrier oxide. Since the diffusion processes are temperature-dependent, oxidation rates increase rapidly with increase in temperature according to: D = D 0 exp (-Q/RT)(
3 Three laws describe the oxidation rates for most metals and alloys: 1. Linear growth x = k l t + x 0 2. Parabolic growth x 2 = k p t + x 0 3. Logarithmic growth x = k e log(at + 1) Weight Gain Time Linear Parabolic Logarithmic x: oxide thickness, t: time, k l, k p, k e, and a are constants. x 0 is oxide thickness at t = 0 The linear rate law is applicable to the formation and development of non-protective oxide layers at high temperature The rate constant, k l, changes with temperature according to an Arrhenius type relationship: x = k l t + x 0 The parabolic rate law assumes that the diffusion of metal cations or oxygen anions is the rate controlling step and is derived from FickF ick s first law of diffusion. This law is applicable to uniform, continuous and protective oxide layers The rate constant, k p, changes with temperature according to an Arrhenius type relationship: x 2 = k p t + x 0 The logarithmic rate law is mainly applicable to thin oxide s (less than 100 nm) ) formed at low temperatures, and therefore rarely applicable to high temperature engineering problems. x = k e log (at + 1) Ideally, the oxidation rate should be relatively slow (parabolic growth law). Such a requirement depends on: 1. Rate of diffusion of reactants (metal cations and through the oxide layer 2. Rate of supply of to the outer surface of the oxide 3. Molar volume ratio of oxide to metal anions) The slowest process at a given temperature will control the rate of oxidation.
4 Types of Oxides Two types of metal oxides 1. Non-protective (unstable) oxides Formation of a porous and cracked oxide film on the metal surface. Because of porosity, penetrates to the metal surface and reacts with the metal to form more oxide Ultimately, the entire metal will be consumed 2. Protective (stable) oxides Formation of a non-porous film on the metal surface can only react with the metal ions through diffusion Growth rate decreases as the oxide thickness increases. This is parabolic growth rate law and is associated with thick oxides An indication whether an oxide film is protective or not is given n by the Pilling Bedworth ratio. This is linear growth rate law and occurs at high temperatures Pilling Bedworth ratio (PB) volume of oxide produced PB = = volume of metal consumed Wd ndw Where: W: : is the molecular weight of oxide, D: : is the density of oxide w: : is the atomic weight of metal, d: : is the density of metal n: : is the number of metal atoms in the oxide (e.g; in Al 2 O 3, n = 2) 1. PB < 1 porous and non-protective oxide (the oxide film is insufficient to fully cover the metal surface 2. PB > 1 protective oxide Oxidation rates at later stages depends on whether the thick film remains continuous and protective as it grows or whether it contains pores or cracks (non- protective) As the oxide film grows and becomes thicker (PB > 2), however, compressive stresses develop in the oxide leading to cracking and then spalling (flake off). This is called breakaway corrosion.
5 List of protective and non-protective protective oxide s Linear Protective oxides Non protective oxides Be 1.59 K 0.45 Cu 1.68 Ag 1.59 Al 1.28 Cd 1.21 Weight Gain Parabolic Breakaway corrosion Cr 1.99 Ti 1.95 Mn 1.79 Mo 3.40 Fe 1.77 Hf 2.61 Co 1.99 Sb 2.35 Ni 1.52 W 3.40 Time Pd 1.60 Ta 2.33 Pb 1.40 U 3.05 Ce 1.16 V 3.18 Some possible forms of oxide films Some possible forms of oxide films Scale Scale Compact and uniform Porous Cracked Compact and uniform Porous Cracked Compound formation in Non-adherent and cracked Compound formation in Non-adherent and cracked
6 Oxidation in Alloys Follow the same principles as for oxidation of pure metals. However, oxidation of alloys is more complex because: An example of the effect of Cr on oxidation is illustrated by one of the common high temperature alloys (Fe Cr). 1. Alloying elements have different affinities for 2. Alloying elements have different diffusion rates in oxides and in i alloy 3. Higher and complex oxides may form 4. Possibility of solid solubility between the oxides Source: D. A. Jones (Principles and Prevention of Corrosion)
What happens if we connect Zn and Pt in HCl solution? Corrosion of platinum (Pt) in HCl. 1. If Zn and Pt are not connected
 Corrosion of platinum (Pt) in HCl Now if we place a piece of Pt in HCl, what will happen? Pt does not corrode does not take part in the electrochemical reaction Pt is a noble metal Pt acts as a reference
Corrosion of platinum (Pt) in HCl Now if we place a piece of Pt in HCl, what will happen? Pt does not corrode does not take part in the electrochemical reaction Pt is a noble metal Pt acts as a reference
Mat E 272 Lecture 26: Oxidation and Corrosion
 Mat E 272 Lecture 26: Oxidation and Corrosion December 11, 2001 Introduction: Environmental degradation of materials is one of the most costly failure modes, accounting for over 5 percent of the total
Mat E 272 Lecture 26: Oxidation and Corrosion December 11, 2001 Introduction: Environmental degradation of materials is one of the most costly failure modes, accounting for over 5 percent of the total
Oxidation of Chromium
 Oxidation of Chromium Oxidation of chromium is very simple as it usually forms a single oxide Cr 2 O 3, It is a p-type of oxide with Cr 3+ ions diffusing outward. Since the defect concentration is so low
Oxidation of Chromium Oxidation of chromium is very simple as it usually forms a single oxide Cr 2 O 3, It is a p-type of oxide with Cr 3+ ions diffusing outward. Since the defect concentration is so low
CHAPTER 23: CORROSION
 CHAPTER 23: CORROSION AND DEGRADATION CORROSION Corrosion: The corrosion is the deterioration of the metals (oxidation) in different environments in presence of oxygen. Anodic reaction: M M +n + ne - Cathodic
CHAPTER 23: CORROSION AND DEGRADATION CORROSION Corrosion: The corrosion is the deterioration of the metals (oxidation) in different environments in presence of oxygen. Anodic reaction: M M +n + ne - Cathodic
CORROSION of Metals CORROSION CORROSION. Outline ISSUES TO ADDRESS... Why does corrosion occur? What metals are most likely to corrode?
 Outline Corrosion - Introduction Corrosion of Metals - e.g. Rusting of iron in water Electrochemical Cell Electrode Potential in Electrochemical Cell Standard Electromotive Force Example Relative Corrosion
Outline Corrosion - Introduction Corrosion of Metals - e.g. Rusting of iron in water Electrochemical Cell Electrode Potential in Electrochemical Cell Standard Electromotive Force Example Relative Corrosion
Effect Of Temperature On Oxidation Kinetics Of Mild Steel
 Effect Of Temperature On Oxidation Kinetics Of Mild Steel Dr. Obotowo W. Obot Mr. Chinda Believe Chibuike Abstract: This research Effect of temperature on oxidation kinetics of mild steel was aimed at
Effect Of Temperature On Oxidation Kinetics Of Mild Steel Dr. Obotowo W. Obot Mr. Chinda Believe Chibuike Abstract: This research Effect of temperature on oxidation kinetics of mild steel was aimed at
Surface films formed during H 2 S corrosion of pipeline steels and the effect on hydrogen permeation
 Surface films formed during H 2 S corrosion of pipeline steels and the effect on hydrogen permeation Frank Cheng Department of Mechanical Engineering University of Calgary June 1, 2017 Pipelines in sour
Surface films formed during H 2 S corrosion of pipeline steels and the effect on hydrogen permeation Frank Cheng Department of Mechanical Engineering University of Calgary June 1, 2017 Pipelines in sour
Therma 4828 EN
 Therma 4828 EN 1.4828 General characteristics Austenitic grade with improved oxidation resistance. Commonly used for furnace equipment (esp. supporting parts), annealing and hardening boxes, air heaters.
Therma 4828 EN 1.4828 General characteristics Austenitic grade with improved oxidation resistance. Commonly used for furnace equipment (esp. supporting parts), annealing and hardening boxes, air heaters.
High Temperature Oxidation Behavior of Flake and Spheroidal Graphite Cast Irons
 Oxid Met (2011) 76:161 168 DOI 10.1007/s11085-011-9244-8 ORIGINAL PAPER High Temperature Oxidation Behavior of Flake and Spheroidal Graphite Cast Irons Meng-Bin Lin Chaur-Jeng Wang Alex A. Volinsky Received:
Oxid Met (2011) 76:161 168 DOI 10.1007/s11085-011-9244-8 ORIGINAL PAPER High Temperature Oxidation Behavior of Flake and Spheroidal Graphite Cast Irons Meng-Bin Lin Chaur-Jeng Wang Alex A. Volinsky Received:
Therma 314/4841 EN , ASTM TYPE 314
 Therma 314/4841 EN 1.4841, ASTM TYPE 314 General characteristics Austenitic heat resisting grade with excellent oxidation resistance. Commonly used for furnace equipment, super heater suspensions, enameling
Therma 314/4841 EN 1.4841, ASTM TYPE 314 General characteristics Austenitic heat resisting grade with excellent oxidation resistance. Commonly used for furnace equipment, super heater suspensions, enameling
Chapter 16 Corrosion and Degradation of Materials
 Chapter 16 Corrosion and Degradation of Materials Concept Check 16.1 Question: Would you expect iron to corrode in water of high purity? Why or why not? Answer: Iron would not corrode in water of high
Chapter 16 Corrosion and Degradation of Materials Concept Check 16.1 Question: Would you expect iron to corrode in water of high purity? Why or why not? Answer: Iron would not corrode in water of high
The Role Of Electroplates In Contact Reliability
 The Role Of Electroplates In Contact Reliability W.H. Abbott Battelle-Columbus Abbott@battelle.org 10/24/02 1 Overview Electroplating Is A Process; i.e. It Should Not Be Viewed As Simply A Material The
The Role Of Electroplates In Contact Reliability W.H. Abbott Battelle-Columbus Abbott@battelle.org 10/24/02 1 Overview Electroplating Is A Process; i.e. It Should Not Be Viewed As Simply A Material The
Interfaces: Corrosion in Pb-alloy cooled nuclear reactors and advanced mitigation measures
 Interfaces: Corrosion in Pb-alloy cooled nuclear reactors and advanced mitigation measures and G. Müller KIT KIT Universität des Landes Baden-Württemberg und nationales Forschungszentrum in der Helmholtz-Gemeinschaft
Interfaces: Corrosion in Pb-alloy cooled nuclear reactors and advanced mitigation measures and G. Müller KIT KIT Universität des Landes Baden-Württemberg und nationales Forschungszentrum in der Helmholtz-Gemeinschaft
Precursors with Metal-Nitrogen Bonds for ALD of Metals, Nitrides and Oxides
 Precursors with Metal-Nitrogen Bonds for ALD of Metals, Nitrides and Oxides Abstract Roy Gordon Gordon@chemistry.harvard.edu, Cambridge, MA To achieve ALD s unique characteristics, ALD precursors must
Precursors with Metal-Nitrogen Bonds for ALD of Metals, Nitrides and Oxides Abstract Roy Gordon Gordon@chemistry.harvard.edu, Cambridge, MA To achieve ALD s unique characteristics, ALD precursors must
Therma 310S/4845 EN , ASTM TYPE 310S / UNS S31008
 Therma 310S/4845 EN 1.4845, ASTM TYPE 310S / UNS S31008 General characteristics Austenitic heat resisting 4845 grade with very good oxidation resistance. This grade 4845 belongs to austenitic chromium-
Therma 310S/4845 EN 1.4845, ASTM TYPE 310S / UNS S31008 General characteristics Austenitic heat resisting 4845 grade with very good oxidation resistance. This grade 4845 belongs to austenitic chromium-
Microstructural Characterization of Reaction Products on Iron Based Alloys Exposed to H 2
 Vol. Materials 5, No. Research, 3, 2002Vol. 5, No. Microstructural 3, 349-355, 2002. Characterization of Reaction Products on Iron Based Alloys Exposed to H 2 S Atmospheres at High Temperatures 2002 349
Vol. Materials 5, No. Research, 3, 2002Vol. 5, No. Microstructural 3, 349-355, 2002. Characterization of Reaction Products on Iron Based Alloys Exposed to H 2 S Atmospheres at High Temperatures 2002 349
Corrosion of Different Types of Steel in Atmospheric and Tidal Marine Environment of Thailand
 Corrosion of Different Types of Steel in Atmospheric and Tidal Marine Environment of Thailand Prasong Permsuwan 1, Pakawat Sancharoen 2, Somnuk Tangtermsirikul 3, Paiboon Sreearunothai 4, and Ekkarut Viyanit
Corrosion of Different Types of Steel in Atmospheric and Tidal Marine Environment of Thailand Prasong Permsuwan 1, Pakawat Sancharoen 2, Somnuk Tangtermsirikul 3, Paiboon Sreearunothai 4, and Ekkarut Viyanit
Corrosion of Archaeological Metals
 Corrosion of Archaeological Metals Professor Michael Notis Archaeometallurgy Laboratory Lehigh University 442 Whitaker Laboratory Bethlehem, PA 10815 USA mrn1@lehigh.edu Kojindani, Sendai, Japan Identical
Corrosion of Archaeological Metals Professor Michael Notis Archaeometallurgy Laboratory Lehigh University 442 Whitaker Laboratory Bethlehem, PA 10815 USA mrn1@lehigh.edu Kojindani, Sendai, Japan Identical
In situ studies of carbon formation leading to metal dusting in syngas processes
 In situ studies of carbon formation leading to metal dusting in syngas processes Olle Söderström Department of Chemical Engineering, Lund University February 2010 Abstract Metal dusting corrosion begins
In situ studies of carbon formation leading to metal dusting in syngas processes Olle Söderström Department of Chemical Engineering, Lund University February 2010 Abstract Metal dusting corrosion begins
THE CORROSION-RESISTANT PROPERTIES OF X33CrNiMn23-8 FOR HIGH-THERMAL LOADED ENGINE OUTLET VALVES. Krzysztof Adamaszek a Zbigniew Jurasz a
 THE CORROSION-RESISTANT PROPERTIES OF X33CrNiMn23-8 FOR HIGH-THERMAL LOADED ENGINE OUTLET VALVES Krzysztof Adamaszek a Zbigniew Jurasz a a Automotive Research and Development Center Bosmal, 43-300 Bielsko-Biała,
THE CORROSION-RESISTANT PROPERTIES OF X33CrNiMn23-8 FOR HIGH-THERMAL LOADED ENGINE OUTLET VALVES Krzysztof Adamaszek a Zbigniew Jurasz a a Automotive Research and Development Center Bosmal, 43-300 Bielsko-Biała,
UNIT-III: ELECTRO CHEMISTRY AND CORROSION
 UNIT-III: ELECTRO CHEMISTRY AND CORROSION Introduction to Electrochemistry - EMF of the cell or Cell potential-electrochemical series and its importance Reference electrodes (SHE and Calomel electrode).
UNIT-III: ELECTRO CHEMISTRY AND CORROSION Introduction to Electrochemistry - EMF of the cell or Cell potential-electrochemical series and its importance Reference electrodes (SHE and Calomel electrode).
CHAPTER 4: Oxidation. Chapter 4 1. Oxidation of silicon is an important process in VLSI. The typical roles of SiO 2 are:
 Chapter 4 1 CHAPTER 4: Oxidation Oxidation of silicon is an important process in VLSI. The typical roles of SiO 2 are: 1. mask against implant or diffusion of dopant into silicon 2. surface passivation
Chapter 4 1 CHAPTER 4: Oxidation Oxidation of silicon is an important process in VLSI. The typical roles of SiO 2 are: 1. mask against implant or diffusion of dopant into silicon 2. surface passivation
Families on the Periodic Table
 Families on the Periodic Table Elements on the periodic table can be grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families
Families on the Periodic Table Elements on the periodic table can be grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families
MSE 352 Engineering Ceramics II
 Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 352 Engineering Ceramics II Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 352 Engineering Ceramics II Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
CORROSION BEHAVIOR OF AUSTENITIC AND FERRITIC STEELS IN SUPERCRITICAL WATER
 CORROSION BEHAVIOR OF AUSTENITIC AND FERRITIC STEELS IN SUPERCRITICAL WATER XIN LUO *, RUI TANG, CHONGSHENG LONG, ZHI MIAO, QIAN PENG and CONG LI 2 National Key Laboratory for Nuclear Fuel and Materials,
CORROSION BEHAVIOR OF AUSTENITIC AND FERRITIC STEELS IN SUPERCRITICAL WATER XIN LUO *, RUI TANG, CHONGSHENG LONG, ZHI MIAO, QIAN PENG and CONG LI 2 National Key Laboratory for Nuclear Fuel and Materials,
Overview of Dual Damascene Cu/Low-k Interconnect
 ERC Retreat Stanford: New Chemistries & Tools for scco 2 Processing of Thin Films Overview of Dual Damascene Cu/Low-k Interconnect P. Josh Wolf 1,4 - Program Manager, Interconnect Div. josh.wolf@sematech.org
ERC Retreat Stanford: New Chemistries & Tools for scco 2 Processing of Thin Films Overview of Dual Damascene Cu/Low-k Interconnect P. Josh Wolf 1,4 - Program Manager, Interconnect Div. josh.wolf@sematech.org
Environmental Interactions
 Environmental Interactions Chemical reaction between the material and its environment Beneficial interactions: materials processing Carburization and nitriding hardens for wear resistance Doping adds electrically
Environmental Interactions Chemical reaction between the material and its environment Beneficial interactions: materials processing Carburization and nitriding hardens for wear resistance Doping adds electrically
Database. Sept , 2014, Aachen, Germany. Thermo-Calc Anwendertreffen
 Database Sept. 11-12, 2014, Aachen, Germany Thermo-Calc Anwendertreffen Thermodynamic and kinetic databases New Databases, June 2014 TCAL3 TCMG3 TCSLD2 TCSI1 TCNI7 MOBNI3 TCAL3.0 TCAL3.0 TCAL1.0 2011.05
Database Sept. 11-12, 2014, Aachen, Germany Thermo-Calc Anwendertreffen Thermodynamic and kinetic databases New Databases, June 2014 TCAL3 TCMG3 TCSLD2 TCSI1 TCNI7 MOBNI3 TCAL3.0 TCAL3.0 TCAL1.0 2011.05
Effects in Ductile Iron
 Summary of Element Effects in Ductile Iron Rick Gundlach Element Materials Technology Wixom Insert Company Logo Here DIS Annual Meeting, June 7, 2012 Muskegon, Michigan Types of Alloying Elements Substitutional
Summary of Element Effects in Ductile Iron Rick Gundlach Element Materials Technology Wixom Insert Company Logo Here DIS Annual Meeting, June 7, 2012 Muskegon, Michigan Types of Alloying Elements Substitutional
Lecture 3: Biomaterials Surfaces: Chemistry
 1 Lecture 3: Biomaterials Surfaces: Chemistry Surfaces are high-energy regions of materials and thereby facilitate chemical reactions that influence performance of biomaterials. We will focus on 3 classes
1 Lecture 3: Biomaterials Surfaces: Chemistry Surfaces are high-energy regions of materials and thereby facilitate chemical reactions that influence performance of biomaterials. We will focus on 3 classes
The early stage oxidation and evaporation of Mg 9%Al 1%Zn alloy
 Corrosion Science 46 (2004) 377 386 www.elsevier.com/locate/corsci The early stage oxidation and evaporation of Mg 9%Al 1%Zn alloy F. Czerwinski * Development Engineering, Husky Injection Molding Systems
Corrosion Science 46 (2004) 377 386 www.elsevier.com/locate/corsci The early stage oxidation and evaporation of Mg 9%Al 1%Zn alloy F. Czerwinski * Development Engineering, Husky Injection Molding Systems
CHM101 SECOND EXAM 162. Chemistry 101 SECOND Exam (162) Name: Date: 30/4/2017
 Chemistry 101 SECOND Exam (162) Name: Date: 30/4/2017 Student no. Section: Useful Information:Gas Constant R= 0.08206 L.atm/K.mol, Specific heat of H 2 O=4.18 J/g. C 1 atm. = 760 mmhg. H 1 He 2 1.000 4
Chemistry 101 SECOND Exam (162) Name: Date: 30/4/2017 Student no. Section: Useful Information:Gas Constant R= 0.08206 L.atm/K.mol, Specific heat of H 2 O=4.18 J/g. C 1 atm. = 760 mmhg. H 1 He 2 1.000 4
Role of Alloying Elements and Carbides in the Chlorine-Induced Corrosion of Steels and Alloys
 Vol. Materials 7, No. Research, 1, 2004 Vol. 7, No. 1, 89-95, Role 2004. of Alloying Elements and Carbides in the Chlorine-Induced Corrosion of Steels and Alloys 2004 89 Role of Alloying Elements and Carbides
Vol. Materials 7, No. Research, 1, 2004 Vol. 7, No. 1, 89-95, Role 2004. of Alloying Elements and Carbides in the Chlorine-Induced Corrosion of Steels and Alloys 2004 89 Role of Alloying Elements and Carbides
1 PASSIVITY By Dr. D. M. Patel Introduction to Passivity: Passivity Alternative Definition of Passivity: Theories of Passivity:
 1 P a g e PASSIVITY By Dr. D. M. Patel Introduction to Passivity: Phenomenon passivity is closely related to protection of metals from corrosion. Term passivity is first utilised by Schonbein in 1836 to
1 P a g e PASSIVITY By Dr. D. M. Patel Introduction to Passivity: Phenomenon passivity is closely related to protection of metals from corrosion. Term passivity is first utilised by Schonbein in 1836 to
Environmental Degradation Fundamentals
 Environmental Degradation Fundamentals R. G. Ballinger Professor Massachusetts Institute of Technology 1 Outline Thermodynamics of Corrosion: Pourbaix Diagrams Corrosion Kinetics Polarization Diagrams
Environmental Degradation Fundamentals R. G. Ballinger Professor Massachusetts Institute of Technology 1 Outline Thermodynamics of Corrosion: Pourbaix Diagrams Corrosion Kinetics Polarization Diagrams
SULFIDATION. From Rolled Alloys Report Number 94-72
 SULFIDATION Environments containing sulfur may rapidly attack high nickel alloys. The problem is more severe under reducing, or low oxygen, environments. The higher the nickel the more sensitive the alloy
SULFIDATION Environments containing sulfur may rapidly attack high nickel alloys. The problem is more severe under reducing, or low oxygen, environments. The higher the nickel the more sensitive the alloy
High-Temperature Oxidation Behavior of a New Ni-Cr-Mo-Si Alloy
 High-Temperature Oxidation Behavior of a New Ni-Cr-Mo-Si B. A. Baker and G. D. Smith Special Metals Corp. 32 Riverside Drive Huntington, WV 2575 B. A. Pint and L. R. Walker Oak Ridge National Laboratory
High-Temperature Oxidation Behavior of a New Ni-Cr-Mo-Si B. A. Baker and G. D. Smith Special Metals Corp. 32 Riverside Drive Huntington, WV 2575 B. A. Pint and L. R. Walker Oak Ridge National Laboratory
Phosphorous Problem. AkMB Rashid Professor, Department of MME BUET, Dhaka
 09 Phosphorous Problem AkMB Rashid Professor, Department of MME BUET, Dhaka Today s Topics Behaviour of phosphorous in metal and slag Oxidation of phosphorous Effect of temperature Effect of metal and
09 Phosphorous Problem AkMB Rashid Professor, Department of MME BUET, Dhaka Today s Topics Behaviour of phosphorous in metal and slag Oxidation of phosphorous Effect of temperature Effect of metal and
Module-13. Corrosion and Degradation of materials
 Module-13 Corrosion and Degradation of materials Contents 1) Corrosion of metals 2) Corrosion of ceramics 3) Degradation of polymers Deterioration of materials Conventional engineering materials are not
Module-13 Corrosion and Degradation of materials Contents 1) Corrosion of metals 2) Corrosion of ceramics 3) Degradation of polymers Deterioration of materials Conventional engineering materials are not
Lecture 3: Biomaterials Surfaces: Chemistry
 1 Lecture 3: Biomaterials Surfaces: Chemistry Surfaces are high-energy regions of materials and thereby facilitate chemical reactions that influence performance of biomaterials. This lecture will focus
1 Lecture 3: Biomaterials Surfaces: Chemistry Surfaces are high-energy regions of materials and thereby facilitate chemical reactions that influence performance of biomaterials. This lecture will focus
Chapter 17: Corrosion and Degradation of Materials
 Chapter 17: Corrosion and Degradation of Materials ISSUES TO ADDRESS... How does corrosion of metals occur? Which metals are more likely to corrode and which less? How do we prevent or control corrosion?
Chapter 17: Corrosion and Degradation of Materials ISSUES TO ADDRESS... How does corrosion of metals occur? Which metals are more likely to corrode and which less? How do we prevent or control corrosion?
E3 Redox: Transferring electrons. Session one of two First hour: Discussion (E1) 2nd and 3rd hour: Lab (E3, Parts 1 and 2A)
 E3 Redox: Transferring electrons Session one of two First hour: Discussion (E) 2nd and 3rd hour: Lab (E3, Parts and 2A) Oxidation-Reduction (Redox) Reactions involve electron transfer. Change in charge
E3 Redox: Transferring electrons Session one of two First hour: Discussion (E) 2nd and 3rd hour: Lab (E3, Parts and 2A) Oxidation-Reduction (Redox) Reactions involve electron transfer. Change in charge
Corrosion response of , 316L and austenitic steels to flowing LBE with 10-7 mass % dissolved oxygen at 400, 450 and 550 C
 12th International Workshop on Spallation Materials Technology 19-23 October 2014, Bregenz, Austria Corrosion response of 1.4970, 316L and 1.4571 austenitic steels to flowing LBE with 10-7 mass % dissolved
12th International Workshop on Spallation Materials Technology 19-23 October 2014, Bregenz, Austria Corrosion response of 1.4970, 316L and 1.4571 austenitic steels to flowing LBE with 10-7 mass % dissolved
Surface Layer Characterization of Atomized Magnesium for use in Powder Metallurgy Products Paul Burke and Georges J. Kipouros
 Surface Layer Characterization of Atomized Magnesium for use in Powder Metallurgy Products Paul Burke and Georges J. Kipouros Materials Engineering Program Process Engineering and Applied Science Dalhousie
Surface Layer Characterization of Atomized Magnesium for use in Powder Metallurgy Products Paul Burke and Georges J. Kipouros Materials Engineering Program Process Engineering and Applied Science Dalhousie
The inhibition of the corrosion of carbon steel by oligomeric corrosion inhibitors in different media
 Plasticheskie Massy, No. 8, 2013, pp. 36 39 The inhibition of the corrosion of carbon steel by oligomeric corrosion inhibitors in different media Kh.S. Beknazarov, A.T. Dzhalilov, U.Yu. Ostanov, and A.M.
Plasticheskie Massy, No. 8, 2013, pp. 36 39 The inhibition of the corrosion of carbon steel by oligomeric corrosion inhibitors in different media Kh.S. Beknazarov, A.T. Dzhalilov, U.Yu. Ostanov, and A.M.
INA-X System for SNMS and SIMS
 Customized Systems and Solutions Nanostructures and Thin Film Deposition Surface Analysis and Preparation Components Surface Science Application INA-X System for SNMS and SIMS Application Notes The quantitative
Customized Systems and Solutions Nanostructures and Thin Film Deposition Surface Analysis and Preparation Components Surface Science Application INA-X System for SNMS and SIMS Application Notes The quantitative
DISSOLVED CONSTITUENTS IN MARINE PORE WATER ... DATA EVALUATION...
 DISSOLVED CONSTITUENTS IN MARINE PORE WATER PORE WATER PROFILES DIFFUSIVE FLUXES... DATA EVALUATION... How to read pore water concentration profiles 1 How to read pore water concentration profiles Consumption
DISSOLVED CONSTITUENTS IN MARINE PORE WATER PORE WATER PROFILES DIFFUSIVE FLUXES... DATA EVALUATION... How to read pore water concentration profiles 1 How to read pore water concentration profiles Consumption
CHAPTER 8. Corrosion 1/1/2016 THE COST OF CORROSION ISSUES TO ADDRESS... CORROSION IN A GRAPEFRUIT. Introduction. Zn Zn 2+ 2e.
 CHAPTER 8 Announcement: Please fill in Class Evaluation Survey (compulsory) http://goo.gl/forms/i8vcyteo0y Access from www.afendirojan.wordpress.com Corrosion 1 2 THE COST OF CORROSION ISSUES TO ADDRESS...
CHAPTER 8 Announcement: Please fill in Class Evaluation Survey (compulsory) http://goo.gl/forms/i8vcyteo0y Access from www.afendirojan.wordpress.com Corrosion 1 2 THE COST OF CORROSION ISSUES TO ADDRESS...
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 7: Chemical systems
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 7: Chemical systems MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 7: Chemical systems MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
SURFACE ANALYSIS OF WATER ATOMISED HIGH CHROMIUM ALLOYED STEEL POWDER BY X-RAY PHOTOELECTRON SPECTROSCOPY
 Powder Metallurgy Progress, Vol.13 (2013), No 2 57 SURFACE ANALYSIS OF WATER ATOMISED HIGH CHROMIUM ALLOYED STEEL POWDER BY X-RAY PHOTOELECTRON SPECTROSCOPY R. Shvab, E. Hryha, E. Dudrová, O. Bergman and
Powder Metallurgy Progress, Vol.13 (2013), No 2 57 SURFACE ANALYSIS OF WATER ATOMISED HIGH CHROMIUM ALLOYED STEEL POWDER BY X-RAY PHOTOELECTRON SPECTROSCOPY R. Shvab, E. Hryha, E. Dudrová, O. Bergman and
Anodizing of aluminium
 Anodizing of aluminium Posted on Nov 04, Posted by P&A International Category General Talk Anodising is a process for producing decorative and protective films on articles made of aluminium and its alloys.
Anodizing of aluminium Posted on Nov 04, Posted by P&A International Category General Talk Anodising is a process for producing decorative and protective films on articles made of aluminium and its alloys.
FRONT END PROCESSES - CLEANING, LITHOGRAPHY, OXIDATION ION IMPLANTATION, DIFFUSION, DEPOSITION AND ETCHING
 Manufacturing, Cleaning, Gettering - Chapter 4 FRONT END PROCESSES - CLEANING, LITHOGRAPHY, OXIDATION ION IMPLANTATION, DIFFUSION, DEPOSITION AND ETCHING Over the next several weeks, we ll study front
Manufacturing, Cleaning, Gettering - Chapter 4 FRONT END PROCESSES - CLEANING, LITHOGRAPHY, OXIDATION ION IMPLANTATION, DIFFUSION, DEPOSITION AND ETCHING Over the next several weeks, we ll study front
Corrosion of disposal canisters
 Corrosion of disposal canisters Fraser King Integrity Corrosion Consulting Ltd, Canada Consultant to Nagra Outline Forms of corrosion - Types of corrosion - Control by design Nature of the disposal environment
Corrosion of disposal canisters Fraser King Integrity Corrosion Consulting Ltd, Canada Consultant to Nagra Outline Forms of corrosion - Types of corrosion - Control by design Nature of the disposal environment
Australian Journal of Basic and Applied Sciences
 AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com The Influence of Molten Salt Deposit in Water Vapor Environment on Corrosion Behaviour
AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com The Influence of Molten Salt Deposit in Water Vapor Environment on Corrosion Behaviour
ECSE-6300 IC Fabrication Laboratory Lecture 4: Dielectrics and Poly-Si Deposition. Lecture Outline
 ECSE-6300 IC Fabrication Laboratory Lecture 4: Dielectrics and Poly-Si Deposition Prof. Rensselaer Polytechnic Institute Troy, NY 12180 Office: CII-6229 Tel.: (518) 276-2909 e-mails: luj@rpi.edu http://www.ecse.rpi.edu/courses/s18/ecse
ECSE-6300 IC Fabrication Laboratory Lecture 4: Dielectrics and Poly-Si Deposition Prof. Rensselaer Polytechnic Institute Troy, NY 12180 Office: CII-6229 Tel.: (518) 276-2909 e-mails: luj@rpi.edu http://www.ecse.rpi.edu/courses/s18/ecse
Thermodynamic conditions for the Mn O system in sintering of manganese steels
 Thermodynamic Conditions for the Mn O system 5 Part I Thermodynamic conditions for the Mn O system in sintering of manganese steels 2 2.1. Manganese in steelmaking Manganese is the most widely used alloying
Thermodynamic Conditions for the Mn O system 5 Part I Thermodynamic conditions for the Mn O system in sintering of manganese steels 2 2.1. Manganese in steelmaking Manganese is the most widely used alloying
Corrosion of copper in final disposal environments. Jari Aromaa Aalto Unversity Hydrometallurgy and Corrosion Research Group
 Corrosion of copper in final disposal environments Jari Aromaa Aalto Unversity Hydrometallurgy and Corrosion Research Group Contents The concept Corrosive environments Copper corrosion mechanisms Copper
Corrosion of copper in final disposal environments Jari Aromaa Aalto Unversity Hydrometallurgy and Corrosion Research Group Contents The concept Corrosive environments Copper corrosion mechanisms Copper
High Temperature Corrosion in Gasifiers
 Vol. Materials 7, No. Research, 1, 2004Vol. 7, No. 1, 53-59, 2004. High Temperature Corrosion in Gasifiers 2004 53 High Temperature Corrosion in Gasifiers Wate Bakker* EPRI 3412 Hillview Avenue Palo Alto,
Vol. Materials 7, No. Research, 1, 2004Vol. 7, No. 1, 53-59, 2004. High Temperature Corrosion in Gasifiers 2004 53 High Temperature Corrosion in Gasifiers Wate Bakker* EPRI 3412 Hillview Avenue Palo Alto,
SCOPE OF ACCREDITATION TO ISO GUIDE 34:2009
 SCOPE OF ACCREDITATION TO ISO GUIDE 34:2009 SCP SCIENCE 21800 Clark Graham Baie d'urfe, Quebec H9X 4B6 CANADA David Smith Phone: 514 457 0701 dsmith@scpscience.com REFERENCE MATERIAL PRODUCER Valid To:
SCOPE OF ACCREDITATION TO ISO GUIDE 34:2009 SCP SCIENCE 21800 Clark Graham Baie d'urfe, Quebec H9X 4B6 CANADA David Smith Phone: 514 457 0701 dsmith@scpscience.com REFERENCE MATERIAL PRODUCER Valid To:
Effect of melt temperature on the oxidation behavior of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres
 available at www.sciencedirect.com www.elsevier.com/locate/matchar Effect of melt temperature on the oxidation behavior of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres Hukui Chen
available at www.sciencedirect.com www.elsevier.com/locate/matchar Effect of melt temperature on the oxidation behavior of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres Hukui Chen
Effect of Thermal Sprayed Al on the Steam Oxidation Resistance of 9Cr-1Mo Steel
 Thermal Spray 2003: Advancing the Science & Applying the Technology, (Ed.) C. Moreau and B. Marple, Published by ASM International, Materials Park, Ohio, USA, 2003 Effect of Thermal Sprayed Al on the Steam
Thermal Spray 2003: Advancing the Science & Applying the Technology, (Ed.) C. Moreau and B. Marple, Published by ASM International, Materials Park, Ohio, USA, 2003 Effect of Thermal Sprayed Al on the Steam
Material Evaporation Application Comment MP P / Optical films, Oxide films, Electrical contacts. Doping, Electrical contacts.
 for vapour Aluminum (Al) -, Optical, Oxide, Electrical BN liners with lid are recommended due to the reactivity and the fact that Al creeps out. Cooling down of the cell with 1K per minute. 660 972 Antimony
for vapour Aluminum (Al) -, Optical, Oxide, Electrical BN liners with lid are recommended due to the reactivity and the fact that Al creeps out. Cooling down of the cell with 1K per minute. 660 972 Antimony
Passivity Definitions and Influencing Parameters
 Lecture 20 Passivity Definitions and Influencing Parameters Keywords: Definition of Passivity, Flade Potential, Anodic Polarization, Critical Anodic Current Density. In the Eh ph diagrams, resistance to
Lecture 20 Passivity Definitions and Influencing Parameters Keywords: Definition of Passivity, Flade Potential, Anodic Polarization, Critical Anodic Current Density. In the Eh ph diagrams, resistance to
Chapter 9 Phase Diagrams. Dr. Feras Fraige
 Chapter 9 Phase Diagrams Dr. Feras Fraige Chapter Outline Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited
Chapter 9 Phase Diagrams Dr. Feras Fraige Chapter Outline Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Passivityof metalsand metallic alloys. Jacek Banaś
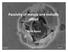 Passivityof metalsand metallic alloys Jacek Banaś Wagner definition of passivation etal is passive when its corrosion in course of chemical or electrochemical reaction is lower at higher affinity of reaction
Passivityof metalsand metallic alloys Jacek Banaś Wagner definition of passivation etal is passive when its corrosion in course of chemical or electrochemical reaction is lower at higher affinity of reaction
Growth Rate and Phase Composition of Oxide Scales during Hot Rolling of Low Carbon Steel
 , pp. 1554 1559 Growth Rate and Phase Composition of Oxide Scales during Hot Rolling of Low Carbon Steel Vladimir V. BASABE and Jerzy A. SZPUNAR Department of Mining, Metals and Materials Engineering,
, pp. 1554 1559 Growth Rate and Phase Composition of Oxide Scales during Hot Rolling of Low Carbon Steel Vladimir V. BASABE and Jerzy A. SZPUNAR Department of Mining, Metals and Materials Engineering,
Lecture 25: Principles of degassing
 Lecture 25: Principles of degassing Contents Introduction Principles Side reactions General considerations Fluid flow in degassing Material balance in de gassing Key words: Degassing, gases in steel, ladle
Lecture 25: Principles of degassing Contents Introduction Principles Side reactions General considerations Fluid flow in degassing Material balance in de gassing Key words: Degassing, gases in steel, ladle
8.1 Introduction 8.2 Summary 8.3 Conclusion. Contents
 Summary and Conclusion Contents Chapter 8 SUMMARY AND CONCLUSION 8.1 Introduction 8.2 Summary 8.3 Conclusion 8.1 Introduction In landfills without liners between the waste and the underlying geology or
Summary and Conclusion Contents Chapter 8 SUMMARY AND CONCLUSION 8.1 Introduction 8.2 Summary 8.3 Conclusion 8.1 Introduction In landfills without liners between the waste and the underlying geology or
Section 4: Thermal Oxidation. Jaeger Chapter 3. EE143 - Ali Javey
 Section 4: Thermal Oxidation Jaeger Chapter 3 Properties of O Thermal O is amorphous. Weight Density =.0 gm/cm 3 Molecular Density =.3E molecules/cm 3 O Crystalline O [Quartz] =.65 gm/cm 3 (1) Excellent
Section 4: Thermal Oxidation Jaeger Chapter 3 Properties of O Thermal O is amorphous. Weight Density =.0 gm/cm 3 Molecular Density =.3E molecules/cm 3 O Crystalline O [Quartz] =.65 gm/cm 3 (1) Excellent
METHODS OF COATING FABRICATION
 METHODS OF COATING FABRICATION Zbigniew Grzesik http://home.agh.edu.pl/~grzesik Department of Physical Chemistry and Modelling DEFINITION The coating is the thin outer layer of the object, which physiochemical
METHODS OF COATING FABRICATION Zbigniew Grzesik http://home.agh.edu.pl/~grzesik Department of Physical Chemistry and Modelling DEFINITION The coating is the thin outer layer of the object, which physiochemical
27-302, Microstructure & Properties II, A.D. Rollett. Fall Homework 2, due Weds, Nov. 6 th
 27-32, Microstructure & Properties II, A.D. Rollett Fall 2 Homework 2, due Weds, Nov. 6 th 1. Materials Design In the first discussion, we discussed materials in preference to an engineered device that
27-32, Microstructure & Properties II, A.D. Rollett Fall 2 Homework 2, due Weds, Nov. 6 th 1. Materials Design In the first discussion, we discussed materials in preference to an engineered device that
Effect of Rare Earth Oxide Additions on Oxidation Behavior of AISI 304L Stainless Steel
 Materials Research, Vol. 9, No. 4, 75-79, 006 006 Effect of Rare Earth Oxide Additions on Oxidation Behavior of AISI 04L Stainless Steel Marina Fuser Pillis*, Edval Gonçalves de Araújo, Lalgudi Venkataraman
Materials Research, Vol. 9, No. 4, 75-79, 006 006 Effect of Rare Earth Oxide Additions on Oxidation Behavior of AISI 04L Stainless Steel Marina Fuser Pillis*, Edval Gonçalves de Araújo, Lalgudi Venkataraman
High Temperature Corrosion of HVAF-Sprayed NiCrAlY Coating Exposed to Various Corrosive Environments
 High Temperature Corrosion of HVAF-Sprayed NiCrAlY Coating Exposed to Various Corrosive Environments Esmaeil Sadeghimeresht a, Reza Jafari b, Taghi Shahrabi Farahani b, Nicolaie Markocsan a, Shrikant Joshi
High Temperature Corrosion of HVAF-Sprayed NiCrAlY Coating Exposed to Various Corrosive Environments Esmaeil Sadeghimeresht a, Reza Jafari b, Taghi Shahrabi Farahani b, Nicolaie Markocsan a, Shrikant Joshi
Thermodynamics and Electrode Potential ME Dr. Zuhair M. Gasem
 Thermodynamics and Electrode Potential ME 472-062 Copyright Dr. Zuhair M. Gasem Corrosion Science and Engineering 2 Corrosion Science Engineering: corrosion forms, and controlling methods Chpater2 Thermodynamics
Thermodynamics and Electrode Potential ME 472-062 Copyright Dr. Zuhair M. Gasem Corrosion Science and Engineering 2 Corrosion Science Engineering: corrosion forms, and controlling methods Chpater2 Thermodynamics
1. Scaling. H.O.: H-5/21, KRISHNA NAGAR, DELHI Tel.: , Fax:
 Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Theoretical Study of Material Removal Mechanism
 Chapter 7 Theoretical Study of Material Removal Mechanism 7.1 Introduction From Chapters 5 and 6, it is known that during dynamic friction polishing of PCD composites, the interface temperature increases
Chapter 7 Theoretical Study of Material Removal Mechanism 7.1 Introduction From Chapters 5 and 6, it is known that during dynamic friction polishing of PCD composites, the interface temperature increases
KINETICS OF IRON SILICIDE DEPOSITED ON AISI D2 STEEL BY PACK METHOD. Ugur SEN, Ozkan OZDEMIR, Senol YILMAZ, Saduman ŞEN
 KINETICS OF IRON SILICIDE DEPOSITED ON AISI D2 STEEL BY PACK METHOD Ugur SEN, Ozkan OZDEMIR, Senol YILMAZ, Saduman ŞEN Sakarya University, Sakarya, Turkey, ugursen@sakarya.edu.tr, Abstract: In this study,
KINETICS OF IRON SILICIDE DEPOSITED ON AISI D2 STEEL BY PACK METHOD Ugur SEN, Ozkan OZDEMIR, Senol YILMAZ, Saduman ŞEN Sakarya University, Sakarya, Turkey, ugursen@sakarya.edu.tr, Abstract: In this study,
Precursors for ceramics synthesis
 Precursors for ceramics synthesis Decrease diffusion lengths by using intimately mixing of cations. Solid precursors containing the desired cations. Coprecipitation: salts of different metals are precipitated
Precursors for ceramics synthesis Decrease diffusion lengths by using intimately mixing of cations. Solid precursors containing the desired cations. Coprecipitation: salts of different metals are precipitated
Corrosion and batteries
 Corrosion and batteries Corrosion is a process of gradual destruction of metal from its surface due to its unwanted chemical or electrochemical interaction of metal. Ore of metal which is stable and is
Corrosion and batteries Corrosion is a process of gradual destruction of metal from its surface due to its unwanted chemical or electrochemical interaction of metal. Ore of metal which is stable and is
Water Vapor and Carbon Nanotubes
 Water Vapor and Carbon Nanotubes Published technical papers on carbon nanotube fabrication point out the need to improve the growth rate and uniformity of Carbon Nanotubes. CNT faces major hurdles in its
Water Vapor and Carbon Nanotubes Published technical papers on carbon nanotube fabrication point out the need to improve the growth rate and uniformity of Carbon Nanotubes. CNT faces major hurdles in its
1. Which of the given statements about the reaction below are incorrect?
 1. Which of the given statements about the reaction below are incorrect? 2PbO(s) + C(s) 2Pb(s) + CO 2 (g) a. Lead is getting reduced b. Carbon dioxide is getting oxidised c. Carbon is getting oxidised
1. Which of the given statements about the reaction below are incorrect? 2PbO(s) + C(s) 2Pb(s) + CO 2 (g) a. Lead is getting reduced b. Carbon dioxide is getting oxidised c. Carbon is getting oxidised
Alkali and zinc chlorides in waterwall tube corrosion:
 Alkali and zinc chlorides in waterwall tube corrosion: Effects of pure salts and mixtures Master of Science Thesis in the Master Degree Programme, Materials Chemistry and Nanotechnology ANDREAS SLOMIAN
Alkali and zinc chlorides in waterwall tube corrosion: Effects of pure salts and mixtures Master of Science Thesis in the Master Degree Programme, Materials Chemistry and Nanotechnology ANDREAS SLOMIAN
STRENGTH OF STEELS EXPOSED TO HEAVY LIQUID METALS
 IAEA INPRO COOL project Activity 6: Components for service in intimate contact with high temperature coolants (LM and MS) 6.1 Experimental study on components and various materials that are in contact
IAEA INPRO COOL project Activity 6: Components for service in intimate contact with high temperature coolants (LM and MS) 6.1 Experimental study on components and various materials that are in contact
POLARIZATION MEASUREMENTS ON IRON IN ALKALINE SOLUTIONS AT HIGH PRESSURES AND HIGH TEMPERATURES.
 POLARIZATION MEASUREMENTS ON IRON IN ALKALINE SOLUTIONS AT HIGH PRESSURES AND HIGH TEMPERATURES. W M. M. HUIJBREGTS, * G. YAN OSCH* A. SNEL * *NV KEMA, Arnhem, The Netherlands During the last few years
POLARIZATION MEASUREMENTS ON IRON IN ALKALINE SOLUTIONS AT HIGH PRESSURES AND HIGH TEMPERATURES. W M. M. HUIJBREGTS, * G. YAN OSCH* A. SNEL * *NV KEMA, Arnhem, The Netherlands During the last few years
Influence on Whiskers:
 Sn Corrosion and It s Influence on Whiskers: J.W.Osenbach 1, J. M. DeLucca, B.D. Potteiger, R.L.Shook, and F.A. Baiocchi Agere Systems, Allentown PA Voice: (610) 712-5469 e-mail: osenbach@agere.com 0 ECTC
Sn Corrosion and It s Influence on Whiskers: J.W.Osenbach 1, J. M. DeLucca, B.D. Potteiger, R.L.Shook, and F.A. Baiocchi Agere Systems, Allentown PA Voice: (610) 712-5469 e-mail: osenbach@agere.com 0 ECTC
Target effective electron emission coefficients during reactive sputtering of Oxides and Nitrides.
 Target effective electron emission coefficients during reactive sputtering of and R. De Gryse, D. Depla, S. Mahieu Department for Solid State Sciences Ghent University (S1) B-9000 Ghent, Belgium X.Y. Li
Target effective electron emission coefficients during reactive sputtering of and R. De Gryse, D. Depla, S. Mahieu Department for Solid State Sciences Ghent University (S1) B-9000 Ghent, Belgium X.Y. Li
Used Fuel Container Designs and Lifetime Prediction
 Used Fuel Container Designs and Lifetime Prediction CNS Conference on Waste Management, Decommissioning and Environmental Restoration for Canada s Nuclear Activities Toronto, Canada September 11-14, 2011
Used Fuel Container Designs and Lifetime Prediction CNS Conference on Waste Management, Decommissioning and Environmental Restoration for Canada s Nuclear Activities Toronto, Canada September 11-14, 2011
E-BRITE E-BRITE. Technical Data Sheet. Stainless Steel: Superferritic GENERAL PROPERTIES PLANAR SOLID OXIDE FUEL CELLS CHEMICAL COMPOSITION
 E-BRITE Stainless Steel: Superferritic (UNS 44627, ASTM Type XM-27) GENERAL PROPERTIES E-BRITE alloy is a high purity ferritic stainless steel which combines excellent resistance to corrosion and oxidation
E-BRITE Stainless Steel: Superferritic (UNS 44627, ASTM Type XM-27) GENERAL PROPERTIES E-BRITE alloy is a high purity ferritic stainless steel which combines excellent resistance to corrosion and oxidation
Corrosion of heat exchanger materials
 Corrosion of heat exchanger materials M. Spiegel Aachen, den 5.06.2008 Introduction HT-Materials Oxidation and Passivation 550 C 600 1200 C > 1200 C Low alloyed steels Ferritic, austenitic steels Ni-based
Corrosion of heat exchanger materials M. Spiegel Aachen, den 5.06.2008 Introduction HT-Materials Oxidation and Passivation 550 C 600 1200 C > 1200 C Low alloyed steels Ferritic, austenitic steels Ni-based
SUB-Programs - Calibration range Fe Base for "PMI-MASTER Pro" Spark - mode Fe 000
 SUB-Programs - Calibration range Fe Base for "PMI-MASTER Pro" Spark - mode Fe 100 Fe 200 *** Fe 250 *** Fe 300 Fe 400 Fe 500 Fe 000 Fe low alloy steel cast iron Cr hard / Ni resist stainless steel tool
SUB-Programs - Calibration range Fe Base for "PMI-MASTER Pro" Spark - mode Fe 100 Fe 200 *** Fe 250 *** Fe 300 Fe 400 Fe 500 Fe 000 Fe low alloy steel cast iron Cr hard / Ni resist stainless steel tool
Corrosion Mechanism of Commercial MgO C Refractories in Contact with Different Gas Atmospheres
 , pp. 760 767 Corrosion Mechanism of Commercial MgO C Refractories in Contact with Different Gas Atmospheres Sune JANSSON, 1) Voicu BRABIE 2) and Pär JÖNSSON 3) 1) Arvika Gjuteri AB, SE-671 82 Arvika,
, pp. 760 767 Corrosion Mechanism of Commercial MgO C Refractories in Contact with Different Gas Atmospheres Sune JANSSON, 1) Voicu BRABIE 2) and Pär JÖNSSON 3) 1) Arvika Gjuteri AB, SE-671 82 Arvika,
UNIT-I ELECTROCHEMISTRY PART-A
 UNIT-I ELECTROCHEMISTRY PART-A 1. What is electrochemistry? 2. What do you understand by electrode potential? 3. Define E.M.F of an electrochemical cell? 4. Define (a) Single electrode potential (b) Standard
UNIT-I ELECTROCHEMISTRY PART-A 1. What is electrochemistry? 2. What do you understand by electrode potential? 3. Define E.M.F of an electrochemical cell? 4. Define (a) Single electrode potential (b) Standard
Electrical conductivity
 Electrical conductivity Ohm's Law: voltage drop (volts = J/C) C = Coulomb A (cross sect. area) ΔV = I R Resistivity, ρ and Conductivity, σ: -- geometry-independent forms of Ohm's Law resistance (Ohms)
Electrical conductivity Ohm's Law: voltage drop (volts = J/C) C = Coulomb A (cross sect. area) ΔV = I R Resistivity, ρ and Conductivity, σ: -- geometry-independent forms of Ohm's Law resistance (Ohms)
Stainless Steel & Stainless Steel Fasteners Chemical, Physical and Mechanical Properties
 Stainless Steel & Stainless Steel Fasteners Chemical, Physical and Mechanical Properties Stainless steel describes a family of steels highly resistant to tarnishing and rusting that contain at least two
Stainless Steel & Stainless Steel Fasteners Chemical, Physical and Mechanical Properties Stainless steel describes a family of steels highly resistant to tarnishing and rusting that contain at least two
Corrosion. Cause of Corrosion: Electrochemical Mechanism of Corrosion (Rusting of Iron)
 Corrosion Any process of deterioration (or destruction) and consequent loss of a solid metallic material, through an unwanted (or unintentional) chemical or electrochemical attack by its environment, starting
Corrosion Any process of deterioration (or destruction) and consequent loss of a solid metallic material, through an unwanted (or unintentional) chemical or electrochemical attack by its environment, starting
SCOPE OF ACCREDITATION TO ISO 17034:2016
 SCOPE OF ACCREDITATION TO ISO 17034:2016 SCP SCIENCE 21800 Clark Graham Baie d'urfe, Quebec H9X 4B6 CANADA David Smith Phone: 514 457 0701 dsmith@scpscience.com REFERENCE MATERIAL PRODUCER Valid To: November
SCOPE OF ACCREDITATION TO ISO 17034:2016 SCP SCIENCE 21800 Clark Graham Baie d'urfe, Quebec H9X 4B6 CANADA David Smith Phone: 514 457 0701 dsmith@scpscience.com REFERENCE MATERIAL PRODUCER Valid To: November
Physical Properties. Can increase the strength by cold working but the recrystallization temperature is 400 to 500 C
 Zirconium Cladding Why? Physical Properties Corrosion Resistance Radiation Effects ----------------------------------------------- In the early 1950Õs the Navy was looking for a material with low σ a high
Zirconium Cladding Why? Physical Properties Corrosion Resistance Radiation Effects ----------------------------------------------- In the early 1950Õs the Navy was looking for a material with low σ a high
Characterization of Coatings on Grey Cast Iron Fabricated by Hot-dipping in Pure Al, AlSi11 and AlTi5 Alloys
 A R C H I V E S o f F O U N D R Y E N G I N E E R I N G Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences ISSN (1897-3310) Volume 14 Issue 1/2014 85 90 20/1 Characterization
A R C H I V E S o f F O U N D R Y E N G I N E E R I N G Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences ISSN (1897-3310) Volume 14 Issue 1/2014 85 90 20/1 Characterization
High Quality Multi-arc Targets
 High Quality Multi-arc Targets IKS provides high-quality multi-arc targets for a wide range of applications for ferromagnetic, complex oxides, and semiconducting films. Our targets are offered in various
High Quality Multi-arc Targets IKS provides high-quality multi-arc targets for a wide range of applications for ferromagnetic, complex oxides, and semiconducting films. Our targets are offered in various
