DEVELOPMENT OF QUANTITATIVE FAST IMAGING WITH STEADY-STATE FREE PRECESSION (FISP) TECHNIQUES FOR HIGH FIELD PRECLINICAL MAGNETIC RESONANCE IMAGING
|
|
|
- Gavin Fox
- 5 years ago
- Views:
Transcription
1 DEVELOPMENT OF QUANTITATIVE FAST IMAGING WITH STEADY-STATE FREE PRECESSION (FISP) TECHNIQUES FOR HIGH FIELD PRECLINICAL MAGNETIC RESONANCE IMAGING by YING GAO Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Chris A. Flask, Ph.D. Department of Biomedical Engineering CASE WESTERN RESERVE UNIVERSITY January 2017
2 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Ying Gao candidate for the degree of PhD *. Committee Chair Efstathios Karathanasis Committee Member Chris A. Flask Committee Member Xin Yu Committee Member Vikas Gulani Committee Member Mitchell L. Drumm Date of Defense September 8, 2016 *We also certify that written approval has been obtained for any proprietary material contained therein.
3 To the best marathon in my life
4 Table of Contents Table of Contents...1 List of Figures...4 Abstract...9 Chapter 1 Introduction Overview of an MRI Signal Acquisition Basic MRI Acquisition Strategies Spin Echo Gradient Echo Echo-Planar Imaging Overview of Quantitative MRI T1 and T2 Quantification Perfusion MRI Compressed Sensing Magnetic Resonance Fingerprinting High-field Preclinical MRI Preclinical Imaging High-field Preclinical MRI Challenges and Opportunities Overview of Dissertation FISP-based Look-Locker T1 Measurement ASL-FISP Preclinical MRF Summary and Future Work
5 Chapter 2 FISP-based Look-Locker T1 Assessment of a Rat Model of Congenital Hepatic Fibrosis Introduction Methods Animal models MRI experiments Histological and biochemical analyses Statistical analyses Results Comparison of T1 relaxation in PCK and SD control rats Comparison of in vivo liver T1 relaxation time with histological and biochemical assessments of biliary dilatation and hepatic fibrosis Discussion...46 Chapter 3 ASL-FISP: a Rapid and Quantitative Perfusion Technique for High-field MRI Introduction Methods In Vivo Comparison of Image Artifacts at 7T ASL-FISP pulse sequence design Initial in vivo ASL-FISP perfusion assessments in mouse brains Additional in vivo ASL-FISP experiments: mouse brain ischemic stroke model, dystrophin-deficient mouse model and healthy mouse kidneys Results
6 3.4 Discussion...65 Chapter 4 Preclinical Magnetic Resonance Fingerprinting (MRF) at 7 T: Effective Quantitative Imaging for Rodent Disease Models Introduction Methods Preclinical MRF Acquisition and Reconstruction Design In vitro MRF assessments of T1 and T2 relaxation times and proton density (M0) Initial in vivo and ex vivo kidney MRF assessments in a healthy mouse Initial MRF Assessments in a Mouse Brain Tumor Model Results Discussion...89 Chapter 5 Summary and Future Work Summary Future Work FISP-based Look-Locker T1 Measurement ASL-FISP Preclinical MRF...99 Bibliography
7 List of Figures Figure 1.1: Schematic diagram of arterial spin labeling-fast imaging with steady-state free precession (ASL-FISP) acquisition. A flow-sensitive alternating inversion recovery (FAIR) ASL preparation is combined with a centrically encoded FISP acquisition. This acquisition is repeated for both a slice-selective inversion (with shaded gradient) and a non-selective inversion (without the shaded gradient) to generate the perfusion contrast. Note that all lines of k-space are acquired following a single ASL preparation. ADC, analog-to-digital converter...27 Figure 2.1: Representative liver Look-Locker T1-weighted images (columns a-d, grayscale) and T1 maps (column e, color scale) from a 3 month-old Sprague-Dawley (SD) control rat (top row) and two age-matched PCK rats (middle and bottom rows). The PCK rat in the bottom row exhibited cholangitis associated with more severe liver disease, which is also observed periodically in ARPKD patients...43 Figure 2.2: Plot of mean liver T1 values for SD (n=6) and PCK (n=4) rats at 3 months of age. The mean T1 values for the PCK rats were significantly increased in comparison to the SD rats (*p = 0.01). The respective T1 value for the PCK rat with cholangitis was further increased (n=1, data not shown, mean T1 = 1413 ms)...44 Figure 2.3: Photomicrographs (10x original magnification) of SD and PCK rat liver specimens stained with Masson s Trichrome to assess periportal fibrosis (in blue) and biliary dilatation (asterisks *). Note the regions of bile duct proliferation and dilatation as well as increased fibrosis in the PCK rats. Periportal fibrosis is especially pronounced in the rat with cholangitis, in which inflammatory cells are evident within a dilated bile duct (arrow)
8 Figure 2.4: Comparison of mean hepatic T1 values for 3-month-old SD rats (black diamonds) and PCK rats (white circles) in: (a) percent bile duct area; (b) percent fibrosis (by Masson s trichrome staining); and (c) biochemical assessments of hepatic hydroxyproline content. All three assessments resulted in significant correlations with mean liver T1 (p = 0.002, 0.004, and 0.01, respectively) suggesting that T1 assessments are an effective imaging marker for ARPKD liver disease Figure 3.1: Representative axial mouse brain images at 7 T using (a) spin echo, (b) FISP, (c) True FISP, and (d) EPI acquisitions. Note the similar lack of artifacts in the spin echo and FISP images in comparison to the True FISP (banding) and EPI (ghosting / distortion) images Figure 3.2: Representative ASL-FISP images of a healthy C57/BL6 mouse brain at 7 T: (a) Slice-selective (bright-blood); (b) non-selective (dark blood) ASL-FISP images (40 averages); (c) M0 image (no inversion); (d) Brain T1 map from Look-Locker acquisition; perfusion map from (e) ASL-FISP and (f) ASL-GRE in ml/min/100g of tissue. Note the lack of distortion and banding artifacts in the M0 FISP image Figure 3.3: (a) Mean C57/BL6 mouse brain perfusion from the ASL-FISP method at 7 T (gray) and 9.4 T (white), respectively. Results are plotted as a function of the number of ASL-FISP averages. No significant differences in mean perfusion were observed for different number of averages (p > 0.6) or field strength (p > 0.2). (b) Mean brain perfusion from a single C57/BL6 mouse at 7 T as a function of the slice thickness ratio (inversion slab thickness / imaging slice thickness) and the number of ASL-FISP averages. Note the large decrease in mean brain perfusion as the slice thickness ratio is 5
9 increased from 1 to 3. The mean perfusion also appears to be more sensitive to the inversion slab thickness than the number of ASL-FISP averages...61 Figure 3.4: ASL-FISP images of an MCAo mouse model of stroke at 7 T. (a) Sliceselective (bright-blood) and (b) non-selective (dark blood) ASL-FISP images; (c) M0 FISP image (no inversion); (d) diffusion-weighted image (b=500 s/mm 2 ) showing right brain infarct; (e) Look-Locker T1 map; and (f) perfusion map. The primary infarct is visible in the right brain in all images. A potential contralateral perfusion deficit is also observed in the perfusion map, but less evident in T1 and diffusion-weighted images...63 Figure 3.5: a-d. Representative perfusion images of 2-month WT (a), 2-month mdx (b), 10-month WT (c), and 10-month mdx (d). e-h. Representative T1-maps of 2-month WT (e), 2-month mdx (f), 10-month WT (g), and 10-month mdx (h). i. Mean cerebral (excluding ventricles) perfusion from the ASL-FISP method in C57/BL6 WT (black) and mdx (white), respectively. There was a significant decrease in mean perfusion as compared to aged WT observed in the aged mdx mice (p < 0.005). There was no significant change in perfusion in young versus aged WT (p > 0.3) or young versus aged mdx (p>0.3), respectively. Data represented as mean ± SD. Colorbar is uniform in each panel...64 Figure 3.6: Kidney ASL-FISP images from a healthy C57/BL6 mouse at 7 T. (a) Sliceselective (bright-blood) and (b) non-selective (dark blood) ASL-FISP images; (c) M0 FISP image (no inversion); (d) Look-Locker T1 map; and (e) perfusion map. Renal arteries (high perfusion) and renal medulla (low perfusion) are clearly visible in the perfusion map
10 Figure 4.1: (A) Schematic of the MRF-FISP pulse sequence with one line of k-space acquired for each of N images in one MRF scan repetition (N=600 for this initial implementation). (B,C) Flip angle and repetition time variation profiles used to create the MRF acquisition and dictionary Figure 4.2: (A) In vitro MRF images from four MnCl2-doped phantoms with varying T1 and T2 relaxation times. Images acquired at multiple timepoints (image 40, 80, and 150) show evolving contrast during the dynamic MRF acquisition. (B) Signal evolution profiles from the four phantoms with acquired data profile (in red) and corresponding matched dictionary profile (in blue). Arrows delineate the specific timepoints for the three phantom images Figure 4.3: (A,B,C) Quantitative axial phantom MRF maps of T1, T2, and M0. (D,E,F) T1, T2 relaxation times and M0 maps from conventional inversion recovery spin echo (IR-SE) and spin echo (SE) acquisitions, respectively for comparison. (G,H,I) Error maps of T1, T2 and M0 (MRF estimate conventional estimate)...82 Figure 4.4: (A) Plots of mean phantom T1, T2 and M0 values from MRF and conventional methods over five scans on different days. Error bars represent one standard deviation in the mean value across all five measurements. (B) Plots of mean voxel-wise standard deviation for T1, T2 and M0 values from MRF and conventional methods as a measure of intrascan uniformity. Error bars represent one standard deviation in the mean value across all five measurements Figure 4.5: MRF T1, T2, M0 maps from (A) in vivo healthy mouse kidneys and (B) the same imaging slice after euthanasia. (C) Acquired (in red) and matched (in blue) MRF signal evolution profiles of mouse kidney cortex from in vivo and (D) ex vivo scans. 7
11 Note the overall similarity of the matched MRF profiles and resistance to respiratory motion spikes (arrows) visible in the acquired in vivo MRF profile, but not visible in the ex vivo MRF profile Figure 4.6: (A,B,C) In vivo MRF T1, T2 and M0 maps of a mouse brain with a GFPexpressing Gli36Δ5 tumor. (D) T2-weighted image as an anatomic and tumor contrast reference. (E) Coronal brightfield image (gray scale) with GFP overlay and (F) H&E image confirming tumor location (arrows)...87 Figure 4.7: MRF T1 (top row), T2 (middle row) relaxation time and M0 (bottom row) maps of the in vivo mouse brain with implanted tumor shown as a function of the number of acquired and matched MRF images. Note that the T1 and T2 maps are consistent with 300 MRF-FISP images suggesting opportunities for significant reduction in the MRF acquisition time...88 Figure 4.8: Ex vivo MRF T1, T2 and M0 maps of an excised mouse brain and glioma using either (A) sinc7 or (B) hermite radiofrequency (RF) excitation pulses for the MRF- FISP acquisition. The RF waveforms and measured slice profiles are also included. Note the sensitivity of the MRF technique, in particular the T2 estimates, to slice profile errors
12 Development of Quantitative Fast Imaging with Steady-State Free Precession (FISP) Techniques for High Field Preclinical Magnetic Resonance Imaging Abstract by YING GAO Preclinical magnetic resonance imaging (MRI) is critical in the investigation of pathophysiology and therapies. High-field ( 4.7 T) preclinical MRI scanners have been developed to quantitatively evaluate disease status and the efficacy of novel therapies in a wide variety of rodent models with rigorous validation. High magnetic fields provide increased signal-to-noise ratio (SNR) that can be traded for spatial / temporal resolution which is extremely valuable in preclinical imaging. However, high-field preclinical MRI systems also face challenges that affect imaging quality, in which B0 inhomogenieties are a major source of artifacts and entail difficulties of conventional clinical acquisitions in low-field settings applied at high fields. In this work, three MRI techniques were developed for high-field preclinical MRI scanners exploiting a fast imaging with steadystate free precession (FISP) acquisition scheme as a technical core to circumvent the significant off-resonance artifacts on high field MRI scanners. First, a FISP-based Look- Locker T1 measurement was developed as a non-invasive and sensitive imaging marker to quantitatively assess autosomal recessive polycystic kidney disease (ARPKD) liver disease in the PCK rat model of ARPKD. Second, a rapid and quantitative arterial spin labeling (ASL)-FISP technique was developed for high-field preclinical MRI scanners to provide perfusion-weighted images in less than 2 s with minimal image artifacts and further investigated in neuroimaging. Third, an initial preclinical 7 T MRI 9
13 implementation of the highly novel magnetic resonance fingerprinting (MRF) methodology was developed and in vivo preclinical MRF results in mouse kidneys and brain tumor models demonstrated an inherent resistance to respiratory motion artifacts as well as sensitivity to known pathology. Overall, FISP-based quantitative MRI techniques developed here will create a wealth of opportunities for preclinical imaging applications and inform future clinical imaging studies. 10
14 Chapter 1 Introduction Magnetic resonance imaging (MRI) is an advanced and versatile imaging modality that has a wealth of clinical and preclinical imaging applications. MRI has a wide range of sensitivities to relaxation, motion, flow, susceptibility, chemical shift, and magnetization transfer, among other physiological and physical parameters. A tremendous amount of MRI technique development has occurred over the past three decades. This chapter introduces the basics of MRI including signal origins, image acquisition, and an overview of quantitative MRI techniques. In addition, challenges and opportunities for high-field preclinical MRI are discussed followed by an overview of this dissertation. 1.1 Overview of an MRI Signal Acquisition The sensitivity of MRI to target tissue properties is controlled by the design of a pulse sequence. A deliberate sequence design is critical to emphasize physical / physiological parameters of interest in imaging for analysis while minimizing others. An MRI pulse sequence has three primary components, which are radiofrequency (RF) excitation / refocusing / preparation pulses, magnetic field gradients, and sampling of the MRI signal. The role they play in MRI signal creation, manipulation, and acquisition is described in this section. RF excitation. The equilibrium magnetization (M0) of the nuclei is aligned along the direction of the static magnetic field (B0) that is usually denoted as the z direction when the subject is positioned in an MRI scanner. The spins of the nucleus of interest precess at an angular frequency ( that is equal to the product of the main field strength (B0) and the nucleus-dependent gyromagnetic ratio () (Eq. 1.1). 11
15 (1.1) An RF field (B1) is produced by the transmit coil (i.e., an RF pulse) for a short time duration () to tip the magnetization to the transverse plane (Mxy, ). The flip angle () is determined by the strength of the B1 field and pulse duration (). The frequency of the RF pulse is tuned to the Larmor frequency () so that the rotation of the precessing spins away from the longitudinal direction is maximally effective. Pulse shape is an important property of RF pulses and it needs to be carefully selected and designed for different applications and pulse sequence optimization. Three major types of RF pulses are rectangular / hard pulses, shaped / soft pulses, and adiabatic pulses. Hard pulses have a rect function-shaped waveform and the pulse duration can be shortened to a very small value. As such, hard pulses have a broad bandwidth and can be used when no spatial selection is required, e.g., 3D volume excitation. In contrast, a shaped pulse is widely used for slice-selective excitation, some examples include: gauss, hermite, and sinc pulses. Among them, sinc pulses with more lobes have a more uniform slice profile. The spatial uniformity of the flip angle across the imaging slice is dependent on the RF pulse shape as well as B1 field inhomogeneities derived from the transmit coil. As discussed later in this chapter, high-field preclinical MRI is challenged by B1 inhomogeneities. Adiabatic pulses are designed to be robust to the variation in B1 field and can be applied to uniformly excite, invert (i.e., 180 excitation from +z to -z axis) and refocus (i.e., 180 rotation in the transverse plane) magnetization. Gradient fields and k-space. Magnetic gradient fields play a pivotal role in linking the MRI signal to the spatial locations of the precessing magnetization, which is the basis of imaging via MRI (1). Specifically, three independent gradient coils are housed within the 12
16 MRI systems and produce orthogonal, linear, and spatially varying magnetic field gradients (Gx, Gy, Gz). The direction of the gradient fields is always parallel to the B0 field and the local Larmor frequency becomes location-dependent with the addition of gradient fields (Eq. 1.2). (1.2) Magnetic field gradients enable the spatial encoding of the MRI signal. MRI signal originating from the precessing transverse magnetization is detected by a receiver coil. The raw MRI signal that corresponds to the spatial frequency and phase information of each pixel in the MRI image is sampled and stored in a diagram called k-space. An MRI image can be reconstructed from the k-space data by the inverse Fourier transform. The center of k-space has low spatial frequency information that composes the main contrast of the image, whereas the edge of k-space contains high spatial frequency information about sharp edges and fine details in the image (2,3). The traversal of k-space is determined by the integral of the gradient waveform over time (Eq. 1.3) and the traversing curve is called the k-space trajectory. (1.3) It s worth noting that the relationship between sampling distance in k-space () and the extent of the imaging subject, known as field of view (FOV), needs to satisfy the Nyquist sampling criterion to avoid the aliasing artifacts (i.e., images overlapping) that is given in Equation 1.4. (1.4) Conventionally, k-space is filled in a line-by-line basis that is referred to as a Cartesian trajectory. Cartesian sampling takes advantage of the grid-like pattern and can be 13
17 implemented and reconstructed in a straightforward procedure. However, Cartesian sampling is sensitive to flow and motion artifacts. On the other hand, non-cartesian trajectories, e.g., radial and spiral trajectories, can be less sensitive to motion and flow (4-7). Non-Cartesian trajectories also have high time efficiency and can be employed in a fast acquisition scheme. FID and relaxation. As alluded to earlier in this section, MRI signals stem from the precession of Mxy at the Larmor frequency. According to the Faraday s law of electromagnetic induction, this changing magnetic field induces a voltage in the receiver coil placed close to the Mxy source. The form of an MRI signal is determined by the design of the RF pulse sequence using a combination of RF pulses and gradients. The simplest MRI experiment that only applies one excitation pulse and detects a global signal with no magnetic field gradients applied is defined as free induction decay (FID). FID signal cannot persist forever due to the relaxation mechanisms in the spin system. The transverse magnetization tends to decay or dephase as a result of thermodynamic effects (T2) and external field inhomogeneities (T2 ). The resultant relaxation time (T2 * ) is shorter than the T2 relaxation time that is given in Equation 1.5. (1.5) In addition to T2 relaxation, the longitudinal magnetization (Mz) tends to regrow to the equilibrium state (M0) through T1 relaxation. More details about T1 and T2 relaxation quantification are presented in Section SNR vs. time vs. resolution. Signal-to-noise ratio (SNR) is a key metric in MRI experiments that depicts to what degree the imaging quality is affected by noise. A high SNR is desirable for tissue differentiation and accurate MRI parameter quantification. 14
18 SNR can be measured by the ratio of the voxel signal (s) to the standard deviation of the background noise (σ) as shown in Equation 1.6. (1.6) SNR on a per voxel basis is dependent on multiple image acquisition parameters including the spatial resolution (), k-space matrix size (), the number of signal averages ( and the readout bandwidth ( as given in Equation 1.7. (1.7) Spatial resolution defines the size of features that can be distinguished in an MRI image. It can be seen from Equation 1.7 that SNR can be traded for high spatial resolution which is critically needed for preclinical MRI applications involving 20 gram mice instead of 70 kg human subjects. In addition, scan time can be reduced by acquiring fewer averages or lines of k-space if SNR is sufficient. In this way, increasing the SNR of MRI images facilitates more effective and accurate tissue characterization. Increased temporal resolution favors a variety of MRI applications such as cardiovascular MRI, functional MRI (fmri), and dynamic contrast-enhanced MRI studies. The interdependent relationship among SNR, scan time, and resolution in high-field preclinical imaging is revisited in Section Basic MRI Acquisition Strategies Efforts have been made to design pulse sequences in order to best delineate tissue of interest by manipulating pulse sequence components and parameters, such as RF pulse shape, echo time (TE), repetition time (TR), etc. Among them, spin echo (SE) and gradient echo (GRE) sequences are the two main classes. In this section, common SE and GRE pulse sequences with different flavors are described at length. 15
19 1.2.1 Spin Echo A spin echo (SE) sequence creates spin echo signals by applying two RF pulses, typically a 90 excitation pulse followed by a 180 refocusing pulse. The refocusing pulse is able to compensate for the phase shift due to static field inhomogeneities by inverting the accumulated phase of the spins. As a result, the spin echo sequence forms an echo that is weighted by T2 relaxation times instead of T2 * (). Image contrast in SE acquisitions is primarily determined by two acquisition parameters: echo time (TE) and repetition time (TR). The time interval between the center of the excitation pulse and the peak signal is defined as TE and the sequence is repeated at each TR to fill one k-space line. A combination of short TR (~1000 ms) and short TE (~10ms) will give a T1-weighted image while long TR (~3000 ms) and long TE (~80 ms) will highlight tissues according to their T2 values. A proton density-weighted image can be acquired by suppressing both T1 and T2 effects with a long TR (~3000 ms) and a short TE (~10ms). Conventional single-echo spin echo sequence recording only one echo signal filling a single line of k-space in each TR interval is limited by a long acquisition time. As such, a fast spin echo (FSE) or turbo SE method was developed to reduce the overall acquisition time (8). In FSE imaging, additional 180 pulses are applied to generate multiple spin echoes and fill other k-space lines in a single TR interval as long as the transverse magnetization has not completely diminished as a result of T2 decay. The number of echoes received during each TR is known as echo train length (ETL). To acquire an image of the same matrix size, FSE requires fewer TR periods than for a single-echo SE acquisition, resulting in reduced total acquisition time. Image contrast in FSE is 16
20 determined by the effective TE (TEeff) when the central k-space line is acquired. In addition, FSE generally has less sensitivity to motion because of its shorter acquisition time. Multi-echo spin echo acquisitions can also be used for T2 mapping. Instead of filling different k-space lines in one image for each TR as in the FSE sequence, a single line of k-space is acquired for multiple images at each TR interval. As a result, more images are acquired with varying TEs. Multi-echo SE has high time efficiency in comparison with sequential SE but reduced accuracy due to imaging artifacts Gradient Echo In gradient echo (GRE) sequences, a dephasing gradient is applied followed by a rephasing gradient with the same strength but reversed polarity to form a gradient echo. GRE imaging is normally T2 * -weighted instead of T2-weighted due to the absence of a 180 refocusing pulse that is employed in the spin echo (SE) sequence to create an echo as well as eliminate off-resonance effects. As a result, GRE is more subject to offresonance effects than SE. One important characteristic of GRE imaging is its flexibility in reducing acquisition time. In GRE sequences, only one RF excitation pulse is applied so that the echo time (TE) during which the peak signal from a gradient echo is achieved can be minimized by sequence optimization. In addition, low-flip-angle excitation in GRE results in increased remnant longitudinal magnetization that allows a faster recovery of magnetization and shorter repetition time (TR). Overall, short TRs enable GRE acquisitions to constitute the basis for many fast imaging techniques. When preceded by magnetization preparation, GRE can be manipulated for multiple quantitative MRI applications with reduced motion 17
21 artifacts, such as diffusion, perfusion, dynamic contrast-enhanced imaging, and cardiac imaging. In fast imaging in the steady state, a steady state of magnetization is approached by continuous perturbation of RF excitation pulses with rapid repetition (9). A finite number of pulses are applied to the spin system before reaching the steady state in a time that depends on the flip angle of RF pulses, TR, and relaxation behaviors of the tissue (T1, T2, and T2 * ). As a result of short repetition time (TR < T2) in a fast imaging setting, the transverse magnetization (Mxy) will not completely decay prior to the new excitation and will participate in the next repetition if not eliminated or spoiled by design. Gradient echo sequences with a steady-state approach are broadly categorized into two classes (coherent and incoherent steady state) depending on whether the residual transverse magnetization contributes to the signal in the next acquisition cycle. Fast low-angle shot (FLASH) is a steady-state incoherent sequence and one of the most commonly used spoiled gradient-echo sequences in fast imaging (10). The transverse magnetization in FLASH is spoiled by variable RF phase and / or varying the spoiler gradients between successive TR cycles. As a result, the longitudinal magnetization reaches a steady state that is determined by flip angle, TR, and T1 values. Heavy T1 contrast is normally seen in FLASH images and fluids with long T1 values, such as cerebrospinal fluid (CSF) and bile, which appear dark on FLASH images. A small flip angle is required to compensate for the reduced signal-to-noise ratio (SNR) in rapid acquisition (short TR). FLASH sequence has been widely used in imaging of the brain, abdomen, breast, etc. Steady-state free precession (SSFP) acquisitions are a class of coherent steady-state 18
22 acquisitions where the remnant Mxy is maintained and involved in signal formation in the next TR (11). In True fast imaging with steady-state free precession (FISP), gradients in all three spatial directions (slice, phase, and frequency encoding) are fully balanced (i.e., a zero net phase) leading to a high SNR / acquisition time. With an extremely short TR (TR T1 or T2), the contrast of True FISP images is dependent on the ratio of T2 / T1. Solid tissues with small T2 / T1 ratio appear dark while fat and fluids (e.g., CSF, blood) are bright and highlighted. TE is usually set to be one half of the TR and this gives true FISP a spin-echo-like behavior and T2 sensitivity rather than T2 *. A challenge in True FISP acquisition is its sensitivity to B0 inhomogenieties resulting in well-known banding artifacts. These artifacts are extremely problematic on high field MRI scanners but can be ameliorated by either shortening the TR, increasing readout bandwidths, and/or optimizing the homogeneity of the main magnetic field. In addition, a reconstruction technique that combines multiple True FISP images acquired with different RF pulse phase profiles can be used eliminate banding artifacts (12). Fast imaging with steady-state free precession (FISP) sequence is another variant in the family of steady-state coherent gradient echo techniques (13). Unlike True FISP with zero gradient moments in three directions, FISP typically only has the gradients refocused in the phase-encoding direction. Image contrast in FISP is dependent on TR, TE, flip angle, as well as T1, T2, and T2 *. FISP is typically implemented with a short TR and TE to provide increased SNR / time and reduced T2 * effects, respectively. A small flip angle makes FISP images largely proton density weighted while image contrast becomes more T2 / T1 dependent as flip angle is increased. FISP has a great advantage in high-field imaging compared to True FISP due to its 19
23 invulnerability to B0 inhomogeneities (14). With prolonged T1 values at high fields, FISP images present better SNR than FLASH. In this work, the FISP acquisition has been employed in multiple MRI technique developments including rapid T1 mapping, arterial spin labeling (ASL), and MR fingerprinting (MRF) Echo-Planar Imaging Echo-planar imaging is an ultrafast imaging technique that enables a 2D image acquisitions < 1 second (15). In a standard gradient-echo (GRE) EPI sequence, a single excitation pulse is followed by recurrent readout gradients switching between negative and positive lobes resulting in a train of gradient echoes. At the same time, a blipped low-amplitude phase-encoding gradient is inserted in between each echo to traverse k- space in the phase encode direction. A single-shot EPI acquisition collects data from the entire k-space in a single excitation while a segment of k-space is traversed in one excitation in a multi-shot EPI. As a trade-off for rapid acquisition, EPI has a high demand for gradient performance because of the fast readout and frequent gradient switching. Most importantly, EPI is highly sensitive to static magnetic field (B0) inhomogeneities, making EPI acquisitions particularly problematic for high-field MRI imaging. Specifically, field variation imposes an extra gradient to the applied gradient that results in echo deviation from its desired spatial location. This k-space shifting results in both image distortion / ghosting. In addition, rapid switching of the magnetic field gradients gives rise to eddy currents that have the same effects as B0 inhomogeneities in EPI images. A standard gradient-echo EPI sequence can be coupled with magnetization preparation modules to produce different image contrast. Spin-echo (SE) EPI and inversion-recovery 20
24 (IR) EPI are two common EPI sequences. In spin-echo EPI, a pair of RF pulses are substituted for a single RF excitation pulse. K-space lines in spin-echo EPI are still sampled with gradient refocusing, but the envelope formed by gradient echo train follows spin echo instead of pure gradient echoes. Due to the superb time resolution of EPI, it has a broad application in rapid imaging applications such as diffusion imaging, perfusion imaging, cardiac imaging, and functional MRI. 1.3 Overview of Quantitative MRI Conventional MRI is excellent at delineating tissues on an anatomical and morphological level. However, signal-intensity-based anatomical MRI can only interpret tissue contrast qualitatively. MR signal intensity is determined by intrinsic tissue properties that include proton density, relaxation times (T1, T2, T2 * ), diffusivity, etc., and how they play into the pulse sequence design. In addition, external factors such as hardware and software performance of the imaging system also influence MR signal, resulting in varied and biased information across subjects or for the same subject enrolled in a longitudinal study. Further, the MRI signal intensity does not have any specific physiological meaning. As a consequence, qualitative MRI provides a very limited opportunity to quantitatively compare different MRI scans. Quantitative MRI measures parameters that directly assess tissue properties such as relaxation times (T1, T2, T2 * ), perfusion, diffusion, etc. In quantitative MRI experiments, multiple images are acquired to estimate the particular MRI parameters using specific acquisitions. Importantly, quantitative MRI provides an objective measurement that can be used as an unbiased link between MRI assessments and pathophysiology. In this section, multiple quantitative MRI techniques including T1 and T2 relaxometry, perfusion 21
25 MRI, compressed sensing, and a novel multi-parametric quantification technique, Magnetic Resonance Fingerprinting (MRF), are discussed T1 and T2 Quantification MRI relaxometry is the quantitative measurement of magnetic relaxation times (T1, T2, T2 * ). T1 relaxation time is defined as the time when the longitudinal magnetization has recovered to 63% of its equilibrium state (i.e., maximum magnetization M0 parallel to static field B0) following a 90 RF pulse due to transfer of energy from the spin system to its external environment. T2 relaxation time is a measure when 63% of the original transverse magnetization has decayed due to dephasing of spins. As discussed above, T2 * is similar to T2 but combines both transverse relaxation (T2) with B0 inhomogeneities. Quantitative relaxometry as a straightforward and fundamental MRI tool has been extensively applied in clinical and preclinical studies for a wide range of diseases in the brain, body, heart, and other tissues. T1 relaxometry plays an important role in cancer imaging. Conventional qualitative T1-weighted images can be acquired rapidly in dynamic contrast-enhanced (DCE) MRI acquisitions to assess tumor location and extent. In addition, these DCE MRI data can be modeled to quantitatively assess tumor vascular permeability and perfusion. In contrast, quantitative T1 mapping offers the opportunity for localization of tumor-tissue boundaries that can be used to guide treatment and/or resection (16,17). T1 measurement also plays a key role in quantitative assessments of tumor perfusion in both DCE MRI and noncontrast arterial spin labeling (ASL) acquisitions (18-20). In addition, T1 quantification can be used to differentiate pathologies (edema, biliary dilatation) with long T1 relaxation times that would otherwise be difficult to distinguish using qualitative imaging 22
26 techniques. Inversion recovery-spin echo (IR-SE) acquisitions are considered the gold standard method for T1 quantification. In this sequence, an inversion (180 ) pulse is applied followed by a conventional SE acquisition with a series of varied inversion times (TI) in between the inversion and acquisition to generate multiple T1-weighted images enable T1 quantification. Based on the Bloch equation, T1 is estimated via curve fitting to Equation 1.8. (1.8) In this exponential model, SI(n) is the measured signal intensity at n th TI; a and b are parameters dependent on equilibrium magnetization and receiver gain. Accurate T1 estimation from the IR-SE acquisition typically requires the repetition time (TR) > T1 for multiple acquisitions leading to considerably long acquisition times. To circumvent this acquisition limitation and adapt T1 measurement to rapidly changing environments such as perfusion and cardiac imaging, efforts have been expanded to develop rapid T1 mapping while maintaining its accuracy. The Look-Locker sequence was developed to rapidly measure T1 values (21,22). Instead of acquiring only one data point after each inversion pulse at a fixed TI, multiple data points along the longitudinal magnetization recovery curve are sampled by a train of radiofrequency (RF) excitation pulses (flip angle ) with a time interval of. Without waiting for equilibrium magnetization before initiating the pulse train, acquisition time can be reduced significantly to the order of seconds. The RF-pulse train drives the longitudinal magnetization to a new steady state that is lower than the original equilibrium state (M0). As such, the measured T1 denoted as T1 * is shorter than the real T1 relaxation time. This 23
27 approach has been applied for a variety of applications and with multiple different models to estimate T1 (23-25). T2 relaxtion time estimates can be obtained from T2-weighted images acquired at multiple echo times. After acquiring images, the T2 relaxation time maps can be estimated by a nonlinear curve fitting to an exponential model as shown in the following Equation: (1.9) where SI(n) is the measured signals at the n th echo time (TE), and T2 are fitting parameters. This model can be simplified to a linear problem by taking the natural logarithm of SI(n). Noteworthy, when the voxels of interest have more than one tissue component, a bi-exponential or multi-exponential model can be used for more accurate parameter estimation. As mentioned in Section 1.2.1, multi-echo spin echo sequence is commonly used for T2 measurement. Instead of repeating the SE experiments at different TEs, recording a train of echoes with consecutive 180 refocusing pulses at each TR can save scan time substantially. Imperfect refocusing pulses due to B1 field inhomogeneity result in unwanted FIDs and stimulated echoes. To eliminate the undesired signals while preserving the spin echo signals, a pair of crusher gradients is employed immediately before and after each refocusing pulse, respectively. In addition, refocusing imperfections lead to reduced transverse magnetization and image artifacts. A phase cycling approach referred to as Carr-Purcell-Meiboom-Gill (CPMG) sequence that shifts the phases of the excitation pulse and all the refocusing pulses by 90 is used to compensate for the signal loss (26). 24
28 1.3.2 Perfusion MRI Perfusion is the physiological process of the body delivering blood to a capillary bed in its tissues (i.e., capillary blood flow). Perfusion is altered in many pathophysiologic conditions including stroke, cancer, neurovascular and neurodegenerative diseases, cardiac disorders, and many other chronic diseases of the body (e.g., chronic kidney, liver, and lung disease). MRI perfusion techniques have been available for over 20 years. They can be classified into two major categories based on the type of contrast agents used for imaging: exogenous contrast agent (dynamic susceptibility contrast-enhanced (DSC) and dynamic contrast-enhanced (DCE) MR perfusion) and endogenous contrast agent (arterial spin labeling (ASL) MR perfusion). DSC and DCE techniques usually use a non-diffusible gadolinium-based contrast agent as an exogenous, intravascular tracer for dynamic MR imaging (27-30). DSC MRI capitalizes on the susceptibility effect of paramagnetic contrast agents and produces T2 * - weighted images with decreased signal intensity dependent on the local concentration. The signal intensity-time curve is measured and transformed into a concentration-time curve to derive blood volume and blood flow in a region of interest (ROI) (27). As described above, DCE MRI acquires serial T1-weighted images before, during, and after injection of the contrast medium (27). Data analysis within the framework of pharmacokinetic modeling is then typically performed to estimate a wide variety of perfusion metrics including K trans (a measure of blood flow and tissue permeability), time to peak image signal intensity, etc (18,19). ASL MRI is a non-contrast perfusion MRI technique that has been shown to provide quantitative assessments of tissue perfusion in multiple clinical imaging applications 25
29 including brain (31-33), kidney (34-37), lung (38-42), and liver (43). One major advantage of ASL over conventional DCE MRI perfusion techniques is the lack of exogenous and potentially toxic, paramagnetic contrast agents. The use of magnetically labeled endogenous blood as signal sources to obtain tissue perfusion information is especially important for imaging patients with chronic kidney diseases, which can be a contraindication for gadolinium-based MRI perfusion methods (44). This attribute is also important for longitudinal preclinical imaging applications, as multiple tail-vein injections and/or catheterizations can cause local inflammation and necrosis resulting in reduced access to veins. ASL MRI techniques generate blood flow contrast between multiple images using a wide variety of blood labeling methods (20,45-49). A typical ASL MRI acquisition combines an ASL preparation phase followed by a rapid imaging readout to capture the blood flow-weighted contrast (Figure 1.1). 26
30 Figure 1.1: Schematic diagram of arterial spin labeling-fast imaging with steady-state free precession (ASL-FISP) acquisition. A flow-sensitive alternating inversion recovery (FAIR) ASL preparation is combined with a centrically encoded FISP acquisition. This acquisition is repeated for both a slice-selective inversion (with shaded gradient) and a non-selective inversion (without the shaded gradient) to generate the perfusion contrast. Note that all lines of k-space are acquired following a single ASL preparation. ADC, analog-to-digital converter. It is important to note that a large majority of the imaging developments have focused on optimizing the preparation phase of the ASL acquisition. As a result, a wide variety of preclinical and clinical ASL MRI techniques have been developed. These ASL techniques can be broadly grouped into continuous (CASL) (45,46,49) and pulsed (PASL) (20,47,48) categories. A hybrid of these two techniques, pseudo-continuous ASL (pcasl) has also been developed recently (50-52). Each of these specialized techniques offers advantages for specific imaging applications Compressed Sensing Compressed sensing (CS) is an emerging signal processing technique that allows reliable signal reconstruction from a desired small number of samples to accelerate image acquisition (53). The Nyquist Shannon sampling theorem requires a minimal sampling 27
31 rate for a perfect signal reconstruction, which restricts the flexibility to reduce acquisition time and data size. The breakthrough CS technique demonstrates that signal with sparsity (i.e., compressibility) in at least one domain and incoherent undersampling artifacts can be retrieved without satisfying the sampling theorem. Most importantly, CS has significantly contributed to the evolution of fast imaging in MRI. MRI images are sparse either in the image domain (e.g., angiograms) or in a transform domain (e.g., by Fourier transform, finite differences, wavelet transform, etc.) (54). In addition to sparsity, the unique acquisition scheme in MRI that employs gradients for spatial encoding in k-space empowers undersampling flexibility, for example, randomly undersampled Cartesian trajactory, radial, variable density spiral trajectories (54). As such, the scan time can be reduced significantly while the image quality is maintained with nonlinear reconstruction. CS MRI has broad applications in brain imaging, dynamic cardiac imaging, 3-D angiography, etc. A model-based CS reconstruction was developed for rapid MRI parameter mapping (55). Specifically, prior knowledge of the signal model can be exploited to learn an overdetermined dictionary and obtain T1 and T2 maps from highly reduced data (55). Its accuracy and efficiency have been validated in both human and small animal imaging (55,56) Magnetic Resonance Fingerprinting As described above, conventional MRI quantification methods are mostly based on linear or nonlinear curve fitting to various MRI models (57,58). The implementation of these established model-based methods, such as T1 and T2 relaxation time estimation, are straightforward. However, these conventional quantification methods are susceptible to 28
32 multiple sources of errors including cardiac and respiratory motion artifacts (59-61) and inhomogeneity in the static magnetic field (62). Importantly, the potential for these errors are significantly increased on high field preclinical MRI scanners where B0 inhomogeneities are increased; rodent heart rates can be as high as beats / minute; and breathholds are not possible. In addition, temporal errors can be observed in preclinical studies that require multiple imaging parameter estimates (e.g. diffusion and perfusion) as extended periods of anesthesia can cause physiologic changes during sequential scans. Magnetic Resonance Fingerprinting (MRF) is an entirely new approach to MR quantification (63) and was pioneered by the MRI research group at CWRU. The original MRF technique was developed for human MRI scanners and was shown to simultaneously generate quantitative maps of T1 and T2 relaxation times and proton density (M0) of human brains in ~10 seconds with resistance to bulk motion artifacts (63). In MRF, several hundred images are acquired rapidly with a priori variation in selected acquisition parameters (i.e., tip angle / repetition time). This variation results in acquired tissue-specific signal evolution profiles for each imaging voxel. These acquired signal evolution profiles are then matched, on a pixel-by-pixel basis, to a single best-matched theoretical signal evolution profile in a pre-calculated database (i.e., MRF dictionary) in a manner similar to human fingerprinting used in law enforcement. In MRF, the matched signal evolution profile provides information on tissue-specific MRI parameters (i.e., T1/T2/M0, tissue perfusion, diffusivity, etc.) that can then be used to identify pathophysiology such as gliomas, liver lesions, acute infections, alterations in pulmonary perfusion and more. 29
33 1.4 High-field Preclinical MRI As discussed in the previous sections, MRI is a versatile imaging technique that allows quantitative tissue characterization with no exposure to ionizing radiation. The past few decades have witnessed a significant revolution in preclinical MRI hardware as well as image acquisition and reconstruction techniques. Specifically, high field ( 4.7 T) preclinical MRI scanners have been developed to provide MRI measures of disease in rodent models. High magnetic fields provide increased SNR that can be traded for spatial / temporal resolution. In addition, preclinical MRI scanners are normally equipped with gradients and RF coils that have improved performance compared with clinical settings. However, high-field preclinical MRI systems also face challenges that affect imaging quality, such as susceptibility to artifacts arising from B0 / B1 field inhomogeneities. In this section, a brief overview of preclinical imaging is presented followed by a discussion of challenges and opportunities of preclinical MRI on high-field scanners Preclinical Imaging As a prerequisite step to clinical trials, preclinical studies that comprise cell studies (i.e., in vitro) and animal studies (i.e., in vivo) are widely employed in drug development and biological investigation of disease and pathophysiology. The primary purpose of preclinical studies is to determine therapeutic efficacy and safety prior to first-in-man studies. The development of animal models replicating human disease creates an opportunity to investigate different therapies (e.g., PCK rat model of autosomal recessive polycystic kidney disease (ARPKD), mdx mouse model of muscular dystrophy, and CNS-1 rodent model of glioblastoma multiforme (GBM)) (64-66). In addition, these 30
34 animal models provide the basis to better understand the cellular and molecular mechanisms underlying disease initiation and progression. The utilization of imaging techniques, including MRI, as outcomes in clinical trials motivates the development of preclinical imaging biomarkers that can be rigorously validated with histological / biochemical assessments. This role makes preclinical MRI research studies almost entirely quantitative by nature in contrast to clinical studies which are largely qualitative. Over the past few decades, preclinical imaging has been increasingly leveraged to advance basic science research in animal models on therapeutic interventions because of its potential to accelerate and optimize the process of drug discovery and development. Many in vivo imaging techniques are either non-invasive or minimally invasive allowing repeated observations and longitudinal imaging studies to monitor in vivo therapeutic response as well as disease progression. Prevalent imaging methodologies include magnetic resonance imaging (MRI), ultrasound, computed tomography (CT), optical imaging (e.g., fluorescence and bioluminescence), positron emission tomography (PET), and single photon emission computed tomography (SPECT). MRI, in particular, is one of the leading preclinical imaging methodologies due to its remarkable soft tissue contrast, lack of ionizing radiation, high spatial resolution (~ microns typical), and opportunities for rapid imaging. As described above, MRI provides not only anatomical and morphological information but also quantitative functional and molecular data (e.g., diffusion and perfusion MRI) High-field Preclinical MRI Challenges and Opportunities As mentioned earlier in this chapter, SNR, spatial resolution, and scan time are three interdependent factors that are balanced in quantitative MRI for accurate and efficient 31
35 measurements. The typical spatial resolution for mouse MRI imaging applications is approximately 100 m 100 m 1 mm, while resolutions of 1 mm 1 mm 5 mm may be typical for human MRI imaging applications. Assuming that all the factors in Equation 1.7 except for the spatial resolution are the same, the SNR/voxel of a human MRI outweighs that of the mouse images by a factor of 500. Overall, the need for improved spatial resolution in mouse imaging applications results in a significant decrease in SNR for mouse MRI. It is this key factor that drives much of the acquisition development in preclinical MRI applications. A straightforward approach to boost SNR is to repeat the experiments by increasing the number of signal averages (). However, since SNR is proportional to the square root of (Eq. 1.7), doubling the SNR requires a quadrupling of the acquisition time. Fortunately, this loss in SNR is offset in part by the use of small RF coils and increased SNR from higher magnetic fields. Accomplishing the resolution and timing goals of preclinical MRI necessitates the use high performance gradients capable of creating strong spatially varying magnetic fields. The small bore diameters ( mm) of preclinical systems enable the installation of gradient coils with increased strengths ranging from 200 mt/m to 1000 mt/m compared to 40 mt/m - 80 mt/m in clinical systems. The benefits of high-performance gradient coils are two-fold. First, for a given k value (), the gradient duration () can be minimized by pushing the gradient amplitude () to its limit. TE and scan time can be shortened as a consequence of reduced gradient duration. Shorter TE is critical in high-field imaging to mitigate the effects of rapid T2 * signal decay. Second, because of the reciprocal relationship between spatial resolution and gradient amplitude for a given gradient 32
36 duration, increased gradient amplitudes and slew rates can be used to achieve desired resolution without onerous increases in acquisition time. B0 inhomogenieties are a major source of artifacts on high field preclinical MRI scanners and make conventional clinical acquisitions in low-field settings difficult at high fields. Specifically, B0 inhomogeneities result in increased distortion / ghosting, and banding artifacts from echo-planar imaging (EPI) and true fast imaging with steady-state free precession (true FISP) imaging techniques, respectively. These artifacts are particularly problematic for rodent body imaging applications, where cardiac and respiratory motion, as well as large adipose tissue depots, can make precise shimming difficult. This study will explore fast imaging with steady-state free precession (FISP) readout as a promising alternative that is immune to B0 inhomogeneities in a variety of MRI techniques and applications at high fields. When the RF field penetrates into the subject, the RF wavelength is decreased due to the dielectric properties of tissues. When the wavelength of RF field is on the order of subject dimension, abnormal dark or bright areas can be seen at the center of the images. Dielectric effects are commonly perceived as the cause of B1 inhomogeneities. Inhomogeneous B1 fields translate to spatial variation in the flip angle that can be detrimental to MRI quantification. In this work, schemes that minimize B1 inhomogeneity effects are incorporated into the sequence design. 1.5 Overview of Dissertation This work is focused on MRI technique developments to overcome challenges faced in high-field preclinical MRI. Specifically, three quantitative MRI techniques including T1 relaxation time quantification, quantitative perfusion assessment, and magnetic resonance 33
37 fingerprinting (MRF) are explored and evaluated in animal models of diseases on highfield preclinical MRI scanners. The following chapters of the dissertation are organized accordingly FISP-based Look-Locker T1 Measurement Chapter 2 describes an initial investigation of T1 relaxation time as a potential imaging biomarker to quantitatively assess the two primary pathologic hallmarks of Autosomal Recessive Polycystic Kidney Disease (ARPKD) liver disease: biliary dilatation and periportal fibrosis in the PCK rat model of ARPKD. ARPKD is a potentially lethal multi-organ disease affecting both the kidneys and the liver. Unfortunately, there are currently no non-invasive methods to monitor liver disease progression in ARPKD patients, limiting the study of potential therapeutic interventions. T1 relaxation time results were obtained for control rats, Sprague-Dawley (SD), and PCK rats at 3 months of age using a FISP-based Look-Locker acquisition on a Bruker Biospec 7 T MRI scanner. Histological and biochemical assessments of bile duct dilatation and hepatic fibrosis were performed for comparison. PCK rats exhibited significantly increased liver T1 values (mean±standard deviation = 935±39 ms) compared to age-matched SD control rats (847±26 ms, p = 0.01). One PCK rat exhibited severe cholangitis (mean T1 = 1413 ms), which occurs periodically in ARPKD patients. The observed increase in the in vivo liver T1 relaxation time correlated significantly with three histological and biochemical indicators of biliary dilatation and fibrosis: bile duct area percent (R=0.85, p=0.002), periportal fibrosis area percent (R=0.82, p=0.004), and hydroxyproline content (R=0.76, p=0.01). These results suggest that hepatic T1 relaxation time may provide a sensitive and non-invasive imaging biomarker to monitor ARPKD liver disease. 34
38 1.5.2 ASL-FISP Chapter 3 details the development of a rapid ASL-FISP MRI acquisition for high field preclinical MRI scanners providing perfusion-weighted images with little or no artifacts in less than 2 seconds. Many previous ASL MRI studies have utilized either EPI or True FISP readouts that are prone to off-resonance artifacts on high field MRI scanners. In this initial implementation, a FAIR ASL preparation was combined with a rapid, centricallyencoded FISP readout. Validation studies on healthy C57/BL6 mice provided consistent estimation of in vivo mouse brain perfusion at 7 T and 9.4 T (249±38 ml/min/100g and 241±17 ml/min/100g, respectively). The utility of this method was further demonstrated in detecting significant perfusion deficits in a C57/BL6 mouse model of ischemic stroke as well as changes in cerebral perfusion in dystrophin-deficient mice (mdx). Reasonable kidney perfusion estimates were also obtained for a healthy C57/BL6 mouse exhibiting differential perfusion in the renal cortex and medulla. Overall, the ASL-FISP technique provides a rapid and quantitative in vivo assessment of tissue perfusion for high field MRI scanners with minimal image artifacts Preclinical MRF Chapter 4 elaborates on the development of an initial preclinical 7 T MRI implementation of the highly novel Magnetic Resonance Fingerprinting (MRF) methodology that has been previously described for clinical imaging applications. Conventional MRI methods are highly susceptible to respiratory and cardiac motion artifacts resulting in potentially inaccurate and misleading data. The MRF technology combines a priori variation in the MRI acquisition parameters with dictionary-based matching of acquired signal evolution profiles to simultaneously generate quantitative maps of T1 and T2 relaxation times and 35
39 proton density. This preclinical MRF acquisition was constructed from a FISP MRI pulse sequence to acquire 600 MRF images with both evolving T1 and T2 weighting in approximately 30 minutes. This initial high field preclinical MRF investigation demonstrated reproducible and differentiated estimates of in vitro phantoms with different relaxation times. In vivo preclinical MRF results in mouse kidneys and brain tumor models demonstrated an inherent resistance to respiratory motion artifacts as well as sensitivity to known pathology. These results suggest that MRF methodology may offer the opportunity for quantification of numerous MRI parameters for a wide variety of preclinical imaging applications Summary and Future Work A summary of this work is presented in Chapter 5 coupled with future directions of further technique developments and potential applications. 36
40 Chapter 2 FISP-based Look-Locker T1 Assessment of a Rat Model of Congenital Hepatic Fibrosis Portions of this chapter were adapted with permission from Gao Y, Erokwu BO, DeSantis DA, Croniger CM, Schur RM, Lu L, Mariappuram J, Dell KM, Flask CA. Initial evaluation of hepatic T1 relaxation time as an imaging marker of liver disease associated with autosomal recessive polycystic kidney disease (ARPKD). NMR in Biomedicine 2016;29(1): Copyright 2015 John Wiley & Sons, Ltd. 2.1 Introduction Autosomal Recessive Polycystic Kidney Disease (ARPKD) is an inherited disease that affects approximately 1/20,000 children in all ethnic groups, and is clinically and histologically distinct from Autosomal Dominant PKD (ADPKD) (67). Fundamentally, ARPKD is a multi-organ disease characterized by both progressive kidney and liver disease. ARPKD kidney disease is characterized by markedly enlarged kidneys with diffuse microscopic collecting duct cysts, usually evident at birth. Approximately 40% of patients develop kidney failure by age 15 (68). ARPKD liver disease is a developmental biliary tract disease characterized by progressive bile duct proliferation and dilatation and dense periportal fibrosis. It is present histologically in all ARPKD patients at birth, but may not be clinically evident until later childhood or early adulthood (69). Importantly, clinically-significant ARPKD liver disease is becoming more prevalent as more patients survive into adulthood following kidney transplantation (70,71). Unfortunately, there are currently no clinically available, disease-specific therapies for ARPKD liver disease and, therefore, treatment is limited to medical or surgical management of complications. Although some novel therapies have shown promise in the PCK rat model of ARPKD (72,73), clinical trials of these therapeutics in ARPKD 37
41 patients have not been possible due to the lack of established methods to safely and accurately monitoring liver disease progression. Liver biopsies are invasive and conventional biochemical clinical measures of liver/biliary tract disease (e.g., serum bilirubin, liver enzymes, serum albumin, gamma-glutamyl transferase), are typically normal until the disease is more advanced (69,74). Alternatively, imaging methodologies, including ultrasound-based elastography, magnetic resonance elastography, and contrastenhanced MRI techniques (such as delayed enhancement MRI), have been used successfully in adults and children to assess liver cirrhosis, predict the presence of esophageal varices, assess hepatic perfusion, and screen for the presence of liver fibrosis in at risk patients (75-80). However, these methodologies have several significant limitations in terms of quantitating the severity of liver disease in ARPKD patients. First, because it is exclusively a fibrocystic biliary tract disease, there is minimal hepatocyte involvement and overt cirrhosis is a very late finding. Second, gadolinium-based contrast studies are not appropriate in ARPKD patients due to concerns about the life-threatening toxicities that can occur from gadolinium chelates in patients with chronic kidney disease (81). Finally, several of these methodologies require specialized equipment and operator expertise that may not be available in many clinical practices. Therefore, a more sensitive, clinically-available, non-invasive marker for assessing liver disease is urgently needed for pediatric and adult ARPKD patients. As described above, one of the key aspects of ARPKD liver disease is increased biliary proliferation and dilatation. Importantly, and in contrast to other more common forms of hepatic fibrosis, the biliary proliferation and dilatation observed in ARPKD liver disease is pathophysiologically linked to periportal fibrosis (74). As the T1 and T2 38
42 relaxation times for bile is significantly higher than liver parenchyma, we hypothesized that MRI relaxometry assessments could be used to provide a straightforward assessment of ARPKD liver disease. In this initial study, we evaluated the capability of T1 MRI to detect liver disease in the PCK rat model of ARPKD (82-84). In vivo T1 relaxometry data were obtained for groups of PCK rats and Sprague-Dawley (SD) control rats at 3 months of age. The T1 data were acquired using a Fast Imaging with Steady-state free Precession (FISP)-based Look- Locker technique that provides T1-weighted images with minimal artifacts on high field MRI scanners (14). The T1 MRI findings were then compared with three histologic and biochemical assays of biliary dilatation and fibrosis for validation. 2.2 Methods All animal experiments were conducted according to approved Institutional Animal Care and Use Committee protocols at Case Western Reserve University Animal models T1 relaxation time assessments were obtained for cohorts of male PCK (n=5) and SD control (n=6) rats at 3 months of age. The PCK rat is a model of human ARPKD, which spontaneously arose in a Sprague Dawley colony. PCK rats harbor a mutation in the rat orthologue of PKHD1, the human gene that encodes fibrocystin. PCK rats develop progressive cystic renal disease as well as biliary dilatation and periportal fibrosis that is consistent with human ARPKD liver disease (82,83). PCK liver disease is evident histologically within a week after birth and becomes severe by 6-7 months of age. A subset of animals develop chronic ascending cholangitis (biliary duct infection) and exhibit more severe, progressive liver disease, which can also occur in humans (85,86). 39
43 All animals were purchased from Charles River Laboratories and were provided with standard rodent chow and water ad libitum. PCK rats and SD rats were scanned at 3 months of age to enable imaging and histological comparisons of PCK rats with normal controls MRI experiments In vivo MRI experiments were conducted on a 7.0 T Bruker Biospec small animal MRI scanner (Bruker Inc., Billerica, MA). Each animal was initially anesthetized with 2-3% isoflurane in oxygen and positioned with its liver at isocenter in a 72-mm cylindrical transmit / receive volume coil to ensure uniform radiofrequency excitation. Animals were provided with 1-3% isoflurane anesthesia continuously throughout the imaging procedure via a nosecone. An animal monitoring and control system (SA Instruments, Stony Brook, NY) was used to maintain each animal s respiration rate (40-60 breaths / minute) and core body temperature (35 ± 1 C). High resolution, axial T2-weighted images were acquired with a multi-echo spin echo acquisition (TR = 2000ms, TE=8ms, spatial resolution = 0.312mm 0.312mm 2mm, number of echoes per excitation = 8) (87). A central region of the liver was selected to limit partial volume effects of the lungs and the gastrointestinal tract, respectively. T1 relaxation time data were acquired with a FISP-based Look-Locker acquisition to limit the image artifacts as well as the effects of noise on the liver T1 assessments (14,65). Briefly, this method combines an initial non-selective Hermite inversion pulse, followed by 8 continuous and sequential FISP image repetitions (linear encoding; single slice; slice thickness, 1.5 mm; matrix, ; field of view, 6 cm 6 cm; spatial resolution, mm mm 1.5 mm; tip angle, 10 ; TR/TE = 3.233/1.616ms; ms/image; 40
44 inversion preparation time = 6.5 ms). Sampling points in the relaxation curve were the time points when the center of k space of the 8 images were acquired (TI = 420 ms, 1248 ms, 2076 ms, 2903 ms, 3731 ms, 4559 ms, 5386 ms, and 6214 ms, respectively). An additional delay of ~13 seconds was implemented between successive averages to allow for full relaxation of the magnetization between averages, thus avoiding systematic errors in T1 estimation and to reduce the gradient duty cycle. A total of 50 averages were acquired over a total acquisition time of 16 minutes and 40 seconds to further limit the effects of noise on the liver T1 data. Respiratory triggering was also applied before the inversion pulse to limit respiratory motion artifacts. Voxel-wise T1 relaxation maps were calculated using previously described methods (23,88): (2.1) where A = M0(T1 * /T1), B = M0(1+T1 * /T1), C = T1 *, Mz is the longitudinal magnetization, TI is the inversion time, M0 is the equilibrium magnetization, and T1 * is the effective relaxation time. A, B and C were obtained with a least-squares error fit to the threeparameter model. T1 values were then calculated by the following equation: (2.2) A region of interest (ROI) was manually selected for each animal covering a large portion of the liver to calculate the mean liver T1 value for each animal. Large blood vessels were avoided in the ROI analysis to limit the effects of vasculature on the T1 relaxation time analysis Histological and biochemical analyses At the completion of the imaging study, animals were euthanized and tissue was collected for histopathologic and biochemical assessments. A portion of the left lateral lobe of the 41
45 liver was excised and fixed in 4% paraformaldehyde and embedded in paraffin as described previously (82). Liver sections (7μm) were stained with Masson s Trichrome (Richard Allan Scientific, Waltham, MA) for detection of collagen. The percentage of collagen staining (for fibrosis assessments) and biliary duct area (for biliary dilatation assessment) were obtained by pixel counting and expressed as the percent of total liver parenchyma. Biochemical hydroxyproline assessments were also performed on the liver specimens, as described in the previous study (89). Cholangitis was identified by visual inspection of the histologic sections for evidence of purulent material or inflammatory cells within the bile ducts (85) Statistical analyses Means and standard deviations (std) of the liver T1 results for the PCK and SD control rats were calculated for each group as a whole. Unpaired 2-tailed Student s t-tests were used to compare the liver T1 results between the two groups. A Pearson correlation coefficient was used to compare the liver T1 relaxation findings from the PCK and SD rats with the quantitative fibrosis scores, bile duct area and hydroxyproline assessments. A p-value of less than 0.05 was considered significant for both the Student s t-tests as well as the Pearson correlation coefficients. 2.3 Results Comparison of T1 relaxation in PCK and SD control rats Representative liver images from the Look-Locker acquisition as well as the final T1 relaxation time maps from a 3-month-old SD control rat (top panel) and two 3-month-old PCK rats (middle and bottom panels) are shown in Figure 2.1. Among the five PCK rats studied, we observed from histology that one of the PCK rats suffered from cholangitis 42
46 (bottom panel) which mirrors the variation in liver disease severity observed in ARPKD patients. Representative T1-weighted images at inversion times of 420 ms, 1248 ms, 2076 ms and 6214 ms for the three rats are shown in columns a-d in Figure 2.1, respectively. The liver T1 maps (column e) for the PCK rat (middle row) show increased hepatic T1 values in comparison to the SD control rat (top row). The PCK rat with severe cholangitis (bottom row) exhibited extensive regions with increased T1 relaxation time values consistent with biliary cysts observed in some ARPKD patients with more severe liver disease. Figure 2.1: Representative liver Look-Locker T1-weighted images (columns a-d, grayscale) and T1 maps (column e, color scale) from a 3 month-old Sprague-Dawley (SD) control rat (top row) and two age-matched PCK rats (middle and bottom rows). The PCK rat in the bottom row exhibited cholangitis associated with more severe liver disease, which is also observed periodically in ARPKD patients. Mean liver T1 values for the cohorts of SD and PCK rats are shown in Figure 2.2. Excluding the severe PCK rat with cholangitis from the analysis (T1 = 1413 ms), the group of PCK rats (n=4) still exhibited a significantly higher mean T1 relaxation 43
47 estimation (mean ± std = 935±39 ms, p = 0.01) when compared with the SD control rats (n=6, mean ± std = 847±26 ms). Figure 2.2: Plot of mean liver T1 values for SD (n=6) and PCK (n=4) rats at 3 months of age. The mean T1 values for the PCK rats were significantly increased in comparison to the SD rats (*p = 0.01). The respective T1 value for the PCK rat with cholangitis was further increased (n=1, data not shown, mean T1 = 1413 ms) Comparison of in vivo liver T1 relaxation time with histological and biochemical assessments of biliary dilatation and hepatic fibrosis Photomicrographs of Masson s Trichrome-stained liver sections from a 3-month-old control SD rat and two 3-month-old PCK rats are shown in Figure 2.3. The PCK rats show increased regions of fibrosis (blue regions staining collagen) around dilated bile ducts (white regions with asterisks) in comparison to the SD rat. It should be noted that the PCK rat with cholangitis exhibited more extensive fibrosis staining and increased bile duct dilatation than both the more typical PCK rat and the SD control rat as previously described in this model (90). 44
48 Figure 2.3: Photomicrographs (10x original magnification) of SD and PCK rat liver specimens stained with Masson s Trichrome to assess periportal fibrosis (in blue) and biliary dilatation (asterisks *). Note the regions of bile duct proliferation and dilatation as well as increased fibrosis in the PCK rats. Periportal fibrosis is especially pronounced in the rat with cholangitis, in which inflammatory cells are evident within a dilated bile duct (arrow). The relationship of mean hepatic T1 relaxation time to bile duct area percent, fibrosis area percent, and hydroxyproline content (μg / mg protein) for each rat (excluding the PCK rat with cholangitis) is shown in Figures 2.4a-c, respectively. Importantly, all three indicators: bile duct area percent (R=0.85, p=0.002), trichrome fibrosis scores (R=0.82, p=0.004) and hydroxyproline content (R=0.76, p=0.01) demonstrated a significant correlation with the mean in vivo liver T1 relaxation assessments. 45
49 Figure 2.4: Comparison of mean hepatic T1 values for 3-month-old SD rats (black diamonds) and PCK rats (white circles) in: (a) percent bile duct area; (b) percent fibrosis (by Masson s trichrome staining); and (c) biochemical assessments of hepatic hydroxyproline content. All three assessments resulted in significant correlations with mean liver T1 (p = 0.002, 0.004, and 0.01, respectively) suggesting that T1 assessments are an effective imaging marker for ARPKD liver disease. 2.4 Discussion In this initial study, we have shown that quantitative T1 relaxation time assessments of the liver in PCK rats can provide a non-invasive and quantitative assessment of ARPKD liver disease. Specifically, the FISP-based Look-Locker technique demonstrated statistically significant increases in hepatic T1 relaxation time in PCK rats in comparison to SD control rats at 3 months of age. Importantly, these hepatic T1 relaxometry assessments resulted in significant correlations with both histologic scores of biliary dilatation and hepatic fibrosis as well as biochemical hydroxyproline content assessing hepatic collagen content. As there are currently no clinical gold standard assessments for ARPKD liver disease, this study suggests that evaluation of T1 relaxation time is a safe and sensitive imaging biomarker for this rare, but potentially lethal pediatric disease. The presented results are significant from multiple aspects. First, the significantly increased liver T1 estimation observed in PCK rats in comparison to SD controls (Figure 2.2) suggests the increased T1 relaxation time can detect ARPKD liver disease. Further, 46
50 the link between T1 and the pathophysiologic changes associated with ARPKD liver disease is supported by the findings that T1 correlates significantly with the primary pathologic hallmarks of disease severity: biliary dilatation and hepatic (periportal) fibrosis. In addition, although not thoroughly studied in this initial investigation, the T1 relaxation time appears to be impacted by cholangitis observed in the single PCK rat (Figure 2.1), which occurs intermittently in ARPKD patients. Altogether, these in vivo results suggest that quantitative T1 relaxation time assessments can provide a noninvasive measure of ARPKD liver disease severity. The T1 relaxometric assessment offers multiple specific advantages for the assessment of ARPKD liver disease. First, this simple T1 measurement is entirely non-invasive and requires no exogenous contrast agents. This method limits the risks of other methodologies to assess liver disease, such as bleeding complications associated with biopsies or toxicities resulting from gadolinium-based MRI imaging contrast agents in patients with chronic kidney disease. This is extremely important for eventual use in ARPKD patients as ARPKD includes both progressive kidney and liver disease components (69,70). In addition, while other techniques such as MR Elastography (MRE) and ultrasound have gained significant momentum in recent years for the assessment of hepatic fibrosis (91,92), T1 relaxation time assessments are already available on virtually all modern MRI scanners offering the potential for rapid dissemination for routine clinical use. The FISP-based acquisition itself also offers reduced image artifacts, especially on high field MRI scanners, in comparison to echo-planar imaging (EPI) and True FISP (14). It is worth noting that the FISP acquisition provides additional T2 weighting in the Look-Locker images in comparison to the previously described spoiled 47
51 gradient echo acquisitions. However, this impact is partially ameliorated by the small tip angle. In this initial preclinical MRI study, 50 averages were acquired over ~16 min for a single imaging slice to obtain high quality T1 relaxation assessments. However, Look- Locker and other previously described T1 relaxation time assessments have already been implemented on lower field human MRI systems to provide quantitative imaging assessments in a single breathhold (93,94). Therefore, T1 relaxometric assessments could provide a safe and effective tool for longitudinal studies in infant and pediatric ARPKD patients. This initial preclinical imaging study had several important limitations and opportunities for future study. The PCK and SD control rats were evaluated only at a single time point (3 months of age), when disease is evident histologically. What remains to be determined is if the T1 relaxation time assessments are sensitive enough to: 1) detect very early-stage ARPKD liver disease (PCK rats at approximately 1 month of age); and 2) monitor liver disease progression over time. As such, additional imaging studies are needed to track liver disease progression in PCK rats over the full course of the disease progression from 1 to 6 months of age. In addition, in this study, we did not perform any comparison with alternative quantitative MRI techniques, which may also be useful in detecting biliary dilatation / hepatic fibrosis. As bile is known to have increased T1 and T2 relaxation times relative to liver parenchyma (95,96), T2 relaxometric assessments may also provide a direct measure of biliary dilatation / proliferation. Other techniques such as Magnetization Transfer and Diffusion MRI assessments may also provide additional information on ARPKD liver disease (97,98). While these various techniques offer specific advantages and disadvantages for both preclinical and future clinical studies, a 48
52 thorough comparison of these methods was beyond the scope of this initial preclinical and histological evaluation. In conclusion, we have performed an initial preclinical study to demonstrate that T1 relaxation time can detect liver disease in the orthologous PCK rat model of ARPKD. We have shown that the straightforward, non-contrast, readily-translatable FISP-based Look-Locker technique can sensitively identify increased liver T1 relaxation in PCK rats in comparison to SD controls and distinguished a PCK rat with cholangitis from other PCK rats not similarly infected. In addition, these liver T1 assessments correlated significantly with two gold-standard histopathologic assessments of hepatic fibrosis and with histologic assessments of biliary dilatation. These initial imaging results, therefore, suggest that T1 relaxation time may provide a useful imaging marker to not only detect ARPKD liver disease, assess severity, and to monitor disease progression. 49
53 Chapter 3 ASL-FISP: a Rapid and Quantitative Perfusion Technique for High-field MRI Portions of this chapter were adapted with permission from Gao Y, Goodnough CL, Erokwu BO, Farr GW, Darrah R, Lu L, Dell KM, Yu X, Flask CA. Arterial spin labelingfast imaging with steady-state free precession (ASL-FISP): a rapid and quantitative perfusion technique for high-field MRI. NMR in Biomedicine 2014;27(8): Copyright 2014 John Wiley & Sons, Ltd. 3.1 Introduction Arterial Spin Labeling (ASL) MRI is a non-contrast perfusion MRI technique that has been shown to provide quantitative assessments of tissue perfusion in multiple clinical imaging applications including brain (31-33), kidney (34-37), lung (38-42), and liver (43). One major advantage of ASL over conventional Dynamic Contrast Enhanced MRI perfusion techniques is the lack of exogenous, and potentially toxic, paramagnetic contrast agents. The use of endogenous blood signal to obtain tissue perfusion information is especially important for imaging patients with chronic kidney diseases, which can be a contraindication for Gadolinium-based MRI perfusion methods (44). This attribute is also important for longitudinal preclinical imaging applications, as multiple tail-vein injections and/or catheterizations can cause local inflammation and necrosis resulting in reduced access to veins. ASL MRI techniques generate blood flow contrast between multiple images using a wide variety of blood labeling methods (20,45-49). A typical ASL MRI acquisition combines an ASL preparation phase followed by a rapid imaging readout to capture the blood flow weighted contrast (Figure 1.1). It is important to note that a large majority of the imaging developments have focused on optimization of the preparation phase of the ASL acquisition. As a result, a wide variety of preclinical and clinical ASL MRI 50
54 techniques have been developed. These ASL techniques can be broadly grouped into continuous (CASL, (45,46,49)) and pulsed (PASL, (20,47,48)) categories. A hybrid of these two techniques, pseudo-continuous ASL (pcasl) has also been recently developed (50-52). Each of these specialized techniques offers advantages for specific imaging applications. At the same time, many of these studies have utilized conventional imaging readouts including Fast Low Angle SHot (FLASH) (99,100), Echo-Planar Imaging (EPI) (101,102), and True Fast Imaging with Steady-State Free Precession (True FISP) (35,42,103,104). Unfortunately, these imaging readouts exhibit significant limitations for high field MRI applications. Specifically, B0 inhomogeneities result in increased distortion / ghosting and banding artifacts from EPI and True FISP imaging techniques, respectively on high field MRI scanners. These artifacts are particularly problematic for body imaging applications where cardiac and respiratory motion as well as adipose tissue can make precise shimming difficult. In addition, the increase in T1 magnetic relaxation times on high field MRI scanners can result in spoiled gradient echo images with a lower signal-to-noise ratio (SNR) relative to these other imaging techniques. A lower SNR is especially problematic for ASL MRI techniques as the differential blood flow signal is typically less than 10% of the mean tissue signal (105,106). Therefore, a need exists to develop a rapid and robust ASL MRI imaging readout that is both sensitive to blood flow labeling from the ASL preparation and also immune to B0 inhomogeneities and motion artifacts on high field MRI scanners. Our group has previously reported on the development of a centrically-encoded FISP imaging technique which can be flexibly combined with conventional diffusion and magnetization transfer (MT) / chemical exchange saturation transfer (CEST) preparation 51
55 methods to rapidly generate quantitative imaging data (107,108). Importantly, the magnetic field gradients for the FISP readout sequence are not completely refocused in either the slice select or frequency encoding directions, resulting in greatly reduced offresonance artifacts in comparison to EPI and True FISP acquisitions. In addition, centric k-space encoding of the FISP readout retains the image contrast generated in the diffusion and MT / CEST preparations. Here, we describe initial in vivo results from an ASL-FISP acquisition on 7T and 9.4T Bruker Biospec small animal MRI scanners. For this initial study, our ASL-FISP implementation combines a FAIR preparation scheme with our rapid centrically encoded FISP imaging readout to generate ASL imaging data (20,35,104,109). The reproducibility of this new ASL-FISP technique was evaluated in brains of healthy C57/BL6 mice. In addition, we demonstrate the effectiveness of this technique for multiple imaging applications including mouse models of ischemic stroke, dystrophin-null mdx mouse model of muscular dystrophy and healthy mouse kidneys. 3.2 Methods In Vivo Comparison of Image Artifacts at 7T Axial FISP images of a healthy C57/BL6 mouse brain were acquired on a 7 T Bruker Biospec MRI scanner (Bruker Inc., Billerica, MA) for comparison to conventional spin echo (TR/TE = 2000/14 ms, 1 average, total scan time = 4 min 16 s), True FISP (TR/TE = 4.0/2.0 ms, 20 averages, tip angle = 60 degrees, total scan time = 11 s), and EPI (TR/TE = 2500/30.8 ms, 4 segments, 2 averages, total scan time = 20 s) imaging readout methods to assess image artifacts. 52
56 3.2.2 ASL-FISP pulse sequence design The ASL-FISP acquisition was implemented on Bruker Biospec 7 T and 9.4 T MRI scanners equipped with 400 mt/m gradient inserts. The ASL-FISP sequence was implemented on both MRI scanners to demonstrate the robustness of the methodology to artifacts on high field MRI scanners. The ASL-FISP pulse sequence was developed by combining a FAIR preparation (slice-selective or non-selective inversion) with a centrically-encoded FISP imaging readout to acquire all lines of k-space following each ASL preparation (Figure 1.1). Note that although a FAIR scheme was implemented herein, the ASL-FISP technique is adaptable to a variety of ASL preparations. A hermite excitation RF pulse of duration 0.5 ms, and a tip angle of 60 degrees was the selected for this implementation. The high tip angle was selected for this FISP acquisition to provide increased SNR in comparison to low tip angles (data not shown). Uniformity of the inversion pulse is essential for accurate and precise quantification of tissue perfusion. Therefore, a hyperbolic secant adiabatic inversion pulse of duration 3.0 ms was used for the FAIR inversion preparation. Magnetic field gradient spoilers were applied after the inversion preparation to limit transverse magnetization prior to the FISP imaging readout. The FISP imaging readout (including 10 dummy scans) provided in vivo images in less than 2 seconds with relatively high SNR in comparison to conventional spoiled gradient echo acquisitions and minimal off-resonance distortion and ghosting artifacts in comparison to EPI (107,108). The FISP imaging readout also prevented banding artifacts typical of balanced SSFP / True FISP acquisitions on high field MRI scanners. The FISP imaging readout was also designed with centric encoding to retain blood flow sensitivity as well as the T1 weighting generated from the FAIR 53
57 inversion preparation Initial in vivo ASL-FISP perfusion assessments in mouse brains All animal studies were conducted in accordance with approved IACUC (Institutional Animal Care and Use Committee) protocols at Case Western Reserve University. The ASL-FISP technique was initially evaluated by assessing brain perfusion in healthy wildtype C57/BL6 mice (The Jackson Laboratory, Bar Harbor, Maine). Each animal was anesthetized in 1-2% isoflurane with supplemented O2 and positioned within a mouse volume coil (inner diameter = 35mm) in a Bruker Biospec MRI scanner (Bruker Inc., Billerica, MA). Each animal s body temperature was maintained at 35±1 C with a warmed air control system. Respiratory triggering was performed through an MRcompatible small animal gating and control system (SA Instruments, Stony Brook, NY). Ten male C57/BL6 10 weeks of age were scanned with the ASL-FISP imaging protocol at 7 T. Five of these mice were scanned with the ASL-FISP imaging protocol at 9.4 T at least one day prior to the 7 T scan for comparison. Rapid localizer scans were first used to identify an appropriate and consistent axial mid-brain imaging slice for the ASL-FISP protocol. After slice positioning, the single-slice ASL-FISP protocol consisted of three sequential scans: 1) ASL-FISP with a slice-selective inversion, 2) ASL-FISP with non-selective inversion, and 3) FISP with no inversion preparation as a reference (M0) scan for blood flow calculation (20). An inversion delay time (TI) of 1420 ms was used for this initial implementation to generate sufficient blood flow contrast between the slice-selective and non-selective ASL-FISP images. Other than the inversion preparation, the FISP imaging readout parameters were identical among these three acquisitions (centric encoding, TR/TE = 2.4/1.2 ms, matrix = 128 x 128, FOV = 3 cm x 3 cm, imaging 54
58 slice thickness = 1.5 mm, tip angle = 60 degrees). These scans were repeated 60 times to determine the effects of image quality on the perfusion calculations. Although not required, for this initial implementation an additional delay time of ~13 seconds was incorporated between each scan repetition to allow the magnetization to return to equilibrium (M0) between repetitions and to limit the duty cycle on the magnetic field gradients. The total acquisition time for one ASL-FISP repetition including the ASL preparation (1420 ms), FISP imaging readout (331 ms), and ~13-second delay was 15 seconds. For comparison, we also implemented an ASL-GRE acquisition by combining an identical FAIR preparation with a gradient echo (GRE) imaging readout (TR/TE = 5000/2 ms, 5 averages, tip angle = 90 degrees). For this simplified ASL-GRE acquisition, only one line of k-space was acquired for each ASL preparation. All other imaging parameters including field of view (FOV) and resolution were identical to the ASL-FISP acquisitions described above. This conventional ASL-GRE acquisition was evaluated on a separate single healthy C57/BL6 mouse brain at 7 T for direct comparison with the ASL-FISP results. Following the ASL-FISP scans, a FISP-based Look-Locker acquisition was then implemented to generate voxel-wise T1 maps according to previously described techniques (23,110). The Look-Locker acquisition consisted of a non-selective adiabatic inversion followed by 10 continuous and sequential FISP image repetitions (linear encoding, tip angle = 10 degrees, TR/TE = 4.0 ms / 2.0 ms, 512 ms / image). The Look- Locker acquisition was repeated at least 40 times to ensure accurate T1 relaxation time estimation. As for the ASL-FISP acquisitions above, a ~7-second delay was introduced 55
59 after each repetition to reduce the duty cycle on the imaging gradients and to allow the magnetization to return to equilibrium (M0). Voxel-wise T1 relaxation maps were then calculated according to previously described methods (23,110). The geometry of the Look-Locker acquisition was identical to the ASL-FISP acquisitions to ensure one-to-one correspondence between the ASL and T1 relaxation data and enable direct calculation of the quantitative ASL maps described below. Quantitative, voxel-wise perfusion maps were generated using previously described methods for the FAIR ASL technique according to Equation 1 below (20,48). (3.1) where f is the tissue perfusion in ml/min/100g of tissue; NS, SS, and M0 are the signal intensities for the non-selective, slice-selective, and reference (M0) ASL-FISP images, respectively; T1 is the longitudinal relaxation time calculated from the Look-Locker acquisition; TI is the inversion time set at 1420 ms; and the tissue-blood partition coefficient (λ) was assumed to be 0.9 ml/g (111). Perfusion maps were calculated using this equation for each ASL-FISP scan. A region-of-interest (ROI) analysis was then used to calculate the mean perfusion over the entire mouse brain visible within the single ASL-FISP imaging slice. Note that these ROIs included both white matter and gray matter, while ventricles were excluded from the analysis. A histogram analysis of the mouse brain ROI was also used to calculate a threshold (mean ± 2 standard deviations) to limit the impact of large vessels (high perfusion values) on the calculation of the mean brain perfusion value for each animal similar to previously described methods (31,35). The mean brain perfusion values for each individual mouse were then used to calculate an overall group average and standard 56
60 deviation at 7 T and 9.4 T respectively, for comparison. This ROI analysis was repeated for 20, 40, and 60 ASL-FISP averages at 7 T and 9.4 T to perform an initial determination of the effects of noise on the brain perfusion assessments. For all C57/BL6 mice, ASL-FISP scans were obtained with an inversion slab thickness three times that of the FISP imaging slice thickness to ensure a uniform inversion over the entire FISP imaging slice. This factor of three was previously described by our group (107) and provides an effective balance between blood flow sensitivity and inversion uniformity. One C57/BL6 mouse was scanned with an inversion slab thickness / imaging slice thickness ratio of 1, 3, 6, and 10 to determine the effects of the relative inversion slab thickness on the perfusion results Additional in vivo ASL-FISP experiments: mouse brain ischemic stroke model, dystrophin-deficient mouse model and healthy mouse kidneys The ASL-FISP acquisition and analysis protocol described above was performed on two additional C57/BL6 mice to evaluate: (1) brain perfusion in the context of known pathology (ischemic stroke); (2) kidney perfusion; and (3) muscle perfusion. Except for the number of signal averages (112), all of the imaging parameters used for these imaging studies were identical to the brain ASL-FISP experiments described above. An ASL-FISP study was performed on a mouse model of ischemic stroke to assess the method s sensitivity to a known pathophysiology. A C57/BL6 mouse (male, 8 weeks of age), was anesthetized with isoflurane. A transient stroke was initiated by inserting a 0.22 mm diameter, silicon-coated filament (Doccol, Corp) into the right carotid artery resulting in an occlusion of the middle cerebral artery (MCAo), while monitoring cerebral blood flow using Laser Doppler flowmetry ( ). The filament was 57
61 removed after one hour of occlusion to allow reperfusion as the animal recovered. At 24 hours post-ictus, the animal was re-anesthetized for ASL-FISP scanning as described above. A diffusion weighted EPI image (TR/TE = 5000 / 31 ms, b=500 s/mm 2 ) was also acquired to confirm the presence of the infarct from the MCAo. A brain perfusion map and a mean perfusion value for the whole brain within the imaging slice were calculated for comparison with healthy C57/BL6 mice. In addition, neuroimaging studies were also performed on young (2-month, n=10) and adult (10-month, n=10) male dystrophin-null (mdx) and wild-type (WT) mice of the C57/BL6 strain. All mice were obtained from Jackson Laboratories (Bar Harbor, ME). All data are reported as mean ± SD. A twotailed, unpaired Student s t-test was performed to compare mdx and WT mice. Statistical significance was established at a level of p < For the kidney and paraspinal muscle perfusion assessment, ASL-FISP was performed on a healthy male 10-week-old C57/BL6 mouse. Respiratory triggering was performed as described above. This study was performed as an initial demonstration of the effectiveness for ASL-FISP for high field body imaging applications. Following the ASL-FISP acquisitions, an ROI analysis was used to calculate the mean renal perfusion values for the left and right kidneys of this mouse and the mean perfusion value of the paraspinal muscles. 3.3 Results Axial mouse brain images from spin echo, FISP, True FISP and EPI imaging techniques at 7 T are shown in Figure 3.1. Banding and ghosting / distortion artifacts are clearly visible in the True FISP and EPI images, respectively. 58
62 Figure 3.1: Representative axial mouse brain images at 7 T using (a) spin echo, (b) FISP, (c) True FISP, and (d) EPI acquisitions. Note the similar lack of artifacts in the spin echo and FISP images in comparison to the True FISP (banding) and EPI (ghosting / distortion) images. By comparison, the spin echo and FISP images show a lack of image artifacts with the FISP images generated in less than 1/10th of the acquisition time (22 seconds vs 4 minutes 16 seconds). Representative brain ASL-FISP image sets, T1 relaxation time maps, and calculated perfusion maps are shown for a healthy C57/BL6 mouse at 7 T (n=10) in Figure 3.2. Qualitatively similar images and maps were obtained from the mice at 9.4 T (n=5, data not shown). 59
63 Figure 3.2: Representative ASL-FISP images of a healthy C57/BL6 mouse brain at 7 T: (a) Slice-selective (bright-blood); (b) non-selective (dark blood) ASL-FISP images (40 averages); (c) M0 image (no inversion); (d) Brain T1 map from Look-Locker acquisition; perfusion map from (e) ASL-FISP and (f) ASL-GRE in ml/min/100g of tissue. Note the lack of distortion and banding artifacts in the M0 FISP image. The mean and standard deviations for the groups of healthy mice scanned at 7 T and 9.4 T are shown in Figure 3.3a as a function of the number of ASL-FISP averages used to calculate the perfusion data (20, 40, and 60 averages). A 2-tailed Student s t-test showed no significant difference between the mean perfusion values at the two magnetic field strengths (p > 0.2) or for 20, 40, and 60 averages (p > 0.6). With 40 signal averages, the mean brain perfusion values for healthy C57/BL6 mice ranged from
64 ml/min/100g of tissue at 7 T and ml/min/100g of tissue at 9.4 T, respectively. The mean ± standard deviation of the mouse brain perfusion (excluding ventricles) at 7 T and 9.4 T were 249 ± 38 ml/min/100g of tissue and 241 ± 17 ml/min/100g of tissue, respectively. Figure 3.3: (a) Mean C57/BL6 mouse brain perfusion from the ASL-FISP method at 7 T (gray) and 9.4 T (white), respectively. Results are plotted as a function of the number of ASL-FISP averages. No significant differences in mean perfusion were observed for different number of averages (p > 0.6) or field strength (p > 0.2). (b) Mean brain perfusion from a single C57/BL6 mouse at 7 T as a function of the slice thickness ratio (inversion slab thickness / imaging slice thickness) and the number of ASL-FISP averages. Note the large decrease in mean brain perfusion as the slice thickness ratio is increased from 1 to 3. The mean perfusion also appears to be more sensitive to the inversion slab thickness than the number of ASL-FISP averages. A secondary ROI analysis of the 7 T mouse brain perfusion data showed differential perfusion values in the cortex (211 ± 30 ml/min/100g) and thalamus (288 ± 48 ml/min/100g), which is consistent with previous studies (115). A perfusion map from the ASL-GRE method (mean cerebral perfusion = 285 ml/min/100g) is shown in Figure 3.2f for comparison. The total imaging time for 5 averages was approximately 4 hours. In order to make a valid comparison between protocols, the time under anesthesia should be consistent to ensure similar perfusion conditions. We chose to evaluate the ASL-GRE method with 2 averages so that the imaging time was approximately the same as ASL- 61
65 FISP. The mean cerebral perfusion value from the ASL-FISP method (40 averages) was 249 ± 38 ml/min/100g, which is quite reasonable compared to the cerebral perfusion value of 240 ml/min/100g from the ASL-GRE method (2 averages). The mean brain perfusion values from a single C57/BL6 mouse using an inversion slab thickness / imaging slice thickness ratio of 1, 3, 6, and 10 and either 5, 10, 20, 40, or 60 ASL-FISP averages are shown in Figure 3.3b. A large decrease in perfusion was observed as the inversion slab thickness / imaging slice thickness ratio was increased from 1 (inversion slab thickness = imaging slice thickness) to 3 due to both increased uniformity of the inversion pulse over the entire imaging slice and reduced perfusion sensitivity. The mean brain perfusion values continued to decrease at a lower rate as the inversion slab thickness / imaging slice thickness ratio increase from 3 to 6 and 10. In addition, only minimal variation was observed in the mean perfusion values as the number of ASL-FISP is reduced from 60 to 5 for each ratio. Axial ASL images, T1 maps, and brain perfusion maps for the middle cerebral artery occlusion (MCAo) model of ischemic stroke in a mouse are shown in Figure 3.4. The region of infarct on the right side of the brain (left side of image) is clearly visible in the ASL-FISP images and the corresponding perfusion map. Note that a smaller secondary infarct is visible on the contralateral side most likely resulting from the extensive cytotoxic edema caused by the induced ischemic stroke. Overall, the mean brain perfusion for the MCAo mouse was measured to be 143 ml/min/100g. 62
66 Figure 3.4: ASL-FISP images of an MCAo mouse model of stroke at 7 T. (a) Sliceselective (bright-blood) and (b) non-selective (dark blood) ASL-FISP images; (c) M0 FISP image (no inversion); (d) diffusion-weighted image (b=500 s/mm 2 ) showing right brain infarct; (e) Look-Locker T1 map; and (f) perfusion map. The primary infarct is visible in the right brain in all images. A potential contralateral perfusion deficit is also observed in the perfusion map, but less evident in T1 and diffusion-weighted images. Representative cerebral perfusion maps for the 2 and 10 month old mdx and WT mice are shown in Figure 3.5a-d. The mean and standard deviations for each group are shown in Figure 3.5e. There was a 15% decrease in cerebral perfusion in 10 month mdx mice as 63
67 compared to WT (Figure 3.5e; p=0.001), without any significant difference in mean cerebral T1 or M0 values (p=n.s.). The mean brain perfusion values for the 2-month old mdx mice were slightly lower than for 2-month old WT mice. However, no significant difference in cerebral perfusion was observed. Figure 3.5: a-d. Representative perfusion images of 2-month WT (a), 2-month mdx (b), 10-month WT (c), and 10-month mdx (d). e-h. Representative T1-maps of 2-month WT (e), 2-month mdx (f), 10-month WT (g), and 10-month mdx (h). i. Mean cerebral (excluding ventricles) perfusion from the ASL-FISP method in C57/BL6 WT (black) and mdx (white), respectively. There was a significant decrease in mean perfusion as compared to aged WT observed in the aged mdx mice (p < 0.005). There was no significant change in perfusion in young versus aged WT (p > 0.3) or young versus aged mdx (p>0.3), respectively. Data represented as mean ± SD. Colorbar is uniform in each panel. Axial ASL images, T1 maps, and perfusion maps for the C57/BL6 mouse kidneys are shown in Figure 3.6. As expected, the aorta and renal arteries show a very bright signal 64
68 in the slice-selective inversion ASL-FISP image (Figure 3.6a), while the arterial blood signal is greatly attenuated for the non-selective ASL-FISP image (Figure 3.6b). Figure 3.6: Kidney ASL-FISP images from a healthy C57/BL6 mouse at 7 T. (a) Sliceselective (bright-blood) and (b) non-selective (dark blood) ASL-FISP images; (c) M0 FISP image (no inversion); (d) Look-Locker T1 map; and (e) perfusion map. Renal arteries (high perfusion) and renal medulla (low perfusion) are clearly visible in the perfusion map. The measured mean renal perfusion values for the left and right kidneys (cortex + medulla + pelvis) were 513 and 560 ml/min/100g, respectively. In addition, the perfusion in the renal cortex was visibly increased relative to the renal medulla as expected. Importantly, mouse kidney perfusion was approximately twice that of the brain perfusion, consistent with previous reports (52,102,116,117). The mean perfusion value in the paraspinal muscles was 81 ml/min/100g, which was significantly lower than that of the kidneys as expected (52). 3.4 Discussion In this study, initial in vivo ASL MRI results were obtained using a rapid and artifactresistant ASL-FISP acquisition on 7 T and 9.4 T small animal MRI scanners. The ASL- 65
69 FISP technique combines an inversion preparation (slice-selective or non-selective) followed by a centrically-encoded FISP imaging readout to provide ASL data with minimal image artifacts in comparison to conventional EPI and True FISP imaging readouts for ASL MRI acquisitions. There are several important design features of the ASL-FISP acquisition. Most importantly, the FISP imaging readout is applied with centric k-space encoding and relatively few (i.e., 10) dummy scans. As shown previously, this centric encoding approach minimizes the loss of perfusion sensitivity caused by additional radiofrequency pulses and gradient lobes encountered in linear k-space encoding (108). A FISP imaging readout was selected for this study instead of a balanced SSFP readout primarily to limit well-known banding artifacts that are significantly increased on high field MRI scanners. An alternative approach using balanced SSFP would be to acquire multiple images with different RF phase variation sequences to reconstruct banding-free image (118,119). This approach may offer increased signal-to-noise, but it may require additional image processing to remove the banding artifacts. As a result, this alternative approach and direct comparison with ASL-FISP was not explored for this initial study. Further studies are also needed to directly compare the FISP and True FISP techniques as the FISP flow sensitivity may be altered due to dephasing (120). Overall, the key advantage of the FISP imaging readout is that it provides perfusion sensitivity with a short acquisition time (~2 seconds / image) with minimal artifacts in comparison to EPI readouts. The ASL-FISP and ASL-GRE acquisitions generated relatively similar perfusion maps (Figure 3.2) and mean brain perfusion values. However, the ASL-GRE method required an acquisition time that was more than 50 times than that 66
70 of the ASL-FISP method. In addition, the FISP imaging readout can easily be coupled with virtually any ASL preparation in order to meet the requirements for specific imaging applications. For this initial implementation, a simple FAIR ASL preparation was implemented with either a slice-selective or non-selective inversion pulse. However, more complex ASL preparations such as pcasl could also be implemented in order to measure transit times and other important perfusion parameters (52). The FISP imaging readout is particularly relevant for FAIR acquisitions as the difference between the images with the slice-selective inversion and non-selective inversion is small relative to the M0 image (typically < 10%). As a result, FAIR-ASL studies generally require numerous signal averages to obtain a reasonable estimate of tissue perfusion. Therefore, acquiring all k-space lines following a single ASL preparation (with minimal artifacts) using the FISP readout results in practical imaging times to acquire sufficient signal averages. While only single slice ASL-FISP results are presented in this initial study, multi-slice and/or 3D ASL-FISP implementations are possible and may have significant advantages for specific imaging applications. Initial in vivo mouse brain perfusion studies demonstrated that the ASL-FISP technique provides reasonable perfusion assessments on high field MRI scanners. ASL- FISP images in the healthy mouse brain and an induced ischemic stroke model show an expected lack of distortion and artifacts and clearly delineated perfusion deficits in the stroke model (Figures 3.2 and 3.4, respectively). Moreover, a first in vivo evaluation of in vivo evaluation of age-dependent alterations in cerebral perfusion demonstrates decreased perfusion in the 10-month mdx mice compared with their age-matched healthy controls. Further, the kidney ASL-FISP images and perfusion maps in Figure 3.6 show differential 67
71 perfusion between the renal cortex and medulla as expected from prior studies (35,121). In addition, the renal arteries are clearly visible in both the slice-selective inversion ASL- FISP images as well as the perfusion maps demonstrating the flow sensitivity of the ASL- FISP technique. Overall, the reliability of the ASL-FISP technique is exhibited by the lack of differences in the perfusion results at 7 T and 9.4 T (Figure 3.3a). Most importantly, the mouse kidney images shown in Figure 3.6 demonstrate that the ASL- FISP technique can provide high quality ASL data for rodent brain and body imaging applications on high field MRI scanners with no distortion and artifacts. The ASL-FISP technique presented herein also has several important limitations. One key observation of these initial results is that the mean perfusion values for mouse brains and kidneys shown here are dependent on the perfusion preparation scheme and inversion pulse design. It has already been shown in multiple studies that the relative thickness between the slice-selective inversion and the imaging readout is directly related to the resulting tissue perfusion estimate (32,34,103,106). For example, a smaller inversion slab thickness (e.g., one times that of the imaging slice) will result in enhanced perfusion sensitivity and erroneously high perfusion estimates. Conversely, a larger inversion slab thickness (ex. 6 x that of the imaging slice) will result in reduced perfusion sensitivity. These results are reflected in Figure 3.3b which shows that an inversion slab thickness / imaging slice ratio 3 is needed to maintain reasonably consistent perfusion results. The perfusion results can also be directly impacted by the shape of the inversion pulse and the excitation pulses of the FISP imaging readout. However, optimization of these pulses was beyond the scope of this initial technical development. Nevertheless, the results shown herein confirm that the ASL-FISP technique can sensitively differentiate normal tissue 68
72 perfusion from pathology (e.g., ischemic stroke) and relative tissue perfusion levels (renal cortex vs. renal medulla vs. skeletal muscle). It is important to note that the ASL-FISP technique may also be sensitive to the effects of pulsatility which may be an underlying cause of the bright CSF signal in the mouse brains. Another observation of these initial ASL-FISP results is the trend towards lower perfusion values at 9.4 T. While not statistically significant, this trend may be due in part to reduced T2 * relaxation times at 9.4 T. For mouse brain imaging, this reduction in T2 * can reduce the SNR of the SS, NS, and M0 images, especially in regions near the ear canals. Fortunately, this potential limitation can be partially mitigated using shorter echo times which would reduce the deleterious T2 * effects at all field strengths. For the FISP acquisition, the reduction in echo time would provide an additional increase in SNR as the repetition time would also be reduced by the same percentage providing an increase in the coherent steady-state magnetization. In conclusion, this study reports a rapid and quantitative ASL-FISP MRI technique for high field MRI scanners. For this initial study, the ASL-FISP technique combines a FAIR ASL preparation with a rapid, centrically-encoded FISP imaging readout to provide perfusion-weighted images in less than 2 seconds with minimal image distortion, ghosting, and banding artifacts in comparison to EPI and balanced SSFP readouts. Initial in vivo ASL-FISP perfusion results in mouse brains were obtained for healthy and ischemic stroke C57/BL6 mouse brains at 7 T and healthy mouse brains at 9.4 T. As a demonstration of the invulnerability of the ASL-FISP technique to off-resonance artifacts, initial in vivo kidney ASL results were also obtained for C57/BL6 mouse at 7 T. This new technique provides an alternative method for many perfusion imaging applications 69
73 on high field MRI scanners where off-resonance artifacts can severely limit the use of EPI and balanced SSFP acquisitions. 70
74 Chapter 4 Preclinical Magnetic Resonance Fingerprinting (MRF) at 7 T: Effective Quantitative Imaging for Rodent Disease Models Portions of this chapter were adapted with permission from Gao Y, Chen Y, Ma D, Jiang Y, Herrmann KA, Vincent JA, Dell KM, Drumm ML, Brady-Kalnay SM, Griswold MA, Flask CA, Lu L. Preclinical MR fingerprinting (MRF) at 7 T: effective quantitative imaging for rodent disease models. NMR in Biomedicine 2015;28(3): Copyright 2015 John Wiley & Sons, Ltd. 4.1 Introduction Over the past three decades, Magnetic Resonance Imaging (MRI) has become an established medical imaging modality due to its superior soft tissue contrast and lack of ionizing radiation. Conventional diagnostic MRI scans are non-quantitative by nature, but have provided clinicians and radiologists with the ability to detect multiple disease pathologies including cancer (122,123), stroke (124,125), musculoskeletal defects (126,127), and cardiovascular disease (128,129), among many others (130). Recently, efforts have been made to establish quantitative MRI assessments as biomarkers for disease detection and progression. These quantitative MRI assessments have included T1 and T2 relaxation times (57,131,132), proton density (57,132), multiple diffusion and perfusion parameters (107,133), as well as chemical exchange and magnetization transfer (108, ). Despite these efforts, the majority of routine clinical MRI scanning remains qualitative. High field ( 4.7 T) preclinical MRI scanners have been developed to provide MRI measures of disease in rodent models. In contrast to clinical MRI scanning, preclinical MRI research studies are almost entirely quantitative by nature and may require assessment of multiple imaging parameters during a single scanning session. These 71
75 quantitative preclinical MRI studies provide the opportunity to assess pathophysiologic changes associated with disease progression and therapeutic efficacy. In addition, rigorous validation of these preclinical MRI assessments has the potential to inform future clinical imaging studies. Therefore, a significant effort is ongoing to develop robust and effective preclinical MRI acquisition and reconstruction techniques. Conventional MRI quantification methods are mostly based on linear or nonlinear curve fitting to various MRI models (57,58). The implementation of these established model-based methods, such as T1 and T2 relaxation time estimation, are straightforward. However, these conventional quantification methods are susceptible to multiple sources of errors including cardiac and respiratory motion artifacts (59-61), as well as inhomogeneity in the main magnetic field (B0) (62). Importantly, the potential for these errors are significantly increased on high field preclinical MRI scanners where B0 inhomogeneities are increased; rodent heart rates can be as high as beats / minute; and breathholds are not possible. In addition, temporal errors can be observed in preclinical studies that require multiple imaging parameter estimates (e.g., diffusion and perfusion) as extended periods of anesthesia can cause physiologic changes during sequential scans. Therefore, new MRI acquisition and reconstruction methods for preclinical imaging applications are needed that are immune to these error sources and can simultaneously obtain estimates of multiple imaging parameters. Over the last few years, a new category of quantification in MRI has emerged which uses dictionary-based methods to match acquired data rather than conventional parameter estimation techniques using error-minimization methods. One of these methods, compressed sensing, has been developed for both clinical and preclinical 72
76 applications and has been shown to limit quantification errors and/or reduce the overall time to acquire quantitative datasets (53,55,56,139). More recently, a new Magnetic Resonance Fingerprinting (MRF) methodology has been proposed (63). MRF uses an entirely unique acquisition and quantification strategy that combines a priori acquisition parameter variation with a dictionary-based matching algorithm to obtain quantitative assessments of multiple imaging parameters simultaneously. The MRF technique was initially developed for low-field (1.5T 3T), clinical MRI scanners and was used to simultaneously generate T1, T2, and M0 maps in healthy human brains. Further, this initial report determined that the MRF technique is inherently resistant to errors from motion artifacts as motion or noise is not included / encoded into the theoretical signal evolution profiles that make up the MRF dictionary (63). Therefore, MRF may provide an ideal basis to generate multi-parametric assessments for preclinical imaging applications with limited impact of motion artifacts. In this study, we have developed an effective MRF acquisition and analysis algorithm for high field, preclinical MRI scanners. This is an initial implementation of the MRF technique on small animal MRI systems. For this initial implementation, we combined a priori variation in flip angles (FA) and repetition time (TR) with a Fast Imaging with Steady-state free Precession (FISP) acquisition (11,140) to simultaneously generate quantitative maps of T1 and T2 relaxation times and proton density (M0) from a single scan. We have evaluated these MRF estimates of T1, T2, and M0 in phantoms in comparison with conventional MRI techniques. We have obtained initial in vivo MRF data from healthy mouse kidneys to verify the robustness of the MRF technique to respiratory motion artifacts. We have also obtained in vivo MRF data from an orthotopic 73
77 mouse glioma model to demonstrate the sensitivity of the MRF technique to known pathology. The impact of RF excitation pulse profile as well as the number of acquired MRF images on the T1, T2, and M0 estimates have also been explored in this initial study. 4.2 Methods All animal studies were conducted in accordance with approved IACUC (Institutional Animal Care and Use Committee) protocols at Case Western Reserve University Preclinical MRF Acquisition and Reconstruction Design The MRF acquisition was implemented on a Bruker Biospec 7 T MRI scanner (Billerica, MA) equipped with a 400 mt/m magnetic field gradient insert. The preclinical MRF acquisition was developed from a FISP acquisition to provide a priori variation in both flip angle (FA) and repetition time (TR) to generate T1 and T2 specific MRF signal evolution profiles as described previously for clinical MRF studies (63,140). A schematic of the MRF pulse sequence is shown in Fig. 4.1A. The MRF acquisition was initiated with an inversion preparation to enhance the overall T1 sensitivity. The inversion preparation was followed by 600 successive FISP acquisition periods with varying excitation flip angle (FA, in degrees) and repetition time (TR, in ms) variation (140). FA and TR variation profiles are shown in Fig. 4.1B and 4.1C, respectively. The echo time was held constant (TE = 3.2 ms) in this MRF implementation. A repeating sinusoidal FA pattern ranging from 0 to 70 degrees was implemented (Figure 4.1B). More FA lobes was used compared to original clinical MRF to provide additional image contrast. The TR pattern selected was a Perlin noise pattern (Figure 4.1C) similar to the original clinical MRF description. However, a higher range of TR values was used for this study (i.e., 12.0 ms to 25.3 ms) to obtain a reasonable signal-to-noise ratio (SNR) for the
78 MRF images (63). Figure 4.1: (A) Schematic of the MRF-FISP pulse sequence with one line of k-space acquired for each of N images in one MRF scan repetition (N=600 for this initial implementation). (B,C) Flip angle and repetition time variation profiles used to create the MRF acquisition and dictionary. To acquire the MRF signal evolutions, the MRF acquisition was designed to acquire the same line of k-space for each of 600 sequential images during each MRF-scan repetition period (11.6 seconds). The FISP-MRF kernel was designed with a filtered 7- lobe sinc radiofrequency (RF) excitation pulse (τrf = 2 ms, bandwidth = 7000 Hz) to ensure a uniform excitation slice profile with limited excitation sidebands. Although not required, a 5-second delay was inserted after each MRF scan repetition (600 MRF-FISP TRs) to allow the magnetization to return to equilibrium prior to the next MRF scan repetition and to limit the duty cycle on the magnetic field gradients. This process was repeated for each line of k-space to generate 600 total temporal MRF images. 75
79 The MRF reconstruction process was implemented in Matlab (The Mathworks, Natick, MA). A fundamental component of the MRF reconstruction is the development of a large dictionary of signal evolution profiles that are subsequently matched to the acquired MRF signal evolution profile for each imaging voxel using vector-based inner product comparisons. The MRF dictionary was created as described previously using Bloch equation simulations of the MRF acquisition (63). The MRF dictionary consisted of 19,634 profiles generated from 199 T1 values (100 to 2000 ms, increment = 10 ms; 2000 to 6000 ms, increment = 500 ms) and 101 T2 values (10 to 150 ms, increment = 2 ms; 150 to 300 ms, increment = 5 ms). Unrealistic profiles with T1 < T2 were excluded. Note that the T1 and T2 increments in the MRF dictionary (2 10ms for low T1/T2 values) are small relative to the expected variation observed for in vivo T1/T2 estimates In vitro MRF assessments of T1 and T2 relaxation times and proton density (M0) Four imaging phantoms with distinctly different T1 and T2 relaxation times were prepared by adding different concentrations of MnCl2 (30, 100, 200, 300µM, respectively) to distilled water. The solutions were added to 200uL centrifuge tubes for imaging. Axial MRF images (600 total MRF images) were obtained for these phantoms. Additional MRF acquisition parameters for the phantom experiments were: FOV = 3 cm 3 cm, matrix = , slice thickness = 1.5 mm, total acquisition time = 35 min 24 s. Although larger than typical, a slice thickness of 1.5mm was used for this initial preclinical MRF development to limit the effects of noise. Conventional inversion-recovery spin echo (IR-SE) (inversion delay times = [50, 200, 350, 500, 650, 800, 1000, 1500, 2000, 2500, 3000, 4000, 5000, 8000, 10,000] ms, TR/TE = 10,000 ms/8.1 ms, one average, total acquisition time = 6.7 h) and spin echo (SE) (echo times = [10, 25, 40, 60, 90, 120, 150, 76
80 300, 400, 500, 800] ms, TR = 10,000 ms, one average, total acquisition time = 3.9 h) methods were also implemented to generate conventional estimates of the phantom T1, T2, and M0 values, respectively for comparison with the MRF findings. All other imaging parameters including field of view and resolution were identical for the MRF, IR-SE (T1), and SE (T2, M0) acquisitions. Quantitative T1 and T2 relaxation times and proton density (M0) maps were obtained from the MRF data. T1 and T2 relaxation time and M0 maps were calculated for the conventional IR-SE and SE acquisitions using established methods (141). The MRF and conventional acquisitions were repeated five times on different days to assess the reproducibility of the MRF estimates. Mean T1, T2, and M0 values for all methods were obtained for each phantom using a region of interest (ROI) analysis. In addition, voxel-wise error maps and standard deviations for T1, T2, and M0 were calculated to assess voxel-wise variation. Two-tailed Student s t-tests were used to compare the mean and voxel-wise variation in the MRF T1, T2, and M0 values with the IR-SE and SE data, respectively Initial in vivo and ex vivo kidney MRF assessments in a healthy mouse In vivo and ex vivo MRF images were acquired for the kidneys of a 12-month old healthy mouse (C57/BL6, Jackson Labs) to assess the impact of respiratory motion. The mouse was initially anesthetized in 1-2% isoflurane with supplemental oxygen and positioned in a mouse volume coil for imaging (inner diameter = 35 mm). The body temperature and breathing rate of the animal were maintained at 35±1 C and breaths/min with adjustable warm air and isoflurane levels, respectively. Single-slice, axial kidney MRF images were obtained for the mouse (FOV = 3 cm 3 cm, matrix = , slice 77
81 thickness = 1.5 mm). Importantly, no respiratory triggering was applied for these MRF acquisitions to demonstrate the robustness of the MRF technique to respiratory motion artifacts. Immediately following the in vivo MRF scans, the isoflurane concentration was increased to 5% for 30 minutes to euthanize the mouse within the MRI scanner with no repositioning. The MRF acquisition was then repeated on the same axial kidney imaging slice to generate an ex vivo MRF dataset with no respiratory motion artifacts. All MRF acquisition and reconstruction parameters for the in vivo and ex vivo kidney experiments were the same Initial MRF Assessments in a Mouse Brain Tumor Model In vivo and ex vivo MRF assessments were also performed on mouse brains orthotopically implanted with a human glioma cell line expressing green fluorescent protein (GFP) to demonstrate the sensitivity of the MRF technique to known pathology and to examine the effects of RF excitation slice profile on the MRF estimates of T1, T2, and M0. To prepare the mouse glioma model, Gli36Δ5 cells were infected with GFP encoding lentivirus, harvested for intracranial implantation by trypsinization, and concentrated to cells/μl in PBS as described previously (142). A six-week old female athymic nude mouse was anesthetized by intraperitoneal administration of 50 mg/kg ketamine/xylazine and fitted into a stereotaxic rodent frame (David Kopf Instruments, Tujunga, CA). Tumor cells were implanted at AP = +0.5 and ML = -2.0 from the bregma in the right striatum at a depth of 3 mm below the dura using a 10-μL syringe (26-gauge needle; Hamilton Co, Reno, NV). A total of 200,000 glioma cells were implanted. In vivo MRF scans were obtained 8 days following inoculation with the tumor cells. 78
82 As for the kidney MRF studies, the animal was anesthetized with 1-2% isoflurane and positioned within a 35-mm inner diameter volume coil within the 7 T Bruker Biospec MRI scanner. Conventional T2-weighted images of the glioma model were obtained from a Rapid Acquisition with Relaxation Enhancement (RARE) acquisition (TR/TE = 3000 ms / 40.0 ms, FOV = 3 3 cm, matrix = , slice thickness = 1.5 mm, three averages, total acquisition time = 8 min) to identify the tumor region (39). The MRF parameters for the in vivo brain tumor assessments were (FOV = 3 cm 3 cm, matrix = , slice thickness = 1.5 mm, excitation sinc7 pulse, total acquisition time = 35 min 24 s). The in vivo brain MRF data were used to generate T1, T2, and M0 maps reconstructed from the full MRF dataset (600 images) as well as subsets of 100, 300, and 500, images respectively to determine the number of MRF images needed for effective quantification. Mean brain T1, T2, and M0 values were obtained from an ROI analysis of each set of MRF reconstructions for comparison. Ex vivo MRF scans of a separate excised mouse brain tumor model were acquired to obtain an initial investigation into the impact of excitation slice profile on MRF estimates. The mouse glioma model was prepared as described above. After 10 days of tumor growth, the animal was sacrificed, and the brain was excised for fluorescence imaging using a Maestro FLEX fluorescence scanner (CRi, Hopkinton, MA) to verify tumor viability. Fluorescence images of the GFP-expressing Gli36Δ5 tumor cells were acquired using standard GFP filters (excitation = nm, emission = 515 nm long-pass filter, acquisition settings = in 10-nm steps). Brightfield images were also acquired to provide an anatomic roadmap. Fluorescence and brightfield exposure durations were 10 milliseconds and 300 milliseconds, respectively. Fluorescence images were background 79
83 subtracted and unmixed, using the Maestro software to spectrally separate brain autofluorescence from GFP-expressing tumor cells. The brain was then placed in neutral buffered 10% formalin (Sigma-Aldrich, Milwaukee, WI, USA) within a 15mL centrifuge tube for ex vivo MRF imaging. The MRF acquisition (FOV = 3 cm 3 cm, matrix = , slice thickness = 1.5 mm, total acquisition time = 35 min 24 s) was repeated using either a filtered 7-lobe sinc radiofrequency (RF) excitation pulse (τrf = 2 ms) or a hermite RF excitation pulse (τrf = 2 ms). The slice profiles for each RF excitation pulse were measured using a conventional Fast Low Angle SHot (FLASH) acquisition (FOV = 1.52 cm 1.52 cm, matrix = , TR/TE = ms/10.0 ms, FA = 10 degrees) by applying the readout gradient along the axial slice-select direction as described previously (143). Although the sinc7 pulse would be expected to provide a more uniform excitation with reduced sidebands, these two pulses were selected as they are commonly used for many preclinical MRI acquisitions. Following the ex vivo MRF acquisition, the mouse brain was sectioned at 8 µm per section and stained with hematoxylin and eosin (H&E) stains to further validate the existence of the brain tumor. 4.3 Results Example in vitro phantom images of MnCl2-doped phantoms at multiple MRF timepoints (image numbers 40, 80, and 150 out of 600 total) and single-voxel signal evolution profiles are shown in Figures 5.2A and 5.2B, respectively. The three selected time points (image 40, 80, 150) are indicated with arrows in Figure 4.2B. Note the varying contrast in the phantom images at the three selected time points. Note the reasonable match between the acquired (in red) and matched dictionary (in blue) MRF signal evolution 80
84 profiles for all four phantoms. Figure 4.2: (A) In vitro MRF images from four MnCl2-doped phantoms with varying T1 and T2 relaxation times. Images acquired at multiple timepoints (image 40, 80, and 150) show evolving contrast during the dynamic MRF acquisition. (B) Signal evolution profiles from the four phantoms with acquired data profile (in red) and corresponding matched dictionary profile (in blue). Arrows delineate the specific timepoints for the three phantom images. Resultant MRF-based T1 (Fig. 4.3A), T2 (Fig. 4.3B), and M0 maps (Fig. 4.3C) for the 81
85 four MnCl2-doped water phantoms are shown in Figure 4.3. Corresponding T1, T2, M0 maps from the conventional IR-SE (T1, Fig. 4.3D) and SE (T2, Fig. 4.3E, M0, Fig. 4.3F) are shown for comparison. Differential error maps (MRF estimate conventional estimate) for T1, T2, and M0 are shown in Figures 4.3G, 4.3H, and 4.3I, respectively. Figure 4.3: (A,B,C) Quantitative axial phantom MRF maps of T1, T2, and M0. (D,E,F) T1, T2 relaxation times and M0 maps from conventional inversion recovery spin echo (IR-SE) and spin echo (SE) acquisitions, respectively for comparison. (G,H,I) Error maps of T1, T2 and M0 (MRF estimate conventional estimate). Quantitative evaluation of these in vitro MRF and conventional maps are shown in Figure 4.4. Mean (± standard deviations) T1, T2 and M0 values for the MRF and conventional methods from an ROI analysis of the five repeat scans are shown in Figure 4.4A. The MRF-based T1 estimates for all four phantoms were not statistically different from the conventional IR-SE T1 maps (p > 0.1), while the mean T2 and M0 MRF 82
86 estimates exhibited some significant differences. The mean T2 estimates from the MRF scans were significantly higher than from the conventional SE methods for three of the four phantoms (p < 0.01) while the phantom with the highest T2 value was significantly lower than the estimates from the conventional SE methods (p < 0.01). Two of the four phantoms had significant higher M0 values from MRF scans compared to SE methods (p < 0.05). Despite these differences, the MRF results showed significant decreases in both T1 and T2 values for increasing MnCl2 concentrations as expected (p < 0.01) (144). A comparison of the mean (± standard deviations) voxel-wise variation in the T1, T2 and M0 maps from the MRF and conventional acquisitions are shown in Figure 4.4B. The voxel-wise variation in the MRF-based T1 estimates was significantly lower than for the conventional IR-SE method for 2 of 4 phantoms suggestive of improved uniformity of the MRF T1 maps (p < 0.05). The voxel-wise variation in the MRF T1 estimates was less than 20 ms for all four phantoms. The voxel-wise variation in the MRF T2 maps was less than 10 ms for all four phantoms, but the variation was significantly increased relative to conventional methods for three of the four phantoms (p < 0.05). No significant differences were observed in the voxel-wise variation in the proton density maps and all mean variation values were less than
87 Figure 4.4: (A) Plots of mean phantom T1, T2 and M0 values from MRF and conventional methods over five scans on different days. Error bars represent one standard deviation in the mean value across all five measurements. (B) Plots of mean voxel-wise standard deviation for T1, T2 and M0 values from MRF and conventional methods as a measure of intrascan uniformity. Error bars represent one standard deviation in the mean value across all five measurements. Axial MRF T1, T2, and M0 maps of the same kidneys of a healthy mouse both before (Fig. 4.5A, in vivo) and after euthanasia (Fig. 4.5B, ex vivo) are shown in Figure 4.5. Importantly, the in vivo kidney T1, T2, and M0 maps are devoid of respiratory motion artifacts typical of conventional in vivo preclinical scans. Hyperintense regions in the T1 and T2 maps were also observed in the renal medulla as expected (145). The in vivo MRF T2 maps showed increased ghosting artifacts near the abdominal aorta possibly due to aortic pulsatility. Otherwise, the in vivo and ex vivo kidney maps are very similar with only regional decreases in the ex vivo T1, T2, and M0 values likely due to the absence of flowing blood. As further verification, MRF signal profiles and respective matched dictionary profiles are shown in Figure 4.5C and 4.5D from an ROI of the right in vivo 84
88 and ex vivo renal cortex (ROI shown in blue in T1 maps). Note the similar shape of the acquired MRF profiles aside from the respiratory motion spikes for the in vivo MRF scan (arrows in Fig. 4.5C). Importantly, the matched MRF profile (in blue) for the in vivo scan appeared to ignore these respiration spikes as the motion is not encoded in the theoretical signal evolution profiles in the MRF dictionary. Figure 4.5: MRF T1, T2, M0 maps from (A) in vivo healthy mouse kidneys and (B) the same imaging slice after euthanasia. (C) Acquired (in red) and matched (in blue) MRF signal evolution profiles of mouse kidney cortex from in vivo and (D) ex vivo scans. Note the overall similarity of the matched MRF profiles and resistance to respiratory motion spikes (arrows) visible in the acquired in vivo MRF profile, but not visible in the ex vivo MRF profile. In vivo MRF-based T1, T2, and M0 maps of a mouse brain orthotopically implanted with a human glioma cell line are shown in Figures 4.6A, 4.6B and 4.6C, respectively. Corresponding T2-weighted anatomic MRI images, combined fluorescence and brightfield images of the GFP expressing glioma cells, and H&E stain validating tumor 85
89 presence and location (arrows in Figure 4.6F) are shown in Figure 4.6D, 4.6E, and 4.6F, respectively. An ROI analysis showed mean T1, T2 and M0 values in the glioma (T1 = 1973 ms, T2 = 82 ms, M0 = 0.5 ms), cerebral cortex (T1 = 1470 ms, T2 = 63 ms, M0 = 0.4) and thalamus (T1 = 1273 ms, T2 = 55 ms, M0 = 0.4 ms) that are comparable to results in previous studies ( ). No ghosting artifacts were observed in these in vivo MRF T2 maps of a mouse brain. 86
90 Figure 4.6: (A,B,C) In vivo MRF T1, T2 and M0 maps of a mouse brain with a GFPexpressing Gli36Δ5 tumor. (D) T2-weighted image as an anatomic and tumor contrast reference. (E) Coronal brightfield image (gray scale) with GFP overlay and (F) H&E image confirming tumor location (arrows). The T1, T2, and M0 maps were reconstructed from subsets of the same in vivo MRF data using the first 100, 300, 500, and 600 images in the MRF profile, respectively (Figure 4.7). The MRF maps obtained from reconstruction of the full dataset (N=600, same as Figure 4.6)) are included as a reference. Mean brain T1, T2, M0 values using the 87
91 first 600, 500, and 300 images were consistent (range of mean T1 = 1436 to 1461 ms; range of mean T2 = ms; range of mean M0 = ). However, the mean values were substantially different using only the first 100 MRF images in the matching process (T1 = 1648 ms, T2 = 52 ms, M0 = 0.8). While not a rigorous evaluation, these initial findings suggest the importance of acquiring a sufficient number of MRF images as well as opportunities for optimizations to reduce the overall MRF acquisition time. Figure 4.7: MRF T1 (top row), T2 (middle row) relaxation time and M0 (bottom row) maps of the in vivo mouse brain with implanted tumor shown as a function of the number of acquired and matched MRF images. Note that the T1 and T2 maps are consistent with 300 MRF-FISP images suggesting opportunities for significant reduction in the MRF acquisition time. MRF T1, T2, and M0 maps were also generated from an ex vivo mouse brain with an implanted glioma using either a sinc7 (Fig. 4.8A) or hermite (Fig. 4.8B) RF excitation pulses in the MRF-FISP acquisition. The magnitude of the RF excitation pulse shape and the corresponding measured slice profiles obtained from the conventional FLASH 88
92 acquisition are shown for both excitation pulses. Note that the sinc7 excitation pulse provides a more uniform tip angle across the entire imaging slice (vertical dashed lines) and reduced side-band excitations in comparison to the hermite excitation pulse, as expected. The MRF T1 and T2 estimates using the sinc7 RF pulse (T1 = 970 ms, T2 = 59 ms, M0 = 0.8) were lower relative to the corresponding values obtained with the hermite pulse (T1 = 1078 ms, T2 = 146 ms, M0 = 0.7), while the M0 values were slightly lower. Overall, the T2 estimates appear to be more sensitive to excitation profile as the estimates obtained from the sinc7 pulse (T2 = 59 ms) were approximately one half that obtained from the hermite pulse (T2 = 146 ms). Figure 4.8: Ex vivo MRF T1, T2 and M0 maps of an excised mouse brain and glioma using either (A) sinc7 or (B) hermite radiofrequency (RF) excitation pulses for the MRF- FISP acquisition. The RF waveforms and measured slice profiles are also included. Note the sensitivity of the MRF technique, in particular the T2 estimates, to slice profile errors. 4.4 Discussion We have developed an initial implementation of the novel Magnetic Resonance Fingerprinting (MRF) technique specifically for high field preclinical MRI scanners. This preclinical MRF implementation builds upon the previously-reported clinical MRF 89
Arterial Spin Labeling (ASL)
 Arterial Spin Labeling (ASL) Imaging Seminars Series Stony Brook University, Health Science Center Stony Brook, NY - December 11 th, 2012 Francesca Zanderigo, PhD Layout BASIC PRINCIPLES ACQUISITION SEQUENCES
Arterial Spin Labeling (ASL) Imaging Seminars Series Stony Brook University, Health Science Center Stony Brook, NY - December 11 th, 2012 Francesca Zanderigo, PhD Layout BASIC PRINCIPLES ACQUISITION SEQUENCES
PRINCIPLES OF CT AND MR IMAGING Marc-André d Anjou, DMV, DACVR Faculty of Veterinary Medicine, University of Montreal Saint-Hyacinthe, Quebec, Canada
 PRINCIPLES OF CT AND MR IMAGING Marc-André d Anjou, DMV, DACVR Faculty of Veterinary Medicine, University of Montreal Saint-Hyacinthe, Quebec, Canada CT and MR imaging offer superior diagnostic possibilities
PRINCIPLES OF CT AND MR IMAGING Marc-André d Anjou, DMV, DACVR Faculty of Veterinary Medicine, University of Montreal Saint-Hyacinthe, Quebec, Canada CT and MR imaging offer superior diagnostic possibilities
Final Project
 Harvard-MIT Division of Health Sciences and Technology HST.584J: Magnetic Resonance Analytic, Biochemical, and Imaging Techniques, Spring 2006 Course Directors: Dr. Bruce Rosen and Dr. Lawrence Wald 22.561
Harvard-MIT Division of Health Sciences and Technology HST.584J: Magnetic Resonance Analytic, Biochemical, and Imaging Techniques, Spring 2006 Course Directors: Dr. Bruce Rosen and Dr. Lawrence Wald 22.561
Artifacts Caused by Eddy Current in Diffusion MRI
 Artifacts Caused by Eddy Current in Diffusion MRI Xi Tan ABSTRACT Magnetic resonance diffusion imaging is potentially an important tool for the noninvasive characterization of normal and pathological tissue.
Artifacts Caused by Eddy Current in Diffusion MRI Xi Tan ABSTRACT Magnetic resonance diffusion imaging is potentially an important tool for the noninvasive characterization of normal and pathological tissue.
MAGNETIC RESONANCE IMAGING OF IN VIVO FLOW PHENOMENA
 MAGNETIC RESONANCE IMAGING OF IN VIVO FLOW PHENOMENA So far we have seen that magnetic resonance can Locate the positions of spins (mainly water) with the aid of one or multiple field gradient: MRI Characterize
MAGNETIC RESONANCE IMAGING OF IN VIVO FLOW PHENOMENA So far we have seen that magnetic resonance can Locate the positions of spins (mainly water) with the aid of one or multiple field gradient: MRI Characterize
Pulsed NMR of Paramagnetic Terbium. Cheyenne Michael Yari
 Pulsed NMR of Paramagnetic Terbium Cheyenne Michael Yari June 26, 2012 1 - Introduction The magnetic properties of atomic nuclei have proven to provide very useful information which can directly be used
Pulsed NMR of Paramagnetic Terbium Cheyenne Michael Yari June 26, 2012 1 - Introduction The magnetic properties of atomic nuclei have proven to provide very useful information which can directly be used
2018 REVIEW CATEGORIES
 2018 REVIEW CATEGORIES 100 Neuro 101 Neuro: Acquisition 102 Neuro: Processing 103 Neuro: Neonatal & Pediatric - Normal Development 104 Neuro: Neonatal & Pediatric - Clinical Studies 105 Neuro: Normal Aging
2018 REVIEW CATEGORIES 100 Neuro 101 Neuro: Acquisition 102 Neuro: Processing 103 Neuro: Neonatal & Pediatric - Normal Development 104 Neuro: Neonatal & Pediatric - Clinical Studies 105 Neuro: Normal Aging
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Anatomy and Morphology In vivo soft tissue imaging for morphological characterization.
Simple, intuitive and accessible MRI solution for preclinical research. M-Series Compact MRI Systems
 Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
Simple, intuitive and accessible MRI solution for preclinical research M-Series Compact MRI Systems Application Oriented Imaging Molecular Imaging Using Contrast Agents Detection and quantification of
The Unique, New MRI Philips Ingenia 3 Tesla is now in Ayios Therissos! The first-ever digital broadband MR system has been installed in Ayios
 The Unique, New MRI Philips Ingenia 3 Tesla is now in Ayios Therissos! The first-ever digital broadband MR system has been installed in Ayios Therissos-Nicosia that delivers crystal clear images, remarkable
The Unique, New MRI Philips Ingenia 3 Tesla is now in Ayios Therissos! The first-ever digital broadband MR system has been installed in Ayios Therissos-Nicosia that delivers crystal clear images, remarkable
ClinScan. think forward. Small Animal MRI Solution for Molecular Imaging and Translational Research. Preclinical MRI
 ClinScan Small Animal MRI Solution for Molecular Imaging and Translational Research think forward Preclinical MRI Be Clinical from the Very Beginning With ClinScan you enter the field of translational
ClinScan Small Animal MRI Solution for Molecular Imaging and Translational Research think forward Preclinical MRI Be Clinical from the Very Beginning With ClinScan you enter the field of translational
CQIE MRI PROCEDURES. American College of Radiology Clinical Research Center. Centers for Quantitative Imaging Excellence LEARNING MODULE
 Centers for Quantitative Imaging Excellence LEARNING MODULE CQIE MRI PROCEDURES American College of Radiology Clinical Research Center Imaging Core Laboratory v.2 Centers for Quantitative Imaging Excellence
Centers for Quantitative Imaging Excellence LEARNING MODULE CQIE MRI PROCEDURES American College of Radiology Clinical Research Center Imaging Core Laboratory v.2 Centers for Quantitative Imaging Excellence
Pre-clinical and postmortem diffusion MRI
 manisha.aggarwal@jhu.edu Pre-clinical and postmortem diffusion MRI Manisha Aggarwal, Ph.D. Department of Radiology Johns Hopkins University School of Medicine Diffusion and tissue microstructure? signal
manisha.aggarwal@jhu.edu Pre-clinical and postmortem diffusion MRI Manisha Aggarwal, Ph.D. Department of Radiology Johns Hopkins University School of Medicine Diffusion and tissue microstructure? signal
BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering
 BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering Outline What is Medical Imaging? History of Medical Imaging X-Ray Imaging
BME101 Introduction to Biomedical Engineering Medical Imaging Özlem BİRGÜL Ankara University Department of Biomedical Engineering Outline What is Medical Imaging? History of Medical Imaging X-Ray Imaging
Magnetic Resonance Spectroscopy from fundamental developments to improved noninvasive diagnosis and characterisation of children s brain tumours.
 Magnetic Resonance Spectroscopy from fundamental developments to improved noninvasive diagnosis and characterisation of children s brain tumours. Martin Wilson IOP Medical Physics Group Scientific and
Magnetic Resonance Spectroscopy from fundamental developments to improved noninvasive diagnosis and characterisation of children s brain tumours. Martin Wilson IOP Medical Physics Group Scientific and
Balancing versatility and value Introducing Optima MR T
 GE Healthcare Balancing versatility and value Introducing Optima MR360 1.5T Optima MR360 1.5T Providing an innovative balance in MR You know what you need from an MR scanner. The new Optima MR360 is engineered
GE Healthcare Balancing versatility and value Introducing Optima MR360 1.5T Optima MR360 1.5T Providing an innovative balance in MR You know what you need from an MR scanner. The new Optima MR360 is engineered
BIOMEDICAL SIGNAL AND IMAGE PROCESSING
 BIOMEDICAL SIGNAL AND IMAGE PROCESSING EE 5390-001 SYLLABUS Instructor: Wei Qian, Ph.D. Professor of Electrical and Computer Engineering Medical Signal and Image Computerized Processing Scheme for Medical
BIOMEDICAL SIGNAL AND IMAGE PROCESSING EE 5390-001 SYLLABUS Instructor: Wei Qian, Ph.D. Professor of Electrical and Computer Engineering Medical Signal and Image Computerized Processing Scheme for Medical
Background. Magnetic Resonance Spectroscopy (MRS) non invasively measures metabolite concentrations
 Applying FID navigators to high field MRS in the body AAPM North Central Chapter Spring Meeting April 7th, 2017 Ryan M. Kalmoe Department of Radiation Oncology, University of Minnesota Advisor: Dr. Gregory
Applying FID navigators to high field MRS in the body AAPM North Central Chapter Spring Meeting April 7th, 2017 Ryan M. Kalmoe Department of Radiation Oncology, University of Minnesota Advisor: Dr. Gregory
Propel your imaging abilities into the future.
 GE Healthcare Propel your imaging abilities into the future. SIGNA CREATOR Imagine what MR can be. Leap ahead and create your future in imaging. Meet SIGNA Creator, named for its ability to help you create
GE Healthcare Propel your imaging abilities into the future. SIGNA CREATOR Imagine what MR can be. Leap ahead and create your future in imaging. Meet SIGNA Creator, named for its ability to help you create
Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow
 Review Article Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow Journal of Cerebral Blood Flow & Metabolism 0(00) 1 20! Author(s) 2017 Reprints and permissions:
Review Article Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow Journal of Cerebral Blood Flow & Metabolism 0(00) 1 20! Author(s) 2017 Reprints and permissions:
Follow this and additional works at: Part of the Physical Sciences and Mathematics Commons
 Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2014 A Feasibility Study of Ultra-Short Echo Time MRI for Positive Contrast Visualization of Prostate Brachytherapy Permanent
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2014 A Feasibility Study of Ultra-Short Echo Time MRI for Positive Contrast Visualization of Prostate Brachytherapy Permanent
Neuro MR: Principles. Review Article. Timothy P.L. Roberts, PhD 1 * and David Mikulis, MD 2
 JOURNAL OF MAGNETIC RESONANCE IMAGING 26:823 837 (2007) Review Article Neuro MR: Principles Timothy P.L. Roberts, PhD 1 * and David Mikulis, MD 2 The principles of contrast mechanisms and fast pulse sequences
JOURNAL OF MAGNETIC RESONANCE IMAGING 26:823 837 (2007) Review Article Neuro MR: Principles Timothy P.L. Roberts, PhD 1 * and David Mikulis, MD 2 The principles of contrast mechanisms and fast pulse sequences
INTRODUCTION. Best Practices in MR Imaging: State-of-the-Art Protocols. 5th Edition. MR Protocols for GE Healthcare Scanners.
 INTRODUCTION These magnetic resonance (MR) protocols were developed by an expert consensus panel for use on General Electric (GE) MR imaging machines, and were developed for high-end platform scanners
INTRODUCTION These magnetic resonance (MR) protocols were developed by an expert consensus panel for use on General Electric (GE) MR imaging machines, and were developed for high-end platform scanners
Preclinical MRI. Solutions for Small Animal Imaging. Molecular Imaging
 Preclinical MRI Solutions for Small Animal Imaging Molecular Imaging The Power of Imaging Applications Resolution Typical resolution in MRI is less than 200μm, to more than 20μm with 2D slices or full
Preclinical MRI Solutions for Small Animal Imaging Molecular Imaging The Power of Imaging Applications Resolution Typical resolution in MRI is less than 200μm, to more than 20μm with 2D slices or full
Principles of translational medicine: imaging, biomarker imaging, theranostics
 Principles of translational medicine: imaging, biomarker imaging, theranostics Compiled by: Endre Mikus PhD, CEO Budapest, 21/9/2015 Imaging and imaging biomarkers An imaging biomarker is an anatomic,
Principles of translational medicine: imaging, biomarker imaging, theranostics Compiled by: Endre Mikus PhD, CEO Budapest, 21/9/2015 Imaging and imaging biomarkers An imaging biomarker is an anatomic,
Clarity CT Technology
 Clarity CT Technology WHITE PAPER January 2013 Using state of the art algorithms Sapheneia Clarity CT allows physicians to lower radiation dose when acquiring CT data while maintaining image quality. The
Clarity CT Technology WHITE PAPER January 2013 Using state of the art algorithms Sapheneia Clarity CT allows physicians to lower radiation dose when acquiring CT data while maintaining image quality. The
Precision in Quantitative Imaging: Trial Development and Quality Assurance
 Precision in Quantitative Imaging: Trial Development and Quality Assurance Susanna I Lee MD, PhD Thanks to: Mitchell Schnall, Mark Rosen. Dan Sullivan, Patrick Bossuyt Imaging Chain: Patient Data Raw data
Precision in Quantitative Imaging: Trial Development and Quality Assurance Susanna I Lee MD, PhD Thanks to: Mitchell Schnall, Mark Rosen. Dan Sullivan, Patrick Bossuyt Imaging Chain: Patient Data Raw data
Supplementary Figure 1 Sizing and Relaxometry of Derivatized Ferumoxytol. a, Dynamic light scattering shows no change in diameter between the
 Supplementary Figure 1 Sizing and Relaxometry of Derivatized Ferumoxytol. a, Dynamic light scattering shows no change in diameter between the clinical specimen (Ferumoxytol) after buffer exchange and the
Supplementary Figure 1 Sizing and Relaxometry of Derivatized Ferumoxytol. a, Dynamic light scattering shows no change in diameter between the clinical specimen (Ferumoxytol) after buffer exchange and the
Overview: Unmet Need: 1 Cell Sense: Enabling in vivo cell tracking
 1 Cell Sense: Enabling in vivo cell tracking Overview: A challenge in the development and translation of new cellular therapies is effective tracking of cells post-transfer in both animal and human subjects.
1 Cell Sense: Enabling in vivo cell tracking Overview: A challenge in the development and translation of new cellular therapies is effective tracking of cells post-transfer in both animal and human subjects.
Magnetic Resonance Imaging at 7T in Glasgow. A unique opportunity!
 Magnetic Resonance Imaging at 7T in Glasgow A unique opportunity! An NHS MR Physicist's perspective... What is different about 7T scanners? What has already been achieved? Imaging Centre of Excellence
Magnetic Resonance Imaging at 7T in Glasgow A unique opportunity! An NHS MR Physicist's perspective... What is different about 7T scanners? What has already been achieved? Imaging Centre of Excellence
Clinical Translation of Tumor Acidosis Measurements with AcidoCEST MRI
 Electronic Supplementary Material Clinical Translation of Tumor Acidosis Measurements with AcidoCEST MRI Journal: Molecular Imaging and Biology Kyle M. Jones, B.S., 1 Edward A. Randtke, Ph.D., 2 Eriko
Electronic Supplementary Material Clinical Translation of Tumor Acidosis Measurements with AcidoCEST MRI Journal: Molecular Imaging and Biology Kyle M. Jones, B.S., 1 Edward A. Randtke, Ph.D., 2 Eriko
Clinical Applications. ImagingRite. Interventional Radiology
 Clinical Applications ImagingRite Interventional Radiology ImagingRite, a comprehensive suite of imaging tools offered with Infinix -i angiographic systems, was designed to assist clinicians in optimizing
Clinical Applications ImagingRite Interventional Radiology ImagingRite, a comprehensive suite of imaging tools offered with Infinix -i angiographic systems, was designed to assist clinicians in optimizing
Supporting Information
 Supporting Information Alenina et al. 10.1073/pnas.0810793106 SI Materials and Methods In Vivo Brain Magnetic Resonance Imaging (MRI). In vivo brain MRI was performed in 6-month-old Tph2 / and control
Supporting Information Alenina et al. 10.1073/pnas.0810793106 SI Materials and Methods In Vivo Brain Magnetic Resonance Imaging (MRI). In vivo brain MRI was performed in 6-month-old Tph2 / and control
MRI Protocols in Experimental Stroke. Xenios Milidonis Centre for Clinical Brain Sciences The University of Edinburgh
 MRI Protocols in Experimental Stroke Xenios Milidonis Centre for Clinical Brain Sciences The University of Edinburgh MultiPART Meeting, Barcelona, 2 October 204 Objective In MultiPART magnetic resonance
MRI Protocols in Experimental Stroke Xenios Milidonis Centre for Clinical Brain Sciences The University of Edinburgh MultiPART Meeting, Barcelona, 2 October 204 Objective In MultiPART magnetic resonance
UNIVERSITY OF CINCINNATI
 UNIVERSITY OF CINCINNATI Date: I,, hereby submit this work as part of the requirements for the degree of: in: It is entitled: This work and its defense approved by: Chair: In Vivo MR Microscopy of Tumor-
UNIVERSITY OF CINCINNATI Date: I,, hereby submit this work as part of the requirements for the degree of: in: It is entitled: This work and its defense approved by: Chair: In Vivo MR Microscopy of Tumor-
Powerful MRI, Simplified. Innovation with Integrity. ICON, compact MRI system. Compact MRI
 Powerful MRI, Simplified ICON, compact MRI system Innovation with Integrity Compact MRI Preclinical MRI with the ICON : Powerful insights, simplicity in operation Traditionally MRI technology has been
Powerful MRI, Simplified ICON, compact MRI system Innovation with Integrity Compact MRI Preclinical MRI with the ICON : Powerful insights, simplicity in operation Traditionally MRI technology has been
Magnetic Resonance Imaging of concrete. Coring. Location of flaws. Assessment of Concrete Structures. Systems to monitor concrete modulus
 Magnetic Resonance Imaging of concrete Dr Chris Burgoyne Department of Engineering University of Cambridge Assessment of Concrete Structures How can we tell what is going on inside concrete? We would like
Magnetic Resonance Imaging of concrete Dr Chris Burgoyne Department of Engineering University of Cambridge Assessment of Concrete Structures How can we tell what is going on inside concrete? We would like
The first MR with IQ Philips Intera 1.5T With SmartExam
 The first MR with IQ Philips Intera 1.5T With SmartExam Smart Exam reproducibility 2 consistency, and efficiency Philips Intera 1.5T gives you the image quality, patient comfort and range of clinical applications
The first MR with IQ Philips Intera 1.5T With SmartExam Smart Exam reproducibility 2 consistency, and efficiency Philips Intera 1.5T gives you the image quality, patient comfort and range of clinical applications
QUANTIFICATION OF PHARMACOKINETICS IN SMALL ANIMALS WITH MOLECULAR IMAGING AND COMPARTMENT MODELING ANALYSIS YU HUA FANG
 QUANTIFICATION OF PHARMACOKINETICS IN SMALL ANIMALS WITH MOLECULAR IMAGING AND COMPARTMENT MODELING ANALYSIS BY YU HUA FANG Submitted in partial fulfillment of the requirements For the degree of Doctor
QUANTIFICATION OF PHARMACOKINETICS IN SMALL ANIMALS WITH MOLECULAR IMAGING AND COMPARTMENT MODELING ANALYSIS BY YU HUA FANG Submitted in partial fulfillment of the requirements For the degree of Doctor
Computer Assisted Surgery Basics of medical imaging
 Computer Assisted Surgery Basics of medical imaging Prof. Leo Joskowicz School of Engineering and Computer Science The Hebrew University of Jerusalem, ISRAEL Medical Image Processing Basics of medical
Computer Assisted Surgery Basics of medical imaging Prof. Leo Joskowicz School of Engineering and Computer Science The Hebrew University of Jerusalem, ISRAEL Medical Image Processing Basics of medical
Introduction: what do we mean by Quantitative MRI?
 Introduction: what do we mean by Quantitative MRI? Paul Tofts PhD formerly Chair in Imaging Physics Brighton and Sussex Medical School, UK ISMRM 2010 Quantitative MRI slide 1 Overview 1. Quantification:
Introduction: what do we mean by Quantitative MRI? Paul Tofts PhD formerly Chair in Imaging Physics Brighton and Sussex Medical School, UK ISMRM 2010 Quantitative MRI slide 1 Overview 1. Quantification:
Master of Molecular Imaging Course Outline
 Master of Molecular Imaging Course Outline Graduate Outcomes On completion of the course, graduates will have achieved the following skills, knowledge and attributes: chemistry/pharmacy physics/engineering
Master of Molecular Imaging Course Outline Graduate Outcomes On completion of the course, graduates will have achieved the following skills, knowledge and attributes: chemistry/pharmacy physics/engineering
Translational & Molecular Imaging Institute
 Translational & Molecular Imaging Institute tmii.mssm.edu Summer 2015 CARDIOVASCULAR IMAGING The Imaging Research Center is the backbone of the Translational & Molecular Imaging Institute at Mount Sinai
Translational & Molecular Imaging Institute tmii.mssm.edu Summer 2015 CARDIOVASCULAR IMAGING The Imaging Research Center is the backbone of the Translational & Molecular Imaging Institute at Mount Sinai
AN AUTOMATED SYRINGE PUMP SYSTEM FOR IMPROVING THE REPRODUCIBILITY OF DYNAMIC HYPERPOLARIZED MRI PHANTOMS
 Texas Medical Center Library DigitalCommons@TMC UT GSBS Dissertations and Theses (Open Access) Graduate School of Biomedical Sciences 8-2016 AN AUTOMATED SYRINGE PUMP SYSTEM FOR IMPROVING THE REPRODUCIBILITY
Texas Medical Center Library DigitalCommons@TMC UT GSBS Dissertations and Theses (Open Access) Graduate School of Biomedical Sciences 8-2016 AN AUTOMATED SYRINGE PUMP SYSTEM FOR IMPROVING THE REPRODUCIBILITY
Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng
 Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
Scientific Reports Multiplexed 3D FRET imaging in deep tissue of live embryos Ming Zhao, Xiaoyang Wan, Yu Li, Weibin Zhou and Leilei Peng 1 Supplementary figures and notes Supplementary Figure S1 Volumetric
MRI. Magnetic Resonance Imaging
 MRI Magnetic Resonance Imaging Key Points MRI: The magnet is always on Know the essential components of an MRI system Know general idea of how MRI captures an image; three steps: Magnetic Alignment, Proton
MRI Magnetic Resonance Imaging Key Points MRI: The magnet is always on Know the essential components of an MRI system Know general idea of how MRI captures an image; three steps: Magnetic Alignment, Proton
High-resolution functional magnetic resonance imaging of the animal brain
 Methods 30 (2003) 28 41 www.elsevier.com/locate/ymeth High-resolution functional magnetic resonance imaging of the animal brain Seong-Gi Kim a, * and Kamil Ugurbil b a Department of Neurobiology, University
Methods 30 (2003) 28 41 www.elsevier.com/locate/ymeth High-resolution functional magnetic resonance imaging of the animal brain Seong-Gi Kim a, * and Kamil Ugurbil b a Department of Neurobiology, University
CT to Ultrasound Registration: A Porcine Phantom Study. J. Xiang, S. Gill, C. Nguan, P. Abolmaesumi and R. N. Rohling University of British Columbia
 CT to Ultrasound Registration: A Porcine Phantom Study J. Xiang, S. Gill, C. Nguan, P. Abolmaesumi and R. N. Rohling University of British Columbia 1 Motivation Ultrasound to CT registration can improve
CT to Ultrasound Registration: A Porcine Phantom Study J. Xiang, S. Gill, C. Nguan, P. Abolmaesumi and R. N. Rohling University of British Columbia 1 Motivation Ultrasound to CT registration can improve
Design and Preliminary Clinical Studies of an MRI-Guided Transrectal Prostate Intervention System
 Design and Preliminary Clinical Studies of an MRI-Guided Transrectal Prostate Intervention System Axel Krieger 1, Peter Guion 2, Csaba Csoma 1, Iulian Iordachita 1, Anurag K. Singh 2,3, Aradhana Kaushal
Design and Preliminary Clinical Studies of an MRI-Guided Transrectal Prostate Intervention System Axel Krieger 1, Peter Guion 2, Csaba Csoma 1, Iulian Iordachita 1, Anurag K. Singh 2,3, Aradhana Kaushal
BIOMEDICAL ENGINEERING (BME)
 Biomedical Engineering (BME) 1 BIOMEDICAL ENGINEERING (BME) BME 500 Introduction to Biomedical Engineering Introduction to the concepts and research in biomedical engineering. Provides an overview of current
Biomedical Engineering (BME) 1 BIOMEDICAL ENGINEERING (BME) BME 500 Introduction to Biomedical Engineering Introduction to the concepts and research in biomedical engineering. Provides an overview of current
First Experiences with the World s First MAGNETOM Vida
 8 Clinical Whole-body Imaging First Experiences with the World s First MAGNETOM Vida Mike Notohamiprodjo, M.D. Associate Chair and Section Chief MRI, Department of Diagnostic and Interventional Radiology,
8 Clinical Whole-body Imaging First Experiences with the World s First MAGNETOM Vida Mike Notohamiprodjo, M.D. Associate Chair and Section Chief MRI, Department of Diagnostic and Interventional Radiology,
Profile: DCE MRI Quantification
 1 2 3 4 5 6 7 8 Profile: DCE MRI Quantification Version 1.0 Reviewed Draft (Public Comments Addressed) July 1, 2012 Page: 1 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34
1 2 3 4 5 6 7 8 Profile: DCE MRI Quantification Version 1.0 Reviewed Draft (Public Comments Addressed) July 1, 2012 Page: 1 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34
OUR WISH LIST RESEARCH EQUIPMENT
 OUR WISH LIST RESEARCH EQUIPMENT WITH YOUR PHILANTHROPIC SUPPORT, WE CAN WORK TOGETHER TO COMPLETE OUR FULLY-FUNCTIONAL FACILITY BY PURCHASING THE CUTTING-EDGE EQUIPMENT AND RESOURCES TO SUPPORT SAHMRI
OUR WISH LIST RESEARCH EQUIPMENT WITH YOUR PHILANTHROPIC SUPPORT, WE CAN WORK TOGETHER TO COMPLETE OUR FULLY-FUNCTIONAL FACILITY BY PURCHASING THE CUTTING-EDGE EQUIPMENT AND RESOURCES TO SUPPORT SAHMRI
OUR WISH LIST RESEARCH EQUIPMENT
 OUR WISH LIST RESEARCH EQUIPMENT YOU CAN MAKE A TANGIBLE DIFFERENCE! The South Australian Health and Medical Research Institute (SAHMRI) is one of the most exciting developments in the field of health
OUR WISH LIST RESEARCH EQUIPMENT YOU CAN MAKE A TANGIBLE DIFFERENCE! The South Australian Health and Medical Research Institute (SAHMRI) is one of the most exciting developments in the field of health
Introduction to Quantitative Imaging as a Biomarker in Clinical Trials
 Quantitative Medical Imaging for Clinical Research and Practice Educational Session ACRIN 2009 Introduction to Quantitative Imaging as a Biomarker in Clinical Trials Katarzyna J. Macura, MD, PhD Johns
Quantitative Medical Imaging for Clinical Research and Practice Educational Session ACRIN 2009 Introduction to Quantitative Imaging as a Biomarker in Clinical Trials Katarzyna J. Macura, MD, PhD Johns
RADIATION ONCOLOGY RESIDENCY PROGRAM Competency Evaluation of Resident
 Resident s Name: RADIATION ONCOLOGY RESIDENCY PROGRAM Competency Evaluation of Resident Rotation: PHYS 705: Clinical Rotation 3 Inclusive dates of rotation: Aug. 25, 2015 Feb. 25, 2016 Director or Associate
Resident s Name: RADIATION ONCOLOGY RESIDENCY PROGRAM Competency Evaluation of Resident Rotation: PHYS 705: Clinical Rotation 3 Inclusive dates of rotation: Aug. 25, 2015 Feb. 25, 2016 Director or Associate
Translational Multimodality Optical Imaging
 Translational Multimodality Optical Imaging Fred S. Azar Xavier Intes Editors 0 ARTECH H O U S E BOSTON LONDON artechhouse.com Contents Foreword Preface xv xvii CHAPTER1 Introduction to Clinical Optical
Translational Multimodality Optical Imaging Fred S. Azar Xavier Intes Editors 0 ARTECH H O U S E BOSTON LONDON artechhouse.com Contents Foreword Preface xv xvii CHAPTER1 Introduction to Clinical Optical
Angio PL.U.S. PLaneWave UltraSensitive TM ultrasound imaging. White Paper
 White Paper Angio PL.U.S. PLaneWave UltraSensitive TM ultrasound imaging Jeremy Bercoff, Vice President of Product Management & Ultrasound Engineering Thomas Frappart, R&D Ultrasound Engineer Introduction
White Paper Angio PL.U.S. PLaneWave UltraSensitive TM ultrasound imaging Jeremy Bercoff, Vice President of Product Management & Ultrasound Engineering Thomas Frappart, R&D Ultrasound Engineer Introduction
Profile: DCE MRI Quantification
 1 2 3 4 5 6 7 Profile: DCE MRI Quantification Version 1.0 May 9, 2012 Page: 1 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Table of Contents I. Executive Summary... 3 II.
1 2 3 4 5 6 7 Profile: DCE MRI Quantification Version 1.0 May 9, 2012 Page: 1 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Table of Contents I. Executive Summary... 3 II.
High-Speed 3T MR Spectroscopic Imaging of Prostate With Flyback Echo-Planar Encoding
 JOURNAL OF MAGNETIC RESONANCE IMAGING 25:1288 1292 (2007) Clinical Note High-Speed 3T MR Spectroscopic Imaging of Prostate With Flyback Echo-Planar Encoding Albert P. Chen, PhD, 1,2 Charles H. Cunningham,
JOURNAL OF MAGNETIC RESONANCE IMAGING 25:1288 1292 (2007) Clinical Note High-Speed 3T MR Spectroscopic Imaging of Prostate With Flyback Echo-Planar Encoding Albert P. Chen, PhD, 1,2 Charles H. Cunningham,
Prof. Steven S. Saliterman
 Department of Biomedical Engineering, University of Minnesota http://saliterman.umn.edu/ Prof. Angela Panoskaltsis-Mortari s BMEn 5361, 3D Bioprinting Tissue engineering Bioprinting Design considerations
Department of Biomedical Engineering, University of Minnesota http://saliterman.umn.edu/ Prof. Angela Panoskaltsis-Mortari s BMEn 5361, 3D Bioprinting Tissue engineering Bioprinting Design considerations
Registration of 3D Ultrasound to Computed Tomography Images of the Kidney
 Registration of 3D Ultrasound to Computed Tomography Images of the Kidney Jing Xiang Supervisors: Dr. Robert Rohling Dr. Purang Abolmaesumi Electrical and Computer Engineering University of British Columbia
Registration of 3D Ultrasound to Computed Tomography Images of the Kidney Jing Xiang Supervisors: Dr. Robert Rohling Dr. Purang Abolmaesumi Electrical and Computer Engineering University of British Columbia
Insight Through In Vivo Imaging
 Insight Through In Vivo Imaging Resolution Revolution in Realtime The Vevo 770 provides anatomical, functional and molecular data in realtime, on an affordable, easy to use and translational platform.
Insight Through In Vivo Imaging Resolution Revolution in Realtime The Vevo 770 provides anatomical, functional and molecular data in realtime, on an affordable, easy to use and translational platform.
BIOMARKER DISCOVERY IN LOCALLY ADVANCED CERVICAL CANCER: IQ-EMBRACE PETRA VAN HOUDT, JESPER KALLEHAUGE, UULKE VAN DER HEIDE, KARI TANDERUP MARCH 23,
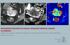 BIOMARKER DISCOVERY IN LOCALLY ADVANCED CERVICAL CANCER: IQ-EMBRACE PETRA VAN HOUDT, JESPER KALLEHAUGE, UULKE VAN DER HEIDE, KARI TANDERUP MARCH 23, 2018 Non-responder Responder DCE-MRI FOR PREDICTION
BIOMARKER DISCOVERY IN LOCALLY ADVANCED CERVICAL CANCER: IQ-EMBRACE PETRA VAN HOUDT, JESPER KALLEHAUGE, UULKE VAN DER HEIDE, KARI TANDERUP MARCH 23, 2018 Non-responder Responder DCE-MRI FOR PREDICTION
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
Advantages and challenges of whole-body MR-PET. Gaspar Delso Axel Martinez-Möller Stephan Nekolla Sibylle Ziegler
 Advantages and challenges of whole-body MR-PET Gaspar Delso Axel Martinez-Möller Stephan Nekolla Sibylle Ziegler Why MR+PET? MR High spatial resolution (~1mm) Good imaging of anatomy. Excellent soft-tissue
Advantages and challenges of whole-body MR-PET Gaspar Delso Axel Martinez-Möller Stephan Nekolla Sibylle Ziegler Why MR+PET? MR High spatial resolution (~1mm) Good imaging of anatomy. Excellent soft-tissue
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
Marco Essig MD, Department of Radiology, German Cancer Research Center, Heidelberg, Germany.
 What is new in contrast enhanced MRI Marco Essig MD, Department of Radiology, German Cancer Research Center, Heidelberg, Germany. Contact: m.essig@dkfz.de To improve sensitivity and specificity of MRI
What is new in contrast enhanced MRI Marco Essig MD, Department of Radiology, German Cancer Research Center, Heidelberg, Germany. Contact: m.essig@dkfz.de To improve sensitivity and specificity of MRI
Acoustically targeted chemogenetics for the non-invasive control of neural circuits
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41551-018-0258-2 In the format provided by the authors and unedited. Acoustically targeted chemogenetics for the non-invasive control of neural
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41551-018-0258-2 In the format provided by the authors and unedited. Acoustically targeted chemogenetics for the non-invasive control of neural
Histology Pattern Recognition Software in Investigative Pathology
 Histology Pattern Recognition Software in Investigative Pathology J. Webster, DVM, PhD, DACVP Laboratory of Cancer Biology and Genetics National Cancer Institute, Bethesda, MD Pathology Visions 2011 November
Histology Pattern Recognition Software in Investigative Pathology J. Webster, DVM, PhD, DACVP Laboratory of Cancer Biology and Genetics National Cancer Institute, Bethesda, MD Pathology Visions 2011 November
Small Animal Imaging Facility Newsletter
 Small Animal Imaging Facility Newsletter Biomedical Research Imaging Center Volume 2. Issue. 2 SAI Facility News and Updates 1. The BRIC website has a new look (http://bric.unc.edu). There were some minor
Small Animal Imaging Facility Newsletter Biomedical Research Imaging Center Volume 2. Issue. 2 SAI Facility News and Updates 1. The BRIC website has a new look (http://bric.unc.edu). There were some minor
Photoacoustic Imaging in Biomedicine Critical Review by Saurabh Vyas Group 9: Interventional Photoacoustic Ultrasound CIS II: 600.
 Photoacoustic Imaging in Biomedicine Critical Review by Saurabh Vyas Group 9: Interventional Photoacoustic Ultrasound CIS II: 600.446, Spring 2011 Introduction Photoacoustic imaging (PA Imaging) is the
Photoacoustic Imaging in Biomedicine Critical Review by Saurabh Vyas Group 9: Interventional Photoacoustic Ultrasound CIS II: 600.446, Spring 2011 Introduction Photoacoustic imaging (PA Imaging) is the
Diagnostic Medical Image Processing
 Diagnostic Medical Image Processing Introduction WS 2010/11 Joachim Hornegger, Dietrich Paulus, Markus Kowarschik Lehrstuhl für Mustererkennung (Informatik 5) Friedrich-Alexander-Universität Erlangen-Nürnberg
Diagnostic Medical Image Processing Introduction WS 2010/11 Joachim Hornegger, Dietrich Paulus, Markus Kowarschik Lehrstuhl für Mustererkennung (Informatik 5) Friedrich-Alexander-Universität Erlangen-Nürnberg
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-07-1-0231 TITLE: Spectroscopic Photoacoustic Tomography of Prostate Cancer PRINCIPAL INVESTIGATOR: Xueding Wang CONTRACTING ORGANIZATION: University Of Michigan Ann Arbor, MI 48109-1274
AD Award Number: W81XWH-07-1-0231 TITLE: Spectroscopic Photoacoustic Tomography of Prostate Cancer PRINCIPAL INVESTIGATOR: Xueding Wang CONTRACTING ORGANIZATION: University Of Michigan Ann Arbor, MI 48109-1274
Fast enough to stop the Capable of delineating Unprecedented imaging power for the. Virtual endoscopy. The gatewa
 Fast enough to stop the Capable of delineating Unprecedented imaging power for the M U L T I S L I C E Virtual endoscopy The gatewa motion of a beating heart. anatomic structures as small as 0.25mm. earliest,
Fast enough to stop the Capable of delineating Unprecedented imaging power for the M U L T I S L I C E Virtual endoscopy The gatewa motion of a beating heart. anatomic structures as small as 0.25mm. earliest,
1. RF ablation guarding circuits for EIT
 1. RF ablation guarding circuits for EIT Synopsis: Intracardiac and endovascular ablation therapies are widespread in their use in the treatment of a variety of cardiac arrhythmias as well as renal artery
1. RF ablation guarding circuits for EIT Synopsis: Intracardiac and endovascular ablation therapies are widespread in their use in the treatment of a variety of cardiac arrhythmias as well as renal artery
GE Healthcare. Introducing Brivo MR T. Bringing high-field MR. within reach
 GE Healthcare Introducing Brivo MR355 1.5T Bringing high-field MR within reach If 1.5T MR technology has been just out of reach for your practice, the Brivo MR355 will be a welcome advancement. The Brivo
GE Healthcare Introducing Brivo MR355 1.5T Bringing high-field MR within reach If 1.5T MR technology has been just out of reach for your practice, the Brivo MR355 will be a welcome advancement. The Brivo
FEASIBILITY STUDY OF MULTI-APPLICATION, MULTI-WALLED CARBON NANOTUBES FOR MAGNETIC RESONANCE TEMPERATURE IMAGING GUIDED LASER INDUCED THERMAL THERAPY
 FEASIBILITY STUDY OF MULTI-APPLICATION, MULTI-WALLED CARBON NANOTUBES FOR MAGNETIC RESONANCE TEMPERATURE IMAGING GUIDED LASER INDUCED THERMAL THERAPY By Xuanfeng Ding A Thesis Submitted to the Graduate
FEASIBILITY STUDY OF MULTI-APPLICATION, MULTI-WALLED CARBON NANOTUBES FOR MAGNETIC RESONANCE TEMPERATURE IMAGING GUIDED LASER INDUCED THERMAL THERAPY By Xuanfeng Ding A Thesis Submitted to the Graduate
Implementation of fast macromolecular proton fraction mapping on 1.5 and 3 Tesla clinical MRI scanners: preliminary experience
 Journal of Physics: Conference Series PAPER OPEN ACCESS Implementation of fast macromolecular proton fraction mapping on 1.5 and 3 Tesla clinical MRI scanners: preliminary experience To cite this article:
Journal of Physics: Conference Series PAPER OPEN ACCESS Implementation of fast macromolecular proton fraction mapping on 1.5 and 3 Tesla clinical MRI scanners: preliminary experience To cite this article:
Imaging INNOVATION. Innovative Technology For Preclinical Imaging. Powerful Yet Simple To Use
 Imaging INNOVATION Innovative Technology For Preclinical Imaging Powerful Yet Simple To Use About MR SOLUTIONS MRS is a world leader in MRI technology and the leading developer and manufacturer of the
Imaging INNOVATION Innovative Technology For Preclinical Imaging Powerful Yet Simple To Use About MR SOLUTIONS MRS is a world leader in MRI technology and the leading developer and manufacturer of the
GENESIS Edition. Transforming CT
 GENESIS Edition Transforming CT Transforming clinical confidence Transforming patient experience Transforming your workspace GENESIS Edition Transforming CT Brought to you by the leaders in area detector
GENESIS Edition Transforming CT Transforming clinical confidence Transforming patient experience Transforming your workspace GENESIS Edition Transforming CT Brought to you by the leaders in area detector
An ultrasonic volumetric scanner for image-guided surgery
 International Congress Series 1230 (2001) 190 196 An ultrasonic volumetric scanner for image-guided surgery Jeremy Johnson a,b, *,Ömer Oralkan b, Kambiz Kaviani b, Utkan Demirci b, Mustafa Karaman b, Pierre
International Congress Series 1230 (2001) 190 196 An ultrasonic volumetric scanner for image-guided surgery Jeremy Johnson a,b, *,Ömer Oralkan b, Kambiz Kaviani b, Utkan Demirci b, Mustafa Karaman b, Pierre
In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating. melanoma cells
 In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells Yun He 1,, Lidai Wang 1,,, Junhui Shi 1,, Junjie Yao 1, Lei Li 1, Ruiying Zhang 1, Chih-Hsien
In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells Yun He 1,, Lidai Wang 1,,, Junhui Shi 1,, Junjie Yao 1, Lei Li 1, Ruiying Zhang 1, Chih-Hsien
1st Faculty of Medicine, Charles University in Prague Center for Advanced Preclinical Imaging (CAPI)
 ADVANTAGES Optical Imaging OI Optical Imaging is based on the detection of weak light by a highly sensitive and high resolution CCD camera DISADVANTAGES High sensitivity Limited penetration depth Easy
ADVANTAGES Optical Imaging OI Optical Imaging is based on the detection of weak light by a highly sensitive and high resolution CCD camera DISADVANTAGES High sensitivity Limited penetration depth Easy
Part 3 Oral Exam Content Guide
 Initial Certification in Medical Physics Part 3 Oral Exam Content Guide The oral examination is designed to test your knowledge and fitness to practice applied medical physics in the specified specialty(ies).
Initial Certification in Medical Physics Part 3 Oral Exam Content Guide The oral examination is designed to test your knowledge and fitness to practice applied medical physics in the specified specialty(ies).
Disclaimer. Fat in the body. Outline. Managing fat in MRI: a technical perspective
 Managing fat in MRI: a technical perspective Jingfei Ma, PhD, DABR Department of Imaging Physics Disclaimer The speaker is an inventor of US patents or patent applications that are licensed to GE Healthcare
Managing fat in MRI: a technical perspective Jingfei Ma, PhD, DABR Department of Imaging Physics Disclaimer The speaker is an inventor of US patents or patent applications that are licensed to GE Healthcare
Arterial Spin Labelling Magnetic Resonance. Imaging of the Brain: Techniques and
 Arterial Spin Labelling Magnetic Resonance Imaging of the Brain: Techniques and Development Ph.D Thesis Jack Anthony Wells 1,2 1 The Advanced Magnetic Resonance Imaging Group Department of Medical Physics
Arterial Spin Labelling Magnetic Resonance Imaging of the Brain: Techniques and Development Ph.D Thesis Jack Anthony Wells 1,2 1 The Advanced Magnetic Resonance Imaging Group Department of Medical Physics
Clinical Applications. ImagingRite. Neuro Intervention. 1 ImagingRite
 Clinical Applications ImagingRite Neuro Intervention 1 ImagingRite ImagingRite, a comprehensive suite of imaging tools offered with Infinix -i angiographic systems, was designed to assist clinicians in
Clinical Applications ImagingRite Neuro Intervention 1 ImagingRite ImagingRite, a comprehensive suite of imaging tools offered with Infinix -i angiographic systems, was designed to assist clinicians in
Long-term dynamics of CA1 hippocampal place codes
 Long-term dynamics of CA1 hippocampal place codes Yaniv Ziv, Laurie D. Burns, Eric D. Cocker, Elizabeth O. Hamel, Kunal K. Ghosh, Lacey J. Kitch, Abbas El Gamal, and Mark J. Schnitzer Supplementary Fig.
Long-term dynamics of CA1 hippocampal place codes Yaniv Ziv, Laurie D. Burns, Eric D. Cocker, Elizabeth O. Hamel, Kunal K. Ghosh, Lacey J. Kitch, Abbas El Gamal, and Mark J. Schnitzer Supplementary Fig.
New Techniques and Optimizations of Short Echo-time 1H MRI with Applications in Murine Lung
 Washington University in St. Louis Washington University Open Scholarship Arts & Sciences Electronic Theses and Dissertations Arts & Sciences Winter 12-15-2016 New Techniques and Optimizations of Short
Washington University in St. Louis Washington University Open Scholarship Arts & Sciences Electronic Theses and Dissertations Arts & Sciences Winter 12-15-2016 New Techniques and Optimizations of Short
Magnetic Resonance Imaging. D. J. McMahon rev cewood
 Magnetic Resonance Imaging D. J. McMahon 150504 rev cewood 2018-02-15 Key Points MRI: The magnet is always on Know the essential components of an MRI system Know general idea of how MRI captures an image;
Magnetic Resonance Imaging D. J. McMahon 150504 rev cewood 2018-02-15 Key Points MRI: The magnet is always on Know the essential components of an MRI system Know general idea of how MRI captures an image;
Quantification in emission tomography: challenges, solutions, performance and impact
 EuroMedIm 2006 Quantification in emission tomography: challenges, solutions, performance and impact Irène Buvat U678 INSERM, Paris buvat@imed.jussieu.fr http://www.guillemet.org/irene EuroMedIm 2006 -
EuroMedIm 2006 Quantification in emission tomography: challenges, solutions, performance and impact Irène Buvat U678 INSERM, Paris buvat@imed.jussieu.fr http://www.guillemet.org/irene EuroMedIm 2006 -
your standard Making high performance Powered by ostream Multiva 1.5T MR systems This material is not for distribution in the USA.
 Multiva 1.5T MR systems This material is not for distribution in the USA. 2015 Koninklijke Philips N.V. All rights reserved. Specifications are subject to change without notice. Trademarks are the property
Multiva 1.5T MR systems This material is not for distribution in the USA. 2015 Koninklijke Philips N.V. All rights reserved. Specifications are subject to change without notice. Trademarks are the property
High quality and fast scanning with Compressed SENSE at KCH
 FieldStrength MRI articles High quality and fast scanning with at KCH FieldStrength MRI magazine User experiences - November 2018 www.philips.com/fieldstrength At Kurashiki Central Hospital, use of resulted
FieldStrength MRI articles High quality and fast scanning with at KCH FieldStrength MRI magazine User experiences - November 2018 www.philips.com/fieldstrength At Kurashiki Central Hospital, use of resulted
Geneva January 10 11, th Swiss Experimental. Surgery Symposium. MGY / 4thSESS /
 4th Swiss Experimental Surgery Symposium Geneva January 10 11, 2008 Source: Reflex issue 2, 2007 The 3 Rs Reduction Replacement Refinement NC3R http://www.nc3rs.org.uk/category.asp?catid =9 No specific
4th Swiss Experimental Surgery Symposium Geneva January 10 11, 2008 Source: Reflex issue 2, 2007 The 3 Rs Reduction Replacement Refinement NC3R http://www.nc3rs.org.uk/category.asp?catid =9 No specific
MRI in Clinical Practice
 MRI in Clinical Practice MRI in Clinical Practice Gary Liney With 62 Figures Gary Liney, PhD MRI Lecturer University of Hull Centre for MR Investigations Hull Royal Infirmary Hull UK British Library Cataloguing
MRI in Clinical Practice MRI in Clinical Practice Gary Liney With 62 Figures Gary Liney, PhD MRI Lecturer University of Hull Centre for MR Investigations Hull Royal Infirmary Hull UK British Library Cataloguing
Reduced Field-of-View MRI Using Outer Volume Suppression for Spinal Cord Diffusion Imaging
 Magnetic Resonance in Medicine 57:625 630 (2007) Reduced Field-of-View MRI Using Outer Volume Suppression for Spinal Cord Diffusion Imaging B.J. Wilm, 1,2 * J. Svensson, 1,3 A. Henning, 2 K.P. Pruessmann,
Magnetic Resonance in Medicine 57:625 630 (2007) Reduced Field-of-View MRI Using Outer Volume Suppression for Spinal Cord Diffusion Imaging B.J. Wilm, 1,2 * J. Svensson, 1,3 A. Henning, 2 K.P. Pruessmann,
Solid stress and elastic energy as measures of tumour mechanopathology
 VOLUME: 1 ARTICLE NUMBER: 0004 In the format provided by the authors and unedited. Solid stress and elastic energy as measures of tumour mechanopathology Hadi T. Nia 1, Hao Liu 1,2, Giorgio Seano 1, Meenal
VOLUME: 1 ARTICLE NUMBER: 0004 In the format provided by the authors and unedited. Solid stress and elastic energy as measures of tumour mechanopathology Hadi T. Nia 1, Hao Liu 1,2, Giorgio Seano 1, Meenal
Physicists Quality Control for MR Equipment
 Physicists Quality Control for MR Equipment Geoffrey D. Clarke, Ph.D. University of Texas Health Science Center at San Antonio 1 Overview ABR and the role of the Qualified Medical Physicist/ MR Scientist
Physicists Quality Control for MR Equipment Geoffrey D. Clarke, Ph.D. University of Texas Health Science Center at San Antonio 1 Overview ABR and the role of the Qualified Medical Physicist/ MR Scientist
