TRELLIS COLLAGEN RIBBON
|
|
|
- Candace Arnold
- 6 years ago
- Views:
Transcription
1 TRELLIS COLLAGEN RIBBON English (en) The following languages are included in this packet: M Wright Medical Technology, Inc Airline Rd. Arlington, TN USA August 2012 Printed in U.S.A.
2
3 Attention Operating Surgeon IMPORTANT MEDICAL INFORMATION WRIGHT MEDICAL TRELLIS Collagen Ribbon OUTLINE: DEFINITIONS GENERAL PRODUCT INFORMATION A. INDICATIONS B. CONTRAINDICATIONS C. POTENTIAL COMPLICATIONS D. PRECAUTIONS E. ADVERSE REACTIONS F. HANDLING & STERILIZATION G. STORAGE CONDITIONS H. DIRECTIONS FOR USE EN 1
4 DEFINITIONS Symbols and abbreviations may be used on the package label. The following table provides the definition of these symbols and abbreviations. Table 1. Definitions of Symbols and Abbreviations Symbol g h D Y i H l p N M I P Definition Batch code Catalog number Do not re-use Caution, consult accompanying documents Consult operating instructions Use by Storage temperature limitation Keep dry Keep away from sunlight Date of manufacture Manufacturer Sterilized using ethylene oxide Do not resterilize For prescription use only Authorized EC Representative in the European Community Do not use if package is damaged 2
5 GENERAL PRODUCT INFORMATION TRELLIS Collagen Ribbon is a sterile, non-perforated processed porcine collagen dermal matrix, which is rehydrated at the time of implant. A. INDICATIONS The TRELLIS Collagen Ribbon is intended to reinforce soft tissue where weakness exists, specifically, for the reinforcement of soft tissue repaired by sutures or suture anchors during tendon repair surgery, including reinforcement of the rotator cuff, patellar, Achilles, biceps, quadriceps, peroneal, posterial tibial, and other tendons. The TRELLIS Collagen Ribbon is not intended to replace normal body structure or provide the full mechanical strength to support tendon repair. Sutures used to repair the tear and suture or bone anchors used to attach the tissue to the bone provide biomechanical strength for the tendon repair. B. CONTRAINDICATIONS TRELLIS Collagen Ribbon is contraindicated for use in any patient with known sensitivity to porcine products, or on patients with history of multiple or serum allergies. Not for reconstruction of cardiovascular defects. Not for reconstruction of central nervous system or peripheral nervous system defects. C. POTENTIAL COMPLICATIONS Proper surgical procedures and techniques are the responsibility of the medical professional. Each surgeon must evaluate the appropriateness of the procedure used based on personal medical training and experience. Although Wright Medical cannot recommend a particular surgical technique suitable for all patients, a detailed surgical technique is available for surgeon reference. D. PRECAUTIONS DO NOT use TRELLIS Collagen Ribbon if either the outer foil bag or either of the inner pouches are damaged or torn. Damaged packaging may result in degradation or contamination of the product. Do not use product beyond indicated expiration date. E. ADVERSE REACTIONS Adverse reactions that are possible with the use of a porcine dermal matrix include, but are not limited to, contamination, infection, inflammation, adhesion, drainage, seroma formation, hematoma, and fever. If an infection or allergic reaction occurs, the entire matrix may have to be revised or removed. 3
6 F. HANDLING AND STERILIZATION TRELLIS Collagen Ribbon is supplied sterile in a sealed package and is intended for single use only. Only the inner-most pouch is sterile. The outer foil bag and middle pouch cannot be placed in the sterile field. Do not use if package is damaged. Immediately return damaged product to Wright Medical Technology, Inc. DO NOT RE-STERILIZE. Re-sterilization may damage the product. Reuse of these devices may potentially result in serious patient harm. Examples of hazards related to the reuse of these devices include, but are not limited to: significant degradation in device performance, cross-infection, and contamination. Strict aseptic technique should be followed when preparing the product for use. G. STORAGE CONDITIONS STORE AT ROOM TEMPERATURE. Do not use product if temperature exceeds 99 F (37 C) or indicator (located on foil bag) is black. Immediately return product to Wright Medical Technology, Inc. H. DIRECTIONS FOR USE 1. Remove from packaging using an aseptic technique. 2. Only the inner-most pouch is sterile. The outer foil bag and middle pouch cannot be placed in the sterile field. 3. The matrix may be trimmed to the required dimension prior to or after rehydration. 4. The matrix must be rehydrated prior to use. The matrix may be rehydrated in sterile saline for a minimum of 10 minutes, but no more than 4 hours. At the surgeon s discretion, the matrix may be rehydrated in autologous blood, which may take up to 50 minutes to completely rehydrate. 5. The matrix is rehydrated when it is soft and pliable throughout. The matrix may require a gentle massage to complete the rehydration process. 6. After use, handle and dispose of any unused portions of the matrix in accordance with accepted medical practice and applicable laws and regulations. CAUTION: Federal Law (U.S.) restricts this device to the sale, distribution, and use by or on the order of a physician. 4
BIOTAPE XM TISSUE MATRIX The following languages are included in this packet:
 BIOTAPE XM TISSUE MATRIX 150866-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -Chinese (sch)
BIOTAPE XM TISSUE MATRIX 150866-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -Chinese (sch)
MIIG INJECTABLE Graft The following languages are included in this packet:
 MIIG INJECTABLE Graft 128801-10 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
MIIG INJECTABLE Graft 128801-10 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: B I O M A T E R I A L en English INSTRUCTIONS FOR USE FOR GORE SYNECOR INTRAPERITONEAL BIOMATERIAL INDICATIONS The GORE SYNECOR Intraperitoneal Biomaterial device is intended
INSTRUCTIONS FOR USE FOR: B I O M A T E R I A L en English INSTRUCTIONS FOR USE FOR GORE SYNECOR INTRAPERITONEAL BIOMATERIAL INDICATIONS The GORE SYNECOR Intraperitoneal Biomaterial device is intended
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM INTRAPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM INTRAPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted
DuraGen Secure. Dural Regeneration Matrix
 DuraGen Secure Dural Regeneration Matrix DESCRIPTION DuraGen Secure Dural Regeneration Matrix is an absorbable implant for the repair of dural defects. DuraGen Secure Dural Regeneration Matrix is an easy
DuraGen Secure Dural Regeneration Matrix DESCRIPTION DuraGen Secure Dural Regeneration Matrix is an absorbable implant for the repair of dural defects. DuraGen Secure Dural Regeneration Matrix is an easy
HANDLING OF WRIGHT MEDICAL DISPOSABLE PROPHECY TM ANKLE INSTRUMENTS
 HANDLING OF WRIGHT MEDICAL DISPOSABLE PROPHECY TM ANKLE INSTRUMENTS 153290-0 The following languages are included in this packet: English (en) For additional languages, visit our website www.wright.com
HANDLING OF WRIGHT MEDICAL DISPOSABLE PROPHECY TM ANKLE INSTRUMENTS 153290-0 The following languages are included in this packet: English (en) For additional languages, visit our website www.wright.com
HANDLING OF WRIGHT DISPOSABLE PROPHECY ANKLE INSTRUMENTS
 HANDLING OF WRIGHT DISPOSABLE PROPHECY ANKLE INSTRUMENTS 150870-0 English (en) The following languages are included in this packet: For additional information and translations please contact the manufacturer
HANDLING OF WRIGHT DISPOSABLE PROPHECY ANKLE INSTRUMENTS 150870-0 English (en) The following languages are included in this packet: For additional information and translations please contact the manufacturer
Repliform. Tissue Regeneration Matrix. Instructions for Use. Distributed by:
 Repliform Tissue Regeneration Matrix Instructions for Use Distributed by: Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA 01752 Processed from Donated Human Tissue by: LifeCell
Repliform Tissue Regeneration Matrix Instructions for Use Distributed by: Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA 01752 Processed from Donated Human Tissue by: LifeCell
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE
 PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 137835-5 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 137835-5 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc.
 for wounds instructions for use Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc. marketed by KCI USA, Inc. San Antonio, TX 78219 USA 800.275.4524 www.kci1.com www.graftjacketbykci.com
for wounds instructions for use Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc. marketed by KCI USA, Inc. San Antonio, TX 78219 USA 800.275.4524 www.kci1.com www.graftjacketbykci.com
CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed physician.
 TM CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed physician. TABLE OF CONTENTS Section Port Styles 4 Description 5 Indications 5 Contraindications 5-6 Information
TM CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed physician. TABLE OF CONTENTS Section Port Styles 4 Description 5 Indications 5 Contraindications 5-6 Information
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English bg Български cz Čeština dk Dansk nl Nederlands ee Eesti fi Suomi fr Français de Deutsch gr Ελληνικά hu Magyar it Italiano lt Lietuvių no Norsk pl Polska pt Português
INSTRUCTIONS FOR USE FOR: en English bg Български cz Čeština dk Dansk nl Nederlands ee Eesti fi Suomi fr Français de Deutsch gr Ελληνικά hu Magyar it Italiano lt Lietuvių no Norsk pl Polska pt Português
ALLODERM SELECT ALLODERM SELECT RESTORE
 ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE
 PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 150842-0 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
PRO-STIM Injectable Inductive Graft Containing DONATED HUMAN TISSUE 150842-0 The following languages are included in this packet: English (en) For additional information please contact the manufacturer
BIOFIBER BIOFIBER CM AMBIENT TEMPERATUR. Absorbable Scaffold. Collagen Coated. Absorbable Scaffold
 BIOFIBER Absorbable Scaffold BIOFIBER CM Collagen Coated Absorbable Scaffold A B S O R B A B L E AMBIENT TEMPERATUR B I O L O G I C S C AAND F F OCRYOPRESERVE L D I N G S OSTORAGE L U T I O N S OPTION
BIOFIBER Absorbable Scaffold BIOFIBER CM Collagen Coated Absorbable Scaffold A B S O R B A B L E AMBIENT TEMPERATUR B I O L O G I C S C AAND F F OCRYOPRESERVE L D I N G S OSTORAGE L U T I O N S OPTION
February 17, DSM Biomedical Ms. Brianna Jordan Regulatory Specialist 735 Pennsylvania Drive Exton, Pennsylvania 19341
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 February 17, 2015 DSM
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 February 17, 2015 DSM
SurgiMend. Product Summary
 SurgiMend Collagen Matrix for Soft Tissue Reconstruction Product Summary Because we are committed to limiting uncertainty, Integra offers SurgiMend in 1, 2, 3 or 4 mm thicknesses and in over sixty configurations,
SurgiMend Collagen Matrix for Soft Tissue Reconstruction Product Summary Because we are committed to limiting uncertainty, Integra offers SurgiMend in 1, 2, 3 or 4 mm thicknesses and in over sixty configurations,
Instructions for Use READY TO USE
 READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
Meso BioMatrix Acellular Peritoneum Matrix RECOMMENDED TECHNIQUE GUIDE. Recreating harmonious bodies for a new vision of rebirth.
 Meso BioMatrix Acellular Peritoneum Matrix RECOMMENDED TECHNIQUE GUIDE Recreating harmonious bodies for a new vision of rebirth. 1 DISCOVER SEBBIN Sebbin is a 30 years-established company with a peerless
Meso BioMatrix Acellular Peritoneum Matrix RECOMMENDED TECHNIQUE GUIDE Recreating harmonious bodies for a new vision of rebirth. 1 DISCOVER SEBBIN Sebbin is a 30 years-established company with a peerless
ASAHI Masters PARKWAY SOFT. ASAHI Masters PARKWAY HF
 ASAHI Masters PARKWAY SOFT Microcatheter ASAHI Masters PARKWAY HF Microcatheter SYMBOLS 1 English 2 AMK-DT207 Ver.1.40 / 11TS028 SYMBOLS Legal manufacturer Consult instructions for use Do not use if package
ASAHI Masters PARKWAY SOFT Microcatheter ASAHI Masters PARKWAY HF Microcatheter SYMBOLS 1 English 2 AMK-DT207 Ver.1.40 / 11TS028 SYMBOLS Legal manufacturer Consult instructions for use Do not use if package
Advancing the Open Ventral Hernia Repair Experience
 Advancing the Open Ventral Hernia Repair Experience SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Absorbable Fixation System Optimized Design for Open Ventral
Advancing the Open Ventral Hernia Repair Experience SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Absorbable Fixation System Optimized Design for Open Ventral
ISO Medical devices symbols to be used with medical device labels, labeling and information to be supplied Part 1: General requirements
 Explanation of the symbols used on Dentsply Sirona Implants packaging Manufacturer Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. This symbol
Explanation of the symbols used on Dentsply Sirona Implants packaging Manufacturer Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. This symbol
CapSure Permanent Fixation System
 CapSure Permanent Fixation System Permanent Fixation Redefined Advancing the Fixation Experience Recipient of 2015 SLS Innovations of the Year recognition. SOFT TISSUE REPAIR Right Procedure. Right Product.
CapSure Permanent Fixation System Permanent Fixation Redefined Advancing the Fixation Experience Recipient of 2015 SLS Innovations of the Year recognition. SOFT TISSUE REPAIR Right Procedure. Right Product.
Ventrio ST Hernia Patch featuring Sepra Technology
 Ventrio ST Hernia Patch featuring Sepra Technology Sepra Technology An extensively studied barrier with more than 10 publications and used clinically since 2007. Unique hydrogel barrier swells to minimize
Ventrio ST Hernia Patch featuring Sepra Technology Sepra Technology An extensively studied barrier with more than 10 publications and used clinically since 2007. Unique hydrogel barrier swells to minimize
ASAHI Masters PARKWAY HF KIT
 ASAHI Masters PARKWAY HF KIT Guide Wire Pre-loaded Microcatheter SYMBOLS 1 English 2 AMK-DT202 Ver.1.30 / 11TS023 SYMBOLS Legal manufacturer GC Minimum guiding catheter I.D. Do not use if package is damaged
ASAHI Masters PARKWAY HF KIT Guide Wire Pre-loaded Microcatheter SYMBOLS 1 English 2 AMK-DT202 Ver.1.30 / 11TS023 SYMBOLS Legal manufacturer GC Minimum guiding catheter I.D. Do not use if package is damaged
Each 7.0 cm x 7.0 cm dressing contains 140 to 260 mg of human fibrinogen and 130 International Units of human thrombin.
 SurgiClot Hemostatic Dressing St. Teresa Medical, Inc. 2915 Waters Road, Suite 108 Eagan, Minnesota 55121 USA Tel: 651-789-6550 www.stteresamedical.com INSTRUCTIONS FOR USE DEVICE DESCRIPTION Each SurgiClot
SurgiClot Hemostatic Dressing St. Teresa Medical, Inc. 2915 Waters Road, Suite 108 Eagan, Minnesota 55121 USA Tel: 651-789-6550 www.stteresamedical.com INSTRUCTIONS FOR USE DEVICE DESCRIPTION Each SurgiClot
Ask your surgeon for more information about Natrelle and visit GummyImplants.com
 The #1 surgeon-preferred gummy implant collection in the US* Two-stage breast reconstruction starts with Natrelle 133 Series Tissue Expanders Natrelle 133 Series Tissue Expanders are made to match Natrelle
The #1 surgeon-preferred gummy implant collection in the US* Two-stage breast reconstruction starts with Natrelle 133 Series Tissue Expanders Natrelle 133 Series Tissue Expanders are made to match Natrelle
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength
 The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
RePlay Hemostasis Clip
 RePlay Hemostasis Clip Instructions for use. Read carefully prior to use. Caution: Federal (U.S.A.) Law restricts this device to sale by or on the order of a physician. Diversatek Healthcare Corporate
RePlay Hemostasis Clip Instructions for use. Read carefully prior to use. Caution: Federal (U.S.A.) Law restricts this device to sale by or on the order of a physician. Diversatek Healthcare Corporate
Platelet Concentrate in Total Knees BIOLOGICS. This brochure is for International use only. It is not for distribution in the United States.
 Platelet Concentrate in Total Knees BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. Platelet Concentrate in Total Knees Total Knee Arthroplasty Total
Platelet Concentrate in Total Knees BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. Platelet Concentrate in Total Knees Total Knee Arthroplasty Total
CLEANING AND HANDLING OF MICROPORT INSTRUMENTS The following languages are included in this packet:
 EN CLEANING AND HANDLING OF MICROPORT INSTRUMENTS 150802-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLEANING AND HANDLING OF MICROPORT INSTRUMENTS 150802-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description
 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing ( Securement System ) is designed
3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing ( Securement System ) is designed
Biological Prostheses
 SAPIENZA UNIVERSITA DI ROMA EHS Dipartimento di Chirurgia Generale Paride Stefanini U.O.C. di Chirurgia Generale D e Day Surgery (Direttore: Prof. P. Negro) PRG M Chirurgia della Parete Addominale (Prof.
SAPIENZA UNIVERSITA DI ROMA EHS Dipartimento di Chirurgia Generale Paride Stefanini U.O.C. di Chirurgia Generale D e Day Surgery (Direttore: Prof. P. Negro) PRG M Chirurgia della Parete Addominale (Prof.
Instructions for Use Reprocessed Electrophysiology Catheter Cables
 Reprocessed by Instructions for Use Reprocessed Electrophysiology Catheter Cables Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed Electrophysiology Catheter Cables Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by
SCIENCE. VALUE. INNOVATION.
 SCIENCE. VALUE. INNOVATION. OUR STORY TELA Bio, a surgical reconstruction company, was created out of the desire to do things differently. Combining the drive and innovation of a start-up with a seasoned
SCIENCE. VALUE. INNOVATION. OUR STORY TELA Bio, a surgical reconstruction company, was created out of the desire to do things differently. Combining the drive and innovation of a start-up with a seasoned
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Universal Trocar Stability Sleeve. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Universal Trocar Stability Sleeve Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Universal Trocar Stability Sleeve Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale
RESPIRATORY SENSING LEAD
 RESPIRATORY SENSING LEAD 4340 Technical Manual ONLY Explanation of symbols on product or package labeling Caution, consult accompanying documents eifu indicator Consult electronic instructions for use
RESPIRATORY SENSING LEAD 4340 Technical Manual ONLY Explanation of symbols on product or package labeling Caution, consult accompanying documents eifu indicator Consult electronic instructions for use
EasyClip Xpress Sterile staple solution. Operative technique
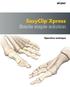 EasyClip Xpress Sterile staple solution Operative technique Sterile staple solution Table of contents Indications 3 Operative technique 4 This publication sets forth detailed recommended procedures for
EasyClip Xpress Sterile staple solution Operative technique Sterile staple solution Table of contents Indications 3 Operative technique 4 This publication sets forth detailed recommended procedures for
RapidPort EZ Port Applier DIRECTIONS FOR USE (DFU)
 RapidPort EZ Port Applier DIRECTIONS FOR USE (DFU) RapidPort EZ Port Applier Ref. No. C-20390 RapidPort EZ Port Applier INTRODUCTION The RapidPort EZ Port Applier is an optional accessory for the LAP-BAND
RapidPort EZ Port Applier DIRECTIONS FOR USE (DFU) RapidPort EZ Port Applier Ref. No. C-20390 RapidPort EZ Port Applier INTRODUCTION The RapidPort EZ Port Applier is an optional accessory for the LAP-BAND
Artist Proof: Clotalyst. Autologous Serum Collection System
 Artist Proof: 01-15-10 Clotalyst Autologous Serum Collection System Clotalyst Autologous Serum The kit is designed for the preparation of autologous serum that is to be mixed with PRP and autograft or
Artist Proof: 01-15-10 Clotalyst Autologous Serum Collection System Clotalyst Autologous Serum The kit is designed for the preparation of autologous serum that is to be mixed with PRP and autograft or
Tendon/Ligament Repair
 Tendon/Ligament Repair Team Collagen Constructs Mario Rossi, Anne Tucker, Brian Ziola Faculty Advisors: Dr. Yawen Li, Dr. Therese Bou-Akl Technical Advisors: Dr. Tristan Maerz, Meagan Salisbury Problem
Tendon/Ligament Repair Team Collagen Constructs Mario Rossi, Anne Tucker, Brian Ziola Faculty Advisors: Dr. Yawen Li, Dr. Therese Bou-Akl Technical Advisors: Dr. Tristan Maerz, Meagan Salisbury Problem
TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing. Technical Q&A
 TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing Technical Q&A TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing
TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing Technical Q&A TissueMend Soft Tissue Repair Matrix For Biologically Inspired Augmentation of Tendon Healing
strength of science Now with sizes up to 25 cm x 45 cm XCM BIOLOGIC Tissue Matrix
 strength of science XCM BIOLOGIC Tissue Matrix Now with sizes up to 25 cm x 45 cm Your trusted partner providing a complete portfolio of biologic meshes. DePuy Synthes Companies of Johnson & Johnson offer
strength of science XCM BIOLOGIC Tissue Matrix Now with sizes up to 25 cm x 45 cm Your trusted partner providing a complete portfolio of biologic meshes. DePuy Synthes Companies of Johnson & Johnson offer
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Dilating Tip Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Dilating Tip Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Dilating Tip Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
For personal use only
 Quarterly Cash Flow Report June 2016 Perth, Australia; 29 April 2016: Orthocell Limited s Quarterly Cash Flow Report for the quarter ended 30 June 2016 is attached. Orthocell is a commercial-stage, regenerative
Quarterly Cash Flow Report June 2016 Perth, Australia; 29 April 2016: Orthocell Limited s Quarterly Cash Flow Report for the quarter ended 30 June 2016 is attached. Orthocell is a commercial-stage, regenerative
Instructions for Use Reusable Electrophysiology Catheter Cables
 Processed by Instructions for Use Reusable Electrophysiology Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized
Processed by Instructions for Use Reusable Electrophysiology Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized
RapidPort EZ Access Port Kit DIRECTIONS FOR USE (DFU)
 RapidPort EZ Access Port Kit DIRECTIONS FOR USE (DFU) RapidPort EZ Access Port Kit Ref. No. C-2304 Standard RapidPort EZ Access Port Kit Ref. No. C-2306 Large RapidPort EZ Access Port Kit INTRODUCTION
RapidPort EZ Access Port Kit DIRECTIONS FOR USE (DFU) RapidPort EZ Access Port Kit Ref. No. C-2304 Standard RapidPort EZ Access Port Kit Ref. No. C-2306 Large RapidPort EZ Access Port Kit INTRODUCTION
ALLOX AND ALLOX 2 TISSUE EXPANDER INSTRUCTIONS FOR USE
 ALLOX AND ALLOX 2 TISSUE EXPANDER INSTRUCTIONS FOR USE DESCRIPTION Specialty Surgical Products (SSP) Tissue Expanders are intended for temporary subcutaneous or submuscular implantation to develop surgical
ALLOX AND ALLOX 2 TISSUE EXPANDER INSTRUCTIONS FOR USE DESCRIPTION Specialty Surgical Products (SSP) Tissue Expanders are intended for temporary subcutaneous or submuscular implantation to develop surgical
Instructions For Use AlloX and AlloX2 Tissue Expanders
 Instructions For Use AlloX and AlloX2 Tissue Expanders Description Sientra Tissue Expanders are intended for temporary subcutaneous or submuscular implantation to develop surgical flaps and additional
Instructions For Use AlloX and AlloX2 Tissue Expanders Description Sientra Tissue Expanders are intended for temporary subcutaneous or submuscular implantation to develop surgical flaps and additional
Instructions for Use Reprocessed Coronary Sinus Diagnostic Electrophysiology Catheter
 Reprocessed by Instructions for Use Reprocessed Coronary Sinus Diagnostic Electrophysiology Catheter Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this
Reprocessed by Instructions for Use Reprocessed Coronary Sinus Diagnostic Electrophysiology Catheter Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this
FINAL DOCUMENT. Global Harmonization Task Force
 GHTF/SG1/N70:2011 FINAL DOCUMENT Global Harmonization Task Force Title: Label and Instructions for Use for Medical Devices Authoring Group: Study Group 1 of the Global Harmonization Task Force Endorsed
GHTF/SG1/N70:2011 FINAL DOCUMENT Global Harmonization Task Force Title: Label and Instructions for Use for Medical Devices Authoring Group: Study Group 1 of the Global Harmonization Task Force Endorsed
Finally! Everything you need when supporting soft tissue; nothing you don t. Strength and Beauty. Inside and Out.
 alatea Surgical Bioresorbable Scaffold Collection a Strength and Beauty. Inside and Out. 3D 3-Dimensional Biologically Derived Monofilament Strong Bioresorbable Finally! Everything you need when supporting
alatea Surgical Bioresorbable Scaffold Collection a Strength and Beauty. Inside and Out. 3D 3-Dimensional Biologically Derived Monofilament Strong Bioresorbable Finally! Everything you need when supporting
MEDICAL DEVICE GUIDANCE
 June 2018 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices Revision 1 CONTENTS PREFACE... 3 1. INTRODUCTION... 4 2. LABELLING REQUIREMENTS... 7 2.1. General Guidelines... 7 2.2.
June 2018 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices Revision 1 CONTENTS PREFACE... 3 1. INTRODUCTION... 4 2. LABELLING REQUIREMENTS... 7 2.1. General Guidelines... 7 2.2.
Tendon/Ligament Repair Utilizing Stromal Derived Factor Infused Alginate Coated Collagen Fibers
 Tendon/Ligament Repair Utilizing Stromal Derived Factor Infused Alginate Coated Collagen Fibers Mario Rossi, Anne Tucker, Brian Ziola Advisors: Dr. Yawen Li, Dr. Therese Bou-Akl, Dr. Tristan Maerz, Meagan
Tendon/Ligament Repair Utilizing Stromal Derived Factor Infused Alginate Coated Collagen Fibers Mario Rossi, Anne Tucker, Brian Ziola Advisors: Dr. Yawen Li, Dr. Therese Bou-Akl, Dr. Tristan Maerz, Meagan
A review of Dr. Dinakar Golla s clinical research with AdMatrix surgical grafts for soft tissue repair
 TEAMeffort: Using aggressive surgical techniques in combination with AdMatrix (Lattice Biologics acellular dermal scaffold product) to heal difficult and persistent wounds A review of Dr. Dinakar Golla
TEAMeffort: Using aggressive surgical techniques in combination with AdMatrix (Lattice Biologics acellular dermal scaffold product) to heal difficult and persistent wounds A review of Dr. Dinakar Golla
LAP-BAND System Access Port II Kit
 LAP-BAND System Access Port II Kit DIRECTIONS FOR USE (DFU) Access Port Needle Access Port Priming Needle Access Port II End Plug Band Priming Needle Stainless Steel Connector Tubing LAP-BAND System Access
LAP-BAND System Access Port II Kit DIRECTIONS FOR USE (DFU) Access Port Needle Access Port Priming Needle Access Port II End Plug Band Priming Needle Stainless Steel Connector Tubing LAP-BAND System Access
Tissue Engineering For Regenerating Life. Cryopreserved Products. Tissue Corporation
 Tissue Engineering For Regenerating Life Cryopreserved Products Tissue Corporation Tissue Corporation TRC (Tissue regeneration corporation) is a multi-facility institute specializing in the preparation
Tissue Engineering For Regenerating Life Cryopreserved Products Tissue Corporation Tissue Corporation TRC (Tissue regeneration corporation) is a multi-facility institute specializing in the preparation
GYNECOLOGIC SURGERY PRODUCT CATALOG
 Urology and Women s Hlth 2018 GYNECOLOGIC SURGERY PRODUCT CATALOG Hysteroscopic Tissue Removal Endometrial Ablation ORDERING NUMBERS Boston Scientific Corporation uses a fully computerized order entry
Urology and Women s Hlth 2018 GYNECOLOGIC SURGERY PRODUCT CATALOG Hysteroscopic Tissue Removal Endometrial Ablation ORDERING NUMBERS Boston Scientific Corporation uses a fully computerized order entry
PRECISION POINT MAGNETIC INJECTION PORT SYSTEM TISSUE EXPANDER INSTRUCTIONS FOR USE
 PRECISION POINT MAGNETIC INJECTION PORT SYSTEM TISSUE EXPANDER INSTRUCTIONS FOR USE DESCRIPTION Specialty Surgical Products (SSP) Tissue Expanders are intended for temporary subcutaneous or submuscular
PRECISION POINT MAGNETIC INJECTION PORT SYSTEM TISSUE EXPANDER INSTRUCTIONS FOR USE DESCRIPTION Specialty Surgical Products (SSP) Tissue Expanders are intended for temporary subcutaneous or submuscular
SYMBOL GLOSSARY Explanation of symbols used in Quidel device labeling. Symbol Symbol Title Explanatory Text Standard Reference
 CE marking Conformité Européene Notified Body Reference no. ### The requirements for accreditation and market surveillance relating to the marketing of products MDD 93/42/EEC IVDD 98/79/EC Article 16.2
CE marking Conformité Européene Notified Body Reference no. ### The requirements for accreditation and market surveillance relating to the marketing of products MDD 93/42/EEC IVDD 98/79/EC Article 16.2
SYMBOL GLOSSARY Explanation of symbols used in Quidel device labeling. Symbol Symbol Title Explanatory Text Standard Reference
 CE marking Conformité Européene Notified Body Reference no. ### The requirements for accreditation and market surveillance relating to the marketing of products MDD 93/42/EEC IVDD 98/79/EC Article 16.2
CE marking Conformité Européene Notified Body Reference no. ### The requirements for accreditation and market surveillance relating to the marketing of products MDD 93/42/EEC IVDD 98/79/EC Article 16.2
NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use
 630 Komas Drive Suite 200 Salt Lake City UT 84108 USA P +1 801.582.5533 F +1 801.582.1509 www.blackrockmicro.com NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use Revision 2.00 / LB-0612
630 Komas Drive Suite 200 Salt Lake City UT 84108 USA P +1 801.582.5533 F +1 801.582.1509 www.blackrockmicro.com NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use Revision 2.00 / LB-0612
INSTRUCTIONS FOR USE
 INSTRUCTIONS FOR USE EZ-Accu Shot Microorganisms EZ-Accu Shot Select Microorganisms INTENDED USE EZ-Accu Shot and EZ-Accu Shot Select microorganisms are lyophilized, enumerated microorganism preparations
INSTRUCTIONS FOR USE EZ-Accu Shot Microorganisms EZ-Accu Shot Select Microorganisms INTENDED USE EZ-Accu Shot and EZ-Accu Shot Select microorganisms are lyophilized, enumerated microorganism preparations
Urology and Women s Health
 Urology and Women s Health GYNECOLOGIC SURGERY PRODUCT CATALOG Hysteroscopic Tissue Removal Endometrial Ablation Introduction The all-in-one Symphion System is designed to transform the way you remove
Urology and Women s Health GYNECOLOGIC SURGERY PRODUCT CATALOG Hysteroscopic Tissue Removal Endometrial Ablation Introduction The all-in-one Symphion System is designed to transform the way you remove
Tornier Medical Education
 Tornier Medical Education D E C E M B E R 7, 2 0 0 9 Orthopaedic Uses of Tissue Matrices in Tendon Repair: Why All Tissue Matrices are Not Created Equal John R. Harper, Ph.D, Research Department, LifeCell
Tornier Medical Education D E C E M B E R 7, 2 0 0 9 Orthopaedic Uses of Tissue Matrices in Tendon Repair: Why All Tissue Matrices are Not Created Equal John R. Harper, Ph.D, Research Department, LifeCell
Matrix HD. wound covering. Sterile, room temperature human dermis graft
 Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD is an acellular human dermis allograft sterilized using
Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD is an acellular human dermis allograft sterilized using
Sterilization in Aesculap Sterilization Containers
 Sterilization in Aesculap Sterilization Containers Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net PKGI-76-D EFFECTIVE 01-2017 English US / PAGE 2 ACUMED STERILIZATION
Sterilization in Aesculap Sterilization Containers Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net PKGI-76-D EFFECTIVE 01-2017 English US / PAGE 2 ACUMED STERILIZATION
TRUFILL DCS ORBIT Detachable Coil System
 ORBIT Conforming to Your Complex Needs Excellent Conformability and Concentric Filling for Outstanding Packing Density ORBIT Full range of Mini Complex and new Tight Distal Loop Technology coils Our Complex
ORBIT Conforming to Your Complex Needs Excellent Conformability and Concentric Filling for Outstanding Packing Density ORBIT Full range of Mini Complex and new Tight Distal Loop Technology coils Our Complex
FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps
 FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps September 27, 2018 Presentation by: Elaine H. Tseng Partner FDA and Life Sciences Group King & Spalding Discussion with: Thomas Poché Vice
FDA Regulatory, Compliance and Policy Developments: 361 HCT/Ps September 27, 2018 Presentation by: Elaine H. Tseng Partner FDA and Life Sciences Group King & Spalding Discussion with: Thomas Poché Vice
Biologic Prosthetics & Beyond
 & Beyond & Beyond: Overview Introduction Prosthetics Overview UCSF Postgraduate Course in General Surgery San Francisco, CA May 18, 2013 Review of Clinical Data Summary Recommendations Hobart W. Harris,
& Beyond & Beyond: Overview Introduction Prosthetics Overview UCSF Postgraduate Course in General Surgery San Francisco, CA May 18, 2013 Review of Clinical Data Summary Recommendations Hobart W. Harris,
Conformability without Compromise
 Conformability without Compromise THE STANDARD IN Conformability AND Designed for flexibility and conformability in tortuous anatomy. Optimized aortic wall apposition in angulated arch anatomy without
Conformability without Compromise THE STANDARD IN Conformability AND Designed for flexibility and conformability in tortuous anatomy. Optimized aortic wall apposition in angulated arch anatomy without
DIRECTIONS FOR USE. NATRELLE 133 Tissue Expanders with Suture Tabs. MAGNA-FINDER Xact. Directions for Use. with Magna-Site.
 DIRECTIONS FOR USE Directions for Use NATRELLE 133 Tissue Expanders with Suture Tabs with Magna-Site Injection Sites MAGNA-FINDER Xact & 21G Needle Infusion Set CAUTION: U.S. Federal law restricts this
DIRECTIONS FOR USE Directions for Use NATRELLE 133 Tissue Expanders with Suture Tabs with Magna-Site Injection Sites MAGNA-FINDER Xact & 21G Needle Infusion Set CAUTION: U.S. Federal law restricts this
Conformability without Compromise
 Conformability without Compromise THE STANDARD IN Conformability AND Designed for flexibility and conformability in tortuous anatomy. Optimized aortic wall apposition in angulated arch anatomy without
Conformability without Compromise THE STANDARD IN Conformability AND Designed for flexibility and conformability in tortuous anatomy. Optimized aortic wall apposition in angulated arch anatomy without
ProCol Vascular Bioprosthesis
 Instructions for Use 1. DEVICE DESCRIPTION The ProCol Vascular Bioprosthesis is derived from bovine mesenteric vein processed with glutaraldehyde. Collateral branches are ligated with surgical suture and
Instructions for Use 1. DEVICE DESCRIPTION The ProCol Vascular Bioprosthesis is derived from bovine mesenteric vein processed with glutaraldehyde. Collateral branches are ligated with surgical suture and
Instructions for Use Reusable Ablation Catheter Cables
 Processed by Instructions for Use Reusable Ablation Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized by Ethylene
Processed by Instructions for Use Reusable Ablation Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized by Ethylene
MENTOR MemoryShape RESTERILIZABLE GEL BREAST IMPLANT SIZER STERILE
 1 Product Insert Data Sheet MENTOR MemoryShape RESTERILIZABLE GEL BREAST IMPLANT SIZER STERILE ENGLISH 112009-001 Rev A Effective May 2017 LAB100300459v2 Caution: Federal (U.S.A.) law restricts this device
1 Product Insert Data Sheet MENTOR MemoryShape RESTERILIZABLE GEL BREAST IMPLANT SIZER STERILE ENGLISH 112009-001 Rev A Effective May 2017 LAB100300459v2 Caution: Federal (U.S.A.) law restricts this device
BAXTER ANNOUNCES AGREEMENT TO BROADEN PORTFOLIO OF INNOVATIVE SURGICAL PRODUCTS
 FOR IMMEDIATE RELEASE Media Contact: Beth Mueller, (224) 948-5353 media@baxter.com Investor Contact: Clare Trachtman, (224) 948-3085 BAXTER ANNOUNCES AGREEMENT TO BROADEN PORTFOLIO OF INNOVATIVE SURGICAL
FOR IMMEDIATE RELEASE Media Contact: Beth Mueller, (224) 948-5353 media@baxter.com Investor Contact: Clare Trachtman, (224) 948-3085 BAXTER ANNOUNCES AGREEMENT TO BROADEN PORTFOLIO OF INNOVATIVE SURGICAL
Page 1 of 6 Reprocessed by. Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 6 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the
Page 1 of 6 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the
PREVELEAK Surgical sealant
 PREVELEAK Surgical sealant Rx only Instructions for Use DEVICE DESCRIPTION PREVELEAK Surgical sealant (PREVELEAK) is a sealant developed to seal suture holes formed during surgical repair of the circulatory
PREVELEAK Surgical sealant Rx only Instructions for Use DEVICE DESCRIPTION PREVELEAK Surgical sealant (PREVELEAK) is a sealant developed to seal suture holes formed during surgical repair of the circulatory
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS Table of Contents GORE ACUSEAL Vascular Graft Properties What is the GORE ACUSEAL Vascular Graft?...2 What is unique about the GORE ACUSEAL Vascular Graft?...3 What are the
FREQUENTLY ASKED QUESTIONS Table of Contents GORE ACUSEAL Vascular Graft Properties What is the GORE ACUSEAL Vascular Graft?...2 What is unique about the GORE ACUSEAL Vascular Graft?...3 What are the
PER F ORM ANC E b y d e s i g n. Pioneering TEVAR Therapy, Time and Time Again. CONFORMABLE
 PER F ORM ANC E b y d e s i g n Pioneering TEVAR Therapy, Time and Time Again. CONFORMABLE T H O R A C I C E N D O P R O S T H E S I S Time-Tested Success For more than 16 years, the GORE TAG Device has
PER F ORM ANC E b y d e s i g n Pioneering TEVAR Therapy, Time and Time Again. CONFORMABLE T H O R A C I C E N D O P R O S T H E S I S Time-Tested Success For more than 16 years, the GORE TAG Device has
Instructions for Use Reprocessed Balloon Inflation Devices
 Reprocessed by Instructions for Use Reprocessed Balloon Inflation Devices Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Reprocessed by Instructions for Use Reprocessed Balloon Inflation Devices Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
FOR IMMEDIATE RELEASE
 FOR IMMEDIATE RELEASE Media Contact: Melanie Battista, Public Relations (TSX-V: LBL) (OTCBB: BLVKF) 16701 N 90th Street, Suite#101 Scottsdale, AZ 85260 480-563-0800 Office News@LatticeBiologics.com www.latticebiologics.com
FOR IMMEDIATE RELEASE Media Contact: Melanie Battista, Public Relations (TSX-V: LBL) (OTCBB: BLVKF) 16701 N 90th Street, Suite#101 Scottsdale, AZ 85260 480-563-0800 Office News@LatticeBiologics.com www.latticebiologics.com
DIRECTIONS FOR USE. NATRELLE 133 Tissue Expanders. MAGNA-FINDER Xact. Directions for Use. with Magna-Site. Injection Sites
 DIRECTIONS FOR USE Directions for Use NATRELLE 133 Tissue Expanders with Magna-Site Injection Sites MAGNA-FINDER Xact & 21G Needle Infusion Set CAUTION: U.S. Federal law restricts this device to sale by
DIRECTIONS FOR USE Directions for Use NATRELLE 133 Tissue Expanders with Magna-Site Injection Sites MAGNA-FINDER Xact & 21G Needle Infusion Set CAUTION: U.S. Federal law restricts this device to sale by
Presenting SURGICEL Powder
 SURGICEL Powder Presenting SURGICEL Powder Built to stop continuous, broad-surface oozing fast1,2 The next generation of SURGICEL Absorbable Hemostats SURGICEL Powder efficiently and effectively controls
SURGICEL Powder Presenting SURGICEL Powder Built to stop continuous, broad-surface oozing fast1,2 The next generation of SURGICEL Absorbable Hemostats SURGICEL Powder efficiently and effectively controls
FORWARD LOOKING STATEMENT
 FORWARD LOOKING STATEMENT Statements made in this presentation that look forward in time or that express management's beliefs, expectations, hopes or predictions of the future are forward-looking statements
FORWARD LOOKING STATEMENT Statements made in this presentation that look forward in time or that express management's beliefs, expectations, hopes or predictions of the future are forward-looking statements
Entellus Medical Sinuscope INSTRUCTIONS FOR USE
 Entellus Medical Sinuscope INSTRUCTIONS FOR USE Table of contents Entellus Medical Sinuscope Instructions for Use 1. About this document... 1 1.1 Purpose... 1 1.2 Symbols used... 1 2. Intended use... 2
Entellus Medical Sinuscope INSTRUCTIONS FOR USE Table of contents Entellus Medical Sinuscope Instructions for Use 1. About this document... 1 1.1 Purpose... 1 1.2 Symbols used... 1 2. Intended use... 2
Instruction for Use of Disposable Endoscopic Hose-type Biopsy Forceps JS-CE-SM-09-01, A/0
 Instruction for Use of Disposable Endoscopic Hose-type Biopsy Forceps JS-CE-SM-09-01, A/0 Instruction for use of Disposable Endoscopic Hose-type Biopsy Forceps Product name Disposable Endoscopic Hose-type
Instruction for Use of Disposable Endoscopic Hose-type Biopsy Forceps JS-CE-SM-09-01, A/0 Instruction for use of Disposable Endoscopic Hose-type Biopsy Forceps Product name Disposable Endoscopic Hose-type
Double Syringe System Autologous Conditioned Plasma
 TM Double Syringe System Autologous Conditioned Plasma Autologous Conditioned Plasma Introduction Autologous blood products have created a growing interest for use in a number of orthopaedic therapies.
TM Double Syringe System Autologous Conditioned Plasma Autologous Conditioned Plasma Introduction Autologous blood products have created a growing interest for use in a number of orthopaedic therapies.
The Ventrio ST Hernia Patch Featuring Sepra Technology. Efficient:
 Ventrio ST Hernia Patch featuring SEPRA Technology NEW Sepra Technology Based on the technology used in Seprafilm with more than 13 years of proven clinical success. Unique hydrogel barrier swells to minimise
Ventrio ST Hernia Patch featuring SEPRA Technology NEW Sepra Technology Based on the technology used in Seprafilm with more than 13 years of proven clinical success. Unique hydrogel barrier swells to minimise
Phasix ST Mesh. Designed to enable functional tissue remodeling for a strong repair. Fully Resorbable Scaffold Featuring Proven Sepra Technology
 Phasix ST Mesh Fully Resorbable Scaffold Featuring Proven Sepra Technology Designed to enable functional tissue remodeling for a strong repair. SOFT TISSUE REPAIR Right Procedure. Right Product. Right
Phasix ST Mesh Fully Resorbable Scaffold Featuring Proven Sepra Technology Designed to enable functional tissue remodeling for a strong repair. SOFT TISSUE REPAIR Right Procedure. Right Product. Right
POSITION PAPER Moving from the MDD to the MDR
 A summary of Key Changes regarding Sterile Packaging and considerations on recommended changes to standards Introduction EN ISO 11607 specifies the requirements and test methods for materials, preformed
A summary of Key Changes regarding Sterile Packaging and considerations on recommended changes to standards Introduction EN ISO 11607 specifies the requirements and test methods for materials, preformed
Median Sternotomy Closure Utilizing Platelet Concentrate BIOLOGICS
 Median Sternotomy Closure Utilizing Platelet Concentrate BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. The Surgical Procedure: Median Sternotomy
Median Sternotomy Closure Utilizing Platelet Concentrate BIOLOGICS This brochure is for International use only. It is not for distribution in the United States. The Surgical Procedure: Median Sternotomy
2/6/2019. Can We Do Better? Tissue Engineering. Tissue Engineering. Tissue Engineering. Mechanism. Why did repair of the ACL fail?
 DISCLOSURES Bridge-Enhanced Repair: From Concept to Clinical Trials Martha M. Murray, MD Professor of Orthopaedic Surgery Boston Children s Hospital Harvard Medical School I, Martha Murray, have relevant
DISCLOSURES Bridge-Enhanced Repair: From Concept to Clinical Trials Martha M. Murray, MD Professor of Orthopaedic Surgery Boston Children s Hospital Harvard Medical School I, Martha Murray, have relevant
LIP IMPLANT UPPER LIP: SIZE MM LOWER LIP: SIZE MM
 INFORMED CONSENT FOR LIP IMPLANT UPPER LIP: SIZE MM LOWER LIP: SIZE MM PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME KAROL A. GUTOWSKI, MD, FACS AESTHETIC SURGERY CERTIFIED
INFORMED CONSENT FOR LIP IMPLANT UPPER LIP: SIZE MM LOWER LIP: SIZE MM PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME KAROL A. GUTOWSKI, MD, FACS AESTHETIC SURGERY CERTIFIED
ARABIC CANADIAN MEDICAL CENTER CSSD POLICIES AND PROCEDURES
 CONTENTS Section Title 2 Disinfection, Packaging and Sterilization 3 Validation of Sterilization (Physical, Chemical and Biological) 4 Sterilizer Requalification 5 Sterile Package Shelf Life 6 Acquisition
CONTENTS Section Title 2 Disinfection, Packaging and Sterilization 3 Validation of Sterilization (Physical, Chemical and Biological) 4 Sterilizer Requalification 5 Sterile Package Shelf Life 6 Acquisition
DESCRIPTION OF THE RESEARCH RESULTS
 REF.: BIO_UAH_02 INDUSTRIAL SECTOR RESEARCHER DEPARTMENT CONTACT DETAILS WEB SITE Biotechnology Juan Manuel Bellón Caneiro Surgery 91 885 45 56 @ juanm.bellon@uah.es Biomaterials and Tisular engineering
REF.: BIO_UAH_02 INDUSTRIAL SECTOR RESEARCHER DEPARTMENT CONTACT DETAILS WEB SITE Biotechnology Juan Manuel Bellón Caneiro Surgery 91 885 45 56 @ juanm.bellon@uah.es Biomaterials and Tisular engineering
3.03 Understand support services
 3.03 Understand support services Clean and Decontaminate the Healthcare Environment Manage Hazardous Materials and Wastes Manage and Store Materials 3.03 Understand support services 2 Clean and Decontaminate
3.03 Understand support services Clean and Decontaminate the Healthcare Environment Manage Hazardous Materials and Wastes Manage and Store Materials 3.03 Understand support services 2 Clean and Decontaminate
A.P.A.G. Activated Plasma Albumin Gel
 A.P.A.G. Activated Plasma Albumin Gel How Does This Work In a new wound, platelets are the first cells which arrive. They aggregate to stop the bleeding and then release substances (growth factors, cytokines,
A.P.A.G. Activated Plasma Albumin Gel How Does This Work In a new wound, platelets are the first cells which arrive. They aggregate to stop the bleeding and then release substances (growth factors, cytokines,
IT S A GLOBAL LEADER. IT S PROVEN.
 isert Preloaded IOL System IT S A GLOBAL LEADER. IT S PROVEN. IT S isert. isert delivers the combination of simple operation, outstanding visual quality, and procedural efficiency. LEADERSHIP, WITH VISION
isert Preloaded IOL System IT S A GLOBAL LEADER. IT S PROVEN. IT S isert. isert delivers the combination of simple operation, outstanding visual quality, and procedural efficiency. LEADERSHIP, WITH VISION
