Mass drug administration for malaria A practical field manual
|
|
|
- Moris McDowell
- 6 years ago
- Views:
Transcription
1 Mass drug administration for malaria A practical field manual Malaria Policy Advisory Committee (MPAC) Meeting March 2017, World Health Organization, Geneva, Switzerland
2 Background on MDA for malaria Over the past decade, mass drug administration (MDA) and other approaches to mass screening and treatment have received increasing interest in the context of malaria elimination and in emergency situations such as the Ebola epidemic in West Africa Mass drug administration (MDA) has played a crucial role in the control and elimination of certain prevalent neglected tropical diseases (NTD s) such as lymphatic filariasis, soil transmitted helminthiasis, onchocerciasis, trachoma and schistosomiasis
3 Large-scale MDA for malaria in Sierra Leone Impact of the MDA for malaria in response to the Ebola outbreak in Sierra Leone. Aregawi et al. Malar J (2016) 15:480
4 WHO recommendations on MDA Based on WHO ERG held in April 2015 and MPAC advice in September who int/malaria/publications/atoz/role-of-mda-for-malaria pdf?ua=1
5 WHO recommendations on MDA (I) Based on a recent evidence review, the WHO Malaria Policy Advisory Committee made the following recommendations on the role of MDA, mass screening and treatment and focal screening and treatment for malaria: 1. Use of MDA for the elimination of P falciparum malaria can be considered in areas approaching interruption of transmission where there is good access to treatment, effective implementation of vector control and surveillance, and a minimal risk of re-introduction of infection 2. Given the threat of multidrug resistance and the WHO call for malaria elimination in the Greater Mekong subregion (GMS), MDA may be considered as a component of accelerated malaria elimination efforts in areas of the GMS with good access to treatment, vector control and surveillance
6 Malaria reported cases in Anjouan, Comores T3 policy Enforcement T3 MDA Artequick LLIN distribution
7 WHO recommendations on MDA (II) 3. Use of time-limited MDA to rapidly reduce malaria morbidity and mortality may be considered for epidemic control as part of the initial response, along with the urgent introduction of other interventions 4. Use of time-limited MDA to reduce malaria morbidity and mortality may be considered in complex emergencies, during exceptional circumstances when the health system is overwhelmed and unable to serve the affected communities 5. In the absence of sufficient evidence, WHO does not recommend the use of MDA in situations other than for areas approaching elimination, epidemics, and complex emergencies, as specified above (see 1-4) 6. Mass primaquine prophylactic treatment, requiring pre-seasonal MDA with daily administration of primaquine for two weeks without G6PD testing, is not recommended for the interruption of vivax transmission
8 WHO recommendations on MDA, MSAT and FSAT 7. Mass screening and treatment and focal screening and treatment for malaria are not recommended as interventions to interrupt malaria transmission 8. Medicines used for MDA must be of proven efficacy in the implementation area and preferably have a long half-life WHO recommends that a medicine different from that used for first line treatment be used for MDA Programs should include monitoring of efficacy, safety and the potential emergence of resistance to the antimalarial medicines deployed for MDA 9. WHO supports the need for more research on the optimum methods of implementing MDA programmes, promoting community participation and compliance with treatment, and evaluating their effectiveness Modelling can help guide the optimum method of administering MDA in different epidemiological circumstances and predict its likely impact
9 MDA: questions from the malaria programs (Dec 2015) Definition/Clarity is required Checklist for countries Analyze if it works operational feasibility Determine % P falciparum in areas with mixed infections (threshold?) Population mobility Not for the whole country, but for specific areas/foci/communities High coverage expected (total eligible population) National treatment guidelines Inclusion of MDA, with indication of medicines to be used for MDA Different chapters for epidemics containment and for malaria elimination (?) P vivax? Impact of MDA on P vivax Use of MDA with CQ for P vivax - Primaquine after G6PD testing
10 Questions on MDA from the programs: planning Data availability Availability of funds Availability of medicines (buffer stock) Timing of operations in relation to the malaria season to be defined Use to control malaria epidemics how to define epidemic thresholds Target population Eligible population: exclude pregnant women or pregnancy testing? Health systems preparedness in detecting & managing ADRs and rumors Dosage for children with complex drug regimens e.g. DHA-piperaquine Detailed (minimal essential) guidelines and SOPs
11 Questions on MDA from the programs: communication Information about MDA, Target communities, health workers, policy makers Not as routine intervention, boost for elimination with other interventions Information about expected and unexpected side-effects Community acceptance 30-50% not willing don t even start the MDA Manage rumors Setting a system for communication in crisis Active detection, management, media briefing and communication dissemination Community ownership and engagement
12 Questions from the programs: monitoring and evaluation Monitoring and Evaluation Measure coverage (household members, dispensed drugs, adherence ) Role of molecular methods for parasite detection Surveillance in elimination settings Define indicators by settings epidemics vs elimination When to stop? Emphasize MDA is time-bound Need effective pharmacovigilance as part of MDA Need for efficacy monitoring, using molecular markers of resistance Minimal reporting format
13 Process for development of the MDA operational manual Post-meeting Meeting Pre-meeting Preparatory phase (before drafting committee meeting) 1 st draft developed by Dr Nanclares based on the MDA experience in Sierra Leone review & inputs by 9 members of drafting committee (October November) 2 nd draft version developed to serve as basis of the drafting commitee Review by drafting committee in 4 thematic groups (22-23 November) Collation of all inputs and development of 3 rd draft version by Rapporteur review & inputs by drafting committee (December January) Collation of all inputs and development of final version by Rapporteur Presentation and discussion at MPAC (March 2017)
14 Composition/affiliations of participants Resource persons participating to the Drafting Committee Large-scale MDA with AS-AQ for malaria in Sierra Leone MDA with Art-PQP-PQ for malaria in Cambodia and Comoros MDA with DHA-PQP in Magude district, Southern Mozambique MDA with DHA-PQP in Southern Province of Zambia Research on MDA with DHA-PQP at Thai-Myanmar border Research on MDA with DHA-PQP in Viet Nam Research on MDA with DHA-PQP in Myanmar Community-based pharmacovigilance of ASAQ Programmatic experience with MDA for the control of NTD Implementation programs Implementation research Intervention studies MDA in the context of transmission reduction for malaria elimination
15 List of contents of the field guide 1. Introduction, background, definitions, objectives, WHO recommendations 2. Organization and implementation of mass drug administration Design phase (macroplanning) Planning and preparation Implementation 33 3 Monitoring and evaluation 43 4 Reporting 51 5 Key steps in a mass drug administration for malaria 53 References List of Annexes
16 Key steps in a MDA campaign for malaria
17 Executive summary Planning and preparation phase This phase involves planning the operational aspects of the framework defined at national level: Conduct micro-planning at province or district level according to the strategies defined by the national task force to guarantee an effective campaign by ensuring adequate distribution of supplies, training of staff, engagement of the community and proper management of resources. The micro-plan should include: demographic information on the province or district eligible for MDA information on the area (e.g. maps, infrastructure, location of health facilities, hard-to-reach areas) timing of MDA in the district delivery strategies human resources (number required, number available) and training plan logistical information social mobilization and communications plan and pharmacovigilance plan.
18 Executive summary (cont'd) Planning and preparation phase (cont'd) Ensure effective logistics, taking into consideration: procurement, storage and distribution of antimalarial medication procurement, storage and distribution of other supplies necessary for MDA transport accessibility to the entire target population, including those in hard-to-reach areas identification and preparation of distribution sites and waste management. Plan human and financial resources: number of teams required and composition training and adequate payment of salaries and per diem. door to door / fixed point urban / rural MDA cards / registration schools, camps, companies
19 Executive summary (cont'd) Human resource requirements Door-to-door strategy: The daily output depend on population density. In urban areas, one team can reach max people per day (average of households of five people, visits lasting min per household). In rural areas, one team can reach max people per day (10 15 households), considering time for transfer and communication to individual households. Centralized, fixed-site strategy: One team can distribute medicines to people per day. As this strategy is likely to miss a higher proportion of the population than door-to door distribution, special activities are required to mobilize and ensure the participation of the population.. The daily output depends on whether MDA cards are issued or a registration book is completed, which is more time-consuming. Specific teams might be considered for distribution in schools, prisons, military camps and orphanages and perhaps for the main local companies, such as factories, mines and plantations.
20 Executive summary (cont'd) Implementation phase The implementation phase involves the actual distribution of antimalarial treatment and includes: stock management: preparation of distribution kits with all the necessary materials ahead of time at the distribution point or peripheral health facility at which supplies are prepositioned distribution of the antimalarial medicine itself, either door to door or at a centralized, fixed site supervision, an essential component to ensure the quality of the campaign: at peripheral, district, regional and national levels data collection: collection and reporting of information on the number of people who receive treatment at community level, adverse drug reactions (ADRs) and analysis and compilation of data at higher levels through a well-established pathway of flow of information and coordination of all actors to monitor activities, detect any difficulties or constraints, address them and react to unforeseen events.
21 Executive summary (cont'd) Monitoring and evaluation intra-campaign monitoring system: a high-quality system for monitoring the campaign allows identification of constraints that require immediate action - can be done by monitors identified within the team or by independent monitors estimate of distribution coverage: the proportion of the target population that has been reached by distribution post-mda survey: recommended, if feasible, after each round or at least at the end of the entire campaign to obtain more reliable information on coverage and to evaluate adherence to treatment, determine reasons for non-participation or non-adherence and evaluate the presentation of ADRs monitoring consumption: daily monitoring of the number of treatments distributed and the number taken
22 Executive summary (cont'd) Monitoring and evaluation (cont'd) pharmacovigilance: a vital component of an MDA, which should be planned to ensure training, detection, reporting, management of follow-up of adverse events and to promote and monitor adherence by both passive and active surveillance. This component is also essential to obtain and maintain good understanding and compliance of the population monitoring drug resistance: one of the main concerns with regard to MDA is the emergence and spread of drug resistance although there is no evidence that MDA of artemisinin-based combined therapy (ACT) at therapeutic doses is related to the emergence of resistance, monitoring of resistance should be an essential component of an MDA campaign evaluation of impact: through routine surveillance and parasitological surveys and reporting: after each round and at the end of the intervention, of the coverage achieved, challenges and difficulties faced and solutions found, lessons learnt, practices with good results, effective social mobilization activities, useful tools and the costs of the intervention.
23 List of Annexes Annex 1. Standard distribution of populations in a developing country Annex 2. Available artemisinin-based combination therapy: dosing, formulation and presentation Annex 3. Example of calculation of orders of antimalarial medicine Annex 4. Example of a chronogram for MDA for malaria (distribution at 8 weeks) Annex 5. Example of micro-planning used in urban Western Area, Sierra Leone Annex 6. Step-by-step procedure for prepositioning supplies Annex 7. Example of radio spot on MDA for malaria used in Sierra Leone Annex 8. Examples of discussion points on MDA for community meetings (adapted from Sierra Leone) Annex 9. Household visit for MDA, step-by-step Annex 10. Example of laminated leaflet used by CHWs in Sierra Leone to explain treatment dosage
24 Examples of useful tools included in the Annexes Household visit guide for drug dispensers (Zambia) ASAQ dose chart for home visits (Sierra Leone)
25 List of Annexes (cont'd) Annex 11. Example of supervisors checklist used in Sierra Leone in Annex 12. Example of MDA card Annex 13. Example of tally sheet (adapted from that used in Sierra Leon ) Annex 14. Example of a household registration form (adapted from the Zambia MDA handbook) Annex 15. Example of daily summary form used in Sierra Leone Annex 16. Example of database for distribution team supervisors (adapted from Sierra Leone) Annex 17. Example of standard template for reporting a suspected adverse drug reaction Annex 18. Example of questionnaire for post-mda survey Annex 19. Example of pharmacovigilance preparedness checklist (used in Sierra Leone) Annex 20. Example of an MDA Pharmacovigilance training module
26 Change in QTc induced by antimalarials ASAQ & DP
27 Change in QTc induced by antimalarials DP in healthy volunteers & DP in malaria patients
28 Relevant findings of the ERG on cardiotoxicity of antimalarials 5. Is the risk of cardiotoxicity after exposure to piperaquine containing medicines higher than that of chloroquine? No. Review of pharmacovigilance, clinical and preclinical data, along with preliminary results of PK/PD modelling, provides no evidence of a significant difference in the risk of cardiotoxicity following exposure to the currently recommended doses of piperaquine, chloroquine or amodiaquine. 6. Is the risk of cardiotoxicity of piperaquine containing medicines higher in healthy volunteers compared to malaria patients? No. Review of pharmacovigilance and clinical data, along with preliminary results from PK/PD modelling, provides no evidence of a difference in the risk of cardiotoxicity of piperaquinecontaining medicines in healthy volunteers compared to malaria patients.
29 Proposed text on cardiotoxicity (to be added in section 3.4.3) WHO has recently reviewed the cardiotoxicity of antimalarial medicines. The full report of the Evidence Review Group meeting and the recommendations provided by the Malaria Policy Advisory Committee are available at the following URLs.. This review has concluded that the cardiovascular risk associated with the antimalarial drugs piperaquine, amodiaquine, chloroquine is considered very low and these medicines can be used in mass drug administration for malaria. As a precautionary principle DHA-PPQ, chloroquine or amodiaquine should not be given for MDA to individuals with a family history of sudden unexplained death consistent with cardiac arrhythmia. Concomitant intake of medicines which prolong the QT interval should be avoided (see Pharmacovigilance should be strengthened to track and investigate the risk factors associated with sudden unexplained deaths or any other adverse events associated with antimalarial drug use.
30 Discussion
Report from the Global Malaria Programme
 Report from the Global Malaria Programme Malaria Policy Advisory Committee Meeting WHO HQ Geneva, 5 March 2015 Pedro Alonso Director, Global Malaria Programme alonsop@who.int On behalf of the global malaria
Report from the Global Malaria Programme Malaria Policy Advisory Committee Meeting WHO HQ Geneva, 5 March 2015 Pedro Alonso Director, Global Malaria Programme alonsop@who.int On behalf of the global malaria
Guidance on temporary malaria control measures in Ebola-affected countries
 GLOBAL MALARIA PROGRAMME Guidance on temporary malaria control measures in Ebola-affected countries Last updated: 13 November 2014 2 1 The effectiveness of the Ebola response in Guinea, Liberia and Sierra
GLOBAL MALARIA PROGRAMME Guidance on temporary malaria control measures in Ebola-affected countries Last updated: 13 November 2014 2 1 The effectiveness of the Ebola response in Guinea, Liberia and Sierra
RAI Regional Steering Committee Regional Component of the Regional Artemisinin-resistance Initiative (RAI2E) Grant CALL FOR PROPOSALS
 RAI Regional Steering Committee Regional Component of the Regional Artemisinin-resistance Initiative (RAI2E) Grant CALL FOR PROPOSALS Package #2: Stimulating operational research and innovation to guide
RAI Regional Steering Committee Regional Component of the Regional Artemisinin-resistance Initiative (RAI2E) Grant CALL FOR PROPOSALS Package #2: Stimulating operational research and innovation to guide
Malaria Research Capability Strengthening in Africa
 July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
Preparations for Ebola Vaccine Deployment
 Preparations for Ebola Vaccine Deployment Ebola vaccine and vaccination - Session 8 Meeting of the Strategic Advisory Group of Experts on Immunization (SAGE) Geneva, 14 16 April 2015 Dr Marie-Pierre Preziosi,
Preparations for Ebola Vaccine Deployment Ebola vaccine and vaccination - Session 8 Meeting of the Strategic Advisory Group of Experts on Immunization (SAGE) Geneva, 14 16 April 2015 Dr Marie-Pierre Preziosi,
Neglected tropical diseases
 EXECUTIVE BOARD EB132/19 132nd session 23 November 2012 Provisional agenda item 9.2 Neglected tropical diseases Prevention, control, elimination and eradication Report by the Secretariat 1. Despite their
EXECUTIVE BOARD EB132/19 132nd session 23 November 2012 Provisional agenda item 9.2 Neglected tropical diseases Prevention, control, elimination and eradication Report by the Secretariat 1. Despite their
What Can I Do With My Data?
 What Can I Do With My Data? Utilizing Existing Data for Analysis and Hypothesis Development Falgunee Parekh, MPH, PhD Agenda My Research Background Background on Analysis of Surveillance (or Initial) Data
What Can I Do With My Data? Utilizing Existing Data for Analysis and Hypothesis Development Falgunee Parekh, MPH, PhD Agenda My Research Background Background on Analysis of Surveillance (or Initial) Data
Report from the Global Malaria Programme
 Report from the Global Malaria Programme Malaria Policy Advisory Committee Geneva, Switzerland Dr Pedro L. Alonso, Director 17 October 2017 GMP Strategy - Core Roles I. Strategic questions Global Vector
Report from the Global Malaria Programme Malaria Policy Advisory Committee Geneva, Switzerland Dr Pedro L. Alonso, Director 17 October 2017 GMP Strategy - Core Roles I. Strategic questions Global Vector
Investing in Research, Development and Innovation for Global Health
 The Malaria Advocacy Working Group (MAWG) of the Roll Back Malaria Partnership s (RBM) contribution to the European Commission consultation on the Green Paper - From Challenges to Opportunities: Towards
The Malaria Advocacy Working Group (MAWG) of the Roll Back Malaria Partnership s (RBM) contribution to the European Commission consultation on the Green Paper - From Challenges to Opportunities: Towards
Progress of Malaria Elimination Efforts in the Greater Mekong Subregion 2016
 Progress of Malaria Elimination Efforts in the Greater Mekong Subregion 216 WHO MPAC BRIEFING GENEVA 14 th September 216 Dr Fred Binka/ ERAR, Dr M Aregawi/ERAR, Dr EM Christophel/SEARO, Dr R Abeyasinghe/WPRO
Progress of Malaria Elimination Efforts in the Greater Mekong Subregion 216 WHO MPAC BRIEFING GENEVA 14 th September 216 Dr Fred Binka/ ERAR, Dr M Aregawi/ERAR, Dr EM Christophel/SEARO, Dr R Abeyasinghe/WPRO
Vector control. REGIONAL COMMITTEE Provisional Agenda item 8.4. SEA/RC70/10 Maldives 6 10 September July Seventieth Session
 REGIONAL COMMITTEE Provisional Agenda item 8.4 Seventieth Session SEA/RC70/10 Maldives 6 10 September 2017 18 July 2017 Vector control Major vector-borne diseases account for an estimated 17% of the global
REGIONAL COMMITTEE Provisional Agenda item 8.4 Seventieth Session SEA/RC70/10 Maldives 6 10 September 2017 18 July 2017 Vector control Major vector-borne diseases account for an estimated 17% of the global
Malaria Control in Zambia. October 27, 2005
 October 27, 2005 Country Background Landlocked country located in south-central Africa Pop. over 10 million, 62% urban, 753,000 sq km. Mostly high plateau with flat or undulating terrain Nine Provinces,
October 27, 2005 Country Background Landlocked country located in south-central Africa Pop. over 10 million, 62% urban, 753,000 sq km. Mostly high plateau with flat or undulating terrain Nine Provinces,
SIAPS assistance is grouped into four technical areas
 Our specific approaches to each of the five result areas follows Governance Under SIAPS, our approach to improving governance and accountability focuses on establishing transparent management systems grounded
Our specific approaches to each of the five result areas follows Governance Under SIAPS, our approach to improving governance and accountability focuses on establishing transparent management systems grounded
Malaria Research Capability Strengthening in Africa
 October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
Process Evaluation. Nampula LLIN Distribution Campaign: Observations and recommendations. November 2016
 Process Evaluation Nampula LLIN Distribution Campaign: Observations and recommendations November 2016 Objectives Identify best practices and draw lessons for the national campaign Understand what processes
Process Evaluation Nampula LLIN Distribution Campaign: Observations and recommendations November 2016 Objectives Identify best practices and draw lessons for the national campaign Understand what processes
Licenced Ebola Vaccine
 The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
Meeting Report. Second Pacific Malaria Drug Resistance Monitoring Network Meeting
 Meeting Report Second Pacific Malaria Drug Resistance Monitoring Network Meeting Manila, Philippines 6 7 May 2013 Participants of the Second Pacific Malaria Drug Resistance Monitoring Network Meeting Manila,
Meeting Report Second Pacific Malaria Drug Resistance Monitoring Network Meeting Manila, Philippines 6 7 May 2013 Participants of the Second Pacific Malaria Drug Resistance Monitoring Network Meeting Manila,
The long walk to a malaria-free world
 1 The long walk to a malaria-free world Message from the Chairman and CEO Mr Ray Chambers Chairman of the Board Dr David Reddy MMV s CEO It always seems S impossible until it s done. Nelson Mandela (1918
1 The long walk to a malaria-free world Message from the Chairman and CEO Mr Ray Chambers Chairman of the Board Dr David Reddy MMV s CEO It always seems S impossible until it s done. Nelson Mandela (1918
Malaria surveillance strengthening in Myanmar
 Malaria surveillance strengthening in Myanmar Implementation plan February 2014 Prepared by Jonathan Co and Steven Mellor CONTENTS 1. BACKGROUND 1 2. KEY AREAS FOR SURVEILLANCE STRENGTHENING 1 3. PATHWAY
Malaria surveillance strengthening in Myanmar Implementation plan February 2014 Prepared by Jonathan Co and Steven Mellor CONTENTS 1. BACKGROUND 1 2. KEY AREAS FOR SURVEILLANCE STRENGTHENING 1 3. PATHWAY
AMP Toolkit A toolkit for mass distribution campaigns to increase coverage and use of long-lasting insecticide-treated nets
 AMP Toolkit 2.0 - A toolkit for mass distribution campaigns to increase coverage and use of long-lasting insecticide-treated nets Presentation to the CORE Group May 23, 2013 Marcy Erskine IFRC Jessica
AMP Toolkit 2.0 - A toolkit for mass distribution campaigns to increase coverage and use of long-lasting insecticide-treated nets Presentation to the CORE Group May 23, 2013 Marcy Erskine IFRC Jessica
Sustainable Health Care Systems: Research and Innovation
 Backgroundpaper B7-Communiqué Sustainable Health Care Systems: Research and Innovation Version II BDI, BUSINESSEUROPE Canadian Chamber of Commerce CBI CONFIDUSTRIA Keidanren MEDEF US Chamber of Commerce
Backgroundpaper B7-Communiqué Sustainable Health Care Systems: Research and Innovation Version II BDI, BUSINESSEUROPE Canadian Chamber of Commerce CBI CONFIDUSTRIA Keidanren MEDEF US Chamber of Commerce
SUMMARY DOCUMENT FOR FROM PIPELINE TO PRODUCT: MALARIA R&D FUNDING NEEDS INTO THE NEXT DECADE
 SUMMARY DOCUMENT FOR FROM PIPELINE TO PRODUCT: MALARIA R&D FUNDING NEEDS INTO THE NEXT DECADE REPORT SUMMARY Introduction Malaria is a major public health challenge that threatens approximately half of
SUMMARY DOCUMENT FOR FROM PIPELINE TO PRODUCT: MALARIA R&D FUNDING NEEDS INTO THE NEXT DECADE REPORT SUMMARY Introduction Malaria is a major public health challenge that threatens approximately half of
Report on meetings of expert committees and study groups 1
 EXECUTIVE BOARD EB137/9 137th session 27 March 2015 Provisional agenda item 10 Report on meetings of expert committees and study groups 1 Report by the Secretariat SPECIFICATIONS FOR PHARMACEUTICAL PREPARATIONS
EXECUTIVE BOARD EB137/9 137th session 27 March 2015 Provisional agenda item 10 Report on meetings of expert committees and study groups 1 Report by the Secretariat SPECIFICATIONS FOR PHARMACEUTICAL PREPARATIONS
JOB DESCRIPTION. Organisational background. Country and project background
 JOB DESCRIPTION Job title: State Technical Officer Location: Kano, Niger and Gomber (3 positions) Department: Technical Length of contract: 2 years Role type: National Grade: 7 Travel involved: In-country
JOB DESCRIPTION Job title: State Technical Officer Location: Kano, Niger and Gomber (3 positions) Department: Technical Length of contract: 2 years Role type: National Grade: 7 Travel involved: In-country
EDCTP perspective on biopreparedness and related capacity development in Africa
 EDCTP perspective on biopreparedness and related capacity development in Africa Consultation workshop on Preparedness for emerging diseases Dr. Michael Makanga EDCTP Executive Director. 29 September 2016
EDCTP perspective on biopreparedness and related capacity development in Africa Consultation workshop on Preparedness for emerging diseases Dr. Michael Makanga EDCTP Executive Director. 29 September 2016
The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines
 The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines Health care uncertainty assessment workshop Session 3: The challenges of health
The EU Risk Management Plan - a tool to address the uncertainties at the time of approval, and manage the risks of medicines Health care uncertainty assessment workshop Session 3: The challenges of health
Summary of STREAM and Delamanid phase III trial results and implications:
 Treating Patient, Not Disease: People-Centered Approach 7th TB Symposium Ministry of Health of the Kyrgyz Republic and Médecins Sans Frontières 1-2 March, 2018, BISHKEK, KYRGYZSTAN Summary of STREAM and
Treating Patient, Not Disease: People-Centered Approach 7th TB Symposium Ministry of Health of the Kyrgyz Republic and Médecins Sans Frontières 1-2 March, 2018, BISHKEK, KYRGYZSTAN Summary of STREAM and
Health Impact of Ebola Crisis. Presentation for LSHTM / Options - Chris Lewis
 Health Impact of Ebola Crisis Presentation for LSHTM / Options - Chris Lewis Outline Introduction Ebola outbreak Impact of the crisis Timeline Early December 2013 - little boy in southern Guinea caught
Health Impact of Ebola Crisis Presentation for LSHTM / Options - Chris Lewis Outline Introduction Ebola outbreak Impact of the crisis Timeline Early December 2013 - little boy in southern Guinea caught
CALL FOR PROPOSALS Operational Research on Access to Morbidity Management & Disability Prevention Services
 CALL FOR PROPOSALS Operational Research on Access to Morbidity Management & Disability Prevention Services A call for proposed studies to be funded with UK aid from the British people through a grant to
CALL FOR PROPOSALS Operational Research on Access to Morbidity Management & Disability Prevention Services A call for proposed studies to be funded with UK aid from the British people through a grant to
SUMMARY OF THE MID-TERM REVIEW OF TRACHOMA ELIMINATION PROGRAMMES IN AFRICA
 SUMMARY OF THE MID-TERM REVIEW OF TRACHOMA ELIMINATION PROGRAMMES IN AFRICA Review conducted by Tropical Health LLP on behalf of The Queen Elizabeth Diamond Jubilee Trust and the UK Department for International
SUMMARY OF THE MID-TERM REVIEW OF TRACHOMA ELIMINATION PROGRAMMES IN AFRICA Review conducted by Tropical Health LLP on behalf of The Queen Elizabeth Diamond Jubilee Trust and the UK Department for International
in Combating Malaria Manos Perros Pfizer Global Research & Development Musée de la Croix-Rouge, Geneva November 12, 2009
 Role of the Private Sector in Combating Malaria Manos Perros Pfizer Global Research & Development Musée de la Croix-Rouge, Geneva November 12, 2009 Drug Development and the Evolving R&D Ecosystem Research
Role of the Private Sector in Combating Malaria Manos Perros Pfizer Global Research & Development Musée de la Croix-Rouge, Geneva November 12, 2009 Drug Development and the Evolving R&D Ecosystem Research
One Year Later, Where Does the U.S. Response to Ebola Stand?
 One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
Developing a 5 Year Strategic Framework: Charting a New Course for Malaria Communication. July 26-27, 2011 Southern Sun Hotel Nairobi, Kenya
 Developing a 5 Year Strategic Framework: Charting a New Course for Malaria Communication July 26-27, 2011 Southern Sun Hotel Nairobi, Kenya Agenda Day 1 Welcome and Introductions Outline and Synopsis of
Developing a 5 Year Strategic Framework: Charting a New Course for Malaria Communication July 26-27, 2011 Southern Sun Hotel Nairobi, Kenya Agenda Day 1 Welcome and Introductions Outline and Synopsis of
Monitoring and Evaluation: the Foundations for Results
 Monitoring and Evaluation: the Foundations for Results Laura B. Rawlings Lead Social Protection Specialist Human Development Network World Bank Beijing, China Impact Evaluation Workshop July 2009 1 Objectives
Monitoring and Evaluation: the Foundations for Results Laura B. Rawlings Lead Social Protection Specialist Human Development Network World Bank Beijing, China Impact Evaluation Workshop July 2009 1 Objectives
Explaining EDCTP2 and its perspective on Capacity Building for PV of products used in managing PRDs
 Explaining EDCTP2 and its perspective on Capacity Building for PV of products used in managing PRDs 16 th Annual meeting of the International Society of Pharmacovigilance Jean Marie Vianney Habarugira,
Explaining EDCTP2 and its perspective on Capacity Building for PV of products used in managing PRDs 16 th Annual meeting of the International Society of Pharmacovigilance Jean Marie Vianney Habarugira,
WHO Medicines Safety & Vigilance (SAV/Rx) Updates:
 Conclusions and Recommendations from the Fourteenth Meeting of the WHO Advisory Committee on Safety of Medicinal Products (ACSoMP) Geneva, Switzerland 25-26 April 2017 The WHO Advisory Committee on Safety
Conclusions and Recommendations from the Fourteenth Meeting of the WHO Advisory Committee on Safety of Medicinal Products (ACSoMP) Geneva, Switzerland 25-26 April 2017 The WHO Advisory Committee on Safety
Manual for Quantification of Malaria Commodities
 Manual for Quantification of Malaria Commodities Rapid Diagnostic Tests and Artemisinin-Based Combination Therapy for First-Line Treatment of Plasmodium Falciparum Malaria This report is made possible
Manual for Quantification of Malaria Commodities Rapid Diagnostic Tests and Artemisinin-Based Combination Therapy for First-Line Treatment of Plasmodium Falciparum Malaria This report is made possible
Manual for Quantification of Malaria Commodities
 Manual for Quantification of Malaria Commodities Rapid Diagnostic Tests and Artemisinin-Based Combination Therapy for First-Line Treatment of Plasmodium Falciparum Malaria This report is made possible
Manual for Quantification of Malaria Commodities Rapid Diagnostic Tests and Artemisinin-Based Combination Therapy for First-Line Treatment of Plasmodium Falciparum Malaria This report is made possible
Monitoring Antimalarial Drug Resistance,
 WHO/CDS/CSR/EPH/2002.17 WHO/CDS/RBM/2002.39 Monitoring Antimalarial Drug Resistance, Report of a WHO consultation Geneva, Switzerland 3 5 December 2001 World Health Organization Department of Communicable
WHO/CDS/CSR/EPH/2002.17 WHO/CDS/RBM/2002.39 Monitoring Antimalarial Drug Resistance, Report of a WHO consultation Geneva, Switzerland 3 5 December 2001 World Health Organization Department of Communicable
Professor PL Chiodini
 Professor PL Chiodini WHO World Malaria Report 2017 WHO World Malaria Report 2017 216 million reported cases in 2016 Increased by 5 million from 2015 445,000 deaths Malaria case incidence has fallen since
Professor PL Chiodini WHO World Malaria Report 2017 WHO World Malaria Report 2017 216 million reported cases in 2016 Increased by 5 million from 2015 445,000 deaths Malaria case incidence has fallen since
PATH/Fatou Kande-Senghor. Zambia: Accelerating Toward Malaria Elimination. Stakeholder Perspectives
 Z AM B I A PATH/Fatou Kande-Senghor Zambia: Accelerating Toward Malaria Elimination Stakeholder Perspectives CO M P L E T E D B Y PAT H M A C E PA I N PA R T N E R S H I P W I T H T H E Z A M B I A M I
Z AM B I A PATH/Fatou Kande-Senghor Zambia: Accelerating Toward Malaria Elimination Stakeholder Perspectives CO M P L E T E D B Y PAT H M A C E PA I N PA R T N E R S H I P W I T H T H E Z A M B I A M I
Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1
 ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL FIXED DOSE COMBINATION PRODUCTS (FDC Products) FOR HIV/AIDS, TUBERCULOSIS, and MALARIA This guideline is intended to provide recommendations to applicants wishing to submit applications
MEDICINES CONTROL COUNCIL FIXED DOSE COMBINATION PRODUCTS (FDC Products) FOR HIV/AIDS, TUBERCULOSIS, and MALARIA This guideline is intended to provide recommendations to applicants wishing to submit applications
Rapid Diagnostic Tests in malaria case management
 Rapid Diagnostic Tests in malaria case management Planning, Procuring and Implementing Suggestions for incorporation of malaria RDT-based diagnosis into proposals to the Global Fund Foundation for Innovative
Rapid Diagnostic Tests in malaria case management Planning, Procuring and Implementing Suggestions for incorporation of malaria RDT-based diagnosis into proposals to the Global Fund Foundation for Innovative
WaterAid and Partners Planning Meeting J&E RESORT, BO.
 WaterAid and Partners Planning Meeting J&E RESORT, BO. Locations: Freetown, Kenema, Pujehun and Bonthe Districts Duration: 3 months i.e. 2 nd January to 31 st March 2015 Project Description Title: Public
WaterAid and Partners Planning Meeting J&E RESORT, BO. Locations: Freetown, Kenema, Pujehun and Bonthe Districts Duration: 3 months i.e. 2 nd January to 31 st March 2015 Project Description Title: Public
Minutes of 5 th TEG on Drug Efficacy and Response meeting. Geneva, October 2017 MPAC meeting
 Minutes of 5 th TEG on Drug Efficacy and Response meeting Geneva, 17-19 October 2017 MPAC meeting Background The TEG DER met 1-2 June 2017 in Geneva TEG members: Arjen DONDORP (chair) Mahidol-Oxford Research
Minutes of 5 th TEG on Drug Efficacy and Response meeting Geneva, 17-19 October 2017 MPAC meeting Background The TEG DER met 1-2 June 2017 in Geneva TEG members: Arjen DONDORP (chair) Mahidol-Oxford Research
Contents. 1. Background 2. Introduction to the manual 3. Purpose 4. The Monitoring System. Annexes:
 Contents 1. Background 2. Introduction to the manual 3. Purpose 4. The Monitoring System 4.1. What to Monitor 4.2. Data Source 4.3. How to Monitor/ Data collection method 4.4. Frequency of Monitoring 4.5.
Contents 1. Background 2. Introduction to the manual 3. Purpose 4. The Monitoring System 4.1. What to Monitor 4.2. Data Source 4.3. How to Monitor/ Data collection method 4.4. Frequency of Monitoring 4.5.
Vivax Working Group Workplan
 Vivax Working Group Workplan 2017-2019 Why Does The VxWG Exist? The choice before us is clear. If we continue with a business as usual approach employing the same level of resources and the same interventions
Vivax Working Group Workplan 2017-2019 Why Does The VxWG Exist? The choice before us is clear. If we continue with a business as usual approach employing the same level of resources and the same interventions
Independent Evaluation (IE) of Phase 1 of the Affordable Medicines Facility - malaria
 Presentation to Institute of Medicine Workshop on Evaluation Methods for Large-Scale, Complex, Multi-National Global Health Initiatives January 7 th, 2014, London Independent Evaluation (IE) of Phase 1
Presentation to Institute of Medicine Workshop on Evaluation Methods for Large-Scale, Complex, Multi-National Global Health Initiatives January 7 th, 2014, London Independent Evaluation (IE) of Phase 1
Emergencies preparedness, response Ebola virus disease Democratic Republic
 1 von 5 05.12.2018, 08:43 Emergencies preparedness, response Ebola virus disease Democratic Republic of the Congo Disease outbreak news: Update 29 November 2018 As the Ebola virus disease (EVD) outbreak
1 von 5 05.12.2018, 08:43 Emergencies preparedness, response Ebola virus disease Democratic Republic of the Congo Disease outbreak news: Update 29 November 2018 As the Ebola virus disease (EVD) outbreak
IMI2 Ebola and other filoviral haemorrhagic fevers programme. Webinar for SRG and SC 3 November 2014
 IMI2 Ebola and other filoviral haemorrhagic fevers programme Webinar for SRG and SC 3 November 2014 Background Ebola virus diseases (EVD) is a rare and deadly disease Filoviruses such as Ebola spread directly
IMI2 Ebola and other filoviral haemorrhagic fevers programme Webinar for SRG and SC 3 November 2014 Background Ebola virus diseases (EVD) is a rare and deadly disease Filoviruses such as Ebola spread directly
RESEARCH DURING PUBLIC HEALTH EMERGENCIES- ETHICAL ISSUES
 RESEARCH DURING PUBLIC HEALTH EMERGENCIES- ETHICAL ISSUES Prof. Suma Krishnasastry Prof of Medicine Govt. T. D. Medical College, Alappuzha, Kerala, INDIA Public health Emergency Hallmark of a Public Health
RESEARCH DURING PUBLIC HEALTH EMERGENCIES- ETHICAL ISSUES Prof. Suma Krishnasastry Prof of Medicine Govt. T. D. Medical College, Alappuzha, Kerala, INDIA Public health Emergency Hallmark of a Public Health
Collaborative clinical research between EU & sub-saharan Africa
 Collaborative clinical research between EU & sub-saharan Africa European & Developing Countries Clinical Trials Partnership Dr Monique Surette, EDCTP Senior Project Officer EC Open Info Day - Horizon 2020
Collaborative clinical research between EU & sub-saharan Africa European & Developing Countries Clinical Trials Partnership Dr Monique Surette, EDCTP Senior Project Officer EC Open Info Day - Horizon 2020
MMV Business Plan MMV Business Plan
 MMV Business Plan 2017 2021 1 MMV Business Plan 2017 21 Executive Summary December 2016 Delivering medicines to secure a future free from malaria MMV Business Plan 2017 2021 3 Contents 4 Statement of intent
MMV Business Plan 2017 2021 1 MMV Business Plan 2017 21 Executive Summary December 2016 Delivering medicines to secure a future free from malaria MMV Business Plan 2017 2021 3 Contents 4 Statement of intent
Pharmacovigilance Approach to Challenges with Use of Medicines. Oksana Lebega USAID SIAPS project Antalya, Turkey December 10-13, 2013
 Pharmacovigilance Approach to Challenges with Use of Medicines Oksana Lebega USAID SIAPS project Antalya, Turkey December 10-13, 2013 Background Development of Pharmacovigilance (1) Growing market of pharmaceutical
Pharmacovigilance Approach to Challenges with Use of Medicines Oksana Lebega USAID SIAPS project Antalya, Turkey December 10-13, 2013 Background Development of Pharmacovigilance (1) Growing market of pharmaceutical
Organizing Framework for a Functional National HIV Monitoring & Evaluation System
 Regional Training on Strategic and Operational Planning in HIV & AIDS Organizing Framework for a Functional National HIV Monitoring & Evaluation System Final Draft for submission to the HIV Monitoring
Regional Training on Strategic and Operational Planning in HIV & AIDS Organizing Framework for a Functional National HIV Monitoring & Evaluation System Final Draft for submission to the HIV Monitoring
Arsenic in Drinking Water and Resulting Arsenic Toxicity in India and Bangladesh: Recommendations for Action
 Página 1 de 8 Arsenic Crisis Info Centre [ Back ] [ Up ] [ Next ] Arsenic in Drinking Water and Resulting Arsenic Toxicity in India and Bangladesh: Recommendations for Action An outcome of the Regional
Página 1 de 8 Arsenic Crisis Info Centre [ Back ] [ Up ] [ Next ] Arsenic in Drinking Water and Resulting Arsenic Toxicity in India and Bangladesh: Recommendations for Action An outcome of the Regional
Liberia s Presentation At the. Global Ebola Vaccine Implementation Team (GEVIT) Regional Workshop October 27-29, 2015 Geneva, Switzerland
 Liberia s Presentation At the Global Ebola Vaccine Implementation Team (GEVIT) Regional Workshop October 27-29, 2015 Geneva, Switzerland Presentation Structure List of participants from Liberia Background
Liberia s Presentation At the Global Ebola Vaccine Implementation Team (GEVIT) Regional Workshop October 27-29, 2015 Geneva, Switzerland Presentation Structure List of participants from Liberia Background
Evaluation: update and proposed workplan for
 EXECUTIVE BOARD 138th session 4 December 2015 Provisional agenda item 12.1 Evaluation: update and proposed workplan for 2016 2017 1. The Executive Board approved the WHO evaluation policy at its 131st
EXECUTIVE BOARD 138th session 4 December 2015 Provisional agenda item 12.1 Evaluation: update and proposed workplan for 2016 2017 1. The Executive Board approved the WHO evaluation policy at its 131st
The Role of the Pharmacist in Pharmacovigilance A Regulatory Perspective
 The Role of the Pharmacist in Pharmacovigilance A Regulatory Perspective Almath Spooner, Irish Medicines Board Pharmaceutical Society of Ireland National Pharmacy Summit, November 2008. Presentation Topics
The Role of the Pharmacist in Pharmacovigilance A Regulatory Perspective Almath Spooner, Irish Medicines Board Pharmaceutical Society of Ireland National Pharmacy Summit, November 2008. Presentation Topics
Classroom Discussion Guide on Ethics and Public Health Emergencies
 Classroom Discussion Guide on Ethics and Public Health Emergencies This guide provides discussion questions and topics based on the Presidential Commission for the Study of Bioethical Issues report, Ethics
Classroom Discussion Guide on Ethics and Public Health Emergencies This guide provides discussion questions and topics based on the Presidential Commission for the Study of Bioethical Issues report, Ethics
SCALING-UP COMMUNITY PARTICIPATION FOR COMMUNICABLE DISEASE CONTROL AND ELIMINATION. Dr Jo-An Atkinson The Australian Prevention Partnership Centre
 SCALING-UP COMMUNITY PARTICIPATION FOR COMMUNICABLE DISEASE CONTROL AND ELIMINATION Dr Jo-An Atkinson The Australian Prevention Partnership Centre Community participation in successful communicable disease
SCALING-UP COMMUNITY PARTICIPATION FOR COMMUNICABLE DISEASE CONTROL AND ELIMINATION Dr Jo-An Atkinson The Australian Prevention Partnership Centre Community participation in successful communicable disease
Ebola outbreak in West Africa : Shift in paradigm
 Ebola outbreak in West Africa : Shift in paradigm Dr S.C Briand, Director Pandemic and Epidemic Diseases department, WHO Geneva EVD West Africa: many "first time" Enormous epidemic in the affected countries
Ebola outbreak in West Africa : Shift in paradigm Dr S.C Briand, Director Pandemic and Epidemic Diseases department, WHO Geneva EVD West Africa: many "first time" Enormous epidemic in the affected countries
Page 1 of 11. WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations.
 Page 1 of 11 WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations November 2016 Page 2 of 11 Target Audience The target audience for this
Page 1 of 11 WHO Target Product Profile for multivalent filovirus vaccines: providing long-term protection to high-risk populations November 2016 Page 2 of 11 Target Audience The target audience for this
Update on the ongoing development of a Global Vector Control Response
 Update on the ongoing development of a Global Vector Control Response Malaria Policy Advisory Committee 15th September 2016 Global Malaria Programme Department of Control of Neglected Tropical Diseases
Update on the ongoing development of a Global Vector Control Response Malaria Policy Advisory Committee 15th September 2016 Global Malaria Programme Department of Control of Neglected Tropical Diseases
Ebola Virus Disease in West Africa: From Crisis to More Resilient Health Systems
 Ebola Virus Disease in West Africa: From Crisis to More Resilient Health Systems Patricio V. Marquez Lead Health Specialist World Bank Health, Nutrition and Population Global Practice April 15, 2015 Outline
Ebola Virus Disease in West Africa: From Crisis to More Resilient Health Systems Patricio V. Marquez Lead Health Specialist World Bank Health, Nutrition and Population Global Practice April 15, 2015 Outline
- Selection of Target Molecular for Drug Discovery by Verifications on the Validity of Target Candidates -
 University of Tokyo and Astellas Enter Collaborative Research for Target Discovery of New Drugs for Neglected Tropical Diseases Caused by Protozoan Parasites - Selection of Target Molecular for Drug Discovery
University of Tokyo and Astellas Enter Collaborative Research for Target Discovery of New Drugs for Neglected Tropical Diseases Caused by Protozoan Parasites - Selection of Target Molecular for Drug Discovery
Module 5. Medicines for Preventive Chemotherapy: Planning, Applying, Managing, Reporting
 : Planning, Applying, Managing, Reporting Sessions 1 & 2. Joint Application Package (JAP) and PART 1. JOINT APPLICATION PACKAGE (JAP) 2 How many forms does the JAP include? 3 Joint Application Package
: Planning, Applying, Managing, Reporting Sessions 1 & 2. Joint Application Package (JAP) and PART 1. JOINT APPLICATION PACKAGE (JAP) 2 How many forms does the JAP include? 3 Joint Application Package
Ebola virus disease outbreak in western Africa
 Action Plan Strategic Objectives 6. Promote innovation and research on new drugs International surveillance networks and information sharing on promising research Active role in research for governments
Action Plan Strategic Objectives 6. Promote innovation and research on new drugs International surveillance networks and information sharing on promising research Active role in research for governments
Furthermore, the level of information and the subsequent assessment will vary on a case by case basis.
 17 March 2016 EMA/352178/2013 Committee for Medicinal Products for Human Use Committee for Medicinal Products for Veterinary Use Points to consider for the overall assessment of a supply shortage of a
17 March 2016 EMA/352178/2013 Committee for Medicinal Products for Human Use Committee for Medicinal Products for Veterinary Use Points to consider for the overall assessment of a supply shortage of a
East Africa: Ebola Preparedness
 East Africa: Ebola Preparedness Ebola Preparedness Fund (EPF) Operation n MDR64007 Date of issue: 13 April, 2015 Date of disaster: Operation manager: Operation start date: 10 February 2015 Operation budget:
East Africa: Ebola Preparedness Ebola Preparedness Fund (EPF) Operation n MDR64007 Date of issue: 13 April, 2015 Date of disaster: Operation manager: Operation start date: 10 February 2015 Operation budget:
WHO Blood Regulators Network (BRN)
 Distribution: General English only WHO Blood Regulators Network (BRN) Position Paper on Use of Convalescent Plasma, Serum or Immune Globulin Concentrates as an Element in Response to an Emerging Virus*
Distribution: General English only WHO Blood Regulators Network (BRN) Position Paper on Use of Convalescent Plasma, Serum or Immune Globulin Concentrates as an Element in Response to an Emerging Virus*
Malaria surveillance system performance in Mozambique: a comprehensive assessment
 TECHNICAL BRIEF Malaria surveillance system performance in Mozambique: a comprehensive assessment Background Despite recent reductions in morbidity and mortality, malaria remains the largest public health
TECHNICAL BRIEF Malaria surveillance system performance in Mozambique: a comprehensive assessment Background Despite recent reductions in morbidity and mortality, malaria remains the largest public health
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE November 2004 Revised June 2009 SEX-RELATED CONSIDERATIONS IN THE CONDUCT OF CLINICAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE November 2004 Revised June 2009 SEX-RELATED CONSIDERATIONS IN THE CONDUCT OF CLINICAL
Data requirements and methods to support the evaluation of new vector control products
 Global Malaria Programme Data requirements and methods to support the evaluation of new vector control products DECEMBER 2017 RECOMMENDATIONS BACKGROUND WHO s process for the evaluation of vector control
Global Malaria Programme Data requirements and methods to support the evaluation of new vector control products DECEMBER 2017 RECOMMENDATIONS BACKGROUND WHO s process for the evaluation of vector control
Improving Access to Malaria Medicines in Zambia
 Logistics Brief Improving Access to Malaria Medicines in Zambia A healthcare worker manages artemisininbased combination therapies at a clinic in Zambia. USAID DELIVER PROJECT 2009 The Essential Medicines
Logistics Brief Improving Access to Malaria Medicines in Zambia A healthcare worker manages artemisininbased combination therapies at a clinic in Zambia. USAID DELIVER PROJECT 2009 The Essential Medicines
Job title: Senior Campaign Manager Location: Yobe. Department: Technical Length of contract: 1-year
 JOB DESCRIPTION Job title: Senior Campaign Manager Location: Yobe Department: Technical Length of contract: 1-year Role type: National Grade: 10 Travel involved: Up to 70% Child safeguarding level: Reporting
JOB DESCRIPTION Job title: Senior Campaign Manager Location: Yobe Department: Technical Length of contract: 1-year Role type: National Grade: 10 Travel involved: Up to 70% Child safeguarding level: Reporting
Action points and notes. UNFPA, UN House, Copenhagen Denmark
 Action points and notes IPC Meeting 30 th November 02 nd December 2011 UNFPA, UN House, Copenhagen Denmark Action points - Share draft documents on diagnostics to be shared with IPC members for review
Action points and notes IPC Meeting 30 th November 02 nd December 2011 UNFPA, UN House, Copenhagen Denmark Action points - Share draft documents on diagnostics to be shared with IPC members for review
UNDERSTANDING SUPPLY AND DEMAND TO IMPROVE HEALTH PRODUCT AVAILABILITY FROM INITIAL RESPONSE THROUGH SUSTAINABLE RECOVERY IN HUMANITARIAN SETTINGS
 July 2018 UNDERSTANDING SUPPLY AND DEMAND TO IMPROVE HEALTH PRODUCT AVAILABILITY FROM INITIAL RESPONSE THROUGH SUSTAINABLE RECOVERY IN HUMANITARIAN SETTINGS Photo: UNFPA.org CONTEXT A changing world is
July 2018 UNDERSTANDING SUPPLY AND DEMAND TO IMPROVE HEALTH PRODUCT AVAILABILITY FROM INITIAL RESPONSE THROUGH SUSTAINABLE RECOVERY IN HUMANITARIAN SETTINGS Photo: UNFPA.org CONTEXT A changing world is
IHR Emergency Committee on the 2014 / 15 Ebola outbreak in West Africa
 IHR Emergency Committee on the 2014 / 15 Ebola outbreak in West Africa April, 2015 Dr Peter Gaturuku Capacity Building and Training Health Security and Emergencies Cluster WHO Regional Office for Africa,
IHR Emergency Committee on the 2014 / 15 Ebola outbreak in West Africa April, 2015 Dr Peter Gaturuku Capacity Building and Training Health Security and Emergencies Cluster WHO Regional Office for Africa,
Quality check of spontaneous adverse drug reaction reporting forms of different countries y
 pharmacoepidemiology and drug safety (2010) Published online in Wiley Online Library (wileyonlinelibrary.com).2004 ORIGINAL REPORT Quality check of spontaneous adverse drug reaction reporting forms of
pharmacoepidemiology and drug safety (2010) Published online in Wiley Online Library (wileyonlinelibrary.com).2004 ORIGINAL REPORT Quality check of spontaneous adverse drug reaction reporting forms of
Technical Assistance to the National Malaria Control Program to Strengthen the Malaria Supply Chain in Niger
 Technical Assistance to the National Malaria Control Program to Strengthen the Malaria Supply Chain in Niger SIAPS Quarterly Progress Report April June 2015 Technical Assistance to the National Malaria
Technical Assistance to the National Malaria Control Program to Strengthen the Malaria Supply Chain in Niger SIAPS Quarterly Progress Report April June 2015 Technical Assistance to the National Malaria
Malaria Elimination Toolkit Malaria Program Efficiency Analysis Tool (MPEAT)
 Malaria Elimination Toolkit Malaria Program Efficiency Analysis Tool (MPEAT) The Malaria Elimination Initiative is a project of the Global Health Group at UCSF Global Health Sciences. shrinkingthemalariamap.org
Malaria Elimination Toolkit Malaria Program Efficiency Analysis Tool (MPEAT) The Malaria Elimination Initiative is a project of the Global Health Group at UCSF Global Health Sciences. shrinkingthemalariamap.org
Statistics for Transparency, Accountability, and Results
 Statistics for Transparency, Accountability, and Results Executive summary Reliable and accessible statistics provide the evidence needed to increase the transparency of policy making, to document results,
Statistics for Transparency, Accountability, and Results Executive summary Reliable and accessible statistics provide the evidence needed to increase the transparency of policy making, to document results,
WHO s IMS Organizational Structure Overview
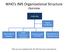 WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
Introduction to Lassa fever
 Introduction to Lassa fever Managing infectious hazards Store food in containers with lids OpenWHO.org WHO2017 1 Learning objectives Describe signs, symptoms, and transmission of Lassa fever List 4 preventive
Introduction to Lassa fever Managing infectious hazards Store food in containers with lids OpenWHO.org WHO2017 1 Learning objectives Describe signs, symptoms, and transmission of Lassa fever List 4 preventive
Opportunities & Challenges in the Landscape of R&D for NTDs
 Opportunities & Challenges in the Landscape of R&D for NTDs South Centre, 31 March 2014 Dr Bernard Pécoul, Executive Director Burden of Neglected Tropical Diseases Buruli Ulcer Chagas disease (American
Opportunities & Challenges in the Landscape of R&D for NTDs South Centre, 31 March 2014 Dr Bernard Pécoul, Executive Director Burden of Neglected Tropical Diseases Buruli Ulcer Chagas disease (American
Emergencies preparedness, response Ebola virus disease Democratic Republic
 1 von 5 26.11.2018, 08:41 Emergencies preparedness, response Ebola virus disease Democratic Republic of the Congo Disease outbreak news: Update 22 November 2018 Containing the ongoing Ebola virus disease
1 von 5 26.11.2018, 08:41 Emergencies preparedness, response Ebola virus disease Democratic Republic of the Congo Disease outbreak news: Update 22 November 2018 Containing the ongoing Ebola virus disease
The WHO Global Observatory on Health Research and Development (R&D)
 The WHO Global Observatory on Health Research and Development (R&D) Taghreed Adam Scientist Research, Ethics, Knowledge Uptake (REK) Health Systems and Innovation Cluster David Schellenberg Scientific
The WHO Global Observatory on Health Research and Development (R&D) Taghreed Adam Scientist Research, Ethics, Knowledge Uptake (REK) Health Systems and Innovation Cluster David Schellenberg Scientific
Global response plan for pfhrp2/3 deletions
 Global response plan for pfhrp2/3 deletions Jane Cunningham MPAC 17-19 October, 2017 The global response plan for pfhrp2/3 mutations that limit the effectiveness of HRP2- based RDTs comprises a global
Global response plan for pfhrp2/3 deletions Jane Cunningham MPAC 17-19 October, 2017 The global response plan for pfhrp2/3 mutations that limit the effectiveness of HRP2- based RDTs comprises a global
Ebola Virus disease in West Africa : challenges and lessons learned
 Ebola Virus disease in West Africa : challenges and lessons learned Pr N. Shindo Dr S.C. Briand Pandemic and Epidemic Diseases Department WHO Geneva EVD outbreak in West Africa in Numbers From24 March
Ebola Virus disease in West Africa : challenges and lessons learned Pr N. Shindo Dr S.C. Briand Pandemic and Epidemic Diseases Department WHO Geneva EVD outbreak in West Africa in Numbers From24 March
Guidelines for price discounts of single-source pharmaceuticals
 WHO/EDM/PAR/2003.3 Guidelines for price discounts of single-source pharmaceuticals World Health Organization Joint United Nations Programme on HIV/AIDS United Nations Children's Fund United Nations Population
WHO/EDM/PAR/2003.3 Guidelines for price discounts of single-source pharmaceuticals World Health Organization Joint United Nations Programme on HIV/AIDS United Nations Children's Fund United Nations Population
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness
 Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
WFP West Africa Regional Bureau REGIONAL SPECIAL OPERATION SO
 WFP West Africa Regional Bureau REGIONAL SPECIAL OPERATION SO 200773 Country: West Africa Regional Bureau (OMD) Ghana, Guinea, Sierra Leone, and Liberia Type of project: Special Operation Title: Logistics
WFP West Africa Regional Bureau REGIONAL SPECIAL OPERATION SO 200773 Country: West Africa Regional Bureau (OMD) Ghana, Guinea, Sierra Leone, and Liberia Type of project: Special Operation Title: Logistics
In vitro Studies into the Genetic Basis of Drug Resistance in Plasmodium falciparum
 In vitro Studies into the Genetic Basis of Drug Resistance in Plasmodium falciparum David A. Fidock, Ph.D. Depts. of Microbiology and Medicine Columbia University MMV Symposium, ASMTH, Nov. 4, 2007 Global
In vitro Studies into the Genetic Basis of Drug Resistance in Plasmodium falciparum David A. Fidock, Ph.D. Depts. of Microbiology and Medicine Columbia University MMV Symposium, ASMTH, Nov. 4, 2007 Global
Experience with obtaining GMP certification and WHO prequalification programme
 Experience with obtaining GMP certification and WHO prequalification programme V. Faillat Proux 1 Mumbai September 29, 2009 COARSUCAM / ASAQ Winthrop ArteSunate + AmodiaQuine, a fixed dose combination
Experience with obtaining GMP certification and WHO prequalification programme V. Faillat Proux 1 Mumbai September 29, 2009 COARSUCAM / ASAQ Winthrop ArteSunate + AmodiaQuine, a fixed dose combination
Ebola Transmission Prevention Two Years into the Post-Ebola Period: Use of Sentinel Sites for Community-Based Surveillance in Guinea
 REFLECTION Ebola Transmission Prevention Two Years into the Post-Ebola Period: Use of Sentinel Sites for Community-Based Surveillance in Guinea INTRODUCTION The Ebola outbreak of 2014 16 in West Africa
REFLECTION Ebola Transmission Prevention Two Years into the Post-Ebola Period: Use of Sentinel Sites for Community-Based Surveillance in Guinea INTRODUCTION The Ebola outbreak of 2014 16 in West Africa
IDSA is pleased to offer the following comments on specific priority areas identified by FDA:
 October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
NEWSLETTER Volume 1, Issue2 May 2017
 NEWSLETTER Volume 1, Issue2 May 2017 SYSTEMS FOR IMPROVED ACCESS TO PHARMACEUTICALS AND SERVICES POST-EBOLA RECOVERY SIERRA LEONE INSIDE THIS ISSUE Updated Treatment Register Streamlines Patient Recording
NEWSLETTER Volume 1, Issue2 May 2017 SYSTEMS FOR IMPROVED ACCESS TO PHARMACEUTICALS AND SERVICES POST-EBOLA RECOVERY SIERRA LEONE INSIDE THIS ISSUE Updated Treatment Register Streamlines Patient Recording
GUIDANCE on policy-making for Integrated Vector Management
 GUIDANCE on policy-making for Integrated Vector Management by Henk van den Berg, Clifford M. Mutero and Kazuyo Ichimori Design & Layout: Patrick Tissot WHO/HTM/NTD GUIDANCE on policy-making for integrated
GUIDANCE on policy-making for Integrated Vector Management by Henk van den Berg, Clifford M. Mutero and Kazuyo Ichimori Design & Layout: Patrick Tissot WHO/HTM/NTD GUIDANCE on policy-making for integrated
