Supplementary Appendix
|
|
|
- Solomon Thompson
- 6 years ago
- Views:
Transcription
1 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Goemans NM, Tulinius M, van den Akker JT, et al. Systemic administration of PRO051 in Duchenne s muscular dystrophy. N Engl J Med 2011;364: DOI: /NEJMoa
2 Supplementary Appendix Systemic Administration of PRO051 in Duchenne s Muscular Dystrophy 1. Methods Patient inclusion/exclusion criteria Patients with Duchenne muscular dystrophy aged 5 16 years and with mutations theoretically correctable by exon 51 skipping were recruited through the neuromuscular clinics of the University Hospitals Leuven, Belgium, and the Queen Silvia Children s Hospital, Gothenburg, Sweden. Inclusion and exclusion criteria were similar to the previous study. 1 An estimated life expectancy of 6 months, no other serious pre-existing medical conditions, no assisted ventilation dependency or FEV 1 or FVC < 60% of predicted, and no severe muscle abnormalities (defined as increased signal intensity in >50% of the tibialis anterior muscle at MRI) were required. Concurrent stable glucocorticosteroid treatment was allowed, recent (6 months) or ongoing treatment with anticoagulants, antithrombotics and antiplatelet agents, or investigational products was not allowed. Patients with known presence of dystrophin in 5% of fibers in a pre-study diagnostic muscle biopsy, with aberrant RNA splicing and/or aberrant response to PRO051 (detected by the in vitro PRO051 assay during screening), with elevated creatinine concentration (above 1.5 times the upper limit of age-corrected normal values), with severe mental retardation, or with symptomatic cardiac myopathy, were excluded. Study drug non-clinical safety Non-clinical safety studies showed that PRO051 was not mutagenic or clastogenic and shares class toxicities common to phosphorothioate oligonucleotides: transient prolongation of activated partial thromboplastin time (aptt); increase in complement split products in non-human primates; pro-inflammatory response, and accumulation of compound in liver and kidney. Specific monitoring of these aspects was conducted during both clinical studies. Pharmacokinetic assessments The pharmacokinetic profile of PRO051 was determined during the dose-escalation phase of the study. Plasma levels of PRO051 were determined using a validated hybridization ligation assay adapted from Yu et al., Plasma samples were incubated with a PRO051-specific capture oligonucleotide probe. After separation, a DIG-labeled oligonucleotide was ligated to the
3 complex and detection followed using an anti-dig antibody linked peroxidase. Noncompartmental pharmacokinetic analysis was performed using WINNONLIN software package (model 200, version 5.2, Pharsight, Mountainview, CA). Assessment of RNA and protein Biopsies were taken from the tibialis anterior muscle. 1 Total muscle RNA was isolated from 10 to 15 mg of muscle tissue, and reverse transcription-polymerase chain reaction and sequence analysis performed as described previously. 1, 3-4 Confirmation of correct splicing of the remainder of the DMD gene and immunofluorescence dystrophin analysis were conducted as previously reported. 1 However, for quantitative image analysis, Leica Qwin Software (Leica Microsystems, Rijswijk, The Netherlands) was used. Entire cross-sections were subdivided into series of 3 to 6 non-overlapping images. Fixed exposure settings were used, and pixel saturation was avoided. Absolute fluorescence intensities above saline-vehicle background were determined for each picture (mean pixel intensity of sarcolemma surface) and compared to healthy control muscle intensities. Western blot analysis was performed according to described methods. 1, 4 Between 75 to 150 µl of concentrated lysate was loaded ( µg) for each patient at both time points, depending on the quality of muscle tissue and/or biopsy as determined, and normalized for, by the signal intensity levels of dysferlin reference (NCL- Hamlet1:200, Novocastra Laboratories, Newcastle, UK). Dystrophin and dysferlin intensities were determined using secondary antibody IRDye800 anti-mouse IgG (1:5000) and the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Methods References 1. van Deutekom J, Janson J, Ginjaar H, et al. Local Dystrophin Restoration with Antisense Oligonucleotide PRO051 N Engl J Med 2007;357(26): Yu RZ, Baker B, Chappell A, Geary RS, Cheung E, Levin AA. Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Analytical biochemistry 2002;304(1): Aartsma-Rus A, Bremmer-Bout M, Janson A, den Dunnen J, van Ommen G, van Deutekom J. Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscul Disord 2002;12 Suppl:S Aartsma-Rus A, Janson AA, Kaman WE, et al. Therapeutic antisense-induced exon skipping in cultured muscle cells from six different DMD patients. Hum Mol Genet 2003;12(8):
4 2. Tables Table 1. Patient Demographics and Characteristics at Baseline by Dosing Group. Patients 0.5 mg/kg Age (yrs) Deletion Stage Tibialis Anterior Involvement (MRI- T1) 6MWT (m) CK (U/L) Stable minimal Stable minimal Non ambulant minimal NA mg/kg Decline moderate Stable minimal Stable minimal mg/kg Stable mild Decline minimal Stable minimal mg/kg Decline mild Decline moderate Stable minimal MWT=6-minute walk test (distance in meters); CK=Creatine kinase levels (U/L), average of 3 baseline samples.
5 3. Figures Figure 1. Figure 1. Mean PRO051 Plasma Concentrations Over Time. Following the first (A) and fifth (B) subcutaneous injections of 0.5, 2, 4, or 6 mg/kg PRO051, mean plasma levels were quantified up to 24 hours. The shape of the individual plasma concentration time profiles was comparable for Day 1 and Day 29, with slightly higher peak levels on Day 29 at the 4 and 6 mg/kg doses. (C) Trough concentrations were measurable for the majority of patients in the higher dose groups up to 13 weeks post-treatment and increased upon repeated dosing. The mean PRO051 plasma concentrations are presented on log-linear scale.
Duchenne Muscular Dystrophy
 Duchenne Muscular Dystrophy Clinical and Commercial Strategy Dr. Leslie Hudson, President and CEO September 10, 2008 Duchenne Muscular Dystrophy (DMD) Defects in the dystrophin gene; no protein expression
Duchenne Muscular Dystrophy Clinical and Commercial Strategy Dr. Leslie Hudson, President and CEO September 10, 2008 Duchenne Muscular Dystrophy (DMD) Defects in the dystrophin gene; no protein expression
Prosensa s continued development of exon-51 skipping with drisapersen after the failure of its phase-iii clinical trial.
 A new research report by Dr. Guenter Scheuerbrandt Prosensa s continued development of exon-51 skipping with drisapersen after the failure of its phase-iii clinical trial. In January 2013 you received
A new research report by Dr. Guenter Scheuerbrandt Prosensa s continued development of exon-51 skipping with drisapersen after the failure of its phase-iii clinical trial. In January 2013 you received
NS-065/NCNP-01 Phase 2 dose finding study
 NS-065/NCNP-01 Phase 2 dose finding study PPMD Webinar 1 February 22, 2017 Introduction NS Pharma, Inc. (Sponsor) is a wholly-owned, US subsidiary of Nippon Shinyaku Co., Ltd. (Kyoto, Japan) National Center
NS-065/NCNP-01 Phase 2 dose finding study PPMD Webinar 1 February 22, 2017 Introduction NS Pharma, Inc. (Sponsor) is a wholly-owned, US subsidiary of Nippon Shinyaku Co., Ltd. (Kyoto, Japan) National Center
-- Study achieved statistical significance on all primary and secondary biological endpoints --
 Sarepta Therapeutics Announces Positive Results in Its Study Evaluating Gene Expression, Dystrophin Production, and Dystrophin Localization in Patients with Duchenne Muscular Dystrophy (DMD) Amenable to
Sarepta Therapeutics Announces Positive Results in Its Study Evaluating Gene Expression, Dystrophin Production, and Dystrophin Localization in Patients with Duchenne Muscular Dystrophy (DMD) Amenable to
Gene therapies for SMA and DMD
 Gene therapies for SMA and DMD Annemieke Aartsma-Rus September 2018 Disclosures Employed by LUMC, which has patents on exon skipping technology, some of which has been licensed to BioMarin and subsequently
Gene therapies for SMA and DMD Annemieke Aartsma-Rus September 2018 Disclosures Employed by LUMC, which has patents on exon skipping technology, some of which has been licensed to BioMarin and subsequently
From Development to Commercial Production: don t contemplate but anticipate
 From Development to Commercial Production: don t contemplate but anticipate Automation in the Pharmaceutical Industry Leiden July 8, 2011 Richard Holslag, VP Manufacturing Our Mission To develop innovative,
From Development to Commercial Production: don t contemplate but anticipate Automation in the Pharmaceutical Industry Leiden July 8, 2011 Richard Holslag, VP Manufacturing Our Mission To develop innovative,
DUCHENNE MUSCULAR DYSTROPHY CLINICAL DEVELOPMENT PROGRAM
 DUCHENNE MUSCULAR DYSTROPHY CLINICAL DEVELOPMENT PROGRAM Dana R. Martin, PharmD Sarepta Therapeutics PPMD ANNUAL CONNECT CONFERENCE ORLANDO, FLORIDA JUNE 27, 2016 FORWARD LOOKING STATEMENTS This presentation,
DUCHENNE MUSCULAR DYSTROPHY CLINICAL DEVELOPMENT PROGRAM Dana R. Martin, PharmD Sarepta Therapeutics PPMD ANNUAL CONNECT CONFERENCE ORLANDO, FLORIDA JUNE 27, 2016 FORWARD LOOKING STATEMENTS This presentation,
Innovative treatments in neuromuscular disorders
 Innovative treatments in neuromuscular disorders Great Ormond Street Hospital for Children NHS Foundation Trust Practical Neurology Study Days 2018 Francesco Muntoni Dubowitz Neuromuscular Centre UCL Institute
Innovative treatments in neuromuscular disorders Great Ormond Street Hospital for Children NHS Foundation Trust Practical Neurology Study Days 2018 Francesco Muntoni Dubowitz Neuromuscular Centre UCL Institute
Spinraza. Spinraza (nusinersen) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.15 Subject: Spinraza Page: 1 of 5 Last Review Date: June 22, 2017 Spinraza Description Spinraza (nusinersen)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.15 Subject: Spinraza Page: 1 of 5 Last Review Date: June 22, 2017 Spinraza Description Spinraza (nusinersen)
CRISPR/Cas9 and genome editing for genetic neuromuscular disorders
 CRISPR/Cas9 and genome editing for genetic neuromuscular disorders Annemieke Aartsma-Rus AFDELING HUMANE GENETICS, LUMC, LEIDEN Disclosures Employed by LUMC, which has patents on exon skipping technology,
CRISPR/Cas9 and genome editing for genetic neuromuscular disorders Annemieke Aartsma-Rus AFDELING HUMANE GENETICS, LUMC, LEIDEN Disclosures Employed by LUMC, which has patents on exon skipping technology,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Table 1 Supplementary Figures 1, 2, 3, 4, 5, 6, 7, 8 and legends Supplementary Video 1, 2 legends AON Sequence Position Length MW tcdna 5 -AACCTCGGCTTACCT-3 +2-13
SUPPLEMENTARY INFORMATION Supplementary Table 1 Supplementary Figures 1, 2, 3, 4, 5, 6, 7, 8 and legends Supplementary Video 1, 2 legends AON Sequence Position Length MW tcdna 5 -AACCTCGGCTTACCT-3 +2-13
Exon Skipping. Wendy Erler Patient Advocacy Wave Life Sciences
 Exon Skipping Wendy Erler Patient Advocacy Wave Life Sciences Forward Looking Statements This document contains forward-looking statements. All statements other than statements of historical facts contained
Exon Skipping Wendy Erler Patient Advocacy Wave Life Sciences Forward Looking Statements This document contains forward-looking statements. All statements other than statements of historical facts contained
Pfizer Program in DMD. Beth Belluscio, MD-Ph.D. Pfizer Rare Disease September 9, 2017
 Pfizer Program in DMD Beth Belluscio, MD-Ph.D. Pfizer Rare Disease September 9, 2017 Myostatin Inhibitor for the Potential Treatment of Duchenne Muscular Dystrophy Disclaimer This presentation includes
Pfizer Program in DMD Beth Belluscio, MD-Ph.D. Pfizer Rare Disease September 9, 2017 Myostatin Inhibitor for the Potential Treatment of Duchenne Muscular Dystrophy Disclaimer This presentation includes
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Eteplirsen (Exondys 51) for DMD Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Eteplirsen (Exondys 51) for Duchenne Muscular Dystrophy Professional
Eteplirsen (Exondys 51) for DMD Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Eteplirsen (Exondys 51) for Duchenne Muscular Dystrophy Professional
Delivering on the Promise of RNA- Based Therapeu;cs
 Delivering on the Promise of RNA- Based Therapeu;cs Parent Project Muscular Dystrophy Webinar June 20, 2011 Forward- Looking Statements This presenta,on includes forward- looking statements, including
Delivering on the Promise of RNA- Based Therapeu;cs Parent Project Muscular Dystrophy Webinar June 20, 2011 Forward- Looking Statements This presenta,on includes forward- looking statements, including
RESEARCH ARTICLE Comparative analysis of antisense oligonucleotide analogs for targeted DMD exon 46 skipping in muscle cells
 (2004) 11, 1391 1398 & 2004 Nature Publishing Group All rights reserved 0969-7128/04 $30.00 www.nature.com/gt RESEARCH ARTICLE Comparative analysis of antisense oligonucleotide analogs for targeted DMD
(2004) 11, 1391 1398 & 2004 Nature Publishing Group All rights reserved 0969-7128/04 $30.00 www.nature.com/gt RESEARCH ARTICLE Comparative analysis of antisense oligonucleotide analogs for targeted DMD
FDA Regulation of Companion Diagnostics
 FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
FDA Regulation of Companion Diagnostics Paul Radensky October 11, 2017 Disclosure + Slideset drawn from Part I of presentation made by Janice Hogan, HoganLovells, October 2016 + Updated where appropriate
Prosensa announces commencement of redosing of drisapersen in North America in patients with Duchenne muscular dystrophy
 Prosensa announces commencement of redosing of drisapersen in North America in patients with Duchenne muscular dystrophy Leiden, The Netherlands, Sept. 17, 2014 (GLOBE NEWSWIRE) -- Prosensa Holding N.V.
Prosensa announces commencement of redosing of drisapersen in North America in patients with Duchenne muscular dystrophy Leiden, The Netherlands, Sept. 17, 2014 (GLOBE NEWSWIRE) -- Prosensa Holding N.V.
Genomics Changing the Way Care is Delivered
 Genomics Changing the Way Care is Delivered Eric P. Hoffman, PhD Director, Center for Genetic Medicine Research A. James Clark Professor of Molecular Genetics Children s National Medical Center Washington,
Genomics Changing the Way Care is Delivered Eric P. Hoffman, PhD Director, Center for Genetic Medicine Research A. James Clark Professor of Molecular Genetics Children s National Medical Center Washington,
SALSA MLPA kit A071 - A078 DMD Lot 0606
 kit A071 - A078 DMD Lot 0606 Please note: This SALSA kit has been developed for the low-resolution detection of DMD (partial) deletions with an agarose gel electrophoresis or Agilent microfluidics. kit
kit A071 - A078 DMD Lot 0606 Please note: This SALSA kit has been developed for the low-resolution detection of DMD (partial) deletions with an agarose gel electrophoresis or Agilent microfluidics. kit
European Medicines Agency decision
 EMA/866546/2015 European Medicines Agency decision P/0029/2016 of 29 January 2016 on the agreement of a paediatric investigation plan and on the granting of a deferral for eteplirsen (EMEA-001722-PIP01-14)
EMA/866546/2015 European Medicines Agency decision P/0029/2016 of 29 January 2016 on the agreement of a paediatric investigation plan and on the granting of a deferral for eteplirsen (EMEA-001722-PIP01-14)
CENTER DIRECTOR DECISIONAL MEMO
 CENTER DIRECTOR DECISIONAL MEMO NDA# 206488 Drug Name EXONDYS 51 (eteplirsen) Indication Duchenne Muscular Dystrophy (DMD) Sponsor Sarepta Author Janet Woodcock, M.D. Director, Center for Drug Evaluation
CENTER DIRECTOR DECISIONAL MEMO NDA# 206488 Drug Name EXONDYS 51 (eteplirsen) Indication Duchenne Muscular Dystrophy (DMD) Sponsor Sarepta Author Janet Woodcock, M.D. Director, Center for Drug Evaluation
Advancing New Treatments for DMD and C. difficile Infection
 Advancing New Treatments for DMD and C. difficile Infection 34 th Annual Canaccord Genuity Growth Conference August 13, 2014 Legal Disclaimer FORWARD-LOOKING STATEMENTS This Document contains forward-looking
Advancing New Treatments for DMD and C. difficile Infection 34 th Annual Canaccord Genuity Growth Conference August 13, 2014 Legal Disclaimer FORWARD-LOOKING STATEMENTS This Document contains forward-looking
Spinraza. Spinraza (nusinersen) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.15 Subject: Spinraza Page: 1 of 4 Last Review Date: March 16, 2017 Spinraza Description Spinraza (nusinersen)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.75.15 Subject: Spinraza Page: 1 of 4 Last Review Date: March 16, 2017 Spinraza Description Spinraza (nusinersen)
A roadmap for bringing FKRP gene therapies into clinical use
 A roadmap for bringing FKRP gene therapies into clinical use Jeffrey S. Chamberlain, Ph.D. McCaw Chair in Muscular Dystrophy Wellstone Muscular Dystrophy Research Center - Seattle Depts. of Neurology,
A roadmap for bringing FKRP gene therapies into clinical use Jeffrey S. Chamberlain, Ph.D. McCaw Chair in Muscular Dystrophy Wellstone Muscular Dystrophy Research Center - Seattle Depts. of Neurology,
SMTC1100: Progressing to Patient Clinical Trials
 SMTC1100: Progressing to Patient Clinical Trials Action Duchenne Conference 9 th November 2013 Legal Disclaimer FORWARD LOOKING STATEMENTS This Document contains forward-looking statements. These statements
SMTC1100: Progressing to Patient Clinical Trials Action Duchenne Conference 9 th November 2013 Legal Disclaimer FORWARD LOOKING STATEMENTS This Document contains forward-looking statements. These statements
Delivering on the promise: the clinical application of new diagnoses and treatments for RD K A T E B U S H B Y N E W C A S T L E U N I V E R S I T Y
 Delivering on the promise: the clinical application of new diagnoses and treatments for RD K A T E B U S H B Y N E W C A S T L E U N I V E R S I T Y PERSPECTIVE/ DISCLAIMERS Doctor with >24 years experience
Delivering on the promise: the clinical application of new diagnoses and treatments for RD K A T E B U S H B Y N E W C A S T L E U N I V E R S I T Y PERSPECTIVE/ DISCLAIMERS Doctor with >24 years experience
ataluren overview A New Approach to Genetic Disorders
 ataluren overview A New Approach to Genetic Disorders Ataluren Scientific Background What is ataluren? Ataluren(formerly PTC124) is the first investigational therapy with the potential to enable the formation
ataluren overview A New Approach to Genetic Disorders Ataluren Scientific Background What is ataluren? Ataluren(formerly PTC124) is the first investigational therapy with the potential to enable the formation
(51) Int Cl.: C12N 15/11 ( ) A61K 31/7088 ( ) C12N 15/86 ( )
 (19) TEPZZ 8_ 7ZB_T (11) EP 2 813 70 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 1.11.17 Bulletin 17/46 (1) Int Cl.: C12N 1/11 (06.01) A61K 31/7088
(19) TEPZZ 8_ 7ZB_T (11) EP 2 813 70 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 1.11.17 Bulletin 17/46 (1) Int Cl.: C12N 1/11 (06.01) A61K 31/7088
6th Form Open Day 15th July 2015
 6th Form Open Day 15 th July 2015 DNA is like a computer program but far, far more advanced than any software ever created. Bill Gates Welcome to the Open Day event at the Institute of Genetic Medicine
6th Form Open Day 15 th July 2015 DNA is like a computer program but far, far more advanced than any software ever created. Bill Gates Welcome to the Open Day event at the Institute of Genetic Medicine
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/4/e1602814/dc1 Supplementary Materials for CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice Yu Zhang, Chengzu Long, Hui
advances.sciencemag.org/cgi/content/full/3/4/e1602814/dc1 Supplementary Materials for CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice Yu Zhang, Chengzu Long, Hui
2111: ALL Post-HCT. Add/ Remove/ Modify. Manual Section. Date. Description. Comprehensive Disease- Specific Manuals
 2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
Safety Assessment of Oligonucleotide Constructs. Scott P. Henry, VP, Preclinical Development, Isis Pharmaceuticals, Inc., USA
 Safety Assessment of ligonucleotide Constructs Scott P. Henry, VP, Preclinical Development, Isis Pharmaceuticals, Inc., USA Drug Development of 2 ME AS Platform Technology 2 Platform technologies offer
Safety Assessment of ligonucleotide Constructs Scott P. Henry, VP, Preclinical Development, Isis Pharmaceuticals, Inc., USA Drug Development of 2 ME AS Platform Technology 2 Platform technologies offer
Muscle-specific CRISPR/Cas9 dystrophin gene editing
 Muscle-specific CRISPR/Cas9 dystrophin gene editing Jeffrey S. Chamberlain, Ph.D. McCaw Endowed Chair in Muscular Dystrophy Director, Wellstone Muscular Dystrophy Research Center Depts. of Neurology, Medicine
Muscle-specific CRISPR/Cas9 dystrophin gene editing Jeffrey S. Chamberlain, Ph.D. McCaw Endowed Chair in Muscular Dystrophy Director, Wellstone Muscular Dystrophy Research Center Depts. of Neurology, Medicine
The connection between genes, proteins and metabolism CAMPBELL BIOLOGY
 Lecture 6 The connection between genes, proteins and metabolism CAMPBELL BIOLOGY 1 What do genes do? What cellular processes do they affect? 1902 - Garrod described: inherited disorders of metabolism also
Lecture 6 The connection between genes, proteins and metabolism CAMPBELL BIOLOGY 1 What do genes do? What cellular processes do they affect? 1902 - Garrod described: inherited disorders of metabolism also
Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles
 Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles Qi Long Lu*, Adam Rabinowitz*, Yun Chao Chen, Toshifumi Yokota*, HaiFang Yin*, Julia Alter*,
Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles Qi Long Lu*, Adam Rabinowitz*, Yun Chao Chen, Toshifumi Yokota*, HaiFang Yin*, Julia Alter*,
Multi-omics analysis powered by massive data integration
 Multi-omics analysis powered by massive data integration RE(ACT) congress 08-02-2018 Kristina Hettne, PhD BioSemantics Group, Human Genetics LEIDEN UNIVERSITY MEDICAL CENTER Duchenne muscular dystrophy
Multi-omics analysis powered by massive data integration RE(ACT) congress 08-02-2018 Kristina Hettne, PhD BioSemantics Group, Human Genetics LEIDEN UNIVERSITY MEDICAL CENTER Duchenne muscular dystrophy
Table S1. Dystrophin antibodies.
 Table S1. Dystrophin antibodies. name clone immunogen (human dystrophin) epitope exons region amino acids vendor (catalogue number) references for characterization of antibodies MANEX1A 4C7 recombinant
Table S1. Dystrophin antibodies. name clone immunogen (human dystrophin) epitope exons region amino acids vendor (catalogue number) references for characterization of antibodies MANEX1A 4C7 recombinant
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin
Received 4 September 2014/ Accepted 9 October 2014
 Kobe J. Med. Sci. Vol. 60, No. 4, pp. E86- E94, 2014 Phosphorothioate Modification of Chimeric 2 -O-Methyl RNA/Ethylene-Bridged Nucleic Acid Oligonucleotides Increases Dystrophin Exon 45 Skipping Capability
Kobe J. Med. Sci. Vol. 60, No. 4, pp. E86- E94, 2014 Phosphorothioate Modification of Chimeric 2 -O-Methyl RNA/Ethylene-Bridged Nucleic Acid Oligonucleotides Increases Dystrophin Exon 45 Skipping Capability
European Medicines Agency decision
 EMA/643929/2016 European Medicines Agency decision P/0279/2016 of 7 October 2016 on the acceptance of a modification of an agreed paediatric investigation plan for Eteplirsen (EMEA- 001722-PIP01-14-M01)
EMA/643929/2016 European Medicines Agency decision P/0279/2016 of 7 October 2016 on the acceptance of a modification of an agreed paediatric investigation plan for Eteplirsen (EMEA- 001722-PIP01-14-M01)
Antisense Oligonucleotides (ASOs): Versatile Tools for Precision Neurology?
 1 Antisense Oligonucleotides (ASOs): Versatile Tools for Precision Neurology? Charles Thornton Dept. of Neurology Wellstone Muscular Dystrophy Cooperative Research Center University of Rochester Disclosure
1 Antisense Oligonucleotides (ASOs): Versatile Tools for Precision Neurology? Charles Thornton Dept. of Neurology Wellstone Muscular Dystrophy Cooperative Research Center University of Rochester Disclosure
Stealth BioTherapeutics Mission:
 The following is a summary of a live presentation offered through joint collaboration with UMDF, MitoAction and the Foundation for Mitochondrial Medicine to the mitochondrial disease patient and family
The following is a summary of a live presentation offered through joint collaboration with UMDF, MitoAction and the Foundation for Mitochondrial Medicine to the mitochondrial disease patient and family
Stem cells in Development
 ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
Stem cells in Development
 ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
ANAT 2341 Embryology Lab 10 8 Oct 2009 Therapeutic Use of Stem Cells Practical Hurdles & Ethical Issues Stem cells in Development Blastocyst Cord blood Antonio Lee PhD Neuromuscular & Regenerative Medicine
Mutagenesis and Expression of Mammalian Clotting Factor IX. Mark McCleland The Children s Hospital of Philadelphia and Lycoming College
 Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Muscular Dystrophy Australia. Research RoadShow
 Muscular Dystrophy Australia Research RoadShow Research at NMDRC Skeletal muscle Development and Hypertrophy Dystrophic Pathology Skeletal Muscle Regeneration Stem cells and Cell Transplantation Identification
Muscular Dystrophy Australia Research RoadShow Research at NMDRC Skeletal muscle Development and Hypertrophy Dystrophic Pathology Skeletal Muscle Regeneration Stem cells and Cell Transplantation Identification
MRC-Holland MLPA. Description version 05; 16 March 2018
 SALSA MLPA probemix P453-A2 GAA Lot A2-0617. As compared to previous version A1 (lot A1-0514), one reference probe has been removed and one has been replaced. The GAA gene encodes the enzyme acid alpha-glycosidase
SALSA MLPA probemix P453-A2 GAA Lot A2-0617. As compared to previous version A1 (lot A1-0514), one reference probe has been removed and one has been replaced. The GAA gene encodes the enzyme acid alpha-glycosidase
Advancing Mitochondrial Medicine. Günther Metz, SVP Business Development
 Advancing Mitochondrial Medicine Günther Metz, SVP Business Development Disclaimer 2 This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to
Advancing Mitochondrial Medicine Günther Metz, SVP Business Development Disclaimer 2 This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to
Site directed mutagenesis, Insertional and Deletion Mutagenesis. Mitesh Shrestha
 Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
Dr. Leslie Hudson President and CEO AVI BioPharma
 Dr. Leslie Hudson President and CEO AVI BioPharma 1 UBS Investor Conference September 25, 2008 Transitioning an Antisense Pioneer into a Leading RNA-based Drug Discovery and Development Company 2 New Investment
Dr. Leslie Hudson President and CEO AVI BioPharma 1 UBS Investor Conference September 25, 2008 Transitioning an Antisense Pioneer into a Leading RNA-based Drug Discovery and Development Company 2 New Investment
-- Sarepta strengthens position as a leader in gene therapy; expands rare disease franchise --
 Sarepta Therapeutics Announces Partnership with Myonexus Therapeutics for the Advancement of Multiple Gene Therapy Programs Aimed at Treating Distinct Forms of Limb-Girdle Muscular Dystrophies -- Sarepta
Sarepta Therapeutics Announces Partnership with Myonexus Therapeutics for the Advancement of Multiple Gene Therapy Programs Aimed at Treating Distinct Forms of Limb-Girdle Muscular Dystrophies -- Sarepta
Positive Pompe Phase 1/2 Functional Data in Initial Patients. Conference Call & Webcast. May 15, 2017
 Positive Pompe Phase 1/2 Functional Data in Initial Patients Conference Call & Webcast May 15, 2017 Introduction 2 Safe Harbor This presentation contains "forward looking statements" within the meaning
Positive Pompe Phase 1/2 Functional Data in Initial Patients Conference Call & Webcast May 15, 2017 Introduction 2 Safe Harbor This presentation contains "forward looking statements" within the meaning
Use of Antisense Oligonucleotides for the Treatment of Inheritable Rare Disorders. C. Frank Bennett Isis Pharmaceuticals
 Use of Antisense ligonucleotides for the Treatment of Inheritable Rare Disorders C. Frank Bennett Isis Pharmaceuticals Agenda Review different antisense strategies Delivery of oligonucleotides to the skin,
Use of Antisense ligonucleotides for the Treatment of Inheritable Rare Disorders C. Frank Bennett Isis Pharmaceuticals Agenda Review different antisense strategies Delivery of oligonucleotides to the skin,
TRANSLATIONAL RESEARCH IN RARE AND NEUROMUSCULAR DISEASES - WHY DATA SHARING MATTERS. H a n n s Lochmüller, Newcastle University
 TRANSLATIONAL RESEARCH IN RARE AND NEUROMUSCULAR DISEASES - WHY DATA SHARING MATTERS H a n n s Lochmüller, Newcastle University Addressing the translational pathway 2 Trials Gene identification/ pathophysiology
TRANSLATIONAL RESEARCH IN RARE AND NEUROMUSCULAR DISEASES - WHY DATA SHARING MATTERS H a n n s Lochmüller, Newcastle University Addressing the translational pathway 2 Trials Gene identification/ pathophysiology
Contents... vii. List of Figures... xii. List of Tables... xiv. Abbreviatons... xv. Summary... xvii. 1. Introduction In vitro evolution...
 vii Contents Contents... vii List of Figures... xii List of Tables... xiv Abbreviatons... xv Summary... xvii 1. Introduction...1 1.1 In vitro evolution... 1 1.2 Phage Display Technology... 3 1.3 Cell surface
vii Contents Contents... vii List of Figures... xii List of Tables... xiv Abbreviatons... xv Summary... xvii 1. Introduction...1 1.1 In vitro evolution... 1 1.2 Phage Display Technology... 3 1.3 Cell surface
Expectations for Biodistribution (BD) Assessments for Gene Therapy (GT) Products
 Expectations for Biodistribution (BD) Assessments for Gene Therapy (GT) Products Approved by the IPRP Management Committee on 3 June 2018 12 April 2018 Table of Contents 1. Position Statement... 3 2. Executive
Expectations for Biodistribution (BD) Assessments for Gene Therapy (GT) Products Approved by the IPRP Management Committee on 3 June 2018 12 April 2018 Table of Contents 1. Position Statement... 3 2. Executive
Mechanisms of muscle membrane repair: The dysferlin model
 Mechanisms of muscle membrane repair: The dysferlin model Eduard Gallardo Laboratori de Neurologia Experimental Institut de Recerca Hospital Sant Pau 2nd MuscleTech Network Workshop September 27 29, 2010
Mechanisms of muscle membrane repair: The dysferlin model Eduard Gallardo Laboratori de Neurologia Experimental Institut de Recerca Hospital Sant Pau 2nd MuscleTech Network Workshop September 27 29, 2010
Session 1 Topics. Vascular Phase of Hemostasis. Coagulation Pathway. Action of Unfractionated Heparin. Laboratory Monitoring of Anticoagulant Therapy
 ~~Marshfield Labs Presents~~ Laboratory Monitoring of Anticoagulant Therapy Session 1 of 4 Session 1 Topics Review of coagulation and the vascular phase of hemostasis Unfractionated heparin Low molecular
~~Marshfield Labs Presents~~ Laboratory Monitoring of Anticoagulant Therapy Session 1 of 4 Session 1 Topics Review of coagulation and the vascular phase of hemostasis Unfractionated heparin Low molecular
DIANA assay for in vitro diagnostics Overview and collaboration proposal
 DIANA assay for in vitro diagnostics Overview and collaboration proposal Institute of Organic Chemistry and Biochemistry AS CR, Prague, Czech Republic DNA-linked Inhibitor ANtibody Assay What is DIANA
DIANA assay for in vitro diagnostics Overview and collaboration proposal Institute of Organic Chemistry and Biochemistry AS CR, Prague, Czech Republic DNA-linked Inhibitor ANtibody Assay What is DIANA
Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides
 Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides Laboratory Sciences, MPI Research, A Charles River Company Amy Smith, BA, Senior Director, Bioanalytical/Analytical
Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides Laboratory Sciences, MPI Research, A Charles River Company Amy Smith, BA, Senior Director, Bioanalytical/Analytical
MRC-Holland MLPA. Description version 16; 29 May 2017
 SALSA MLPA probemix P029-C1 Williams-Beuren Syndrome Lot C1-0517. As compared to version B1 (lot B1-0315, B1-1211), two target probes have been removed, three reference probes have been replaced and three
SALSA MLPA probemix P029-C1 Williams-Beuren Syndrome Lot C1-0517. As compared to version B1 (lot B1-0315, B1-1211), two target probes have been removed, three reference probes have been replaced and three
INTERNATIONAL SYMPOSIUM ON USHER SYNDROME
 (Annamarie Dillon) OK, we are on? Everybody can hear me? Great, first I would like to thank the organizers for the opportunity to come here today to provide you with short updates on our QR-421a program
(Annamarie Dillon) OK, we are on? Everybody can hear me? Great, first I would like to thank the organizers for the opportunity to come here today to provide you with short updates on our QR-421a program
In-Cell Western Kits I and II
 Odyssey and Aerius Infrared Imaging Systems In-Cell Western Assay Kits I and II Published November, 2006. The most recent version of this protocol is posted at http://biosupport.licor.com/protocols.jsp
Odyssey and Aerius Infrared Imaging Systems In-Cell Western Assay Kits I and II Published November, 2006. The most recent version of this protocol is posted at http://biosupport.licor.com/protocols.jsp
Giardia RNAi Paper Discussion. Supplemental - VSG clonality. Variant-specific surface protein VSP9B10
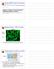 Giardia RNAi Paper Discussion Supplemental - VSG clonality VSP9B10 Green - VSP9B10 antibody Blue - DAPI Demonstration that cell line expresses a single VSP Variant-specific surface protein Outside Inside
Giardia RNAi Paper Discussion Supplemental - VSG clonality VSP9B10 Green - VSP9B10 antibody Blue - DAPI Demonstration that cell line expresses a single VSP Variant-specific surface protein Outside Inside
Technical Note. Housekeeping Protein Validation Protocol
 Technical Note Housekeeping Protein Validation Protocol Published March 2017. The most recent version of this Technical Note is posted at licor.com/bio/support. Visit us on protocols.io! Explore an interactive
Technical Note Housekeeping Protein Validation Protocol Published March 2017. The most recent version of this Technical Note is posted at licor.com/bio/support. Visit us on protocols.io! Explore an interactive
AAV Platform and Pipeline Expansion: Vectorized Antisense to Treat Duchenne Muscular Dystrophy and Myotonic Dystrophy.
 AAV Platform and Pipeline Expansion: Vectorized Antisense to Treat Duchenne Muscular Dystrophy and Myotonic Dystrophy April 8, 2019 Safe Harbor Except for statements of historical fact, any information
AAV Platform and Pipeline Expansion: Vectorized Antisense to Treat Duchenne Muscular Dystrophy and Myotonic Dystrophy April 8, 2019 Safe Harbor Except for statements of historical fact, any information
Supplementary Figure 1. α-synuclein is truncated in PD and LBD brains. Nature Structural & Molecular Biology: doi: /nsmb.
 Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
Supplementary Figure 1 α-synuclein is truncated in PD and LBD brains. (a) Specificity of anti-n103 antibody. Anti-N103 antibody was coated on an ELISA plate and different concentrations of full-length
MRC-Holland MLPA. Description version 35b; 11 August 2017
 SALSA MLPA probemix P021-A2 SMA Lot A2-0316, Lot A2-0415, Lot A2-0613, Lot A2-0511: As compared to the previous version A1 (lots A1-0910, A1-1209, A1-0809, A1-1208, A1-0808, A1-0208 & A1-0807), two control
SALSA MLPA probemix P021-A2 SMA Lot A2-0316, Lot A2-0415, Lot A2-0613, Lot A2-0511: As compared to the previous version A1 (lots A1-0910, A1-1209, A1-0809, A1-1208, A1-0808, A1-0208 & A1-0807), two control
Prosensa Therapeutics R&D in ultra-rare disease
 Prosensa Therapeutics R&D in ultra-rare disease European Business Development Conference Dusseldorf, September 24, 2013 Tina C Flatau VP Alliances and Project Management Forward-Looking Statements This
Prosensa Therapeutics R&D in ultra-rare disease European Business Development Conference Dusseldorf, September 24, 2013 Tina C Flatau VP Alliances and Project Management Forward-Looking Statements This
Supplementary Figure S1. The tetracycline-inducible CRISPR system. A) Hela cells stably
 Supplementary Information Supplementary Figure S1. The tetracycline-inducible CRISPR system. A) Hela cells stably expressing shrna sequences against TRF2 were examined by western blotting. shcon, shrna
Supplementary Information Supplementary Figure S1. The tetracycline-inducible CRISPR system. A) Hela cells stably expressing shrna sequences against TRF2 were examined by western blotting. shcon, shrna
In addition, 9 reference probes are included in the P116 probemix, detecting several autosomal locations.
 mix P116-B1 SGC Lot B1-0115, B1-0511. As compared to version A1 (lot A1-0708), one extra probe each for SGCB, SGCD and FKRP have been included. Five reference probes and the control fragments have been
mix P116-B1 SGC Lot B1-0115, B1-0511. As compared to version A1 (lot A1-0708), one extra probe each for SGCB, SGCD and FKRP have been included. Five reference probes and the control fragments have been
Gene therapy for retinitis pigmentosa
 Gene therapy for retinitis pigmentosa Robert E MacLaren Professor of Ophthalmology University of Oxford & Honorary Consultant Vitreoretinal Surgeon Oxford University Hospitals, Moorfields Eye Hospital
Gene therapy for retinitis pigmentosa Robert E MacLaren Professor of Ophthalmology University of Oxford & Honorary Consultant Vitreoretinal Surgeon Oxford University Hospitals, Moorfields Eye Hospital
The SCN5A protein is found primarily in cardiac muscle and mediates the voltage-dependent sodium ion permeability of excitable membranes.
 SALSA MLPA probemix P108-B3 SCN5A Lot B3-0916. As compared to the previous lot (B2-0312), two reference probes have been replaced and two probe lengths have been adjusted. The Brugada syndrome-1 and long
SALSA MLPA probemix P108-B3 SCN5A Lot B3-0916. As compared to the previous lot (B2-0312), two reference probes have been replaced and two probe lengths have been adjusted. The Brugada syndrome-1 and long
SALSA MLPA probemix P176-C2 CAPN3 Lot C2-0813: Compared to previous lot C1-0213, one reference probe has been removed.
 SALSA MLPA probemix P176-C2 CAPN3 Lot C2-0813: Compared to previous lot C1-0213, one reference probe has been removed. Limb-girdle muscular dystrophies (LGMD) are a group of phenotypically and genotypically
SALSA MLPA probemix P176-C2 CAPN3 Lot C2-0813: Compared to previous lot C1-0213, one reference probe has been removed. Limb-girdle muscular dystrophies (LGMD) are a group of phenotypically and genotypically
Expression Array System
 Integrated Science for Gene Expression Applied Biosystems Expression Array System Expression Array System SEE MORE GENES The most complete, most sensitive system for whole genome expression analysis. The
Integrated Science for Gene Expression Applied Biosystems Expression Array System Expression Array System SEE MORE GENES The most complete, most sensitive system for whole genome expression analysis. The
Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP.
 Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP. Supplementary Figure 2. Synthesis of CP-PTX conjugate. Supplementary
Supplementary Figure 1. Determination of the purity of CP. a, SDS-PAGE of CP and CP- PTX conjugate, and b, HPLC trace of purified CP. Supplementary Figure 2. Synthesis of CP-PTX conjugate. Supplementary
Drug Development and Clinical Trials Pat Furlong Parent Project Muscular Dystrophy
 Drug Development and Clinical Trials Pat Furlong Parent Project Muscular Dystrophy ParentProjectMD.org -LATE 1990 s Where Are We? - Total NIH funding for MD is $17M -No new drugs in development -Average
Drug Development and Clinical Trials Pat Furlong Parent Project Muscular Dystrophy ParentProjectMD.org -LATE 1990 s Where Are We? - Total NIH funding for MD is $17M -No new drugs in development -Average
Using Sapphire700 Stain and DRAQ5 Stain for Cell Number Normalization
 Using Sapphire700 Stain and DRAQ5 Stain for Cell Number Normalization Developed for: Aerius, Odyssey Classic, Odyssey CLx, and Odyssey Sa Infrared Imaging Systems Please refer to your manual to confirm
Using Sapphire700 Stain and DRAQ5 Stain for Cell Number Normalization Developed for: Aerius, Odyssey Classic, Odyssey CLx, and Odyssey Sa Infrared Imaging Systems Please refer to your manual to confirm
CAP-1002: HOPE Clinical Trials PPMD Annual Conference 2018
 CAP-1002: HOPE Clinical Trials PPMD Annual Conference 2018 1 Capricor, Inc. PPMD Annual Conference June 2018 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and
CAP-1002: HOPE Clinical Trials PPMD Annual Conference 2018 1 Capricor, Inc. PPMD Annual Conference June 2018 Forward-Looking Statements Statements in this presentation regarding the efficacy, safety, and
FSHD: Clinical Trial Preparedness
 FSHD: Clinical Trial Preparedness Rabi Tawil, MD University of Rochester Medical Center FSHD Clinical Trials Readiness Workshop Newcastle, October 31, 2013 Developing an FSHD Clinical Trial Toolkit Why
FSHD: Clinical Trial Preparedness Rabi Tawil, MD University of Rochester Medical Center FSHD Clinical Trials Readiness Workshop Newcastle, October 31, 2013 Developing an FSHD Clinical Trial Toolkit Why
Inherited Neuromuscular Diseases: Translation From Pathomechanisms To Therapies (Advances In Experimental Medicine And Biology)
 Inherited Neuromuscular Diseases: Translation From Pathomechanisms To Therapies (Advances In Experimental Medicine And Biology) people with slowly progressive neuromuscular diseases. and Treatment of Peripheral
Inherited Neuromuscular Diseases: Translation From Pathomechanisms To Therapies (Advances In Experimental Medicine And Biology) people with slowly progressive neuromuscular diseases. and Treatment of Peripheral
Experimental genetics - I
 Experimental genetics - I Examples of diseases with genetic-links Hemophilia (complete loss or altered form of factor VIII): bleeding disorder Duchenne muscular dystrophy (altered form of dystrophin) muscle
Experimental genetics - I Examples of diseases with genetic-links Hemophilia (complete loss or altered form of factor VIII): bleeding disorder Duchenne muscular dystrophy (altered form of dystrophin) muscle
Tumor tissues or cells were homogenized and proteins were extracted using
 SUPPLEMENTAL MATERIALS AND METHODS Western Blotting Tumor tissues or cells were homogenized and proteins were extracted using T-PER tissue protein extraction buffer. Protein concentrations were determined
SUPPLEMENTAL MATERIALS AND METHODS Western Blotting Tumor tissues or cells were homogenized and proteins were extracted using T-PER tissue protein extraction buffer. Protein concentrations were determined
Solutions will be posted on the web.
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 7 FRIDAY December 3,
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 7 FRIDAY December 3,
TITLE: Translational Studies of GALGT2 Gene Therapy for Duchenne Muscular Dystrophy
 AD Award Number: W81XWH-12-1-0416 TITLE: Translational Studies of GALGT2 Gene Therapy for Duchenne Muscular Dystrophy PRINCIPAL INVESTIGATOR: Paul T. Martin, Ph.D. CONTRACTING ORGANIZATION: The Research
AD Award Number: W81XWH-12-1-0416 TITLE: Translational Studies of GALGT2 Gene Therapy for Duchenne Muscular Dystrophy PRINCIPAL INVESTIGATOR: Paul T. Martin, Ph.D. CONTRACTING ORGANIZATION: The Research
MRC-Holland MLPA. Description version 11; 7 September 2017
 SALSA MLPA mix P268-A2 DYSF Lot A2-1115, A2-0312. Compared to previous version A1-0608, two reference s have been replaced and one reference has been added. In addition, the control fragments have been
SALSA MLPA mix P268-A2 DYSF Lot A2-1115, A2-0312. Compared to previous version A1-0608, two reference s have been replaced and one reference has been added. In addition, the control fragments have been
Pre-Clinical Evaluation of ALN-AAT to Ameliorate Liver Disease Associated with Alpha-1 Antitrypsin Deficiency
 Pre-Clinical Evaluation of ALN-AAT to Ameliorate Liver Disease Associated with Alpha-1 Antitrypsin Deficiency A. Sehgal 1, K. Blomenkamp 2, K Qian 1, A. Simon 1, P. Haslett, S. Barros 1, J. Teckman 2 May
Pre-Clinical Evaluation of ALN-AAT to Ameliorate Liver Disease Associated with Alpha-1 Antitrypsin Deficiency A. Sehgal 1, K. Blomenkamp 2, K Qian 1, A. Simon 1, P. Haslett, S. Barros 1, J. Teckman 2 May
Type of intervention Treatment; secondary prevention. Economic study type Cost-effectiveness analysis.
 Coagulation disorders induced by L-asparaginase: correction with and without fresh-frozen plasma Glasmacher A, Kleinschmidt R, Unkrig C, Mezger J, Scharf R E Record Status This is a critical abstract of
Coagulation disorders induced by L-asparaginase: correction with and without fresh-frozen plasma Glasmacher A, Kleinschmidt R, Unkrig C, Mezger J, Scharf R E Record Status This is a critical abstract of
HELICA BIOSYSTEMS, INC. HIGH SENSITIVITY HUMAN C-REACTIVE PROTEIN FOR RESEARCH USE ONLY (Not for in vitro diagnostic use)
 INTENDED USE HELICA BIOSYSTEMS, INC. HIGH SENSITIVITY HUMAN C-REACTIVE PROTEIN FOR RESEARCH USE ONLY (Not for in vitro diagnostic use) The Helica C-reactive protein assay is intended for the detection
INTENDED USE HELICA BIOSYSTEMS, INC. HIGH SENSITIVITY HUMAN C-REACTIVE PROTEIN FOR RESEARCH USE ONLY (Not for in vitro diagnostic use) The Helica C-reactive protein assay is intended for the detection
Sarepta Therapeutics, Inc. (SRPT-NASDAQ)
 January 27, 2015 Sarepta Therapeutics, Inc. (SRPT-NASDAQ) Current Recommendation SUMMARY DATA NEUTRAL Prior Recommendation Outperform Date of Last Change 08/12/2013 Current Price (01/26/15) $12.44 Target
January 27, 2015 Sarepta Therapeutics, Inc. (SRPT-NASDAQ) Current Recommendation SUMMARY DATA NEUTRAL Prior Recommendation Outperform Date of Last Change 08/12/2013 Current Price (01/26/15) $12.44 Target
Results to be Presented at LDN WORLD Symposium in February Initiation of Repeat-Dose Pompe Study Anticipated in 3Q13
 Amicus Therapeutics Announces Positive Results from All Four Cohorts in Phase 2 Chaperone-Enzyme Replacement Therapy (ERT) Co-Administration Study for Pompe Disease Strong Proof-of-Concept Data for Chaperone
Amicus Therapeutics Announces Positive Results from All Four Cohorts in Phase 2 Chaperone-Enzyme Replacement Therapy (ERT) Co-Administration Study for Pompe Disease Strong Proof-of-Concept Data for Chaperone
MPCR Kit for Human DMD/BMD Set I Cat No. MP-70053: 50 reactions Cat No. MP-70052: 100 reactions
 Maxim Biotech, Inc. 780 Dubuque Avenue So. San Francisco, CA 94080, U.S.A. Tel: (800) 989-6296 / Fax:(650)871-2857 http://www.maximbio.com E-mail: mbi@maximbio.com MPCR Kit for Human DMD/BMD Set I Cat
Maxim Biotech, Inc. 780 Dubuque Avenue So. San Francisco, CA 94080, U.S.A. Tel: (800) 989-6296 / Fax:(650)871-2857 http://www.maximbio.com E-mail: mbi@maximbio.com MPCR Kit for Human DMD/BMD Set I Cat
Supplementary Online Content
 Supplementary Online Content Scher HI, Lu D, Schreiber NA, et al. Association of on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate
Supplementary Online Content Scher HI, Lu D, Schreiber NA, et al. Association of on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate
SALSA MLPA probemix P074-A3 Androgen Receptor (AR) Lot A Compared to previous lot A2-0712, three reference probes have been replaced.
 SALSA MLPA probemix P074-A3 Androgen Receptor (AR) Lot A3-0814. Compared to previous lot A2-0712, three reference probes have been replaced. The androgen insensitivity syndrome (AIS), formerly known as
SALSA MLPA probemix P074-A3 Androgen Receptor (AR) Lot A3-0814. Compared to previous lot A2-0712, three reference probes have been replaced. The androgen insensitivity syndrome (AIS), formerly known as
What determines if a mutation is deleterious, neutral, or beneficial?
 BIO 184 - PAL Problem Set Lecture 6 (Brooker Chapter 18) Mutations Section A. Types of mutations Define and give an example the following terms: allele; phenotype; genotype; Define and give an example
BIO 184 - PAL Problem Set Lecture 6 (Brooker Chapter 18) Mutations Section A. Types of mutations Define and give an example the following terms: allele; phenotype; genotype; Define and give an example
Product Description SALSA MLPA Probemix P438-D2 HLA
 Product Description SALSA Probemix P438-D2 HLA To be used with the MLPA General Protocol. Version D2. Catalogue numbers: P438-025R: SALSA MLPA Probemix P438 HLA, 25 reactions. P438-050R: SALSA MLPA Probemix
Product Description SALSA Probemix P438-D2 HLA To be used with the MLPA General Protocol. Version D2. Catalogue numbers: P438-025R: SALSA MLPA Probemix P438 HLA, 25 reactions. P438-050R: SALSA MLPA Probemix
Convoy TM Transfection Reagent
 Convoy TM Transfection Reagent Catalog No.11103 0.25ml (40-80 transfections in 35mm dishes) Catalog No.11105 0.5 ml (80-165 transfections in 35mm dishes) Catalog No.11110 1.0 ml (165-330 transfections
Convoy TM Transfection Reagent Catalog No.11103 0.25ml (40-80 transfections in 35mm dishes) Catalog No.11105 0.5 ml (80-165 transfections in 35mm dishes) Catalog No.11110 1.0 ml (165-330 transfections
Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge
 Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge G. Scott*, H. Gaus #, B. Rivera*, and M. McGinley* *Phenomenex,
Rapid Extraction of Therapeutic Oligonucleotides from Primary Tissues for LC/ MS Analysis Using Clarity OTX, an Oligonucleotide Extraction Cartridge G. Scott*, H. Gaus #, B. Rivera*, and M. McGinley* *Phenomenex,
