MONITORING THE SUSTAINABLE MANAGEMENT OF NUTRIENTS ON C&H FARM IN BIG CREEK WATERSHED
|
|
|
- Dustin Austin
- 6 years ago
- Views:
Transcription
1 Big Creek Research & Extension Team Quarterly Report Big Creek Research and Extension Team University of Arkansas System Division of Agriculture Quarterly Report January 1 to March 31, 2018 MOITORIG THE SUSTAIABLE MAAGEMET OF UTRIETS O C&H FARM I BIG CREEK WATERSHED Mission of the University of Arkansas System Division of Agriculture The mission of the Division of Agriculture is to advance the stewardship of natural resources and the environment, cultivate the improvement of agriculture and agribusiness, develop leadership skills and productive citizenship among youth and adults, enhance economic security and financial responsibility among the citizens of the state, ensure a safe, nutritious food supply, improve the quality of life in communities across Arkansas, and strengthen Arkansas families. Dr. Mark J. Cochran Vice President for Agriculture January 1 to March 31, 2018 Page 1
2 Big Creek Research & Extension Team Quarterly Report Executive Summary This is the first Quarterly Report of 2018 for the Big Creek Research and Extension Team that details activities and progress made from October 1 through December 31, We continue to collect weekly base flow and periodic stormflow water samples from Big Creek above and below the C&H Farm, along with water from a spring (reflecting shallow aquifer flow), ephemeral stream (reflecting landscape drainage from the area of the holding ponds and operational facilities), surface runoff sites on Fields 1, 5a, and 12, two interceptor trenches below the slurry holding ponds (reflecting subsurface flow below the holding ponds), and house well (reflecting deeper ground water) for chemical and bacterial analysis. 2. This Quarterly Report contains certified analyses of samples collected between January 1, 2017 and March 31, The surface runoff of nutrients and sediment form the three monitored fields (i.e., Fields 1, 5a, and 12) are presented along with runoff volumes for each storm flow event in 2014, 2015, 2016, and From these flows and concentrations, the amount of nutrients and sediment runoff from each field are estimated and presented. We determined the catchment area contributing runoff to the flumes and from this, calculated the amounts of nutrient and sediment transported from each field. Additionally, from the amount of slurry and commercial fertilizer applied, the amount of applied P and transported from the fields was calculated. Losses tend to be dominated by twice the annual rainfall in 2015 than the other years of monitoring, which led to high runoff volumes. 4. The concentration of P,, sediment, E. coli, coliform, chloride, and electrical conductivity from well, ephemeral stream, and trench sites since sampling began, are presented. Any seasonal or annual trends will be statistically evaluated when a 5 year period of record has been obtained. Analysis of seasonal or annual trends over a shorter period can lead to incorrect interpretations. 5. Although not part of the BCRET work plan, dissolved oxygen concentrations were monitored between 2014 and 2017 at the site downstream of the C&H (i.e., USGS Big Creek near Mt. Judea). These data are given in this report and have been made available to ADEQ and on the BCRET website. January 1 to March 31, 2018 Page 2
3 Big Creek Research & Extension Team Quarterly Report Big Creek Science Team Andrew Sharpley, Ph.D., TEAM LEADER Distinguished Professor Soil science, water quality, soil phosphorus chemistry, agricultural management Kris Brye, Ph.D., Professor Effects of land application of poultry litter on in situ nutrient leaching, effects of land use and management practices on soil physical, chemical, and biological properties related to soil quality and sustainability Mike Daniels, Ph.D., Professor Extension water quality and nutrient management specialist Ed Gbur, Ph.D., Professor and Director, Agricultural Statistics Laboratory Experimental design, linear and generalized linear mixed models, regression, agricultural applications of statistics. Brian Haggard, Ph.D., Professor Ecological engineering, environmental soil and water sciences, water quality chemistry, water quality monitoring and modeling, algal nutrient limitation, pollutant transport in aquatic systems Phil Hays, Ph.D. Ground Water Specialist, U.S. Geological Survey and Research Professor with Geosciences Dept., University of Arkansas, application of stable isotopes and other geochemical indicators in delineating movement and behavior of contaminants in ground water systems Mary Savin, Ph.D. Structure and function of microbial communities in natural and managed ecosystems, microorganisms in nutrient cycling, contaminant degradation Karl VanDevender, Ph.D. and P.E., Professor Extension Engineer, Livestock and poultry manure and mortality management, nutrient management planning Jun Zhu, PhD., Professor Biological and agricultural engineering, agricultural sustainability, manure treatment technologies Adam Willis, M.Sc., ewton County Extension Agent Agriculture Field Technicians The Big Creek Research and Extension Team are ably supported by several outstanding and dedicated Program Technicians based in Fayetteville and Little Rock. January 1 to March 31, 2018 Page 3
4 Big Creek Research & Extension Team Quarterly Report Table of Contents Executive Summary... 2 Big Creek Science Team... 3 List of Tables... 5 List of Figures... 7 Water Sampling and Analytical Methods Sampling Locations Sampling Protocols and Analyses Big Creek Research and Extension Team Monitoring Data utrients, Sediment, and Bacteria by Date of Sampling utrients, Sediment, and Bacteria by Date Spring, Upstream, and Downstream Sites utrients, Sediment, and Bacteria by Site for Ephemeral Stream, Trenches, Left Fork and Field Runoff Water ph, Alkalinity, Chloride, Electrical Conductivity, and Solids for Several Big Creek Sites Discharge at USGS Site Downstream of C&H Operation Surface Runoff from Fields 1, 5a and Field Site Description Surface Runoff of utrient and Sediment Temporal Trends in Phosphorus, itrogen, Bacteria, and Chloride in Big Creek Above and Below the C&H Farm utrient Concentrations in Well, Ephemeral Stream, and Trenches Big Creek Oxygen Regulation Establishing Oxygen Standards for Surface Waters of the State of Arkansas Field Determination and Methodology Big Creek Oxygen January 1 to March 31, 2018 Page 4
5 Big Creek Research & Extension Team Quarterly Report List of Tables Table 1. Location of sampling sites on the Big Creek Research and Extension Team project Table 2. Parameters used to enable ISCO auto samplers at BCRET edge of field sites Field 1, 5a, and Table 3. Parameters used to enable ISCO auto samplers at BCRET stream sites BC4, BC6, and BC Table 4. Minimum detection limits (MDLs) for each chemical and biological constituent Table 5. Water quality analyses at each sample site since January 2017, with those collected since the last report noted. Coliform units are Most Probable umber (MP) per 100 ml of water Table 6. Water quality analyses in Big Creek upstream and downstream of the C&H Farm boundary of permitted land application since January 2017, with those collected since the last report noted Table 7. Water quality analyses at the ephemeral stream draining the subwatershed containing the production houses and manure holding ponds, and surface runoff from Fields 1, 5a, and 12 since January, 2017, with those collected since the last report noted Table 8. The ph, Chloride concentration, and electrical conducting of water samples collected at upstream, downstream, spring, ephemeral stream, house well and trench sites, initiated at the beginning of 2017, with those collected since the last report noted Table 9. Area of Field 1, 5a, and 12 monitored for surface runoff, area of flume catchment, area of buffers where no slurry is applied, and area of flume receiving slurry Table 10. Slurry (i.e., Fields 1 and 12) and fertilizer (i.e., Fields 5a) application to the monitored fields for 2014 to Table 11. Flow and flow weighted concentration of phosphorus and nitrogen in each runoff event for Fields 1, 5a, and 12 in 2014, 2015, 2016, and Table 12. Loss of phosphorus and nitrogen in each runoff event as pounds per acre for Fields 1, 5a, and 12 in 2014, 2015, 2016, and Table 13. Loss of phosphorus and nitrogen in each runoff event as grams per hectare for Fields 1, 5a, and 12 in 2014, 2015, 2016, and Table 14. Annual flow and flow weighted concentrations of phosphorus, nitrogen, and sediment in runoff for 2014, 2015, 2016, and Table 15. Annual loss of phosphorus and nitrogen in surface runoff from Fields 1, 5a, and 12 for 2014, 2015, 2016, and Table 16. Amount of phosphorus and nitrogen applied to the flume catchment area of Fields 1, 5a, and 12, loss in runoff, and percent of applied lost in runoff for 2014, 2015, 2016, and Table 17. oxygen criteria for area rivers and streams January 1 to March 31, 2018 Page 5
6 Big Creek Research & Extension Team Quarterly Report Table 18. Big Creek Oxygen concentrations Table 19. Big Creek Oxygen concentrations downstream of C&H, as a function of stream temperature January 1 to March 31, 2018 Page 6
7 Big Creek Research & Extension Team Quarterly Report List of Figures Figure 1. Location of sampling sites for the Big Creek Research and Extension Team project Figure 2. Discharge in Big Creek downstream of the C&H Farm for the period of monitoring; December 1, 2017 to April 1, Figure 3. Map of Field 1 showing catchment area for surface runoff flume and buffers where no slurry can be applied Figure 4. Map of Field 5a showing catchment area for surface runoff flume and buffers where no slurry can be applied Figure 5. Map of Field 12 showing catchment area for surface runoff flume and buffers where no slurry can be applied Figure 6. P concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR Figure 7. P concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR Figure 8. itrate concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR Figure 9. concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR Figure 10. E. coli numbers at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR Figure 11. coliform numbers at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR Figure 12. Chloride concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR Figure 13. Phosphorus and nitrogen concentrations in house well samples over the period of monitoring Figure 14. E. coli, total coliform, electrical conductivity, chloride concentrations in house well samples over the period of monitoring Figure 15. Phosphorus and nitrogen concentrations in the ephemeral stream over the period of monitoring Figure 16. E. coli, total coliform, electrical conductivity, chloride concentrations in the ephemeral stream over the period of monitoring Figure 17. Phosphorus and nitrogen concentrations in trench 1 over the period of monitoring January 1 to March 31, 2018 Page 7
8 Big Creek Research & Extension Team Quarterly Report Figure 18. E. coli, total coliform, electrical conductivity, chloride concentrations in trench 1 over the period of monitoring Figure 19. Phosphorus and nitrogen concentrations in trench 2 over the period of monitoring Figure 20. E. coli, total coliform, electrical conductivity, chloride concentrations in trench 2 over the period of monitoring Figure 21. Flow, water temperature, and dissolved oxygen concentration downstream of the C&H Farm between September 9, 2014 and December 11, Figure 22. Flow and dissolved oxygen concentration downstream of the C&H Farm between September 9 and ovember 13, Figure 23. Water temperature and dissolved oxygen concentration downstream of the C&H Farm between September 9 and ovember 13, Figure 24. Flow and dissolved oxygen concentration downstream of the C&H Farm between S April 10 and October 16, Figure 25. Water temperature and dissolved oxygen concentration downstream of the C&H Farm between April 10 and October 16, Figure 26. Flow and dissolved oxygen concentration downstream of the C&H Farm between May 16 and December 11, Figure 27. Water temperature and dissolved oxygen concentration downstream of the C&H Farm between May 16 and December 11, Figure 28. Water temperature and dissolved oxygen concentration upstream and downstream of the C&H Farm between October 22 and ovember 13, Figure 29. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between September 9 and December 11, Figure 30. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between September 9 and ovember 13, Figure 31. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between April 10 and October 16, Figure 32. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between May 15 and December 11, Figure 33. Water temperature and dissolved oxygen saturation upstream and downstream of the C&H Farm between October 22 and ovember 13, Figure 34. oxygen concentration and % saturation downstream of the C&H Farm between 2014 and Figure 35. oxygen concentration and % saturation downstream of the C&H Farm between September 9 and ovember 13, January 1 to March 31, 2018 Page 8
9 Big Creek Research & Extension Team Quarterly Report Figure 36. oxygen concentration and % saturation downstream of the C&H Farm between April 10 and October 16, Figure 37. oxygen concentration and % saturation downstream of the C&H Farm between May 16 and December 11, Figure 38. oxygen concentration and % saturation upstream and downstream of the C&H Farm between October 22, 2014 and ovember 13, Figure 39. oxygen concentration at the USGS Carver site (USGA: ) for June 3 to May 1, January 1 to March 31, 2018 Page 9
10 Big Creek Research & Extension Team Quarterly Report Water Sampling and Analytical Methods Sampling Locations Water quality monitoring sites detailed in Table 1 and Figure 1, are: Site 1. Edge of field monitoring on Field 1 permitted to receive slurry. Site 2. Edge of field monitoring on Field 5a excluded from receiving slurry. Site 3. Edge of field monitoring on Field 12 permitted to receive slurry. Site 4. Ephemeral stream flow draining a subwatershed containing the production facilities. Site 5. Spring below Field 1. Site 6. Big Creek upstream of the C&H Farm operation. Site 7. Big Creak downstream of the C&H Farm operation. Site 8. Left Fork downstream of the C&H Farm operation. Site 9. orth interceptor trench below the manure holding ponds. Site 10. South interceptor trench below the manure holding ponds. Site 11. House well at animal facility. Table 1. Location of sampling sites on the Big Creek Research and Extension Team project. Site description Latitude Longitude Elevation, ft Field Field 5a Field Ephemeral stream Spring Big Creek upstream of Big Creek downstream of Left Fork Trench 1 (south) Trench 2 (north) House well January 1 to March 31, 2018 Page 10
11 Big Creek Research & Extension Team Quarterly Report Figure 1. Location of sampling sites for the Big Creek Research and Extension Team project. January 1 to March 31, 2018 Page 11
12 Big Creek Research & Extension Team Quarterly Report Sampling Protocols and Analyses The following protocols were used to collect, prepare, and analyze all water samples: 1. One liter acid washed bottles were used to collect the stream samples for nutrient analyses. 2. Water was collected from just beneath the surface, where the stream was actively moving and wellmixed. 3. The bottle was rinsed with stream water before collecting the sample. 4. Sterilized specimen cups were used to collect samples for bacterial evaluation. 5. Time of collection was noted, and samples placed in a cooler on ice to preserve them until processed and were submitted to the Arkansas Water Resources Center Water Quality Lab on the day of collection for analyses. 6. The ISCO autosamplers collect storm flow samples at sites edge of field sites on Fields 1, 5a, and 12, and at the ephemeral stream, upstream of the C&H Farm, and downstream of the C&H Farm sites (i.e., BC1, BC2, BC3, BC4, BC 6, and BC 7, respectively). Water sample collection criteria for each site are detailed in Tables 2 and Analyses included Alkalinity (APHA 2320 B), Ammonia (EPA 351.2), Chloride (EPA 300.0), Phosphorus (EPA 365.2), E. coli (APHA 9223 B), Electrical Conductivity (EPA 120.1), itrate (EPA 300.0), ph (EPA 150.1), Coliforms (APHA 9223 B), Solids (EPA 160.1), itrogen (APHA 4500 P J), Phosphorus (APHA 4500 P J), and Suspended Solids (EPA 160.2). APHA is American Public Health Association from the Wadeable Streams Assessment, Water Chemistry Laboratory Manual 8. Prior to collection of a house well water sample, the well is purged and water temperature, ph, and electrical conductivity measured on site every 30 seconds until all values stabilize (primarily water temperature). At that point, a sample of water is collected in a 1 L acid washed bottle. This method is taken from USGS and EPA well water sampling protocols. See USGS methods for sampling at Specific and detailed guidance on the collected of water quality data can be found in the USGS ational Field Manual at file:///u:/words/c&h%20farm/publications/planning/usgs%20ational%20field%20mannual_co mplete% pdf The U.S. EPA also recommend that selected water quality parameters can be monitored during lowrate purging, with stabilization of these parameters indicating when the discharge water represents aquifer water or source well water. See: nd%20barcelona%201996%20low%20flow%20sampling.pdf and situ.com/wpcontent/uploads/2015/01/low Flow Groundwater Sampling Techniques Improve Sample Qualityand Reduce Monitoring Program Costs Case Study.pdf 9. Minimum detection limits (MDLs) for each chemical and biological constituent are listed in Table 4. Some constituent concentrations were reported by the laboratory as less than the MDL but greater January 1 to March 31, 2018 Page 12
13 Big Creek Research & Extension Team Quarterly Report than zero. Those values are given in subsequent tables but have less confidence in their accuracy than concentrations above the MDL. 10. Chemical and biological analyses of samples collected from the beginning of 2017 to the current date are given in Tables 5, 6, 7, and 8. Table 2. Parameters used to enable ISCO auto samplers at BCRET edge of field sites Field 1, 5a, and 12. Site Identifier ISCO enabled when stage height (inches) above Volume pacing, 100 ml water collected per gallon of water Rainfall, inches < to 4 >4 Field 1 BC1 > ,000 5,000 Field 5a BC2 > ,000 10,000 50,000 Field 12 BC3 > ,000 5,000 Table 3. Parameters used to enable ISCO auto samplers at BCRET stream sites BC4, BC6, and BC7. Site Identifier ISCO enabled when, over a 30 minute period, stage height (inches) increases by Volume pacing, 100 ml water collected per gallon of water Rainfall, inches < to 4 >4 Ephemeral stream BC4 > 2.0 * 25,000 50, ,000 Upstream Big Creek BC ,000,000 50,000,000 70,000,000 Downstream Big Creek BC ,000,000 80,000, ,000,000 * For ephemeral stream stage height increases >2.0 inches over a 30 min period. January 1 to March 31, 2018 Page 13
14 Big Creek Research & Extension Team Quarterly Report Table 4. Minimum detection limits (MDLs) for each chemical and biological constituent. Constituent Minimum detection limit 1 Alkalinity, mg/l as CaCO 3 2 Chloride, mg/l P, mg/l Conductivity, us/cm 1 Ammonia, mg/l 0.03 organic carbon, mg/l 0.18 E. coli, MP/100 ml 1 itrate, mg/l ph 0.1 coliform, MP/100 ml 1 dissolved solids, mg/l 15.22, mg/l P, mg/l suspended solids, mg/l MDL the Minimum Detection Limit of an analyte that can be measured and reported with 99% confidence that the analyte concentration is greater than zero. Further information is available at 193/detection.html January 1 to March 31, 2018 Page 14
15 Big Creek Research and Extension Team Monitoring Data utrients, Sediment, and Bacteria by Date of Sampling Table 5. Water quality analyses at each sample site since January 2017, with those collected since the last report noted. Coliform units are Most Probable umber (MP) per 100 ml of water. Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform mg/l MP/100 ml 1/5/2017 1/25/2017 Grab sample 12:28 15:25 Spring :12 15:25 12:00 15:25 Upstream Downstream :42 15:25 Left Fork :47 15:25 House well <1.0 <1.0 1/19/2017 1/19/2017 Grab sample 10:41 14:30 Spring < :27 14:30 10:30 14:30 Upstream Downstream > :10 14:30 Left Fork > :00 14:30 House well <1.0 <1.0 January 1 to March 31, 2018 Page 15
16 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 2/2/2017 2/2/2017 Grab sample 10:45 14:30 Spring < :20 14:30 10:30 14:30 Upstream Downstream < > > :15 14:30 Left Fork > :57 14:30 House well <1.0 <1.0 2/15/2017 2/15/2017 Grab sample 11:50 15:35 Spring :30 15:35 11:24 15:35 12:08 15:35 Upstream Downstream Ephemeral stream :11 15:35 Left Fork :46 15:35 Trench :56 15:35 Trench :25 15:35 House well <1.0 <1.0 3/1/2017 3/1/2017 Grab sample January 1 to March 31, 2018 Page 16
17 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 12:38 14:55 11:18 14:55 11:43 14:55 Upstream Downstream Ephemeral stream :00 14:55 Left Fork :16 14:55 Trench :52 14:55 House well <1.0 <1.0 3/16/2017 3/16/2017 Grab sample 7:30 11:45 Spring < > :38 11:45 7:13 11:45 7:38 11:45 Upstream Downstream Ephemeral stream < < < :00 11:45 Left Fork < > :00 11:45 Trench < < :46 11:45 House well < <1.0 <1.0 3/27/2017 3/27/2017 Grab sample 11:27 15:40 Spring < January 1 to March 31, 2018 Page 17
18 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 12:51 15:40 10:51 15:40 Upstream Downstream :37 15:40 Left Fork :50 15:40 Trench :55 15:40 Trench :38 15:40 House well /27/2017 3/27/ :40 15:40 Storm sample Ephemeral stream :05 15:40 Field :15 15:40 Field 5a :06 15:40 Field /30/2017 3/30/ :15 14:15 Storm sample Ephemeral stream 4/6/2017 4/6/2017 Grab sample D D 11:40 15:25 Spring :30 15:25 Upstream > January 1 to March 31, 2018 Page 18
19 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:55 15:25 11:20 15:25 Downstream Ephemeral stream > < :50 15:25 Left Fork :20 15:25 Trench /6/2017 4/6/ :15 15:25 Storm sample Ephemeral stream 4/13/2017 4/13/2017 Grab sample D D 12:22 15:30 Spring < :05 15:30 11:56 15:30 12:50 15:30 Upstream Downstream Ephemeral stream < > < :33 15:30 Left Fork < > :35 15:30 House well < < /17/2017 4/17/2017 Grab sample 12:02 14:55 Spring :45 14:55 Upstream < January 1 to March 31, 2018 Page 19
20 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 10:51 14:55 11:10 14:55 Downstream Ephemeral stream < :40 14:55 Left Fork :25 14:55 House well < /24/2017 4/24/ :50 15:30 Storm sample Ephemeral stream D D 11:15 15:30 Field D D 11:35 15:30 Field 5a D D 12:15 15:30 Trench D D 12:20 15:30 Trench D D 4/27/2017 4/27/2017 Grab sample 11:05 16:25 Spring < > :10 16:25 10:35 16:25 Upstream Downstream < < :20 16:25 Left Fork < :30 16:25 House well < January 1 to March 31, 2018 Page 20
21 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:43 16:25 Trench /27/2017 4/27/ :52 16:25 Storm sample Ephemeral stream > :50 16:25 Field D D 11:15 16:25 Field 5a D D 13:40 16:25 Field D D 11:40 16:25 Trench /1/2017 5/1/2017 Grab sample 11:35 15:45 Spring < :05 15:45 11:01 15:45 12:54 15:45 Upstream Downstream Ephemeral stream < < < :50 15:45 Left Fork < :15 15:45 House well < :30 15:45 Trench < :40 15:45 Trench < January 1 to March 31, 2018 Page 21
22 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 5/1/2017 5/1/2017 Storm sample 11:20 15:45 Field D D 12:00 15:45 Field 5a D D 13:15 15:45 Field D D 12:30 15:45 Trench D D 12:40 15:45 Trench D D 5/11/2017 5/11/2017 Grab sample 7:40 12:05 Spring < :25 12:05 7:24 12:05 7:50 12:05 Upstream Downstream Ephemeral stream < < < :13 12:05 Left Fork < :55 12:05 House well < < /18/2017 5/18/2017 Grab sample 10:55 15:00 Spring < :40 15:00 Upstream < > January 1 to March 31, 2018 Page 22
23 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 10:45 15:00 11:05 15:00 Downstream Ephemeral stream < < :30 15:00 Left Fork < :15 15:00 House well < /25/2017 5/25/2017 Grab sample 12:16 15:27 Spring :06 15:27 11:35 15:27 12:25 15:27 Upstream Downstream Ephemeral stream D D > > :18 15:27 Left Fork :48 15:27 House well < /25/2017 5/25/ :35 15:27 Storm sample Downstream 5/31/2017 5/31/2017 Grab sample < D D 10:38 13:50 Spring < :05 13:50 Upstream < January 1 to March 31, 2018 Page 23
24 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 10:30 13:50 10:55 13:50 Downstream Ephemeral stream < < :20 13:50 Left Fork < :51 13:50 House well < < /5/2017 6/5/2017 Grab sample 10:53 14:35 Spring :51 14:35 10:35 14:35 11:03 14:35 Upstream Downstream Ephemeral stream < :25 14:35 Left Fork :09 14:35 House well :30 14:35 Trench > /6/2017 6/6/2017 Storm sample 11:17 15:30 Field D D 12:12 15:30 Field 5a < D D 12:46 15:30 Field D D January 1 to March 31, 2018 Page 24
25 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:30 15:30 11:02 15:30 Ephemeral stream Downstream 6/12/2017 6/12/2017 Grab sample D D D D 10:27 14:25 Spring :02 14:25 10:14 14:25 10:35 14:25 Upstream Downstream Ephemeral stream < :05 14:25 Left Fork < :40 14:25 House well < /19/2017 6/19/2017 Grab sample 10:45 14:25 Spring :17 14:25 10:35 14:25 Upstream Downstream < :25 14:25 Left Fork :00 14:25 House well <1.0 <1.0 6/29/2017 6/29/2017 Grab sample January 1 to March 31, 2018 Page 25
26 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:47 14:55 Spring :19 14:55 11:35 14:55 Upstream Downstream :24 14:55 Left Fork :01 14:55 House well /5/2016 7/5/2016 Grab sample 11:25 14:55 Spring < :15 14:55 11:05 14:55 Upstream Downstream < < :55 14:55 Left Fork < :30 14:55 House well < /11/2017 7/11/2017 Grab sample 10:40 13:48 Spring < > :23 13:48 10:26 13:48 Upstream Downstream < :17 13:48 Left Fork January 1 to March 31, 2018 Page 26
27 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 10:50 13:48 House well < /19/2017 7/19/2017 Grab sample 10:51 15:30 Spring :04 15:30 10:26 15:30 Upstream Downstream :12 15:30 Left Fork :40 15:30 House well <1.0 <1.0 7/26/2017 7/26/2017 Grab sample 7:28 11:40 Spring > :08 11:40 7:05 11:40 Upstream Downstream :51 11:40 Left Fork :44 11:40 House well <1.0 <1.0 8/3/2017 8/3/2017 Grab sample 11:52 15:08 Spring :32 15:08 Upstream Farm < January 1 to March 31, 2018 Page 27
28 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:25 15:08 Downstream Farm :12 15:08 Left Fork :09 15:08 House well < /3/2017 8/3/ :25 15:08 Storm sample Downstream 8/9/2017 8/9/2017 Grab sample D D 11:56 15:00 Spring > :36 15:00 11:29 15:00 Upstream Downstream :11 15:00 Left Fork :10 15:00 House well <1.0 <1.0 8/16/2017 8/16/2017 Grab sample 7:04 11:50 Spring :58 11:50 6:47 11:50 Upstream Downstream < < :29 11:50 Left Fork January 1 to March 31, 2018 Page 28
29 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 7:24 11:50 House well < < /24/2016 8/24/2016 Grab sample 11:58 14:55 Spring < :38 14:55 11:23 14:55 Upstream Downstream < :11 14:55 Left Fork < :15 14:55 House well < < /24/2017 8/24/ :23 14:55 Storm sample Downstream 8/31/2017 8/31/2017 Grab sample < D D 11:28 14:15 Spring :55 14:15 11:18 14:15 Upstream Downstream < :00 14:15 Left Fork < :41 14:15 House well /6/2017 9/6/2017 Grab sample January 1 to March 31, 2018 Page 29
30 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 8:41 12:15 Spring < :20 12:15 8:22 12:15 Upstream Downstream < :06 12:15 Left Fork :57 12:15 House well <1.0 <1.0 9/13/2017 9/13/2017 Grab sample 10:02 13:25 Spring :30 13:25 9:46 13:25 Upstream Downstream :36 13:25 Left Fork :15 13:25 House well <1.0 <1.0 9/21/2017 9/21/2017 Grab sample 10:50 14:40 Downstream :34 14:40 Left Fork < :25 14:40 House well < < /28/2017 9/28/2017 Grab sample January 1 to March 31, 2018 Page 30
31 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:29 14:19 Downstream :17 14:19 Left Fork :45 14:19 House well <1.0 <1.0 10/5/ /5/2017 Grab sample 9:15 13:20 Downstream :03 13:20 Left Fork :46 13:20 House well < /12/ /12/2017 Grab sample 8:13 12:40 Downstream :01 12:40 Left Fork < :40 12:40 House well < <1.0 <1.0 10/18/ /18/2017 Grab sample 12:12 15:00 Downstream :59 15:00 Left Fork :35 15:00 House well < /23/ /23/2017 Grab sample 12:00 15:20 Spring January 1 to March 31, 2018 Page 31
32 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 12:52 15:20 11:28 15:20 Upstream Downstream :10 15:20 Left Fork :31 15:20 House well < < /23/ /23/ :16 15:20 Storm sample Ephemeral stream 11/1/ /1/2017 Grab sample 8:06 12:15 Downstream D D :51 12:15 Left Fork :43 12:15 House well <1.0 <1.0 11/9/ /9/2017 Grab sample 7:59 12:05 Downstream :42 12:05 Left Fork :30 12:05 House well <1.0 <1.0 11/15/ /15/2017 Grab sample 7:36 13:15 Downstream January 1 to March 31, 2018 Page 32
33 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 7:24 13:15 Left Fork :54 13:15 House well < :10 13:15 Trench /15/ /15/2017 8:37 13:15 Storm sample Downstream :27 13:15 Left Fork /30/ /30/2017 Grab sample 7:45 12:20 Downstream :28 12:20 Left Fork :24 12:20 House well <1.0 <1.0 12/13/ /13/2017 Grab sample 8:15 12:35 7:25 12:35 Upstream Downstream Samples collected and analyzed since the last quarterly report > :04 12:35 Left Fork :57 12:35 House well < /18/ /18/2017 Grab sample January 1 to March 31, 2018 Page 33
34 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 12:02 14:43 Downstream > :15 14:43 Left Fork > :33 14:43 House well <1.0 <1.0 1/4/2018 1/4/2018 Grab sample 12:45 15:20 12:05 15:20 Upstream Downstream :52 15:20 Left Fork :/22 15:20 House well < /18/2018 1/18/2018 Grab sample 11:50 14:45 11:01 14:45 Upstream Downstream > :45 14:45 Left Fork :24 14:45 House well <1.0 <1.0 1/30/2018 1/30/2018 Grab sample 12:13 14:30 11:36 14:30 Upstream Downstream January 1 to March 31, 2018 Page 34
35 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:24 14:30 Left Fork :00 14:30 House well <1.0 <1.0 2/14/2018 2/14/2018 Grab sample 10:46 13:20 10:00 13:20 Upstream Downstream :44 13:20 Left Fork :31 13:20 House well <1.0 <1.0 2/21/2018 2/21/2018 Storm sample 11:32 15:32 Field 5a D D 2/22/2018 2/22/2018 Grab sample 11:16 14:35 Spring > :16 14:35 11:00 14:35 12:04 14:35 Upstream Downstream Ephemeral stream > :52 14:35 Left Fork :38 14:35 House well <1.0 <1.0 January 1 to March 31, 2018 Page 35
36 Time sample collected Time laboratory Sample location P P Ammonia itrate suspended solids Organic Carbon E. coli coliform 11:43 14:35 Trench Values proceeded by < were reported by the analytical laboratory as zero and the minimum detection limit is given. D is o Data, due to coliform not measured on water samples collected automatically by non sterilized ISCO sampler. Storm sample collected by hand a er a 30 minute storm in the watershed at 7:35 on 11/15/2017. January 1 to March 31, 2018 Page 36
37 utrients, Sediment, and Bacteria by Date Spring, Upstream, and Downstream Sites Table 6. Water quality analyses in Big Creek upstream and downstream of the C&H Farm boundary of permitted land application since January 2017, with those collected since the last report noted. Sample location P P Ammonia itrate suspended solids Organic C E. coli coliform mg/l MP/100 ml 1/5/2017 Upstream Downstream /19/2017 Upstream Downstream /2/2017 Upstream < > Downstream > /15/2017 Upstream Downstream /1/2017 Upstream January 1 to March 31, 2018 Page 37
38 Sample location P P Ammonia itrate suspended solids Organic C E. coli coliform Downstream /16/2017 Upstream < Downstream < /27/2017 Upstream Downstream /6/2017 Upstream > Downstream > /13/2017 Upstream < Downstream > /17/2017 Upstream < Downstream /27/2017 Upstream < January 1 to March 31, 2018 Page 38
39 Sample location P P Ammonia itrate suspended solids Organic C E. coli coliform Downstream < /18/2017 Upstream < > Downstream < /31/2017 Upstream Downstream /5/2017 Upstream Downstream /12/2017 Upstream Downstream /19/2017 Upstream < Downstream /29/2017 Upstream January 1 to March 31, 2018 Page 39
40 Sample location P P Ammonia itrate suspended solids Organic C E. coli coliform Downstream /5/2016 Upstream < Downstream < /11/2017 Upstream Downstream < /19/2017 Upstream Downstream /26/2017 Upstream Downstream /3/2017 Upstream Downstream D D 8/9/2017 Upstream January 1 to March 31, 2018 Page 40
41 Sample location P P Ammonia itrate suspended solids Organic C E. coli coliform Downstream /16/2017 Upstream < Downstream < /24/2017 Upstream < Downstream /31/2017 Upstream Downstream < /6/2017 Upstream < Downstream /13/2017 Upstream Downstream /23/2017 Upstream January 1 to March 31, 2018 Page 41
42 Sample location P P Ammonia itrate suspended solids Organic C E. coli coliform Downstream /13/2017 Samples collected and analyzed since the last quarterly report Upstream > Downstream /4/2018 Upstream Downstream /18/2018 Upstream Downstream > /30/2018 Upstream Downstream /14/2018 Upstream Downstream /22/2018 January 1 to March 31, 2018 Page 42
43 Upstream > Downstream Values proceeded by < were reported by the analytical laboratory as zero and the Minimum detection limit is given. D is o Data, due to coliform not measured on water samples collected automatically by non sterilized ISCO sampler. January 1 to March 31, 2018 Page 43
44 utrients, Sediment, and Bacteria by Site for Ephemeral Stream, Trenches, Left Fork and Field Runoff Table 7. Water quality analyses at the ephemeral stream draining the subwatershed containing the production houses and manure holding ponds, and surface runoff from Fields 1, 5a, and 12 since January, 2017, with those collected since the last report noted. Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform mg/l MP/100 ml Ephemeral stream 2/15/ /1/ /16/ < /27/ /30/ /6/ < /6/ D D 4/17/ < /24/ D D 4/27/ > /18/ < /31/ < January 1 to March 31, 2018 Page 44
45 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 6/5/ < /6/ D D 6/12/ < /23/ D D Samples collected and analyzed since the last quarterly report 2/22/ House well 1/5/ <1.0 <1.0 1/19/ <1.0 <1.0 2/2/ <1.0 <1.0 2/15/ <1.0 <1.0 3/1/ <1.0 <1.0 3/16/ < < /27/ /13/ < < /17/ < January 1 to March 31, 2018 Page 45
46 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 4/27/ < /18/ < /31/ < < /5/ /12/ < < /19/ <1.0 <1.0 6/29/ /5/ < /11/ < /19/ <1.0 <1.0 7/26/ <1.0 <1.0 8/3/ < /9/ < <1.0 <1.0 8/16/ < < /24/ < < /31/ /6/ <1.0 <1.0 January 1 to March 31, 2018 Page 46
47 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 9/13/ <1.0 <1.0 9/21/ < < /28/ <1.0 <1.0 10/5/ < /12/ < <1.0 <1.0 10/18/ < /23/ < < /1/ <1.0 <1.0 11/9/ <1.0 <1.0 11/15/ < /30/ <1.0 <1.0 Samples collected and analyzed since the last quarterly report 12/13/ < /18/ <1.0 <1.0 1/4/ < /18/ <1.0 <1.0 1/30/ <1.0 <1.0 January 1 to March 31, 2018 Page 47
48 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 2/14/ <1.0 <1.0 2/22/ <1.0 <1.0 Interceptor Trench 1 (South) 2/15/ /16/ < < /27/ /6/ /24/ D D 4/27/ Samples collected and analyzed since the last quarterly report 2/22/ Interceptor Trench 2 (orth) 2/15/ /1/ /27/ /24/ D D January 1 to March 31, 2018 Page 48
49 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 4/27/ /5/ /15/ o samples collected since the last quarterly report Left Fork 1/5/ /19/ > /2/ > /15/ /1/ /16/ < > /27/ /6/ /13/ < > /17/ January 1 to March 31, 2018 Page 49
50 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 4/27/ < /18/ < /31/ /5/ /12/ < /19/ /29/ /11/ /19/ /26/ /3/ /9/ /16/ /31/ < /6/ January 1 to March 31, 2018 Page 50
51 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 9/13/ /21/ < /28/ /5/ /12/ < /18/ /23/ /1/ /9/ /15/ /30/ Samples collected and analyzed since the last quarterly report 12/13/ /18/ > /4/ January 1 to March 31, 2018 Page 51
52 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 1/18/ /30/ /14/ /22/ Field 1 10/13/ S..S. 3/27/ /24/ D D 4/27/ D D 6/6/ D D o samples collected and analyzed since the last quarterly report Field 5a 3/31/ D D 5/10/ D D 3/27/ January 1 to March 31, 2018 Page 52
53 Date sample collected P P Ammonia itrate suspended solids Organic C E. coli coliform 4/24/ D D 4/27/ D D 6/6/ < D D Samples collected and analyzed since the last quarterly report 2/21/ D D Field 12 3/10/ D D 5/10/ D D 3/27/ /27/ D D 6/6/ D D o samples collected since the last quarterly report Values proceeded by < were reported by the analytical laboratory as zero and the minimum detection limit is given. D is o Sample. E. coli and total coliform were not measured on surface runoff samples collected by ISCO samplers when sample holding time exceeded the required 8 hour threshold. January 1 to March 31, 2018 Page 53
54 Big Creek Research & Extension Team Quarterly Report Water ph, Alkalinity, Chloride, Electrical Conductivity, and Solids for Several Big Creek Sites At the beginning of 2015, the ph, alkalinity, chloride concentration, electrical conductivity and total dissolved solids were determined on water samples collected at the upstream and downstream sites, spring, house well, and trenches, to build a data base that will enable to eventually source track the major water source pathways at these sites. These values are given below in Table 8. Table 8. The ph, Chloride concentration, and electrical conducting of water samples collected at upstream, downstream, spring, ephemeral stream, house well and trench sites, initiated at the beginning of 2017, with those collected since the last report noted. Date ph Chloride mg/l Electrical conductivity µs/cm Upstream 1/5/ /19/ /2/ /15/ /1/ /16/ /27/ /6/ /13/ /17/ /27/ /18/ /31/ /5/ /12/ /19/ January 1 to March 31, 2018 Page 54
55 Big Creek Research & Extension Team Quarterly Report Date ph Chloride Electrical conductivity 6/29/ /5/ /11/ /19/ /26/ /3/ /9/ /16/ /24/ /31/ /6/ /13/ /23/ Samples collected and analyzed since the last quarterly report 12/13/ /4/ /18/ /30/ /14/ /22/ Downstream 1/5/ /19/ /2/ /15/ /1/ January 1 to March 31, 2018 Page 55
56 Big Creek Research & Extension Team Quarterly Report Date ph Chloride Electrical conductivity 3/16/ /27/ /6/ /13/ /17/ /27/ /18/ /31/ /5/ /12/ /19/ /29/ /5/ /11/ /19/ /26/ /3/ /9/ /16/ /24/ /31/ /6/ /13/ /21/ /28/ /5/ /12/ January 1 to March 31, 2018 Page 56
57 Big Creek Research & Extension Team Quarterly Report Date ph Chloride Electrical conductivity 10/18/ /23/ /1/ /9/ /15/ /30/ Samples collected and analyzed since the last quarterly report 12/13/ /18/ /4/ /18/ /30/ /14/ /22/ Spring 1/5/ /19/ /2/ /15/ /16/ /27/ /6/ /13/ /17/ /27/ /18/ January 1 to March 31, 2018 Page 57
58 Big Creek Research & Extension Team Quarterly Report Date ph Chloride Electrical conductivity 5/31/ /5/ /12/ /19/ /29/ /5/ /11/ /19/ /26/ /3/ /9/ /16/ /24/ /31/ /6/ /13/ /23/ Samples collected and analyzed since the last quarterly report 2/22/ Ephemeral Stream 2/15/ /1/ /16/ /27/ /30/ /6/ January 1 to March 31, 2018 Page 58
59 Big Creek Research & Extension Team Quarterly Report Date ph Chloride Electrical conductivity 4/13/ /17/ /23/ /27/ /18/ /31/ /5/ /12/ /23/ Samples collected since the last quarterly report 2/22/ House Well 1/5/ /19/ /2/ /15/ /1/ /16/ /27/ /13/ /17/ /27/ /1/ /11/ /18/ /25/ /31/ January 1 to March 31, 2018 Page 59
60 Big Creek Research & Extension Team Quarterly Report Date ph Chloride Electrical conductivity 6/5/ /12/ /19/ /29/ /5/ /11/ /19/ /26/ /3/ /9/ /16/ /24/ /31/ /13/ /21/ /28/ /5/ /12/ /18/ /23/ /1/ /9/ /15/ /30/ Samples collected and analyzed since the last quarterly report 12/13/ /18/ January 1 to March 31, 2018 Page 60
61 Big Creek Research & Extension Team Quarterly Report Date ph Chloride Electrical conductivity 1/4/ /18/ /30/ /14/ /22/ Trench 1 2/15/ /16/ /27/ /6/ /24/ /27/ /1/ Samples collected and analyzed since the last quarterly report 2/22/ Trench 2 2/15/ /1/ /27/ /6/ /24/ /27/ /1/ /5/ /15/ o samples collected since the last quarterly report January 1 to March 31, 2018 Page 61
62 Big Creek Research & Extension Team Quarterly Report Discharge at USGS Site Downstream of C&H Operation Discharge downstream of the C&H Farm (USGS station Big Creek near Mt. Judea, AR) is available at default&period=&begin_date= &end_date= &site_no= Figure 2. Discharge in Big Creek downstream of the C&H Farm for the period of monitoring; December 1, 2017 to April 1, January 1 to March 31, 2018 Page 62
63 Big Creek Research & Extension Team Quarterly Report Surface Runoff from Fields 1, 5a and 12 Field Site Description Surface runoff from Fields 1, 5a, and 12 has been collected after storm rainfall induced runoff. The catchment area for each flume located on Fields 1, 5a, and 12 is depicted in Figures 3, 4, and 5, respectively. The catchment area is that that which contributes runoff water to our monitoring site. Due to natural slope and elevation changes in all fields, the flumes do not collect runoff water from the entire field. Also depicted on Figures 3, 4, and 5, are buffers imposed by the C&H ADEQ permit for ponds, school, slope, and stream, where no slurry can be applied to Fields 1, 5a, and 12, respectively. On field 12, the owners have implemented a 100 ft buffer along the south neighboring Field 5. The field area, flume catchment area, and area to which slurry can be applied to Fields 1, 5a, and 12 is given in Table 9. Annual amounts and rates of commercial fertilizer (Field 5a) and slurry from the C&H operation (Fields 1 and 12) are given in Table 10. The slurry rates are obtained from ADEQ annual management reports for the and commercial fertilizer application from the landowner. Surface Runoff of utrient and Sediment The flow weighted event concentration of nutrients and sediment in surface runoff from each Field are detailed in Table 11, along with runoff volume. As noted earlier in the Methods section, all surface runoff samples are collected by ISCO autosamplers programed to initiate sample collection when a critical stage height is exceeded (see Table 2). Pacing of sample collection is subsequently programmed to a specific volume of flow, as detailed in Table 2. This standard operating procedure for ISCO autosamplers results in the collection of one flow weighted sample, which is subsequently analyzed. Based on flow weighted concentration and flow for each surface runoff event, the amount of nutrients and sediment based on flume catchment area are determined and presented in Table 12 in English units (i.e., lbs/acre) and in Table 13 in metric units (i.e., g/ha). There was no surface runoff as measured at the flume for Field 12 in The annual flow and mean annual flow weighted concentrations of P,, and sediment in runoff for 2014, 2015, 2016, and 2017 are given in Table 14. The annual loss of P,, and sediment in surface runoff from Fields 1, 5a, and 12 for 2014, 2015, 2016, and 2017 is given Table 15 in both English and metric units. Finally, the amount of P and applied to the flume catchment area of Fields 1, 5a, and 12, loss in runoff, and percent of applied lost in runoff for 2014, 2015, 2016, and 2017 are given in Table 16. Losses are dominated by high rainfall in 2015 than the other years of monitoring, which led to large runoff volumes (Table 16). January 1 to March 31, 2018 Page 63
64 Big Creek Research & Extension Team Quarterly Report Figure 3. Map of Field 1 showing catchment area for surface runoff flume and buffers where no slurry can be applied. January 1 to March 31, 2018 Page 64
65 Big Creek Research & Extension Team Quarterly Report Figure 4. Map of Field 5a showing catchment area for surface runoff flume and buffers where no slurry can be applied. January 1 to March 31, 2018 Page 65
66 Big Creek Research & Extension Team Quarterly Report Figure 5. Map of Field 12 showing catchment area for surface runoff flume and buffers where no slurry can be applied. January 1 to March 31, 2018 Page 66
67 Table 9. Area of Field 1, 5a, and 12 monitored for surface runoff, area of flume catchment, area of buffers where no slurry is applied, and area of flume receiving slurry. Site Site ID Field area Flume catchment area Buffer Flume catchment area minus buffer Flume catchment receiving slurry Area of flume catchment in designated field acres hectares acres hectares acres hectares acres hectares % acres hectares Field 1 BC Field 5a BC Field 12 BC Slurry has not been applied to Field 5a or the adjacent Field 5. January 1 to March 31, 2018 Page 67
68 Big Creek Research & Extension Team Quarterly Report Table 10. Slurry (i.e., Fields 1 and 12) and fertilizer (i.e., Fields 5a) application to the monitored fields for 2014 to Site Slurry applied, gals Field 1 46,000 48,000 78,000 60,000 Field 12 48,000 93, ,000 90,000 utrients applied in slurry, lbs/1000 gallons P P P P Field Field utrients applied to field, lbs/acre Field Field 5a Field utrients applied to flume catchment, lbs Field Field 5a Field utrients applied to field, kg/ha Field Field 5a Field utrients applied to flume catchment, kg Field Field 5a Field utrient applied as mineral fertilizer (i.e., 19%, 19% P 2 O 5, and 19% K 2 O) in early spring at a rate of 300 lbs/acre. January 1 to March 31, 2018 Page 68
69 Table 11. Flow and flow weighted concentration of phosphorus and nitrogen in each runoff event for Fields 1, 5a, and 12 in 2014, 2015, 2016, and Date Flow Flow Flow Flow P P Ammonia itrate Solids gal L gal/acre L/ha mg/l Field 1 4/4/ ,795 78,709 11, , /9/ ,956 60,393 9,066 84, /13/ ,420 58,365 8,761 81, /28/ ,600 66,616 10,000 93, /24/2014 1,440 5, , /27/ , ,623 23, , /25/2014 4,920 18,622 2,795 26, /14/ , , /25/2015 4,642 17,570 2,638 24, /8/ ,510 47,350 7,108 66, /11/ , ,265 30, , /18/ , , January 1 to March 31, 2018 Page 69
70 Date Flow Flow Flow Flow P P Ammonia itrate Solids 5/26/2015 6,010 22,748 3,415 31, /29/2015 6,133 23,214 3,485 32, /7/ ,060 75,927 11, , /13/2016 3,755 14,212 2,133 19, /27/ ,430 73,543 11, , /24/ ,120 79,939 12, , /27/ , ,321 18, , /1/ , ,569 28, , /6/ ,309 2,116, ,789 2,972, Field 5a 6/27/ ,630 78,085 2,154 20, /25/2014 2,000 7, , /13/ ,720 44,360 1,224 11, January 1 to March 31, 2018 Page 70
71 Date Flow Flow Flow Flow P P Ammonia itrate Solids 3/26/ , ,782 4,463 41, /11/2015 5,158, ,525, ,621 5,038, /7/ , ,135 16, , /31/ , ,045 14, , /10/ /24/ , ,525 4,870 45, /27/ , ,587 26, , /1/ ,570 1,444,242 39, , /6/ ,490 1,360,670 37, , Field 12 5/8/ ,630 51,590 16, , /11/ ,555 3,230,706 1,016,137 9,504, /1/ , /29/ , , January 1 to March 31, 2018 Page 71
72 Date Flow Flow Flow Flow P P Ammonia itrate Solids 7/6/15 7,000 26,495 8,333 77, /10/2016 2,496 9,445 2,971 27, /2/ , /10/ , /27/ , ,674 87, , /1/ , , ,333 2,519, /6/ , , ,583 1,146, Flow measurement by the flume on Field 5a was affected by Big Creek breeching its banks during the rainfall runoff event. January 1 to March 31, 2018 Page 72
73 Big Creek Research & Extension Team Quarterly Report Table 12. Loss of phosphorus and nitrogen in each runoff event as pounds per acre for Fields 1, 5a, and 12 in 2014, 2015, 2016, and Date P P Ammonia itrate Solids lbs/ac Field 1 4/4/ /9/ /13/ /28/ /24/ /27/ /25/ /14/ /25/ /8/ /11/ /18/ /26/ /29/ /7/ /13/ /27/ January 1 to March 31, 2018 Page 73
74 Big Creek Research & Extension Team Quarterly Report Date P P Ammonia itrate Solids 4/24/ /27/ /1/ /6/ Field 5a 6/27/ /25/ /13/ /26/ /11/ /7/ /31/ /10/ /24/ /27/ /1/ /6/ Field 12 5/8/ /11/ /1/ January 1 to March 31, 2018 Page 74
75 Big Creek Research & Extension Team Quarterly Report Date P P Ammonia itrate Solids 6/29/ /6/ /10/ /2/ /10/ /27/ /1/ /6/ January 1 to March 31, 2018 Page 75
76 Big Creek Research & Extension Team Quarterly Report Table 13. Loss of phosphorus and nitrogen in each runoff event as grams per hectare for Fields 1, 5a, and 12 in 2014, 2015, 2016, and Date P P Ammonia itrate Solids g/ha Field 1 4/4/ , /9/ , /13/ , /28/ , /24/ /27/ , /25/ /14/ /25/ , /8/ , /11/ , /18/ /26/ , /29/ /7/ /13/ , /27/ ,846.2 January 1 to March 31, 2018 Page 76
77 Big Creek Research & Extension Team Quarterly Report Date P P Ammonia itrate Solids 4/24/ , /27/ , /1/ , /6/2017 2, , , , , ,464.5 Field 5a 6/27/ /25/ /13/ /26/ , /11/2015 1, , , , ,612, /7/ , /31/ , /10/ /24/ /27/ , /1/ , /6/ ,511.0 Field 12 5/8/ , /11/2015 1, , , , ,829.2 January 1 to March 31, 2018 Page 77
78 Big Creek Research & Extension Team Quarterly Report Date P P Ammonia itrate Solids 6/1/ /29/ /6/ , /10/ , /2/ /10/ /27/ , /1/ , , /6/ , ,975.7 January 1 to March 31, 2018 Page 78
79 Table 14. Annual flow and flow weighted concentrations of phosphorus, nitrogen, and sediment in runoff for 2014, 2015, 2016, and Flow Flow Flow Flow P TP Ammonia itrate Solids gal L gal/acre L/ha mg/l Field , ,451 67, , , ,708 58, , ,755 14,212 2,133 19, ,789 2,584, ,948 3,628, Field 5a , ,015 3,587 33, ,357,063 20,276, ,335 5,231, , ,625 14, , ,039,108 3,933, ,494 1,014, Field D. 1.D..D..D ,765 3,310,986 1,041,387 9,741, January 1 to March 31, 2018 Page 79
80 Flow Flow Flow Flow P TP Ammonia itrate Solids ,888 10,931 3,438 32, ,100 1,525, ,881 4,488, o runoff occurred from Field 12, while the site was operational in January 1 to March 31, 2018 Page 80
81 Big Creek Research & Extension Team Quarterly Report Table 15. Annual loss of phosphorus and nitrogen in surface runoff from Fields 1, 5a, and 12 for 2014, 2015, 2016, and Date P P Ammonia itrate Solids lbs/ac Field Field 5a , Field D. 1.D..D..D..D..D g/ha Field , , , , , , , , ,052 January 1 to March 31, 2018 Page 81
82 Big Creek Research & Extension Team Quarterly Report Date P P Ammonia Field 5a itrate Solids , , , , , ,655, , , , ,700 Field D..D..D..D..D..D , , , , , , , , , ,434 1 o runoff occurred from Field 12, while the site was operational in January 1 to March 31, 2018 Page 82
83 Big Creek Research & Extension Team Quarterly Report Table 16. Amount of phosphorus and nitrogen applied to the flume catchment area of Fields 1, 5a, and 12, loss in runoff, and percent of applied lost in runoff for 2014, 2015, 2016, and Date Rain Applied Phosphorus Loss in runoff Percent applied lost in runoff Applied itrogen Loss in runoff Percent applied lost in runoff inches lbs % lbs % Field Field 5a Field January 1 to March 31, 2018 Page 83
84 Big Creek Research & Extension Team Quarterly Report Temporal Trends in Phosphorus, itrogen, Bacteria, and Chloride in Big Creek Above and Below the C&H Farm The concentration of dissolved P, total P, nitrate, total, bacteria and chloride in Big Creek above and below the C&H Farm are presented in subsequent figures to show the season / temporal trends in measured concentrations (Figures 3, 4, 5, 6, 7, 8, and 9). Figure 6. P concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR. January 1 to March 31, 2018 Page 84
85 Figure 7. P concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR. January 1 to March 31, 2018 Page 85
86 Figure 8. itrate concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR. January 1 to March 31, 2018 Page 86
87 Figure 9. concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR. January 1 to March 31, 2018 Page 87
88 Figure 10. E. coli numbers at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR. January 1 to March 31, 2018 Page 88
89 Figure 11. coliform numbers at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR. January 1 to March 31, 2018 Page 89
90 Figure 12. Chloride concentration at the Big Creek monitoring site up and downstream of the C&H Farm, ewton County, AR. January 1 to March 31, 2018 Page 90
91 Big Creek Research & Extension Team Quarterly Report utrient Concentrations in Well, Ephemeral Stream, and Trenches The concentration of P,, sediment, E. coli, coliform, chloride, and electrical conductivity from well, ephemeral stream, and trench sites with time since sampling began are presented in Figures 13 to 20. Any seasonal or annual trends will be statistically evaluated when a 5 year period of record has been obtained. Analysis of seasonal or annual trends over a shorter period can lead to incorrect interpretations. January 1 to March 31, 2018 Page 91
92 Big Creek Research & Extension Team Quarterly Report HOUSE WELL W1 Figure 13. Phosphorus and nitrogen concentrations in house well samples over the period of monitoring. January 1 to March 31, 2018 Page 92
93 Big Creek Research & Extension Team Quarterly Report HOUSE WELL W2 Figure 14. E. coli, total coliform, electrical conductivity, chloride concentrations in house well samples over the period of monitoring. January 1 to March 31, 2018 Page 93
94 Big Creek Research & Extension Team Quarterly Report EPHEMERAL STREAM BC4 Figure 15. Phosphorus and nitrogen concentrations in the ephemeral stream over the period of monitoring. January 1 to March 31, 2018 Page 94
95 Big Creek Research & Extension Team Quarterly Report EPHEMERAL STREAM BC4 Figure 16. E. coli, total coliform, electrical conductivity, chloride concentrations in the ephemeral stream over the period of monitoring. January 1 to March 31, 2018 Page 95
96 Big Creek Research & Extension Team Quarterly Report TRECH 1 T1 Figure 17. Phosphorus and nitrogen concentrations in Trench 1 over the period of monitoring. January 1 to March 31, 2018 Page 96
97 Big Creek Research & Extension Team Quarterly Report TRECH 1 T1 Figure 18. E. coli, total coliform, electrical conductivity, chloride concentrations in Trench 1 over the period of monitoring. January 1 to March 31, 2018 Page 97
98 Big Creek Research & Extension Team Quarterly Report TRECH 2 T2 Figure 19. Phosphorus and nitrogen concentrations in Trench 2 over the period of monitoring. January 1 to March 31, 2018 Page 98
99 Big Creek Research & Extension Team Quarterly Report TRECH 2 T2 Figure 20. E. coli, total coliform, electrical conductivity, chloride concentrations in Trench 2 over the period of monitoring. January 1 to March 31, 2018 Page 99
100 Big Creek Research & Extension Team Quarterly Report Big Creek Oxygen oxygen is an important indicator of aquatic ecosystem health and as such is a key waterquality parameter measured in many water studies and is a guiding regulatory standard for evaluating and protecting stream health. Arkansas Pollution Control and Ecology Commission Regulation establishes the standards for surface water dissolved oxygen values. Regulation Establishing Oxygen Standards for Surface Waters of the State of Arkansas Arkansas Pollution Control and Ecology Commission Regulation o. 2, as amended by Pollution Control and Ecology Commission # from Arkansas Department of Environmental Quality (ADEQ), 2018, pages of Assessment methodology: For the Preparation of the 2016 Integrated Water Quality Monitoring and Assessment Report pursuant to the Clean Water Act Sections 303(d) and 305(b). Final Draft pages total. Available at draft 2018 am_10oct2017 (2).pdf provides the specific dissolved oxygen standard for surface waters in the State. oxygen levels are specified in water quality standards. In the 2 to 5 mg/l range, most fish and aquatic life will survive, but will not thrive. At less than 2 mg/l, mortality begins. The following dissolved oxygen standards are applicable for rivers and streams (Table 7). Table 17. oxygen criteria for area rivers and streams Watershed area, mi 2 Ozark Highlands Boston Mountains Primary 1 Critical 2 Primary 1 Critical 2 mg/l <10 mi 2 watershed to 100 mi 2 watershed 6 5 one one >100 mi 2 watershed Primary season is defined as when water temperatures are at or below 22 C. 2. Critical season is defined as when water temperatures are greater 22 C. In streams with watersheds of less than 10 mi 2, it is assumed that insufficient water exists to support aquatic life during the critical season. During this time, a dissolved oxygen standard of 2 mg/l will apply to prevent nuisance conditions. However, field verification is required in January 1 to March 31, 2018 Page 100
101 Big Creek Research & Extension Team Quarterly Report areas suspected of having significant groundwater flows or enduring pools, which may support unique aquatic biota. In such waters, the critical season standard for the next size category of stream shall apply. All streams with watersheds of less than 10 mi 2 are expected to support aquatic life during the primary season when stream flows, including discharges, equal or exceed 1 cubic foot per second (cfs). However, when site verification indicates that aquatic life exists at flows below 1 cfs, such aquatic biota will be protected by the primary standard (refer to the State of Arkansas Continuing Planning Process for field verification requirements). Also, in these streams with watersheds of less than 10 mi 2, where waste discharges are 1 cfs or more, they are assumed to provide sufficient water to support aquatic life and, therefore, must meet the dissolved oxygen standards of the next size category of streams. For purposes of determining effluent discharge limits, the following conditions apply: A. The primary season dissolved oxygen standard is to be met at a water temperature of 22 C (71.5 F) and at the minimum stream flow for that season. At water temperatures of 10 C (50 F), the dissolved oxygen standard is 6.5 mg/l. B. During March, April and May, when background stream flows are 15 cfs or higher, the dissolved oxygen standard is 6.5 mg/l in all areas except the Delta Ecoregion, where the primary season dissolved oxygen standard will remain at 5 mg/l. C. The critical season dissolved oxygen standard is to be met at maximum allowable water temperatures and at Q7 10 flows. However, when water temperatures exceed 22 C (71.6 F), a 1 mg/l diurnal depression will be allowed below the applicable critical standard for no more than 8 hours during any 24 hour period. Field Determination and Methodology oxygen and temperature are determined in situ using a luminescent dissolved oxygen sensor integrated to a Hydrolab HL4 sonde 1. In situ measurement entails no holding time for samples, and the act of collecting a sample can change the oxygen level, a direct probe reading is often the preferred method. The probe is air calibrated at monthly intervals when data are downloaded from the sonde data logger. Data quality are assured through recording calibration accuracy and conducting a field duplicate to determine precision. See U.S. EPA Field Measurement of Oxygen for details 07/documents/field_do_measurement106_af.r4.pdf. Sonde readings were recorded every hour for the time they were deployed in Big Creek. 1 Mention of trade names does not imply endorsement by the Division of Agriculture, University of Arkansas Systems. Information on the Hydrolab HL4 sonde is available at quality 2/hydrolab hl4 multiparameter sonde 54/ January 1 to March 31, 2018 Page 101
102 Big Creek Research & Extension Team Quarterly Report Big Creek Oxygen The following tables and Figures detail the dissolved oxygen concentration in Big Creek upstream of the C&H October 22 to ovember 13, 2014 and downstream of the from September 9 to ovember 11, 2014, April 10 to October 16, 2015, and May 16 to December 11, Measurements upstream of the were not taken after 2014 due to the sonde probe being dislodged and lost during a large storm even in the spring of It was not replaced until early 2018, when a secure site on the low water bridge at the upstream site on Big Creek was made available. Measurements at the downstream site were not taken in 2016, again due to loss of the sonde at that site during an early spring storm event. Loss of the two sonde units are indicative of the significant amount of scouring and stream bed modification that can take place in large storm events. Also, measurements were not recorded between September 16 and October 17, 2017 due the sonde malfunctioning. Diurnal and seasonal fluctuations in dissolved oxygen concentrations are apparent from measurements in Big Creek. See Figures 1 through 8. The diurnal fluctuations in dissolved oxygen concentration are typically a function of photosynthesis during daylight hours (which releases oxygen); removal of dissolved oxygen by microbial respiration (satisfying microbial and chemical oxygen demands, either in the water column or through interaction with the bed sediments); and exchange of oxygen at the water surface (i.e., reaeration) (O Connor and Di Toro, ; Williams et al., ). The diurnal and seasonal fluctuations of dissolved oxygen concentration are clearly dependent on many factors (Williams et al., ). The influence of water column temperature on oxygen solubility can be eliminated by converting dissolved oxygen concentration in mg/l to % saturation. See Figures 9 to 12. Additional breaks in dissolved oxygen concentrations were determined with flow less and greater than 15 cfs for monitoring in 2014, 2015, and 2017 and given in Table 2. oxygen was measured at both upstream and downstream from October 22 to ovember 13, 2014 and is presented in Figures 13 to 18. Finally, dissolved oxygen at the USGS Carver gaging station is presented in Figure 19 for June 3, 2014 to May 1, The information on dissolved oxygen in Big Creek downstream of the C&H Farm at USGS Big Creek near Mt. Judea given here is made available to ADEQ and on the BCRET website. Interpretation of dissolved oxygen concentrations in terms of water quality standards is deferred to ADEQ. 2 O Connor, D.J., and D.M. Di Toro Photosynthesis and oxygen balance in streams. J. Sanit. Eng. Div. ASCE, 98: Williams, R.J., C. White, M.L. Harrow, and C. eal Temporal and small scale spatial variations of dissolved oxygen in the River Thames, Pang and Kennet, UK. Sci.. Environ. 251/252: January 1 to March 31, 2018 Page 102
103 Metric Table 18. Big Creek Oxygen concentrations. Upstream 1 Downstream 2 10/22/ /13/ /22/ /13/2014 9/9/ /13/2014 4/8/ /16/2015 5/16/ /11/2017 9/9/ /11/2017 Average Minimum Maximum Median Observations 525 1,557 4,524 4,093 10, Watershed area is miles Watershed area is miles 2. January 1 to March 31, 2018 Page 103
104 Table 19. Big Creek Oxygen concentrations downstream of C&H, as a function of stream temperature. Metric 9/9/ /13/2014 4/10/ /16/2015 5/16/ /11/2017 9/9/ /11/2017 Temperature oxygen Temperature oxygen Temperature oxygen Temperature oxygen C mg/l C mg/l C mg/l C mg/l All observed water temperatures Average Minimum Maximum Median Observations 1,557 4,525 4,093 10,175 Observations <6 mg/l DO Observations <6 mg/l DO, % Water temperature > 22 C Average Minimum Maximum Median Observations ,090 January 1 to March 31, 2018 Page 104
105 Metric 9/9/ /13/2014 4/10/ /16/2015 5/16/ /11/2017 9/9/ /11/2017 Observations <6 mg/l DO Flow oxygen Flow oxygen Flow oxygen Flow oxygen cfs mg/l cfs mg/l cfs mg/l cfs mg/l Stream flow <15 cfs Average Minimum Maximum Median Observations Stream flow >15 cfs Average Minimum Maximum Median Observations January 1 to March 31, 2018 Page 105
106 Figure 21. Flow, water temperature, and dissolved oxygen concentration downstream of the C&H Farm between September 9, 2014 and December 11, January 1 to March 31, 2018 Page 106
107 Figure 22. Flow and dissolved oxygen concentration downstream of the C&H Farm between September 9 and ovember 13, January 1 to March 31, 2018 Page 107
108 Figure 23. Water temperature and dissolved oxygen concentration downstream of the C&H Farm between September 9 and ovember 13, January 1 to March 31, 2018 Page 108
109 Figure 24. Flow and dissolved oxygen concentration downstream of the C&H Farm between S April 10 and October 16, January 1 to March 31, 2018 Page 109
110 Figure 25. Water temperature and dissolved oxygen concentration downstream of the C&H Farm between April 10 and October 16, January 1 to March 31, 2018 Page 110
111 Figure 26. Flow and dissolved oxygen concentration downstream of the C&H Farm between May 16 and December 11, January 1 to March 31, 2018 Page 111
112 Figure 27. Water temperature and dissolved oxygen concentration downstream of the C&H Farm between May 16 and December 11, January 1 to March 31, 2018 Page 112
113 Figure 28. Water temperature and dissolved oxygen concentration upstream and downstream of the C&H Farm between October 22 and ovember 13, January 1 to March 31, 2018 Page 113
114 Figure 29. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between September 9 and December 11, January 1 to March 31, 2018 Page 114
115 Figure 30. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between September 9 and ovember 13, January 1 to March 31, 2018 Page 115
116 Figure 31. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between April 10 and October 16, January 1 to March 31, 2018 Page 116
117 Figure 32. Water temperature and dissolved oxygen saturation downstream of the C&H Farm between May 15 and December 11, January 1 to March 31, 2018 Page 117
118 Figure 33. Water temperature and dissolved oxygen saturation upstream and downstream of the C&H Farm between October 22 and ovember 13, January 1 to March 31, 2018 Page 118
119 Figure 34. oxygen concentration and % saturation downstream of the C&H Farm between 2014 and January 1 to March 31, 2018 Page 119
120 Figure 35. oxygen concentration and % saturation downstream of the C&H Farm between September 9 and ovember 13, January 1 to March 31, 2018 Page 120
121 Figure 36. oxygen concentration and % saturation downstream of the C&H Farm between April 10 and October 16, January 1 to March 31, 2018 Page 121
122 Figure 37. oxygen concentration and % saturation downstream of the C&H Farm between May 16 and December 11, January 1 to March 31, 2018 Page 122
123 Figure 38. oxygen concentration and % saturation upstream and downstream of the C&H Farm between October 22, 2014 and ovember 13, January 1 to March 31, 2018 Page 123
124 Figure 39. oxygen concentration at the USGS Carver site (USGA: ) for June 3 to May 1, January 1 to March 31, 2018 Page 124
125 Big Creek Research & Extension Team Quarterly Report The University of Arkansas System Division of Agriculture offers its programs to all eligible persons regardless of race, color, sex, gender identity, sexual orientation, national origin, religion, age, disability, marital or veteran status, genetic information, or any other legally protected status, and is an Affirmative Action/Equal Opportunity Employer. January 1 to March 31, 2018 Page 125
MONITORING THE SUSTAINABLE MANAGEMENT OF NUTRIENTS ON C&H FARM IN BIG CREEK WATERSHED
 Big Creek Research & Extension Team Quarterly Report Big Creek Research and Extension Team University of Arkansas System Division of Agriculture Quarterly Report July 1 to September 30, 2017 MOITORIG THE
Big Creek Research & Extension Team Quarterly Report Big Creek Research and Extension Team University of Arkansas System Division of Agriculture Quarterly Report July 1 to September 30, 2017 MOITORIG THE
Arkansas Water Resources Center
 Arkansas Water Resources Center WATER SAMPLING, ANALYSIS AND ANNUAL LOAD DETERMINATIONS FOR TSS, NITROGEN AND PHOSPHORUS AT THE L ANGUILLE RIVER NEAR PALESTINE Submitted to the Arkansas Soil and Water
Arkansas Water Resources Center WATER SAMPLING, ANALYSIS AND ANNUAL LOAD DETERMINATIONS FOR TSS, NITROGEN AND PHOSPHORUS AT THE L ANGUILLE RIVER NEAR PALESTINE Submitted to the Arkansas Soil and Water
How to Collect Your Water Sample and Interpret the Results for the Poultry Analytical Package
 How to Collect Your Water Sample and Interpret the Results for the Poultry Analytical Package Bradley J. Austin, Josh B. Payne, Susan E. Watkins, Mike Daniels, and Brian E. Haggard Arkansas Water Resources
How to Collect Your Water Sample and Interpret the Results for the Poultry Analytical Package Bradley J. Austin, Josh B. Payne, Susan E. Watkins, Mike Daniels, and Brian E. Haggard Arkansas Water Resources
Sampling Surface and Ground Water Sources Compiled by Deanne Meyer and Patricia Price Department of Animal Science, UC Davis, September 2012
 CDQAP North Coast Reference Binder TAB 15.1 September, 2012 Sampling Surface and Ground Water Sources Compiled by Deanne Meyer and Patricia Price Department of Animal Science, UC Davis, September 2012
CDQAP North Coast Reference Binder TAB 15.1 September, 2012 Sampling Surface and Ground Water Sources Compiled by Deanne Meyer and Patricia Price Department of Animal Science, UC Davis, September 2012
Long-Term Volunteer Lake Monitoring in the Upper Woonasquatucket Watershed
 Long-Term Volunteer Lake Monitoring in the Upper Woonasquatucket Watershed Linda Green URI Watershed Watch 1-87-95, lgreen@uri.edu WATER QUALITY IS A REFLECTION OF THE ACTIVITIES IN THE LANDS AND WATERS
Long-Term Volunteer Lake Monitoring in the Upper Woonasquatucket Watershed Linda Green URI Watershed Watch 1-87-95, lgreen@uri.edu WATER QUALITY IS A REFLECTION OF THE ACTIVITIES IN THE LANDS AND WATERS
MARK CREEK DEMONSTRATION PROJECT
 MARK CREEK DEMONSTRATION PROJECT OCC Tasks 20 and 21 FY 1990 319(h) Task 210 EPA Grant # C9-006704-90-0 Submitted by: Oklahoma Conservation Commission Water Quality Division 413 NW 12 th Oklahoma City,
MARK CREEK DEMONSTRATION PROJECT OCC Tasks 20 and 21 FY 1990 319(h) Task 210 EPA Grant # C9-006704-90-0 Submitted by: Oklahoma Conservation Commission Water Quality Division 413 NW 12 th Oklahoma City,
DEMONSTRATING AND MONITORING THE SUSTAINABLE MANAGEMENT OF NUTRIENTS ON C&H FARM IN BIG CREEK WATERSHED
 Big Creek Research and Extension Team University of Arkansas System Division of Agriculture - October 2013 to December 2013 DEMONSTRATING AND MONITORING THE SUSTAINABLE MANAGEMENT OF NUTRIENTS ON C&H FARM
Big Creek Research and Extension Team University of Arkansas System Division of Agriculture - October 2013 to December 2013 DEMONSTRATING AND MONITORING THE SUSTAINABLE MANAGEMENT OF NUTRIENTS ON C&H FARM
How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package
 How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package Bradley J. Austin, Dirk Philipp, Mike Daniels, and Brian E. Haggard Arkansas Water Resources Center University
How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package Bradley J. Austin, Dirk Philipp, Mike Daniels, and Brian E. Haggard Arkansas Water Resources Center University
Clyde Mine Discharge/Tenmile Creek Water Quality Final Report
 Clyde Mine Discharge/Tenmile Creek Water Quality Final Report November 01, 2016 Background In follow-up to the Pennsylvania Department of Environmental Protection s (DEP) December 15, 2015, Tenmile Creek
Clyde Mine Discharge/Tenmile Creek Water Quality Final Report November 01, 2016 Background In follow-up to the Pennsylvania Department of Environmental Protection s (DEP) December 15, 2015, Tenmile Creek
CONTINUOUS TURBIDITY MONITORING AND REGRESSION ANALYSIS TO ESTIMATE TOTAL SUSPENDED SOLIDS AND FECAL COLIFORM BACTERIA LOADS IN REAL TIME
 CONTINUOUS TURBIDITY MONITORING AND REGRESSION ANALYSIS TO ESTIMATE TOTAL SUSPENDED SOLIDS AND FECAL COLIFORM BACTERIA LOADS IN REAL TIME By Victoria G. Christensen, Andrew C. Ziegler, and Xiaodong Jian
CONTINUOUS TURBIDITY MONITORING AND REGRESSION ANALYSIS TO ESTIMATE TOTAL SUSPENDED SOLIDS AND FECAL COLIFORM BACTERIA LOADS IN REAL TIME By Victoria G. Christensen, Andrew C. Ziegler, and Xiaodong Jian
How to Collect Your Water Sample and Interpret the Results for the Fish Pond Analytical Package
 How to Collect Your Water Sample and Interpret the Results for the Fish Pond Analytical Package Bradley, J. Austin, Amit Sinha, Nathan Stone, W. Reed Green, Mike Daniels and Brian E. Haggard Arkansas Water
How to Collect Your Water Sample and Interpret the Results for the Fish Pond Analytical Package Bradley, J. Austin, Amit Sinha, Nathan Stone, W. Reed Green, Mike Daniels and Brian E. Haggard Arkansas Water
Hydrology and Water Quality. Water. Water 9/13/2016. Molecular Water a great solvent. Molecular Water
 Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Re-plumbing Roadside Ditch Networks
 Re-plumbing Roadside Ditch Networks Ditches Improving management to reduce flooding, water pollution, and in-stream erosion and habitat degradation Rebecca Schneider Dept. Natural Resources Cornell University,
Re-plumbing Roadside Ditch Networks Ditches Improving management to reduce flooding, water pollution, and in-stream erosion and habitat degradation Rebecca Schneider Dept. Natural Resources Cornell University,
SOIL P-INDEXES: MINIMIZING PHOSPHORUS LOSS. D. Beegle, J. Weld, P. Kleinman, A. Collick, T. Veith, Penn State & USDA-ARS
 SOIL P-INDEXES: MINIMIZING PHOSPHORUS LOSS D Beegle, J Weld, P Kleinman, A Collick, T Veith, Penn State & USDA-ARS Part of a larger national effort to improve the P Index and P Management Chesapeake Bay
SOIL P-INDEXES: MINIMIZING PHOSPHORUS LOSS D Beegle, J Weld, P Kleinman, A Collick, T Veith, Penn State & USDA-ARS Part of a larger national effort to improve the P Index and P Management Chesapeake Bay
Clean Water Legacy Project Final Report
 Clean Water Legacy Project Final Report Project Title: Evaluation of alternative surfacewater monitoring protocols for use in agriculture TMDL load allocation and BMP evaluation. Submission Date: August
Clean Water Legacy Project Final Report Project Title: Evaluation of alternative surfacewater monitoring protocols for use in agriculture TMDL load allocation and BMP evaluation. Submission Date: August
Water Resources on PEI: an overview and brief discussion of challenges
 Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
6. Pollutant Sources in
 6. Pollutant Sources in 45 6. Pollutant Sources in the Plum Creek Watershed The LDC analysis for Plum Creek indicates that both point and nonpoint sources contribute pollutants in the watershed. Identifying
6. Pollutant Sources in 45 6. Pollutant Sources in the Plum Creek Watershed The LDC analysis for Plum Creek indicates that both point and nonpoint sources contribute pollutants in the watershed. Identifying
Jordan River Total Maximum Daily Load Study. Presented By: James Harris Utah Division of Water Quality
 Jordan River Total Maximum Daily Load Study Presented By: James Harris Utah Division of Water Quality Jordan River Watershed TMDL Study Area No Introduction Necessary A Few Things to Point Out... Lower
Jordan River Total Maximum Daily Load Study Presented By: James Harris Utah Division of Water Quality Jordan River Watershed TMDL Study Area No Introduction Necessary A Few Things to Point Out... Lower
Regional Watershed Planning. Calumet Summit 2010: A Call to Connect Calumet Conference Center April 27, 2010
 Regional Watershed Planning Calumet Summit 2010: A Call to Connect Calumet Conference Center April 27, 2010 Basins The Calumet Region Watershed Communities Land Cover- 2006 Impervious Cover 303d Listed
Regional Watershed Planning Calumet Summit 2010: A Call to Connect Calumet Conference Center April 27, 2010 Basins The Calumet Region Watershed Communities Land Cover- 2006 Impervious Cover 303d Listed
Eutrophication: Tracing Nutrient Pollution Back to Penns Creek
 Eutrophication: Tracing Nutrient Pollution Back to Penns Creek Nutrients are substances that life depends on for growth and reproduction. However, when these nutrients enter our planets waterways in excess,
Eutrophication: Tracing Nutrient Pollution Back to Penns Creek Nutrients are substances that life depends on for growth and reproduction. However, when these nutrients enter our planets waterways in excess,
Grants Pass Water Quality Monitoring
 Grants Pass Water Quality Monitoring 2003-2005 Rogue Valley Council of Governments April 2005 Rogue Valley Council of Governments Natural Resources Department 155 North First Street Central Point, Oregon
Grants Pass Water Quality Monitoring 2003-2005 Rogue Valley Council of Governments April 2005 Rogue Valley Council of Governments Natural Resources Department 155 North First Street Central Point, Oregon
SUSQUEHANNA RIVER MONITORING:
 SUSQUEHANNA RIVER MONITORING: Monitoring the fecal coliform bacteria and water quality in the Upper Susquehanna River, summer 2002 Selinda Schlierman 1 ABSTRACT As part of a long-term, ongoing study by
SUSQUEHANNA RIVER MONITORING: Monitoring the fecal coliform bacteria and water quality in the Upper Susquehanna River, summer 2002 Selinda Schlierman 1 ABSTRACT As part of a long-term, ongoing study by
WATERSHED. Maitland Valley. Report Card 201
 Maitland Valley WATERSHED Report Card 201 The Maitland Valley Conservation Authority has prepared this report card as a summary on the state of our forests, wetlands, surface water, and ground water resources.
Maitland Valley WATERSHED Report Card 201 The Maitland Valley Conservation Authority has prepared this report card as a summary on the state of our forests, wetlands, surface water, and ground water resources.
Winter Manure Application
 Winter Manure Application Developing Best Management Practices to Minimize the Water Quality Impacts for Winter Manure Application Nathan Brandenburg, Graduate Student, Agric. Biosyst. Engrg. SDSU Jeppe
Winter Manure Application Developing Best Management Practices to Minimize the Water Quality Impacts for Winter Manure Application Nathan Brandenburg, Graduate Student, Agric. Biosyst. Engrg. SDSU Jeppe
Saturated Buffer. Subsurface Drainage PURPOSE N REDUCTION LOCATION COST BARRIERS
 Saturated Buffer To intercept tile drainage prior to discharging to surface waters and redistribute the water laterally in the soil profile of the streamside buffer. Subsurface drainage, also known as
Saturated Buffer To intercept tile drainage prior to discharging to surface waters and redistribute the water laterally in the soil profile of the streamside buffer. Subsurface drainage, also known as
Part B: Phosphorus Loss Potential due to Management Practices and P Source Characteristics
 not suffer from such impacts. Surface waters having high water quality may require the implementation of policies and management practices to protect them from deterioration due to excess inputs of nutrients.
not suffer from such impacts. Surface waters having high water quality may require the implementation of policies and management practices to protect them from deterioration due to excess inputs of nutrients.
MURPHY DRAIN CATCHMENT
 The RVCA produces individual reports for 16 catchments in the Lower Rideau subwatershed. Using data collected and analysed by the RVCA through its watershed monitoring and land cover classification programs,
The RVCA produces individual reports for 16 catchments in the Lower Rideau subwatershed. Using data collected and analysed by the RVCA through its watershed monitoring and land cover classification programs,
Past experience in Arkansas Sally Entrekin University of Central Arkansas
 Past experience in Arkansas Sally Entrekin University of Central Arkansas Collaborators Funding through Arkansas Game and Fish Commission State Wildlife Grant # 223017 & 223023 University of Central Arkansas,
Past experience in Arkansas Sally Entrekin University of Central Arkansas Collaborators Funding through Arkansas Game and Fish Commission State Wildlife Grant # 223017 & 223023 University of Central Arkansas,
Strategies for nitrate reduction: The Cedar River Case Study
 2010 Integrated Crop Management Conference - Iowa State University 195 Strategies for nitrate : The Cedar River Case Study Matthew J. Helmers, associate professor, Agricultural and Biosystems Engineering,
2010 Integrated Crop Management Conference - Iowa State University 195 Strategies for nitrate : The Cedar River Case Study Matthew J. Helmers, associate professor, Agricultural and Biosystems Engineering,
EVALUATION OF METAL TRANSPORT INTO AND OUT OF TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, APRIL 1994 THROUGH MARCH 1995
 EVALUATION OF METAL TRANSPORT INTO AND OUT OF TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, APRIL 1994 THROUGH MARCH 1995 By Sheryl A. Ferguson U.S. Geological Survey, WRD 201 W. 8th Street, Suite 200 Pueblo,
EVALUATION OF METAL TRANSPORT INTO AND OUT OF TERRACE RESERVOIR, CONEJOS COUNTY, COLORADO, APRIL 1994 THROUGH MARCH 1995 By Sheryl A. Ferguson U.S. Geological Survey, WRD 201 W. 8th Street, Suite 200 Pueblo,
USING MICROBIAL SOURCE TRACKING TO IDENTIFY FECAL CONTAMINATION SOURCES IN BOZEMAN CREEK BOZEMAN, MONTANA
 USING MICROBIAL SOURCE TRACKING TO IDENTIFY FECAL CONTAMINATION SOURCES IN BOZEMAN CREEK BOZEMAN, MONTANA Prepared by: Elizabeth Wilson, Big Sky Watershed Corps Member Gallatin Local Water Quality District
USING MICROBIAL SOURCE TRACKING TO IDENTIFY FECAL CONTAMINATION SOURCES IN BOZEMAN CREEK BOZEMAN, MONTANA Prepared by: Elizabeth Wilson, Big Sky Watershed Corps Member Gallatin Local Water Quality District
Read: Case Study: America s First River : A Success Story Summarize the story of the Hudson River and PCB s:
 Botkin & Keller: Environmental Science: Earth as a Living Planet- 8th Ed. APES- Chapter #19- Water Pollution and Treatment- Guided Reading Name: Brandon Tran Learning Objectives: Degradation of our surface-water
Botkin & Keller: Environmental Science: Earth as a Living Planet- 8th Ed. APES- Chapter #19- Water Pollution and Treatment- Guided Reading Name: Brandon Tran Learning Objectives: Degradation of our surface-water
SSO 700 Integrated Watershed Action Plan: Development of a Water Quality Sampling Program
 SSO 700 Integrated Watershed Action Plan: Development of a Water Quality Sampling Program OWEA June 29, 2016 Presented by Kathleen A. Bollmer, P.E. CH2M Agenda Project Overview/Goals Task Background Task
SSO 700 Integrated Watershed Action Plan: Development of a Water Quality Sampling Program OWEA June 29, 2016 Presented by Kathleen A. Bollmer, P.E. CH2M Agenda Project Overview/Goals Task Background Task
fcgov.com/water-quality Water Quality Update Summer 2017 Monitoring and Protecting Our Water Sources
 Water Quality Update Summer 217 Monitoring and Protecting Our Water Sources SOURCE WATER MONITORING The Upper Cache la Poudre (UCLP) Watershed Collaborative Monitoring Program was established in 28 between
Water Quality Update Summer 217 Monitoring and Protecting Our Water Sources SOURCE WATER MONITORING The Upper Cache la Poudre (UCLP) Watershed Collaborative Monitoring Program was established in 28 between
GULF OF MEXICO - SEGMENT 2501
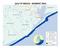 GULF OF MEXICO - SEGMENT 2501 GULF OF MEXICO - SEGMENT 2501 LAND COVER BACTERIA CHLOROPHYLL A Impairment Concern No Impairments or Concerns GULF OF MEXICO - SEGMENT 2501 OTHER IMPAIRMENTS Bays & Estuaries
GULF OF MEXICO - SEGMENT 2501 GULF OF MEXICO - SEGMENT 2501 LAND COVER BACTERIA CHLOROPHYLL A Impairment Concern No Impairments or Concerns GULF OF MEXICO - SEGMENT 2501 OTHER IMPAIRMENTS Bays & Estuaries
What s In your Watershed Reeder Creek
 What s In your Watershed Reeder Creek Stephen Penningroth, Ph.D. Executive Director, Community Science Institute, Ithaca, NY Three Brothers Winery, Geneva, NY October 29, 2015 Sponsored by the Seneca Lake
What s In your Watershed Reeder Creek Stephen Penningroth, Ph.D. Executive Director, Community Science Institute, Ithaca, NY Three Brothers Winery, Geneva, NY October 29, 2015 Sponsored by the Seneca Lake
(1)(A)Inventory of the following existing natural resources on the USFSP Campus or within the context area adjacent to the University.
 13. 6C-21.208 CONSERVATION ELEMENT Information Sources George F. Young, Interview notes, June 2002 Sasaki Associates, Inc., interview notes, April, 2000 Interviews between DRMP Staff and USF Staff, May,
13. 6C-21.208 CONSERVATION ELEMENT Information Sources George F. Young, Interview notes, June 2002 Sasaki Associates, Inc., interview notes, April, 2000 Interviews between DRMP Staff and USF Staff, May,
Delaware River Basin Commission
 Delaware River Basin Commission Special Protection Waters: 2009-2011 Lower Delaware Measurable Change Assessment Delaware Watershed Research Conference 2017 Elaine Panuccio, Water Resource Scientist November
Delaware River Basin Commission Special Protection Waters: 2009-2011 Lower Delaware Measurable Change Assessment Delaware Watershed Research Conference 2017 Elaine Panuccio, Water Resource Scientist November
Pembina River Watershed Water Quality Report
 Pembina River Watershed Water Quality Report January 29 Prepared by: Manitoba Water Stewardship 16-123 Main St. Winnipeg, MB. Canada State of the Watershed Report 5OA and 5OB Pembina River Watershed -
Pembina River Watershed Water Quality Report January 29 Prepared by: Manitoba Water Stewardship 16-123 Main St. Winnipeg, MB. Canada State of the Watershed Report 5OA and 5OB Pembina River Watershed -
Water Quality Conditions & Management on Rangelands
 Water Quality Conditions & Management on Rangelands Ken Tate, Leslie Roche, and Rob Atwill UC Davis and UC Cooperative Extension Grazing Workshop Mariposa County UCCE March 2014 CA Rangelands Safe Water
Water Quality Conditions & Management on Rangelands Ken Tate, Leslie Roche, and Rob Atwill UC Davis and UC Cooperative Extension Grazing Workshop Mariposa County UCCE March 2014 CA Rangelands Safe Water
Kapil Arora, Carl Pederson, Dr. Matt Helmers, and Dr. Ramesh Kanwar. DATE SUBMITTED: October 23, INDUSTRY SUMMARY
 TITLE: Evaluating Nutrient (nitrogen and ortho-phosphate) Export with Subsurface Drainage Water from Spring Applied Swine Manure to Soybean Planted Micro-watersheds - NPB #12-117 INVESTIGATORS: INSTITUTION:
TITLE: Evaluating Nutrient (nitrogen and ortho-phosphate) Export with Subsurface Drainage Water from Spring Applied Swine Manure to Soybean Planted Micro-watersheds - NPB #12-117 INVESTIGATORS: INSTITUTION:
Operations Report. September 10, 2015
 Operations Report September 1, 215 Plant Loading and Operations Summary August 215 Biochemical Oxygen Demand, 5-Day (Avg. 2,151 lb./day) Total Suspended Solids (Avg. 33,197 lb./day) Average Daily Flow
Operations Report September 1, 215 Plant Loading and Operations Summary August 215 Biochemical Oxygen Demand, 5-Day (Avg. 2,151 lb./day) Total Suspended Solids (Avg. 33,197 lb./day) Average Daily Flow
Nutrient Management in. A presentation to the West Metro Water Alliance
 Nutrient Management in Developing and Agricultural Areas A presentation to the West Metro Water Alliance Chris Meehan, P.E. May 25, 2011 Agenda Physical Improvements Planning Improvements How to get it
Nutrient Management in Developing and Agricultural Areas A presentation to the West Metro Water Alliance Chris Meehan, P.E. May 25, 2011 Agenda Physical Improvements Planning Improvements How to get it
Status and Trends of Water Quality in Wisconsin s Lakes, Streams, and Rivers
 Status and Trends of Water Quality in Wisconsin s Lakes, Streams, and Rivers Tim Asplund, Matt Diebel, Katie Hein, and Mike Shupryt Water Resources Program, WDNR AWRA Wisconsin Section Annual Meeting March
Status and Trends of Water Quality in Wisconsin s Lakes, Streams, and Rivers Tim Asplund, Matt Diebel, Katie Hein, and Mike Shupryt Water Resources Program, WDNR AWRA Wisconsin Section Annual Meeting March
Grand River Monitoring
 City of Grand Rapids Environmental Services Grand River Monitoring by Mike Lunn Environmental Services Department Manager Grand River Monitoring History 1968 Grand River Monitoring Network Since 1988 Data
City of Grand Rapids Environmental Services Grand River Monitoring by Mike Lunn Environmental Services Department Manager Grand River Monitoring History 1968 Grand River Monitoring Network Since 1988 Data
Nutrient Management Planning
 Nutrient Management Planning Gordon Fairchild, Ph.D., P.Ag. Soils specialist Eastern Canada Soil and Water Conservation Centre Improving Management Practices in the Livestock Sector March 4-5, 2003. Moncton,NB
Nutrient Management Planning Gordon Fairchild, Ph.D., P.Ag. Soils specialist Eastern Canada Soil and Water Conservation Centre Improving Management Practices in the Livestock Sector March 4-5, 2003. Moncton,NB
 Good Morning! Bruce Gilman Department of Environmental Conservation and Horticulture Finger Lakes Community College 3325 Marvin Sands Drive Canandaigua, New York 14424 585-785- 1255 gilmanba@flcc.edu Northern
Good Morning! Bruce Gilman Department of Environmental Conservation and Horticulture Finger Lakes Community College 3325 Marvin Sands Drive Canandaigua, New York 14424 585-785- 1255 gilmanba@flcc.edu Northern
The Next Generation of Stormwater Management and Site Design. Melanie R. Grigsby, P.E. Stormwater Resource Manager, City of Fort Myers
 The Next Generation of Stormwater Management and Site Design Melanie R. Grigsby, P.E. Stormwater Resource Manager, City of Fort Myers History of WMD Stormwater Rule Florida passed the first rule requiring
The Next Generation of Stormwater Management and Site Design Melanie R. Grigsby, P.E. Stormwater Resource Manager, City of Fort Myers History of WMD Stormwater Rule Florida passed the first rule requiring
Natural Resources & Environmental Stewardship
 Natural Resources & Environmental Stewardship Fundamentals of Nutrient Management Melissa L. Wilson Department of Environmental Science & Technology Ag Nutrient Management Program University of Maryland,
Natural Resources & Environmental Stewardship Fundamentals of Nutrient Management Melissa L. Wilson Department of Environmental Science & Technology Ag Nutrient Management Program University of Maryland,
Chronic Stream Pollution in a CAFO Rich Watershed in Duplin County, NC
 Chronic Stream Pollution in a CAFO Rich Watershed in Duplin County, NC Michael A. Mallin, Matthew R. McIver, Amanda Kahn Dickens and Anna R. Robuck Center for Marine Sciences University of North Carolina
Chronic Stream Pollution in a CAFO Rich Watershed in Duplin County, NC Michael A. Mallin, Matthew R. McIver, Amanda Kahn Dickens and Anna R. Robuck Center for Marine Sciences University of North Carolina
Phosphorus Loading to Western Lake Erie: Trends and Sources
 Phosphorus Loading to Western Lake Erie: Trends and Sources Western Lake Erie Conference Toledo Yacht Club March 13, 28 Dr. David B. Baker National Center for Water Quality Research Heidelberg College
Phosphorus Loading to Western Lake Erie: Trends and Sources Western Lake Erie Conference Toledo Yacht Club March 13, 28 Dr. David B. Baker National Center for Water Quality Research Heidelberg College
Our Eastern Shore Groundwater Part IV Groundwater Quality on the Eastern Shore: How safe is our groundwater and are there ways we can protect it?
 Our Eastern Shore Groundwater Part IV Groundwater Quality on the Eastern Shore: How safe is our groundwater and are there ways we can protect it? Britt McMillan, Malcolm Pirnie, Inc. Mercury Venus Mars
Our Eastern Shore Groundwater Part IV Groundwater Quality on the Eastern Shore: How safe is our groundwater and are there ways we can protect it? Britt McMillan, Malcolm Pirnie, Inc. Mercury Venus Mars
Nutrient BMP Evaluation Project: Preliminary Conclusions
 Nutrient BMP Evaluation Project: Preliminary Conclusions Manure Management Update 2013 Conference Lethbridge, AB January 14, 2013 Beneficial Management Practices How effective are they, what are the costs,
Nutrient BMP Evaluation Project: Preliminary Conclusions Manure Management Update 2013 Conference Lethbridge, AB January 14, 2013 Beneficial Management Practices How effective are they, what are the costs,
2014 ASSINIBOINE RIVER MONITORING REPORT
 October 15, 2014 Temperature º C 9.7 9.6 9.6 9.6 9.7 9.7 10.1 10.2 10.1 Dissolved Oxygen mg/l 10.3 10.2 10.2 10.3 10.1 10.2 10.2 10.2 10.2 Conductivity ms/cm 1.262 1.235 1.253 1.307 1.315 1.307 1.322 1.32
October 15, 2014 Temperature º C 9.7 9.6 9.6 9.6 9.7 9.7 10.1 10.2 10.1 Dissolved Oxygen mg/l 10.3 10.2 10.2 10.3 10.1 10.2 10.2 10.2 10.2 Conductivity ms/cm 1.262 1.235 1.253 1.307 1.315 1.307 1.322 1.32
Lake Elsinore and Canyon Lake Watersheds Nutrient TMDL Monitoring Annual Report
 Lake Elsinore and Canyon Lake sheds Nutrient TMDL Monitoring 2016 2017 Annual Report Prepared for: Lake Elsinore & San Jacinto sheds Project Authority 11615 Sterling Avenue Riverside, California 92503
Lake Elsinore and Canyon Lake sheds Nutrient TMDL Monitoring 2016 2017 Annual Report Prepared for: Lake Elsinore & San Jacinto sheds Project Authority 11615 Sterling Avenue Riverside, California 92503
Farming Challenges and Opportunities
 RURAL Farming Challenges and Opportunities Managing water and nutrient at the farm and paddock scale Roger MacGibbon National Technical Director Opus Rural November 2016 Opus Rural Our Rural team comprises
RURAL Farming Challenges and Opportunities Managing water and nutrient at the farm and paddock scale Roger MacGibbon National Technical Director Opus Rural November 2016 Opus Rural Our Rural team comprises
Phosphorus Dynamics and Mitigation in Soils
 Phosphorus Dynamics and Mitigation in Soils Umass Extension - Managing Phosphorus in Organic Residuals Applied to Soils: Composts, Biosolids, Manures and Others November 2, 2016 - Marlborough, MA Jennifer
Phosphorus Dynamics and Mitigation in Soils Umass Extension - Managing Phosphorus in Organic Residuals Applied to Soils: Composts, Biosolids, Manures and Others November 2, 2016 - Marlborough, MA Jennifer
Riparian Buffers and Stream Restoration
 Riparian Buffers and Stream Restoration Why focus on riparian areas? Link land and water on any given site and link landscapes together in a watershed Riparian corridors protect the circulatory system
Riparian Buffers and Stream Restoration Why focus on riparian areas? Link land and water on any given site and link landscapes together in a watershed Riparian corridors protect the circulatory system
Orange County Fertilizer Application Education Course for Citizens
 Orange County Fertilizer Application Education Course for Citizens Thanks for taking the time to learn how to keep Central Florida beautiful while protecting our water quality! Why is Orange County Requiring
Orange County Fertilizer Application Education Course for Citizens Thanks for taking the time to learn how to keep Central Florida beautiful while protecting our water quality! Why is Orange County Requiring
Manitoba Water Quality Standards, Objectives, and Guidelines Dwight Williamson, Manager Water Quality Management Section Manitoba Conservation
 Manitoba Water Quality Standards, Objectives, and Guidelines 2002 Dwight Williamson, Manager Water Quality Management Section Manitoba Conservation 1 Outline Brief history Review process Overview of proposed
Manitoba Water Quality Standards, Objectives, and Guidelines 2002 Dwight Williamson, Manager Water Quality Management Section Manitoba Conservation 1 Outline Brief history Review process Overview of proposed
Watercourses and Wetlands and Agricultural Activities
 Watercourses and Wetlands and Agricultural Activities Watercourses and regulated wetlands can be found throughout New Brunswick, including on agricultural lands. The Clean Water Act defines a watercourse
Watercourses and Wetlands and Agricultural Activities Watercourses and regulated wetlands can be found throughout New Brunswick, including on agricultural lands. The Clean Water Act defines a watercourse
Nitrate in Trout Brook
 Nitrate in Trout Brook Dakota County, Minnesota Prepared by Dakota County Soil and Water Conservation District, March 2014 Introduction Trout Brook is a trout stream in Dakota County, Minnesota. It is
Nitrate in Trout Brook Dakota County, Minnesota Prepared by Dakota County Soil and Water Conservation District, March 2014 Introduction Trout Brook is a trout stream in Dakota County, Minnesota. It is
3/23/2015. Bunker Silage Storage Leachate and Runoff Management. Bunker Silage Storage. Silage
 Bunker Silage Storage Leachate and Runoff Management Aaron Wunderlin, Discovery Farms Becky Larson, Assistant Professor UW-Madison Eric Cooley, Discovery Farms Mike Holly, PhD Student UW-Madison Bunker
Bunker Silage Storage Leachate and Runoff Management Aaron Wunderlin, Discovery Farms Becky Larson, Assistant Professor UW-Madison Eric Cooley, Discovery Farms Mike Holly, PhD Student UW-Madison Bunker
THE THREE PATHWAYS FOR THE
 THE THREE PATHWAYS FOR THE REMOVAL OF AMMOIA-ITROGE I AQUACULTURE SYSTEMS James M. Ebeling, Ph.D. Research Engineer Aquaculture Systems Technologies, LLC Michael B. Timmons, Ph.D. Professor Dept. of Bio.
THE THREE PATHWAYS FOR THE REMOVAL OF AMMOIA-ITROGE I AQUACULTURE SYSTEMS James M. Ebeling, Ph.D. Research Engineer Aquaculture Systems Technologies, LLC Michael B. Timmons, Ph.D. Professor Dept. of Bio.
Study Justification. Coordinated Resource Management Project Little Snake River Conservation District
 Trends in Surface-Water Quality of the Muddy Creek asin: Carbon County, Wyoming C.. Ellison, Q. D. Skinner and L. S. Hicks Department of Renewable Resources University of Wyoming Funded by: Little Snake
Trends in Surface-Water Quality of the Muddy Creek asin: Carbon County, Wyoming C.. Ellison, Q. D. Skinner and L. S. Hicks Department of Renewable Resources University of Wyoming Funded by: Little Snake
Mystic River Watershed. Baseline Index Report
 Mystic River Watershed Baseline Index Report 2001 2007 ABR049 ABR028 MIB001 Mill Brook WIB001 ABR006 UPL001 MYR071 MEB001 ALB006 Alewife Brook MAR036 Map of watershed with Mystic Monitoring Network baseline
Mystic River Watershed Baseline Index Report 2001 2007 ABR049 ABR028 MIB001 Mill Brook WIB001 ABR006 UPL001 MYR071 MEB001 ALB006 Alewife Brook MAR036 Map of watershed with Mystic Monitoring Network baseline
Modeling Hydrology, Sediment, and Nutrients in the Flathead Lake Watershed Appendix C
 APPENDIX C. SOUTH FORK FLATHEAD RIVER BOUNDARY CONDITION CONTENTS Introduction... C 3 Available Data... C 6 Available Flow Data... C 6 Available Suspended Sediment Concentration Data... C 8 Available Nitrogen
APPENDIX C. SOUTH FORK FLATHEAD RIVER BOUNDARY CONDITION CONTENTS Introduction... C 3 Available Data... C 6 Available Flow Data... C 6 Available Suspended Sediment Concentration Data... C 8 Available Nitrogen
Edge-of-Field Monitoring
 Edge-of-Field Monitoring Karma Anderson Water Quality Specialist National Water Quality and Quantity Team NRCS Edge-of-Field Monitoring NRCS WQ monitoring first introduced in 2010 as Interim CPS 799 in
Edge-of-Field Monitoring Karma Anderson Water Quality Specialist National Water Quality and Quantity Team NRCS Edge-of-Field Monitoring NRCS WQ monitoring first introduced in 2010 as Interim CPS 799 in
Antonio Mallarino Professor, Department of Agronomy. Introduction
 2003 Integrated Crop Management Conference - Iowa State University 121 USING THE IOWA PHOSPHORUS INDEX FOR AGRONOMIC AND ENVIRONMENTAL MANAGEMENT OF FERTILIZER AND MANURE PHOSPHORUS Antonio Mallarino Professor,
2003 Integrated Crop Management Conference - Iowa State University 121 USING THE IOWA PHOSPHORUS INDEX FOR AGRONOMIC AND ENVIRONMENTAL MANAGEMENT OF FERTILIZER AND MANURE PHOSPHORUS Antonio Mallarino Professor,
Salinity TMDL Development and Modeling in the Otter Creek Watershed. Erik Makus DEQ Hydrologist June 6, 2013
 Salinity TMDL Development and Modeling in the Otter Creek Watershed 1 Erik Makus DEQ Hydrologist June 6, 2013 Outline for Today: Otter Creek and the Tongue River Previous salinity modeling efforts Existing
Salinity TMDL Development and Modeling in the Otter Creek Watershed 1 Erik Makus DEQ Hydrologist June 6, 2013 Outline for Today: Otter Creek and the Tongue River Previous salinity modeling efforts Existing
Tools to Improve Water Quality
 Tools to Improve Water Quality SARA Water Quality Modeling Tool Development Client: San Antonio River Authority (SARA) Firm: Lockwood, Andrews & Newnam, Inc. Category A: Studies, Research and Consulting
Tools to Improve Water Quality SARA Water Quality Modeling Tool Development Client: San Antonio River Authority (SARA) Firm: Lockwood, Andrews & Newnam, Inc. Category A: Studies, Research and Consulting
LAVACA BASIN SUMMARY REPORT
 LAVACA BASIN SUMMARY REPORT July, 2002 Lavaca-Navidad River Authority PO Box 429 Edna, Texas 77957 Paul Price Associates, Inc. 3006 Bee Caves Road, Suite D-230 Austin, Texas 78746 Prepared in cooperation
LAVACA BASIN SUMMARY REPORT July, 2002 Lavaca-Navidad River Authority PO Box 429 Edna, Texas 77957 Paul Price Associates, Inc. 3006 Bee Caves Road, Suite D-230 Austin, Texas 78746 Prepared in cooperation
BIG ROCHE A CRI LAKE CHARACTERISTICS
 BIG ROCHE A CRI LAKE CHARACTERISTICS Lake Description Big Roche Cri Lake is located in north central Adams County and has a surface area of 205 acres, a shoreline length of 6.1 mile, a volume of 6,993
BIG ROCHE A CRI LAKE CHARACTERISTICS Lake Description Big Roche Cri Lake is located in north central Adams County and has a surface area of 205 acres, a shoreline length of 6.1 mile, a volume of 6,993
Modeling Nutrient and Sediment Losses from Cropland D. J. Mulla Dept. Soil, Water, & Climate University of Minnesota
 Modeling Nutrient and Sediment Losses from Cropland D. J. Mulla Dept. Soil, Water, & Climate University of Minnesota Watershed Management Framework Identify the problems and their extent Monitor water
Modeling Nutrient and Sediment Losses from Cropland D. J. Mulla Dept. Soil, Water, & Climate University of Minnesota Watershed Management Framework Identify the problems and their extent Monitor water
Municipal Stadium Wetland
 W A T E R S H E D F A C T S Total watershed area 140 mi 2 Average annual rainfall 38 in Average annual temperature 58 F Elevation Max 1321 ft Min 899 ft C. J. Brown Reservoir controls flow from 83 mi 2
W A T E R S H E D F A C T S Total watershed area 140 mi 2 Average annual rainfall 38 in Average annual temperature 58 F Elevation Max 1321 ft Min 899 ft C. J. Brown Reservoir controls flow from 83 mi 2
Water Quality Study In the Streams of Flint Creek and Flint River Watersheds For TMDL Development
 Water Quality Study In the Streams of Flint Creek and Flint River Watersheds For TMDL Development Idris Abdi Doctoral Dissertation Presentation Major Advisor: Dr. Teferi Tsegaye April 18, 2005 Alabama
Water Quality Study In the Streams of Flint Creek and Flint River Watersheds For TMDL Development Idris Abdi Doctoral Dissertation Presentation Major Advisor: Dr. Teferi Tsegaye April 18, 2005 Alabama
POLLUTANT REMOVAL EFFICIENCIES FOR TYPICAL STORMWATER MANAGEMENT SYSTEMS IN FLORIDA
 POLLUTANT REMOVAL EFFICIENCIES FOR TYPICAL STORMWATER MANAGEMENT SYSTEMS IN FLORIDA Presented at the Fourth Biennial Stormwater Research Conference Clearwater, FL October 18-20, 1995 Sponsored By: The
POLLUTANT REMOVAL EFFICIENCIES FOR TYPICAL STORMWATER MANAGEMENT SYSTEMS IN FLORIDA Presented at the Fourth Biennial Stormwater Research Conference Clearwater, FL October 18-20, 1995 Sponsored By: The
Newmont Mining Corporation Water Management Standard
 Page No: Page 1 of 5 1 PURPOSE AND OBJECTIVES This Global Standard sets the minimum Newmont requirements to proactively plan and manage water from exploration to post-closure in accordance with Newmont
Page No: Page 1 of 5 1 PURPOSE AND OBJECTIVES This Global Standard sets the minimum Newmont requirements to proactively plan and manage water from exploration to post-closure in accordance with Newmont
Illinois River and Baron Fork Watershed Implementation Project
 Illinois River and Baron Fork Watershed Implementation Project OCC Task 113 FY 1999 319(h) Task 800 Submitted by: Oklahoma Conservation Commission Water Quality Division 2401 Lincoln Boulevard, Will Rogers
Illinois River and Baron Fork Watershed Implementation Project OCC Task 113 FY 1999 319(h) Task 800 Submitted by: Oklahoma Conservation Commission Water Quality Division 2401 Lincoln Boulevard, Will Rogers
BMP Verification: What is it and How Will it Impact Pennsylvania?
 BMP Verification: What is it and How Will it Impact Pennsylvania? Chesapeake Bay Program Conservation District and Agency Staff Meeting March 13, 2017 + over 400 other unique practices approved by the
BMP Verification: What is it and How Will it Impact Pennsylvania? Chesapeake Bay Program Conservation District and Agency Staff Meeting March 13, 2017 + over 400 other unique practices approved by the
Objective 1: Manage the demonstration site using common agricultural practices and monitor runoff quantity and quality.
 Appendix B Objectives/Tasks Accomplishments By J. Kjaersgaard, South Dakota State University. Objective 1: Manage the demonstration site using common agricultural practices and monitor runoff quantity
Appendix B Objectives/Tasks Accomplishments By J. Kjaersgaard, South Dakota State University. Objective 1: Manage the demonstration site using common agricultural practices and monitor runoff quantity
Evaluating Soil Phosphorus and Surface Runoff Phosphorus Relationships at the Plot and Watershed Scale
 Evaluating Soil Phosphorus and Surface Runoff Phosphorus Relationships at the Plot and Watershed Scale David R. German Dr. Ronald H. Gelderman James R. Gerwing South Dakota State University Dr. Frank V.
Evaluating Soil Phosphorus and Surface Runoff Phosphorus Relationships at the Plot and Watershed Scale David R. German Dr. Ronald H. Gelderman James R. Gerwing South Dakota State University Dr. Frank V.
Factsheet: Town of Deep River Water Quality and Stormwater Summary
 79 Elm Street Hartford, CT 06106-5127 www.ct.gov/deep Affirmative Action/Equal Opportunity Employer Factsheet: Town of Deep River Water Quality and Stormwater Summary This document was created for each
79 Elm Street Hartford, CT 06106-5127 www.ct.gov/deep Affirmative Action/Equal Opportunity Employer Factsheet: Town of Deep River Water Quality and Stormwater Summary This document was created for each
Susquehanna River Monitoring
 Susquehanna River Monitoring Monitoring water quality and fecal coliform bacteria in the Upper Susquehanna River, summer 2008 Jessie E. Matus 1 ABSTRACT During the summer of 2008, the Upper Susquehanna
Susquehanna River Monitoring Monitoring water quality and fecal coliform bacteria in the Upper Susquehanna River, summer 2008 Jessie E. Matus 1 ABSTRACT During the summer of 2008, the Upper Susquehanna
Bacteria Characterization of Big Creek and Buffalo River near Carver
 Bacteria Characterization of Big Creek and Buffalo River near Carver Boone County Library September 23, 2014 Faron D. Usrey, Aquatic Ecologist Presentation Presuppositions (Acknowledging the Elephant in
Bacteria Characterization of Big Creek and Buffalo River near Carver Boone County Library September 23, 2014 Faron D. Usrey, Aquatic Ecologist Presentation Presuppositions (Acknowledging the Elephant in
Lower Mission Creek Watershed Status Survey 2002
 Lower Mission Creek Watershed Status Survey 2002 Prepared For: Mosquito Creek Stewardship Society Prepared By: Fernando F. Junqueira, Ph.D. August 16, 2002 North Vancouver, August 16, 2002 ENVIRONMENTAL
Lower Mission Creek Watershed Status Survey 2002 Prepared For: Mosquito Creek Stewardship Society Prepared By: Fernando F. Junqueira, Ph.D. August 16, 2002 North Vancouver, August 16, 2002 ENVIRONMENTAL
New Practices for Nutrient Reduction: STRIPs and Saturated Buffers. Matthew Helmers and Tom Isenhart Iowa State University
 New Practices for Nutrient Reduction: STRIPs and Saturated Buffers Matthew Helmers and Tom Isenhart Iowa State University Situation Increasing concern for local and regional waters Substantial demand for
New Practices for Nutrient Reduction: STRIPs and Saturated Buffers Matthew Helmers and Tom Isenhart Iowa State University Situation Increasing concern for local and regional waters Substantial demand for
CONSTRUCTION AND DEMOLITION DEBRIS DISPOSAL SITE GUIDELINES
 Nova Scotia Environment and Labour CONSTRUCTION AND DEMOLITION DEBRIS DISPOSAL SITE GUIDELINES Approval Date: October 24, 1997 Effective Date: October 24, 1997 Approved By: Peter Underwood Version Control:
Nova Scotia Environment and Labour CONSTRUCTION AND DEMOLITION DEBRIS DISPOSAL SITE GUIDELINES Approval Date: October 24, 1997 Effective Date: October 24, 1997 Approved By: Peter Underwood Version Control:
Soil Health and Water Quality at St. Andrew s School, Delaware. Final Project Report. Submitted to: GreenWatch Institute, Inc.
 Soil Health and Water Quality at St. Andrew s School, Delaware Final Project Report Submitted to: GreenWatch Institute, Inc. Submitted by: Kyle Castillo, College of Engineering and Ms. Maria Pautler and
Soil Health and Water Quality at St. Andrew s School, Delaware Final Project Report Submitted to: GreenWatch Institute, Inc. Submitted by: Kyle Castillo, College of Engineering and Ms. Maria Pautler and
POLICY FOR NATURAL RESOURCE MANAGEMENT AREAS
 POLICY FOR NATURAL RESOURCE MANAGEMENT AREAS I. POLICY STATEMENT Auburn University's (the University's) "Policy for Natural Resource Management Areas" implements the Campus Master Plan Land Use Element
POLICY FOR NATURAL RESOURCE MANAGEMENT AREAS I. POLICY STATEMENT Auburn University's (the University's) "Policy for Natural Resource Management Areas" implements the Campus Master Plan Land Use Element
EUTROPHICATION. Student Lab Workbook
 EUTROPHICATION Student Lab Workbook THE SCIENTIFIC METHOD 1. Research Background literature research about a topic of interest 2. Identification of a problem Determine a problem (with regards to the topic)
EUTROPHICATION Student Lab Workbook THE SCIENTIFIC METHOD 1. Research Background literature research about a topic of interest 2. Identification of a problem Determine a problem (with regards to the topic)
The Water We Drink. Why is drinking water important to you? How much drinking water do we have? Where We Find the Earth's Water
 Written for youth in grades 6-12, this tool introduces public and private water systems and includes an activity on how to read a water quality test report. Why is drinking water important to you? You
Written for youth in grades 6-12, this tool introduces public and private water systems and includes an activity on how to read a water quality test report. Why is drinking water important to you? You
ATLANTIC SOIL AND WATER CONSERVATION CHALLENGES
 ATLANTIC SOIL AND WATER CONSERVATION CHALLENGES Gordon Fairchild, Ph.D., P.Ag., Soils Specialist, ECSWCC and Jean-Louis Daigle, P.Eng., P.Ag., Director, ECSWCC Environmental Farm Planning Worshop Crystal
ATLANTIC SOIL AND WATER CONSERVATION CHALLENGES Gordon Fairchild, Ph.D., P.Ag., Soils Specialist, ECSWCC and Jean-Louis Daigle, P.Eng., P.Ag., Director, ECSWCC Environmental Farm Planning Worshop Crystal
Non-point source pollution now accounts for over 75% of Pennsylvania s total water pollution
 2. Non-point Source Pollution Living on the Landscape and How We Affect Our Shared Water Resources by John Jose, Watershed Specialist, Pike County Conservation District Water is the most critical resource
2. Non-point Source Pollution Living on the Landscape and How We Affect Our Shared Water Resources by John Jose, Watershed Specialist, Pike County Conservation District Water is the most critical resource
Report Date: September 30, 2009 Technical Report #
 Bacterial Assessment: Tributaries to Horn Pond in Woburn April 29, 2009 Report Date: September 30, 2009 Technical Report # 0909-004 Summary: This report documents the results of a monitoring event that
Bacterial Assessment: Tributaries to Horn Pond in Woburn April 29, 2009 Report Date: September 30, 2009 Technical Report # 0909-004 Summary: This report documents the results of a monitoring event that
Evaluation of Pollutants in Wastewater Produced from Air Conditioning Cleaning Operations
 2017 APWA NC Stormwater Management Division Conference Greenville, NC Evaluation of Pollutants in Wastewater Produced from Air Conditioning Cleaning Operations City of Durham Public Works Department -
2017 APWA NC Stormwater Management Division Conference Greenville, NC Evaluation of Pollutants in Wastewater Produced from Air Conditioning Cleaning Operations City of Durham Public Works Department -
Memorandum. To: From: Date: July 10, Cc: Re: Introduction. to control. This time. primarily. excavated
 Memorandum To: From: Date: Cc: Dave Taylor, Madison Metropolitan Sewerage District Rob Montgomery July 10, 2012 Kevin Connors, Dane County Peter Hughes, USGS Re: DRAFT description of Alum treatment demonstration
Memorandum To: From: Date: Cc: Dave Taylor, Madison Metropolitan Sewerage District Rob Montgomery July 10, 2012 Kevin Connors, Dane County Peter Hughes, USGS Re: DRAFT description of Alum treatment demonstration
Field Verification of Performance of an Engineered Phosphorus Removal Media Deployed in a Flow Based Stormwater Filter
 Field Verification of Performance of an Engineered Phosphorus Removal Media Deployed in a Flow Based Stormwater Filter Gretchen Tellessen 1, Vaikko Allen 2* 1 Contech Engineered Solutions, Portland, Oregon.
Field Verification of Performance of an Engineered Phosphorus Removal Media Deployed in a Flow Based Stormwater Filter Gretchen Tellessen 1, Vaikko Allen 2* 1 Contech Engineered Solutions, Portland, Oregon.
