Frequently Asked Questions. Neuraxial Connectors (ISO ) October 19, 2016 (v2)
|
|
|
- Branden Lynch
- 6 years ago
- Views:
Transcription
1 Frequently Asked Questions Neuraxial Connectors (ISO ) October 19, 2016 (v2) 1
2 Frequently Asked Questions Neuraxial Connectors (ISO ) 1. What is a small-bore connector? 2. What changes are coming to small-bore connectors? 3. Will new neuraxial medical connectors have distinct names? 4. Why is industry adopting the new ISO neuraxial connector? 5. What are the implications of these new standards? 6. What connectors are affected by the ISO (NRFit) connector standard? 7. Which devices are affected by the ISO standard? 8. Who developed the proposed new ISO connector design? 9. Why should we adopt the new connector? 10. What makes the new ISO connector different from the current Luer system? 11. When will the new ISO connector be available? 12. How will the new ISO connector be introduced into my healthcare organization? 13. If we use Neuraxial parts from another manufacturer s set, how will we know the products will work together? 14. Is it mandatory to transition to the new ISO connector? 15. When will the neuraxial devices with current connectors be discontinued? 16. Will there be new item numbers or SKUs for the new sets? 17. If applicable, when will the new item numbers be available and how will we know when to order the new item numbers (SKUs)? 18. Will there be a price increase? 19. Will there be a standard color for the connector? 20. What is the role of StayConnected? 21. Does this new system require that devices using the Luer connector become obsolete? 22. Will there be adapters for different kinds of syringes? 2
3 1. What is a small-bore connector? A small-bore connector is a connector with an inner diameter of less than 8.5mm used to connect medical devices, components and accessories for the purposes of delivering fluids or gases. 2. What changes are coming to small-bore connectors? The International Organization for Standardization (ISO 1 ) has developed a series of standards for small-bore connectors, known as ISO series. These standards will provide design and performance specifications for a range of connectors which can be used for different medical device applications ( enteral ENFit, neuraxial and major regional NRFit, intravascular and hypodermic Luer, etc.) and which will reduce the risks of misconnections between applications section Use Common name -2 Breathing systems and driving gases -3 Enteral applications ENFit -4 Urethral and urinary applications -5 Limb cuff inflation -6 Neuraxial applications and major NRFit regional anesthesia -7 intravascular or hypodermic applications Luer 3. Will new neuraxial medical connectors have distinct names? Connector naming is not included in the ISO series of standards. Therefore, companies are free to name the new connectors as they choose. GEDSA and members of the ISO joint working group have proposed the use of the name NRFit (pronounced ner-fit ) for the new connectors used for neuraxial and major regional applications. NRFit is a name that will be used to identify devices that comply with the ISO standard. 4. Why is industry adopting the new ISO neuraxial connector? The standard small-bore connector in the medical device field for many years has been the Luer connector. Because the Luer connector was such an effective and reliable design, it has been used in many different types of device applications, such as vascular, enteral, respiratory, epidural, and intrathecal. The consequence of this has been misconnection with devices that were not meant to connect, such as the delivery of toxic chemotherapy drugs into the spinal canal. As well as preventing the delivery of IV drugs into the neuraxial space, use of the ISO connectors will also prevent wrong-route delivery of neuraxial medicines (such as bupivacaine) intravenously. 1 ISO (International Organization for Standardization) is the world s largest developer of voluntary International Standards. It has published more than 19,500 International Standards covering 3
4 5. What are the implications of these new standards? Unique connector designs promote patient safety and helps ensure that connectors for unrelated applications are incompatible. This reduces the chances of wrong route injections, infusions and the harm associated with these incidents. Syringes used for intravascular and hypodermic access will no longer be interchangeable with neuraxial syringes. For manufacturers and suppliers this may/ cause temporary inconvenience as procedure packs may be more difficult to customize. Clinicians need to be aware of what extra items ( drop-ins 2 ) they use, as those will need to be ordered specifically: pulling those from intravenous supplies stock will no longer suffice. Pharmacy departments preparing medicines for intrathecal and epidural use (for example, aseptic preparation of intrathecal chemotherapy preparations) will also have to ensure they have appropriate stocks of syringes, syringe caps, filling devices, filters and other equipment necessary to fill and dispense using the new connectors. 6. What connectors are affected by the ISO (NRFit) connector standard? applications involve the use of medical devices to administer medications to and take samples from neuraxial sites (central nervous system including intracranial and spinal canal such as epidural and intrathecal), major peripheral nerve local anaesthetic blockade and continuous wound infiltration. This includes anaesthetic delivery, monitoring cerebrospinal fluid (CSF) pressure, and removing CSF for therapeutic or diagnostic purposes. 7. Which devices are affected by the ISO standard? Neuraxial devices and devices for major regional anesthesia that currently use Luer connectors will, over the next few years, incorporate the connectors. 8. Who developed the proposed new ISO connector design? This new connector design was developed by ISO through the combined global effort of clinicians, regulators, test houses and industry. The ISO connector has undergone a rigorous validation process including computer aided design (CAD) misconnection tests, laboratory leak testing, human factors and usability assessment. 2 Drop in is a phrase used by industry for devices from other suppliers that are added by clinicians to procedure packs to make a complete kit. 4
5 9. Why should we adopt the new connector? The ISO connector provides a simple way to reduce the risk of neuraxial misconnections and improve patient safety. The new connector reduces the chance of an unintentional cross-connection with any other connector intended for non-neuraxial routes. In some countries, the legal systems expect clinical staff to take all reasonable steps to mitigate the risk of cross-connection incidents, and therefore may expect clinical staff to use devices that use these application specific connectors. Non-adoption of the new connectors may expose clinical staff and organizations to legal challenges if further wrong-route incidents take place which could have been prevented by use of ISO compliant devices. 10. What makes the new ISO connector different from the current Luer system? The new ISO connector looks like a Luer connector, but is about 20% smaller and has a unique design that reduces the risk of cross connection with other connectors developed under the same series of standards, especially with Luer connectors. There is one visible difference: slip males will have a collar surrounding the connector. 11. When will the new ISO connector be available? Neuraxial devices with the ISO connector are expected to be available starting in the U.S., Canada and other markets in The ISO connector should be introduced into use with minimal disruption. Timing is subject to change pending regulatory agency clearance and each manufacturer s readiness. Check with your supplier representative for precise timing and device-specific details. 12. How will the new ISO connector be introduced into my healthcare organization? This will vary depending on where you are based. In some countries, it will be under the direction of regulatory authorities and practice experts. In other countries the decision of when to deploy ISO compliant devices may be up to local healthcare organizations or Governments. No matter who is driving the process, healthcare facilities and providers will be able to call upon industry to help them with a careful transition plan to replace Luer devices with the new ISO connector. Although each company may follow their own device and market launch timeline, it is expected that there will be substantial efforts to coordinate the availability of devices in a way that minimizes confusion and logistical problems. To that end, the global industry group has agreed to: Develop and execute a coordinated communications initiative Use a common brand name for the ISO connector NRFit. Use the same time frame to introduce neuraxial devices with the new connector 5
6 13. If we use Neuraxial parts from another manufacturer s set, how will we know the products will work together? As long as the connectors have been produced in compliance with the ISO standard, then it is expected that the devices will connect together effectively and safely. All major neuraxial device manufacturers are expected to comply with the proposed new ISO standards to help ensure neuraxial components fit together as a system. Many compatible devices will be marked with the NRFit label or with the standard number (ISO ). For clinician convenience, some items, such as syringe plungers, may be colored yellow. 14. Is it mandatory to transition to the new ISO connector? This varies by jurisdiction. For example, in California all epidural use must be transitioned by January 1, In the UK, the National Health Service (NHS) has already started planning for the introduction of connectors within 6 months of the California deadline. In Europe and other markets throughout the world, many manufacturers and suppliers are following the subject closely, and plan to adopt the same new global standard connector system. The deployment of devices with the connector may be on different timelines in different global markets, but the goal remains the same to align to a common neuraxial connector across the globe to improve patient safety. 15. When will the neuraxial devices with current connectors be discontinued? Discontinuation of items is at the sole discretion of manufacturers. For precise timing of item discontinuation, contact your supplier representative. The Luer connector for neuraxial devices will be phased out of hospitals on varying timelines. It is important to understand that some devices, such as spinal needles, are presently used for non-neuraxial applications, such as amniocentesis and joint injections. The use of long needles with a Luer connector will still be required after the change to connectors, and some manufacturers have declared their intent to market such devices to meet clinical needs. You should start contacting the suppliers of the needles you presently use and ask them what plans they have for long needles with Luer connectors in the future. If this does not happen, then there is a risk that many common clinical procedures will find themselves in difficulty after the withdrawal of Luer-based spinal needles from the healthcare setting. 16. Will there be new item numbers or SKUs for the new sets? Introduction of new items and related issues such as new item numbers are at the sole discretion of manufacturers. For precise answers relative to new item introductions, contact your supplier representative. In the UK, clinical incidents have already arisen over confusion between proprietary neuraxial connector devices and standard Luer devices, resulting in delays to treatments. It is anticipated that many manufacturers will use product codes that allow individuals to distinguish easily between Luer and ISO devices to avoid such incidents. In countries where proprietary non-luer connectors are already in use the codes will also be different, but there will be need for additional vigilance in these settings. 6
7 17. If applicable, when will the new item numbers be available and how will we know when to order the new item numbers (SKUs)? Introduction of new items and related issues, such as new item numbers, are at the sole discretion of manufacturers. For precise answers relative to new item introductions, contact your supplier representative. 18. Will there be a price increase? Pricing is at the sole discretion of device manufacturers. 19. Will there be a standard color for the connector? Color-coding is not included in the ISO series of standards. The standards will only address the shape and size of the new connectors. These engineering controls make it unlikely that two unintended connectors will fit together. While you might see a consistent color used for devices with neuraxial connectors, it is not a requirement. There is, however, a trend in parts of the world to use the color yellow to indicate neuraxial routes. This feature would be provided for convenience, not safety. 20. What is the role of StayConnected? StayConnected is the implementation branch of the Global Enteral Device Supplier Association (GEDSA) which is a federally tax exempt non-profit trade association StayConnected was established in part to help introduce the new ISO standard connectors and facilitate adoption of the new connectors with the healthcare community. GEDSA, which is composed of leading manufacturers and distributors of medical devices, is united by a shared desire to increase patient safety and optimal delivery of neuraxial treatments and procedures. GEDSA speaks with a unified voice to communicate with government agencies, professional associations, healthcare institutions and member suppliers regarding issues that face device manufacturers, suppliers and distributors. GEDSA will lead StayConnected, a joint communication initiative on behalf of the industry to ensure consistency and avoid any confusion as new, safer connectors are introduced in market. 21. Does this new system require that devices using the Luer connector become obsolete? The Luer connector will still be used for intravascular and hypodermic access and now has its own standard in the ISO series ISO It is, however, expected that availability of neuraxial devices using Luer connectors will decline rapidly because of the significant patient safety benefits associated with a connector which will not cross-connect with Luer. One of the most effective ways to comprehensively reduce the risk of misconnections and enhance patient safety is to ensure that connectors of different delivery systems (i.e., neuraxial and intravascular) are not compatible. 22. Will there be adapters for different kinds of syringes? There will be no adapters for ISO syringes. Syringes will be supplied with the application specific connector. 7
8 692 N. High St. Suite 304 Columbus, OH USA gedsa.org Prepared by GEDSA 8
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 80369-7 First edition 2016-10-15 Corrected version 2016-12-01 Small-bore connectors for liquids and gases in healthcare applications Part 7: Connectors for intravascular or hypodermic
INTERNATIONAL STANDARD ISO 80369-7 First edition 2016-10-15 Corrected version 2016-12-01 Small-bore connectors for liquids and gases in healthcare applications Part 7: Connectors for intravascular or hypodermic
To: Commissioners of Prefectural/Cities with Established Health Centers/Special District Health Department (Bureau)
 HPB/GAD Notification No. 1227-1 PSEHB/PED Notification No. 1227-1 PSEHB/MDED Notification No. 1227-1 PSEHB/PSD Notification No. 1227-1 December 27, 2017 To: Commissioners of Prefectural/Cities with Established
HPB/GAD Notification No. 1227-1 PSEHB/PED Notification No. 1227-1 PSEHB/MDED Notification No. 1227-1 PSEHB/PSD Notification No. 1227-1 December 27, 2017 To: Commissioners of Prefectural/Cities with Established
ICAHN Targeted Focus Area: Medication Devices and Technology
 ICAHN Targeted Focus Area: Medication Devices and Technology Matthew Fricker, RPh, MS, FASHP Program Director, ISMP Rebecca Lamis, PharmD, FISMP Medication Safety Analyst, ISMP April 24, 2014 1 Objectives
ICAHN Targeted Focus Area: Medication Devices and Technology Matthew Fricker, RPh, MS, FASHP Program Director, ISMP Rebecca Lamis, PharmD, FISMP Medication Safety Analyst, ISMP April 24, 2014 1 Objectives
Your Complete Outsourcing Partner EXPERTISE TO TURN YOUR IDEAS INTO REALITY
 Your Complete Outsourcing Partner EXPERTISE TO TURN YOUR IDEAS INTO REALITY The difference between a vendor and a partner THERE ARE OEM SUPPLIERS. THEN THERE S B. BRAUN S OEM DIVISION. We set ourselves
Your Complete Outsourcing Partner EXPERTISE TO TURN YOUR IDEAS INTO REALITY The difference between a vendor and a partner THERE ARE OEM SUPPLIERS. THEN THERE S B. BRAUN S OEM DIVISION. We set ourselves
Advancing the Delivery of Home Infusion Therapy curlinpump.com
 Advancing the Delivery of Home Infusion Therapy curlinpump.com Innovative, multi-therapy pump designed for more precise and efficient infusions Home healthcare and drug advancements place new demands on
Advancing the Delivery of Home Infusion Therapy curlinpump.com Innovative, multi-therapy pump designed for more precise and efficient infusions Home healthcare and drug advancements place new demands on
with Nemera s extensive experience in developing and manufacturing parenteral drug delivery devices
 Co-Development INJECTING NEW IDEAS Empower patients through good design Parenteral administration of a drug exposes users (patients and healthcare professionals) to numerous hazards. In designing a medical
Co-Development INJECTING NEW IDEAS Empower patients through good design Parenteral administration of a drug exposes users (patients and healthcare professionals) to numerous hazards. In designing a medical
The Device Side of Combination Products
 The Device Side of Combination Products Technical and Regulatory Challenges in Life Cycle Management Bob Laughner Associate Director, Combination Products 04 May 2016 What are combination products? Combination
The Device Side of Combination Products Technical and Regulatory Challenges in Life Cycle Management Bob Laughner Associate Director, Combination Products 04 May 2016 What are combination products? Combination
DIFFERENTIATION WITHOUT DISRUPTION
 DIFFERENTIATION WITHOUT DISRUPTION In this article, Alan Shortall, Chief Executive Officer, Unilife Corporation, describes a new paradigm now driving the pharmaceutical market for advanced drug delivery
DIFFERENTIATION WITHOUT DISRUPTION In this article, Alan Shortall, Chief Executive Officer, Unilife Corporation, describes a new paradigm now driving the pharmaceutical market for advanced drug delivery
Drug Products, Labeling, and Packaging
 442 Pharmaceutical Industry: Drug Products, Labeling, and Packaging Positions Drug Products, Labeling, and Packaging Ready-to-Administer Packaging for Hazardous Drug Products Intended for Home Use (1711)
442 Pharmaceutical Industry: Drug Products, Labeling, and Packaging Positions Drug Products, Labeling, and Packaging Ready-to-Administer Packaging for Hazardous Drug Products Intended for Home Use (1711)
ChemoClave System Oncology Kits and Multi-Packs
 A complete line of pre-packed oncology solutions that are safe and easy to use during the entire hazardous drug handling process. ChemoClave System Oncology Kits and Multi-Packs > > Save time by eliminating
A complete line of pre-packed oncology solutions that are safe and easy to use during the entire hazardous drug handling process. ChemoClave System Oncology Kits and Multi-Packs > > Save time by eliminating
Contents. Introduction
 Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
TECHNICAL DATA SHEET BD Emerald Syringe with and without needle Sterile, Single Use, Latex free
 BD Sàrl TECHNICAL DATA SHEET BD Emerald Syringe with and without needle Sterile, Single Use, Latex free 1.0 General Information 1.1 General The BD Emerald syringe is a medical single use product for injection
BD Sàrl TECHNICAL DATA SHEET BD Emerald Syringe with and without needle Sterile, Single Use, Latex free 1.0 General Information 1.1 General The BD Emerald syringe is a medical single use product for injection
Value Plastics, inc. SPECIALISTS IN FLUID CONNECTIONS
 Value Plastics, inc. SERVICE QUALITY DELIVERY PROVEN PRECISION INNOVATION TRUSTED LEADERSHIP DIRECT SALES CUSTOM PARTS QUICK CONNECT FITTINGS SANITARY FITTINGS LUERS TUBE-TO-TUBE BLOOD PRESSURE THREADED
Value Plastics, inc. SERVICE QUALITY DELIVERY PROVEN PRECISION INNOVATION TRUSTED LEADERSHIP DIRECT SALES CUSTOM PARTS QUICK CONNECT FITTINGS SANITARY FITTINGS LUERS TUBE-TO-TUBE BLOOD PRESSURE THREADED
Venue. Ultrasound for the critical moment. Simple. Fast. Precise. gehealthcare.com
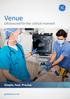 Venue Ultrasound for the critical moment Simple. Fast. Precise. gehealthcare.com In the critical moment, every second matters. Seamless flat display Make cleaning a breeze. Convenient button probe Control
Venue Ultrasound for the critical moment Simple. Fast. Precise. gehealthcare.com In the critical moment, every second matters. Seamless flat display Make cleaning a breeze. Convenient button probe Control
PROPOSED DOCUMENT. Global Harmonization Task Force. Title: Reportable Events During Pre-Market Clinical Investigations
 SG2&5(PD1)N5R10 PROPOSED DOCUMENT Global Harmonization Task Force Title: Reportable Events During Pre-Market Clinical Investigations Authoring Group: Study Groups 2 and 5 Date: 7 June 2011 CONTENTS Reportable
SG2&5(PD1)N5R10 PROPOSED DOCUMENT Global Harmonization Task Force Title: Reportable Events During Pre-Market Clinical Investigations Authoring Group: Study Groups 2 and 5 Date: 7 June 2011 CONTENTS Reportable
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids
 Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
Streamlined Blow-Fill-Seal Insertion Technology Increases Flexibility and Safety in Aseptic Packaging of Pharmaceutical Liquids Isolators adapted specifically for blow-fill-seal insertion applications
CASE STUDY Recalled Blood Monitoring Medical Device
 CASE STUDY Recalled Blood Monitoring Medical Device In-home medical devices are among the most complicated products to recall because unlike, say, food or consumer products abruptly pulling a product off
CASE STUDY Recalled Blood Monitoring Medical Device In-home medical devices are among the most complicated products to recall because unlike, say, food or consumer products abruptly pulling a product off
SECTION: PATIENT CARE Number:
 SUBJECT: Safe Handling of Hazardous Drugs Page: 1 of 10 INTRODUCTION Purpose: This policy has been developed to promote safe work practices for all employees who prepare or administer hazardous drugs or
SUBJECT: Safe Handling of Hazardous Drugs Page: 1 of 10 INTRODUCTION Purpose: This policy has been developed to promote safe work practices for all employees who prepare or administer hazardous drugs or
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB
 Translated English of Chinese Standard: GB18671-2009 www.chinesestandard.net Sales@ChineseStandard.net ICS 11.040.20 NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB C 31 Replacing GB 18671-2002
Translated English of Chinese Standard: GB18671-2009 www.chinesestandard.net Sales@ChineseStandard.net ICS 11.040.20 NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB C 31 Replacing GB 18671-2002
Part 4: Prefilled syringes
 INTERNATIONAL STANDARD ISO 11040-4 Third edition 2015-04-01 Prefilled syringes Part 4: Glass barrels for injectables and sterilized subassembled syringes ready for filling Seringues préremplies Partie
INTERNATIONAL STANDARD ISO 11040-4 Third edition 2015-04-01 Prefilled syringes Part 4: Glass barrels for injectables and sterilized subassembled syringes ready for filling Seringues préremplies Partie
How are GS1 and IHTSDO standards working together?
 How are GS1 and IHTSDO standards working together? Mark Brommeyer Manager Supply Chain Global GS1 Healthcare Conference, Buenos Aires 25 April 2013 0 National E-Health Transition Authority Agenda 1. AMT
How are GS1 and IHTSDO standards working together? Mark Brommeyer Manager Supply Chain Global GS1 Healthcare Conference, Buenos Aires 25 April 2013 0 National E-Health Transition Authority Agenda 1. AMT
New product code functionality starting Multiple product codes for each article number
 To our submitters of medicines New product code functionality starting 1.1.2018 Multiple product codes can be added to each article number in VareWeb Product codes for inner packs can be registered in
To our submitters of medicines New product code functionality starting 1.1.2018 Multiple product codes can be added to each article number in VareWeb Product codes for inner packs can be registered in
FDA s Bar Code Label Requirements for Human Drug Products. General Questions Related to Drugs and Biologics
 FDA s Bar Code Label Requirements for Human Drug Products General Questions Related to Drugs and Biologics 1. Which medical products should carry a bar code? For example, should all prescription and over-the-counter
FDA s Bar Code Label Requirements for Human Drug Products General Questions Related to Drugs and Biologics 1. Which medical products should carry a bar code? For example, should all prescription and over-the-counter
BRITISH GENERIC MANUFACTURERS ASSOCIATION
 BRITISH GENERIC MANUFACTURERS ASSOCIATION Response by the British Generic Manufacturers Association (BGMA) to the Department of Health Consultation on Amendments to the Statutory Scheme to Control the
BRITISH GENERIC MANUFACTURERS ASSOCIATION Response by the British Generic Manufacturers Association (BGMA) to the Department of Health Consultation on Amendments to the Statutory Scheme to Control the
Teleflex Incorporated (NYSE: TFX) Investor Presentation
 Teleflex Incorporated (NYSE: TFX) Investor Presentation Important Information 2 Forward Looking Statements This presentation and our discussion contain forward-looking information and statements, which
Teleflex Incorporated (NYSE: TFX) Investor Presentation Important Information 2 Forward Looking Statements This presentation and our discussion contain forward-looking information and statements, which
510(k) Summary. 510(k) Number: K Summary Prepared: August 31, 2017
 510(k) Summary 510(k) Number: K162613 Summary Prepared: August 31, 2017 Submitter: Dr. Fred Ma Repro Med System, Inc., D/B/A RMS Medical Products 24 Carpenter Road, Chester, NY 10918 Tel: 845-469-2042
510(k) Summary 510(k) Number: K162613 Summary Prepared: August 31, 2017 Submitter: Dr. Fred Ma Repro Med System, Inc., D/B/A RMS Medical Products 24 Carpenter Road, Chester, NY 10918 Tel: 845-469-2042
FDA PLANS TO REGULATE TESTS AS DEVICES. August 2010 SPECIAL REPRINT. By Ellen Flannery and Scott Danzis
 August 2010 SPECIAL REPRINT FDA PLANS TO REGULATE LABORATOR ORY DEVELOPED TESTS AS DEVICES By Ellen Flannery and Scott Danzis Reproduced with the kind permission of Global Regulatory Press from the Journal
August 2010 SPECIAL REPRINT FDA PLANS TO REGULATE LABORATOR ORY DEVELOPED TESTS AS DEVICES By Ellen Flannery and Scott Danzis Reproduced with the kind permission of Global Regulatory Press from the Journal
Automated compounding for intravenous chemotherapy
 Automated compounding for intravenous chemotherapy Not valid for printing! Low resolution PDF file. For high resolution files, please contact Communication & Image Dept. The correct preparation and administration
Automated compounding for intravenous chemotherapy Not valid for printing! Low resolution PDF file. For high resolution files, please contact Communication & Image Dept. The correct preparation and administration
March 4, Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
March 4, 2014 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2013-N-1523: Request for Nominations: Drug Products that
Solutions for All Your Aseptic Parenteral Drug Formulation/Filling Needs. BUBBLE-FREE FILLING : A New Option in Prefilled Syringe Filling
 WHITEPAPER Hyaluron Contract Manufacturing Solutions for All Your Aseptic Parenteral Drug Formulation/Filling Needs BUBBLE-FREE FILLING : A New Option in Prefilled Syringe Filling Introduction The advent
WHITEPAPER Hyaluron Contract Manufacturing Solutions for All Your Aseptic Parenteral Drug Formulation/Filling Needs BUBBLE-FREE FILLING : A New Option in Prefilled Syringe Filling Introduction The advent
Safe Medication. Disposable Syringes
 Safe Medication Disposable Syringes Disposable Syringes Safe Medication Safety, reliability, and accuracy down to the smallest detail as with all other CODAN products, this commitment also holds true for
Safe Medication Disposable Syringes Disposable Syringes Safe Medication Safety, reliability, and accuracy down to the smallest detail as with all other CODAN products, this commitment also holds true for
Supply of aseptically - prepared doses of IMPs across legal boundaries. Edition 1. December 2017
 Supply of aseptically - prepared doses of IMPs across legal boundaries Edition 1 December 2017 Endorsed and supported by: NHS Pharmaceutical Quality Assurance Committee 2017 with National Pharmacy Clinical
Supply of aseptically - prepared doses of IMPs across legal boundaries Edition 1 December 2017 Endorsed and supported by: NHS Pharmaceutical Quality Assurance Committee 2017 with National Pharmacy Clinical
CADTH COMMON DRUG REVIEW. Procedure for the CADTH Common Drug Review
 CADTH COMMON DRUG REVIEW Procedure for the CADTH Common Drug Review AUGUST 2014 RECORD OF UPDATES TO THE PROCEDURE FOR THE CADTH COMMON DRUG REVIEW Update Original June 2003 1 January 2005 2 July 2005
CADTH COMMON DRUG REVIEW Procedure for the CADTH Common Drug Review AUGUST 2014 RECORD OF UPDATES TO THE PROCEDURE FOR THE CADTH COMMON DRUG REVIEW Update Original June 2003 1 January 2005 2 July 2005
Easy EHR Issue Reporting Challenge. June 7, 2018
 Easy EHR Issue Reporting Challenge June 7, 2018 On Today s Call Challenge Team: Adam Wong, Office of the National Coordinator for Health IT Marcy Opstal, Office of the National Coordinator for Health IT
Easy EHR Issue Reporting Challenge June 7, 2018 On Today s Call Challenge Team: Adam Wong, Office of the National Coordinator for Health IT Marcy Opstal, Office of the National Coordinator for Health IT
CANADA (HEALTH CANADA)
 1 GMP GAZETTE TM March 2015 HPFBI CANADA (HEALTH CANADA) Notice, Re: Regulatory Decision Summaries and Submissions Under Review Who s affected? Manufacturers of prescription drugs (pharmaceuticals and
1 GMP GAZETTE TM March 2015 HPFBI CANADA (HEALTH CANADA) Notice, Re: Regulatory Decision Summaries and Submissions Under Review Who s affected? Manufacturers of prescription drugs (pharmaceuticals and
SAN TM PRODUCT FAMILY SAFE AUTO-NEEDLES TM. Better injectors. Better compliance. Better care.
 SAFE AUTO-NEEDLES TM SAN TM PRODUCT FAMILY Better injectors. Better compliance. Better care. Addressing patient-centered challenges in injectable drug delivery Compatible with: Prefilled syringes (luer
SAFE AUTO-NEEDLES TM SAN TM PRODUCT FAMILY Better injectors. Better compliance. Better care. Addressing patient-centered challenges in injectable drug delivery Compatible with: Prefilled syringes (luer
Guideline on the label design for Injections (Draft for Comments)
 Guideline on the label design for Injections (Draft for Comments) 2018.3 Content Foreword... 3 1. Scope... 4 2. Terms and definitions... 4 3. Basic requirements for labels... 4 4. Design principles of
Guideline on the label design for Injections (Draft for Comments) 2018.3 Content Foreword... 3 1. Scope... 4 2. Terms and definitions... 4 3. Basic requirements for labels... 4 4. Design principles of
Cardinal Health overview and strategic priorities
 Cardinal Health overview and strategic priorities Steve Inacker President, Channel Management Medical Segment Copyright 2011, Cardinal Health, Inc. or one of its subsidiaries. All rights reserved. Cardinal
Cardinal Health overview and strategic priorities Steve Inacker President, Channel Management Medical Segment Copyright 2011, Cardinal Health, Inc. or one of its subsidiaries. All rights reserved. Cardinal
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 7864 Fourth edition 2016-08-01 Sterile hypodermic needles for single use Requirements and test methods Aiguilles hypodermiques stériles, non réutilisables Exigences et méthodes
INTERNATIONAL STANDARD ISO 7864 Fourth edition 2016-08-01 Sterile hypodermic needles for single use Requirements and test methods Aiguilles hypodermiques stériles, non réutilisables Exigences et méthodes
Leading Pharmacy Benefits Manager (PBM) Implements ProcurePort esourcing to Efficiently Source Prescription Drugs
 Leading Pharmacy Benefits Manager (PBM) Implements ProcurePort esourcing to Efficiently Source Prescription Drugs Company Profile Leading pharmacy benefits manager (PBM) that partners with leading health
Leading Pharmacy Benefits Manager (PBM) Implements ProcurePort esourcing to Efficiently Source Prescription Drugs Company Profile Leading pharmacy benefits manager (PBM) that partners with leading health
Manufacturers. Factsheet for. of Medical Devices. Medical Devices Regulation (MDR) background. Act now to be ready on time!
 Ref. Ares(2018)3873669-20/07/2018 Factsheet for Manufacturers of Medical Devices This Factsheet is aimed at manufacturers of medical devices. For a general overview of the impact of the In-Vitro Medical
Ref. Ares(2018)3873669-20/07/2018 Factsheet for Manufacturers of Medical Devices This Factsheet is aimed at manufacturers of medical devices. For a general overview of the impact of the In-Vitro Medical
Technologies to Prevent Medication Errors in Hospitals
 Technologies to Prevent Medication Errors in Hospitals CSHP Professional Practice Conference February 2, 2004 David U President and CEO, ISMP Canada ISMP CANADA Vision:! ISMP Canada is an independent Canadian
Technologies to Prevent Medication Errors in Hospitals CSHP Professional Practice Conference February 2, 2004 David U President and CEO, ISMP Canada ISMP CANADA Vision:! ISMP Canada is an independent Canadian
-- Study achieved statistical significance on all primary and secondary biological endpoints --
 Sarepta Therapeutics Announces Positive Results in Its Study Evaluating Gene Expression, Dystrophin Production, and Dystrophin Localization in Patients with Duchenne Muscular Dystrophy (DMD) Amenable to
Sarepta Therapeutics Announces Positive Results in Its Study Evaluating Gene Expression, Dystrophin Production, and Dystrophin Localization in Patients with Duchenne Muscular Dystrophy (DMD) Amenable to
Management. National Standards Authority of Ireland
 Medical Equipment Quality Management NSAI: Role and Structure of Standards Brian Cunningham 2009-03-26 National Standards Authority of Ireland Statutory body Formed in 1947 as IIRS 1996 NSAI Act Functions
Medical Equipment Quality Management NSAI: Role and Structure of Standards Brian Cunningham 2009-03-26 National Standards Authority of Ireland Statutory body Formed in 1947 as IIRS 1996 NSAI Act Functions
ENABLE INJECTIONS AND FLEX SET AMBITIOUS PLANS FOR LARGE VOLUME DRUG DELIVERY
 ENABLE INJECTIONS AND FLEX SET AMBITIOUS PLANS FOR LARGE VOLUME DRUG DELIVERY The delivery of high volumes of biologics outside of the clinical setting remains a key challenge in the industry. Here, John
ENABLE INJECTIONS AND FLEX SET AMBITIOUS PLANS FOR LARGE VOLUME DRUG DELIVERY The delivery of high volumes of biologics outside of the clinical setting remains a key challenge in the industry. Here, John
S P O N S O R E D B Y
 S P O N S O R E D B Y The Value of Visibility Data Seeing and Benefiting from Data after its Capture Example: Preparing for Serialization and Pedigree within the U.S. Pharmaceutical Supply Chain Agenda
S P O N S O R E D B Y The Value of Visibility Data Seeing and Benefiting from Data after its Capture Example: Preparing for Serialization and Pedigree within the U.S. Pharmaceutical Supply Chain Agenda
THE SARSTEDT GROUP. Your Partner in Medicine. and Science Worldwide
 THE SARSTEDT GROUP Your Partner in Medicine and Science Worldwide 1 THE SARSTEDT GROUP WORLDWIDE Your Partner in Medicine and Science Worldwide The SARSTEDT Group, with headquarters in Nümbrecht, Germany,
THE SARSTEDT GROUP Your Partner in Medicine and Science Worldwide 1 THE SARSTEDT GROUP WORLDWIDE Your Partner in Medicine and Science Worldwide The SARSTEDT Group, with headquarters in Nümbrecht, Germany,
Public Consultation on Annual Review and Proposal for Fees For Financial Year Medical Devices
 Public Consultation on Annual Review and Proposal for Fees For Financial Year 2018 Medical Devices 8th May 2018 CONTENTS 1 INTRODUCTION 3 2 REVIEW OF THE 2017 FEES 3 2.1 The 2017 fees 3 2.2 The 2018 fees
Public Consultation on Annual Review and Proposal for Fees For Financial Year 2018 Medical Devices 8th May 2018 CONTENTS 1 INTRODUCTION 3 2 REVIEW OF THE 2017 FEES 3 2.1 The 2017 fees 3 2.2 The 2018 fees
A New Era of Clinical Diagnostics: How the Business Model is Changing.
 A New Era of Clinical Diagnostics: How the Business Model is Changing. Nancy J Kelley, President and CEO of Nancy J Kelley & Associates Remarks to BTIG Emerging Technologies in Healthcare Diagnostics September
A New Era of Clinical Diagnostics: How the Business Model is Changing. Nancy J Kelley, President and CEO of Nancy J Kelley & Associates Remarks to BTIG Emerging Technologies in Healthcare Diagnostics September
Environmental Services Conference
 Sharps Compliance Corp. NASDAQ: SMED Credit Suisse Industrial and Environmental Services Conference May 24, 2011 Diana P. Diaz Vice President and Chief Financial Officer 1 Safe Harbor Statement These slides
Sharps Compliance Corp. NASDAQ: SMED Credit Suisse Industrial and Environmental Services Conference May 24, 2011 Diana P. Diaz Vice President and Chief Financial Officer 1 Safe Harbor Statement These slides
NEW EU Regulations and the EU UDI system
 NEW EU Regulations and the EU UDI system 24 October 2017, Brussels Géraldine Lissalde-Bonnet Director Public Policy, GS1 Global Office Disclaimer Neither GS1 nor its member organizations nor their staffs
NEW EU Regulations and the EU UDI system 24 October 2017, Brussels Géraldine Lissalde-Bonnet Director Public Policy, GS1 Global Office Disclaimer Neither GS1 nor its member organizations nor their staffs
Consulted With; Post/Committee/Group Date Haematology Dr Shereen Elshazly (Lead for Anticoagulation) January 2016 Department of Anaesthesia
 Peri-procedural anticoagulation in Adult patients taking Warfarin and novel oral anti-coagulants (NOACS) Clinical Guideline Register No: 16005 Status: Public Developed in response to: Contributes to CQC
Peri-procedural anticoagulation in Adult patients taking Warfarin and novel oral anti-coagulants (NOACS) Clinical Guideline Register No: 16005 Status: Public Developed in response to: Contributes to CQC
Scan4Safety. Implications on Hospital Pharmacy and effect on delivery of healthcare November 2017
 Scan4Safety Implications on Hospital Pharmacy and effect on delivery of healthcare November 2017 Evolution NHS eprocurement strategy GS1 and PEPPOL Scan4Safety Audience Patients Trusts Suppliers Technology
Scan4Safety Implications on Hospital Pharmacy and effect on delivery of healthcare November 2017 Evolution NHS eprocurement strategy GS1 and PEPPOL Scan4Safety Audience Patients Trusts Suppliers Technology
Product Catalog (Canada) Conventional Needles I.V. Catheters Micro-Collection Products Safety Products Winged Infusion Sets
 Product Catalog (Canada) Conventional Needles I.V. Catheters Micro-Collection Products Safety Products Winged Infusion Sets Table of Contents Hypodermics SurGuard 3 Safety Hypodermic Needle 3-4 Terumo
Product Catalog (Canada) Conventional Needles I.V. Catheters Micro-Collection Products Safety Products Winged Infusion Sets Table of Contents Hypodermics SurGuard 3 Safety Hypodermic Needle 3-4 Terumo
Compounding Questions and Answers
 Compounding Questions and Answers JANUARY 10, 2011 1. Question: What is a reliable supplier? Answer: Some examples of reliable suppliers are FDA licensed manufacturers, CA Department of Public Health Food
Compounding Questions and Answers JANUARY 10, 2011 1. Question: What is a reliable supplier? Answer: Some examples of reliable suppliers are FDA licensed manufacturers, CA Department of Public Health Food
BUSINESS OBJECTIVES AND FUTURE PLANS
 BUSINESS OBJECTIVES AND STRATEGIES Our objectives are to consolidate and strengthen our position to become one of the leading distributors of pharmaceutical products in Zhejiang province. To further develop
BUSINESS OBJECTIVES AND STRATEGIES Our objectives are to consolidate and strengthen our position to become one of the leading distributors of pharmaceutical products in Zhejiang province. To further develop
Business Continuity Management Policy
 Business Continuity Management Policy Version FINAL 1.0 Ratified by Dudley CCG Audit Committee Date ratified 17/03/16 Name of originator(s) / author(s) David Morris, Midlands and Lancashire CSU/ Sue Johnson,
Business Continuity Management Policy Version FINAL 1.0 Ratified by Dudley CCG Audit Committee Date ratified 17/03/16 Name of originator(s) / author(s) David Morris, Midlands and Lancashire CSU/ Sue Johnson,
MEDICAL WASTE MANAGEMENT STUDY NOTES
 MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
Anhydrous Ammonia Code of Practice Frequently Asked Questions
 Anhydrous Ammonia Code of Practice Frequently Asked Questions 1. I have an audit scheduled this fall (between July 1 and December 31, 2016). Will I be audited to the revised January 2017 Ammonia Code?
Anhydrous Ammonia Code of Practice Frequently Asked Questions 1. I have an audit scheduled this fall (between July 1 and December 31, 2016). Will I be audited to the revised January 2017 Ammonia Code?
Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC
 Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC U.S. House of Representatives Energy and Commerce Committee Subcommittee on Health Subcommittee January 30, 2018
Testimony of Molly Ventrelli Vice President, Regulatory Affairs Fresenius Kabi USA, LLC U.S. House of Representatives Energy and Commerce Committee Subcommittee on Health Subcommittee January 30, 2018
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 5361 Third edition 2016-09-01 Anaesthetic and respiratory equipment Tracheal tubes and connectors Matériel d anesthésie et de réanimation respiratoire Sondes trachéales et raccords
INTERNATIONAL STANDARD ISO 5361 Third edition 2016-09-01 Anaesthetic and respiratory equipment Tracheal tubes and connectors Matériel d anesthésie et de réanimation respiratoire Sondes trachéales et raccords
Supply Chain Congress 21 August 2013 Megan Main, Chief Executive
 Reforming the health supply chain down under Supply Chain Congress 21 August 2013 Megan Main, Chief Executive Health Purchasing Victoria (HPV) 1. HPV overview 2. Becoming more strategic 3. Global trends
Reforming the health supply chain down under Supply Chain Congress 21 August 2013 Megan Main, Chief Executive Health Purchasing Victoria (HPV) 1. HPV overview 2. Becoming more strategic 3. Global trends
USP800: A quick summary and disposal. Brenda Denson, Pharm.D.
 USP800: A quick summary and disposal Brenda Denson, Pharm.D. Objectives Review current USP800 guidelines on destruction of hazardous medications. Illustrate how a local pharmacy demonstrates compliance
USP800: A quick summary and disposal Brenda Denson, Pharm.D. Objectives Review current USP800 guidelines on destruction of hazardous medications. Illustrate how a local pharmacy demonstrates compliance
Infusion management. Complete administration of the medication without rest volumes
 Infusion management Complete administration of the medication without rest volumes Drip SWAN and CODAN Guard Complete administration Do you want your patient to receive the whole medication and at the
Infusion management Complete administration of the medication without rest volumes Drip SWAN and CODAN Guard Complete administration Do you want your patient to receive the whole medication and at the
Re: SUPPLY DISRUPTION - Baxter Colleague pump compatible IV administration sets
 For the Attention of the Medical Director URGENT FOR ACTION 10 April 2015 Re: SUPPLY DISRUPTION - Baxter Colleague pump compatible IV administration sets Baxter Healthcare has notified us that they are
For the Attention of the Medical Director URGENT FOR ACTION 10 April 2015 Re: SUPPLY DISRUPTION - Baxter Colleague pump compatible IV administration sets Baxter Healthcare has notified us that they are
GS1 Guide on Unique Device Identification (UDI) implementation
 GS1 Guide on Unique Device Identification (UDI) implementation This document aims at providing clarification to questions raised by the industry as well as implementation guidance on the use of GS1 standards.
GS1 Guide on Unique Device Identification (UDI) implementation This document aims at providing clarification to questions raised by the industry as well as implementation guidance on the use of GS1 standards.
GUIDELINES FOR REGISTRATION OF IMPORTERS AND FOR THE IMPORTATION OF MEDICINES AND RELATED PRODUCTS
 MEDICINES CONTROL AGENCY 54 Kairaba Avenue, Pipeline, The Gambia. Tel.no. +220 4380632 GUIDELINES FOR REGISTRATION OF IMPORTERS AND FOR THE IMPORTATION OF MEDICINES AND RELATED PRODUCTS Document no: MCA/IMPG/2017/1
MEDICINES CONTROL AGENCY 54 Kairaba Avenue, Pipeline, The Gambia. Tel.no. +220 4380632 GUIDELINES FOR REGISTRATION OF IMPORTERS AND FOR THE IMPORTATION OF MEDICINES AND RELATED PRODUCTS Document no: MCA/IMPG/2017/1
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL MEDICAL DEVICES and IVDs ESSENTIAL PRINCIPLES of SAFETY & PERFORMANCE This guideline is intended to provide recommendations to applicants wishing to sell medical devices and IVDs
MEDICINES CONTROL COUNCIL MEDICAL DEVICES and IVDs ESSENTIAL PRINCIPLES of SAFETY & PERFORMANCE This guideline is intended to provide recommendations to applicants wishing to sell medical devices and IVDs
THE PARTNER FOR INNOVATIVE SOLUTIONS
 04 THE PARTNER FOR INNOVATIVE SOLUTIONS THE COMPANY WHO ARE WE? Over the past 30 years, ROVIPHARM has become the specialist manufacturer of medical and dosing devices for the pharmaceutical and healthcare
04 THE PARTNER FOR INNOVATIVE SOLUTIONS THE COMPANY WHO ARE WE? Over the past 30 years, ROVIPHARM has become the specialist manufacturer of medical and dosing devices for the pharmaceutical and healthcare
VALUE WITHOUT COMPARISON DIAGNOSTIC PROCEDURE TRAYS. Quality Without Compromise VALUE WITHOUT COMPARISON
 Quality Without Compromise Value Without Comparison VALUE WITHOUT COMPARISON Quality Without Compromise QUALITY WITHOUT COMPROMISE VALUE WITHOUT COMPARISON VALUE WITHOUT COMPARISON DIAGNOSTIC PROCEDURE
Quality Without Compromise Value Without Comparison VALUE WITHOUT COMPARISON Quality Without Compromise QUALITY WITHOUT COMPROMISE VALUE WITHOUT COMPARISON VALUE WITHOUT COMPARISON DIAGNOSTIC PROCEDURE
DEMONSTRATING YOUR MEDICINE S VALUE TO ALL STAKEHOLDERS TRUSTED COMMERCIALIZATION AND MARKET ACCESS EXPERTISE
 CATALYSTS DRIVING SUCCESSFUL DECISIONS IN LIFE SCIENCES DEMONSTRATING YOUR MEDICINE S VALUE TO ALL STAKEHOLDERS TRUSTED COMMERCIALIZATION AND MARKET ACCESS EXPERTISE WHEN COMMERCIALIZING A MEDICINE, IT
CATALYSTS DRIVING SUCCESSFUL DECISIONS IN LIFE SCIENCES DEMONSTRATING YOUR MEDICINE S VALUE TO ALL STAKEHOLDERS TRUSTED COMMERCIALIZATION AND MARKET ACCESS EXPERTISE WHEN COMMERCIALIZING A MEDICINE, IT
UPS Healthcare Supply Chain Vital Signs
 UPS Study Executive September 2017 START 2017 UPS Survey 2017 United Parcel Service of America, Inc. 1 An industry growing more complex: managing competition, compliance and costs Pharmacies are grappling
UPS Study Executive September 2017 START 2017 UPS Survey 2017 United Parcel Service of America, Inc. 1 An industry growing more complex: managing competition, compliance and costs Pharmacies are grappling
Global Medical Device Nomenclature (GMDN)
 Global Medical Device Nomenclature (GMDN) GMDN A Requirement for UDI Edward Glenn, Term Developer - GMDN Agency What we will be discussing Why is the GMDN needed GMDN data structure How to find GMDN terms
Global Medical Device Nomenclature (GMDN) GMDN A Requirement for UDI Edward Glenn, Term Developer - GMDN Agency What we will be discussing Why is the GMDN needed GMDN data structure How to find GMDN terms
Cancer Vanguard. Biosimilars Trust Policy Template
 Cancer Vanguard Biosimilars Trust Policy Template Aim of this document: The document provides generic guidance and outline for the development of local trust policies in relation to the adoption of biosimilars
Cancer Vanguard Biosimilars Trust Policy Template Aim of this document: The document provides generic guidance and outline for the development of local trust policies in relation to the adoption of biosimilars
PUTTING PATIENTS FIRST: INNOVATING DRUG CONTAINMENT AND DELIVERY
 PUTTING PATIENTS FIRST: INNOVATING DRUG CONTAINMENT AND DELIVERY Innovations in self-administered drug delivery systems are supporting care for a variety of medical conditions transitioning out of hospitals
PUTTING PATIENTS FIRST: INNOVATING DRUG CONTAINMENT AND DELIVERY Innovations in self-administered drug delivery systems are supporting care for a variety of medical conditions transitioning out of hospitals
Anaesthetic and respiratory equipment Tracheostomy tubes and connectors
 INTERNATIONAL STANDARD ISO 5366 First edition 2016-10-01 Anaesthetic and respiratory equipment Tracheostomy tubes and connectors Matériel d anesthésie et de réanimation respiratoire Raccords et tubes de
INTERNATIONAL STANDARD ISO 5366 First edition 2016-10-01 Anaesthetic and respiratory equipment Tracheostomy tubes and connectors Matériel d anesthésie et de réanimation respiratoire Raccords et tubes de
GUIDE HEA
 Companies are often overwhelmed by the field of digital health. From digital and med device companies to biotechnology and pharmaceutical companies, there is a struggle to cut through the noise of numerous
Companies are often overwhelmed by the field of digital health. From digital and med device companies to biotechnology and pharmaceutical companies, there is a struggle to cut through the noise of numerous
Medicines Barcoding and Serialization Guideline. National Health Regulatory Authority (NHRA) Kingdom of Bahrain
 edicines Barcoding and Serialization Guideline National Health Regulatory Authority (NHRA) Kingdom of Bahrain August 2018 Version 1.0 Chief of Pharmaceutical Product Regulation: Dr / Roaya Al Abbasi Date:
edicines Barcoding and Serialization Guideline National Health Regulatory Authority (NHRA) Kingdom of Bahrain August 2018 Version 1.0 Chief of Pharmaceutical Product Regulation: Dr / Roaya Al Abbasi Date:
The Art of Clinical Supply Planning : Quality Product in the Right Place at the Right Time
 The Art of Clinical Supply Planning : Quality Product in the Right Place at the Right Time Clinical Trial Supply Southeast 2015 July 15 th July 16 th RTP, North Carolina Presenters: Eva Allen Heather Phillips
The Art of Clinical Supply Planning : Quality Product in the Right Place at the Right Time Clinical Trial Supply Southeast 2015 July 15 th July 16 th RTP, North Carolina Presenters: Eva Allen Heather Phillips
The future of biologics: reverse engineering for successful product supply
 The future of biologics: reverse engineering for successful product supply CORNELL STAMORAN VP, STRATEGY & CORPORATE DEV T. 03.10.11 Catalent s biologics presence Advanced GPEx cell line engineering technology
The future of biologics: reverse engineering for successful product supply CORNELL STAMORAN VP, STRATEGY & CORPORATE DEV T. 03.10.11 Catalent s biologics presence Advanced GPEx cell line engineering technology
34 th Annual J.P. Morgan Healthcare Conference
 34 th Annual J.P. Morgan Healthcare Conference Vincent A. Forlenza Chairman, Chief Executive Officer and President January 13, 2016 Forward-Looking Statements These materials include forward-looking statements
34 th Annual J.P. Morgan Healthcare Conference Vincent A. Forlenza Chairman, Chief Executive Officer and President January 13, 2016 Forward-Looking Statements These materials include forward-looking statements
U.S. Hospitals Study Executive Summary
 UPS Healthcare Study Executive September 2017 START 2017 Survey 2017 United Parcel Service of America, Inc. 1 An industry facing disruptive change: managing consumerization, clinical outcomes and costs
UPS Healthcare Study Executive September 2017 START 2017 Survey 2017 United Parcel Service of America, Inc. 1 An industry facing disruptive change: managing consumerization, clinical outcomes and costs
REQUEST FOR PROPOSALS SUPPLY OF NEEDLES AND SYRINGES (INCLUDING SOME SPECIALTY PRODUCTS)
 03 November 2017 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF NEEDLES AND SYRINGES (INCLUDING SOME SPECIALTY PRODUCTS) PHARMAC invites proposals for the supply of Needles and Syringes in New Zealand District
03 November 2017 Dear Supplier REQUEST FOR PROPOSALS SUPPLY OF NEEDLES AND SYRINGES (INCLUDING SOME SPECIALTY PRODUCTS) PHARMAC invites proposals for the supply of Needles and Syringes in New Zealand District
Purchasing for Safety Policy for Pharmaceuticals January Page 1 of 8 (Purchasing for Safety Policy for Pharmaceuticals)
 Purchasing for Safety Policy for s January 2010 Page 1 of 8 (Purchasing for Safety Policy for s) Policy Title Purchasing for Safety Policy for s Policy Reference Number Acute10/006 Implementation Date
Purchasing for Safety Policy for s January 2010 Page 1 of 8 (Purchasing for Safety Policy for s) Policy Title Purchasing for Safety Policy for s Policy Reference Number Acute10/006 Implementation Date
INFLATIONARY INDICES JULY 1, DECEMBER 31, 2012
 INFLATIONARY INDICES JULY 1, 2011 - DECEMBER 31, 2012 AMERINET INFLATIONARY INDICES Amerinet s Customer-Focused Portfolio Get on the fast track to savings. A typical healthcare provider can reduce costs
INFLATIONARY INDICES JULY 1, 2011 - DECEMBER 31, 2012 AMERINET INFLATIONARY INDICES Amerinet s Customer-Focused Portfolio Get on the fast track to savings. A typical healthcare provider can reduce costs
Regulatory Requirements & Recent Changes, including expectations for APIs & IMPs
 Regulatory Requirements & Recent Changes, including expectations for APIs & IMPs Neil Raw - GMP Inspector Richard Andrews - Operations Manager 11 th November 2008 Programme: Regulatory Requirements Neil
Regulatory Requirements & Recent Changes, including expectations for APIs & IMPs Neil Raw - GMP Inspector Richard Andrews - Operations Manager 11 th November 2008 Programme: Regulatory Requirements Neil
Manufacturers. Factsheet for. of Medical Devices. Medical Devices Regulation (MDR) background. Act now to be ready on time!
 European Commission Factsheet for Manufacturers of Medical Devices This Factsheet is aimed at manufacturers of medical devices. For a general overview of the impact of the In Vitro Medical Devices Regulation
European Commission Factsheet for Manufacturers of Medical Devices This Factsheet is aimed at manufacturers of medical devices. For a general overview of the impact of the In Vitro Medical Devices Regulation
Guide for Class I Manufacturers on Compliance with European Communities (Medical Devices) Regulations, 1994
 Guide for Class I Manufacturers on Compliance with European Communities (Medical Devices) Regulations, 1994 SUR-G0006-2 27 AUGUST 2010 This guide does not purport to be an interpretation of law and/or
Guide for Class I Manufacturers on Compliance with European Communities (Medical Devices) Regulations, 1994 SUR-G0006-2 27 AUGUST 2010 This guide does not purport to be an interpretation of law and/or
Product Catalog (US) Conventional Needles I.V. Catheters Micro-Collection Products Safety Products Winged Infusion Sets
 Product Catalog (US) Conventional Needles I.V. Catheters Micro-Collection Products Safety Products Winged Infusion Sets Table of Contents Hypodermics SurGuard 3 Safety Hypodermic Needle 3-4 Terumo Standard
Product Catalog (US) Conventional Needles I.V. Catheters Micro-Collection Products Safety Products Winged Infusion Sets Table of Contents Hypodermics SurGuard 3 Safety Hypodermic Needle 3-4 Terumo Standard
Guideline for Theraputic Plasma Exchange
 Guideline for Theraputic Plasma Exchange Therapeutic plasma exchange (TPE) removes large-molecular-weight substances such as harmful antibodies from the plasma. It is usually carried out using an automated
Guideline for Theraputic Plasma Exchange Therapeutic plasma exchange (TPE) removes large-molecular-weight substances such as harmful antibodies from the plasma. It is usually carried out using an automated
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program?
 Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL GUIDELINE FOR A LICENCE TO MANUFACTURE, IMPORT, EXPORT OR DISTRIBUTE MEDICAL DEVICES & IVDs This guideline is intended to provide recommendations to applicants wishing to submit
MEDICINES CONTROL COUNCIL GUIDELINE FOR A LICENCE TO MANUFACTURE, IMPORT, EXPORT OR DISTRIBUTE MEDICAL DEVICES & IVDs This guideline is intended to provide recommendations to applicants wishing to submit
P E D A L A P P R O A C H
 PEDAL APPROACH PEDAL APPROACH SET-UP & ACCESS ANGIOGRAPHY INTERVENTION HEMOSTASIS INTEGRATE Merit Medical offers the most comprehensive suite of products for pedal access. From Set Up & Access to Hemostasis,
PEDAL APPROACH PEDAL APPROACH SET-UP & ACCESS ANGIOGRAPHY INTERVENTION HEMOSTASIS INTEGRATE Merit Medical offers the most comprehensive suite of products for pedal access. From Set Up & Access to Hemostasis,
U.S. Food and Drug Administration (FDA) Regulatory Pathways for Medical Devices
 Lisa L. Michels General Counsel & Regulatory Expert Susan J. Schniepp, Distinguished Fellow Regulatory Compliance Associates Inc. U.S. Food and Drug Administration (FDA) Regulatory Pathways for Medical
Lisa L. Michels General Counsel & Regulatory Expert Susan J. Schniepp, Distinguished Fellow Regulatory Compliance Associates Inc. U.S. Food and Drug Administration (FDA) Regulatory Pathways for Medical
Perspective: Convergence of CLIA and FDA Requirements A Rational Shift in the Regulatory Paradigm
 Perspective: Convergence of CLIA and FDA Requirements A Rational Shift in the Regulatory Paradigm Planning for Efficiencies of Data, Resources, and Timelines A PRECISION BRIEF Introduction As precision
Perspective: Convergence of CLIA and FDA Requirements A Rational Shift in the Regulatory Paradigm Planning for Efficiencies of Data, Resources, and Timelines A PRECISION BRIEF Introduction As precision
Developing a European First-in-Human Study: Three Key Decisions
 Developing a European First-in-Human Study: Three Key Decisions By Nicole Feist, BA Clinical A key step in the translational medicine benchtop to bedside process model is the move from research and preclinical
Developing a European First-in-Human Study: Three Key Decisions By Nicole Feist, BA Clinical A key step in the translational medicine benchtop to bedside process model is the move from research and preclinical
GS1 Healthcare - Discussion paper on multi-market packs for pharmaceutical products
 Purpose This GS1 Healthcare paper has been written to help demonstrate ways in which GS1 bar codes can be used to minimise the need for multiple bar codes to appear on product packaging while still enabling
Purpose This GS1 Healthcare paper has been written to help demonstrate ways in which GS1 bar codes can be used to minimise the need for multiple bar codes to appear on product packaging while still enabling
DRAFTS IN WIDE CIRCULATION DOCUMENT DESPATCH ADVICE MHD 10/ T- 2,3,41,33,43,44,45,46,47,48 10/02/2015 TECHNICAL COMMITTEE: MHD 10
 DRAFTS IN WIDE CIRCULATION TECHNICAL COMMITTEE: MHD 10 DOCUMENT DESPATCH ADVICE Ref Date MHD 10/ T- 2,3,41,33,43,44,45,46,47,48 10/02/2015 ADDRESSED TO: 1. All members of Medical Laboratory Instruments
DRAFTS IN WIDE CIRCULATION TECHNICAL COMMITTEE: MHD 10 DOCUMENT DESPATCH ADVICE Ref Date MHD 10/ T- 2,3,41,33,43,44,45,46,47,48 10/02/2015 ADDRESSED TO: 1. All members of Medical Laboratory Instruments
Comfort your patient deserves. Strength you demand.
 POLYSITE LOW PROFILE HYBRID PORTS Comfort your patient deserves. Strength you demand. We never settle because you never settle. Throughout our 35-year history, clinicians and patients have benefited from
POLYSITE LOW PROFILE HYBRID PORTS Comfort your patient deserves. Strength you demand. We never settle because you never settle. Throughout our 35-year history, clinicians and patients have benefited from
Healthcare Sector. - Upcoming legislations - GS1 standards. Ann Luyckx Healthcare Manager. Phi Data Event 2018
 Healthcare Sector - Upcoming legislations - GS1 standards Ann Luyckx Healthcare Manager Phi Data Event 2018 Anti-trust GS1 Belgium & Luxembourg will not enter into any discussion, activity or conduct that
Healthcare Sector - Upcoming legislations - GS1 standards Ann Luyckx Healthcare Manager Phi Data Event 2018 Anti-trust GS1 Belgium & Luxembourg will not enter into any discussion, activity or conduct that
