Solubility Product Constant Lab Calcium Hydroxide Results
|
|
|
- Bennett Hines
- 6 years ago
- Views:
Transcription
1 Constant Lab Calcium Hydroxide Results Free PDF ebook Download: Constant Lab Calcium Hydroxide Results Download or Read Online ebook solubility product constant lab calcium hydroxide results in PDF Format From The Best User Guide Database Lab: Determination of the Constant of an Ionic Compound. Introduction: product constant will be determined for the compound calcium hydroxide.. What additional steps could be taken to give a more accurate result? Experiment 6 AND THE SOLUBILITY PRODUCT CONSTANT OF CALCIUM HYDROXIDE. As a result, it is possible to directly compare the Ksp values of. Calcium hydroxide is an ionic solid that is sparingly soluble in water. A saturated The solubility product expression describes, in mathematical terms, the equilibrium that is. The constant that illustrates a substance's solubility in water is calle Make a data/results table with 3 separate sections, one for each of the three different methods. In this LAD, the solubility constant of calcium hydroxide will be determined by several different methods and the results. AT Center LAB Bench.. ebooks docs Bellow will provide you all similar to solubility product constant lab calcium hydroxide results! the solubility product constant of calcium hydroxide Product Constant Of Calcium Hydroxide equilibrium constant whose values are used to express the solubility of a similar experiment designed to determine the solubility product constant of lead (II). This PDF book include solubility product constant lab calcium hydroxide results information. To download free the solubility product constant of calcium hydroxide you need to
2 Exp 5 The of Calcium Hydroxide Exp 5 The Of Calcium Hydroxide In this experiment, we will measure the concentration of Ca2+(aq) in equilibrium with modestly soluble Ca(OH)2(s) and then calculate the Ksp for Ca(OH)2. This PDF book incorporate solubility product constant lab calcium hydroxide results guide. To download free exp 5 the solubility product of calcium hydroxide you need to LAD F.1 Ksp for Calcium Hydroxide LAD F.1 Ksp For Calcium Hydroxide Make a data/results table with 3 separate sections, one for each of the three different methods. In this LAD, the solubility constant of calcium hydroxide will be determined by several different methods and the results. AT Center LAB Bench. This PDF book incorporate solubility product constant lab calcium hydroxide results conduct. To download free lad f.1 ksp solubility product for calcium hydroxide you need to for Calcium Hydroxide WebAssign For Calcium Hydroxide WebAssign understand solubility equilibria, acid-base neutralization, and the chemistry of lime A saturated solution of Ca(OH)2 can be prepared by the reaction of calcium metal with water. Complete your lab summary or write a report (as instructed). This PDF book contain solubility of calcium hydroxide lab report document. To download free solubility product for calcium hydroxide webassign you need to The Constant of Calcium Iodate Product Constant Of Calcium Iodate The Constant of Calcium Iodate. Background. Weak acids and For example, in this experiment the sparingly soluble salt calcium iodate, Ca(IO3)2, is used. In water this salt would. The Report. Your initial calculations. This PDF book incorporate calcium iodate solubility equilibrium lab report information. To download free the solubility product constant of calcium iodate you need to of Calcium Iodate classes link 1 Of Calcium Iodate Classes Link 1 Introduction: The equilibrium process in this experiment is a saturated aqueous calcium ion, or the molar concentration iodate ion, the solubility product. This PDF book contain calcium iodate solubility equilibrium lab report conduct. To download free solubility product of calcium iodate classes link 1 you need to The of Calcium Iodate Grossmont College Product Of Calcium Iodate Grossmont College Chemistry 142 Grossmont College EXPERIMENT 3. The of. Calcium Iodate. Introduction. The solubility product constant is simply the This PDF book include calcium iodate solubility equilibrium lab report document. To download free the solubility product of calcium iodate grossmont college you need to
3 Constant Constant In general, the solubility product constant (Ksp), is the equilibrium constant for the. (a) Calculate the molar solubility of barium fluoride, BaF2, in water at 25oC. of certain ions in drinking water or surface water can be determined by. This PDF book contain determination of the solubility product of ba information. To download free solubility product constant you need to EXPERIMENT 3.6 SOLUBILITY PRODUCT CONSTANT EXPERIMENT 3.6 SOLUBILITY PRODUCT CONSTANT Solubility equilibria can be solved using the same calculation strategy as In this experiment, you will attempt to estimate the solubility product constant for the. as potassium nitrate is also used as a blank liquid to add to solutions so that a. This PDF book incorporate ksp of lead iodide lab calculations guide. To download free experiment 3.6 solubility product constant you need to Lab: Constant Home Lab: Solubility Product Constant Home Lab: Determination of the Constant of an Ionic Compound. Introduction: product constant will be determined for the compound calcium hydroxide.. What additional steps could be taken to give a more accurate result? This PDF book incorporate solubility product constant lab calcium hydroxide results conduct. To download free lab: solubility product constant home you need to Determination of a Constant and the Determination Of A Constant And The In this experiment we will determine the solubility product constant, Ksp, of the For example, the equilibrium expression for barium sulfate dissolving in water is. This PDF book incorporate determination of the solubility product of ba document. To download free determination of a solubility product constant and the you need to Constant, Pre-Lab Ventura College Constant, Pre-Lab Ventura College Constant, Pre-Lab. Chem1BL, General Chemistry II Lab. 1. As solutions get more dilute, the activity coefficients get closer and closer to 1,. This PDF book include solubility product lab answers information. To download free solubility product constant, pre-lab ventura college you need to Constant, K Illinois Central College Constant, K Illinois Central College The objective of this experiment is to illustrate the use the In general, the solubility product constant (Ksp), is the equilibrium constant for the. is to be used, material is to be handled to avoid loss, and standard solutions prev This PDF book provide solubility product constant lab 17a answers conduct. To download free solubility product constant, k illinois central college you need to
4 Determining the Ksp of Calcium Hydroxide Vernier Software Determining The Ksp Of Calcium Hydroxide Vernier Software Calcium hydroxide is an ionic solid that is sparingly soluble in water. A saturated The solubility product expression describes, in mathematical terms, the equilibrium that is. The constant that illustrates a substance's solubility in water is calle This PDF book contain solubility product constant lab calcium hydroxide results information. To download free determining the ksp of calcium hydroxide vernier software you need to Solubility of Aluminum in the Presence of Hydroxide Fluoride Solubility Of Aluminum In The Presence Of Hydroxide Fluoride estimate aluminum solubility, at 25C and 1 atmosphere total pressure, when the. ph of the gibbsite and their solubilities also are shown on ph-[f] diagrams. This PDF book include aluminum solubility ph guide. To download free solubility of aluminum in the presence of hydroxide fluoride you need to Chem(Bio) Week 11 Ksp and the solubility of Calcium Chem(Bio) Week 11 Ksp And The Solubility Of Calcium Water and possibly harvest plants (details provided in lab). Ksp and this experiment, the solubility of calcium hydroxide, Ca(OH)2, will be determined at. group on the calculations but each person should submit the answers in their own. This PDF book contain solubility of calcium hydroxide lab report information. To download free chem(bio) week 11 ksp and the solubility of calcium you need to CHM 106 of Calcium Iodate BACKGROUND CHM 106 The Solubility Of Calcium Iodate BACKGROUND of Calcium Iodate The purpose of this experiment is to measure the solubility product constant of calcium iodate at. The concentration of the calcium A. Average together all good trials, and report an average value for the. This PDF book include calcium iodate solubility equilibrium lab report guide. To download free chm 106 the solubility of calcium iodate background you need to Calcium Phosphate Solubility LDT Health Solutions, Inc. Calcium Phosphate Solubility LDT Health Solutions, Inc. Mar 5, Baxter's Premasol Amino Acid Solutions. David L. Calcium Phosphate Solubility with B. Braun Medical's TrophAmine and Baxter's. This PDF book incorporate baxter amino acid calcium phosphate solubility conduct. To download free calcium phosphate solubility ldt health solutions, inc. you need to Modelling of calcium sulphate solubility in concentrated Modelling Of Calcium Sulphate Solubility In Concentrated Meissner or Pitzer models to predict the solubility of calcium sulphate perature up to C. The regressed solubility curve is presented in Fig. 2. Contrary. This PDF book incorporate model 2 solubility curves information. To download free modelling of calcium sulphate solubility in concentrated you need to
5 Solubility and Structure of Calcium Silicate Hydrate Civil Solubility And Structure Of Calcium Silicate Hydrate Civil Calcium silicate hydrates possess a remarkable level of structural complexity. The CaO layers in jennite have the empirical formula Ca2O5 and are highly. This PDF book incorporate empirical formula calcium silicate conduct. To download free solubility and structure of calcium silicate hydrate civil you need to Solubility Constant Solubility Constant In today's experiment, you will calculate the solubility of the ionic compound, calcium iodate, Ca(IO3)2. The solution equilibrium for the substance is: Ca(IO3)2(s) This PDF book include calcium iodate solubility equilibrium lab report document. To download free solubility constant you need to Part One: Solubility Equilibria A. Ksp, the Part One: Solubility Equilibria A. Ksp, Product CHAPTER 17: SOLUBILITY AND COMPLEX ION EQUILIBRIA. Part One: Note - in all saturated aqueous solutions of BaSO4, no matter what other materials are. For Bi2S3 with molar solubility = s (See lab manual - Experiments #21-26). This PDF book incorporate experiment 26 solubility product lab answers information. To download free part one: solubility equilibria a. ksp, the solubility product you need to Solubility and Complex-Ion Equilibria The Solubility And Complex-Ion Equilibria The W rite the solubility product (Ksp) expression for: Calculate the solubility of barium sulfate (a. Determine the amount of I- in the solution and extent of dilution:. This PDF book incorporate determination of the solubility product of ba guide. To download free solubility and complex-ion equilibria the solubility product you need to Determining the Solubility Constant for a Slightly Soluble Salt Determining The Solubility Constant For A Slightly Soluble Salt Constant Lab. Text reference: Chapter 18, pp Pre-Lab Discussion. There are many ionic compounds considered to be insoluble This PDF book incorporate soluble and insoluble salts lab guide. To download free determining the solubility constant for a slightly soluble salt you need to 14.4 (Chp16) Solubility and 14.4 (Chp16) Solubility And Mar 1, What is the solubility product for the following reaction: Ag. 3. PO How can one predict if a precipitate forms when mixing solutions? Solubility. This PDF book include experiment 26 solubility product lab answers document. To download free 14.4 (chp16) solubility and solubility product you need to Product Safety Summary of Calcium Sulfate Product Safety Summary Of Calcium Sulfate Nov 1, provide a general overview of the chemical substance. Calcium sulphate (CaSO4) is a white, odorless,. Molecular weight: 136 g/mol. Uses. This PDF book include chemical structure of calcium sulphate guide. To download free product safety summary of calcium sulfate you need to
6 Unit 2 Day #2 Solubility Curves Investigation Results.pdf Unit 2 Day #2 Solubility Curves Investigation Results.pdf Unit #2: INVESTIGATION #1: SOLUBILITY OF POTASSIUM NITRATE g , g , g , Can't answer from graph as it is based on 100g of water or 100 ml and this asked for 20. This PDF book contain unit 8 solubility and solubility curve answers conduct. To download free unit 2 day #2 solubility curves investigation results.pdf you need to LAB 3.2: THE SOLUBILITY PRODUCT OF Ca(OH)2 LAB 3.2: THE SOLUBILITY PRODUCT OF Ca(OH)2 LAB 3.2: THE SOLUBILITY PRODUCT OF Ca(OH)2 Since Ksp is an equilibrium constant, its value should remain constant even when the. 1- Calculate the [OH'] in the saturated solution of Ca(OH)2 in pure water from the results of the. This PDF book contain solubility product constant lab calcium hydroxide results information. To download free lab 3.2: the solubility product of ca(oh)2 you need to Measuring the of Ca(OH)2 Measuring The Of Ca(OH)2 Lab: Measuring the of Ca(OH)2 Because the equilibrium expression is a "product" (the result of multiplying numbers together),. equilibrium constant is called the "solubility product", and is represented by the symb This PDF book contain solubility product constant lab calcium hydroxide results document. To download free measuring the solubility product of ca(oh)2 you need to DETERMINATION OF THE SOLUBILITY PRODUCT OF DETERMINATION OF THE SOLUBILITY PRODUCT OF In this experiment you will determine the Ksp of three (essentially) insoluble. Would you be able to determine the solubility product of barium sulfate using this. This PDF book incorporate determination of the solubility product of ba guide. To download free determination of the solubility product of you need to SOLUBILITY PRODUCT CONSTANTS SOLUBILITY PRODUCT CONSTANTS SOLUBILITY PRODUCT CONSTANTS. Compound. Formula. Ksp (25 C). Aluminium hydroxide. Al(OH) Aluminium phosphate. AlPO This PDF book contain crc handbook ksp values conduct. To download free solubility product constants you need to The of PbCl2 from Electrochemical Product Of PbCl2 From Electrochemical The experiment presented in this article is not found in physical chemistry laboratory textbooks; however, it reinforces rium constant of a reaction are related to the emf, at different istry, 3rd ed.; Holt, Rinehart and Winston: New York, 1976;. This PDF book provide solubility product constant lab holt chemistry conduct. To download free the solubility product of pbcl2 from electrochemical you need to
7 Experiment # 10: Determination Experiment # 10: Determination when the value of Q exceeds the solubility product value of lead iodide (i.e., when Q > Ksp). A single-page report will be submitted for this experiment. This PDF book incorporate ksp of lead iodide lab calculations document. To download free experiment # 10: solubility product determination you need to Worksheet Answers Worksheet Answers Solubility product constants are usually specified for 250 C. Why does the. Ksp value for a chemical compound depend on the temperature? 5). The Ksp for This PDF book include solubility and temperature answers guide. To download free solubility product worksheet answers you need to Constants WebAssign Constants WebAssign 3 To calculate the solubility product constant (Ksp) of a sparingly soluble salt from its molar Earlier this semester in the Solutions and Spectroscopy lab, you prepared a. In this experiment, you will prepare a calibration curve for Cu(NH3)4 26 Collec This PDF book provide experiment 26 solubility product lab answers conduct. To download free solubility product constants webassign you need to Experiment 24 Determination of Pblz Experiment 24 Determination Of Pblz Solubility Product experiment date: Page 1 of 1. Experiment 24 - Determination of Pblz Constant Place test tubes in the special bin in the back of the lab. This PDF book contain experiment 26 solubility product lab answers information. To download free experiment 24 determination of pblz solubility product you need to Determination of the of an Ionic Compound Determination Of Product Of An Ionic Compound In this experiment you will determine the solubility and solubility product of a slightly soluble salt. INTRODUCTION. When a salt of low solubility dissolves in water, equilibrium is established In this laboratory you will determine the Ksp for the very This PDF book include calcium iodate solubility equilibrium lab report conduct. To download free determination of the solubility product of an ionic compound you need to 39 Laboratory Manual Chapter 18 A 39 Laboratory Manual Chapter 18 A Solubility Product dissolved substance in the solution? One way is to use the Ksp, the solubility product constant. In this experiment, you will find the Ksp of a slightly soluble salt,. This PDF book provide experiment 17 solubility product document. To download free 39 laboratory manual chapter 18 a solubility product you need to
8 Gravimetric Analysis of Calcium as Calcium Oxalate Gravimetric Analysis Of Calcium As Calcium Oxalate Gravimetric Determination of Calcium as Calcium Oxalate Monohydrate.pdf Put your water bottle into the ice bath so you'll have cold water for step minutes or until the indicator turns yellow (the yellow color may be hard to see but. This PDF book incorporate gravimetric analysis of calcium and hard water information. To download free gravimetric analysis of calcium as calcium oxalate you need to Gravimetric Determination of Calcium as Calcium Oxalate Gravimetric Determination Of Calcium As Calcium Oxalate Gravimetric Determination of Calcium as Calcium Oxalate Monohydrate. Introduction: Calcium ion can be analyzed by precipitation with oxalate in basic solution This PDF book incorporate gravimetric determination of calcium document. To download free gravimetric determination of calcium as calcium oxalate you need to Calcium Gluconate Chemical Name: Calcium Enzymax Calcium Gluconate Chemical Name: Calcium Enzymax Synonyms: Calcium d-clugconate;calcium gluconate;calcium gluconate;gluconic adci calcium salt. Molecular formula: C12H22CaO14. Molecular weight: This PDF book include chemical structure of calciumgluconate guide. To download free calcium gluconate chemical name: calcium enzymax you need to to see some examples of product testing results from To See Some Examples Of Product Testing Results From A8 Eureka. A] HOOVGT A3 Hoover WindTunnel Bagless. bagless vac; NA indicates bag type not available. Under plus manual controls and more zoom than most. 16 Pentaxoptiopzo loo E zorjq Q Q Q Q zoo 5 0. This PDF book include eureka 411b bagless manual information. To download free to see some examples of product testing results from you need to mybooklibrary.com
Solubility Product Constant Ksp Of Kno3
 Constant Ksp Of Kno3 Free PDF ebook Download: Constant Ksp Of Kno3 Download or Read Online ebook solubility product constant ksp of kno3 in PDF Format From The Best User Guide Database The objective of
Constant Ksp Of Kno3 Free PDF ebook Download: Constant Ksp Of Kno3 Download or Read Online ebook solubility product constant ksp of kno3 in PDF Format From The Best User Guide Database The objective of
Solubility Equilibrium
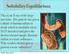 Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
Solubility Equilibrium This is an X-ray of the large intestine. The patient was given a drink of barium sulfate to drink which is insoluble (does NOT dissolve) and gives the doctor a better image. Barium
Chapter 17 Solubility and Complex Ion Equilibria
 Chem 1046 General Chemistry by Ebbing and Gammon, 8th Edition Prof George W.J. Kenney, Jr Last Update: 27Nov2008 Chapter 17 Solubility and Complex Ion Equilibria These Notes are to SUPPLIMENT the Text,
Chem 1046 General Chemistry by Ebbing and Gammon, 8th Edition Prof George W.J. Kenney, Jr Last Update: 27Nov2008 Chapter 17 Solubility and Complex Ion Equilibria These Notes are to SUPPLIMENT the Text,
Equilibria of Slightly Soluble Ionic Compounds: We lied to you in chem 115
 Precipitation Reactions Predicting Whether a Precipitate Will Form Note the ions present in the reactants Consider all possible cation-anion combinations Use the solubility rules to decide whether any
Precipitation Reactions Predicting Whether a Precipitate Will Form Note the ions present in the reactants Consider all possible cation-anion combinations Use the solubility rules to decide whether any
Chapter 9. Dissolution and Precipitation Equilibria
 Chapter 9 Dissolution and Precipitation Equilibria 9-1 The Nature of Solubility Equilibria 9-2 The Solubility of Ionic Solids 9-3 Precipitation and the Solubility Product 9-4 The Effects of ph on Solubility
Chapter 9 Dissolution and Precipitation Equilibria 9-1 The Nature of Solubility Equilibria 9-2 The Solubility of Ionic Solids 9-3 Precipitation and the Solubility Product 9-4 The Effects of ph on Solubility
Solutions Unit Exam Name Date Period
 Name Date Period Ms. Roman Page 1 Regents Chemistry 1. Which mixture can be separated by using the equipment shown below? 6. Which ion, when combined with chloride ions, Cl, forms an insoluble substance
Name Date Period Ms. Roman Page 1 Regents Chemistry 1. Which mixture can be separated by using the equipment shown below? 6. Which ion, when combined with chloride ions, Cl, forms an insoluble substance
2/24/2003 OFB Chapter 9 1
 2/24/2003 OFB Chapter 9 1 Chapter 9 Dissolution and Precipitation Equilibria 9-1 The Nature of Solubility Equilibria 9-2 The Solubility of Ionic Solids 9-3 Precipitation and the Solubility Product 9-4
2/24/2003 OFB Chapter 9 1 Chapter 9 Dissolution and Precipitation Equilibria 9-1 The Nature of Solubility Equilibria 9-2 The Solubility of Ionic Solids 9-3 Precipitation and the Solubility Product 9-4
AP*Chemistry Solubility Equilibria
 AP*Chemistry Solubility Equilibria SOLUBILITY EQUILIBRIA (K sp, THE SOLUBILITY PRODUCT) Saturated solutions of salts are another type of chemical equilibria. Remember those solubility rules? The fine print
AP*Chemistry Solubility Equilibria SOLUBILITY EQUILIBRIA (K sp, THE SOLUBILITY PRODUCT) Saturated solutions of salts are another type of chemical equilibria. Remember those solubility rules? The fine print
Chapter 9 Systematic Equilibrium
 Chapter 9 Systematic Equilibrium 1 What we Skipped Ch. 8 discussed Activity (γ) This is what should be used in equilibrium calculations rather than conc. (molarity) In Ch. 9 in later chapters, you will
Chapter 9 Systematic Equilibrium 1 What we Skipped Ch. 8 discussed Activity (γ) This is what should be used in equilibrium calculations rather than conc. (molarity) In Ch. 9 in later chapters, you will
Chapter six. Precipitate is separated by filtration, washing,drying and finally is accurately weighed.
 Gravimetric Analysis Chapter six Gravimetric Analysis :- Is one of the most accurate and precise methods of quantative analysis.it is based on the measurements of the mass of solid product of a chemical
Gravimetric Analysis Chapter six Gravimetric Analysis :- Is one of the most accurate and precise methods of quantative analysis.it is based on the measurements of the mass of solid product of a chemical
SOLUBILITY STUDY GUIDE- Multiple Choice Section
 SOLUBILITY STUDY GUIDE- Multiple Choice Section Multiple Choice Section: This study guide is a compilation of questions from provincial exams since 2000. I urge you to become intimately familiar with question
SOLUBILITY STUDY GUIDE- Multiple Choice Section Multiple Choice Section: This study guide is a compilation of questions from provincial exams since 2000. I urge you to become intimately familiar with question
Lecture 11. Solubility of Ionic Compounds in Water
 Lecture 11 Solubility of Ionic Compounds in Water Soluble Compounds Exceptions Acetates, Nitrates, Chlorates Na +, K +, NH 4 + compounds None None Halides ( Cl -, Br -, I - ) Ag +, Hg 2 2+, Cu +, Pb 2+
Lecture 11 Solubility of Ionic Compounds in Water Soluble Compounds Exceptions Acetates, Nitrates, Chlorates Na +, K +, NH 4 + compounds None None Halides ( Cl -, Br -, I - ) Ag +, Hg 2 2+, Cu +, Pb 2+
Solubility Equi librium Ksp. Chapter 17 Sections
 Solubility Equi librium Ksp Chapter 17 Sections 17.4 17.6 1 Precipitation Reactions Earlier in this course, we considered precipitation reactions zinc nitrate reacts with sodium hydroxide Zn(NO3)2 + 2NaOH
Solubility Equi librium Ksp Chapter 17 Sections 17.4 17.6 1 Precipitation Reactions Earlier in this course, we considered precipitation reactions zinc nitrate reacts with sodium hydroxide Zn(NO3)2 + 2NaOH
Chapter 18 Homework Answers
 Chapter 18 Homework Answers 18.19 (a) AgI(s) Ag + + I K sp = [Ag + ][I ] (b) Ag PO (s) Ag + + PO K sp = [Ag + ] [PO ] (c) PbCrO (s) Pb + + CrO K sp = [Pb + ][CrO ] (d) Al(OH) (s) Al + + OH K sp = [Al +
Chapter 18 Homework Answers 18.19 (a) AgI(s) Ag + + I K sp = [Ag + ][I ] (b) Ag PO (s) Ag + + PO K sp = [Ag + ] [PO ] (c) PbCrO (s) Pb + + CrO K sp = [Pb + ][CrO ] (d) Al(OH) (s) Al + + OH K sp = [Al +
Metal Hydroxides Solubility Curve With Ph
 Metal Hydroxides Solubility Curve With Ph Free PDF ebook Download: Metal Hydroxides With Ph Download or Read Online ebook metal hydroxides solubility curve with ph in PDF Format From The Best User Guide
Metal Hydroxides Solubility Curve With Ph Free PDF ebook Download: Metal Hydroxides With Ph Download or Read Online ebook metal hydroxides solubility curve with ph in PDF Format From The Best User Guide
Please write the balanced net ionic reaction for each one. Then answer the accompanying question.
 AP Chemistry Net Ionic Rx Practice Test A CLASS SET PLEASE RETURN!! Please write the balanced net ionic reaction for each one. Then answer the accompanying question. 1) A piece of potassium is dropped
AP Chemistry Net Ionic Rx Practice Test A CLASS SET PLEASE RETURN!! Please write the balanced net ionic reaction for each one. Then answer the accompanying question. 1) A piece of potassium is dropped
1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is.. The precipitate which forms first is
 CHEMISTRY 12 UNIT 3 - REVIEW Ksp Part A - Multiple Choice 1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is A. Fe 2 (SO 4 ) 3 (s) 3 Fe 2+ (aq) + 2 SO 4 3 (aq)
CHEMISTRY 12 UNIT 3 - REVIEW Ksp Part A - Multiple Choice 1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is A. Fe 2 (SO 4 ) 3 (s) 3 Fe 2+ (aq) + 2 SO 4 3 (aq)
Solubility Equi librium Ksp. Chapter 17 Sections
 Solubility Equi librium Ksp Chapter 17 Sections 17.4 17.6 Precipitation Reactions Earlier in this course, we considered precipitation reactions zinc nitrate reacts with sodium hydroxide Zn(NO3)2 + 2NaOH
Solubility Equi librium Ksp Chapter 17 Sections 17.4 17.6 Precipitation Reactions Earlier in this course, we considered precipitation reactions zinc nitrate reacts with sodium hydroxide Zn(NO3)2 + 2NaOH
NANDI NORTH DISTRICT JOINT MOCK EVALUATION TEST 2013
 NAME:. SIGNATURE: INDEX NO:. DATE :.. 233/1 CHEMISTRY PAPER 1 THEORY JULY / AUGUST 2013 TIME: 2 HOURS NANDI NORTH DISTRICT JOINT MOCK EVALUATION TEST 2013 Kenya Certificate of Secondary Education (K.C.S.E.)
NAME:. SIGNATURE: INDEX NO:. DATE :.. 233/1 CHEMISTRY PAPER 1 THEORY JULY / AUGUST 2013 TIME: 2 HOURS NANDI NORTH DISTRICT JOINT MOCK EVALUATION TEST 2013 Kenya Certificate of Secondary Education (K.C.S.E.)
Tutorial 3 Unit 3 - Solubility
 Tutorial 3 Unit 3 - Solubility The Answer is A Answer A is the primary condition for solubility equilibrium. Remember the solution is also called saturated. B, of course is wrong because these opposing
Tutorial 3 Unit 3 - Solubility The Answer is A Answer A is the primary condition for solubility equilibrium. Remember the solution is also called saturated. B, of course is wrong because these opposing
2.4 Period 3. Na Mg Al Si P S Cl Ar
 2.4 Period 3 Period 3 Na Mg Al Si P S Cl Ar Periodicity: Periodicity: The repeating trends in physical and chemical properties of elements as you go across the Periodic Table Periods often show gradual
2.4 Period 3 Period 3 Na Mg Al Si P S Cl Ar Periodicity: Periodicity: The repeating trends in physical and chemical properties of elements as you go across the Periodic Table Periods often show gradual
3. [7 points] How many significant figures should there be in the answer to the following problem?
![3. [7 points] How many significant figures should there be in the answer to the following problem? 3. [7 points] How many significant figures should there be in the answer to the following problem?](/thumbs/83/87492512.jpg) 1 of 6 10/20/2009 3:52 AM 1. [7 points] What is the correct name for ZnF 2? (a) zinc fluorine (b) zinc (II) fluoride (c) zinc fluoride (d) fluoride zinc 2. [7 points] What is the correct name for P 2 O
1 of 6 10/20/2009 3:52 AM 1. [7 points] What is the correct name for ZnF 2? (a) zinc fluorine (b) zinc (II) fluoride (c) zinc fluoride (d) fluoride zinc 2. [7 points] What is the correct name for P 2 O
Ksp Take Home Recitation
 Ksp Take Home Recitation Names: Compound Formula Ksp at 298 K, 1 atm Aluminum hydroxide Al(OH)3 1.3x10-33 Aluminum phosphate Al(PO4)3 9.84x10-21 Aluminum nitrate Al(NO3)3 1.6x10-2 Barium carbonate BaCO3
Ksp Take Home Recitation Names: Compound Formula Ksp at 298 K, 1 atm Aluminum hydroxide Al(OH)3 1.3x10-33 Aluminum phosphate Al(PO4)3 9.84x10-21 Aluminum nitrate Al(NO3)3 1.6x10-2 Barium carbonate BaCO3
Quantitive Chemistry Question paper
 Quantitive Chemistry Question paper Level Subject Exam Board Topic Sub-Topic Booklet GCSE Chemistry CCEA Quantitative Chemistry Quantitive Chemistry Question paper Time Allowed: 93 minutes Score: /77 Percentage:
Quantitive Chemistry Question paper Level Subject Exam Board Topic Sub-Topic Booklet GCSE Chemistry CCEA Quantitative Chemistry Quantitive Chemistry Question paper Time Allowed: 93 minutes Score: /77 Percentage:
Properties Of Metals And Nonmetals If8767
 And Nonmetals If8767 Free PDF ebook Download: And Nonmetals If8767 Download or Read Online ebook properties of metals and nonmetals if8767 in PDF Format From The Best User Guide Database metals, nonmetals
And Nonmetals If8767 Free PDF ebook Download: And Nonmetals If8767 Download or Read Online ebook properties of metals and nonmetals if8767 in PDF Format From The Best User Guide Database metals, nonmetals
CHEMISTRY. SCIENCE Paper 2. (Two hours) You will not be allowed to write during the first 15 minutes.
 CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
CHEMISTRY SCIENCE Paper 2 (Two hours) Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
14.4 (Chp16) Solubility and Solubility Product
 14.4 (Chp16) Solubility and Solubility Product What drives substances to dissolve and others to remain as a precipitate? Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Solubility and Solubility
14.4 (Chp16) Solubility and Solubility Product What drives substances to dissolve and others to remain as a precipitate? Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Solubility and Solubility
Lafayette College Department of Civil and Environmental Engineering
 Lafayette College Department of Civil and Environmental Engineering CE 321: Environmental Engineering and Science Fall 2017 Homework #12 Due Tuesday: 12/12/17 SOLUTIONS The town of Easton is considering
Lafayette College Department of Civil and Environmental Engineering CE 321: Environmental Engineering and Science Fall 2017 Homework #12 Due Tuesday: 12/12/17 SOLUTIONS The town of Easton is considering
9.2.1 Similarities and trends in the properties of the Group II metals magnesium to barium and their compounds
 9.2 Group II Content 9.2.1 Similarities and trends in the properties of the Group II metals magnesium to barium and their compounds Learning Outcomes Candidates should be able to: (a) (b) (c) (d) describe
9.2 Group II Content 9.2.1 Similarities and trends in the properties of the Group II metals magnesium to barium and their compounds Learning Outcomes Candidates should be able to: (a) (b) (c) (d) describe
Department of Chemistry University of Texas at Austin
 Colligative Properties Supplemental Worksheet PROBLEM #1: Give the ecular formula, the van t hoff factor for the following Ionic Compounds as well as guess the solubility of the compounds. If you cannot
Colligative Properties Supplemental Worksheet PROBLEM #1: Give the ecular formula, the van t hoff factor for the following Ionic Compounds as well as guess the solubility of the compounds. If you cannot
Compounds & Reactions Week 1. Writing Formulas & Balancing Equations. Write the chemical formula for each molecular (covalent) compound.
 Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Solution 2: Class X Chapter-4 Analytical Chemistry Chemistry. Book Name: Selina Concise EXERCISE- 1 (A)
 EXERCISE- 1 (A) Book Name: Selina Concise Question 1: Write the probable colour of the following salts. (a) Ferrous salts (b) Ammonium salts (c) Cupric salts (d) Calcium salts (e) Aluminium Salts Solution
EXERCISE- 1 (A) Book Name: Selina Concise Question 1: Write the probable colour of the following salts. (a) Ferrous salts (b) Ammonium salts (c) Cupric salts (d) Calcium salts (e) Aluminium Salts Solution
Slide 1. Slide 2. Slide 3 Equilibrium problems involve 3 parts: The End of Equilibrium! K sp. (well, for us!) What is the solubility of FeCO 3?
 Slide 1 The End of Equilibrium! (well, for us!) 1 Slide 2 K sp What is the solubility of FeCO 3? Solubility = MAXIMUM amount of a compound that can dissolve in water. This is actually an equilibrium. 2
Slide 1 The End of Equilibrium! (well, for us!) 1 Slide 2 K sp What is the solubility of FeCO 3? Solubility = MAXIMUM amount of a compound that can dissolve in water. This is actually an equilibrium. 2
Gypsum Solubility Simulating Real World Aqueous Problems
 Simulating Real World Aqueous Problems or Getting an edge on the competition! Reliable simulation of complex processes can provide insights for process optimization and reduced operating costs. The solubility
Simulating Real World Aqueous Problems or Getting an edge on the competition! Reliable simulation of complex processes can provide insights for process optimization and reduced operating costs. The solubility
Exercises. Solubility Equilibria. a. AgC 2 H 3 O 2 b. Al(OH) 3 c. Ca 3 (PO 4 ) Write balanced equations for the dissolution reactions and the
 766 Active Learning Questions These questions are designed to be used by groups of students in class. 1. Which of the following will affect the total amount of solute that can dissolve in a given amount
766 Active Learning Questions These questions are designed to be used by groups of students in class. 1. Which of the following will affect the total amount of solute that can dissolve in a given amount
Topic 2.6 GROUP 2, THE ALKALINE EARTH METALS
 Topic 2.6 GROUP 2, THE ALKALINE EARTH METALS Trends in size, first ionization energy and electronegativity Trends in reaction with water Trends in solubility of group 2 sulphates and hydroxides PROPERTIES
Topic 2.6 GROUP 2, THE ALKALINE EARTH METALS Trends in size, first ionization energy and electronegativity Trends in reaction with water Trends in solubility of group 2 sulphates and hydroxides PROPERTIES
BORABU-MASABA DISTRICTS JOINT EVALUATION TEST 2012 Kenya Certificate of Secondary Education (K.C.S.E)
 Name. School Candidate s Signature. Index No /. Date. 233/2 CHEMISTRY Paper 2 (Theory) JULY / AUGUST - 2012 Time: 2 Hours BORABU-MASABA DISTRICTS JOINT EVALUATION TEST 2012 Kenya Certificate of Secondary
Name. School Candidate s Signature. Index No /. Date. 233/2 CHEMISTRY Paper 2 (Theory) JULY / AUGUST - 2012 Time: 2 Hours BORABU-MASABA DISTRICTS JOINT EVALUATION TEST 2012 Kenya Certificate of Secondary
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET
 INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
ICSE-Science 2 (Chemistry) 2004
 ICSE-Science 2 (Chemistry) 2004 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
ICSE-Science 2 (Chemistry) 2004 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
Q1. From the following list of substances, choose the substances which meet the description given in parts (i) to (v) below :
 Questions:- Q1. From the following list of substances, choose the substances which meet the description given in parts (i) to (v) below : Ammonium chloride, ammonium nitrate, chlorine, dilute hydrochloric
Questions:- Q1. From the following list of substances, choose the substances which meet the description given in parts (i) to (v) below : Ammonium chloride, ammonium nitrate, chlorine, dilute hydrochloric
*20GSD5201* Double Award Science: Chemistry. Unit C2 Higher Tier WEDNESDAY 15 JUNE 2016, AFTERNOON [GSD52] *GSD52* *G5802* TIME 1 hour 15 minutes.
![*20GSD5201* Double Award Science: Chemistry. Unit C2 Higher Tier WEDNESDAY 15 JUNE 2016, AFTERNOON [GSD52] *GSD52* *G5802* TIME 1 hour 15 minutes. *20GSD5201* Double Award Science: Chemistry. Unit C2 Higher Tier WEDNESDAY 15 JUNE 2016, AFTERNOON [GSD52] *GSD52* *G5802* TIME 1 hour 15 minutes.](/thumbs/84/89360002.jpg) Centre Number Candidate Number General Certificate of Secondary Education 2016 Double Award Science: Chemistry Unit C2 Higher Tier [GSD52] *GSD52* *G5802* *GSD52* WEDNESDAY 15 JUNE 2016, AFTERNOON TIME
Centre Number Candidate Number General Certificate of Secondary Education 2016 Double Award Science: Chemistry Unit C2 Higher Tier [GSD52] *GSD52* *G5802* *GSD52* WEDNESDAY 15 JUNE 2016, AFTERNOON TIME
1. Nickel has a face-centered-cubic unit cell. The density of nickel is 6.84 g/cm 3. Calculate the atomic radius of nickel.
 ADDITIONAL PRACTICE ON UNIT CELLS 1. Nickel has a face-centered-cubic unit cell. The density of nickel is 6.84 g/cm 3. Calculate the atomic radius of nickel. 2. Tungsten metal exists in a body-centered-cubic
ADDITIONAL PRACTICE ON UNIT CELLS 1. Nickel has a face-centered-cubic unit cell. The density of nickel is 6.84 g/cm 3. Calculate the atomic radius of nickel. 2. Tungsten metal exists in a body-centered-cubic
21. sodium nitrite 31. potassium carbonate. 23. aluminum hydroxide 33. nickel (II) carbonate. 24. ammonium hydroxide 34.
 N.E. Packet 1 Nomenclature WS 1 (Ionic Compounds) Name: Date: Per: Write the name for each of the following compounds. 1. CaCO 3 11. BaSO 4 2. FeO 12. Zn(NO 3 ) 2 3. K 2 SO 3 13. CuSO 4 4. AgCl 14. AlCl
N.E. Packet 1 Nomenclature WS 1 (Ionic Compounds) Name: Date: Per: Write the name for each of the following compounds. 1. CaCO 3 11. BaSO 4 2. FeO 12. Zn(NO 3 ) 2 3. K 2 SO 3 13. CuSO 4 4. AgCl 14. AlCl
1. What volume of water is required to make a 4.65 M solution from 5.2 g of NaBr (MM = g/mol)?
 CHEMISTRY 110 EXAM II Answer Key March 22, 2013 1. What volume of water is required to make a 4.65 M solution from 5.2 g of NaBr (MM = 102.894 g/mol)? A. 4.65 ml B. 10.9 ml C. 11 ml D. 235 ml E. 240 ml
CHEMISTRY 110 EXAM II Answer Key March 22, 2013 1. What volume of water is required to make a 4.65 M solution from 5.2 g of NaBr (MM = 102.894 g/mol)? A. 4.65 ml B. 10.9 ml C. 11 ml D. 235 ml E. 240 ml
ADVANCED AP PLACEMENT CHEMISTRY. Activity Series. Introduction. Objective. Chemicals and Equipment
 ADVANCED AP PLACEMENT CHEMISTRY Introduction Activity Series An activity series of metals is a table of metals arranged in the order of their decreasing chemical activity or the ease at which the metal
ADVANCED AP PLACEMENT CHEMISTRY Introduction Activity Series An activity series of metals is a table of metals arranged in the order of their decreasing chemical activity or the ease at which the metal
Duncan. UNIT 8 - Chemical Equations BALANCING EQUATIONS PRACTICE WORKSHEET 14.) C2H6 + O2 CO2 + H2O. 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.
 BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
FACTFILE: GCE CHEMISTRY
 FACTFILE: GCE CHEMISTRY 2.11 GROUP II ELEMENTS AND THEIR COMPOUNDS Learning Outcomes Students should be able to: 2.11.1 explain why these are regarded as s-block elements; 2.11.2 recall and explain the
FACTFILE: GCE CHEMISTRY 2.11 GROUP II ELEMENTS AND THEIR COMPOUNDS Learning Outcomes Students should be able to: 2.11.1 explain why these are regarded as s-block elements; 2.11.2 recall and explain the
JSUNIL TUTORIAL, SAMASTIPUR
 Chapter 1 Chemical Reactions and Equations Q 1. Why should a magnesium ribbon be cleaned before burning in air? Ans. Before burning in air, the magnesium ribbon is cleaned by rubbing with a sandpaper.
Chapter 1 Chemical Reactions and Equations Q 1. Why should a magnesium ribbon be cleaned before burning in air? Ans. Before burning in air, the magnesium ribbon is cleaned by rubbing with a sandpaper.
GEOCHEMICAL MODULE FOR AMDTreat
 GEOCHEMICAL MODULE FOR AMDTreat Charles A. Cravotta III and David L. Parkhurst, U.S. Geological Survey Brent P. Means, Robert M. McKenzie, and Bill Arthur, U.S. Office of Surface Mining Reclamation and
GEOCHEMICAL MODULE FOR AMDTreat Charles A. Cravotta III and David L. Parkhurst, U.S. Geological Survey Brent P. Means, Robert M. McKenzie, and Bill Arthur, U.S. Office of Surface Mining Reclamation and
Sodium Peroxides (Na 2 O 2 ): Preparation: It is formed by heating the metal in excess of air or oxygen at 300, which is free from
 S-Block Elements Generally one question was asked every year from this topic. This is completely theoretical and little memory based. Last minute revision generally helps. The general trends in the properties
S-Block Elements Generally one question was asked every year from this topic. This is completely theoretical and little memory based. Last minute revision generally helps. The general trends in the properties
Unit F FR (part B) Solubility Equilibrium, Ksp (pg 1 of 16)
 Unit F FR (part B) Solubility Equilibrium, Ksp (pg 1 of 16) 1. Answer the following questions about the solubility of some fluoride salts of alkaline earth metals. (a) A student prepares 100. ml of a saturated
Unit F FR (part B) Solubility Equilibrium, Ksp (pg 1 of 16) 1. Answer the following questions about the solubility of some fluoride salts of alkaline earth metals. (a) A student prepares 100. ml of a saturated
Page 2. Q is calcium or magnesium 1. M1.(a) bromide 1. R is aluminium 1. chloride 1. S is iron(iii) 1. sulfate. Mark this question independently
 M.(a) Q is calcium or magnesium bromide R is aluminium chloride S is iron(iii) sulfate Mark this question independently (b) Ba 2+ + SO 4 2 BaSO 4 [Fe(H 2O) 6] 3+ + 3OH Fe(H 2O) 3(OH) 3 + 3H 2O 2[Fe(H 2O)
M.(a) Q is calcium or magnesium bromide R is aluminium chloride S is iron(iii) sulfate Mark this question independently (b) Ba 2+ + SO 4 2 BaSO 4 [Fe(H 2O) 6] 3+ + 3OH Fe(H 2O) 3(OH) 3 + 3H 2O 2[Fe(H 2O)
TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION
 EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
EXPERIMENT 10 (2 Weeks) Chemistry 100 Laboratory TYPES OF CHEMICAL REACTIONS PART I INTRODUCTION It is useful to classify reactions into different types, because products of reactions can be predicted.
Compiled by Rahul Arora What do you mean by corrosion? How can you prevent it?
 Rahul Arora 12. What do you mean by corrosion? How can you prevent it? 13. MnO2 + 4HCl MnCl2 + 2H2O + Cl2 In the above equation, name the compound which is oxidized and which is reduced? 14. Match the
Rahul Arora 12. What do you mean by corrosion? How can you prevent it? 13. MnO2 + 4HCl MnCl2 + 2H2O + Cl2 In the above equation, name the compound which is oxidized and which is reduced? 14. Match the
MR. D HR UV AS HE R I.C.S.E. BOA RD PAP ER ICSE-2005
 MR D HR UV AS HE R ICSE BOA RD PAP ER 200 5 1 ICSE-2005 Section A (40 Marks) (Attempt all questions from this section) Question 1 (a) Write balanced equation s for the following reactions: - [5] (i) Potassium
MR D HR UV AS HE R ICSE BOA RD PAP ER 200 5 1 ICSE-2005 Section A (40 Marks) (Attempt all questions from this section) Question 1 (a) Write balanced equation s for the following reactions: - [5] (i) Potassium
MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE. CHEMISTRY OCTOBER hour
 MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE CANDIDATE NAME SCHOOL NAME CLASS/SECTION NATIONAL ASSESSMENT AT FORM III CHEMISTRY
MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE CANDIDATE NAME SCHOOL NAME CLASS/SECTION NATIONAL ASSESSMENT AT FORM III CHEMISTRY
Water Chemistry and Plant Performance
 Plant Operations and Laboratory Analysis Specialty Workshop Water Chemistry and Plant Performance September 1, 2010 Dan Miklos Water Environment Association Preserving & Enhancing Ohio s Water Environment
Plant Operations and Laboratory Analysis Specialty Workshop Water Chemistry and Plant Performance September 1, 2010 Dan Miklos Water Environment Association Preserving & Enhancing Ohio s Water Environment
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION
 GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
CH 112 Special Assignment #4 Chemistry to Dye for: Part A
 CH 112 Special Assignment #4 Chemistry to Dye for: Part A PRE-LAB ASSIGNMENT: Make sure that you read this handout and bring the essentials to lab with you. Here are the pre-lab questions for this week.
CH 112 Special Assignment #4 Chemistry to Dye for: Part A PRE-LAB ASSIGNMENT: Make sure that you read this handout and bring the essentials to lab with you. Here are the pre-lab questions for this week.
Some Basic Concepts of Chemistry
 Q 1. Some Basic Concepts of Chemistry What weight of AgCI will be precipitated when a solution containing 4.77 g of NaCI is added to a solution of 5.77 g of AgNO 3? (IIT JEE 1978 3 Marks) Q 2. One gram
Q 1. Some Basic Concepts of Chemistry What weight of AgCI will be precipitated when a solution containing 4.77 g of NaCI is added to a solution of 5.77 g of AgNO 3? (IIT JEE 1978 3 Marks) Q 2. One gram
Angel International School - Manipay 1 st Term Examination November, 2017
 Grade 10 Angel International School - Manipay 1 st Term Examination November, 2017 CHEMISTRY Duration: 2.30 Hours Index No:- Part 1 Choose the correct answer and circle the number neatly 1) As we move
Grade 10 Angel International School - Manipay 1 st Term Examination November, 2017 CHEMISTRY Duration: 2.30 Hours Index No:- Part 1 Choose the correct answer and circle the number neatly 1) As we move
to water. You add 1 drop of phenolphthalein and the solution turns pink. This observation tells you Na 2
 1A End of Lab Questions Notes 9/11/18 Lab 4 1 You add 100 g of to water You add 1 drop of phenolphthalein and the solution turns pink This observation tells you is a base 2 You add vinegar (09 M acetic
1A End of Lab Questions Notes 9/11/18 Lab 4 1 You add 100 g of to water You add 1 drop of phenolphthalein and the solution turns pink This observation tells you is a base 2 You add vinegar (09 M acetic
ICSE-Science 2 (Chemistry) 1996
 ICSE-Science 2 (Chemistry) 1996 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
ICSE-Science 2 (Chemistry) 1996 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
DURATION: 1 hour 30 minutes
 1 Our country, our future 545/1 S4 CHEMISTRY Exam 14 PAPER 1 DURATION: 1 hour 30 minutes Instructions - This paper consists of 50 compulsory objective questions - Answer the questions by writing the correct
1 Our country, our future 545/1 S4 CHEMISTRY Exam 14 PAPER 1 DURATION: 1 hour 30 minutes Instructions - This paper consists of 50 compulsory objective questions - Answer the questions by writing the correct
Kenya Certificate of Secondary Education (K.C.S.E.)
 Name: Index No. School:. Candidate s Sign.... Date:... 233/2 CHEMISTRY PAPER 2 JULY/AUGUST 2011 TIME: 2 HOURS Kenya Certificate of Secondary Education (K.C.S.E.) Chemistry Paper 2 INSTRUCTIONS TO CANDIDATES:
Name: Index No. School:. Candidate s Sign.... Date:... 233/2 CHEMISTRY PAPER 2 JULY/AUGUST 2011 TIME: 2 HOURS Kenya Certificate of Secondary Education (K.C.S.E.) Chemistry Paper 2 INSTRUCTIONS TO CANDIDATES:
Ph Of Calcium Carbonate Solution
 We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with ph of calcium carbonate
We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer, you have convenient answers with ph of calcium carbonate
McCord CH302 Exam 1 Spring 2017
 112 version last name first name signature McCord CH302 Exam 1 Spring 2017 50375 / 50380 Reminder: Be sure and correctly bubble in your name, uteid, and version number on your bubblesheet. The Periodic
112 version last name first name signature McCord CH302 Exam 1 Spring 2017 50375 / 50380 Reminder: Be sure and correctly bubble in your name, uteid, and version number on your bubblesheet. The Periodic
Copper Odyssey. Chemical Reactions of Copper
 Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Name Lab Partner(s) Copper Odyssey Chemical Reactions of Copper Date Period Elemental copper metal will be converted into copper (II) ion and then brought through a series of compound conversions until
Chemical reactions and electrolysis
 Chemical reactions and electrolysis Higher Revision Questions Name: Class: Date: Time: 95 minutes Marks: 95 marks Comments: Page of 29 (a) Magnesium metal is shaped to make magnesium ribbon. Explain why
Chemical reactions and electrolysis Higher Revision Questions Name: Class: Date: Time: 95 minutes Marks: 95 marks Comments: Page of 29 (a) Magnesium metal is shaped to make magnesium ribbon. Explain why
Opera Chemisol India Private Limited
 +91-8071802256 Opera Chemisol India Private Limited https://www.indiamart.com/jan-enterprises/ We are one of the leading traders and suppliers of a wide range of Chemicals and Dyes procured from reputed
+91-8071802256 Opera Chemisol India Private Limited https://www.indiamart.com/jan-enterprises/ We are one of the leading traders and suppliers of a wide range of Chemicals and Dyes procured from reputed
AP* CHEMISTRY EQUATIONS BY TYPE
 AP* CHEMISTRY EQUATIONS BY TYPE Double Replacement 1. Hydrogen sulfide is bubbled through a solution of silver nitrate. 2. An excess of sodium hydroxide solution is added to a solution of magnesium nitrate.
AP* CHEMISTRY EQUATIONS BY TYPE Double Replacement 1. Hydrogen sulfide is bubbled through a solution of silver nitrate. 2. An excess of sodium hydroxide solution is added to a solution of magnesium nitrate.
MR. D HR UV AS HE R I.C.S.E. BOA RD PAP ER ICSE
 MR D HR UV AS HE R ICSE BOA RD PAP ER 200 4 1 ICSE-2004 Section A (40 Marks) (Attempt all questions from this section) Question 1 (a) Choose the letters A,B,C or D to match the descriptions (i) to (iv)
MR D HR UV AS HE R ICSE BOA RD PAP ER 200 4 1 ICSE-2004 Section A (40 Marks) (Attempt all questions from this section) Question 1 (a) Choose the letters A,B,C or D to match the descriptions (i) to (iv)
SCHOOL CHEMICALS FROM SCRAP COKE COLA CANS AND CALCIUM CARBIDE-WATER REACTION RESIDUE
 SCHOOL CHEMICALS FROM SCRAP COKE COLA CANS AND CALCIUM CARBIDE-WATER REACTION RESIDUE By RCE PORT HARCOURT ERONDU, CHINONSO NGOZI (YOUTH COORDINATOR) RCE PORT HARCOURT, NIGERIA PRESENTED AT THE 7TH AFRICAN
SCHOOL CHEMICALS FROM SCRAP COKE COLA CANS AND CALCIUM CARBIDE-WATER REACTION RESIDUE By RCE PORT HARCOURT ERONDU, CHINONSO NGOZI (YOUTH COORDINATOR) RCE PORT HARCOURT, NIGERIA PRESENTED AT THE 7TH AFRICAN
GRADE: 10 CHEMISTRY MCQ (TERM-1)
 GRADE: 10 CHEMISTRY MCQ (TERM-1) 1 When ferrous sulphate crystals are heated, the colour of the residue formed is : (a) red (b) brown (c) orange (d) green. 2 A small amount of quick lime is taken in a
GRADE: 10 CHEMISTRY MCQ (TERM-1) 1 When ferrous sulphate crystals are heated, the colour of the residue formed is : (a) red (b) brown (c) orange (d) green. 2 A small amount of quick lime is taken in a
AQA Chemistry A-level
 AQA Chemistry A-level Required Practical 10 Preparation of a pure organic solid, test of its purity, and preparation of a pure organic liquid Reflux Reflux: continuous boiling and condensing of a reaction
AQA Chemistry A-level Required Practical 10 Preparation of a pure organic solid, test of its purity, and preparation of a pure organic liquid Reflux Reflux: continuous boiling and condensing of a reaction
OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/02. TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Higher Tier)
 OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/02 TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Higher Tier) TUESDAY 10 JUNE 2014: Afternoon DURATION: 1 hour plus your
OXFORD CAMBRIDGE AND RSA EXAMINATIONS GCSE A172/02 TWENTY FIRST CENTURY SCIENCE CHEMISTRY A/ADDITIONAL SCIENCE A Modules C4 C5 C6 (Higher Tier) TUESDAY 10 JUNE 2014: Afternoon DURATION: 1 hour plus your
Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons?
 Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons? b) Electrons? c) Neutrons? 1 CH3OS Warm Up (Sept 13) 1. What is an isotope? 2. Neon has two major isotopes, Neon 20 and
Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons? b) Electrons? c) Neutrons? 1 CH3OS Warm Up (Sept 13) 1. What is an isotope? 2. Neon has two major isotopes, Neon 20 and
NATIONAL BUSINESS AND TECHNICAL EXAMINATIONS BOARD (GENERAL EDUCATION EXAMINATION) MAY/JUNE 2007 SECTION B CHEMISTRY (ESSAY) TIME: 1 HOUR 40 MINUTES
 NATIONAL BUSINESS AND TECHNICAL EXAMINATIONS BOARD (GENERAL EDUCATION EXAMINATION) MAY/JUNE 2007 SECTION B CHEMISTRY (ESSAY) TIME: 1 HOUR 40 MINUTES 1. (a) Give THREE differences between a physical and
NATIONAL BUSINESS AND TECHNICAL EXAMINATIONS BOARD (GENERAL EDUCATION EXAMINATION) MAY/JUNE 2007 SECTION B CHEMISTRY (ESSAY) TIME: 1 HOUR 40 MINUTES 1. (a) Give THREE differences between a physical and
Thermal decomposition. Metal carbonates
 Decomposition reactions Copy correctly Up to 3% of a workbook Copying or scanning from ESA workbooks is subject to the New Zealand Copyright Act which limits copying to 3% of this workbook. Many compounds
Decomposition reactions Copy correctly Up to 3% of a workbook Copying or scanning from ESA workbooks is subject to the New Zealand Copyright Act which limits copying to 3% of this workbook. Many compounds
Our country, our future S2 CHEMISTRY DURATION: 2 HOUR
 Our country, our future S2 CHEMISTRY Exam 1 DURATION: 2 HOUR INSTRUCTIONS: This paper consists of two sections A and B, Attempt all questions in section A and B For section A, circle the most correct alternative
Our country, our future S2 CHEMISTRY Exam 1 DURATION: 2 HOUR INSTRUCTIONS: This paper consists of two sections A and B, Attempt all questions in section A and B For section A, circle the most correct alternative
Double Award Science: Chemistry Unit C2 Higher Tier
 Centre Number 71 Candidate Number General Certificate of Secondary Education 2013 Double Award Science: Chemistry Unit C2 Higher Tier GSD52 [GSD52] MONDAY 10 JUNE 2013, AFTERNOON TIME 1 hour 15 minutes.
Centre Number 71 Candidate Number General Certificate of Secondary Education 2013 Double Award Science: Chemistry Unit C2 Higher Tier GSD52 [GSD52] MONDAY 10 JUNE 2013, AFTERNOON TIME 1 hour 15 minutes.
Precipitation Reactions and Gravimetric Analysis
 Precipitation Reactions and Gravimetric Analysis Titrations where the titrant forms a precipitate with the analyte. Not always so straightforward a number of requirements need to be met. Precipitation
Precipitation Reactions and Gravimetric Analysis Titrations where the titrant forms a precipitate with the analyte. Not always so straightforward a number of requirements need to be met. Precipitation
JACOBS NEW PROCESS FOR REMOVING IRON FROM PHOSPHORIC ACID FINAL REPORT
 JACOBS NEW PROCESS FOR REMOVING IRON FROM PHOSPHORIC ACID FINAL REPORT Stephen W. Hilakos Process Engineer 3149 Winter Lake Road, Lakeland, FL 33803 P.O. Box 2008, Lakeland, FL 33806-2008 Prepared for
JACOBS NEW PROCESS FOR REMOVING IRON FROM PHOSPHORIC ACID FINAL REPORT Stephen W. Hilakos Process Engineer 3149 Winter Lake Road, Lakeland, FL 33803 P.O. Box 2008, Lakeland, FL 33806-2008 Prepared for
Year 7 Chemistry HW Questions
 Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
The Study of Method for Complex Processing Turgay Sub-Standard Aluminum-Containing Raw Materials
 Advances in Materials Physics and Chemistry, 2012, 2, 216-220 doi:10.4236/ampc.2012.24b055 Published Online December 2012 (http://www.scirp.org/journal/ampc) The Study of Method for Complex Processing
Advances in Materials Physics and Chemistry, 2012, 2, 216-220 doi:10.4236/ampc.2012.24b055 Published Online December 2012 (http://www.scirp.org/journal/ampc) The Study of Method for Complex Processing
1. Which of the following elements has the highest percentage by mass in nature? A. Oxygen B. Aluminium C. Nitrogen D. Silicon
 Class: F.3 ( ) Baptist Lui Ming Choi Secondary School First Term Examination (2013-2014) Date: 6 / 12 / 2013 Name: Form 3 Chemistry Time: 10:20-11:05 a.m. Answer ALL the questions. For Section A, choose
Class: F.3 ( ) Baptist Lui Ming Choi Secondary School First Term Examination (2013-2014) Date: 6 / 12 / 2013 Name: Form 3 Chemistry Time: 10:20-11:05 a.m. Answer ALL the questions. For Section A, choose
Experiment 18 Determination of Iron by Visible Spectrophotometry
 Experiment 18 Determination of Iron by Visible Spectrophotometry PRELAB DISCUSSION Visible spectrophotometry (spectral analysis) is a method of measuring the concentration of colored solutions by the amount
Experiment 18 Determination of Iron by Visible Spectrophotometry PRELAB DISCUSSION Visible spectrophotometry (spectral analysis) is a method of measuring the concentration of colored solutions by the amount
IGCSE(A*-G) Edexcel - Chemistry
 IGCSE(A*-G) Edexcel - Chemistry Principles of Chemistry States of Matter NOTES 1.1 Understand the arrangement, movement and energy of the particles in each of the three states of matter: solid, liquid
IGCSE(A*-G) Edexcel - Chemistry Principles of Chemistry States of Matter NOTES 1.1 Understand the arrangement, movement and energy of the particles in each of the three states of matter: solid, liquid
CO forms CO 2. forms. (a) The coke reacts with the oxygen in the air to form carbon dioxide. C + O 2
 1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
Approved for NPDES (Editorial Revision 1978) Silica, Dissolved (Colorimetric)
 METHOD #: 370.1 TITLE: Approved for NPDES (Editorial Revision 1978) Silica, Dissolved (Colorimetric) ANALYTE: Silica, SiO 2 INSTRUMENTATION: Spectrophotometer STORET No. Dissolved 00955 1.0 Scope and Application
METHOD #: 370.1 TITLE: Approved for NPDES (Editorial Revision 1978) Silica, Dissolved (Colorimetric) ANALYTE: Silica, SiO 2 INSTRUMENTATION: Spectrophotometer STORET No. Dissolved 00955 1.0 Scope and Application
Applications of Oxidation/Reduction Titrations. Lecture 6
 Applications of Oxidation/Reduction Titrations Lecture 6 Pretreatmentauxiliary oxidizing/reducing reagent Ex: when a sample containing iron is dissolved, the resulting solution usually contains a mixture
Applications of Oxidation/Reduction Titrations Lecture 6 Pretreatmentauxiliary oxidizing/reducing reagent Ex: when a sample containing iron is dissolved, the resulting solution usually contains a mixture
Suggest one reason why spoons are electroplated. ... Why is hydrogen produced at the negative electrode and not sodium?
 Q1.This question is about electrolysis. (a) Metal spoons can be coated with silver. This is called electroplating. Suggest one reason why spoons are electroplated. (b) When sodium chloride solution is
Q1.This question is about electrolysis. (a) Metal spoons can be coated with silver. This is called electroplating. Suggest one reason why spoons are electroplated. (b) When sodium chloride solution is
INTERPRETING CHEMICAL FORMULAS AND SYMBOLS
 INTERPRETING CHEMICAL FORMULAS AND SYMBOLS THE FORMULA OR SYMBOL REPRESENTS: CHEMICAL FORMULA OR SYMBOL NUMBER OF ATOMS TOTAL CHARGE NUMBER OF PROTONS NUMBER OF ELECTRONS Atom Molecule Monatomic Cation
INTERPRETING CHEMICAL FORMULAS AND SYMBOLS THE FORMULA OR SYMBOL REPRESENTS: CHEMICAL FORMULA OR SYMBOL NUMBER OF ATOMS TOTAL CHARGE NUMBER OF PROTONS NUMBER OF ELECTRONS Atom Molecule Monatomic Cation
ICSE-Science 2 (Chemistry) 2000
 ICSE-Science 2 (Chemistry) 2000 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
ICSE-Science 2 (Chemistry) 2000 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
(a) To find out which is the more reactive metal, zinc or tin, the following experiment could be carried out. piece of zinc shiny surface
 1 The reactivity series lists metals in order of reactivity. (a) To find out which is the more reactive metal, zinc or tin, the following experiment could be carried out. piece of zinc shiny surface tin(ii)
1 The reactivity series lists metals in order of reactivity. (a) To find out which is the more reactive metal, zinc or tin, the following experiment could be carried out. piece of zinc shiny surface tin(ii)
COPPER CYCLE EXPERIMENT 3
 COPPER CYCLE EXPERIMENT 3 INTRODUCTION One simple way to state the aim of chemistry is: The study of matter and its transformations. In this experiment, a copper sample will appear in five different forms
COPPER CYCLE EXPERIMENT 3 INTRODUCTION One simple way to state the aim of chemistry is: The study of matter and its transformations. In this experiment, a copper sample will appear in five different forms
Chemical Precipitation
 Chapter 9 Chemical Precipitation 9.1 INTRODUCTION The main chemical processes used in water and wastewater treatment practice include (1) Chemical coagulation, which is used to remove colloidal material
Chapter 9 Chemical Precipitation 9.1 INTRODUCTION The main chemical processes used in water and wastewater treatment practice include (1) Chemical coagulation, which is used to remove colloidal material
1. Name the first element by its name. 2. The second element has the ending ide. 3. The number of atoms of each element is indicated with Greek
 Binary compounds containing two nonmetals 1. Name the first element by its name. 2. The second element has the ending ide. 3. The number of atoms of each element is indicated with Greek prefixes. Greek
Binary compounds containing two nonmetals 1. Name the first element by its name. 2. The second element has the ending ide. 3. The number of atoms of each element is indicated with Greek prefixes. Greek
Name: Mods: Date Agenda Homework
 Name: Mods: UNIT 6 FORMULA WRITING, FORMULA WEIGHTS, and PERCENT COMPOSITION Date Agenda Homework Wed Dec 3 Thurs Dec 4 Fri Dec 5 Mon Dec 8 Tues Dec 9 Wed Dec 10 (1/2 Day) Thurs Dec 11 Fri Dec 12 Mon Dec
Name: Mods: UNIT 6 FORMULA WRITING, FORMULA WEIGHTS, and PERCENT COMPOSITION Date Agenda Homework Wed Dec 3 Thurs Dec 4 Fri Dec 5 Mon Dec 8 Tues Dec 9 Wed Dec 10 (1/2 Day) Thurs Dec 11 Fri Dec 12 Mon Dec
