EFFECTS OF CARBONATION ON THE LONG-TERM LEACHING PERFORMANCE OF CEMENTITIOUS WASTEFORMS
|
|
|
- Holly Gilbert
- 6 years ago
- Views:
Transcription
1 EFFECTS OF CARBONATION ON THE LONG-TERM LEACHING PERFORMANCE OF CEMENTITIOUS WASTEFORMS N. Gutierrez 1, M.D.S. Bin-Shafique 1, J.C. Walton 1, A. Tarquin 1, R. Smith 2, P. Sheeley 1, M. Rodriguez 1, and R. Andrade 1, 1 University of Texas at El Paso, Department of Civil Engineering, El Paso, TX, and 2 Idaho National Engineering Laboratory, Idaho Falls, ID ABSTRACT Cement-based wasteforms are among the most commonly used waste disposal and site remediation options. However, concrete knowledge of the processes controlling long-term performance of the wasteforms is lacking. This research evaluates the effect of carbonation on cementitious wasteforms. Wasteforms were reacted with carbon dioxide for a prolonged period and then subjected to leaching tests. Ions tested were nitrate, strontium, cadmium, cobalt, calcium, and lead. Results indicate that carbonation causes both physical and chemical changes to the concrete. The leaching rate was higher in the carbonated wasteforms for all the ions except strontium. Thus, effects of carbonation can vary depending on the chemical species and other physical and chemical effects. KEYWORDS: cementitious wasteforms, carbonation INTRODUCTION The use of cement-based wasteforms for solidification and stabilization of waste has been rapidly increasing in the United States. In fact, it is one of the most commonly used technologies for site remediation and treatment of hazardous wastes. Cement as a binder is an attractive option over others due to its superior performance and economic attractiveness. Examples of its advantages include: readily available materials, low cost, flexibility in tailoring properties for varying wastes, and high strength. In addition, it is a fairly simple material to produce and form, offering a wide range of disposal options. The performance of cement as a solidification and stabilization agent has been evaluated by the U.S. Government. The tests used, however, are short-term laboratory tests which do not mimic the actual service environment of the cementitious wasteforms. These tests such as the Toxicity Characteristic Leaching Procedure (TCLP) give information on the characteristics of cement wasteforms, but give no indication as to their expected performance in the actual soil environment. One main reason for this is that these tests specify water saturated conditions which are unrepresentative of the actual disposal environment. Because the law requires that wastes be placed in the vadose zone where soil gases are present, it is important to research the extent to which soil gases might affect the physical and chemical properties of the wasteforms. Thus, the reaction of cement with soil gases and its effect on the long-term leaching performance of wasteforms needs to be evaluated. A better understanding of this can aid in the development of standardized leach tests, necessary for making the adequate choices for the proper and safe disposal of wastes.
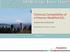
2 CO 2 Diffusion Carbonated Zone Unaltered Zone wasteforms. Permeability and diffusivity will decrease due to the obstruction of pores from calcium carbonate accumulation. CO2 will also cause a decrease in the ph of the system, which in turn alters the solubility of the waste metals, either increasing or decreasing it. Additionally, carbonation may cause certain ions to go into solid solution in the calcium carbonate, which may limit the release of such ions. FIGURE 1. CARBONATION OF WASTEFORM. In terms of long-term performance, the vadose zone offers a very aggressive environment due to the presence of soil gases such as carbon dioxide or CO2 and oxygen, in addition to variable contact with water. Due to the nature of cement, carbonation is a potentially significant process affecting the leaching behavior of wasteforms. Carbonation can take place when soil or atmospheric carbon dioxide reacts with the cementitious materials resulting in the formation of calcium carbonate. This can lead to physical as well as chemical changes which in turn may affect the leaching rate. The major reactions of Portland cement with carbon dioxide are as follows: Ca(OH) 2(s) + CO 2(g) CaCO 3(s) + H 2O CSH(s) + CO 2(g) CaCO 3 (s) + SiO 2 nh 2O(s) + H 2O(l) (silica) All the calcium-containing phases existent in the cement are subject to carbonation, with calcium hydroxide being the more rapidly reacting compound. These reactions show the formation of calcium carbonate, silica gel, and metallic oxides, which will eventually begin accumulating. Carbonation can result in physical and chemical changes to cementitious Carbonation occurs through the mechanism of diffusion and proceeds as a reaction front passing through the concrete wasteform. As the gas diffuses through, it alters the concrete. Figure 1 illustrates the conceptual model of this process. The outer ring is the carbonated zone, where the concrete has been altered. This surrounds an inner zone of intact concrete not yet reached by the carbonation front. In addition, the rate of carbonation is dependent on the relative humidity, and has been found to occur more rapidly at a value of 50%. The shrinking core model can be used to approximate the rate of penetration of the reaction front. The equation describing this is as follows: x( t ) = C D φ τ t CSCa 2 HCO 3 HCO 3 where Χ represents the thickness of the carbonated shell, CHCO3 = aqueous concentration of bicarbonate at the outer edge of the wasteform, DHCO3 = aqueous diffusion coefficient of bicarbonate ion in concrete pore fluid, CSCa = concentration of calcium in the solid concrete, φ = porosity of the wasteform, τ = tortuosity factor - a ratio of thickness to length of the sinuous path, and t = time. The leaching of wasteforms can be a complex process to quantify, but is best described through the use of mass transport models. The mechanisms for mass transport
3 are diffusion and convection. For our purposes, convective transport is neglected due to low permeability of the wasteforms; therefore, diffusion is the controlling factor for describing leachability. Several diffusional models exist ranging from the less complex, such as Fick s First Law, to extensively detailed models which incorporate factors such as porosity and tortuosity of the medium. One of the most widely used models is that of diffusion through semi-infinite media. In this model, the wasteform is looked at as a semi-infinite domain with a constant diffusion coefficient. This model assumes that the contaminant concentration in the solid remains uniform, that leaching is controlled by diffusion, and that the concentration at the solid-liquid interface is zero. In addition, this model applies to situations where the cumulative fractional release is less than 20%. Using this model, the cumulative fractional release of contaminant can be predicted. The expression is as follows: an V Dt e = 2 Ao S π where an = total amount of material released during time interval n; Ao = initial amount of material in wasteform; V = volume of wasteform; S = surface area of the wasteform; De = effective diffusion coefficient; and t = time period for leaching. As can be seen from this equation, the fractional cumulative release (FCR) is the first term of the equation, and it has a square root of time dependence. This is important to note because other processes follow the same time dependence. Because this expression is valid only when the cumulative fractional release is less than 20%, other models must be used in cases where it approaches a value of one. Modeling the release in this case can be done using a more complex model for diffusion through finite media proposed by Pescatore [1]. Another important parameter that needs to be considered in studying leachability is solubility. This is important because, fundamentally, any constituent must first be dissolved in the pore water before it can leach. Solubility of different species varies, and it is related to reaction kinetics and other factors. The solubility product of a reaction can be calculated using the Gibb s Free Energy and the following equation: o G r = RT ln Ksp where Gºr = standard free energy change of the reaction; R = universal gas constant; and T = absolute temperature Kº. Another parameter related to solubility is the distribution coefficient, Kd. This coefficient represents the nature of partitioning of the ion between the solid and liquid phase. It is actually the ratio of ion concentration in the solid phase over the ion concentration in the liquid phase. Whether an ion will partition into the solid or liquid phase is determined partly by this coefficient. The value of Kd varies with the type of chemical and solid being considered. The present research examines the role of carbonation in affecting the long-term leaching behavior of cementitious wasteforms. Leaching tests are performed on wasteforms made from Portland cement cured with a highly concentrated waste solution. The research combines the experimental results with modeling programs to evaluate the factors affecting the leaching behavior.
4 EXPERIMENTAL METHODS Portland cement was used to form the wasteforms used in this experiment. The wasteforms are of cylindrical shape with dimensions of 3.1 cm diameter and 6.7 cm height. The wasteforms were cast using a synthetic waste water with high ion concentrations as illustrated in Table 1. Enough waste water was added to result in a water to cement ratio of 0.6, the ratio found to give optimum results in terms of porosity and strength. The mixture was poured into polyvinylchloride molds and allowed to cure for seven days. The wasteforms were then reacted with CO2. This process was accomplished by placing a number of the wasteforms in stacks in two large beakers. Both sets of beakers were placed in an environmental control chamber to maintain a constant temperature of 50ºC. The relative humidity of the system was also maintained at an approximate value of 50% by bubbling of gases through deionized water and into the beakers using diffuser tubes. One of the beakers was bubbled using a gas mixture of 50% CO2. These were the carbonated wasteforms and are referred to as the experimental wasteforms. The other set of wasteforms were used as controls and were reacted with CO2-removed air by passing of air through sodium hydroxide before bubbling into the deionized water. This reaction stage was continued and TABLE 1. SYNTHETIC WASTE WATER. Salt CdCl 2 Co Cl 2 Pb(NO 3) 2 Sr(NO 3) 2 ZnCl 2 Ion Conc. (mg/l) 3,000 (Cd) 3,000 (Co) 3,000 (Pb) 1,000 (Sr) 3,000 (Zn) carefully monitored for 26 days. Following the reaction stage, the wasteforms were leached. Two separate leaching tests were conducted. In the first test, wasteforms were leached in 150 ml of deionized water. Five samples each of the experimental and controls were all leached for time intervals of 2, 7, 24, and 48 hours and 4, 7, 14, and 39 days. The leachate water was collected after each of the intervals and replaced with clean water. In the second test a 0.5 Normal acetic acid solution was used as the leachant, but the same time intervals were used except for the last of the series which was leached for 14 days instead of 39. The leachate water was analyzed by atomic absorption spectrophotometry and ion chromatography for presence of the ions introduced in the synthetic waste, following standard methods. Following the leaching tests, the wasteforms were subjected to re-carbonation tests. In this procedure the wasteforms were digested in a closed Erlenmeyer flask using a 0.5 Normal sulfuric acid solution. This resulted in release of gas from the wasteforms which was collected and measured simply by using an inverted graduated cylinder in water, which was connected to the flask by plastic tubing (Figure 2). The collected gas was then analyzed in a carbon analyzer to determine the CO2 content. Knowing the CO2 concentration, the calcite concentration in the wasteforms can be calculated using the gas laws. To ascertain the extent of carbonation occurring in the cement, the thickness of the carbonated shell was determined by performing a phenolphthalein test. A wasteform from each of the leaching tests was cut in half and topically dosed with a solution of 1% phenolphthalein in 70% ethyl alcohol. Phenolphthalein serves as an indicator; for ph values over 9.2 it turns red
5 FIGURE 2. SCHEMATIC OF RE-CARBONATION TEST. on contact whereas it remains uncolored for low ph values. RESULTS AND DISCUSSION Wasteforms were measured and weighed to determine their physical properties. In the case of the experimental wasteforms, the final porosity was 35.39%, and they were found to be fully carbonated with an average bulk calcium carbonate concentration of 9 x 10-4 mol/cm 3. The non-carbonated control samples had a porosity of 48.11%. Thus, it is evident that carbonation results in reduced porosity due to the larger molar volume of calcite (CaCO3) relative to the initial portlandite (Ca(OH)2) in the cement. First leaching test Leaching results were obtained only for strontium and nitrates. Results for the other ions couldn t be obtained because their concentrations were below detection limits. Graphs of the fractional cumulative release (FCR) vs. square root of time were drawn in order to determine the apparent diffusion coefficient of the different species. The square root of time was used due to the fact that several processes including simple diffusional release lead to a square root of time dependence for total contaminant release. The theoretical model of FCR vs. time is non-linear, but plotting FCR vs. the FIGURE 3. FRACTIONAL CUMULATIVE RELEASE (FCR) OF NITRATE. square root of time results in a straight line, indicating that the wasteform acts like a semi-infinite domain during early time periods. The line then curves towards the right as the FCR approaches a value of 1. Solubility graphs were also constructed. These are, of course, essential in determining the species present at varying ph. Other important parameters in determining the apparent diffusion coefficient are the retardation factor and tortuosity, which are related to the apparent diffusion coefficient by the following [2]: D a D = τ R d where τ = tortuosity; Da = apparent diffusion coefficient; D = diffusion coefficient in water; Rd = retardation factor of the ion; ρb = bulk density(g/cm 3 ); Kd = distribution coefficient; and θ = volumetric water content. The results of the nitrate analysis are indicated in Figure 3. Each of the points represents an average of five replicates; the line indicates the non-linear fit to the finite domain diffusion equation [1]. The
6 FIGURE 4. FRACTIONAL CUMULATIVE RELEASE (FCR) OF STRONTIUM. calculated apparent diffusion coefficients for nitrate were 2.55 x cm 2 /s for the controls and 2.59 x cm 2 /s for the carbonated wasteforms. The leaching rate of nitrate is rapid. For this reason, nitrate was placed in the wasteforms so as to assess the diffusion rate of a chemical species which would not interact chemically with the wasteforms. This would indicate physical changes taking place in the wasteform. Our results show that the carbonated wasteforms had a higher leaching rate than the controls. This is contrary to what was expected, since the carbonated wasteforms have a lower porosity which should lead to a lower diffusion coefficient. A reason for this discrepancy could be that increased microcracking from the expansive forces generated in the carbonated samples led to higher initial leaching rates or resulted in a modification of the pore distribution favoring a larger average pore size. The results of the strontium analysis are indicated in Figure 4. Here we see that the leaching of strontium from the carbonated wasteforms is lower than from the controls. The apparent diffusion coefficients are 2.55 x cm 2 /s for the controls and 2.59 x cm 2 /s for the experimental. In this case FIGURE 5. SOLUBILITY OF STRONTIUM. results are consistent with other factors such as the retardation factor which had values of 4.32 for the controls and 1, for the experimental. The distribution coefficient was also consistent with a higher index of leachability of the controls, giving values of 0.84 ml/g for the controls and ml/g for the experimental. This indicates a higher concentration of strontium in the pore water than in the solid. Figure 5 shows the equilibrium diagram of strontium, which indicates that strontium is of higher solubility at lower ph. This is consistent with our results. Second leaching test The use of acetic acid as the leachant resulted in higher leaching rates for all the species in the second leaching test. In this case, results were obtained for a number of ions including cadmium, calcium, lead, and cobalt. The results of the nitrate analysis are similar to those in the first test. Figure 6 shows the results for nitrate. As can be seen, the experimental samples leached at a higher rate than the controls. The curves for each set of data are the non-linear best fit model and result in apparent diffusion coefficient
7 FIGURE 6. FRACTIONAL CUMULATIVE RELEASE (FCR) OF NITRATE IN ACETIC ACID. FIGURE 7. FRACTIONAL CUMULATIVE RELEASE (FCR) OF STRONTIUM IN ACETIC ACID. values of 1.77 x 10-8 for the controls and 1.14 x 10-7 for the carbonated samples. The strontium results also supported those of the first test. Figure 7 shows that the leaching rate of the controls is higher than that of the carbonated samples. The apparent diffusion coefficients based upon the non-linear best fit model were found to be 6.61 x 10-8 for the controls and 1.74 x 10-8 for the carbonated samples. Again, in this case, the other parameters of the retardation factor and distribution coefficient were consistent with the results. The retardation factors are for the controls and 2.38 for the carbonated samples. The distribution coefficients are and 0.38 ml/g for the controls and carbonated samples, respectively. The negative value of the distribution coefficient is not theoretically possible and could be due to the fact that the molecular diffusion coefficient of strontium in water instead of acidic water was used to calculate it. Analyses were also performed for ions of calcium, cadmium, cobalt, and lead. For all of these ions, the leaching rate was higher in the carbonated wasteforms than the controls. This might indicate that factors other than diffusion are dominating. It follows that the apparent diffusion coefficients calculated for each of these ions is larger in the carbonated wasteforms. The graphs of the FCR and solubility for these ions are contained in Figures The calculated porosities, however, are inconsistent with these results, being greater in the controls than the experimental wasteforms. CONCLUSION The presented research was done with the goal of reproducing as closely as possible one of the fundamental environmental processes encountered by wasteforms in the vadose zone, carbonation. It is clearly evident that carbonation leads to physical and chemical changes in cementitious wasteforms. The results indicate that carbonation indeed does affect the leaching rate, but does not affect it in the same manner all the time. Although it leads to decreased porosity, it also resulted in higher leaching rates for some ions. It is believed this is due to microcracking in the carbonated samples. Microcracks may
8 FIGURE 8. FRACTIONAL CUMULATIVE RELEASE (FCR) OF COBALT. FIGURE 11. SOLUBILITY OF CADMIUM. FIGURE 9. SOLUBILITY OF COBALT. FIGURE 12. FRACTIONAL CUMULATIVE RELEASE (FCR) OF LEAD. FIGURE 10. FRACTIONAL CUMULATIVE RELEASE (FCR) OF CADMIUM. provide a conduit through which the contaminants leach faster. FIGURE 13. SOLUBILITY OF LEAD. Leaching can be affected by a combination of physical and chemical effects. Strong chemical effects in the carbonated wasteforms are believed to limit the leaching rate. In our research, however, there seems
9 to be a crossover between physical and chemical effects, leading to increased leaching from carbonation for certain species. What net effect it will have is dependent on the contaminant species itself. Other than strontium, carbonation resulted in higher leaching rates for all the other chemical species. Thus, for the ions tested, carbonation seems to have a negative effect on the leaching characteristics of cementitious wasteforms. 5. R.W. Smith and J.C. Walton, Materials Research Society Symposium Proceedings, 294 (1993) L.H. Weitzman, L.E. Hamel, and E.F. Barth, Evaluation of Solidification/Stabilization as a Best Demonstrated Available Technology for Contaminated Soils, EPA/600/52-89/013, ACKNOWLEDGMENT This work was supported by the University of Texas at El Paso s Center for Environmental Resource Management through funding from the Office of Exploratory Research of the U.S. Environmental Protection Agency (cooperative agreement CR ). REFERENCES 1. C. Pescatore, Improved expression for modeling diffusive, fractional, cumulative leaching from finite-size wasteforms, Waste Management, 10 (1990) J.C. Walton, Performance of Intact and Partially Degraded Concrete Barriers in Limiting Mass Transport, NUREG/CR- 5445, EGG-2662, M.D.S. Bin-Shafique, Long Term Performance of Cementitious Wasteforms: The Role of Soil Gases, M.S. Thesis, University of Texas at El Paso, R.W. Smith and J.C. Walton, The effect of calcite solid solution formation on the transient release of radionuclides from concrete barriers, Materials Research Society, 212 (1991)
Evaluation Methods of Concrete Carbonation Suppressive Performance of Surface Coating
 Evaluation Methods of Concrete Carbonation Suppressive Performance of Surface Coating Kenji Motohashi Shibaura Institute of Technology, Japan Toyosu 3-7-, Koto-Ku, 13-848 Japan, motoken@shibaura-it.ac.jp
Evaluation Methods of Concrete Carbonation Suppressive Performance of Surface Coating Kenji Motohashi Shibaura Institute of Technology, Japan Toyosu 3-7-, Koto-Ku, 13-848 Japan, motoken@shibaura-it.ac.jp
Comparison of Carbonation Models
 Comparison of Carbonation Models I. Galan and C. Andrade Eduardo Torroja Institute IETcc-CSIC, Madrid, Spain ABSTRACT: In order to describe the CO 2 diffusion process into the concrete, several carbonation
Comparison of Carbonation Models I. Galan and C. Andrade Eduardo Torroja Institute IETcc-CSIC, Madrid, Spain ABSTRACT: In order to describe the CO 2 diffusion process into the concrete, several carbonation
Durability Predictions Using Early-Age Durability Index Testing
 Durability Predictions Using Early-Age Durability Index Testing JR Mackechnie & MG Alexander Summary: Durability of reinforced concrete structures is often dependent on the corrosion of reinforcement due
Durability Predictions Using Early-Age Durability Index Testing JR Mackechnie & MG Alexander Summary: Durability of reinforced concrete structures is often dependent on the corrosion of reinforcement due
FACTFILE: GCE CHEMISTRY
 FACTFILE: GCE CHEMISTRY 2.11 GROUP II ELEMENTS AND THEIR COMPOUNDS Learning Outcomes Students should be able to: 2.11.1 explain why these are regarded as s-block elements; 2.11.2 recall and explain the
FACTFILE: GCE CHEMISTRY 2.11 GROUP II ELEMENTS AND THEIR COMPOUNDS Learning Outcomes Students should be able to: 2.11.1 explain why these are regarded as s-block elements; 2.11.2 recall and explain the
bleeding during sample preparation. The specimens were demolded at hours and sealed with aluminum foil. Afterward, the sealed samples were stored at C
 コンクリート工学年次論文集,Vol.3,No.1,1 - Technical Paper - CHEMICAL EVOLUTION OF CEMENT-BASED MATERIALS IN SODIUM AND MAGNESIUM SULFATE SOLUTIONS Yogarajah ELAKNESWARAN *1, Tetsuya ISHIDA * ABSTRACT This paper presents
コンクリート工学年次論文集,Vol.3,No.1,1 - Technical Paper - CHEMICAL EVOLUTION OF CEMENT-BASED MATERIALS IN SODIUM AND MAGNESIUM SULFATE SOLUTIONS Yogarajah ELAKNESWARAN *1, Tetsuya ISHIDA * ABSTRACT This paper presents
DURABILITY of CONCRETE STRUCTURES
 DURABILITY of CONCRETE STRUCTURES Assist. Prof. Dr. Mert Yücel YARDIMCI This presentation covers the subjects in CEB Durable Concrete Structures Guideline and has been prepared by the graduate students
DURABILITY of CONCRETE STRUCTURES Assist. Prof. Dr. Mert Yücel YARDIMCI This presentation covers the subjects in CEB Durable Concrete Structures Guideline and has been prepared by the graduate students
Solidification and Stabilization of Fly Ash from Mixed Hazardous Waste Incinerator Using Ordinary Portland Cement
 Environmental Sciences, 13, 5 (2006) 289 296 A. Pariatamby et al. 289 MYU Tokyo ES633 Solidification and Stabilization of Fly Ash from Mixed Hazardous Waste Incinerator Using Ordinary Portland Cement Agamuthu
Environmental Sciences, 13, 5 (2006) 289 296 A. Pariatamby et al. 289 MYU Tokyo ES633 Solidification and Stabilization of Fly Ash from Mixed Hazardous Waste Incinerator Using Ordinary Portland Cement Agamuthu
Oxidation Reactions. This oxide will from only if thermodynamics favour a reaction of the form: M + O 2 = MO 2. Which must form rapidly (favourable(
 Oxidation of s Oxidation is a general term used to define the reaction between a metal or alloy and its environment. s or alloys are oxidised when heated to elevated temperatures es in air or highly oxidised
Oxidation of s Oxidation is a general term used to define the reaction between a metal or alloy and its environment. s or alloys are oxidised when heated to elevated temperatures es in air or highly oxidised
Water permeability and chloride penetrability of lightweight aggregate concrete. Chen Chung Kho
 ABSTRACT Water permeability and chloride penetrability of lightweight aggregate concrete Chen Chung Kho Department of Civil Engineering, National University of Singapore, Engineering Drive 2, Singapore
ABSTRACT Water permeability and chloride penetrability of lightweight aggregate concrete Chen Chung Kho Department of Civil Engineering, National University of Singapore, Engineering Drive 2, Singapore
Ins & outs of carbonation of concrete
 Ins & outs of carbonation of concrete Lecture Adv. Concrete technology, 24-28 feb 2014, Indian Institute of Technology, Madras. 2 Ins & outs of carbonation of concrete Contents: 1. Introduction: what is
Ins & outs of carbonation of concrete Lecture Adv. Concrete technology, 24-28 feb 2014, Indian Institute of Technology, Madras. 2 Ins & outs of carbonation of concrete Contents: 1. Introduction: what is
Concrete Pipe in Acid Sulfate Soil Conditions
 TECHNICAL BRIEF Concrete Pipe in Acid Sulfate Soil Conditions Concrete Pipe Association of Australasia Concrete Pipe in Acid Sulfate Soil Conditions There are widespread areas in Australia where the soil
TECHNICAL BRIEF Concrete Pipe in Acid Sulfate Soil Conditions Concrete Pipe Association of Australasia Concrete Pipe in Acid Sulfate Soil Conditions There are widespread areas in Australia where the soil
Chemical Compatibility of a Polymer-Modified GCL
 Chemical Compatibility of a Polymer-Modified GCL SWANA NW SYMPOSIUM PRESENTED BY: Melody A. Adams May 1, 2015 INTRODUCTION Waste product at a mine site regulated under EPA Subtitle D regulations Waste
Chemical Compatibility of a Polymer-Modified GCL SWANA NW SYMPOSIUM PRESENTED BY: Melody A. Adams May 1, 2015 INTRODUCTION Waste product at a mine site regulated under EPA Subtitle D regulations Waste
MODELLING THE DURABILITY OF CONCRETE FOR NUCLEAR WASTE DISPOSAL FACILITIES
 MODELLING THE DURABILITY OF CONCRETE FOR NUCLEAR WASTE DISPOSAL FACILITIES Olli-Pekka J. Kari (1), Jari A. Puttonen (1) (1) Helsinki University of Technology, Department of Structural Engineering and Building
MODELLING THE DURABILITY OF CONCRETE FOR NUCLEAR WASTE DISPOSAL FACILITIES Olli-Pekka J. Kari (1), Jari A. Puttonen (1) (1) Helsinki University of Technology, Department of Structural Engineering and Building
Uncertainty Impact of the Effective Diffusion Coefficient on the Concrete Chemical Degradation
 American Journal of Engineering and Applied Sciences Original Research Paper Uncertainty Impact of the Effective Diffusion Coefficient on the Concrete Chemical Degradation A. Badaoui National School of
American Journal of Engineering and Applied Sciences Original Research Paper Uncertainty Impact of the Effective Diffusion Coefficient on the Concrete Chemical Degradation A. Badaoui National School of
Performance of Cement for Immobilizing Strontium Waste in Saline Environment
 Materials Sciences and Applications, 013, 4, 7-11 Published Online December 013 (http://www.scirp.org/journal/msa) http://dx.doi.org/10.436/msa.013.41a00 7 Performance of Cement for Immobilizing Strontium
Materials Sciences and Applications, 013, 4, 7-11 Published Online December 013 (http://www.scirp.org/journal/msa) http://dx.doi.org/10.436/msa.013.41a00 7 Performance of Cement for Immobilizing Strontium
DIFFUSION OF Cl - IONS IN PARTIALLY DRY AND SATURATED CRACKED REINFORCED CONCRETE MEMBERS
 - Technical paper - DIFFUSION OF Cl - IONS IN PARTIALLY DRY AND SATURATED CRACKED REINFORCED CONCRETE MEMBERS Pa Pa WIN* 1, Atsuhiko MACHIDA*, Daisuke MORI* 3 and Hansu PARK* ABSTRACT: Both of the ingression
- Technical paper - DIFFUSION OF Cl - IONS IN PARTIALLY DRY AND SATURATED CRACKED REINFORCED CONCRETE MEMBERS Pa Pa WIN* 1, Atsuhiko MACHIDA*, Daisuke MORI* 3 and Hansu PARK* ABSTRACT: Both of the ingression
Chapter 2. Literature Review
 Chapter 2 Literature Review 2.1 Outline In this chapter, literature review about definition and risk of a concrete carbonation, durability degradation mechanisms of a concrete carbonation, substance for
Chapter 2 Literature Review 2.1 Outline In this chapter, literature review about definition and risk of a concrete carbonation, durability degradation mechanisms of a concrete carbonation, substance for
Eric Samson Cementitious Barriers Partnership SIMCO Technologies Inc. August 2014
 SIMCO Experimental Results Eric Samson Cementitious Barriers Partnership SIMCO Technologies Inc. August 2014 Summary Concrete mixture characterization Saltstone characterization Effect of damage on transport
SIMCO Experimental Results Eric Samson Cementitious Barriers Partnership SIMCO Technologies Inc. August 2014 Summary Concrete mixture characterization Saltstone characterization Effect of damage on transport
CHAPTER 6 POLYPROPYLENE FIBRE REINFORCED GEOPOLYMER CONCRETE COMPOSITES
 113 CHAPTER 6 POLYPROPYLENE FIBRE REINFORCED GEOPOLYMER CONCRETE COMPOSITES 6.1 GENERAL This chapter describes the effect of addition of polypropylene fibres on the strength characteristics of geopolymer
113 CHAPTER 6 POLYPROPYLENE FIBRE REINFORCED GEOPOLYMER CONCRETE COMPOSITES 6.1 GENERAL This chapter describes the effect of addition of polypropylene fibres on the strength characteristics of geopolymer
A MODEL FOR SOIL OXYGEN DELIVERY TO WASTEWATER INFILTRATION SURFACES. J. Erickson, E. J. Tyler* ABSTRACT
 #4.44 A MODEL FOR SOIL OXYGEN DELIVERY TO WASTEWATER INFILTRATION SURFACES J. Erickson, E. J. Tyler* ABSTRACT Soil could accept onsite wastewater at rates two to three orders of magnitude higher than the
#4.44 A MODEL FOR SOIL OXYGEN DELIVERY TO WASTEWATER INFILTRATION SURFACES J. Erickson, E. J. Tyler* ABSTRACT Soil could accept onsite wastewater at rates two to three orders of magnitude higher than the
CONSIDERING SUSTAINABLE DEVELOPMENT DECISION IN LIFE CYCLE ANALYSES AND CONCRETE TRANSPORTATION. Liv Haselbach
 CONSIDERING SUSTAINABLE DEVELOPMENT DECISION IMPACTS IN LIFE CYCLE ANALYSES AND TESTING METHODOLOGIES FOR CONCRETE TRANSPORTATION MATERIALS Liv Haselbach Civil and Environmental Engineering US-Japan Workshop
CONSIDERING SUSTAINABLE DEVELOPMENT DECISION IMPACTS IN LIFE CYCLE ANALYSES AND TESTING METHODOLOGIES FOR CONCRETE TRANSPORTATION MATERIALS Liv Haselbach Civil and Environmental Engineering US-Japan Workshop
Characteristic and efficiency of PEM fuel cell and PEM electrolyser
 Related topics Electrolysis, electrode polarisation, decomposition voltage, galvanic elements, Faraday s law. Principle and task In a PEM electrolyser, the electrolyte consists of a protonconducting membrane
Related topics Electrolysis, electrode polarisation, decomposition voltage, galvanic elements, Faraday s law. Principle and task In a PEM electrolyser, the electrolyte consists of a protonconducting membrane
Effect of curing conditions on freeze-thaw de-icing salt resistance of blast furnace slag cement mortars
 Effect of curing conditions on freeze-thaw de-icing salt resistance of blast furnace slag cement mortars O. Çopuroğlu, A. L. A. Fraaij & J. M. J. M. Bijen Section of Materials Science and Sustainable Construction,
Effect of curing conditions on freeze-thaw de-icing salt resistance of blast furnace slag cement mortars O. Çopuroğlu, A. L. A. Fraaij & J. M. J. M. Bijen Section of Materials Science and Sustainable Construction,
Electricity. Characteristic and efficiency of PEM fuel cell and PEM electrolyser Stationary currents. What you need:
 Stationary currents Electricity Characteristic and efficiency of PEM fuel cell and PEM electrolyser What you can learn about Electrolysis Electrode polarisation Decomposition voltage Galvanic elements
Stationary currents Electricity Characteristic and efficiency of PEM fuel cell and PEM electrolyser What you can learn about Electrolysis Electrode polarisation Decomposition voltage Galvanic elements
Saltstone Oxidation Study: Leaching Method
 ABSTRACT Saltstone Oxidation Study: Leaching Method - 13092 C. A. Langton, D. B. Stefanko, and H. H. Burns, Savannah River National Laboratory, Savannah River Remediation, LLC Savannah River Site, Aiken,
ABSTRACT Saltstone Oxidation Study: Leaching Method - 13092 C. A. Langton, D. B. Stefanko, and H. H. Burns, Savannah River National Laboratory, Savannah River Remediation, LLC Savannah River Site, Aiken,
1. CORROSION OF REINFORCEMENT
 MAB 1033 Structural Assessment and Repair 1. CORROSION OF REINFORCEMENT Professor Dr. Mohammad bin Ismail C09-313 Learning Outcome At the end of the course students should be able to understand Mechanism
MAB 1033 Structural Assessment and Repair 1. CORROSION OF REINFORCEMENT Professor Dr. Mohammad bin Ismail C09-313 Learning Outcome At the end of the course students should be able to understand Mechanism
SiC crystal growth from vapor
 SiC crystal growth from vapor Because SiC dissolves in Si and other metals can be grown from melt-solutions: Liquid phase epitaxy (LPE) Solubility of C in liquid Si is 0.029% at 1700oC high T process;
SiC crystal growth from vapor Because SiC dissolves in Si and other metals can be grown from melt-solutions: Liquid phase epitaxy (LPE) Solubility of C in liquid Si is 0.029% at 1700oC high T process;
SULFATE AND CHLORIDE RESISTANCE PROPERTIES OF PORTLAND CEMENT BLENDS
 Proceedings of the 4 th International Conference on Civil Engineering for Sustainable Development (ICCESD 2018), 9~11 February 2018, KUET, Khulna, Bangladesh (ISBN-978-984-34-3502-6) SULFATE AND CHLORIDE
Proceedings of the 4 th International Conference on Civil Engineering for Sustainable Development (ICCESD 2018), 9~11 February 2018, KUET, Khulna, Bangladesh (ISBN-978-984-34-3502-6) SULFATE AND CHLORIDE
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Application of New Leaching Protocols for Assessing Beneficial Use of Solid Wastes in Florida. Technical Awareness Group Meeting June 30 th, 2015
 Application of New Leaching Protocols for Assessing Beneficial Use of Solid Wastes in Florida Technical Awareness Group Meeting June 30 th, 2015 Background Historically a number of different tests have
Application of New Leaching Protocols for Assessing Beneficial Use of Solid Wastes in Florida Technical Awareness Group Meeting June 30 th, 2015 Background Historically a number of different tests have
DOWNLOAD OR READ : THE LEACHING RATE OF TIN METAL IN OXYGENATED SODIUM HYDROXIDE SOLUTIONS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : THE LEACHING RATE OF TIN METAL IN OXYGENATED SODIUM HYDROXIDE SOLUTIONS PDF EBOOK EPUB MOBI Page 1 Page 2 the leaching rate of tin metal in oxygenated sodium hydroxide solutions the
DOWNLOAD OR READ : THE LEACHING RATE OF TIN METAL IN OXYGENATED SODIUM HYDROXIDE SOLUTIONS PDF EBOOK EPUB MOBI Page 1 Page 2 the leaching rate of tin metal in oxygenated sodium hydroxide solutions the
Chemical Admixtures for Concrete. ACCELERATORS Özge Andiç Çakır, PhD
 Chemical Admixtures for Concrete ACCELERATORS Özge Andiç Çakır, PhD Accelerators: Definition An accelerating admixture is a material that is added to concrete for reducing the time of setting and accelerating
Chemical Admixtures for Concrete ACCELERATORS Özge Andiç Çakır, PhD Accelerators: Definition An accelerating admixture is a material that is added to concrete for reducing the time of setting and accelerating
Chemical interaction between amorphous silica mineral and highly alkaline plume with Ca ion
 Chemical interaction between amorphous silica mineral and highly alkaline plume with Ca ion Usui H. 1, Niibori Y. 1, Kadowaki J. 1, Tanaka K. 1, Tochiyama O. 2, Mimura H. 1 1 Department of Quantum Science
Chemical interaction between amorphous silica mineral and highly alkaline plume with Ca ion Usui H. 1, Niibori Y. 1, Kadowaki J. 1, Tanaka K. 1, Tochiyama O. 2, Mimura H. 1 1 Department of Quantum Science
Electrolysis, electrode polarisation, decomposition voltage, galvanic elements, Faraday s law.
 Characteristics and efficiency of PEM fuel cell TEP Related Topics Electrolysis, electrode polarisation, decomposition voltage, galvanic elements, Faraday s law. Principle In a PEM electrolyser, the electrolyte
Characteristics and efficiency of PEM fuel cell TEP Related Topics Electrolysis, electrode polarisation, decomposition voltage, galvanic elements, Faraday s law. Principle In a PEM electrolyser, the electrolyte
ANALYSIS OF LONG-TERM INFLUENCE OF CHLORIDE AGGRESSIVE ENVIRONMENT ON THE UHPC
 Proceedings of the 6th International Conference on Mechanics and Materials in Design, Editors: J.F. Silva Gomes & S.A. Meguid, P.Delgada/Azores, 26-30 July 2015 PAPER REF: 5573 ANALYSIS OF LONG-TERM INFLUENCE
Proceedings of the 6th International Conference on Mechanics and Materials in Design, Editors: J.F. Silva Gomes & S.A. Meguid, P.Delgada/Azores, 26-30 July 2015 PAPER REF: 5573 ANALYSIS OF LONG-TERM INFLUENCE
TESTING STABILIZATION/SOLIDIFICATION PROCESSES FOR MIXED WASTE. Tri T. Hoang (U. S. Environmental Protection Agency, Washington, DC)
 TESTING STABILIZATION/SOLIDIFICATION PROCESSES FOR MIXED WASTE Tri T. Hoang (U. S. Environmental Protection Agency, Washington, DC) ABSTRACT Stabilization/Solidification technologies reduce the mobility
TESTING STABILIZATION/SOLIDIFICATION PROCESSES FOR MIXED WASTE Tri T. Hoang (U. S. Environmental Protection Agency, Washington, DC) ABSTRACT Stabilization/Solidification technologies reduce the mobility
Table of Contents. Preface...
 Preface... xi Chapter 1. Metallurgical Thermochemistry... 1 1.1. Introduction... 1 1.2. Quantities characterizing the state of a system and its evolution... 3 1.2.1. The types of operations... 3 1.2.2.
Preface... xi Chapter 1. Metallurgical Thermochemistry... 1 1.1. Introduction... 1 1.2. Quantities characterizing the state of a system and its evolution... 3 1.2.1. The types of operations... 3 1.2.2.
Optimization Of Silica Fume, Fly Ash And Cement Mixes For High Performance Concrete
 Optimization Of Silica Fume, Fly Ash And Cement Mixes For High Performance Concrete Richard A. Livingston 1 and Walairat Bumrongjaroen 2 1 Federal Highway Administration, Office of Infrastructure R&D,
Optimization Of Silica Fume, Fly Ash And Cement Mixes For High Performance Concrete Richard A. Livingston 1 and Walairat Bumrongjaroen 2 1 Federal Highway Administration, Office of Infrastructure R&D,
Solutions Unit Exam Name Date Period
 Name Date Period Ms. Roman Page 1 Regents Chemistry 1. Which mixture can be separated by using the equipment shown below? 6. Which ion, when combined with chloride ions, Cl, forms an insoluble substance
Name Date Period Ms. Roman Page 1 Regents Chemistry 1. Which mixture can be separated by using the equipment shown below? 6. Which ion, when combined with chloride ions, Cl, forms an insoluble substance
CO forms CO 2. forms. (a) The coke reacts with the oxygen in the air to form carbon dioxide. C + O 2
 1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
1 Iron is extracted from the ore hematite in the Blast Furnace. waste gases firebrick lining raw materials: coke, C iron ore, Fe 2 O 3 limestone, CaCO 3 CO forms air slag molten iron CO 2 forms (a) The
SP2 Report on. Service Model of Calcareous Aggregate Cement Based on Field Test. Author and Chief Investigator: M. Valix
 SP2 Report on Service Model of Calcareous Aggregate Cement Based on Field Test Author and Chief Investigator: M. Valix Summary This is a service life model based on the kinetic analysis of calcareous aggregate
SP2 Report on Service Model of Calcareous Aggregate Cement Based on Field Test Author and Chief Investigator: M. Valix Summary This is a service life model based on the kinetic analysis of calcareous aggregate
Resistance of cracked concrete to chloride attack
 Resistance of cracked concrete to chloride attack Mathias Maes 1* and Nele De Belie 1* 1 Magnel Laboratory for Concrete Research, Department of Structural Engineering, Ghent University, Technologiepark
Resistance of cracked concrete to chloride attack Mathias Maes 1* and Nele De Belie 1* 1 Magnel Laboratory for Concrete Research, Department of Structural Engineering, Ghent University, Technologiepark
The Release of Base Metals During Acidic Leaching of Fly Ash
 The Release of Base Metals During Acidic Leaching of Fly Ash George Kazonich and Ann G. Kim U.S. Department of Energy Federal Energy Technology Center P.O. Box 19 Pittsburgh, PA 153 ABSTRACT Since 199,
The Release of Base Metals During Acidic Leaching of Fly Ash George Kazonich and Ann G. Kim U.S. Department of Energy Federal Energy Technology Center P.O. Box 19 Pittsburgh, PA 153 ABSTRACT Since 199,
LEACHING PERFORMANCE TEST ASSESSING DURABILITY OF CONCRETE EXPOSED TO CHEMICAL ATTACK
 LEACHING PERFORMANCE TEST ASSESSING DURABILITY OF CONCRETE EXPOSED TO CHEMICAL ATTACK François Jacquemot (1) and other members of GEF 8 leaching test working group (2) (1) CERIB (Centre d Études et de
LEACHING PERFORMANCE TEST ASSESSING DURABILITY OF CONCRETE EXPOSED TO CHEMICAL ATTACK François Jacquemot (1) and other members of GEF 8 leaching test working group (2) (1) CERIB (Centre d Études et de
8.0 FEASIBILITY OF PASSIVE SITE REMEDIATION. 8.1 Introduction
 8.0 FEASIBILITY OF PASSIVE SITE REMEDIATION 8.1 Introduction The purpose of this chapter is to evaluate the feasibility of passive site remediation based on the results of strength testing of stabilized
8.0 FEASIBILITY OF PASSIVE SITE REMEDIATION 8.1 Introduction The purpose of this chapter is to evaluate the feasibility of passive site remediation based on the results of strength testing of stabilized
Experiment 18 Determination of Iron by Visible Spectrophotometry
 Experiment 18 Determination of Iron by Visible Spectrophotometry PRELAB DISCUSSION Visible spectrophotometry (spectral analysis) is a method of measuring the concentration of colored solutions by the amount
Experiment 18 Determination of Iron by Visible Spectrophotometry PRELAB DISCUSSION Visible spectrophotometry (spectral analysis) is a method of measuring the concentration of colored solutions by the amount
CO 2 Utilization in Concrete
 CO 2 Utilization in Concrete Yixin Shao 1* and Hilal El-Hassan 2 1 McGill University, Canada 2 American University of Dubai, UAE * Department of Civil Engineering, McGill University, Montreal, Canada H3A
CO 2 Utilization in Concrete Yixin Shao 1* and Hilal El-Hassan 2 1 McGill University, Canada 2 American University of Dubai, UAE * Department of Civil Engineering, McGill University, Montreal, Canada H3A
WM 03 Conference, February 23-27, 2003, Tucson, AZ IN-SITU CHEMICAL OXIDATION OF CHLORINATED HYDROCARBONS IN THE PRESENCE OF RADIONUCLIDES
 IN-SITU CHEMICAL OXIDATION OF CHLORINATED HYDROCARBONS IN THE PRESENCE OF RADIONUCLIDES ABSTRACT Duane K. Root, Shaw Environmental & Infrastructure Treatability testing for In Situ Chemical Oxidation was
IN-SITU CHEMICAL OXIDATION OF CHLORINATED HYDROCARBONS IN THE PRESENCE OF RADIONUCLIDES ABSTRACT Duane K. Root, Shaw Environmental & Infrastructure Treatability testing for In Situ Chemical Oxidation was
Non-Steady-State Chloride Migration Test on Mortar with Supplementary Cementitious Materials
 Non-Steady-State Chloride Migration Test on Mortar with Supplementary Cementitious Materials Eisuke Nakamura 1, Satoshi Suzuki, and Hiroshi Watanabe 3 1 Public Works Research Institute, Japan 1-6 Minamihara,
Non-Steady-State Chloride Migration Test on Mortar with Supplementary Cementitious Materials Eisuke Nakamura 1, Satoshi Suzuki, and Hiroshi Watanabe 3 1 Public Works Research Institute, Japan 1-6 Minamihara,
Lecture 11. Solubility of Ionic Compounds in Water
 Lecture 11 Solubility of Ionic Compounds in Water Soluble Compounds Exceptions Acetates, Nitrates, Chlorates Na +, K +, NH 4 + compounds None None Halides ( Cl -, Br -, I - ) Ag +, Hg 2 2+, Cu +, Pb 2+
Lecture 11 Solubility of Ionic Compounds in Water Soluble Compounds Exceptions Acetates, Nitrates, Chlorates Na +, K +, NH 4 + compounds None None Halides ( Cl -, Br -, I - ) Ag +, Hg 2 2+, Cu +, Pb 2+
Laboratory Investigation on Effect of Phosphate Ions on Concrete C. H. Mattus L. R. Dole D.J. Naus
 1. Introduction Laboratory Investigation on Effect of Phosphate Ions on Concrete C. H. Mattus L. R. Dole D.J. Naus At the 499th Advisory Committee on Reactor Safeguards (ACRS) meeting, the staff presented
1. Introduction Laboratory Investigation on Effect of Phosphate Ions on Concrete C. H. Mattus L. R. Dole D.J. Naus At the 499th Advisory Committee on Reactor Safeguards (ACRS) meeting, the staff presented
Experimental Investigation on Diffusion Coefficient of Carbon Dioxide for Sustainable Construction Materials
 Experimental Investigation on Diffusion Coefficient of Carbon Dioxide for Sustainable Construction Materials Sang Hwa Jung 1, Myung Kue Lee 2, Seong Lo Lee 3, and Byung Hwan Oh 4 1 Korea Institute of Construction
Experimental Investigation on Diffusion Coefficient of Carbon Dioxide for Sustainable Construction Materials Sang Hwa Jung 1, Myung Kue Lee 2, Seong Lo Lee 3, and Byung Hwan Oh 4 1 Korea Institute of Construction
Leaching Tests Supported by TestAmerica Overview of Leaching
 Leaching Tests Supported by TestAmerica Current Methods SW-846 3 & 32; Low-level Radioactive Wastes ANSI/ANS-6.; LEAF EPA Methods 33, 34, 35 & 36 Leaching tests are tools to estimate the potential release
Leaching Tests Supported by TestAmerica Current Methods SW-846 3 & 32; Low-level Radioactive Wastes ANSI/ANS-6.; LEAF EPA Methods 33, 34, 35 & 36 Leaching tests are tools to estimate the potential release
Contaminant Release from Cementitious Materials: Savannah River Practice
 Contaminant Release from Cementitious Materials: Savannah River Practice Greg Flach July 14, 2009 Salt Lake City Performance Assessment Community of Practice Technical Exchange Meeting Modeling the Performance
Contaminant Release from Cementitious Materials: Savannah River Practice Greg Flach July 14, 2009 Salt Lake City Performance Assessment Community of Practice Technical Exchange Meeting Modeling the Performance
EXPERIMENTAL ANALYSIS OF WATER AND WATER VAPOR TRANSPORT IN COATING- SUBSTRATE SYSTEMS
 EXPERIMENTAL ANALYSIS OF WATER AND WATER VAPOR TRANSPORT IN COATING- SUBSTRATE SYSTEMS Milena Jiřičková, Robert Černý Czech Technical University, Faculty of Civil Engineering, Department of Structural
EXPERIMENTAL ANALYSIS OF WATER AND WATER VAPOR TRANSPORT IN COATING- SUBSTRATE SYSTEMS Milena Jiřičková, Robert Černý Czech Technical University, Faculty of Civil Engineering, Department of Structural
Ceramic Processing Research
 Journal of Ceramic Processing Research. Vol. 16, Special. 1, pp. s40~s44 (2015) J O U R N A L O F Ceramic Processing Research Influence of lead, chromium and zinc ions as toxic heavy metals between C-S-H
Journal of Ceramic Processing Research. Vol. 16, Special. 1, pp. s40~s44 (2015) J O U R N A L O F Ceramic Processing Research Influence of lead, chromium and zinc ions as toxic heavy metals between C-S-H
EFFECT OF CARBONATION ON THE PROPERTIES OF CONCRETE
 International Journal of Civil Engineering and Technology (IJCIET) Volume 9, Issue 7, July 2018, pp. 1605 1611, Article ID: IJCIET_09_07_172 Available online at http://www.iaeme.com/ijciet/issues.asp?jtype=ijciet&vtype=9&itype=7
International Journal of Civil Engineering and Technology (IJCIET) Volume 9, Issue 7, July 2018, pp. 1605 1611, Article ID: IJCIET_09_07_172 Available online at http://www.iaeme.com/ijciet/issues.asp?jtype=ijciet&vtype=9&itype=7
University of Arizona Department of Hydrology and Water Resources Dr. Marek Zreda
 University of Arizona Department of Hydrology and Water Resources Dr. Marek Zreda HWR431/531 - Hydrogeology Final exam - 12 May 1997 Open books and notes The test contains 8 problems on 7 pages. Read the
University of Arizona Department of Hydrology and Water Resources Dr. Marek Zreda HWR431/531 - Hydrogeology Final exam - 12 May 1997 Open books and notes The test contains 8 problems on 7 pages. Read the
Claudia Cardona, Yelena Katsenovich, Leonel Lagos Applied Research Center Florida International University. Session # 064, Abstract # 14434
 Claudia Cardona, Yelena Katsenovich, Leonel Lagos Applied Research Center Florida International University March 2 March 6, 2014 Phoenix, Arizona Session # 064, Abstract # 14434 Outline Background Objective
Claudia Cardona, Yelena Katsenovich, Leonel Lagos Applied Research Center Florida International University March 2 March 6, 2014 Phoenix, Arizona Session # 064, Abstract # 14434 Outline Background Objective
INFLUENCE OF POZZOLANIC PROPERTIES OF RED MUD WASTE AND HYDRATION PRODUCTS IN THE DURABILITY AND RETENTION OF HEAVY METALS IN MORTARS
 INFLUNC OF POZZOLANIC PROPRTIS OF RD MUD WAST AND HYDRATION PRODUCTS IN TH DURABILITY AND RTNTION OF HAVY MTALS IN MORTARS liz P. Manfroi (1), Malik Cheriaf () and Janaíde C. Rocha () (1, ) Civil ngineering,
INFLUNC OF POZZOLANIC PROPRTIS OF RD MUD WAST AND HYDRATION PRODUCTS IN TH DURABILITY AND RTNTION OF HAVY MTALS IN MORTARS liz P. Manfroi (1), Malik Cheriaf () and Janaíde C. Rocha () (1, ) Civil ngineering,
DISSOLVED CONSTITUENTS IN MARINE PORE WATER ... DATA EVALUATION...
 DISSOLVED CONSTITUENTS IN MARINE PORE WATER PORE WATER PROFILES DIFFUSIVE FLUXES... DATA EVALUATION... How to read pore water concentration profiles 1 How to read pore water concentration profiles Consumption
DISSOLVED CONSTITUENTS IN MARINE PORE WATER PORE WATER PROFILES DIFFUSIVE FLUXES... DATA EVALUATION... How to read pore water concentration profiles 1 How to read pore water concentration profiles Consumption
1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is.. The precipitate which forms first is
 CHEMISTRY 12 UNIT 3 - REVIEW Ksp Part A - Multiple Choice 1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is A. Fe 2 (SO 4 ) 3 (s) 3 Fe 2+ (aq) + 2 SO 4 3 (aq)
CHEMISTRY 12 UNIT 3 - REVIEW Ksp Part A - Multiple Choice 1. The equation that represents the equilibrium in a saturated solution of Fe 2 (SO 4 ) 3 is A. Fe 2 (SO 4 ) 3 (s) 3 Fe 2+ (aq) + 2 SO 4 3 (aq)
Effect of Moisture Conditioning and Handling on Leaching and Physical Properties of Sodium Bicarbonate Flue Gas Desulfurization Materials
 2017 World of Coal Ash (WOCA) Conference in Lexington, KY - May 9-11, 2017 http://www.flyash.info/ Effect of Moisture Conditioning and Handling on Leaching and Physical Properties of Sodium Bicarbonate
2017 World of Coal Ash (WOCA) Conference in Lexington, KY - May 9-11, 2017 http://www.flyash.info/ Effect of Moisture Conditioning and Handling on Leaching and Physical Properties of Sodium Bicarbonate
THE LEACHING EFFECT OF CONCRETE IMMERSED IN AMMONIUM NITRATE SOLUTION
 THE LEACHING EFFECT OF CONCRETE IMMERSED IN AMMONIUM NITRATE SOLUTION U. Schneider and S.-W. Chen Institute of Building Construction and Technology, Vienna University of Technology, Austria Abstract This
THE LEACHING EFFECT OF CONCRETE IMMERSED IN AMMONIUM NITRATE SOLUTION U. Schneider and S.-W. Chen Institute of Building Construction and Technology, Vienna University of Technology, Austria Abstract This
SECTION B3 DIFFUSION OF GASES IN FORMATE BRINES
 FORMATE TECHNICAL MANUAL C A B O T COMPATIBILITIES AND INTERACTIONS SECTION B3 DIFFUSION OF GASES IN FORMATE BRINES B3.1 Introduction...2 B3.2 The diffusion model...2 B3.3 Diffusion of CH 4 in formate
FORMATE TECHNICAL MANUAL C A B O T COMPATIBILITIES AND INTERACTIONS SECTION B3 DIFFUSION OF GASES IN FORMATE BRINES B3.1 Introduction...2 B3.2 The diffusion model...2 B3.3 Diffusion of CH 4 in formate
INTERDISCIPLINARY INVESTIGATION (IDI)-LAB LABORATORY HANDOUT
 INTERDISCIPLINARY INVESTIGATION (IDI)-LAB LABORATORY HANDOUT Research-based Interdisciplinary Science Education ION CHROMATOGRAPHY TO INVESTIGATE WATER QUALITY ISSUES: FLUORIDE IN DRINKING WATER Introduction
INTERDISCIPLINARY INVESTIGATION (IDI)-LAB LABORATORY HANDOUT Research-based Interdisciplinary Science Education ION CHROMATOGRAPHY TO INVESTIGATE WATER QUALITY ISSUES: FLUORIDE IN DRINKING WATER Introduction
Geology 627, Hydrogeology Review questions for final exam h t 1/ 2
 Geology 67, Hydrogeology Review questions for final exam 004 Multiple choice and fill in the blank. There may be more than one correct choice for each question. 1. Which hydrogeologic quantities are represented
Geology 67, Hydrogeology Review questions for final exam 004 Multiple choice and fill in the blank. There may be more than one correct choice for each question. 1. Which hydrogeologic quantities are represented
Erik Lindeberg and Per Bergmo. SINTEF Petroleum Research, NO-7465 Trondheim, Norway
 THE LONG-TERM FATE OF CO 2 INJECTED INTO AN AQUIFER Erik Lindeberg and Per Bergmo SINTEF Petroleum Research, NO-7465 Trondheim, Norway ABSTRACT Assuming that an underground aquifer is capped by a capillary
THE LONG-TERM FATE OF CO 2 INJECTED INTO AN AQUIFER Erik Lindeberg and Per Bergmo SINTEF Petroleum Research, NO-7465 Trondheim, Norway ABSTRACT Assuming that an underground aquifer is capped by a capillary
Prediction of Chloride Permeability of High Performance Concrete
 Prediction of Chloride Permeability of High Performance Concrete M. Iqbal KHAN Saleh ALSAYED Assistant Professor Professor King Saud University King Saud University Riyadh 1141, KSA Riyadh 1141, KSA Summary
Prediction of Chloride Permeability of High Performance Concrete M. Iqbal KHAN Saleh ALSAYED Assistant Professor Professor King Saud University King Saud University Riyadh 1141, KSA Riyadh 1141, KSA Summary
Effect of high temperature on immobilization of heavy metals in concrete with an addition of galvanic sludge
 Waste Management and the Environment IV 331 Effect of high temperature on immobilization of heavy metals in concrete with an addition of galvanic sludge A. Król Faculty of Environmental Engineering, Opole
Waste Management and the Environment IV 331 Effect of high temperature on immobilization of heavy metals in concrete with an addition of galvanic sludge A. Król Faculty of Environmental Engineering, Opole
NORDTEST METHOD NT BUILD NT BUILD
 NORDTEST METHOD NT BUILD NT BUILD 492 1 492 Approved 1999 11 1(8) CONCRETE, MORTAR AND CEMENT-BASED REPAIR MATERIALS: CHLORIDE MIGRATION COEFFICIENT FROM NON-STEADY-STATE MIGRATION EXPERIMENTS UDC 691.32/691.53/691.54
NORDTEST METHOD NT BUILD NT BUILD 492 1 492 Approved 1999 11 1(8) CONCRETE, MORTAR AND CEMENT-BASED REPAIR MATERIALS: CHLORIDE MIGRATION COEFFICIENT FROM NON-STEADY-STATE MIGRATION EXPERIMENTS UDC 691.32/691.53/691.54
Assoc.Prof.BOONCHAI STITMANNAITHUM. Department of Civil Engineering, Faculty of Engineering Chulalongkorn University, Thailand
 Modeling of Chloride Penetration into Concrete Structures under Flexural Cyclic Load and Tidal Environment Assoc.Prof.BOONCHAI STITMANNAITHUM Department of Civil Engineering, Faculty of Engineering Chulalongkorn
Modeling of Chloride Penetration into Concrete Structures under Flexural Cyclic Load and Tidal Environment Assoc.Prof.BOONCHAI STITMANNAITHUM Department of Civil Engineering, Faculty of Engineering Chulalongkorn
1. Scaling. H.O.: H-5/21, KRISHNA NAGAR, DELHI Tel.: , Fax:
 Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Determining hydraulic properties of concrete and mortar by inverse modelling
 Mater. Res. Soc. Symp. Proc. Vol. 1475 2012 Materials Research Society DOI: 10.1557/opl.2012. 601 Determining hydraulic properties of concrete and mortar by inverse modelling Sébastien Schneider 1, Dirk
Mater. Res. Soc. Symp. Proc. Vol. 1475 2012 Materials Research Society DOI: 10.1557/opl.2012. 601 Determining hydraulic properties of concrete and mortar by inverse modelling Sébastien Schneider 1, Dirk
Pozzolanic reaction of glass powder and its influences on concrete properties
 Southern Cross University epublications@scu 23rd Australasian Conference on the Mechanics of Structures and Materials 214 Pozzolanic reaction of glass powder and its influences on concrete properties Hongjian
Southern Cross University epublications@scu 23rd Australasian Conference on the Mechanics of Structures and Materials 214 Pozzolanic reaction of glass powder and its influences on concrete properties Hongjian
Technology, Japan ABSTRACT
 Control of crystal polymorphism of CaCO 3 generated by self-healing under controlled temperature and ph *Risa Sengoku 1), Heesup Choi 2) and Masumi Inoue 3) 1), 2), 3) Department of Civil and Environmental
Control of crystal polymorphism of CaCO 3 generated by self-healing under controlled temperature and ph *Risa Sengoku 1), Heesup Choi 2) and Masumi Inoue 3) 1), 2), 3) Department of Civil and Environmental
Chapter 4. Prediction of the Service Life of. RC Structures Considering. Concrete Carbonation
 Chapter 4 Prediction of the Service Life of RC Structures Considering Concrete Carbonation 4.1 Outline In this chapter, FEMA is used in order to predict carbonation depth quantitatively because it can
Chapter 4 Prediction of the Service Life of RC Structures Considering Concrete Carbonation 4.1 Outline In this chapter, FEMA is used in order to predict carbonation depth quantitatively because it can
Effect of Acidic Curing Environment on the Strength and Durability of Concrete
 Effect of Acidic Curing Environment on the Strength and Durability of Concrete Taku, Kumator Josiphiah Department of Civil Engineering, University of Agriculture Makurdi, Nigeria Amartey, D Yusuf Department
Effect of Acidic Curing Environment on the Strength and Durability of Concrete Taku, Kumator Josiphiah Department of Civil Engineering, University of Agriculture Makurdi, Nigeria Amartey, D Yusuf Department
EXPERIMENT 5. Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline
 EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
Study of different assisting agents for the removal of heavy metals from MSW fly ashes
 Study of different assisting agents for the removal of heavy metals from MSW fly ashes C. Ferreiral 2, A. B, Ribeiro3, L. M. 0ttosen2 1Escola Sup. Agrdria de Coimbra, Portugal. 2Dep. of Civil Engineering,
Study of different assisting agents for the removal of heavy metals from MSW fly ashes C. Ferreiral 2, A. B, Ribeiro3, L. M. 0ttosen2 1Escola Sup. Agrdria de Coimbra, Portugal. 2Dep. of Civil Engineering,
IAEA-TECDOC-482 PREVENTION AND MITIGATION OF GROUNDWATER CONTAMINATION FROM RADIOACTIVE RELEASES
 IAEA-TECDOC-482 PREVENTION AND MITIGATION OF GROUNDWATER CONTAMINATION FROM RADIOACTIVE RELEASES A TECHNICAL DOCUMENT ISSUED BY THE INTERNATIONAL ATOMIC ENERGY AGENCY, VIENNA, 1988 PREVENTION AND MITIGATION
IAEA-TECDOC-482 PREVENTION AND MITIGATION OF GROUNDWATER CONTAMINATION FROM RADIOACTIVE RELEASES A TECHNICAL DOCUMENT ISSUED BY THE INTERNATIONAL ATOMIC ENERGY AGENCY, VIENNA, 1988 PREVENTION AND MITIGATION
Shrinkage Development in High Performance Concrete. Ammar Yahia, P.Eng., Ph.D.,
 Shrinkage Development in High Performance Concrete Ammar Yahia, P.Eng., Ph.D., Outlines 1. Introduction 2. High-performance concrete 3. Autogenous shrinkage 4. Autogenous shrinkage stress 5. Autogenous
Shrinkage Development in High Performance Concrete Ammar Yahia, P.Eng., Ph.D., Outlines 1. Introduction 2. High-performance concrete 3. Autogenous shrinkage 4. Autogenous shrinkage stress 5. Autogenous
Cementitious Waste Form Formulation and Testing Status. David Swanberg February 2018
 Cementitious Waste Form Formulation and Testing Status David Swanberg February 2018 1 Development Focus Generate data to support disposal facility performance assessments (PAs) Conduct screening tests
Cementitious Waste Form Formulation and Testing Status David Swanberg February 2018 1 Development Focus Generate data to support disposal facility performance assessments (PAs) Conduct screening tests
PERFORMANCE STUDY ON NO FINES CONCRETE
 Proceedings of the 3 rd International Conference on Civil Engineering for Sustainable Development (ICCESD 2016), 12~14 February 2016, KUET, Khulna, Bangladesh (ISBN: 978-984-34-0265-3) PERFORMANCE STUDY
Proceedings of the 3 rd International Conference on Civil Engineering for Sustainable Development (ICCESD 2016), 12~14 February 2016, KUET, Khulna, Bangladesh (ISBN: 978-984-34-0265-3) PERFORMANCE STUDY
CONCRETE TEST AND CALCIUM TREATMENT BEFORE THE APPLICATION OF PENESEAL TM PRO (RTU) ON OLD SURFACES LIKELY TO HAVE UNDERGONE CARBONATION
 CONCRETE TEST AND CALCIUM TREATMENT BEFORE THE APPLICATION OF PENESEAL TM PRO (RTU) ON OLD SURFACES LIKELY TO HAVE UNDERGONE CARBONATION 1. CONCRETE Concrete is basically made up of sand, cement, aggregate
CONCRETE TEST AND CALCIUM TREATMENT BEFORE THE APPLICATION OF PENESEAL TM PRO (RTU) ON OLD SURFACES LIKELY TO HAVE UNDERGONE CARBONATION 1. CONCRETE Concrete is basically made up of sand, cement, aggregate
Analysis of the Rapid Chloride Migration test
 Analysis of the Rapid Chloride Migration test P. Spiesz and H. J. H. Brouwers Eindhoven University of Technology, the Netherlands ABSTRACT: In this study the Rapid Chloride Migration test (RCM) standardized
Analysis of the Rapid Chloride Migration test P. Spiesz and H. J. H. Brouwers Eindhoven University of Technology, the Netherlands ABSTRACT: In this study the Rapid Chloride Migration test (RCM) standardized
Duncan. UNIT 8 - Chemical Equations BALANCING EQUATIONS PRACTICE WORKSHEET 14.) C2H6 + O2 CO2 + H2O. 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.
 BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
BALANCING EQUATIONS PRACTICE WORKSHEET 1.) CH4 + O2 CO2 + H2O 2.) Na + I2 NaI 3.) N2 + O2 N2O 4.) N2 + H2 NH3 5.) KI + Cl2 KCl + I2 6.) HCl + Ca(OH)2 CaCl2 + H2O 7.) KClO3 KCl + O2 8.) K3PO4 + HCl KCl
Slide 1. Slide 2. Slide 3. Hardness. Concentration is. What s the concentration of red triangles? What s in your pipes? 500 ml
 Slide 1 Hardness What s in your pipes? Slide 2 What s the concentration of red triangles? 500 ml 1 g 1 g 1 g A. 10 B. 10 C. D. 1 g 1 g It s all of the above! Slide 3 Concentration is any statement of the
Slide 1 Hardness What s in your pipes? Slide 2 What s the concentration of red triangles? 500 ml 1 g 1 g 1 g A. 10 B. 10 C. D. 1 g 1 g It s all of the above! Slide 3 Concentration is any statement of the
RemTech October 12, 2017 B.J. Min, M.Eng., P.Eng.
 Soil Stabilization /Solidification (S/S) Technology as a Cost Effective Tool for Heavy Metal Risk Management RemTech 2017 October 12, 2017 B.J. Min, M.Eng., P.Eng. AGENDA Risk based Approach for Heavy
Soil Stabilization /Solidification (S/S) Technology as a Cost Effective Tool for Heavy Metal Risk Management RemTech 2017 October 12, 2017 B.J. Min, M.Eng., P.Eng. AGENDA Risk based Approach for Heavy
Recycling Contaminated Spent Blasting Abrasives in Portland Cement Mortars
 TRANSPORTATION RESEARCH RECORD 1458 73 Recycling Contaminated Spent Blasting Abrasives in Portland Cement Mortars BRYAN K. SALT, ANDRE G. GARNER, DAVID W. FOWLER, RAYMOND C. LOEHR, AND RAMON L. CARRASQUILLO
TRANSPORTATION RESEARCH RECORD 1458 73 Recycling Contaminated Spent Blasting Abrasives in Portland Cement Mortars BRYAN K. SALT, ANDRE G. GARNER, DAVID W. FOWLER, RAYMOND C. LOEHR, AND RAMON L. CARRASQUILLO
Evaluation of cementation effect of sand mediated by enzymeinduced calcium carbonate precipitation
 The 2012 World Congress on Advances in Civil, Environmental, and Materials Research (ACEM 12) Seoul, Korea, August 26-30, 2012 Evaluation of cementation effect of sand mediated by enzymeinduced calcium
The 2012 World Congress on Advances in Civil, Environmental, and Materials Research (ACEM 12) Seoul, Korea, August 26-30, 2012 Evaluation of cementation effect of sand mediated by enzymeinduced calcium
Lecture (Part-2) Portland Cement Based Paste Systems
 Hydration, Porosity and Strength of Cementitious Materials Prof. Sudhir Mishra and Prof. K. V. Harish Department of Civil Engineering Indian Institute of Technology, Kanpur Lecture - 16-18 (Part-2) Portland
Hydration, Porosity and Strength of Cementitious Materials Prof. Sudhir Mishra and Prof. K. V. Harish Department of Civil Engineering Indian Institute of Technology, Kanpur Lecture - 16-18 (Part-2) Portland
Demonstration of osmosis
 Demonstration of osmosis We will try to carry out a classical experiment on demonstration of osmosis. The principle is shown in the figure. Water moves from the solution of lower osmolality, across the
Demonstration of osmosis We will try to carry out a classical experiment on demonstration of osmosis. The principle is shown in the figure. Water moves from the solution of lower osmolality, across the
Study on Leaching of Hexavalent Chromium from Hardened Concretes Using Tank Leaching Test
 Journal of Advanced Concrete Technology Vol. 5, No., -7, June 7 / Copyright 7 Japan Concrete Institute Scientific paper Study on Leaching of Hexavalent Chromium from Hardened Concretes Using Tank Leaching
Journal of Advanced Concrete Technology Vol. 5, No., -7, June 7 / Copyright 7 Japan Concrete Institute Scientific paper Study on Leaching of Hexavalent Chromium from Hardened Concretes Using Tank Leaching
NSF Carbon Sequestration. Concrete Masonry Block. 2 nd Trial. This report compares testing of carbon sequestration at low pressure CO2 (5psi) with a
 NSF Carbon Sequestration Concrete Masonry Block 2 nd Trial Purpose: This report compares testing of carbon sequestration at low pressure CO2 (5psi) with a fine water mist addition. CO2 CHAMBER, MATERIALS
NSF Carbon Sequestration Concrete Masonry Block 2 nd Trial Purpose: This report compares testing of carbon sequestration at low pressure CO2 (5psi) with a fine water mist addition. CO2 CHAMBER, MATERIALS
Some studies on the reaction between fly ash and lime
 Bull. Mater. Sci., Vol. 28, No. 2, April 2005, pp. 131 136. Indian Academy of Sciences. Some studies on the reaction between fly ash and lime A BASUMAJUMDAR, A K DAS, N BANDYOPADHYAY and S MAITRA* College
Bull. Mater. Sci., Vol. 28, No. 2, April 2005, pp. 131 136. Indian Academy of Sciences. Some studies on the reaction between fly ash and lime A BASUMAJUMDAR, A K DAS, N BANDYOPADHYAY and S MAITRA* College
Blast Furnace Slag Cements
 REFERENCE DATA SHEET 3-2011 Blast Furnace Slag Cements FLY ASH REFERENCE Properties, Characteristics and Applications DATA SHEET No. 1 August 2009 1. INTRODUCTION This data sheet reviews in some detail
REFERENCE DATA SHEET 3-2011 Blast Furnace Slag Cements FLY ASH REFERENCE Properties, Characteristics and Applications DATA SHEET No. 1 August 2009 1. INTRODUCTION This data sheet reviews in some detail
Experimental research for the determination of some parameters needed for the calculation of life-cycle CO 2 emission of reinforced concrete buildings
 Experimental research for the determination of some parameters needed for the calculation of life-cycle CO 2 emission of reinforced concrete buildings Dan Paul GEORGESCU, Adelina APOSTU, Radu PASCU Reinforced
Experimental research for the determination of some parameters needed for the calculation of life-cycle CO 2 emission of reinforced concrete buildings Dan Paul GEORGESCU, Adelina APOSTU, Radu PASCU Reinforced
with SF. Table 1 shows details of physical properties of concrete mix components. 2.3 Selection of water to cement ratio Mortar mixing was firstly con
 コンクリート工学年次論文集,Vol.36,No.1,214 -Technical Paper- STRENGTH CHARACTERISTICS AND EFFECTIVE CHLORIDE DIFFUSION COEFFICIENT OF RUBBERIZED CONCRETE Nurazuwa MD NOOR *1, Hidenori HAMADA *2, Yasutaka SAGAWA *3
コンクリート工学年次論文集,Vol.36,No.1,214 -Technical Paper- STRENGTH CHARACTERISTICS AND EFFECTIVE CHLORIDE DIFFUSION COEFFICIENT OF RUBBERIZED CONCRETE Nurazuwa MD NOOR *1, Hidenori HAMADA *2, Yasutaka SAGAWA *3
6 AN ORIGINAL METHOD TO DETERMINE THE NON LINEAR CHLORIDE BINDING ISOTHERM FROM BULK SPECIMENS OF MORTAR
 6 AN ORIGINAL METHOD TO DETERMINE THE NON LINEAR CHLORIDE BINDING ISOTHERM FROM BULK SPECIMENS OF MORTAR J.P. BIGAS1, F. LAMBERT2, J.P. OLLIVIER3 ' LMSC, Cergy Pontoise, France DESD SCCD, CEA, Cadarache,
6 AN ORIGINAL METHOD TO DETERMINE THE NON LINEAR CHLORIDE BINDING ISOTHERM FROM BULK SPECIMENS OF MORTAR J.P. BIGAS1, F. LAMBERT2, J.P. OLLIVIER3 ' LMSC, Cergy Pontoise, France DESD SCCD, CEA, Cadarache,
