Study the Effect of adding Ascorbic acid, Folic acid and Calcium on Stability Iron(II) in Physiological media
|
|
|
- Derrick Preston
- 6 years ago
- Views:
Transcription
1 International Journal of ChemTech Research CODEN( US): IJCRGG ISSN : Vol.6, No.2, pp , pril-june 2014 Study the Effect of adding scorbic acid, Folic acid and Calcium on Stability Iron(II) in Physiological media Th. l-ghabra 1 *, L. Mamoli 1 1 Department of Chemistry, Faculty of Sciences, University of Damascus, Syria * Corres.author: ghabra2004@yahoo.com Telephone : , Mobile Number : bstract: The aim of this research is a chemical study of the absorption Iron(II) in physiological media in the human body and to determine the optimum conditions for the absorption within the duodenum.the study has been carried on to pharmaceuticals that contain a high percentage of iron in laboratory conditions resemble physiological conditions within the duodenum including ph and temperature.lso to study the effect of adding some materials on stability of Iron II in physiological media to decide Whether this effect is positive or negative such as ascorbic acid, folic acid and calcium.we have also put optimum conditions of substances that increase the absorption of Iron (II) for substances that have a positive effect on the stability of Iron(II) such as ascorbic acid and folic acid and we have studied separation time between doses of iron and other materials that have a negative effect on the stability of Iron(II) in the duodenum such as calcium. Key words: physiological media duodenum- Stability iron (II) - folic acid - ascorbic acid - separation time. Introduction: Iron exists in the structure of many of the cells in the human body, such as hemoglobin (Hb), muscle tissue (meloglobin) responsible for storing oxygen in the muscles and the enzymes responsible for the oxidation of carbohydrates and fat within the body.nd is one of the most important metal ions in the human body. Intestine absorbs iron on duo/dual forms because it is the least availabe form and not constant.¹ We study the factors that increase the stability of duo iron that lead to increase the absorption process in the intestine and enable the human body to benefit from it optimallyto the most. Mechanism of iron absorption in the human body: Iron is absorbed in the upper part of the intestine by 5-10% of the iron exists in food in the presence of a factor returns such as vitamin C.The remaining iron becomes a burden on the colon.² Iron is absorbed in the wall of the intestine in duo form, and if the proportion of duo forms is increased the absorption of iron will be increased within intestine, after the duo iron crossing the wall of intestine it is reoxidized again to triple iron and associated with a protein called ferritin because it is not allowed to triple iron
2 Th. l-ghabra et al /Int.J. ChemTech Res.2014,6(2),pp to be free without link in the bloodstream and then travels through the blood to be used in the body such as the liver, bone marrow and other organs. Upon its arrival to the centers of its use, triple iron liberates from ferritin to be used in the synthesis of red blood cells, or to be used in other uses. Figure(1) absorption of duo iron in the human body.¹ Study Reference : (although I made a wide search,i could not find a rsearch pertaining to this studyafter 2008) study is published in Human Nutrition Journal In 1986 : s a result 299 studies presented in this area. Meals containing non hemi iron and knighted radioactive isotope of iron found that diets did not contain ascorbic acid, iron absorption was less absorbed than that contained ascorbic acid.³ study published in the Journal of Clinic Nutrition in 2001: bsorption of iron was studied in the presence of vitamin C on three periods:long-term,medium-term and short-term. The absorption of iron Increased to 14% in long-term, the absorption of iron Increased to 14% in medium-term and increased to 12% in short-term.⁴ study published in 1965 in the British medical journal This study showed that there is a relationship between iron deficiency in pregnant women and folic acid found that giving doses of folic acid reduces the likelihood of anemia in pregnant women.⁵ study was conducted by the University of Kansas in the United States in 1991: Used 3 types of calcium salts- Calcium phosphate calcium carbonate and, calcium citrate The first reduced iron absorption about 62-65%,the second reduced iron absorption about 49-50% and the third reduced iron absorption about 20-25%. Therefore proposed the use of calcium carbonate as a dietary supplement.⁶
3 Th. l-ghabra et al /Int.J. ChemTech Res.2014,6(2),pp study was published in Food Chemistry in 2006: It studied suitabl ph on the stability of duo iron that has been added to fruit juices. It used reagent pyridine derivatives in the measurement method to determine the optimal conditions to realize the stabilization of the added iron and color consistency of the juice that contains citric acid. the result of the study showed that the proper ph range is between 5-8.⁷ study was published in Meta Science in 2007: It studied the effect of adding ascorbic acid and citric acid on the stability of beef coulor. It showed that the color stability increases when ascorbic acid is added for meat.this stability was increased when the of ascorbic acid was raised from 3% to 10% this result attributed to the reduction properties of this acid. By adding the combination of ascorbic acid and citric acid the stability of chromatography of beef decreased because of the oxidizing properties of citric acid.⁸ Research Materials and Methods First- Ferrous Sulfate Heptahydrate (FeSo4.7H2O) SECOND-PR Reagent: 4-(2-Pyridylazo)resorcinol monosodium salt mono hydrate indicator reagent. We use sodium salt of PR which has the outlined formula C11H8N3NaO2 *H2O that reacts with Duo Iron to make complex by the following equation: 4-(2-pyridylazo)resorcinol= H2L. MX2 + 2H2L M(HL)2+ 2HX where M = FeII (X = Cl) Figure (2) Chemical formula of PR complex.⁹ Third- (borax 0.05 Molar): Sodium salt of boric acid (tetra sodium borate) prepared by mixing sodium hydroxide with boric acid. Na2B4O7.10H2O Borax Mouka solution is used in the measurement method. Fourth- folic acid 0.1 Molar C19H17N7O6 Fifth- ascorbic acid (0.1 Molar) Outlined Formula: C6H8O6 Sixth- EDT solution 0.1 Molar used in the method of measurement (masking reagent)
4 Th. l-ghabra et al /Int.J. ChemTech Res.2014,6(2),pp Methods of Measurement: There are several ways to measure duo iron one of them: 1-The method uses ortho phenanthroline, that makes orange complex with duo iron. 2-The method uses reagent of pyridine derivatives which is more selective and welknown : Highly selective and sensitive spectrophotometric determination of Iron(II)with 4-(2-Pyridylazo) resorcinol(pr): This method has been used in the measurement for duo iron to high selectivity, sensitivity and stability colorimetric with duo iron bar complexes. This method is still being used to determinate duo iron in analytical laboratories in pharmaceutical plants.¹⁰ Results and Discussion: Study the effect of ascorbic acid on the stability of duo iron in similar conditions of physiological media: Standard series was prepared containing 10 ml of Ferrous Sulfate Heptahydrate 0.1 Molar where the degree of PH 3-4 then added a mixture of borax and sodium hydroxide as buffer solution to become PH 6.5 similar degree of biological media in the intestinal then added different s of ascorbic acid and measured absorption at the wavelength of 520 nm using PR Reagent 0.1 Molar at a temperature of 37 C, ph 6.5 and added the PR after one of the mixing process. Table (1) Shows the values of the absorption of duo iron with different s of ascorbic acid Volume (ml) ferrous sulphate 0.1 M dded ascorbic acid bsorption reading 1 bsorption reading 2 bsorption reading 3 bsorption reading (verage) We noted from the table (1) when the of ascorbic acid is increased the duo iron absorbance is increased as well. This result is attributable to the reduction properties of ascorbic acid.we noticed by increasing the of ascorbic acid to more than 0.03 mol / liter. So it is in vain to increase ascorbic acid higher than its limit to increase the stability of duo iron because the duo iron absorbance does not increase.
5 Th. l-ghabra et al /Int.J. ChemTech Res.2014,6(2),pp Study time effect on different s of ascorbic acid on duo iron absorption when the temperature and ph similar to the physiological media: series of standard containing 10 ml of Ferrous Sulfate Heptahydrate 0.1 Molar was prepared a mixture of borax and sodium hydroxide then was added to become the PH of the media similar to duodenum-about 6.5; then different s of ascorbic acid added. We measured absorption at the wavelength of 520 nm using PR reagent 0.1 Molar at a temperature of 37 C and ph 6.5, PR was added after 15, 30, 45, 60 and 90. Table (2) shows the values of the absorption of duo iron after mixing it with different s of ascorbic acid and different time periods Volume (ml) ferrous sulphate 0.1 M dded ascorbic acid fter 15 fter 30 fter 45 fter 60 fter , We can deduce from the table (2) that when you increase the stay time of ascorbic acid with iron sulfate the absorption of duo iron increases but when we increase the stay time more than an hour process becomes useless. Study the effect of folic acid on the stability of duo iron in similar conditions of physiological media: Standard series was prepared containing 10 ml of Ferrous Sulfate Heptahydrate 0.1 Molar where the degree of ph 3-4 then added a mixture of borax and sodium hydroxide as buffer solution to become ph 6.5 similar degree of biological media in the intestinal then added different s of folic acid and measured absorption at the wavelength of 520 nm using PR reagent Molar 0.1 at a temperature of 37 C, ph 6.5 and added the PR after one of the mixing process. Table (3) Shows the values of the absorption of duo iron with different s of folic acid Volume (ml) ferrous sulphate 0.1 M dded Folic acid verage
6 Th. l-ghabra et al /Int.J. ChemTech Res.2014,6(2),pp We note from the table (3) above that the absorption of duo iron increased with increasing of folic acid, but increased it more than 0.03 mol / liter become useless.this result is attributable to reduction properties of folic acid but emanated from a certain percentage become useless. Study time effect on different s of folic acid on duo iron absorption when the temperature and ph similar to the physiological media: series of standard containing 10 ml of Ferrous Sulfate Heptahydrate 0.1 Molar was prepared a mixture of borax and sodium hydroxide then was added to become the ph of the media similar to duodenum-about 6.5 ; then different s of folic acid added. We measured absorption at the wavelength of 520 nm using PR reagent 0.1 Molar at a temperature of 37 C and ph 6.5, PR was added after 15, 30, 45, 60 and 90. Table (4) shows the values of the absorption of duo iron after mixing it with different s of folic acid and different time periods Volume (ml) ferrous sulphate 0.1 M dded folic acid fter 15 fter 30 fter 45 fter 60 fter We note from the table (4) useless of mixing duo iron sulfate solution 0.1 Molar with folic acid solution more than 30 s. Study the effect of calcium gluconate on the stability of duo Iron in similar conditions of physiological media: Standard series was prepared containing 10 ml of Ferrous Sulfate Heptahydrate 0.1 Molar where the degree of PH 3-4 then added a mixture of borax and sodium hydroxide as buffer solution to become PH 6.5 similar degree of biological media in the intestinal then added different s of calcium gluconate and measured absorption at the wavelength of 520 nm using PR Reagent 0.1 Molar at a temperature of 37 C, ph 6.5 and added the PR after one of the mixing process.. Table (5) Shows the values of the absorption of duo iron with different s of gluconate calcium Volume (ml) dded calcium ferrous sulphate gluconate M verage
7 Th. l-ghabra et al /Int.J. ChemTech Res.2014,6(2),pp We note from the table (5) there was positive absorption for iron duo when there is no calcium gluconate but when you add a 0.01 mol/l of calcium gluconate the absorption became negative suggesting a negative effect of ions of calcium on duo iron absorption in low s but when the gradual increase of the of calcium gluconate the absorption of duo iron increased. That's a negative effect on calcium absorption of duo iron in low s and this effect turns into a positive effect in high s but it was immediately measured without leaving enough time to mix the two components (Duo iron sulfate 0.1 Molar with calcium gluconate 0.1 Molar). Study time effect on different s of calcium gluconate on duo iron absorption when the temperature and PH similar to the physiological media: series of standard containing 10 ml of Ferrous Sulfate Heptahydrate 0.1 Molar was prepared a mixture of borax and sodium hydroxide then was added to become the PH of the media similar to duodenum-about 6.5; then different s of calcium gluconate added. We measured absorption at the wavelength of 520 nm using PR reagent 0.1 Molar at a temperature of 37 C and ph 6.5, PR was added after 15, 30, 45, 60 and 90. Table (6) shows the values of the absorption of duo iron after mixing it with different s of calcium gluconate and different time periods Volume (ml) ferrous sulphate 0.1 M dded calcium gluconate fter 15 fter 30 fter 45 fter 60 fter We note from the table (6) negative effect of duo iron absorption after periods of mixing it with different of calcium gluconate. By increasing the of calcium gluconate the negative effect increases. There was no remarkable difference after 30, 60 and 90. Hence,the calcium gluconate needs to stay with duo ferrous sulphate half an hour to give this negative effect on the absorption of duo iron. Recommendations & Conclusions First- Duo iron increases by adding ascorbic acid.so the absorption of duo iron increases in the duodenum due to reduction ability of ascorbic acid.the continuous increase of the of ascorbic acid is useless in the absorption of iron. Second- to increase the time of stay of ascorbic acid with iron sulfate the absorption of duo iron increases. But to increase the time of stay more than an hour, the process becomes useless. so it is preferable to give vitamin C simultaneously with an iron supplement. Third-Duo iron increases by adding folic acid so the absorption of duo iron increases in the duodenum and continues to increase. The continuous increase of the of folic acid is useless in the absorption of iron.
8 Th. l-ghabra et al /Int.J. ChemTech Res.2014,6(2),pp Fourth- increasing stay time of folic acid with iron sulfate increases the absorption of duo iron and stability in duodenum. Increasing the stay time to more than 30 s becomes useless. so it is advisable to give folic tablets simultaneously with an iron supplement. Fifth- Calcium affects negatively on the stability of duo iron so its absorption in the duodenum decreases. It is recommended to separate the intake time of calcium supplement from iron supplement no less than two hours to avoid the occurrence of negative impact. References 1. Marshall, W., Nancy, J., Morck, L., ndrews,., Clinical Chemistry sixth edition,,london Oxford (2008), Muir,., Hopfer, U., Regional specificity of iron uptake by smal intestinal brush-boarder membranes from normal and iron deficient mice. Gastrointestinal and Liver Pathology 11, (1985), Hallberg L., Brune, M., Rossander, L., Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum Nutr ppl Nutr, pr 40(2) (1986 ), Cook, J., Reddy,M., Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet1,2, The merican Society for Clinical Nutrition journal vol 73,no 1 (2001), Chanarin, I., Rothmant,., Berry, V., Iron Deficiency and its Relation to Folic-acid Status in Pregnancy, Results of a Clinical Trial, Brit. med. Journal 1, (1965), Cook, J., Dassenko, S., Whittaker, P., Calcium supplementation: effect on iron absorption, by The merican Society for Clinical Nutrition January vol 53 no 1 (1991), Vicente, H J., Martínez, GC., Ros, G., Optimisation of in vitro measurement of available iron from different fortificants in citric fruit juices Food ChemistryVolume 98, Issue 4, (2006), Mancinia, R., Huntb, M., Seyfertb, M., Kropfb, D., Hachmeisterb, K., Heraldb,T., Johnsonc, D., Effects of ascorbic and citric acid on beef lumbar vertebrae marrow colour Meat ScienceVolume 76, Issue 3, (2007), Karipcin.F., Kabalcilar, E., Spectroscopic and Thermal Studies on Solid Complexes of 4-(2-pyridylazo) resorcinol with Some Transition Metals cta Chim. Slov,(2007), 54, Sandell, E.,Colorimetric Determination of Traces of Metals, 2 nd ed,interescience,new York (1950), pp. 49. *****
Chem 2115 Experiment #9. Consumer Chemistry: Determining the Iron Content in Supplements
 Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
IRON METABOLISM. Harper s Illustrated Biochemistry chapter 50
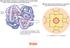
 IRON METABOLISM Harper s Illustrated Biochemistry chapter 50 IRON 26th element in the periodic table Chemical Symbol: Fe MW = 55.85 Electron Configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 Fourth most
IRON METABOLISM Harper s Illustrated Biochemistry chapter 50 IRON 26th element in the periodic table Chemical Symbol: Fe MW = 55.85 Electron Configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 Fourth most
Hypochromic/Microcytic Anemias
 Hypochromic/Microcytic Anemias (NORMO)/HYPOCHROMIC &/or (NORMO)/MICROCYTIC ANEMIAS 1. Disorders of iron utilization a. iron deficiency b. anemia of chronic disease 2. Disorders of globin synthesis a. Thalassemias
Hypochromic/Microcytic Anemias (NORMO)/HYPOCHROMIC &/or (NORMO)/MICROCYTIC ANEMIAS 1. Disorders of iron utilization a. iron deficiency b. anemia of chronic disease 2. Disorders of globin synthesis a. Thalassemias
Determination of Iron Content in Different Hemoglobin Samples from Some Patients by UV-Visible Spectrophotometer
 Advances in Analytical Chemistry 2016, 6(2): 35-40 DOI: 10.5923/j.aac.20160602.01 Determination of Iron Content in Different Hemoglobin Samples from Some Patients by UV-Visible Spectrophotometer Isam Eldin
Advances in Analytical Chemistry 2016, 6(2): 35-40 DOI: 10.5923/j.aac.20160602.01 Determination of Iron Content in Different Hemoglobin Samples from Some Patients by UV-Visible Spectrophotometer Isam Eldin
H-ferritin (Human) ELISA Kit
 H-ferritin (Human) ELISA Kit Catalog Number KA0211 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
H-ferritin (Human) ELISA Kit Catalog Number KA0211 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
THE UTILIZATION OF IRON AND THE RAPIDITY OF HEMOGLOBIN FORMATION IN ANEMIA DUE TO BLOOD LOSS
 Published Online: 1 June, 1940 Supp Info: http://doi.org/10.1084/jem.71.6.731 Downloaded from jem.rupress.org on April 30, 2018 THE UTILIZATION OF IRON AND THE RAPIDITY OF HEMOGLOBIN FORMATION IN ANEMIA
Published Online: 1 June, 1940 Supp Info: http://doi.org/10.1084/jem.71.6.731 Downloaded from jem.rupress.org on April 30, 2018 THE UTILIZATION OF IRON AND THE RAPIDITY OF HEMOGLOBIN FORMATION IN ANEMIA
Name(s) Food science, 20 points possible NAILS FOR BREAKFAST
 Name(s) Food science, 20 points possible NAILS FOR BREAKFAST Breakfast cereals may be fortified with a variety of vitamins and minerals to ensure a completely balanced nutritional meal. In general, a fortified
Name(s) Food science, 20 points possible NAILS FOR BREAKFAST Breakfast cereals may be fortified with a variety of vitamins and minerals to ensure a completely balanced nutritional meal. In general, a fortified
IRON METABOLISM & CLINICAL IMPORTANCE
 Iron IRON METABOLISM & CLINICAL IMPORTANCE IRON METABOLISM LEARNING OBJECTIVES At the end of the lecture, student should be able to; Know the biochemical proper1es of iron Enlist the func1ons of iron in
Iron IRON METABOLISM & CLINICAL IMPORTANCE IRON METABOLISM LEARNING OBJECTIVES At the end of the lecture, student should be able to; Know the biochemical proper1es of iron Enlist the func1ons of iron in
EDITORIALS REGULATION OF IRON ABSORPTION
 GSTROETEROOGY Copyright 1966 by The Williams & Wilkins Co. Vol. 50, o.6 Printed in U.S.. EDITORIS REGUTIO OF IRO BSORPTIO lthough a number of factors influence the amount of dietary iron absorbed, regulation
GSTROETEROOGY Copyright 1966 by The Williams & Wilkins Co. Vol. 50, o.6 Printed in U.S.. EDITORIS REGUTIO OF IRO BSORPTIO lthough a number of factors influence the amount of dietary iron absorbed, regulation
EXPERIMENT 5. Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline
 EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons?
 Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons? b) Electrons? c) Neutrons? 1 CH3OS Warm Up (Sept 13) 1. What is an isotope? 2. Neon has two major isotopes, Neon 20 and
Warm Up (Sept 12) How will an atom change if you change the number of: a) Protons? b) Electrons? c) Neutrons? 1 CH3OS Warm Up (Sept 13) 1. What is an isotope? 2. Neon has two major isotopes, Neon 20 and
Test sticks and test papers for semi-quantitative determinations
 QUANTOFIX test sticks for semi-quantitative determinations QUANTOFIX test sticks meet the most important requirements for a modern quick-test: rapid dip and read convenient the analysis can be carried
QUANTOFIX test sticks for semi-quantitative determinations QUANTOFIX test sticks meet the most important requirements for a modern quick-test: rapid dip and read convenient the analysis can be carried
Irish Medicines Board
 IRISH MEDICINES BOARD ACTS 1995 AND 2006 MEDICINAL PRODUCTS(CONTROL OF PLACING ON THE MARKET)REGULATIONS,2007 (S.I. No.540 of 2007) PA0949/003/001 Case No: 2064627 The Irish Medicines Board in exercise
IRISH MEDICINES BOARD ACTS 1995 AND 2006 MEDICINAL PRODUCTS(CONTROL OF PLACING ON THE MARKET)REGULATIONS,2007 (S.I. No.540 of 2007) PA0949/003/001 Case No: 2064627 The Irish Medicines Board in exercise
64 CuCl 2 in 50 µl 0.1N NaOAc buffer, and 20 µg of each DOTA-antibody conjugate in 40 µl
 Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
Number of DOTA per antibody The average number of DOTA chelators per antibody was measured using a reported procedure with modifications (1,2). Briefly, nonradioactive CuCl 2 (80-fold excess of DOTA antibodies)
Cell Growth and DNA Extraction- Technion igem HS
 Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
AP*Chemistry Solubility Equilibria
 AP*Chemistry Solubility Equilibria SOLUBILITY EQUILIBRIA (K sp, THE SOLUBILITY PRODUCT) Saturated solutions of salts are another type of chemical equilibria. Remember those solubility rules? The fine print
AP*Chemistry Solubility Equilibria SOLUBILITY EQUILIBRIA (K sp, THE SOLUBILITY PRODUCT) Saturated solutions of salts are another type of chemical equilibria. Remember those solubility rules? The fine print
FECAL OCCULT BLOOD DETECTION (HEMOCCULT TEST)
 University of California, San Francisco -- Department of Laboratory Medicine San Francisco General Hospital 1001 Potrero Avenue, San Francisco CA 94110 Clinical Laboratory - Eberhard Fiebig, M.D., Director
University of California, San Francisco -- Department of Laboratory Medicine San Francisco General Hospital 1001 Potrero Avenue, San Francisco CA 94110 Clinical Laboratory - Eberhard Fiebig, M.D., Director
(06) WMP/Jun10/CHEM5
 Period 3 Elements 6 2 Sodium, aluminium and silicon are solid elements with a silver colour. These elements react with oxygen to form oxides with high melting points. Aluminium is a reactive metal, but
Period 3 Elements 6 2 Sodium, aluminium and silicon are solid elements with a silver colour. These elements react with oxygen to form oxides with high melting points. Aluminium is a reactive metal, but
Specification and Certificate of Analysis
 Reagents Green chemistry For decades, the Tintometer Group has been known as a producer of reagents for water analysis, which are supplied under the brand name Lovibond. The wide range of applications
Reagents Green chemistry For decades, the Tintometer Group has been known as a producer of reagents for water analysis, which are supplied under the brand name Lovibond. The wide range of applications
Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women 1 3
 Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women 1 3 Penni Davila-Hicks, Elizabeth C Theil, and Bo Lönnerdal ABSTRACT Background: Recent studies in humans suggest
Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women 1 3 Penni Davila-Hicks, Elizabeth C Theil, and Bo Lönnerdal ABSTRACT Background: Recent studies in humans suggest
Rev Experiment 10
 Experiment 10 SPECTROPHOTOMETRIC DETERMINATION OF IRON IN DRINKING WATER 2 lab periods Reading: 1) Chapter 17, pg 393-403, Quantitative Chemical Analysis, 8 h Edition, Daniel C. Harris (7 th Edition: Chapter
Experiment 10 SPECTROPHOTOMETRIC DETERMINATION OF IRON IN DRINKING WATER 2 lab periods Reading: 1) Chapter 17, pg 393-403, Quantitative Chemical Analysis, 8 h Edition, Daniel C. Harris (7 th Edition: Chapter
Accutest ValuPak Immunological Fecal Occult Blood Test
 INTENDED USE The Accutest ValuPak Immunological Fecal Occult Blood (IFOB) Test is a rapid chromatographic immunoassay for the qualitative detection of human occult blood in human fecal specimens. The device
INTENDED USE The Accutest ValuPak Immunological Fecal Occult Blood (IFOB) Test is a rapid chromatographic immunoassay for the qualitative detection of human occult blood in human fecal specimens. The device
4-1 Cell biology Trilogy
 4- Cell biology Trilogy.0 Figure shows cells containing and surrounded by oxygen molecules. Oxygen can move into cells or out of cells. Figure Cell A Cell B Cell C Oxygen molecules Cell D. Into which cell,
4- Cell biology Trilogy.0 Figure shows cells containing and surrounded by oxygen molecules. Oxygen can move into cells or out of cells. Figure Cell A Cell B Cell C Oxygen molecules Cell D. Into which cell,
Acta Medica Okayama. Iron metabolism of in-testinal mucosa in various blood diseases JUNE Ikuro Kimura Junichiro Tsuchida Hidenari Kodani
 Acta Medica Okayama Volume 18, Issue 3 1964 Article 3 JUNE 1964 Iron metabolism of in-testinal mucosa in various blood diseases Ikuro Kimura Junichiro Tsuchida Hidenari Kodani Okayama University, Okayama
Acta Medica Okayama Volume 18, Issue 3 1964 Article 3 JUNE 1964 Iron metabolism of in-testinal mucosa in various blood diseases Ikuro Kimura Junichiro Tsuchida Hidenari Kodani Okayama University, Okayama
SPECTROPHOTOMETRIC DETERMINATION OF IRON
 SPECTROPHOTOMETRIC DETERMINATION OF IRON In this experiment you will determine trace amounts of iron using spectrophotometric methods. BACKGROUND In solution ferrous iron combines with 2,2 bipyridyl to
SPECTROPHOTOMETRIC DETERMINATION OF IRON In this experiment you will determine trace amounts of iron using spectrophotometric methods. BACKGROUND In solution ferrous iron combines with 2,2 bipyridyl to
Method (0.1 to 8.0 mg/l Cu) TNTplus 860
 Chlorine, Total, Bulk Powder, 8167 DOC316.53.01255 Bathocuproine Method 1 Method 10238 (0.1 to 8.0 mg/l Cu) TNTplus 860 Scope and Application: For water, wastewater and process water. Digestion may be
Chlorine, Total, Bulk Powder, 8167 DOC316.53.01255 Bathocuproine Method 1 Method 10238 (0.1 to 8.0 mg/l Cu) TNTplus 860 Scope and Application: For water, wastewater and process water. Digestion may be
IRON ABSORPTION IN DOGS DURING ANEMIA DUE TO ACETYLPHENYLHYDRAZINE
 IRON ABSORPTION IN DOGS DURING ANEMIA DUE TO ACETYLPHENYLHYDRAZINE W. B. Stewart,, Philip S. Vassar, Robert S. Stone J Clin Invest. 1953;32(12):1225-1228. https://doi.org/10.1172/jci102850. Research Article
IRON ABSORPTION IN DOGS DURING ANEMIA DUE TO ACETYLPHENYLHYDRAZINE W. B. Stewart,, Philip S. Vassar, Robert S. Stone J Clin Invest. 1953;32(12):1225-1228. https://doi.org/10.1172/jci102850. Research Article
What Is The Chemical Formula For Copper 2 Sulfate
 What Is The Chemical Formula For Copper 2 Sulfate Free Download WHAT IS THE CHEMICAL FORMULA FOR COPPER 2 SULFATE WHAT IS THE FORMULA FOR COPPER(II) SULFATE? REFERENCE Sun, 17 Dec 2017 13:50:00 GMT the
What Is The Chemical Formula For Copper 2 Sulfate Free Download WHAT IS THE CHEMICAL FORMULA FOR COPPER 2 SULFATE WHAT IS THE FORMULA FOR COPPER(II) SULFATE? REFERENCE Sun, 17 Dec 2017 13:50:00 GMT the
CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Name KEY Period CRHS Academic Chemistry Unit 1 Matter and Change HOMEWORK Due Date Assignment On-Time (100) Late (70) 1.1 1.2 1.3 Warm Ups Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic
Polyvidone Polyvinylpyrrolidone H 2 C H C N
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 (C 6 H 9 NO)n [9003-39-8] Poly [(2-oxo-1-pyrrolidinyl) ethylene] Povidone (Rev. 1, Stage 4)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 (C 6 H 9 NO)n [9003-39-8] Poly [(2-oxo-1-pyrrolidinyl) ethylene] Povidone (Rev. 1, Stage 4)
Some Basic Concepts of Chemistry
 Q 1. Some Basic Concepts of Chemistry What weight of AgCI will be precipitated when a solution containing 4.77 g of NaCI is added to a solution of 5.77 g of AgNO 3? (IIT JEE 1978 3 Marks) Q 2. One gram
Q 1. Some Basic Concepts of Chemistry What weight of AgCI will be precipitated when a solution containing 4.77 g of NaCI is added to a solution of 5.77 g of AgNO 3? (IIT JEE 1978 3 Marks) Q 2. One gram
CHEMILUMINESCENCE ENZYME IMMUNOASSAY (CLIA) THYROID STIMULATING HORMONE (FERRITIN) Ferritin
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills CA 91367 USA Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills CA 91367 USA Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
ICSE-Science 2 (Chemistry) 2004
 ICSE-Science 2 (Chemistry) 2004 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
ICSE-Science 2 (Chemistry) 2004 Answers to this Paper must be written on the paper provided separately. You will not be allowed to write during the first 15 minutes. This time is to be spent in reading
Appendix C1: Batch Kinetics Tests
 Appendix C1: Batch Kinetics Tests This appendix contains the entire data set for the batch kinetics tests for the potential biofilter components. These tests were performed to provide estimates of optimal
Appendix C1: Batch Kinetics Tests This appendix contains the entire data set for the batch kinetics tests for the potential biofilter components. These tests were performed to provide estimates of optimal
Fisher BioReagents Buffers for Life Science Research
 Fisher BioReagents Buffers for Life Science Research The Water Makes a Difference. CellPURE Biological Buffers are of the highest quality (purity 99%). They are tested for low heavy metal content and the
Fisher BioReagents Buffers for Life Science Research The Water Makes a Difference. CellPURE Biological Buffers are of the highest quality (purity 99%). They are tested for low heavy metal content and the
MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE. CHEMISTRY OCTOBER hour
 MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE CANDIDATE NAME SCHOOL NAME CLASS/SECTION NATIONAL ASSESSMENT AT FORM III CHEMISTRY
MINISTRY OF EDUCATION AND HUMAN RESOURCES, TERTIARY EDUCATION AND SCIENTIFIC RESEARCH MAURITIUS EXAMINATIONS SYNDICATE CANDIDATE NAME SCHOOL NAME CLASS/SECTION NATIONAL ASSESSMENT AT FORM III CHEMISTRY
Fisher BioReagents Buffers for Life Science Research
 Fisher BioReagents Buffers for Life Science Research The Water Makes a Difference. CellPURE Biological Buffers are of the highest quality (purity 99%). They are tested for low heavy metal content and the
Fisher BioReagents Buffers for Life Science Research The Water Makes a Difference. CellPURE Biological Buffers are of the highest quality (purity 99%). They are tested for low heavy metal content and the
SCIENCE REVISION CARDS For KS3 and Common Entrance
 SCIENCE REVISION CARDS For KS3 and Common Entrance I recommend printing the following pages onto card and cutting them into 4 equal pieces. They will then fit into a file box for revision purposes. Many
SCIENCE REVISION CARDS For KS3 and Common Entrance I recommend printing the following pages onto card and cutting them into 4 equal pieces. They will then fit into a file box for revision purposes. Many
Enzyme Linked Immunosorbent Assay for Horseradish Peroxidase Labeled Antibodies. (Cat. # )
 115PR G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name femtoelisa HRP Kit Enzyme Linked Immunosorbent Assay for Horseradish Peroxidase Labeled
115PR G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name femtoelisa HRP Kit Enzyme Linked Immunosorbent Assay for Horseradish Peroxidase Labeled
Iron filings (Fe) 56g IRON + SULPHUR IRON SULPHIDE
 W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
Iron Deficiency Anemia
 Diagnosis of Iron Deficiency Anemia Using the Abaxis VetScan HM2 Hematology System Contributing Authors: Mary Anna Thrall, BA, DVM, MS, DACVP; Barton Gillespie, BS, DVM Ross University School of Veterinary
Diagnosis of Iron Deficiency Anemia Using the Abaxis VetScan HM2 Hematology System Contributing Authors: Mary Anna Thrall, BA, DVM, MS, DACVP; Barton Gillespie, BS, DVM Ross University School of Veterinary
CytoScan WST 1 Cell Cytotoxicity Assay
 087PR G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name CytoScan WST 1 Cell Cytotoxicity Assay Colorimetric Assay for Quantitation of Cellular
087PR G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name CytoScan WST 1 Cell Cytotoxicity Assay Colorimetric Assay for Quantitation of Cellular
TREATMENT OF DAIRY WASTE WATER BY MORINGA OLEIFERA AS NATURAL COAGULANT
 . TREATMENT OF DAIRY WASTE WATER BY MORINGA OLEIFERA AS NATURAL COAGULANT Neethu.P 1, Navami.D 2, Anitha.K 3 1 P.G Student, Environmental Engineering, MCET, Kerala, India 2 P.G Student, Environmental Engineering,
. TREATMENT OF DAIRY WASTE WATER BY MORINGA OLEIFERA AS NATURAL COAGULANT Neethu.P 1, Navami.D 2, Anitha.K 3 1 P.G Student, Environmental Engineering, MCET, Kerala, India 2 P.G Student, Environmental Engineering,
Transferrin ELISA. For the quantitative determination of transferrin in human biological samples
 Transferrin ELISA For the quantitative determination of transferrin in human biological samples Please see Appendix A for Reference Serum information. For Research Use Only. Not For Use In Diagnostic Procedures.
Transferrin ELISA For the quantitative determination of transferrin in human biological samples Please see Appendix A for Reference Serum information. For Research Use Only. Not For Use In Diagnostic Procedures.
Influences of organic pollutants in water on antioxidant enzyme in zebra fish
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(4):1014-1021 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Influences of organic pollutants in water on antioxidant
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(4):1014-1021 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Influences of organic pollutants in water on antioxidant
Glucose-6-Phosphate Dehydrogenase (G6PDH) Activity Assay Kit
 Product Manual Glucose-6-Phosphate Dehydrogenase (G6PDH) Activity Assay Kit Catalog Number MET-5081 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Glucose-6-phosphate
Product Manual Glucose-6-Phosphate Dehydrogenase (G6PDH) Activity Assay Kit Catalog Number MET-5081 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Glucose-6-phosphate
Paper 2. Science test. Remember. First name. Last name. School KEY STAGE 3 TIER 5 7
 Sc KEY STAGE 3 TIER 5 7 Science test Paper 2 First name Last name School 2009 Remember The test is 1 hour long. You will need: pen, pencil, rubber, ruler, protractor and calculator. The test starts with
Sc KEY STAGE 3 TIER 5 7 Science test Paper 2 First name Last name School 2009 Remember The test is 1 hour long. You will need: pen, pencil, rubber, ruler, protractor and calculator. The test starts with
METALS
 METALS 3 Gallium is a metallic element in Group III. It has similar properties to aluminium. (a) (i) Describe the structure and bonding in a metallic element. You should include a labelled diagram in your
METALS 3 Gallium is a metallic element in Group III. It has similar properties to aluminium. (a) (i) Describe the structure and bonding in a metallic element. You should include a labelled diagram in your
Appendix. Medium Composition. Peptone - 0.5gm (gram) Yeast extract - 0.5gm. Beef extract - 0.1gm. NaCl - 0.5g. Agar - 2gm. ph Starch - 0.
 Appendix Medium Composition Nutrient Agar Peptone - 0.5gm (gram) Yeast extract - 0.5gm Beef extract - 0.1gm NaCl - 0.5g Agar - 2gm Distilled water - 100ml ph - 7.0 Starch Agar Starch - 0.5 Peptone - 0.5
Appendix Medium Composition Nutrient Agar Peptone - 0.5gm (gram) Yeast extract - 0.5gm Beef extract - 0.1gm NaCl - 0.5g Agar - 2gm Distilled water - 100ml ph - 7.0 Starch Agar Starch - 0.5 Peptone - 0.5
1. Which of the given statements about the reaction below are incorrect?
 1. Which of the given statements about the reaction below are incorrect? 2PbO(s) + C(s) 2Pb(s) + CO 2 (g) a. Lead is getting reduced b. Carbon dioxide is getting oxidised c. Carbon is getting oxidised
1. Which of the given statements about the reaction below are incorrect? 2PbO(s) + C(s) 2Pb(s) + CO 2 (g) a. Lead is getting reduced b. Carbon dioxide is getting oxidised c. Carbon is getting oxidised
Solutions Unit Exam Name Date Period
 Name Date Period Ms. Roman Page 1 Regents Chemistry 1. Which mixture can be separated by using the equipment shown below? 6. Which ion, when combined with chloride ions, Cl, forms an insoluble substance
Name Date Period Ms. Roman Page 1 Regents Chemistry 1. Which mixture can be separated by using the equipment shown below? 6. Which ion, when combined with chloride ions, Cl, forms an insoluble substance
Influence Factors and Kinetics on Crystal Violet Degradation by Fenton and Optimization Parameters using Response Surface Methodology
 2011 International Conference on Environmental and Agriculture Engineering IPCBEE vol.15(2011) (2011) IACSIT Press, Singapore Influence Factors and Kinetics on Crystal Violet Degradation by Fenton and
2011 International Conference on Environmental and Agriculture Engineering IPCBEE vol.15(2011) (2011) IACSIT Press, Singapore Influence Factors and Kinetics on Crystal Violet Degradation by Fenton and
WST-1 CTLL-2 cell proliferation Kit (ready-to-use)
 Immunservice WST-1 CTLL-2 cell proliferation Kit (ready-to-use) Optimized for applications with CTLL-2 cells USER MANUAL For research use only. Not intended for diagnostic or therapeutic procedures 1.
Immunservice WST-1 CTLL-2 cell proliferation Kit (ready-to-use) Optimized for applications with CTLL-2 cells USER MANUAL For research use only. Not intended for diagnostic or therapeutic procedures 1.
FERROUS SULFATE HEPTAHYDRATE. Level 8, 1 Nicholson Street East Melbourne Victoria 3002 Australia (ALL HOURS)
 1. IDENTIFICATION OF THE MATERIAL AND SUPPLIER Product Name: Other name(s): FERROUS SULFATE HEPTAHYDRATE Ferrous sulphate heptahydrate; Iron sulphate heptahydrate; Iron sulfate heptahydrate; Iron protosulfate;
1. IDENTIFICATION OF THE MATERIAL AND SUPPLIER Product Name: Other name(s): FERROUS SULFATE HEPTAHYDRATE Ferrous sulphate heptahydrate; Iron sulphate heptahydrate; Iron sulfate heptahydrate; Iron protosulfate;
YEAR 9 (13+) SCHOLARSHIP. February 2013 for entry in September 2013 SCIENCE FACULTY 1. Biology, Chemistry, Physics. Your Name: Your School:..
 YEAR 9 (13+) SCHOLARSHIP February 2013 for entry in September 2013 SCIENCE FACULTY 1 Biology, Chemistry, Physics Your Name: Your School:.. Time allowed: 1 hour Total marks: 90 Equipment needed: Pen, pencil
YEAR 9 (13+) SCHOLARSHIP February 2013 for entry in September 2013 SCIENCE FACULTY 1 Biology, Chemistry, Physics Your Name: Your School:.. Time allowed: 1 hour Total marks: 90 Equipment needed: Pen, pencil
INSTRUCTIONS: CHLORIDE AND WATER HARDNESS (VERSENATE) METHOD PART No
 OFI Testing Equipment Chloride & Water Hardness Method Instructions Page 1 of 3 INSTRUCTIONS: CHLORIDE AND WATER HARDNESS (VERSENATE) METHOD PART No. 144-70 EQUIPMENT: QUANTITY DESCRIPTION PART NO. 1 Titration
OFI Testing Equipment Chloride & Water Hardness Method Instructions Page 1 of 3 INSTRUCTIONS: CHLORIDE AND WATER HARDNESS (VERSENATE) METHOD PART No. 144-70 EQUIPMENT: QUANTITY DESCRIPTION PART NO. 1 Titration
Modified Thiocyanate Method
 DefSci J, Vol38, NO. 2, April 1988, pp 177-182 Rapid Determination of Iron in Water by Modified Thiocyanate Method D.C. Goswami and H. Kalita Defence Research Laboratory, Tezpur-784 001 ABSTRACT A rapid
DefSci J, Vol38, NO. 2, April 1988, pp 177-182 Rapid Determination of Iron in Water by Modified Thiocyanate Method D.C. Goswami and H. Kalita Defence Research Laboratory, Tezpur-784 001 ABSTRACT A rapid
JSUNIL TUTORIAL, SAMASTIPUR
 Chapter 1 Chemical Reactions and Equations Q 1. Why should a magnesium ribbon be cleaned before burning in air? Ans. Before burning in air, the magnesium ribbon is cleaned by rubbing with a sandpaper.
Chapter 1 Chemical Reactions and Equations Q 1. Why should a magnesium ribbon be cleaned before burning in air? Ans. Before burning in air, the magnesium ribbon is cleaned by rubbing with a sandpaper.
Q1. The data in the table below show the melting points of oxides of some Period 3 elements. O 10 O P (Extra space) (2)......
 Q1. The data in the table below show the melting points of oxides of some Period 3 elements. Na 2 O P 4 O 10 SO 2 T m /K 1548 573 200 (a) In terms of structure and bonding, explain why (i) sodium oxide
Q1. The data in the table below show the melting points of oxides of some Period 3 elements. Na 2 O P 4 O 10 SO 2 T m /K 1548 573 200 (a) In terms of structure and bonding, explain why (i) sodium oxide
Iron Deficiency & Performance
 Iron Deficiency & Performance Does anemia matter? Does frank iron deficiency matter? Does a low ferritin 30-100 matter? Does a high hemoglobin matter? Does an even higher ferritin matter? Jeannie Callum,
Iron Deficiency & Performance Does anemia matter? Does frank iron deficiency matter? Does a low ferritin 30-100 matter? Does a high hemoglobin matter? Does an even higher ferritin matter? Jeannie Callum,
AnaTag HiLyte Fluor 647 Protein Labeling Kit
 AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
AnaTag HiLyte Fluor 647 Protein Labeling Kit Catalog # 72049 Kit Size 3 Conjugation Reactions This kit is optimized to conjugate HiLyte Fluor 647 SE to proteins (e.g., IgG). It provides ample materials
The oxides of nitrogen and the details of the oxygen states of nitrogen and the N:O ratio can be presented in a tabular form as:
 Oxides of Nitrogen Introduction to oxides of nitrogen Nirogen has a position in second period of group V in the modern periodic table. It has molecular formula N2. It has atomic number 7 and atomic weight
Oxides of Nitrogen Introduction to oxides of nitrogen Nirogen has a position in second period of group V in the modern periodic table. It has molecular formula N2. It has atomic number 7 and atomic weight
Edexcel GCSE Chemistry. Topic 4: Extracting metals and equilibria. Obtaining and using metals. Notes.
 Edexcel GCSE Chemistry Topic 4: Extracting metals and equilibria Obtaining and using metals Notes 4.1 Deduce the relative reactivity of some metals, by their reactions with water, acids and salt solutions
Edexcel GCSE Chemistry Topic 4: Extracting metals and equilibria Obtaining and using metals Notes 4.1 Deduce the relative reactivity of some metals, by their reactions with water, acids and salt solutions
Laser ACB 50 Product Code: Revised Date: 03/17/2009. Laser ACB 50
 DESCRIPTION Laser ACB 50 Laser ACB 50 is a peroxide/sulfuric acid system that replaces bichromate, chromic acid, or nitricsulfuric acid pickles commonly used for pickling copper, brass and bronze alloys.
DESCRIPTION Laser ACB 50 Laser ACB 50 is a peroxide/sulfuric acid system that replaces bichromate, chromic acid, or nitricsulfuric acid pickles commonly used for pickling copper, brass and bronze alloys.
Biotechnology Explorer
 Biotechnology Explorer Size Exclusion Chromatography Instruction Manual Catalog Number 166-0008-EDU www.bio-rad.com For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD
Biotechnology Explorer Size Exclusion Chromatography Instruction Manual Catalog Number 166-0008-EDU www.bio-rad.com For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD
1082 M. C. Fidler et al. fortified with hydrogen-reduced Fe (Fairweather-Tait et al. 2001), or when added to a typical Guatemalan meal fortified with
 British Journal of Nutrition (2003), 90, 1081 1085 q The Authors 2003 DOI: 10.1079/BJN2003995 Iron absorption from ferrous fumarate in adult women is influenced by ascorbic acid but not by Na 2 EDTA Meredith
British Journal of Nutrition (2003), 90, 1081 1085 q The Authors 2003 DOI: 10.1079/BJN2003995 Iron absorption from ferrous fumarate in adult women is influenced by ascorbic acid but not by Na 2 EDTA Meredith
HEPARIN CALCIUM. Heparinum calcicum
 Pharmeuropa 25.1 1 Reference: PA/PH/Exp. 6/T (12) 38 ANP NOTE ON THE MONOGRAPH Definition. It is proposed to restrict the scope to heparin material of porcine origin since some of the latest requirements
Pharmeuropa 25.1 1 Reference: PA/PH/Exp. 6/T (12) 38 ANP NOTE ON THE MONOGRAPH Definition. It is proposed to restrict the scope to heparin material of porcine origin since some of the latest requirements
Complement C4 Universal Kit
 Order references Reagents CONT C4TUR-B00 Universal kit 1 x 50 ml R1 + 1 x 5 ml R2 C4TUR-00 Universal kit 2 x 50 ml R1 + 1 x 15 ml R2 C4TUR-C00 Universal kit 1 x 12 ml R1 + 1 x 2 ml R2 Other necessary products
Order references Reagents CONT C4TUR-B00 Universal kit 1 x 50 ml R1 + 1 x 5 ml R2 C4TUR-00 Universal kit 2 x 50 ml R1 + 1 x 15 ml R2 C4TUR-C00 Universal kit 1 x 12 ml R1 + 1 x 2 ml R2 Other necessary products
Measuring Manganese Concentration Using Spectrophotometry
 Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
Design and Dosage Form. Dr. Deny Susanti
 Design and Dosage Form Dr. Deny Susanti Example 1 Aspirin tablet is stable but not as a liquid dosage form How to design liquid form? Soluble or dispersible aspirin tablets-to be dissolved in water Note:
Design and Dosage Form Dr. Deny Susanti Example 1 Aspirin tablet is stable but not as a liquid dosage form How to design liquid form? Soluble or dispersible aspirin tablets-to be dissolved in water Note:
Sunlight (solar energy) CO2 + H2O. Cellular Respiration (mitochondria) 36 ATP
 Aerobic & Anaerobic Conditions HASPI Medical Biology Lab 11 Background/Introduction The Flow of Energy & Cycling of Matter Photosynthesis and cellular respiration (including aerobic and anaerobic respiration)
Aerobic & Anaerobic Conditions HASPI Medical Biology Lab 11 Background/Introduction The Flow of Energy & Cycling of Matter Photosynthesis and cellular respiration (including aerobic and anaerobic respiration)
TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY. Unit 2 Metals
 TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY Unit 2 Metals Part 2 Metals differ in their reactivity with other chemicals and this influences their uses. Describe observable changes when metals react
TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY Unit 2 Metals Part 2 Metals differ in their reactivity with other chemicals and this influences their uses. Describe observable changes when metals react
DNA-RNA EXTRACTION. Dr. Amira A. T. AL-Hosary Lecturer of infectious diseases, Faculty of Veterinary Medicine, Assiut University, Egypt
 DNA-RNA EXTRACTION Dr. Amira A. T. AL-Hosary Lecturer of infectious diseases, Faculty of Veterinary Medicine, Assiut University, Egypt Nucleic Acids (DNA & RNA) DNA and RNA Breaks (Nucleotides) I. DNA
DNA-RNA EXTRACTION Dr. Amira A. T. AL-Hosary Lecturer of infectious diseases, Faculty of Veterinary Medicine, Assiut University, Egypt Nucleic Acids (DNA & RNA) DNA and RNA Breaks (Nucleotides) I. DNA
Gastrointestinal Cancer Antigen CA 19-9 ELISA Kit Protocol. (Cat. No.:EK )
 Gastrointestinal Cancer Antigen CA 19-9 ELISA Kit Protocol (Cat. No.:EK-310-17) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com
Gastrointestinal Cancer Antigen CA 19-9 ELISA Kit Protocol (Cat. No.:EK-310-17) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com
Year 7 Chemistry HW Questions
 Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Year 7 Chemistry HW Questions 37 minutes 56 marks Page 1 of 15 Q1. Molly used a ph sensor to test different liquids. She dipped the probe of the sensor into each liquid and recorded the ph value in a table.
Fibrinogen Universal Kit
 Order references Reagents CONT FITUR-B00 Universal kit 1 x 50 ml R1 + 1 x 5 ml R2 FITUR-00 Universal kit 2 x 50 ml R1 + 1 x 15 ml R2 FITUR-C00 Universal kit 2 x 17 ml R1 + 1 x 4 ml R2 Other necessary products
Order references Reagents CONT FITUR-B00 Universal kit 1 x 50 ml R1 + 1 x 5 ml R2 FITUR-00 Universal kit 2 x 50 ml R1 + 1 x 15 ml R2 FITUR-C00 Universal kit 2 x 17 ml R1 + 1 x 4 ml R2 Other necessary products
Application Notes for COD Analysis DETERMINATION OF CHEMICAL OXYGEN DEMAND (COD) IN WATER AND WASTE WATER.
 DETERMINATION OF CHEMICAL OXYGEN DEMAND (COD) IN WATER AND WASTE WATER. INTRODUCTION The chemical oxygen demand can be considered as an approximate measurement of the theoretical oxygen consumption, i.e.,
DETERMINATION OF CHEMICAL OXYGEN DEMAND (COD) IN WATER AND WASTE WATER. INTRODUCTION The chemical oxygen demand can be considered as an approximate measurement of the theoretical oxygen consumption, i.e.,
LDH-Cytotoxicity Assay Kit II
 LDH-Cytotoxicity Assay Kit II Catalog Number KA0786 500 assays Version: 08 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
LDH-Cytotoxicity Assay Kit II Catalog Number KA0786 500 assays Version: 08 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Surname. Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE G623 APPLIED SCIENCE. Cells and Molecules
 Candidate Forename Centre Number Candidate Surname Candidate Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE G623 APPLIED SCIENCE Cells and Molecules TUESDAY 12 JANUARY 2010: Morning
Candidate Forename Centre Number Candidate Surname Candidate Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE G623 APPLIED SCIENCE Cells and Molecules TUESDAY 12 JANUARY 2010: Morning
Covered with a thin layer of oxide at ordinary temperatures.
 1 More about Metals Physical properties of metals In general metals have luster, are malleable and ductile, good conductors of heat and electricity and have high boiling and melting points and nonmetals
1 More about Metals Physical properties of metals In general metals have luster, are malleable and ductile, good conductors of heat and electricity and have high boiling and melting points and nonmetals
LDH-Cytox Assay Kit. A Colorimetric Cytotoxicity Measuring Kit. Cat. No LDH-Cytox Assay Kit can be used to measure cytotoxicity in vitro
 A Colorimetric Cytotoxicity Measuring Kit Cat. No. 426401 LDH-Cytox Assay Kit can be used to measure cytotoxicity in vitro BioLegend, Inc Biolegend.com It is highly recommended that this manual be read
A Colorimetric Cytotoxicity Measuring Kit Cat. No. 426401 LDH-Cytox Assay Kit can be used to measure cytotoxicity in vitro BioLegend, Inc Biolegend.com It is highly recommended that this manual be read
BIOO RESEARCH PRODUCTS. Alkaline Phosphatase (AP) Color Endpoint Assay Kit Manual Catalog #:
 BIOO RESEARCH PRODUCTS Alkaline Phosphatase (AP) Color Endpoint Assay Kit Manual Catalog #: 3460-09 BIOO Scientific Corp. 2011 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure
BIOO RESEARCH PRODUCTS Alkaline Phosphatase (AP) Color Endpoint Assay Kit Manual Catalog #: 3460-09 BIOO Scientific Corp. 2011 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure
for production of 2-[ F]-fluoro-2-deoxy-D-glucose with the
![for production of 2-[ F]-fluoro-2-deoxy-D-glucose with the for production of 2-[ F]-fluoro-2-deoxy-D-glucose with the](/thumbs/78/76807906.jpg) Reagents Kit 18 for production of 2-[ F]-fluoro-2-deoxy-D-glucose with the GE TRACERlab MX FDG Module Catalog No.: RK-100 Date of Manufacturing: Sep.16 2008 Lot No.: RK-100-080916 Expiry Date: Sep.15 2009
Reagents Kit 18 for production of 2-[ F]-fluoro-2-deoxy-D-glucose with the GE TRACERlab MX FDG Module Catalog No.: RK-100 Date of Manufacturing: Sep.16 2008 Lot No.: RK-100-080916 Expiry Date: Sep.15 2009
Chemical Testing of Drinking Water
 Chemical Testing of Drinking Water Adapted from: An original Creek Connections activity. Water Chemistry Grade Level: all Duration: 50 minutes Setting: lab or classroom Summary: Students will conduct chemistry
Chemical Testing of Drinking Water Adapted from: An original Creek Connections activity. Water Chemistry Grade Level: all Duration: 50 minutes Setting: lab or classroom Summary: Students will conduct chemistry
Chapter 12 Reactivity of Metals 12.1 Different Reactivities of Metals Recall an experiment performed in F.3
 Chapter 12 Reactivity of Metals 12.1 Different Reactivities of Metals Recall an experiment performed in F.3 p.1/9 When freshly cut, potassium has a shiny surface and it reacts vigorously with water, giving
Chapter 12 Reactivity of Metals 12.1 Different Reactivities of Metals Recall an experiment performed in F.3 p.1/9 When freshly cut, potassium has a shiny surface and it reacts vigorously with water, giving
Laboratory Reagents and Raw Materials for Production.
 Laboratory Reagents and Raw Materials for Production www.itwreagents.com The Origin of Our Industrial Character ITW Illinois Tool Works Inc. (NYSE: ITW) is a diversified manufacturing company that delivers
Laboratory Reagents and Raw Materials for Production www.itwreagents.com The Origin of Our Industrial Character ITW Illinois Tool Works Inc. (NYSE: ITW) is a diversified manufacturing company that delivers
Compounds & Reactions Week 1. Writing Formulas & Balancing Equations. Write the chemical formula for each molecular (covalent) compound.
 Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Colorimetric Microplate Assay for Total Antioxidant Power For Research Use Only INTRODUCTION
 Colorimetric Microplate Assay for Total Antioxidant Power For Research Use Only INTRODUCTION Total Antioxidant Power Kit Product Number: TA02 Store at 4 C FOR RESEARCH USE ONLY Document Control Number:
Colorimetric Microplate Assay for Total Antioxidant Power For Research Use Only INTRODUCTION Total Antioxidant Power Kit Product Number: TA02 Store at 4 C FOR RESEARCH USE ONLY Document Control Number:
 Stoichiometric Calculations 1. The weight of calcium carbonate required to produce carbon-dioxide that is sufficient for conversion of one 0.1 mole sodium carbonate to sodium bicarbonate is 1) 1gm 2) 10gm
Stoichiometric Calculations 1. The weight of calcium carbonate required to produce carbon-dioxide that is sufficient for conversion of one 0.1 mole sodium carbonate to sodium bicarbonate is 1) 1gm 2) 10gm
MR. D HR UV AS HE R I.C.S.E. BOA RD PAP ER ICSE
 MR D HR UV AS HE R ICSE BOA RD PAP ER 200 4 1 ICSE-2004 Section A (40 Marks) (Attempt all questions from this section) Question 1 (a) Choose the letters A,B,C or D to match the descriptions (i) to (iv)
MR D HR UV AS HE R ICSE BOA RD PAP ER 200 4 1 ICSE-2004 Section A (40 Marks) (Attempt all questions from this section) Question 1 (a) Choose the letters A,B,C or D to match the descriptions (i) to (iv)
look down at cross on paper paper cross on paper
 1 The equation for the reaction between sodium thiosulfate and hydrochloric acid is given below. Na 2 S 2 O 3 (aq) + 2HCl (aq) 2NaCl (aq) + S(s) + SO 2 (g) + H 2 O(l) The speed of this reaction was investigated
1 The equation for the reaction between sodium thiosulfate and hydrochloric acid is given below. Na 2 S 2 O 3 (aq) + 2HCl (aq) 2NaCl (aq) + S(s) + SO 2 (g) + H 2 O(l) The speed of this reaction was investigated
PHYSICOCHEMICAL TREATMENT OF DAIRY PLANT WASTEWATER USING FERROUS SULFATE AND FERRIC CHLORIDE COAGULANTS
 International Journal of Basic and Applied Chemical Sciences ISSN: 2277-273 (Online) PHYSICOCHEMICAL TREATMENT OF DAIRY PLANT WASTEWATER USING FERROUS SULFATE AND FERRIC CHLORIDE COAGULANTS *Yogesh M.
International Journal of Basic and Applied Chemical Sciences ISSN: 2277-273 (Online) PHYSICOCHEMICAL TREATMENT OF DAIRY PLANT WASTEWATER USING FERROUS SULFATE AND FERRIC CHLORIDE COAGULANTS *Yogesh M.
AssayMax Human Transferrin ELISA Kit
 AssayMax Human Transferrin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
AssayMax Human Transferrin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
AssayMax Mouse Transferrin ELISA Kit
 AssayMax Mouse Transferrin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
AssayMax Mouse Transferrin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
Coagulation and Flocculation: Color Removal
 Coagulation and Flocculation: Color Removal Submitted to: Dr. Hashsham Research Complex Engineering Department of Civil and Environmental Engineering Michigan State University East Lansing, MI 48824 Authors
Coagulation and Flocculation: Color Removal Submitted to: Dr. Hashsham Research Complex Engineering Department of Civil and Environmental Engineering Michigan State University East Lansing, MI 48824 Authors
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Shardlow Business Park London Road Shardlow Derby Derbyshire DE72 2GD Contact: Sarah Ferguson Tel: +44 (0)1332-793000 Fax: +44 (0)1332-799263
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Shardlow Business Park London Road Shardlow Derby Derbyshire DE72 2GD Contact: Sarah Ferguson Tel: +44 (0)1332-793000 Fax: +44 (0)1332-799263
Approved for NPDES (Editorial Revision 1978) Silica, Dissolved (Colorimetric)
 METHOD #: 370.1 TITLE: Approved for NPDES (Editorial Revision 1978) Silica, Dissolved (Colorimetric) ANALYTE: Silica, SiO 2 INSTRUMENTATION: Spectrophotometer STORET No. Dissolved 00955 1.0 Scope and Application
METHOD #: 370.1 TITLE: Approved for NPDES (Editorial Revision 1978) Silica, Dissolved (Colorimetric) ANALYTE: Silica, SiO 2 INSTRUMENTATION: Spectrophotometer STORET No. Dissolved 00955 1.0 Scope and Application
Distillery Wastewater Decontamination by the Fenton Advanced Oxidation Method
 International Journal of Research in Engineering and Science (IJRES) ISSN (Online): 2320-9364, ISSN (Print): 2320-9356 Volume 3 Issue 2 ǁ February. 2015 ǁ PP.29-34 Distillery Wastewater Decontamination
International Journal of Research in Engineering and Science (IJRES) ISSN (Online): 2320-9364, ISSN (Print): 2320-9356 Volume 3 Issue 2 ǁ February. 2015 ǁ PP.29-34 Distillery Wastewater Decontamination
Human Peptide YY EIA
 KAMIYA BIOMEDICAL COMPANY Human Peptide YY EIA For the quantitative determination of Peptide YY in human serum and plasma. Cat. No. KT-378 For Research Use Only. 1 Rev. 9822378 PRODUCT INFORMATION Human
KAMIYA BIOMEDICAL COMPANY Human Peptide YY EIA For the quantitative determination of Peptide YY in human serum and plasma. Cat. No. KT-378 For Research Use Only. 1 Rev. 9822378 PRODUCT INFORMATION Human
