POINT DEFECTS IN CRYSTALS
|
|
|
- Meghan Woods
- 5 years ago
- Views:
Transcription
1 POINT DEFECTS IN CRYSTALS Overview Vacancies & their Clusters Interstitials Defects in Ionic Crytals Frenkel defect Shottky defect Part of MATERIALS SCIENCE & ENGINEERING A Learner s Guide AN INTRODUCTORY E-BOOK Anandh Subramaniam & Kantesh Balani Materials Science and Engineering (MSE) Indian Institute of Technology, Kanpur anandh@iitk.ac.in, URL: home.iitk.ac.in/~anandh Advanced Reading Point Defects in Materials F. Agullo-Lopez, C.R.A. Catlow, P.D. Townsend Academic Press, London (1988)
2 Point defects can be considered as 0D (zero dimensional) defects. The more appropriate term would be point like as the influence of 0D defects spreads into a small region around the defect. Point defects could be associated with stress fields and charge Point defects could associate to form larger groups/complexes the behaviour of these groups could be very different from an isolated point defect In the case of vacancy clusters in a crystal plane the defect could be visualized as an edge dislocation loop Point defects could be associated with other defects (like dislocations, grain boundaries etc.) Segregation of Carbon to the dislocation core region gives rise to yield point phenomenon Impurity /solute atoms may segregate to the grain boundaries Based on Origin Point defects could be Random (statistically stored) or Structural More in the next slide Based on Position Point defects could be Random (based on position) or Ordered More in the next slide
3 Point defects can be classified as below from two points of view The behaviour of a point defect depends on the class (as below) a point defect belongs to Based on origin Statistical Point Defects Structural Arise in the crystal for thermodynamic reasons Arise due to off-stoichiometry in an compound (e.g. in NiAl with B2 structure Al rich compositions result from vacant Ni sites) Based on position Random Point Defects Ordered Occupy random positions in a crystal Occupy a specific sublattice Vacancy ordered phases in Al-Cu-Ni alloys (V 6 C 5, V 8 C 7)
4 Based on source Intrinsic No additional foreign atom involved Point Defects Extrinsic Atoms of another species involved Vacancies Self Interstitials Anti-site defects In ordered alloys/compounds Note: Presence of a different isotope may also be considered as a defect
5 0D (Point defects) Non-ionic crystals Ionic crystals Vacancy Impurity Frenkel defect Schottky defect Interstitial Substitutional Other ~ Imperfect point-like regions in the crystal about the size of 1-2 atomic diameters Point defects can be created by removal, addition or displacement of an atomic species (atom, ion) Defect structures in ionic crystals can be more complex and are not discussed in detail in the elementary introduction
6 Vacancy Missing atom from an atomic site Atoms around the vacancy displaced Stress field produced in the vicinity of the vacancy Based on their origin vacancies can be Random/Statistical (thermal vacancies, which are required by thermodynamic equilibrium) or Structural (due to off-stoichiometry in a compound) Based on their position vacancies can be random or ordered Vacancies play an important role in diffusion of substitutional atoms Vacancies also play an important role in some forms of creep Non-equilibrium concentration of vacancies can be generated by: quenching from a higher temperature or by bombardment with high energy particles
7 Impurity Or alloying element Interstitial Substitutional Relative size Compressive & Shear Stress Fields Compressive stress fields SUBSTITUTIONAL IMPURITY/ELEMENT Foreign atom replacing the parent atom in the crystal E.g. Cu sitting in the lattice site of FCC-Ni INTERSTITIAL IMPURITY/ELEMENT Foreign atom sitting in the void of a crystal E.g. C sitting in the octahedral void in HT FCC-Fe Tensile Stress Fields
8 In some situations the same element can occupy both a lattice position and an interstitial position e.g. B in steel
9 Interstitial C sitting in the octahedral void in HT FCC-Fe r Octahedral void / r FCC atom = r Fe-FCC = 1.29 Å r Octahedral void = x 1.29 = 0.53 Å r C = 0.71 Å Compressive strains around the C atom Solubility limited to 2 wt% (9.3 at%) Interstitial C sitting in the octahedral void in LT BCC-Fe r Tetrahedral void / r BCC atom = 0.29 r C = 0.71 Å r Fe-BCC = Å r Tetrahedral void = 0.29 x = Å But C sits in smaller octahedral void- displaces fewer atoms Severe compressive strains around the C atom Solubility limited to wt% (0.037 at%)
10 Why are vacancies preferred in a crystal (at T> 0K)? Formation of a vacancy leads to missing bonds and distortion of the lattice The potential energy (Enthalpy) of the system increases Work required for the formation of a point defect Enthalpy of formation (H f ) [kj/mol or ev/defect] Though it costs energy to form a vacancy, its formation leads to increase in configurational entropy (the crystal without vacancies represents just one state, while the crystal with vacancies can exist in many energetically equivalent states, corresponding to various positions of the vacancies in the crystal the system becomes configurationally rich ) above zero Kelvin there is an equilibrium concentration/number of vacancies These type of vacancies are called Thermal Vacancies (and will not leave the crystal on annealing at any temperature Thermodynamically stable) Note: up and above the equilibrium concentration of vacancies there might be a additional nonequilibrium concentration of vacancies which are present. This can arise by quenching from a high temperature, irradiation with ions, cold work etc. When we quench a sample from high temperature part of the higher concentration of vacancies present (at higher temperature there is a higher equilibrium concentration of vacancies present) may be quenched-in at low temperature
11 Enthalpy of formation of vacancies (H f ) Crystal Kr Cd Pb Zn Mg Al Ag Cu Ni kj / mol ev / vacancy
12 Calculation of equilibrium concentration of vacancies Let n be the number of vacancies, N the number of sites in the lattice Assume that concentration of vacancies is small i.e. n/n << 1 the interaction between vacancies can be ignored H formation (n vacancies) = n. H formation (1 vacancy) Let H f be the enthalpy of formation of 1 mole of vacancies G = H T S G n H f S = S configurational H n n f zero T S n config G (putting n vacancies) = nh f T S config S config n k ln N n n For minimum H f N Exp 1 kt n G 0 n H f kt N n ln n H Assuming n << N n f exp N kt User R instead of k if H f is in J/mole
13 Variation of G with vacancy concentration at a fixed temperature T (ºC) n/n x x x x 10 3 H f = 1 ev/vacancy = 0.16 x J/vacancy Close to the melting point in FCC metals Au, Ag, Cu the fraction of vacancies is about 10 4 (i.e. one in 10,000 lattice sites are vacant)
14 Even though it costs energy to put vacancies into a crystal (due to broken bonds ), the Gibbs free energy can be lowered by accommodating some vacancies into the crystal due to the configurational entropy benefit that this provides Hence, certain equilibrium concentration/number of vacancies are preferred at T > 0K
15 Ionic Crystals In ionic crystal, during the formation of the defect the overall electrical neutrality has to be maintained (or to be more precise the cost of not maintaining electrical neutrality is high) Frenkel defect Cation being smaller get displaced to interstitial voids E.g. AgI, CaF 2 Ag interstitial concentration near melting point: in AgCl of 10 3 in AgBr of 10 2 This kind of self interstitial costs high energy in simple metals and is not usually found [H f (vacancy) ~ 1eV; H f (interstitial) ~ 3eV]
16 Schottky defect Pair of anion and cation vacancies E.g. Alkali halides Missing Anion Missing Cation
17 Other defects due to charge balance (/neutrality condition) If Cd 2+ replaces Na + one cation vacancy is created Schematic
18 Defects due to off stiochiometry ZnO heated in Zn vapour Zn y O (y >1) The excess cations occupy interstitial voids The electrons (2e ) released stay associated to the interstitial cation Schematic
19 Other defect configurations: association of ions with electrons and holes M 2+ cation associated with an electron X 2 anion associated with a hole
20 How do colours in some crystals arise due to colour centres? Colour centres (F Centre) Actually the distribution of the excess electron (density) is more on the +ve metal ions adjacent to the vacant site Violet colour of CaF 2 missing F with an electron in lattice Ionic Crystal F centre absorption energy (ev) LiCl 3.1 NaCl 2.7 KCl 2.2 CsCl 2.0 KBr 2.0 LiF 5.0 E h E c h 19 EKBr 2eV 2 ( ) J 34 8 absorption ( )(310 ) 7 KBr m 19 2 ( ) Visible spectrum: nm 620nm Red
21 Some more complications: an example of defect association Two adjacent F centres giving rise to a M centre
22 Structural Point defects In ordered NiAl (with ordered B2 structure) Al rich compositions result from vacancies in Ni sublattice In Ferrous Oxide (Fe 2 O) with NaCl structure there is a large concentration of cation vacancies. Some of the Fe is present in the Fe 3+ state correspondingly some of the positions in the Fe sublattice is vacant leads to off stoichiometry (Fe x O where x can be as low as 0.9 leading to considerable concentration of nonequilibrium vacancies) In NaCl with small amount of Ca 2+ impurity: for each impurity ion there is a vacancy in the Na + sublattice Antisite on Al sublattice Ni rich side NiAl Al rich side vacancies in Ni sublattice Antisite on Al sublattice Fe rich side FeAl Al rich side antisite in Fe sublattice The choice of antisite or vacancy is system specific
23 FeO heated in oxygen atmosphere Fe x O (x <1) Vacant cation sites are present Charge is compensated by conversion of ferrous to ferric ion: Fe 2+ Fe 3+ + e For every vacancy (of Fe cation) two ferrous ions are converted to ferric ions provides the 2 electrons required by excess oxygen
24 Point Defect ordering Using the example of vacancies we illustrate the concept of defect ordering As shown before, based on position vacancies can be random or ordered Ordered vacancies (like other ordered defects) play a different role in the behaviour of the material as compared to random vacancies
25 Origin of A sublattice Origin of B sublattice Schematic Crystal with vacancies As the vacancies are in the B sublattice these vacancies lead to off stoichiometry and hence are structural vacancies Vacancy ordering Examples of Vacancy Ordered Phases: V 6 C 5, V 8 C 7
26 Vacancy Ordered Phases (VOP) Me 6 C 5 trigonal ordered structures (e.g. V 6 C 5 ordered trigonal structure exists between ~ K) (The disordered structure is of NaCl type (FCC lattice) with C in non-metallic sites) Space group: P3 1 The disordered FCC basis vectors are related to the ordered structure by: a trigonal b c trigonal trigonal FCC FCC FCC Atom Wyckoff Position x y z Vacancy 3(a) 1/9 8/9 1/6 C1 3(a) 4/9 5/9 1/6 C2 3(a) 7/9 2/9 1/6 C3 3(a) 1/9 5/9 1/3 C4 3(a) 4/9 2/9 1/3 C5 3(a) 7/9 8/9 1/3 V1 3(a) 1/9 5/9 1/12 V2 3(a) 4/9 2/9 1/12 V3 3(a) 7/9 8/9 1/12 V4 3(a) 1/9 2/9 1/6 V5 3(a) 4/9 8/9 1/6 V6 3(A) 7/9 5/9 1/6
27 Complex and Associated Point Defects
28 Association of Point defects (especially vacancies) Point defects can occur in isolation or could get associated with each other (we have already seen some examples of these). If the system is in equilibrium then the enthalpic and entropic effects (i.e. on G) have to be considered in understanding the association of vacancies. If two vacancies get associated with each other (forming a di-vacancy) then this can be visualized as a reduction in the number of bonds broken, leading to an energy benefit (in Au this binding energy is ~ 0.3 ev). but this reduces the number of configurations possible with only dissociated vacancies. The ratio of vacancies to divacancies decreases with increasing temperature. Similarly an interstitial atom and a vacancy can come together to reduce the energy of the crystal would preferred to be associated. Non-equilibrium concentration of interstitials and vacancies can condense into larger clusters. In some cases these can be visualized as prismatic dislocation loop or stacking fault tetrahedron). Point defects can also be associated with other defects like dislocations, grain boundaries etc. We had considered a divacancy. Similar considerations come into play for tri-vacancy formation etc. Concept of Defect in a Defect & Hierarchy of Defects Click here to know more about Association of Defects Click here to know more about Defect in a Defect
29 Complex Point Defect Structures: an example The defect structures especially ionic solids can be much more complicated than the simple picture presented before. Using an example such a possibility is shown. In transition metal oxides the composition is variable In NiO and CoO fractional deviations from stoichiometry ( ) accommodated by introduction of cation vacancies In FeO larger deviations from stoichiometry is observed At T > 570C the stable composition is Fe (1x) O [x (0.05, 0.16)] Such a deviation can in principle be accommodated by Fe 2+ vacancies or O 2 interstitials In reality the situation is more complicated and the iron deficient structure is the 4:1 cluster 4 Fe 2+ vacancies as a tetrahedron + Fe 3+ interstitial at centre of the tetrahedron + additional neighbouring Fe 3+ interstitials These 4:1 clusters can further associate to form 6:2 and 13:4 aggregates Note: these are structural vacancies Continued
30 Schematic 4:1 cluster 4 Fe 2+ vacancies as a tetrahedron + Fe 3+ interstitial at centre of the tetrahedron + additional neighbouring Fe 3+ interstitials The figure shows an ideal starting configuration- the actual structure will be distorted with respect to this depiction
31 Methods of producing point defects Growth and synthesis Impurities may be added to the material during synthesis Thermal & thermochemical treatments and other stimuli Heating to high temperature and quench Heating in reactive atmosphere Heating in vacuum e.g. in oxides it may lead to loss of oxygen Etc. Plastic Deformation Ion implantation and irradiation Electron irradiation (typically >1MeV) Direct momentum transfer or during relaxation of electronic excitations) Ion beam implantation (As, B etc.) Neutron irradiation
32 Solved Example What is the equilibrium concentration of vacancies at 800K in Cu Data for Cu: Melting point = 1083 C = 1356K H f (Cu vacancy) = J/mole k (Boltzmann constant) = J/K R (Gas constant) = J/mole/K First point we note is that we are below the melting point of Cu 800K ~ 0.59 T m (Cu) n N exp H f RT n N exp exp( 18.04) If we increase the temperature to 1350K (near MP of copper) n N exp exp( 10.69) Experimental value:
33 Solved Example If a copper rod is heated from 0K to 1250K increases in length by ~2%. What fraction of this increase in length is due to the formation of vacancies? Data for Cu: H f (Cu vacancy) = J/mole R (Gas constant) = J/mole/K Cu is FCC (n = 4) L L 0 Fractional increase in length = 0.02 L0 to the 0K state. There are two contributions to this increase in length ( L 0.02 L 0, where subscript 0 refers L ): (i) from thermal expansion ( LTE ) and (ii) from increase in fraction of vacancies due to heating ( LV ). The vacancies are created by atoms migrating to the surface leading to an increase in volume of the material. The vacancies are incorporated in the crystal due to the entropic stabilization that it provides (which more than offsets the increase in enthalpy caused by broken bonds). V = L 3 dv = 3L 2 dl dv dl 3 V L or in terms of finite differences: 3 (1) V L V L The fraction required to be calculated is f L L v 3 n v exp exp( 11.54) N (= x) Continued
34 1 unit cell gives a volume of a 3 4 vacancies give a volume of a 3 n v vacancies give a volume of n v a 4 3 V v Vv 3Lv Equation (1) V L 0 0. Where V 0 is given by: V 0 Na o 4 3 n a xn a 3 3 v 0 V 4 4 v 3 3 V0 N0a N0a 4 4 3Lv = x L 0 Lv x x, L0 3 f x L0 3 L xl L 3(0.02) 0 4 this effect is about 1 in 10 4 as compared to thermal expansion due to atomic vibrations!
TOPIC 2. STRUCTURE OF MATERIALS III
 Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Universidad Carlos III de Madrid www.uc3m.es MATERIALS SCIENCE AND ENGINEERING TOPIC 2. STRUCTURE OF MATERIALS III Topic 2.3: Crystalline defects. Solid solutions. 1 PERFECT AND IMPERFECT CRYSTALS Perfect
Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities)
 Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities) 1 Structural Imperfections A perfect crystal has the lowest internal energy E Above absolute zero
Imperfections: Good or Bad? Structural imperfections (defects) Compositional imperfections (impurities) 1 Structural Imperfections A perfect crystal has the lowest internal energy E Above absolute zero
Crystal Defects. Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K)
 Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
Crystal Defects Perfect crystal - every atom of the same type in the correct equilibrium position (does not exist at T > 0 K) Real crystal - all crystals have some imperfections - defects, most atoms are
Lecture 3: Description crystal structures / Defects
 Lecture 3: Description crystal structures / Defects Coordination Close packed structures Cubic close packing Hexagonal close packing Metallic structures Ionic structures with interstitial sites Important
Lecture 3: Description crystal structures / Defects Coordination Close packed structures Cubic close packing Hexagonal close packing Metallic structures Ionic structures with interstitial sites Important
A great many properties of crystals are determined by imperfections.
 Defect in ceramics A great many properties of crystals are determined by imperfections. Electrical conductivity Diffusion transport imperfection Optical properties Rate of kinetic process Precipitation
Defect in ceramics A great many properties of crystals are determined by imperfections. Electrical conductivity Diffusion transport imperfection Optical properties Rate of kinetic process Precipitation
Imperfections, Defects and Diffusion
 Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Imperfections, Defects and Diffusion Lattice Defects Week5 Material Sciences and Engineering MatE271 1 Goals for the Unit I. Recognize various imperfections in crystals (Chapter 4) - Point imperfections
Defect in crystals. Primer in Materials Science Spring
 Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
Defect in crystals Primer in Materials Science Spring 2017 11.05.2017 1 Introduction The arrangement of the atoms in all materials contains imperfections which have profound effect on the behavior of the
From sand to silicon wafer
 From sand to silicon wafer 25% of Earth surface is silicon Metallurgical grade silicon (MGS) Electronic grade silicon (EGS) Polycrystalline silicon (polysilicon) Single crystal Czochralski drawing Single
From sand to silicon wafer 25% of Earth surface is silicon Metallurgical grade silicon (MGS) Electronic grade silicon (EGS) Polycrystalline silicon (polysilicon) Single crystal Czochralski drawing Single
Module 10. Crystal Defects in Metals I. Lecture 10. Crystal Defects in Metals I
 Module 10 Crystal Defects in Metals I Lecture 10 Crystal Defects in Metals I 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Introduction Keywords:
Module 10 Crystal Defects in Metals I Lecture 10 Crystal Defects in Metals I 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Introduction Keywords:
Order in materials. Making Solid Stuff. Primary Bonds Summary. How do they arrange themselves? Results from atomic bonding. What are they?
 Making Solid Stuff Primary Bonds Summary What are they? Results from atomic bonding So the atoms bond together! Order in materials No long range order to atoms Gases little or no interaction between components
Making Solid Stuff Primary Bonds Summary What are they? Results from atomic bonding So the atoms bond together! Order in materials No long range order to atoms Gases little or no interaction between components
Materials Science. Imperfections in Solids CHAPTER 5: IMPERFECTIONS IN SOLIDS. Types of Imperfections
 In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
In the Name of God Materials Science CHAPTER 5: IMPERFECTIONS IN SOLIDS ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects
Learning Objectives. Chapter Outline. Solidification of Metals. Solidification of Metals
 Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Unit-1 THE SOLID STATE QUESTIONS VSA QUESTIONS (1 - MARK QUESTIONS)
 Unit-1 THE SOLID STATE QUESTIONS VSA QUESTIONS (1 - MARK QUESTIONS) 1. What are anistropic substances. 2. Why are amorphous solids isotropic in nature?. Why glass is regarded as an amorphous solid? 4.
Unit-1 THE SOLID STATE QUESTIONS VSA QUESTIONS (1 - MARK QUESTIONS) 1. What are anistropic substances. 2. Why are amorphous solids isotropic in nature?. Why glass is regarded as an amorphous solid? 4.
Point Defects LATTICE VACANCIES 585. DIFFUSION 588 Metals 591. COLOR CENTERS 592 F centers 592 Other centers in alkali halides 593 PROBLEMS 595
 ch20.qxd 9/22/04 5:27 PM Page 583 20 Point Defects LATTICE VACANCIES 585 DIFFUSION 588 Metals 591 COLOR CENTERS 592 F centers 592 Other centers in alkali halides 593 PROBLEMS 595 1. Frenkel defects 595
ch20.qxd 9/22/04 5:27 PM Page 583 20 Point Defects LATTICE VACANCIES 585 DIFFUSION 588 Metals 591 COLOR CENTERS 592 F centers 592 Other centers in alkali halides 593 PROBLEMS 595 1. Frenkel defects 595
Mechanisms of Diffusion II. Ionic Crystals L5 11/3/03-1-
 Mechanisms of Diffusion II. Ionic Crystals 3.05 L5 11/3/03-1- Charges on point imperfections Point imperfections in ionic crystals are generally electrically charged. 3.05 L5 11/3/03 - (a) Unit cell in
Mechanisms of Diffusion II. Ionic Crystals 3.05 L5 11/3/03-1- Charges on point imperfections Point imperfections in ionic crystals are generally electrically charged. 3.05 L5 11/3/03 - (a) Unit cell in
Defects and Diffusion
 Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Defects and Diffusion Goals for the Unit Recognize various imperfections in crystals Point imperfections Impurities Line, surface and bulk imperfections Define various diffusion mechanisms Identify factors
Point Defects. Vacancies are the most important form. Vacancies Self-interstitials
 Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
Grain Boundaries 1 Point Defects 2 Point Defects A Point Defect is a crystalline defect associated with one or, at most, several atomic sites. These are defects at a single atom position. Vacancies Self-interstitials
Dept.of BME Materials Science Dr.Jenan S.Kashan 1st semester 2nd level. Imperfections in Solids
 Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
Why are defects important? Imperfections in Solids Defects have a profound impact on the various properties of materials: Production of advanced semiconductor devices require not only a rather perfect
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS ev /atom = exp. kt ( =
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
NPTEL COURSE ADVANCED CERAMICS FOR STRATEGIC APPLICATIONS QUESTIONS AND ANSWERS
 NPTEL COURSE ADVANCED CERAMICS FOR STRATEGIC APPLICATIONS QUESTIONS AND ANSWERS Q1: What do you understand by Ceramics? Ans: Ceramics are a group of chemical compounds, either simple (consisting of only
NPTEL COURSE ADVANCED CERAMICS FOR STRATEGIC APPLICATIONS QUESTIONS AND ANSWERS Q1: What do you understand by Ceramics? Ans: Ceramics are a group of chemical compounds, either simple (consisting of only
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 5.1 Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084 C (1357 K). Assume
Chapter 5. Imperfections in Solids
 Chapter 5 Imperfections in Solids Chapter 5 2D Defects and Introduction to Diffusion Imperfections in Solids Issues to Address... What types of defects arise in solids? Can the number and type of defects
Chapter 5 Imperfections in Solids Chapter 5 2D Defects and Introduction to Diffusion Imperfections in Solids Issues to Address... What types of defects arise in solids? Can the number and type of defects
Point Defects in Metals
 CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Point Defects in Metals 5.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327 C (600 K). Assume an energy
CHAPTER 5 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Point Defects in Metals 5.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327 C (600 K). Assume an energy
1. Use the Ellingham Diagram (reproduced here as Figure 0.1) to answer the following.
 315 Problems 1. Use the Ellingham Diagram (reproduced here as Figure 0.1) to answer the following. (a) Find the temperature and partial pressure of O 2 where Ni(s), Ni(l), and NiO(s) are in equilibrium.
315 Problems 1. Use the Ellingham Diagram (reproduced here as Figure 0.1) to answer the following. (a) Find the temperature and partial pressure of O 2 where Ni(s), Ni(l), and NiO(s) are in equilibrium.
(a) Would you expect the element P to be a donor or an acceptor defect in Si?
 MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
MSE 200A Survey of Materials Science Fall, 2008 Problem Set No. 2 Problem 1: At high temperature Fe has the fcc structure (called austenite or γ-iron). Would you expect to find C atoms in the octahedral
E45 Midterm 01 Fall 2007! By the 0.2% offset method (shown on plot), YS = 500 MPa
 1.!Mechanical Properties (20 points) Refer to the following stress-strain plot derived from a standard uniaxial tensile test of a high performance titanium alloy to answer the following questions. Show
1.!Mechanical Properties (20 points) Refer to the following stress-strain plot derived from a standard uniaxial tensile test of a high performance titanium alloy to answer the following questions. Show
CHAPTER 6 OUTLINE. DIFFUSION and IMPERFECTIONS IN SOLIDS
 CHAPTER 6 DIFFUSION and IMPERFECTIONS IN SOLIDS OUTLINE 1. TYPES OF DIFFUSIONS 1.1. Interdiffusion 1.2. Selfdiffusion 1.3.Diffusion mechanisms 1.4.Examples 2. TYPES OF IMPERFECTIONS 2.1.Point Defects 2.2.Line
CHAPTER 6 DIFFUSION and IMPERFECTIONS IN SOLIDS OUTLINE 1. TYPES OF DIFFUSIONS 1.1. Interdiffusion 1.2. Selfdiffusion 1.3.Diffusion mechanisms 1.4.Examples 2. TYPES OF IMPERFECTIONS 2.1.Point Defects 2.2.Line
Single vs Polycrystals
 WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
WEEK FIVE This week, we will Learn theoretical strength of single crystals Learn metallic crystal structures Learn critical resolved shear stress Slip by dislocation movement Single vs Polycrystals Polycrystals
Material Science. Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore India
 Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 3. Imperfections in Solids Learning objectives:
Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 3. Imperfections in Solids Learning objectives:
Chapter 4: Imperfections (Defects) in Solids
 Chapter 4: Imperfections (Defects) in Solids ISSUES TO ADDRESS... What types of defects exist in solids? How do defects affect material properties? Can the number and type of defects be varied and controlled?
Chapter 4: Imperfections (Defects) in Solids ISSUES TO ADDRESS... What types of defects exist in solids? How do defects affect material properties? Can the number and type of defects be varied and controlled?
Imperfections in the Atomic and Ionic Arrangements
 Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point
 Defects in solids http://www.bath.ac.uk/podcast/powerpoint/inaugural_lecture_250407.pdf http://www.materials.ac.uk/elearning/matter/crystallography/indexingdirectionsandplanes/indexing-of-hexagonal-systems.html
Defects in solids http://www.bath.ac.uk/podcast/powerpoint/inaugural_lecture_250407.pdf http://www.materials.ac.uk/elearning/matter/crystallography/indexingdirectionsandplanes/indexing-of-hexagonal-systems.html
Stacking Oranges. Packing atoms together Long Range Order. What controls the nearest number of atoms? Hard Sphere Model. Hard Sphere Model.
 { Stacking atoms together Crystal Structure Stacking Oranges Packing atoms together Long Range Order Crystalline materials... atoms pack in periodic, 3D arrays typical of: -metals -many ceramics -some
{ Stacking atoms together Crystal Structure Stacking Oranges Packing atoms together Long Range Order Crystalline materials... atoms pack in periodic, 3D arrays typical of: -metals -many ceramics -some
Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties
 Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties A perfect crystal, with every atom of the same type in the correct position, does not exist. There always exist crystalline defects,
Dr. Ali Abadi Chapter Three: Crystal Imperfection Materials Properties A perfect crystal, with every atom of the same type in the correct position, does not exist. There always exist crystalline defects,
Material Science. Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore India
 Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 5. Diffusion Learning objectives: - To know the
Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore 560012 India Chapter 5. Diffusion Learning objectives: - To know the
General Characteristics of Solid State
 General Characteristics of Solid State (i) They have definite mass, volume and shape. (ii) Intermolecular distances are short. (iii) Intermolecular forces are strong. (iv) Their constituent particles (atoms,
General Characteristics of Solid State (i) They have definite mass, volume and shape. (ii) Intermolecular distances are short. (iii) Intermolecular forces are strong. (iv) Their constituent particles (atoms,
Chapter 12: Structures & Properties of Ceramics
 Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Review of structures for ceramics How are impurities accommodated in the ceramic lattice? In what ways are ceramic phase diagrams similar
Chapter 12: Structures & Properties of Ceramics ISSUES TO ADDRESS... Review of structures for ceramics How are impurities accommodated in the ceramic lattice? In what ways are ceramic phase diagrams similar
IMPERFECTIONSFOR BENEFIT. Sub-topics. Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface
 IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
IMPERFECTIONSFOR BENEFIT Sub-topics 1 Point defects Linear defects dislocations Plastic deformation through dislocations motion Surface IDEAL STRENGTH Ideally, the strength of a material is the force necessary
CHAPTER 5: DIFFUSION IN SOLIDS
 CHAPTER 5: DIFFUSION IN SOLIDS ISSUES TO ADDRESS... How does diffusion occur? Why is it an important part of processing? How can the rate of diffusion be predicted for some simple cases? How does diffusion
CHAPTER 5: DIFFUSION IN SOLIDS ISSUES TO ADDRESS... How does diffusion occur? Why is it an important part of processing? How can the rate of diffusion be predicted for some simple cases? How does diffusion
Intrinsic Defects and Defect
 Chapter 4 Intrinsic Defects and Defect Concentrations in MgAl 2 O 4 Spinel 4.1 Introduction MgAl 2 O 4 spinel is an important industrial ceramic with a range of applications that take advantage of its
Chapter 4 Intrinsic Defects and Defect Concentrations in MgAl 2 O 4 Spinel 4.1 Introduction MgAl 2 O 4 spinel is an important industrial ceramic with a range of applications that take advantage of its
Chapter Outline Dislocations and Strengthening Mechanisms. Introduction
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
These metal centres interact through metallic bonding
 The structures of simple solids The majority of inorganic compounds exist as solids and comprise ordered arrays of atoms, ions, or molecules. Some of the simplest solids are the metals, the structures
The structures of simple solids The majority of inorganic compounds exist as solids and comprise ordered arrays of atoms, ions, or molecules. Some of the simplest solids are the metals, the structures
3. Solidification & Crystalline Imperfections
 3. Solidification & Crystalline Imperfections solidification (casting process) of metals divided into two steps (1) nucleation formation of stable nuclei in the melt (2) growth of nuclei into crystals
3. Solidification & Crystalline Imperfections solidification (casting process) of metals divided into two steps (1) nucleation formation of stable nuclei in the melt (2) growth of nuclei into crystals
Physics of Transition Metal Oxides
 Physics of Transition Metal Oxides Lecture 11 Defects in oxides Defects in oxides: We have looked at a variety of defects already. Today we discuss structural defects, giving rise to distinct phases impurity
Physics of Transition Metal Oxides Lecture 11 Defects in oxides Defects in oxides: We have looked at a variety of defects already. Today we discuss structural defects, giving rise to distinct phases impurity
Unit 1 The Solid State
 Points to Remember Amorphous and Crystalline Solids Unit 1 The Solid State Amorphous- short range order, Irregular shape eg-glass Crystalline Solids- long range order, regular shape eg : NaCl Molecular
Points to Remember Amorphous and Crystalline Solids Unit 1 The Solid State Amorphous- short range order, Irregular shape eg-glass Crystalline Solids- long range order, regular shape eg : NaCl Molecular
Point defects. process complicated. Now we shall discuss on defects, which will help to understand the atomic mechanism of diffusion.
 Point defects Diffusion of elements is possible because of the presence of defects. For example, substitutional diffusion occurs because of exchange of an atom with acancies. Further, impurities are present
Point defects Diffusion of elements is possible because of the presence of defects. For example, substitutional diffusion occurs because of exchange of an atom with acancies. Further, impurities are present
ME 254 MATERIALS ENGINEERING 1 st Semester 1431/ rd Mid-Term Exam (1 hr)
 1 st Semester 1431/1432 3 rd Mid-Term Exam (1 hr) Question 1 a) Answer the following: 1. Do all metals have the same slip system? Why or why not? 2. For each of edge, screw and mixed dislocations, cite
1 st Semester 1431/1432 3 rd Mid-Term Exam (1 hr) Question 1 a) Answer the following: 1. Do all metals have the same slip system? Why or why not? 2. For each of edge, screw and mixed dislocations, cite
Diffusion & Crystal Structure
 Lecture 5 Diffusion & Crystal Structure Diffusion of an interstitial impurity atom in a crystal from one void to a neighboring void. The impurity atom at position A must posses an energy E to push the
Lecture 5 Diffusion & Crystal Structure Diffusion of an interstitial impurity atom in a crystal from one void to a neighboring void. The impurity atom at position A must posses an energy E to push the
Introduction to Engineering Materials ENGR2000 Chapter 4: Imperfections in Solids. Dr. Coates
 Introduction to Engineering Materials ENGR000 Chapter 4: Imperfections in Solids Dr. Coates Learning Objectives 1. Describe both vacancy and self interstitial defects. Calculate the equilibrium number
Introduction to Engineering Materials ENGR000 Chapter 4: Imperfections in Solids Dr. Coates Learning Objectives 1. Describe both vacancy and self interstitial defects. Calculate the equilibrium number
Ph.D. Admission 20XX-XX Semester X
 Ph.D. Admission 20XX-XX Semester X Written Examination Materials Science & Engineering Department, IIT Kanpur Date of Examination: XX XXXX 20XX Timing: XX:XX XX:XX XX Application# Please read these instructions
Ph.D. Admission 20XX-XX Semester X Written Examination Materials Science & Engineering Department, IIT Kanpur Date of Examination: XX XXXX 20XX Timing: XX:XX XX:XX XX Application# Please read these instructions
Point coordinates. x z
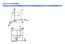 Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
Point coordinates c z 111 a 000 b y x z 2c b y Point coordinates z y Algorithm 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a,
Introduction to Point Defects. Version 2.1. Andrew Green, MATTER John Humphreys, UMIST/University of Manchester Ross Mackenzie, Open University
 Introduction to Point Defects Version 2.1 Andrew Green, MATTER John Humphreys, UMIST/University of Manchester Ross Mackenzie, Open University October 1997 Assumed Pre-knowledge This module has been developed
Introduction to Point Defects Version 2.1 Andrew Green, MATTER John Humphreys, UMIST/University of Manchester Ross Mackenzie, Open University October 1997 Assumed Pre-knowledge This module has been developed
HOMEWORK 6. solutions
 HOMEWORK 6. SCI 1410: materials science & solid state chemistry solutions Textbook Problems: Imperfections in Solids 1. Askeland 4-67. Why is most gold or siler jewelry made out of gold or siler alloyed
HOMEWORK 6. SCI 1410: materials science & solid state chemistry solutions Textbook Problems: Imperfections in Solids 1. Askeland 4-67. Why is most gold or siler jewelry made out of gold or siler alloyed
CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS
 MODULE ONE CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS CRYSTAL STRUCTURE Metallic crystal structures; BCC, FCC and HCP Coordination number and Atomic Packing Factor (APF) Crystal imperfections:
MODULE ONE CRYSTAL STRUCTURE, MECHANICAL BEHAVIOUR & FAILURE OF MATERIALS CRYSTAL STRUCTURE Metallic crystal structures; BCC, FCC and HCP Coordination number and Atomic Packing Factor (APF) Crystal imperfections:
EE CRYSTAL GROWTH, WAFER FABRICATION AND BASIC PROPERTIES OF Si WAFERS- Chapter 3. Crystal Structure z a
 1 EE 1 FALL 1999-00 CRYSTAL GROWTH, WAFER FABRICATION AND BASIC PROPERTIES OF Si WAFERS- Chapter 3 z a B Crystal Structure z a z a C y y y A x x Cubic BCC FCC x Crystals are characterized by a unit cell
1 EE 1 FALL 1999-00 CRYSTAL GROWTH, WAFER FABRICATION AND BASIC PROPERTIES OF Si WAFERS- Chapter 3 z a B Crystal Structure z a z a C y y y A x x Cubic BCC FCC x Crystals are characterized by a unit cell
STRUCTURE OF MATERIALS The Key to its Properties A Multiscale Perspective
 STRUCTURE OF MATERIALS The Key to its Properties A Multiscale Perspective Anandh Subramaniam Materials and Metallurgical Engineering INDIAN INSTITUTE OF TECHNOLOGY KANPUR Kanpur- 208016 Email: anandh@iitk.ac.in
STRUCTURE OF MATERIALS The Key to its Properties A Multiscale Perspective Anandh Subramaniam Materials and Metallurgical Engineering INDIAN INSTITUTE OF TECHNOLOGY KANPUR Kanpur- 208016 Email: anandh@iitk.ac.in
Point coordinates. Point coordinates for unit cell center are. Point coordinates for unit cell corner are 111
 Point coordinates c z 111 Point coordinates for unit cell center are a/2, b/2, c/2 ½ ½ ½ Point coordinates for unit cell corner are 111 x a z 000 b 2c y Translation: integer multiple of lattice constants
Point coordinates c z 111 Point coordinates for unit cell center are a/2, b/2, c/2 ½ ½ ½ Point coordinates for unit cell corner are 111 x a z 000 b 2c y Translation: integer multiple of lattice constants
ISSUES TO ADDRESS...
 Chapter 5: IMPERFECTIONS IN SOLIDS School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 4-1 ISSUES TO ADDRESS... What are the solidification mechanisms? What types
Chapter 5: IMPERFECTIONS IN SOLIDS School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 4-1 ISSUES TO ADDRESS... What are the solidification mechanisms? What types
Definition and description of different diffusion terms
 Definition and description of different diffusion terms efore proceeding further, it is necessary to introduce different terms frequently used in diffusion studies. Many terms will be introduced, which
Definition and description of different diffusion terms efore proceeding further, it is necessary to introduce different terms frequently used in diffusion studies. Many terms will be introduced, which
Chapter 7 Dislocations and Strengthening Mechanisms. Dr. Feras Fraige
 Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
Chapter 7 Dislocations and Strengthening Mechanisms Dr. Feras Fraige Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and
Chapter 2. Ans: e (<100nm size materials are called nanomaterials)
 Chapter 2 1. Materials science and engineering include (s) the study of: (a) metals (b) polymers (c) ceramics (d) composites (e) nanomaterials (f) all of the above Ans: f 2. Which one of the following
Chapter 2 1. Materials science and engineering include (s) the study of: (a) metals (b) polymers (c) ceramics (d) composites (e) nanomaterials (f) all of the above Ans: f 2. Which one of the following
Imperfections in atomic arrangements
 MME131: Lecture 9 Imperfections in atomic arrangements Part 2: 1D 3D Defects A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Classifications and characteristics of 1D 3D defects
MME131: Lecture 9 Imperfections in atomic arrangements Part 2: 1D 3D Defects A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Classifications and characteristics of 1D 3D defects
Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number:
 Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number: Instructions: Answer all questions and show your work. You will not receive partial credit unless you show your work.
Engineering 45: Properties of Materials Final Exam May 9, 2012 Name: Student ID number: Instructions: Answer all questions and show your work. You will not receive partial credit unless you show your work.
atoms = 1.66 x g/amu
 CHAPTER 2 Q1- How many grams are there in a one amu of a material? A1- In order to determine the number of grams in one amu of material, appropriate manipulation of the amu/atom, g/mol, and atom/mol relationships
CHAPTER 2 Q1- How many grams are there in a one amu of a material? A1- In order to determine the number of grams in one amu of material, appropriate manipulation of the amu/atom, g/mol, and atom/mol relationships
Chapter 12 Metals. crystalline, in which particles are in highly ordered arrangement. (Have MP.)
 Chapter 12 Metals 12.1 Classification of Solids Covalent Ionic Molecular Metallic Solids Solids Solids Solids Molecular consist of molecules held next to each other by IMF s. Relatively low to moderate
Chapter 12 Metals 12.1 Classification of Solids Covalent Ionic Molecular Metallic Solids Solids Solids Solids Molecular consist of molecules held next to each other by IMF s. Relatively low to moderate
10/7/ :43 AM. Chapter 5. Diffusion. Dr. Mohammad Abuhaiba, PE
 10/7/2013 10:43 AM Chapter 5 Diffusion 1 2 Why Study Diffusion? Materials of all types are often heat-treated to improve their properties. a heat treatment almost always involve atomic diffusion. Often
10/7/2013 10:43 AM Chapter 5 Diffusion 1 2 Why Study Diffusion? Materials of all types are often heat-treated to improve their properties. a heat treatment almost always involve atomic diffusion. Often
Lecture # 11 References:
 Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC Dr.Haydar Al-Ethari
Lecture # 11 - Line defects (1-D) / Dislocations - Planer defects (2D) - Volume Defects - Burgers vector - Slip - Slip Systems in FCC crystals - Slip systems in HCP - Slip systems in BCC Dr.Haydar Al-Ethari
Module-6. Dislocations and Strengthening Mechanisms
 Module-6 Dislocations and Strengthening Mechanisms Contents 1) Dislocations & Plastic deformation and Mechanisms of plastic deformation in metals 2) Strengthening mechanisms in metals 3) Recovery, Recrystallization
Module-6 Dislocations and Strengthening Mechanisms Contents 1) Dislocations & Plastic deformation and Mechanisms of plastic deformation in metals 2) Strengthening mechanisms in metals 3) Recovery, Recrystallization
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 6 THE IMPERFECT SOLID STATE
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
N = N A ρ Pb A Pb. = ln N Q v kt. 지난문제. Below are shown three different crystallographic planes for a unit cell of some hypothetical metal.
 지난문제. Below are shown three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms: (a) To what crystal system does the unit cell belong? (b) What would
지난문제. Below are shown three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms: (a) To what crystal system does the unit cell belong? (b) What would
CHAPTER 4 DIFFUSIVITY AND MECHANISM
 68 CHAPTER 4 DIFFUSIVITY AND MECHANISM 4.1 INTRODUCTION The various elements present in the alloys taken for DB joining diffuse in varying amounts. The diffusivity of elements into an alloy depends on
68 CHAPTER 4 DIFFUSIVITY AND MECHANISM 4.1 INTRODUCTION The various elements present in the alloys taken for DB joining diffuse in varying amounts. The diffusivity of elements into an alloy depends on
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 6 THE IMPERFECT SOLID STATE
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
3.091 Introduction to Solid State Chemistry Lecture Notes No. 6 THE IMPERFECT SOLID STATE 1. INTRODUCTION Real crystals are never perfect: they always contain a considerable density of defects and imperfections
INGE Engineering Materials. Chapter 3 (cont.)
 Some techniques used: Chapter 3 (cont.) This section will address the question how do we determine the crystal structure of a solid sample? Electron microscopy (by direct and indirect observations) Scanning
Some techniques used: Chapter 3 (cont.) This section will address the question how do we determine the crystal structure of a solid sample? Electron microscopy (by direct and indirect observations) Scanning
12/10/09. Chapter 4: Imperfections in Solids. Imperfections in Solids. Polycrystalline Materials ISSUES TO ADDRESS...
 Chapter 4: ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects be varied and controlled? How do defects affect material
Chapter 4: ISSUES TO ADDRESS... What are the solidification mechanisms? What types of defects arise in solids? Can the number and type of defects be varied and controlled? How do defects affect material
Imperfections in Solids. Imperfections in Solids. Point Defects. Types of Imperfections
 Imperfections in Solids In this topic we will try to answer the following questions: What types of defects arise in solids? Are these defects undesirable? How do defects affect material properties? Can
Imperfections in Solids In this topic we will try to answer the following questions: What types of defects arise in solids? Are these defects undesirable? How do defects affect material properties? Can
MSE 351 Engineering Ceramics I
 Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 351 Engineering Ceramics I Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
Kwame Nkrumah University of Science & Technology, Kumasi, Ghana MSE 351 Engineering Ceramics I Ing. Anthony Andrews (PhD) Department of Materials Engineering Faculty of Mechanical and Chemical Engineering
Physics of Materials: Defects in Solids. Dr. Anurag Srivastava. Indian Institute of Information Technology and Manegement, Gwalior
 : Defects in Solids Dr. Anurag Srivastava Atal Bihari Vajpayee Indian Institute of Information Technology and Manegement, Gwalior What you have learnt so far? Properties of Materials affected by defects
: Defects in Solids Dr. Anurag Srivastava Atal Bihari Vajpayee Indian Institute of Information Technology and Manegement, Gwalior What you have learnt so far? Properties of Materials affected by defects
Student Name: ID Number:
 Student Name: ID Number: DEPARTMENT OF MECHANICAL ENGINEERING CONCORDIA UNIVERSITY MATERIALS SCIENCE - MECH 1/ - Sections T & X MIDTERM 003 Instructors: Dr. M.Pugh & Dr. M.Medraj Time Allowed: one (1)
Student Name: ID Number: DEPARTMENT OF MECHANICAL ENGINEERING CONCORDIA UNIVERSITY MATERIALS SCIENCE - MECH 1/ - Sections T & X MIDTERM 003 Instructors: Dr. M.Pugh & Dr. M.Medraj Time Allowed: one (1)
Bulk Diffusion in Alumina: Solving the Corundum Conundrum
 Bulk Diffusion in Alumina: Solving the Corundum Conundrum Nicholas D.M. Hine 1,2,3 K. Frensch 3, W.M.C Foulkes 1,2, M.W. Finnis 2,3, A. H. Heuer 3,4 1 Theory of Condensed Matter Group, Cavendish Laboratory,
Bulk Diffusion in Alumina: Solving the Corundum Conundrum Nicholas D.M. Hine 1,2,3 K. Frensch 3, W.M.C Foulkes 1,2, M.W. Finnis 2,3, A. H. Heuer 3,4 1 Theory of Condensed Matter Group, Cavendish Laboratory,
Engineering 45 The Structure and Properties of Materials Midterm Examination October 26, 1987
 Engineering 45 The Structure and Properties of Materials Midterm Examination October 26, 1987 Problem 1: (a) The compound CsCl is an ordered arrangement of Cs and Cl over the sites of a BCC lattice. Draw
Engineering 45 The Structure and Properties of Materials Midterm Examination October 26, 1987 Problem 1: (a) The compound CsCl is an ordered arrangement of Cs and Cl over the sites of a BCC lattice. Draw
The story so far: Isolated defects
 The story so far: Infinite, periodic structures have Bloch wave single-particle states, labeled by a wavenumber k. Translational symmetry of the lattice + periodic boundary conditions give discrete allowed
The story so far: Infinite, periodic structures have Bloch wave single-particle states, labeled by a wavenumber k. Translational symmetry of the lattice + periodic boundary conditions give discrete allowed
Chapter 2 Structure of Materials
 Chapter 2 Structure of Materials In this chapter, we will briefly go through the hierarchical structure of materials. First, the atomic bonding and crystal structures are covered briefly. Then, the emphasis
Chapter 2 Structure of Materials In this chapter, we will briefly go through the hierarchical structure of materials. First, the atomic bonding and crystal structures are covered briefly. Then, the emphasis
C h a p t e r 4 : D e f e c t s i n C r y s t a l s
 C h a p t e r 4 : D e f e c t s i n C r y s t a l s...perfection's a gift of The gods, few can boast they possess it - and most Of you, my dears, don't. - Ovid, The Art of Love Chapter 4: Defects in Crystals...
C h a p t e r 4 : D e f e c t s i n C r y s t a l s...perfection's a gift of The gods, few can boast they possess it - and most Of you, my dears, don't. - Ovid, The Art of Love Chapter 4: Defects in Crystals...
Continuous Cooling Diagrams
 Continuous Cooling Diagrams Isothermal transformation (TTT) diagrams are obtained by rapidly quenching to a given temperature and then measuring the volume fraction of the various constituents that form
Continuous Cooling Diagrams Isothermal transformation (TTT) diagrams are obtained by rapidly quenching to a given temperature and then measuring the volume fraction of the various constituents that form
Chapter 8 Strain Hardening and Annealing
 Chapter 8 Strain Hardening and Annealing This is a further application of our knowledge of plastic deformation and is an introduction to heat treatment. Part of this lecture is covered by Chapter 4 of
Chapter 8 Strain Hardening and Annealing This is a further application of our knowledge of plastic deformation and is an introduction to heat treatment. Part of this lecture is covered by Chapter 4 of
CHAPTER THE SOLID STATE
 133 CHAPTER THE SOLID STATE 1. The ability of a substances to assume two or more crystalline structures is called [1990] Isomerism Polymorphism Isomorphism Amorphism 2. Most crystals show good cleavage
133 CHAPTER THE SOLID STATE 1. The ability of a substances to assume two or more crystalline structures is called [1990] Isomerism Polymorphism Isomorphism Amorphism 2. Most crystals show good cleavage
10/8/2016 8:29 PM. Chapter 5. Diffusion. Mohammad Suliman Abuhaiba, Ph.D., PE
 Chapter 5 Diffusion 1 2 Home Work Assignments 10, 13, 17, 21, 27, 31, D1 Due Tuesday 18/10/2016 3 rd Exam on Sunday 23/10/2016 3 Why Study Diffusion? Materials of all types are often heattreated to improve
Chapter 5 Diffusion 1 2 Home Work Assignments 10, 13, 17, 21, 27, 31, D1 Due Tuesday 18/10/2016 3 rd Exam on Sunday 23/10/2016 3 Why Study Diffusion? Materials of all types are often heattreated to improve
Question Grade Maximum Grade Total 100
 The Islamic University of Gaza Industrial Engineering Department Engineering Materials, EIND 3301 Final Exam Instructor: Dr. Mohammad Abuhaiba, P.E. Exam date: 31/12/2013 Final Exam (Open Book) Fall 2013
The Islamic University of Gaza Industrial Engineering Department Engineering Materials, EIND 3301 Final Exam Instructor: Dr. Mohammad Abuhaiba, P.E. Exam date: 31/12/2013 Final Exam (Open Book) Fall 2013
ASTM Conference, May , Hilton Head Island, SC
 ASTM Conference, May 17 2016, Hilton Head Island, SC Understanding Irradiation Growth through Atomistic Simulations: Defect Diffusion and Clustering in Alpha-Zirconium and the Influence of Alloying Elements
ASTM Conference, May 17 2016, Hilton Head Island, SC Understanding Irradiation Growth through Atomistic Simulations: Defect Diffusion and Clustering in Alpha-Zirconium and the Influence of Alloying Elements
Introduction to Materials Science
 EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
EPMA Powder Metallurgy Summer School 27 June 1 July 2016 Valencia, Spain Introduction to Materials Science Prof. Alberto Molinari University of Trento, Italy Some of the figures used in this presentation
Atomic Transport & Phase Transformations. PD Dr. Nikolay Zotov
 Atomic Transport & Phase Transformations PD Dr. Nikolay Zotov Atomic Transport & Phase Transformations Part II Lecture Diffusion Short Description 1 Introduction; Non-equilibrium thermodynamics 2 Diffusion,
Atomic Transport & Phase Transformations PD Dr. Nikolay Zotov Atomic Transport & Phase Transformations Part II Lecture Diffusion Short Description 1 Introduction; Non-equilibrium thermodynamics 2 Diffusion,
Impurities in Solids. Crystal Electro- Element R% Structure negativity Valence
 4-4 Impurities in Solids 4.4 In this problem we are asked to cite which of the elements listed form with Ni the three possible solid solution types. For complete substitutional solubility the following
4-4 Impurities in Solids 4.4 In this problem we are asked to cite which of the elements listed form with Ni the three possible solid solution types. For complete substitutional solubility the following
FINAL EXAM KEY. Professor Buhro. ID Number:
 FINAL EXAM Chemistry 465 KEY 10 May 011 Professor Buhro KEY Signature KEY Print Name Clearly ID Number: Information. This is a closed-book exam; no books, notes, other students, other student exams, or
FINAL EXAM Chemistry 465 KEY 10 May 011 Professor Buhro KEY Signature KEY Print Name Clearly ID Number: Information. This is a closed-book exam; no books, notes, other students, other student exams, or
CRYSTAL GROWTH, WAFER FABRICATION AND BASIC PROPERTIES OF Si WAFERS- Chapter 3. Crystal Structure z a
 CRYSTAL GROWTH, WAFER FABRICATION AND BASIC PROPERTIES OF Si WAFERS- Chapter 3 Crystal Growth, Si Wafers- Chapter 3 z a C y B z a y Crystal Structure z a y Crystals are characterized by a unit cell which
CRYSTAL GROWTH, WAFER FABRICATION AND BASIC PROPERTIES OF Si WAFERS- Chapter 3 Crystal Growth, Si Wafers- Chapter 3 z a C y B z a y Crystal Structure z a y Crystals are characterized by a unit cell which
3. Anisotropic blurring by dislocations
 Dynamical Simulation of EBSD Patterns of Imperfect Crystals 1 G. Nolze 1, A. Winkelmann 2 1 Federal Institute for Materials Research and Testing (BAM), Berlin, Germany 2 Max-Planck- Institute of Microstructure
Dynamical Simulation of EBSD Patterns of Imperfect Crystals 1 G. Nolze 1, A. Winkelmann 2 1 Federal Institute for Materials Research and Testing (BAM), Berlin, Germany 2 Max-Planck- Institute of Microstructure
Diffusion phenomenon
 Module-5 Diffusion Contents 1) Diffusion mechanisms and steady-state & non-steady-state diffusion 2) Factors that influence diffusion and nonequilibrium transformation & microstructure Diffusion phenomenon
Module-5 Diffusion Contents 1) Diffusion mechanisms and steady-state & non-steady-state diffusion 2) Factors that influence diffusion and nonequilibrium transformation & microstructure Diffusion phenomenon
Materials and their structures
 Materials and their structures 2.1 Introduction: The ability of materials to undergo forming by different techniques is dependent on their structure and properties. Behavior of materials depends on their
Materials and their structures 2.1 Introduction: The ability of materials to undergo forming by different techniques is dependent on their structure and properties. Behavior of materials depends on their
ASTM Conference, Feb , Hyderabad, India
 ASTM Conference, Feb 6 2013, Hyderabad, India Effect of Hydrogen on Dimensional Changes of Zirconium and the Influence of Alloying Elements: First-principles and Classical Simulations of Point Defects,
ASTM Conference, Feb 6 2013, Hyderabad, India Effect of Hydrogen on Dimensional Changes of Zirconium and the Influence of Alloying Elements: First-principles and Classical Simulations of Point Defects,
Physical Metallurgy Friday, January 28, 2011; 8:30 12:00 h
 Physical Metallurgy Friday, January 28, 2011; 8:30 12:00 h Always motivate your answers All sub-questions have equal weight in the assessment Question 1 Precipitation-hardening aluminium alloys are, after
Physical Metallurgy Friday, January 28, 2011; 8:30 12:00 h Always motivate your answers All sub-questions have equal weight in the assessment Question 1 Precipitation-hardening aluminium alloys are, after
